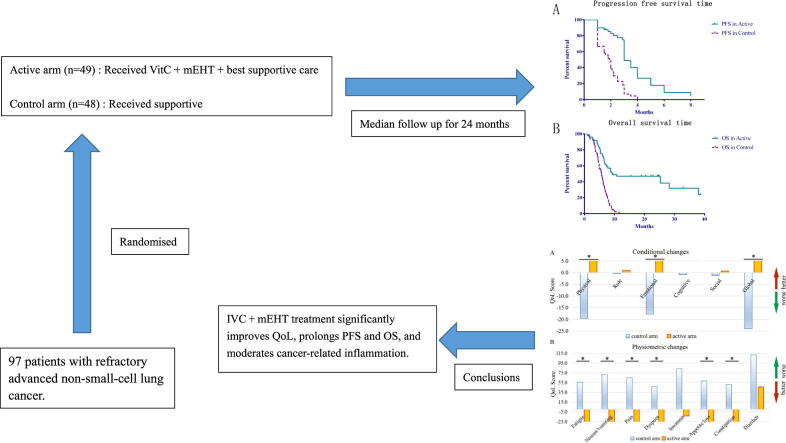
Graphical abstract
Keywords: Vitamin C, Modulated electrohyperthermia, Non-small-cell lung cancer, Overall survival, Quality of life, Remission rate
Abbreviations: IVC, intravenous vitamin C; HT, hyperthermia; mEHT, modulated electrohyperthermia; NSCLC, non-small-cell lung cancer; PFS, progression-free survival; OS, overall survival; QoL, quality of life; TKIs, tyrosine kinase inhibitors; BSC, best supportive care; AUC, area under the curve; PR, partial response; SD, stable disease; PD, progressive disease; ECOG, Eastern Cooperative Oncology Group; RECIST, Response Evaluation Criteria in Solid Tumors; G6PD, glucose 6-phosphate dehydrogenase; DCR, disease control rate; CT, computed tomography; CR, complete response; QLQ-C30, Quality of Life Questionnaire; CI, confidence interval; EGFR, epidermal growth factor receptor; CEA, carcinoembryonic antigen; SCC, squamous cell carcinoma antigen; CA15-3, carbohydrate antigen 15-3; CYFRA21-1, cytokeratin-19 fragments; IL-6, interleukin- 6; CRP, C-reactive protein; TNF-α, Tumor Necrosis Factor-α
Abstract
Our previous study indicated that intravenous vitamin C (IVC) treatment concurrent with modulated electrohyperthermia (mEHT) was safe and improved the quality of life (QoL) of non-small-cell lung cancer (NSCLC) patients. The aim of this trial was to further verify the efficacy of the above combination therapy in previously treated patients with refractory advanced (stage IIIb or IV) NSCLC. A total of 97 patients were randomized to receive IVC and mEHT plus best supportive care (BSC) (n = 49 in the active arm, receiving 1 g/kg * d IVC concurrently with mEHT, three times a week for 25 treatments in total) or BSC alone (n = 48 in the control arm). After a median follow-up of 24 months, progression-free survival (PFS) and overall survival (OS) were significantly prolonged by combination therapy compared to BSC alone (PFS: 3 months vs 1.85 months, P < 0.05; OS: 9.4 months vs 5.6 months, P < 0.05). QoL was significantly increased in the active arm despite the advanced stage of disease. The 3-month disease control rate after treatment was 42.9% in the active arm and 16.7% in the control arm (P < 0.05). Overall, IVC and mEHT may have the ability to improve the prognosis of patients with advanced NSCLC.
Introduction
Lung cancer is the most common cancer type and the leading cause of cancer mortality in China [1], accounting for 19.6% of all newly diagnosed cancer cases [2]. Nearly 85% of lung cancers are non-small-cell lung cancer (NSCLC), which has a 5-year survival rate of 17.1%. The majority of patients diagnosed with NSCLC are found to be at an advanced stage. The overall survival (OS) of patients who fail to respond to conventional anticancer therapies (chemotherapy, radiotherapy, targeted therapy, immunotherapy, etc.) remains unsatisfactory.
The application of vitamin C for malignant diseases has had a renaissance [3]. Studies [4], [5] have found that high-dose intravenous pharmacological administration of vitamin C produces plasma concentrations 100–1000 times higher than those of healthy nutritional levels and up to 100-fold higher than the maximally tolerated oral intake [6]. Phase I clinical trials show its safety, high tolerability and relief from the side effects of chemotherapy [7], [8]. Clinical trials indicated the potential efficacy of intravenous vitamin C (IVC), with improved performance status or prolonged disease progression/overall time in ovarian [9] and pancreatic cancers [10]. Its synergy with chemotherapy improves quality of life (QoL) [10].
High-dose vitamin C is also applied for lung cancer. It decreases cell proliferation in lung cancer cell lines [11], including mechanisms of cell cycle arrest [12] and apoptosis [13]. Clinical studies [9] suggested that a large dose of IVC can increase the efficacy or reduce the toxic side effects of chemotherapy when used in synergy with chemotherapy. Recently, Schoenfeld [14] presented a phase II study of advanced-stage NSCLC patients (n = 14) treated with IV carboplatin (area under the curve (AUC), 6; 4 cycles), IV paclitaxel (200 mg/m2, 4 cycles), and IVC (75 g twice a week, four cycles). No grade 3 or 4 toxicities related to vitamin C were reported. Four out of the 14 patients showed a partial response (PR), 9 out of the 14 patients showed stable disease (SD), and one showed progressive disease (PD), which indicated the potential efficacy of IVC in NSCLC therapy.
Hyperthermia (HT) is a method of treating tumors at the lesion site, which is mainly divided into local, regional, and whole-body HT. It is a complementary cancer treatment, often used in association with chemotherapy or radiotherapy, increasing the efficacy and prolonging the survival time [15], [16]. Takayuki et al [17] suggested that HT and radiotherapy exerted a synergistic effect in the treatment of NSCLC. Modulated electro-hyperthermia (mEHT) is a regional electromagnetic HT method. The major advantage of mEHT is the nano-range energy liberation, rather than overall heating of the target [18]. Due to its high efficacy [18] and the synergy of the electric field [19], the targeted cancer cells absorb the heat that raises the temperature 3 °C higher than the enviorment [20]. Studies have found that the antitumor mechanism of mEHT is as follows: inducing cell apoptosis, improving tumor perfusion, inhibiting tumor angiogenesis and resolving tumor hypoxia [18], [20], [21], [22], [23]. Clinical data show that mEHT has long been used in clinical practice for various malignant diseases, and has clinical results for NSCLC [24], [25], [26]. mEHT can be used alone or in combination with radiotherapy (RT), chemotherapy, and chemoradiotherapy, and a growing number of studies are exploring combinations of mEHT and other therapies [27], [28], [29]. In a retrospective study, 93 patients with advanced NSCLC (stage IIIB-IV) were divided into HT combined with chemotherapy and chemotherapy groups, and the results indicated that HT combined with chemotherapy might lead to the development of a better therapeutic strategy for advanced NSCLC patients with malignant pleural effusion and greatly reduce the toxic effects of chemotherapy on the incidence of weakness and gastrointestinal adverse reactions in advanced NSCLC patients [30]. A multi-institutional prospective randomized trial observed that RT + HT improved local PFS in the treatment of locally advanced NSCLC [31].
In our previous phase I clinical study [32], we found that IVC with simultaneous mEHT is safe and well tolerated, and concomitant application significantly increases the plasma vitamin C level. The average scores for the functioning scale increased continuously, and the average values for symptoms decreased gradually, which indicates that QoL is improved when patients receive the above treatments.
Therefore, we conducted a randomized phase II trial to evaluate the effect of best supportive care (BSC) with or without IVC combined with simultaneous mEHT on tumor response, progression-free survival (PFS) and OS in previously treated patients with refractory advanced (stage IIIb or IV) NSCLC. Herein, we present the results of this trial.
Materials and methods
Patient recruitment
Eligible patients were adults (≥18 years ≤70 years) who had an Eastern Cooperative Oncology Group (ECOG) performance status of 0–2; who had a histologically proven diagnosis of primary NSCLC, stage IIIb or IV; who were not curable with surgery or showed radiographically confirmed PD during previous radiotherapy and/or four to six cycles of platinum-based chemotherapy (mostly cisplatin/carboplatin in combination with vinblastine, etoposide, or paclitaxel); who had failed to respond to targeted therapy or immunotherapy or were intolerant of their latest anticancer therapy regimen; and who showed at least one measurable disease according to the Response Evaluation Criteria in Solid Tumors (RECIST) (Table 1).
Table 1.
Patient baseline characteristics.
| Characteristics | Active arm (n = 49) | Control arm (n = 48) |
|---|---|---|
| Age (years) | ||
| Median | 62 | 63 |
| Range | 42–72 | 43–72 |
| Sex | ||
| Male | 38 | 37 |
| Female | 11 | 11 |
| ECOG performance status | ||
| Grade 0 | 25 | 26 |
| Grade 1 | 12 | 11 |
| Grade 2 | 12 | 11 |
| Stage at study entry | ||
| Stage IIIB | 25 | 25 |
| Stage IV | 24 | 23 |
| Pathology | ||
| Squamous cell carcinoma | 24 | 25 |
| Adenocarcinoma | 23 | 23 |
| EGFR in Adenocarcinoma | 2 | 0 |
| EGFR in Adenocarcinoma | ||
| EGFR(−) | 13 | 6 |
| EGFR(+) | 10 | 17 |
| Smoking status | ||
| Current | 3 | 4 |
| Prior | 36 | 33 |
| Never | 10 | 11 |
| Unknown | 0 | 0 |
| Reason for failure of last anticancer therapy | ||
| Refractory | 45 | 43 |
| Intolerant | 4 | 5 |
ECOG: Eastern Cooperative Oncology Group.
Patients were excluded if they showed G6PD deficiency or a history of oxalosis by urinalysis; were receiving anticancer therapies; were diagnosed with a comorbid condition that would affect survival, such as end-stage congestive heart failure, unstable angina or myocardial infarction within 6 weeks prior to the study; or had metallic implants or replacements in the treatment area or implanted electronic devices anywhere in the body.
All patients provided written informed consent. The study was approved by the Ethics Committee of the Clifford Hospital affiliated with Jinan University. All patients provided written informed consent according to Good Clinical Practice (GCP) and national regulations [No: 2/2015-10].
Study design and treatment
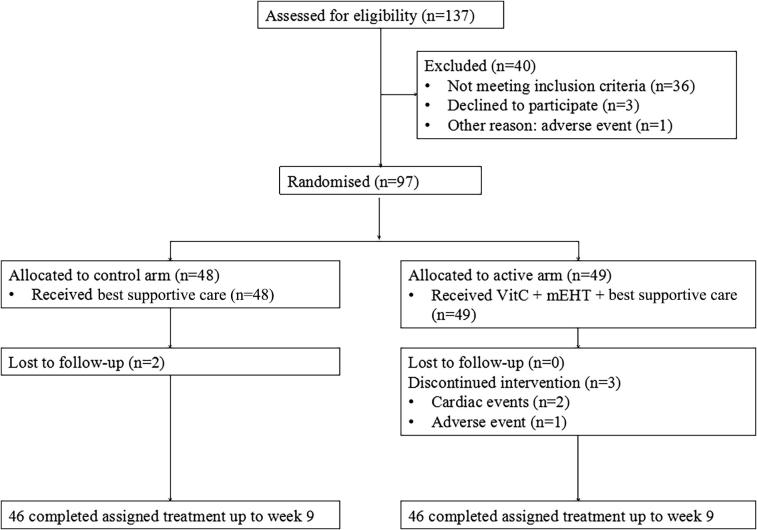
The study was a single-center, Phase II, randomized clinical trial. Trial Registration: ClinicalTrials.gov, NCT02655913; registration date, 7th Jan 2016. The date of enrollment of the first and last participants in the trial was 17th Jan 2016 and 17th July 2017, respectively, and all participants were recruited by the Clifford Hospital affiliated with Jinan University.
Eligible patients were randomized to receive IVC + mEHT + BSC (active arm) or BSC alone (control arm) (Fig. 1). BSC included multidisciplinary care, BSC documentation, symptom assessment and symptom management [32]. In the active arm, patients received IVC 1 g/kg·d three times a week for 25 treatments in total. Each milliliter of vitamin C injection contained 3 g of sodium ascorbate and water for injection, with the pH adjusted to 6.5–8.0 with sodium bicarbonate. Vitamin C was infused for 120 min. We used the mEHT method for HT treatment with the EHY2000+ device. This impedance-coupled device works with an amplitude-modulated 13.56 MHz carrier frequency, and its principles and practice are described in our previous study [32]. The treatment regimen of mEHT was 60 min/session; the power of mEHT was gradually increased from 135 W to 150 W depending on the patient’s actual tolerance. The applicator used was 7.1 dm2. The applied energy range in one session was between 486 kJ and 540 kJ. The patients were placed lying in the prone position, and the treatment covered the complete lung (30 cm diameter circle). The temperature of the treatment area was in the range of 40–42 °C, calculated indirectly by the treatment device. BSC focuses on helping patients obtain relief from symptoms such as nausea, pain, fatigue or shortness of breath.
Fig. 1.
Study design and patient disposition: Eligible patients were randomized to receive IVC + mEHT + best supportive care (active arm) or best supportive care alone (control arm).
The primary endpoint of this study was OS assessed by an independent investigator. Secondary endpoints included PFS, the 3-month disease control rate (DCR) that was defined as the proportion of patients with a complete response (CR) or PR or SD, QoL, and the association between biomarkers and treatment outcome.
Randomization and masking
We used a computer-generated random sequence to allocate patients (nonmasked) to BSC (control arm) or IVC + mEHT + BSC (active arm). The minimization method was used for randomization. When a new subject was added, the unevenness of the distribution of influencing factors in each group was calculated, and then the group of the subject was determined with different probabilities to ensure that the unevenness of the distribution of influencing factors was minimized. Patients were stratified by histology (adenocarcinoma or squamous cell carcinoma), ECOG performance status (ECOG score 0, 1, or 2), Epithelial growth factor receptor (EGFR) mutation in adenocarcinoma, medical records of anticancer therapies in the past 6 months, and stage of cancer.
Best supportive care
Since BSC was the control arm in our clinical trial, we designed a BSC program based on the recommendations from Zafar [33]. Patients from the BSC arm received appropriate treatments judged by the team including nurses, physicians, psychologist, and dietitians. Therapeutic measures included antibiotics, analgesic drugs, and dietetic assistance according to actual situations of patients. All the symptoms, supportive or palliative care methods and results were documented. Symptoms were assessed at baseline and throughout the trial in person. The symptom assessment was followed up by telephone every two weeks. Clinical assessment was performed during each hospitalization. Tumor-control assessment was assessed by radiographic examination every three months. Assessment methods are detailed in the study assessments section below. Symptom management was based on the National Comprehensive Cancer Network (NCCN) guidelines.
Study assessments
Enhanced chest and abdomen CT scans, brain MRI and bone scans were carried out at baseline and every 4 weeks for the first 12 weeks from the start of the study. All scans were assessed by an independent central radiology review. Response measurements were carried out according to RECIST 1.1. PFS was defined as the time from the onset of the study until disease progression or death from any cause. Three-month DCR was measured 3 months after therapy and defined as the percentage of subjects with a CR, a PR or SD at 3 months relative to all randomly assigned patients. We categorized patients as nonresponding when they had PD; otherwise, patients were categorized as responding. OS was defined as the time from randomization to death due to any cause. Adverse events were recorded, and their severity was assessed according to the Common Terminology Criteria for Adverse Events, version 3.0. To evaluate the maintenance of improvement in the QoL, the European Organization for the Research and Treatment of Cancer Quality of Life Questionnaire (QLQ-C30) was used.
Statistical analysis
The statistical systems GraphPad Prism 6 and PASS 15 were used for modeling and analysis. The sample size was determined to ensure that appropriate conclusions could be drawn with sufficient confidence. At least eighty-nine candidates were required, considering that a one-sided log-rank test with 45 active participants and 44 control participants achieves 85% power at a 0.05% significance level to detect a hazard ratio (HR) of 0.48 with a median survival time of 5.5 in the control arm for patients of Asian origin [34]. Survival estimates were analyzed using the log-rank test and the Kaplan–Meier method. Evaluation of short term response effects in two arms were examined by χ2 test and T test. Comparisons of the study arms considering selected tumor markers and immune-associated factors were conducted using T test and Wilcoxon test. Descriptive statistics were used for treatment administration and safety.
Results
Patient characteristics
Between 2016 and 2017, 97 patients were randomly assigned to receive IVC + mEHT + BSC (n = 49) or BSC alone (n = 48) (Fig. 1). Demographics and baseline tumor characteristics were comparable between the groups (Table 1). The most common histologies were adenocarcinoma and squamous cell carcinoma. Two cases were adenosquamous carcinoma. EGFR exons 19 (n = 4) and 21 (n = 6) were mutated in the active arm.
Efficacy
The median follow-up time was 24 months. A total of five patients dropped out. Of them, two patients in the active arm experienced cardiac events; one patient suffered severe diarrhea. Two patients were lost to follow-up in the control arm. Efficacy analyses were performed in a modified intention-to-treat population of patients who did not receive other anticancer therapy before the cutoff date (May 1, 2019). Ultimately, based on the intent-to-treat principle, 97 patients were analyzed.
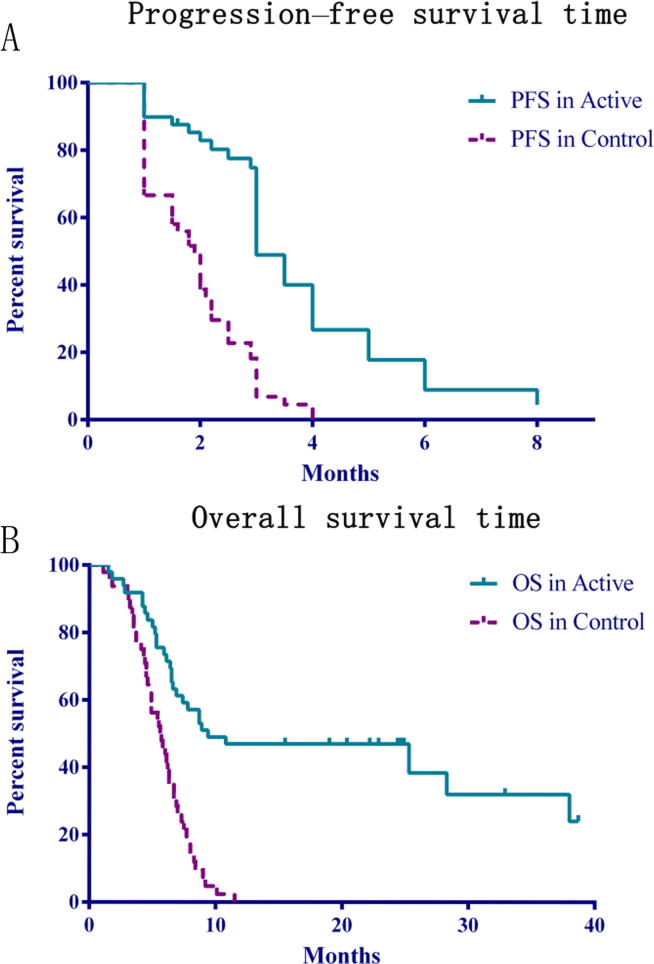
The log-rank test and Kaplan–Meier plots of OS and PFS showed highly significant differences (P < 0.05) between the active and control arms. The median OS was 9.4 months for the active arm and 5.6 months for the control arm [HR = 0.3268; 95% CI, 0.1582–0.4105; P < 0.0001]. The median PFS was 3.0 months for the active arm and 1.85 months for the control arm (HR = 0.3294; 95% CI, 0.1222–0.3166; P < 0.0001; Fig. 2). Neither OS nor PFS were affected by the pathological type of carcinoma (P > 0.05) (Table 2).
Fig. 2.
Progression-free survival time (A) and overall survival time (B): Kaplan–Meier plots for progression-free and overall survival. A. The log-rank test for PFS for the two comparisons: active arm vs control arm [HR = 0.3294; 95% CI, 0.1222–0.3166; P < 0.0001]. B. The log-rank test for OS for the two comparisons: active arm vs control arm [HR = 0.3268; 95% CI, 0.1582–0.4105; P < 0.0001].
Table 2.
Short-term response effects of squamous cell carcinoma and adenocarcinoma patients in the active arm.
| Parameters | Squamous cell carcinoma (n = 24) |
Adenocarcinoma (n = 23) |
P value* |
|---|---|---|---|
| 3-Month Response | |||
| PR | 3 | 4 | 0.563 |
| SD | 9 | 5 | |
| PD | 12 | 14 | |
| 3-Month DCR (PR + SD) | 12 | 9 | 0.561 |
| PFS (Median) | 3 (months) | 2.9 (months) | 0.293 |
| OS (Median) | 12.45 (months) | 10.8 (months) | 0.616 |
Abbreviations: PR, partial response; SD, stable disease; PD, progressive disease; DCR, disease control rate; PFS, progression-free survival; OS, overall survival.
Response effects of squamous cell carcinoma and adenocarcinoma patients were examined by χ2 test and T test; P < 0 0.05 indicates statistically significant difference.
By using the RECIST 1.1 criteria, 5 of 49 (10.2%) subjects in the active arm had PR, while no PR was observed in the control arm; 16 of 49 (32.7%) subjects in the active arm and 8 of 48 (16.7%) subjects in the control arm had SD; and 28 of 49 (57.1%) subjects in the active arm and 40 of 48 (83.3%) subjects in the control arm had PD. No CR was observed in both two arms. The 3-month DCR was 42.9% in the treatment arm and 16.7% in the control arm (odds, 95% CI, P = 0.0073) (Table 3).
Table 3.
Evaluation of short-term response effects in the active arm and control arm.
| Parameters | Active arm (n = 49) |
Control arm (n = 48) |
P value* |
|---|---|---|---|
| Number of deaths (%) | 30 (61.2) | 46 (95.8) | <0.001 |
| 3-Month Response | |||
| PR (%) | 5 (10.2) | 0 (0) | 0.004 |
| SD (%) | 16 (32.7) | 8 (16.7) | |
| PD (%) | 28 (57.1) | 40 (83.3) | |
| 3-Month DCR (PR + SD) (%) | 21 (42.9) | 8 (16.7) | 0.0073 |
Abbreviations: PR, partial response; SD, stable disease; PD, progressive disease; DCR, disease control rate.
Response effects in the the active arm and control arm were examined by χ2 test and T test; P < 0 0.05 indicates statistically significant difference.
There were no significant differences in 3-month DCR, PFS or OS between adenocarcinoma and squamous cell carcinoma (Table 2) or between EGFR(+) and EGFR(–) subjects (Table 4).
Table 4.
Short-term response effects of EGFR(+) and EGFR(−) patients in the active arm.
| EGFR in Adenocarcinoma | EGFR(+) (n = 10) |
EGFR(−) n = 13 |
P value* | |
|---|---|---|---|---|
| 19 (+) (n = 4) |
21 (+) (n = 6) |
|||
| 3-Month Response | ||||
| PR | 3 | 0 | 0 | 0.100 |
| SD | 1 | 2 | 3 | |
| PD | 0 | 4 | 10 | |
| 3-Month DCR (PR + SD) | 6 | 3 | 0.072 | |
| PFS (Median) | 3 (months) | 2.9 (months) | 0.805 | |
| OS (Median) | 21.8 (months) | 7.8 (months) | 0.253 | |
Abbreviations: PR, partial response; SD, stable disease; PD, progressive disease; DCR, disease control rate; PFS, progression-free survival; OS, overall survival.
Response effects of EGFR(+) and EGFR(-) patients in the active arm were examined by χ2 test and T test; P < 0 0.05 indicates statistically significant difference.
None of the patients received further chemotherapy, radiotherapy, targeted therapy or immune therapy. However, in the active arm, four patients received a total of 50 follow-up IVC + mEHT treatments, and three patients received a total of 25 follow-up treatments (once a week).
Adverse effects and toxicity
The overall adverse effects of IVC and mEHT were marginal. Thirst was the major symptom during all of the treatments. Adverse effects were measured in 22/49 (44.9%) of subjects in the active arm. Symptoms disappeared when the treatments ended, except for one patient who experienced severe diarrhea (Table S1). This patient was withdrawn from the study at the stage when he ended the second combined treatment. Acute toxicity was not observed in other patients at any stage of treatment. No significant differences were registered in full blood count or biochemical and hematologic profiles before and after the treatment.
Quality of life
The QLQ-C30 scores were recorded over the full cycle of the study. The average scores for the functioning scales increased continuously, so QoL improved (Table 5).
Table 5.
Function subscale and psychometric parameters.
| Parameters | Prior treatment | Post treatment | P value* |
P value (Active vs Control)# |
|
|---|---|---|---|---|---|
| Mean ± SD | Mean ± SD | Prior vs Post | Prior | Post | |
| Physical | |||||
| Active arm | 77.69 ± 16.70 | 85.71 ± 15.39 | <0.0001 | 0.0533 | <0.0001 |
| Control arm | 74.44 ± 13.21 | 59.93 ± 15.35 | <0.0001 | ||
| Role | |||||
| Active arm | 72.79 ± 24.70 | 73.54 ± 24.31 | 0.5000 | 0.8119 | 0.6919 |
| Control arm | 71.67 ± 23.43 | 71.39 ± 23.81 | >0.9999 | ||
| Emotional | |||||
| Active arm | 84.01 ± 20.33 | 88.61 ± 15.75 | 0.2633 | 0.4408 | <0.0001 |
| Control arm | 83.68 ± 17.36 | 68.86 ± 19.20 | <0.0001 | ||
| Cognitive | |||||
| Active arm | 85.03 ± 18.40 | 85.03 ± 19.02 | >0.9999 | 0.1862 | 0.1026 |
| Control arm | 81.25 ± 18.07 | 80.55 ± 17.97 | 0.5000 | ||
| Social | |||||
| Active arm | 77.89 ± 22.15 | 78.43 ± 21.07 | 0.7500 | 0.2452 | 0.3953 |
| Control arm | 82.99 ± 19.90 | 81.94 ± 19.70 | 0.5000 | ||
| Global | |||||
| Active arm | 46.25 ± 20.85 | 74.76 ± 20.11 | <0.0001 | 0.0635 | <0.0001 |
| Control arm | 52.77 ± 22.12 | 40.49 ± 22.77 | <0.0001 | ||
| Fatigue | |||||
| Active arm | 46.48 ± 17.52 | 20.63 ± 18.14 | <0.0001 | 0.0770 | <0.0001 |
| Control arm | 39.93 ± 20.59 | 61.34 ± 25.32 | <0.0001 | ||
| Nausea/vomiting | |||||
| Active arm | 24.83 ± 22.08 | 11.56 ± 26.18 | 0.0008 | 0.1460 | <0.0001 |
| Control arm | 18.63 ± 20.26 | 31.94 ± 28.94 | 0.0007 | ||
| Pain | |||||
| Active arm | 31.18 ± 21.21 | 25.51 ± 27.45 | 0.0205 | 0.4413 | <0.0001 |
| Control arm | 28.82 ± 20.84 | 47.45 ± 24.55 | <0.0001 | ||
| Dyspnea | |||||
| Active arm | 38.09 ± 23.57 | 27.21 ± 22.23 | <0.0001 | 0.4542 | <0.0001 |
| Control arm | 34.03 ± 23.31 | 50.23 ± 26.61 | 0.0003 | ||
| Insomnia | |||||
| Active arm | 35.37 ± 37.52 | 30.61 ± 30.30 | 0.2781 | 0.2068 | 0.0772 |
| Control arm | 23.84 ± 26.43 | 43.75 ± 33.09 | <0.0001 | ||
| Appetite loss | |||||
| Active arm | 29.93 ± 24.76 | 10.20 ± 20.64 | <0.0001 | 0.4090 | <0.0001 |
| Control arm | 25.00 ± 24.31 | 39.58 ± 26.32 | <0.0001 | ||
| Constipation | |||||
| Active arm | 23.81 ± 26.35 | 4.761 ± 11.78 | <0.0001 | 0.1395 | <0.0001 |
| Control arm | 17.36 ± 27.50 | 26.16 ± 31.38 | 0.0097 | ||
| Diarrhea | |||||
| Active arm | 8.843 ± 20.16 | 12.92 ± 24.36 | 0.3283 | 0.7753 | 0.3014 |
| Control arm | 7.870 ± 19.71 | 7.870 ± 19.71 | 0.0112 | ||
| Financial problems | |||||
| Active arm | 40.14 ± 35.99 | 21.09 ± 20.06 | <0.0001 | 0.7496 | <0.0001 |
| Control arm | 38.19 ± 30.74 | 56.94 ± 27.47 | <0.0001 | ||
T test was used when data of the two group fit the normal distribution, and Wilcoxon test was used when data didn’t conform to the normal distribution; P < 0 0.05 indicates statistically significant difference.
In comparison, the differences in physical, emotional and global improvement after 9 weeks of therapy between the control and the active arms were significant. The psychometric parameters (symptoms) decreased gradually in the active arm of the study, despite the advanced NSCLC and the short (nine week) period of study. The symptoms in the control arm became stronger with time. Fatigue, nausea, pain, dyspnea, appetite loss and constipation were decreased significantly between the groups post treatment (negatively, corresponding to a decrease in symptoms). Note that no significant difference between the groups prior to treatment was observed.
Biomarker analysis
No significant differences in tumor markers, such as CEA, SCC, CA15-3, and CYFRA21-1, were observed before and after treatment or between the treatment and control arms (Table S2).
Inflammation markers
The statistical evaluation shows some significant changes in inflammatory immune factors. The complete comparison of the arms to each other shows more significance than the changes in the individual groups. IL-6 was not different in the two arms before the treatment (P = 0.9413) but differed significantly after therapy (P = 0.0033) and was lower in the active arm (Table 6). The difference originated from the active arm therapy (P = 0.0046), while the value in the control arm was nearly constant (P = 0.1317) (Table 6). The same was also observed for C-reactive protein (CRP); prior to therapy, the two arms were equal (P = 0.7835), but after therapy, they were significantly different (P = 0.0205) (Table 6). The value in the control arm was also unchanged (P = 0.0729). TNF-α did not significantly change between evaluations prior to and after treatment or between the arms of the study after therapy (Table 6).
Table 6.
Inflammation markers in the active arm and control arm.
| Prior treatment | Post treatment | P value* |
P value (Active vs Control)# |
||
|---|---|---|---|---|---|
| Mean ± SD | Mean ± SD | Prior vs Post | Prior | Post | |
| IL-6 | |||||
| Active arm | 9.962 ± 6.408 | 6.674 ± 4.536 | 0.0046 | 0.9413 | 0.0033 |
| Control arm | 10.03 ± 6.506 | 10.08 ± 6.436 | 0.1317 | ||
| CRP | |||||
| Active arm | 24.42 ± 28.45 | 14.43 ± 24.70 | 0.0134 | 0.7835 | 0.0205 |
| Control arm | 24.99 ± 28.68 | 25.30 ± 29.21 | 0.0729 | ||
| TNF-α | |||||
| Active arm | 10.68 ± 23.38 | 8.777 ± 7.771 | 0.4930 | 0.7180 | 0.6782 |
| Control arm | 8.827 ± 10.35 | 8.963 ± 10.34 | 0.1012 | ||
T test was used when data of the two group fit the normal distribution, and Wilcoxon test was used when data didn’t conform to the normal distribution; P < 0.05 indicates statistically significant difference.
Discussion
IVC and mEHT are widely used by integrative cancer practitioners for many years. To our knowledge, no studies have been reported on mEHT combined with high-dose vitamin C in the treatment of tumors. Our phase I clinical study demonstrated that mEHT significantly improved QoL of NSCLC patients with less side effects [32].
This study shows that PFS and OS in the active arm were significantly improved compared with those in the control arm. The overall 3-month DCR was 42.9% with combination therapy, which was significantly higher than that with BSC alone (16.7%), indicating that our active therapy of IVC + mEHT may be an option for advanced NSCLC patients.
The reasons why there is a significant survival benefit are unclear, and we suspect two possible explanations. The first possibility is that the concomitant application of mEHT with IVC significantly increases the plasma concentration of vitamin C compared to that in the sole or nonconcomitant application of the treatments, which was proven by our phase I clinical trial [32]. Previous studies [12], [35] demonstrated that vitamin C in pharmacologic concentrations generated H2O2, which selectively affected cancer cell lines but not normal cells. The increased VitC level can generate a high concentration of H2O2, which can react with the increased labile iron pools in cancer cells to mediate Fenton chemistry and cause oxidative damage to cellular DNA, protein, and lipids, resulting in an energy crisis and cell death [14]. Saitoh et al found that vitamin C combined with HT inhibited the growth of Ehrlich ascites tumor (EAT) cells through G2/M arrest and apoptosis induction via H2O2 generation at lower vitamin C concentrations, but the same concentration of vitamin C alone didn’t exert the carcinostatic effect [36]. The results show that the combination of vitamin C and HT can induce synergic carcinostatic effects. Conventional HT often induces massive necrosis, while mEHT may avoide this outcome by its highly-selective nanoscopic heating [19]. One study indicated that mEHT produced a much higher apoptosis rate by selectively depositing energy on the cell membrane, compared with conventional capacitive coupling hyperthermia [21]. We suspect that the concentration of VitC is significantly increased by mEHT, which is key to attacking cancer cells.
However, in the active arm, we did not find any differences in 3-month DCR, PFS or OS between adenocarcinoma and squamous cell carcinoma or between EGFR(+) and EGFR(-) subjects. The mechanisms need to be addressed, which may be due to the small sample size of each group after stratification.
The second possibility is that IVC + mEHT can modulate the cancer inflammatory microenvironment. The cytokine IL-6 is the bridge connecting cancer cells to the inflammatory environment [37]. Clinical studies have indicated that an increased concentration of IL-6 is strongly associated with increased tumor size and poor prognosis in patients sufering from NSCLC [38], [39], so it may be a potential target in cancer therapy. Cancer inflammation is accompanied by angiogenesis and an inflammatory microenvironment, which is also an independent prognostic marker of poor clinical outcome in NSCLC patients [38], [39]. Welc et al detected HT up-regulated IL-6 level in an animal model [40]. While some studies indicated vitamin C treatment attenuated synthesis of IL-6 [41], [42]. In this study, we found that IL-6 level significantly decreased after 25 treatments in the active arm, and was significantly lower than that in the control arm.
Marsik [43] indicated that candidates with an increased level of CRP have a 28-fold increase in cancer-related death risk. Our study showed that CRP level also significantly decreased after 25 treatments, compared with the control arm. This is similar to the result observed by Mikirova [44], who found that IVC can suppress inflammation, as indicated by reduced CRP levels.
Meanwhile IVC + mEHT could significantly increase the functional scales and significantly decrease the symptom scales, so that QoL improved in these advanced NSCLC patients. Only mild adverse symptoms, such as thirst, fatigue and diarrhea were seen in the active arm. Symptoms (except for one patient with diarrhea) disappeared when the treatments ended.
In addition, 7 patients in the active arm felt better when they finished 25 treatments, and they spontaneously came to our center to receive another 25 to 50 follow-up treatments (once a week). We noticed that 4 of them (2 received 25 follow-up treatments and 2 received 50 follow-up treatments) had a tendency of longer survival time (OS: 38, 38, 37, and 32 months) than other candidates.
Conclusion
Overall, IVC has been shown to be safe and can produce various beneficial effects in nearly all kinds of cancer patients alone and in combination with chemotherapies. To our knowledge, this is the first study to evaluate the efficacy of IVC + mEHT for previously treated patients with refractory advanced (stage IIIb or IV) NSCLC who received BSC treatment. In summary, IVC + mEHT is well tolerated, significantly improves QoL, prolongs PFS and OS, and moderates cancer-related inflammation, so it is a feasible treatment in advanced NSCLC.
Declaration of Competing Interest
The authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.
Acknowledgments
Acknowledgments
The authors sincerely thank the patients and investigators.
The study was financed with institutional funds from Clifford L.K. Pang Funding, China [Grant number: 2016-01], and the Major Medical and Health Project of the Department of Science, Technology, Industry, Commerce and Information Bureau in Panyu of Guangzhou [Grant number: 2018-Z04-05].
Consent for publication
Not applicable.
Availability of data and materials
The datasets used and/or analyzed during the current study are available from the corresponding author on reasonable request.
Footnotes
Peer review under responsibility of Cairo University.
Supplementary data to this article can be found online at https://doi.org/10.1016/j.jare.2020.03.004.
Appendix A. Supplementary material
The following are the Supplementary data to this article:
References
- 1.Chen W., Zheng R., Baade P.D., Zhang S., Zeng H., Bray F. Cancer statistics in China 2015. CA Cancer J Clin. 2016;66(2):115–132. doi: 10.3322/caac.21338. [DOI] [PubMed] [Google Scholar]
- 2.Chen W., Zheng R., Zeng H., Zhang S. Epidemiology of lung cancer in China. Thorac Cancer. 2015;6(2):209–215. doi: 10.1111/1759-7714.12169. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Shenoy N., Creagan E., Witzig T., Levine M. Ascorbic acid in cancer treatment: let the phoenix fly. Cancer Cell. 2018;34(5):700–706. doi: 10.1016/j.ccell.2018.07.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Welsh J.L., Wagner B.A., van’t Erve T.J., Zehr P.S., Berg D.J., Halfdanarson T.R. Pharmacological ascorbate with gemcitabine for the control of metastatic and node-positive pancreatic cancer (PACMAN): results from a phase I clinical trial. Cancer Chemother Pharmacol. 2013;71(3):765–775. doi: 10.1007/s00280-013-2070-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Levine M., Wang Y., Padayatty S.J., Morrow J. A new recommended dietary allowance of vitamin C for healthy young women. Proc Natl Acad Sci U S A. 2001;98(17):9842–9846. doi: 10.1073/pnas.171318198. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Creagan E.T., Moertel C.G., O'Fallon J.R., Schutt A.J., O'Connell M.J., Rubin J. Failure of high-dose vitamin C (ascorbic acid) therapy to benefit patients with advanced cancer. A controlled trial. N Engl J Med. 1979;301(13):687–690. doi: 10.1056/NEJM197909273011303. [DOI] [PubMed] [Google Scholar]
- 7.Stephenson C.M., Levin R.D., Spector T., Lis C.G. Phase I clinical trial to evaluate the safety, tolerability, and pharmacokinetics of high-dose intravenous ascorbic acid in patients with advanced cancer. Cancer Chemother Pharmacol. 2013;72(1):139–146. doi: 10.1007/s00280-013-2179-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Riordan H.D., Casciari J.J., Gonzalez M.J., Riodan N.H., Miranda-Massari J.R., Taylor P. A pilot clinical study of continuous intravenous ascorbate in terminal cancer patients. P R Health Sci J. 2005;24(4):269–276. [PubMed] [Google Scholar]
- 9.Ma Y., Chapman J., Levine M., Polireddy K., Drisko J., Chen Q. High-dose parenteral ascorbate enhanced chemosensitivity of ovarian cancer and reduced toxicity of chemotherapy. Sci Transl Med. 2014;6(222):222ra18. doi: 10.1126/scitranslmed.3007154. [DOI] [PubMed] [Google Scholar]
- 10.Vollbracht C., Schneider B., Leendert V., Weiss G., Auerbach L., Beuth J. Intravenous vitamin C administration improves quality of life in breast cancer patients during chemo-/radiotherapy and aftercare: results of a retrospective, multicentre, epidemiological cohort study in Germany. Vivo. 2011;25(6):983–990. [PubMed] [Google Scholar]
- 11.Chen Q., Espey M.G., Sun A.Y., Pooput C., Kirk K.L., Krishna M.C. Pharmacologic doses of ascorbate act as a prooxidant and decrease growth of aggressive tumor xenografts in mice. Proc Natl Acad Sci U S A. 2008;105(32):11105–11109. doi: 10.1073/pnas.0804226105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Chen Q., Espey M.G., Krishna M.C., Mitchell J.B., Corpe C.P., Buettner G.R. Pharmacologic ascorbic acid concentrations selectively kill cancer cells: action as a pro-drug to deliver hydrogen peroxide to tissues. Proc Natl Acad Sci U S A. 2005;102(38):13604–13609. doi: 10.1073/pnas.0506390102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Carosio R., Zuccari G., Orienti I., Mangraviti S., Montaldo P.G. Sodium ascorbate induces apoptosis in neuroblastoma cell lines by interfering with iron uptake. Mol Cancer. 2007;6:55. doi: 10.1186/1476-4598-6-55. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Schoenfeld J.D., Sibenaller Z.A., Mapuskar K.A., Wagner B.A., Cramer-Morales K.L., Furqan M. O2(−) and H2O2− mediated disruption of Fe metabolism causes the differential susceptibility of NSCLC and GBM cancer cells to pharmacological ascorbate. Cancer Cell. 2017;32(2):268. doi: 10.1016/j.ccell.2017.07.008. [DOI] [PubMed] [Google Scholar]
- 15.Soares P.I., Ferreira I.M., Igreja R.A., Novo C.M., Borges J.P. Application of hyperthermia for cancer treatment: recent patents review. Recent Pat Anticancer Drug Discov. 2012;7(1):64–73. doi: 10.2174/157489212798358038. [DOI] [PubMed] [Google Scholar]
- 16.Cheng Y., Weng S., Yu L., Zhu N., Yang M., Yuan Y. The role of hyperthermia in the multidisciplinary treatment of malignant tumors. Integr Cancer Ther. 2019;18 doi: 10.1177/1534735419876345. 1534735419876345. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Ohguri T., Imada H., Yahara K., Moon S.D., Yamaguchi S., Yatera K. Re-irradiation plus regional hyperthermia for recurrent non-small cell lung cancer: a potential modality for inducing long-term survival in selected patients. Lung Cancer. 2012;77(1):140–145. doi: 10.1016/j.lungcan.2012.02.018. [DOI] [PubMed] [Google Scholar]
- 18.Szasz A.M., Minnaar C.A., Szentmártoni G., Szigeti G.P., Dank M. Review of the clinical evidences of modulated electro-hyperthermia (mEHT) method: an update for the practicing oncologist. Front Oncol. 2019;9:1012. doi: 10.3389/fonc.2019.01012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Andocs G., Renner H., Balogh L., Fonyad L., Jakab C., Szasz A. Strong synergy of heat and modulated electro-magnetic field in tumor cell killing. Strahlenther Onkol. 2009;185:120–126. doi: 10.1007/s00066-009-1903-1. [DOI] [PubMed] [Google Scholar]
- 20.Andocs G., Rehman M.U., Zhao Q.L., Papp E., Kondo T., Szasz A. Nanoheating without artificial nanoparticles part II. Experimental support of the nanoheating concept of the modulated electro-hyperthermia method, using U937 cell suspension model. Biol Med (Aligarh) 2015;7:1–9. [Google Scholar]
- 21.Yang K.L., Huang C.C., Chi M.S., Chiang H.C., Wang Y.S., Hsia C.C. In vitro comparison of conventional hyperthermia and modulated electro-hyperthermia. Oncotarget. 2016;7(51):84082–84092. doi: 10.18632/oncotarget.11444. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Lee S.Y., Kim J.H., Han Y.H., Cho D.H. The effect of modulated electro-hyperthermia on temperature and blood flow in human cervical carcinoma. Int J Hyperthermia. 2018;34(7):953–960. doi: 10.1080/02656736.2018.1423709. [DOI] [PubMed] [Google Scholar]
- 23.Vancsik T., Kovago C., Kiss E., Papp E., Forika G., Benyo Z. Modulated electro-hyperthermia induced loco-regional and systemic tumor destruction in colorectal cancer allografts. J Cancer. 2018;9(1):41–53. doi: 10.7150/jca.21520. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Yeo S.G. Definitive radiotherapy with concurrent oncothermia for stage IIIB non-small-cell lung cancer: a case report. Exp Ther Med. 2015;10(2):769–772. doi: 10.3892/etm.2015.2567. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Rubovszky G., Nagy T., Godeny M., Szasz A., Lang I. Successful treatment of solitary bone metastasis of non-small cell lung cancer with bevacizumab and hyperthermia. Pathol Oncol Res. 2013;19(1):119–122. doi: 10.1007/s12253-012-9551-7. [DOI] [PubMed] [Google Scholar]
- 26.Szasz A. Current status of oncothermia therapy for lung cancer. Korean J Thorac Cardiovasc Surg. 2014;47(2):77–93. doi: 10.5090/kjtcs.2014.47.2.77. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Ariyafar T., Mahdavi S.R., Geraily G., Fadavi P., Farhood B., Najafi M. Evaluating the effectiveness of combined radiotherapy and hyperthermia or the treatmentresponse of patients with painful bony metastases: a phase 2 clinical trial. J Therm Biol. 2019;84:129–135. doi: 10.1016/j.jtherbio.2019.06.003. [DOI] [PubMed] [Google Scholar]
- 28.Issels R.D., Lindner L.H., Verweij J., Wessalowski R., Reichardt P., Wust P. Effect of neoadjuvant chemotherapy plus regional hyperthermia on long-term outcomes among patients with localized high-risk soft tissue sarcoma: the EORTC 62961-ESHO 95 randomized clinical trial. JAMA Oncol. 2018;4(4):483–492. doi: 10.1001/jamaoncol.2017.4996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Wittlinger M., Rödel C.M., Weiss C., Krause S.F., Kühn R., Fietkau R. Quadrimodal treatment of high-risk T1 and T2 bladder cancer: transurethral tumor resection followed by concurrent radiochemotherapy and regional deep hyperthermia. Radiother Oncol. 2009;93(2):358–363. doi: 10.1016/j.radonc.2009.09.018. [DOI] [PubMed] [Google Scholar]
- 30.Yang W.H., Xie J., Lai Z.Y., Yang M.D., Zhang G.H., Li Y. Radiofrequency deep hyperthermia combined with chemotherapy in the treatment of advanced non-small cell lung cancer. Chin Med J (Engl) 2019;132(8):922–927. doi: 10.1097/CM9.0000000000000156. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Mitsumori M., Zeng Z.F., Oliynychenko P., Park J.H., Choi I.B., Tatsuzaki H. Regional hyperthermia combined with radiotherapy for locally advanced non-small cell lung cancers: a multi-institutional prospective randomized trial of the International Atomic Energy Agency. Int J Clin Onco. 2007;12(3):192–198. doi: 10.1007/s10147-006-0647-5. [DOI] [PubMed] [Google Scholar]
- 32.Ou J., Zhu X., Lu Y., Zhao C., Zhang H., Wang X. The safety and pharmacokinetics of high dose intravenous ascorbic acid synergy with modulated electrohyperthermia in Chinese patients with stage III-IV non-small cell lung cancer. Eur J Pharm Sci. 2017;109:412–418. doi: 10.1016/j.ejps.2017.08.011. [DOI] [PubMed] [Google Scholar]
- 33.Zafar S.Y., Currow D.C., Cherny N., Strasser F., Fowler R., Abernethy A.P. Consensus-based standards for best supportive care in clinical trials in advanced cancer. Lancet Oncol. 2012;13(2):e77–e82. doi: 10.1016/S1470-2045(11)70215-7. [DOI] [PubMed] [Google Scholar]
- 34.Thatcher N., Chang A., Parikh P., Pereira J.R., Ciuleanu T., Von Pawel J. Gefitinib plus best supportive care in previously treated patients with refractory advanced non-small-cell lung cancer: results from a randomised, placebo-controlled, multicentre study (Iressa Survival Evaluation in Lung Cancer) Lancet. 2005;366(9496):1527–1537. doi: 10.1016/S0140-6736(05)67625-8. [DOI] [PubMed] [Google Scholar]
- 35.Chen Q., Espey M.G., Sun A.Y., Lee J.H., Krishna M.C., Shacter E. Ascorbate in pharmacologic concentrations selectively generates ascorbate radical and hydrogen peroxide in extracellular fluid in vivo. Proc Natl Acad Sci U S A. 2007;104(21):8749–8754. doi: 10.1073/pnas.0702854104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Saitoh Y., Yoshimoto T., Kato S., Miwa N. Synergic carcinostatic effects of ascorbic acid and hyperthermia on Ehrlich ascites tumor cell. Exp Oncol. 2015;37(2):94–99. [PubMed] [Google Scholar]
- 37.Lippitz B.E. Cytokine patterns in patients with cancer: a systematic review. Lancet Oncol. 2013;14(6):e218–e228. doi: 10.1016/S1470-2045(12)70582-X. [DOI] [PubMed] [Google Scholar]
- 38.Silva E.M., Mariano V.S., Pastrez P.R.A., Pinto M.C., Castro A.G., Syrjanen K.J. High systemic IL-6 is associated with worse prognosis in patients with non-small cell lung cancer. PLoS ONE. 2017;12(7):e0181125. doi: 10.1371/journal.pone.0181125. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Tang H., Bai Y., Pan G., Wang X., Wei Y., Yang Z. Interleukin-6 and insulin-like growth factor-1 synergistically promote the progression of NSCLC. Autoimmunity. 2018;51(8):399–407. doi: 10.1080/08916934.2018.1550079. [DOI] [PubMed] [Google Scholar]
- 40.Welc S.S., Phillips N.A., Oca-Cossio J., Wallet S.M., Chen D.L., Clanton T.L. Hyperthermia increases interleukin-6 in mouse skeletal muscle. Am J Physiol Cell Physiol. 2012;303(4):C455–C466. doi: 10.1152/ajpcell.00028.2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Chen Y., Luo G., Yuan J., Wang Y., Yang X., Wang X. Vitamin C mitigates oxidative stress and tumor necrosis factor-alpha in severe community-acquired pneumonia and LPS-induced macrophages. Mediators Inflamm. 2014;2014:426740. doi: 10.1155/2014/426740. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Portugal C.C., Socodato R., Canedo T., Silva C.M., Martins T., Coreixas V.S. Caveolin-1–mediated internalization of the vitamin C transporter SVCT2 in microglia triggers an inflammatory phenotype. Sci. Signal. 2017;10(472) doi: 10.1126/scisignal.aal2005. pii: eaal2005. [DOI] [PubMed] [Google Scholar]
- 43.Marsik C., Kazemi-Shirazi L., Schickbauer T., Winkler S., Joukhadar C., Wagner O.F. C-reactive protein and all-cause mortality in a large hospital-based cohort. Clin Chem. 2008;54(2):343–349. doi: 10.1373/clinchem.2007.091959. [DOI] [PubMed] [Google Scholar]
- 44.Mikirova N., Casciari J., Rogers A., Taylor P. Effect of high-dose intravenous vitamin C on inflammation in cancer patients. J Transl Med. 2012;10:189. doi: 10.1186/1479-5876-10-189. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
The datasets used and/or analyzed during the current study are available from the corresponding author on reasonable request.