Abstract
Malaria is a complex disease and its distribution is not random in endemic areas, and hence areas with low malaria transmission require fine spatial sampling and careful follow-up to identify the hot spots for effective resource utilization to control malaria. The present study is aimed to assess malaria infection in both humans and mosquitoes in a small residential lowland area of southern Ethiopia from July to December 2016. A repeated cross-sectional household survey was conducted in Kolla-Shara Kebele (village) to describe the distribution of malaria and infectious mosquitoes. For the parasitological surveys, a total of 90 households were randomly selected from five sub-villages in equal proportion. About a quarter of the total households included for the surveys were randomly selected for entomological surveys. A P-value of <0.05 was used as a cut-off point for statistical significance. More than a third (35.1%, 46 of 131) febrile cases were microscopically confirmed malaria positive. Above half (58.7%, 27 of 46) of those positive cases were due to P. falciparum and the rest (41.3%, 19 of 46) were due to P. vivax. This study identified two of the five sub-villages as independent clusters with higher risk of malaria infection. Four times higher relative risk (RR) of malaria infection was documented in Abullo sub-village compared to the others (RR = 3.87; P = 0.002). Most of the falciparum malaria cases were aggregated in these sub-villages. About six infectious bites of An. arabiensis per person was recorded during the survey. The infectious bite per person was 17.0 in Abullo and 10.6 in Erze clusters where higher human infections were detected. It is clearly indicated that a smaller portion of the population carry higher malaria cases and infectious bites. Malaria interventions targeting such areas could be effective in the context of malaria elimination strategy in Ethiopia, which consider district as a planning and implementing unit. Future research would preferably be designed to perform long duration of follow-up to identify the appropriate period for interventions and more participants with more heterogeneous villages and districts.
Keywords: Malaria infection, Malaria clustering, Anopheles arabiensis, Infectious bites, Kolla-Shara village
1. Introduction
Malaria transmission varies in space and time where a small proportion of the community members receive majority of infections. Previous studies attested that 20% of the population receives 80% of the infectious bites of malaria transmitting mosquitoes (Clark et al., 2008; Bousema et al., 2012). There are some factors accounting for the variation. For instance, distance from the nearest mosquito breeding sites, wind direction, and the human behavioural factors (Clark et al., 2008; Baidjoe et al., 2016; Midega et al., 2012) can result in variation to human exposure to malaria over a small area and create malaria hotspots, where transmission intensity is higher than in the surrounding areas (Kreuels et al., 2008; Bousema et al., 2012). Since hotspots are the primary source of infection, interventions that aimed at reducing transmission at community level preferred targeting them. Thus, targeted interventions are effective and efficient enough especially in malaria elimination.
Characteristically malaria transmission in Ethiopia is seasonal and unstable, which is amenable to variation with space and time. Following its remarkable reduction the country embarked on elimination of malaria, which is believed to rely on local evidence at lower administrative units like village. Thus, understanding the malaria infection micro-epidemiology i.e., where it is concentrated helpful to guide program in resource limited settings. Previous studies showed spatial and temporal variation of malaria in low transmission settings of highlands and (Yeshiwondim et al., 2009; Woyessa et al., 2012) and moderate transmission areas in lowlands (Loha et al., 2012). At present, evidence-based decision is a vital step for district level plan for malaria elimination strategy. However, there is scarce information in this regard. Thus, the present study is aimed at describing the fine scale spatial distribution of malaria infection in a lowland area of Ethiopia.
2. Materials and methods
2.1. Study area description
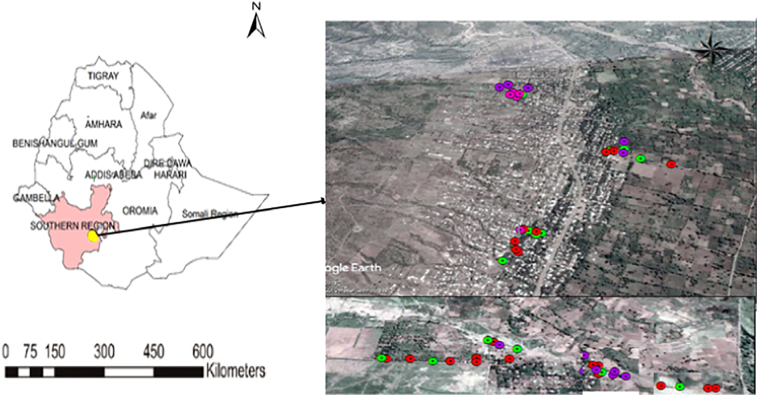
This study was conducted in Kolla-Shara village situated 490 Km southwest of Addis Ababa. It is found 6 km north of Arba Minch town (Fig. 1). The study village has a total area of 58 Km2 with an altitude range of 1170–1390 m above sea level (masl).
Fig. 1.
Map of Ethiopia, location of Kolla Shara village (yellow dot in Ethiopia map), southwestern Ethiopia: legend in the google map indicates study households. (For interpretation of the references to colour in this figure legend, the reader is referred to the web version of this article.)
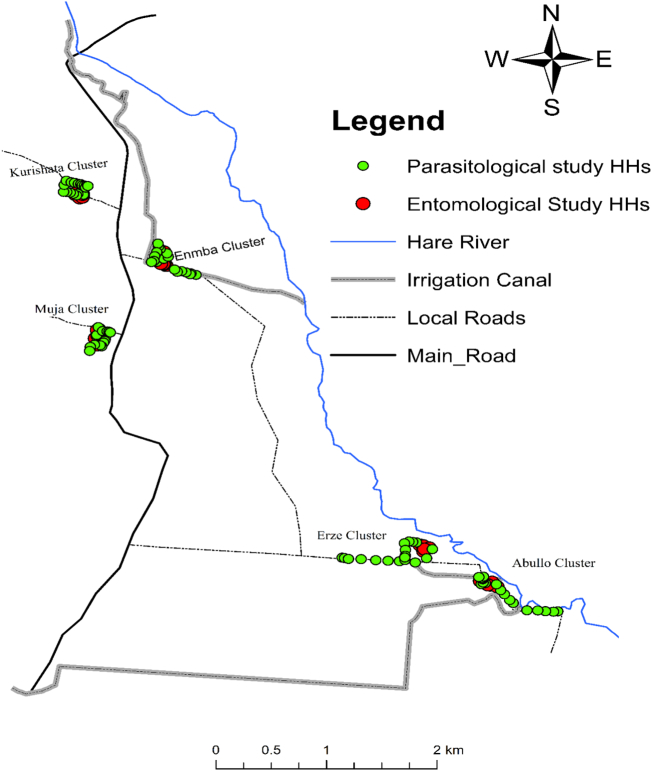
The mean annual rainfall of the area is estimated to be 750–1060 mm and minimum and maximum temperature of 18 °C and 35 °C, respectively (Agricultural Office Data). The total population of the village is approximately above 10 thousand and an average of 4.7 family size is documented (Unpublished District Health Office Data, 2016). A health post is established to serve the local population in malaria diagnosis and treatment using the national guide line. It also provides another basic health services. The farmers in the village depend on the Hare River which bisects the village nearer to the eastern border and detours to the southeast direction that empty to the Lake Abaya (Fig. 2). The residents depend on the river for irrigation to grow vegetables and fruits as well as crops. The river also creates potential mosquito breeding places.
Fig. 2.
Location of households selected for epidemiological and entomological sampling in the five study sub-villages of Kolla Shara village, southwestern Ethiopia.
The main type of housing in this village is corrugated iron roofed houses with separate bed room and kitchen. The typical rural huts constructed of wood, and grass thatched roof are also found in the village. Number of grass thatched roof houses are common in two of the sub-villages located adjacent to Hare River. Houses in the other sub-villages are commonly corrugated iron roofed. Long-lasting insecticidal nets (LLINs) and indoor residual spraying (IRS) are the principal vector control tools. All sub-villages are included in this study (Fig. 2).
2.2. Study design and sample size determination
A community-based repeated cross sectional survey was employed to obtain both parasitological and entomological information at household level. The whole family members were screened for fever and malaria parasite in each visit.
A single population proportion formula was employed to compute sample size required for parasitological data with the assumptions of 95% confidence interval level (CI), 50% expected prevalence of malaria (p = 0.5) to maximize the sample size, and 5% margin of error (d = 0.05). The 50% assumption was considered due to the extreme heterogeneity and variability of malaria transmission even in small geographical locations. By using the above assumptions, the sample size (n) was calculated as: n = z2p (1-p)/d2, where n = sample size, z = z statistic for a level of confidence (z = 1.96 at 95% CI), p = expected prevalence or proportion (p = 0.5), d = precision/marginal error (if 5%, d = 0.05), we obtained a sample size of 384. In this survey, 10% compensation for non-response rate of participants (additional 38 participants) was computed. Therefore, a total sample size was 422. The estimated population size per household in the village was 4.7 (Unpublished District Health Office Data, 2016).
2.3. Sampling and selection of households
On the basis of 4.7 family size a total of 90 households were randomly selected to achieve the estimated sample size. Equal proportion of households was allocated to the five sub-villages namely Muja, Enmba, Kurishata, Erze and Abullo in the study village. The study sampling frame (a list of family members) was obtained from the village administration office. Systematic random sampling procedure was employed and 18 households were selected in each sub-village. During July 2016, the study team enumerated all family members of the selected households and well informed them about the study.
2.3.1. Parasitological sampling
For parasitological sampling, a unique identification number was assigned to each household and posted it on a metal plate at the main entrance of each house. All study participants in the selected households were measured for auxiliary temperature using thermometer by clinical nurse. Second visit was arranged to screen the participants missed in the first visit to ensure maximum response.
All selected households were visited twice per month to screen each member of a family for fever episodes. This was performed from July to December 2016. A febrile case is defined as an individual whose auxiliary temperature is ≥37.5 °C at the time of visit or a history of fever or other malaria-related symptoms such as chills, severe malaise, headache, or vomiting in 24–48 h prior to the visit. Trained health personnel collected blood specimen from febrile cases and screened for malaria using RDT (Malaria HRP2/pLDH (Pf/PAN) Ag COMBO test). Microscopic confirmation was performed for positive cases using thick and thin blood film. The positive cases were treated with first line anti-malarial drug according to the national treatment guideline for both P. falciparum and P. vivax.
2.3.2. Entomological sampling and mosquito processing
For entomological survey only five households were selected in each sub-village and a total of 25 households were included for mosquito sampling. A buffer zone of 2 km radius of the distance between sub-villages was taken into account by considering the average flight range of mosquitoes. Moreover, the houses were selected from the center of each sub-village to avoid contamination from neighboring sub-villages that might occur due to the overlapping flight ranges mosquitoes. This is applied based on similar study that conducted to investigating variation among villages in smaller areas elsewhere (Bousema et al., 2010).
Mosquito sampling was carried out by Center for Disease Control and Prevention (CDC) light traps (Model 512; John W. Hock Company, Gainesville, FL, USA) from July to December 2016. It was operated two times per month for six months in each house selected for the study. The traps were switched on at 18:00 pm and switch off at 6:00 am hours. Each CDC light trap was hanged on a wall or roof at about 1 m above the floor at the foot end of a person who protected by untreated bed net (Lines et al., 1991). Other occupants in the houses were left to use the bed nets provided by the district health office as part of the routine malaria control program. Mosquitoes in the traps were removed from the bags, counted and sorted out into species by a morphological key (Gillies and Coetzee, 1987). Live Anopheles mosquitoes were killed by freezing. Female Anopheles mosquitoes were preserved individually over silica gel in vials and kept at room temperature for circum-sporozoite proteins (CSPs) test.
The head and thorax of dried female An. arabiensis and An. pharoensis were tested for P. falciparum and P. vivax_210 CSPs using Enzyme Linked Immuno-Sorbent Assay (ELISA) (Beier et al., 1987) in Arba Minch University Medical Entomology Laboratory. Demographic data on socio-demographic characteristics such as name, gender and age of each participant from sampled households was carefully recorded. This information was used for each visit and intended blood collection for malaria parasite screening with fever. A total of 623 study participants were enumerated from 90 households. Most of the study participants (96%, 599 of 623) were included during the beginning of the study period, July 2016.
2.4. Geo-location of houses
The geographic coordinates of sampled houses recorded using single hand held Global Positioning System 60™ (GPS) (GARMIN, Olathe, KS Venture) at ±5 m precision. Houses selected for both entomological and malaria incidence study and the main vector breeding sites were recorded. The epidemiological and entomological study results were linked to each household's GPS data. In addition, the centroid for each sub-village was extracted using ArcGIS 10.1 software to verify data recorded using hand held GPS units. All the relevant shapes of geographical features were re-projected to WGS_1984_UTM_Zone_37N to integrate the dataset into one geodatabase, a data structure used in ArcGIS.
2.5. Quality control
Two experienced laboratory technicians read each slide independently. A third reader confirmed a discordant reading. All readers were blinded to the readings of the other and RDT results. Sporozoite positive samples were re-tested and confirmed. GPS points were uploaded to database system and cleaned for duplicates.
2.6. Data management and statistical analysis
Study participants were coded and data entered into computer program IBM® SPSS® version 20 (Armonk, New York: IBM Corporation) for analysis. Data cleaning performed and ready for analysis. Descriptive statistics was performed to describe characteristics of study participants. SaTScan software version 9.4 was employed to perform spatial analysis to detect malaria case clustering at sub-village levels. A statistical significance was reported at P < 0.05.
Sporozoite rate was calculated as the proportion of mosquitoes' positive for P. falciparum and P. vivax in their salivary glands among the total number tested by ELISA. Entomological inoculation rate (EIR) is the infective bites per person/night/in a specific period of time, which was calculated for each sub-village. It is calculated using the formula applied elsewhere (Kilama et al., 2014). Plasmodium falciparum EIR of An. arabiensis was computed at the 95% confidence interval as the number of days in six months divided by number of catches multiplied by number of P. falciparum positive mosquito from CDC light trap plus number of P. falciparum positive mosquitoes divided by number ELISA tested multiplied by number of An. arabiensis collected from CDC light trap minus number of ELISA tested.
A model was fit using Poisson regression with a generalized estimating equation (GEE) to analyze difference in malaria incidence among sub-villages. Pearson Chi-Square was used as the scale parameter and robust estimator for the covariance matrix. The hypothesis test was based on Wald Chi-Square. The number of weeks an individual has been observed was considered as a scale weight variable. Analysis of variance (ANOVA) was used to determine the differences in Anopheles density in the five study sub-villages.
Maps of the sub-villages were generated using ArcGIS software (version 10.1; ESRI, City, Country). This map helped to clarify the clustering of malaria episodes, Anopheles mosquito density and EIR in each sub-village.
2.7. Ethical approval and consent to participate
This study obtained ethical approval from Institutional Ethics Review Committee of Arba Minch University (Ref.No.1466/111). The study procedures were explained, and written and informed verbal consent were obtained from the study participants. For minors, consent was obtained from their caregivers or legal guardians. The blood samples were collected aseptically using sterile blood lancet for single use only. Malaria positive cases were treated immediately following RDT results. Local health authorities and community were informed about the study using official letters and written permission was sought before commencement of the study. Informed consent was obtained from the head of study households for CDC light trap mosquito collection.
3. Results
3.1. Socio-demographic characteristics
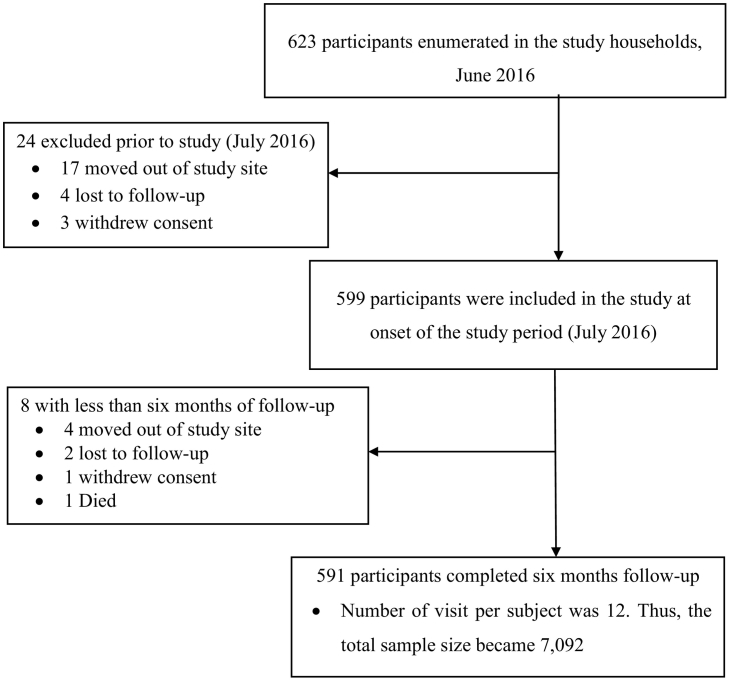
Most of the study participants (98.7%, 591 of 599) recruited in the study completed the follow up (Fig. 3). The average age of the participants was 19.7 (standard deviation = 15.1) years. Of those above half of the study participants (51.3%, n = 303) were ≥15 years old and more than a third (35.3%, n = 209) were 5–14 years old and the rest were children <5 years (13.4%, n = 79). Ratio of male to female participants was about a unity. About similar proportion of participants were recruited from each sub-village, ranging from 18.6% to 21.8%.
Fig. 3.
Study flow chart: distribution of study participants from enrollment to the end of six months follow-up period in the Kolla Shara village, southwestern Ethiopia.
3.2. Malaria cases and incidence
Overall, fever incidence was 1.8% (131 of 7188) within six months of follow-up period. Of those more than a third (35.1%; 46 of 131) were microscopically confirmed. Similarly, RDT also detected a slightly higher proportion (38.2%, 50 of 131). Among microscopically confirmed, P. falciparum accounted more than half (58.7%, 27 of 46) and the rest were due to P. vivax (41.3%, 19 of 46). Mixed infection was not recorded.
Overall, above 277 person-year observation time was recorded throughout the study period. The overall incidence of 0.10 episodes per person-year yielded from 27 microscopically confirmed P. falciparum cases in the village. Table 1 shows variation of malaria cases among the sub-villages. Both Abullo and Erze sub-villages accounted higher incidence risk ratio of P. falciparum compared to the rest.
Table 1.
Malaria incidence rate ratio (IRR) of P. falciparum among in five sub-villages of Kolla-Shara village in southwestern Ethiopia, July to December 2016.
| Sub-village | P. falciparum cases (n = 27) | Person years of contribution (n = 277.4) | Malaria incidence per person-year | IRR (95% CI) | P-value |
|---|---|---|---|---|---|
| Abullo | 16 (59.3%) | 55.1 | 0.29 | 16.0 (3.6–71.2) | 0.0001 |
| Erze | 10 (37.0%) | 60.4 | 0.17 | 9.1 (1.7–49.4) | 0.005 |
| Enmbaa | 1 (3.7%) | 55.0 | 0.02 | 1 | – |
| Muja | 0 | 55.1 | 0.0 | 0 | – |
| Kurishata | 0 | 51.8 | 0.0 | 0 | – |
Reference sub-village; CI = Confidence interval; IRR = incidence rate ratio.
3.3. Age distribution of malaria incidence
Malaria incidence found to be varying among different age groups. Only pooled data for age-specific incidence analysis was used in the village level due to the limited number of malaria cases. More than four episodes per person-year were detected in 5–14 years old. The difference in malaria incidence among age groups 5–14 years old was statistically significant compared to the other age groups (IRR: 4.1; 95% CI:0.7–9.1; P = 0.003) (Table 2).
Table 2.
Malaria cases incidence rate ratio (IRR) of P. falciparum among different age groups and gender in southwestern Ethiopia, July to December 2016.
| Variable | No. participants | Pf cases (n = 27) | Person years of contribution (n = 277.4) | Malaria incidence per person-year | IRR (95% CI) | P value | |
|---|---|---|---|---|---|---|---|
| Age groups | <5 years | 79 | 4 | 37.0 | 0.11 | 2.6 (0.7–9.1) | 0.15 |
| 5–14 years | 209 | 17 | 98.1 | 0.17 | 4.1 (1.6–10.4) | 0.003 | |
| ≥15 yearsa | 303 | 6 | 142.3 | 0.04 | 1 | ||
| Sex | Male | 292 | 17 | 137.0 | 0.12 | 1.7 (0.8–3.8) | 0.16 |
| Femalea | 299 | 10 | 140.4 | 0.07 | 1 | ||
Reference group, CI = Confidence interval, IRR = incidence rate ratio, Pf = Plasmodium falciparum.
3.4. Malaria parasite species among sub-villages
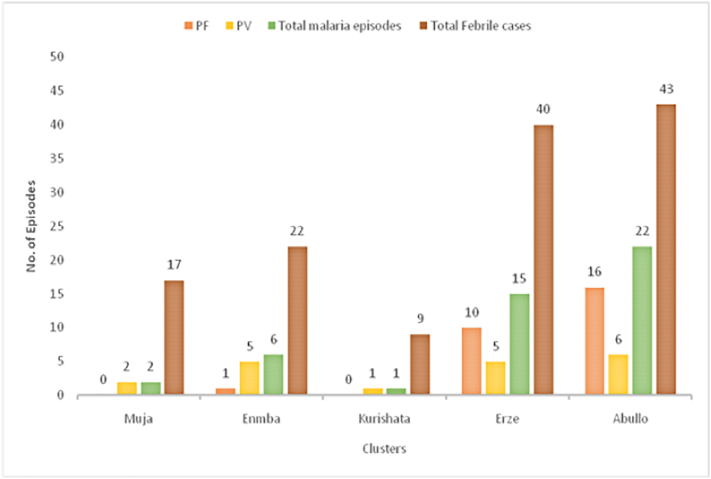
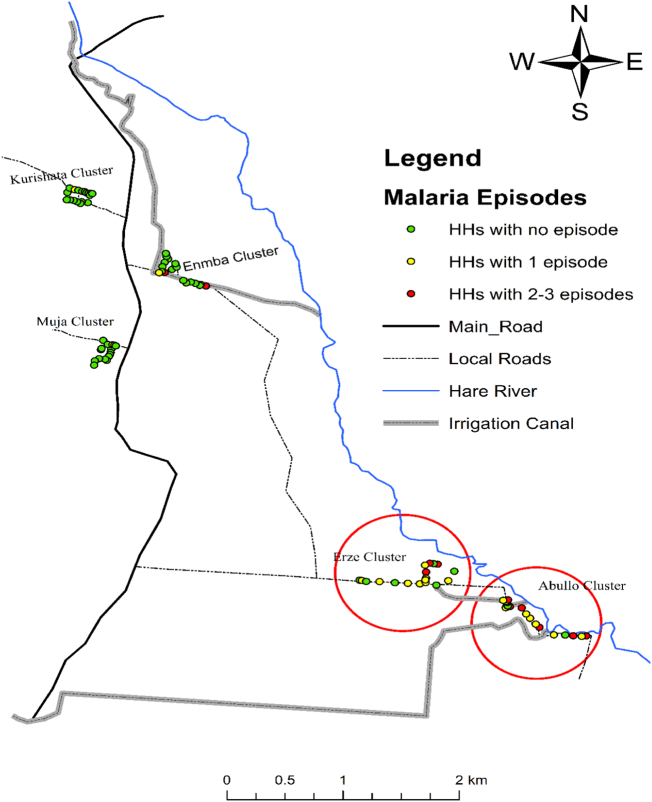
Plasmodium falciparum was the dominant malaria parasite in Abullo (72.7%; 16 of 22) and in Erze sub-villages (66.7%; 10 of 15), whereas P. vivax (83.3%; 5 of 6) was common in Enmba sub-village. Only P. vivax malaria cases were identified in Muja and Kurishata sub-villages. Majority of the malaria cases (80.4%; 37 of 46) were reported from few houses (30% households) from the two malaria hot spot sub-villages. Only a few households in the other three sub-villages had malaria cases. The number of malaria episodes was high in Abullo and Erze sub-villages (Fig. 4, Fig. 5).
Fig. 4.
Number of febrile cases and malaria episodes during the six months of follow-up period in the five sub-villages of Kolla Shara village, southwestern Ethiopia.
Fig. 5.
The number of malaria case per household during the follow-up period in the five different sub-villages of Kolla Shara village, southwestern Ethiopia.
3.5. and density of Anopheles species
1086 Anopheles mosquitoes sampled throughout the survey period. Although seven anopheline species (An. gambiae s. l (An. arabiensis), An. pharoensis, An. tenebrosus, An. funestus-group, An. ziemanni, An. demeilloni and An. pretoriensis) were identified, An. arabiensis was the most abundant species (70.5%, 766 of 1086), followed by An. pharoensis (10%; 109 of 1086). The rest of the small proportion was accounted for An. tenebrosus, An. funestus-group, An. ziemanni, An. demeilloni and An. pretoriensis (Table 3).
Table 3.
Anopheles mosquito species composition and density in five sub-villages of Kolla Shara village, southwestern Ethiopia, July to December 2016.
| Sub-villages |
Anopheles species (%) |
Total | ||||||
|---|---|---|---|---|---|---|---|---|
| Anarb | Anpha | Anten | Anfun | Anzim | Andem | Anprt | ||
| Abullo | 339 (44.2) | 66 (60.5) | 69 (71.1) | 17 (23.6) | 19 (86.4) | 0 (0.0) | 1 (20) | 511 (47.1) |
| Erze | 260 (33.9) | 34 (31.2) | 28 (28.9) | 22 (30.6) | 3 (13.6) | 0 (0.0) | 2 (40) | 348 (32) |
| Enmba | 143 (18.7) | 7 (6.4) | 0 (0.0) | 31 (43.0) | 0 (0.0) | 13 (86.6) | 1 (20) | 196 (18) |
| Kurishata | 1 (0.13) | 0 (0) | 0 (0.0) | 0 (0.0) | 0 (0.0) | 1 (6.7) | 0 (0.0) | 2 (0.2) |
| Muja | 23 (3.0) | 2 (1.8) | 0 (0.0) | 2 (2.8) | 0 (0.0) | 1 (6.7) | 1 (20) | 29 (2.7) |
| Total | 766 (70.5) | 109 (10) | 97 (8.9) | 72 (6.6) | 22 (2.0) | 15 (1.4) | 5 (0.5) | 1086 |
Anarb = An. arabiensis, Anpha = An. pharoensis, Anten = An. tenebrosus, Anfun = An. funestus-group, Anzim = An. ziemanni, Andem = An. demeilloni, Anprt = An. pretoriensis.
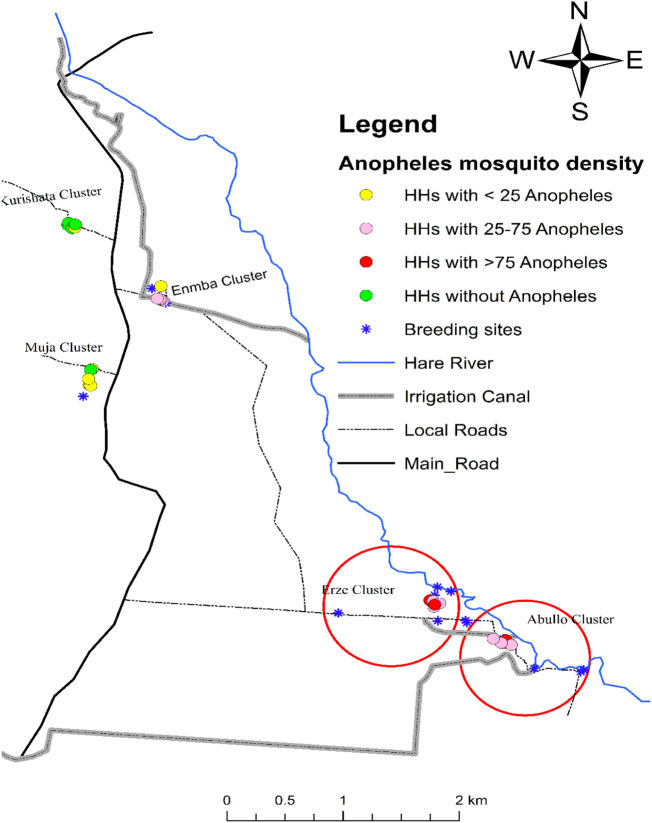
In addition, Anopheles mosquito density in Kolla Shara village varied among the sub-villages. About half of the Anopheles mosquitoes were collected from Abullo (47.1%: 511 of 1086) and followed by Erze (32.0%: 348 of 1086) sub-villages. The difference in mosquito density was statistically significant (F = 4.4, DF = 4, P = 0.001). In Erze and Abullo sub-villages, all the sampling houses were positive for Anopheles mosquitoes (Fig. 6).
Fig. 6.
The distribution of Anopheles mosquitoes per household in the five sub-villages of Kolla Shara village, southwestern Ethiopia. Anopheles mosquito density was higher in circled sub-villages.
3.6. Plasmodium infection rates in Anopheles mosquitoes.
733 (645 An. arabiensis and 88 An. pharoensis) mosquitoes were tested for Plasmodium CSPs. A substantial number of An. arabiensis was found to be positive for P. falciparum (1.2%, 8 of 645). Both Abullo (1.7%, 5 of 299) and Erze (1.4%, 3 of 220) sub-villages accounted for the falciparum cases. This result is consistent with the human infection detected. None was positive in the case of An. pharoensis. This study reported an overall CSP rate of 1.1% (8 of 733). No An. arabiensis was positive for P.vivax_210 CSP. The other sub-villages remained negative for Plasmodium CSPs throughout the survey period (Table 4).
Table 4.
Infection rate of An. arabiensis and An. pharoensis in five sub-villages, of Kolla Shara village, southwestern Ethiopia, July to December 2016.
| Sub-villages |
An. arabiensis |
An. pharoensis |
Total Anopheles tested | No. of CSP positive An. arabiensis (%) | Overall CSP rate (%; 95% CI) | ||
|---|---|---|---|---|---|---|---|
| No. collected | No tested | No. collected | No tested | ||||
| Abullo | 339 | 299 | 66 | 56 | 355 | 5 (1.7) | 1.4 (0.5–3.3) |
| Erze | 260 | 220 | 34 | 23 | 243 | 3 (1.4) | 1.2 (0.3–3.6) |
| Enmba | 143 | 106 | 7 | 7 | 113 | 0.0 | 0.0 |
| Kurishata | 1 | 1 | 0 | 0 | 1 | 0.0 | 0.0 |
| Muja | 23 | 19 | 2 | 2 | 21 | 0.0 | 0.0 |
| Total | 766 | 645 | 109 | 88 | 733 | 8 (1.2) | 1.1 (0.5–2.1) |
3.7. Computed entomological inoculation rate
The overall estimated P. falciparum EIR of An. arabiensis in the Kolla Shara village was 5.7 infectious bites per person (ib/p)/six-months. There was variation in EIR between sub-villages. The EIR of An. arabiensis was 17.0 ib/p/6 months in Abullo, whereas it was 10.6 ib/p/6 months in Erze sub-village (Table 5). These two sub-villages also had a higher density of malaria vectors.
Table 5.
The entomological inoculation rate (EIR) of An. arabiensis in five sub-villages of Kolla Shara village, southwestern Ethiopia, July to December 2016.
| Sub-villages | No. collected | No. assayed for CSP | No. positive (CSP rate %) | PfEIR (95% CI) |
|---|---|---|---|---|
| Abullo | 339 | 299 | 5 (1.7) | 17.0 (15.6–19.7) |
| Erze | 260 | 220 | 3 (1.4) | 10.6 (9.4–13.7) |
| Enmba | 143 | 106 | 0 (0.0) | 0.0 |
| Kurishata | 1 | 1 | 0 (0.0) | 0.0 |
| Muja | 23 | 19 | 0 (0.0) | 0.0 |
| PfEIR of village | 766 | 645 | 8 (1.2) | 5.7 (5.2–6.5) |
Pf EIR = (No. days in six month/total no. of catches) × (No. Pf positive An. arabiensis from CDC light trap + (No. Pf positive mosquito/No. ELISA tested) × (No. An. arabiensis collected from CDC light trap - No. ELISA tested). CI = Confidence interval; CSP = Circum-sporozoite protein; SR = Sporozoite rate; PfEIR = P. falciparum entomological inoculation rate.
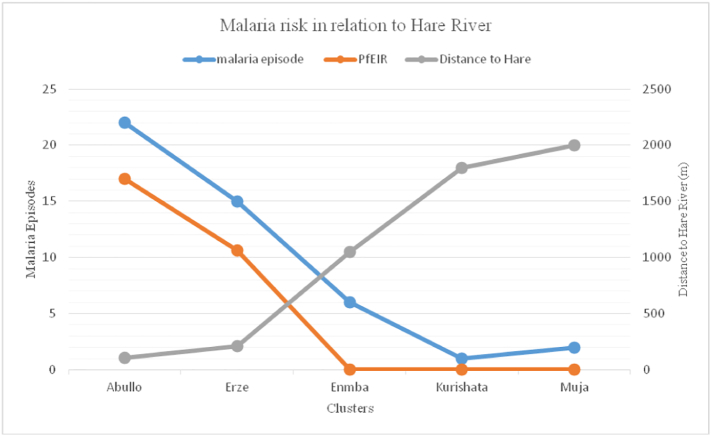
3.8. Malaria risk in relation to proximity to Hare River
The malaria risk was higher in two sub-villages which are close to the river Hare than did Muja, Kurishata and Enmba sub-villages (Fig. 7).
Fig. 7.
Malaria cases and Pf EIR in relation to proximity to Hare River among the five study sub-villages of Kolla Shara village, southwestern Ethiopia.
4. Discussion
The present study demonstrated that malaria infection is clustered in smaller residential areas in both human and mosquito hosts in lowland southwestern Ethiopia. Malaria risk appears to be higher in school-age children and most of the cases were accounted by two sub-villages. About a third of the study households from two sub-villages were sources of cumulative malaria incidence in six months follow up study. As a caveat of this study that could be addressed in future research is considering a longer duration of follow up and covering more areas.
Malaria infection is clustered in smaller residential areas in both human and mosquito hosts. This phenomenon is widely reported elsewhere (Clark et al., 2008; Bousema et al., 2010; Creasey et al., 2004). The heterogeneity of malaria exposure within short distances was reported in previous studies (Bousema et al., 2010; Cook et al., 2016). Furthermore, this finding is in line with other studies that showed clustering of malaria episodes at the level of an individual households (Gaudart et al., 2006), between adjoining villages (Oduro et al., 2013) and larger geographical areas (Bousema et al., 2010; Bejon et al., 2010). The current spatial heterogeneity in malaria risk may be justified by the relative distance of the household from malaria vectors breeding sites (the distance from the Hare River). This finding was supported by the analysis showing the significant effect of proximity of breeding sites on the malaria infection. The strong relation between malaria incidence and the distance to a river (as a potential vector breeding site) has been also observed elsewhere (Machault et al., 2009). Hence, since the end game will likely require efforts to be concentrated mostly in communities that live close to and primarily benefit from the river.
In the present study, P. falciparum and P. vivax were the two common cause of malaria infection in the village, but, P. falciparum was slightly dominant compared to P. vivax. This scenario was comparable to the report from Ethiopia (FMOH, 2012). A similar finding was also reported from the nearby village in south-west Ethiopia (Loha and Lindtjørn, 2012). The incidence of malaria species however varied between study sub-villages that P. falciparum was the dominant parasite species in sub-villages with higher malaria incidence (Abullo and Erze sub-villages). This dominance indicates active malaria transmission in these sub-villages. We also confirmed the existence of active malaria transmission by incriminating sporozoite positive malaria vector. On the other hand, in the Enmba sub-village P. vivax was the dominant species which accounted for 83.3% of total malaria episodes. The high P. vivax parasite may not indicate active transmission as it can survive in the host for a long time and relapse (Mendis et al., 2001; Wells et al., 2010). Moreover, we didn't find CSP positive malaria vectors in these sub-villages.
Although the malaria incidences were observed in all age groups, the greater malaria risk was observed in the children age group 5–14 years whereas fewer malaria cases were documented in the age group ≥15 years. This finding was comparable with previous studies in south-central and south-west Ethiopia (Peterson et al., 2009; Woyessa et al., 2013). The higher risk of malaria infection in this age group may be associated with lower bed net use rate (Loha et al., 2012; Rulisa et al., 2013). Additionally, there is a greater likelihood of younger age groups (<5-year-olds) using bed nets compared to their older age groups (Loha et al., 2012). Generally, the findings have been indicated that there is a shift in the rate of acquiring malaria to this age group and they may play a role as a reservoir of infection and fuel transmission.
The entomological survey also revealed variation in the density of malaria vectors a small village. The relative density of Anopheles mosquitoes was high in Abullo, Erze and Enmbasub-villages which are close to Hare River; the potential breeding sites to mosquitoes (Massebo et al., 2013). The Hare River is located within the distance of 60-250 m to the immediate households from the Abullo, Erze and Enmbasub-villages. Moreover, the CSP rate was varied between the study sub-villages. Abullo and Erze sub-villages had higher P. falciparum CSP rate. The same sub-villages had higher EIR than other three sub-villages. These findings clearly show an increased exposure to infectious mosquito bites is evidence of the higher malaria transmission. The variation in the level of exposure to the malaria parasite-infected mosquito in small scale has been described elsewhere (Bousema et al., 2010; Kilama et al., 2014). Thus, EIR is a sensitive and candid tool to identify small geographical areas with higher malaria transmission intensity that measures the intensity of malaria parasite transmission by vectors and also in monitoring the impact of malaria vector control interventions (Shaukat et al., 2010). Moreover, the overall estimated P. falciparum EIR of An. arabiensis (5.7 ib/p/6 months) indicating that the village is endemic for malaria transmission as documented in the nearby village in the same region (Abraham et al., 2017). Generally, the findings of the current study confirm clustering of malaria exposure at a small village which may help to provide better knowledge on how the disease transmission is interrupted by targeting those at higher malaria risk.
As it is a case in other studies this study has its own strengths and limitations. As a strength, malaria infection in human and detection of infectious mosquito was done prospectively using active case surveillance. In addition, most of the participants completed their follow up survey. In contrast, the limitations of this study include the relatively small sample size used for parasitological study and the limited period of observation which may not allow assessing the stability of clusters. This was due to limited resources.
5. Conclusions
More clustering of malaria infection is observed and hence focused interventions in areas with a higher risk of infection may enhance the effective use of limited resources. This study also confirmed the importance of spatial analysis that employ geographical information tools in improving public health actions mainly in malaria control. Future studies could emphasize on longer duration of follow up to see the stability of the clusters and considering more sites that improve the number of participants.
Abbreviations
Funding
This study obtained financial support from The Norwegian Programme for Capacity Development in Higher Education and Research for Development (ETH-13/0025). The funding body played no role in study design, field data collection, data analysis and interpretation, and reporting.
Authors' contributions
EE: participated in the planning and implementation of the study, data collection and entry, analyzed the data, drafting the manuscript. AW: participated in planning the study, and critically commented on the draft manuscript. FM: conceived the study, participated in planning and implementation and data analysis and, gave critical comment on the draft manuscript. All authors read and approved the final manuscript.
Declaration of competing interest
The authors have no competing interests.
Acknowledgements
The authors thank Nigatu Eligo and Gemachu Leta for technical support in mosquito analysis for CSPs. We acknowledge Nigatu Girma for assisting in mosquito collection. We thank the communities of KollaShara for allowing us to collect mosquitoes. Wasu Manawokew is acknowledged for assisting in mapping the study area and households.
References
- Abraham M., Massebo F., Lindtjørn B. High entomological inoculation rate of malaria vectors in area of high coverage of interventions in southwest Ethiopia: implication for residual malaria transmission. Parasite Epidemiol. Control. 2017;2:61–69. doi: 10.1016/j.parepi.2017.04.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Baidjoe A., Stevenson J.P.K., Stone W., Stresman G., Osoti V., Makori E., Owaga C., Odongo W., China P. Factors associated with high heterogeneity of malaria at fine spatial scale in the Western Kenyan highlands. Malar. J. 2016;15:307. doi: 10.1186/s12936-016-1362-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Beier J.C., Perkins P.V., Wirtz R.A., Whitmire R.E., Mugambi M., Hockmeyer W.T. Field evaluation of an Enzyme-Linked Immunosorbent Assay (ELISA) for Plasmodium falciparumsporozoite detection in anopheline mosquitoes from Kenya. Am. J. Trop. Med. Hyg. 1987;36:459–468. doi: 10.4269/ajtmh.1987.36.459. [DOI] [PubMed] [Google Scholar]
- Bejon P., Williams T., Liljander A., Noor A., Wambua J., Ogada E. Stable and unstable malaria hotspots in longitudinal cohort studies in Kenya. PLoS Med. 2010;7 doi: 10.1371/journal.pmed.1000304. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bousema T., Drakeley C., Gesase S., Hashim R., Magesa S., Mosha F. Identification of hotspots of malaria transmission for targeted malaria control. J. Infect. Dis. 2010;201:1764–1774. doi: 10.1086/652456. [DOI] [PubMed] [Google Scholar]
- Bousema T., Griffin J.T., Sauerwein R.W., Smith D.L., Churcher T.S., Takken W. Hitting hotspots: spatial targeting of malaria for control and elimination. PLoS Med. 2012;9 doi: 10.1371/journal.pmed.1001165. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Clark T.D., Greenhouse B., Njama-Meya D., Nzarubara B., Maiteki-Sebuguzi C., Staedke S.G. Factors determining the heterogeneity of malaria incidence in children in Kampala, Uganda. J. Infect. Dis. 2008;198:393–400. doi: 10.1086/589778. [DOI] [PubMed] [Google Scholar]
- Cook J., Grignard L., Al-Eryani S.M.A., Al-Selwei M., Mnzava A.P., Al-Yarie H. High heterogeneity of malaria transmission and a large sub-patent and diverse reservoir of infection in Wusab As Safil district, Republic of Yemen. Malar. J. 2016;15:193. doi: 10.1186/s12936-016-1249-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Creasey A., Giha H., Hamad A.A., El Hassan I.M., Theander T.G., Arnot D.E. Eleven years of malaria surveillance in a Sudanese village highlights unexpected variation in individual disease susceptibility and outbreak severity. Parasitology. 2004;129:263–271. doi: 10.1017/s0031182004005724. [DOI] [PubMed] [Google Scholar]
- Gaudart J., Poudiougou B., Dicko A., Ranque S., Toure O., Sagara I. Spacetime clustering of childhood malaria at the household level: a dynamic cohort in a Mali village. BMC Public Health. 2006;6:286. doi: 10.1186/1471-2458-6-286. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gillies M.T., Coetzee M. A supplement to the Anophelinea of Africa south of the Sahara (Afrotropical region) Publications of the South African Institute for Medical Research. 1987;55:1–143. [Google Scholar]
- Kilama M., Smith D., Hutchinson R., Kigozi R., Yeka A., Lavoy G. Estimating the annual entomological inoculation rate for Plasmodium falciparum transmitted by Anopheles gambiae s.l. using three sampling methods in three sites in Uganda. Malar. J. 2014;13:111. doi: 10.1186/1475-2875-13-111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kreuels B., Kobbe R., Adjei S., Kreuzberg C., von Reden C., Bäter K. Spatial variation of malaria incidence in young children from a geographically homogeneous area with high endemicity. J. Infect. Dis. 2008;197:85–93. doi: 10.1086/524066. [DOI] [PubMed] [Google Scholar]
- Lines J., Curtis C., Wilkes T., Njunwa K. Monitoring humanbiting mosquitoes (Diptera: Culicidae) in Tanzania with light traps hung beside mosquito nets. Bull. Entomol. Res. 1991;81:77–84. [Google Scholar]
- Loha E., Lindtjørn B. Predictors of Plasmodium falciparummalaria incidence in Chano Mille, south Ethiopia: a longitudinal study. Am. J. Trop. Med. Hyg. 2012;87:450–459. doi: 10.4269/ajtmh.2012.12-0155. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Loha E., Lunde T.M., Lindtjørn B. Effect of bed nets and indoor residual spraying on spatio-temporal clustering of malaria in a village in South Ethiopia: alongitudinal study. PLoS One. 2012;7 doi: 10.1371/journal.pone.0047354. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Machault V., Gadiaga L., Vignolles C., Jarjaval F., Bouzid S., Sokhna C. Highly focused anopheline breeding sites and malaria transmission in Dakar. Malar. J. 2009;8:138. doi: 10.1186/1475-2875-8-138. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Massebo F., Balkew M., Gebre-Michael T., Lindtjørn B. Entomologic inoculation rates of Anopheles arabiensis in South-Western Ethiopia. Am. J. Trop. Med. Hyg. 2013;89:466–473. doi: 10.4269/ajtmh.12-0745. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mendis K., Sina B., Marchesini P., Carter R. The neglected burden of Plasmodium vivax malaria. Am. J. Trop. Med. Hyg. 2001;64:97–106. doi: 10.4269/ajtmh.2001.64.97. [DOI] [PubMed] [Google Scholar]
- Midega J., Smith D., Olotu A., Mwangangi J., Nzovu J., Wambua J. Wind direction and proximity to larval sites determines malaria risk in Kilifi District in Kenya. Nat. Commun. 2012;3:674. doi: 10.1038/ncomms1672. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Oduro A.R., Conway D.J., Schellenberg D., Satoguina J., Greenwood B.M., Bojang K.A. Seroepidemiological and parasitological evaluation of the heterogeneity of malaria infection in the Gambia. Malar. J. 2013;12:222. doi: 10.1186/1475-2875-12-222. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Peterson I., Borrell L., El-Sadr W., Teklehaimanot A. Individual and household level factors associated with malaria incidence in a highland region of Ethiopia: amultilevel analysis. Am. J. Trop. Med. Hyg. 2009;80:103–111. [PubMed] [Google Scholar]
- Rulisa S., Kateera F., Bizimana J.P., Agaba S., Dukuzumuremyi J., Baas L. Malaria prevalence, spatial clustering and risk factors in a low endemic area of eastern Rwanda: across-sectional study. PLoS One. 2013;8 doi: 10.1371/journal.pone.0069443. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shaukat A., Breman J., Mckenzie E. Using the entomological inoculation rate to assess the impact of vector control on malaria parasite transmission and elimination. Malar. J. 2010;9:122. doi: 10.1186/1475-2875-9-122. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wells T., Burrows J., Baird J. Targeting the hypnozoite reservoir of Plasmodium vivax: the hidden obstacle to malaria elimination. Trends Parasitol. 2010;26:145–151. doi: 10.1016/j.pt.2009.12.005. [DOI] [PubMed] [Google Scholar]
- Woyessa A., Deressa W., Ali A., Lindtjørn B. Prevalence of malaria infection in Butajira area, south-central Ethiopia. Malar. J. 2012;11:84. doi: 10.1186/1475-2875-11-84. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Woyessa A., Deressa W., Ali A., Lindtjørn B. Malaria risk factors in Butajira area, south-central Ethiopia: a multilevel analysis. Malar. J. 2013;12:273. doi: 10.1186/1475-2875-12-273. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yeshiwondim A., Gopal S., Hailemariam A., Dengela D., Patel H. Spatial analysis of malaria incidence at the village level in areas with unstable transmission in Ethiopia. Int. J. Health Geogr. 2009;8:5. doi: 10.1186/1476-072X-8-5. [DOI] [PMC free article] [PubMed] [Google Scholar]