Abstract
Genic male sterility (GMS) is a common and important trait, which is widely used for the production of hybrid seeds. However, the molecular mechanism of GMS in watermelon remains poorly understood. In this study, we comparatively analyzed the transcriptome profiles of sterile and fertile floral buds using the bulked segregant analysis (BSA) and transcriptome sequencing (RNA-seq). A total of 2507 differentially expressed genes (DEGs) including 593 up-regulated and 1914 down-regulated, were identified to be related to male sterility in watermelon line Se18. Gene ontology (GO) analysis showed that 57 GO terms were significantly enriched, while Kyoto Encyclopedia of Genes and Genomes (KEGG) pathway enrichment analysis revealed plant hormone signal transduction, glycolysis/gluconeogenesis, starch and sucrose metabolism, plant–pathogen interaction, phenylpropanoid biosynthesis pathways were obviously enriched. Furthermore, the efficiency of the RNA-seq analysis was validated by quantitative real-time PCR (qRT-PCR). Among the DEGs, some valuable candidate genes involved in pollen development were identified, such as gene Cla000029, a bHLH transcription factor and homologous to MS1 in Arabidopsis. Moreover, other DEGs including MYB gene Cla012590 (MYB26), Cla017100 (MYB21), etc., also provide useful information for further understanding the function of key genes involved in pollen development. This study provides new insights into the global network of male sterility in watermelon.
Electronic supplementary material
The online version of this article (10.1007/s13205-020-02208-2) contains supplementary material, which is available to authorized users.
Keywords: Watermelon, Male sterile, Transcriptome, Degs, BSR-seq
Introduction
Sterility of male plants is a common phenomenon in nature and refers to the lack of functional pollen production in plants. Sterility can be harnessed as an important tool for heterosis (Perez-Prat and Campagne 2002), and can also be used to providing ideal materials in the study of plant reproductive development functional networks (Gómez et al. 2015). According to inheritance or origin, the types of male sterility can be classified into three categories: genic male sterility (GMS), cytoplasmic male sterility (CMS), and cytoplasmic–genic male sterility (CGMS) (Rhee et al. 2015). GMS is an important and inherited trait that widely occurs in plants, and can be characterized by an inability to produce functional pollen, while leaving female flower fertility intact (Fang et al. 1995). It has been occasionally implemented as an effective and economic system for hybrid seed production in many plants (Kley 1954) such as cabbage (Guo et al. 2016), pepper (Chen et al. 2015), and tomato (Atanassova 2000). In addition to changes in cytological characteristics and stamens structures, the process of pollen and male gametophyte dysplasia in GMS is complex involving numerous gene expressions. Changes in expression pattern of these genes leads to changes at various metabolic levels, including the protein, starch and sugar, cell wall synthesis and extension, sugar and lipid transport, endogenous hormones, and free amino acid levels, which eventually results in sterility of male plants (Zhao and Ma 2000; Liu et al. 2014; Wu et al. 2014).
In melon (Cucumis melo L.), aborted microspores (AMS) were predicted as a candidate gene, responsible for single nuclear male sterility. This gene encodes a basic helix–loop–helix (bHLH) transcription factor, which is required for tapetal cell development and postmeiotic microspore formation (Sheng et al. 2017). The recessive gene BnMs3 controls the GMS line 7365AB, which is an epistatic gene (BnRf) in B. napus. Moreover, BnMs3 is essential for both tapetal function and microspore development in B. napus (Dun et al. 2011; Zhou et al. 2011). In Arabidopsis thaliana, previous studies reported that anther development involved numerous important genes. The gene Sporocyteless/nozzle (SPL/NZZ) is involved in cell differentiation and division of anther cell walls and encodes a protein related to MADS box transcription factors (Ito et al. 2004; Liu et al. 2009). In conclusion, these studies identified and analyzed numerous genes related to pollen development, and revealed that the effect of certain important genes could result in male sterility.
The watermelon (Citrullus lanatus (Thunb.) Matsum. & Nakai) is an important summer fruit crop around the world with high economic and nutritional value (Guo et al. 2011). For production, the application of heterosis in watermelon plants is apparent for both improved fruit quality and increased fruit yield; and the utilization of male sterile lines is effective for the production of hybrid seeds (Guo et al. 2016). However, very few studies have focused on the molecular mechanism of GMS in watermelon during the developmental stage of pollen. In this study, we adopted the use of BSA and RNA-seq technologies (Liu et al. 2012). We collected floral buds during a certain period to form sample pools for transcriptome sequencing, and analysis of GMS-related gene expressions and pathways. This study obtained reliable differentially expressed genes, and laid a specific foundation for both subsequent infertility gene identification and cloning.
Materials and methods
Plant materials
The spontaneous male sterile mutant Se18 from watermelon inbred line ‘Sugarlee’ (Zhang et al. 2005a) was found in 1997 in Shaanxi Province of China, which has been crossed with its sister fertile individuals for more than 20 generations to generate near-isogenic lines. Then, the dominant heterozygotes (male fertile) were used to cross the recessive homozygotes (male sterile) to produce BC1 segregating populations. Floral buds (2–3 mm) (Zhang et al. 2005b; Wang et al. 2016) (Fig. 1) collected from 30 sterile and 30 fertile individuals of a BC1 population were pooled independently to constitute male sterile (Se18-MS) and fertile (Se18-MF) bulks (2 biological replicates for each bulk), which were immediately frozen in liquid nitrogen and then stored at − 80 °C for RNA extraction.
Fig. 1.
Morphologic characteristics of flowers and buds of fertile and sterile watermelon lines. a Se18-MF, genic male fertile; b Se18-MS, genic male sterile. Floral buds are marked by red arrows. Bar = 10 mm
Library construction and RNA-seq analysis
Total RNA was extracted from four floral bud bulks (Se18-MS-1, Se18-MS-2, Se18-MF-1 and Se18-MF-2) using the TRIzol reagent (Invitrogen, Carlsbad, CA, USA) according to the manufacturer’s instructions. All contaminating genomic DNA was removed via RNase-free DNaseI (Takara, Dalian, China). Then, RNA quality was checked using a NanoDrop 2000C Spectrophotometer (Thermo Fisher Scientific, USA) and RNase-free agarose gel electrophoresis. Four libraries (Se18-MS-1, Se18-MS-2, Se18-MF-1 and Se18-MF-2) were constructed with 3 μg RNA per sample, following the protocol of the Gene Expression Sample Prep Kit (Illumina, San Diego, CA, USA). RNA sequencing of four independent libraries was performed on an Illumina HiSeq™ 2500 system (Illumina, San Diego, CA, USA) with paired-end and 125 bp mode by Breeding Biotechnologies Co. (Shaanxi, China). The raw sequence data were deposited in the short-read archive (SRA) of the National Centre for Biotechnology (NCBI) under BioProject accession PRJNA394044.
Data processing and analysis
After removing adapters and low-quality reads, the high-quality clean reads obtained were mapped to the watermelon reference genome “97103” (https://cucurbitgenomics.org/v1) using top-hat software with default parameters (Kim et al. 2013). The mapped clean reads of each gene were counted and standardized into a reads per million sequenced reads (RPKM) value via Cufflinks (Trapnell et al. 2010). DEGs were determined with the software DESeq (Anders and Huber 2010). In this study, an FDR < 0.01 and a fold change ≥ 2 were used to judge the significance of gene expression differences (Wang et al. 2013; Fang et al. 2015; Qu et al. 2015; Su et al. 2015; Liu et al. 2016a, b; Han et al. 2018).
The DEGs were retrieved via BLAST2GO (Conesa et al. 2005) in the GO database and functional categorization was performed via the WEGO software (Ye et al. 2006). Gene ontology (GO) analyses and GO enrichment were implemented via the GO database (Ashburner et al. 2000). Further BLASTX against the Clusters of Orthologous Groups (COG) database was performed to predict and classify functions of the assembled unitranscripts, using Autofact tool (Koski et al. 2005). The Kyoto Encyclopedia of Genes and Genomics (KEGG) orthology database was utilized for pathway mapping (Kanehisa et al. 2004).
The analysis of pollen development related to DEGs were done by the identification of 117 genes related to pollen development from Arabidopsis database (https://www.arabidopsis.org/) using the keyword. Then, these genes were used as query sequences to search against the watermelon database (https://cucurbitgenomics.org/v1) using the BLASTP program with default settings (1e−2). Finally, homologous DEGs associated with pollen development were found.
Transcription factor analysis was performed using the BMKCloud cloud server (https://www.biocloud.net/). TFs were annotated by searching against the Plant Transcription Factor Database (https://planttfdb.cbi.pku.edu.cn/) using the BLAST program with e value setting to 1e−10.
Quantitative real-time (qRT-PCR) analysis
The reliability of DEGs was validated by qRT-PCR using the SYBR Premix Ex TaqTM kit (Takara) on a CFX96 real-time PCR System (Bio-RAD, USA). The total RNA of three independent biological replicates of floral buds from Se18-MS and Se18-MF were extracted and used for the qRT-PCR. cDNA was synthesized from the total RNA. The sequences of the selected genes were retrieved from the watermelon reference genome. The specific primers were designed using Primer Premier 6.0 (Premier Biosoft International, Palo Alto, CA) and ClACT was used as an internal control (Kong et al. 2014) (Table S1). All the qRT-PCR reactions were done in a 20 μl reaction volume, which contained 10.0 μl SYBR Green Premix, 0.8 μl of each primer (10 μM), 1.0 μl cDNA template (80 ng/μl), and 7.4 μl ddH2O. All reactions were carried out in triplicate. The amplification was arranged with the following cycling parameters: 95 °C for 10 min, followed by 40 cycles of: 95 °C for 15 s and 60 °C for 60 s. Expression levels of the relative gene were quantified via the 2–ΔΔCt method (Livak and Schmittgen 2001).
Results
Transcriptome sequencing
Four independent mRNA libraries (Se18-MS-1, Se18-MS-2, Se18-MF-1, and Se18-MF-2) with two biological replicates for each MS and MF sample were sequenced by a service provider Breeding Biotechnologies Co. (Shaanxi, China). Each replicate contained 30 plants. After removing the adaptors and low-quality sequences, a total of 336,574,566 clean reads were obtained (Table 1). The ratio of unique mapped reads varied from 90.43% to 91.68% and the Q30 values of four libraries ranged above 93.86%, indicating that the clean reads had high quality. In addition, the Pearson correlation coefficient was used to further validate the reliability of the obtained four RNA-seq data (Fig. S1). The libraries for the same line (two biological replicates) showed a highly correlated relationship (R2 > 0.9).
Table 1.
Output statistics of sequencing analysis
| Sample | Clean reads | Mapped reads | Unique mapped reads | Unique mapped ratio (%) | Q30 percentage (%) |
|---|---|---|---|---|---|
| Se18-MS-1 | 84,540,406 | 78,968,766 | 77,504,648 | 91.68 | 94.19 |
| Se18-MS-2 | 86,612,632 | 80,504,203 | 78,946,056 | 91.15 | 93.86 |
| Se18-MF-1 | 86,302,804 | 80,467,663 | 78,393,219 | 90.84 | 94.05 |
| Se18-MF-2 | 79,118,724 | 73,675,514 | 71,549,722 | 90.43 | 94.57 |
Functional annotation and classification of differentially expressed genes (DEGs)
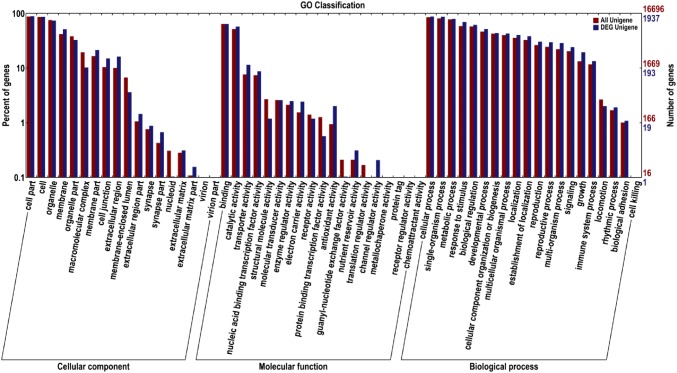
Using the FDR < 0.01 and fold change ≥ 2 as thresholds, a total of 2507 DEGs were identified between sterile (Se18-MS) and fertile (Se18-MF) lines. These included 593 up-regulated and 1914 down-regulated genes in Se18-MS (Table S2). Among the 2507 DEGs, 2443 were annotated via search against the GO (1937), KEGG (451), COG (946), Swiss-Prot (2030), and NR (2443) databases. 1937 DEGs were categorized into three types, namely cellular components (1765), molecular function (1715), and biological processes (1822) (Fig. 2). Moreover, via enrichment analysis, all GO terms were subdivided into 57 sub-categories (corrected P value ≤ 0.05) (Table S3). In the category of cellular components, the three largest annotated groups were “plasma membrane” (GO: 0005886), “plasmodesma” (GO: 0009506), and “membrane” (GO: 0016020). The “iron ion binding” (GO: 0005506), “heme binding” (GO: 0020037), and “peroxidase activity” (GO: 0004601) accounted for the main molecular function of the category. Within the category of biological processes, the three major common functions were “response to salt stress” (GO: 0009651), “oxidation–reduction process” (GO: 0055114), and “response to water deprivation” (GO: 0009414).
Fig. 2.
Gene Ontology Classification of DEGs of the watermelon floral bud transcriptome. The x-axis indicates the GO term. The right side of the y-axis shows the numbers of genes, while the left side of the y-axis shows the percent of genes
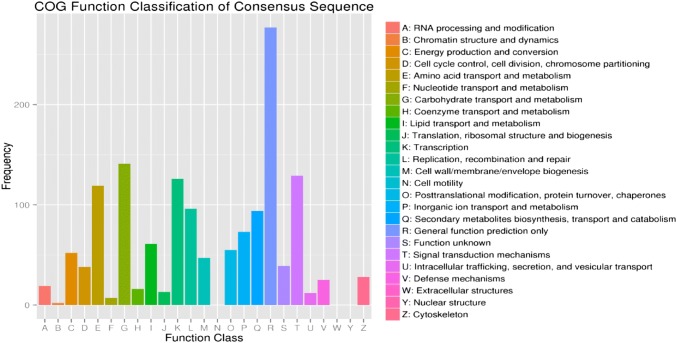
COG analysis was applied in a functional classification and a sum of 946 DEGs were annotated into 22 functional COG categories (e value ≤ 1.0e–5) (Fig. 3). The following top five group categories were found: “general functional predictions only” (277), “carbohydrate transport and metabolism” (141), “signal transduction mechanisms” (129), “transcription” (126), and “amino acid transport and metabolism” (119).
Fig. 3.
Cluster of Orthologous Groups (COG) categories of differentially expressed genes
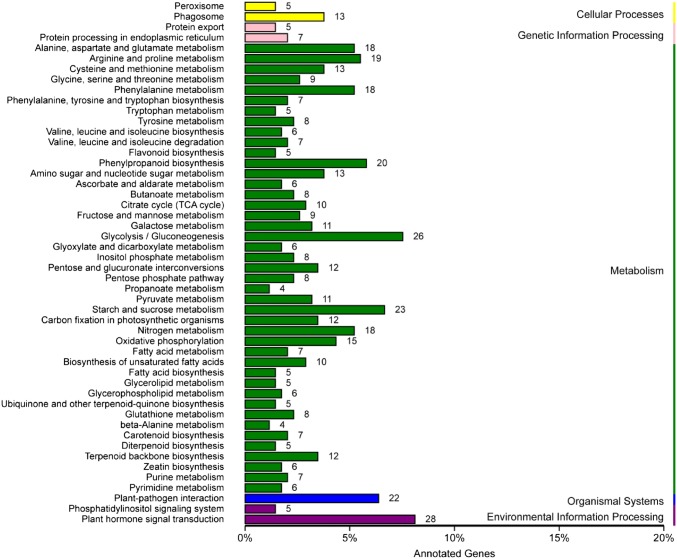
KEGG pathway enrichment analysis was carried out to further evaluate the roles of DEGs for various biological purposes. In this study, a total of 451 DEGs could be assigned to 95 different biochemical pathways (Table S4). The top five pathways that contained the most DEGs were “plant hormone signal transduction” (n = 28; 6.2%), “glycolysis/gluconeogenesis” (n = 26; 5.8%), “starch and sucrose metabolism” (n = 23; 5.1%), “plant–pathogen interaction” (n = 22; 4.9%) and “phenylpropanoid biosynthesis” (n = 20; 4.4%) (Fig. 4).
Fig. 4.
Classification based on the categories of KEGG pathways. The x-axis indicates the number of DEGs in a function class and the number of gene accounts for the total number of genes in the annotation; the y-axis indicates the function classes
Analysis of plant hormone-related differentially expressed genes
Plant hormones play central roles in the regulation of male sterility (Nakajima et al. 1991; Gaillard et al. 1998; Cheng et al. 2004; Depuydt and Hardtke 2011). Thus, a total of 28 DEGs (that had been identified via KEGG analysis) were further found to be involved in phytohormone signal transduction, including auxin (11 genes), cytokinin (6 genes), abscisic acid (4 genes), ethylene (2 genes), jasmonic acid (2 genes), brassinosteroid (2 genes), and salicylic acid (1 gene) related pathways (Table S5).
The auxin-responsive protein (AUX/IAA) is known to regulate auxin-responsive genes (Tiwari et al. 2004). In our study, an auxin-responsive gene AUX/IAA (Cla015414) was found to be down-regulated. The transcript levels of genes that were involved in the ethylene-signaling pathway were up-regulated in Se18-MS, such as ethylene-insensitive 3 (EIN3) (Cla013662) and ethylene response factor 1/2 (ERF1/2) (Cla021525). In the present study, the gene Cla016899 was found to be down-regulated in the sterile plants Se18-MS. It is homologous to the cytokinin receptor gene CRE1, which could be activated by cytokines to initiate phosphorelay signaling (Inoue et al. 2001). Moreover, both Cla001487 and Cla018445 share high similarity with the jasmonic acid co-receptor protein JAZ (Kazan and Manners 2012) and were also down-regulated in Se18-MS. In addition, the transcripts of biosynthetic pathways of brassinosteroid, abscisic acid, and salicylic acid were also affected in Se18-MS.
Analysis of DEGs related to floral development
Pollen development is a complex process that plays a central role in plant reproductive growth. Using genes that function in the pollen development of Arabidopsis as queries, a series of homologous DEGs have been found in watermelon, such as Cla000029 (MS1), Cla011600 (MS2), Cla004641 (AGP6), Cla012590 (MYB26), Cla007809 (CalS5), Cla019144 (TDF1), Cla021361 (CER1), Cla017100 (MYB21), Cla022956 (ACOS5), Cla003353 (CYP704B1) and Cla004479 (WBC27) (Table 2), and so on. Male sterility 1 (MS1), tapetal development function 1 (TDF1), ABC trans-porters family genes (WBC27), AGP6, MYB26 and MYB21, etc., play important roles in tapetal development, pollen wall development and pollen production (Yang et al. 2007a; Coimbra et al. 2009; Song et al. 2011; Xu et al. 2014; Qu et al. 2015), were down-regulated. Male sterility 2 (MS2), Acyl-CoA synthetase 5 (ACOS5) and CYP704B1 shown to be essential for the biosynthesis of sporopollenin during another development (Aarts et al. 1997; De et al. 2009; Dobritsa et al. 2009; Chen et al. 2011), were also down-regulated in Se18-MS. Callose synthase 5 (CalS5) was down-regulated and shown to be essential for the completion of the outer wall of the pollen wall (Dong et al. 2005). CER1 which plays an important role in wax deposition of pollen epidermis (Aarts et al. 1995), was also down-regulated. With all these genes proven to show an association with pollen development, their apparent downregulation in Se18-MS might result in pollen abortion and male sterility.
Table 2.
Functional analysis of homologous genes involved in some genes related to flower development
| Gene ID | FDR | log2FC | Arabidopsis genes | Function of homologous gene |
|---|---|---|---|---|
| Cla000029 | 9.91E−145 | − 8.77804 | MS1 | Sporophytic factor controlling anther and pollen development. Mutants fail to make functional pollen; pollen degeneration occurs after microspore release and the tapetum also appears abnormally vacuolated. Similar to PHD-finger motif transcription factors |
| Cla011600 | 5.84E−204 | − 9.92015 | MS2 |
Jojoba acyl-CoA reductase-related male sterility protein Similar to fatty acid reductases |
| Cla004641 | 4.28E−20 | − 9.86469 | AGP6 | Encodes an arabinogalactan protein that is expressed in pollen, pollen sac and pollen tube. Loss of AGP6 function results in decreased fertility due to defects in pollen tube growth |
| Cla012590 | 2.15E−36 | − 5.06038 | MYB26 | Encodes a putative transcription factor (MYB26) |
| Cla007809 | 2.19E−42 | − 2.47509 | CalS5 | Responsible for the synthesis of callose deposited at the primary cell wall of meiocytes, tetrads and microspores. Required for exine formation during microgametogenesis and for pollen viability. Highest expression in meiocytes, tetrads, microspores and mature pollen |
| Cla019144 | 5.07E−42 | − Inf | TDF1 | Member of the R2R3 factor gene family |
| Cla021361 | 3.34E−07 | − 1.05674 | CER1 | Expression of the CER1 gene associated with production of stem epicuticular wax and pollen fertility. Biochemical studies showed that cer1 mutants are blocked in the conversion of stem wax C30 aldehydes to C29 alkanes, and they also lack the secondary alcohols and ketones. These suggested the CER1 protein is an aldehyde decarbonylase, but the exact molecular function of this protein remains to be determined |
| Cla017100 | 1.44E−93 | − 3.96942 | MYB21 | Encodes a member of the R2R3-MYB transcription factor gene family. Induced by jasmonate. Involved in jasmonate response during stamen development |
| Cla022956 | 2.55E−196 | − 7.35899 | ACOS5 | Encodes an acyl-CoA synthetase, has in vitro activity towards medium- to long-chain fatty acids and their hydroxylated derivatives. Expressed in the tapetum. Involved in pollen wall exine formation. Null mutants were devoid of pollen grains at anther maturity and were completely male sterile |
| Cla003353 | 2.56E−15 | − 12.0827 | CYP704B1 | Encodes a cytochrome P450, designated CYP704B1. Expressed in the developing anthers. Essential for pollen exine development. Mutations in CYP704B1 result in impaired pollen walls that lack a normal exine layer and exhibit a characteristic striped surface, termed zebra phenotype. Heterologous expression of CYP704B1 in yeast cells demonstrated that it catalyzes omega-hydroxylation of long-chain fatty acids, implicating these molecules in sporopollenin synthesis |
| Cla004479 | 1.64E−127 | − 5.94523 | WBC27 | Encodes an ATP-binding cassette transporter G26 (ABCG26) involved in tapetal cell and pollen development. Required for male fertility and pollen exine formation |
Analysis of DEGs related to transcription factors
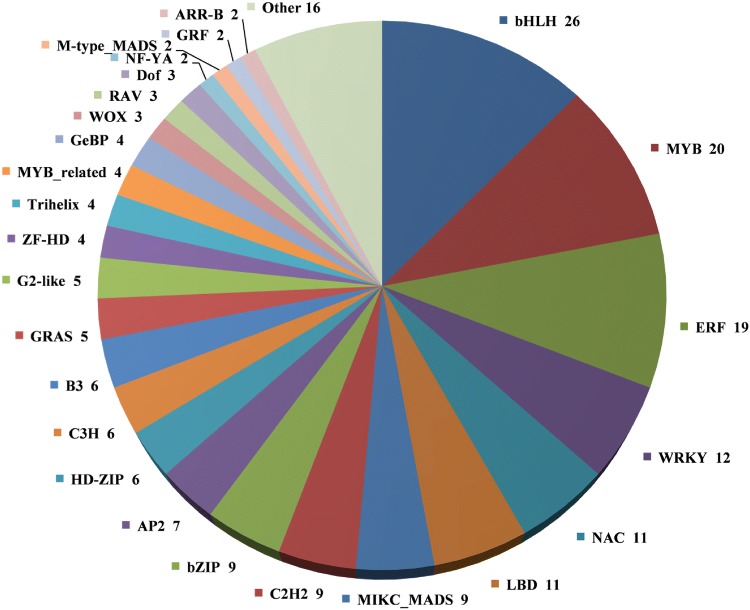
Based on the plant transcription factor database PlnTFDB, 210 DEGs were annotated as transcription factor genes, which belong to 41 families such as bHLHs, MYBs, ERFs, WRKYs, NACs, LBDs, bZIPs, and C2H2s (Fig. 5, Table S6). Among them, a total of 26 DEGs were annotated to the bHLH transcription family, which was the transcription family with the most DEGs. In the MYB transcription family, 20 DEGs were annotated, including 13 down-regulated and 7 up-regulated genes in the sterile plants Se18-MS. There were 19 DEGs annotated into the ERF transcription family. In the WRKY transcription family, 12 DEGs were annotated, including 1 down-regulated and 11 up-regulated genes in Se18-MS. As well as NAC and LBD transcription family, 11 DEGs were annotated, respectively.
Fig. 5.
Family assignment of the 210 differentially expressed TFs
Real-time quantitative PCR validation of the RNA-seq results
To confirm the expression profiles obtained via Illumina sequencing 20 randomly selected genes related to transcriptional regulation, hormone effect, antioxidant activity and pollen development were selected to carry out quantitative real-time PCR (qRT-PCR) for the validation of RNA-seq data, including 12 up-regulated and 8 down-regulated genes in the sterile plants Se18-MS. The data indicated that the results of qRT-PCR were generally uniform with the transcriptome sequencing data. In Fig. 6, the relative expression levels of the 12 genes were significantly increased in Se18-MS, reaching a significant level (P < 0.05). The relative expression levels of the eight genes were significantly reduced in Se18-MS (P < 0.05).
Fig. 6.
Validation of gene expression via qRT-PCR. The relative expression levels of 20 DEGs are shown. Student’s t test: *P < 0.05. Error bars indicate mean ± SE
Discussion
Watermelon is an important summer crop around the world with high economic and nutritional value. The exploitation of heterosis in watermelon can not only efficiently increase the yield, but also improve tolerance against environmental stresses. Genic male sterility (GMS) is a common and important trait in crops, which is widely used to produce hybrid watermelon seeds.
Validation of RNA-seq data and identification of candidate gene
In this study, we identified 2507 DEGs between the male sterile watermelon line Se18-MS and its near isogenic fertile line Se18-MF using Bulked Segregant RNA-Seq (BSR-Seq). We verified the reliability of the four RNA-seq data obtained by Pearson correlation coefficient. The libraries for the same line (two biological replicates) showed a highly correlated relationship (R2 > 0.9). The weak correlation across different lines (sterile and fertile) suggests that the expression levels of a large number of genes affected was between Se18-MS and Se18-MF genotypes. Furthermore, the reliability of the transcriptome data obtained by Illumina sequencing in this study was confirmed by the expression pattern of all the selected genes measured in qRT-PCR according to that described by Rhee et al. (2015). The BSA used in this study to select the material having different backgrounds, obtained more reliable differentially expressed genes, and laid a specific foundation for both subsequent infertility gene identification and cloning.
Fertility in plants has always been a major point of interest in the field of plant reproductive biology. In plants, all mutations that occur in the process of male organ development might lead to the phenomenon of male sterility (Feys et al. 1994). Based on the anther development period and impairment, several major processes can lead to male sterility, including abnormal meiosis, callose metabolism disorder, tapetum dysplasia, pollen sac wall dysplasia, and abnormal anther dehiscence (Liu et al. 2016a, b). Several important genes have been identified in Arabidopsis which have been shown to play an important role in tapetum development and pollen production. In this study, the Cla000029 (MS1), Cla011600 (MS2), Cla004641 (AGP6), Cla012590 (MYB26), Cla007809 (CalS5), Cla019144 (TDF1), Cla021361 (CER1), Cla017100 (MYB21), Cla022956 (ACOS5), Cla003353 (CYP704B1) and Cla004479 (WBC27) were found to be down-regulated in Se18-MS when compared to Se18-MF. MS1 and TDF1 play important roles in tapetal development and programmed cell death (Wilson and Da-Bing 2009), and studies have revealed that MS1 plays crucial roles during the early stages of anther development in Arabidopsis (Yang et al. 2007a). TDF1 encodes an R2R3 MYB transcription factor, which is highly expressed in the tapetum, sex mother cells, and microspores during the development of anthers. It is a key gene that controls the breakdown of callose and affects the differentiation and function of the tapetum (Zhu et al. 2008). WBC27 is essential for pollen wall development and associated with secretion of sporopollenin during flowering (Xu et al. 2010, 2014). MS2, ACOS5 and CYP704B1 were shown to be essential for the biosynthesis of sporopollenin during another development (Aarts et al. 1997; De et al. 2009; Chen et al. 2011; Dobritsa et al. 2009), with CYP704B1 associated with secretion of sporopollenin during flowering. MS2 and ACOS5 which are involved in another development (Wijeratne et al. 2007). In addition, MS1, MS2, ACOS5 and WBC27, which are involved in the fatty acid biosynthesis and metabolism and related to the synthesis of sporopollenin precursors or pollen wall development by regulating lipid acyl metabolism (Yang et al. 2007a; Chen et al. 2011; De et al. 2009; Xu et al. 2010, 2014). CalS5 encodes a callose synthase, which is related to the formation of callus in the blastoblast, tetrad, and microspore primary cell wall, and the expression of CalS5 is necessary for the construction of the outer wall of the pollen wall (Dong et al. 2005). Arabinogalactan proteins (AGPs) have been implicated in different developmental processes, including pollen tube growth. AGP6 encodes cell wall-associated AGPs and research showed that AGP6 was specifically expressed in stamens, pollen grains, and pollen tubes in Arabidopsis. Consequently, AGP6 gene has been demonstrated to have a key role in pollen tube growth and stamen function (Levitin et al. 2008). MYB26 plays a critical role in the anther endothecium and succeeding dehiscence in the development of secondary thickening, and its expression was down-regulated in the Arabidopsis ms35 mutation (Steiner‐Lange et al. 2003; Yang et al. 2007b). CER1 has an important role in wax deposition of pollen epidermis and pollen fertility (Aarts et al. 1995). MYB21 was shown to regulate stamen development and plays a dominant role in stamen filament elongation (Mandaokar et al. 2006; Cheng et al. 2009) and research has shown that the myb21 myb24 double mutant exhibited defects specifically in pollen maturation, anther dehiscence, and filament elongation leading to male sterility (Song et al. 2011). Overall, these genes play important roles in flowering, and would therefore enable a better understanding of gene expression patterns during flowering in watermelon. Importantly, these genes provide clues as to the identities of genes involved in genic male sterility (Fig. 7).
Fig. 7.
Proposed model for sterility of watermelon
Role of plant endogenous hormones
Plant endogenous hormones play a regulatory function in the occurrence of male sterility (Gaillard et al. 1998; Nakajima et al. 1991). Some studies indicated that many phytohormones could also affect male fertility, including auxin, cytokinins, ethylene, and jasmonic acids (Kieber et al. 1993; Park et al. 2002; Huang et al. 2003; Cecchetti 2008), and many biosynthetic or signaling mutants of phytohormones commonly reduce male fertility (Ye et al. 2010). In this study, 11 DGEs from the auxin metabolism pathway were found; these genes were annotated as AUX1, AUX/IAA, GH3, and SAUR. AUX1 is a member of the auxin influx carrier family, which is a receptor for auxin signaling transduction (Parry et al. 2001; Vandenbussche et al. 2003). AUX/IAA genes have typically been classified as primary auxin response genes (Hagen and Guilfoyle 2002) and these also contain GH3 and SAUR (Park et al. 2007). GH3 is a key enzyme for the synthesis of indole-3-acetic acid-amido (Guo et al. 2016). SAUR encodes short transcripts that rapidly accumulate after treatment with auxin (Park et al. 2007). The majority of early auxin response genes in the auxin metabolic pathway were down-regulated in sterile Se18-MS plants in comparison to fertile Se18-MF plants, implying that auxin signaling may be decreased in Se18-MS. This was in accordance with previous studies that indicated that the auxin content was lowered in sterile plants compared to fertile plants, suggesting that male sterility was linked with auxin shortage (Fig. 7), and with the shortage of indole-3-acetic acid in particular (Hamdi et al. 1987).
Cytokinins are a type of the plant hormones that play an important role in the regulation of cell division and cell differentiation (Skoog and Miller 1957). In this study, DEGs involved in the cytokinin-signaling pathway were A-ARR and B-ARR as well as CRE1 and AHP. Previous research showed that type-B ARRs played a key role during the early plant response to cytokinin (Argyros et al. 2008). CRE1 has been reported as a cytokinin receptor and cytokinins could activate CRE1 to initiate phosphorelay signaling (Inoue et al. 2001). Furthermore, AHP has been suggested as a positive regulator of cytokinin signaling (Hutchison et al. 2006). Abscisic acid is an important growth inhibitor in plants and could inhibit cell division and elongation. PYR/PYL is an ABA-receptor and can control abscisic acid signaling by inhibiting PP2Cs (a type 2 C protein phosphatases) (Park et al. 2009). The kinase SnRK2 is a positive regulator of ABA signaling (Fujii et al. 2007). Salicylic acid is important for the regulation of gene expression related to cell growth, flowering, and aging (Vlot et al. 2009). In conclusion, these results demonstrated that levels of cytokinin, abscisic acid, and salicylic acid might be different in Se18-MS (Fig. 7). Although the specific contents of these plant endogenous hormones have been studied, their roles still remain unclear (Shukla and Sawhney 1993, 1994).
Ye et al. (2010) indicated that brassinosteroids control male fertility by regulating the expression of key genes involved in the development of both anther and pollen in Arabidopsis. In our study, we found that, several genes were also differentially expressed in the brassinosteroid metabolism pathway, implying that the brassinosteroid signaling pathway might participate in the abortion of the anther and pollen development in sterile plants (Fig. 7).
Jasmonic acid is a common plant hormone signal and plays an important role in male sterility (Stintzi 2000). In this study, the gene JAZ from the jasmonic acid-signaling pathway was down-regulated in Se18-MS (Fig. 7). It has previously been reported that the JAZ family proteins act as jasmonic acid co-receptors and transcriptional repressors in jasmonic acid signaling in Arabidopsis (Kazan and Manners 2012).
Ethylene is a major plant hormone that regulates plant development. In this study, both EIN3 and ERF1/2 genes (both in the ethylene-signaling pathway) were up-regulated in Se18-MS. EIN3 is a key transcriptional regulator in the ethylene-signaling pathway (Chao et al. 1997; Solano et al. 1998) and ethylene signal transduction has been reported to be involved in anther dehiscence (Rieu et al. 2003). Thus, ethylene might also play a central role in the regulation of the anther and pollen development in sterile watermelon plants (Fig. 7).
Functional proteins
TFs are a type of important proteins that mediate the regulation of gene expression in various biochemical plant processes, including the process of flower development (Riechmann et al. 2000). Previous studies have reported that TFs were closely related to pollen development, which also correlated with microsporocyte development, pollen sac and tapetum development, and growth of the pollen tube (Vizcay-Barrena 2006; Yang et al. 2007b; Zhang et al. 2006; Zou et al. 2010). At least 608 TFs from 34 gene families were identified in the male gametophyte of Arabidopsis (Honys and Twell 2004). In this study, 210 transcription factor genes, that belong to 41 transcription families, were differentially expressed, of which bHLH was the most differentially expressed with a total of 26, the second most differentially expressed was MYB (20), followed by ERF (19), and WRKY (12). In Arabidopsis, many bHLH factors were involved in regulating anther microspore development or tapetum development (Dukowic-Schulze et al. 2014; Sorensen et al. 2010; Zhang et al. 2006). Previous studies have reported about two bHLH factors (AMS and DYT1) that take part in tapetum development (Sorensen et al. 2010; Zhang et al. 2006). In the present study, 15 bHLH genes were found to be down-regulated in GMS lines.
Many MYB genes have been shown to play important roles in pollen and anther development (Yang et al. 2007c). AtMYB32 is required for normal pollen development, and it has been reported that the atmyb32 mutant produced more than half of all aberrant pollen grains (Preston et al. 2004). AtMYB103, AtMYB26, and AtMYB21 were also important for pollen and anther development, and their abnormal expression caused early tapetum degeneration, abnormal another development, and abnormal flower development, respectively (Higginson et al. 2010; Shin et al. 2010; Steiner-Lange et al. 2003). In the current study, Cla012590 (AtMYB26) and Cla017100 (AtMYB21) were found to be down-regulated in Se18-MS.
The ethylene response factor (ERF) family is a large gene family of TFs and is a subfamily of the AP2/ERF superfamily (Riechmann et al. 2000). It has been shown that AP2/ERF proteins play important roles in regulating various biological processes related to growth and development (Nakano et al. 2006). Genes in the AP2 family have been reported to participate in the regulation of flower development (Elliott et al. 1996). In the present study, 10 ERF genes were upregulated in GMS lines of watermelon. Consequently, it is worth studying how these genes function in watermelon GMS.
WRKY TFs have diverse functions, and the number of publications indicates that WRKY proteins are key regulators of several developmental processes (Zou et al. 2010). WRKY2 and WRKY34 have been shown to be responsible for male gametogenesis, which is required for pollen development during the early stage (Guan et al. 2014). In this study, 11 WRKY genes were up-regulated in Se18-MS. Although the function of WRKY proteins in pollen remains unclear, our data suggested these WRKY genes to be very valuable for further study of their roles in watermelon GMS.
In brief, these TFs were essential for the regulation of plant gene expression. Furthermore, changes in gene transcription were linked to changes in the expression of TFs (Yan et al. 2013). In current study, the expression of interfering TF genes might be related to the development of flower organs, which supposedly resulted in the observed male sterility of Se18-MS (Fig. 7).
Conclusion
In this study, we successfully detected DEGs associated with genic male sterility by comparing floral buds of male sterile and fertile near-isogenic lines of watermelon to improve understanding of the molecular mechanisms in watermelon. The male sterile line Se18-MS and near isogenic male fertile Se18-MF line of watermelon cultivar were used as an experimental material. Based on the transcriptome data analysis in this study, we have suggested a role for genes like Cla000029, Cla004641 in pollen development and a possible role in genic male sterility in watermelon. This study provides a scope for more research that can be done for TFs like WRKY for their role in pollen and flower sex differentiation.
Electronic supplementary material
Below is the link to the electronic supplementary material.
Acknowledgements
This study was supported by the Modern Agro-industry Technology Research System of China (No. CARS-25), the Scientific Startup Foundation for Doctors of Northwest A&F University (No. Z109021604), the Basal Research Foundation of Northwest A&F University (No. Z109021612), the Science and Technological of Shaanxi Province (No. 2015KTTSNY03-04) and the Key Project of Shaanxi Province (2017ZDXM-NY-025).
Author contributions
YW, CW and XZ conceived and designed the experiments. YW and XY sequenced data analysis, and qPCR validation of gene expression. YW wrote the paper. ZW, RZ, JC and YY participated in the data analysis. HL, YZ, JM and VY provided helpful advice on data analysis. YM and CW revised the paper. XZ provided the materials, revised the paper and supervised the research.
Compliance with ethical standards
Conflict of interest
The authors declare that they have no conflict of interest.
Footnotes
Yongqi Wang and Xiaozhen Yang contributed equally to this work.
Contributor Information
Chunhua Wei, Email: xjwend020405@163.com.
Xian Zhang, Email: zhangxian@nwsuaf.edu.cn.
References
- Aarts MG, Keijzer CJ, Stiekema WJ, Pereira A. Molecular characterization of the CER1 gene of arabidopsis involved in epicuticular wax biosynthesis and pollen fertility. Plant Cell. 1995;7(12):2115–2127. doi: 10.1105/tpc.7.12.2115. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Aarts MGM, Hodge R, Kalantidis K, Florack D, Pereira A. The Arabidopsis MALE STERILITY 2 protein shares similarity with reductases in elongation/condensation complexes. Plant J. 1997;12(3):615–623. doi: 10.1046/j.1365-313x.1997.00615.x. [DOI] [PubMed] [Google Scholar]
- Anders S, Huber W. Differential expression analysis for sequence count data. Genome Biol. 2010;11(10):R106. doi: 10.1186/gb-2010-11-10-r106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Argyros RD, Mathews DE, Chiang Y-H, Palmer CM, Thibault DM, Etheridge N, Argyros DA, Mason MG, Kieber JJ, Schaller GE. Type B response regulators of Arabidopsis play key roles in cytokinin signaling and plant development. Plant Cell. 2008;20(8):2102–2116. doi: 10.1105/tpc.108.059584. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ashburner M, Ball CA, Blake JA, Botstein D, Butler H, Cherry JM, Davis AP, Dolinski K, Dwight SS, Eppig JT. Gene Ontology: tool for the unification of biology. Nat Genet. 2000;25(1):25–29. doi: 10.1038/75556. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Atanassova B. Functional male sterility in tomato (Lycopersicon esculentum Mill.) and its application in hybrid seed production. Acta Physiol Plant. 2000;22(3):221–225. [Google Scholar]
- Cecchetti V. Auxin regulates Arabidopsis anther dehiscence, pollen maturation, and filament elongation. Plant Cell. 2008;20(7):1760–1774. doi: 10.1105/tpc.107.057570. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chao Q, Rothenberg M, Solano R, Roman G, Terzaghi W, Ecker JR. Activation of the ethylene gas response pathway in Arabidopsis by the nuclear protein ETHYLENE-INSENSITIVE3 and related proteins. Cell. 1997;89(7):1133–1144. doi: 10.1016/s0092-8674(00)80300-1. [DOI] [PubMed] [Google Scholar]
- Chen W, Yu XH, Zhang K, Shi J, De Oliveira S, Schreiber L, Shanklin J, Zhang D. Male Sterile 2 encodes a Plastid-Localized Fatty Acyl carrier protein reductase required for pollen exine development in Arabidopsis. Plant Physiol. 2011;157(2):842–853. doi: 10.1104/pp.111.181693. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen C, Chen G, Cao B, Lei J. Transcriptional profiling analysis of genic male sterile–fertile Capsicum annuum reveal candidate genes for pollen development and maturation by RNA-Seq technology. Plant Cell Tissue Organ Culture (PCTOC) 2015;122:465–476. [Google Scholar]
- Cheng H, Qin LJ, Lee S, Fu XD, Richards DE, Cao DN, et al. Gibberellin regulates Arabidopsis floral development via suppression of DELLA protein function. Development. 2004;131(5):1055–1064. doi: 10.1242/dev.00992. [DOI] [PubMed] [Google Scholar]
- Cheng H, Song SS, Xiao LT, Soo HM, Cheng ZW, Xie DX, Peng JR. Gibberellin acts through jasmonate to control the expression of MYB21, MYB24, and MYB57 to promote stamen filament growth in Arabidopsis. PLoS Genetics. 2009;5(3):e1000440. doi: 10.1371/journal.pgen.1000440. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Coimbra S, Costa M, Jones B, Mendes MA, Pereira LG. Pollen grain development is compromised in Arabidopsis agp6 agp11 null mutants. J Exp Bot. 2009;60(11):3133–3142. doi: 10.1093/jxb/erp148. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Conesa A, Götz S, García-Gómez JM, Terol J, Talón M, Robles M. Blast2GO: a universal tool for annotation, visualization and analysis in functional genomics research. Bioinformatics. 2005;21(18):3674–3676. doi: 10.1093/bioinformatics/bti610. [DOI] [PubMed] [Google Scholar]
- De Azevedo Souza C, Kim SS, Koch S, Kienow L, Schneider K, McKim SM, Haughn GW, Kombrink E, Douglas CJ. A novel fatty Acyl-CoA synthetase is required for pollen development and sporopollenin biosynthesis in Arabidopsis. Plant Cell. 2009;21(2):507–525. doi: 10.1105/tpc.108.062513. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Depuydt S, Hardtke CS. Hormone signalling crosstalk in plant growth regulation. Curr Biol Cb. 2011;21(9):365–373. doi: 10.1016/j.cub.2011.03.013. [DOI] [PubMed] [Google Scholar]
- Dobritsa AA, Shrestha J, Morant M, Pinot F, Matsuno M, Swanson R, Moller BL, Preuss D. CYP704B1 is a long-chain fatty acid ω-Hydroxylase essential for sporopollenin synthesis in pollen of Arabidopsis. Plant Physiol. 2009;151(2):574–589. doi: 10.1104/pp.109.144469. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dong X, Hong Z, Sivaramakrishnan M, Mahfouz M, Verma DPS. Callose synthase (CalS5) is required for exine formation during microgametogenesis and for pollen viability in Arabidopsis. Plant J. 2005;42(3):315–328. doi: 10.1111/j.1365-313X.2005.02379.x. [DOI] [PubMed] [Google Scholar]
- Dukowic-Schulze S, Harris A, Li J, Sundararajan A, Mudge J, Retzel EF, Pawlowski WP, Chen C. Comparative transcriptomics of early meiosis in Arabidopsis and Maize. J Genet Genomics. 2014;41(3):139–152. doi: 10.1016/j.jgg.2013.11.007. [DOI] [PubMed] [Google Scholar]
- Dun X, Zhou Z, Xia S, Wen J, Fu T. BnaC.Tic40, a plastid inner membrane translocon originating from. Plant J. 2011;68(3):532–545. doi: 10.1111/j.1365-313X.2011.04708.x. [DOI] [PubMed] [Google Scholar]
- Elliott RC, Betzner AS, Huttner E, Oakes MP, Tucker W, Gerentes D, Perez P, Smyth DR. AINTEGUMENTA, an APETALA2-like gene of Arabidopsis with pleiotropic roles in ovule development and floral organ growth. Plant Cell. 1996;8(2):155–168. doi: 10.1105/tpc.8.2.155. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fang ZY, Peitian S, Liu LM, Yang LM, Hou AF, Wang XW, et al. Preliminary study on the inheritance of male sterility in cabbage line 79–399-438. Acta Hort. 1995;402:414–417. [Google Scholar]
- Fang WP, Zhao FA, Sun Y, Xie DY, Sun L, Xu ZZ, et al. Transriptomic profiling reveals complex molecular regulation in cotton genic male sterile mutant Yu98-8A. PLoS ONE. 2015;10(9):e0133425. doi: 10.1371/journal.pone.0133425. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Feys BJ, Benedetti CE, Penfold CN, Turner JG. Arabidopsis mutants selected for resistance to the phytotoxin coronatine are male sterile, insensitive to methyl jasmonate, and resistant to a bacterial pathogen. Plant Cell. 1994;6(5):751–759. doi: 10.1105/tpc.6.5.751. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fujii H, Verslues PE, Zhu JK. Identification of two protein kinases required for abscisic acid regulation of seed germination, root growth, and gene expression in Arabidopsis. Plant Cell. 2007;19(2):485–494. doi: 10.1105/tpc.106.048538. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gaillard C, Moffatt BA, Blacker M, Laloue M. Male sterility associated with APRT deficiency in Arabidopsis thaliana results from a mutation in the gene APT1. Mol Gen Genet Mgg. 1998;257(3):348–353. doi: 10.1007/s004380050656. [DOI] [PubMed] [Google Scholar]
- Gómez JF, Talle B, Wilson ZA. Anther and pollen development: a conserved developmental pathway. J Integr Plant Biol. 2015;57(11):876–891. doi: 10.1111/jipb.12425. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Guan Y, Meng X, Khanna R, LaMontagne E, Liu Y, Zhang S. Phosphorylation of a WRKY transcription factor by MAPKs is required for pollen development and function in Arabidopsis. PLoS Genet. 2014;10(5):e1004384. doi: 10.1371/journal.pgen.1004384. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Guo S, Liu J, Zheng Y, Huang M, Zhang H, Gong G, He H, Ren Y, Zhong S, Fei Z. Characterization of transcriptome dynamics during watermelon fruit development: sequencing, assembly, annotation and gene expression profiles. BMC Genomics. 2011;12(1):454. doi: 10.1186/1471-2164-12-454. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Guo J, Zhang Y, Hui M, Cheng Y, Zhang E, Xu Z. Transcriptome sequencing and de novo analysis of a recessive genic male sterile line in cabbage (Brassica oleracea L. var. capitata) Mol Breed. 2016;36(8):117–131. [Google Scholar]
- Hagen G, Guilfoyle T. Auxin-responsive gene expression: genes, promoters and regulatory factors. Plant Mol Biol. 2002;49(3–4):373–385. [PubMed] [Google Scholar]
- Hamdi S, Teller G, Louis J-P. Master regulatory genes, auxin levels, and sexual organogeneses in the dioecious plant Mercurialis annua. Plant Physiol. 1987;85(2):393–399. doi: 10.1104/pp.85.2.393. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Han YK, Wang XY, Zhao FY, Gao S, Wei AM, Chen ZW, et al. Transriptomic analysis of differentially expressed genes in flower-buds of genetic male sterile and wild type cucumber by RNA sequencing. Physiol Mol Biol Plants. 2018;24:359–367. doi: 10.1007/s12298-018-0515-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Higginson T, Li SF, Parish RW. AtMYB103 regulates tapetum and trichome development in Arabidopsis thaliana. Plant J. 2010;35(2):177–192. doi: 10.1046/j.1365-313x.2003.01791.x. [DOI] [PubMed] [Google Scholar]
- Honys D, Twell D. Transcriptome analysis of haploid male gametophyte development in Arabidopsis. Genome Biol. 2004;5(11):R85. doi: 10.1186/gb-2004-5-11-r85. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Huang S, Cerny RE, Qi Y, Bhat D, Aydt CM, Hanson DD, Malloy KP, Ness LA. Transgenic studies on the involvement of cytokinin and gibberellin in male development. Plant Physiol. 2003;131(3):1270–1282. doi: 10.1104/pp.102.018598. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hutchison CE, Li J, Argueso C, Gonzalez M, Lee E, Lewis MW, Maxwell BB, Perdue TD, Schaller GE, Alonso JM. The Arabidopsis histidine phosphotransfer proteins are redundant positive regulators of cytokinin signaling. Plant Cell. 2006;18(11):3073–3087. doi: 10.1105/tpc.106.045674. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Inoue T, Higuchi M, Hashimoto Y, Seki M, Kobayashi M, Kato T, Tabata S, Shinozaki K, Kakimoto T. Identification of CRE1 as a cytokinin receptor from Arabidopsis. Nature. 2001;409(6823):1060–1063. doi: 10.1038/35059117. [DOI] [PubMed] [Google Scholar]
- Ito T, Wellmer F, Yu H, Das P, Ito N, Alves-Ferreira M, Riechmann JL, Meyerowitz EM. The homeotic protein AGAMOUS controls microsporogenesis by regulation of SPOROCYTELESS. Nature. 2004;430(6997):356–360. doi: 10.1038/nature02733. [DOI] [PubMed] [Google Scholar]
- Kanehisa M, Goto S, Kawashima S, Okuno Y, Hattori M. The KEGG resource for deciphering the genome. Nucleic Acids Res. 2004;32(suppl 1):277–280. doi: 10.1093/nar/gkh063. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kazan K, Manners JM. JAZ repressors and the orchestration of phytohormone crosstalk. Trends Plant Sci. 2012;17(1):22–31. doi: 10.1016/j.tplants.2011.10.006. [DOI] [PubMed] [Google Scholar]
- Kieber JJ, Rothenberg M, Roman G, Feldmann KA, Ecker JR. CTR1, a negative regulator of the ethylene response pathway in Arabidopsis, encodes a member of the Raf family of protein kinases. Cell. 1993;72(3):427–441. doi: 10.1016/0092-8674(93)90119-b. [DOI] [PubMed] [Google Scholar]
- Kim D, Pertea G, Trapnell C, Pimentel H, Kelley R, Salzberg SL. TopHat2: accurate alignment of transcriptomes in the presence of insertions, deletions and gene fusions. Genome Biol. 2013;14(4):R36. doi: 10.1186/gb-2013-14-4-r36. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kley FKVD. Male sterility and its importance in breeding heterosis varieties. Euphytica. 1954;3(2):117–124. [Google Scholar]
- Kong Q, Yuan J, Gao L, Zhao S, Jiang W, Huang Y, Bie Z. Identification of suitable reference genes for gene expression normalization in qRT-PCR analysis in watermelon. PLoS ONE. 2014;9(2):e90612. doi: 10.1371/journal.pone.0090612. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Koski LB, Gray MW, Lang BF, Burger G. AutoFACT: an automatic functional annotation and classification tool. BMC Bioinf. 2005;6(1):1–11. doi: 10.1186/1471-2105-6-151. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Levitin B, Richter D, Markovich I, Zik M. Arabinogalactan proteins 6 and 11 are required for stamen and pollen function in Arabidopsis. Plant J. 2008;56(3):351–363. doi: 10.1111/j.1365-313X.2008.03607.x. [DOI] [PubMed] [Google Scholar]
- Liu X, Huang J, Parameswaran S, Ito T, Seubert B, Auer M, Rymaszewski A, Jia G, Owen HA, Zhao D. The SPOROCYTELESS/NOZZLE gene is involved in controlling stamen identity in Arabidopsis. Plant Physiol. 2009;151(3):1401–1411. doi: 10.1104/pp.109.145896. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu S, Yeh CT, Tang HM, Dan N, Schnable PS. Gene mapping via bulked segregant RNA-Seq (BSR-Seq) PLoS ONE. 2012;7(5):e36406. doi: 10.1371/journal.pone.0036406. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu HY, Kun WU, Yang MM, Zhou XA, Zhao YZ. Variation of soluble sugar, starch and plant hormones contents in sesame dominant genic male sterile line during bud development. Chin J Oil Crop Sci. 2014;36(2):175–180. [Google Scholar]
- Liu C, Liu Z, Li C, Zhang Y, Feng H. Comparative transcriptome analysis of fertile and sterile buds from a genetically male sterile line of Chinese cabbage. Vitro Cell Dev Biol-Plant. 2016;52(2):130–139. [Google Scholar]
- Liu QC, Lan YP, Wen CL, Zhao H, Wang J, Wang YQ. Transcriptome sequencing analyses between the cytoplasmic male sterile line and its maintainer line in welsh onion (Allium fistulosum L.) Int J Mol Sci. 2016;17(7):1058. doi: 10.3390/ijms17071058. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Livak KJ, Schmittgen TD. Analysis of relative gene expression data using real-time quantitative PCR and the 2− ΔΔCT method. Methods. 2001;25(4):402–408. doi: 10.1006/meth.2001.1262. [DOI] [PubMed] [Google Scholar]
- Mandaokar A, Thines B, Shin B, Lange BM, Choi G, Koo YJ, Yoo YJ, Choi YD, Choi G, Browse J. Transcriptional regulators of stamen development in Arabidopsis identified by transcriptional profiling. Plant J. 2006;46(6):984–1008. doi: 10.1111/j.1365-313X.2006.02756.x. [DOI] [PubMed] [Google Scholar]
- Nakajima M, Yamaguchi I, Kizawa S, Murofushi N, Takahashi N. Semi-quantification of GAX and GA4 in male-sterile anthers of rice byradioimmunoassay. Plant Cell Physiol. 1991;32(4):511–513. [Google Scholar]
- Nakano T, Suzuki K, Fujimura T, Shinshi H. Genome-wide analysis of the ERF gene family in Arabidopsis and rice. Plant Physiol. 2006;140(2):411–432. doi: 10.1104/pp.105.073783. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Park JH, Halitschke R, Kim HB, Baldwin IT, Feyereisen R. A knock-out mutation in allene oxide synthase results in male sterility and defective wound signal transduction in Arabidopsis due to a block in jasmonic acid biosynthesis. Plant J. 2002;31(1):1–12. doi: 10.1046/j.1365-313x.2002.01328.x. [DOI] [PubMed] [Google Scholar]
- Park JE, Kim YS, Yoon HK, Park CM. Functional characterization of a small auxin-up RNA gene in apical hook development in Arabidopsis. Plant Sci. 2007;172(1):150–157. [Google Scholar]
- Park SY, Fung P, Nishimura N, Jensen DR, Fujii H, Zhao Y, Lumba S, Santiago J, Rodrigues A, T-fF C. Abscisic acid inhibits PP2Cs via the PYR/PYL family of ABA-binding START proteins. Science (New York, NY) 2009;324(5930):1068. doi: 10.1126/science.1173041. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Parry G, Delbarre A, Marchant A, Swarup R, Napier R, Perrot-Rechenmann C, Bennett MJ. Novel auxin transport inhibitors phenocopy the auxin influx carrier mutation aux1. Plant J. 2001;25(4):399–406. doi: 10.1046/j.1365-313x.2001.00970.x. [DOI] [PubMed] [Google Scholar]
- Perez-Prat E, Campagne MML. Hybrid seed production and the challenge of propagating male-sterile plants. Trends Plant Sci. 2002;7(5):199–203. doi: 10.1016/s1360-1385(02)02252-5. [DOI] [PubMed] [Google Scholar]
- Preston J, Wheeler J, Heazlewood J, Li SF, Parish RW. AtMYB32 is required for normal pollen development in Arabidopsis thaliana. Plant J. 2004;40(6):979–995. doi: 10.1111/j.1365-313X.2004.02280.x. [DOI] [PubMed] [Google Scholar]
- Qu CM, Fu FY, Liu M, Zhao HY, Liu C, Li JN, et al. Comparative transcriptome analysis of recessive male sterility (RGMS) in sterile and Fertile Brassica napus lines. PLoS ONE. 2015;10(12):e0144118. doi: 10.1371/journal.pone.0144118. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rhee SJ, Seo M, Jang YJ, Cho S, Lee GP. Transcriptome profiling of differentially expressed genes in floral buds and flowers of male sterile and fertile lines in watermelon. BMC Genomics. 2015;16(1):914. doi: 10.1186/s12864-015-2186-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Riechmann JL, Heard J, Martin G, Reuber L, Jiang C-Z, Keddie J, Adam L, Pineda O, Ratcliffe O, Samaha R. Arabidopsis transcription factors: genome-wide comparative analysis among eukaryotes. Science. 2000;290(5499):2105–2110. doi: 10.1126/science.290.5499.2105. [DOI] [PubMed] [Google Scholar]
- Rieu I, Wolters-Arts M, Derksen J, Mariani C, Weterings K. Ethylene regulates the timing of anther dehiscence in tobacco. Planta. 2003;217(1):131–137. doi: 10.1007/s00425-003-0976-9. [DOI] [PubMed] [Google Scholar]
- Sheng Y, Yudan W, Shiqi J, Yazhong J, Peng J, Feishi L. Mapping and preliminary analysis of ABORTED MICROSPORES (AMS) as the candidate gene underlying the male sterility (MS-5) mutant in melon (Cucumis melo L.) Front Plant Sci. 2017;8:902. doi: 10.3389/fpls.2017.00902. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shin B, Choi G, Yi H. AtMYB21, a gene encoding a flower-specific transcription factor, is regulated by COP1. Plant J. 2010;30(1):23–32. doi: 10.1046/j.1365-313x.2002.01264.x. [DOI] [PubMed] [Google Scholar]
- Shukla A, Sawhney V. Metabolism of dihydrozeatin in floral buds of wild-type and a genic male sterile line of rapeseed (Brassica napus L.) J Exp Bot. 1993;44(9):1497–1505. [Google Scholar]
- Shukla A, Sawhney V. Abscisic acid: one of the factors affecting male sterility in Brassica napus. Physiol Plant. 1994;91(3):522–528. [Google Scholar]
- Skoog F, Miller C. Chemical regulation of growth and organ formation in plant tissues cultured in vitro. Symp Soc Exp. 1957;11(21):118. [PubMed] [Google Scholar]
- Solano R, Stepanova A, Chao Q, Ecker JR. Nuclear events in ethylene signaling: a transcriptional cascade mediated by ethylene-insensitive3 and ethylene-response-factor1. Genes Dev. 1998;12(23):3703–3714. doi: 10.1101/gad.12.23.3703. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Song S, Qi T, Huang H, Ren Q, Wu D, Chang C, Peng W, Liu Y, Peng J, Xie D. The Jasmonate-ZIM domain proteins interact with the R2R3-MYB transcription factors MYB21 and MYB24 to affect jasmonate-regulated stamen development in Arabidopsis. Plant Cell. 2011;23(3):1000–1013. doi: 10.1105/tpc.111.083089. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sorensen AM, Kröber S, Unte US, Huijser P, Dekker K, Saedler H. The Arabidopsis ABORTED MICROSPORES (AMS) gene encodes a MYC class transcription factor. Plant J Cell Mol Biol. 2010;33(2):413–423. doi: 10.1046/j.1365-313x.2003.01644.x. [DOI] [PubMed] [Google Scholar]
- Steiner-Lange S, Unte US, Eckstein L, Yang C, Wilson ZA, Schmelzer E, Dekker K, Saedler H. Disruption of Arabidopsis thaliana MYB26 results in male sterility due to non-dehiscent anthers. Plant J. 2003;34(4):519–528. doi: 10.1046/j.1365-313x.2003.01745.x. [DOI] [PubMed] [Google Scholar]
- Stintzi A. The Arabidopsis male-sterile mutant, opr3, lacks the 12-oxophytodienoic acid reductase required for jasmonate synthesis. Proc Natl Acad Sci. 2000;97(19):10625–10630. doi: 10.1073/pnas.190264497. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Su AG, Song W, Xing JF, Zhao YX, Zhang RY, Li CH, et al. Identification of genes potentially associated with the fertility instability of S-type cytoplasm male sterility in maize via bulked segregant RNA-Seq. PLoS ONE. 2015;11(9):e0163489. doi: 10.1371/journal.pone.0163489. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tiwari SB, Hagen G, Guilfoyle TJ. AUX/IAA proteins contain a potent transcriptional repression domain. Plant Cell. 2004;16(2):533–543. doi: 10.1105/tpc.017384. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Trapnell C, Williams BA, Pertea G, Mortazavi A, Kwan G, Van Baren MJ, Salzberg SL, Wold BJ, Pachter L. Transcript assembly and quantification by RNA-Seq reveals unannotated transcripts and isoform switching during cell differentiation. Nat Biotechnol. 2010;28(5):511–515. doi: 10.1038/nbt.1621. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vandenbussche F, Smalle J, Le J, Saibo NJM, De Paepe A, Chaerle L, Tietz O, Smets R, Laarhoven LJ, Harren FJ. The Arabidopsis mutant alh1 illustrates a cross talk between ethylene and auxin. Plant Physiol. 2003;131(3):1228–1238. doi: 10.1104/pp.010850. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vizcay-Barrena G. Altered tapetal PCD and pollen wall development in the Arabidopsis ms1 mutant. J Exp Bot. 2006;57(11):2709. doi: 10.1093/jxb/erl032. [DOI] [PubMed] [Google Scholar]
- Vlot AC, Dempsey DMA, Klessig DF. Salicylic acid, a multifaceted hormone to combat disease. Annu Rev Phytopathol. 2009;47:177–206. doi: 10.1146/annurev.phyto.050908.135202. [DOI] [PubMed] [Google Scholar]
- Wang WJ, Wang FJ, Sun XT, Liu FL, Liang ZR. Comparison of transcriptome under red and blue light culture of Saccharina japonica (Phaeophyceae) Planta. 2013;237(4):1123–1133. doi: 10.1007/s00425-012-1831-7. [DOI] [PubMed] [Google Scholar]
- Wang YQ, Yang XZ, Mo YL, Zheng JQ, Zhang Y, Ma JX, et al. Analysis of the changes in antioxidant enzymes activities and endogenous hormones contents in watermelon male sterile line Se18 during bud development. Acta Horticulturae Sinica. 2016;43(11):2161–2172. [Google Scholar]
- Wijeratne AJ, Zhang W, Sun Y, Liu W, Albert R, Zheng Z, Oppenheimer DG, Zhao D, Ma H. Differential gene expression in Arabidopsis wild-type and mutant anthers: insights into anther cell differentiation and regulatory networks. Plant J. 2007;52(1):14–29. doi: 10.1111/j.1365-313X.2007.03217.x. [DOI] [PubMed] [Google Scholar]
- Wilson ZA, Da-Bing Z. From Arabidopsis to rice: pathways in pollen development. J Exp Bot. 2009;5:5. doi: 10.1093/jxb/erp095. [DOI] [PubMed] [Google Scholar]
- Wu K, Liu H, Zuo Y, Yang M, Zhao Y. Histological and transcriptional characterization of a novel recessive genic male sterility mutant in sesame (Sesamum indicum L.) Acta Physiol Plant. 2014;36(2):421. [Google Scholar]
- Xu J, Yang C, Yuan Z, Zhang D, Gondwe MY, Ding Z, Liang W, Zhang D, Wilson ZA. The ABORTED MICROSPORES regulatory network is required for postmeiotic male reproductive development in Arabidopsis thaliana. Plant Cell. 2010;22(1):91–107. doi: 10.1105/tpc.109.071803. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xu J, Ding Z, Vizcay-Barrena G, Shi J, Liang W, Yuan Z, Werck-Reichhart D, Schreiber L, Wilson ZA, Zhang D. ABORTED MICROSPORES Acts as a master regulator of pollen wall formation in Arabidopsis. Plant Cell. 2014;26(4):1544–1556. doi: 10.1105/tpc.114.122986. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yan X, Dong C, Yu J, Liu W, Jiang C, Liu J, Hu Q, Fang X, Wei W. Transcriptome profile analysis of young floral buds of fertile and sterile plants from the self-pollinated offspring of the hybrid between novel restorer line NR1 and Nsa CMS line in Brassica napus. BMC Genomics. 2013;14(1):26. doi: 10.1186/1471-2164-14-26. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yang C, Vizcay-Barrena G, Conner K, Wilson ZA. MALE STERILITY1 is required for tapetal development and pollen wall biosynthesis. Plant Cell. 2007;19(11):3530–3548. doi: 10.1105/tpc.107.054981. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yang C, Xu Z, Song J, Conner K, Barrena GV, Wilson ZA. Arabidopsis MYB26/MALE STERILE35 regulates secondary thickening in the endothecium and is essential for anther dehiscence. Plant Cell. 2007;19(2):534–548. doi: 10.1105/tpc.106.046391. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yang X, Li J, Pei M, Gu H, Chen Z, Qu LJ. Over-expression of a flower-specific transcription factor gene AtMYB24 causes aberrant anther development. Plant Cell Rep. 2007;26(2):219–228. doi: 10.1007/s00299-006-0229-z. [DOI] [PubMed] [Google Scholar]
- Ye J, Fang L, Zheng H, Zhang Y, Chen J, Zhang Z, Wang J, Li S, Li R, Bolund L. WEGO: a web tool for plotting GO annotations. Nucleic Acids Res. 2006;34:293–297. doi: 10.1093/nar/gkl031. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ye Q, Zhu W, Li L, Zhang S, Yin Y, Ma H, Wang X. Brassinosteroids control male fertility by regulating the expression of key genes involved in Arabidopsis anther and pollen development. Proc Natl Acad Sci USA. 2010;107(13):6100–6105. doi: 10.1073/pnas.0912333107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang X, Yang JQ, Zhang JS, Zhang Y, Ma JX, Hou P. Studies on botanical character and genetic model of Se18 watermelon male sterile material. China Cucurbits Vegetables. 2005;5:3–6. [Google Scholar]
- Zhang X, Wang M, Ma JX, Zhang JS, Yang JQ. Cytological studies on recessive nuclease male sterile lines in watermelon. J Northwest A&F Univ (Nat Sci Ed) 2005;33(1):71–74. [Google Scholar]
- Zhang W, Sun Y, Timofejeva L, Chen C, Ma H. Regulation of Arabidopsis tapetum development and function by DYSFUNCTIONAL TAPETUM1 (DYT1) encoding a putative bHLH transcription factor. Development. 2006;133(16):3085–3095. doi: 10.1242/dev.02463. [DOI] [PubMed] [Google Scholar]
- Zhao D, Ma H. Male fertility: a case of enzyme identity. Curr Biol. 2000;10(24):904–907. doi: 10.1016/s0960-9822(00)00848-4. [DOI] [PubMed] [Google Scholar]
- Zhou Z, Xiaoling D, Xia S, Shi D, Qin M, Yi B, et al. BnMs3 is required for tapetal differentiation and degradation, microspore separation, and pollen-wall biosynthesis in Brassica napus. J Exp Bot. 2011;63(5):2041–2058. doi: 10.1093/jxb/err405. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhu J, Chen H, Li H, Gao JF, Jiang H, Wang C, Guan YF, Yang ZN. Defective in tapetal development and function 1 is essential for another development and tapetal function for microspore maturation in Arabidopsis. Plant J. 2008;55(2):266–277. doi: 10.1111/j.1365-313X.2008.03500.x. [DOI] [PubMed] [Google Scholar]
- Zou C, Jiang W, Yu D. Male gametophyte-specific WRKY34 transcription factor mediates cold sensitivity of mature pollen in Arabidopsis. J Exp Bot. 2010;61:3901–3914. doi: 10.1093/jxb/erq204. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.