Abstract
Purpose: To aid in the selection of a suitable combination of irradiation mode and jaw width in helical tomotherapy (HT) for the treatment of nasopharyngeal carcinoma (NPC).
Materials and Methods: Twenty patients with NPC who underwent radiotherapy were retrospectively selected. Four plans using a jaw width of 2.5 or 5-cm in dynamic jaw (DJ) or fix jaw (FJ) modes for irradiation were designed (2.5DJ, 2.5FJ, 5.0DJ, and 5.0FJ). The dose parameters of planning target volume (PTV) and organs at risk (OARs) of the plans were compared and analyzed, as well as the beam on time (BOT) and monitor unit (MU). The plans in each group were ranked by scoring the doses received by the OARs and the superity was assessed in combination with the planned BOT and MU.
Results: The prescribed dose coverage of PTV met the clinical requirements for all plans in the four groups. The groups using a 2.5-cm jaw width or a DJ mode provided better protection to most OARs, particularly for those at the longitudinal edges of the PTV (P < 0.05). The 2.5DJ group had the best ranking for OAR-dose, followed by the 2.5FJ and 5.0DJ groups with a same score. The BOT and MU of the groups using a 5.0-cm jaw width reduced nearly 45% comparing to those of the 2.5-cm jaw groups.
Conclusion: 2.5DJ has the best dose distribution, while 5.0DJ has satisfactory dose distribution and less BOT and MU that related to the leakage dose. Both 2.5DJ or 5DJ were recommended for HT treatment plan for NPC based on the center workload.
Keywords: helical tomotherapy, nasopharyngeal carcinoma, dynamic jaw, irradiation mode, jaw width
Introduction
Helical tomotherapy (HT) utilizes the opening and closing of a 64-leaf, pneumatically powered, binary multileaf collimator with 51 equally-spaced beam angles at 360° and translational motion of the treatment couch at a constant speed to achieve a high degree of freedom and power in dose optimization (1). Since HT can attain superior conformity of the dose distribution and homogeneity of the target dose, it has been widely adopted as a radiotherapy modality for various malignant tumors (2–5). HT is particularly suitable for the treatment of patients with nasopharyngeal carcinoma (NPC), who have target volumes with complex shapes and numerous organs at risk (OARs) in the surrounding area (6, 7). Dose optimization for HT is achieved by adjusting specific parameters of the plan, primarily the modulation factor, jaw width, and pitch (1).
A radiotherapy plan in clinical practice should be selected considering not only the quality of dose distribution but also the delivery time and treatment efficiency, which is related to the patient motion and target displacement during treatment, as well as the total number of monitor units (MUs) associated with leakage dose received by the patient. In NPC, many OARs are present adjacent to the edges of the target volume in the longitudinal direction, such as the hippocampus, temporal lobe, and optic nerve. The use of a narrower jaw width for beam delivery can provide better conformity of dose distribution, which can better protect the aforementioned organs while markedly increasing beam-on time (BOT) and number of MUs. Conversely, when a wider jaw width is used for beam delivery, the BOT and number of MUs are greatly reduced; however, the conformity of dose distribution may be unsatisfactory. This may increase the doses received by normal organs, such as the hippocampus and temporal lobe. The hippocampus is an important organ for memory and cognitive function in humans. The control and reduction of radiation dose received by the hippocampus are critical for the quality of long-term survival in patients with NPC who achieve satisfactory therapeutic effects (8–10). Therefore, to ensure better protection of OARs, clinical planning of HT for NPC often involves using a jaw width of 2.5-cm and partly sacrifices the delivery efficiency when using the conventional fixed jaw mode.
Conventional HT utilizes the fixed jaw mode of the collimator for beam delivery. The collimator jaw of the machine is fully opened to the predetermined width as soon as one of the edges of the target volume in the longitudinal direction enters the beam and is only completely closed when the other edge of the target volume exits the beam. Therefore, the width of the penumbra of the target volume in the longitudinal direction completely depends on the selected jaw width (11), and using a wider jaw width in this fixed jaw mode will lead to a considerable dose of scatter radiation in the craniocaudal edges of the target volume. The recently released novel Precision tomotherapy platform provides a dynamic jaw (DJ) mode that can deliver a radiation beam dynamically to the superior and inferior borders of the target volume by using narrower jaw widths (12). Several studies have reported an improved HT efficiency for the treatment of thoracic, abdominal, total marrow, and prostate diseases using a combination of relatively large jaw width and DJ mode, followed by the dynamic reduction of jaw width at the edges of the target volume in the longitudinal direction. Moreover, the penumbras at the edges of the target volumes in the longitudinal direction are significantly reduced (13–17).
NPC is a common malignant tumor in China and Southeast Asia, with intensity-modulated radiation therapy being the primary treatment modality. Due to the complex shapes of the target volumes and the proximity to many important normal tissues and organs in NPC, the dose conformity requirement when using radiotherapy is extremely high. Thus, NPC is particularly suitable for HT. However, there are few reports on the application and parameter selection in tomotherapy using DJ technology for NPC. This study compared the effects of different irradiation modes and jaw widths in HT on the quality of dose distribution, delivery efficiency, and MUs for the treatment of NPC. The objective of this study was to provide a reference for the selection of a combination of irradiation mode and jaw width in HT suitable for the clinical treatment of NPC.
Materials and Methods
Patients' Clinical Characteristics
Twenty patients with NPC who underwent radiotherapy between 2017 and 2019 were retrospectively and randomly selected. All patients had pathologically confirmed, poorly differentiated squamous cell carcinoma. The 19 males and 1 female were 28–69 years of age, with a median age of 50.5 years.
Image Acquisition at Simulation
All patients were placed in a supine position and a thermoplastic mask was fixed in place. Contrast-enhanced helical computed tomography (CT) scans (Somatom Sensation Open, Siemens AG) were then performed under the following conditions: 140 kV, 280 mAs, a scan and reconstruction slice thickness of 3-mm, and a pitch of 1:1. The scan range was from the top of the head to 2-cm below the clavicle. Each patient also underwent magnetic resonance imaging (MRI) (Ingenia 3.0T, Philips) using the same immobilization and the same scan range to acquire images for localization. MRI sequences, including T1, T2, contrast-enhanced T1, and fat-suppressed T1, were acquired. The CT and MR images acquired as described above were transmitted to the treatment planning system (Monaco version 5.1, Elekta) for target volume and OAR delineation.
Delineations of Target Volumes and OARs
The target volumes and OARs for all patients were delineated by radiation oncologists based on the MRI and contrast-enhanced CT simulation images, in accordance with the ICRU50 (18) and ICRU62 reports (19), which included the gross tumor target volume in the nasopharynx (GTVnx), the nodal target volume in the neck (GTVnd), the high-risk clinical target volume (CTV1), and the preventive clinical target volume (CTV2). Following the delineations of the above target volumes, the corresponding planning target volumes (PTVs) were generated by the treatment planning system (TPS) through margin expansion to account for positioning errors and were defined correspondingly as PTVnx, PTVnd, PTV1, and PTV2, respectively. OARs were delineated based on the ICRU83 report (20) and primarily included the brain stem, spinal cord, lens, optic nerve, optic chiasm, hippocampus, pituitary, temporal lobe, inner ear, parotid gland, oral cavity, larynx, mandible, and temporomandibular joint. The corresponding planning organ at risk volumes were generated by the TPS through margin expansion to account for positioning errors.
Plan Designs
The CT simulation images and contoured structures of each patient were transmitted to the treatment planning workstation (Precision 1.1.1.0; Accuray, Sunnyvale, CA, USA) for plan design. Four HT plans were designed for each patient: 2.5-cm-fixed jaw (2.5FJ), 2.5-cm-dynamic jaw (2.5DJ), 5.0-cm-fixed jaw (5.0FJ), and 5.0-cm-dynamic jaw (5.0DJ). All plans utilized a pitch of 0.287 and a modulation factor of 3.0. The dose calculation algorithm utilized convolution superposition and fine-matrix calculations were performed. The output dose rate was 1,180 MU/min. The prescribed doses to the target volumes were as follows: PTVnx, 70 Gy; PTVnd, 66 Gy; PTV1, 60 Gy; and PTV2, 54 Gy. All plans included 33 fractions and required that the prescribed dose coverage of the target volume be not <95% of the PTV and the maximum dose should not exceed 110% of the prescribed dose. All dose constraints for OARs were based on the ICRU83 report (20), the RTOG0615 protocol (21) and the international guidelines on dose prioritization and acceptance criteria in radiation therapy planning for nasopharyngeal carcinoma (22). Since there was a 30–40% of the parotid volume overlapped with the PTV2 in those cases of advanced staging. The center criteria of acceptance, mead dose of parotid gland <40 Gy (T3) and <45 Gy (T4) for advanced patients were used in the assessment and were verified by clinicians. All plans were optimized using the same dose constraints, and an identical and sufficient number of iterative optimizations were performed.
Assessment of Plan Parameters
Dose volume histograms were used to assess the dose distributions in the target volumes and the dose volumes received by the OARs.
The parameters assessed for target volumes included the conformity index (CI) and heterogeneity index (HI) of the target volume, dose received by 98% of the target volume (D98%), percentage of target volume covered by the prescribed dose (V100%), maximum dose (Dmax), and mean dose (Dmean). Since multiple dose gradients were used for NPC, the CI and HI were only calculated for PVTnx. The CI and HI were calculated using the following formula (23, 24):
where TV is the volume of the PTV (cm3), VRI is the volume encompassed by the prescription isodose (cm3), TVRI is the target volume covered by the prescription isodose (cm3); D2% is the dose received by 2% of the volume of the PTV, D98% is the dose received by 98% of the volume of the PTV, and DP is the prescribed dose. A CI closer to 1 denotes better dose conformity of the target volume and a lower HI value indicates a more homogenous dose distribution within the target volume.
The parameters assessed for the OARs included the maximum dose to 1 cc volume (D1cc) of the brain stem, spinal cord, and temporal lobe; maximum dose (Dmax) to the brain stem, spinal cord, lens, optic nerve, optic chiasm, pituitary, and hippocampus; and the mean dose (Dmean) to the optic nerve, optic chiasm, eyes, parotid gland, and hippocampus. The planned MUs and delivery time were recorded to evaluate the beam utilization and execution efficiency of the four plans.
Statistical Analysis
Pairwise comparisons of dosimetric parameters in the four plans were analyzed using paired t-tests. All statistical analyses were performed using SPSS version 20.0 for Windows (IBM Corp., Armonk, NY, USA). Analysis items with P < 0.05 were considered statistically significant.
Results
Comparisons of Dosimetric Parameters of Target Volumes
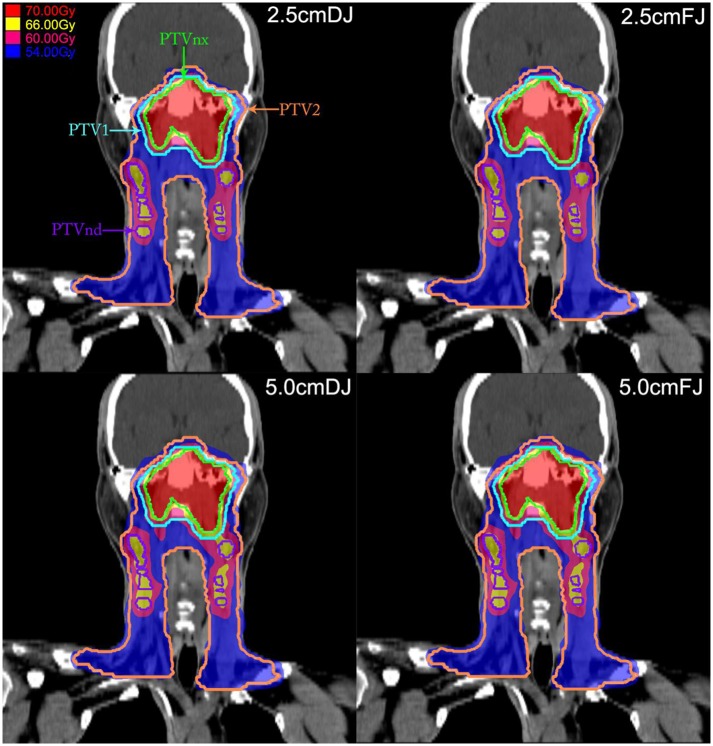
All the four groups of treatment plan met the requirement of a 95% prescribed dose coverage of the target volume. The target dose CI showed greater differences between the 2.5-cm and 5-cm jaw width groups, irrespective of the FJ or DJ irradiation mode. The CI of the 2.5-cm jaw width groups were significantly better than those in the 5.0-cm jaw groups (P < 0.05). However, apart from the HI of the PTVnx which was slightly better in the 2.5-cm jaw width groups, the differences in the remaining dosimetric parameters of target volumes in the four plans, including the V100%, D98%, Dmax, and Dmean, were <1% among the groups. Since such minor differences did not have practical clinical significance, the target doses of the plans in the four groups were all acceptable. The comparisons of the dosimetric parameters of the target volumes in each group are shown in Table 1. Figure 1 shows the dose distributions in the coronal plane of the four plans for one patient.
Table 1.
Comparisons of dosimetric parameters of target volumes among the four plans in 20 patients.
| PTVs | Parameters | 2.5FJ | 2.5DJ | 5.0FJ | 5.0DJ | P2.5 | P5.0 | PF | PD | P* |
|---|---|---|---|---|---|---|---|---|---|---|
| PTVnx | D98% (Gy) | 69.99 ± 0.19 | 69.99 ± 0.17 | 70.00 ± 0.24 | 69.99 ± 0.23 | 0.453 | 0.47 | 0.711 | 0.899 | 0.766 |
| V100% (%) | 97.96 ± 1.03 | 97.92 ± 1.00 | 97.94 ± 1.09 | 97.90 ± 1.08 | 0.176 | 0.494 | 0.706 | 0.688 | 0.263 | |
| Dmean (Gy) | 71.66 ± 0.35 | 71.65 ± 0.35 | 72.00 ± 0.50 | 71.99 ± 0.45 | 0.043 | 0.635 | 0 | 0 | 0 | |
| Dmax (Gy) | 74.32 ± 1.15 | 74.31 ± 1.15 | 74.54 ± 0.95 | 74.58 ± 0.86 | 0.624 | 0.352 | 0.227 | 0.156 | 0.165 | |
| CI | 0.91 ± 0.02 | 0.91 ± 0.02 | 0.86 ± 0.04 | 0.86 ± 0.03 | 1 | 0.891 | 0 | 0 | 0 | |
| HI | 0.042 ± 0.009 | 0.042 ± 0.009 | 0.050 ± 0.012 | 0.050 ± 0.012 | 0.297 | 0.577 | 0 | 0 | 0 | |
| PTVnd | D98% (Gy) | 66.11 ± 0.25 | 66.09 ± 0.26 | 65.96 ± 0.27 | 65.97 ± 0.23 | 0.119 | 0.947 | 0.011 | 0.012 | 0.003 |
| Dmean (Gy) | 66.77 ± 0.25 | 66.76 ± 0.25 | 67.98 ± 0.32 | 67.99 ± 0.29 | 0.012 | 0.696 | 0.005 | 0 | 0 | |
| Dmax (Gy) | 70.74 ± 1.17 | 70.72 ± 1.17 | 71.13 ± 1.31 | 71.17 ± 1.27 | 0.032 | 0.53 | 0.008 | 0 | 0.001 | |
| PTV1 | D98% (Gy) | 60.39 ± 0.55 | 60.36 ± 0.51 | 60.99 ± 0.86 | 60.99 ± 0.83 | 0.056 | 0.904 | 0 | 0 | 0 |
| V100% (%) | 98.44 ± 1.27 | 98.41 ± 1.18 | 99.00 ± 1.02 | 99.00 ± 1.05 | 0.467 | 0.881 | 0 | 0 | 0 | |
| PTV2 | D98% (Gy) | 54.13 ± 0.42 | 54.13 ± 0.40 | 54.01 ± 0.62 | 53.99 ± 0.58 | 0.789 | 0.33 | 0.068 | 0.029 | 0.02 |
| V100% (%) | 98.20 ± 1.09 | 98.16 ± 1.14 | 97.74 ± 1.31 | 97.80 ± 1.21 | 0.088 | 0.419 | 0.001 | 0 | 0 |
P2.5: 2.5FJ vs. 2.5DJ; P5.0: 5.0FJ vs. 5.0DJ; PF: 2.5FJ vs. 5.0FJ; PD: 2.5DJ vs. 5.0DJ; P*: 2.5FJ vs. 5.0DJ.
Figure 1.
Dose distributions in the target volume in the coronal plane of the four plans in a patient with nasopharyngeal carcinoma (NPC). In these four plans, the 70-Gy dose cloud covers the planning target volume (PTV)nx (green line), the 66-Gy dose cloud covers the PTVnd (purple line), the 60-Gy dose cloud covers the PTV1 (cyan line), and the 54-Gy dose cloud covers the PTV2 (orange line).
Comparisons of Dosimetric Parameters of OARs
Based on the same jaw width, the doses received by OARs in the DJ groups were all lower than or equivalent to those in the FJ groups. In the DJ groups, the doses received by OARs adjacent to the edges of the target volumes in the longitudinal (craniocaudal) direction, including the Dmax and Dmean of the optic nerve, optic chiasm, and pituitary gland, Dmean of the eyes and temporal lobe, and D1cc of the hippocampus, were all significantly lower than those in the FJ groups with the same jaw width (P < 0.05). No significant differences were observed for the other dosimetric parameters of the OARs between the DJ and FJ groups (P > 0.05).
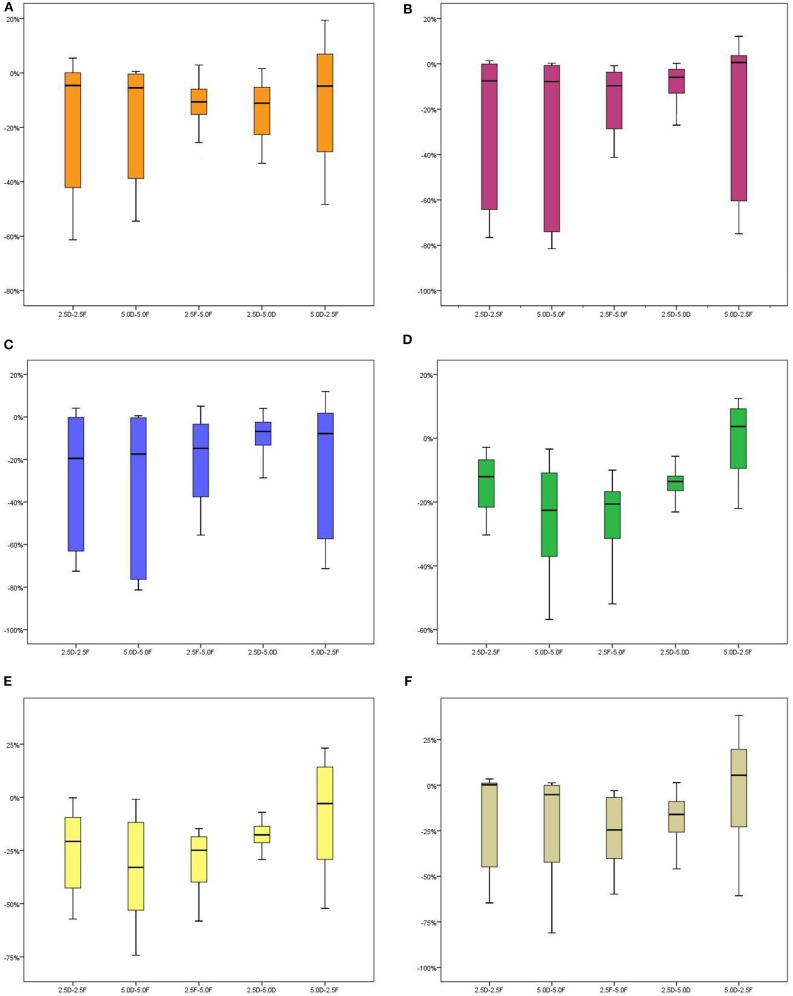
Based on the same irradiation mode, the doses received by almost all OARs in the 2.5-cm jaw width groups were lower than those in the 5.0-cm jaw width groups (P < 0.05). The groups using a narrow jaw width showed better OAR protection, particularly for OARs adjacent to the edges of the target volumes in the longitudinal direction. In the FJ mode, the Dmean of the optic nerve, Dmax and Dmean of the optic chiasm, Dmean of the temporal lobe, and D1cc and Dmean of the hippocampus were significantly lower in the 2.5FJ group than in the 5.0FJ group, with respective reductions of 11.04, 15.93, 20.44, 24.20, 25.40, and 30.15%. In the DJ mode, the Dmean of the optic nerve, Dmean of the temporal lobe, D1cc and Dmean of the hippocampus in the 2.5DJ group were reduced by 12.92, 13.87, 17.09, and 17.47%, respectively, compared to those in the 5.0DJ group (Table 2 and Figure 2).
Table 2.
Comparisons of dosimetric parameters of OARs among the 4 HT plans in 20 patients.
| OARs | Parameters | 2.5FJ | 2.5DJ | 5.0FJ | 5.0DJ | P2.5 | P5.0 | PF | PD | P* |
|---|---|---|---|---|---|---|---|---|---|---|
| Spinal cord | Dmax (Gy) | 38.20 ± 6.06 | 38.22 ± 6.05 | 38.19 ± 5.77 | 38.22 ± 5.75 | 0.436 | 0.51 | 0.91 | 0.978 | 0.888 |
| D1cc (Gy) | 34.50 ± 1.87 | 34.51 ± 2.16 | 33.78 ± 2.16 | 33.82 ± 2.15 | 0.891 | 0.277 | 0.001 | 0.002 | 0.002 | |
| Brain stem | Dmax (Gy) | 60.42 ± 6.54 | 60.66 ± 6.27 | 61.82 ± 5.60 | 61.94 ± 5.49 | 0.033 | 0.117 | 0.01 | 0.016 | 0.006 |
| D1cc (Gy) | 53.93 ± 5.65 | 54.11 ± 5.48 | 55.45 ± 5.81 | 55.56 ± 5.66 | 0.012 | 0.178 | 0 | 0 | 0 | |
| Lens | Dmax (Gy) | 6.10 ± 1.01 | 6.06 ± 1.03 | 6.39 ± 1.04 | 6.33 ± 1.04 | 0.443 | 0.157 | 0.003 | 0.003 | 0.025 |
| Optic nerve | Dmax (Gy) | 49.69 ± 10.46 | 43.76 ± 17.00 | 52.29 ± 9.29 | 46.82 ± 15.08 | 0.002 | 0.002 | 0.002 | 0 | 0.052 |
| Dmean (Gy) | 33.77 ± 9.00 | 28.06 ± 14.33 | 37.58 ± 7.25 | 30.91 ± 13.18 | 0.001 | 0.001 | 0 | 0 | 0.046 | |
| Optic chiasm | Dmax (Gy) | 44.02 ± 15.66 | 35.69 ± 24.21 | 51.04 ± 11.91 | 38.35 ± 24.82 | 0.001 | 0.001 | 0 | 0 | 0.017 |
| Dmean (Gy) | 38.54 ± 16.34 | 31.38 ± 22.99 | 46.56 ± 11.06 | 33.36 ± 23.27 | 0 | 0 | 0 | 0.002 | 0.005 | |
| Eyes | Dmean (Gy) | 9.96 ± 2.46 | 9.26 ± 2.87 | 10.83 ± 2.61 | 9.73 ± 3.21 | 0 | 0 | 0 | 0.003 | 0.351 |
| Parotid | Dmean (Gy) | 39.56 ± 2.87 | 39.53 ± 2.88 | 40.01 ± 3.05 | 39.99 ± 3.04 | 0.033 | 0.43 | 0 | 0 | 0 |
| Pituitary | Dmax (Gy) | 51.84 ± 13.35 | 48.75 ± 16.87 | 56.30 ± 1.00 | 51.73 ± 15.29 | 0.003 | 0.006 | 0 | 0.001 | 0.919 |
| Dmean (Gy) | 47.75 ± 14.11 | 43.32 ± 18.96 | 53.22 ± 10.19 | 46.23 ± 17.56 | 0.001 | 0.002 | 0 | 0 | 0.162 | |
| Temporal lobe | D1cc (Gy) | 62.99 ± 6.99 | 62.98 ± 6.99 | 65.16 ± 5.52 | 65.13 ± 5.55 | 0.68 | 0.567 | 0 | 0 | 0 |
| Dmean (Gy) | 18.67 ± 5.41 | 16.31 ± 6.25 | 24.43 ± 5.16 | 18.91 ± 7.02 | 0 | 0 | 0 | 0 | 0.61 | |
| V60Gy (%) | 1.96 ± 2.39 | 1.95 ± 2.39 | 2.92 ± 3.05 | 2.88 ± 3.02 | 0.163 | 0.069 | 0 | 0 | 0 | |
| Hippocampus | Dmax (Gy) | 49.06 ± 13.54 | 46.70 ± 17.00 | 53.60 ± 10.13 | 49.28 ± 16.25 | 0.066 | 0.02 | 0.001 | 0 | 0.852 |
| D1cc (Gy) | 29.45 ± 13.38 | 26.41 ± 16.25 | 37.57 ± 10.66 | 31.31 ± 17.56 | 0.007 | 0.002 | 0 | 0 | 0.237 | |
| Dmean (Gy) | 17.97 ± 6.42 | 14.60 ± 8.02 | 25.15 ± 5.38 | 17.75 ± 9.73 | 0 | 0 | 0 | 0 | 0.796 |
P2.5: 2.5FJ vs. 2.5DJ; P5.0: 5.0FJ vs. 5.0DJ; PF: 2.5FJ vs. 5.0FJ; PD: 2.5DJ vs. 5.0DJ; P*: 2.5FJ vs. 5.0DJ.
Figure 2.
Stem-and-leaf displays of percentage differences in dosimetric parameters of several OARs among the four plans in 20 patients. Percentage difference . For example, the percentage difference between the 2.5DJ and 2.5FJ groups was calculated as the difference between the 2.5DJ dose minus the 2.5FJ dose, divided by the 2.5DJ dose, multiplied by 100%. [(A) Dmean of optic nerves, (B) Dmax of optic chiasma, (C) Dmean of optic chiasma, (D) Dmean of temporal lobes, (E) Dmean of hippocampus, (F) D1cc of hippocampus].
All Tested Plans Passed the Acceptance Criteria
To better assess the quality of dose distributions in the four plans, we rounded the mean values of the dosimetric parameters of all OARs in each plan and ranked each parameter in ascending order by dose values. Then, we assigned a score according to the ranking: the group with the lowest dose was ranked first and was assigned a corresponding score of 1, while the group with the highest dose was ranked fourth and assigned a score of 4. Two groups with identical dose values were assigned the same rank and the same score (e.g., if they were both ranked first, they would both be assigned a score of 1). Finally, the scores of each group were calculated, with the group with the lowest score considered the best. The ranking of the overall scores showed that among the four groups, the 2.5DJ group had the best overall score, followed by the 2.5FJ and 5.0DJ groups with the same score and the 5.0FJ group with the worst score (Tables 3, 4).
Table 3.
Mean values of dosimetric parameters of organs at risk (OARs) among the four plans in 20 patients (rounded).
| OARs | Parameters | 2.5FJ | 2.5DJ | 5.0FJ | 5.0DJ |
|---|---|---|---|---|---|
| Spinal cord | Dmax (Gy) | 38 | 38 | 38 | 38 |
| D1cc (Gy) | 35 | 35 | 34 | 34 | |
| Brainstem | Dmax (Gy) | 60 | 61 | 62 | 62 |
| D1cc (Gy) | 54 | 54 | 55 | 55 | |
| Lens | Dmax (Gy) | 6 | 6 | 6 | 6 |
| Optic nerve | Dmax (Gy) | 50 | 44 | 52 | 47 |
| Dmean (Gy) | 34 | 28 | 38 | 31 | |
| Optic chiasm | Dmax (Gy) | 44 | 36 | 51 | 38 |
| Dmean (Gy) | 39 | 31 | 47 | 33 | |
| Eyes | Dmean (Gy) | 10 | 9 | 11 | 10 |
| Parotid | Dmean (Gy) | 40 | 40 | 40 | 40 |
| Pituitary | Dmax (Gy) | 52 | 49 | 56 | 52 |
| Dmean (Gy) | 48 | 43 | 53 | 46 | |
| Temporal lobe | D1cc (Gy) | 63 | 63 | 65 | 65 |
| Dmean (Gy) | 19 | 16 | 24 | 19 | |
| V60Gy (%) | 2 | 2 | 3 | 3 | |
| Hippocampus | Dmax (Gy) | 49 | 47 | 54 | 49 |
| D1cc (Gy) | 29 | 26 | 38 | 31 | |
| Dmean (Gy) | 18 | 15 | 25 | 18 |
Table 4.
Ranks and scores based on the mean values of dosimetric parameters of organs at risk (OARs) among the four plans in 20 patients.
| OARs | Parameters | 2.5FJ | 2.5DJ | 5.0FJ | 5.0DJ |
|---|---|---|---|---|---|
| Spinal cord | Dmax (Gy) | 1 | 1 | 1 | 1 |
| D1cc (Gy) | 2 | 2 | 1 | 1 | |
| Brainstem | Dmax (Gy) | 1 | 2 | 3 | 3 |
| D1cc (Gy) | 1 | 1 | 2 | 2 | |
| Lens | Dmax (Gy) | 1 | 1 | 1 | 1 |
| Optic nerve | Dmax (Gy) | 3 | 1 | 4 | 2 |
| Dmean (Gy) | 3 | 1 | 4 | 2 | |
| Optic chiasm | Dmax (Gy) | 3 | 1 | 4 | 2 |
| Dmean (Gy) | 3 | 1 | 4 | 2 | |
| Eyes | Dmean (Gy) | 2 | 1 | 3 | 2 |
| Parotid | Dmean (Gy) | 1 | 1 | 1 | 1 |
| Pituitary | Dmax (Gy) | 2 | 1 | 3 | 2 |
| Dmean (Gy) | 3 | 1 | 4 | 2 | |
| Temporal lobe | D1cc (Gy) | 1 | 1 | 2 | 2 |
| Dmean (Gy) | 2 | 1 | 3 | 2 | |
| V60Gy (%) | 1 | 1 | 2 | 2 | |
| Hippocampus | Dmax (Gy) | 2 | 1 | 3 | 2 |
| D1cc (Gy) | 2 | 1 | 4 | 3 | |
| Dmean (Gy) | 2 | 1 | 3 | 2 | |
| Total score | 36 | 21 | 52 | 36 | |
| Overall ranking | 2 | 1 | 3 | 2 |
Comparisons of BOTs and MUs
The delivery time (BOT) and MU for each plan was based on the calculation of the treatment planning system. Since the dose rate of HT was fixed, the proportions of the differences in the mean BOTs and numbers of MUs between the FJ and DJ groups were also the same. The BOT and MU values of the FJ and DJ modes were more similar when the jaw width was the same. However, regardless of irradiation mode, the BOT and MU were reduced by ~45% for a 5.0-cm jaw width compared to those for a 2.5-cm jaw width (Table 5).
Table 5.
Mean beam-on time(s) (BOTs) and monitor units (MUs) in each plan group.
| Group | 2.5FJ | 2.5DJ | 5.0FJ | 5.0DJ |
|---|---|---|---|---|
| Time (s) | 389 | 390 | 215 | 216 |
| Monitor units (MUs) | 7,644 | 7,671 | 4,237 | 4,247 |
Discussion
Precision, a novel tomotherapy technology platform, offers a DJ mode for beam delivery, which effectively improves the issue of scatter radiation doses at the edges of the irradiation field (25). This study compared the differences in dosimetric parameters for four plans with different combinations of jaw width and irradiation mode, including 2.5FJ, 2.5DJ, 5.0FJ, and 5.0DJ, in the treatment of NPC. The effects of the different combinations on dose distribution quality, delivery efficiency, and MU of HT plans for NPC treatment were analyzed. Furthermore, an overall score was assigned to the four combinations for superiority evaluation. The optimization algorithm of the Precision planning system prioritizes target doses. The target dose requirement must first be satisfied before the doses to OARs could be optimized based on predetermined constraints. This study repeatedly optimized the plans used for the same patient using the same optimization parameters. The results obtained from sufficient iterative optimizations of the plans were stable, which ensured the comparability of the four plans assessed in this study.
The results of this study showed that all four combinations of jaw width and irradiation mode for HT treatment of NPC met the prescribed dose coverage of the target volume requirement. The mean differences in dosimetric parameters of the target volumes among the plans were <1%. Such minor differences could be ignored during actual treatment in clinical practice. The comparison of the doses received by the OARs showed that a narrower jaw width or a DJ mode provided better protection to most OARs, particularly those at the edges of the target volumes in the longitudinal direction, including normal tissues such as the optic nerve, pituitary gland, temporal lobe, and hippocampus. Treatment using a wider jaw width in the FJ mode may result in out-of-field doses in extended regions due to the larger penumbra and higher doses of scatter radiation. This would, in turn, increase the out-of-field irradiated volume. Thus, the doses received and irradiated volumes of organs and tissues adjacent to the edges of the target volume in the longitudinal direction also increase (26–28). When a narrow jaw width was used in the FJ mode or when a narrow jaw opening was used at the edges of the target volume in the longitudinal direction in the DJ mode, a steeper dose gradient was formed outside the borders of the target volumes along the Y-axis. This overcame the pitfall of dose extension in the longitudinal direction caused by a wide jaw width in the FJ mode (28).
In NPC, many important OARs are adjacent to the edges of the target volumes in the longitudinal direction. Zeng et al. (10) established a clinical dose association model and performed an analysis using the equivalent dose in 2-Gy fractions (EQD2), reporting a biologically-equivalent tolerance dose of D1cc for the development of temporal lobe injury of 62.83 Gy. Hsiao et al. (9) reported that patients with NPC showed significant cognitive decline after receiving radiotherapy when the Dmean of the temporal lobe was >36 Gy. The results of the present study indicated that reducing the jaw width or using the DJ mode for beam delivery significantly reduced the D1cc of the temporal lobe while also significantly reducing the Dmean of the temporal lobe. Furthermore, Gondi et al. (29) demonstrated that an EQD2 to 40% of the bilateral hippocampi >7.3 Gy was associated with long-term impairment in list-learning and delayed recall. The anatomic location of the hippocampus is adjacent to or overlaps with the target volume of NPC in the longitudinal direction. Therefore, the hippocampus can be selectively protected during HT planning. The results of the present study showed mean doses to the hippocampus in the 2.5FJ, 2.5DJ, 5.0FJ, and 5.0DJ groups of 17.97 ± 6.42, 14.60 ± 8.02, 25.15 ± 5.38, and 17.75 ± 9.73 Gy, respectively. The reduction in jaw width or use of the DJ mode could effectively reduce the mean doses to the hippocampus. Due to the numerous organs surrounding the target volume in NPC, it is necessary to consider the doses received by the target volume and OARs collectively during treatment planning or plan selection. Therefore, in this study, we comprehensively evaluated the quality of the four plans with different combinations by ranking and assigning scores to each based on the doses received by the OARs. The results showed that the 2.5DJ group had the best (lowest) overall score, followed by the 2.5FJ and 5.0DJ groups, with the same scores, the 5.0FJ group with the worst score. Furthermore, regardless of irradiation mode, the BOT and MU were reduced by ~45% when a 5.0-cm jaw width was used compared to those for a 2.5-cm jaw width. Although the 2.5DJ group exhibited the best dose distribution, the delivery time and MU were 45% higher than those in the 5.0DJ group. An increase in delivery time implies an increased probability of patient position and target displacements during treatment, while an increased number of MUs results in increased leakage radiation, thereby increasing the risk of second primary malignancies (SPMs) (30). The incidence of SPM has always been underestimated in the past, mainly because most patients have a relatively short life expectancy after treatment or because the follow-up period was <15 years. With the possibility of longer-term survival and longer follow-up times, the incidence of SPM among patients who have undergone radiation therapy can be up to 20%. The time between radiotherapy and SPM may be at least 10 years and even up to 50 years in some cases (31). Since NPC is a tumor that can almost be completely cured and is associated with long-term survival (32), the issue of SPM in patients with cannot be ignored. The present study considered the quality of dose distribution, BOT, and number of MUs in the four plans, and concluded that both the 2.5 DJ and 5.0DJ group provided an excellent combination for use as an HT plan for NPC.
In summary, the use of a narrower jaw width or DJ mode in HT plans for NPC can provide better protection for OARs. The 5-cm jaw width can reduce the delivery time and number of MUs by 45%. We recommend both the 2.5DJ and 5.0DJ mode for the clinical HT treatment of NPC patients based on center work load. Although the 2.5DJ plan perform the best in the test, the 5.0DJ mode can be chosen as an alternative selection when considering the balance of treatment efficiency and plan quality or concern of the out-of-field dose (related to delivery MUs) for those patients with expected long-term survival. According to reported literatures, out-of-field radiation doses to normal tissues may be associated with an increased risk of secondary malignancies, particularly in long-term survivors.
In this study, for simplifying the effect to plan quality and focus on the combination of jaw width and delivery mode, we simply chose the fix pitch of 0.287 and consistent modulation factor of 3. However, these parameters should be chosen based on the size, length or shape of the PTV to get an optimal dose distribution. This might bring bios in real clinical application, and need further study on particular classified group testing.
Data Availability Statement
The datasets of this research are backed up on the Research Data Deposit (RDD, https://www.researchdata.org.cn, approval number: RDDA2019001300) and are available on reasonable request.
Ethics Statement
Our study was reviewed and approved by the IRB committee of Sun Yat-sen University Cancer Center, with the approval number of YB2018-40. As this study is only a retrospective analysis, informed consent exemption was approved (shown in the attached IRB document.
Author Contributions
XD and LZ contributed conception and design of the study. JZha, SD, JZhu, and YL organized the database. JZha, YP, MC, and WS performed the statistical analysis. JZha wrote the first draft of the manuscript. JZha and YP wrote sections of the manuscript. All authors contributed to manuscript revision, read, and approved the submitted version.
Conflict of Interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Footnotes
Funding. This work was jointly supported by the National Key R&D Program of China (2017YFC0113200), Science and Technology program of Guangdong Province, China (2015B020214002).
References
- 1.Mackie TR. History of tomotherapy. Phys Med Biol. (2006) 51:R427–53. 10.1088/0031-9155/51/13/R24 [DOI] [PubMed] [Google Scholar]
- 2.Fiandra C, Filippi AR, Catuzzo P, Botticella A, Ciammella P, Franco P, et al. Different IMRT solutions vs. 3D-conformal radiotherapy in early stage Hodgkin's lymphoma: dosimetric comparison and clinical considerations. Radiat Oncol. (2012) 7:186. 10.1186/1748-717X-7-186 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Bijdekerke P, Verellen D, Tournel K, Vinh-Hung V, Somers F, Bieseman P, et al. TomoTherapy: implications on daily workload and scheduling patients. Radiother Oncol. (2008) 86:224–30. 10.1016/j.radonc.2007.10.036 [DOI] [PubMed] [Google Scholar]
- 4.Sugie C, Shibamoto Y, Ayakawa S, Mimura M, Komai K, Ishii M, et al. Craniospinal irradiation using helical tomotherapy: evaluation of acute toxicity and dose distribution. Technol Cancer Res Treat. (2011) 10:187–95. 10.7785/tcrt.2012.500194 [DOI] [PubMed] [Google Scholar]
- 5.Sugie C, Manabe Y, Hayashi A, Murai T, Takaoka T, Hattori Y, et al. Efficacy of the dynamic jaw mode in helical tomotherapy with static ports for breast cancer. Technol Cancer Res Treat. (2014) 90:459–65. 10.1177/1533034614558746 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Du L, Zhang XX, Feng LC, Chen J, Yang J, Liu HX, et al. Treatment of nasopharyngeal carcinoma using simultaneous modulated accelerated radiation therapy via helical tomotherapy: a phase II study. Radiol Oncol. (2016) 50:218–25. 10.1515/raon-2016-0001 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Du L, Zhang XX, Ma L, Feng LC, Li F, Zhou GX, et al. Clinical study of nasopharyngeal carcinoma treated by helical tomotherapy in China: 5-Year outcomes. Biomed Res Int. (2014) 2014:980767. 10.1155/2014/980767 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Khodayari B, Michaud AL, Stanic S, Wooten OH, Dublin A, Purdy JA, et al. Evaluation of hippocampus dose for patients undergoing intensity-modulated radiotherapy for nasopharyngeal carcinoma. Br J Radiol. (2014) 87:20130474. 10.1259/bjr.20130474 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Hsiao KY, Yeh SA, Chang CC, Tsai PC, Wu JM, Gau JS. Cognitive function before and after intensity-modulated radiation therapy in patients with nasopharyngeal carcinoma: a prospective study. Int J Radiat Oncol Biol Phys. (2010) 77:722–6. 10.1016/j.ijrobp.2009.06.080 [DOI] [PubMed] [Google Scholar]
- 10.Zeng L, Huang SM, Tian YM, Sun XM, Han F, Lu TX, et al. Normal tissue complication probability model for radiation-induced temporal lobe injury after intensity-modulated radiation therapy for nasopharyngeal carcinoma. Radiology. (2015) 276:243–9. 10.1148/radiol.14141721 [DOI] [PubMed] [Google Scholar]
- 11.Rong Y, Chen Y, Shang L, Zuo L, Lu W, Chen Q. Helical tomotherapy with dynamic running-start-stop delivery compared to conventional tomotherapy delivery. Med Phys. (2014) 41:051709. 10.1118/1.4870987 [DOI] [PubMed] [Google Scholar]
- 12.Manabe Y, Shibamoto Y, Sugie C, Hayashi A, Murai T, Yanagi T. Helical and static-port tomotherapy using the newly-developed dynamic jaws technology for lung cancer. Technol Cancer Res Treat. (2015) 14:583–91. 10.7785/tcrtexpress.2013.600280 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Krause S, Beck S, Kai S, Lissner S, Hui S, Herfarth K, et al. Accelerated large volume irradiation with dynamic jaw/dynamic couch helical tomotherapy. Radiat Oncol. (2012) 7:191. 10.1186/1748-717X-7-191 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Krause S, Beck S, Schramm O, Schubert K, Hauswald H, Zabel-du Bois A, et al. Tomotherapy radiosurgery for arteriovenous malformations–current possibilities and future options with helical tomotherapy dynamic jaws? Technol Cancer Res Treat. (2013) 12:421–8. 10.7785/tcrt.2012.500335 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Chen Y, Chen Q, Chen M, Lu W. Dynamic tomotherapy delivery. Med Phys. (2011) 38:3013–24. 10.1118/1.3584198 [DOI] [PubMed] [Google Scholar]
- 16.van Vulpen M, Field C, Raaijmakers CP, Parliament MB, Terhaard CH, MacKenzie MA, et al. Comparing step-and-shoot IMRT with dynamic helical tomotherapy IMRT plans for head-and-neck cancer. Int J Radiat Oncol Biol Phys. (2005) 62:1535–9. 10.1016/j.ijrobp.2005.04.011 [DOI] [PubMed] [Google Scholar]
- 17.Wiezorek T, Brachwitz T, Georg D, Blank E, Fotina I, Habl G, et al. Rotational IMRT techniques compared to fixed gantry IMRT and Tomotherapy: multi-institutional planning study for head-and-neck cases. Radiat Oncol. (2011) 6:20. 10.1186/1748-717X-6-20 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.ICRU Prescribing, Recording, and Reporting Photon Beam Therapy. ICRU Report 50. Bethesda, MD: International Commission on Radiation Units and Measurements; (1993). [Google Scholar]
- 19.Wambersie A, Landberg T. ICRU Report 62, Prescribing, Recording and Reporting Photon Beam Therapy (Supplement to ICRU Report 50). ICRU News; (1999) [Google Scholar]
- 20.International Commission on Radiation Units and Measurements Report 83. Prescribing, Recording, and Reporting Photon- Beam Intensity-Modulated Radiation Therapy (IMRT). Oxford: Pergamon Press; (2010). [Google Scholar]
- 21.Lee N, Pfister DG, Garden A, Ang K, Kim J, Chan A, et al. RTOG 0615. A Phase II Study of Concurrent Chemoradiotherapy Using Three-Dimensional Conformal Radiotherapy (3D-CRT) or Intensity-Modulated Radiation Therapy (IMRT) + Bevacizumab (BV) [NSC 708865; IND 7921] for Locally or Regionally Advanced Nasopharyngeal Cancer. RTOG Foundation. [Google Scholar]
- 22.Lee AW, Ng WT, Pan JJ, Chiang CL, Poh SS, Choi HC, et al. International guideline on dose prioritization and acceptance criteria in radiation therapy planning for nasopharyngeal carcinoma. Int J Radiat Oncol Biol Phys. (2019) 105:567–80. 10.1016/j.ijrobp.2019.09.030 [DOI] [PubMed] [Google Scholar]
- 23.Feuvret L, Noël G, Mazeron JJ, Bey P. Conformity index: a review. Int J Radiat Oncol Biol Phys. (2006) 64:333–42. 10.1016/j.ijrobp.2005.09.028 [DOI] [PubMed] [Google Scholar]
- 24.Kataria T, Sharma K, Subramani V, Karrthick KP, Bisht SS. Homogeneity index: an objective tool for assessment of conformal radiation treatments. J Med Phys. (2012) 37:207–13. 10.4103/0971-6203.103606 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Sterzing F, Uhl M, Hauswald H, Schubert K, Sroka-Perez G, Chen Y, et al. Dynamic jaws and dynamic couch in helical tomotherapy. Int J Radiat Oncol Biol Phys. (2010) 76:1266–73. 10.1016/j.ijrobp.2009.07.1686 [DOI] [PubMed] [Google Scholar]
- 26.Mackie TR, Holmes T, Swerdloff S, Reckwerdt P, Deasy JO, Yang J, et al. Tomotherapy: a new concept for the delivery of dynamic conformal radiotherapy. Med Phys. (1993) 20:1709–19. 10.1118/1.596958 [DOI] [PubMed] [Google Scholar]
- 27.Han EY, Kim DW, Zhang X, Penagaricano J, Liang X, Hardee M, et al. Dosimetric effect on pediatric conformal treatment plans using dynamic jaw with tomotherapy HDA. Med Dos. (2015) 40:244–7. 10.1016/j.meddos.2015.01.007 [DOI] [PubMed] [Google Scholar]
- 28.Xie C, Xu S, Ge R, Xu W, Cong X, Ju Z, et al. Study of dosimetric of volumetric modulated arc therapy and helical tomotherapy for patient with locally advanced laryngeal and hypopharyngeal carcinoma. Prac J Cancer. (2016) 31:120–3. 10.3969/j.issn.1001-5930.2016.01.036 [DOI] [Google Scholar]
- 29.Gondi V, Hermann BP, Mehta MP, Tome WA. Hippocampal dosimetry predicts neurocognitive function impairment after fractionated stereotactic radiotherapy for benign or low-grade adult brain tumors. Int J Radiat Oncol Biol Phys. (2012) 83:e487–93. 10.1016/j.ijrobp.2011.10.021 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Kry SF, Salehpour M, Followill DS, Stovall M, Kuban DA, White RA, et al. The calculated risk of fatal secondary malignancies from intensity-modulated radiation therapy. Int J Radiat Oncol Biol Phys. (2005) 62:1195–1203. 10.1016/j.ijrobp.2005.03.053 [DOI] [PubMed] [Google Scholar]
- 31.Tubiana M. Can we reduce the incidence of second primary malignancies occurring after radiotherapy? A critical review. Radiother Oncol. (2009) 91:4–15. 10.1016/j.radonc.2008.12.016 [DOI] [PubMed] [Google Scholar]
- 32.Lee CC, Huang TT, Lee MS, Su YC, Chou P, Hsiao SH, et al. Survival rate in nasopharyngeal carcinoma improved by high caseload volume: a nationwide population-based study in Taiwan. Radiat. Oncol. (2011) 6:92. 10.1186/1748-717X-6-92 [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
The datasets of this research are backed up on the Research Data Deposit (RDD, https://www.researchdata.org.cn, approval number: RDDA2019001300) and are available on reasonable request.