Among hospitalized patients with high-risk conditions (eg, stem cell or solid organ transplants), invasive aspergillosis is infrequent but is associated with increases in hospital mortality and 30-day readmission rates and costs, costing the US healthcare system approximately $600 billion annually.
Keywords: invasive aspergillosis, epidemiology, outcomes, hospital
Abstract
Background
Though invasive aspergillosis (IA) complicates care of up to 13% of patients with immunocompromise, little is known about its morbidity and mortality burden in the United States.
Methods
We analyzed the Health Care Utilization Project’s data from the Agency for Healthcare Research and Quality for 2009–2013. Among subjects with high-risk conditions for IA, IA was identified via International Classification of Diseases, Ninth Revision, Clinical Modification codes 117.3, 117.9, and 484.6. We compared characteristics and outcomes between those with (IA) and without IA (non-IA). Using propensity score matching, we calculated the IA-associated excess mortality and 30-day readmission rates, length of stay, and costs.
Results
Of the 66634683 discharged patients meeting study inclusion criteria, 154888 (0.2%) had a diagnosis of IA. The most common high-risk conditions were major surgery (50.1%) in the non-IA and critical illness (41.0%) in the IA group. After propensity score matching, both mortality (odds ratio, 1.43; 95% confidence interval, 1.36–1.51) and 30-day readmission (1.39; 1.34–1.45) rates were higher in the IA group. IA was associated with 6.0 (95% confidence interval, 5.7–6.4) excess days in the hospital and $15542 ($13869–$17215) in excess costs per hospitalization.
Conclusions
Although rare even among high-risk groups, IA is associated with increased hospital mortality and 30-day readmission rates, excess duration of hospitalization, and costs. Given nearly 40000 annual admissions for IA in the United States, the aggregate IA-attributable excess costs may reach $600 million annually.
Although invasive aspergillosis (IA) occurs infrequently among immunocompetent individuals, it remains a major issue in the care of patients who have undergone either stem cell or solid organ transplantation, with the prevalence over 10% in select populations [1–7]. There are few broadly generalizable data available on the current morbidity and mortality burden from IA hospitalizations in the United States. One analysis based on a national sample of all US hospitalizations from >2 decades ago indicated that IA-associated mortality neared 20% [8]. However, few large analyses, if any, have examined this issue in more recent years. Furthermore, and underscoring the burden of IA, that analysis estimated that the national costs related to IA hospitalizations topped $600 million.
Such older estimates regarding outcomes related to IA require reassessment. Much has changed in the last 20 years, in terms of IA prevalence and severity, its underlying associated conditions, and its treatment. Several reports suggest that IA has increased in prevalence, a phenomenon most likely due to several factors, including improved diagnostics, an overall escalation in the use of immunosuppressive therapies, and an increased number of organ transplantations performed in recent decades [9–11]. Given the expanded spectrum of disease severity among patients now eligible for aggressive immunosuppressive treatments, and newer treatment options for IA, it is unclear what these countervailing factors imply for outcomes associated with IA. Therefore, to derive more recent estimates of the prevalence and costs related to acute hospitalizations associated with IA, we examined a nationally representative sample of patients admitted to acute-care hospitals in the United States with a diagnosis of IA.
METHODS
Study Design
We performed a retrospective cohort study to explore the epidemiology and outcomes of hospitalizations with IA in the United States. The outcomes of interest were hospital mortality rate, length of stay (LOS), costs, and 30-day readmission rate.
Data Sources
We analyzed the National (formerly “Nationwide”) Inpatient Sample (NIS), part of the Health Care Utilization Project administered by the Agency for Healthcare Research and Quality for the years 2010–2013. The NIS is a large, all-payer database of inpatient hospitalizations, which can be used to derive nationally representative estimates of hospital inpatient stays. (For more information on the NIS database, see https://www.hcup-us.ahrq.gov/nisoverview.jsp.) The NIS consists of a stratified sample of hospital discharge records from approximately 1000 participating facilities, representing about 20% of all acute-care hospitals in the United States.
The unit of reporting in the NIS database is a hospital discharge. The database includes data on patient demographics, diagnoses, and procedures and in-hospital mortality rates, as well as hospital charges and LOS for each discharge. Additional data files, linkable to discharges in the NIS database, provide data on hospital characteristics, illness severity measures, and cost-to-charge conversion coefficients for each individual institution in the database. Complex survey methods exist to develop national and regional estimates for conditions addressed in the database. The Agency for Healthcare Research and Quality undertakes an assessment of completeness and data quality, and documentation is provided with the data set. Data quality checks are limited to logical issues (eg, birth date precedes age at hospital admission; excessively low total charges or long LOS; age <10 or >55 years on a maternal record; mixed neonatal and maternal records). No chart reviews are undertaken by the Agency.
Because the NIS does not report 30-day readmission rates, we relied on data in the State Inpatient Databases (SID), also part of the Health Care Utilization Project, from 4 geographically diverse US states (California [available only for 2009–2011], Florida, Iowa, and New York) for 2009–2013. Although these databases are similar to the NIS, they are confined to the individual states. However, it is possible to identify readmissions owing to the way individual patients are tracked within the state, provided that they occurred within the same state and year as the index hospitalization.
Case Identification
IA hospitalizations were identified using International Classification of Diseases, Ninth Revision, Clinical Modification (ICD-9-CM) codes 117.3, 117.9, and 484.6, shown to be sensitive in detecting IA [12]. We used ICD-9-CM codes, procedure codes, and diagnosis-related groups to identify discharged patients at high risk for IA [13, 14] (see Supplementary Material). These categories were as follows: stem cell transplant, solid organ transplant, critical illness (including mechanical ventilation, trauma, sepsis), major surgery, mild-to-moderate immunocompromise (includes renal disease, lupus and a variety of blood disorders), severe immunocompromise (including leukemia, lymphoma, and chemotherapy), and other (including pneumonia, chronic obstructive pulmonary disease, and human immunodeficiency virus infection). Discharges falling outside these definitions were excluded from analyses.
Outcome Variables and Follow-up
Hospital mortality rate served as the primary end point of the study. As secondary outcomes, we analyzed hospital LOS in days and hospital costs in US dollars. In the SID analysis only, we examined 30-day readmission rates among survivors of the index hospitalization. The groups were followed up until discharge from or death in the hospital. For the 30-day readmission, survivors of the index hospitalization were followed up for another 30 days.
Statistical Analyses
We conducted the following analyses in the NIS, and repeated them in the SID data with the addition of the 30-day readmission outcome. We compared hospital mortality rates, LOS, costs and 30-day readmission rates among all high-risk discharged patients with concomitant diagnosis of IA and those without IA (non-IA). We examined demographic, clinical, hospital, and discharge characteristics in these groups. Means (with standard deviation) and medians (with interquartile range) were calculated for continuous variables, and counts and proportions for categorical variables. All statistics took the weighted nature of the data into account. Continuous variables were compared between the 2 groups using Student t or the Wald tests, and categorical variables using χ2 tests. All inferences were 2 tailed. Statistical significance was defined as P values <.05.
To adjust for confounding in all outcomes, we developed a single propensity score using a probit regression model with IA status as the outcome based on patient demographics (age, race, sex), patient type (urgent, emergent elective, trauma), admission source, weekend admission status, comorbid conditions (based on the Elixhauser classification [15]), number of discharge diagnoses and procedures, the defined high-risk groups, and hospital characteristics (hospital size, location, teaching status, and urbanicity). Propensity score matching was conducted using a greedy 5:3 digit algorithm [16, 17]. The covariates in the propensity regression model were compared after matching to see if they were significantly different, and standardized differences between covariates by IA status were also derived. Covariate balance was a priori determined to exist if all covariates were nonsignificantly different after matching (P < .05) or, if they were significantly different, the standardized difference was <0.1.
For mortality and 30-day readmission outcomes, we constructed a weighted conditional logistic regression model on the matched pairs, with the predictor being IA status. As a sensitivity analysis, we repeated the analysis using logistic regression for all patients who met our study criteria, incorporating all the predictors used to estimate the propensity of IA along with a new variable denoting IA status. In this sensitivity analysis, we examined both spline terms for age and clinically plausible interactions during the construction of the logistic regression model. The model fit was assessed with the Hosmer-Lemeshow goodness-of-fit for model calibration and the area under the receiver operating curve for model discrimination.
For the continuous outcomes of LOS and hospital costs, the method was repeated after taking into account the continuous nature of the outcomes. Namely, a weighted linear regression model on the matched pairs was constructed as the principal analysis. As a sensitivity analysis, generalized linear models were used on all patients who met our study criteria with the covariates in the propensity score along with a variable for IA status. Because the continuous outcome data were skewed, a logarithmic link was used, along with a gamma distribution to improve model fit. Statistical analyses were performed using Stata/MP software (version 13.1 for Windows; StataCorp).
We report in detail the NIS findings since the SID analyses were done largely to explore the 30-day readmission outcome (unavailable in the NIS) and to confirm the findings observed in the NIS data set. Consequently, the details of the SID-based cohort can be found in the Supplementary Material.
RESULTS
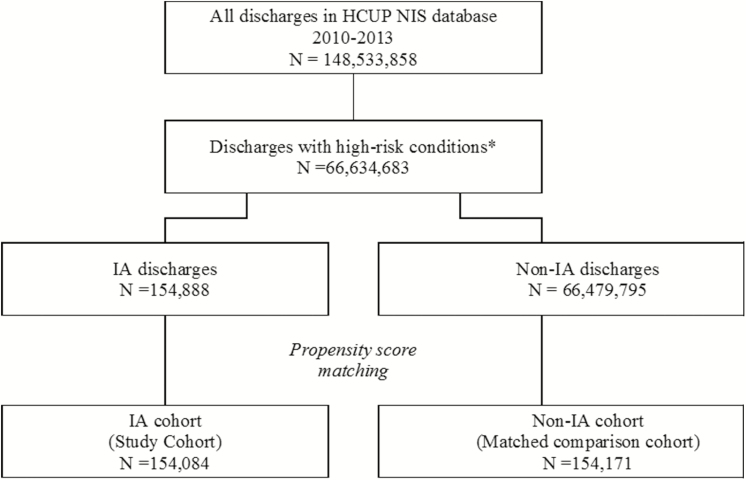
Among a total of 148533858 patients discharged from years 2010 through 2013 in the NIS database, 66634683 met the study inclusion criteria, of whom 154888 (0.2%) had a diagnosis of IA (Figure 1). The baseline characteristics of the 2 groups are shown in Table 1. The discharges were spread evenly across the 4 years of the study period in both IA and non-IA groups. Although the mean ages (and standard deviations) in the 2 groups were similar, the small difference was statistically significant (P < .001), owing in part to the large sample size. Patients with IA were more likely to be male (50.9% in the IA vs 46.7% in non-IA group; P < .001) and African-American (15.3% vs 12.5%; P < .001) (Table 1).
Figure 1.
Study enrollment flow chart. High-risk conditions include stem cell and solid organ transplants, severe immunocompromise (leukemia/lymphoma, chemotherapy, other immune disorders, and long-term steroid use), critical illness (trauma, septicemia, necrotizing fasciitis, intracranial hemorrhage, and mechanical ventilation for ≥96 hours), mild-to- moderate immunocompromise (end-stage renal disease or hemodialysis, lupus, malignant solid tumors, diabetic ketoacidosis, and blood disorders), major surgery, and other conditions (pneumonia, chronic obstructive pulmonary disease, and human immunodeficiency virus infection). Abbreviations: HCUP, Health Care Utilization Project; IA, invasive aspergillosis; NIS, National Inpatient Sample.
Table 1.
Baseline Characteristics, National Inpatient Sample Analysisa
| Characteristic | Patients, No. (%) | P Value | |
|---|---|---|---|
| Non-IA Group (n = 66479795) | IA Group (n = 154888) | ||
| Demographic characteristics | |||
| Sex | |||
| Male | 31042952 (46.7) | 78770 (50.9) | <.001 |
| Female | 35405502 (53.3) | 76118 (49.1) | |
| Age, y | |||
| Mean (SD) | 62.6 (17.9) | 61.8 (16.8) | <.001 |
| Median (IQR) | 64 (51–77) | 64 (52–74) | |
| Race | |||
| White | 44526259 (67.0) | 10068 (65.0) | <.001 |
| Black | 8320004 (12.5) | 23631 (15.3) | |
| Hispanic | 5248792 (7.9) | 12228 (7.9) | |
| Asian or Pacific Islander | 1170046 (1.8 | 3889 (2.5) | |
| Native American | 383561 (0.6) | 676 (0.4) | |
| Other | 1561063 (2.3) | 4030 (2.6) | |
| Data missing | 5270070 (7.9) | 9752 (6.3) | |
| Primary expected payer | |||
| Medicare | 36495542 (54.9) | 87822 (56.7) | <.001 |
| Medicaid | 6949615 (10.5) | 20611 (13.3) | |
| Private | 17239637 (25.9) | 36877 (23.8) | |
| Self-pay | 3056800 (4.6) | 4653 (3.0) | |
| No charge | 329595 (0.5) | 612 (0.4) | |
| Other | 2253531 (3.4) | 4104 (2.6) | |
| Data missing | 155074 (0.2) | 209 (0.1) | |
| Year of admission | |||
| 2010 | 17287324 (26.0) | 39284 (25.4) | .56 |
| 2011 | 17695983 (26.6) | 40364 (26.1) | |
| 2012 | 15807892 (23.8) | 38390 (24.8) | |
| 2013 | 15688596 (23.6) | 36850 (23.8) | |
| Admission characteristics | |||
| Admission type | |||
| Emergency | 17288605 (26.0) | 41892 (27.0) | <.001 |
| Urgent | 5116128 (7.7) | 15200 (9.8) | |
| Elective | 8731135 (13.1) | 12089 (7.8) | |
| Delivery | 332851 (0.5) | 221 (0.1) | |
| Trauma center | 3073 (0.0) | <10b (0.0) | |
| Other/data missing | 35008003 (52.7) | 85481 (55.2) | |
| Emergency department service | 38184223 (57.4) | 92437 (59.7) | <.001 |
| Weekend admission | 12463539 (18.7) | 31548 (20.4) | <.001 |
| Hospital characteristics | |||
| Census region | |||
| Northeast | 12497745 (18.8) | 28761 (18.6) | .007 |
| Midwest | 15659097 (23.6) | 32394 (20.9) | |
| West | 25910920 (39.0) | 62268 (40.2) | |
| South | 12412034 (18.7) | 31466 (20.3) | |
| Location/teaching status | |||
| Rural | 7705468 (11.6) | 10152 (6.6) | |
| Urban nonteaching | 26165688 (39.4) | 52016 (33.6) | <.001 |
| Urban teaching | 32185686 (48.4) | 91927 (59.4) | |
| Data missing | 422953 (0.6) | 794 (0.5) | |
| Size | |||
| Small (1–49 beds) | 8538228 (12.8) | 15168 (9.8) | <.001 |
| Medium (50–99 beds) | 16162746 (24.3) | 32689 (21.1) | |
| Large (≥100 beds) | 41355868 (62.2) | 106238 (68.6) | |
| Data missing | 422953 (0.6) | 794 (0.5) | |
| Hospital IA caseload, % | |||
| Mean (SD) | 0.2 (0.2) | 0.5 (0.8) | … |
| Median (IQR) | 0.2 (0.1–0.3) | 0.3 (0.2–0.6) | <.001 |
| Clinical characteristics | |||
| High-risk conditions | <.001 | ||
| Stem cell transplant | 193249 (0.3) | 5305 (3.4) | |
| Solid organ transplant | 875378 (1.3) | 7708 (5.0) | |
| Critical illness | 14175190 (21.3) | 63465 (41.0) | |
| Major surgery | 33281840 (50.1) | 54799 (35.4) | |
| Severe immunocompromise | 6619094 (10.0) | 12711 (8.2) | |
| Mild-to-moderate immunocompromise | 3508553 (5.3) | 3883 (2.5) | |
| Otherc | 7826491 (11.8) | 10018 (6.5) | |
| Elixhauser comorbid conditionsd | |||
| AIDS/HIV | 294562 (0.4) | 1153 (0.7) | <.001 |
| Alcohol abuse | 3039272 (4.6) | 6132 (4.0) | <.001 |
| Blood loss anemia | 1136933 (1.7) | 2307 (1.5) | <.001 |
| Chronic pulmonary disease | 18218914 (27.4) | 50872 (32.8) | <.001 |
| Coagulopathy | 4103153 (6.2) | 26129 (16.9) | <.001 |
| Congestive heart failure | 7650797 (11.5) | 27690 (17.9) | <.001 |
| Deficiency anemia | 13992274 (21.0) | 50308 (32.5) | <.001 |
| Depression | 7998384 (12.0) | 19398 (12.5) | <.001 |
| Diabetes, complicated | 3694396 (5.6) | 11452 (7.4) | <.001 |
| Diabetes, uncomplicated | 14062429 (21.2) | 34321 (22.2) | <.001 |
| Drug abuse | 2132535 (3.2) | 5941 (3.8) | <.001 |
| Fluid and electrolyte disorders | 18524222 (27.9) | 81222 (52.4) | <.001 |
| Hypertension | 37299088 (56.1) | 76275 (49.2) | <.001 |
| Hypothyroidism | 8314191 (12.5) | 17152 (11.1) | <.001 |
| Liver disease | 2379727 (3.6) | 8723 (5.6) | <.001 |
| Lymphoma | 662042 (1.0) | 4752 (3.1) | <.001 |
| Metastatic cancer | 2206932 (3.3) | 7438 (4.8) | <.001 |
| Neurodegenerative disorders | 5470468 (8.2) | 15927 (10.3) | <.001 |
| Obesity | 8096351 (12.2) | 15438 (10.0) | <.001 |
| Paralysis | 1845579 (2.8) | 8371 (5.4) | <.001 |
| Peptic ulcer disease, no bleeding | 29401 (0.0) | 116 (0.1) | .007 |
| Peripheral vascular disorders | 5022891 (7.6) | 12471 (8.1) | .007 |
| Psychosis | 3025718 (4.6) | 7640 (4.9) | .003 |
| Pulmonary circulation disorders | 1948873 (2.9) | 10934 (7.1) | <.001 |
| Renal failure | 10182469 (15.3) | 33219 (21.4) | <.001 |
| Rheumatoid arthritis/collagen vascular diseases | 2303887 (3.5) | 6761 (4.4) | <.001 |
| Solid tumor without metastasis | 1987297 (3.0) | 6081 (3.9) | <.001 |
| Valvular disease | 2938867 (4.4) | 8180 (5.3) | <.001 |
| Weight loss | 4377595 (6.6) | 40076 (25.9) | <.001 |
| Elixhauser comorbid conditions, total No. | |||
| Mean (SD) | 3.1 (2.2) | 4.3 (2.3) | … |
| Median (IQR | 3 (1–5) | 4 (3–6) | <.001 |
| Discharge diagnoses, No. | |||
| Mean (SD) | 11.3 (6.0) | 17.5 (6.3) | … |
| Median (IQR) | 10 (7–15) | 17 (13–23) | <.001 |
| Procedures, No. | |||
| Mean (SD) | 2.2 (2.4) | 4.5 (4.5) | … |
| Median (IQR) | 2 (1–3) | 3 (1–6) | <.001 |
| Abdominal procedures/ operations | 5738103 (8.6) | 6373 (4.1) | <.001 |
Since the distributions were non-normal, the appropriate comparison values are provided for the median but not the mean values.
Abbreviations: HIV, human immunodeficiency virus; IA, invasive aspergillosis; IQR, interquartile range; SD, standard deviation.
aData represent No. (%) of patients unless otherwise specified.
bThe Agency for Healthcare Research and Quality prohibits reporting on <10 cases.
cIncludes pneumonia, chronic obstructive pulmonary disease, and HIV infection.
dTotal adds up to >100% because it is possible to have >1 comorbid condition per discharge.
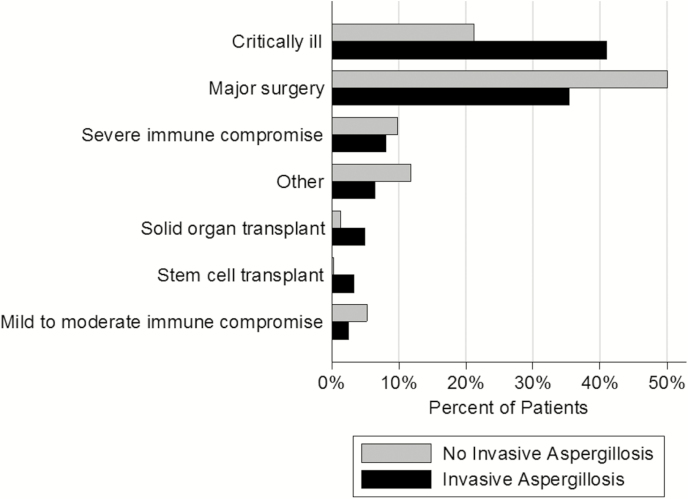
As expected, there were significant differences between groups in terms of high-risk condition distributions (Figure 2). For example, the most common reason for identification as high risk in the non-IA group was major surgery (50.1%), whereas in the IA group it was critical illness (41.0%). Stem cell transplant (0.3%) and mild-to-moderate immunocompromise (2.5%) were the least likely criteria for being high risk in non-IA and IA groups, respectively. The burden of both chronic conditions (median number of Elixhauser comorbid conditions [interquartile range], 3 [1–5] in non-IA vs 4 [3–6] in IA group; P < .001) and the number of procedures done during hospitalization (median, 2 [1–3] vs 3 [1–6], respectively ; P < .001) was higher in the IA than in the non-IA group. Geographically, the plurality of all discharges occurred in the Western region in both groups. Despite the statistically significant differences, there were no clinically notable differences between regional contributions to the IA and non-IA groups (Table 1). Although urban teaching hospitals provided the bulk of discharges in both groups, the proportion coming from this type of institution in the IA group was higher than in the non-IA group (59.4% vs 48.4%, respectively; P < .001, Table 1). Larger hospitals were more likely than smaller ones to contribute IA cases (Table 1).
Figure 2.
High-risk conditions in the comparator groups in the National Inpatient Sample. The invasive aspergillosis (IA) and non-IA groups differed significantly (P < .001). The “Other” designation includes pneumonia, chronic obstructive pulmonary disease, and human immunodeficiency virus infection.
The unadjusted outcomes for non-IA and IA groups are shown in Table 2. The hospital mortality rate among patients with IA (14.1%) was 4-fold higher than in the non-IA group (3.4%; P < .001). Similarly, there were large differences between groups in the LOS, hospital charges, and costs, with each being 2.5–4 times higher in the IA than in the non-IA group (Table 2).
Table 2.
Unadjusted Hospital Outcomes
| Outcome | Non-IA Group (n = 66479795) | IA Group (n = 154888) |
P Value |
|---|---|---|---|
| Mortality rate, No. (%) | 2254516 (3.4) | 21870 (14.1) | <.001 |
| Length of stay, d | |||
| Mean (SD) | 5.6 (6.9) | 17.9 (20.2) | … |
| Median (IQR) | 4 (2–7) | 12 (6–24) | <.001 |
| Total charges, $ | |||
| Mean (SD) | 53133 (78632) | 185626 (287748) | … |
| Median (IQR) | 32401 (17483–60128) | 91978 (40329–215168) | <.001 |
| Total costs, $ | |||
| Mean (SD) | 14860 (20102) | 50661 (74719) | … |
| Median (IQR) | 9772 (5695–16838) | 25823 (12171–59895) | <.001 |
Since the distributions were non-normal, the appropriate comparison values are provided for the median but not the mean values.
Abbreviations: IA, invasive aspergillosis; IQR, interquartile range; SD, standard deviation.
Except for a handful of characteristics, propensity matching of 154081 IA discharges (99.5%) eliminated intergroup differences (Table 3). Even where the differences remained statistically significant, no standardized difference was >0.034. Table 4 lists hospital outcomes after propensity matching. Adjusted mortality rates remained significantly higher with IA than without (14.1% vs 10.3%; P < .001; odds ratio, 1.43; 95% confidence interval [CI], 1.36–1.51). Similarly, relative to no IA, IA was associated with an excess attributable LOS of 6.0 days (95% CI, 5.7–6.4 days) and an excess cost of $15542 ($13869–$17215).
Table 3.
Characteristics of Groups After Propensity Matching
| Characteristic | Patients, No. (%)a | P Value | |
|---|---|---|---|
| Non-IA Group (n = 154186) | IA Group (n = 154081) | ||
| Demographic characteristics | |||
| Year of admission | |||
| 2010 | 39142 (25.4) | 38916 (25.3) | .98 |
| 2011 | 40225 (26.1) | 39930 (25.9) | |
| 2012 | 38025 (24.7) | 38385 (24.9) | |
| 2013 | 36795 (23.9) | 36850 (23.9) | |
| Sex | |||
| Male | 77720 (50.4) | 78357 (50.9) | .30 |
| Female | 76466 (49.6) | 75724 (49.1) | |
| Age, y | |||
| Mean (SD) | 62.9 (17.6) | 61.9 (16.7) | <.001 |
| Median (IQR) | 64 (52–77) | 64 (52–75) | .22 |
| Race | |||
| White | 99406 (64.5) | 100138 (65.0) | .84 |
| Black | 23817 (15.4) | 23552 (15.3) | |
| Hispanic | 12014 (7.8) | 12135 (7.9) | |
| Asian or Pacific Islander | 3900 (2.5) | 3884 (2.5) | |
| Native American | 647 (0.4) | 619 (0.4) | |
| Other | 4256 (2.8) | 4011 (2.6) | |
| Data missing | 10147 (6.6) | 9742 (6.3) | |
| Primary expected payer | |||
| Medicare | 88315 (57.3) | 87387 (56.7) | .80 |
| Medicaid | 20340 (13.2) | 20522 (13.3) | |
| Private | 36203 (23.5) | 36692 (23.8) | |
| Self-pay | 4364 (2.8) | 4597 (3.0) | |
| No charge | 535 (0.3) | 590 (0.4) | |
| Other | 4236 (2.7) | 4084 (2.7) | |
| Data missing | 193 (0.1) | 209 (0.1) | |
| Admission characteristics | |||
| Admission type | |||
| Emergency | 41796 (27.1) | 41451 (26.9) | <.001 |
| Urgent | 12866 (8.3) | 14962 (9.7) | |
| Elective | 14850 (9.6) | 11985 (7.8) | |
| Delivery | 964 (0.6) | 221 (0.1) | |
| Trauma center | <10b (0.0) | <10b (0.0) | |
| Other/data missing | 83701 (54.3) | 85457 (55.5) | |
| Emergency department service | 94055 (61.0) | 93046 (60.4) | .33 |
| Weekend admission | 32113 (20.8) | 31382 (20.4) | .17 |
| Clinical characteristics | |||
| High-risk conditions | |||
| Stem cell transplant | 4636 (3.0) | 5271 (3.4) | .26 |
| Solid organ transplant | 7576 (4.9) | 7698 (5.0) | |
| Critical illness | 63659 (41.3) | 63091 (40.9) | |
| Major surgery | 51254 (33.2) | 51560 (33.5) | |
| Severe immunocompromise | 13257 (8.6) | 12670 (8.2) | |
| Mild-to-moderate immunocompromise | 3889 (2.5) | 3848 (2.5) | |
| Otherc | 9915 (6.4) | 9942 (6.5) | |
| Elixhauser comorbid conditionsd | |||
| AIDS/HIV | 1329 (0.9) | 1149 (0.7) | .18 |
| Alcohol abuse | 6089 (3.9) | 6098 (4.0) | .96 |
| Blood loss anemia | 2460 (1.6) | 2294 (1.5) | .32 |
| Chronic pulmonary disease | 52134 (33.8) | 50602 (32.8) | .03 |
| Coagulopathy | 26132 (16.9) | 26009 (16.9) | .84 |
| Congestive heart failure | 27881 (18.1) | 27577 (17.9) | .58 |
| Deficiency anemia | 52785 (34.2) | 50137 (32.5) | <.001 |
| Depression | 19702 (12.8) | 19315 (12.5) | .39 |
| Diabetes, complicated | 34308 (22.3) | 34195 (22.2) | .87 |
| Diabetes, uncomplicated | 11614 (7.5) | 11401 (7.4) | .58 |
| Drug abuse | 6029 (3.9) | 5896 (3.8) | .61 |
| Fluid and electrolyte disorders | 81453 (52.8) | 80763 (52.4) | .41 |
| Hypertension | 75429 (48.9) | 75987 (49.3) | .41 |
| Hypothyroidism | 17144 (11.1) | 17080 (11.1) | .90 |
| Liver disease | 8788 (5.7) | 8651 (5.6) | .67 |
| Lymphoma | 4721 (3.1) | 4730 (3.1) | .96 |
| Metastatic cancer | 7464 (4.8) | 7394 (4.8) | .81 |
| Neurodegenerative disorders | 16022 (10.4) | 15868 (10.3) | .72 |
| Obesity | 15415 (10.0) | 15365 (10.0) | .92 |
| Paralysis | 8573 (5.6) | 8347 (5.4) | .47 |
| Peptic ulcer disease, no bleeding | 121 (0.1) |
116 (0.1) | .88 |
| Peripheral vascular disorders | 12270 (8.0) | 12416 (8.1) | .67 |
| Psychosis | 7446 (4.8) | 7593 (4.9) | .58 |
| Pulmonary circulation disorders | 11210 (7.3) | 10891 (7.1) | .34 |
| Renal failure | 34368 (22.3) | 33067 (21.5) | .02 |
| Rheumatoid arthritis/collagen vascular diseases | 6463 (4.2) | 6703 (4.4) | .36 |
| Solid tumor without metastasis | 6150 (4.0) | 6057 (3.9) | .72 |
| Valvular disease | 8289 (5.4) | 8123 (5.3) | .59 |
| Weight loss | 40301 (26.1) | 39803 (25.8) | .48 |
| Elixhauser comorbid conditions, total No. | |||
| Mean (SD) | 4.4 (2.5) | 4.3 (2.3) | .05 |
| Median (IQR) | 4 (3–6) | 4 (3–6) | |
| Discharge diagnoses, No. | |||
| Mean (SD) | 14.3 (6.6) | 17.5 (6.3) | <.001 |
| Median (IQR) | 14 (9–18) | 17 (13–23) | |
| Procedures, No. | |||
| Mean (SD) | 2.9 (3.2) | 4.5 (4.5) | <.001 |
| Median (IQR) | 2 (1–4) | 3 (1–6) | |
| Abdominal procedures/ operations | 6084 (3.9) | 6335 (4.1) | .32 |
| Hospital characteristics | |||
| Census region | .72 | ||
| Northeast | 28080 (18.2) | 28761 (18.7) | |
| Midwest | 31179 (20.2) | 31817 (20.7) | |
| West | 63478 (41.1) | 62151 (40.3) | |
| South | 31594 (20.5) | 31317 (20.3) | |
| Location/teaching status | |||
| Rural | 9817 (6.4) | 10152 (6.6) | .38 |
| Urban nonteaching | 53367 (34.6) | 52016 (33.8) | |
| Urban teaching | 91002 (59.0) | 91913 (59.7) | |
| Hospital size | |||
| Small (1–49 beds) | 16480 (10.7) | 15168 (9.8) | .10 |
| Medium (50–99 beds) | 32986 (21.4) | 32689 (21.2) | |
| Large (≥100 beds) | 104720 (67.9) | 106224 (68.9) | |
| Hospital IA caseload, %e | |||
| Mean (SD) | 0.3 (0.3) | 0.5 (0.8) | … |
| Median (IQR) | 0.2 (0.1–0.4) | 0.4 (0.2–0.6) | <.001 |
Since the distributions were non-normal, the appropriate comparison values are provided for the median but not the mean values.
Abbreviations: HIV, human immunodeficiency virus; IA, invasive aspergillosis; IQR, interquartile range; SD, standard deviation.
aData represent No. (%) of patients unless otherwise specified.
bThe Agency for Healthcare Research and Quality prohibits reporting on <10 cases.
cIncludes pneumonia, chronic obstructive pulmonary disease, and HIV infection.
dTotal adds up to >100% because it is possible to have >1 comorbid condition per discharge.
eFactor not matched on because it was defined based on the exposure of interest.
Table 4.
Propensity-Adjusted Hospital Outcomes
| Outcome | Non-IA Group (n = 154186) | IA Group (n = 154081) | P Value |
|---|---|---|---|
| Mortality rate, No. (%) | 15902 (10.3) | 21748 (14.1) | <.001 |
| Length of stay, d | |||
| Mean (SD) | 11.8 (15.6) | 17.9 (20.1) | |
| Median (IQR) | 7 (4–15) | 12 (6–24) | <.001 |
| Total charges, $ | |||
| Mean (SD) | 126385 (204977) | 185529 (287276) | |
| Median (IQR) | 59831 (27503–139724) | 92048 (40354–215034) | <.001 |
| Total costs, $ | |||
| Mean (SD) | 35038 (53301) | 50519 (73716) | |
| Median (IQR) | 17134 (8555–38962) | 25836 (12177–59880) | <.001 |
Abbreviations: IA, invasive aspergillosis; IQR, interquartile range; SD, standard deviation.
The results from the SID analyses revealed similar findings and outcomes by IA status compared with those seen in the NIS (Supplementary Material). The propensity-adjusted 30-day readmission rate was higher in the IA than in the non-IA group (18.0% vs 13.7%; P < .001). In a logistic regression, IA was associated with significantly higher odds of readmission within 30 days (odds ratio, 1.39; 95% CI, 1.34–1.45), relative to no IA.
DISCUSSION
We demonstrate that IA remains a rare event among patients hospitalized in the United States, even in the presence of a high-risk condition. At the same time, IA adds substantially not only to the risk of death, but also to the expenses of hospitalization. Namely, IA contributes 6 additional days, and >$15000 in additional costs, per case. Furthermore, a diagnosis of IA is associated with a significant increase (approximately 40%) in the odds of a 30-day rehospitalization among survivors.
Few current data are available on the clinical and economic outcomes of hospitalizations involving IA. A study using 1996 NIS data provides the most generalizable results on the outcomes we evaluated [8]. Compared with the findings from that earlier study, we document a marked rise in the prevalence of IA among patients undergoing acute hospitalizations. Although 1996 saw approximately 10000 IA hospitalizations, our data suggest a quadrupling of this volume, with the attendant increase in the incidence from 3/10000 to 10/10000 discharges overall. At the same time, crude mortality has dropped from 19% to 14%, and excess LOS and costs have also fallen from 12 to 6 days and from $51000 to $15000 per admission, respectively [8].
It is precisely this drop in hospital utilization that seems responsible for the overall stability of total national costs for IA hospitalizations at $600 million in the face of a dramatic rise in prevalence. That is, although the incidence of IA has increased, outcomes for these patients seem to have improved substantially. Consequently, the net total economic burden has probably fallen when one incorporates the impact of healthcare inflation, because our findings are reported in nominal dollars. As mentioned above, it is important to reiterate that the increase in the volume of IA may be due to improved diagnostics, an overall escalation in the use of immunosuppressive therapies, and an increased number of organ transplantations performed in recent decades [9–11].
Our finding of an approximate 40% increase in the odds of 30-day readmission associated with IA represents a novel and important observation, particularly in the context of current cost-containment efforts and dwindling hospital reimbursements. The passage of the Affordable Care Act created the Hospital Readmission Reduction Program, under which the Centers for Medicare and Medicaid Services (CMS) is mandated to help reduce hospital readmissions for select conditions [18]. Although the initial focus of the program was on acute myocardial infarction, congestive heart failure, and pneumonia, the list of conditions has been expanding over time and is expected to continue to do so [18, 19]. Under this regulation, hospitals incur reimbursement cuts if their readmission rates exceed those expected. Although IA is not included on the most recent list of conditions under CMS scrutiny, it is very likely that patients with IA require readmissions for many of the conditions already tracked by the CMS. Thus, hospitals may suffer financial punishment simply for caring for an increasingly complex cohort of patients. This further reinforces the imperative to focus on preventive efforts for IA.
Our study has a number of strengths. Because we relied on a large nationally representative sample of high-risk discharges, our results are broadly generalizable to all US hospitals. Although the data supporting the examination of 30-day readmission rates among patients discharged alive after an IA hospitalization are limited to 4 states, the states were chosen based on their geographic diversity, which increases the generalizability of the findings [20]. In addition, the sample size and number of covariates in the data set allowed us to adjust for confounding in a statistically rigorous manner. It is also important to stress that no other systematic information exists regarding the risk for readmission in persons with IA.
At the same time, our analysis suffers from a number of potential limitations. First, we relied on administrative codes, and not on clinical data, to identify both the high-risk groups and IA. This may predispose our case definitions to errors of misclassification, though methods similar to ours have been used successfully in both high-risk patients and IA [8, 12, 14]. Findings from another study, however, indicate that the IA ICD-9-CM codes are prone to overestimate the prevalence of IA [12]. This type of misclassification would be expected to reduce the observed differences between IA and non-IA groups. Consequently, IA may indeed have an even greater impact on the outcomes than we were able to detect. This idea is supported by the fact that studies relying on clinical definition for IA consistently report higher associated mortality rates [21].
Second, because of the cross-sectional nature of the data set, it was not possible to ascertain the temporal connection between the high-risk condition of interest and IA. Third, because we were unable to pinpoint the time of onset of IA, the disparities in the adjusted hospital LOS and costs cannot be directly associated with IA itself; that is, it is possible that at least some of the increase in the LOS (and related costs) in the IA group was a risk factor for, rather than a consequence of, IA. At the same time, at least some of the adjusted LOS and cost differences are likely to be directly attributable to IA, because nosocomial complications in general are known to affect LOS and costs. Moreover, it is reassuring that propensity matching greatly reduced confounding from the variables in the database between comparator groups.
Fourth, no treatment information is available in the current data set, so we were unable to explore its potential impact on the outcomes. Specifically, we cannot stratify by the timing of treatment onset or by the specific options used. Fifth, though we note a dramatic reduction in the IA-associated LOS compared with prior studies, our analysis reflects only acute-care hospitalization, excluding any downstream expenses associated either with chronic care facility or home health care. Given advances in the continuum of healthcare delivery since 1996, it is likely that some of the expenditures in the older estimates include the chronic care days that are missing from the current acute-care data set.
Sixth, it is not possible to differentiate in the NIS database initial versus repeated hospitalization. Because the patients who require readmission may differ systematically from those who do not, this may be a source of potential bias. However, given that SID analyses, in which readmissions can be differentiated from index hospitalizations, broadly confirmed the associations noted in the NIS data, this is not likely to present a significant threat to validity. Finally, as in any cohort study, residual confounding is a risk due to unmeasured variables not found in this database, although our propensity scores were based on a large number of covariates.
In summary, although rare even among high-risk groups, IA is associated with high hospital mortality rates, excess duration of hospitalization, and a 40% relative increase in the odds of 30-day readmission. Given nearly 40000 annual IA admissions in the United States, at an excess cost of $15000, the aggregate IA-attributable excess costs in the United States may reach $600 million annually.
Supplementary Data
Supplementary materials are available at Clinical Infectious Diseases online. Consisting of data provided by the authors to benefit the reader, the posted materials are not copyedited and are the sole responsibility of the authors, so questions or comments should be addressed to the corresponding author.
Notes
Author contributions. M. D. Z., R. H., J. R. S., and A. F. S. contributed substantially to the study design, data interpretation, and the writing of the manuscript. B. H. N. had full access to all of the data in the study and takes responsibility for the integrity of the data and the accuracy of the data analysis; he contributed substantially to the study design, data interpretation, and the writing of the manuscript. No one other than the listed authors participated in the study design, analysis, interpretation or manuscript drafting. M. D. Z. takes responsibility for the content of the manuscript, including the data and analysis.
Disclaimer. R. H. and J. R. S., as investigators and authors, were involved in the conception, design, analysis and reporting of the study, and manuscript development. Other scientific representatives of Astellas Pharma Global Development were involved in the review of the study and manuscript. Representatives of commercial and marketing divisions of Astellas had no input to the study or the manuscript.
Financial support. This work was supported by Astellas Pharma and by the medical and development division of Astellas, Astellas Pharma Global Development.
Potential conflicts of interest. M. D. Z. reports grants from EviMed Research Group and Astellas Pharma during the conduct of the study, personal fees from Paratek, Tetraphase, Melinta, Achaogen, Pfizer, Shionogi, and Cleveland Clinic, grants from Merck and The Medicines Company, and stock ownership from Johnson & Johnson, outside the submitted work. B. H. N. is an employee of OptiStatim, which has received funding from EviMed Research Group to conduct this study. R. H. is a former employee of Astellas Pharma Global Development. J. R. S. is an employee of Astellas Pharma Global Development. A. F. S. is a consultant to and has received research grant support from Astellas Pharma and has received grant support and/or has personal fees for consultancy from Merck, Tetraphase, Pfizer, The Medicines Company, Shionogi, and Theravance. All authors have submitted the ICMJE Form for Disclosure of Potential Conflicts of Interest. Conflicts that the editors consider relevant to the content of the manuscript have been disclosed.
Presented in part at IDWeek, 4–8 October 2017, San Diego, CA. Poster 189.
References
- 1. Minari A, Husni R, Avery RK, et al. The incidence of invasive aspergillosis among solid organ transplant recipients and implications for prophylaxis in lung transplants. Transpl Infect Dis 2002; 4:195–200. [DOI] [PubMed] [Google Scholar]
- 2. Montoya JG, Chaparro SV, Celis D, et al. Invasive aspergillosis in the setting of cardiac transplantation. Clin Infect Dis 2003; 37(suppl 3):S281–92. [DOI] [PubMed] [Google Scholar]
- 3. Paterson DL, Singh N. Invasive aspergillosis in transplant recipients. Medicine (Baltimore) 1999; 78:123–38. [DOI] [PubMed] [Google Scholar]
- 4. Singh N, Arnow PM, Bonham A, et al. Invasive aspergillosis in liver transplant recipients in the 1990s. Transplantation 1997; 64:716–20. [DOI] [PubMed] [Google Scholar]
- 5. Marr KA, Carter RA, Boeckh M, Martin P, Corey L. Invasive aspergillosis in allogeneic stem cell transplant recipients: changes in epidemiology and risk factors. Blood 2002; 100:4358–66. [DOI] [PubMed] [Google Scholar]
- 6. Martino R, Subirá M, Rovira M, et al. ; alloPBSCT Infectious/Non-infectious Complications Subcommittees of the Grupo Español de Trasplante Hematopoyético (GETH) Invasive fungal infections after allogeneic peripheral blood stem cell transplantation: incidence and risk factors in 395 patients. Br J Haematol 2002; 116:475–82. [DOI] [PubMed] [Google Scholar]
- 7. Morgan J, Wannemuehler KA, Marr KA, et al. Incidence of invasive aspergillosis following hematopoietic stem cell and solid organ transplantation: interim results of a prospective multicenter surveillance program. Med Mycol 2005; 43(suppl 1):S49–58. [DOI] [PubMed] [Google Scholar]
- 8. Dasbach EJ, Davies GM, Teutsch SM. Burden of aspergillosis-related hospitalizations in the United States. Clin Infect Dis 2000; 31:1524–8. [DOI] [PubMed] [Google Scholar]
- 9. Health Resources and Services Administration, US Department of Health & Human Services. Organ Procurement and Transplantation Network: view data reports Available at: https://optn.transplant.hrsa.gov/data/view-data-reports/. Accessed 14 May 2017.
- 10. Hope WW, Walsh TJ, Denning DW. Laboratory diagnosis of invasive aspergillosis. Lancet Infect Dis 2005; 5:609–22. [DOI] [PubMed] [Google Scholar]
- 11. Vallabhaneni S, Benedict K, Derado G, Mody RK. Trends in hospitalizations related to invasive aspergillosis and mucormycosis in the United States, 2000–2013. Open Forum Infect Dis 2017; 4:ofw268. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Chang DC, Burwell LA, Lyon GM, et al. Comparison of the use of administrative data and an active system for surveillance of invasive aspergillosis. Infect Control Hosp Epidemiol 2008; 29:25–30. [DOI] [PubMed] [Google Scholar]
- 13. Baddley JW. Clinical risk factors for invasive aspergillosis. Med Mycol 2011; 49(suppl 1):S7–S12. [DOI] [PubMed] [Google Scholar]
- 14. Zilberberg MD, Shorr AF, Huang H, Chaudhari P, Paly VF, Menzin J. Hospital days, hospitalization costs, and inpatient mortality among patients with mucormycosis: a retrospective analysis of US hospital discharge data. BMC Infect Dis 2014; 14:310. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Elixhauser A, Steiner C, Harris DR, Coffey RM. Comorbidity measures for use with administrative data. Med Care 1998; 36:8–27. [DOI] [PubMed] [Google Scholar]
- 16. Dehejia RH, Wahba S. Propensity score matching methods for nonexperimental causal studies. Rev Economics Statistics 2002; 84:151–61. [Google Scholar]
- 17. D’Agostino RB., Jr Propensity score methods for bias reduction in the comparison of a treatment to a non-randomized control group. Stat Med 1998; 17:2265–81. [DOI] [PubMed] [Google Scholar]
- 18. Leppin AL, Gionfriddo MR, Kessler M, et al. Preventing 30-day hospital readmissions: a systematic review and meta-analysis of randomized trials. JAMA Intern Med 2014; 174:1095–107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Marks E. Complexity science and the readmission dilemma. JAMA Intern Med 2013; 173:629–31. [DOI] [PubMed] [Google Scholar]
- 20. Goodwin AJ, Rice DA, Simpson KN, Ford DW. Frequency, cost, and risk factors of readmissions among severe sepsis survivors. Crit Care Med 2015; 43:738–46. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Steinbach WJ, Marr KA, Anaissie EJ, et al. Clinical epidemiology of 960 patients with invasive aspergillosis from the PATH Alliance registry. J Infect 2012; 65:453–64. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.