Abstract
Circadian rhythms impact a variety of behavioral and physiological functions contributing to longevity and successful reproduction. In their natural environments, individuals of a species are faced with a multitude of challenges and the coordination of internal processes and behavior with external pressures has been hypothesized to be an important target of natural selection. Several lines of evidence from cyanobacteria, Drosophila, and plants provide strong support for an important role of the circadian clock in survival and reproductive success. Similarly in mammals, disruptions in circadian function markedly impact reproduction and lifespan. The present review discusses research outlining the proximate and ultimate mechanisms responsible for the central and peripheral control of the reproductive axis. Because precise temporal coordination of the endocrine system is particularly crucial for reproduction by females, the present overview focuses on the role of circadian timing in this sex.
Introduction
In order to survive and successfully reproduce, animals in their natural environments must satisfy a variety of competing behavioral and physiological demands on a daily basis. Because selection pressures vary over the course of the day, and all requirements cannot be filled concomitantly, the most successful individuals of a species are those whose behavioral and physiological functions are organized temporally. As a result, organisms have evolved in ways that restrict behavioral activities such as foraging, defending territory, seeking and competing for mates, and sleeping, each to their own specific temporal niche. Not surprisingly, the internal central and peripheral physiological processes subserving these behaviors exhibit the same systematic orchestration. The harmony maintained among organismal events and the environment is coordinated by a clock in the brain, located in the suprachiasmatic nucleus (SCN) of the anterior hypothalamus (Fig. 1). This overview examines the organization of the circadian system and its role in driving appropriate timing of endocrine-mediated imperatives. Via the cerebrospinal fluid (CSF) and the bloodstream, hormones are in a position to have widespread influence over the central nervous system (CNS) and periphery. Presumably, most endocrine mediators acting on the periphery have also been co-opted throughout evolutionary time to drive behaviors associated with systemic alterations. For example, the hormones responsible for ovulation (i.e., estrogen and progesterone) commonly trigger sexual motivation and associated species-specific behavior across taxa. As a result, the timing of endocrine function represents an ideal model system in which to explore the proximate and ultimate mechanisms underlying circadian organization.
Fig. 1.

The mammalian circadian clock is located in the suprachiasmatic nucleus (SCN) of the anterior hypothalamus. The SCN is situated at the base of the brain directly above the optic chiasm (oc) and surrounding the third ventricle (3V). The image depicts a low-power photomicrograph of the SCN of a Syrian hamster (Mesocricetus auratus) stained immunohistochemically for FOS protein as previously described (Gibson et al., 2008). Arrows point to the lateral borders of the SCN.
Evolution and adaptive significance of the circadian system
The pervasiveness of circadian clocks among prokaryotic and eukaryotic organisms living longer than a day has been used to argue for the adaptive significance of circadian function, although this hypothesis went largely untested until the late 1990s (Ouyang et al., 1998; Sharma, 2003; Yan et al., 2008). Although these studies are still in their infancy, several lines of evidence suggest a role for the circadian system in reproductive fitness. Interested readers are directed to several excellent reviews on this topic (Sharma, 2003; Johnson, 2005). One early study examined survivorship among free-living chipmunks in the Allegheny Mountains following destruction of the circadian clock versus a sham procedure. Loss of circadian function led to nighttime restlessness and increased susceptibility to terrestrial predators, suggesting an important advantage of fitness in avoiding predators (DeCoursey et al., 2000). These data must be interpreted cautiously as such naturalistic studies do not afford the control of variables possible in the laboratory. Although a correlation exists between clock disruption and nighttime restlessness, it is not possible to determine whether or not nighttime activity resulted in predation. Likewise, because the surgeries took place in the laboratory, and animals were released into their natural habitats after recovery, it is possible that re-establishment of territory contributed to mortality. Sham surgeries were conducted to control for this, but it is possible that the greater destruction of the brain in lesioned animals made re-acclimation to the environment more difficult. Although this study did not uncover increased mortality resulting from “natural” causes in arrhythmic animals, studies across species, including Drosophila melanogaster (Pittendrigh and Minis, 1972; Klarsfeld and Rouyer, 1998) and Syrian hamsters (Mesocricetus auratus) (Hurd and Ralph, 1998) demonstrated that adult longevity is increased when animals are housed in environments in which the period of the light:dark cycle matches the endogenous period of an individual's clock. Presumably, a circadian clock allows organisms to anticipate daily environmental change and synchronize internal processes accordingly. As more findings emerge in both laboratory and natural settings, a clearer role of the circadian clock in effective adaptation can be more firmly established.
In addition to alterations in lifespan, a functioning circadian clock can contribute to an organism's fitness by affecting reproductive output. In studies of Drosophila with mutations of core clock genes controlling circadian function at the cellular level, those animals with dysfunctional clocks exhibited a 40% reduction in the production of progeny relative to flies with functional clocks (Beaver et al., 2002). This reduction in reproductive fitness was the result of reduced output of eggs and an increase in the number of unfertilized eggs. These latter deficits were associated with abnormal reproductive function of males, with the authors noting reduced production and transport of sperm, presumably due to loss of clock gene function in the testes and seminal vesicles. Additionally, when clock-mutant males were mated with wild-type (WT) females, reductions in reproductive capacity remained, further indicating a role for cellular clocks in the fertility of males. Importantly, when clock function was rescued in male flies, reproductive abnormalities were abolished (Beaver et al., 2002). Analogous results were obtained in studies of the pitcher-plant mosquito, Wyeomyia smithii, in which incongruence between the free-running period of these insects and the light:dark cycle resulted in reductions in the fecundity of females (mean number of eggs in which eclosion was observed) (Emerson et al., 2008).
The adaptive significance of circadian function has perhaps been best demonstrated using asexual strains of cyanobacteria, Synechococcus, which differ in free-running periods (Ouyang et al., 1998; Johnson and Golden, 1999; Woelfle et al., 2004). In these studies, strains of cyanobacteria with a circadian period closest to the light:dark cycle of the environment have greater reproductive success when examined in studies of competition (Ouyang et al., 1998). Strains whose clocks were disrupted were defeated by strains with a functional clock, but only when held in a light:dark cycle; any competitive advantage was lost under constant conditions (Woelfle et al., 2004). Similar results have been obtained in the plant, Arabidopsis thaliana, where plants with periods closest to the natural light:dark cycle contain more chlorophyll, fix more carbon, grow faster, and survive better (Green et al., 2002; Dodd et al., 2005a; Dodd et al., 2005b). Together, these findings indicate a selective advantage of having an internal circadian clock when living in a rhythmic environment, presumably by allowing organisms to anticipate environmental change and initiate appropriate functions in advance of the optimal time of day.
Mammalian reproduction as a model system
All of the studies investigating the evolutionary/adaptive significance of clock function to date have explored these questions by either using organisms whose clock is disrupted at the cellular level or whose master circadian clock has been destroyed. Our approach to investigating the significance of the circadian clock in reproductive success has been to examine the role of circadian mechanisms in the neural circuitry driving sexual motivation, ovulation, and maintenance of pregnancy in rodents. In most laboratory rodents (e.g., rats, Syrian hamsters, and mice), an intact circadian system is essential for females’ reproductive success (Kriegsfeld and Silver, 2006). Lesions of the SCN result in arrhythmicity in all behavioral and physiological parameters, including endocrine function (Moore and Eichler, 1972; Stephan and Zucker, 1972; Wiegand and Terasawa, 1982). With regard to endocrine function specifically, SCN lesions or mutation of “clock” genes driving rhythms at the cellular level result in marked deficits in ovulation and fecundity in most rodents (Nunez and Stephan, 1977; Wiegand and Terasawa, 1982; Miller et al., 2004). In mice, rats, and hamsters the circadian clock is essential for normal ovulation, whereas ovulation and sexual behavior in primates is under less stringent temporal control (Yamaji et al., 1971; Knobil, 1974).
Because ovulation and sexual behavior are tightly controlled by the circadian system in rodents in the laboratory, one can exploit this tractable model system to explore the importance of endogenous daily timing for reproductive success. Throughout the rodent ovulatory cycle, concentrations of sex steroids are controlled by a neuroendocrine cascade beginning with the secretion of hypothalamic gonadotropin-releasing hormone (GnRH) into the hypophyseal portal system. In turn, GnRH acts on the anterior pituitary to stimulate the synthesis and secretion of the gonadotropins, luteinizing hormone (LH) and follicle-stimulating hormone (FSH). LH and FSH then act on the gonads to regulate steroidogenesis and gametogensis, respectively. The activity of the reproductive axis is controlled through the action of negative feedback from sex steroids, with this mechanism maintaining LH at low concentrations throughout most of the ovulatory cycle. At the time of the LH surge, however, high concentrations of estradiol are required for the SCN to trigger ovulation (Legan and Karsch, 1975; Kriegsfeld and Silver, 2006). The mechanisms mediating both the positive and negative feedback of estradiol are complex and not fully understood, though evidence points to the importance of ERα receptors in mediating estradiol negative and positive feedback in regions upstream of GnRH, especially in the AVPV (Herbison et al., 1992; Dorling et al., 2003; Wintermantel et al., 2006a). Likewise, it is unclear whether a single system switches from negative to positive feedback, whether two independent systems (one positive and one negative) differentially dominate throughout the cycle, or a combination of the two mechanisms mediate the switch from negative to positive feedback, thereby allowing the LH surge. Below, we highlight the role of two hypothalamic RFamide (Arg-Phe-NH2) peptides, kisspeptin and RFamide-related peptide (RFRP; also known as gonadotropin-inhibitory hormone or GnIH), in the temporal coordination of the activity of the HPG axis required for successful reproduction (Kriegsfeld, 2006; Kriegsfeld et al., 2006; Smith et al., 2006a; Adachi et al., 2007).
RFamide peptide control of the timing of the reproductive axis
Discovery and functions of kisspeptin and RFRP
Kisspeptin and RFRP have pronounced, but opposing, actions on the reproductive axis (Gottsch et al., 2004; Irwig et al., 2004; Navarro et al., 2005; Kriegsfeld, 2006; Johnson et al., 2007). Likewise, there is significant evidence that both systems are targeted by the SCN and are key regulators of ovulation (Gibson et al., 2008; Williams et al., 2008). Through coordinated regulation of these opposing peptidergic systems, the circadian clock is in a position to maintain the reproductive axis within optimal operating limits in males and allow for the LH surge in females.
Numerous studies across a variety of species indicate a striking stimulatory role for kisspeptin in the reproductive axis (de Roux et al., 2003; Funes et al., 2003; Seminara et al., 2003; Gottsch et al., 2004; Irwig et al., 2004; Navarro et al., 2004; Thompson et al., 2004; Shahab et al., 2005; Gottsch et al., 2006; Smith et al., 2006a; Smith, 2009). Early evidence for a stimulatory role of kisspeptin was revealed with the identification of GPR54, the orphan G-protein-coupled receptor for the kisspeptin ligand. In humans, a congenital mutation of the GPR54 gene is associated with hypogonadotropic hypogonadism (de Roux et al., 2003; Semple et al., 2005). In Gpr54 knockout (KO) mice, a similar phenotype to those patients with mutations in Gpr54 is observed (Funes et al., 2003), whereas kisspeptin administration to female rats advances vaginal opening and induces precocious puberty (Navarro et al., 2004). Kisspeptin mRNA and protein are localized to hypothalamic areas associated with reproductive function, including the anteroventral periventricular (AVPV) and arcuate (ARC) nuclei in mice, rats, and hamsters (Gottsch et al., 2004; Irwig et al., 2004; Kinoshita et al., 2005; Greives et al., 2007; Mason et al., 2007; Revel et al., 2008), the preoptic area (POA) and ARC in sheep (Franceschini et al., 2006; Smith et al., 2008a), and the infundibular nucleus in humans and monkeys (Rometo et al., 2007). Administration of kisspeptin stimulates GnRH activity and the secretion of gonadotropins across species (Dhillo et al., 2005; Messager et al., 2005; Navarro et al., 2005; Shahab et al., 2005; Dhillo et al., 2007). Kisspeptinergic neurons project to GnRH cells that express GPR54 (Irwig et al., 2004; Han et al., 2005; Kinoshita et al., 2005; Clarkson and Herbison, 2006; Smith et al., 2008a), suggesting a direct effect of kisspeptin on the GnRH system. GnRH antagonists block the stimulatory actions of kisspeptin on LH in vivo, suggesting that kisspeptin does not act at the level of the anterior pituitary (Gottsch et al., 2004; Greives et al., 2007; Irwig et al., 2004). However, it is conceivable that there are subtle effects of kisspeptin on secretion of LH by the pituitary as Kiss1 and Gpr54 have been localized to a subset of LHβ-immunoreactive (ir) cells (Richard et al., 2008) and kisspeptin can elicit LH secretion in primary pituitary cultures collected during the follicular phase of the ovulatory cycle (Smith et al., 2008b). In contrast to these positive results, other studies find no effect of kisspeptin on secretion of pituitary LH (Matsui et al., 2004). It is undeniable that kisspeptin, at least in part, modulates the reproductive axis by stimulating the release of GnRH, but determining whether or not kisspeptin also acts at the level of the pituitary requires further investigation.
In contrast to kisspeptin, RFRP has been shown to have rapid, pronounced inhibitory effects on the reproductive axis across species. RFRP was first identified in the avian brain and was named GnIH due to its suppressive actions on secretion of LH by the pituitary (Tsutsui et al., 2000; Satake et al., 2001; Bentley et al., 2003). In birds, GnIH inhibits synthesis (Ciccone et al., 2004) and secretion (Tsutsui et al., 2000; Osugi et al., 2004) of gonadotropin as well as gonadal development and maintenance (Ubuka et al., 2006). GnIH-ir cells project to GnRH neurons that express the receptor for this peptide (Bentley et al., 2003; Bentley et al., 2006; Ubuka et al., 2006; Ubuka et al., 2008), suggesting a direct effect on the GnRH system in addition to the pituitary. The mammalian ortholog is typically referred to as RFRP because there is equivocal evidence regarding its effects on the pituitary (see below). In rodents, RFRP is expressed principally in the dorsomedial hypothalamus (DMH), with direct projections to GnRH cells (Kriegsfeld et al., 2006; Johnson et al., 2007; Gibson et al., 2008). In vivo administration of RFRP markedly attenuates LH secretion and inhibits sexual behavior in rodents (Kriegsfeld et al., 2006; Johnson et al., 2007). In one recent study, application of RFRP to GnRH cells in brains slices from male and female mice decreased neural activity in a subset of cells (Ducret et al., 2009), further supporting a suppressive role for this peptide on GnRH. As suggested previously, it is unclear whether RFRP acts at the level of the pituitary in mammals. Suggestive evidence for this possibility comes from studies showing that the RFRP receptor (GPR147) is localized to rat and Syrian hamster pituitaries (Hinuma et al., 2000; Gibson et al., 2008), and RFRP-ir fibers have been reported to extend into the external layer of the median eminence in hamsters (Gibson et al., 2008). Additionally, recent studies of rats suggest that RFRP inhibits GnRH-elicited release of LH at the level of the pituitary (Murakami et al., 2008). In other studies using a different antiserum to RFRP, RFRP-ir was not observed in the median eminence of rat brain, and intraperitoneal injections of the retrograde tracer Fluorogold only labeled a small number of RFRP cell bodies (Rizwan et al., 2009), suggesting that most of these cells do not reach the hypophyseal portal system. However, intravenously administered RFRP rapidly (within 3 min) inhibited GnRH-induced release of LH (Rizwan et al., 2009) as also occurs in birds (Osugi et al., 2004). These data indicate the potential for RFRP to act on the release of pituitary gonadotropin. Whether or not endogenous RFRP acts on the pituitary in addition to GnRH cells in mammalian species requires further investigation to clarify whether discrepant findings represent interspecies differences or result from technical variation across studies.
Kisspeptin and RFRP in ovulatory control
The timing of the LH surge is tightly regulated by the circadian system in Syrian hamsters. In this species, the LH surge occurs 4 h prior to the onset of locomotor activity, with a cessation of the surge occurring 2 h later (Stetson, 1978). GnRH neurons exhibit robust increases in immediate-early gene expression one hour after the peak in the LH surge (Lee et al., 1990; Lee et al., 1992; Hoffman et al., 1993). Hamsters housed in a light:dark (LD) schedule exhibit one activity bout every 24 h. This activity is associated with symmetrical, bilateral activation of the SCN and GnRH system, which elicits an LH surge every 24 h in ovariectomized, estradiol-treated animals. When housed in constant light, some hamsters exhibit a splitting of behavior, with two daily activity bouts, each reflecting an anti-phase oscillation of the left and right sides of the bilateral SCN (de la Iglesia et al., 2000; Yan et al., 2005). Notably, behaviorally split animals exhibit two daily surges of LH (Swann and Turek, 1985) as well as asymmetrical activation of the GnRH system (de la Iglesia et al., 2000; de la Iglesia et al., 2003; Gibson et al., 2008). We examined the possibility that, in addition to driving the LH surge positively, the SCN concomitantly coordinates the removal of steroid-mediated RFRP inhibition of the gonadotropic axis to permit the surge (Fig. 2). The SCN forms close appositions with RFRP cells, suggesting the possibility for direct temporal control of RFRP activity (Gibson et al., 2008). During the time of the surge in LH, immediate-early gene expression is reduced in RFRP cells. Additionally, in split animals, activation of the RFRP system is asymmetrical. Importantly, this asymmetry is opposite to the state of the GnRH system (Gibson et al., 2008). Together, these findings suggest that the SCN acts to temporally balance the contribution of negative and positive inputs to the GnRH system, thereby permitting the LH surge and ultimately ovulation.
Fig. 2.

Lateralization of GnRH and RFRP Activational Patterns in ‘Split’ Hamsters is Associated with the LH Surge. (A and B) Actograms of wheel-running activity in estradiol-implanted, ovariectomized hamsters kept under constant light conditions (LL). (A) Non-split (NS) hamsters were sacrificed (*) 1 h or 13 h prior to the onset of the activity bout. (B) Split hamsters were sacrificed (*) 1 h prior to the onset of one of the two activity bouts. (C and D) Photomicrographs of FOS activation in SCN and GnRH cells (insets) of NS and split hamsters. (E and F) Mean (±SEM) percentage of FOS-ir GnRH cells in non-split hamsters sacrificed 1 h (NS1) or 13 h (before the surge; NS13) prior to their activity bout and split hamsters sacrificed 1 h prior to one of their activity bouts. (G and H) Photomicrographs of FOS-ir RFRP cells in the DMH of NS and split hamsters. (I and J) Mean (±SEM) percentage of FOS-ir RFRP cells in NS1, NS13 and split hamsters. Asterisk indicates significantly different from all other values, P < 0.05. Reprinted with permission from (Gibson et al., 2008). Copyright: The Endocrine Society.
In addition to the direct stimulatory actions of the circadian system on GnRH described previously, the SCN may stimulate the LH surge indirectly through kisspeptin (Fig. 3). Early evidence suggested an important role for the AVPV in the initiation of the LH surge, although the cell phenotype mediating these actions remained elusive (Le et al., 1997; Wiegand et al., 1978; Le et al., 1999; Wintermantel et al., 2006b). In the AVPV, Kiss1 mRNA is downregulated following ovariectomy and upregulated by estradiol treatment, suggesting a role for this peptide in the positive feedback effects of estradiol. The opposite pattern is seen in the ARC of these animals (Smith et al., 2005). A majority of kisspeptin-ir cells co-express ERα, the receptor subtype implicated in estrogen positive feedback (Franceschini et al., 2006; Wintermantel et al., 2006b), and exhibit increases in FOS-ir around the time of the LH surge (Smith et al., 2006b; Clarkson et al., 2008). Gpr54- and Kiss1-null mice do not exhibit LH surges or FOS induction in GnRH cells (Clarkson et al., 2008). This study contrasts with previous work suggesting that Gpr54 mutant mice could initiate an LH surge (Dungan et al., 2007). The conflicting evidence for the necessity of GPR54 in the generation of the preovulatory LH surge may be a result of the mutant mouse models used by each group. Dungan and colleagues employed a retroviral insertion, with no loss in the coding sequence of Gpr54. This mutation might result in residual gene function and may explain why a subset of animals maintain the ability to generate an LH surge. Conversely, the Gpr54 mouse line used by Clarkson and colleagues had a complete knockout of Gpr54 (Clarkson et al., 2008). Administration of kisspeptin upregulates the release of GnRH and LH in sheep (Messager et al., 2005), thus stimulating ovulation in anestrus ewes (Caraty et al., 2007). In addition to direct actions of kisspeptin on GnRH cells (Irwig et al., 2004; Han et al., 2005; Kinoshita et al., 2005; Clarkson and Herbison, 2006; Smith et al., 2008a), kisspeptin may stimulate the release of GnRH via direct actions on GnRH terminals in mice (d'Anglemont de Tassigny et al., 2008). Taken together, these studies suggest an important role for kisspeptin as an integration point for circadian and estrogenic signals and its role as an essential stimulatory element in generation of the LH surge.
Fig. 3.
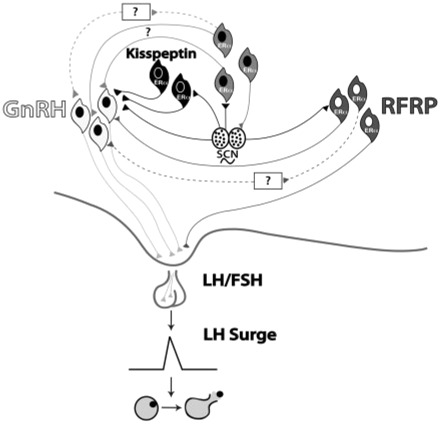
Integration of RFRP and kisspeptin in control of the LH surge. A proposed model incorporating a role for the RFRP and kisspeptin systems in regulation of the ovulatory circuitry. Black lines depict projections from the circadian system to GnRH neurons and to neurons containing estrogen receptors (de la Iglesia et al., 1995) as well as to the RFRP system (Gibson et al., 2008) and the AVPV, including the kisspeptin system (Watson et al., 1995; Williams et al., 2008) reported in rats, mice, and hamsters. Kisspeptin cells in the AVPV are active at the time of the LH surge (Irwig et al., 2004, Kauffman et al., 2007). Neurons containing ER-α in the preoptic area and elsewhere are known to project to the SCN (de la Iglesia et al., 1999) and to the vicinity of GnRH neurons (Simonian et al., 1999) and may play a role in mediating the circadian signal to GnRH neurons directly and/or indirectly. While ER-responsive cells have not been definitively shown to project specifically to GnRH neurons, the emergence and sexual dimorphism of kisspeptin cells and fibers that project to GnRH cell bodies provide compelling evidence for the direct connection between these two neural phenotypes (Clarkson and Herbison, 2006). Future studies are necessary to determine whether neurons in the AVPV project to GnRH cells or adjacent interneurons in the POA. Connections between the RFRP and GnRH systems (Kriegsfeld et al., 2006; Gibson et al., 2008,) may indicate a putative role for RFRP in modulating the negative-feedback effects of estrogen (Kriegsfeld et al., 2006), with SCN communication allowing for removal of negative feedback on the reproductive axis during the time of the LH surge. Connections between kisspeptin and GnRH cells indicate a possible role for the kisspeptin system in mediation of the GnRH system (Irwig et al., 2004). Dashed lines indicate indirect connections between systems that have not yet been explored. According to this model, RFRP cells respond to estradiol with inhibition of the HPG axis during most of the estrous cycle. At the time of the LH surge, however, the SCN signals the RFRP system to “ignore” estradiol input and remove the influences of negative feedback on the HPG axis while simultaneously signaling activation of the kisspeptin system. This allows for activation of the GnRH system directly or indirectly via the circadian system and/or through activation of the kisspeptin system.
Aging as a tool for understanding reproductive control
Reproductive senescence in females is defined as the inability to produce viable oocytes. Historically, it was generally accepted that the transition to reproductive senescence resulted from a depletion of ovarian follicles and that changes in the CNS components of the reproductive axis resulted from reductions in ovarian estrogen (Mandl and Shelton, 1959; Nelson et al., 1987; Richardson et al., 1987; Hansen et al., 2008). Whereas this mechanism drives the transition to menopause in primates, results from numerous studies suggest that aging causes changes in the brain that precede deficits at the level of the ovary in rodents (van der Schoot, 1976; Cooper et al., 1980; Sopelak and Butcher, 1982; Wise, 1982b; Felicio et al., 1983; Lloyd et al., 1994; Lu et al., 1994; Nelson et al., 1995). Thus, it is likely that the transition to reproductive senescence results from dysregulation of the reproductive axis at the levels of both the brain and the ovaries, at least in rodents (Rubin, 2000). Importantly, because ovarian follicles are the primary source of estrogen, many non-reproductive functions are also compromised following the transition to reproductive senescence, including: the regulation both of cardiovascular function and bone metabolism as well as protection from neurodegenerative diseases such as Alzheimer's and Parkinson's disease (Downs and Wise, 2009). By unraveling the contributions of the brain in the transition to reproductive senescence, insight into the general process of aging of the brain can be gained, as reproductive aging occurs in advance of many other pathological changes that tend to confound gerontological studies (Wise, 1999).
Peripheral control of reproductive aging
As suggested above, reduction in the number of ovarian follicles is associated with reproductive aging in rats (Mandl and Shelton, 1959; LaPolt et al., 1998), mice (Nelson et al., 1987) and humans (Hansen et al., 2008), and is considered to be the hallmark of reproductive senescence. These peripheral changes in ovarian function can have a marked impact on the HPG axis. For example, when follicular reserves are experimentally reduced in young rats, reproductive deficiencies that are normally seen in aged animals emerge, including deficits in secretion of gonadotropin and abnormal estrous cycles (Meredith and Butcher, 1985). Conversely, slowing the loss of ovarian follicles through chronic treatment with progesterone (LaPolt et al., 1998) or through dietary restriction (Nelson et al., 1985) can delay age-related anomalies in estrous cycles. Moreover, experimental reduction in follicular reserves reduces the magnitude of the preovulatory LH surge, ovulation rate and estrous cyclicity in both young and middle-aged rats, further suggesting that with advancing age ovarian decline impacts hypothalamo–pituitary function (Anzalone et al., 1998). Importantly, animals with experimental reduction in follicular pools have unaltered estradiol profiles throughout their cycles, suggesting that the reduction in follicular pool-size affects the central neuroendocrine response independent of changes in gonadal steroids.
Role of ovarian oscillators in reproductive decline
The molecular machinery that drives circadian rhythms in cells of the SCN also operates relatively ubiquitously throughout the brain and periphery (Hardin, 1994; Tosini and Menaker, 1996; Sun et al., 1997; Balsalobre et al., 1998; Zylka et al., 1998; Yamazaki et al., 2000). The intracellular clockwork consists of interacting transcriptional and translational feedback loops that operate with a period of about 24 h (for review see Okamura, 2007). Briefly, the transcription of three Per genes (1–3) and two Cry genes (1 and 2) is driven by the dimerization of the bHLH/PAS containing CLOCK and BMAL1 transcription factors (Gekakis et al., 1998; Takahata et al., 1998; Shearman et al., 2000). To complete the cycle, PER and CRY proteins translocate into the nucleus as multimeric complexes to discontinue CLOCK::BMAL-mediated transcription (Shearman et al., 2000). A number of cytoplasmic enzymes phosphorylate the PER and CRY proteins and play an important role in maintaining the precise timing of feedback loops (Lowrey et al., 2000). The SCN synchronizes independent oscillators in peripheral tissues, with loss of synchrony among independent cellular oscillators in the absence of SCN input (Welsh et al., 2004). Thus, the SCN acts as a “master clock” that synchronizes molecular rhythms in the periphery that, in turn, regulate the timing of a vast array of physiological and behavioral rhythms (Pando et al., 2002; Schibler and Sassone-Corsi, 2002; Guo et al., 2006).
The ovaries have been shown to express daily rhythms in the clock genes Per1 and Per2 (Fahrenkrug et al., 2006; Karman and Tischkau, 2006). The integrity of these local clocks may be important for normal ovarian function; genes contributing to the initiation of ovarian steroid production are rhythmic in the human ovary (Bao et al., 2003; Foster et al., 2005). Ovarian clock genes may also have a functional role in the synthesis of progesterone. In domestic chickens, the largest and most mature preovulatory follicle expresses diurnal changes in Per2 and Per3, whereas smaller follicles do not exhibit changes in clock-gene expression (Nakao et al., 2007). These changes are concomitant with increases in StAR, a steroidogenic acute regulatory protein that increases synthesis of progesterone (Nakao et al., 2007). Importantly, StAR genes contain E-Box enhancers (Christenson and Strauss, 2001), DNA elements that are direct targets of the molecular clock. Although correlational, these studies suggest a potential role for local timing mechanisms in ovulatory control. Although previous work has confirmed the existence of rhythmically expressed clock genes in the ovarian cells of rats, the role of murine ovarian circadian clock genes is largely speculative (Fahrenkrug et al., 2006). Whether or not alterations in ovarian clock genes contribute to the maintenance of ovarian function and/or reproductive decline with age remains an open question.
Central control of reproductive aging
Whereas alterations at the level of the ovary play an integral role in reproductive decline associated with advancing age, a number of lines of evidence suggest that central mechanisms also contribute to these changes. The timing of neural signals that control the release of gonadotropin exhibit marked changes in middle-aged women and non-human animals, and these modifications may accelerate the loss of follicles with advancing age (Nass et al., 1984; Wise, 1993; Hall et al., 2000; Gore et al., 2004). Transplanting the ovaries of old rats into young, ovariectomized rats restores follicular development and ovulation, indicating that aged ovaries maintain ovulatory function in some cases (Krohn, 1955; Peng and Huang, 1972). Likewise in young rats, electrolytic lesions of the medial preoptic area (POA), where GnRH neurons reside, result in irregular estrous cycling akin to that seen in older rats (Clemens and Bennett, 1977). Conversely, stimulation of the medial POA in old, acyclic rats results in an enhanced LH response (Wuttke and Meites, 1973). Together, these studies suggest that the brain contributes to aging of the reproductive axis and represents an important consideration for further investigation.
One caveat to consider when generalizing from rodent models to humans is that changes at the level of the brain may not be equivalent. In postmenopausal women, for example, gonadotropin levels are high from loss of negative feedback, whereas aged acyclic rats have normal levels of LH (Lu et al., 1994; Wise et al., 1999). Furthermore, follicular loss beginning in middle-aged women eventually leads to the complete depletion of ovarian follicles. In aged, acyclic rats, however, remaining follicles have been reported (Wise, 1999). These findings suggest that the contribution of hypothalamic decline versus the exhaustion of ovarian follicles to reproductive senescence likely differs between rodents and humans. Indeed, estrogen and progesterone administration in postmenopausal women can still generate or inhibit LH and FSH surges, depending on the timing and level of gonadal steroid treatment, suggesting the maintenance of positive and negative feedback in postmenopausal women (Gill et al., 2002; Ottowitz et al., 2008). Despite these differences, rodent models can provide important insight into human menopause. In both rats and humans, for example, a rise in concentration of FSH remains a hallmark of impending reproductive decline (DePaolo, 1987; Klein et al., 1996), though a rise in human FSH has been primarily linked to a decrease in inhibin B, the main ovarian-derived negative feedback peptide in humans, through depletion in follicle numbers (Burger, 1993; Burger et al., 2000). The pattern of LH secretion also changes in both perimenopausal humans and in rats transitioning into acyclicity. The duration of LH pulses increases and the frequency decreases in both premenopausal women and middle-aged, regularly cycling rats (Scarbrough and Wise, 1990; Matt et al., 1998). The ability of estradiol to induce LH surges becomes attenuated in perimenopausal women (van Look et al., 1977) and middle-aged rats (Wise, 1984). The studies by van Look and colleagues and Matt and colleagues, however, take place in women that are well in to the transition to menopause and therefore have a marked decrease in follicle number, which alters levels of ovarian hormones such as inhibin B and may result in changes in cyclicity that precede any alterations in the brain's responsiveness to positive feedback. Therefore, while changes in the brain's ability to control the timing of the LH surge and respond to the negative and positive feedback of circulating estradiol contribute to the desynchrony of gonadotropin secretion in rats transitioning into senescence, the impact of changes in positive and negative feedback on the transition to menopause in humans remains controversial (Hall, 2007). This underscores the idea that the origin of the dysregulation of the brain's control of the HPG axis differs between rats and humans. In rats, changes in the hypothalamus contribute to reproductive senescence, while alterations in gonadotropin secretion in humans transitioning to menopause are preceded by the elimination of negative feedback originating from follicle depletion. Although the mechanisms differ, the resulting impact on general HPG axis activity affects both aged rodents and primates. Recent studies indicate that the increase in circulating gonadotropins may be a result of hypertrophy of kisspeptin neurons in the infundibular nucleus (Rance, 2009). These results are almost identical to rodent studies of arcuate kisspeptin neurons after ovariectomy, and may constitute a neural compensatory mechanism to ovarian failure (Rance, 2009). Further research is needed to fully elucidate the neural mechanisms underlying HPG activity during the transition to menopause. Thus, using rodent models of hypothalamic decline in reproductive aging can lend insight into human menopause, as reliable endocrine markers for predicting the full transition to anovulation (including final menses) in humans have yet to be specified (Burger et al., 2008).
One of the first indicators of reproductive decline in rodents involves an attenuated and delayed LH surge on the day of proestrus (Wise, 1982a). This decreased LH response occurs prior to any changes in pituitary responsiveness to GnRH or reductions in GnRH cell numbers (Wise, 1982a; Lloyd et al., 1994; Krajnak et al., 2001), although a decrease in neuronal activation is seen in GnRH neurons in middle-aged, regularly cycling rats (Lloyd et al., 1994). It is likely that the decreased and delayed LH surge seen in middle-aged animals stems from an alteration in the timed activation pattern of GnRH neurons (Lloyd et al., 1994; Rubin and King, 1994; Krajnak et al., 2001). This decline in GnRH activation may reflect alterations in the strength and timing of the afferent inputs to this system originating from the circadian clock (Krajnak et al., 1998). Additionally, significant alterations in SCN rhythmicity are observed with advancing age and may contribute to deficits in the timing of GnRH neuronal activation at the time of the LH surge (Wise et al., 1988). Furthermore, unlike the condition in young animals, rhythms in vasoactive intestinal polypeptide (VIP) mRNA are not apparent in middle-aged female rodents (Krajnak et al., 1998). Suppression of VIP in the SCN through the administration of antisense oligonucleotides also leads to an aging-like attenuation of cyclic secretion of LH (Harney et al., 1996). Together, these findings suggest that changes in the timing and amplitude of the preovulatory LH surge may result from age-related changes in the SCN and in its neurochemical output.
Role of central oscillators in reproductive decline
Perhaps the most noticeable alteration in behavior associated with advancing age is in the quality, quantity and phase of sleep (Dijk et al., 1999). Whether such age-related changes are the result of alterations at the level of the SCN or extra-SCN clocks (e.g., clocks in the reticular activating system) remains a topic of intense interest. Several lines of evidence suggest that age-related changes in the SCN (Sutin et al., 1993; Benloucif et al., 1997; Aujard et al., 2001) are responsible for the decline in behavioral and physiological rhythms seen in aged animals (Yamazaki et al., 2002), and that these changes may contribute to reductions in fertility. For example, the amplitude of electrical activity in SCN slices taken from aged rats is dampened relative to that seen in the SCN of young rats (Watanabe et al., 1995). Furthermore, the number of SCN neurons expressing arginine vasopressin (AVP) and vasoactive intestinal polypeptide (VIP), two key SCN neuropeptides, decreases in aged rats (Roozendaal et al., 1987; Chee et al., 1988). Because both of these neurochemicals have been implicated in the circadian control of ovulation (van der Beek et al., 1997; Kalsbeek and Buijs, 2002), the integrity of these neurons in the SCN may be critical for the maintenance of reproductive functioning with age.
Studies investigating age-related changes in the molecular components of the circadian clock have uncovered equivocal results. In rats, the rhythm of SCN Per1 and Per2 expression does not differ between young and old animals (Asai et al., 2001; Yamazaki et al., 2002). However, in mice, the day-night rhythm of SCN Per2 is blunted in aged animals (Weinert et al., 2001). Some findings suggest that reductions in clock-gene amplitude with advanced age may be due to changes in the synchrony of the internal clock with external time. The expression of Bmal1 in old hamsters, for example, is lower than in young hamsters during the subjective night, when Bmal1 expression is normally at its peak (Kolker et al., 2003). These subtle changes in Bmal1 may have marked consequences for circadian function, as Bmal1 is the only single clock-gene deletion that causes loss of rhythmic function (Bunger et al., 2000). Aging does not seem to affect other clock genes in the SCN, as Per1 and Per2 rhythms do not diminish in the aged SCN (Davidson et al., 2008) and mice with Clock mutations do not exhibit an increase in the effects of age on circadian rhythms over WT littermates (Kolker et al., 2004). Therefore, the reduction in the expression of Bmal1 in aged rats may principally account for the diminished molecular rhythms in the SCN.
As mentioned previously, the SCN is essential for ovulation in a number of rodent species and AVP may be an important output signal triggering the LH surge. Injections of AVP into estradiol-treated, SCN-lesioned rats induce an LH surge (Grace et al., 1999; Palm et al., 1999). Notably, rhythms in AVP expression are regulated by the core molecular clock through an E-BOX motif (Grace et al., 1999). Clock mutant mice have blunted AVP rhythms (Jin et al., 1999; Silver et al., 1999) that presumably impact ovulatory function negatively (Miller et al., 2006). Because AVP is regulated by the core molecular clockwork, age-related disruptions in SCN cellular function can clearly impact the output/timing of this peptide and, ultimately, ovulatory functioning. Interestingly, the diurnal expression of AVP mRNA within the SCN is maintained in aged animals (Krajnak et al., 1998), although it is possible that, with age, cells targeted by AVP, upstream of the GnRH system, become insensitive to this peptide. Neurons in the AVPV that play a critical role in the initiation of the LH surge (Le et al., 1997; Le et al., 1999; Terasawa et al., 1980) express V1a receptors (Ostrowski et al., 1994) and are synaptic targets of the SCN (Watson et al., 1995). Importantly, these cells are upregulated in the presence of estradiol in young rats but not middle-aged rats (Funabashi et al., 2000).
Timed coordination of RFamide peptides during aging
As described previously, the cellular activity of the inhibitory peptide, RFRP, appears to be orchestrated by the SCN in ways that remove negative input to the GnRH system during the LH surge (Gibson et al., 2008). Likewise, the kisspeptin system represents a potential key integration point for estrogenic and circadian signals necessary to stimulate the LH surge (Smith et al., 2006b; Williams et al., 2008). Because the precision in the coordination of these neuropeptides is important for ovulatory control in young animals, we examined whether changes in the expression or timing of RFRP and kisspeptin are associated with reproductive decline in middle-aged Syrian hamsters. Young (60–90 days of age) and middle-aged (12–13 months of age) animals were ovariectomized and implanted with Silastic capsules containing estradiol to remove potential effects of reduced gonadal steroids in middle-aged animals. Brains and serum samples were collected two, six, ten, and twelve hours after light onset (light:dark cycle = 14L:10D) to capture time points before, during, and after the LH surge. Brains were labeled immunofluorescently for RFRP and FOS or kisspeptin and FOS and serum samples were assayed for LH as previously described (Greives et al., 2007; Gibson et al., 2008).
Although the timing of the LH surge was unaffected in middle-aged females, the amplitude was significantly lower in middle-aged relative to young animals (P < 0.05; Fig. 4). In both young and middle-aged animals, the number of RFRP cells visualized with immunohistochemistry was greatest during the LH surge (ZT10; P < 0.05 in both cases) and the percentage of cells expressing FOS was lowest during the LH surge in both age groups (P < 0.05 in both cases; Fig. 5). In contrast, the number of RFRP cells labeled and the percentage of RFRP cells expressing FOS was unaffected by age (P > 0.05 in all cases; Fig. 5). It is unclear whether or not more pronounced changes in the RFRP system would have been observed in aged hamsters had the animals been left intact. However, as middle-aged females were cycling irregularly, or became anestrus, it was impossible to select appropriate time-points for comparisons without ovariectomy and replacement of estradiol.
Fig. 4.

The preovulatory LH surge is blunted in middle-aged hamsters. LH concentrations are shown relative to zeitgeber (light: dark cycle) time, with ZT0 = lights on and ZT14 = lights off. Concentrations of LH in the serum were measured in duplicate in a single RIA with reagents obtained from the National Institutes of Health (Bethesda, MD) as previously described. The antiserum was rLH-S-11, and the standard was rLH-RP3. The sensitivity was 0.01 ng/tube, and the intraassay coefficient of variation was 2.8% for the low pool and 8.4% for the high pool. The antisera were highly specific for the hormones measured, with low cross-reactivity with other hormones. *Significantly greater than all other groups, P < 0.05.
Fig. 5.

Number of, and percent of FOS-positive, RFRP-ir cells are associated with the timing of the LH surge. The mean (±SEM) number of RFRP-ir cells and percent (±SEM) of FOS-positive RFRP-ir cells do not differ in young and middle-aged animals at any time-point (P > 0.05 in all cases). Data are shown relative to zeitgeber (light: dark cycle) time, with ZT0 = lights on and ZT14 = lights off. RFRP cells and FOS were labeled immunohistochemically as previously described (Gibson et al., 2008). *Significantly greater (top) or less (bottom) than all other time-points in the same age group P < 0.05. **Significantly less than all other time-points from the same age group, excluding ZT6, P < 0.05.
Numbers of AVPV kisspeptin cells were reduced during the LH surge in young animals (P < 0.05) but not in middle-aged (P > 0.05) ones (Fig. 6). In both age groups, the percentage of cells expressing kisspeptin was increased at ZT10/ZT12 relative to time-points prior to the LH surge (P < 0.05 in all cases; Fig. 6). There were no age differences in the percentage of cells expressing FOS at any time-point (P > 0.05 in all cases; Fig. 6). As with RFRP, it is possible that changes in kisspeptin may have been observed had animals been examined under conditions of endogenous estrogen exposure, particularly given that the activity of kisspeptin is sensitive to estradiol (Smith et al., 2006b; Roa et al., 2008).
Fig. 6.

Number and percent of FOS-positive, kisspeptin-ir cells are associated with the timing of the LH surge. The mean (±SEM) number of kisspeptin-ir cells and percent (±SEM) of FOS-positive kisspeptin-ir cells do not differ in young and middle-aged animals at any time-point (P > 0.05 in all cases). Data are shown relative to zeitgeber (light: dark cycle) time, with ZT0 = lights on and ZT14 = lights off. Kisspeptin cells and FOS were labeled immunohistochemically as previously described (Williams et al., 2008; Greives et al., 2007). *Significantly less than all other groups, P < 0.05. **Significantly greater than values at ZT2 and ZT6, P < 0.05.
Given the relatively small differences in kisspeptin and RFRP expression in young and middle-aged animals, it is unlikely that alterations in these systems account entirely for the reduction in the amplitude of LH surge in older animals. Both in young and middle-aged animals, it appears that the circadian system is equally capable of removing the influence of RFRP inhibition at the time of the surge. In young animals, there is a reduction in kisspeptin cell numbers at the time of the surge, possibly due to increased transport and release in this age group relative to those in middle-aged animals. Likewise, we have recently shown that the GnRH system is differentially responsive to kisspeptin stimulation across the day, presumably due to local regulation by clock genes (Zhao and Kriegsfeld, 2009). Thus, it is also possible that middle-aged animals lose the ability to time the responsiveness of GnRH to kisspeptin to coincide with times of RFRP disinhibition and concomitant stimulation by estradiol.
In agreement with results from studies of the effects of estradiol on kisspeptin neurons in the arcuate of Syrian hamsters (Smith 2005; Franceschini 2006; Roa 2006), postmenopausal rhesus monkeys exhibit an increase in Kiss1 gene expression and cell size relative to reproductively competent animals. The authors speculated that this increase is due to decreased negative feedback control by ovarian steroids (Rometo et al., 2007; Kim et al., 2009). In humans, the number and size of kisspeptin mRNA neurons increases in postmenopausal women (Rometo et al., 2007). In primates, reproductive decline is primarily due to ovarian aging, whereas reproductive aging in rodents may be the result of alterations both in the brain and ovary (Yin and Gore, 2006). Results in Syrian hamsters indicate that kisspeptin expression is higher during the LH surge in middle-aged animals relative to young animals. Whether this increased expression pattern represents a reduction in release with continued mRNA turnover, or an increase in mRNA transcription/translation, requires further examination. However, because estrogen concentrations were clamped at equivalent values both in young and middle-aged animals, these changes are not the result of alterations in estrogen negative feedback as might occur in human/non-human primates. Instead, these findings suggest that kisspeptin regulation may change independently of decreasing ovarian steroids.
Conclusions and considerations
The control of reproduction by the circadian system represents an exciting opportunity for exploring the role of endogenous timing mechanisms in reproductive success. In females, the RFamide peptides, kisspeptin and RFRP, represent important intermediary systems in the circadian control of the preovulatory surge in LH. The SCN projects to both the kisspeptin and RFRP systems, and the activity of these neuronal populations is coordinated with the time of the LH surge. Presumably, activity of the kisspeptin system is upregulated at the time of the LH surge whereas that of the RFRP system is downregulated. In middle-aged animals, although significant suppression of the amplitude of the surge in LH is observed, there is little evidence that alterations in the RFRP system contribute to this deficit in Syrian hamsters. In contrast, changes in the positive drive by the kisspeptin system, independent of gonadal steroids, may impact reproductive aging. Additional exploration of the role of the circadian timing system in the neural circuits regulating the LH surge and ovulatory function is necessary to further understand how aging might impact timing mechanisms contributing to reproductive senescence.
Funding
National Institutes of Health Grant HD050470 (to L.J.K.) and NSF grant IOS-0641188 (G.E.B.).
Footnotes
From the symposium “Evolution of Mechanisms Controlling Timing of Breeding in Animals” presented at the annual meeting of the Society for Integrative and Comparative Biology, January 3–7, 2009, at Boston, Massachusetts.
References
- Adachi S, Yamada S, Takatsu Y, Matsui H, Kinoshita M, Takase K, Sugiura H, Ohtaki T, Matsumoto H, Uenoyama Y, Tsukamura H, Inoue K, Maeda K. Involvement of anteroventral periventricular metastin/kisspeptin neurons in estrogen positive feedback action on luteinizing hormone release in female rats. J Reprod Dev. 2007;53:367–78. doi: 10.1262/jrd.18146. [DOI] [PubMed] [Google Scholar]
- Anzalone CR, Lu JK, LaPolt PS. Influences of age and reproductive status on ovarian ovulatory responsiveness to gonadotropin stimulation. Proc Soc Exp Biol Med. 1998;217:455–60. doi: 10.3181/00379727-217-44257. [DOI] [PubMed] [Google Scholar]
- Asai M, Yoshinobu Y, Kaneko S, Mori A, Nikaido T, Moriya T, Akiyama M, Shibata S. Circadian profile of Per gene mRNA expression in the suprachiasmatic nucleus, paraventricular nucleus, and pineal body of aged rats. J Neurosci Res. 2001;66:1133–9. doi: 10.1002/jnr.10010. [DOI] [PubMed] [Google Scholar]
- Aujard F, Herzog ED, Block GD. Circadian rhythms in firing rate of individual suprachiasmatic nucleus neurons from adult and middle-aged mice. Neuroscience. 2001;106:255–61. doi: 10.1016/s0306-4522(01)00285-8. [DOI] [PubMed] [Google Scholar]
- Balsalobre A, Damiola F, Schibler U. A serum shock induces circadian gene expression in mammalian tissue culture cells. Cell. 1998;93:929–37. doi: 10.1016/s0092-8674(00)81199-x. [DOI] [PubMed] [Google Scholar]
- Bao AM, Liu RY, van Someren EJ, Hofman MA, Cao YX, Zhou JN. Diurnal rhythm of free estradiol during the menstrual cycle. Eur J Endocrinol. 2003;148:227–32. doi: 10.1530/eje.0.1480227. [DOI] [PubMed] [Google Scholar]
- Beaver LM, Gvakharia BO, Vollintine TS, Hege DM, Stanewsky R, Giebultowicz JM. Loss of circadian clock function decreases reproductive fitness in males of Drosophila melanogaster. Proc Natl Acad Sci USA. 2002;99:2134–9. doi: 10.1073/pnas.032426699. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Benloucif S, Masana MI, Dubocovich ML. Light-induced phase shifts of circadian activity rhythms and immediate early gene expression in the suprachiasmatic nucleus are attenuated in old C3H/HeN mice. Brain Res. 1997;747:34–42. doi: 10.1016/s0006-8993(96)01182-1. [DOI] [PubMed] [Google Scholar]
- Bentley GE, Kriegsfeld LJ, Osugi T, Ukena K, O'Brien S, Perfito N, Moore IT, Tsutsui K, Wingfield JC. Interactions of gonadotropin-releasing hormone (GnRH) and gonadotropin-inhibitory hormone (GnIH) in birds and mammals. J Exp Zoolog A Comp Exp Biol. 2006;305:807–14. doi: 10.1002/jez.a.306. [DOI] [PubMed] [Google Scholar]
- Bentley GE, Perfito N, Ukena K, Tsutsui K, Wingfield JC. Gonadotropin-inhibitory peptide in song sparrows (Melospiza melodia) in different reproductive conditions, and in house sparrows (Passer domesticus) relative to chicken-gonadotropin-releasing hormone. J Neuroendocrinol. 2003;15:794–802. doi: 10.1046/j.1365-2826.2003.01062.x. [DOI] [PubMed] [Google Scholar]
- Bunger MK, Wilsbacher LD, Moran SM, Clendenin C, Radcliffe LA, Hogenesch JB, Simon MC, Takahashi JS, Bradfield CA. Mop3 is an essential component of the master circadian pacemaker in mammals. Cell. 2000;103:1009–17. doi: 10.1016/s0092-8674(00)00205-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Burger HG. Evidence for a negative feedback role of inhibin in follicle stimulating hormone regulation in women. Hum Reprod. 1993;8(Suppl 2):129–32. doi: 10.1093/humrep/8.suppl_2.129. [DOI] [PubMed] [Google Scholar]
- Burger HG, Dudley E, Mamers P, Groome N, Robertson DM. Early follicular phase serum FSH as a function of age: the roles of inhibin B, inhibin A and estradiol. Climacteric. 2000;3:17–24. doi: 10.3109/13697130009167595. [DOI] [PubMed] [Google Scholar]
- Burger HG, Hale GE, Dennerstein L, Robertson DM. Cycle and hormone changes during perimenopause: the key role of ovarian function. Menopause. 2008;15:603–12. doi: 10.1097/gme.0b013e318174ea4d. [DOI] [PubMed] [Google Scholar]
- Caraty A, Smith JT, Lomet D, Ben Said S, Morrissey A, Cognie J, Doughton B, Baril G, Briant C, Clarke IJ. Kisspeptin synchronizes preovulatory surges in cyclical ewes and causes ovulation in seasonally acyclic ewes. Endocrinology. 2007;148:5258–67. doi: 10.1210/en.2007-0554. [DOI] [PubMed] [Google Scholar]
- Chee CA, Roozendaal B, Swaab DF, Goudsmit E, Mirmiran M. Vasoactive intestinal polypeptide neuron changes in the senile rat suprachiasmatic nucleus. Neurobiol Aging. 1988;9:307–12. doi: 10.1016/s0197-4580(88)80070-8. [DOI] [PubMed] [Google Scholar]
- Christenson LK, Strauss JF., 3rd Steroidogenic acute regulatory protein: an update on its regulation and mechanism of action. Arch Med Res. 2001;32:576–86. doi: 10.1016/s0188-4409(01)00338-1. [DOI] [PubMed] [Google Scholar]
- Ciccone NA, Dunn IC, Boswell T, Tsutsui K, Ubuka T, Ukena K, Sharp PJ. Gonadotrophin inhibitory hormone depresses gonadotrophin alpha and follicle-stimulating hormone beta subunit expression in the pituitary of the domestic chicken. J Neuroendocrinol. 2004;16:999–1006. doi: 10.1111/j.1365-2826.2005.01260.x. [DOI] [PubMed] [Google Scholar]
- Clarkson J, d'Anglemont de Tassigny X, Moreno AS, Colledge WH, Herbison AE. Kisspeptin-GPR54 signaling is essential for preovulatory gonadotropin-releasing hormone neuron activation and the luteinizing hormone surge. J Neurosci. 2008;28:8691–7. doi: 10.1523/JNEUROSCI.1775-08.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Clarkson J, Herbison AE. Postnatal development of kisspeptin neurons in mouse hypothalamus; sexual dimorphism and projections to gonadotropin-releasing hormone neurons. Endocrinology. 2006;147:5817–25. doi: 10.1210/en.2006-0787. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Clemens JA, Bennett DR. Do aging changes in the preoptic area contribute to loss of cyclic endocrine function? J Gerontol. 1977;32:19–24. doi: 10.1093/geronj/32.1.19. [DOI] [PubMed] [Google Scholar]
- Cooper RL, Conn PM, Walker RF. Characterization of the LH surge in middle-aged female rats. Biol Reprod. 1980;23:611–5. doi: 10.1095/biolreprod23.3.611. [DOI] [PubMed] [Google Scholar]
- d'Anglemont de Tassigny X, Fagg LA, Carlton MB, Colledge WH. Kisspeptin can stimulate gonadotropin-releasing hormone (GnRH) release by a direct action at GnRH nerve terminals. Endocrinology. 2008;149:3926–32. doi: 10.1210/en.2007-1487. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Davidson AJ, Yamazaki S, Arble DM, Menaker M, Block GD. Resetting of central and peripheral circadian oscillators in aged rats. Neurobiol Aging. 2008;29:471–7. doi: 10.1016/j.neurobiolaging.2006.10.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- de la Iglesia HO, Blaustein JD, Bittman EL. Oestrogen receptor-alpha-immunoreactive neurones project to the suprachiasmatic nucleus of the female Syrian hamster. J Neuroendocrinology. 1999;11:481–90. doi: 10.1046/j.1365-2826.1999.00341.x. [DOI] [PubMed] [Google Scholar]
- de la Iglesia HO, Meyer J, Carpino A, Jr, Schwartz WJ. Antiphase oscillation of the left and right suprachiasmatic nuclei. Science. 2000;290:799–801. doi: 10.1126/science.290.5492.799. [DOI] [PubMed] [Google Scholar]
- de la Iglesia HO, Meyer J, Schwartz WJ. Lateralization of circadian pacemaker output: Activation of left- and right-sided luteinizing hormone-releasing hormone neurons involves a neural rather than a humoral pathway. J Neurosci. 2003;23:7412–4. doi: 10.1523/JNEUROSCI.23-19-07412.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- de Roux N, Genin E, Carel JC, Matsuda F, Chaussain JL, Milgrom E. Hypogonadotropic hypogonadism due to loss of function of the KiSS1-derived peptide receptor GPR54. Proc Natl Acad Sci U S A. 2003;100:10972–6. doi: 10.1073/pnas.1834399100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- DeCoursey PJ, Walker JK, Smith SA. A circadian pacemaker in free-living chipmunks: essential for survival? J. Comparative Phy. a-Neuroethology Sensory Neural Behavioral Phy. 2000;186:169–80. doi: 10.1007/s003590050017. [DOI] [PubMed] [Google Scholar]
- DePaolo LV. Age-associated increases in serum follicle-stimulating hormone levels on estrus are accompanied by a reduction in the ovarian secretion of inhibin. Exp Aging Res. 1987;13:3–7. doi: 10.1080/03610738708259293. [DOI] [PubMed] [Google Scholar]
- Dhillo WS, Chaudhri OB, Patterson M, Thompson EL, Murphy KG, Badman MK, McGowan BM, Amber V, Patel S, Ghatei MA, Bloom SR. Kisspeptin-54 stimulates the hypothalamic-pituitary gonadal axis in human males. J Clin Endocrinol Metab. 2005;90:6609–15. doi: 10.1210/jc.2005-1468. [DOI] [PubMed] [Google Scholar]
- Dhillo WS, Chaudhri OB, Thompson EL, Murphy KG, Patterson M, Ramachandran R, Nijher GK, Amber V, Kokkinos A, Donaldson M, Ghatei MA, Bloom SR. Kisspeptin-54 stimulates gonadotropin release most potently during the preovulatory phase of the menstrual cycle in women. J Clin Endocrinol Metab. 2007;92:3958–66. doi: 10.1210/jc.2007-1116. [DOI] [PubMed] [Google Scholar]
- Dijk DJ, Duffy JF, Riel E, Shanahan TL, Czeisler CA. Ageing and the circadian and homeostatic regulation of human sleep during forced desynchrony of rest, melatonin and temperature rhythms. J Physiol. 1999;516(Pt 2):611–627. doi: 10.1111/j.1469-7793.1999.0611v.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dodd AN, Love J, Webb AA. The plant clock shows its metal: circadian regulation of cytosolic free Ca(2+) Trends Plant Sci. 2005;10:15–21. doi: 10.1016/j.tplants.2004.12.001. [DOI] [PubMed] [Google Scholar]
- Dodd AN, Salathia N, Hall A, Kevei E, Toth R, Nagy F, Hibberd JM, Millar AJ, Webb AA. Plant circadian clocks increase photosynthesis, growth, survival, and competitive advantage. Science. 2005;309:630–3. doi: 10.1126/science.1115581. [DOI] [PubMed] [Google Scholar]
- Dorling AA, Todman MG, Korach KS, Herbison AE. Critical role for estrogen receptor alpha in negative feedback regulation of gonadotropin-releasing hormone mRNA expression in the female mouse. Neuroendocrinology. 2003;78:204–9. doi: 10.1159/000073703. [DOI] [PubMed] [Google Scholar]
- Downs JL, Wise PM. The role of the brain in female reproductive aging. Mol Cell Endocrinol. 2009;299:32–8. doi: 10.1016/j.mce.2008.11.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ducret E, Anderson GM, Herbison AE. RFamide-related peptide-3 (RFRP-3), a mammalian gonadotropin-inhibitory hormone ortholog, regulates gonadotropin-releasing hormone (GnRH) neuron firing in the mouse. Endocrinology. 2009;150:2799–804. doi: 10.1210/en.2008-1623. [DOI] [PubMed] [Google Scholar]
- Dungan HM, Gottsch ML, Zeng H, Gragerov A, Bergmann JE, Vassilatis DK, Clifton DK, Steiner RA. The role of kisspeptin-GPR54 signaling in the tonic regulation and surge release of gonadotropin-releasing hormone/luteinizing hormone. J Neurosci. 2007;27:12088–95. doi: 10.1523/JNEUROSCI.2748-07.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Emerson KJ, Bradshaw WE, Holzapfel CM. Concordance of the circadian clock with the environment is necessary to maximize fitness in natural populations. Evolution. 2008;62:979–83. doi: 10.1111/j.1558-5646.2008.00324.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fahrenkrug J, Georg B, Hannibal J, Hindersson P, Gras S. Diurnal rhythmicity of the clock genes Per1 and Per2 in the rat ovary. Endocrinology. 2006;147:3769–76. doi: 10.1210/en.2006-0305. [DOI] [PubMed] [Google Scholar]
- Felicio LS, Nelson JF, Gosden RG, Finch CE. Restoration of ovulatory cycles by young ovarian grafts in aging mice: potentiation by long-term ovariectomy decreases with age. Proc Natl Acad Sci U S A. 1983;80:6076–80. doi: 10.1073/pnas.80.19.6076. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Foster CM, Olton PR, Padmanabhan V. Diurnal changes in FSH-regulatory peptides and their relationship to gonadotrophins in pubertal girls. Hum Reprod. 2005;20:543–8. doi: 10.1093/humrep/deh607. [DOI] [PubMed] [Google Scholar]
- Franceschini I, Lomet D, Cateau M, Delsol G, Tillet Y, Caraty A. Kisspeptin immunoreactive cells of the ovine preoptic area and arcuate nucleus co-express estrogen receptor alpha. Neurosci Lett. 2006;401:225–30. doi: 10.1016/j.neulet.2006.03.039. [DOI] [PubMed] [Google Scholar]
- Funabashi T, Shinohara K, Mitsushima D, Kimura F. Estrogen increases arginine-vasopressin V1a receptor mRNA in the preoptic area of young but not of middle-aged female rats. Neurosci Lett. 2000;285:205–8. doi: 10.1016/s0304-3940(00)01069-7. [DOI] [PubMed] [Google Scholar]
- Funes S, Hedrick JA, Vassileva G, Markowitz L, Abbondanzo S, Golovko A, Yang S, Monsma FJ, Gustafson EL. The KiSS-1 receptor GPR54 is essential for the development of the murine reproductive system. Biochem Biophys Res Commun. 2003;312:1357–63. doi: 10.1016/j.bbrc.2003.11.066. [DOI] [PubMed] [Google Scholar]
- Gekakis N, Staknis D, Nguyen HB, Davis FC, Wilsbacher LD, King DP, Takahashi JS, Weitz CJ. Role of the CLOCK protein in the mammalian circadian mechanism. Science. 1998;280:1564–9. doi: 10.1126/science.280.5369.1564. [DOI] [PubMed] [Google Scholar]
- Gibson EM, Humber SA, Jain S, Williams WP, 3rd, Zhao S, Bentley GE, Tsutsui K, Kriegsfeld LJ. Alterations in RFamide-related peptide expression are coordinated with the preovulatory luteinizing hormone surge. Endocrinology. 2008;149:4958–69. doi: 10.1210/en.2008-0316. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gill S, Lavoie HB, Bo-Abbas Y, Hall JE. Negative feedback effects of gonadal steroids are preserved with aging in postmenopausal women. J Clin Endocrinol Metab. 2002;87:2297–302. doi: 10.1210/jcem.87.5.8510. [DOI] [PubMed] [Google Scholar]
- Gore AC, Windsor-Engnell BM, Terasawa E. Menopausal increases in pulsatile gonadotropin-releasing hormone release in a nonhuman primate (Macaca mulatta) Endocrinology. 2004;145:4653–9. doi: 10.1210/en.2004-0379. [DOI] [PubMed] [Google Scholar]
- Gottsch ML, Clifton DK, Steiner RA. Kisspepeptin-GPR54 signaling in the neuroendocrine reproductive axis. Mol Cell Endocrinol. 2006;254-255:91–6. doi: 10.1016/j.mce.2006.04.030. [DOI] [PubMed] [Google Scholar]
- Gottsch ML, Cunningham MJ, Smith JT, Popa SM, Acohido BV, Crowley WF, Seminara S, Clifton DK, Steiner RA. A role for kisspeptins in the regulation of gonadotropin secretion in the mouse. Endocrinology. 2004;145:4073–7. doi: 10.1210/en.2004-0431. [DOI] [PubMed] [Google Scholar]
- Grace CO, Fink G, Quinn JP. Characterization of potential regulatory elements within the rat arginine vasopressin proximal promoter. Neuropeptides. 1999;33:81–90. doi: 10.1054/npep.1999.0018. [DOI] [PubMed] [Google Scholar]
- Green RM, Tingay S, Wang ZY, Tobin EM. Circadian rhythms confer a higher level of fitness to Arabidopsis plants. Plant Physiol. 2002;129:576–84. doi: 10.1104/pp.004374. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Greives TJ, Mason AO, Scotti MA, Levine J, Ketterson ED, Kriegsfeld LJ, Demas GE. Environmental control of kisspeptin: Implications for seasonal reproduction. Endocrinology. 2007;148:1158–66. doi: 10.1210/en.2006-1249. [DOI] [PubMed] [Google Scholar]
- Guo H, Brewer JM, Lehman MN, Bittman EL. Suprachiasmatic regulation of circadian rhythms of gene expression in hamster peripheral organs: effects of transplanting the pacemaker. J Neurosci. 2006;26:6406–12. doi: 10.1523/JNEUROSCI.4676-05.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hall JE. Neuroendocrine changes with reproductive aging in women. Semin Reprod Med. 2007;25:344–51. doi: 10.1055/s-2007-984740. [DOI] [PubMed] [Google Scholar]
- Hall JE, Lavoie HB, Marsh EE, Martin KA. Decrease in gonadotropin-releasing hormone (GnRH) pulse frequency with aging in postmenopausal women. J Clin Endocrinol Metab. 2000;85:1794–800. doi: 10.1210/jcem.85.5.6612. [DOI] [PubMed] [Google Scholar]
- Han SK, Gottsch ML, Lee KJ, Popa SM, Smith JT, Jakawich SK, Clifton DK, Steiner RA, Herbison AE. Activation of gonadotropin-releasing hormone neurons by kisspeptin as a neuroendocrine switch for the onset of puberty. J Neurosci. 2005;25:11349–56. doi: 10.1523/JNEUROSCI.3328-05.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hansen KR, Knowlton NS, Thyer AC, Charleston JS, Soules MR, Klein NA. A new model of reproductive aging: the decline in ovarian non-growing follicle number from birth to menopause. Hum Reprod. 2008;23:699–708. doi: 10.1093/humrep/dem408. [DOI] [PubMed] [Google Scholar]
- Hardin PE. Analysis of period mRNA cycling in Drosophila head and body tissues indicates that body oscillators behave differently from head oscillators. Mol Cell Biol. 1994;14:7211–8. doi: 10.1128/mcb.14.11.7211. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Harney JP, Scarbrough K, Rosewell KL, Wise PM. In vivo antisense antagonism of vasoactive intestinal peptide in the suprachiasmatic nuclei causes aging-like changes in the estradiol-induced luteinizing hormone and prolactin surges. Endocrinology. 1996;137:3696–701. doi: 10.1210/endo.137.9.8756535. [DOI] [PubMed] [Google Scholar]
- Herbison AE, Augood SJ, McGowan EM. Expression of glutamic acid decarboxylase messenger RNA in rat medial preoptic area neurones during the oestrous cycle and after ovariectomy. Brain Res Mol Brain Res. 1992;14:310–6. doi: 10.1016/0169-328x(92)90098-v. [DOI] [PubMed] [Google Scholar]
- Hinuma S, Shintani Y, Fukusumi S, Iijima N, Matsumoto Y, Hosoya M, Fujii R, Watanabe T, Kikuchi K, Terao Y, Yano T, Yamamoto T, Kawamata Y, Habata Y, Asada M, Kitada C, Kurokawa T, Onda H, Nishimura O, Tanaka M, Ibata Y, Fujino M. New neuropeptides containing carboxy-terminal RFamide and their receptor in mammals. Nat Cell Biol. 2000;2:703–8. doi: 10.1038/35036326. [DOI] [PubMed] [Google Scholar]
- Hoffman GE, Smith MS, Verbalis JG. c-Fos and related immediate early gene products as markers of activity in neuroendocrine systems. Front Neuroendocrinol. 1993;14:173–213. doi: 10.1006/frne.1993.1006. [DOI] [PubMed] [Google Scholar]
- Hurd MW, Ralph MR. The significance of circadian organization for longevity in the golden hamster. J Biol Rhythms. 1998;13:430–6. doi: 10.1177/074873098129000255. [DOI] [PubMed] [Google Scholar]
- Irwig MS, Fraley GS, Smith JT, Acohido BV, Popa SM, Cunningham MJ, Gottsch ML, Clifton DK, Steiner RA. Kisspeptin activation of gonadotropin releasing hormone neurons and regulation of KiSS-1 mRNA in the male rat. Neuroendocrinology. 2004;80:264–72. doi: 10.1159/000083140. [DOI] [PubMed] [Google Scholar]
- Jin X, Shearman LP, Weaver DR, Zylka MJ, de Vries GJ, Reppert SM. A molecular mechanism regulating rhythmic output from the suprachiasmatic circadian clock. Cell. 1999;96:57–68. doi: 10.1016/s0092-8674(00)80959-9. [DOI] [PubMed] [Google Scholar]
- Johnson CH. Testing the adaptive value of circadian systems. Methods Enzymol. 2005;393:818–37. doi: 10.1016/S0076-6879(05)93043-7. [DOI] [PubMed] [Google Scholar]
- Johnson CH, Golden SS. Circadian programs in cyanobacteria: adaptiveness and mechanism. Annu Rev Microbiol. 1999;53:389–409. doi: 10.1146/annurev.micro.53.1.389. [DOI] [PubMed] [Google Scholar]
- Johnson MA, Tsutsui K, Fraley GS. Rat RFamide-related peptide-3 stimulates GH secretion, inhibits LH secretion, and has variable effects on sex behavior in the adult male rat. Horm Behav. 2007;51:171–80. doi: 10.1016/j.yhbeh.2006.09.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kalsbeek A, Buijs RM. Output pathways of the mammalian suprachiasmatic nucleus: coding circadian time by transmitter selection and specific targeting. Cell Tissue Res. 2002;309:109–18. doi: 10.1007/s00441-002-0577-0. [DOI] [PubMed] [Google Scholar]
- Karman BN, Tischkau SA. Circadian clock gene expression in the ovary: Effects of luteinizing hormone. Biol Reprod. 2006;75:624–32. doi: 10.1095/biolreprod.106.050732. [DOI] [PubMed] [Google Scholar]
- Kauffman AS, Clifton DK, Steiner RA. Emerging ideas about kisspeptin-GPR54 signaling in the neuroendocrine regulation of reproduction. Trends Neurosci. 2007;10:504–11. doi: 10.1016/j.tins.2007.08.001. [DOI] [PubMed] [Google Scholar]
- Kim W, Jessen HM, Auger AP, Terasawa E. Postmenopausal increase in KiSS-1, GPR54, and luteinizing hormone releasing hormone (LHRH-1) mRNA in the basal hypothalamus of female rhesus monkeys. Peptides. 2009;30:103–10. doi: 10.1016/j.peptides.2008.06.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kinoshita M, Tsukamura H, Adachi S, Matsui H, Uenoyama Y, Iwata K, Yamada S, Inoue K, Ohtaki T, Matsumoto H, Maeda K. Involvement of central metastin in the regulation of preovulatory luteinizing hormone surge and estrous cyclicity in female rats. Endocrinology. 2005;146:4431–6. doi: 10.1210/en.2005-0195. [DOI] [PubMed] [Google Scholar]
- Klarsfeld A, Rouyer F. Effects of circadian mutations and LD periodicity on the life span of Drosophila melanogaster. J Biol Rhythms. 1998;13:471–8. doi: 10.1177/074873098129000309. [DOI] [PubMed] [Google Scholar]
- Klein NA, Battaglia DE, Fujimoto VY, Davis GS, Bremner WJ, Soules MR. Reproductive aging: accelerated ovarian follicular development associated with a monotropic follicle-stimulating hormone rise in normal older women. J Clin Endocrinol Metab. 1996;81:1038–45. doi: 10.1210/jcem.81.3.8772573. [DOI] [PubMed] [Google Scholar]
- Knobil E. On the control of gonadotropin secretion in the rhesus monkey. Recent Prog Horm Res. 1974;30:1–46. doi: 10.1016/b978-0-12-571130-2.50005-5. [DOI] [PubMed] [Google Scholar]
- Kolker DE, Fukuyama H, Huang DS, Takahashi JS, Horton TH, Turek FW. Aging alters circadian and light-induced expression of clock genes in golden hamsters. J Biol Rhythms. 2003;18:159–69. doi: 10.1177/0748730403251802. [DOI] [PubMed] [Google Scholar]
- Kolker DE, Vitaterna MH, Fruechte EM, Takahashi JS, Turek FW. Effects of age on circadian rhythms are similar in wild-type and heterozygous Clock mutant mice. Neurobiol Aging. 2004;25:517–23. doi: 10.1016/j.neurobiolaging.2003.06.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Krajnak K, Kashon ML, Rosewell KL, Wise PM. Aging alters the rhythmic expression of vasoactive intestinal polypeptide mRNA but not arginine vasopressin mRNA in the suprachiasmatic nuclei of female rats. J Neurosci. 1998;18:4767–74. doi: 10.1523/JNEUROSCI.18-12-04767.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Krajnak K, Rosewell KL, Wise PM. Fos-induction in gonadotropin-releasing hormone neurons receiving vasoactive intestinal polypeptide innervation is reduced in middle-aged female rats. Biol Reprod. 2001;64:1160–4. doi: 10.1095/biolreprod64.4.1160. [DOI] [PubMed] [Google Scholar]
- Kriegsfeld LJ. Driving reproduction: RFamide peptides behind the wheel. Horm Behav. 2006;50:655–66. doi: 10.1016/j.yhbeh.2006.06.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kriegsfeld LJ, Mei DF, Bentley GE, Ubuka T, Mason AO, Inoue K, Ukena K, Tsutsui K, Silver R. Identification and characterization of a gonadotropin-inhibitory system in the brains of mammals. Proc Natl Acad Sci USA. 2006;103:2410–5. doi: 10.1073/pnas.0511003103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kriegsfeld LJ, Silver R. The regulation of neuroendocrine function: Timing is everything. Horm Behav. 2006;49:557–74. doi: 10.1016/j.yhbeh.2005.12.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Krohn PL. Ovarian homotransplantation. Ann N Y Acad Sci. 1955;59:443–7. doi: 10.1111/j.1749-6632.1955.tb45958.x. [DOI] [PubMed] [Google Scholar]
- LaPolt PS, Matt DW, Lu JK. Progesterone implants delay age-related declines in regular estrous cyclicity and the ovarian follicular reserve in Long-Evans rats. Biol Reprod. 1998;59:197–201. doi: 10.1095/biolreprod59.1.197. [DOI] [PubMed] [Google Scholar]
- Le WW, Attardi B, Berghorn KA, Blaustein J, Hoffman GE. Progesterone blockade of a luteinizing hormone surge blocks luteinizing hormone-releasing hormone Fos activation and activation of its preoptic area afferents. Brain Res. 1997;778:272–80. doi: 10.1016/s0006-8993(97)00971-2. [DOI] [PubMed] [Google Scholar]
- Le WW, Berghorn KA, Rassnick S, Hoffman GE. Periventricular preoptic area neurons coactivated with luteinizing hormone (LH)-releasing hormone (LHRH) neurons at the time of the LH surge are LHRH afferents. Endocrinology. 1999;140:510–9. doi: 10.1210/endo.140.1.6403. [DOI] [PubMed] [Google Scholar]
- Lee WS, Abbud R, Smith MS, Hoffman GE. LHRH neurons express cJun protein during the proestrous surge of luteinizing hormone. Endocrinology. 1992;130:3101–3. doi: 10.1210/endo.130.5.1572316. [DOI] [PubMed] [Google Scholar]
- Lee WS, Smith MS, Hoffman GE. Luteinizing hormone-releasing hormone neurons express Fos protein during the proestrous surge of luteinizing hormone. Proc Natl Acad Sci U S A. 1990;87:5163–7. doi: 10.1073/pnas.87.13.5163. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Legan SJ, Karsch FJ. A daily signal for the LH surge in the rat. Endocrinology. 1975;96:57–62. doi: 10.1210/endo-96-1-57. [DOI] [PubMed] [Google Scholar]
- Lloyd JM, Hoffman GE, Wise PM. Decline in immediate early gene expression in gonadotropin-releasing hormone neurons during proestrus in regularly cycling, middle-aged rats. Endocrinology. 1994;134:1800–5. doi: 10.1210/endo.134.4.8137745. [DOI] [PubMed] [Google Scholar]
- Lowrey PL, Shimomura K, Antoch MP, Yamazaki S, Zemenides PD, Ralph MR, Menaker M, Takahashi JS. Positional syntenic cloning and functional characterization of the mammalian circadian mutation tau. Science. 2000;288:483–92. doi: 10.1126/science.288.5465.483. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lu JK, Anzalone CR, LaPolt PS. Relation of neuroendocrine function to reproductive decline during aging in the female rat. Neurobiol Aging. 1994;15:541–4. doi: 10.1016/0197-4580(94)90094-9. [DOI] [PubMed] [Google Scholar]
- Mandl AM, Shelton M. A quantitative study of oocytes in young and old nulliparous laboratory rats. J Endocrinol. 1959;18:444–50. doi: 10.1677/joe.0.0180444. [DOI] [PubMed] [Google Scholar]
- Mason AO, Greives TJ, Scotti MA, Levine J, Frommeyer S, Ketterson ED, Demas GE, Kriegsfeld LJ. Suppression of kisspeptin expression and gonadotropic axis sensitivity following exposure to inhibitory day lengths in female Siberian hamsters. Horm Behav. 2007;52:492–8. doi: 10.1016/j.yhbeh.2007.07.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Matsui H, Takatsu Y, Kumano S, Matsumoto H, Ohtaki T. Peripheral administration of metastin induces marked gonadotropin release and ovulation in the rat. Biochem Biophys Res Commun. 2004;320:383–8. doi: 10.1016/j.bbrc.2004.05.185. [DOI] [PubMed] [Google Scholar]
- Matt DW, Gilson MP, Sales TE, Krieg RJ, Kerbeshian MC, Veldhuis JD, Evans WS. Characterization of attenuated proestrous luteinizing hormone surges in middle-aged rats by deconvolution analysis. Biol Reprod. 1998;59:1477–82. doi: 10.1095/biolreprod59.6.1477. [DOI] [PubMed] [Google Scholar]
- Meredith S, Butcher RL. Role of decreased numbers of follicles on reproductive performance in young and aged rats. Biol Reprod. 1985;32:788–94. doi: 10.1095/biolreprod32.4.788. [DOI] [PubMed] [Google Scholar]
- Messager S, Chatzidaki EE, Ma D, Hendrick AG, Zahn D, Dixon J, Thresher RR, Malinge I, Lomet D, Carlton MB, Colledge WH, Caraty A, Aparicio SA. Kisspeptin directly stimulates gonadotropin-releasing hormone release via G protein-coupled receptor 54. Proc Natl Acad Sci USA. 2005;102:1761–6. doi: 10.1073/pnas.0409330102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Miller BH, Olson SL, Levine JE, Turek FW, Horton TH, Takahashi JS. Vasopressin regulation of the proestrous luteinizing hormone surge in wild-type and Clock mutant mice. Biol Reprod. 2006;75:778–84. doi: 10.1095/biolreprod.106.052845. [DOI] [PubMed] [Google Scholar]
- Miller BH, Olson SL, Turek FW, Levine JE, Horton TH, Takahashi JS. Circadian clock mutation disrupts estrous cyclicity and maintenance of pregnancy. Curr Biol. 2004;14:1367–73. doi: 10.1016/j.cub.2004.07.055. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Moore RY, Eichler VB. Loss of a circadian adrenal corticosterone rhythm following suprachiasmatic lesions in the rat. Brain Res. 1972;42:201–6. doi: 10.1016/0006-8993(72)90054-6. [DOI] [PubMed] [Google Scholar]
- Murakami M, Matsuzaki T, Iwasa T, Yasui T, Irahara M, Osugi T, Tsutsui K. Hypophysiotropic role of RFamide-related peptide-3 in the inhibition of LH secretion in female rats. J Endocrinol. 2008;199:105–12. doi: 10.1677/JOE-08-0197. [DOI] [PubMed] [Google Scholar]
- Nakao N, Yasuo S, Nishimura A, Yamamura T, Watanabe T, Anraku T, Okano T, Fukada Y, Sharp PJ, Ebihara S, Yoshimura T. Circadian clock gene regulation of steroidogenic acute regulatory protein gene expression in preovulatory ovarian follicles. Endocrinology. 2007;148:3031–8. doi: 10.1210/en.2007-0044. [DOI] [PubMed] [Google Scholar]
- Nass TE, LaPolt PS, Judd HL, Lu JK. Alterations in ovarian steroid and gonadotrophin secretion preceding the cessation of regular oestrous cycles in ageing female rats. J Endocrinol. 1984;100:43–50. doi: 10.1677/joe.0.1000043. [DOI] [PubMed] [Google Scholar]
- Navarro VM, Castellano JM, Fernandez-Fernandez R, Tovar S, Roa J, Mayen A, Barreiro ML, Casanueva FF, Aguilar E, Dieguez C, Pinilla L, Tena-Sempere M. Effects of KiSS-1 peptide, the natural ligand of GPR54, on follicle-stimulating hormone secretion in the rat. Endocrinology. 2005;146:1689–97. doi: 10.1210/en.2004-1353. [DOI] [PubMed] [Google Scholar]
- Navarro VM, Fernandez-Fernandez R, Castellano JM, Roa J, Mayen A, Barreiro ML, Gaytan F, Aguilar E, Pinilla L, Dieguez C, Tena-Sempere M. Advanced vaginal opening and precocious activation of the reproductive axis by KiSS-1 peptide, the endogenous ligand of GPR54. J Physiol. 2004;561:379–86. doi: 10.1113/jphysiol.2004.072298. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nelson JF, Bergman MD, Karelus K, Felicio LS. Aging of the hypothalamo-pituitary-ovarian axis: hormonal influences and cellular mechanisms. J Steroid Biochem. 1987;27:699–705. doi: 10.1016/0022-4731(87)90139-7. [DOI] [PubMed] [Google Scholar]
- Nelson JF, Gosden RG, Felicio LS. Effect of dietary restriction on estrous cyclicity and follicular reserves in aging C57BL/6J mice. Biol Reprod. 1985;32:515–22. doi: 10.1095/biolreprod32.3.515. [DOI] [PubMed] [Google Scholar]
- Nelson JF, Karelus K, Bergman MD, Felicio LS. Neuroendocrine involvement in aging: evidence from studies of reproductive aging and caloric restriction. Neurobiol Aging. 1995;16:837–43. doi: 10.1016/0197-4580(95)00072-m. discussion 855–856. [DOI] [PubMed] [Google Scholar]
- Nunez AA, Stephan FK. The effects of hypothalamic knife cuts on drinking rhythms and the estrus cycle of the rat. Behav Biol. 1977;20:224–34. doi: 10.1016/s0091-6773(77)90786-6. [DOI] [PubMed] [Google Scholar]
- Okamura H. Suprachiasmatic nucleus clock time in the mammalian circadian system. Cold Spring Harb Symp Quant Biol. 2007;72:551–6. doi: 10.1101/sqb.2007.72.033. [DOI] [PubMed] [Google Scholar]
- Ostrowski NL, Lolait SJ, Young WS., 3rd Cellular localization of vasopressin V1a receptor messenger ribonucleic acid in adult male rat brain, pineal, and brain vasculature. Endocrinology. 1994;135:1511–28. doi: 10.1210/endo.135.4.7925112. [DOI] [PubMed] [Google Scholar]
- Osugi T, Ukena K, Bentley GE, O'Brien S, Moore IT, Wingfield JC, Tsutsui K. Gonadotropin-inhibitory hormone in Gambel's white-crowned sparrow (Zonotrichia leucophrys gambelii): cDNA identification, transcript localization and functional effects in laboratory and field experiments. J Endocrinol. 2004;182:33–42. doi: 10.1677/joe.0.1820033. [DOI] [PubMed] [Google Scholar]
- Ottowitz WE, Dougherty DD, Fischman AJ, Hall JE. [18F]2-fluoro-2-deoxy-D-glucose positron emission tomography demonstration of estrogen negative and positive feedback on luteinizing hormone secretion in women. J Clin Endocrinol Metab. 2008;93:3208–14. doi: 10.1210/jc.2008-0203. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ouyang Y, Andersson CR, Kondo T, Golden SS, Johnson CH. Resonating circadian clocks enhance fitness in cyanobacteria. Proc Natl Acad Sci U S A. 1998;95:8660–4. doi: 10.1073/pnas.95.15.8660. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Palm IF, Van Der Beek EM, Wiegant VM, Buijs RM, Kalsbeek A. Vasopressin induces a luteinizing hormone surge in ovariectomized, estradiol-treated rats with lesions of the suprachiasmatic nucleus. Neuroscience. 1999;93:659–66. doi: 10.1016/s0306-4522(99)00106-2. [DOI] [PubMed] [Google Scholar]
- Pando MP, Morse D, Cermakian N, Sassone-Corsi P. Phenotypic rescue of a peripheral clock genetic defect via SCN hierarchical dominance. Cell. 2002;110:107–17. doi: 10.1016/s0092-8674(02)00803-6. [DOI] [PubMed] [Google Scholar]
- Peng MT, Huang HH. Aging of hypothalamic-pituitary-ovarian function in the rat. Fertil Steril. 1972;23:535–42. doi: 10.1016/s0015-0282(16)39131-2. [DOI] [PubMed] [Google Scholar]
- Petersen SL, Ottem EN, Carpenter CD. Direct and indirect regulation of gonadotropin-releasing hormone neurons by estradiol. Biol Reprod. 2003;69:1771–8. doi: 10.1095/biolreprod.103.019745. [DOI] [PubMed] [Google Scholar]
- Pittendrigh CS, Minis DH. Circadian systems: longevity as a function of circadian resonance in Drosophila melanogaster. Proc Natl Acad Sci USA. 1972;69:1537–9. doi: 10.1073/pnas.69.6.1537. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rance NE. Menopause and the human hypothalamus: evidence for the role of kisspeptin/neurokinin B neurons in the regulation of estrogen negative feedback. Peptides. 2009;30:111–22. doi: 10.1016/j.peptides.2008.05.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Revel FG, Saboureau M, Pevet P, Simonneaux V, Mikkelsen JD. RFamide-related peptide gene is a melatonin-driven photoperiodic gene. Endocrinology. 2008;149:902–12. doi: 10.1210/en.2007-0848. [DOI] [PubMed] [Google Scholar]
- Richard N, Galmiche G, Corvaisier S, Caraty A, Kottler ML. KiSS-1 and GPR54 genes are co-expressed in rat gonadotrophs and differentially regulated in vivo by oestradiol and gonadotrophin-releasing hormone. J Neuroendocrinol. 2008;20:381–93. doi: 10.1111/j.1365-2826.2008.01653.x. [DOI] [PubMed] [Google Scholar]
- Richardson SJ, Senikas V, Nelson JF. Follicular depletion during the menopausal transition: evidence for accelerated loss and ultimate exhaustion. J Clin Endocrinol Metab. 1987;65:1231–7. doi: 10.1210/jcem-65-6-1231. [DOI] [PubMed] [Google Scholar]
- Rizwan MZ, Porteous R, Herbison AE, Anderson GM. Cells expressing RFamide-related peptide-1/3, the mammalian gonadotropin-inhibitory hormone orthologs, are not hypophysiotropic neuroendocrine neurons in the rat. Endocrinology. 2009;150:1413–20. doi: 10.1210/en.2008-1287. [DOI] [PubMed] [Google Scholar]
- Roa J, Aguilar E, Dieguez C, Pinilla L, Tena-Sempere M. New frontiers in kisspeptin/GPR54 physiology as fundamental gatekeepers of reproductive function. Front Neuroendocrinol. 2008;29:48–69. doi: 10.1016/j.yfrne.2007.07.002. [DOI] [PubMed] [Google Scholar]
- Rometo AM, Krajewski SJ, Voytko ML, Rance NE. Hypertrophy and increased kisspeptin gene expression in the hypothalamic infundibular nucleus of postmenopausal women and ovariectomized monkeys. J Clin Endocrinol Metab. 2007;92:2744–50. doi: 10.1210/jc.2007-0553. [DOI] [PubMed] [Google Scholar]
- Roozendaal B, van Gool WA, Swaab DF, Hoogendijk JE, Mirmiran M. Changes in vasopressin cells of the rat suprachiasmatic nucleus with aging. Brain Res. 1987;409:259–64. doi: 10.1016/0006-8993(87)90710-4. [DOI] [PubMed] [Google Scholar]
- Rubin BS. Hypothalamic alterations and reproductive aging in female rats: evidence of altered luteinizing hormone-releasing hormone neuronal function. Biol Reprod. 2000;63:968–76. doi: 10.1095/biolreprod63.4.968. [DOI] [PubMed] [Google Scholar]
- Rubin BS, King JC. The number and distribution of detectable luteinizing hormone (LH)-releasing hormone cell bodies changes in association with the preovulatory LH surge in the brains of young but not middle-aged female rats. Endocrinology. 1994;134:467–74. doi: 10.1210/endo.134.1.8275960. [DOI] [PubMed] [Google Scholar]
- Satake H, Hisada M, Kawada T, Minakata H, Ukena K, Tsutsui K. Characterization of a cDNA encoding a novel avian hypothalamic neuropeptide exerting an inhibitory effect on gonadotropin release. Biochem J. 2001;354:379–85. doi: 10.1042/0264-6021:3540379. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Scarbrough K, Wise PM. Age-related changes in pulsatile luteinizing hormone release precede the transition to estrous acyclicity and depend upon estrous cycle history. Endocrinology. 1990;126:884–90. doi: 10.1210/endo-126-2-884. [DOI] [PubMed] [Google Scholar]
- Schibler U, Sassone-Corsi P. A web of circadian pacemakers. Cell. 2002;111:919–22. doi: 10.1016/s0092-8674(02)01225-4. [DOI] [PubMed] [Google Scholar]
- Seminara SB, Messager S, Chatzidaki EE, Thresher RR, Acierno JS, Jr, Shagoury JK, Bo-Abbas Y, Kuohung W, Schwinof KM, Hendrick AG, Zahn D, Dixon J, Kaiser UB, Slaugenhaupt SA, Gusella JF, O'Rahilly S, Carlton MB, Crowley WF, Jr, Aparicio SA, Colledge WH. The GPR54 gene as a regulator of puberty. N Engl J Med. 2003;349:1614–27. doi: 10.1056/NEJMoa035322. [DOI] [PubMed] [Google Scholar]
- Semple RK, Achermann JC, Ellery J, Farooqi IS, Karet FE, Stanhope RG, O'Rahilly S, Aparicio SA. Two novel missense mutations in g protein-coupled receptor 54 in a patient with hypogonadotropic hypogonadism. J Clin Endocrinol Metab. 2005;90:1849–55. doi: 10.1210/jc.2004-1418. [DOI] [PubMed] [Google Scholar]
- Shahab M, Mastronardi C, Seminara SB, Crowley WF, Ojeda SR, Plant TM. Increased hypothalamic GPR54 signaling: a potential mechanism for initiation of puberty in primates. Proc Natl Acad Sci U S A. 2005;102:2129–34. doi: 10.1073/pnas.0409822102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sharma VK. Adaptive significance of circadian clocks. Chronobiol Int. 2003;20:901–19. doi: 10.1081/cbi-120026099. [DOI] [PubMed] [Google Scholar]
- Shearman LP, Sriram S, Weaver DR, Maywood ES, Chaves I, Zheng B, Kume K, Lee CC, van der Horst GT, Hastings MH, Reppert SM. Interacting molecular loops in the mammalian circadian clock. Science. 2000;288:1013–9. doi: 10.1126/science.288.5468.1013. [DOI] [PubMed] [Google Scholar]
- Silver R, Sookhoo AI, LeSauter J, Stevens P, Jansen HT, Lehman MN. Multiple regulatory elements result in regional specificity in circadian rhythms of neuropeptide expression in mouse SCN. Neuroreport. 1999;10:3165–74. doi: 10.1097/00001756-199910190-00008. [DOI] [PubMed] [Google Scholar]
- Simonian SX, Spratt DP, Herbison AE. Identification and characterization of estrogen receptor alpha-containing neurons projecting to the vicinity of the gonadotropin-releasing hormone perikarya in the rostral preoptic area of the rat. J Comp Neurol. 1999;411:346–58. doi: 10.1002/(sici)1096-9861(19990823)411:2<346::aid-cne13>3.0.co;2-s. [DOI] [PubMed] [Google Scholar]
- Smith JT. Sex steroid control of hypothalamic Kiss1 expression in sheep and rodents: comparative aspects. Peptides. 2009;30:94–102. doi: 10.1016/j.peptides.2008.08.013. [DOI] [PubMed] [Google Scholar]
- Smith JT, Clifton DK, Steiner RA. Regulation of the neuroendocrine reproductive axis by kisspeptin-GPR54 signaling. Reproduction. 2006;131:623–30. doi: 10.1530/rep.1.00368. [DOI] [PubMed] [Google Scholar]
- Smith JT, Coolen LM, Kriegsfeld LJ, Sari IP, Jaafarzadehshirazi MR, Maltby M, Bateman K, Goodman RL, Tilbrook AJ, Ubuka T, Bentley GE, Clarke IJ, Lehman MN. Variation in kisspeptin and RFamide-related peptide (RFRP) expression and terminal connections to gonadotropin-releasing hormone neurons in the brain: a novel medium for seasonal breeding in the sheep. Endocrinology. 2008;149:5770–82. doi: 10.1210/en.2008-0581. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Smith JT, Dungan HM, Stoll EA, Gottsch ML, Braun RE, Eacker SM, Clifton DK, Steiner RA. Differential regulation of KiSS-1 mRNA expression by sex steroids in the brain of the male mouse. Endocrinology. 2005;146:2976–84. doi: 10.1210/en.2005-0323. [DOI] [PubMed] [Google Scholar]
- Smith JT, Popa SM, Clifton DK, Hoffman GE, Steiner RA. Kiss1 neurons in the forebrain as central processors for generating the preovulatory luteinizing hormone surge. J Neurosci. 2006;26:6687–94. doi: 10.1523/JNEUROSCI.1618-06.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Smith JT, Rao A, Pereira A, Caraty A, Millar RP, Clarke IJ. Kisspeptin is present in ovine hypophysial portal blood but does not increase during the preovulatory luteinizing hormone surge: evidence that gonadotropes are not direct targets of kisspeptin in vivo. Endocrinology. 2008;149:1951–9. doi: 10.1210/en.2007-1425. [DOI] [PubMed] [Google Scholar]
- Sopelak VM, Butcher RL. Contribution of the ovary versus hypothalamus-pituitary to termination of estrous cycles in aging rats using ovarian transplants. Biol Reprod. 1982;27:29–37. doi: 10.1095/biolreprod27.1.29. [DOI] [PubMed] [Google Scholar]
- Stephan FK, Zucker I. Circadian rhythms in drinking behavior and locomotor activity of rats are eliminated by hypothalamic lesions. Proc Natl Acad Sci USA. 1972;69:1583–6. doi: 10.1073/pnas.69.6.1583. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stetson MH. Circadian organization and female reproductive cyclicity. Adv Exp Med Biol. 1978;108:251–74. doi: 10.1007/978-1-4757-4460-6_11. [DOI] [PubMed] [Google Scholar]
- Sun ZS, Albrecht U, Zhuchenko O, Bailey J, Eichele G, Lee CC. RIGUI, a putative mammalian ortholog of the Drosophila period gene. Cell. 1997;90:1003–11. doi: 10.1016/s0092-8674(00)80366-9. [DOI] [PubMed] [Google Scholar]
- Sutin EL, Dement WC, Heller HC, Kilduff TS. Light-induced gene expression in the suprachiasmatic nucleus of young and aging rats. Neurobiol Aging. 1993;14:441–6. doi: 10.1016/0197-4580(93)90102-h. [DOI] [PubMed] [Google Scholar]
- Swann JM, Turek FW. Multiple circadian oscillators regulate the timing of behavioral and endocrine rhythms in female golden hamsters. Science. 1985;228:898–900. doi: 10.1126/science.4001926. [DOI] [PubMed] [Google Scholar]
- Takahata S, Sogawa K, Kobayashi A, Ema M, Mimura J, Ozaki N, Fujii-Kuriyama Y. Transcriptionally active heterodimer formation of an Arnt-like PAS protein, Arnt3, with HIF-1a, HLF, and clock. Biochem Biophys Res Commun. 1998;248:789–94. doi: 10.1006/bbrc.1998.9012. [DOI] [PubMed] [Google Scholar]
- Terasawa E, Wiegand SJ, Bridson WE. A role for medial preoptic nucleus on afternoon of proestrus in female rats. Am J Physiol. 1980;238:E533–9. doi: 10.1152/ajpendo.1980.238.6.E533. [DOI] [PubMed] [Google Scholar]
- Thompson EL, Patterson M, Murphy KG, Smith KL, Dhillo WS, Todd JF, Ghatei MA, Bloom SR. Central and peripheral administration of kisspeptin-10 stimulates the hypothalamic-pituitary-gonadal axis. J Neuroendocrinol. 2004;16:850–8. doi: 10.1111/j.1365-2826.2004.01240.x. [DOI] [PubMed] [Google Scholar]
- Tosini G, Menaker M. Circadian rhythms in cultured mammalian retina. Science. 1996;272:419–21. doi: 10.1126/science.272.5260.419. [DOI] [PubMed] [Google Scholar]
- Tsutsui K, Saigoh E, Ukena K, Teranishi H, Fujisawa Y, Kikuchi M, Ishii S, Sharp PJ. A novel avian hypothalamic peptide inhibiting gonadotropin release. Biochem Biophys Res Commun. 2000;275:661–7. doi: 10.1006/bbrc.2000.3350. [DOI] [PubMed] [Google Scholar]
- Ubuka T, Kim S, Huang YC, Reid J, Jiang J, Osugi T, Chowdhury VS, Tsutsui K, Bentley GE. Gonadotropin-inhibitory hormone neurons interact directly with gonadotropin-releasing hormone-I and -II neurons in European starling brain. Endocrinology. 2008;149:268–78. doi: 10.1210/en.2007-0983. [DOI] [PubMed] [Google Scholar]
- Ubuka T, Ukena K, Sharp PJ, Bentley GE, Tsutsui K. Gonadotropin-inhibitory hormone inhibits gonadal development and maintenance by decreasing gonadotropin synthesis and release in male quail. Endocrinology. 2006;147:1187–94. doi: 10.1210/en.2005-1178. [DOI] [PubMed] [Google Scholar]
- van der Beek EM, Wiegant VM, van Oudheusden HJ, van der Donk HA, van den Hurk R, Buijs RM. Synaptic contacts between gonadotropin-releasing hormone-containing fibers and neurons in the suprachiasmatic nucleus and perichiasmatic area: an anatomical substrate for feedback regulation? Brain Res. 1997;755:101–11. doi: 10.1016/s0006-8993(97)00086-3. [DOI] [PubMed] [Google Scholar]
- van der Schoot P. Changing pro-oestrous surges of luteinizing hormone in ageing 5-day cyclic rats. J Endocrinol. 1976;69:287–8. doi: 10.1677/joe.0.0690287. [DOI] [PubMed] [Google Scholar]
- van Look PF, Lothian H, Hunter WM, Michie EA, Baird DT. Hypothalamic-pituitary-ovarian function in perimenopausal women. Clin Endocrinol (Oxf) 1977;7:13–31. doi: 10.1111/j.1365-2265.1977.tb02936.x. [DOI] [PubMed] [Google Scholar]
- Watanabe A, Shibata S, Watanabe S. Circadian rhythm of spontaneous neuronal activity in the suprachiasmatic nucleus of old hamster in vitro. Brain Res. 1995;695:237–9. doi: 10.1016/0006-8993(95)00713-z. [DOI] [PubMed] [Google Scholar]
- Watson RE, Jr, Langub MC, Jr, Engle MG, Maley BE. Estrogen-receptive neurons in the anteroventral periventricular nucleus are synaptic targets of the suprachiasmatic nucleus and peri-suprachiasmatic region. Brain Res. 1995;689:254–64. doi: 10.1016/0006-8993(95)00548-5. [DOI] [PubMed] [Google Scholar]
- Weinert H, Weinert D, Schurov I, Maywood ES, Hastings MH. Impaired expression of the mPer2 circadian clock gene in the suprachiasmatic nuclei of aging mice. Chronobiol Int. 2001;18:559–65. doi: 10.1081/cbi-100103976. [DOI] [PubMed] [Google Scholar]
- Welsh DK, Yoo SH, Liu AC, Takahashi JS, Kay SA. Bioluminescence imaging of individual fibroblasts reveals persistent, independently phased circadian rhythms of clock gene expression. Curr Biol. 2004;14:2289–95. doi: 10.1016/j.cub.2004.11.057. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wiegand SJ, Terasawa E. Discrete lesions reveal functional heterogeneity of suprachiasmatic structures in regulation of gonadotropin secretion in the female rat. Neuroendocrinology. 1982;34:395–404. doi: 10.1159/000123335. [DOI] [PubMed] [Google Scholar]
- Wiegand SJ, Terasawa E, Bridson WE. Persistent estrus and blockade of progesterone-induced LH release follows lesions which do not damage the suprachiasmatic nucleus. Endocrinology. 1978;102:1645–8. doi: 10.1210/endo-102-5-1645. [DOI] [PubMed] [Google Scholar]
- Williams WP, 3rd, Mikkelsen JD, Kriegsfeld LJ. Circadian control of kisspeptin in the regulation of the preovulatory luteinizing hormone surge In: Society for Neuroscience. DC: Washington; 2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wintermantel TM, Campbell RE, Porteous R, Bock D, Grone HJ, Todman MG, Korach KS, Greiner E, Perez CA, Schutz G, Herbison AE. Definition of estrogen receptor pathway critical for estrogen positive feedback to gonadotropin-releasing hormone neurons and fertility. Neuron. 2006;52:271–80. doi: 10.1016/j.neuron.2006.07.023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wintermantel TM, Elzer J, Herbison AE, Fritzemeier KH, Schutz G. Genetic dissection of estrogen receptor signaling in vivo. Ernst Schering Found Symp Proc. 2006;00:25–44. doi: 10.1007/2789_2006_015. [DOI] [PubMed] [Google Scholar]
- Wise PM. a. Alterations in proestrous LH, FSH, and prolactin surges in middle-aged rats. Proc Soc Exp Biol Med. 1982;169:348–54. doi: 10.3181/00379727-169-41356. [DOI] [PubMed] [Google Scholar]
- Wise PM. Alterations in the proestrous pattern of median eminence LHRH, serum LH, FSH, estradiol and progesterone concentrations in middle-aged rats. Life Sci. 1982;31:165–73. doi: 10.1016/0024-3205(82)90429-5. [DOI] [PubMed] [Google Scholar]
- Wise PM. Estradiol-induced daily luteinizing hormone and prolactin surges in young and middle-aged rats: correlations with age-related changes in pituitary responsiveness and catecholamine turnover rates in microdissected brain areas. Endocrinology. 1984;115:801–9. doi: 10.1210/endo-115-2-801. [DOI] [PubMed] [Google Scholar]
- Wise PM. Neuroendocrine ageing: its impact on the reproductive system of the female rat. J Reprod Fertil Suppl. 1993;46:35–46. [PubMed] [Google Scholar]
- Wise PM. Neuroendocrine modulation of the “menopause”: insights into the aging brain. Am J Physiol. 1999;277:E965–70. doi: 10.1152/ajpendo.1999.277.6.E965. [DOI] [PubMed] [Google Scholar]
- Wise PM, Cohen IR, Weiland NG, London ED. Aging alters the circadian rhythm of glucose utilization in the suprachiasmatic nucleus. Proc Natl Acad Sci USA. 1988;85:5305–9. doi: 10.1073/pnas.85.14.5305. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wise PM, Smith MJ, Dubal DB, Wilson ME, Krajnak KM, Rosewell KL. Neuroendocrine influences and repercussions of the menopause. Endocr Rev. 1999;20:243–248. doi: 10.1210/edrv.20.3.0364. [DOI] [PubMed] [Google Scholar]
- Woelfle MA, Ouyang Y, Phanvijhitsiri K, Johnson CH. The adaptive value of circadian clocks: an experimental assessment in cyanobacteria. Curr Biol. 2004;14:1481–6. doi: 10.1016/j.cub.2004.08.023. [DOI] [PubMed] [Google Scholar]
- Wuttke W, Meites J. Effects of electrochemical stimulation of medial preoptic area on prolactin and luteinizing hormone release in old female rats. Pflugers Arch. 1973;341:1–6. doi: 10.1007/BF00587324. [DOI] [PubMed] [Google Scholar]
- Yamaji T, Dierschke DJ, Hotchkiss J, Bhattacharya AN, Surve AH, Knobil E. Estrogen induction of LH release in the rhesus monkey. Endocrinology. 1971;89:1034–41. doi: 10.1210/endo-89-4-1034. [DOI] [PubMed] [Google Scholar]
- Yamazaki S, Numano R, Abe M, Hida A, Takahashi R, Ueda M, Block GD, Sakaki Y, Menaker M, Tei H. Resetting central and peripheral circadian oscillators in transgenic rats. Science. 2000;288:682–5. doi: 10.1126/science.288.5466.682. [DOI] [PubMed] [Google Scholar]
- Yamazaki S, Straume M, Tei H, Sakaki Y, Menaker M, Block GD. Effects of aging on central and peripheral mammalian clocks. Proc Natl Acad Sci U S A. 2002;99:10801–6. doi: 10.1073/pnas.152318499. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yan J, Wang H, Liu Y, Shao C. Analysis of gene regulatory networks in the mammalian circadian rhythm. PLoS Comput Biol. 2008;4:e1000193. doi: 10.1371/journal.pcbi.1000193. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yan L, Foley NC, Bobula JM, Kriegsfeld LJ, Silver R. Two antiphase oscillations occur in each suprachiasmatic nucleus of behaviorally split hamsters. J Neurosci. 2005;25:9017–26. doi: 10.1523/JNEUROSCI.2538-05.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yin W, Gore AC. Neuroendocrine control of reproductive aging: roles of GnRH neurons. Reproduction. 2006;131:403–14. doi: 10.1530/rep.1.00617. [DOI] [PubMed] [Google Scholar]
- Zhao S, Kriegsfeld LJ. Daily changes in GT1-7 cell sensitivity to GnRH secretagogues that trigger ovulation. Neuroendocrinology. 2009 doi: 10.1159/000192370. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zylka MJ, Shearman LP, Weaver DR, Reppert SM. Three period homologs in mammals: differential light responses in the suprachiasmatic circadian clock and oscillating transcripts outside of brain. Neuron. 1998;20:1103–10. doi: 10.1016/s0896-6273(00)80492-4. [DOI] [PubMed] [Google Scholar]


