Abstract
Nerve growth factor (NGF) regulates many aspects of neuronal biology by retrogradely propagating signals along axons to the targets of those axons. How this occurs when axons contain a plethora of proteins that can silence those signals has long perplexed the neurotrophin field. In this issue of the JCI, Li et al. suggest an answer to this vexing problem, while exploring why the Elp1 gene that is mutated in familial dysautonomia (FD) causes peripheral neuropathy. They describe a distinctive function of Elp1 as a protein that is required to sustain NGF signaling by blocking the activity of its phosphatase that shuts off those signals. This finding helps explain the innervation deficits prominent in FD and reveals a unique role for Elp1 in the regulation of NGF-dependent TrkA activity.
Innervation deficits in familial dysautonomia
Familial dysautonomia (FD), also called hereditary sensory and autonomic neuropathy type III, is a genetic disorder that affects the development and survival of nerve growth factor–dependent (NGF-dependent) sympathetic and sensory neurons of the peripheral nervous system. Individuals with FD exhibit a range of perturbed autonomic (hence dysautonomia) and nociceptive phenotypes (1). FD is caused by mutations in the Elp1 (IKBKAP) gene, best known as a scaffolding component of the Elongator complex that regulates tRNA modification and therefore translation, and for its role in normal transcriptional elongation (1–3). Relevant to neuronal function, Elongator is also required for appropriate neuronal branching, organization of actin networks, and acetylation of α-tubulin. Loss of Elp1 results in mitochondrial dysfunction (1–4). A locus of action outside the nucleus is supported by evidence that Elp1 is readily detected in the cytosol. FD mouse models in which Elp1 is mutated or deleted show profound peripheral nervous system (PNS) and central nervous system (CNS) innervation deficits that recapitulate the FD human phenotype (5, 6). Given the many consequences of Elp1 mutation, it has been difficult to determine how and why the PNS is severely disrupted in FD.
The neurotrophic factor hypothesis is a classic concept in neurobiology first proposed by Levi-Montalcini and Hamburger (7) that states that developing neurons compete with each other for a limited supply of a neurotrophic factor provided by the target tissue. Successful competitors survive and innervate the target tissue, while unsuccessful neurons die. NGF is the prototypical target-derived factor, binding receptors at axon terminals innervating the target and transmitting its signals down the axon toward the cell body to support the survival of neuronal cell bodies and locally to support sprouting. Similarly, adult sensory, sympathetic, and basal forebrain cholinergic neurons depend on target-derived NGF for axon and dendrite growth and the acquisition and lifelong maintenance of neuronal specification and neurotransmitter phenotypes. For decades, the neurotrophin field has focused on how retrograde signals are transmitted over remarkably long distances. For example, the cell bodies of sensory axons that innervate our fingertips and are responsible for thermo- and pain sensations are located near the spinal cord, up to a meter or more away (think of a giraffe). How does this extended signaling occur? NGF first binds to the TrkA receptor tyrosine kinase at nerve terminals, which changes the conformation of the TrkA dimer. This enables TrkA to transphosphorylate tyrosine residues on each monomer of the receptor, including on key tyrosine residues that function as recognition sites for intracellular signaling proteins that associate with and are phosphorylated and activated by TrkA (8, 9). The best known TrkA-bound protein is Shc, which activates the Ras-MAP kinase and Ras-PI3-kinase signaling pathways that mediate axonal and dendrite growth and cell survival (10). How then does target-derived NGF transmit its signals over long distances? Upon internalization, the NGF-TrkA complex is localized to membrane-bound organelles that are retrogradely transported toward the cell body by a microtubule-based motor system (11–13). TrkA is oriented with its ligand-binding domain inside, and its cytoplasmic kinase and substrate-bound signaling proteins outside of the organelle. In this manner, TrkA signals locally as it travels down the axon to stimulate axonal growth. Upon arriving at the cell body, the signaling endosome releases its contents to activate gene expression responsible for survival, growth, and neuronal specification. Disruption of activated TrkA endocytosis, transport, or activity during the transport process results in cell body apoptosis, indicating the key role of retrograde signaling endosomes — as essential carriers of NGF signals. Notably, a portion of the activated TrkA-containing endosomes traverses the cell body and translocates to dendrites where it mediates circuit formation and synapse maintenance (14).
Maintaining TrkA activity through distance and time
A puzzling question, however, is how TrkA activity is maintained during endosomal transport from nerve terminals to cell bodies over long distances and in a process whose duration can be many hours. All signaling processes reversibly regulated by phosphorylation require a kinase that adds phosphates to proteins and a corresponding phosphatase that removes phosphates and inactivates the kinase-activated signaling protein. Indeed, inhibition of tyrosine phosphatase activity elevates and sustains TrkA signaling both in the presence and absence of NGF, indicating that phosphatases normally keep TrkA in the inactive state when not bound to NGF and modulate and prevent TrkA from overactivity following NGF binding (15). Overactive TrkA that induces aberrant sprouting and nociceptive responses is deleterious. TrkA possesses several important tyrosine phosphorylation sites, and at least eight different protein tyrosine phosphatases are known to dephosphorylate TrkA and suppress signaling. Even more problematic is the NGF-dependent association of TrkA with SHP1 (also called PTPN6), a potent phosphatase. SHP1 keeps TrkA in an off-state in the absence of NGF and modulates TrkA activity after activation by NGF (15). One way to maintain TrkA phosphorylation is to shift the balance of the kinase-phosphatase relationship that occurs when NGF binds and activates TrkA kinase activity, overwhelming the activity of nearby phosphatases. However, since as few as one NGF molecule are present in a signaling endosome that presumably contains many more activated TrkA receptors (16), another mechanism must suppress the activity of TrkA phosphatases during the retrograde transport process.
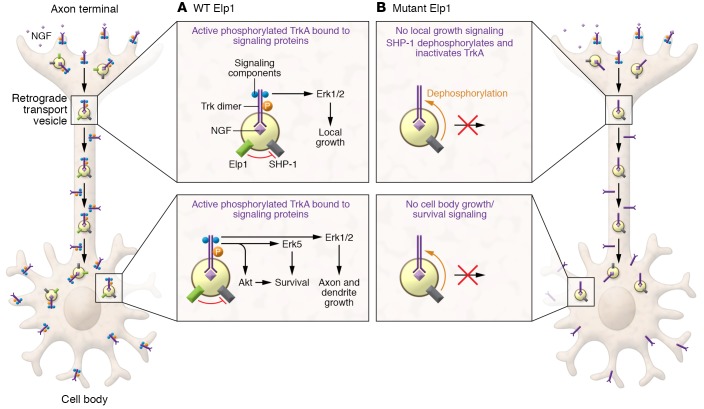
The correspondence between the sensory and sympathetic neurons affected in FD patients and those neurons known to be responsive to NGF produced in their targets of innervation has long suggested a role for NGF and NGF signaling in this disorder (1). In this issue of the JCI, Li et al. (17) explored the role of Elp1 in retrograde neuronal signaling and survival. The researchers generated mice engineered to express the human Elp1 mutation together with the WT Elp1 gene. The WT Elp1 gene was subjected to conditional ablation by tamoxifen addition to cultured sympathetic neurons from the mice. Neurons only expressing the mutant Elp1 failed to survive to the same extent as WT neurons when NGF was added to axon terminals in compartmented chambers, and survival to WT levels by retrograde NGF signaling was rescued when WT Elp1 was reexpressed in those neurons. While NGF induced the same tyrosine transphosphorylation and internalization of TrkA at axon terminals and TrkA retrograde endosomal transport in mutant and WT Elp1 neurons, TrkA was rapidly dephosphorylated only in the Elp1 mutant neurons. The appearance of specific phosphorylated and activated signaling proteins in the cell body, indicates retrograde NGF signaling. However, none of these phosphorylated proteins (Erk1/2, Erk5, Akt and CREB, which are required for axonal growth or survival) were observed in the Elp1 mutant cell bodies following application of NGF to the distal axons. In contrast, TrkA and TrkA substrate phosphorylation was maintained for up to five hours in WT neurons. Elp1 is thus required to propagate NGF-dependent retrograde signals and neuronal survival (17). How then does Elp1 function to sustain NGF signaling? The authors intuited that Elp1 might suppress TrkA phosphatase activity, and they focused on the previously identified TrkA-associated tyrosine phosphatase SHP1 (15). NGF induced the association of Elp1 and SHP1 with TrkA and their recruitment to TrkA-containing transport endosomes. Though SHP1 phosphatase was still recruited to the TrkA signaling complex in Elp1 mutant neurons, it was hyperactive; and inhibiting SHP1 activity or expression rescued the deficits in retrograde NGF-mediated survival in these neurons. Thus, at least in culture, the consequence of the FD Elp1 mutation was SHP1 hyperactivation that dephosphorylated retrogradely transported TrkA, resulting in decreased NGF-mediated survival (ref. 17 and Figure 1).
Figure 1. Elp1 in the regulation of NGF-dependent TrkA activity.
(A) Retrograde signaling in WT axons. Elp1 suppresses SHP1 phosphatase activity and enables NGF-mediated neuronal innervation and survival. (B) In the absence of Elp1, SHP1 dephosphorylates retrogradely transported TrkA and inhibits NGF-mediated neuronal innervation and survival.
A prominent human FD phenotype, however, is deficits in sympathetic target innervation. To begin to address whether hyperactive SHP1 is responsible for this phenotype, the authors examined neonatal mice with Elp1 specifically ablated in sympathetic neurons. The neurons in those Elp1-deficient mice showed diminished TrkA phosphorylation and innervation defects. Remarkably, infusion of a SHP1-selective inhibitor rescued the deficits in TrkA phosphorylation and sympathetic neuron innervation, consistent with a unique and required role for Elp1 in regulating NGF-dependent TrkA activity and neuronal innervation in FD.
Is the hyperactivation of SHP1 activity the major consequence of the FD Elp1 mutation on peripheral neuron innervation? A reduced velocity of retrogradely transported NGF has been noted in sensory neurons lacking Elp1, possibly due to dysregulated Elongator activity that can hyperacetylate α-tubulin and alter protein translation (18). However, in the present study, no deficits were observed in the expression of TrkA and TrkA signaling proteins, and there was no substantial reduction in the amount of retrogradely transported TrkA. The widespread CNS phenotypes of patients with FD, including seizures, visual and learning impairments, heightened anxiety, and white and grey matter structural deficits, suggest that the Elp1 modulation of Elongator that mediates many general cell functions (1, 3, 5) is more likely to be mechanistically responsible for these perturbations.
Next steps
The next step is understanding how Elp1 maintains TrkA activity during retrograde transport. For example, does Elp1 inhibit SHP1 activity directly, outcompete SHP1 from binding to TrkA at its association site (Y490), or sterically bind to and prevent SHP1 from dephosphorylating TrkA? Retrogradely transported TrkA is not only protected from SHP1 by Elp1, but from all of the many TrkA phosphatases. This protection suggests that Elp1 has a more general role as an inhibitor of TrkA dephosphorylation. Whether Elp1 also inhibits TrkA dephosphorylation by the SHP1 relative and TrkA phosphatase SHP2 (19) would be helpful to know. Other questions are: How are Elp1 levels and association with TrkA regulated by NGF? Does Elp1 in the retrograde transport complex function as an Elongator regulator when it arrives in the TrkA signaling endosome at the cell body? Finally, is Elp1 involved in determining the levels and temporal activity of TrkA in other neurons, including NGF-dependent basal forebrain cholinergic neurons important for attentive learning and memory or neurons that utilize TrkB for synaptic plasticity? Elp1 emerges as a prime candidate to not only preserve TrkA activity during long-distance transport, but as a fine-tuner of Trk activity by regulating Trk phosphatases. It will be exciting to further explore the spectrum of Elp1-interacting receptor tyrosine kinases, and what other Elp1-like proteins are recruited to modulate TrkA activation.
The present study adds important insights into the extraordinary ability of neuronal cell bodies to sustain innervation of distant targets through formation and transport of signaling endosomes. Especially given these new disease-relevant observations, this study makes a strong case for continued attention to further elucidating this fundamental aspect of neuronal function.
Version 1. 04/13/2020
Electronic publication
Version 2. 05/01/2020
Print issue publication
Version 3. 05/11/2020
Corrected text related to reference 7.
Footnotes
Conflict of interest: DRK has received consulting/board service funds from Reveille Inc., and holds the following US patents: 5231001, 5817471, and 5811396. WCM owns shares of Cortexyme Inc., received consulting funds from Samumed Inc. and Koren-Tillery LLP, and has received or currently receives research support from QPharma and AC Immune. WCM holds the following US patents: 5134121, 9072730, 9072746, 9938263, and 10472346.
Copyright: © 2020, American Society for Clinical Investigation.
Reference information: J Clin Invest. 2020;130(5):2195–2198. https://doi.org/10.1172/JCI136162.
See the related article at Retrograde nerve growth factor signaling abnormalities in familial dysautonomia.
References
- 1.Tourtellotte WG. Axon transport and neuropathy: relevant perspectives on the etiopathogenesis of familial dysautonomia. Am J Pathol. 2016;186(3):489–499. doi: 10.1016/j.ajpath.2015.10.022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Kojic M, Wainwright B. The many faces of elongator in neurodevelopment and disease. Front Mol Neurosci. 2016;9:115. doi: 10.3389/fnmol.2016.00115. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Kojic M, et al. Elongator mutation in mice induces neurodegeneration and ataxia-like behavior. Nat Commun. 2018;9(1):3195. doi: 10.1038/s41467-018-05765-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Close P, et al. Transcription impairment and cell migration defects in elongator-depleted cells: implication for familial dysautonomia. Mol Cell. 2006;22(4):521–531. doi: 10.1016/j.molcel.2006.04.017. [DOI] [PubMed] [Google Scholar]
- 5.Lefcort F, Mergy M, Ohlen SB, Ueki Y, George L. Animal and cellular models of familial dysautonomia. Clin Auton Res. 2017;27(4):235–243. doi: 10.1007/s10286-017-0438-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Jackson MZ, Gruner KA, Qin C, Tourtellotte WG. A neuron autonomous role for the familial dysautonomia gene ELP1 in sympathetic and sensory target tissue innervation. Development. 2014;141(12):2452–2461. doi: 10.1242/dev.107797. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Yuen EC, Howe CL, Li Y, Holtzman DM, Mobley WC. Nerve growth factor and the neurotrophic factor hypothesis. Brain Dev. 1996;18(5):362–368. doi: 10.1016/0387-7604(96)00051-4. [DOI] [PubMed] [Google Scholar]
- 8.Kaplan DR, Miller FD. Neurotrophin signal transduction in the nervous system. Curr Opin Neurobiol. 2000;10(3):381–391. doi: 10.1016/S0959-4388(00)00092-1. [DOI] [PubMed] [Google Scholar]
- 9.Huang EJ, Reichardt LF. Trk receptors: roles in neuronal signal transduction. Annu Rev Biochem. 2003;72:609–642. doi: 10.1146/annurev.biochem.72.121801.161629. [DOI] [PubMed] [Google Scholar]
- 10.Miller FD, Kaplan DR. Signaling mechanisms underlying dendrite formation. Curr Opin Neurobiol. 2003;13(3):391–398. doi: 10.1016/S0959-4388(03)00072-2. [DOI] [PubMed] [Google Scholar]
- 11.Delcroix JD, et al. Trafficking the NGF signal: implications for normal and degenerating neurons. Prog Brain Res. 2004;146:3–23. doi: 10.1016/s0079-6123(03)46001-9. [DOI] [PubMed] [Google Scholar]
- 12.Zweifel LS, Kuruvilla R, Ginty DD. Functions and mechanisms of retrograde neurotrophin signalling. Nat Rev Neurosci. 2005;6(8):615–625. doi: 10.1038/nrn1727. [DOI] [PubMed] [Google Scholar]
- 13.Cosker KE, Courchesne SL, Segal RA. Action in the axon: generation and transport of signaling endosomes. Curr Opin Neurobiol. 2008;18(3):270–275. doi: 10.1016/j.conb.2008.08.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Lehigh KM, West KM, Ginty DD. Retrogradely transported TrkA endosomes signal locally within dendrites to maintain sympathetic neuron synapses. Cell Rep. 2017;19(1):86–100. doi: 10.1016/j.celrep.2017.03.028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Marsh HN, et al. SHP-1 negatively regulates neuronal survival by functioning as a TrkA phosphatase. J Cell Biol. 2003;163(5):999–1010. doi: 10.1083/jcb.200309036. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Cui B, et al. One at a time, live tracking of NGF axonal transport using quantum dots. Proc Natl Acad Sci U S A. 2007;104(34):13666–13671. doi: 10.1073/pnas.0706192104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Li L, et al. Retrograde nerve growth factor signaling abnormalities in familial dysautonomia. J Clin Invest. 2020;130(5):2478–2487. doi: 10.1172/JCI130401. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Naftelberg S, et al. Phosphatidylserine ameliorates neurodegenerative symptoms and enhances axonal transport in a mouse model of familial dysautonomia. PLoS Genet. 2016;12(12):e1006486. doi: 10.1371/journal.pgen.1006486. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Fujita Y, Endo S, Takai T, Yamashita T. Myelin suppresses axon regeneration by PIR-B/SHP-mediated inhibition of Trk activity. EMBO J. 2011;30(7):1389–1401. doi: 10.1038/emboj.2011.55. [DOI] [PMC free article] [PubMed] [Google Scholar]