Abstract
Background and aim
Objective and more rigorous therapeutic outcomes are emerging as novel targets in Crohn’s disease (CD). We investigated the association between maintenance serum infliximab trough concentrations and biochemical, endoscopic, or histologic remission in CD.
Methods
This retrospective multicenter study involved consecutive CD patients treated with infliximab who had a serum C-reactive protein (CRP) measured within 1 week or endoscopic evaluation within 12 weeks of therapeutic drug monitoring between January 2010 and June 2016. Biochemical remission was defined as a normal CRP (≤5 mg/L). Endoscopic remission was defined as absence of any mucosal break (ulceration or erosion) or for patients with an ileocolonic resection, a Rutgeerts score of ≤i1. Histologic remission was defined as absence of active inflammation.
Results
Seventy-one CRP levels and 96 colonoscopies from 110 CD patients were evaluated. Based on ROC analyses, infliximab concentration thresholds of 2.2, 9.7, and 9.8 μg/mL were found to be related with biochemical, endoscopic, and histologic remission, respectively. Multiple logistic regression analyses identified infliximab concentration ≥2.2 (OR 6.4; 95% CI, 1.5–27.1; P = 0.011), ≥9.7 (OR 3.6; 95% CI, 1.4–9; P = 0.006) and ≥9.8 μg/mL (OR 3.2; 95% CI, 1.3–7.9; P = 0.011) as variables independently associated with biochemical, endoscopic, and histologic remission, respectively.
Conclusions
This study showed that higher maintenance infliximab trough concentrations are associated with more favorable rates of biochemical, endoscopic, or histologic remission in CD patients and that infliximab concentrations may differ based on the treatment goal.
Keywords: antibodies to infliximab, biological therapy, IBD, objective therapeutic outcomes, therapeutic drug monitoring
INTRODUCTION
Infliximab, an antitumor necrosis factor (anti-TNF) agent, is an established treatment for moderate to severe Crohn’s disease (CD).1 Stringent therapeutic goals, including biochemical, endoscopic, and histologic remission, are associated with a more favorable course of the inflammatory bowel disease (IBD) with less relapses, steroid use, hospitalizations, and surgeries and are currently considered more desirable goals of anti-TNF therapy for both CD and ulcerative colitis (UC).2, 3
Besides biomarkers, such as faecal calprotectin and C-reactive protein (CRP),4 therapeutic drug monitoring (TDM) has shown great promise for optimization of anti-TNF therapy in IBD, as association studies have shown a positive correlation between serum anti-TNF drug levels and favorable therapeutic outcomes.5–17 Nevertheless, the range of therapeutic drug concentration to aim for achieving objective therapeutic outcomes is still largely unknown.17, 18 Moreover, to our knowledge, there are no published data relating infliximab concentrations with histologic remission in patients with CD.
We investigated the association between maintenance serum infliximab trough concentrations and biochemical, endoscopic, or histologic remission in CD patients and identified variables associated with these outcomes.
MATERIALS AND METHODS
Study Design, Population, and Definitions
This was a retrospective multicenter study. Eligible patients were consecutive CD patients treated with infliximab who had a serum C-reactive protein (CRP) measured within 1 week or endoscopic evaluation within 12 weeks of therapeutic drug monitoring (TDM) between January 2010 and June 2016. We excluded patients with an ostomy or ileal pouch-anal anastomosis. Patients had an endoscopy either for surveillance of dysplasia or for disease activity and mucosal healing assessment. A patient could be assessed more than once if the time interval among procedures was more than 6 months and each colonoscopy was linked to an adjacent serum sample. Biochemical remission was defined as a normal CRP (≤5 mg/L). Endoscopic remission was defined as absence of any mucosal break (ulceration or erosion), or for patients who had undergone ileocolonic resection, a Rutgeerts score of ≤i1. Histologic remission was defined as absence of active inflammation.
All biochemical, endoscopic, and histologic data was retrieved from the patients’ online medical records. The Institutional Review Boards of the Beth Israel Deaconess Medical Center and the University of Pennsylvania approved the study.
Therapeutic Drug Monitoring
Serum infliximab trough concentrations and antibodies to infliximab (ATI) were evaluated by Prometheus Laboratories (enzyme-linked immunosorbent assay [ELISA], until July 2012 and homogeneous mobility shift assay [HMSA]).19 Infliximab concentrations of <1 and 1.4 µg/mL and ATI <3.1 U/mL and 1.7 µg/mL equivalents were considered undetectable for the HMSA and ELISA, respectively.
Statistical Analysis
Categorical variables were reported as frequencies, and percentages and continuous variables were reported as medians with interquartile range (IQR). A receiver-operating characteristic (ROC) analysis was used to find infliximab concentration thresholds associated with biochemical, endoscopic, or histologic remission based on the Youden index.13 Sensitivity (SN), specificity (SP), positive predictive value (PPV) and negative predictive value (NPV) were also provided. The Mann-Whitney U test was used to compare infliximab concentrations between groups. Univariate analyses were performed using the Mann-Whitney U test and the χ2 or the Fisher exact tests to identify continous or categorical variables, respectively, associated with biochemical, endoscopic, or histologic remission. Variables with a P value < 0.1 from univariate analysis entered the multivariate binary logistic regression analysis to indentify independently associated variables with the outcomes of interest, using the Backward Wald selection method. Variables included age at diagnosis, age at infliximab initiation, gender, body mass index, duration of the disease, CD behavior and location, history of prior anti-TNF therapy, perianal fistulizing CD, ileocolonic resection, smoking status, infliximab regimen different than 5 mg/kg every 8 weeks at the time of TDM, use of concomitant immonumodulators, type of assay, ATI, and infliximab concentration as a categorical variable (based on thresholds identified by ROC analyses). The results were expressed as odds ratio (OR) with 95% confidence intervals (CI) and corresponding P value . We performed an incremental gain analysis to find the range of maximal increase in the biochemical, endoscopic, or histologic remission rate with any rise in infliximab concentrations. These rates were compared using a χ2 test (linear-by-linear association). Results were considered statistically significant if P < 0.05. All statistical analyses were performed using the SPSS 24.0 software (SPSS, Chicago, Illinois, USA) and GraphPad Prism version 5.03 for Windows (GraphPad Software, San Diego CA, USA).
RESULTS
Study Population
Seventy-one CRP evaluations and 96 colonoscopies (surveillance colonoscopies, n = 76 [70%]) from 110 patients with CD (male, n = 59 [54%]) were evaluated. Fifty-four patients had 1 endoscopy, 12 patients had 2 endoscopies, and 6 patients had 3 endoscopies. Demographic and clinical data of the patients are reported in Table 1.
Table 1:
Patients’ Demographic and Clinical Characteristics
| Patients’ Demographic and Clinical Characteristics | N = 110 |
|---|---|
| Male, (%) | 59 (54) |
| Age at diagnosis, median (IQR), y | 23 (18–30) |
| Age at infliximab initiation, median (IQR), y | 25 (32–43) |
| Disease duration: median (IQR), y | 8 (3–16) |
| Time from start of infliximab to first TDM: median (IQR), months | 15.3 (6.9–42.6) |
| Behavior: B1 / B2 / B3a, (%) | 54 (49) / 18 (16) / 38 (35) |
| Location: L1 / L2 / L3 / L4a, (%), n = 109 | 20 (18) / 25 (23) / 59 (54) / 5 (5) |
| Anti-TNF naïve, (%) | 102 (93) |
| Smoking at first TDM, (%) | 19 (17) |
| Infliximab dosing other than 5mg/Kg every 8 weeks at first TDM, (%) | 47 (43) |
| Concomitant IMM at first TDM, (%) | 28 (26) |
| BMI at first TDM, median (IQR), Kg/m2, n = 80 | 24.8 (21.6–29.1) |
| Perianal fistulizing CD, (%) | 43 (39) |
| Prior ileocolonic resection, (%) | 20 (18) |
| CRP at first TDM, median (IQR), mg/L, n = 49 | 2.5 (0.7–6.6) |
| HMSA at first TDM, (%) | 72 (66) |
| Infliximab TC at first TDM, median (IQR), μg/mL | 6.8 (3.2–12.2) |
| ATI at first TDM, (%) | 9 (8) |
aaccording to Montreal classification; bthiopurines
y: years
Biochemical Remission and Infliximab Concentration
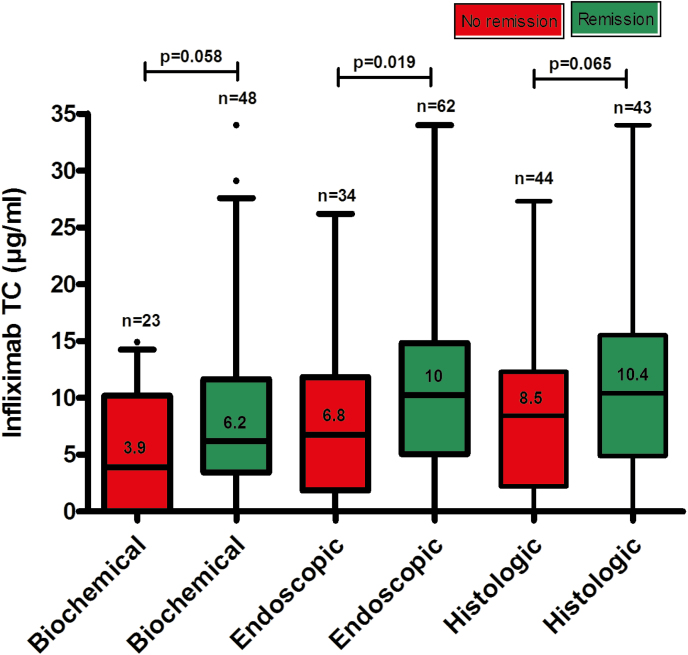
Biochemical remission was found in 48 of 71 (68%) of CRP evaluations. Patients with biochemical remission had numerically higher serum infliximab trough concentration (median [IQR]) compared with those without (6.2 [3.5–14.5] vs 3.9 [0–10.2] μg/mL; P = 0.058) (Fig. 1). Receiver-operating characteristic analysis showed an infliximab concentration ≥2.2 μg/mL associated with biochemical remission (SN 92%, SP 35%, PPV 75%, NPV 67%) (Fig. 2A). In multivariate analysis, infliximab concentration ≥2.2 μg/mL (OR 6.4; 95% CI, 1.5–27.1; P = 0.011), infliximab dosing other than 5 mg/kg every 8 weeks at the time of TDM (OR 0.3; 95% CI 0.1–0.8; P = 0.021), and the use of concomitant immunomodulators (OR 0.3; 95% CI 0.1–0.9; P = 0.040) were identified as the only independent variables associated with biochemical remission (Table 2A). An incremental gain analysis is depicted in Fig. 3.
FIGURE 1.
Distribution of serum infliximab trough concentrations during maintenance therapy based on biochemical, endoscopic, or histologic remission in patients with Crohn’s disease. Box plots (5%–95%) show the median (solid line within box), interquartile range (upper and lower box boundaries), standard deviation (whiskers), and outliers (black dot). TC, trough concentation.
FIGURE 2.
Receiver-operator curve (ROC) analysis for infliximab serum trough concentrations during maintenance therapy stratifying patients with CD with and without biochemical (A), endoscopic (B), or histologic (C) remission. AUC, area under the curve.
Table 2:
Variables Associated (P < 0.1) with Biochemical (A), Endoscopic (B), and Histologic (C) Remission
| Univariable analysis | Multivariable analysis | ||||||
|---|---|---|---|---|---|---|---|
| Variables | OR | 95%CI | P | OR | 95%CI | P | |
| A. Biochemical remission | |||||||
| Infliximab dosing other than 5mg/Kg every 8 weeks at time of TDM | 0.3 | 0.1–0.8 | 0.020 | 0.3 | 0.1–0.8 | 0.021 | |
| Concomitant IMM | — | — | 0.098 | 0.3 | 0.1–0.9 | 0.040 | |
| Infliximab TC ≥2.2 μg/mL | 5.9 | 1.5–22.3 | 0.014 | 6.4 | 1.5–27.1 | 0.011 | |
| B. Endoscopic remission | |||||||
| Concomitant IMM | — | — | 0.065 | — | — | — | |
| Infliximab TC ≥ 9.7 μg/mL | 3.6 | 1.4–9 | 0.006 | 3.6 | 1.4–9 | 0.006 | |
| B. Histologic remission | |||||||
| Male | 0.4 | 0.2–0.9 | 0.032 | 0.4 | 0.2–0.9 | 0.035 | |
| Infliximab TC ≥ 9.8 μg/mL | 3.3 | 1.4–7.9 | 0.010 | 3.2 | 1.3–7.9 | 0.011 | |
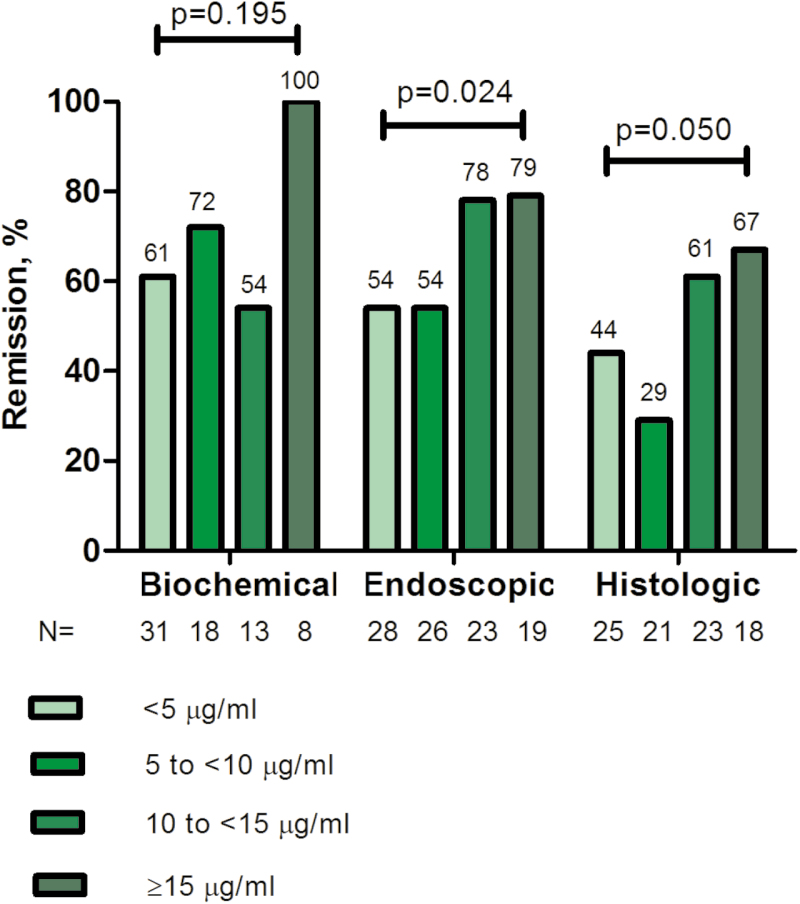
FIGURE 3.
Incremental gain in biochemical, endoscopic, and histologic remission rates in relation to serum infliximab trough concentrations during maintenance therapy in patients with CD. Increments of 5 μg/mL infliximab trough concentration were used to define the range of maximal increase in the rate of biochemical, endoscopic, or histological remission with any increase in drug concentration during maintenance therapy. P value: linear-by-linear association.
Endoscopic Remission and Infliximab Concentration
Endoscopic remission was observed in 62 of 96 (65%) of colonoscopies. Patients with endoscopic remission had statistically significantly higher infliximab concentration (median [IQR]) compared with those without (10 [5.1–15] vs 6.8 [1.9–11.8] μg/mL, P = 0.019) (Fig. 1). ROC analysis identified an infliximab concentration of ≥9.7 μg/mL to be associated with endoscopic remission (SN 57%, SP 73%, PPV 80%, NPV 48%) (Fig. 2B). Multivariate analysis identified infliximab concentration ≥9.7 μg/mL as the only independent variable associated with endoscopic remission (OR 3.6; 95% CI, 1.4–9; P = 0.006) (Table 2B). Based on incremental gain analysis, an infliximab concentration range of 10–15 μg/mL was related to an endoscopic remission rate >70% (Fig. 3).
Histologic Remission and Infliximab Concentration
Histologic remission was found in 43 of 87 (49%) of colonoscopies. Patients with histologic remission had numerically higher serum infliximab concentration (median [IQR]) compared with those without (10.4 [4.9–15.5] vs 8.5 [2.2–12.3] μg/mL, P = 0.065) (Fig. 1). Based on ROC analysis, an infliximab concentration ≥9.8 μg/mL was related to histologic remission (SN 63%, SP 66%, PPV 64%, NPV 64%) (Fig. 2C). In multivariate analysis, infliximab concentration ≥9.8 μg/mL (OR 3.2; 95% CI, 1.3–7.9; P = 0.011) and male sex (OR 0.4; 95% CI, 0.2–0.9); P = 0.035) were identified as the only variables independently associated with histologic remission (Table 2C). Based on incremental gain analysis, an infliximab concentration range of 10–15 μg/mL was shown to be associated with a histologic remission rate over 60% (Fig. 3).
DISCUSSION
This retrospective multicenter study demonstrated that higher maintenance serum infliximab trough concentrations were linked to increased rates of biochemical, endoscopic, and histologic remission in CD. Infliximab concentrations of ≥2.2, 9.7, and 9.8 μg/ml were independently associated with biochemical, endoscopic, and histologic remission, respectively, with higher concentrations related to endoscopic or histologic remission than with biochemical remission.
This is in line with prior studies demonstrating that there is a positive correlation between anti-TNF concentrations and favorable objective therapeutic outcomes in IBD6–17 and that drug levels may differ depending on the treatment goal.7, 9, 14, 20, 21 A recent study by Roblin et al showed that higher infliximab levels were related to CRP normalization (>2.9 μg/mL), fecal calprotectin (>3.9 μg/mL), or both (>4.9 μg/mL) in IBD.7 We have also recently demonstrated that infliximab concentrations of <1.85, 3.55, 4.65, and 6.35 μg/mL were independently associated with treatment failure, ATI, IBD-related hospitalization and serious infusion reaction, respectively, in patients with IBD.9
We identified that a serum infliximab trough concentration range of 10–15 μg/mL was associated with endoscopic or histologic remission in CD with rates of >70% or 60%, respectively. The finding that even higher infliximab concentrations are linked to more rigorous outcome measures is in line with the results of 2 other recent studies. Ungar et al reported that over 80% of patients with IBD can achieve mucosal healing when infliximab levels are higher than 6 μg/mL.11 Similarly, Yarur et al demonstarted that infliximab concentration ≥10.1 μg/mL is related with healing of fistulas in CD.12 Given these observations, it is entirely possible that higher concentrations of infliximab are required to achieve more rigorous outcomes, such as endoscopic or histologic healing. Conversely, it is also possible that only with a more healed mucosa can higher concentrations of infliximab be achieved, as infliximab may be cleared via a severely inflamed mucosa.22
Interestingly, we observed that an infliximab regimen different than 5 mg/kg every 8 weeks at the time of TDM and the use of concomitant immunomodulators were associated with a lack of endoscopic remission. We suspect that this may be due to the likelihood that these patients had a high disease burden with rapid clearance of infliximab. Similarly, we suspect that the same phenemonon occurred in male patients in this study, who were found to have lower rates of histologic remission and have previously been shown to have increased drug clearance.23
This study is limited by the retrospective design and the absence of central reading of colonoscopies and histology specimens. Nevertheless, the endoscopists who performed the examinations and pathologists who read the slides are all IBD specialists with years of experience. Moreover, 2 different assays were used (ELISA and HMSA), although no significant discrepancies have been noted in terms of infliximab concentrations between these 2 assays.24 Finally, based on our study, only association can be established and not causality.
To conclude, we showed that higher serum maintenance infliximab concentrations were associated with biochemical, endoscopic, and histologic remission in CD patients, while infliximab concentrations may differ based on the treatment goal. Large prospective studies to confirm these results are certainly warranted.
Glossary
Abbreviations:
- ATI
antibodies to infliximab
- AUC
area under curve
- BMI
body mass index
- CI
confidence interval
- CRP
C-reactive protein
- ELISA
enzyme-linked immunosorbent assay
- HMSA
homogeneous mobility shift assay
- IBD
inflammatory bowel disease
- IMM
immunomodulators
- IQR
interquartile range
- NPV
negative predictive value
- OR
odds ratio
- PPV
positive predictive value
- ROC
receiver-operating characteristic
- SN
sensitivity
- SP
specificity
- TDM
therapeutic drug monitoring
- TNF
antitumor necrosis factor
- UC
ulcerative colitis
- CD
Crohn’s disease
Conflicts of interest: ASC received consultancy fees from AbbVie, Janssen, Takeda, Ferring, Miraca, AMAG, Innovation Pharma, and Pfizer; MTO received consultancy fees from Janssen, AbbVie, UCB, Takeda, Pfizer, Merck, and Lycera and received research grant support from UCB. The remaining authors disclose no conflicts of interest.
Author contributions: KP conceived and designed the study, acquired data, analyzed and interpreted data, performed statistical analysis, and wrote the manuscript. SR acquired and interpreted data and critically revised the manuscript. CR acquired data and critically revised the manuscript. ASC and MTO conceived and designed the study, acquired and interpreted data, and critically revised the manuscript. All the authors approved of the final draft.
Supported by a Ruth L. Kirschstein NRSA Institutional Research Training Grant (5T32DK007760-18) given to KP. The content of this project is solely the responsibility of the authors and do not necessarily represent the official views of the NIH.
REFERENCES
- 1. Miligkos M, Papamichael K, Vande Casteele N, et al. Efficacy and safety profile of antitumor necrosis factor-α versus anti-integrin agents for the treatment of Crohn’s disease: a network meta-analysis of indirect comparisons. Clin Ther. 2016;38:1342–58.e6. [DOI] [PubMed] [Google Scholar]
- 2. Colombel JF, Narula N, Peyrin-Biroulet L. Management strategies to improve outcomes of patients with inflammatory bowel diseases. Gastroenterology. 2017;152:351–61.e5. [DOI] [PubMed] [Google Scholar]
- 3. Pineton de Chambrun G, Blanc P, Peyrin-Biroulet L. Current evidence supporting mucosal healing and deep remission as important treatment goals for inflammatory bowel disease. Expert Rev Gastroenterol Hepatol. 2016;10:915–27. [DOI] [PubMed] [Google Scholar]
- 4. Colombel JF, Panaccione R, Bossuyt P, et al. Effect of tight control management on Crohn’s disease (CALM): a multicentre, randomised, controlled phase 3 trial. Lancet. 2018;390:2779–89. [DOI] [PubMed] [Google Scholar]
- 5. Papamichael K, Cheifetz AS. Therapeutic drug monitoring in IBD: the new standard-of-care for anti-TNF therapy. Am J Gastroenterol. 2017;112:673–6. [DOI] [PubMed] [Google Scholar]
- 6. Kelly OB, Donnell SO, Stempak JM, et al. Therapeutic drug monitoring to guide infliximab dose adjustment is associated with better endoscopic outcomes than clinical decision making alone in active inflammatory bowel disease. Inflamm Bowel Dis. 2017;23:1202–9. [DOI] [PubMed] [Google Scholar]
- 7. Roblin X, Boschetti G, Duru G, et al. Distinct thresholds of infliximab trough level are associated with different therapeutic outcomes in patients with inflammatory bowel disease: a prospective observational study. Inflamm Bowel Dis. 2017;23:2048–53. [DOI] [PubMed] [Google Scholar]
- 8. Papamichael K, Cheifetz AS. Higher adalimumab drug levels are associated with mucosal healing in patients with Crohn’s disease. J Crohns Colitis. 2016;10:507–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Papamichael K, Chachu KA, Vajravelu RK, et al. Improved long-term outcomes of patients with inflammatory bowel disease receiving proactive compared with reactive monitoring of serum concentrations of infliximab. Clin Gastroenterol Hepatol. 2017;15:1580–8.e3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Cornillie F, Hanauer SB, Diamond RH, et al. Postinduction serum infliximab trough level and decrease of C-reactive protein level are associated with durable sustained response to infliximab: a retrospective analysis of the ACCENT I trial. Gut. 2014;63:1721–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Ungar B, Levy I, Yavne Y, et al. Optimizing anti-TNF-α therapy: serum levels of infliximab and adalimumab are associated with mucosal healing in patients with inflammatory bowel diseases. Clin Gastroenterol Hepatol. 2016;14:550–7.e2. [DOI] [PubMed] [Google Scholar]
- 12. Yarur AJ, Jain A, Hauenstein SI, et al. Higher adalimumab levels are associated with histologic and endoscopic remission in patients with Crohn’s disease and ulcerative colitis. Inflamm Bowel Dis. 2016;22:409–15. [DOI] [PubMed] [Google Scholar]
- 13. Papamichael K, Van Stappen T, Vande Casteele N, et al. Infliximab concentration thresholds during induction therapy are associated with short-term mucosal healing in patients with ulcerative colitis. Clin Gastroenterol Hepatol. 2016;14:543–9. [DOI] [PubMed] [Google Scholar]
- 14. Ward MG, Warner B, Unsworth N, et al. Infliximab and adalimumab drug levels in Crohn’s disease: contrasting associations with disease activity and influencing factors. Aliment Pharmacol Ther. 2017;46:150–61. [DOI] [PubMed] [Google Scholar]
- 15. Yarur AJ, Kanagala V, Stein DJ, et al. Higher infliximab trough levels are associated with perianal fistula healing in patients with Crohn’s disease. Aliment Pharmacol Ther. 2017;45:933–40. [DOI] [PubMed] [Google Scholar]
- 16. Peyrin-Biroulet L, Reinisch W, Colombel JF, et al. Clinical disease activity, C-reactive protein normalisation and mucosal healing in Crohn’s disease in the SONIC trial. Gut. 2014;63:88–95. [DOI] [PubMed] [Google Scholar]
- 17. Papamichael K, Cheifetz AS. Use of anti-TNF drug levels to optimise patient management. Frontline Gastroenterol. 2016;7:289–300. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Papamichael K, Casteele NV, Ferrante M, et al. Therapeutic drug monitoring during induction of antitumor necrosis factor therapy in inflammatory bowel disease: defining a therapeutic drug window. Inflamm Bowel Dis. 2017;23:1510–15. [DOI] [PubMed] [Google Scholar]
- 19. Wang SL, Ohrmund L, Hauenstein S, et al. Development and validation of a homogeneous mobility shift assay for the measurement of infliximab and antibodies-to-infliximab levels in patient serum. J Immunol Methods. 2012;382:177–88. [DOI] [PubMed] [Google Scholar]
- 20. Gonczi L, Vegh Z, Golovics PA, et al. Prediction of short- and medium-term efficacy of biosimilar infliximab therapy. Do trough levels and antidrug antibody levels or clinical and biochemical markers play the more important role?J Crohns Colitis. 2017;11:697–705. [DOI] [PubMed] [Google Scholar]
- 21. Imaeda H, Bamba S, Takahashi K, et al. Relationship between serum infliximab trough levels and endoscopic activities in patients with Crohn’s disease under scheduled maintenance treatment. J Gastroenterol. 2014;49:674–82. [DOI] [PubMed] [Google Scholar]
- 22. Brandse JF, van den Brink GR, Wildenberg ME, et al. Loss of infliximab into feces is associated with lack of response to therapy in patients with severe ulcerative colitis. Gastroenterology. 2015;149:350–5.e2. [DOI] [PubMed] [Google Scholar]
- 23. Ordás I, Feagan BG, Sandborn WJ. Therapeutic drug monitoring of tumor necrosis factor antagonists in inflammatory bowel disease. Clin Gastroenterol Hepatol. 2012;10:1079–87; quiz e85. [DOI] [PubMed] [Google Scholar]
- 24. Steenholdt C, Bendtzen K, Brynskov J, et al. Clinical implications of measuring drug and anti-drug antibodies by different assays when optimizing infliximab treatment failure in Crohn’s disease: post hoc analysis of a randomized controlled trial. Am J Gastroenterol. 2014;109:1055–64. [DOI] [PubMed] [Google Scholar]