Abstract
Probiotics are known as “live microorganisms” and have been proven to have a health effect on hosts at the proper dose. Recently, a kind of probiotic mixture including eight live bacterial strains, VSL#3, has attracted considerable attention for its combined effect. VSL#3 is the only probiotic considered as a kind of medical food; it mainly participates in the regulation of the intestinal barrier function, including improving tight junction protein function, balancing intestinal microbial composition, regulating immune-related cytokine expression and so on. The objective of this review is to discuss the treatment action and mechanism for the administration of VSL#3 in chronic diseases of animals and humans (including children). We found that VSL#3 has a therapeutic or preventive effect in various systemic diseases per a large number of studies, including digestive systemic diseases (gastrointestinal diseases and hepatic diseases), obesity and diabetes, allergic diseases, nervous systemic diseases, atherosclerosis, bone diseases, and female reproductive systemic diseases.
Keywords: VSL#3, Intestinal barrier function, Chronic diseases, Intestinal microbial balance, Cytokines, Therapeutic use
Core tip: The imbalance of intestinal microbiota is one of the important factors in multiple diseases. Probiotics have a benefit on human health as live microorganisms that can positively regulate the intestinal microbial composition. One probiotic mixture consisting of eight live bacterial strains, VSL#3, plays an essential function in preventing and treating digestive systemic and other systemic diseases in animals and humans. There is increasing evidence that VSL#3 works by modulating intestinal barrier function. It is able to improve tight junction protein function and the composition of intestinal microbiota and regulate immune-related cytokine expression. This review seeks to provide an overview of the role of VSL#3 in various kinds of diseases and its potential for clinical use in the future.
INTRODUCTION
The internationally endorsed definition of probiotics is live microorganisms which, when administered in adequate amounts, confer a health benefit to the host[1]. VSL#3 is a commercial probiotic mixture consisting of eight bacterial strains: Four strains of Lactobacillus (Lactobacillus acidophilus, Lactobacillus plantarum, Lactobacillus casei, and Lactobacillus delbrueckii subspecies bulgaricus), three strains of Bifidobacterium (Bifidobacterium breve, Bifidobacterium longum, and Bifidobacterium infantis), and one strain of Streptococcus (Streptococcus salivarius subspecies thermophilus). Different kinds of bacterial strains exert different effects. The gene clusters of S. thermophilus are forecasted to code most of the defense systems. The gene clusters of Bifidobacterium are forecasted to code tight adherence pili in order to promote intestinal barrier integrity, and the genomes of Lactobacillus are predicted to encode signaling proteins[2]. VSL#3 has a protective effect on intestinal barrier function (IBF), which is one of the important functions for treating multiple chronic diseases. This article provides insight into the physiological characteristics of VSL#3 and its involvement in the treatment of chronic diseases. Furthermore, we review the results from a large number of basic and clinical studies about digestive systemic diseases and the use of VSL#3 for other systemic diseases (Figure 1), which are indicative of future directions for VSL#3-based therapy. VSL#3 is a kind of formula probiotic with sufficient evidence-based medical evidence in some digestive systemic diseases, but evidence is insufficient in many other systemic diseases. We need to observe whether VSL#3 is effective in these diseases in the future.
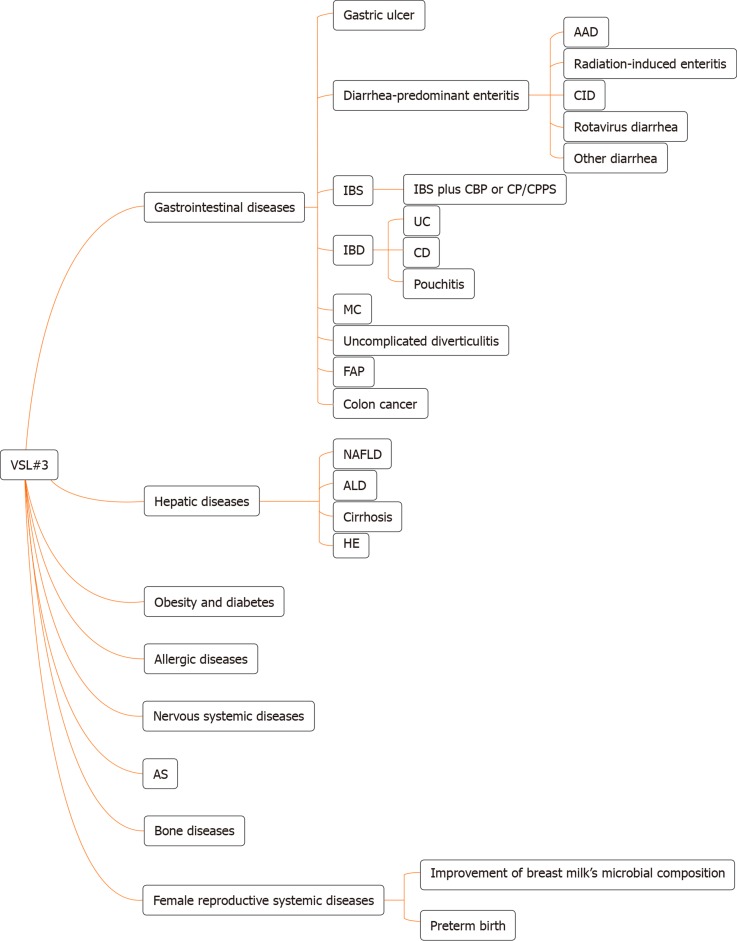
Figure 1.
The types of disease for which VSL#3 can work. AAD: Antibiotic-associated diarrhea; CID: Chemotherapy-induced diarrhea; IBS: Irritable bowel syndrome; CBP: Chronic bacterial prostatitis; CP: Chronic prostatitis; CPPS: Chronic pelvic pain syndrome; IBD: Inflammatory bowel disease; UC: Ulcerative colitis; CD: Crohn’s disease; MC: Microscopic colitis; FAP: Familial adenomatous polyposis; NAFLD: Non-alcoholic fatty liver disease; ALD: Alcoholic liver disease; HE: Hepatic encephalopathy; AS: Atherosclerosis.
EFFECTS OF VSL#3 ON IBF
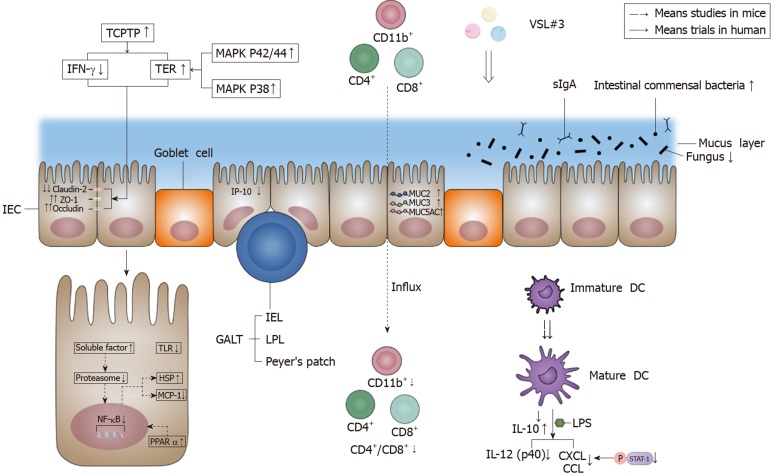
Several factors have been identified to be responsible for IBF: The mechanical barrier, biological barrier, chemical barrier, and immune barrier. The effects of VSL#3 on IBF are shown in Figure 2.
Figure 2.
Effects of VSL#3 on intestinal barrier function. VSL#3 acts on the four components of the intestinal barrier: The mechanical barrier, biological barrier, chemical barrier, and immune barrier. In terms of the mechanical barrier, VSL#3 can increase occludin and zonula occludens-1 and decrease claudin-2 in order to improve tight junction protein function, and the effect is achieved by increasing the activity of T-cell protein tyrosine phosphatase, which is able to decrease T-cell protein tyrosine phosphatase-dependent interferon-γ signaling and increase transepithelial electrical resistance[5-7]. VSL#3 can increase transepithelial electrical resistance by activating the mitogen-activated protein kinase p42/44 and p38 pathway[9]. In terms of the biological barrier, VSL#3 can increase the amount of intestinal commensal bacteria and decrease the amount of fungi[12]. In terms of the chemical barrier, VSL#3 can increase MUC2, MUC3 and MUC5AC gene expression to regulate mucus secretion[9]. In terms of the immune barrier, VSL#3 can inhibit the proinflammatory nuclear factor-κB (NF-κB) pathway, such as inducing heat shock protein (HSP) and reducing monocyte chemoattractant protein-1 (MCP-1). The action mechanism is via the early inhibition of proteasome by producing soluble factors[21]. VSL#3 also up-regulates the peroxisome proliferator-activated receptor α (PPARα) signaling pathway to antagonize the NF-κB pathway[32]. An appropriate dose of VSL#3 can induce the maturation of dendrite cells (DC)[27,28], and VSL#3 can inhibit interferon-inducible protein-10 (IP-10) in intestinal epithelial cells (IEC)[22-24] and the lipopolysaccharide (LPS)-induced expression of chemokines (CXCL9, CXCL10, CCL2, CCL7, and CCL8) by inhibiting STAT-1 phosphorylation[27]. VSL#3 is also able to decrease interleukin (IL)-12 (p40) production induced by LPS[30]. Moreover, VSL#3 can induce IL-10 produced by DC and decrease the influx of innate immune cells (CD11b+) and adaptive immune cells (CD4+/CD8+)[30,31]. The down-regulation of the signaling pathway of Toll-like receptors (TLR) by VSL#3 also has benefits for the intestinal immune barrier[32]. IEC: Intestinal epithelial cells; ZO-1: Zonula occludens-1; TCPTP: T-cell protein tyrosine phosphatase; IFN-γ: Interferon-γ; TER: Transepithelial electrical resistance; MAPK: Mitogen-activated protein kinase; GALT: Gut-associated lymphoid tissue; IEL: Intraepithelial lymphocytes; LPL: Lamina propria lymphocytes; sIgA: secreted immunoglobulin A; NF-κB: Nuclear factor-κB; IL: Interleukin; MCP-1: Monocyte chemoattractant protein-1; HSP: Heat shock protein; PPARα: Peroxisome proliferator-activated receptor α; IP-10: Interferon-inducible protein-10; DC: Dendrite cells; LPS: Lipopolysaccharide; TLR: Toll-like receptors.
Effects of VSL#3 on mechanical barrier function
The mechanical barrier is mainly comprised of intestinal epithelial cells (IEC) and the protein networks between IEC, including desmosomes, adherent junctions, and tight junctions. Tight junctions maintain epithelial polarity and regulate selective paracellular ion solute transport[3,4]. Among all the components in the intestinal epithelium, VSL#3 mainly affects tight junctions. VSL#3 could lead to a recovery of tight junction protein injury with significantly increased occludin and zonula occludens-1 (ZO-1) and significantly decreased claudin-2 in the intestinal mucosa[5-7]. The influence of VSL#3 on tight junction proteins is achieved by increasing the protein level and enzymatic activity of T-cell protein tyrosine phosphatase (TCPTP), which is the protein product of the tyrosine-protein phosphatase non-receptor type 2 (PTPN2) gene, and PTPN2 has a protective effect against inflammatory bowel disease (IBD)[5,8]. Then, VSL#3 mediates its beneficial effect by reducing interferon-γ (IFN-γ) signaling in a TCPTP-dependent manner and correcting the reduction in transepithelial electrical resistance (TER)[5]. VSL#3 probably increased TER by activating the mitogen-activated protein kinase p42/44 and p38 pathway in T84 cells[9]. Besides, the increase of phosphorylated-p38 (P-p38) and phosphorylated-extracellular signal-regulated kinase (P-ERK) signaling pathways by VSL#3 is also important in improving tight junction protein expression and IBF in rats[7].
Effects of VSL#3 on biological barrier function
The biological barrier is formed by the resident intestinal microbiota co-inhabiting the intestinal cavity or colonizing the surface of the intestinal mucosa[3]. Most of the intestinal bacteria groups are members of Bacteroidetes and Firmicutes phyla[10]. Moreover, large numbers of anaerobes grow on the surface of the intestinal mucosa, such as Bifidobacteria, which can resist the invasion of foreign pathogens[3]. The production of short-chain fatty acids (SCFAs) by gut microbial fermentation reduces intestinal pH and plays an important role in maintaining intestinal biological barrier function[3,11]. VSL#3 has been reported to increase the richness and diversity of the bacteria in the intestinal mucosa, mainly by increasing the total concentration of anaerobes (including Lactobacillus and Bifidobacteria) and reducing fungal diversity. However, the alteration in bacterial diversity was not caused by the colonization of bacterial strains in VSL#3 and had an independent effect[12]. The change of Bifidobacterium concentration was particularly obvious in individuals with a low endogenous bifidobacterial concentration, but no obvious changes were shown in individuals with a high endogenous bifidobacterial concentration[13]. In addition, the fecal concentrations of Enterococci, Clostridia, Bacteroides, and Coliforms were not altered significantly, which indicated that the beneficial effect of VSL#3 was not mediated by inhibiting the endogenous microbiota[14]. The positive effects of VSL#3 treatment were also mediated by SCFA acetate. Acetate significantly reduced colonic permeability by reducing colonic inflammation and promoting growth of the commensal Lactobacillus[11].
Effects of VSL#3 on chemical barrier function
The chemical barrier includes bile, digestive fluid, lysozymes, mucopolysaccharides, other chemical substances, and antibacterial substances secreted by the intestinal tract[3]. Mucus secreted by goblet cells in the intestinal epithelium forms a first-line protective mucus layer which is able to prevent foreign pathogens from penetrating the barrier into deep tissue[15]. VSL#3 was able to increase basal luminal mucin content by 60% in Wistar rats, and it was suggested that VSL#3 regulated mucin gene expression and mucus secretion by increasing MUC2, MUC3, and MUC5AC gene expression in IEC. The changes in MUC2 (3.5 fold) and MUC5AC (4.5 fold) were more prominent than MUC3 (three-fold)[9,16]. Besides, VSL#3 was reported to induce ileal bile acid deconjugation and fecal bile acid secretion. It exerted this effect by inhibiting the intestine-liver farnesoid X receptor-fibroblast growth factor 15 (FXR-FGF15) axis, which resulted in increased cholesterol-7α-hydroxylase (Cyp7a1) and sterol-12α-hydroxylase (Cyp8b1) gene expression. Besides, fecal bile acid secretion was associated with increased bile salt hydrolase (BSH) transcription and enzymatic activity in VSL#3-treated mice[17].
Effects of VSL#3 on immune barrier function
The immune barrier comprises gut-associated lymphoid tissue (GALT) and disseminated immune cells, which are the effector sites of intestinal mucosal immunity[3]. GALT includes intraepithelial lymphocytes (IEL), lamina propria lymphocytes (LPL), and Peyer’s patches. Most IEL and LPL are CD103+ and CD3+ T cells, but the number of CD20+ B cells is significantly small[18]. Furthermore, secreted immunoglobulin A (sIgA) is the main humoral immune component, and it is the first line of defense to prevent intestinal mucosal adhesion and colonization[3]. It has been shown that VSL#3 could normalize intestine epithelial barrier integrity and dampen inflammatory responses partly by decreasing the mucosal secretion of the proinflammatory cytokines tumor necrosis factor-α (TNF-α) and IFN-γ in interleukin (IL)-10 gene-deficient mice[19,20]. Similarly, VSL#3 exerted its anti-inflammatory cell protective effect by inhibiting proinflammatory nuclear factor-κB (NF-κB) pathway activation. For instance, VSL#3 decreased NF-κB stimulated endogenous immune response gene monocyte chemoattractant protein-1 (MCP-1), inhibited the degradation of NF-κB inhibitor IκB, and induced heat shock protein (HSP) at the transcriptional level in murine colonic epithelial cells. The mechanism of action was the early inhibition of the chymotrypsin-like activity of the proteasome via soluble factors produced by VSL#3[21]. Except for the pro-inflammatory cytokine pathway, the inhibition of proinflammatory chemokines in IEC is also important in maintaining intestinal immune barrier function. Studies demonstrated that Lactobacillus contained in VSL#3 induced the inhibition of proinflammatory T-cell chemokine interferon-inducible protein-10 (IP-10) secretion in IEC, probably associated with inducing IP-10 ubiquitination, secreting prtP-encoded lactocepin (protective bacterial protease), and destroying the vesicular pathway[22-24]. VSL#3 DNA was shown to inhibit epithelial IL-8 secretion in response to Salmonella DNA and proinflammatory stimuli (such as TNF-α)[20].
However, one study showed an opposite mechanism of VSL#3. It demonstrated that a high dose of VSL#3 (5 × 1010 CFU/d) locally stimulated epithelial innate immunity by increasing the production of TNF-α and activating the NF-κB pathway to restore IBF. The stimulation effect required the temporary colonization of VSL#3 in the intestinal lumen[25]. On the other hand, another study also indicated that VSL#3 exerted its beneficial effect by producing the proinflammatory cytokines IL-6 and IL-12 (p40) in bone marrow-derived macrophages isolated from Balb/c mice[26].
Dendrite cells (DC) are the key cells in the production of immune responses and the modulation of inflammatory signaling pathways. The stimulation of human DC with an appropriate dose of VSL#3 will induce the maturation of immature DC[27,28]. However, VSL#3 (105 organisms/mL) did not change the immature phenotype and costimulatory molecule expression of DC, and VSL#3 (107 organisms/mL) was shown to increase the expression of CD40, CD80, CD86, and major histocompatibility complex (MHC) class II I-Ad during the last 3 d of culture in bone marrow-derived dendritic cells[29]. The effects of VSL#3 on DC maturation were dose- and time-dependent, and reached the maximum at the 107 dose after 18 h of co-culture[28]. Besides, VSL#3 has been shown to decrease the expression of CD80 and increase the expression of CD83, but it had no effect on CD86[30]. In terms of cytokine expression, VSL#3 inhibited the lipopolysaccharide (LPS)-induced expression of chemokines (CXCL9, CXCL10, CCL2, CCL7, and CCL8) mainly by inhibiting STAT-1 phosphorylation under the stimulation of human monocyte-derived DC with LPS and VSL#3[27]. VSL#3 also decreased IL-12 (p40) production induced by LPS, and was a potent inducer of IL-10 produced by DC from blood and intestinal tissue[30]. Additionally, one study showed that VSL#3 had an anti-inflammatory effect by decreasing the influx of innate immune cells (CD11b+) and adaptive immune cells (CD4+/CD8+) in the intestinal mucosa and reduced proinflammatory serum cytokines (IL-17, IL-1α, IL-1β, and granulocyte-macrophage colony-stimulating factor [GM-CSF])[31]. Then, a study showed that VSL#3 down-regulated the signaling pathway of Toll-like receptors (TLR), mainly TLR4, and the related effector pathways such as T-cell and B-cell receptor signaling in IL-10 knockout (KO) mice. Meanwhile, VSL#3 up-regulated the peroxisome proliferator-activated receptor α (PPARα) signaling pathway and lipid signaling genes, which had a potent antagonistic action on the NF-κB inflammatory process[32].
EFFECTS OF VSL#3 ON GASTROINTESTINAL DISEASES
Gastrointestinal diseases are a worldwide problem, and the imbalance of intestinal microbiota is one of the important factors in multiple gastrointestinal diseases. Thus, probiotics play a significant role in these diseases by modulating intestinal microbiota. Studies have indicated that VSL#3 provides benefits in gastric ulcer, diarrhea-predominant enteritis, irritable bowel syndrome (IBS), ulcerative colitis (UC), pouchitis, and colitis in animals and humans.
Gastric ulcer
Gastric ulcer healing is a complex process, involving the filling of mucosal defects through the proliferation and migration of epithelial cells and connective tissue cells to restore the mucosal architecture[33]. A study demonstrated that VSL#3 is effective at high concentrations (1.2 × 1010 bacteria) in enhancing acetic acid-induced gastric ulcer healing by stimulating the expression and production of angiogenesis promoting growth factors, mainly vascular endothelial growth factor, in rats[34]. However, there are no related trials in humans.
Diarrhea-predominant enteritis
The occurrence of diarrhea is closely related to the change of gastrointestinal (GI) motility as a result of enteritis. Different bacterial components in VSL#3 showed different effects on GI motility in guinea-pig isolated tissue. Lactobacillus in VSL#3 stimulated the contraction of ileum segment. All bacteria and non-protein cytoplasm components (not DNA) of VSL#3 were able to induce reverse proximal colon relaxation and then decrease fecal frequency to improve enteritis[35]. Table 1 shows the clinical and patient-reported characteristics of trials in various kinds of diarrhea-predominant enteritis.
Table 1.
Trials assessing the effect of VSL#3 in patients with diarrhea-predominant enteritis
| Ref. | Design | n | VSL#3 intake |
| Delia et al[40], 2002 | Not mentioned | 190 patients receiving radiotherapy on the pelvic area (including 95 who received radiotherapy alone and 95 treated with VSL#3) | 6 to 7 wk with one bag three times/d |
| Delia et al[39], 2007 | RCT | 490 with postoperative radiation therapy | 1 sachet (4.5 × 1011 viable lyophilized bacteria/g) three times a day starting from the first day of radiation therapy until the end of radiation therapy |
| Dubey et al[44], 2008 | RCT | 230 rotavirus-positive acute diarrhea children; 224 children completed the study (113 in the VSL#3 group and 111 in the placebo group) | 4 d, < 5 kg children, 2 sachets a day; 5 to 10 kg children, 4 sachets a day; each sachet of VSL#3 contained 9 × 1010 bacteria |
| Frohmader et al[46], 2010 | RCT | 45 who needed enteral nutrition for more than 3 d (including 20 intervention and 25 control) | Not clear |
| Selinger et al[37], 2013 | RCT | 229 exposed to systemic antibiotics (including 117 with VSL#3 and 112 with placebo) | 1 sachet (4.5 × 1011 live bacteria per sachet) twice a day during the antibiotic course and for a further 7 d |
| Lacouture et al[47], 2016 | Randomized, multicenter, triple cohort, phase II trial | Patients with advanced NSCLC treated with dacomitinib were enrolled in two cohorts (cohort I: 114 patients, including 56 in the doxycycline group, 58 in the placebo group; cohort II: 59 patients with VSL#3 plus topical alclometasone) | 5 wk with 4 capsules once daily (patients in the United States) or 1 sachet daily (patients in South Korea) in cohort II |
RCT: Randomized controlled trial; NSCLC: Non-small-cell lung cancer.
Antibiotic-associated diarrhea: Diarrhea is a common adverse event of antibiotic medication. The occurrence of diarrhea is probably due to disruption of the normal gastrointestinal microbiota. The effect of probiotics deserves discussion[36]. Clostridium difficile-associated diarrhea (CDAD) is the most serious form of antibiotic-associated diarrhea (AAD). A trial suggested that VSL#3 could prevent the occurrence of AAD significantly in patients exposed to systemic antibiotics in an average-risk hospital. A shorter hospital stay was related to the reduction of AAD incidence, but it was not significant[37]. The effect of VSL#3 on CDAD remained unknown because no CDAD cases were included in this trial.
Radiation-induced enteritis: Enteritis and colitis characterized by diarrhea are severe complications of radiotherapy that may lead to the development of bacterial overgrowth in patients who have cancer[38]. Two trials have addressed the utility of adding VSL#3 to radiation-induced enteritis therapy. They demonstrated that VSL#3 was a great approach to prevent the occurrence and reduce the severity of radiation-induced enteritis with good tolerance[39,40]. One trial showed that more patients treated with placebo had radiation-induced enteritis (51.8% vs 31.6%) and suffered grade 3 or 4 diarrhea (55.4% and 1.4%) compared with patients treated with VSL#3. The up-regulation of the innate immune reaction against invasive microbiota is probably one of the important mechanisms underlying the therapeutic effect of VSL#3 in patients with radiation-induced enteritis[39]. Recommendations for probiotic use from the Yale/Harvard workshop showed that the recommended level for VSL#3 use in radiation-induced enteritis was a C rating[41].
Chemotherapy-induced diarrhea: Chemotherapy can cause diarrhea, which worsens the quality of life of cancer patients. An animal study demonstrated that VSL#3 with irinotecan was able to prevent severe diarrhea following chemotherapy and weight loss in female rats[42]. However, it is still uncertain whether VSL#3 is effective in clinical application.
Rotavirus diarrhea: Rotavirus is one of the main causes of severe gastroenteritis in children[43]. It is transmitted directly through the fecal-oral route, and the main symptom of rotavirus infection is diarrhea, which is usually accompanied by abdominal pain, vomiting, and fever. One randomized controlled trial (RCT) indicated that the administration of VSL#3 resulted in better overall recovery rates and reduced frequency of oral rehydration salts and intravenous fluid use in children with acute rotavirus diarrhea[44].
Other diarrhea: Gastrointestinal complications are common complications in enterally-fed critically ill patients. They will prolong hospitalization and increase the mortality of patients[45]. A pilot trial indicated that VSL#3 had benefits in reducing the frequency of liquid and loose stool and stool weight, further minimizing diarrhea in enterally-fed critically ill patients[46].
A randomized phase II trial demonstrated that VSL#3 had no effect on the incidence of all-grade diarrhea adverse events and the quality of life of advanced non-small-cell lung cancer patients treated with dacomitinib, which is a tyrosine kinase inhibitor[47,48]. An improvement may have been induced by VSL#3 in tissue functions, but this requires confirmation.
IBS
IBS is a common chronic gastrointestinal functional disease characterized by sudden changes of two major symptoms: Constipation and diarrhea. The pathogenesis of IBS remains unclear, but an improvement of colon dysmotility and visceral hypersensitivity may play an important role in treating it[49]. One trial published the results of 25 patients with diarrhea-predominant IBS who consumed VSL#3 or placebo for 8 wk. It demonstrated that VSL#3 might relieve abdominal bloating, especially in patients with higher bloating scores, but VSL#3 had no significant effect on colonic transit and bowel function[50]. Another study showed that VSL#3 was able to dampen clinical symptoms by improving the mechanical distensions of the colonic wall in patients with diarrhea-predominant IBS[49]. In terms of bloating-predominant IBS, VSL#3 resulted in a reduction of flatulence scores during the first 4 wk (VSL#3 30.8 ± 2.5 vs placebo 40.1 ± 2.5) and retard of colonic transit compared with placebo, but no significant alteration in other individual symptoms was observed in patients administered with VSL#3[51]. In addition, another trial suggested that VSL#3 was able to improve IBS symptoms significantly by increasing salivary morning melatonin, particularly in males and individuals with normal circadian rhythm[52]. Furthermore, VSL#3 was superior to placebo in improving symptoms including abdominal pain and abdominal bloating in children with IBS[53]. But the mechanism has not been clearly studied. The recommended level for VSL#3 use in IBS was a B rating[41].
In a rat model of IBS, one study demonstrated that VSL#3 could reverse visceral hyperalgesia and allodynia and reset gene expression related to pain and inflammation (such as tryptophan hydroxylase 1 [TPH1])[54]. Also, VSL#3 treatment decreased visceral hypersensitivity probably by decreasing the number of mast cells in the colon and decreasing protease-activated receptor 2 and transient receptor potential vanilloid type 1 expression in dorsal root ganglia in an experimental rat model of IBS[55]. Furthermore, nitric oxide (NO) might participate in the beneficial effect of VSL#3 to some extent[56].
IBS plus chronic bacterial prostatitis or chronic prostatitis/chronic pelvic pain syndrome: In general, IBS has a large number of complications including gastrointestinal and non-gastrointestinal complications such as chronic pelvic pain[57]. Moreover, patients with IBS and chronic bacterial prostatitis (CBP) had a significantly greater number of male accessory gland infections compared with patients with CBP alone[58]. Two trials considered the use of rifaximin followed by VSL#3 in patients with IBS and CBP or chronic prostatitis/chronic pelvic pain syndrome (CP/CPPS)[59,60]. The first trial showed that the therapy may improve urinary and gastrointestinal symptoms with significantly reducing total National Institute of Health Chronic Prostatitis Symptom Index scores in patients with diarrhea-predominant IBS plus CP/CPPS[59]. Besides, the second trial demonstrated that long-term treatment with VSL#3 and rifaximin was effective in reducing the evolution of chronic prostatitis into more complicated infections of male accessory glands, such as chronic microbial prostate-vesiculitis or prostate-vesiculo-epididymitis (PVE), in infertile patients with IBS plus cured CBP[60]. All the information for the human trials above is shown in Table 2.
Table 2.
Trials assessing the effect of VSL#3 in patients with irritable bowel syndrome
| Ref. | Design | n | VSL#3 intake |
| Bazzocchi et al[49], 2002 | Open non-controlled trial | 42 with diarrhea-predominant IBS | 3 g (3 × 1011 cells/g), administered to each patient in the morning, in fasting conditions, for 20 d |
| Kim et al[50], 2003 | RCT | 25 with diarrhea-predominant IBS (including 13 with placebo and 12 with VSL#3) | 8 wk with 4.5 × 1011 lyophilized bacteria a day |
| Kim et al[51], 2005 | RCT | 48 with IBS and significant bloating (including 24 with placebo and 24 with VSL# 3) | Twice a day (31 patients received 4 wk and 17 patients received 8 wk), the daily dose was not clear |
| Guandalini et al[53], 2010 | RCT | 59 children completed the study | 6 wk with 1 sachet of VSL#3 (once a day for children 4 to 11 yr old; twice a day for those 12 to 18 yr old) |
| Vicari et al[60], 2014 | Not clear | 106 infertile male patients with CBP and IBS; 95 completed | 6 to 12 mo with 4.5 × 1011 CFU a day following rifaximin |
| Wong et al[52], 2015 | RCT | 42 with IBS (including 20 with VSL#3 and 22 with placebo) | 6 wk with 4 capsules (each capsule contained 1.125 × 1011 viable lyophilized bacteria) twice a day |
| Vicari et al[59], 2017 | Not mentioned | 85 patients with CP/CPPS (45 with subtype IIIa and 40 with subtype IIIb) plus diarrhea-predominant IBS and 75 patients with diarrhea-predominant IBS alone | 4.5 × 1011 CFU a day following rifaximin, the number of days was not clear |
IBS: Irritable bowel syndrome; CFU: Colony-forming unit; RCT: Randomized controlled trial; CBP: Chronic bacterial prostatitis; CP: Chronic prostatitis; CPPS: Chronic pelvic pain syndrome.
IBD
IBD consists of UC, Crohn’s disease (CD), and a kind of undefined enteritis. The etiology and pathogenesis of IBD are not quite clear at present. It is currently thought that possible factors include environmental factors, genetic susceptibility, infection factors, and a dysregulated immune system. An improper immune response to the intestinal microbiota probably contributes to the occurrence of IBD in genetically susceptible hosts. Dietary factors among environmental factors might influence immune function and could also be some of the risk factors for the occurrence of IBD[61,62]. One study indicated that milk and fried food were associated with an increased risk of IBD[63]. The treatment of IBD may be associated with multiple mechanisms, such as by altering the intestinal microbial composition to improve IBF.
UC: UC is a kind of chronic inflammatory colonic disease. In a systemic review, Eubacterium rectale and Akkermansia were decreased in all three studies, and Escherichia coli (E. coli) was increased in four of nine studies for patients with UC[64]. Traditionally, UC can be treated or maintained with immunomodulatory or anti-inflammatory drugs, such as 5-aminosalicylic acid (5-ASA)[65]. Also, VSL#3 has been reported to have a maintenance effect. One trial enrolled 20 patients with UC in remission who were treated with VSL#3 and identified that 15 patients were still in remission. The underlying mechanism is not completely understood, but it was shown that VSL#3 was able to induce a significant increase of protective bacterial strains (Streptococcus salivarius subspecies thermophilus, Lactobacillus, and Bifidobacteria) compared to their basal levels in feces of patients. Furthermore, VSL#3 could colonize the intestine and decrease fecal pH from the 20th day of treatment period and keep it stable[66].
Moreover, there are several trials (Table 3) demonstrating the therapeutic action of VSL#3 in adults with mild-to-moderate UC. In two trials, VSL#3 was both capable of reducing the UC disease activity index (DAI) scores and ameliorating clinical symptoms significantly compared with placebo[67,68]. One trial indicated that there were 42.9% of patients given VSL#3 who achieved remission and only 15.7% of patients given placebo who achieved remission[68]. Besides, VSL#3 and the traditional drugs seem to have a synergistic action. The mechanism is unclear, but it was possible that VSL#3 could increase the anti-inflammatory action of 5-ASA, which could reduce free radical production and then leukotriene and IL-1 production[67,69]. One trial also demonstrated that the combination therapy of low-dose balsalazide and VSL#3 was more effective than balsalazide or mesalazine alone in obtaining the remission of acute mild-to-moderate UC[70]. A longer treatment with VSL#3 may provide more of an improvement. It was shown that VSL#3 could improve more parameters (such as stool frequency) significantly in a 12-wk trial than in an 8-wk trial[67,68]. In addition, an open-label trial indicated that treatment with VSL#3 would induce remission (DAI ≤ 2) and response (decrease in DAI ≥ 3, but final score ≥ 3) in 77% of patients with active mild-to-moderate UC. Two bacterial components (S. salivarius subspecies thermophilus and B. infantis) in VSL#3 played an important role in inducing the remission by reaching the diseased bowel site[71]. Then, the effect of alkaline sphingomyelinase was investigated in 15 UC patients treated with VSL#3 for 5 wk. The results clarified that VSL#3 up-regulated mucosal alkaline sphingomyelinase activity and improved UC[72].
Table 3.
Trials assessing the effect of VSL#3 in patients with ulcerative colitis
| Ref. | Design | n | VSL#3 intake |
| Venturi et al[66], 1999 | Not mentioned | 20 with UC who were intolerant or allergic to 5-ASA | 12 mo with 3 g, 5 × 1011 cells/g, twice a day |
| Tursi et al[70], 2004 | Multicenter, randomized trial | 90 with newly diagnosed or recently relapsed mild-to-moderate UC | 8 wk with 3 g a day, 1 g bags contained 3 × 1011 viable lyophilized bacteria |
| Bibiloni et al[71], 2005 | Open-label trial | 34 ambulatory patients with active UC; 32 patients completed | 6 wk with 3.6 × 1012 bacteria a day in two divided doses |
| Soo et al[72], 2008 | Not mentioned | 15 patients with UC | 5 wk with 1 sachet (containing 9 × 1011 lyophilized bacteria) twice a day |
| Sood et al[68], 2009 | RCT | 147 with active mild-to-moderate UC (including 77 with VSL#3 and 70 with placebo) | 12 wk with 3.6 × 1012 CFU twice a day |
| Huynh et al[74], 2009 | Open-label study | 18 patients between ages of 3 and 17 with mild-to-moderate acute UC | One dose of VSL#3 sachet containing 4.5 × 1011 viable lyophilized bacteria; patients were treated twice daily for 8 wk with a dose of VSL#3 based on their age, range: one-half sachet to two and one-half sachets |
| Miele et al[73], 2009 | RCT | 29 consecutive patients (mean age: 9.8 yr; range: 1.7 to 16.1 yr) with newly diagnosed UC (VSL#3 group: n = 14; placebo group: n = 15) | Weight-based dose, range: 4.5 × 1011 to 1.8 × 1012 bacteria a day, the treatment time was not clear |
| Tursi et al[67], 2010 | RCT | 144 with relapsing UC (including 71 with VSL#3 and 73 with placebo); 65 patients with VSL#3 and 66 with placebo completed | 8 wk with 3.6 × 1012 CFU a day |
UC: Ulcerative colitis; CFU: Colony-forming unit; RCT: Randomized controlled trial; 5-ASA: 5-aminosalicylic acid.
VSL#3 also has a beneficial effect in children with UC. One RCT indicated that VSL#3 was effective in the maintenance of remission and reduction of recurrence in children with active UC[73]. Except for the role in maintaining remission, another pilot study demonstrated that therapy with VSL#3 resulted in a remission of the disease in children with mild-to-moderate acute UC[74]. In addition, the recommended level for VSL#3 use in maintaining the remission of UC was an A rating and the recommended level in inducing remission was a B rating[41].
Several studies have provided evidence about the effect of VSL#3 in dextran sulphate sodium (DSS)-induced colitis. One study demonstrated that VSL#3 (0.5 mL/d) decreased intestinal bacteria diversity related to tissue injury. It also locally produced conjugated linoleic acid which targets the myeloid cell peroxisome proliferator-activated receptor γ (PPARγ) in the colon to inhibit colitis[75]. Besides, one placebo-controlled study showed an obvious decrease in inflammation and a reduction of epithelial permeability upon administration of high-dose VSL#3 (15 mg/d for 7 d) to patients[76]. However, one study showed that VSL#3 (0.2 mL/d, containing 4 × 109 CFU of freeze-dried VSL#3 for 14 d) was not able to heal DSS-induced murine colitis or reinforce the mucus barrier. It revealed that VSL#3 had a tendency to decrease histological inflammation but not change colon inflammatory characteristics. Furthermore, the increase of Bifidobacteria was not enough to repair the intestinal barrier impairment[77]. The above results are not exactly consistent, and the negative result may be related to the sex of mice and the induced pattern of DSS colitis. The dose, mode of administration, and treatment course of VSL#3 also impact the result to some extent. In addition, the anti-inflammatory effect is still the main therapeutic mechanism of VSL#3 in DSS-induced rat colitis. Both heat-killed and live VSL#3 could reduce DSS-induced rat colitis by reducing IL-23, IL-6, STAT3 and phosphorylated-STAT3 (P-STAT3) expression in colonic tissue[78,79]. The expression of inflammatory-related mediators (inducible nitric oxide synthase [iNOS], cyclooxygenase-2 [COX-2], and NF-κB) was also inhibited by VSL#3[80]. In DSS-induced murine colitis, VSL#3 was shown to reduce the production of proinflammatory chemokine KC and macrophage inflammatory protein-2 (MIP-2) and up-regulate transforming growth factor-β (TGF-β), fibroblast growth factor-1, and vascular endothelial growth factor-A (VEGF-A) in Muc2-deficient mice[11]. Furthermore, VSL#3 improved various parameters of DSS-induced colitis (such as myeloperoxidase [MPO]) in weanling rats[81]. TLR9 signaling and myeloid differentiation marker 88 (MyD88) also played an important role in mediating the anti-inflammatory effect of VSL#3[26].
CD: CD is a kind of patchy transmural inflammation affecting any part of the intestinal tract. The pathogenesis of CD is associated with the defect of mucosal innate immunity accompanied by the failure of clearing bacteria and bacterial debris, which leads to an improper adaptive immune response to the intestinal commensal bacteria. The increase of T-helper 1 (Th1) cytokines by LPL was observed in human and murine CD[82,83]. In a systemic review, Christensenellaceae and Coriobacteriaceae in all three studies and Faecalibacterium prausnitzii in six of eleven studies were decreased compared with controls, and Actinomyces, Veillonella, and E. coli were increased in two studies for patients with CD[64]. Some patients with CD will require an operation, but surgery cannot cure it, and post-operative recurrence is common[84]. One RCT assessed the ability of VSL#3 to prevent human CD endoscopic recurrence after surgery. In this trial, 10% of patients in the early VSL#3 group (administration of VSL#3 for the whole 365 d) did not have severe lesions at the 90th day but developed severe recurrence at the 365th day compared with 26% of patients in the late VSL#3 group (administration of VSL#3 from the 90th day to the 365th day)[85]. This finding indicated that the exposure time of VSL#3 is closely associated with its therapeutic effect. However, for the two groups, the Crohn’s Disease Activity Index and Inflammatory Bowel Disease Questionnaire (IBDQ) scores were similar[85].
Moreover, several studies elucidated the protective effect of VSL#3 in 2,4,6-trinitrobenzene sulfonic acid (TNBS)-induced colitis from different mechanisms. For instance, VSL#3 was proven to reduce the up-regulation of gene clusters associated with mast cells in TNBS colitis by suppressing chemokine gene expression[31]. Recently, a study demonstrated a different protective mechanism of VSL#3 mediated by the increase of IL-10 and IL-10-dependent TGF-β-bearing regulatory cells in a latent-associated protein form[86]. Then, one study showed that Bifidobacteria and VSL#3 had similar effects in ameliorating intestinal inflammation by decreasing the concentrations of serum and fecal high mobility group box 1, and both probiotic treatment might decrease F4/80+ levels in lamina propria mononuclear cells in TNBS-induced murine colitis[87]. Besides, it was shown that VSL#3 resulted in lower macroscopic and microscopic damage in the proximal colon and lower macrophage infiltration in rat acute TNBS-induced colitis[88].
Pouchitis: Pouchitis is a nonspecific inflammation of the ileal reservoir, which is known as a long-term complication after ileal pouch anal anastomosis (IPAA) surgery. It is characterized clinically by abdominal pain, bloating, and increased stool frequency[89]. Four clinical trials in Table 4 demonstrated that VSL#3 could prevent or maintain remission of chronic pouchitis[12,14,90,91]. It was reported that 10% of patients in the VSL#3 group had an onset of acute pouchitis compared with 40% of patients in the placebo group after IPAA for UC, and more patients treated with VSL#3 (17 patients, 85%) maintained antibiotic-induced remission of pouchitis compared with patients treated with placebo (one patient, 6%)[14,90]. The recommended level was an A rating[41]. In addition, an open-label trial clarified that high-dose VSL#3 (3.6 × 1012 bacteria/d) was effective in treating mildly active pouchitis. In this trial, almost 70% of patients achieved complete remission after treatment with VSL#3[92]. The recommended level was a C rating[41]. It suggested that the recommended level of VSL#3 in inducing the remission of pouchitis was not as good as that in preventing and maintaining the remission of pouchitis. Also, VSL#3 could improve the quality of life of patients by improving IBDQ scores significantly[14,92]. The potential mechanism of VSL#3 may be mediated by improving IBF. Although VSL#3 was effective in the therapy of chronic pouchitis, an open-label trial indicated that most patients with antibiotic-dependent pouchitis were not capable of accessing the long-term therapy of VSL#3, mainly due to recurrent symptoms[93].
Table 4.
Trials assessing the effect of VSL#3 in patients with pouchitis
| Ref. | Design | n | VSL#3 intake |
| Gionchetti et al[91], 2000 | RCT | 40 patients in clinical and endoscopic remission of pouchitis | 9 mo with 6 g, 5 × 1011 viable lyophilized bacteria/g, a day |
| Gionchetti et al[14], 2003 | RCT | 40 patients undergoing IPAA for UC (including 20 with VSL#3 and 20 with placebo) | 12 mo with 9 × 1011 bacteria a day |
| Mimura et al[90], 2004 | Not clear | 36 with pouchitis: 20 with VSL#3 and 16 with placebo | 12 mo with 6 g a day, 3 × 1011 bacteria/g |
| Shen et al[93], 2005 | Not mentioned | 31 patients with antibiotic-dependent pouchitis | 8 mo with 6 g/d |
| Kühbacher et al[12], 2006 | RCT | 15 patients with pouchitis: 10 with VSL#3 and 5 with placebo | 12 mo with 6 g, 3 × 1011 viable lyophilized bacteria/g, once a day |
| Gionchetti et al[92], 2007 | Not mentioned | 23 consecutive patients with active mild pouchitis | 4 wk with 3.6 × 1012 bacteria a day |
RCT: Randomized controlled trial; IPAA: Ileal pouch anal anastomosis; UC: Ulcerative colitis.
Microscopic colitis
Microscopic colitis is a chronic inflammatory disease of the intestine characterized by chronic diarrhea with a normal endoscopic appearance of the colon[94]. A randomized, open-label trial demonstrated that VSL#3 had a benefit in inducing and maintaining short-term clinical remission of active microscopic colitis compared with mesalamine. In this trial, 46% of patients treated with VSL#3 achieved clinical remission compared with 8% of patients treated with mesalamine with a significantly lowering stool weight, mucus, and diarrheal rate. Furthermore, a significant decrease of iNOS scores in the VSL#3 group suggested a decrease in the inflammatory status of the disease[95]. The trial had limitations probably due to a small sample size.
Uncomplicated diverticulitis
Diverticulitis is an inflammation related to the diverticula of the colon. An open, pilot clinical trial demonstrated that a combination therapy of VSL#3 and balsalazide had a better effect than VSL#3 treatment alone in preventing relapse and maintaining the remission of uncomplicated diverticulitis[96].
Familial adenomatous polyposis
Familial adenomatous polyposis (FAP) is an inherited autosomal dominant disorder characterized by high proliferation of epithelial cells in the pouch mucosa[97]. A randomized, pilot trial demonstrated that sulindac therapy alone and treatment with VSL#3 and inulin tended to reduce cell proliferation and increase glutathione S-transferase (GST) enzyme activity in patients with FAP and IPAA, while the combination treatment with sulindac, inulin, and VSL#3 had no effect[98]. However, the trial had such a small number of patients enrolled that the conclusion might not be stable or reliable.
Colon cancer
Colitis is a chronic inflammatory colonic disease, and patients have a higher risk of suffering from colorectal cancer (CRC) compared with healthy people. It was reported that the risk of CRC had a 2.4-fold increase when UC existed in population-based cohorts[99]. Three studies showed the inhibitory or preventive effect of VSL#3 on azoxymethane (AOM)/DSS-induced murine colitis-associated carcinogenesis by comparing VSL#3 with other traditional drugs (metformin, balsalazide, and 5-ASA)[100-102]. The combination of metformin and VSL#3 was able to decrease inflammation and tumor progression by inhibiting macrophage infiltration, and the positive effect of VSL#3 against murine UC-associated carcinogenesis was achieved by adjusting the proportion of beneficial and harmful bacteria in feces and the intestinal mucosa[100,101]. One study showed that VSL#3 could retard the development of colonic inflammation to dysplasia and cancer, accompanied by an increase in antiangiogenic factor vitamin D receptor (VDR) in a rat model of colitis-associated CRC[103]. However, in terms of murine colitis-associated CRC, two studies showed contrasting results. The first study showed that VSL#3 had excellent anti-carcinogenic and anti-inflammatory activities by regulating mucosal CD4+ T cell responses, involving increasing IL-17-expressing CD4+ T cells in mesenteric lymph nodes. Interestingly, the study showed an increased concentration of colonic TNF-α in mice treated with VSL#3 compared to controls[104]. In contrast, the second study showed that VSL#3 was not able to protect against colitis-associated CRC. VSL#3 produced the effect of enhancing tumorigenesis probably by inducing the depletion of protective commensal bacteria. However, the effect was not related to inflammatory cytokines[105]. The different results may be associated with the type of mice and the induced pattern of murine CRC used in the two studies. The dose, mode of administration, and treatment course of VSL#3 may also have impacted the results. In the first study, wild-type C57BL/6 mice were treated with AOM (10 mg/kg) at the sixth week, followed by a 2.0% DSS treatment for a week, and IL-10-deficient 129/SvEv mice were treated with 5 × 107 CFU Helicobacter typhlonius by using oral gavage. VSL#3 was administered daily by orogastric gavage using a ball tip gavage needle at a dose of 1.2 × 109 bacteria/mouse/d[104]. In the second study, IL-10-deficient 129/SvEv mice were treated with specific pathogen free (SPF) bacteria for 7 wk, then mice received an injection of AOM (10 mg/kg) for 6 wk. The oral dose of VSL#3 was 109 CFU per mouse/d[105].
EFFECTS OF VSL#3 ON HEPATIC DISEASES
There is a close relationship between the liver and intestine tract. The metabolism of bile acid is tightly connected with the intestinal microbiota. The probiotic mixture VSL#3 was shown to modulate intestinal microbiota, thus down-regulating the intestinal-liver FXR-FGF15 axis to induce ileal bile acid deconjugation, fecal bile acid excretion, and the synthesis of bile acid in the liver[17]. Hence, VSL#3 may have a potential for treatment of chronic liver diseases and their complications.
Non-alcoholic fatty liver disease
Non-alcoholic fatty liver disease (NAFLD) is an increasing problem worldwide, and is now one of the leading causes of chronic liver diseases in Western countries[106]. It encompasses a spectrum of histopathological changes from simple liver steatosis to steatohepatitis, advanced fibrosis, and cirrhosis, and NAFLD may be caused by intestinal dysbiosis, which increases intestinal permeability to bacterial products and harmful substances[107,108]. Intestinal dysbiosis can also cause intestinal dysmotility, inflammation, and other immune responses that may contribute to hepatic inflammation and fibrosis[108]. One study in Table 5 showed that VSL#3 could decrease liver damage in NAFLD and hepatitis C virus-related hepatitis patients[109]. In addition, pediatric NAFLD has also been proven to be responsive to VSL#3, as shown in Table 5. VSL#3 could decrease the severity of NAFLD, mainly by increasing the levels of glucagon-like peptide 1 (GLP-1) and activated GLP-1 (aGLP-1)[110,111]. It was shown that the increase of SCFA butyrate by VSL#3 was able to stimulate the secretion of GLP-1 from intestinal L-cells which were responsible for synthesizing the pre-propetide of GLP-1[110,112]. GLP-1 could stimulate the endocrine pancreas, thus improving insulin sensitivity in order to improve glucose and fat metabolism[110]. Besides, it was suggested that the administration of VSL#3, by increasing GLP-1, was able to regulate the metabolic flux of muscle mitochondria, make valine be completely degraded, and finally produce succinyl-coenzyme A, the intermediate product of citric acid cycle, which is used for energy metabolism[111]. These improvements of energy metabolism provide benefits for the recovery of childhood NAFLD. The recommended level for VSL#3 use in NAFLD (including adults and children) was a C rating[41].
Table 5.
Trials assessing the effect of VSL#3 in patients with non-alcoholic fatty liver disease
| Ref. | Design | n | VSL#3 intake |
| Loguercio et al[109], 2005 | Not mentioned | 78 patients: 22 with NAFLD, 20 with alcoholic cirrhosis, 20 with HCV-related chronic hepatitis, and 16 with HCV-related cirrhosis | 3 mo, the daily dose was not clear |
| Alisi et al[110], 2014 | RCT | 48 randomized children; 44 (22 with VSL#3 and 22 with placebo) completed the study | 4 mo with 1 sachet a day (less than 10 yr old) or 2 sachets a day (older than 10 yr old) |
| Miccheli et al[111], 2015 | RCT | 31 pediatric NAFLD patients | 4 mo with 1 sachet a day (less than 10 yr old) or 2 sachets a day (older than 10 yr old) |
NAFLD: Non-alcoholic fatty liver disease; HCV: Hepatitis C virus; RCT: Randomized controlled trial.
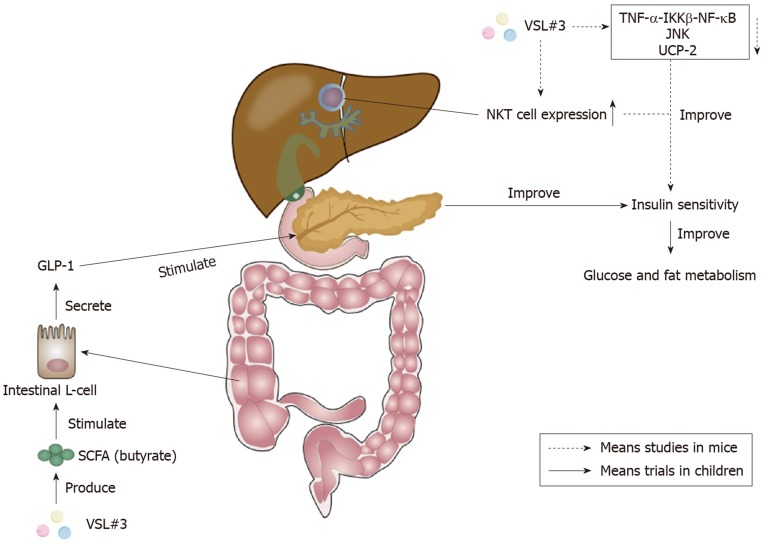
Although there are nearly no clinical trials clarifying the effect of VSL#3 on adult NAFLD, there are several studies demonstrating that VSL#3 was able to improve murine NAFLD. It acts by improving liver histology and liver insulin resistance, and decreasing the level of serum alanine aminotransferase[113]. VSL#3 was proven to be able to improve hepatic insulin resistance by reducing the TNF-α-IκB kinase β-NF-κB pathway, the activity of TNF-regulated kinase Jun N-terminal kinase, and uncoupling protein-2[113-115]. TNF-regulated kinase Jun N-terminal kinase could promote insulin resistance by increasing the serine phosphorylation of insulin receptor substrate-1[116]. The increase of hepatic natural kill T cell expression stimulated by lipids extracted from VSL#3 also contributed to the improvement of hepatic insulin resistance[117,118]. The action mechanism of VSL#3 on insulin sensitivity is shown in Figure 3.
Figure 3.
Effects of VSL#3 on insulin sensitivity. The increase of short-chain fatty acid (SCFA) butyrate caused by VSL#3 is able to stimulate the secretion of glucagon-like peptide 1 (GLP-1) from intestinal L-cells[112]. GLP-1 can stimulate the pancreas and ameliorate insulin sensitivity to improve glucose and fat metabolism[110]. Furthermore, VSL#3 can improve hepatic insulin resistance by reducing tumor necrosis factor-α (TNF-α)-IκB kinase β (IKKβ)-nuclear factor-κB (NF-κB) pathway, the activity of TNF-regulated kinase Jun N-terminal kinase (JNK), and uncoupling protein-2[113-115]. The increase of hepatic natural kill T (NKT) cells caused by VSL#3 also plays a significant role in the improvement of hepatic insulin sensitivity[117,118]. SCFA: Short-chain fatty acid; GLP-1: Glucagon-like peptide 1; TNF-α: Tumor necrosis factor-α; IKKβ: IκB kinase β; NF-κB: Nuclear factor-κB; JNK: Jun N-terminal kinase; UCP-2: Uncoupling protein-2; NKT: Natural kill T.
Non-alcoholic steatohepatitis (NASH) is the advanced stage of NAFLD, and metabolic syndrome increases the risk of having NASH[119]. VSL#3 was not able to prevent liver steatosis or inflammation but was able to reduce hepatic fibrosis and the accumulation of collagen and α-smooth muscle actin (α-SMA) in methionine choline-deficient (MCD) diet-induced murine NASH[120]. Furthermore, VSL#3 significantly increased the expression of liver PPARα and PPARγ, which was inhibited by TNF-α, and decreased the expression of pro-collagen and matrix metalloproteinases compared to an MCD diet alone[120,121]. Besides, VSL#3 maintained MCD diet-induced expression of TGF-β but increased the expression of bone morphogenic protein and activin membrane-bound inhibitor (Bambi), having a negative regulatory effect on TGF-β-family signaling[120].
Alcoholic liver disease
Intestinal barrier dysfunction may be related to endotoxemia and eventually cause hepatic inflammation and the development of alcoholic liver disease (ALD)[122]. One trial indicated that VSL#3 could mitigate liver damage in patients with alcoholic liver cirrhosis by decreasing the plasma levels of malondialdehyde, 4-hydroxynonenal, and S-nitrosothiols. The plasma levels of cytokines (TNF-α, IL-6, and IL-10) also improved[109]. Besides, one study demonstrated that VSL#3 was as effective as glutamine in rats with experimental acute ALD, and the combination therapy was more effective. The action mechanism mainly proceeded by reducing intestinal permeability[122]. The recommended level for VSL#3 use in ALD was a C rating[41].
Cirrhosis
Impaired IBF, bacterial overgrowth, and disturbance of local immunity contribute to bacterial translocation (BT) in cirrhosis; BT is one of the major causes of many complications of cirrhosis[123,124]. Gram-negative enteric bacilli, such as E. coli, are relatively effective actors for BT to the mesenteric lymph nodes[125]. Also, some pro-inflammatory pathways (such as TNF-α) worsen liver dysfunction[126]. Two clinical trials, as shown in Table 6, showed that VSL#3 led to the amelioration of hepatic and systemic hemodynamic disturbances accompanied by decreased hepatic venous pressure gradient in order to improve the symptoms in patients with cirrhosis[127,128]. One trial demonstrated that adjunctive VSL#3 significantly improved the response rate (percentage of patients with a decrease in hepatic venous pressure gradient from baseline of ≥ 20% or to ≤ 12 mmHg) compared with the control group (58% vs 31%). VSL#3 could lead to a reduction in pro-inflammatory cytokine TNF-α plasma levels. However, no differences could be observed in the plasma level of IL-6 or NO[127]. Another trial observed a significant decrease in plasma aldosterone levels induced by VSL#3, but the changes in the other measured parameters, including the stool microbiota, were not significant[129]. Apart from the above-mentioned plasma component changes, VSL#3 treatment induced an increase in the plasma levels of albumin and hemoglobin, which might lead to lower model for end-stage liver disease scores in patients with decompensated liver cirrhosis[130]. The effect of VSL#3 was also proven in a rat model of biliary cirrhosis with portal hypertension. VSL#3 prevented endothelial dysfunction by improving vascular oxidative stress (reducing BT) and the local angiotensin system[131].
Table 6.
Trials assessing the effect of VSL#3 in patients with cirrhosis
| Ref. | Design | n | VSL#3 intake |
| Gupta et al[127], 2013 | RCT | 94 with cirrhosis | 2 mo with 9 × 1011 CFU a day |
| Jayakumar et al[129], 2013 | RCT | 17 with decompensated cirrhosis; 15 completed | 2 mo with 3.6 × 1012 bacteria a day |
| Rincón et al[128], 2014 | Not mentioned | 17 with cirrhosis and ascites; 12 completed | 6 wk, the dose was not clear |
| Marlicz et al[130], 2016 | Not mentioned | 20 with cirrhosis (13 with compensated liver cirrhosis, 7 with decompensated liver cirrhosis), and 10 healthy controls | Daily for 28 d, the daily dose was not clear |
RCT: Randomized controlled trial; CFU: Colony-forming unit.
Hepatic encephalopathy
Patients with liver cirrhosis could cause systemic inflammation, which may be associated with hepatic encephalopathy (HE)[132]. HE, including minimal HE (MHE) and overt HE (OHE), is a common complication of cirrhosis with impaired quality of life. Sometimes MHE can develop into OHE. The development of HE could be related to alterations in the intestinal microbiota (higher Veillonellaceae, Alcaligeneceae, and Enterobacteriaceae), ammonia, endotoxemia, and inflammation (IL-2, IL-6, IL-13, and TNF-α)[133,134]. Two trials, as shown in Table 7, investigated the effect of VSL#3 compared with other traditional medicines in MHE. They demonstrated that VSL#3 had similar efficacy in improvement of MHE with lactulose and L-ornithine L-aspartate[135,136]. MHE improved in 62.5% of patients taking lactulose and 69.7% of patients taking VSL#3, and the amelioration of MHE was associated with decreased serum ammonia levels[135].
Table 7.
Trials assessing the effect of VSL#3 in patients with hepatic encephalopathy
| Ref. | Design | n | VSL#3 intake |
| Mittal et al[136], 2011 | RCT | 322 patients were screened for MHE; 160 patients were found to have MHE and included in the trial | 3 mo with 1.1 × 1011 CFU twice a day |
| Lunia et al[137], 2014 | RCT | 160 with cirrhosis without OHE (including 86 in treatment group, 42 with MHE; 74 in control group, 33 with MHE) | 3 mo with 1 capsule 3 times a day, the dose was not clear |
| Dhiman et al[138], 2014 | RCT | 130 with cirrhosis who had recovered from HE (including 66 with VSL#3, 64 with placebo) | 6 mo with 9 × 1011 CFU a day |
| Pratap Mouli et al[135], 2015 | RCT | 227 with CLD were screened for MHE; 120 were diagnosed with MHE; 40 in the lactulose group and 33 in the VSL#3 group completed | 2 mo with 4.5 × 1011 CFU a day |
HE: Hepatic encephalopathy; RCT: Randomized controlled trial; MHE: Minimal hepatic encephalopathy; CFU: Colony-forming unit; OHE: Overt hepatic encephalopathy; CLD: Chronic liver diseases.
Also, there have been two trials, as shown in Table 7, investigating the preventive effect of VSL#3 against OHE[137,138]. An open-label RCT investigated the effect of VSL#3 in primary prophylaxis of OHE. Seven patients in the VSL#3 group (n = 86, 42 with MHE) and 14 in the control group (n = 74, 33 with MHE) developed OHE. This change is probably related to the significant decrease in the number of patients with MHE and the small intestinal bacterial overgrowth in the VSL#3 group[137]. The secondary prophylaxis efficacy of VSL#3 was investigated in another trial in patients with cirrhosis who have recovered from an episode of HE. There was a tendency to reduce the development of OHE in the VSL#3 group. Fewer patients hospitalized for HE were observed in the VSL#3 group compared with the placebo group (19.7% vs 42.2%) over a 6-mo period. The results also indicated that VSL#3 improved systemic inflammatory response syndrome and model for end-stage liver disease scores, and decreased plasma indole, renin, aldosterone, and brain natriuretic peptide levels significantly[138]. The recommended level for VSL#3 use in HE was an A rating[41].
VSL#3 administration in chronic liver diseases still has its own limitations. We need further studies to clarify its therapeutic effect in portal hypertension and its complications.
EFFECTS OF VSL#3 ON OBESITY AND DIABETES
VSL#3 has been shown to significantly decrease body and fat mass during a high-fat diet compared with placebo[139,140]. In overweight (body mass index [BMI] > 25) adults without diabetes, VSL#3 (one capsule every day before any meal or breakfast, 1.125 × 1011 CFU/capsule) was able to significantly reduce total cholesterol, triglyceride, low-density lipoprotein, and very-low-density lipoprotein concentrations and increase high-density lipoprotein concentration[141].
At present, there are no definite clinical trials about the effect of VSL#3 on diabetes mellitus. However, the efficacy of VSL#3 on diabetes has been researched in obesity and non-obesity murine models. Increasing evidence supports the idea that impaired IBF promotes the access of infected pathogens to mucosal immune elements, which may eventually lead to diabetogenic immune responses and insulin resistance[142]. Two studies demonstrated that oral administration of VSL#3 was able to prevent spontaneous autoimmune diabetes in non-obese diabetic (NOD) mice[143,144]. The prevention of autoimmune diabetes was related to increased IL-10 production from the Peyer's patch, spleen, and pancreas and decreased β-cell destruction[143]. VSL#3 treatment increased the release of protolerogenic components of the inflammatory corpuscles, such as indoleamine 2,3-dioxygenase (IDO) and IL-33. These changes regulate intestinal immunity in VSL#3-treated NOD mice by promoting the differentiation of tolerant dendritic cells and reducing the differentiation of Th1 and T-helper 17 (Th17) cells in the intestinal mucosa[144]. Besides, IDO inhibited T cell proliferation by limiting the local tryptophan concentration directly, thus reducing Th1 and Th17 cell differentiation[145]. In addition, VSL#3 treatment increased protolerogenic species, and decreased the relative abundance of species, such as Bacteroidetes S24-7, in NOD mice[144]. However, one study showed that VSL#3 could not delay diabetes probably because it colonized the intestine poorly and could not overcome the effect of diabetogenic microbiota[146]. The result contradicts previous studies and the reasons for this need to be further studied. Moreover, one study demonstrated that VSL#3 was able to prevent diabetes in high fat diet-induced obesity mice by inhibiting weight gain and insulin resistance. Increased transcript levels of free fatty acid receptor 3 (Ffar3) were observed in VSL#3-treated murine intestine. VSL#3 could protect against glucose intolerance and obesity in Lepob/ob mice, and significantly increase proopiomelanocortin levels and decrease neuropeptide Y and agouti-related protein levels in the hypothalamus, which are related to energy homeostasis[112,147].
EFFECTS OF VSL#3 ON ALLERGIC DISEASES
It is known that VSL#3 has the capacity to regulate immune responses, so it has been considered to treat allergic diseases. There have not been definite conclusions about the efficacy of VSL#3 on allergic diseases in clinical trials, but a potential effect of VSL#3 has been shown in animal studies. Several studies demonstrated that the oral administration of VSL#3 had a beneficial effect in ameliorating anaphylactic symptoms in murine food allergy induced by shrimp tropomyosin (ST) or peanut by inhibiting T-helper 2 (Th2) immune reactions[148-150]. Meanwhile, the administration of VSL#3 had a regulating effect on allergen-induced Th2 responses by turning primary immune reactions to a T regulatory (Treg)/T-helper 0 (Th0)-type profile[28]. Th2-secreted cytokines IL-5 and IL-13 decreased and Treg/Th1 cytokines IL-10 and IFN-γ increased in the presence of VSL#3[149]. TGF-β, induced by VSL#3 supplementation, was capable of reducing the Th2 inflammation related to food anaphylaxis in a mouse model of peanut sensitization. It acted through the induction or maintenance of regulatory T cells expressing FOXP3[150]. The administration of VSL#3 (1.5 × 1010 CFU in 250 µL of PBS daily) inhibited the β-lactoglobulin (BLG)-induced allergic reaction, mainly by increasing intestinal sIgA in BLG-sensitized mice[151].
EFFECTS OF VSL#3 ON NERVOUS SYSTEMIC DISEASES
Two studies showed that VSL#3 was able to improve some types of nervous systemic disorders and related brain mechanisms through the gut-brain axis when dysbiosis caused by a cafeteria diet or the ageing process occurred in rats[152,153]. VSL#3 was proven to prevent diet-induced memory deficits on the place task but had no effect on anxiety-like behaviors on the elevated plus maze (EPM) in rats[152]. Furthermore, one study indicated that VSL#3 improved the age-related deficits in long-term potentiation in aged rats. The effect was accompanied by a decrease of microglial activation markers and an increase of brain-derived neurotrophic factor and synapsin. The effects above might be due to the increase in the amounts of Bacteroidetes and Actinobacteria caused by VSL#3[153]. One study showed that the effects of VSL#3 on the nervous system are independent of the changes in the intestinal microbiota in rats. VSL#3 could significantly improve sickness behaviors and brain dysfunction, probably by decreasing microglial activation and brain inflammatory monocyte infiltration in bile duct ligation mice with liver inflammation[154]. Furthermore, one study demonstrated that the post-injury treatment of VSL#3 improved locomotor recovery after murine spinal cord injury and activated neuroprotective mucosal immune cells[155].
EFFECTS OF VSL#3 ON ATHEROSCLEROSIS
Atherosclerosis (AS) is a chronic disease that might be associated with the bacteria from the oral cavity and even the gut[156]. Two studies assessed the ability of VSL#3 to protect against AS in ApoE−/− mice. They demonstrated that VSL#3 had a beneficial effect on AS and perhaps worked by significantly reducing the development of atherosclerotic or aortic plaques and biomarkers of vascular inflammation. Vascular cell adhesion molecules and intercellular adhesion molecules were shown to be reduced by VSL#3 in both two studies[157,158]. One study indicated that VSL#3 (2.78 × 1011 CFU/d for 12 wk) was able to significantly lower gelatinase matrix metalloproteinase-9 , but another plasma atherosclerotic biomarker, total plasminogen activator inhibitor-1, was significantly elevated by the combination of telmisartan (positive control drug) and VSL#3[157]. The reason for this is not clear. The administration of VSL#3 (2 × 1010 CFU/kg/d, 6 d per week for 12 wk) effectively decreased the percentage of CD36 positive cells among circulating macrophages[158].
EFFECTS OF VSL#3 ON BONE DISEASES
It has been reported that the oral administration of VSL#3 (twice a week with 1 × 109 CFU) could protect against ovariectomy (OVX)-induced bone loss in sex steroid-deficient mice by increasing intestinal barrier integrity and inhibiting intestinal and bone marrow inflammation[159].
EFFECTS OF VSL#3 ON FEMALE REPRODUCTIVE SYSTEMIC DISEASES
Diseases of the female reproductive system include various kinds of diseases, such as gynecological inflammation, gynecological tumor, menstrual disorder, and infertility. In this review, we mainly investigated the effect of VSL#3 on breast milk’s microbial composition and preterm birth.
Improvement of breast milk’s microbial composition
Breast milk provides nutritious substances for infant growth and development, and it also provides several altered beneficial microbiota including Staphylococci, Streptococci, Lactobacilli, and Bifidobacteria[160,161]. These beneficial bacteria in maternal milk will transfer from mother to infant and colonize the infant intestine during lactation[161]. One RCT demonstrated that oral VSL#3 was able to improve breast milk’s microbial composition with a significant increase of the levels of Bifidobacteria and Lactobacilli compared with placebo in mothers during the perinatal period. The improvement in breast milk’s microbial composition caused by VSL#3 was not associated with differences in the milk concentrations of oligosaccharides and lactoferrin, and the results indicated that the administered VSL#3 did not pass from the maternal intestine to mammary gland through an endogenous approach, but had a systemic effect[162].
Preterm birth
The balance of vaginal microbiota is important during pregnancy, and abnormal vaginal communities (such as bacterial vaginosis) may be associated with preterm delivery and perinatal complications[163]. One trial showed the potential implications of VSL#3 in preventing preterm birth via an anti-inflammatory effect on vaginal immunity in healthy pregnant women. The consumption of VSL#3 led to a decrease of the pro-inflammatory chemokine Eotaxin. There were no significant alterations in the amount of the principal vaginal bacteria in women orally administered with VSL#3. However, VSL#3 played a potential role in counteracting the decrease in Bifidobacteria and the increase in Atopobium. VSL#3 was also capable of modulating the Lactobacillus population, which was associated with the decrease of L. helveticus species[164]. One study indicated that VSL#3 was able to survive and stay stable in a continuous culture system simulating the vaginal environment and inhibit the vaginal vault pathogen Gardnerella vaginalis[165].
SIDE EFFECTS AND LIMITS RELATED TO VSL#3
There are almost no serious side effects related to the use of VSL#3 in most diseases. Among these side effects, only very few are intolerable. A meta-analysis showed that 33 patients experienced mild side effects (mainly bloating) out of 353 UC patients treated with VSL#3[166]. Three patients experienced abdominal pain out of 60 patients with HE who received VSL#3[135]. One patient was reported to experience abdominal cramps, vomiting, and diarrhea out of 20 patients with pouchitis who received VSL#3[90]. Finally, of the 31 pouchitis patients treated with VSL#3, two experienced intolerable side effects: One experienced bloody bowel movements and the other experienced severe constipation, bloating, and gas[93].
The use of VSL#3 also has some limits. VSL#3 contains only eight bacterial strains, and the intestinal microecosystem of each patient is complex, so these strains may not be suitable for all patients. Achieving the one-to-one treatment of formula probiotics and individuals is difficult. The results mentioned above of VSL#3 in the treatment of various diseases are not exactly consistent, which may have been caused by different sources of VSL#3. One study indicated that proteomic analyses showed differences in protein abundances, identities, and origins of VSL#3 from different sites[167]. Therefore, we suggest that the protein abundances should be identified through proteomic analyses in the clinical and basic studies of VSL#3 in the future.
CONCLUSION
In the past, it has been reported that VSL#3 has a profound effect on digestive systemic disorders, especially gastrointestinal disorders. Apart from this, many studies demonstrated that VSL#3 has a beneficial effect on obesity and diabetes, allergic diseases, nervous systemic diseases, AS, bone diseases, and female reproductive systemic diseases. In most cases, the use of VSL#3 is safe and well-tolerated, but more precise mechanisms and effects of VSL#3 still need to be studied. To achieve one-to-one individualized treatment, we need to prepare exclusive probiotics according to the real-time monitoring of patients' intestinal microbiota. However, the current detection technology is not accurate enough. The study of the intestinal microbiota is challenging since most cannot be cultivated[10]. Thus, the solutions to the above problems will hopefully provide a new direction for precise medicine in the future.
Footnotes
Conflict-of-interest statement: The authors disclose no potential competing interests.
Manuscript source: Invited manuscript
Peer-review started: December 30, 2019
First decision: February 19, 2020
Article in press: April 8, 2020
Specialty type: Medicine, research and experimental
Country/Territory of origin: China
Peer-review report’s scientific quality classification
Grade A (Excellent): 0
Grade B (Very good): B, B
Grade C (Good): 0
Grade D (Fair): 0
Grade E (Poor): 0
P-Reviewer: Bourgoin SG, Villéger R S-Editor: Ma YJ L-Editor: Wang TQ E-Editor: Liu JH
Contributor Information
Fang-Shu Cheng, Department of Dermatology, Shengjing Hospital of China Medical University, Shenyang 110004, Liaoning Province, China; Class 85 of 101k, China Medical University, Shenyang 110004, Liaoning Province, China.
Dan Pan, Department of Geriatrics, the First Affiliated Hospital, China Medical University, Shenyang 110001, Liaoning Province, China.
Bing Chang, Department of Gastroenterology, the First Affiliated Hospital, China Medical University, Shenyang 110001, Liaoning Province, China.
Min Jiang, Department of Gastroenterology, the First Affiliated Hospital, China Medical University, Shenyang 110001, Liaoning Province, China.
Li-Xuan Sang, Department of Geriatrics, the First Affiliated Hospital, China Medical University, Shenyang 110001, Liaoning Province, China. sanglixuan2008@163.com.
References
- 1.Sanders ME. Probiotics: definition, sources, selection, and uses. Clin Infect Dis. 2008;46 Suppl 2:S58–S61; discussion S144-S151. doi: 10.1086/523341. [DOI] [PubMed] [Google Scholar]
- 2.Douillard FP, Mora D, Eijlander RT, Wels M, de Vos WM. Comparative genomic analysis of the multispecies probiotic-marketed product VSL#3. PLoS One. 2018;13:e0192452. doi: 10.1371/journal.pone.0192452. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Ren Z, Guo C, Yu S, Zhu L, Wang Y, Hu H, Deng J. Progress in Mycotoxins Affecting Intestinal Mucosal Barrier Function. Int J Mol Sci. 2019;20:2777. doi: 10.3390/ijms20112777. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Capaldo CT, Farkas AE, Nusrat A. Epithelial adhesive junctions. F1000Prime Rep. 2014;6:1. doi: 10.12703/P6-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Krishnan M, Penrose HM, Shah NN, Marchelletta RR, McCole DF. VSL#3 Probiotic Stimulates T-cell Protein Tyrosine Phosphatase-mediated Recovery of IFN-γ-induced Intestinal Epithelial Barrier Defects. Inflamm Bowel Dis. 2016;22:2811–2823. doi: 10.1097/MIB.0000000000000954. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Corridoni D, Pastorelli L, Mattioli B, Locovei S, Ishikawa D, Arseneau KO, Chieppa M, Cominelli F, Pizarro TT. Probiotic bacteria regulate intestinal epithelial permeability in experimental ileitis by a TNF-dependent mechanism. PLoS One. 2012;7:e42067. doi: 10.1371/journal.pone.0042067. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Dai C, Zhao DH, Jiang M. VSL#3 probiotics regulate the intestinal epithelial barrier in vivo and in vitro via the p38 and ERK signaling pathways. Int J Mol Med. 2012;29:202–208. doi: 10.3892/ijmm.2011.839. [DOI] [PubMed] [Google Scholar]
- 8.Spalinger MR, McCole DF, Rogler G, Scharl M. Protein tyrosine phosphatase non-receptor type 2 and inflammatory bowel disease. World J Gastroenterol. 2016;22:1034–1044. doi: 10.3748/wjg.v22.i3.1034. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Otte JM, Podolsky DK. Functional modulation of enterocytes by gram-positive and gram-negative microorganisms. Am J Physiol Gastrointest Liver Physiol. 2004;286:G613–G626. doi: 10.1152/ajpgi.00341.2003. [DOI] [PubMed] [Google Scholar]
- 10.Eckburg PB, Bik EM, Bernstein CN, Purdom E, Dethlefsen L, Sargent M, Gill SR, Nelson KE, Relman DA. Diversity of the human intestinal microbial flora. Science. 2005;308:1635–1638. doi: 10.1126/science.1110591. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Kumar M, Kissoon-Singh V, Coria AL, Moreau F, Chadee K. Probiotic mixture VSL#3 reduces colonic inflammation and improves intestinal barrier function in Muc2 mucin-deficient mice. Am J Physiol Gastrointest Liver Physiol. 2017;312:G34–G45. doi: 10.1152/ajpgi.00298.2016. [DOI] [PubMed] [Google Scholar]
- 12.Kühbacher T, Ott SJ, Helwig U, Mimura T, Rizzello F, Kleessen B, Gionchetti P, Blaut M, Campieri M, Fölsch UR, Kamm MA, Schreiber S. Bacterial and fungal microbiota in relation to probiotic therapy (VSL#3) in pouchitis. Gut. 2006;55:833–841. doi: 10.1136/gut.2005.078303. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Brigidi P, Swennen E, Vitali B, Rossi M, Matteuzzi D. PCR detection of Bifidobacterium strains and Streptococcus thermophilus in feces of human subjects after oral bacteriotherapy and yogurt consumption. Int J Food Microbiol. 2003;81:203–209. doi: 10.1016/s0168-1605(02)00245-3. [DOI] [PubMed] [Google Scholar]
- 14.Gionchetti P, Rizzello F, Helwig U, Venturi A, Lammers KM, Brigidi P, Vitali B, Poggioli G, Miglioli M, Campieri M. Prophylaxis of pouchitis onset with probiotic therapy: a double-blind, placebo-controlled trial. Gastroenterology. 2003;124:1202–1209. doi: 10.1016/s0016-5085(03)00171-9. [DOI] [PubMed] [Google Scholar]
- 15.Peterson LW, Artis D. Intestinal epithelial cells: regulators of barrier function and immune homeostasis. Nat Rev Immunol. 2014;14:141–153. doi: 10.1038/nri3608. [DOI] [PubMed] [Google Scholar]
- 16.Caballero-Franco C, Keller K, De Simone C, Chadee K. The VSL#3 probiotic formula induces mucin gene expression and secretion in colonic epithelial cells. Am J Physiol Gastrointest Liver Physiol. 2007;292:G315–G322. doi: 10.1152/ajpgi.00265.2006. [DOI] [PubMed] [Google Scholar]
- 17.Degirolamo C, Rainaldi S, Bovenga F, Murzilli S, Moschetta A. Microbiota modification with probiotics induces hepatic bile acid synthesis via downregulation of the Fxr-Fgf15 axis in mice. Cell Rep. 2014;7:12–18. doi: 10.1016/j.celrep.2014.02.032. [DOI] [PubMed] [Google Scholar]
- 18.Veazey RS, Rosenzweig M, Shvetz DE, Pauley DR, DeMaria M, Chalifoux LV, Johnson RP, Lackner AA. Characterization of gut-associated lymphoid tissue (GALT) of normal rhesus macaques. Clin Immunol Immunopathol. 1997;82:230–242. doi: 10.1006/clin.1996.4318. [DOI] [PubMed] [Google Scholar]
- 19.Madsen K, Cornish A, Soper P, McKaigney C, Jijon H, Yachimec C, Doyle J, Jewell L, De Simone C. Probiotic bacteria enhance murine and human intestinal epithelial barrier function. Gastroenterology. 2001;121:580–591. doi: 10.1053/gast.2001.27224. [DOI] [PubMed] [Google Scholar]
- 20.Jijon H, Backer J, Diaz H, Yeung H, Thiel D, McKaigney C, De Simone C, Madsen K. DNA from probiotic bacteria modulates murine and human epithelial and immune function. Gastroenterology. 2004;126:1358–1373. doi: 10.1053/j.gastro.2004.02.003. [DOI] [PubMed] [Google Scholar]
- 21.Petrof EO, Kojima K, Ropeleski MJ, Musch MW, Tao Y, De Simone C, Chang EB. Probiotics inhibit nuclear factor-kappaB and induce heat shock proteins in colonic epithelial cells through proteasome inhibition. Gastroenterology. 2004;127:1474–1487. doi: 10.1053/j.gastro.2004.09.001. [DOI] [PubMed] [Google Scholar]
- 22.Hörmannsperger G, Clavel T, Hoffmann M, Reiff C, Kelly D, Loh G, Blaut M, Hölzlwimmer G, Haller D. Posttranslational inhibition of proinflammatory chemokine secretion in intestinal epithelial cells: implications for specific IBD indications. J Clin Gastroenterol. 2010;44 Suppl 1:S10–S15. doi: 10.1097/MCG.0b013e3181e102c1. [DOI] [PubMed] [Google Scholar]
- 23.Hoermannsperger G, Clavel T, Hoffmann M, Reiff C, Kelly D, Loh G, Blaut M, Hölzlwimmer G, Laschinger M, Haller D. Post-translational inhibition of IP-10 secretion in IEC by probiotic bacteria: impact on chronic inflammation. PLoS One. 2009;4:e4365. doi: 10.1371/journal.pone.0004365. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.von Schillde MA, Hörmannsperger G, Weiher M, Alpert CA, Hahne H, Bäuerl C, van Huynegem K, Steidler L, Hrncir T, Pérez-Martínez G, Kuster B, Haller D. Lactocepin secreted by Lactobacillus exerts anti-inflammatory effects by selectively degrading proinflammatory chemokines. Cell Host Microbe. 2012;11:387–396. doi: 10.1016/j.chom.2012.02.006. [DOI] [PubMed] [Google Scholar]
- 25.Pagnini C, Saeed R, Bamias G, Arseneau KO, Pizarro TT, Cominelli F. Probiotics promote gut health through stimulation of epithelial innate immunity. Proc Natl Acad Sci USA. 2010;107:454–459. doi: 10.1073/pnas.0910307107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Rachmilewitz D, Katakura K, Karmeli F, Hayashi T, Reinus C, Rudensky B, Akira S, Takeda K, Lee J, Takabayashi K, Raz E. Toll-like receptor 9 signaling mediates the anti-inflammatory effects of probiotics in murine experimental colitis. Gastroenterology. 2004;126:520–528. doi: 10.1053/j.gastro.2003.11.019. [DOI] [PubMed] [Google Scholar]
- 27.Mariman R, Tielen F, Koning F, Nagelkerken L. The probiotic mixture VSL#3 dampens LPS-induced chemokine expression in human dendritic cells by inhibition of STAT-1 phosphorylation. PLoS One. 2014;9:e115676. doi: 10.1371/journal.pone.0115676. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Mastrangeli G, Corinti S, Butteroni C, Afferni C, Bonura A, Boirivant M, Colombo P, Di Felice G. Effects of live and inactivated VSL#3 probiotic preparations in the modulation of in vitro and in vivo allergen-induced Th2 responses. Int Arch Allergy Immunol. 2009;150:133–143. doi: 10.1159/000218116. [DOI] [PubMed] [Google Scholar]
- 29.Drakes M, Blanchard T, Czinn S. Bacterial probiotic modulation of dendritic cells. Infect Immun. 2004;72:3299–3309. doi: 10.1128/IAI.72.6.3299-3309.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Hart AL, Lammers K, Brigidi P, Vitali B, Rizzello F, Gionchetti P, Campieri M, Kamm MA, Knight SC, Stagg AJ. Modulation of human dendritic cell phenotype and function by probiotic bacteria. Gut. 2004;53:1602–1609. doi: 10.1136/gut.2003.037325. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Mariman R, Kremer B, van Erk M, Lagerweij T, Koning F, Nagelkerken L. Gene expression profiling identifies mechanisms of protection to recurrent trinitrobenzene sulfonic acid colitis mediated by probiotics. Inflamm Bowel Dis. 2012;18:1424–1433. doi: 10.1002/ibd.22849. [DOI] [PubMed] [Google Scholar]
- 32.Reiff C, Delday M, Rucklidge G, Reid M, Duncan G, Wohlgemuth S, Hörmannsperger G, Loh G, Blaut M, Collie-Duguid E, Haller D, Kelly D. Balancing inflammatory, lipid, and xenobiotic signaling pathways by VSL#3, a biotherapeutic agent, in the treatment of inflammatory bowel disease. Inflamm Bowel Dis. 2009;15:1721–1736. doi: 10.1002/ibd.20999. [DOI] [PubMed] [Google Scholar]
- 33.Tarnawski A, Szabo IL, Husain SS, Soreghan B. Regeneration of gastric mucosa during ulcer healing is triggered by growth factors and signal transduction pathways. J Physiol Paris. 2001;95:337–344. doi: 10.1016/s0928-4257(01)00046-8. [DOI] [PubMed] [Google Scholar]
- 34.Dharmani P, De Simone C, Chadee K. The probiotic mixture VSL#3 accelerates gastric ulcer healing by stimulating vascular endothelial growth factor. PLoS One. 2013;8:e58671. doi: 10.1371/journal.pone.0058671. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Massi M, Ioan P, Budriesi R, Chiarini A, Vitali B, Lammers KM, Gionchetti P, Campieri M, Lembo A, Brigidi P. Effects of probiotic bacteria on gastrointestinal motility in guinea-pig isolated tissue. World J Gastroenterol. 2006;12:5987–5994. doi: 10.3748/wjg.v12.i37.5987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Giannelli FR. Antibiotic-associated diarrhea. JAAPA. 2017;30:46–47. doi: 10.1097/01.JAA.0000524721.01579.c9. [DOI] [PubMed] [Google Scholar]
- 37.Selinger CP, Bell A, Cairns A, Lockett M, Sebastian S, Haslam N. Probiotic VSL#3 prevents antibiotic-associated diarrhoea in a double-blind, randomized, placebo-controlled clinical trial. J Hosp Infect. 2013;84:159–165. doi: 10.1016/j.jhin.2013.02.019. [DOI] [PubMed] [Google Scholar]
- 38.Donner CS. Pathophysiology and therapy of chronic radiation-induced injury to the colon. Dig Dis. 1998;16:253–261. doi: 10.1159/000016873. [DOI] [PubMed] [Google Scholar]
- 39.Delia P, Sansotta G, Donato V, Frosina P, Messina G, De Renzis C, Famularo G. Use of probiotics for prevention of radiation-induced diarrhea. World J Gastroenterol. 2007;13:912–915. doi: 10.3748/wjg.v13.i6.912. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Delia P, Sansotta G, Donato V, Messina G, Frosina P, Pergolizzi S, De Renzis C. Prophylaxis of diarrhoea in patients submitted to radiotherapeutic treatment on pelvic district: personal experience. Dig Liver Dis. 2002;34 Suppl 2:S84–S86. doi: 10.1016/s1590-8658(02)80173-6. [DOI] [PubMed] [Google Scholar]
- 41.Floch MH, Walker WA, Sanders ME, Nieuwdorp M, Kim AS, Brenner DA, Qamar AA, Miloh TA, Guarino A, Guslandi M, Dieleman LA, Ringel Y, Quigley EM, Brandt LJ. Recommendations for Probiotic Use--2015 Update: Proceedings and Consensus Opinion. J Clin Gastroenterol. 2015;49 Suppl 1:S69–S73. doi: 10.1097/MCG.0000000000000420. [DOI] [PubMed] [Google Scholar]
- 42.Bowen JM, Stringer AM, Gibson RJ, Yeoh AS, Hannam S, Keefe DM. VSL#3 probiotic treatment reduces chemotherapy-induced diarrhea and weight loss. Cancer Biol Ther. 2007;6:1449–1454. doi: 10.4161/cbt.6.9.4622. [DOI] [PubMed] [Google Scholar]
- 43.Parashar UD, Hummelman EG, Bresee JS, Miller MA, Glass RI. Global illness and deaths caused by rotavirus disease in children. Emerg Infect Dis. 2003;9:565–572. doi: 10.3201/eid0905.020562. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Dubey AP, Rajeshwari K, Chakravarty A, Famularo G. Use of VSL[sharp]3 in the treatment of rotavirus diarrhea in children: preliminary results. J Clin Gastroenterol. 2008;42 Suppl 3 Pt 1:S126–S129. doi: 10.1097/MCG.0b013e31816fc2f6. [DOI] [PubMed] [Google Scholar]
- 45.Montejo JC. Enteral nutrition-related gastrointestinal complications in critically ill patients: a multicenter study. The Nutritional and Metabolic Working Group of the Spanish Society of Intensive Care Medicine and Coronary Units. Crit Care Med. 1999;27:1447–1453. doi: 10.1097/00003246-199908000-00006. [DOI] [PubMed] [Google Scholar]
- 46.Frohmader TJ, Chaboyer WP, Robertson IK, Gowardman J. Decrease in frequency of liquid stool in enterally fed critically ill patients given the multispecies probiotic VSL#3: a pilot trial. Am J Crit Care. 2010;19:e1–11. doi: 10.4037/ajcc2010976. [DOI] [PubMed] [Google Scholar]
- 47.Lacouture ME, Keefe DM, Sonis S, Jatoi A, Gernhardt D, Wang T, Doherty JP, Giri N, Nadanaciva S, O'Connell J, Sbar E, Piperdi B, Garon EB. A phase II study (ARCHER 1042) to evaluate prophylactic treatment of dacomitinib-induced dermatologic and gastrointestinal adverse events in advanced non-small-cell lung cancer. Ann Oncol. 2016;27:1712–1718. doi: 10.1093/annonc/mdw227. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Mok T, Lee K, Tang M, Leung L. Dacomitinib for the treatment of advanced or metastatic non-small-cell lung cancer. Future Oncol. 2014;10:813–822. doi: 10.2217/fon.14.22. [DOI] [PubMed] [Google Scholar]
- 49.Bazzocchi G, Gionchetti P, Almerigi PF, Amadini C, Campieri M. Intestinal microflora and oral bacteriotherapy in irritable bowel syndrome. Dig Liver Dis. 2002;34 Suppl 2:S48–S53. doi: 10.1016/s1590-8658(02)80164-5. [DOI] [PubMed] [Google Scholar]
- 50.Kim HJ, Camilleri M, McKinzie S, Lempke MB, Burton DD, Thomforde GM, Zinsmeister AR. A randomized controlled trial of a probiotic, VSL#3, on gut transit and symptoms in diarrhoea-predominant irritable bowel syndrome. Aliment Pharmacol Ther. 2003;17:895–904. doi: 10.1046/j.1365-2036.2003.01543.x. [DOI] [PubMed] [Google Scholar]
- 51.Kim HJ, Vazquez Roque MI, Camilleri M, Stephens D, Burton DD, Baxter K, Thomforde G, Zinsmeister AR. A randomized controlled trial of a probiotic combination VSL# 3 and placebo in irritable bowel syndrome with bloating. Neurogastroenterol Motil. 2005;17:687–696. doi: 10.1111/j.1365-2982.2005.00695.x. [DOI] [PubMed] [Google Scholar]
- 52.Wong RK, Yang C, Song GH, Wong J, Ho KY. Melatonin regulation as a possible mechanism for probiotic (VSL#3) in irritable bowel syndrome: a randomized double-blinded placebo study. Dig Dis Sci. 2015;60:186–194. doi: 10.1007/s10620-014-3299-8. [DOI] [PubMed] [Google Scholar]
- 53.Guandalini S, Magazzù G, Chiaro A, La Balestra V, Di Nardo G, Gopalan S, Sibal A, Romano C, Canani RB, Lionetti P, Setty M. VSL#3 improves symptoms in children with irritable bowel syndrome: a multicenter, randomized, placebo-controlled, double-blind, crossover study. J Pediatr Gastroenterol Nutr. 2010;51:24–30. doi: 10.1097/MPG.0b013e3181ca4d95. [DOI] [PubMed] [Google Scholar]
- 54.Distrutti E, Cipriani S, Mencarelli A, Renga B, Fiorucci S. Probiotics VSL#3 protect against development of visceral pain in murine model of irritable bowel syndrome. PLoS One. 2013;8:e63893. doi: 10.1371/journal.pone.0063893. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Li YJ, Dai C, Jiang M. Mechanisms of Probiotic VSL#3 in a Rat Model of Visceral Hypersensitivity Involves the Mast Cell-PAR2-TRPV1 Pathway. Dig Dis Sci. 2019;64:1182–1192. doi: 10.1007/s10620-018-5416-6. [DOI] [PubMed] [Google Scholar]
- 56.Dai C, Guandalini S, Zhao DH, Jiang M. Antinociceptive effect of VSL#3 on visceral hypersensitivity in a rat model of irritable bowel syndrome: a possible action through nitric oxide pathway and enhance barrier function. Mol Cell Biochem. 2012;362:43–53. doi: 10.1007/s11010-011-1126-5. [DOI] [PubMed] [Google Scholar]
- 57.Whitehead WE, Palsson O, Jones KR. Systematic review of the comorbidity of irritable bowel syndrome with other disorders: what are the causes and implications? Gastroenterology. 2002;122:1140–1156. doi: 10.1053/gast.2002.32392. [DOI] [PubMed] [Google Scholar]
- 58.Vicari E, Calogero AE, Condorelli RA, Vicari LO, La Vignera S. Male accessory gland infection frequency in infertile patients with chronic microbial prostatitis and irritable bowel syndrome. Int J Androl. 2012;35:183–189. doi: 10.1111/j.1365-2605.2011.01216.x. [DOI] [PubMed] [Google Scholar]
- 59.Vicari E, Salemi M, Sidoti G, Malaguarnera M, Castiglione R. Symptom Severity Following Rifaximin and the Probiotic VSL#3 in Patients with Chronic Pelvic Pain Syndrome (Due to Inflammatory Prostatitis) Plus Irritable Bowel Syndrome. Nutrients. 2017;9 doi: 10.3390/nu9111208. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Vicari E, La Vignera S, Castiglione R, Condorelli RA, Vicari LO, Calogero AE. Chronic bacterial prostatitis and irritable bowel syndrome: effectiveness of treatment with rifaximin followed by the probiotic VSL#3. Asian J Androl. 2014;16:735–739. doi: 10.4103/1008-682X.131064. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Khalili H, Chan SSM, Lochhead P, Ananthakrishnan AN, Hart AR, Chan AT. The role of diet in the aetiopathogenesis of inflammatory bowel disease. Nat Rev Gastroenterol Hepatol. 2018;15:525–535. doi: 10.1038/s41575-018-0022-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Porter CK, Tribble DR, Aliaga PA, Halvorson HA, Riddle MS. Infectious gastroenteritis and risk of developing inflammatory bowel disease. Gastroenterology. 2008;135:781–786. doi: 10.1053/j.gastro.2008.05.081. [DOI] [PubMed] [Google Scholar]
- 63.Wang ZW, Ji F, Teng WJ, Yuan XG, Ye XM. Risk factors and gene polymorphisms of inflammatory bowel disease in population of Zhejiang, China. World J Gastroenterol. 2011;17:118–122. doi: 10.3748/wjg.v17.i1.118. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Pittayanon R, Lau JT, Leontiadis GI, Tse F, Yuan Y, Surette M, Moayyedi P. Differences in Gut Microbiota in Patients With vs Without Inflammatory Bowel Diseases: A Systematic Review. Gastroenterology. 2020;158:930–946.e1. doi: 10.1053/j.gastro.2019.11.294. [DOI] [PubMed] [Google Scholar]
- 65.Travis SP, Stange EF, Lémann M, Oresland T, Bemelman WA, Chowers Y, Colombel JF, D'Haens G, Ghosh S, Marteau P, Kruis W, Mortensen NJ, Penninckx F, Gassull M European Crohn's and Colitis Organisation (ECCO) European evidence-based Consensus on the management of ulcerative colitis: Current management. J Crohns Colitis. 2008;2:24–62. doi: 10.1016/j.crohns.2007.11.002. [DOI] [PubMed] [Google Scholar]
- 66.Venturi A, Gionchetti P, Rizzello F, Johansson R, Zucconi E, Brigidi P, Matteuzzi D, Campieri M. Impact on the composition of the faecal flora by a new probiotic preparation: preliminary data on maintenance treatment of patients with ulcerative colitis. Aliment Pharmacol Ther. 1999;13:1103–1108. doi: 10.1046/j.1365-2036.1999.00560.x. [DOI] [PubMed] [Google Scholar]
- 67.Tursi A, Brandimarte G, Papa A, Giglio A, Elisei W, Giorgetti GM, Forti G, Morini S, Hassan C, Pistoia MA, Modeo ME, Rodino' S, D'Amico T, Sebkova L, Sacca' N, Di Giulio E, Luzza F, Imeneo M, Larussa T, Di Rosa S, Annese V, Danese S, Gasbarrini A. Treatment of relapsing mild-to-moderate ulcerative colitis with the probiotic VSL#3 as adjunctive to a standard pharmaceutical treatment: a double-blind, randomized, placebo-controlled study. Am J Gastroenterol. 2010;105:2218–2227. doi: 10.1038/ajg.2010.218. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Sood A, Midha V, Makharia GK, Ahuja V, Singal D, Goswami P, Tandon RK. The probiotic preparation, VSL#3 induces remission in patients with mild-to-moderately active ulcerative colitis. Clin Gastroenterol Hepatol. 2009;7:1202–1209, 1209.e1. doi: 10.1016/j.cgh.2009.07.016. [DOI] [PubMed] [Google Scholar]
- 69.Greenfield SM, Punchard NA, Teare JP, Thompson RP. Review article: the mode of action of the aminosalicylates in inflammatory bowel disease. Aliment Pharmacol Ther. 1993;7:369–383. doi: 10.1111/j.1365-2036.1993.tb00110.x. [DOI] [PubMed] [Google Scholar]
- 70.Tursi A, Brandimarte G, Giorgetti GM, Forti G, Modeo ME, Gigliobianco A. Low-dose balsalazide plus a high-potency probiotic preparation is more effective than balsalazide alone or mesalazine in the treatment of acute mild-to-moderate ulcerative colitis. Med Sci Monit. 2004;10:PI126–PI131. [PubMed] [Google Scholar]
- 71.Bibiloni R, Fedorak RN, Tannock GW, Madsen KL, Gionchetti P, Campieri M, De Simone C, Sartor RB. VSL#3 probiotic-mixture induces remission in patients with active ulcerative colitis. Am J Gastroenterol. 2005;100:1539–1546. doi: 10.1111/j.1572-0241.2005.41794.x. [DOI] [PubMed] [Google Scholar]
- 72.Soo I, Madsen KL, Tejpar Q, Sydora BC, Sherbaniuk R, Cinque B, Di Marzio L, Cifone MG, Desimone C, Fedorak RN. VSL#3 probiotic upregulates intestinal mucosal alkaline sphingomyelinase and reduces inflammation. Can J Gastroenterol. 2008;22:237–242. doi: 10.1155/2008/520383. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Miele E, Pascarella F, Giannetti E, Quaglietta L, Baldassano RN, Staiano A. Effect of a probiotic preparation (VSL#3) on induction and maintenance of remission in children with ulcerative colitis. Am J Gastroenterol. 2009;104:437–443. doi: 10.1038/ajg.2008.118. [DOI] [PubMed] [Google Scholar]
- 74.Huynh HQ, deBruyn J, Guan L, Diaz H, Li M, Girgis S, Turner J, Fedorak R, Madsen K. Probiotic preparation VSL#3 induces remission in children with mild to moderate acute ulcerative colitis: a pilot study. Inflamm Bowel Dis. 2009;15:760–768. doi: 10.1002/ibd.20816. [DOI] [PubMed] [Google Scholar]
- 75.Bassaganya-Riera J, Viladomiu M, Pedragosa M, De Simone C, Carbo A, Shaykhutdinov R, Jobin C, Arthur JC, Corl BA, Vogel H, Storr M, Hontecillas R. Probiotic bacteria produce conjugated linoleic acid locally in the gut that targets macrophage PPAR γ to suppress colitis. PLoS One. 2012;7:e31238. doi: 10.1371/journal.pone.0031238. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Mennigen R, Nolte K, Rijcken E, Utech M, Loeffler B, Senninger N, Bruewer M. Probiotic mixture VSL#3 protects the epithelial barrier by maintaining tight junction protein expression and preventing apoptosis in a murine model of colitis. Am J Physiol Gastrointest Liver Physiol. 2009;296:G1140–G1149. doi: 10.1152/ajpgi.90534.2008. [DOI] [PubMed] [Google Scholar]
- 77.Gaudier E, Michel C, Segain JP, Cherbut C, Hoebler C. The VSL# 3 probiotic mixture modifies microflora but does not heal chronic dextran-sodium sulfate-induced colitis or reinforce the mucus barrier in mice. J Nutr. 2005;135:2753–2761. doi: 10.1093/jn/135.12.2753. [DOI] [PubMed] [Google Scholar]
- 78.Sang LX, Chang B, Dai C, Gao N, Liu WX, Jiang M. Heat-killed VSL#3 ameliorates dextran sulfate sodium (DSS)-induced acute experimental colitis in rats. Int J Mol Sci. 2013;15:15–28. doi: 10.3390/ijms15010015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Sang LX, Chang B, Wang BY, Liu WX, Jiang M. Live and heat-killed probiotic: effects on chronic experimental colitis induced by dextran sulfate sodium (DSS) in rats. Int J Clin Exp Med. 2015;8:20072–20078. [PMC free article] [PubMed] [Google Scholar]
- 80.Dai C, Zheng CQ, Meng FJ, Zhou Z, Sang LX, Jiang M. VSL#3 probiotics exerts the anti-inflammatory activity via PI3k/Akt and NF-κB pathway in rat model of DSS-induced colitis. Mol Cell Biochem. 2013;374:1–11. doi: 10.1007/s11010-012-1488-3. [DOI] [PubMed] [Google Scholar]
- 81.Fitzpatrick LR, Hertzog KL, Quatse AL, Koltun WA, Small JS, Vrana K. Effects of the probiotic formulation VSL#3 on colitis in weanling rats. J Pediatr Gastroenterol Nutr. 2007;44:561–570. doi: 10.1097/MPG.0b013e31803bda51. [DOI] [PubMed] [Google Scholar]
- 82.Cobrin GM, Abreu MT. Defects in mucosal immunity leading to Crohn's disease. Immunol Rev. 2005;206:277–295. doi: 10.1111/j.0105-2896.2005.00293.x. [DOI] [PubMed] [Google Scholar]
- 83.Marks DJ, Segal AW. Innate immunity in inflammatory bowel disease: a disease hypothesis. J Pathol. 2008;214:260–266. doi: 10.1002/path.2291. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Rutgeerts P, Geboes K, Vantrappen G, Beyls J, Kerremans R, Hiele M. Predictability of the postoperative course of Crohn's disease. Gastroenterology. 1990;99:956–963. doi: 10.1016/0016-5085(90)90613-6. [DOI] [PubMed] [Google Scholar]
- 85.Fedorak RN, Feagan BG, Hotte N, Leddin D, Dieleman LA, Petrunia DM, Enns R, Bitton A, Chiba N, Paré P, Rostom A, Marshall J, Depew W, Bernstein CN, Panaccione R, Aumais G, Steinhart AH, Cockeram A, Bailey RJ, Gionchetti P, Wong C, Madsen K. The probiotic VSL#3 has anti-inflammatory effects and could reduce endoscopic recurrence after surgery for Crohn's disease. Clin Gastroenterol Hepatol. 2015;13:928–35.e2. doi: 10.1016/j.cgh.2014.10.031. [DOI] [PubMed] [Google Scholar]
- 86.Di Giacinto C, Marinaro M, Sanchez M, Strober W, Boirivant M. Probiotics ameliorate recurrent Th1-mediated murine colitis by inducing IL-10 and IL-10-dependent TGF-beta-bearing regulatory cells. J Immunol. 2005;174:3237–3246. doi: 10.4049/jimmunol.174.6.3237. [DOI] [PubMed] [Google Scholar]
- 87.Chen X, Fu Y, Wang L, Qian W, Zheng F, Hou X. Bifidobacterium longum and VSL#3® amelioration of TNBS-induced colitis associated with reduced HMGB1 and epithelial barrier impairment. Dev Comp Immunol. 2019;92:77–86. doi: 10.1016/j.dci.2018.09.006. [DOI] [PubMed] [Google Scholar]
- 88.Isidro RA, Lopez A, Cruz ML, Gonzalez Torres MI, Chompre G, Isidro AA, Appleyard CB. The Probiotic VSL#3 Modulates Colonic Macrophages, Inflammation, and Microflora in Acute Trinitrobenzene Sulfonic Acid Colitis. J Histochem Cytochem. 2017;65:445–461. doi: 10.1369/0022155417718542. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Wall GC, Schirmer LL, Anliker LE, Tigges AE. Pharmacotherapy for acute pouchitis. Ann Pharmacother. 2011;45:1127–1137. doi: 10.1345/aph.1P790. [DOI] [PubMed] [Google Scholar]
- 90.Mimura T, Rizzello F, Helwig U, Poggioli G, Schreiber S, Talbot IC, Nicholls RJ, Gionchetti P, Campieri M, Kamm MA. Once daily high dose probiotic therapy (VSL#3) for maintaining remission in recurrent or refractory pouchitis. Gut. 2004;53:108–114. doi: 10.1136/gut.53.1.108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Gionchetti P, Rizzello F, Venturi A, Brigidi P, Matteuzzi D, Bazzocchi G, Poggioli G, Miglioli M, Campieri M. Oral bacteriotherapy as maintenance treatment in patients with chronic pouchitis: a double-blind, placebo-controlled trial. Gastroenterology. 2000;119:305–309. doi: 10.1053/gast.2000.9370. [DOI] [PubMed] [Google Scholar]
- 92.Gionchetti P, Rizzello F, Morselli C, Poggioli G, Tambasco R, Calabrese C, Brigidi P, Vitali B, Straforini G, Campieri M. High-dose probiotics for the treatment of active pouchitis. Dis Colon Rectum. 2007;50:2075–82; discussion 2082-2084. doi: 10.1007/s10350-007-9068-4. [DOI] [PubMed] [Google Scholar]
- 93.Shen B, Brzezinski A, Fazio VW, Remzi FH, Achkar JP, Bennett AE, Sherman K, Lashner BA. Maintenance therapy with a probiotic in antibiotic-dependent pouchitis: experience in clinical practice. Aliment Pharmacol Ther. 2005;22:721–728. doi: 10.1111/j.1365-2036.2005.02642.x. [DOI] [PubMed] [Google Scholar]
- 94.Pardi DS. Microscopic colitis: an update. Inflamm Bowel Dis. 2004;10:860–870. doi: 10.1097/00054725-200411000-00020. [DOI] [PubMed] [Google Scholar]
- 95.Rohatgi S, Ahuja V, Makharia GK, Rai T, Das P, Dattagupta S, Mishra V, Garg SK. VSL#3 induces and maintains short-term clinical response in patients with active microscopic colitis: a two-phase randomised clinical trial. BMJ Open Gastroenterol. 2015;2:e000018. doi: 10.1136/bmjgast-2014-000018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96.Tursi A, Brandimarte G, Giorgetti GM, Elisei W, Aiello F. Balsalazide and/or high-potency probiotic mixture (VSL#3) in maintaining remission after attack of acute, uncomplicated diverticulitis of the colon. Int J Colorectal Dis. 2007;22:1103–1108. doi: 10.1007/s00384-007-0299-6. [DOI] [PubMed] [Google Scholar]
- 97.Friederich P, van Heumen BW, Nagtegaal ID, Berkhout M, van Krieken JH, Peters WH, Nagengast FM. Increased epithelial cell proliferation in the ileal pouch mucosa of patients with familial adenomatous polyposis. Virchows Arch. 2007;451:659–667. doi: 10.1007/s00428-007-0451-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98.Friederich P, Verschuur J, van Heumen BW, Roelofs HM, Berkhout M, Nagtegaal ID, van Oijen MG, van Krieken JH, Peters WH, Nagengast FM. Effects of intervention with sulindac and inulin/VSL#3 on mucosal and luminal factors in the pouch of patients with familial adenomatous polyposis. Int J Colorectal Dis. 2011;26:575–582. doi: 10.1007/s00384-010-1127-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99.Jess T, Rungoe C, Peyrin-Biroulet L. Risk of colorectal cancer in patients with ulcerative colitis: a meta-analysis of population-based cohort studies. Clin Gastroenterol Hepatol. 2012;10:639–645. doi: 10.1016/j.cgh.2012.01.010. [DOI] [PubMed] [Google Scholar]
- 100.Chung EJ, Do EJ, Kim SY, Cho EA, Kim DH, Pak S, Hwang SW, Lee HJ, Byeon JS, Ye BD, Yang DH, Park SH, Yang SK, Kim JH, Myung SJ. Combination of metformin and VSL#3 additively suppresses western-style diet induced colon cancer in mice. Eur J Pharmacol. 2017;794:1–7. doi: 10.1016/j.ejphar.2016.11.012. [DOI] [PubMed] [Google Scholar]
- 101.Wang CS, Li WB, Wang HY, Ma YM, Zhao XH, Yang H, Qian JM, Li JN. VSL#3 can prevent ulcerative colitis-associated carcinogenesis in mice. World J Gastroenterol. 2018;24:4254–4262. doi: 10.3748/wjg.v24.i37.4254. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 102.Do EJ, Hwang SW, Kim SY, Ryu YM, Cho EA, Chung EJ, Park S, Lee HJ, Byeon JS, Ye BD, Yang DH, Park SH, Yang SK, Kim JH, Myung SJ. Suppression of colitis-associated carcinogenesis through modulation of IL-6/STAT3 pathway by balsalazide and VSL#3. J Gastroenterol Hepatol. 2016;31:1453–1461. doi: 10.1111/jgh.13280. [DOI] [PubMed] [Google Scholar]
- 103.Appleyard CB, Cruz ML, Isidro AA, Arthur JC, Jobin C, De Simone C. Pretreatment with the probiotic VSL#3 delays transition from inflammation to dysplasia in a rat model of colitis-associated cancer. Am J Physiol Gastrointest Liver Physiol. 2011;301:G1004–G1013. doi: 10.1152/ajpgi.00167.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 104.Bassaganya-Riera J, Viladomiu M, Pedragosa M, De Simone C, Hontecillas R. Immunoregulatory mechanisms underlying prevention of colitis-associated colorectal cancer by probiotic bacteria. PLoS One. 2012;7:e34676. doi: 10.1371/journal.pone.0034676. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 105.Arthur JC, Gharaibeh RZ, Uronis JM, Perez-Chanona E, Sha W, Tomkovich S, Mühlbauer M, Fodor AA, Jobin C. VSL#3 probiotic modifies mucosal microbial composition but does not reduce colitis-associated colorectal cancer. Sci Rep. 2013;3:2868. doi: 10.1038/srep02868. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 106.Younossi Z, Anstee QM, Marietti M, Hardy T, Henry L, Eslam M, George J, Bugianesi E. Global burden of NAFLD and NASH: trends, predictions, risk factors and prevention. Nat Rev Gastroenterol Hepatol. 2018;15:11–20. doi: 10.1038/nrgastro.2017.109. [DOI] [PubMed] [Google Scholar]
- 107.Angulo P. Nonalcoholic fatty liver disease. N Engl J Med. 2002;346:1221–1231. doi: 10.1056/NEJMra011775. [DOI] [PubMed] [Google Scholar]
- 108.Leung C, Rivera L, Furness JB, Angus PW. The role of the gut microbiota in NAFLD. Nat Rev Gastroenterol Hepatol. 2016;13:412–425. doi: 10.1038/nrgastro.2016.85. [DOI] [PubMed] [Google Scholar]
- 109.Loguercio C, Federico A, Tuccillo C, Terracciano F, D'Auria MV, De Simone C, Del Vecchio Blanco C. Beneficial effects of a probiotic VSL#3 on parameters of liver dysfunction in chronic liver diseases. J Clin Gastroenterol. 2005;39:540–543. doi: 10.1097/01.mcg.0000165671.25272.0f. [DOI] [PubMed] [Google Scholar]
- 110.Alisi A, Bedogni G, Baviera G, Giorgio V, Porro E, Paris C, Giammaria P, Reali L, Anania F, Nobili V. Randomised clinical trial: The beneficial effects of VSL#3 in obese children with non-alcoholic steatohepatitis. Aliment Pharmacol Ther. 2014;39:1276–1285. doi: 10.1111/apt.12758. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 111.Miccheli A, Capuani G, Marini F, Tomassini A, Praticò G, Ceccarelli S, Gnani D, Baviera G, Alisi A, Putignani L, Nobili V. Urinary (1)H-NMR-based metabolic profiling of children with NAFLD undergoing VSL#3 treatment. Int J Obes (Lond) 2015;39:1118–1125. doi: 10.1038/ijo.2015.40. [DOI] [PubMed] [Google Scholar]
- 112.Yadav H, Lee JH, Lloyd J, Walter P, Rane SG. Beneficial metabolic effects of a probiotic via butyrate-induced GLP-1 hormone secretion. J Biol Chem. 2013;288:25088–25097. doi: 10.1074/jbc.M113.452516. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 113.Li Z, Yang S, Lin H, Huang J, Watkins PA, Moser AB, Desimone C, Song XY, Diehl AM. Probiotics and antibodies to TNF inhibit inflammatory activity and improve nonalcoholic fatty liver disease. Hepatology. 2003;37:343–350. doi: 10.1053/jhep.2003.50048. [DOI] [PubMed] [Google Scholar]
- 114.Yuan M, Konstantopoulos N, Lee J, Hansen L, Li ZW, Karin M, Shoelson SE. Reversal of obesity- and diet-induced insulin resistance with salicylates or targeted disruption of Ikkbeta. Science. 2001;293:1673–1677. doi: 10.1126/science.1061620. [DOI] [PubMed] [Google Scholar]
- 115.Stancovski I, Baltimore D. NF-kappaB activation: the I kappaB kinase revealed? Cell. 1997;91:299–302. doi: 10.1016/s0092-8674(00)80413-4. [DOI] [PubMed] [Google Scholar]
- 116.Aguirre V, Uchida T, Yenush L, Davis R, White MF. The c-Jun NH(2)-terminal kinase promotes insulin resistance during association with insulin receptor substrate-1 and phosphorylation of Ser(307) J Biol Chem. 2000;275:9047–9054. doi: 10.1074/jbc.275.12.9047. [DOI] [PubMed] [Google Scholar]
- 117.Ma X, Hua J, Li Z. Probiotics improve high fat diet-induced hepatic steatosis and insulin resistance by increasing hepatic NKT cells. J Hepatol. 2008;49:821–830. doi: 10.1016/j.jhep.2008.05.025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 118.Liang S, Webb T, Li Z. Probiotic antigens stimulate hepatic natural killer T cells. Immunology. 2014;141:203–210. doi: 10.1111/imm.12180. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 119.Neuschwander-Tetri BA. Nonalcoholic steatohepatitis and the metabolic syndrome. Am J Med Sci. 2005;330:326–335. doi: 10.1097/00000441-200512000-00011. [DOI] [PubMed] [Google Scholar]
- 120.Velayudham A, Dolganiuc A, Ellis M, Petrasek J, Kodys K, Mandrekar P, Szabo G. VSL#3 probiotic treatment attenuates fibrosis without changes in steatohepatitis in a diet-induced nonalcoholic steatohepatitis model in mice. Hepatology. 2009;49:989–997. doi: 10.1002/hep.22711. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 121.Sung CK, She H, Xiong S, Tsukamoto H. Tumor necrosis factor-alpha inhibits peroxisome proliferator-activated receptor gamma activity at a posttranslational level in hepatic stellate cells. Am J Physiol Gastrointest Liver Physiol. 2004;286:G722–G729. doi: 10.1152/ajpgi.00411.2003. [DOI] [PubMed] [Google Scholar]
- 122.Chang B, Sang L, Wang Y, Tong J, Zhang D, Wang B. The protective effect of VSL#3 on intestinal permeability in a rat model of alcoholic intestinal injury. BMC Gastroenterol. 2013;13:151. doi: 10.1186/1471-230X-13-151. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 123.Wiest R, Garcia-Tsao G. Bacterial translocation (BT) in cirrhosis. Hepatology. 2005;41:422–433. doi: 10.1002/hep.20632. [DOI] [PubMed] [Google Scholar]
- 124.Inamura T, Miura S, Tsuzuki Y, Hara Y, Hokari R, Ogawa T, Teramoto K, Watanabe C, Kobayashi H, Nagata H, Ishii H. Alteration of intestinal intraepithelial lymphocytes and increased bacterial translocation in a murine model of cirrhosis. Immunol Lett. 2003;90:3–11. doi: 10.1016/j.imlet.2003.05.002. [DOI] [PubMed] [Google Scholar]
- 125.Steffen EK, Berg RD, Deitch EA. Comparison of translocation rates of various indigenous bacteria from the gastrointestinal tract to the mesenteric lymph node. J Infect Dis. 1988;157:1032–1038. doi: 10.1093/infdis/157.5.1032. [DOI] [PubMed] [Google Scholar]
- 126.Riordan SM, Williams R. Mechanisms of hepatocyte injury, multiorgan failure, and prognostic criteria in acute liver failure. Semin Liver Dis. 2003;23:203–215. doi: 10.1055/s-2003-42639. [DOI] [PubMed] [Google Scholar]
- 127.Gupta N, Kumar A, Sharma P, Garg V, Sharma BC, Sarin SK. Effects of the adjunctive probiotic VSL#3 on portal haemodynamics in patients with cirrhosis and large varices: a randomized trial. Liver Int. 2013;33:1148–1157. doi: 10.1111/liv.12172. [DOI] [PubMed] [Google Scholar]
- 128.Rincón D, Vaquero J, Hernando A, Galindo E, Ripoll C, Puerto M, Salcedo M, Francés R, Matilla A, Catalina MV, Clemente G, Such J, Bañares R. Oral probiotic VSL#3 attenuates the circulatory disturbances of patients with cirrhosis and ascites. Liver Int. 2014;34:1504–1512. doi: 10.1111/liv.12539. [DOI] [PubMed] [Google Scholar]
- 129.Jayakumar S, Carbonneau M, Hotte N, Befus AD, St Laurent C, Owen R, McCarthy M, Madsen K, Bailey RJ, Ma M, Bain V, Rioux K, Tandon P. VSL#3 ® probiotic therapy does not reduce portal pressures in patients with decompensated cirrhosis. Liver Int. 2013;33:1470–1477. doi: 10.1111/liv.12280. [DOI] [PubMed] [Google Scholar]
- 130.Marlicz W, Wunsch E, Mydlowska M, Milkiewicz M, Serwin K, Mularczyk M, Milkiewicz P, Raszeja-Wyszomirska J. The effect of short term treatment with probiotic VSL#3 on various clinical and biochemical parameters in patients with liver cirrhosis. J Physiol Pharmacol. 2016;67:867–877. [PubMed] [Google Scholar]
- 131.Rashid SK, Idris-Khodja N, Auger C, Alhosin M, Boehm N, Oswald-Mammosser M, Schini-Kerth VB. Probiotics (VSL#3) prevent endothelial dysfunction in rats with portal hypertension: role of the angiotensin system. PLoS One. 2014;9:e97458. doi: 10.1371/journal.pone.0097458. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 132.Shawcross DL, Sharifi Y, Canavan JB, Yeoman AD, Abeles RD, Taylor NJ, Auzinger G, Bernal W, Wendon JA. Infection and systemic inflammation, not ammonia, are associated with Grade 3/4 hepatic encephalopathy, but not mortality in cirrhosis. J Hepatol. 2011;54:640–649. doi: 10.1016/j.jhep.2010.07.045. [DOI] [PubMed] [Google Scholar]
- 133.Dhiman RK. Gut microbiota and hepatic encephalopathy. Metab Brain Dis. 2013;28:321–326. doi: 10.1007/s11011-013-9388-0. [DOI] [PubMed] [Google Scholar]
- 134.Bajaj JS, Ridlon JM, Hylemon PB, Thacker LR, Heuman DM, Smith S, Sikaroodi M, Gillevet PM. Linkage of gut microbiome with cognition in hepatic encephalopathy. Am J Physiol Gastrointest Liver Physiol. 2012;302:G168–G175. doi: 10.1152/ajpgi.00190.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 135.Pratap Mouli V, Benjamin J, Bhushan Singh M, Mani K, Garg SK, Saraya A, Joshi YK. Effect of probiotic VSL#3 in the treatment of minimal hepatic encephalopathy: A non-inferiority randomized controlled trial. Hepatol Res. 2015;45:880–889. doi: 10.1111/hepr.12429. [DOI] [PubMed] [Google Scholar]
- 136.Mittal VV, Sharma BC, Sharma P, Sarin SK. A randomized controlled trial comparing lactulose, probiotics, and L-ornithine L-aspartate in treatment of minimal hepatic encephalopathy. Eur J Gastroenterol Hepatol. 2011;23:725–732. doi: 10.1097/MEG.0b013e32834696f5. [DOI] [PubMed] [Google Scholar]
- 137.Lunia MK, Sharma BC, Sharma P, Sachdeva S, Srivastava S. Probiotics prevent hepatic encephalopathy in patients with cirrhosis: a randomized controlled trial. Clin Gastroenterol Hepatol. 2014;12:1003–8.e1. doi: 10.1016/j.cgh.2013.11.006. [DOI] [PubMed] [Google Scholar]
- 138.Dhiman RK, Rana B, Agrawal S, Garg A, Chopra M, Thumburu KK, Khattri A, Malhotra S, Duseja A, Chawla YK. Probiotic VSL#3 reduces liver disease severity and hospitalization in patients with cirrhosis: a randomized, controlled trial. Gastroenterology. 2014;147:1327–37.e3. doi: 10.1053/j.gastro.2014.08.031. [DOI] [PubMed] [Google Scholar]
- 139.Osterberg KL, Boutagy NE, McMillan RP, Stevens JR, Frisard MI, Kavanaugh JW, Davy BM, Davy KP, Hulver MW. Probiotic supplementation attenuates increases in body mass and fat mass during high-fat diet in healthy young adults. Obesity (Silver Spring) 2015;23:2364–2370. doi: 10.1002/oby.21230. [DOI] [PubMed] [Google Scholar]
- 140.Boutagy NE, Neilson AP, Osterberg KL, Smithson AT, Englund TR, Davy BM, Hulver MW, Davy KP. Probiotic supplementation and trimethylamine-N-oxide production following a high-fat diet. Obesity (Silver Spring) 2015;23:2357–2363. doi: 10.1002/oby.21212. [DOI] [PubMed] [Google Scholar]
- 141.Rajkumar H, Mahmood N, Kumar M, Varikuti SR, Challa HR, Myakala SP. Effect of probiotic (VSL#3) and omega-3 on lipid profile, insulin sensitivity, inflammatory markers, and gut colonization in overweight adults: a randomized, controlled trial. Mediators Inflamm. 2014;2014:348959. doi: 10.1155/2014/348959. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 142.de Kort S, Keszthelyi D, Masclee AA. Leaky gut and diabetes mellitus: what is the link? Obes Rev. 2011;12:449–458. doi: 10.1111/j.1467-789X.2010.00845.x. [DOI] [PubMed] [Google Scholar]
- 143.Calcinaro F, Dionisi S, Marinaro M, Candeloro P, Bonato V, Marzotti S, Corneli RB, Ferretti E, Gulino A, Grasso F, De Simone C, Di Mario U, Falorni A, Boirivant M, Dotta F. Oral probiotic administration induces interleukin-10 production and prevents spontaneous autoimmune diabetes in the non-obese diabetic mouse. Diabetologia. 2005;48:1565–1575. doi: 10.1007/s00125-005-1831-2. [DOI] [PubMed] [Google Scholar]
- 144.Dolpady J, Sorini C, Di Pietro C, Cosorich I, Ferrarese R, Saita D, Clementi M, Canducci F, Falcone M. Oral Probiotic VSL#3 Prevents Autoimmune Diabetes by Modulating Microbiota and Promoting Indoleamine 2,3-Dioxygenase-Enriched Tolerogenic Intestinal Environment. J Diabetes Res. 2016;2016:7569431. doi: 10.1155/2016/7569431. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 145.Matteoli G, Mazzini E, Iliev ID, Mileti E, Fallarino F, Puccetti P, Chieppa M, Rescigno M. Gut CD103+ dendritic cells express indoleamine 2,3-dioxygenase which influences T regulatory/T effector cell balance and oral tolerance induction. Gut. 2010;59:595–604. doi: 10.1136/gut.2009.185108. [DOI] [PubMed] [Google Scholar]
- 146.Brown K, Godovannyi A, Ma C, Zhang Y, Ahmadi-Vand Z, Dai C, Gorzelak MA, Chan Y, Chan JM, Lochner A, Dutz JP, Vallance BA, Gibson DL. Prolonged antibiotic treatment induces a diabetogenic intestinal microbiome that accelerates diabetes in NOD mice. ISME J. 2016;10:321–332. doi: 10.1038/ismej.2015.114. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 147.Belgardt BF, Okamura T, Brüning JC. Hormone and glucose signalling in POMC and AgRP neurons. J Physiol. 2009;587:5305–5314. doi: 10.1113/jphysiol.2009.179192. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 148.Di Felice G, Barletta B, Butteroni C, Corinti S, Tinghino R, Colombo P, Boirivant M. Use of probiotic bacteria for prevention and therapy of allergic diseases: studies in mouse model of allergic sensitization. J Clin Gastroenterol. 2008;42 Suppl 3 Pt 1:S130–S132. doi: 10.1097/MCG.0b013e318169c463. [DOI] [PubMed] [Google Scholar]
- 149.Schiavi E, Barletta B, Butteroni C, Corinti S, Boirivant M, Di Felice G. Oral therapeutic administration of a probiotic mixture suppresses established Th2 responses and systemic anaphylaxis in a murine model of food allergy. Allergy. 2011;66:499–508. doi: 10.1111/j.1398-9995.2010.02501.x. [DOI] [PubMed] [Google Scholar]
- 150.Barletta B, Rossi G, Schiavi E, Butteroni C, Corinti S, Boirivant M, Di Felice G. Probiotic VSL#3-induced TGF-β ameliorates food allergy inflammation in a mouse model of peanut sensitization through the induction of regulatory T cells in the gut mucosa. Mol Nutr Food Res. 2013;57:2233–2244. doi: 10.1002/mnfr.201300028. [DOI] [PubMed] [Google Scholar]
- 151.Thang CL, Boye JI, Zhao X. Low doses of allergen and probiotic supplementation separately or in combination alleviate allergic reactions to cow β-lactoglobulin in mice. J Nutr. 2013;143:136–141. doi: 10.3945/jn.112.169466. [DOI] [PubMed] [Google Scholar]
- 152.Beilharz JE, Kaakoush NO, Maniam J, Morris MJ. Cafeteria diet and probiotic therapy: cross talk among memory, neuroplasticity, serotonin receptors and gut microbiota in the rat. Mol Psychiatry. 2018;23:351–361. doi: 10.1038/mp.2017.38. [DOI] [PubMed] [Google Scholar]
- 153.Distrutti E, O'Reilly JA, McDonald C, Cipriani S, Renga B, Lynch MA, Fiorucci S. Modulation of intestinal microbiota by the probiotic VSL#3 resets brain gene expression and ameliorates the age-related deficit in LTP. PLoS One. 2014;9:e106503. doi: 10.1371/journal.pone.0106503. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 154.D'Mello C, Ronaghan N, Zaheer R, Dicay M, Le T, MacNaughton WK, Surrette MG, Swain MG. Probiotics Improve Inflammation-Associated Sickness Behavior by Altering Communication between the Peripheral Immune System and the Brain. J Neurosci. 2015;35:10821–10830. doi: 10.1523/JNEUROSCI.0575-15.2015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 155.Kigerl KA, Hall JC, Wang L, Mo X, Yu Z, Popovich PG. Gut dysbiosis impairs recovery after spinal cord injury. J Exp Med. 2016;213:2603–2620. doi: 10.1084/jem.20151345. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 156.Koren O, Spor A, Felin J, Fåk F, Stombaugh J, Tremaroli V, Behre CJ, Knight R, Fagerberg B, Ley RE, Bäckhed F. Human oral, gut, and plaque microbiota in patients with atherosclerosis. Proc Natl Acad Sci USA. 2011;108 Suppl 1:4592–4598. doi: 10.1073/pnas.1011383107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 157.Chan YK, El-Nezami H, Chen Y, Kinnunen K, Kirjavainen PV. Probiotic mixture VSL#3 reduce high fat diet induced vascular inflammation and atherosclerosis in ApoE(-/-) mice. AMB Express. 2016;6:61. doi: 10.1186/s13568-016-0229-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 158.Mencarelli A, Cipriani S, Renga B, Bruno A, D'Amore C, Distrutti E, Fiorucci S. VSL#3 resets insulin signaling and protects against NASH and atherosclerosis in a model of genetic dyslipidemia and intestinal inflammation. PLoS One. 2012;7:e45425. doi: 10.1371/journal.pone.0045425. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 159.Li JY, Chassaing B, Tyagi AM, Vaccaro C, Luo T, Adams J, Darby TM, Weitzmann MN, Mulle JG, Gewirtz AT, Jones RM, Pacifici R. Sex steroid deficiency-associated bone loss is microbiota dependent and prevented by probiotics. J Clin Invest. 2016;126:2049–2063. doi: 10.1172/JCI86062. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 160.Ballard O, Morrow AL. Human milk composition: nutrients and bioactive factors. Pediatr Clin North Am. 2013;60:49–74. doi: 10.1016/j.pcl.2012.10.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 161.Fernández L, Langa S, Martín V, Maldonado A, Jiménez E, Martín R, Rodríguez JM. The human milk microbiota: origin and potential roles in health and disease. Pharmacol Res. 2013;69:1–10. doi: 10.1016/j.phrs.2012.09.001. [DOI] [PubMed] [Google Scholar]
- 162.Mastromarino P, Capobianco D, Miccheli A, Praticò G, Campagna G, Laforgia N, Capursi T, Baldassarre ME. Administration of a multistrain probiotic product (VSL#3) to women in the perinatal period differentially affects breast milk beneficial microbiota in relation to mode of delivery. Pharmacol Res. 2015;95-96:63–70. doi: 10.1016/j.phrs.2015.03.013. [DOI] [PubMed] [Google Scholar]
- 163.Donati L, Di Vico A, Nucci M, Quagliozzi L, Spagnuolo T, Labianca A, Bracaglia M, Ianniello F, Caruso A, Paradisi G. Vaginal microbial flora and outcome of pregnancy. Arch Gynecol Obstet. 2010;281:589–600. doi: 10.1007/s00404-009-1318-3. [DOI] [PubMed] [Google Scholar]
- 164.Vitali B, Cruciani F, Baldassarre ME, Capursi T, Spisni E, Valerii MC, Candela M, Turroni S, Brigidi P. Dietary supplementation with probiotics during late pregnancy: outcome on vaginal microbiota and cytokine secretion. BMC Microbiol. 2012;12:236. doi: 10.1186/1471-2180-12-236. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 165.Onderdonk AB. Probiotics for women's health. J Clin Gastroenterol. 2006;40:256–259. doi: 10.1097/00004836-200603000-00018. [DOI] [PubMed] [Google Scholar]
- 166.Mardini HE, Grigorian AY. Probiotic mix VSL#3 is effective adjunctive therapy for mild to moderately active ulcerative colitis: a meta-analysis. Inflamm Bowel Dis. 2014;20:1562–1567. doi: 10.1097/MIB.0000000000000084. [DOI] [PubMed] [Google Scholar]
- 167.Razafindralambo H, Correani V, Fiorucci S, Mattei B. Variability in Probiotic Formulations Revealed by Proteomics and Physico-chemistry Approach in Relation to the Gut Permeability. Probiotics Antimicrob Proteins. 2019 doi: 10.1007/s12602-019-09590-1. [DOI] [PubMed] [Google Scholar]