Abstract
BACKGROUND
Adherence to antihypertensives is suboptimal, but previous methods of quantifying adherence fail to account for varying patterns of use over time. We sought to improve classification of antihypertensive adherence using group-based trajectory models, and to determine whether individual factors predict adherence trajectories.
METHODS
We identified older adults initiating antihypertensive therapy during 2008–2011 using a 20% sample of Medicare (federal health insurance available to US residents over the age of 65) beneficiaries enrolled in parts A (inpatient services), B (outpatient services), and D (prescription medication). We developed monthly adherence indicators using prescription fill dates and days supply data in the 12 months following initiation. Adherence was defined as having at least 80% of days covered. Logistic models were used to identify trajectory groups. Bayesian information criterion and trajectory group size were used to select the optimal trajectory model. We compared the distribution of covariates across trajectory groups using multivariable logistic regression.
RESULTS
During 2008–2011, 282,520 Medicare beneficiaries initiated antihypertensive therapy (mean age 75 years, 60% women, 84% White). Six trajectories were identified ranging from perfect adherence (12-month adherence of 0.97, 40% of beneficiaries) to immediate stopping (12-month adherence of 0.10, 18% of beneficiaries). The strongest predictors of nonadherence were initiation with a single antihypertensive class (adjusted odds ratio = 2.08 (95% confidence interval: 2.00–2.13)), Hispanic (2.93 (2.75–3.11)) or Black race/ethnicity (2.04 (1.95–2.13)), and no prior history of hypertension (2.04 (2.00–2.08)) (Area under the receiving operating characteristic curve: 0.53).
CONCLUSIONS
There is substantial variation in antihypertensive adherence among older adults. Certain patient characteristics are likely determinants of antihypertensive adherence trajectories.
Keywords: antihypertensive adherence, blood pressure, epidemiology, hypertension, older adults
An estimated 65% of older adults have elevated blood pressure (hypertension) or take antihypertensive medications.1 The prevalence of hypertension increases with age due to changes in metabolic and vascular functioning.2,3 Hypertension increases the risk for cardiovascular diseases, kidney disease, and death.2,4 Antihypertensive medications reduce the risk of cardiovascular disease among hypertensive patients,5–7 yet few older adults are adherent to these medications.8 A meta-analysis reported antihypertensive adherence of 49% after 1 year.9 Failure to remain adherent can lead to increased risk of cardiovascular disease, hospitalizations, and mortality.5,10–12 Older adults are at greater risk of nonadherence due to polypharmacy and increased comorbidities.2 Female gender, low income, presence of comorbidities, mental health disorders, and cognitive impairment are associated with nonadherence.2,9,13,14
Commonly used adherence measures, such as proportion days covered (PDC) and the medication possession ratio, quantify the number of days covered with medications over a defined period of time,8,13 but miss the time-varying nature of medication adherence.8,15 Good control of this variability in medication adherence may be critical in studies of factors that strongly depend on age. Group-based trajectory models (GBTM) can quantify these time-varying patterns,16–18 accounting for dynamic patterns of medication use without assumptions about trajectory shape.15,19 In a study of adults initiating statins, GBTMs distinguished between adherent and nonadherent users better than time-static adherence measures.15
Despite these advantages, no prior study has used GBTMs to model antihypertensive adherence trajectories among older adults initiating therapy. Our objectives were to (i) use GBTMs to identify antihypertensive adherence trajectories in the first year following initiation, (ii) compare adherence trajectories to traditional adherence measures, and (iii) examine whether patient characteristics predict adherence trajectories.
METHODS
Data
We used a 20% nationwide, random sample of fee-for-service Medicare beneficiaries who were enrolled at least 1 month in Medicare parts A (inpatient care), B (outpatient care), and D (prescription drug) coverage between 2007 and 2011. Medicare is the federally provided health insurance available to all US residents ≥65 years old and fee-for-service is the part of Medicare where individual insurance claims are sent directly to the Centers for Medicare and Medicaid Services (CMS). Data were obtained under an agreement between CMS and the University of North Carolina at Chapel Hill (UNC). The study protocol was approved by the UNC’s Institutional Review Board (#15–1704).
Cohort
The cohort included Medicare beneficiaries initiating antihypertensive therapy during 2008–2011 who were continuously enrolled in Medicare Parts A, B, and D for at least 12 months prior to initiation (index date). New use was defined as no prior prescription in the last 12 months of angiotensin-converting enzyme inhibitors, angiotensin receptor blockers, beta blockers, calcium channel blockers, or thiazide diuretics (Supplementary Table 1A).
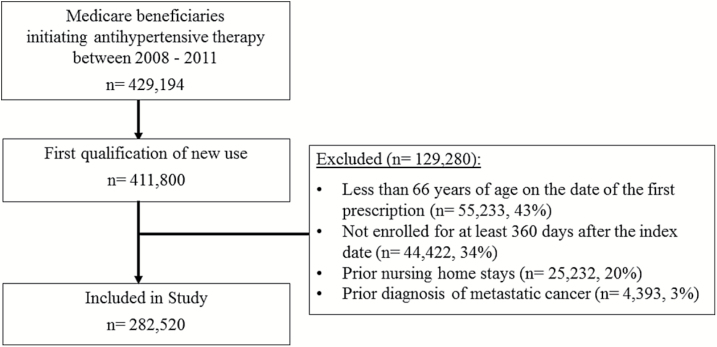
We limited the cohort to first time new users of antihypertensives. To ensure 1 full year of Medicare enrollment prior to initiation, beneficiaries were >66 years old. Beneficiaries with nursing home stays or metastatic cancer claims in the last 12 months were excluded since these factors could affect medication adherence. To capture patterns of antihypertensive use, only beneficiaries enrolled in Medicare for ≥1 year following initiation were included (Figure 1).
Figure 1.
Eligibility flow chart for the study cohort.
Antihypertensive adherence
Patterns of antihypertensive use were defined using date of dispensing and days supply data. Starting on the index date, we counted the number of days each month a beneficiary was covered by an antihypertensive drug class recommended for hypertension treatment in older adults (angiotensin-converting enzyme inhibitors, angiotensin receptor blockers, beta blockers, calcium channel blockers, or thiazide diuretics).2 If a new prescription was filled prior to the end of the last day’s supply, the day of the new prescription began the day after the prior prescription would have ended.
After counting days covered each month, binary indicator variables specified whether coverage by an antihypertensive occurred for ≥24 of 30 days (80%). The 80% threshold has high sensitivity (92%) and specificity (89%) for distinguishing adherent from nonadherent antihypertensive patients20 and is associated with improved cardiovascular health.12 We calculated 2 common adherence measures: proportion months covered (PMC) and PDC. PMC was the number of months a beneficiary had ≥80% days covered divided by 12 (total follow-up months). PDC was the number of days covered with an antihypertensive medication divided by 360 (total follow-up days).
Predictors of adherence trajectories
Potential predictors of antihypertensive adherence trajectories were selected based on literature2,9,13,21,22 and defined based on claims during the 12 months prior to initiation. Demographics (age, gender, and race/ethnicity) were identified using the Medicare Denominator File. We categorized antihypertensive medication initiated on the index date as combination therapy (more than one class of antihypertensive) or monotherapy. Concurrent medication use was the number of distinct drugs prescribed in the 14 days prior to the index date. We identified whether beneficiaries were in the Medicare coverage gap during the baseline period. Medicare covers most drug-related expenses until a beneficiary reaches a threshold amount each year, at which time Medicare no longer covers these expenses unless the costs exceed another threshold amount.23 We identified whether beneficiaries were eligible for the Medicare low-income subsidy (LIS) program (proxy for sociodemographic status), which offers medication at a reduced cost for beneficiaries that are eligible due to income, family size, and household resources. Finally, we identified whether beneficiaries had prescriptions for loop diuretics, antiarrhythmics, antidepressants, antiepileptics, anxiolytics, benzodiazepines, opioids, and hypnotics.
Chronic health predictors included: diabetes, chronic kidney disease, Parkinson’s disease, Alzheimer’s disease, chronic obstructive pulmonary disease, congestive heart failure, arrhythmia, osteoarthritis, rheumatoid arthritis, stroke, myocardial infarction, hypertension, obesity, and fractures (Supplementary Table 2A).
We used the frailty index score (FIS) as a proxy measure of frailty.24 FIS was developed using Medicare data to predict limitations in activities of daily living based on factors associated with frailty including: demographics, chronic health conditions, geriatric syndromes, medical equipment use, and health screenings. We examined variables positively (ambulance transfer, wheelchair/walker use, home oxygen use, hospital bed, difficulty walking, and vertigo) and inversely (cancer screenings) associated with limitations in activities of daily living.24 Finally, we assessed examined hospital admissions, long-term hospital stays, and short-term hospital stays in the year prior to the index date.
ANALYSIS
Trajectory models
We used GBTMs to group beneficiaries by patterns of antihypertensive use. GBTMs are a type of mixed models originally developed to model changes in behavior.19,25 We chose to use GBTMs over other modeling techniques because GBTMs do not require prior assumptions about trajectory shapes.19
GBTMs were estimated using logistic regression models. Dependent variables were the monthly binary indicators of antihypertensive use, and the independent variables were months since initiation. GBTMs were not adjusted for baseline covariates. Time was modeled using linear and cubic terms. We started with a 2-group model and subsequently added up to 7 groups. The maximum of 7 groups was imposed to avoid small group sizes. We used Bayesian information criterion, group size, and the average posterior probability to identify the optimal number of groups. Bayesian information criterion is a measure of model fit with lower scores signifying better fit. The average posterior probability signifies how well beneficiaries fit within the trajectory group they are assigned (typical threshold for defining good fit is 0.7).19
We examined spaghetti plots (stacked individual line plots of the number of days covered with antihypertensives each month) for a random sample of 500 beneficiaries to verify that the average trends of use aligned with the trajectories identified with the best-fitting GBTM (results not shown). As a sensitivity analysis, we repeated the GBTM analyses removing beneficiaries who were in the Medicare insurance gap during the follow-up period to verify that the GBTM results were not driven by these individuals. Trajectories were defined using “Proc Traj”.26
Comparison of adherence measures
We compared the GBTM results to traditional static adherence measures, PDC and PMC, using the monthly binary indicators as the gold standard. We separated months into adherent vs. nonadherent and assigned adherence measures to each month. GBTM values varied across months, but PDC and PMC did not. Area under the receiving operating characteristic curves compared adherence measures ability to discriminate between adherent vs. nonadherent months, with a value of one indicating perfect discrimination.27–29
Predictors of adherence
We evaluated predictors of adherence by first examining the distribution of covariates across trajectory groups. Next, we used multivariable logistic models to examine associations between baseline covariates and trajectory groups. Adjusting for all baseline covariates, we calculated odds ratios (ORs) and 95% confidence intervals (95% CIs). The outcome of interest was being in a specific trajectory group vs. the most adherent group. Strength of the ORs were determined by examining the distance from the null value (OR = 1) and by examining their precision (width of the 95% CIs). Area under the receiving operating characteristic curve statistics quantified the ability of the predictors to discriminate between trajectory groups.
Since previous medication persistence is predictive of future use,30,31 we examined whether prior persistence with statins (medications frequently prescribed to older adults) improved prediction of antihypertensive trajectories in a subgroup of beneficiaries who had any statin filled ≥180 days prior to the index date. Statin persistence was defined as ≥180 days continuously covered by a statin, allowing for a 30-day grace period between prescription fills.
Lastly, we conducted post-hoc subgroup analyses stratified by Medicare race/ethnicity (White, Black, and Hispanic) to see if the GBTMs and adjusted multivariable models differed according across racial/ethnic groups.
RESULTS
During 2008–2011, 282,520 Medicare beneficiaries initiated antihypertensive therapy. On average beneficiaries were 75 years old, 60% were women, and 84% were White. Most beneficiaries initiated therapy with 1 antihypertensive class (86%) and the mean days supplied on the index date was 32.
Antihypertensive adherence trajectories
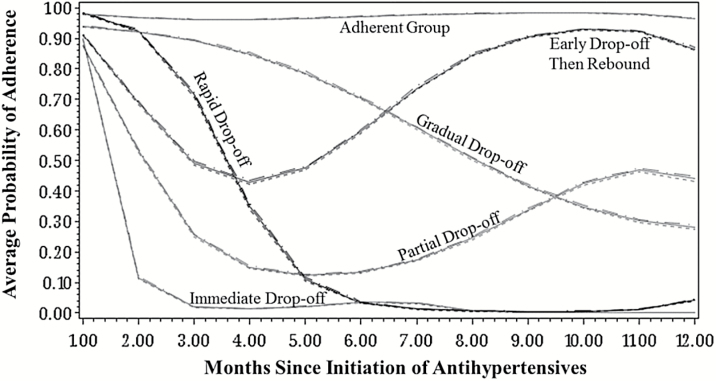
After fitting GBTMs with different groupings, the 6-group trajectory model was the best fit (Figure 2, Supplementary Table 3A). Beneficiaries were grouped as adherent (40%, mean adherence: 0.97); early drop-off then rebound to almost full adherence (10%, mean adherence: 0.73); partial drop-off (10%, mean adherence: 0.35); gradual drop-off (14%, mean adherence: 0.63); rapid drop-off (8%, mean adherence: 0.27); and immediate drop-off (18%, mean adherence: 0.10) (Table 1). When we removed beneficiaries in the insurance gap period during follow-up (n = 43,595, 15%), the 6-group model remained the best-fitting model and the trajectories were similar to those from the full cohort (Supplementary Figure 1A).
Figure 2.
Antihypertensive adherence trajectories in the 12 months following initiation of therapy.
Table 1.
Antihypertensive adherence trajectories in the 12 months following initiation according to adherence measures
| Group size | Average probability of adherence a | Proportion days covered (PDC) | Proportion months covered (PMC) | Average posterior probability b | ||||||
|---|---|---|---|---|---|---|---|---|---|---|
| Trajectory group | N | % | Mean | Std | Mean | Std | Mean | Std | Mean | Std |
| Immediate drop-off | 50,797 | 18.0 | 0.095 | 0.257 | 0.136 | 0.092 | 0.099 | 0.050 | 0.887 | 0.174 |
| Rapid drop-off | 22,404 | 7.9 | 0.267 | 0.385 | 0.318 | 0.093 | 0.281 | 0.064 | 0.856 | 0.177 |
| Gradual drop-off | 39,953 | 14.1 | 0.629 | 0.258 | 0.708 | 0.137 | 0.636 | 0.135 | 0.855 | 0.170 |
| Partial drop-off | 29,429 | 10.4 | 0.346 | 0.226 | 0.465 | 0.147 | 0.352 | 0.118 | 0.865 | 0.161 |
| Early drop-off then rebound | 28,304 | 10.0 | 0.733 | 0.196 | 0.789 | 0.100 | 0.720 | 0.100 | 0.818 | 0.151 |
| Adherent | 111,633 | 39.5 | 0.973 | 0.016 | 0.979 | 0.031 | 0.975 | 0.043 | 0.956 | 0.086 |
| Comparison of adherence measures ability to distinguish between adherent and nonadherent months | ||||||||||
| Adherence measure | AUCc | 95% Confidence interval (CI) | ||||||||
| PDC | 0.914 | 0.914, 0.914 | ||||||||
| PMC | 0.918 | 0.918, 0.919 | ||||||||
| Six-group trajectory model | 0.954 | 0.954, 0.955 | ||||||||
Overall model BIC for 6-group trajectory model: -1300277. BIC is used as a measure of model fit. Lower BIC values signify better model fit. Logistic regression models were used to identify trajectory groups. The dependent variables were the monthly binary indicators of antihypertensive use and months since start of antihypertensive therapy were the independent variables. Time was modeled using cubic terms. Abbreviations: BIC, Bayesian information criterion.
aAverage probability of being at least 80% adherent over 12 months of follow-up.
bIndicates how well beneficiaries fit in their assigned group. 0.70 is typically used as a threshold to signify good model fit.
cArea under the curve (AUC) statistics are used to quantify the ability of the measures to discriminate between adherent and nonadherent months. Values of 1 symbolize perfect discrimination.
Comparison of GBTMs to traditional adherence measures
The 6-group trajectory model discriminated better between adherent and nonadherent months than PDC and PMC (Area under the receiving operating characteristic curve 95%, 91%, and 92%, respectively; Table 1). In results stratified according to trajectory group, the trajectory model outperformed PDC and PMC for all groups except the adherent group (Area under the receiving operating characteristic curve 66%, 87%, and 89%, respectively; Supplementary Table 4A).
Predictors of adherence trajectories
Individual factors that varied between trajectory groups were race/ethnicity, initiation with combination therapy, days supply on the index date, opioid use, history of chronic obstructive pulmonary disease or cardiovascular disease (e.g., arrhythmia, hypertension, or myocardial infarction), vertigo, prior cancer screenings, and hospital utilization (Table 2). In the adjusted, multivariable analysis, factors most predictive of being nonadherent were: initiation with monotherapy vs. more than one class of antihypertensive drug (adjusted [aOR]: 2.08, 95% CI: 2.00–2.13), non-White race/ethnicity (Black vs. White aOR: 2.04, 95% CI: 1.95–2.13, Hispanic vs. White aOR: 2.93, 95% CI: 2.75–3.11), and having no prior history of hypertension (aOR: 2.04, 95% CI: 2.00–2.08) or myocardial infarction (aOR: 2.00, 95% CI: 1.85–2.17) (OR > 1.0 indicates nonadherence). Other factors strongly predictive of nonadherence were having a high probability of being frail, Parkinson’s disease, opioid use, no prior history of being in the Medicare insurance gap, vertigo, chronic obstructive pulmonary disease, and no prior history of having hospital admissions during baseline (Table 3).
Table 2.
Distribution of baseline characteristics according to adherence trajectory among Medicare beneficiaries initiating antihypertensives
| Trajectory group, % | ||||||
|---|---|---|---|---|---|---|
| Immediate drop-off | Rapid drop-off | Gradual drop-off | Partial drop-off | Early drop-off then rebound | Adherent | |
| Covariates | n = 50,797 | n = 22,404 | n = 39,953 | n = 29,429 | n = 28,304 | n = 111,633 |
| Female gender | 59.9 | 57.3 | 58.9 | 59.8 | 61.1 | 60.8 |
| Mean age (std) | 75.3 (7.1) | 75.0 (7.0) | 75.0 (7.0) | 75.1 (7.0) | 75.2 (7.1) | 75.0 (7.1) |
| Race/ethnicity | ||||||
| White | 80.8 | 82.2 | 83.6 | 77.9 | 83.4 | 88.6 |
| African American | 8.4 | 6.8 | 6.9 | 10.4 | 7.6 | 5.2 |
| Hispanic | 4.8 | 4.7 | 3.6 | 5.0 | 3.5 | 1.9 |
| Othera | 6.0 | 6.4 | 5.9 | 6.7 | 5.6 | 4.3 |
| Initiated with combination therapy | 8.7 | 12.1 | 13.6 | 11.8 | 13.5 | 17.7 |
| Average days supply on index date (std) | 27.3 (9.0) | 40.6 (25.9) | 35.1 (21.9) | 29.6 (15.1) | 30.6 (17.5) | 32.6 (19.7) |
| Medication use 14 days prior to index date | ||||||
| 1–2 Meds | 55.0 | 56.0 | 55.6 | 57.0 | 55.3 | 53.5 |
| 3–4 Meds | 30.1 | 29.3 | 28.9 | 29.1 | 29.1 | 29.1 |
| 5 or More meds | 15.0 | 14.7 | 15.5 | 13.9 | 15.6 | 17.3 |
| Insurance gap during baseline | 16.0 | 15.7 | 16.4 | 14.5 | 16.5 | 16.7 |
| Eligible for low-income subsidy | 5.6 | 5.3 | 5.6 | 6.1 | 5.7 | 5.6 |
| Loop diuretic | 9.5 | 9.6 | 10.4 | 9.4 | 10.7 | 11.1 |
| Antiarrhythmic | 5.2 | 4.5 | 4.5 | 4.7 | 4.8 | 4.6 |
| Antidepressantb | 17.4 | 17.1 | 17.7 | 16.5 | 17.8 | 16.8 |
| Antiepileptic | 10.5 | 10.1 | 9.6 | 9.2 | 10.2 | 9.1 |
| Anxiolytic | 4.9 | 4.4 | 4.2 | 4.4 | 4.1 | 3.7 |
| Benzodiazepine | 1.5 | 1.3 | 1.2 | 1.2 | 1.2 | 1.2 |
| Opioid | 37.1 | 33.2 | 33.0 | 34.2 | 34.0 | 30.2 |
| Hypnotic | 8.5 | 8.0 | 7.3 | 7.5 | 7.7 | 6.7 |
| Diabetes | 24.8 | 28.3 | 27.6 | 28.3 | 28.6 | 25.5 |
| Chronic kidney disease | 11.9 | 11.8 | 12.0 | 11.8 | 12.4 | 11.5 |
| Parkinson’s disease | 2.0 | 1.7 | 1.7 | 1.5 | 1.7 | 1.5 |
| Alzheimer’s disease | 3.3 | 2.8 | 3.3 | 2.7 | 3.3 | 3.5 |
| COPD | 19.5 | 17.3 | 17.5 | 17.3 | 17.7 | 16.4 |
| Congestive heart failure | 10.1 | 9.8 | 10.7 | 9.9 | 10.6 | 11.5 |
| Arrhythmia | 20.9 | 20.5 | 20.9 | 18.3 | 20.4 | 23.4 |
| Osteoarthritis | 18.7 | 18.0 | 17.4 | 18.5 | 18.2 | 16.1 |
| Rheumatoid arthritis | 3.8 | 3.5 | 3.5 | 3.8 | 3.5 | 3.1 |
| Stroke | 16.1 | 15.9 | 16.1 | 15.2 | 16.8 | 16.6 |
| Myocardial infarction | 1.8 | 2.0 | 2.4 | 1.5 | 2.1 | 3.8 |
| Hypertension | 66.4 | 73.2 | 77.8 | 74.6 | 79.1 | 79.6 |
| Obesity | 4.6 | 4.8 | 5.0 | 4.8 | 5.3 | 5.0 |
| Fracture history | 9.8 | 8.2 | 8.4 | 8.8 | 8.7 | 7.9 |
| Average frailty predictor index (std)c | 0.11 (0.1) | 0.10 (0.1) | 0.10 (0.1) | 0.10 (0.1) | 0.11 (0.1) | 0.10 (0.1) |
| Home oxygen use | 5.1 | 4.5 | 4.2 | 4.3 | 4.4 | 4.1 |
| Walker or wheelchair use | 4.5 | 4.0 | 3.9 | 4.2 | 4.2 | 3.8 |
| Hospital bed | 1.2 | 0.8 | 1.0 | 1.0 | 1.0 | 0.9 |
| Difficulty walking | 11.4 | 10.6 | 10.5 | 10.4 | 11.0 | 10.3 |
| Vertigo | 15.9 | 14.6 | 13.7 | 14.5 | 14.0 | 12.7 |
| Ambulance transport | 14.8 | 11.8 | 12.6 | 11.7 | 12.9 | 13.7 |
| Cancer screenings | 34.6 | 35.5 | 37.3 | 34.8 | 37.7 | 39.5 |
| Hospital admissions | 25.2 | 21.2 | 21.5 | 19.6 | 21.7 | 23.8 |
| Long stay admissions | 2.1 | 1.5 | 1.6 | 1.5 | 1.8 | 1.8 |
| Short-term hospital stays | 24.3 | 20.6 | 20.8 | 18.8 | 20.8 | 23.0 |
Abbreviations: COPD, chronic obstructive pulmonary disease; Index date, start of antihypertensive therapy; std, standard deviation.
aOther includes Asian, North American Native, and other race/ethnicities.
bAntidepressants include selective serotonin reuptake inhibitors, tricyclics, monoamine oxidase inhibitors, serotonin, and norepinephrine inhibitors.
cHigher scores denote a higher probability of being frail.
Table 3.
Strongest predictors of antihypertensive nonadherence among Medicare beneficiaries initiating antihypertensive therapy
| Multivariable odds ratios (ORs) and 95% confidence intervals (CIs) | ||||||
|---|---|---|---|---|---|---|
| Immediate drop-off | Rapid drop-off | Gradual drop-off | Partial drop-off | Early drop-off then rebound | Adherent | |
| Initiation with monotherapya | 2.08 (2.00, 2.13) | 1.49 (1.43, 1.56) | 1.33 (1.28, 1.37) | 1.54 (1.47, 1.59) | 1.32 (1.27, 1.37) | 1 |
| Race/ethnicity | ||||||
| White race | Ref | Ref | Ref | Ref | Ref | 1 |
| Black race | 2.04 (1.95, 2.13) | 1.69 (1.59, 1.79) | 1.51 (1.44, 1.59) | 2.42 (2.30, 2.54) | 1.59 (1.51, 1.68) | 1 |
| Hispanic race | 2.93 (2.75, 3.11) | 2.77 (2.56, 2.99) | 2.02 (1.88, 2.16) | 2.85 (2.66, 3.06) | 1.88 (1.74, 2.03) | 1 |
| Other raceb | 1.66 (1.58, 1.74) | 1.61 (1.51, 1.72) | 1.48 (1.40, 1.56) | 1.81 (1.71, 1.91) | 1.44 (1.35, 1.53) | 1 |
| No history of hypertension | 2.04 (2.00, 2.08) | 1.49 (1.45, 1.56) | 1.15 (1.12, 1.19) | 1.41 (1.37, 1.45) | 1.08 (1.04, 1.11) | 1 |
| No history of myocardial infarction | 2.00 (1.85, 2.17) | 1.52 (1.35,1.67) | 1.28 (1.19, 1.39) | 1.92 (1.72, 2.13) | 1.49 (1.35, 1.64) | 1 |
| Frailty predictor index | 1.32 (1.16, 1.47) | 1.20 (1.03, 1.43) | 1.11 (0.98, 1.27) | 1.59 (1.37, 1.85) | 1.32 (1.15, 1.52) | 1 |
| Parkinson’s disease | 1.37 (1.26, 1.49) | 1.27 (1.13, 1.44) | 1.18 (1.07, 1.30) | 1.18 (1.05, 1.32) | 1.20 (1.08, 1.34) | 1 |
| No history of arrhythmia | 1.30 (1.25, 1.33) | 1.15 (1.10, 1.19) | 1.14 (1.10, 1.16) | 1.32 (1.27, 1.35) | 1.18 (1.14, 1.22) | 1 |
| Opioid use | 1.33 (1.30, 1.36) | 1.18 (1.14, 1.22) | 1.16 (1.13, 1.19) | 1.25 (1.22, 1.29) | 1.18 (1.15, 1.22) | 1 |
| No history of being in the insurance gap | 1.25 (1.22, 1.30) | 1.14 (1.09, 1.19) | 1.09 (1.04, 1.12) | 1.27 (1.22, 1.32) | 1.12 (1.08, 1.16) | 1 |
| Vertigo | 1.26 (1.22, 1.30) | 1.21 (1.15. 1.26) | 1.10 (1.06, 1.14) | 1.17 (1.13, 1.22) | 1.08 (1.04, 1.13) | 1 |
| COPD | 1.24 (1.20, 1.28) | 1.15 (1.10, 1.20) | 1.15 (1.11, 1.19) | 1.17 (1.13, 1.22) | 1.14 (1.09, 1.18) | 1 |
| No history of hospital admissions | 1.23 (1.05, 1.43) | 1.43 (1.12, 1.82) | 1.22 (1.02, 1.47) | 1.19 (0.96, 1.47) | 1.19 (0.98, 1.45) | 1 |
Odds ratios and 95% CIs are adjusted for all baseline covariates. ORs >1 are predictive of nonadherence. AUC-statistic for fully adjusted model: 0.525. Only the strongest predictors of nonadherence are shown, see Table 5A in Supplementary for full listing of baseline covariates. Prevalence of baseline characteristics were assessed in the 12 months prior to initiation. Abbreviation: AUC, Area under the receiving operating characteristic curves; COPD, chronic obstructive pulmonary disease.
aInitiated with more than one class of antihypertensive drug vs. more than one class of antihypertensive drug. We did not distinguish between single and combination therapy medications.
bOther includes Asian, North American Native, and other race/ethnicities.
In the post-hoc stratified analysis, we found similar adherence trajectories across racial/ethnic groups, but the distribution of beneficiaries within trajectory groups varied (Supplementary Figure 2A, Table 6A). The proportion in the adherent group was higher for White than Black and Hispanic beneficiaries (45% vs. 32% and 26%, respectively). In multivariable models stratified by race/ethnicity, overall the same covariates were predictive of being adherent across race/ethnic groups (Supplementary Table 7A and 8A). However, in contrast to White beneficiaries, female gender and history of being in the low-income subsidy were strongly associated with being adherent among Black and Hispanic beneficiaries.
Prior statin persistence and adherence trajectories
Statins were dispensed ≥180 days prior to the start of antihypertensive use for 25% of beneficiaries (n = 69,668). Of those, 68% (n = 47,668) were persistent for ≥180 days. Prior statin persistence was predictive of being more adherent (Table 4). After adjustment, prior statin persistence was strongly associated with not being in the partial drop-off group vs. adherent (aOR: 0.40, 95% CI 0.38–0.42).
Table 4.
Influence of prior statin persistence on antihypertensive adherence trajectories following initiation of antihypertensive therapy
| Statin persist subcohort (n = 69,668) | Trajectory group (n, %) | |||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Immediate drop-off | Rapid drop-off | Gradual drop-off | Partial drop-off | Early drop-off then rebound | Adherent | |||||||
| Persistent ≥ 180 days (n = 47,668) | 6,727 | 14.1 | 3,295 | 6.9 | 6,517 | 13.7 | 3,781 | 7.9 | 4,759 | 10.0 | 22,589 | 47.4 |
| Not persistent at least 180 days (n = 22,000) | 4,215 | 19.2 | 2,003 | 9.1 | 3,501 | 15.9 | 2,973 | 13.5 | 2,454 | 11.2 | 6,854 | 31.2 |
| Baseline prediction model + prior statin persistence | ||||||||||||
| Multivariable odds ratio (95% CI)a | 0.48 (0.46, 0.51) | 0.49 (0.46, 0.53) | 0.56 (0.54, 0.59) | 0.40 (0.38, 0.42) | 0.60 (0.57, 0.64) | Referent | ||||||
AUC-statistic adjusted for all baseline covariates and prior statin persistence: 0.531. Abbreviation: AUC, Area under the receiving operating characteristic curves; CI, confidence interval; OR, odds ratio.
aOdds ratio comparing prior statin persistence and the odds of belonging to the adherent group. Adjusted for all baseline covariates. ORs <1 are predictive of being more adherent. Sixty-eight observations were removed due to missing beneficiary race/ethnicity.
DISCUSSION
Overall, GBTMs are effective for identifying patterns of antihypertensive adherence among older adults initiating therapy. We identified 6 adherence trajectories ranging from fully adherent to beneficiaries who never returned after their first prescription. Nearly half of beneficiaries remained adherent in the year following initiation. Compared to traditional adherence measures, GBTMs were better at distinguishing fluctuating adherence patterns. Individual factors predictive of adherence included initiation with combination therapy, White race, and history of cardiovascular disease.
To our knowledge, no previous study has used GBTMs to identify antihypertensive adherence trajectories among Medicare beneficiaries initiating therapy. The 6 trajectories identified are similar to previous studies that used GBTMs to model medication adherence to other drugs.15,16,31,32 Similar to other studies,15,32 GBTMs were better than PMC and PDC at distinguishing between adherent and nonadherent patients, especially for beneficiaries with fluctuating adherence. Physician visits, health screenings, and hospitalizations can influence patients stopping and reinitiating with statins,33 and these factors should be confirmed for other chronic medications. Future research could use GBTMs to identify time-dependent factors influencing fluctuations in medication behavior.
Similar to past studies,21,22 beneficiaries with cardiovascular diseases and those who initiated therapy with more than one class of antihypertensive were more likely to be adherent. These older adults may be more aware of the importance of being adherent due to more severe hypertension and a history of cardiovascular disease. However, a large proportion of beneficiaries with a history of cardiovascular disease were not adherent. For instance, 21% of beneficiaries in the immediate drop-off group had arrhythmia and 10% had congestive heart failure. Our results suggest clinicians should encourage older adults with cardiovascular disease to remain adherent to antihypertensives.
Our results that prior statin persistence predicted antihypertensive adherence confirm that past medication behavior predicts future medication adherence.30,31 Bushnell et al. found that prior persistence to chronic medication was associated with improved antidepressant persistence among adults.30 Similarly, Franklin et al. found that initial statin adherence improved the prediction of future statin adherence trajectories.31 Prior persistence to other chronic medications may help clinicians identify patients who are more or less likely to remain adherent to antihypertensives therapy.
In our post-hoc analysis, we found similar adherence trajectories across racial/ethnic groups; however, the distribution of beneficiaries within these trajectories varied. These differences are likely explained by intrinsic variations in barriers to adherence across racial/ethnic groups. For instance, history of cardiovascular disease was predictive of being adherent across race/ethnic groups. However, the strength of these associations was strongest among White beneficiaries. Despite having the highest prevalence of stroke, hypertension, and congestive heart failure, Black beneficiaries had fewer adherent beneficiaries. Other factors, besides prior health experiences, may be stronger predictors of adherence among Black beneficiaries. Previous studies found social support, health literacy, and access to a primary care to be strong predictors of adherence among Black hypertensive adults.34,35 Cost was cited a stronger barrier to adherence than medication-related side effects and perceived need for the medication among Black and Hispanic older adults.36 However, these barriers cannot be examined in Medicare claims data. Given that hypertension prevalence is highest among Non-Hispanic Blacks,1 more research is needed to identify differences in barriers to antihypertensive adherence across various race/ethnicity subpopulations. Expanding access to the Medicare low-income subsidy may be one mechanism to improve adherence among racial minority groups.
This study has limitations. An antihypertensive prescription dispensed does not guarantee that the beneficiary is taking the medication as prescribed. However, since a copayment is required for most dispensed prescriptions, it is reasonable to assume that patients actually take their antihypertensives after the first refill. Antihypertensive medications obtained outside of Part D (e.g., medication purchased out-of-pocket, through private insurance, or samples provided by physicians) were not captured. Fortunately, most antihypertensives are generic and sample use is less likely.37,38 Additionally, multiple- vs. single-pill combination therapy were not distinguished. Adherence tends to decrease with increasing treatment complexity14; therefore, beneficiaries initiating with single-pill combination therapy may have been more likely to remain adherent compared to beneficiaries initiating combination therapy with multiple pills.
Our results may be subject to residual confounding related to uncontrolled frailty measures and time-varying factors affecting medication adherence. Physician visits, frailty, and medication-related adverse events are time-varying factors that likely affect antihypertensive adherence. For instance, a history of fractures was weakly associated with being nonadherent, potentially because individuals with prior fractures stop use of antihypertensive medication due to unwanted side effects. A dramatic decline in blood pressure (e.g., hypotension or hypoperfusion) with initiation of antihypertensives may increase the risk of falls and subsequent fractures, and older adults may discontinue use of these new medications.39 Further research is needed to examine the impact of time-varying covariates, including those affected by prior treatment, on antihypertensive adherence trajectories.
Lastly, results of traditional adherence measures vs. GBTMs should be interpreted with caution. We included PDC and PMC to highlight that these static adherence measures fall short of separating adherence from persistence. Further research using external indicators, such as mortality outcomes or cardiovascular events, could validate the use of GBTMs over traditional adherence measures.
Our finding that nearly half of Medicare beneficiaries were adherent to their antihypertensive medication in the year following initiation is encouraging. GBTMs are an effective tool for visualizing and capturing patterns of antihypertensive use among older adult populations. Future studies can use GBTMs to identify factors related to a return to adherence after an initial decline and to assess the link of adherence trajectories with improved clinical outcomes. Interventions for improving antihypertensive medication adherence may need to be tailored for subpopulations of patients with hypertension. These results may guide researchers and clinicians in identifying older adult populations for interventions to increase adherence.
SUPPLEMENTARY MATERIAL
Supplementary data are available at American Journal of Hypertension online.
DISCLOSURE
T.S. receives investigator-initiated research funding and support as Principal Investigator from the National Institute on Aging (R01/56 AG023178), and as Co-Investigator, National Institutes of Health (NIH). He also receives salary support as Director of the Comparative Effectiveness Research (CER) Strategic Initiative, NC TraCS Institute, UNC Clinical and Translational Science Award (UL1TR001111) and as Director of the Center for Pharmacoepidemiology (current members: GlaxoSmithKline, UCB BioSciences, Merck) and research support from pharmaceutical companies (Amgen, AstraZeneca) to the Department of Epidemiology, University of North Carolina at Chapel Hill. Dr. Stürmer does not accept personal compensation of any kind from any pharmaceutical company. He owns stock in Novartis, Roche, BASF, AstraZeneca, and Novo Nordisk. Other authors declared no conflict of interest.
Supplementary Material
ACKNOWLEDGMENTS
The database infrastructure used for this project was funded by the Pharmacoepidemiology Gillings Innovation Lab (PEGIL) for the Population-Based Evaluation of Drug Benefits and Harms in Older US Adults (GIL200811.0010); the Center for Pharmacoepidemiology, Department of Epidemiology, UNC Gillings School of Global Public Health; the CER Strategic Initiative of UNC’s Clinical Translational Science Award (UL1TR001111); the Cecil G. Sheps Center for Health Services Research, UNC, and the UNC School of Medicine.
REFERENCES
- 1. Yoon SS, Carroll MD, Fryar CD. Hypertension prevalence and control among adults: United states, 2011–2014. NCHS Data Brief. 2015;220:1–8. [PubMed] [Google Scholar]
- 2. Aronow WS, Fleg JL, Pepine CJ, Artinian NT, Bakris G, Brown AS, Ferdinand KC, Ann Forciea M, Frishman WH, Jaigobin C, Kostis JB, Mancia G, Oparil S, Ortiz E, Reisin E, Rich MW, Schocken DD, Weber MA, Wesley DJ. ACCF/AHA 2011 expert consensus document on hypertension in the elderly: a report of the American College of Cardiology Foundation Task Force on Clinical Expert Consensus documents developed in collaboration with the American Academy of Neurology, American Geriatrics Society, American Society for Preventive Cardiology, American Society of Hypertension, American Society of Nephrology, Association of Black Cardiologists, and European Society of Hypertension. J Am Coll Cardiol 2011; 57:2037–2114. [DOI] [PubMed] [Google Scholar]
- 3. Rosenthal T, Nussinovitch N. Managing hypertension in the elderly in light of the changes during aging. Blood Press 2008; 17:186–194. [DOI] [PubMed] [Google Scholar]
- 4. Go AS, Mozaffarian D, Roger VL, Benjamin EJ, Berry JD, Borden WB, Bravata DM, Dai S, Ford ES, Fox CS, Franco S, Fullerton HJ, Gillespie C, Hailpern SM, Heit JA, Howard VJ, Huffman MD, Kissela BM, Kittner SJ, Lackland DT, Lichtman JH, Lisabeth LD, Magid D, Marcus GM, Marelli A, Matchar DB, McGuire DK, Mohler ER, Moy CS, Mussolino ME, Nichol G, Paynter NP, Schreiner PJ, Sorlie PD, Stein J, Turan TN, Virani SS, Wong ND, Woo D, Turner MB; American Heart Association Statistics Committee and Stroke Statistics Subcommittee Executive summary: heart disease and stroke statistics–2013 update: a report from the American Heart Association. Circulation 2013; 127:143–152. [DOI] [PubMed] [Google Scholar]
- 5. Corrao G, Rea F, Ghirardi A, Soranna D, Merlino L, Mancia G. Adherence with antihypertensive drug therapy and the risk of heart failure in clinical practice. Hypertension 2015; 66:742–749. [DOI] [PubMed] [Google Scholar]
- 6. James PA, Oparil S, Carter BL, Cushman WC, Dennison-Himmelfarb C, Handler J, Lackland DT, LeFevre ML, MacKenzie TD, Ogedegbe O, Smith SC, Jr, Svetkey LP, Taler SJ, Townsend RR, Wright JT, Jr, Narva AS, Ortiz E. 2014 evidence-based guideline for the management of high blood pressure in adults: report from the panel members appointed to the Eighth Joint National Committee (JNC 8). JAMA 2014; 311:507–520. [DOI] [PubMed] [Google Scholar]
- 7. Law MR, Morris JK, Wald NJ. Use of blood pressure lowering drugs in the prevention of cardiovascular disease: meta-analysis of 147 randomised trials in the context of expectations from prospective epidemiological studies. BMJ 2009; 338:b1665. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Kronish IM, Ye S. Adherence to cardiovascular medications: lessons learned and future directions. Prog Cardiovasc Dis 2013; 55:590–600. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Lemstra M, Alsabbagh MW. Proportion and risk indicators of nonadherence to antihypertensive therapy: a meta-analysis. Patient Prefer Adherence 2014; 8:211–218. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Kim S, Shin DW, Yun JM, Hwang Y, Park SK, Ko YJ, Cho B. Medication adherence and the risk of cardiovascular mortality and hospitalization among patients with newly prescribed antihypertensive medications. Hypertension 2016; 67:506–512. [DOI] [PubMed] [Google Scholar]
- 11. Chowdhury R, Khan H, Heydon E, Shroufi A, Fahimi S, Moore C, Stricker B, Mendis S, Hofman A, Mant J, Franco OH. Adherence to cardiovascular therapy: a meta-analysis of prevalence and clinical consequences. Eur Heart J 2013; 34:2940–2948. [DOI] [PubMed] [Google Scholar]
- 12. Ho PM, Bryson CL, Rumsfeld JS. Medication adherence: its importance in cardiovascular outcomes. Circulation 2009; 119:3028–3035. [DOI] [PubMed] [Google Scholar]
- 13. Lehmann A, Aslani P, Ahmed R, Celio J, Gauchet A, Bedouch P, Bugnon O, Allenet B, Schneider MP. Assessing medication adherence: options to consider. Int J Clin Pharm 2014; 36:55–69. [DOI] [PubMed] [Google Scholar]
- 14. Burnier M. Medication adherence and persistence as the cornerstone of effective antihypertensive therapy. Am J Hypertens 2006; 19:1190–1196. [DOI] [PubMed] [Google Scholar]
- 15. Franklin JM, Shrank WH, Pakes J, Sanfélix-Gimeno G, Matlin OS, Brennan TA, Choudhry NK. Group-based trajectory models: a new approach to classifying and predicting long-term medication adherence. Med Care 2013; 51:789–796. [DOI] [PubMed] [Google Scholar]
- 16. Bateman BT, Franklin JM, Bykov K, Avorn J, Shrank WH, Brennan TA, Landon JE, Rathmell JP, Huybrechts KF, Fischer MA, Choudhry NK. Persistent opioid use following cesarean delivery: patterns and predictors among opioid-naïve women. Am J Obstet Gynecol 2016; 215:353.e1–353.e18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Franklin JM, Krumme AA, Tong AY, Shrank WH, Matlin OS, Brennan TA, Choudhry NK. Association between trajectories of statin adherence and subsequent cardiovascular events. Pharmacoepidemiol Drug Saf 2015; 24:1105–1113. [DOI] [PubMed] [Google Scholar]
- 18. Newman-Casey PA, Blachley T, Lee PP, Heisler M, Farris KB, Stein JD. Patterns of glaucoma medication adherence over four years of follow-up. Ophthalmology 2015; 122:2010–2021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Nagin DS, Odgers CL. Group-based trajectory modeling in clinical research. Annu Rev Clin Psychol 2010; 6:109–138. [DOI] [PubMed] [Google Scholar]
- 20. Hansen RA, Kim MM, Song L, Tu W, Wu J, Murray MD. Comparison of methods to assess medication adherence and classify nonadherence. Ann Pharmacother 2009; 43:413–422. [DOI] [PubMed] [Google Scholar]
- 21. Mazzaglia G, Ambrosioni E, Alacqua M, Filippi A, Sessa E, Immordino V, Borghi C, Brignoli O, Caputi AP, Cricelli C, Mantovani LG. Adherence to antihypertensive medications and cardiovascular morbidity among newly diagnosed hypertensive patients. Circulation 2009; 120:1598–1605. [DOI] [PubMed] [Google Scholar]
- 22. Chapman RH, Petrilla AA, Benner JS, Schwartz JS, Tang SS. Predictors of adherence to concomitant antihypertensive and lipid-lowering medications in older adults: a retrospective, cohort study. Drugs Aging 2008; 25:885–892. [DOI] [PubMed] [Google Scholar]
- 23. Centers for Medicare and Medicaid Services. Costs in the coverage gap. Medicare.gov Web site <https://www.medicare.gov/part-d/costs/coverage-gap/part-d-coverage-gap.html> 2016. Accessed 24 June 2016.
- 24. Faurot KR, Jonsson Funk M, Pate V, Brookhart MA, Patrick A, Hanson LC, Castillo WC, Stürmer T. Using claims data to predict dependency in activities of daily living as a proxy for frailty. Pharmacoepidemiol Drug Saf 2015; 24:59–66. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Muthén B, Shedden K. Finite mixture modeling with mixture outcomes using the EM algorithm. Biometrics 1999; 55:463–469. [DOI] [PubMed] [Google Scholar]
- 26. Jones BL, Nagin DS, Roeder K. A SAS procedure based on mixture models for estimating developmental trajectories. Socio Meth Res 2001;29:374–393. [Google Scholar]
- 27. DeLong ER, DeLong DM, Clarke-Pearson DL. Comparing the areas under two or more correlated receiver operating characteristic curves: a nonparametric approach. Biometrics 1988; 44:837–845. [PubMed] [Google Scholar]
- 28. Hanley JA, McNeil BJ. The meaning and use of the area under a receiver operating characteristic (ROC) curve. Radiology 1982; 143:29–36. [DOI] [PubMed] [Google Scholar]
- 29. Harrell FE, Jr, Califf RM, Pryor DB, Lee KL, Rosati RA. Evaluating the yield of medical tests. JAMA 1982; 247:2543–2546. [PubMed] [Google Scholar]
- 30. Bushnell GA, Stürmer T, White A, Pate V, Swanson SA, Azrael D, Miller M. Predicting persistence to antidepressant treatment in administrative claims data: Considering the influence of refill delays and prior persistence on other medications. J Affect Disord 2016; 196:138–147. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Franklin JM, Krumme AA, Shrank WH, Matlin OS, Brennan TA, Choudhry NK. Predicting adherence trajectory using initial patterns of medication filling. Am J Manag Care 2015; 21:e537–e544. [PubMed] [Google Scholar]
- 32. Li Y, Zhou H, Cai B, Kahler KH, Tian H, Gabriel S, Arcona S. Group-based trajectory modeling to assess adherence to biologics among patients with psoriasis. Clinicoecon Outcomes Res 2014; 6:197–208. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Brookhart MA, Patrick AR, Schneeweiss S, Avorn J, Dormuth C, Shrank W, van Wijk BL, Cadarette SM, Canning CF, Solomon DH. Physician follow-up and provider continuity are associated with long-term medication adherence: a study of the dynamics of statin use. Arch Intern Med 2007; 167:847–852. [DOI] [PubMed] [Google Scholar]
- 34. Gerber BS, Cho YI, Arozullah AM, Lee SY. Racial differences in medication adherence: a cross-sectional study of Medicare enrollees. Am J Geriatr Pharmacother 2010; 8:136–145. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Schoenthaler AM, Butler M, Chaplin W, Tobin J, Ogedegbe G. Predictors of changes in medication adherence in Blacks with hypertension: moving beyond cross-sectional data. Ann Behav Med 2016; 50:642–652. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. Gellad WF, Haas JS, Safran DG. Race/ethnicity and nonadherence to prescription medications among seniors: results of a national study. J Gen Intern Med 2007; 22:1572–1578. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37. Li X, Camelo Castillo W, Stürmer T, Pate V, Gray CL, Simpson RJ, Jr, Setoguchi S, Hanson LC, Jonsson Funk M. Use of combination antihypertensive therapy initiation in older Americans without prevalent cardiovascular disease. J Am Geriatr Soc 2014; 62:1729–1735. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38. Hampp C, Greene P, Pinheiro SP. Use of prescription drug samples in the USA: a descriptive study with considerations for pharmacoepidemiology. Drug Saf 2016; 39:261–270. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39. Butt DA, Harvey PJ. Benefits and risks of antihypertensive medications in the elderly. J Intern Med 2015; 278:599–626. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.