Abstract
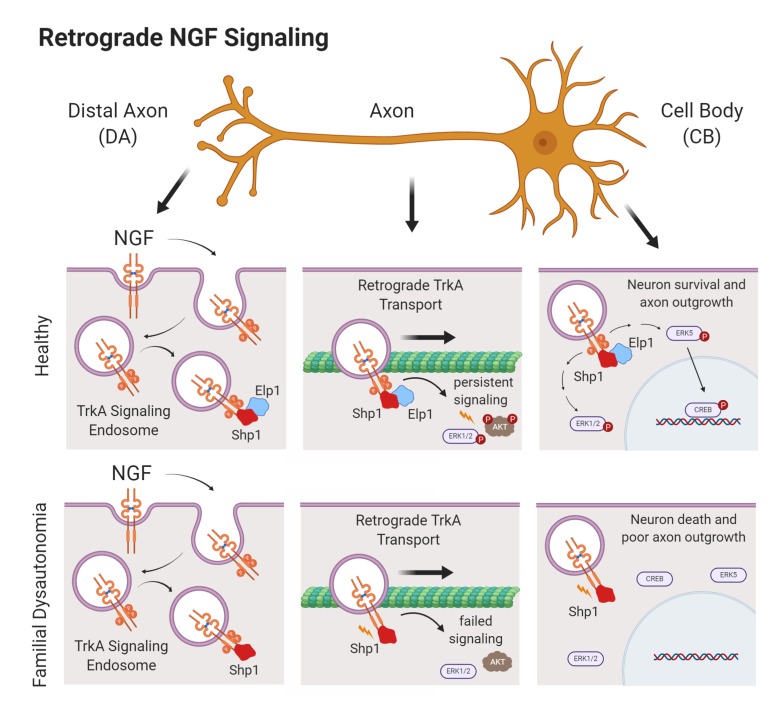
Familial dysautonomia (FD) is the most prevalent form of hereditary sensory and autonomic neuropathy (HSAN). In FD, a germline mutation in the Elp1 gene leads to Elp1 protein decrease that causes sympathetic neuron death and sympathetic nervous system dysfunction (dysautonomia). Elp1 is best known as a scaffolding protein within the nuclear hetero-hexameric transcriptional Elongator protein complex, but how it functions in sympathetic neuron survival is very poorly understood. Here, we identified a cytoplasmic function for Elp1 in sympathetic neurons that was essential for retrograde nerve growth factor (NGF) signaling and neuron target tissue innervation and survival. Elp1 was found to bind to internalized TrkA receptors in an NGF-dependent manner, where it was essential for maintaining TrkA receptor phosphorylation (activation) by regulating PTPN6 (Shp1) phosphatase activity within the signaling complex. In the absence of Elp1, Shp1 was hyperactivated, leading to premature TrkA receptor dephosphorylation, which resulted in retrograde signaling failure and neuron death. Inhibiting Shp1 phosphatase activity in the absence of Elp1 rescued NGF-dependent retrograde signaling, and in an animal model of FD it rescued abnormal sympathetic target tissue innervation. These results suggest that regulation of retrograde NGF signaling in sympathetic neurons by Elp1 may explain sympathetic neuron loss and physiologic dysautonomia in patients with FD.
Keywords: Cell Biology, Neuroscience
Keywords: Neurological disorders, Signal transduction, growth factors
Introduction
Humans with FD have debilitating sympathetic nervous system dysfunction (dysautonomia) that impairs normal cardiovascular, renal, gastrointestinal, and pulmonary function, and most patients afflicted by the disease have a shortened lifespan despite aggressive symptomatic treatment (1, 2). FD is caused by a homozygous splice-site mutation in the Elp1 gene (also known as IKBKAP), which leads to nonsense-mediated mRNA degradation and reduced Elp1 protein (3, 4). Elp1 was identified as a noncatalytic scaffolding protein within the heterohexameric transcriptional Elongator complex (5) and therefore, abnormalities in gene transcription have been thought to be central to the pathogenesis of dysautonomia in FD (6–8). However, most Elp1 protein is localized in the cytoplasm of cells, suggesting that it may also have a function apart from transcription. Previous studies have shown that Elp1, as part of the Elongator complex, may have a role in alpha-tubulin acetylation to regulate cortical neuron migration and differentiation (9, 10). In addition, many studies have shown that Elongator has a role in mRNA translation by modifying tRNA wobble uridines involved in codon bias and translational efficiency (11–16), but whether alterations in transcription or translation of select genes and proteins in sympathetic neurons explains their death in FD is not known. Previous studies have shown that Elp1-deficient sympathetic neurons can survive normally in vitro when immersed in media containing their obligate survival factor, nerve growth factor (NGF) (17), suggesting that broad abnormalities in gene transcription or translation may not adequately explain sympathetic neuron death in patients with FD.
During their development, sympathetic neurons acquire NGF from target tissues, which is required for their survival and target tissue innervation. NGF binds to TrkA (NTRK1) receptors on axon terminals, causing receptor homodimerization and autophosphorylation of specific endodomain tyrosine residues, which serve as docking sites for essential signal transduction mediators. The ligand bound and phosphorylated receptors are internalized and incorporated into NGF/TrkA signaling endosomes that are retrogradely transported to the neuron cell body to engage signal transduction pathways required for target tissue innervation and sympathetic neuron survival (18–20). Sympathetic and some sensory neurons that depend on NGF signaling for survival are particularly affected in FD, raising the possibility that NGF signaling abnormalities may have a role in disease pathogenesis (21, 22). Moreover, patients afflicted by other rare forms of HSAN have sympathetic and sensory nervous system abnormalities caused by gene mutations known to affect NGF signaling. For example, HSAN5 (OMIM 608654) and HSAN4 (OMIM 256800) are caused by rare germline mutations in the NGF and TrkA genes, respectively. Here, we identified an unexpected and essential role for cytoplasmic Elp1 in retrograde NGF signaling that may elucidate the mechanism by which sympathetic neurons die in patients with FD.
Results
Elp1 is ubiquitously expressed in all cells and ablating it in the germline in mice leads to early embryonic lethality (23). Humans with FD have a germline mutation in the Elp1 gene with a phenotype largely restricted to sympathetic and some sensory neurons. This is because the mutation causes Elp1 splicing abnormalities, leading to exon 20 skipping and protein loss that is more severe in these neurons relative to other cells (24). We previously generated mice to recapitulate the FD mutation by flanking exon 20 in the Elp1 gene with loxP sites in the mouse germline (Elp1+/f20 mice) (17). To explore the mechanism of Elp1 function in sympathetic neurons, Elp1+/f20 mice were mated to tamoxifen-dependent cre-recombinase–expressing mice (Rosa+/CreERT2 mice) so that normal sympathetic neurons (TCtl and TElp1f20/f20; Supplemental Figure 1, A and B; supplemental material available online with this article; https://doi.org/10.1172/JCI130401DS1) could be isolated from newborn mice and endogenous Elp1 protein could be ablated within 24 hours of adding 4-hydroxy-tamoxifen (4-OHT) to cultured neurons (TcKO) under various experimental conditions and at different stages during NGF-dependent differentiation in vitro (Supplemental Figure 1C). Dissociated TcKO sympathetic neurons lacking Elp1 survived normally when completely immersed in NGF-containing culture medium, as previously reported by us for neurons derived from Elp1-sympathetic neuron-specific (conditional) knockout mice (17) and similar to wild type sympathetic neurons (25).
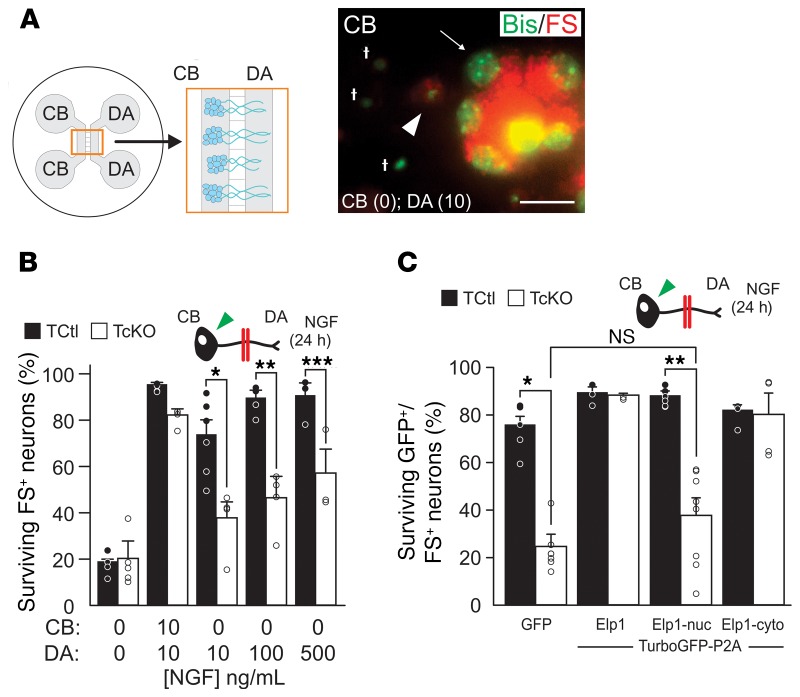
Immersing dissociated sympathetic neurons in NGF-containing medium bypasses their need to transport NGF from distal axons (DAs) for survival and it does not reflect the way NGF is normally acquired by axons as they innervate target tissues during development in vivo. To more closely emulate these conditions and to interrogate retrograde NGF signaling in Elp1-deficient neurons, we established partitioned cultures (26), which have been extensively used to define the mechanisms of retrograde NGF/TrkA signaling in sympathetic neurons (reviewed in ref. 27). TCtl and TcKO neurons were grown in partitioned culture, bisbenzamide (Bis) staining was used to distinguish live from dead neurons, and fluorescent microspheres (FS) were added to the DA compartment medium to identify retrogradely labeled neurons that extended axons into the DA compartment (Figure 1A). In previous studies, we identified a highly significant retrograde survival abnormality of TcKO neurons relative to TCtl neurons when NGF was restricted to the DA compartment. By contrast, no survival differences between TCtl and TcKO neurons were identified when NGF was present in both CB and DA compartments (28), similar to previous results with dissociated Elp1-deficient sympathetic neurons immersed in NGF-containing medium and where dependence on retrograde NGF signaling is bypassed (17). Here, we found that significant retrograde survival differences were identified between TCtl and TcKO neurons even when the NGF concentration was elevated 50-fold (from 10 ng/mL to 500 ng/mL) in the DA compartment. Since NGF is produced in limiting quantities by peripheral tissues in vivo, these results suggest that impaired retrograde NGF signaling in TcKO neurons could underly sympathetic neuron survival and target tissue innervation abnormalities in patients with FD (Figure 1B).
Figure 1. Cytoplasmic Elp1 is essential for NGF-dependent retrograde sympathetic neuron survival.
(A) Partitioned neuron cultures, which isolate cell bodies (CB) from distal axons (DA), were used to assess retrograde neuron survival when NGF was applied to the microfluidically isolated DA compartment using Bisbenzimide (Bis) to label nuclear DNA (green) and fluorescent microspheres (FS) to identify neurons that extended axons into the DA compartment and retrogradely transported them (red). Apoptotic neurons were identified by condensed, fragmented or absent nuclear DNA. Apoptotic cells not containing retrogradely transported FS (†) were not scored, but apoptotic FS-containing neurons (FS+, arrowhead) and healthy FS+ neurons with open chromatin and punctate nucleoli (arrow) were scored. Scale bar: 20 μM. (B) Whereas most neurons survived when 10 ng/mL NGF was present in the DA compartment in TCtl neurons, there is significantly less survival of TcKO neurons even when NGF was escalated 50-fold in the DA compartment (Student’s t test; *P = 0.007, n = 3–6; **P = 0.004, n = 4; ***P = 0.04, n = 3). (C) TcKO neurons differentiated in partitioned cultures and infected with doxycycline-inducible adenoviruses showed highly significant NGF-dependent survival abnormalities when only GFP was expressed in them (Student’s t test; *P < 0.0001, n = 7), and their retrograde survival was completely rescued when Elp1 expression was restored (n = 3). Expression of nuclear localized Elp1 (Elp1-nuc) showed highly significant retrograde survival abnormalities (Student’s t test; **P < 0.001, n = 7) and no significant rescue of retrograde survival relative to GFP+/FS+ infected TcKO neurons (Student’s t test; NS, P = 0.12). Expression of cytoplasm localized Elp1 (Elp1-cyto) completely rescued retrograde neuron survival (n = 4). For C, results were considered significant if the Bonferroni’s corrected P value was less than 0.013.
Elp1 may have a role in transcriptional regulation in the nucleus as a constituent of the transcriptional Elongator protein complex (9). However, in sympathetic neurons almost all detectable Elp1 protein is localized outside of the nucleus (Supplemental Figure 2A) and in a distinctly punctate pattern in the cytoplasm and in axons (Supplemental Figure 2B), that we previously identified as Rab7-positive late endosomes thought to, at least in part, also represent NGF-signaling endosomes (28, 29). Moreover, consistent with the concept that at least some Elp1-containing endosomes may also represent NGF-signaling endosomes, there was a significant increase in Elp1-positive endosomes in proximal axons 5 hours after NGF treatment of DAs (Supplemental Figure 2C). To examine whether cytoplasmic Elp1 has a function in retrograde NGF-dependent survival signaling in sympathetic neurons, we generated doxycycline-inducible adenoviruses to reconstitute either nuclear-localized (Elp1-nuc) or cytoplasm-localized (Elp1-cyto) forms of Elp1 in sympathetic neurons lacking endogenous Elp1 protein (Supplemental Figure 3). Titrating the concentration of doxycycline in the culture medium made it possible to reexpress the exogenous molecules at near physiologic levels (Supplemental Figure 4). GFP+/FS+ TcKO neurons infected with virus expressing only GFP showed retrograde survival abnormalities relative to GFP+/FS+ TCtl neurons, as we previously reported for TcKO sympathetic neurons (ref. 28 and Figure 1C). However, in GFP+/FS+ TcKO neurons that reexpressed GFP and WT Elp1, retrograde neuron survival was completely rescued, as expected. Reexpression of Elp1-nuc in TcKO sympathetic neurons showed no significant rescue of retrograde survival in GFP+/FS+ neurons, whereas reexpression of Elp1-cyto completely rescued retrograde survival signaling (Figure 1C). Thus, normal NGF-dependent retrograde survival of sympathetic neurons requires Elp1 function in the cytoplasm.
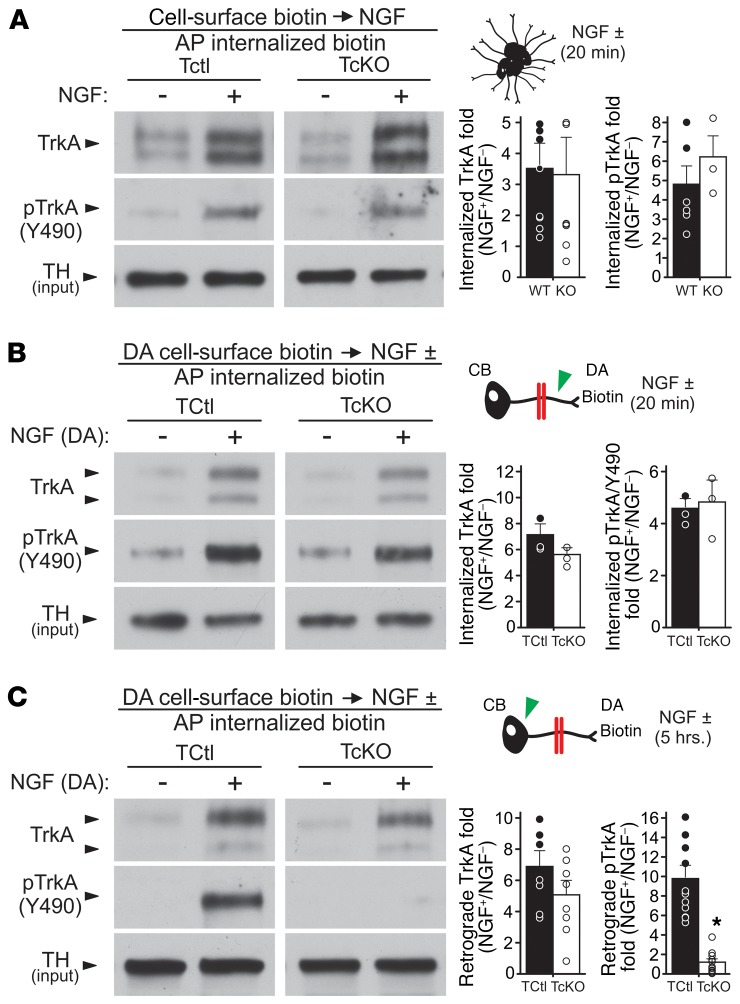
Retrograde NGF signaling in sympathetic neurons is a complex process. Elp1 could be involved in many aspects of NGF/TrkA signaling, including TrkA expression on the surface of axons, internalization of the NGF/TrkA receptor complex, phosphorylation (activation) of the TrkA receptor, and incorporation of the NGF/TrkA complex into signaling endosomes and/or their retrograde transport to the cell body to engage downstream signaling pathways. Cell-surface biotinylation of bulk-cultured sympathetic neurons showed normal cell-surface TrkA expression in TcKO compared with TCtl neurons (Supplemental Figure 5). Moreover, consistent with our previous results showing that Elp1-deficient sympathetic neurons survive normally when immersed in NGF-containing medium (17), we observed normal cell-surface TrkA receptor internalization and phosphorylation at Y490 of the receptor endodomain in bulk-cultured neurons treated with NGF (Figure 2A). To better recapitulate in vivo retrograde NGF signaling mechanisms, compartmentalized cultures and axon surface protein biotinylation in the DA compartment were used to examine NGF-dependent TrkA receptor internalization, activation, and trafficking from DAs to neuron cell bodies. Twenty minutes after NGF treatment in the DA compartment, both TCtl and TcKO axons internalized cell-surface TrkA receptors. The internalized receptors were similarly phosphorylated at Y490 (Figure 2B) and Y674/5 (Supplemental Figure 6A). By 5 hours after treatment of DAs with NGF, biotinylated axon–localized surface TrkA receptors in both TCtl and TcKO neurons showed similar retrograde transport of TrkA to the cell body (Figure 2C). NGF-dependent retrograde TrkA signaling endosome transport into proximal axons was also confirmed to be similar between TCtl and TcKO neurons by a second method tracing TrkA-GFP labeled endosomes in transfected neurons challenged with NGF treatment of DAs (Supplemental Figure 7). Although axon surface TrkA receptor trafficking to the cell body was similar between TCtl and TcKO neurons, NGF-dependent TrkA phosphorylation at both Y490 (Figure 2C) and Y674/5 (Supplemental Figure 6B) was completely absent compared with TCtl neurons, which maintained TrkA receptor phosphorylation to the cell bodies for at least 5 hours after NGF treatment of DAs. Thus, while Elp1 has no appreciable role in sympathetic neuron cell-surface expression of TrkA receptors, their NGF-dependent internalization, their initial phosphorylation/activation at critical tyrosine residues in axons, or retrograde transport of the receptors to the neuron cell body, it is essential for maintaining TrkA receptor phosphorylation/activation during retrograde transport.
Figure 2. Abnormal retrograde TrkA receptor phosphorylation (activation) in the absence of Elp1.
(A) TCtl and TcKO neurons grown in bulk culture and differentiated for 5–7 days were deprived of NGF for 24 hours using NGF-free medium, anti-NGF antibody (0.05 μg/mL), and BAF (50 μM) to prevent neuronal apoptosis. Cell-surface proteins were biotinylated at 4°C and the neurons were either left unchallenged or they were challenged with NGF (10 ng/mL) for 20 minutes at 37°C. Excess cell-surface biotin was stripped from the live neurons and total cellular and membrane protein lysates were obtained. Internalized biotinylated proteins were isolated by streptavidin affinity purification (AP) and the proteins were subjected to Western blotting and film densitometry. There was no detectable abnormality in TrkA receptor internalization in response to NGF in TcKO neurons (Student’s t test; P = 0.89, n = 7–9), and internalized TrkA receptors showed no difference in phosphorylation at Y490 20 minutes after NGF treatment (Student’s t test; P = 0.38, n = 3–6). (B) Similarly, DA cell-surface biotinylation in partitioned neuron cultures showed that TCtl and TcKO neurons were similar in their ability to internalize cell-surface TrkA receptors (Student’s t test; NS, P = 0.19, n = 3) and phosphorylate the receptors at Y490 within axons (green arrowhead) 20 minutes after NGF treatment (Student’s t test; NS, P = 0.92, n = 3). (C) Five hours after NGF treatment and DA cell-surface biotinylation, internalization and retrograde transport of biotinylated TrkA receptors to the cell body (green arrowhead) was similar between TCtl and TcKO neurons (Student’s t test; NS, P = 0.19, n = 8). However, the retrogradely transported TrkA axon-surface receptors were not phosphorylated in TcKO neurons compared with TCtl neurons (Student’s t test; *P < 0.00002, n = 11).
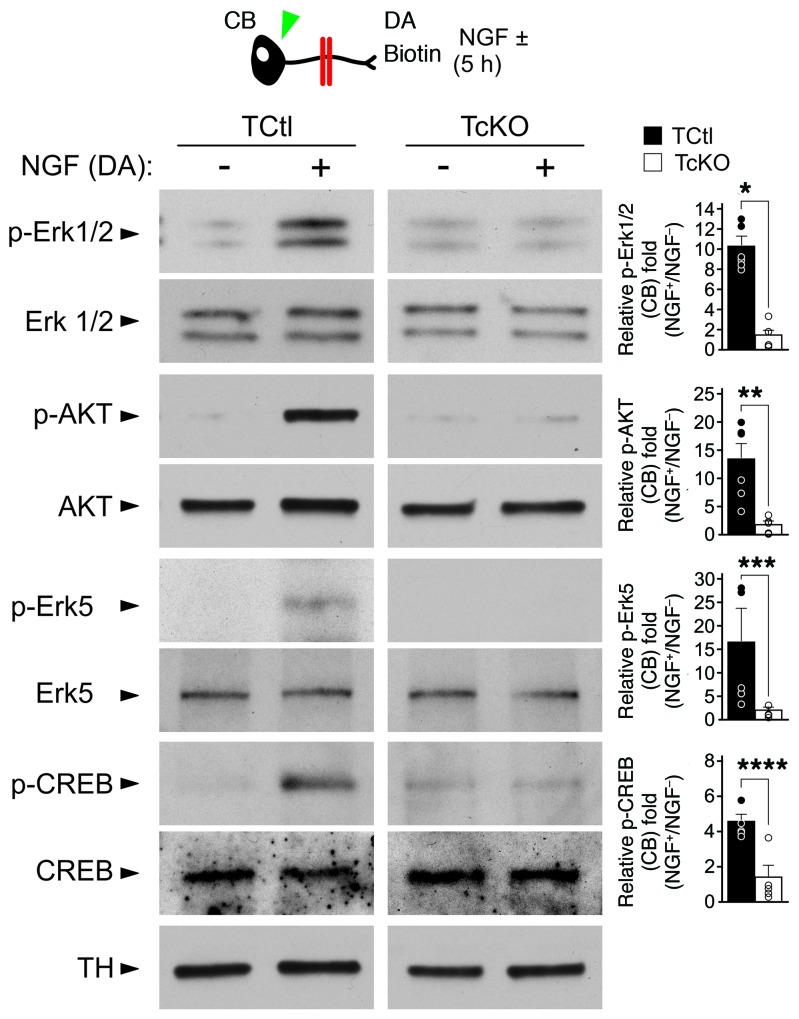
NGF-induced phosphorylation of the TrkA endodomain at Y490 creates a well-characterized phosphotyrosine docking site that is essential to engage Ras-MAPK and Ras-PI3K signaling pathways, both of which are essential for sympathetic neuron survival and differentiation. Signaling endosomes containing NGF bound and activated TrkA receptors maintain prolonged activation of these pathways during their retrograde transport to the cell body. A failure to maintain TrkA phosphorylation would be expected to impair the activation of critical effector signaling pathways required for sympathetic neuron survival and differentiation (for review see ref. 30). Consistent with this hypothesis, we found that essential downstream effectors of retrograde TrkA receptor signaling, such as Erk1/2, AKT, Erk5, and CREB, were not retrogradely phosphorylated (activated) by NGF acquired from DAs in TcKO neurons lacking Elp1, like they were in TCtl neurons (Figure 3). Since activation of these signaling pathways is essential for sympathetic neuron survival and differentiation (31–33), failure to retrogradely propagate terminal axon–derived NGF signaling in the absence of Elp1 defines a clear mechanism to explain sympathetic neuron death and abnormal target tissue innervation in patients with FD.
Figure 3. Impaired retrograde TrkA receptor activation in Elp1-deficient neurons is associated with impaired activation of effector signaling pathways required for their differentiation and survival.
TcKO sympathetic neurons failed to maintain retrograde TrkA receptor phosphorylation after NGF treatment of DAs, which was associated with failure to engage several well-established signaling pathways that are activated by retrograde TrkA signaling. For example, whereas Erk1/2, AKT, Erk5 and CREB were all phosphorylated in neuron cell bodies (green arrowhead) 5 hours after NGF treatment of DAs in TCtl neurons, significant abnormalities in their phosphorylation were observed in TcKO neurons after similar treatment (blot images and quantitative results are from multiple different experiments using an identical experiment protocol; Student’s t test; pErk 1/2, *P < 0.001, n = 6; pAKT, **P < 0.00002, n = 6; pErk5, ***P < 0.01, n = 5–6; pCREB, ****P < 0.003, n = 5).
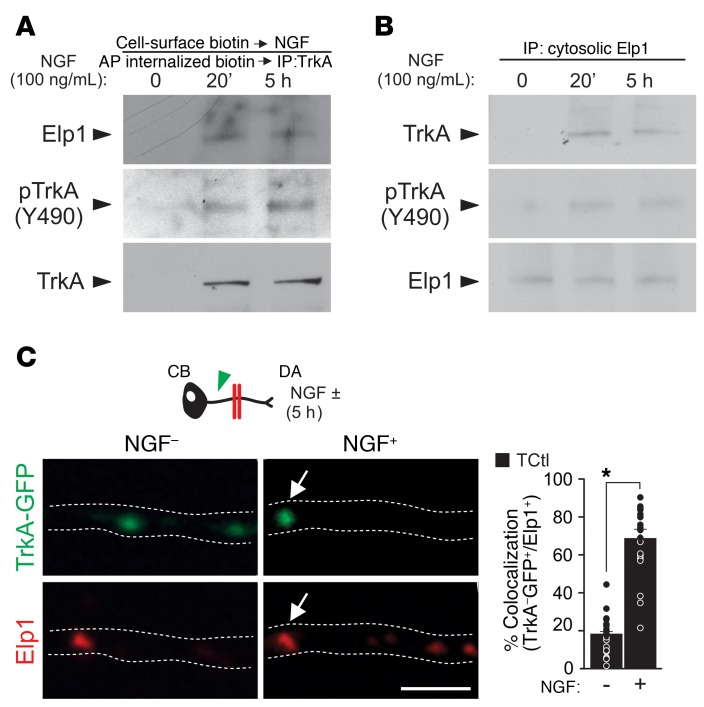
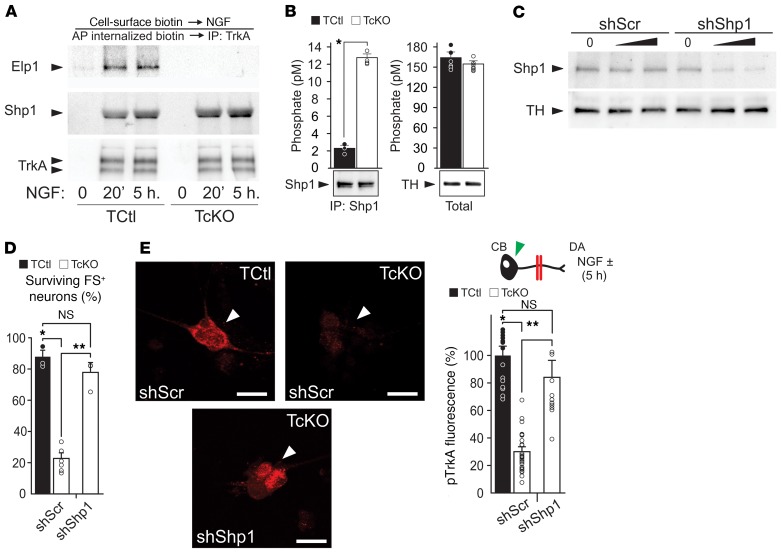
To examine how Elp1 maintains TrkA phosphorylation during retrograde NGF signaling, we first examined whether Elp1 was recruited to internalized TrkA receptors when sympathetic neurons were exposed to NGF. We found that Elp1 was recruited to internalized cell-surface biotinylated TrkA receptors (Figure 4A), and that recruitment was NGF dependent (Figure 4B). This was confirmed using another method in neurons expressing TrkA-GFP, in which Elp1 association with GFP-labeled TrkA signaling endosomes in proximal axons was increased when NGF was presented to DAs in partitioned cultures (Figure 4C). Previous studies showed that Shp1 (PTPN6) phosphatase associates with TrkA in an NGF-dependent manner and it regulates TrkA signaling and sympathetic neuron survival by regulating TrkA phosphorylation (34). We confirmed NGF-dependent Shp1 recruitment to the TrkA signaling complex in sympathetic neurons, as previously reported (ref. 34 and Figure 5A), suggesting that Elp1 could have a role in TrkA signaling by regulating Shp1 activity and TrkA phosphorylation status during retrograde transport. Although Shp1 recruitment to the TrkA signaling complex still occurred in the absence of Elp1 (Figure 5A), we found that Shp1 phosphatase activity was significantly increased in sympathetic neurons lacking Elp1, which was clearly detectable under Shp1 protein–enrichment conditions using immunoprecipitation (Figure 5B). To determine whether Shp1 hyperactivity is responsible for the abnormal regulation of NGF/TrkA phosphorylation and decreased retrograde neuron survival in the absence of Elp1, previously characterized dominant-negative Shp1 (dnShp1) and small hairpin Shp1 RNA (shShp1) molecules were expressed to specifically abrogate Shp1 function (35). Adenovirus-mediated expression of dnShp1 in TcKO Elp1-deficient sympathetic neurons was able to significantly, but incompletely, rescue retrograde NGF-mediated sympathetic neuron survival (Supplemental Figure 8). The incomplete rescue of retrograde neuron survival by dnShp1 may be due to incomplete inhibition of Shp1. To test this hypothesis, expression of shShp1, which resulted in a more than 80% reduction in endogenous Shp1 protein (Figure 5C), completely rescued retrograde neuron survival (Figure 5D). As expected, retrograde pTrkA levels in the cell body were significantly elevated (Figure 5E). These results suggest that failure of retrograde NGF/TrkA signaling in the absence of Elp1 is a consequence of Shp1 hyperactivation, which leads to precocious TrkA dephosphorylation and impaired activation of downstream survival and differentiation signal transduction pathways.
Figure 4. Elp1 binds to the NGF/TrkA signaling complex and is associated with NGF signaling endosomes.
(A) Consecutive cell-surface biotinylation, affinity purification of internalized biotinylated proteins, and TrkA immunoprecipitation from TCtl sympathetic neurons showed that Elp1 was bound to the internalized NGF/TrkA signaling complex and that (B) cytosolic Elp1 was recruited to phosphorylated TrkA receptors after NGF treatment (results representative of n = 3 replicates). (C) In TCtl neurons, NGF treatment of DAs significantly increased the recruitment of Elp1 to TrkA-GFP labeled signaling endosomes (arrowhead) (Student’s t test; *P < 0.0001, n = 23–29). Scale bar: 2 μm.
Figure 5. Elp1 maintains TrkA phosphorylation by negatively regulating Shp1 phosphatase activity.
(A) Shp1 and Elp1 recruitment to a protein complex containing TrkA in TCtl neurons is NGF-dependent and binding of Shp1 to TrkA is independent of Elp1 (results representative of n = 3 replicates). (B) Although no detectable differences in total phosphatase activity were identified between TCtl and TcKO neurons, immunoprecipitation of Shp1 showed it was significantly hyperactivated in the absence of Elp1 in TcKO neurons compared with TCtl neurons (Student’s t test; *P < 0.00001, n = 4). (C) Adenovirus-mediated expression of a short hairpin RNA (shShp1) reduced the level of Shp1 protein more than 80% relative to expression of a scrambled (shScr) shRNA in TCtl neurons. (D) Whereas TcKO neurons expressing shScr showed significant retrograde NGF-dependent survival abnormalities (Student’s t test; *P < 0.001, n = 3–6), expression of shShp1 significantly and completely rescued retrograde NGF-dependent survival (Student’s t test; **P < 0.000001, n = 4). (E) Accordingly, the level of retrograde pTrkA in cell bodies was highly decreased in TcKO neurons compared with TCtl neurons infected with adenovirus expressing shScr (Student’s t test; *P < 0.000005, n = 28) and it was completely rescued by infection with shShp1-expressing adenovirus (Student’s t test; **P < 0.0001, n = 11; arrowheads, results represent pTrkA fluorescence). Scale bars: 25 μM. For D and E, the results were considered significant if the Bonferroni’s corrected P value was less than 0.017.
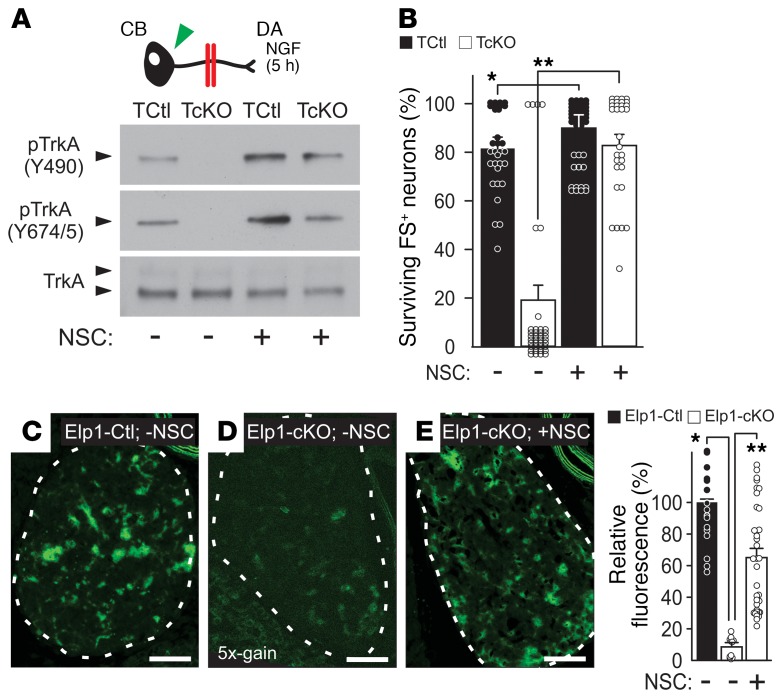
Hyperactivation of Shp1 phosphatase in sympathetic neurons may explain the impaired retrograde NGF/TrkA signaling in Elp1-deficient neurons, suggesting that pharmacological inhibition of Shp1 might also be useful to rescue disease-relevant sympathetic neuron death. To test this hypothesis, TCtl and TcKO neurons were grown in partitioned cultures with NGF in the DA compartment together with Shp1 phosphatase inhibitors NSC87877 (NSC; Figure 6, A and B) or sodium stibogluconate (SSG; Supplemental Figure 9) in both compartments. Whereas in the absence of either inhibitor, retrograde TrkA phosphorylation at Y490 and Y674/5 was completely absent in TcKO neurons as previously shown, it was rescued by the presence of either Shp1 phosphatase inhibitor (Figure 6A and Supplemental Figure 9). Moreover, increased TrkA phosphorylation was accompanied by a small but significant increase in NGF-dependent survival in TCtl neurons as previously reported (34), and retrograde neuron survival was completely rescued in TcKO neurons by pharmacological Shp1 phosphatase inhibition (Figure 6B).
Figure 6. The phosphatase inhibitor NSC87877 rescues retrograde TrkA phosphorylation and neuron survival.
(A) Pharmacologic inhibition of Shp1 using the phosphatase inhibitor NSC87877 (NSC) rescued retrograde TrkA Y490 and Y674/5 phosphorylation (pTrkA) in sympathetic neurons grown in partitioned cultures when challenged with NGF at their terminals (results representative of n = 3 replicates). (B) NSC slightly enhanced retrograde neuron survival of TCtl neurons as previously reported for Shp1-inhibition (34) (Student’s t test; *P < 0.001, n = 30–37 images from 3 experimental replicates) and it completely rescued retrograde NGF-dependent neuron survival in TcKO neurons (Student’s t test; **P < 0.000001, n = 3–37 images from 3 experimental replicates). pTrkA was also rescued in vivo by NSC treatment. Compared with (C) PBS-treated (-NSC) Elp1-Ctl mice, (D) PBS-treated Elp1-cKO mice showed markedly diminished pTrkA in SCG sympathetic neurons (Student’s t test; *P < 0.000001, n = 18 images from 3 animals; image gain shown in D magnified 5 times to visualize low signal). (E) Treatment with NSC significantly rescued pTrkA in Elp1-cKO mice compared with PBS-treated Elp1-cKO mice (Student’s t test; **P < 0.000001, n = 36 images from 3 animals). Scale bars in C–E: 50 μm. For B, the results were considered significant if the Bonferroni’s corrected P value was less than 0.012 and for C if it was less than 0.017.
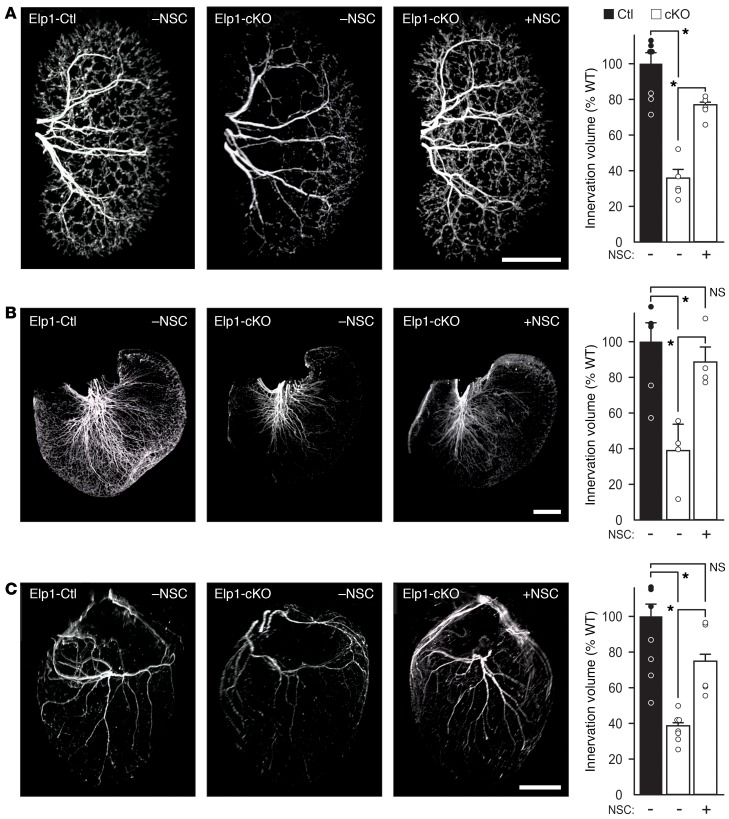
To examine whether Shp1 phosphatase inhibition can protect sympathetic neurons lacking Elp1 in vivo, pregnant mice were implanted with subcutaneous osmotic pumps to systemically deliver Shp1 phosphatase inhibitor NSC or PBS starting at embryonic day (E) 11.5. Embryos received drug or PBS in utero, and after birth the neonatal mice continued to receive drug from lactating dams before they were examined at postnatal day 5. Neonatal mice with Elp1 loss specifically in sympathetic neurons (DβH-iCre+ Elp1f20/f20 [Elp1-cKO]) and control neonatal mice (DβH-iCre+ Elp1+/+ [Elp1-Ctl]) were examined (Supplemental Figure 10). Compared with sympathetic neurons from the SCG of PBS treated Elp1-Ctl mice (Figure 6C), SCG neurons from PBS-treated Elp1-cKO mice (Figure 6D) showed a highly significant loss of TrkA phosphorylation that was significantly rescued after NSC treatment (Figure 6E). Accordingly, compared with PBS-treated Elp1-Ctl mice that showed robust sympathetic innervation to kidney (Figure 7A and Supplemental Video 1), stomach (Figure 7B and Supplemental Video 2), and heart (Figure 7C and Supplemental Video 3), Elp1-cKO mice showed significant attenuation of target tissue innervation that was rescued by NSC treatment. Similarly, SCG and stellate ganglion (STG) atrophy in Elp1-ckO mice (Figure 8A) was rescued by NSC treatment (Figure 8B and Supplemental Video 4), consistent with its role in rescuing retrograde NGF/TrkA signaling in the absence of Elp1.
Figure 7. The phosphatase inhibitor NSC87877 (NSC) rescues sympathetic target tissue innervation in a mouse model of FD.
Sympathetic innervation to (A) kidneys, (B) stomach, and (C) heart, as representative organs regulated by the sympathetic nervous system, were examined using tyrosine hydroxylase immunofluorescence, whole organ tissue clearing, light sheet confocal microscopy and 3D reconstruction with volumetric masking to quantify total organ innervation volume. A significant decrease in sympathetic innervation was observed in PBS treated Elp1-cKO mice in all organs (Student’s t test; *P < 0.01, kidney, n = 5, stomach, n = 4, and heart, n = 7), and in Elp1-cKO mice treated with NSC there was a significant rescue of innervation (Student’s t test; *P < 0.01, Kidney, n = 5, Stomach, n = 4, and Heart, n = 5). Scale bar: 1 mm. For A–C, the results were considered significant if the Bonferroni’s corrected P value was less than 0.017.
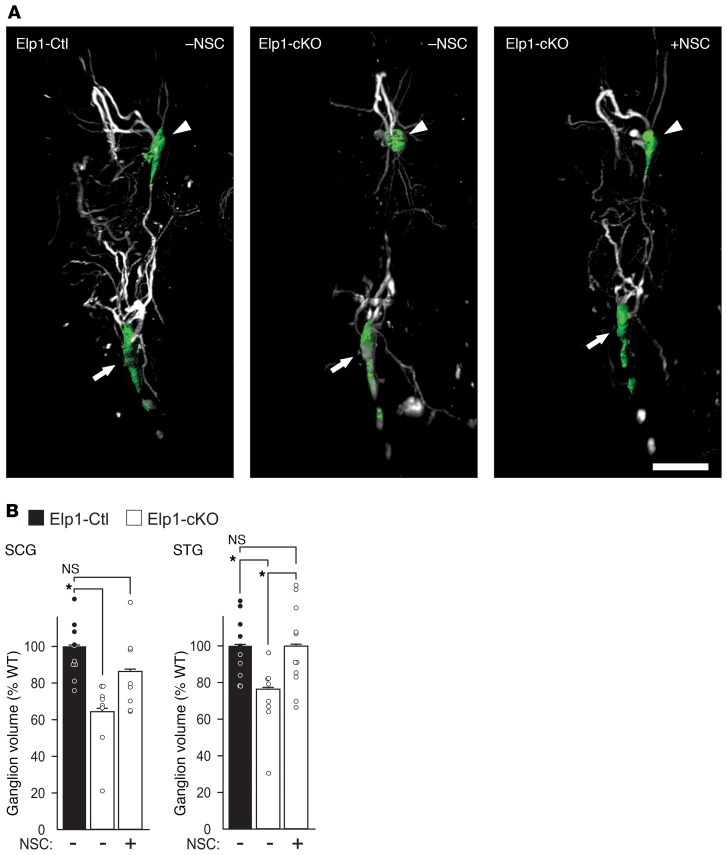
Figure 8. The Shp1 phosphatase inhibitor, NSC87877 (NSC) rescues sympathetic ganglion volume in a mouse model of FD.
(A) Sympathetic ganglion (SCG arrowhead/green mask and stellate ganglion/green mask; STG arrow) volume was measured using tyrosine hydroxylase immunofluorescence, whole organ tissue clearing, light sheet confocal microscopy and 3D reconstruction with volumetric masking. (B) A significant decrease in ganglion volume was observed in PBS-treated Elp1-cKO (SCG, Student’s t test, *P = 0.001, n = 10; STG, Student’s t test, *P = 0.003, n = 10). Elp1-cKO mice treated with NSC showed a significant rescue in ganglionic volume (SCG, Student’s t test, NS, P = 0.166, n = 10; STG, Student’s t test, *P = 0.01, n = 11). Scale bar: 1 mm. The results were considered significant if the Bonferroni’s corrected P value was less than 0.017.
Discussion
A systematic and mechanistic analysis of disease-relevant sympathetic neurons isolated from mice with acutely ablated Elp1 has identified an NGF signaling abnormality that may explain sympathetic neuron death and sympathetic dysautonomia in patients with FD. After NGF binding to cell-surface TrkA receptors on sympathetic axons in target tissues, Elp1 protein is recruited to NGF-bound and -internalized receptors, which also recruit the TrkA regulatory Shp1 phosphatase. In WT sympathetic neurons, NGF/TrkA phosphorylation is maintained in signaling endosomes by Elp1-mediated negative regulation of Shp1 during retrograde transport to the cell body, and downstream signaling mediators required for survival and differentiation, such as Erk1/2, AKT, Erk5 and CREB, are phosphorylated and activated. In the absence of Elp1, however, Shp1 bound to the NGF/TrkA signaling complex becomes hyperactivated, leading to premature TrkA dephosphorylation and a failure to engage essential retrograde downstream NGF-dependent signaling events. Decreasing Shp1 phosphatase activity using molecular or pharmacological approaches reverses the TrkA phosphorylation abnormalities and rescues NGF-dependent retrograde neuron death in Elp1-deficient sympathetic neurons in vitro and in vivo.
Animal models generated to examine the function of Elp1 in FD have been useful for understanding the role of Elp1 in sensory and sympathetic neuron development. A previous study reported decreased neuron precursor proliferation in sensory ganglia that was thought to account for loss of TrkA-positive sensory neurons in the absence of Elp1 (36). We examined this question in detail in a previous study using Wnt1-Cre driver mice to specifically ablate Elp1 in neural crest cells before their fate specification, migration, and formation of sympathetic and sensory ganglia. We found that Elp1-deficient sensory and sympathetic neurons normally migrated from the neural crest. There was no identifiable abnormality in total neuron number, proliferation, or apoptosis in the primordial ganglia at a developmental time point before their innervation of target tissues and dependency on NGF signaling. Interestingly, we identified markedly elevated neuronal apoptosis in sympathetic and sensory ganglia at birth, when sensory and sympathetic neurons are actively innervating target tissues and they depend on target tissue–derived NGF signaling for survival (37). These results are consistent with the hypothesis proposed many decades ago suggesting that NGF signaling may have a role in sympathetic and sensory neuron loss in the pathogenesis of FD (21, 22).
Retrograde NGF transport abnormalities were previously identified in mice lacking Elp1 in DRG sensory neurons (38). These studies showed decreased acetylated alpha-tubulin and reduced velocity of retrogradely transported NGF from distal neurites in Elp1-deficient sensory neurons. Similarly, previous studies suggested that microtubule disorganization or altered acetylation dynamics in the absence of Elp1 could lead to abnormal transport of endosome cargo within axons (17, 39, 40). Although we did not explicitly examine the rate of retrograde NGF/TrkA signaling endosome transport in axons from sympathetic neurons acutely depleted of Elp1 in this study, the quantity of TrkA receptors retrogradely transported from DAs to cell bodies was similar between TCtl and TcKO neurons 5 hours after NGF treatment of their DAs. Instead, we found clear evidence of abnormal phosphorylation of retrogradely transported TrkA receptors and downstream signaling mediators required for NGF-dependent survival signaling. Thus, it seems unlikely that a reduction in retrograde TrkA transport capacity or velocity, if it exists in sympathetic neurons, could account for impaired activation of TrkA and downstream NGF-dependent signaling pathways observed in sympathetic neurons acutely depleted of Elp1. It is possible that cells depleted of Elp1 for longer periods of time may acquire cytoskeletal abnormalities that could affect other aspects of protein trafficking.
The cytoplasmic localization of Elp1 has prompted an intensive search for its function apart from nuclear transcription. Interestingly, Elp1 depletion, which destabilizes the Elongator (Elp1–Elp6) complex, was found to alter the modification of wobble nucleosides in 2 tRNA species that modulate the protein translation efficiency of a subset of transcripts in fission yeast (11, 16). There is now widespread consensus that this function is evolutionarily conserved in eukaryotes, where it may have a role in regulating the translation efficiency of a broad number of transcripts (12, 13, 41). Of particular relevance, a recent study reported widespread abnormalities in protein translation in Elp1-deficient sensory neurons that may have a role in TrkA-expressing nociceptive neuron survival in FD during development (14). Although the role of Elp1 in modulating protein translation efficiency seems clear, the extent to which this may explain neuron death in sensory neurons in FD is somewhat less clear from this study, considering the likelihood that maladaptive developmental regulatory mechanisms may have confounded the proteomic and expression analyses that were performed on E17.5 neurons, long after Elp1 was ablated in vivo by Wnt1-Cre at approximately E9.5 gestation (42). Nevertheless, it seems plausible that protein translation may be altered in sympathetic neurons lacking Elp1, but we did not identify differences between TCtl and TcKO neurons in the level of any of the specific proteins that we examined (TH, Tuj1, TrkA, Shp1, Erk 1/2, AKT, Erk5, or CREB) when normalized for total protein. Importantly, immunoprecipitated Shp1 protein complexes that contained similar amounts of Shp1 protein were tested in the phosphatase assays, which showed a clear increase in Shp1 phosphatase activity in TcKO neurons, eliminating the possibility that the result was due to altered Shp1 protein translation in TcKO neurons relative to TCtl neurons.
Elp1 interacts with the previously characterized NGF/TrkA/Shp1 signaling complex, but our results do not address whether the regulatory interaction is direct or indirect. Therefore, it is possible that loss of Elp1 may alter translational efficiency of other proteins that bind within the complex or outside of the complex to regulate Shp1 phosphatase activity. Indeed, Elp1 has previously been shown to primarily localize to intracellular endosomes (43) and in particular Rab7-positive endosomes (28). Rab7-positive endosomes are known to represent, at least in part, TrkA signaling endosomes (29, 44) and they are platforms for local protein translation in axons (45), raising the possibility that protein translation may be modulated by Elp1 within them. It is interesting that TrkA colocalizes with only about 50% of the Elp1-positive endosomes in proximal axons after NGF treatment of DAs, suggesting that Elp1 has a broader role in endosomal function in sympathetic neurons beyond its role in regulating TrkA signaling.
Elp1 interacts with the NGF/TrkA/Shp1 signaling complex to regulate Shp1 activity, but whether it has a similar function in NGF-dependent nociceptive sensory neurons that also degenerate in FD is currently unknown. Shp1 contains 2 Src homology 2 (SH2) protein-protein interaction domains that regulate its phosphatase activity through coordinated interaction with binding partner proteins. Elp1 may have a role in coordinating these protein interactions to regulate its function, but the specific binding partner proteins and interaction domains have not yet been characterized. Elp1 appears to have an essential function in regulating Shp1 phosphatase activity during retrograde NGF signaling, and it may regulate other proteins in sympathetic neurons and in other cells. Early embryonic lethality in mice lacking Elp1 in all cells clearly shows it has a widespread function beyond its role in regulating TrkA receptor signaling in sympathetic neurons. Shp1 is broadly expressed and has a role in regulating many tyrosine kinase receptors, and it appears to be a tumor suppressor gene in lymphoma, leukemia, and a variety of solid tumors (46). Whether Elp1 has a role in regulating Shp1 phosphatase activity to modulate tyrosine kinase receptor signaling in other cell types and their growth, differentiation, and oncogenic potential will be interesting to explore in future studies.
Methods
Methods, including statements of reagent availability and references, are available in the Supplemental Methods.
Study approval.
All animal procedures were reviewed and approved by the Institutional Animal Care and Use Committee (IACUC) at Cedars Sinai Medical Center in Los Angeles California (approval IACUC007292).
Author contributions
WGT conceived the project and designed the studies. LL, KG, and WGT performed experiments. WGT and LL analyzed the data, and WGT wrote the manuscript.
Supplementary Material
Acknowledgments
We thank T. Fu and C. Re Bogguri for providing technical assistance. R. Kuruvilla, D. Ginty, and J. Levitt provided recombinant adenovirus vectors. This work was supported by NIH grants R21-HD064918, K26-OD010945, R56-NS089626, and R01-NS095894.
Version 1. 04/13/2020
Electronic publication
Version 2. 05/01/2020
Print issue publication
Footnotes
Conflict of interest: The authors have declared that no conflict of interest exists.
Copyright: © 2020, American Society for Clinical Investigation.
Reference information: J Clin Invest. 2020;130(5):2478–2487.https://doi.org/10.1172/JCI130401.
See the related Commentary at (H)Elping nerve growth factor: Elp1 inhibits TrkA’s phosphatase to maintain retrograde signaling.
References
- 1.Axelrod FB, Nachtigal R, Dancis J. Familial dysautonomia: diagnosis, pathogenesis and management. Adv Pediatr. 1974;21:75–96. [PubMed] [Google Scholar]
- 2.Axelrod FB. Familial dysautonomia. Muscle Nerve. 2004;29(3):352–363. doi: 10.1002/mus.10499. [DOI] [PubMed] [Google Scholar]
- 3.Anderson SL, et al. Familial dysautonomia is caused by mutations of the IKAP gene. Am J Hum Genet. 2001;68(3):753–758. doi: 10.1086/318808. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Slaugenhaupt SA, et al. Tissue-specific expression of a splicing mutation in the IKBKAP gene causes familial dysautonomia. Am J Hum Genet. 2001;68(3):598–605. doi: 10.1086/318810. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Winkler GS, et al. RNA polymerase II elongator holoenzyme is composed of two discrete subcomplexes. J Biol Chem. 2001;276(35):32743–32749. doi: 10.1074/jbc.M105303200. [DOI] [PubMed] [Google Scholar]
- 6.Close P, et al. Transcription impairment and cell migration defects in elongator-depleted cells: implication for familial dysautonomia. Mol Cell. 2006;22(4):521–531. doi: 10.1016/j.molcel.2006.04.017. [DOI] [PubMed] [Google Scholar]
- 7.Cheishvili D, Maayan C, Smith Y, Ast G, Razin A. IKAP/hELP1 deficiency in the cerebrum of familial dysautonomia patients results in down regulation of genes involved in oligodendrocyte differentiation and in myelination. Hum Mol Genet. 2007;16(17):2097–2104. doi: 10.1093/hmg/ddm157. [DOI] [PubMed] [Google Scholar]
- 8.Cohen-Kupiec R, Pasmanik-Chor M, Oron-Karni V, Weil M. Effects of IKAP/hELP1 deficiency on gene expression in differentiating neuroblastoma cells: implications for familial dysautonomia. PLoS ONE. 2011;6(4):e19147. doi: 10.1371/journal.pone.0019147. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Creppe C, et al. Elongator controls the migration and differentiation of cortical neurons through acetylation of alpha-tubulin. Cell. 2009;136(3):551–564. doi: 10.1016/j.cell.2008.11.043. [DOI] [PubMed] [Google Scholar]
- 10.Solinger JA, et al. The Caenorhabditis elegans Elongator complex regulates neuronal alpha-tubulin acetylation. PLoS Genet. 2010;6(1):e1000820. doi: 10.1371/journal.pgen.1000820. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Esberg A, Huang B, Johansson MJ, Byström AS. Elevated levels of two tRNA species bypass the requirement for elongator complex in transcription and exocytosis. Mol Cell. 2006;24(1):139–148. doi: 10.1016/j.molcel.2006.07.031. [DOI] [PubMed] [Google Scholar]
- 12.Chen C, Tuck S, Byström AS. Defects in tRNA modification associated with neurological and developmental dysfunctions in Caenorhabditis elegans elongator mutants. PLoS Genet. 2009;5(7):e1000561. doi: 10.1371/journal.pgen.1000561. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Karlsborn T, Tükenmez H, Chen C, Byström AS. Familial dysautonomia (FD) patients have reduced levels of the modified wobble nucleoside mcm(5)s(2)U in tRNA. Biochem Biophys Res Commun. 2014;454(3):441–445. doi: 10.1016/j.bbrc.2014.10.116. [DOI] [PubMed] [Google Scholar]
- 14.Goffena J, et al. Elongator and codon bias regulate protein levels in mammalian peripheral neurons. Nat Commun. 2018;9(1):889. doi: 10.1038/s41467-018-03221-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Johansson MJO, Xu F, Byström AS. Elongator-a tRNA modifying complex that promotes efficient translational decoding. Biochim Biophys Acta Gene Regul Mech. 2018;1861(4):401–408. doi: 10.1016/j.bbagrm.2017.11.006. [DOI] [PubMed] [Google Scholar]
- 16.Huang B, Johansson MJ, Byström AS. An early step in wobble uridine tRNA modification requires the Elongator complex. RNA. 2005;11(4):424–436. doi: 10.1261/rna.7247705. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Jackson MZ, Gruner KA, Qin C, Tourtellotte WG. A neuron autonomous role for the familial dysautonomia gene ELP1 in sympathetic and sensory target tissue innervation. Development. 2014;141(12):2452–2461. doi: 10.1242/dev.107797. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Glebova NO, Ginty DD. Heterogeneous requirement of NGF for sympathetic target innervation in vivo. J Neurosci. 2004;24(3):743–751. doi: 10.1523/JNEUROSCI.4523-03.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Smeyne RJ, et al. Severe sensory and sympathetic neuropathies in mice carrying a disrupted Trk/NGF receptor gene. Nature. 1994;368(6468):246–249. doi: 10.1038/368246a0. [DOI] [PubMed] [Google Scholar]
- 20.Crowley C, et al. Mice lacking nerve growth factor display perinatal loss of sensory and sympathetic neurons yet develop basal forebrain cholinergic neurons. Cell. 1994;76(6):1001–1011. doi: 10.1016/0092-8674(94)90378-6. [DOI] [PubMed] [Google Scholar]
- 21.Bell J, Gruenthal M, Finger S, Lundberg P, Johnson E. Behavioral effects of early deprivation of nerve growth factor: some similarities with familial dysautonomia. Brain Res. 1982;234(2):409–421. doi: 10.1016/0006-8993(82)90880-0. [DOI] [PubMed] [Google Scholar]
- 22.Siggers DC, et al. Increased nerve-growth-factor beta-chain cross-reacting material in familial dysautonomia. N Engl J Med. 1976;295(12):629–634. doi: 10.1056/NEJM197609162951201. [DOI] [PubMed] [Google Scholar]
- 23.Chen YT, et al. Loss of mouse Ikbkap, a subunit of elongator, leads to transcriptional deficits and embryonic lethality that can be rescued by human IKBKAP. Mol Cell Biol. 2009;29(3):736–744. doi: 10.1128/MCB.01313-08. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Cuajungco MP, et al. Tissue-specific reduction in splicing efficiency of IKBKAP due to the major mutation associated with familial dysautonomia. Am J Hum Genet. 2003;72(3):749–758. doi: 10.1086/368263. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Kuruvilla R, Ye H, Ginty DD. Spatially and functionally distinct roles of the PI3-K effector pathway during NGF signaling in sympathetic neurons. Neuron. 2000;27(3):499–512. doi: 10.1016/S0896-6273(00)00061-1. [DOI] [PubMed] [Google Scholar]
- 26.Campenot RB. Local control of neurite development by nerve growth factor. Proc Natl Acad Sci U S A. 1977;74(10):4516–4519. doi: 10.1073/pnas.74.10.4516. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Harrington AW, Ginty DD. Long-distance retrograde neurotrophic factor signalling in neurons. Nat Rev Neurosci. 2013;14(3):177–187. doi: 10.1038/nrn3253. [DOI] [PubMed] [Google Scholar]
- 28.Tourtellotte WG. Axon transport and neuropathy: relevant perspectives on the etiopathogenesis of familial dysautonomia. Am J Pathol. 2016;186(3):489–499. doi: 10.1016/j.ajpath.2015.10.022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Saxena S, Bucci C, Weis J, Kruttgen A. The small GTPase Rab7 controls the endosomal trafficking and neuritogenic signaling of the nerve growth factor receptor TrkA. J Neurosci. 2005;25(47):10930–10940. doi: 10.1523/JNEUROSCI.2029-05.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Marlin MC, Li G. Biogenesis and function of the NGF/TrkA signaling endosome. Int Rev Cell Mol Biol. 2015;314:239–257. doi: 10.1016/bs.ircmb.2014.10.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Watson FL, Heerssen HM, Bhattacharyya A, Klesse L, Lin MZ, Segal RA. Neurotrophins use the Erk5 pathway to mediate a retrograde survival response. Nat Neurosci. 2001;4(10):981–988. doi: 10.1038/nn720. [DOI] [PubMed] [Google Scholar]
- 32.Riccio A, Pierchala BA, Ciarallo CL, Ginty DD. An NGF-TrkA-mediated retrograde signal to transcription factor CREB in sympathetic neurons. Science. 1997;277(5329):1097–1100. doi: 10.1126/science.277.5329.1097. [DOI] [PubMed] [Google Scholar]
- 33.Philpott KL, McCarthy MJ, Klippel A, Rubin LL. Activated phosphatidylinositol 3-kinase and Akt kinase promote survival of superior cervical neurons. J Cell Biol. 1997;139(3):809–815. doi: 10.1083/jcb.139.3.809. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Marsh HN, et al. SHP-1 negatively regulates neuronal survival by functioning as a TrkA phosphatase. J Cell Biol. 2003;163(5):999–1010. doi: 10.1083/jcb.200309036. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Ramachandran IR, et al. The phosphatase SRC homology region 2 domain-containing phosphatase-1 is an intrinsic central regulator of dendritic cell function. J Immunol. 2011;186(7):3934–3945. doi: 10.4049/jimmunol.1001675. [DOI] [PubMed] [Google Scholar]
- 36.George L, et al. Familial dysautonomia model reveals Ikbkap deletion causes apoptosis of Pax3+ progenitors and peripheral neurons. Proc Natl Acad Sci U S A. 2013;110(46):18698–18703. doi: 10.1073/pnas.1308596110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Jackson M, Tourtellotte W. Neuron culture from mouse superior cervical ganglion. Bio Protoc. 2014;4(2):e1035. doi: 10.21769/bioprotoc.1035. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Naftelberg S, et al. Phosphatidylserine ameliorates neurodegenerative symptoms and enhances axonal transport in a mouse model of familial dysautonomia. PLoS Genet. 2016;12(12):e1006486. doi: 10.1371/journal.pgen.1006486. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Cheishvili D, et al. IKAP/Elp1 involvement in cytoskeleton regulation and implication for familial dysautonomia. Hum Mol Genet. 2011;20(8):1585–1594. doi: 10.1093/hmg/ddr036. [DOI] [PubMed] [Google Scholar]
- 40.Gardiner J, Barton D, Marc J, Overall R. Potential role of tubulin acetylation and microtubule-based protein trafficking in familial dysautonomia. Traffic. 2007;8(9):1145–1149. doi: 10.1111/j.1600-0854.2007.00605.x. [DOI] [PubMed] [Google Scholar]
- 41.Karlsborn T, Tükenmez H, Mahmud AK, Xu F, Xu H, Byström AS. Elongator, a conserved complex required for wobble uridine modifications in eukaryotes. RNA Biol. 2014;11(12):1519–1528. doi: 10.4161/15476286.2014.992276. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Wilson YM, Richards KL, Ford-Perriss ML, Panthier JJ, Murphy M. Neural crest cell lineage segregation in the mouse neural tube. Development. 2004;131(24):6153–6162. doi: 10.1242/dev.01533. [DOI] [PubMed] [Google Scholar]
- 43.Lefler S, et al. Familial dysautonomia (FD) human embryonic stem cell derived PNS neurons reveal that synaptic vesicular and neuronal transport genes are directly or indirectly affected by IKBKAP downregulation. PLoS ONE. 2015;10(10):e0138807. doi: 10.1371/journal.pone.0138807. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Zhang K, et al. Defective axonal transport of Rab7 GTPase results in dysregulated trophic signaling. J Neurosci. 2013;33(17):7451–7462. doi: 10.1523/JNEUROSCI.4322-12.2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Cioni JM, et al. Late endosomes act as mRNA translation platforms and sustain mitochondria in axons. Cell. 2019;176(1-2):56–72.e15. doi: 10.1016/j.cell.2018.11.030. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Chong ZZ, Maiese K. The Src homology 2 domain tyrosine phosphatases SHP-1 and SHP-2: diversified control of cell growth, inflammation, and injury. Histol Histopathol. 2007;22(11):1251–1267. doi: 10.14670/hh-22.1251. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.