Abstract
Epigenetic events like DNA methylation and histone modification can alter heritable phenotypes. Zinc is required for the activity of various epigenetic enzymes, such as DNA methyltransferases (DNMTs), histone acetyltransferases (HATs), histone deacetylases (HDACs), and histone demethylases, which possess several zinc binding sites. Thus, the dysregulation of zinc homeostasis can lead to epigenetic alterations. Zinc homeostasis is regulated by Zinc Transporters (ZnTs), Zrt- and Irt-like proteins (ZIPs), and the zinc storage protein metallothionein (MT). Recent advances revealed that ZIPs modulate epigenetics. ZIP10 deficiency was found to result in reduced HATs, confirming its involvement in histone acetylation for rigid skin barrier formation. ZIP13 deficiency, which is associated with Spondylocheirodysplastic Ehlers-Danlos syndrome (SCD-EDS), increases DNMT activity, leading to dysgenesis of dermis via improper gene expressions. However, the precise molecular mechanisms remain to be elucidated. Future molecular studies investigating the involvement of zinc and its transporters in epigenetics are warranted.
Keywords: epigenetics, zinc, zinc transporter
INTRODUCTION
Zinc is an essential trace metal found in the tissues and fluids of living organisms (Chasapis and Loutsidou, 2012). It is well known that zinc is a key component of many proteins. Several transcription factors need zinc to bind directly to specific regions of DNA (Coleman, 1992). For this, zinc finger domains (ZnDs) act as a binding site and facilitate transcriptional activity (Razin et al., 2012). Additionally, over 300 enzymes require zinc for their function (Vallee and Auld, 1990). DNA polymerase needs zinc for DNA replication (Macdonald, 2000). Taken together, it is predicted that about 10% of the genes in the human genome bind to zinc directly or indirectly (Andreini et al., 2006).
Furthermore, zinc also plays a role in the immune system, influencing signaling molecules under immune-related extracellular stimulation (Bin et al., 2018a; Rink and Gabriel, 2000). Zinc acts as a secondary messenger, with the ability to deliver signals to immune cells. In this process, a “zinc wave” is released from the perinuclear area, including the endoplasmic reticulum (Yamasaki et al., 2007). Zinc signals allow fundamental cell functions, such as proliferation, differentiation, survival, and migration (Bin et al., 2016; Fukunaka et al.,2017).
ZINC HOMEOSTASIS AND ZINC TRANSPORTERS
In mammalian cells, intracellular zinc homeostasis is tightly regulated by membrane transporting importers and exporters, namely zinc transporters (Eide, 2006). Zinc transporters are divided into two distinct families with contrasting functions: the ZIP and ZnT families (Fig. 1).
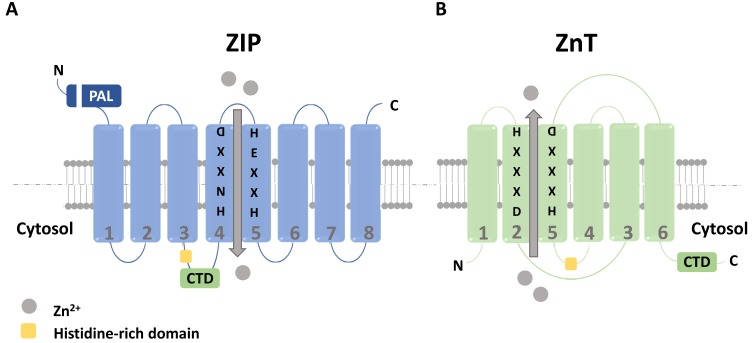
Fig. 1. The structures of zinc transporters.
(A) Structural model of ZIP. The ZIP protein family is composed of 8 transmembrane domains (TM), with the N- and C-termini facing the extracellular/luminal space. A proline-alanine-leucine (PAL) motif is conserved in each N-terminus, while a cytoplasmic domain (CTD) and a histidine-rich domain is common to TM3 and TM4. Zinc binds to TM4 and TM5 through the HNXXD motif in TM4 and the HEXXH motif in TM5. (B) Structural model of ZnT. The ZnT protein family is composed of 6 TMs. A histidine-rich domain is common to present between TM4 and TM5, with a CTD in the C-terminus. TM2 and TM5 share the zinc binding site through their HXXXD motifs.
Firstly, the ZIP family, which is named after the first identified Zrtand Irt-like proteins (ZIPs; zinc-regulated transporter and iron-regulated transporter-like proteins, encoded by SLC39A genes) (Jeong and Eide, 2013), is comprised of 14 ZIP transporters that mediate the influx of zinc into the cytoplasm, resulting in an increased level of intracellular zinc. The members of the ZIP family possess eight transmembrane domains (TM), which form a pore for passing zinc ion, with both the Nand C-termini facing the extracellular or luminal side (Fig. 1A) (Bin et al., 2011; Guerinot, 2000). In mammals, the members of the ZIP family evolved into LZT proteins; the LIV-1 subfamily of zinc transporters conserves a proline-alanine-leucine (PAL) motif in each N-terminus, a cytoplasmic domain (CTD), and a histidine-rich domain (Bin et al., 2018a). Zinc binding occurs through the HNXXD motif in TM4 and HEXXH motif in TM5. The LZT proteins contain long extracellular and intracellular domains that are reportedly involved in cellular signaling via phosphorylation and cleavage (Taylor and Nicholson, 2003). LZT proteins are associated with several human diseases, including breast and pancreatic cancers, rheumatism, and hemochromatosis, as well as innate immune and tissue developments, suggesting that LZT proteins play important roles in human health (Taylor and Nicholson, 2003; Walker and Black, 2004).
Secondly, the ZnT family (encoded by SLC30A genes) act to reduce intracellular zinc by promoting its efflux from cells or intracellular vesicles (Fig. 1B) (Kambe et al., 2015). At least 10 members of the ZnT family have been identified in humans, of which ZnT9 is not thought to play a role as a zinc transporter (Perez et al., 2017). The members of the ZnT family possess six transmembrane domains (except for ZnT5, which possesses additional domains) and Nand C-termini facing the cytoplasm (Bin et al., 2018a; Fukada et al., 2011). The members of this family possess HXXXD motifs in TM2 and TM5, through which zinc binds to the protein, as well as a histidine-rich domain between TM4 and TM5. Furthermore, the ZnT proteins contain a large CTD at the C-terminus that shares a high structural similarity with the copper chaperone (Bin et al., 2018a; Lu and Fu, 2007). The CTD is essential for dimeric formation and is predicted to be involved in zinc sensing. Many mutations have been found in ZnTs; their clinical features are dysarthria and hypertonia in ZnT10 mutations, erosive dermatitis in ZnT2 mutations, and diabetes in ZnT8 (Bin et al., 2018b; Kambe et al., 2017).
Additionally, zinc homeostasis is also regulated by the intracellular zinc storage protein, metallothionein (MT) (Roesijadi, 1996; Ruttkay-Nedecky et al., 2013). MT is composed of 6168 amino acids with 20-21 cysteines and can bind to 7 zinc ions via multi-conserved cysteine residues. This zinc-binding protein is involved in responses to metal toxicity and oxidative stress, protecting cells from DNA damage. Hence, MT acts as a scavenger when zinc is present in high concentrations, as well as a zinc reservoir to supply zinc when it is deficient (Kelly et al., 1996; Suhy et al., 1999).
Zinc homeostasis is essential for cellular events and its dysfunction can lead to several human disorders. In this review, we focus on recent advances concerning the relationship between zinc, zinc transporters, and epigenetics.
EPIGENETIC REGULATION
Epigenetics is involved in many cellular processes and causes stable heritable phenotypes by changing chromosomes without altering the DNA sequence (Berger et al., 2009). This takes place via the occurrence of epigenetic marks, such as DNA methylation and histone modification, which regulate gene expression and transposon activity (Du et al., 2015; Slotkin and Martienssen, 2007). Many enzymes involved in those epigenetic regulations require zinc (Fig. 2).
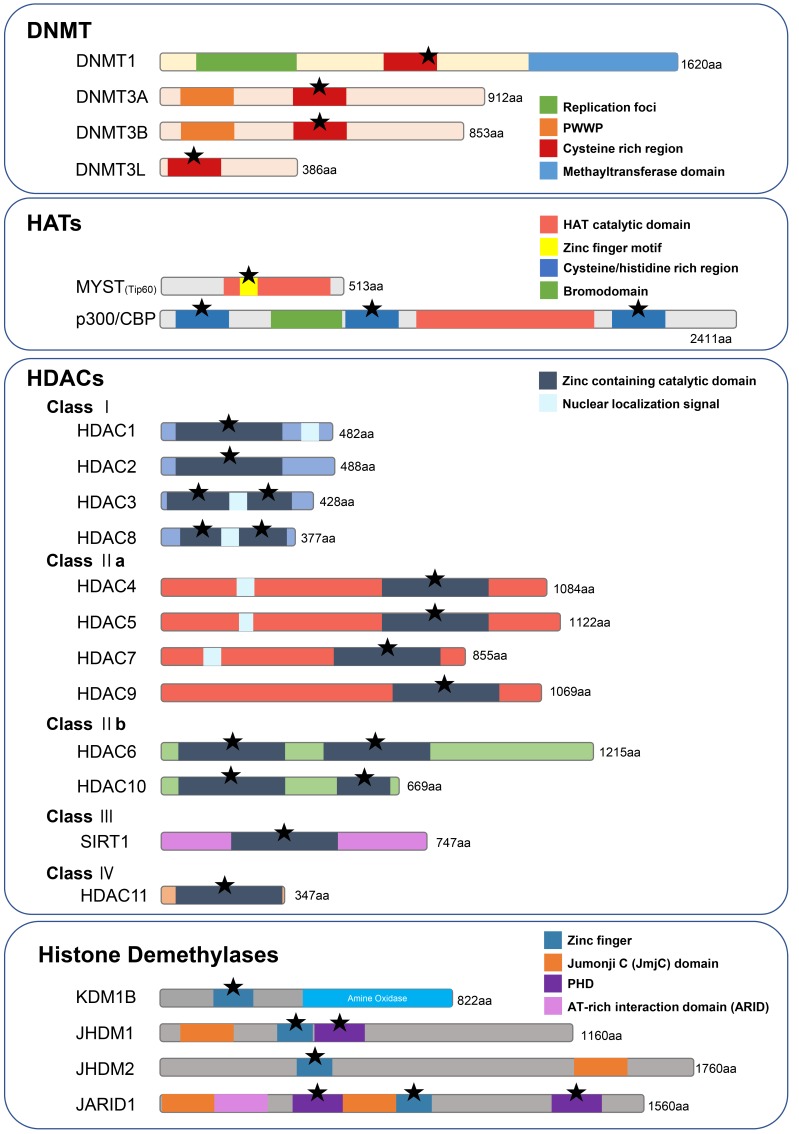
Fig. 2. Epigenetic enzymes possessing zinc-binding sites.
Asterisks denote the zinc-binding sites.
ZINC AND DNA METHYLATION ENZYMES
The DNA methylation pathway is an epigenetic mechanism that allows for the recruitment of proteins involved in gene repression or the inhibition of transcription factor binding to DNA (Moore et al., 2013). Given the important roles of DNA methylation in cellular processes, there is a strong correlation between aberrant DNA methylation and a wide number of human diseases (Robertson, 2005). This post-replicative modification involves the transfer of methyl groups (CH3) to DNA, specifically CpG dinucleotides, resulting in changes in molecular activity via the suppression of gene transcription, and thus its expression levels (Laird and Jaenisch, 1996; Robertson, 2005). In mammals, methylation takes place in the fifth carbon (5-C) position of the cytosine ring of DNA, resulting in the formation of 5-methylcytosine (5-mC) (Moore et al., 2013).
One of the essential components of methylation is the DNA methyltransferase (DNA MTase) family (Lyko, 2017). The DNA MTase family is comprised of key enzymes (commonly abbreviated as DNMTs) whose role is to mediate the transfer of the methyl group to DNA. In this process, S-adenosyl methionine (SAM) acts as the methyl donor to cytosine (Klimasauskas et al., 1994). DNMTs are mainly comprised of two parts: an N-terminus and a C-terminus (Jurkowska et al., 2011). At least 3 DNMTs are found in humans, namely DNMT1, DNMT3a, and DNMT3b (Gowher and Jeltsch, 2018). The N-terminus in DNMT1 is constituted of a proliferating cell domain (PbD) and a replication-foci-targeting domain (RFTD), which serve to suppress de novo methylation (Zhang et al., 2015). Additionally, it contains a CXXC domain, which binds to unmethylated DNA and comprises the ZnD (Jeltsch and Jurkowska, 2016; Zhang et al., 2015). Furthermore, the C-terminal part contains repeated DNA-(cytosine-5)-MTase motifs, as the catalytic domain (CatD); the interaction between ZnD and CatD is critical for the allosteric activation of DNMT1 (Algahtani and Shirah, 2017; Fatemi et al., 2001). DNMT2 shows a high similarity to other DNMTs, but mediates the methylation of aspartic acid transfer RNA and does not methylate DNA. For this reason, its name was subsequently revised to tRNA aspartic acid methyltransferase (TRDMT1) (Goll et al., 2006). DNMT1 is mainly required for establishing and maintaining DNA methylation, while DNMT3a, DNMT3b, and cofactor DNMT3L seem to mediate the patterns of de novo DNA methylation (Cui and Xu, 2018; Zeng and Chen, 2019). In addition, DNMT3-like protein (DNMT3L) is a regulatory factor that does not contain CatD. However, it also possesses Cys-rich areas that mediate its interactions with the amino tail of histone 3 for de novo DNA methylation (Chédin et al., 2002; Ooi et al., 2007).
ZINC AND HISTONE MODIFICATION ENZYMES
Histone modifications are biological processes that modify chromatin structure either by acetylating or deacetylating the lysines found in the N-terminal tail of the histones bound to the DNA molecule (Ahn et al., 2019; Kuo and Allis, 1998; Verdone et al., 2006). During acetylation, the addition of acetyl groups to lysine residues removes their positive charge and reduces the overall positive charge of the histone proteins, thereby decreasing the interactions between the N-termini of these proteins and DNA, which has a negative charge (Chrun et al., 2017). This process allows for the deconstruction of chromatin (euchromatin) due to the neutralization of histones, facilitating transcription. Deacetylation represents the opposite process, whereby chromatin is condensed (heterochromatin), resulting in the repression of gene transcription. These mechanisms are catalyzed by the enzymes histone acetyltransferase (HAT) and histone deacetylase (HDAC), occurring as a natural part of gene regulation.
HATs mediate the transfer of acetyl groups from acetyl-CoA to N-lysine in both histone and non-histone proteins (Roth et al., 2001). Many HAT families have been identified, such as Gcn5-related N-acetyltransferases (GNATs), MYSTs, p300/CBP, nuclear receptor coactivators (SRCs), TFIIIC, and CLOCK, among others (Yuan and Marmorstein, 2013). The members of the MYST family have diverse range of architectures, some of which contain zinc within a ZnD or a plant homeodomain-linked zinc finger domain (PHD) (Avvakumov and Côté, 2007). PHD is conserved in both monocytic leukemia zinc-finger protein (MOZ) and MOZ-related factor (MORF), also known as MYST4. These zinc binding motifs are known to promote substrate access and recognition. Furthermore, p300/CREB-binding protein (CBP) acetylates non-histone substrates, including transcription factors, receptors, enzymes, and structural proteins, containing three cysteine/ histidine-rich domains that interact with other molecules and DNA (Dancy and Cole, 2015).
As for HDACs, they are responsible for the deacetylation of both histones and non-histone proteins, removing the acetyl group from the N-acetyl lysine amino acid on target proteins (Wade et al., 1997). There are 4 classes of HDACs (I, II, III, and IV), which are highly conserved in eukaryotes, and all contain zinc in their catalytic domain (Seto and Yoshida, 2014). Class III HDACs, also known as sirtuins, do not share sequence or structural homologies with the other class members, although they appear to be zinc-independent in some cases (Martínez-Redondo and Vaquero, 2013). Thus, whether class III HDACs require zinc for their proper function in vivo remains controversial. However, other HDACs require zinc ions to facilitate the nucleophilic attack of a water molecule in order to initiate the catalytic reaction (Bottomley et al., 2008; Schuetz et al., 2008; Vannini et al., 2004). Zinc ions within active sites coordinate aspartic acid and histidine, which are often found within the zinc binding sites of general zinc-binding proteins.
Furthermore, histone methylation/demethylation is also crucial for epigenetic reprogramming and is involved in tumorigenesis (Ahn et al., 2019). In this process, demethylases mediate demethylation by removing the methyl groups in histones and other proteins. Two types of demethylases have been identified according to their mechanism, namely flavin adenine dinucleotide (FAD)-dependent amine oxidases (flavin-dependent, lysine-specific protein demethylases [KDM] subfamily 1), and Fe(II) and α-ketoglutarate-dependent hydroxylases (KDM subfamily 2-6) (Shi and Whetstine, 2007).
In the former, KDM1B (also known as a lysine-specific demethylase [LSD2]) contains a zinc finger at the N-terminus, which is comprised of three parts, namely C4H2C2, CWtype zinc finger (Zf-CW), derived from the conserved Cys and Trp residues, and linker domains (Burg et al., 2015). Both C4H2C2 and Zf-CW are zinc fingers that coordinate substrate specificity and catalytic activity by interacting with the demethylase substrate, similar to other regulators. Mutations in the transcriptional machinery of these motifs lead to loss of function and mislocalization (Zhang et al., 2013). Moreover, the histone lysine demethylase KDM2B (also known as JmjC domain-containing histone demethylase 1B [JHDM1B]), possesses a CXXC zinc-finger motif between the N-terminal Jumonji C (JmjC) domain and the C-terminal PHD/F-box (Yan et al., 2018). Additionally, the CXXC motif serves for DNA binding by recognizing CpG islands and mediates the formation of complexes comprised of polycomb repressors (Frauer et al., 2011). The PHD domain is also known to have a finger-like structure composed of cysteines and histidines, which coordinates zinc ion binding (Rössler and Marschalek, 2013). The PHD domain works as an E3 ligase or a histone modification reader (Ivanov et al., 2007; Li et al., 2006). These findings indicate that zinc is an essential factor in various methylation enzymes, playing a critical role in their activity, substrate recognition, and structural changes.
ZINC TRANSPORTERS AND EPIGENETICS
Considering that zinc is a modulator, since it acts as a cofactor for epigenetic enzymes and binds to active or allosteric sites, it is expected that zinc transporter proteins also play pivotal roles in epigenetic regulation. However, the relationship between zinc transporters and epigenetics has not been well-studied. Recently, two zinc importing proteins, ZIP10 and ZIP13, were reported to be involved in epigenetic regulation (Fig. 3).
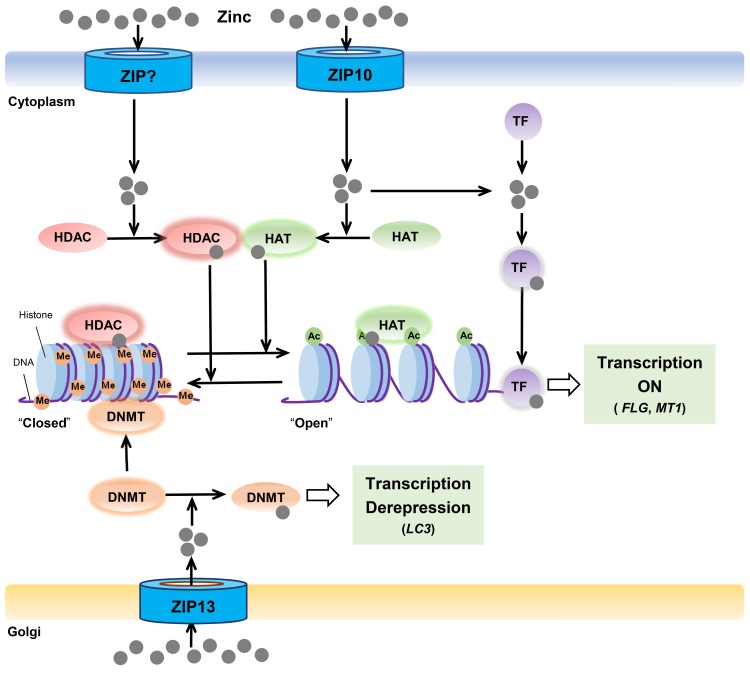
Fig. 3. Zinc transporters in epigenetic regulations.
The zinc-ZIP10-HAT axis is crucial for the gene expressions for skin integrity like FLG and MT1. The zinc-ZIP13-DNMT axis modulates the LC3 expression for autophagy.
ZIP10 was initially known to be involved in humoral immunity, in the regulation of B cell development, and signal strength by mediating the importation of zinc through the plasma membrane (Hojyo et al., 2014; Miyai et al., 2014). Recently, it has been reported that ZIP10 is expressed in the epidermis of skin at considerable levels (Bin et al., 2017; 2018b). In this study, ZIP10 deficiency induced a reduction in the activities of HATs, resulting in the downregulation of transcription of diverse genes, including FLG, KRT1, DSP, TGM1, and AQP3, which are essential for the epidermal stratification, providing the foundation for the rigid skin barrier (Bin et al., 2019). Reduced HAT activity was recovered by zinc treatment, while the chelation of zinc ions reduced the levels of HAT activity. These findings indicate that ZIP10-mediated zinc supply is essential for epidermal organization via the regulation of HATs.
In addition, ZIP10 deficiency has been observed in atopic dermatitis patients with severe epidermal barrier defects (Nakajima et al., 2020). Notably, ZIP10 deficiency did not affect the activity of HDACs in keratinocytes. It is possible that there is a specific axis for zinc transporters to epigenetic enzymes, and epigenetic enzymes may have different sensitivity to zinc. In addition, cell type, species, and state may also affect the association between zinc transporters and epigenetic enzymes, wherein Hdac1-knockout (KO) mice revealed no skin phenotype. However, severe dysgenesis was observed in C57BL/6J-derived Hdac1-KO mice.
ZIP13 is found in connective tissues and is localized in the Golgi apparatus (Bin et al., 2014; Fukada et al., 2008; Lee and Bin, 2019). It is a key partner of SMAD proteins located in connective tissue, forming cells by supplying them with zinc and promoting transcriptional activities for the production of collagen. In previous studies, the nuclear translocation of SMAD proteins for transcription in ZIP13-deficient cells was prohibited. However, zinc treatment did not recover the nuclear translocation of SMAD proteins, implying that ZIP13 may not directly regulate the nuclear translocation of SMAD proteins.
Moreover, ZIP13 has been shown to function in modulation of DNMTs (Lee et al., 2019). The increased DNMT activity is observed in ZIP13-deficient cells, which is recovered by zinc treatment. Zinc binding to the Cys-rich region of DNMTs may inhibit its activity. As expected, both cells from the cohort of Spondylocheirodysplastic Ehlers–Danlos syndrome (SCD-EDS) patient and the Zip13-KO mice having a significant decrease in cellular zinc levels, show a substantial increase of DNMT activity, indicating the disturbance of the DNMT activity by ZIP13 deficiency.
CONCLUSIONS
A diet containing an adequate amount of zinc is essential for human health since many epigenetic enzymes require zinc for their activities. For instance, HATs use zinc for DNA binding via their zinc finger motif and HDACs contain zinc in their active sites, which is needed for hydrolase reaction. Moreover, DNMTs are known to possess several zinc binding sites. Similarly, it is expected that several other epigenetic enzymes may also possess zinc binding sites, even though their precise structure and function remain unclear. Recent advances have demonstrated that zinc transporters are essential for cellular zinc homeostasis and for the modulation of the activity of epigenetic enzymes. Therefore, controlling zinc level and its transporters represents a potential therapeutic approach for epigenetic-associated diseases.
ACKNOWLEDGMENTS
This study was supported by National Research Foundation of Korea (NRF) grants funded by the Korean government (MSIT) (2019005607, 2017M2A2A7A01021034, and 2017R1A2B4010146).
Footnotes
AUTHOR CONTRIBUTIONS
S.B., M.G.L., B.H.B., and J.S.L. conceived and wrote the manuscript. B.H.B. and J.S.L. secured funding.
CONFLICT OF INTEREST
The authors have no potential conflicts of interest to disclose.
REFERENCES
- Ahn H.J., Hwang S.Y., Nguyen N.H., Lee I.J., Lee E.J., Seong J., Lee J.S. Radiation-induced CXCL12 upregulation via histone modification at the promoter in the tumor microenvironment of hepatocellular carcinoma. Mol. Cells. 2019;42:530. doi: 10.14348/molcells.2019.2280. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Algahtani H., Shirah B. A novel mutation in the DNMT1 gene in a patient presenting with pure cerebellar ataxia. J. Genet. Med. 2017;14:71–74. doi: 10.5734/JGM.2017.14.2.71. [DOI] [Google Scholar]
- Andreini C., Banci L., Bertini I., Rosato A. Counting the zinc-proteins encoded in the human genome. J. Proteome Res. 2006;5:196–201. doi: 10.1021/pr050361j. [DOI] [PubMed] [Google Scholar]
- Avvakumov N., Côté J. The MYST family of histone acetyltransferases and their intimate links to cancer. Oncogene. 2007;26:5395–5407. doi: 10.1038/sj.onc.1210608. [DOI] [PubMed] [Google Scholar]
- Berger S.L., Kouzarides T., Shiekhattar R., Shilatifard A. An operational definition of epigenetics. Genes Dev. 2009;23:781–783. doi: 10.1101/gad.1787609. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bin B.H., Bhin J., Kim N.H., Lee S., Jung H., Kim D., Hwang D., Fukada T., Lee A., Lee T.R., et al. An acrodermatitis enteropathica-associated Zn transporter, ZIP4, regulates human epidermal homeostasis. J. Investig. Dermatol. 2016;137:874–883. doi: 10.1016/j.jid.2016.11.028. [DOI] [PubMed] [Google Scholar]
- Bin B.H., Bhin J., Takaishi M., Toyoshima K.E., Kawamata S., Ito K., Hara T., Watanabe T., Irié T., Takagashi T., et al. Requirement of zinc transporter ZIP10 for epidermal development: implication of the ZIP10-p63 axis in epithelial homeostasis. Proc. Natl. Acad. Sci. U. S. A. 2017;114:12243–12248. doi: 10.1073/pnas.1710726114. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bin B.H., Fukada T., Hosaka T., Yamasaki S., Ohashi W., Hojyo S., Miyai T., Nishida K., Yokoyama S., Hirano T. Biochemical characterization of human ZIP13 protein: a homo-dimerized zinc transporter involved in the spondylocheiro dysplastic Ehlers-Danlos syndrome. J. Biol. Chem. 2011;286:40255–40265. doi: 10.1074/jbc.M111.256784. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bin B.H., Hojyo S., Hosaka T., Bhin J., Kano H., Miyai T., Ikeda M., Kimura-Someya T., Shirouzu M., Cho E.G., et al. Molecular pathogenesis of spondylocheirodysplastic Ehlers-Danlos syndrome caused by mutant ZIP13 proteins. EMBO Mol. Med. 2014;6:1028–1042. doi: 10.15252/emmm.201303809. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bin B.H., Hojyo S., Seo J., Hara T., Takagishi T., Mishima K., Fukada T. The role of the Slc39a family of zinc transporters in zinc homeostasis in skin. Nutrients. 2018b;10:219. doi: 10.3390/nu10020219. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bin B.H., Lee S.H., Bhin J., Irié T., Kim S., Seo J., Mishima K., Lee T.R., Hwang D., Fukada T., et al. The epithelial zinc transporter ZIP10 epigenetically regulates human epidermal homeostasis by modulating histone acetyltransferase activity. Br. J. Dermatol. 2019;180:869–880. doi: 10.1111/bjd.17339. [DOI] [PubMed] [Google Scholar]
- Bin B.H., Seo J., Kim S.T. Function, structure, and transport aspects of ZIP and ZnT Zinc transporters in immune cells. J. Immunol. Res. 2018a;2018:9365747. doi: 10.1155/2018/9365747. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bottomley M.J., Surdo P.L., Giovine D., Cirillo A., Scarpelli R., Ferrigno F., Jones P., Neddermann P., De Francesco R., Steinkühler C., et al. Structural and functional analysis of the human HDAC4 catalytic domain reveals a regulatory structural zinc-binding domain. J. Biol. Chem. 2008;283:26694–26704. doi: 10.1074/jbc.M803514200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Burg J.M., Link J.E., Morgan B.S., Heller F.J., Hargrove A.E., Mccafferty D.G. KDM1 class flavin-dependent protein lysine demethylases. Biopolymers. 2015;104:213–246. doi: 10.1002/bip.22643. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chasapis C.T., Loutsidou A.C. Zinc and human health: an update. Arch. Toxicol. 2012;86:521–534. doi: 10.1007/s00204-011-0775-1. [DOI] [PubMed] [Google Scholar]
- Chédin F., Lieber M.R., Hsieh C.L. The DNA methyltransferase-like protein DNMT3L stimulates de novo methylation by Dnmt3a. Proc. Natl. Acad. Sci. U. S. A. 2002;99:16916–16921. doi: 10.1073/pnas.262443999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chrun E.S., Modolo F., Daniel F.I. Histone modifications: a review about the presence of this epigenetic phenomenon in carcinogenesis. Pathol. Res. Pract. 2017;213:1329–1339. doi: 10.1016/j.prp.2017.06.013. [DOI] [PubMed] [Google Scholar]
- Coleman J.E. Zinc proteins: enzymes, storage replication proteins. Annu. Rev. Biochem. 1992;61:897–946. doi: 10.1146/annurev.bi.61.070192.004341. [DOI] [PubMed] [Google Scholar]
- Cui D., Xu X. DNA methyltransferases, DNA methylation, and age-associated cognitive function. Int. J. Mol. Sci. 2018;19:1315. doi: 10.3390/ijms19051315. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dancy B.M., Cole P.A. Protein lysine acetylation by p300/CBP. Chem. Rev. 2015;115:2419–2452. doi: 10.1021/cr500452k. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Du J., Johnson L.M., Jacobsen S.E., Patel D.J. DNA methylation pathways and their crosstalk with histone methylation. Nat. Rev. Mol. Cell Biol. 2015;16:519–532. doi: 10.1038/nrm4043. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Eide D.J. Zinc transporters and the cellular trafficking of zinc. Biochim. Biophys. Acta - Mol. Cell Res. 2006;1763:711–722. doi: 10.1016/j.bbamcr.2006.03.005. [DOI] [PubMed] [Google Scholar]
- Fatemi M., Hermann A., Pradhan S., Jeltsch A. The activity of the murine DNA methyltransferase Dnmt1 is controlled by interaction of the catalytic domain with the N-terminal part of the enzyme leading to an allosteric activation of the enzyme after binding to methylated DNA. J. Mol. Biol. 2001;309:1189–1199. doi: 10.1006/jmbi.2001.4709. [DOI] [PubMed] [Google Scholar]
- Frauer C., Rottach A., Meilinger D., Bultmann S., Fellinger K., Hasenöder S., Wang M., Qin W., Söding J., Spada F., et al. Different binding properties and function of CXXC zinc finger domains in Dnmt1 and Tet1. PLoS One. 2011;6:e16627. doi: 10.1371/journal.pone.0016627. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fukada T., Civic N., Furuichi T., Shimoda S., Mishima K., Higashiyama H., Idaira Y., Asada Y., Kitamura H., Yamasaki H., et al. The zinc transporter SLC39A13/ZIP13 is required for connective tissue development; its involvement in BMP/TGF-β signaling pathways. PLoS One. 2008;3:e3642. doi: 10.1371/annotation/a6c35a12-e8eb-43a0-9d00-5078fa6da1bb. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fukada T., Yamasaki S., Nishida K., Murakami M., Hirano T. Zinc homeostasis and signaling in health and diseases. J. Biol. Inorg. Chem. 2011;16:1123–1134. doi: 10.1007/s00775-011-0797-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fukunaka A., Fukada T., Bhin J., Suzuki L., Tsuzuki T., Takamine Y., Bin B.H., Yoshihara T., Ichinoseki-Sekine N., Naito H., et al. Zinc transporter ZIP13 suppresses beige adipocyte biogenesis and energy expenditure by regulating C/EBP-β expression. PLoS Genet. 2017;13:e1006950. doi: 10.1371/journal.pgen.1006950. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Goll M.G., Kirpekar F., Maggert K.A., Yoder J.A., Hsieh C.L., Zhang X., Golic K.G., Jacobsen S.E., Bestor T.H. Methylation of tRNAAsp by the DNA methyltransferase homolog Dnmt2. Science. 2006;311:395–398. doi: 10.1126/science.1120976. [DOI] [PubMed] [Google Scholar]
- Gowher H., Jeltsch A. Mammalian DNA methyltransferases: new discoveries and open questions. Biochem. Soc. Trans. 2018;46:1191–1202. doi: 10.1042/BST20170574. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Guerinot M.L. The ZIP family of metal transporters. Biomembr. 2000;1465:190–198. doi: 10.1016/S0005-2736(00)00138-3. [DOI] [PubMed] [Google Scholar]
- Hojyo S., Miyai T., Fujishiro H., Kawamura M., Yasuda T., Hijikata A., Bin B.H., Irié T., Tanaka J., Atsumi T., et al. Zinc transporter SLC39A10/ZIP10 controls humoral immunity by modulating B-cell receptor signal strength. Proc. Natl. Acad. Sci. U. S. A. 2014;111:11786–11791. doi: 10.1073/pnas.1323557111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ivanov A.V., Peng H., Yurchenko V., Yap K.L., Negorev D.G., Schultz D.C., Psulkowski E., Fredericks W.J., White D.E., Maul G.G., et al. PHD domain-mediated E3 ligase activity directs intramolecular sumoylation of an adjacent bromodomain required for gene silencing. Mol. Cell. 2007;28:823–837. doi: 10.1016/j.molcel.2007.11.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jeltsch A., Jurkowska R.Z. Allosteric control of mammalian DNA methyltransferases - a new regulatory paradigm. Nucleic Acids Res. 2016;44:8556–8575. doi: 10.1093/nar/gkw723. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jeong J., Eide D.J. The SLC39 family of zinc transporters. Mol. Aspects Med. 2013;34:612–619. doi: 10.1016/j.mam.2012.05.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jurkowska R.Z., Jurkowski T.P., Jeltsch A. Structure and function of mammalian DNA methyltransferases. ChemBioChem. 2011;12:206–222. doi: 10.1002/cbic.201000195. [DOI] [PubMed] [Google Scholar]
- Kambe T., Nishito Y., Fukue K. Zinc transporters in health and disease. In: J. Collins., editor. Molecular Genetic, and Nutritional Aspects of Major and Trace Minerals. Academic Press; Cambridge MA: 2017. pp. 283–291. [DOI] [Google Scholar]
- Kambe T., Tsuji T., Hashimoto A., Itsumura N. The physiological, biochemical, and molecular roles of zinc transporters in zinc homeostasis. Physiol. Rev. 2015;95:749–784. doi: 10.1152/physrev.00035.2014. [DOI] [PubMed] [Google Scholar]
- Kelly E.J., Quaife C.J., Froelick G.J., Palmiter R.D. Metallothionein I and II protect against zinc deficiency and zinc toxicity in mice. J. Nutr. 1996;126:1782–1790. doi: 10.1093/jn/126.7.1782. [DOI] [PubMed] [Google Scholar]
- Klimasauskas S., Kumar S., Roberts R.J., Cheng X. Hhal methyltransferase flips its target base out of the DNA helix. Cell. 1994;76:357–369. doi: 10.1016/0092-8674(94)90342-5. [DOI] [PubMed] [Google Scholar]
- Kuo M.H., Allis C.D. Roles of histone acetyltransferases and deacetylases in gene regulation. BioEssays. 1998;20:615–626. doi: 10.1002/(SICI)1521-1878(199808)20:8<615::AID-BIES4>3.0.CO;2-H. [DOI] [PubMed] [Google Scholar]
- Laird P.W., Jaenisch R. The role of DNA methylation in cancer. Annu. Rev. Genet. 1996;30:441–464. doi: 10.1146/annurev.genet.30.1.441. [DOI] [PubMed] [Google Scholar]
- Lee M.G., Bin B.H. Different actions of intracellular zinc transporters ZIP7 and ZIP13 are essential for dermal development. Int. J. Mol. Sci. 2019;20:3941. doi: 10.3390/ijms20163941. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lee M.G., Choi M.A., Chae S., Kang M.A., Jo H., Baek J., In K.R., Park H., Heo H., Jang D., et al. Loss of the dermis zinc transporter ZIP13 promotes the mildness of fibrosarcoma by inhibiting autophagy. Sci. Rep. 2019;9:1–11. doi: 10.1038/s41598-019-51438-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li H., Ilin S., Wang W., Duncan E.M., Wysocka J., Allis C.D., Patel D.J. Molecular basis for site-specific read-out of histone H3K4me3 by the BPTF PHD finger of NURF. Nature. 2006;442:91–95. doi: 10.1038/nature04802. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lu M., Fu D. Structure of the zinc transporter YiiP. Science. 2007;317:1746–1748. doi: 10.1126/science.1143748. [DOI] [PubMed] [Google Scholar]
- Lyko F. The DNA methyltransferase family: a versatile toolkit for epigenetic regulation. Nat. Rev. Genet. 2017;19:81–92. doi: 10.1038/nrg.2017.80. [DOI] [PubMed] [Google Scholar]
- Macdonald R.S. The role of zinc in growth and cell proliferation. J. Nutr. 2000;130:1500S–1508S. doi: 10.1093/jn/130.5.1500S. [DOI] [PubMed] [Google Scholar]
- Martínez-redondo P., Vaquero A. The diversity of histone versus nonhistone sirtuin substrates. Genes Cancer. 2013;4:148–163. doi: 10.1177/1947601913483767. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Miyai T., Hojyo S., Ikawa T., Kawamura M., Irié T., Ogura H., Hijikata A., Bin B.H., Yasuda T., Kitamura H., et al. Zinc transporter SLC39A10/ZIP10 facilitates antiapoptotic signaling during early B-cell development. Proc. Natl. Acad. Sci. U. S. A. 2014;111:11780–11785. doi: 10.1073/pnas.1323549111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Moore L.D., Le T., Fan G. DNA methylation and its basic function. Neuropsychopharmacology. 2013;38:23–38. doi: 10.1038/npp.2012.112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nakajima K., Lee M.G., Bin B.H., Hara T., Takagishi T., Chae S., Sano S., Fukada T. Possible involvement of zinc transporter ZIP10 in atopic dermatitis. J. Dermatol. 2020;47:e51–e53. doi: 10.1111/1346-8138.15190. [DOI] [PubMed] [Google Scholar]
- Ooi S.K., Qiu C., Bernstein E., Li K., Jia D., Yang Z., Erdjument-Bromage H., Tempst P., Lin S., Allis C., et al. DNMT3L connects unmethylated lysine 4 of histone H3 to de novo methylation of DNA. Nature. 2007;448:714–717. doi: 10.1038/nature05987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Perez Y., Shorer Z., Liani-Leibson K., Chabosseau P., Kadir R., Volodarsky M., Halperin D., Barber-Zucker S., Shalev H., Schreiber R., et al. SLC30A9 mutation affecting intracellular zinc homeostasis causes a novel cerebro-renal syndrome. Brain. 2017;140:928–939. doi: 10.1093/brain/awx013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Razin S.V., Borunova V.V., Maksimenko O.G., Kantidze O.L. Cys2His2 zinc finger protein family: classification, functions, and major members. Biochemistry Mosc. 2012;77:217–226. doi: 10.1134/S0006297912030017. [DOI] [PubMed] [Google Scholar]
- Rink L., Gabriel P. Zinc and the immune system. Proc. Nutr. Soc. 2000;59:541–552. doi: 10.1017/S0029665100000781. [DOI] [PubMed] [Google Scholar]
- Robertson K.D. DNA methylation and human disease. Nat. Rev. Genet. 2005;6:597. doi: 10.1038/nrg1655. [DOI] [PubMed] [Google Scholar]
- Roesijadi G. Metallothionein and its role in toxic metal regulation. Comp. Biochem. Physiol. C Pharmacol. Toxicol. Endocrinol. 1996;113:117–123. doi: 10.1016/0742-8413(95)02077-2. [DOI] [PubMed] [Google Scholar]
- Rössler T., Marschalek R. An alternative splice process renders the MLL protein either into a transcriptional activator or repressor. Pharmazie. 2013;68:601–607. doi: 10.1055/s-0033-1343653. [DOI] [PubMed] [Google Scholar]
- Roth S.Y., Denu J.M., Allis C.D. Histone acetyltransferases. Annu. Rev. Biochem. 2001;70:81–120. doi: 10.1146/annurev.biochem.70.1.81. [DOI] [PubMed] [Google Scholar]
- Ruttkay-Nedecky B., Nejdl L., Gumulec J., Zitka O., Masarik M., Eckschlager T., Stiborova M., Adam V., Kizek R. The role of metallothionein in oxidative stress. Int. J. Mol. Sci. 2013;14:6044–6066. doi: 10.3390/ijms14036044. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schuetz A., Min J., Allali-hassani A., Schapira M., Shuen M., Loppnau P., Mazitschek R., Kwiatkowski N.P., Lewis T.A., Maglathin R.L., et al. Human HDAC7 harbors a class IIa histone deacetylase-specific zinc binding motif and cryptic deacetylase activity. J. Biol. Chem. 2008;283:11355–11363. doi: 10.1074/jbc.M707362200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Seto E., Yoshida M. Erasers of histone acetylation: the histone deacetylase enzymes. Cold Spring Harb. Perspect. Biol. 2014;6:a018713. doi: 10.1101/cshperspect.a018713. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shi Y., Whetstine J.R. Dynamic regulation of histone lysine methylation by demethylases. Mol. Cell. 2007;25:1–14. doi: 10.1016/j.molcel.2006.12.010. [DOI] [PubMed] [Google Scholar]
- Slotkin R.K., Martienssen R. Transposable elements and the epigenetic regulation of the genome. Nat. Rev. Genet. 2007;8:272–285. doi: 10.1038/nrg2072. [DOI] [PubMed] [Google Scholar]
- Suhy D.A., Simon K.D., Linzer D.I.H., Halloran T.V.O. Metallothionein is part of a zinc-scavenging mechanism for cell survival under conditions of extreme zinc deprivation. J. Biol. Chem. 1999;274:9183–9192. doi: 10.1074/jbc.274.14.9183. [DOI] [PubMed] [Google Scholar]
- Taylor K.M., Nicholson R.I. The LZT proteins; the LIV-1 subfamily of zinc transporters. Biochim. Biophys. ActaBiomembr. 2003;1611:16–30. doi: 10.1016/s0005-2736(03)00048-8. [DOI] [PubMed] [Google Scholar]
- Vallee B.L., Auld D.S. Zinc coordination, function, and structure of zinc enzymes and other proteins. Biochemistry. 1990;29:5647–5659. doi: 10.1021/bi00476a001. [DOI] [PubMed] [Google Scholar]
- Vannini A., Volpari C., Filocamo G., Casavola E.C., Brunetti M., Renzoni D., Chakravarty P., Paolini C., De Francesco R., Gallinari P., et al. Crystal structure of a eukaryotic zinc-dependent histone deacetylase, human HDAC8, complexed with a hydroxamic acid inhibitor. Proc. Natl. Acad. Sci. U. S. A. 2004;101:15064–15069. doi: 10.1073/pnas.0404603101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Verdone L., Agricola E., Caserta M., Di Mauro E. Histone acetylation in gene regulation. Brief. Funct. Genomic. Proteomic. 2006;5:209–221. doi: 10.1093/bfgp/ell028. [DOI] [PubMed] [Google Scholar]
- Wade P.A., Pruss D., Wolffe A.P. Histone acetylation: chromatin in action. Trends Biochem. Sci. 1997;22:128–132. doi: 10.1016/S0968-0004(97)01016-5. [DOI] [PubMed] [Google Scholar]
- Walker C.F., Black R.E. Zinc and the risk for infectious disease. Annu. Rev. Nutr. 2004;24:255–275. doi: 10.1146/annurev.nutr.23.011702.073054. [DOI] [PubMed] [Google Scholar]
- Yamasaki S., Sakata-Sogawa K., Hasegawa A., Suzuki T., Kabu K., Sato E., Kurosaki T., Yamashita S., Tokunaga M., Nishida K., et al. Zinc is a novel intracellular second messenger. J. Cell Biol. 2007;177:637–645. doi: 10.1083/jcb.200702081. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yan M., Yang X., Wang H., Shao Q. The critical role of histone lysine demethylase KDM2B in cancer. Am. J. Transl. Res. 2018;10:2222. [PMC free article] [PubMed] [Google Scholar]
- Yuan H., Marmorstein R. Histone acetyltransferases: rising ancient counterparts to protein kinases. Biopolymers. 2013;99:98–111. doi: 10.1002/bip.22128. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zeng Y., Chen T. DNA methylation reprogramming during mammalian development. Genes. 2019;10:257. doi: 10.3390/genes10040257. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang Q., Qi S., Xu M., Yu L., Tao Y., Deng Z., Wu W., Li J., Chen Z., Wong J. Structure-function analysis reveals a novel mechanism for regulation of histone demethylase LSD2/AOF1/KDM1b. Cell Res. 2013;23:225–241. doi: 10.1038/cr.2012.177. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang Z.M., Liu S., Lin K., Luo Y., Perry J.J., Wang Y., Song J. Crystal structure of human DNA methyltransferase 1. J. Mol. Biol. 2015;427:2520–2531. doi: 10.1016/j.jmb.2015.06.001. [DOI] [PMC free article] [PubMed] [Google Scholar]