Abstract
Background: Tai Chi Chuan(TCC), as a mind-body exercise, may have a positive impact on physical function and psychological well-being in breast cancer patients. The latest systematic review and meta-analysis of TCC for breast cancer was made 4 years ago and some new clinical trials about it were published. We remade a systematic review and meta-analysis to evaluate the effect of TCC in breast cancer patients.
Methods: In this systematic review and meta-analysis, we searched MEDLINE (via PubMed), EMBASE (via embase.com), CENTRAL, CNKI, COVIP, Wanfang, Chaoxing, CiNii, J-SSTAGE, DBpia, and ThaiJO with no language restrictions from inception to December 31, 2018 (updated on February 16, 2020), for randomized clinical trials comparing TCC with non-exercised therapy in breast cancer patients. The primary outcome was quality of life in patients with breast cancer and data pooled by a random-effects model. Subgroup analyses were conducted to estimate the effect of different durations of TCC for breast cancer patients. This study was registered in PROSPERO, number CRD 4201810326.
Results: Fifteen articles involving a total of 885 breast cancer participants were included in this review. Compared with non-exercised therapy, TCC had a significant effect on quality of life in breast cancer patients (SMD = 0.37, 95% CI 0.15–0.59, p = 0.001), and subgroup analysis found that TCC showed beneficial effect in 12 weeks and 25 weeks (12 weeks: SMD = 0.40, 95% CI 0.19–0.62, p = 0.0003; 25 weeks: SMD = 0.38, 95% CI 0.15–0.62, p = 0.002). Meta-analyses of secondary outcomes showed that 3 weeks TCC increased shoulder function (SMD = 1.08, 95% CI 0.28–1.87, p = 0.008), 12 weeks TCC improved pain (SMD = 0.30, 95% CI 0.08–0.51, p = 0.007), shoulder function (SMD = 1.34, 95% CI 0.43–2.25, p = 0.004), strength of arm (SMD = 0.44, 95% CI 0.20–0.68, p = 0.0004), and anxiety (MD = −4.90, 95% CI −7.83 to −1.98, p = 0.001) in breast cancer patients compared with the control group.
Conclusions: TCC appears to be effective on some physical and psychological symptoms and improves the quality of life in patients with breast cancer. Additional randomized controlled trials with a rigorous methodology and low risk of bias are needed to provide more reliable evidence.
Keywords: Tai Chi Chuan, breast cancer, physical and psychological symptoms, quality of life, meta-analysis
Introduction
Although breast cancer is the most commonly diagnosed cancer and being the leading cause of cancer-related mortality in female (1–3), the long-term survival rates after a diagnosis of breast cancer are steadily rising in recent years (4–6). In the meantime, more patients with breast cancer are facing persistent symptoms and side-effects after diagnosis and treatment, such as fatigue (7–10), cognitive limitations (7, 9), depression (7, 9), anxiety (9), sleep problems (7, 9), and pain (9, 11). To address the persistent symptoms, complementary and alternative medicine (CAM), a non-mainstream medicine used together with or in place of conventional medicine, is recommended as supportive care strategies during and following the treatment (12).
Complementary therapies, especially mind-body practices, are effective approaches to manage breast cancer symptoms, and side-effects of treatment (12). For breast cancer patients, physical and psychosocial therapies improve physical function, and emotional disorders (13–15), and moreover, post-diagnostic physical activity reduces cancer-related mortality (16–18). Tai Chi Chuan (TCC) and Qigong are complementary therapies involving physical and psychological aspects (19). They are ancient Chinese mind-body exercises that both combine meditation, breathing, relaxation, and physical activity (20–22). Traditional TCC is usually a series of elaborate, lengthy, and complex movements, while Qigong is a simpler and more repetitive exercise (22, 23). TCC is a martial art that was gradually simplified and made into a common sport in 1950s (24). Nowadays, TCC as a sport focuses more on body environment and mind-body interaction (25). When TCC is performed for health and energy enhancement, it is a form of Qigong (26). Qigong produces more than a dozen forms to keep people healthy (22). Because other forms of Qigong are different from the way of practicing TCC, this review only focused on the effects of TCC on breast cancer patients.
TCC with slow, supple, graceful, curved, spiral and sequential motions, is a mild-to-moderate intensive whole-body exercise incorporating meditation into breathing control (20, 27, 28). TCC improves pain and anxiety in patients with fibromyalgia (29), exercise self-efficacy and mood disturbance in patients with chronic heart failure (30), depression and physical function in patients with osteoarthritis (31), and balance and tumble in patients with Parkinson's disease (32). TCC for more than 8 weeks reduces cancer-related fatigue, especially in breast or lung cancer patients (33, 34). In senior female cancer survivors, TCC affects systolic blood pressure and cortisol area-under-curve which may regulate the endocrine system (35). In general, TCC plays a good role in improving the quality of life (QOL) in cancer patients (36). TCC is recommended to patients with chronic conditions for multi-effects, easily learning, good safety, and low-cost (28).
Breast cancer is a chronical clinical setting with persistent symptoms of body and mind, and TCC may have a positive effect on it. Several clinical trials found TCC reduced inflammatory responses and improved quality of life, muscle strength, shoulder function, bone formation, and insomnia in breast cancer patients (37–41). Two early reviews published in 2007 and 2010, respectively, both found that the effect of TCC for breast cancer patients to improve QOL and symptoms was not definite (42, 43). In a systematic review and meta-analysis published in 2015, Pan and colleagues considered TCC had a significant effect on improving handgrip strength and limb elbow flexion, but failed to improve QOL, physical and emotional well-being, pain, and body mass index (44).
Previous reviews pooled studies ignoring the influence of diverse exercise durations and control therapies, and found limited evidence that TCC was beneficial on physical and psychosocial capacity in breast cancer patients. Additionally, some new clinical trials explored TCC for breast cancer have been published. Therefore, a systematic review and meta-analysis including the latest randomized clinical trials of TCC for breast cancer is necessary to be done. This systematic review and meta-analysis are performed to evaluate the effect of TCC on QOL and psychosomatic outcomes in breast cancer patients.
Methods
Search Strategy
The report of this systematic review and meta-analysis is followed by the Preferred Reporting Items for Systematic Reviews and Meta-analyses (PRISMA) (45). The checklist of PRISMA is in Supplement Table 1. We searched English databases (MEDLINE, EMBASE, and CENTRAL), Chinese databases (CNKI, CQVIP, Wanfang, Chaoxing), Japanese databases (CiNii, and J-SSTAGE), Korean database (DBpia), and Thai database (ThaiJO) from inception to December 31, 2018 (updated on February 16, 2020). MEDLINE and EMBASE were available for consultation through PubMed and embase.com, respectively. The following search terms in various relevant combinations were used to screen potential studies: tai*ji*, tai*chi*, breast cancer, breast tumor, breast neoplasm, breast carcinoma. Example of search strategy is in Supplement Table 2. Language restrictions were not part of data searches. The reference lists of identified original or review studies were searched manually for further articles.
Selection Criteria
The following inclusive selection criteria were applied: (I) participants were adult female patients who were diagnosed breast cancer through pathology with any tumor stage; (II) intervention measure was TCC, such as Yang-style TCC, Chen-style TCC, Wu-style TCC, Sun-style TCC, 24 simplified TCC, or movements of TCC; (III) compared intervention was non-exercised treatment, such as standard support therapy (a psychotherapy for education and peer discussion on nutrition, exercise, stress, cancer risk, and fatigue) (46, 47), usual health care (regular check-ups, medication, and health education by health care workers), or blank control; (IV) primary outcome was QOL and the measurement was not limited [e.g., the Medical Outcomes Study 36-Item Short-Form Health Survey (MOS SF-36), the World Health Organization Quality of Life Brief Questionnaire (WHOQOL-BREF), the Functional Assessment of Chronic Illness Therapy-Fatigue (FACIT–F), the Functional Assessment of Cancer Therapy–Breast (FACT-B), and the Generic Quality of life Inventory 74 (GQOLL 74)]; secondary outcomes were pain, shoulder function, strength of arm, anxiety, and other clinical outcomes; (V) only randomized controlled trial (RCT) was included. Exclusive selection criteria: (I) participants were not only breast cancer patients; (II) experimental intervention was TCC combining other exercise; (III) control intervention included other exercise methods, such as yoga, physical activity, aerobics; (IV) data of outcomes couldn't be acquired; (V) non-randomized clinical trial.
Data Extraction and Quality Assessment
Information extracted from each eligible study through electronic form containing the first author, year of publication, country of origin, number of participants, age, status of cancer, current treatment, experimental intervention, duration and frequency of TCC, controlled intervention, and outcomes. Data from eligible studies were extracted independently by two investigators (XL, LS). The risk of bias was assessed by the Cochrane Collaboration's tool with seven items: random sequence generation, allocation concealment, blinding of participants and personnel, blinding of outcome assessment, incomplete outcome data, selective reporting, and other bias (48). We used the Grades of Recommendations Assessment, Development and Evaluation (GRADE) system to evaluate the certainty of outcomes (49). Any disagreements were resolved by discussion and consensus.
Data Analysis
We tried to make meta-analysis when the number of eligible studies was more than one in each outcome and data were combined by Review Manager (version 5.3, Cochrane Library). We selected a random-effect model to pool data (50–53). Mean difference (MD)/standardized mean difference (SMD) and 95% confidence interval (CI) were calculated. If outcome with the same measurement method, we selected MD, otherwise chosen SMD. Heterogeneity across studies was tested by the I2 statistic. I2 is regarded of 25, 50, and 75% as low, moderate, and high amount of heterogeneity, respectively (54). In the Cochrane Handbook, more than 50% of I2 are regarded as may material heterogeneity (55). We did subgroup analysis based on different TCC durations. A two-tailed p ≤ 0.05 was considered as a criterion for statistical significance.
Registration
This study was registered in PROSPERO, number CRD 4201810326.
Results
Study Selection and Characteristics
By searching English, Chinese, Japanese, Korean, and Thai databases, we obtained 606 records, of which 409 records were excluded for irrelevance, or duplication through the titles and abstracts. The full-text of the remaining 197 articles were retrieved for more detailed evaluation, and 182 articles were excluded (Figure 1). Fifteen articles (40, 41, 56–68) met the inclusion criteria containing 885 participants (447 in the TCC group, 438 in the control group). Six articles (40, 41, 65–68) came from the USA, eight (56–61, 63, 64) from China and one (62) from Thailand. The types of TCC were 15-move short-form of Yang-style TCC (40, 65–68), Chen-style TCC (58, 60), Tai Chi Yunshou (63), 18-form TCC (62), 24-form TCC (57, 59, 61, 64), 8-form TCC (56), and the other article (41) didn't mention the specific form of TCC. Duration of TCC was from 12 weeks to 6 months. The frequency of TCC was 120 min per week (41), 40–60 min per session and three sessions a week (40, 61, 62, 65–68), 20–30 min per session and two sessions per day (57–60, 63, 64), and at least 40 min per session for twice a day (56). TCC was performed during chemotherapy or after conventional therapy. Some trials (56–58, 61, 63, 64) used TCC intervention after surgery to alleviate the side effects of operation. The interventions of control groups were cognitive behavioral therapy (41), standard or psychosocial support therapy (40, 65–68), usual care (56, 62), and routine rehabilitation training (57–61, 63, 64). The interest control treatments had cognitive behavioral therapy (41), standard or psychosocial support therapy (40, 65–68), and blank control (56–64). The cognitive behavioral therapy involves re-establishing a consistent sleep-wake schedule, sleep restriction, relaxation, sleep hygiene education and cognitive procedures (69). The extracted information of eligible studies is in Table 1.
Figure 1.
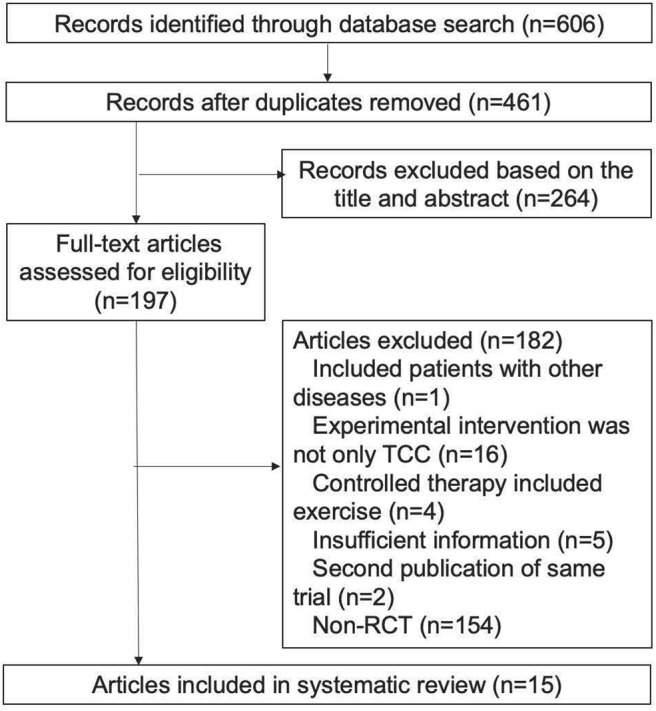
Literature search and screening process.
Table 1.
Characteristics of the eligible articles.
| Study | Country | Patients (N) | Mean age(y) | Status | Treatment condition | TCC intervention | Controlled intervention | Frequency Duration | Outcomes | ||
|---|---|---|---|---|---|---|---|---|---|---|---|
| TCC | CON | TCC | CON | ||||||||
| Han et al. (56) | China | 23 | 21 | 46.39 | 45.52 | I-III | After modified radical mastectomy and chemotherapy | Usual care+8-form TCC | Usual care | At least 40 min/session and 2 sessions /day, 5 days/week, 12 weeks | 1. Fatigue; 2. Adverse event |
| Irwin et al. (41) | USA | 45 | 45 | 59·6 | 60·0 | – | Treatment at least 6 months before enrollment | Tai Chi Chih | Cognitive behavioral therapy | 120 min/session, 3 sessions/week, 3 months | 1. Insomnia treatment response; 2. Insomnia remission; 3. Sleep quality; 4. Sleep continuity |
| Wang et al. (57) | China | 45 | 41 | 50·5 | I-III | 10 days after modified radical mastectomy | RRT + 24-form TCC | RRT | 20 min/session, every morning and evening, 6 months | 1. QOL(WHOQOLBREF); 2. Fatigue; 3. Sleep quality; 4. Anxiety; 5. Depression | |
| Wang et al. (58) | China | 44 | 46 | 53·64 | 51·74 | – | 10 days after modified radical mastectomy | RRT + Chen-style TCC | RRT | 20 min/session, every morning and evening, 3 months | 1. QOL(FACT-B); 2. Neer shoulder function score |
| Zhu et al. (59) | China | 47 | 48 | 45·51 | – | After modified radical mastectomy | RRT +24-form simplified TCC | RRT | 20 min/session, every morning and evening, 3 months | 1. Pain; 2. Activities of daily living; 3. Range of motion; 4. Strength of arm | |
| Wang et al. (60) | China | 75 | 74 | 51·4 | 50·58 | – | 10 days after modified radical mastectomy | RRT + Chen-style TCC | RRT | 20 min/session, every morning and evening, 6 months | Self-rating anxiety scale |
| Lv et al. (61) | China | 50 | 49 | 48·61 | – | After modified radical mastectomy andchemotherapy | RRT + 24-form simplified TCC | RRT | 90 min/session, 3 sessions/week, 6 months | 1. QOL(MOSSF-36); 2. Pain; 3. Activities of daily living; 4. Range of motion; 5. Strength of arm | |
| Thongteratham et al. (62) | Thailand | 15 | 15 | – | 0-IIIb | Completion of treatment at least 1 year before enrollment | Usual care + 18-form TCC | Usual care | 60 min/session, 3 sessions/week, 12 weeks | 1. QOL(FACT-B); 2. Rosenberg Self-esteem; 3. Fatigue Symptom Inventory; 4. Cortisol | |
| Li et al. (63) | China | 29 | 28 | 47·56 | 0-IIIb | 7 days after modified radical mastectomy | RRT + Tai Chi Yunshou | RRT | 30 min/session, at 7:00 and 17:00, 6 months | 1. QOL(WHOQOLBREF); 2. Edema of upper extremity; 3. Function of shoulder joint; 4. Muscle strength | |
| Wang et al. (64) | China | 63 | 71 | 47·19 | I-III | 10 days after modified radical mastectomy | RRT + 24-form simplified TCC | RRT | 20 min/session, every morning and evening, 180 days | 1. QOL(WHOQOLBREF); 2. Pain; 3. Activities of daily living; 4. Range of motion; 5. Strength of arm | |
| Sprod et al. (65) | USA | 11 | 10 | 54·33 | 52·70 | 0–IIIb | Chemotherapy, radiotherapy, hormone therapy, or none | A 15-move short-form of Yang-style TCC | Standard support therapy | 60 min/session, 3 sessions/week, 12 weeks | 1. QOL(MOSSF-36); 2. Pain; 3. Biomarkers (IL-6, IL-8, IGF-1, IGFBP-1, IGFBP-3, glucose, insulin, cortisol) |
| Janelsins et al. (66) | USA | 9 | 10 | 54·33 | 52·70 | 0–IIIb | Chemotherapy, radiotherapy, hormone therapy, or none | A 15-move short-form of Yang-style TCC | Psychosocial support therapy | 60 min/session, 3 sessions/week, 12 weeks | 1. Biomarkers (insulin, IGF-1, IGFBP-1, IGFBP-3, IL-6, IL-2, IFN-γ); 2. BMI; 3. Body composition |
| Peppone et al. (40) | USA | 7 | 9 | 53·8 | 52·6 | 0–IIIb | Treatment completed from 1 to 30 months before enrollment | A 15-move short-form of Yang-style TCC | Standard support therapy | 60 min/session, 3 sessions/week, 12 weeks | 1. Bone-specific alkaline phosphatase; 2. N-telopeptides of type I collagen; 3. Bone Remodeling Index; 4. Biomarkers (IGFBP-1, IGFBP-3, cortisol, IL-2, IL-6, IL-8) |
| Mustian et al. (67) | USA | 11 | 10 | 52 | 0–IIIb | Chemotherapy, radiotherapy, hormone therapy, or none | A 15-move short-form of Yang-style TCC | Psychosocial support therapy | 60 min/session, 3 sessions/week, 12 weeks | 1. QOL(FACIT-F); 2. Strength; 3. Flexibility; 4. Aerobic capacity | |
| Mustian et al. (68) | USA | 11 | 10 | 52 | 0–IIIb | Chemotherapy, radiotherapy, hormone therapy, or none | A 15-move short-form of Yang-style TCC | Psychosocial support therapy | 60 min/session, 3 sessions/week, 12 weeks | 1. Aerobic capacity; 2. Strength; 3. Flexibility; 4. Weight; 5. BMI;6. Body composition | |
TCC, Tai Chi Chuan; CON, control; SD, standard deviation; QOL, quality of life; MOSSF-36, Medical Outcomes Study 36-Item Short-Form Health Survey; IL-6, interleukin-6; IL-8, interleukin-8; IGF-1, insulin-like growth factor-1; IGFBP-1, insulin-like growth factor-binding protein-1; IGFBP-3, insulin-like growth factor-binding protein-3; IL-2, interleukin-2; IFN-γ, interferon- γ; BMI, body mass index; FACIT-F, Functional Assessment of Chronic Illness Therapy-Fatigue; RRT, routine rehabilitation training; WHOQOLBREF, World Health Organization Quality of Life Brief Questionnaire; FACT-B, Functional Assessment of Cancer Therapy–Breast.
Risk of Bias
According to the Cochrane Collaboration's tool, risk of bias analysis was assessed to evaluate the quality of included studies. For eligible articles, 80% had low risk of random sequence generation and 54% had low risk of allocation concealment in selection bias; 73% had unclear risk of blinding both in performance and detection bias; 87% had low risk of incomplete outcome data in attrition bias; reporting bias was unclear because the proposals of these trials were not available (Figure 2). Publication bias was not analyzed because the number of eligible studies in each meta-analysis was <10 and conceivable publication bias existed in each meta-analysis.
Figure 2.

Risk of bias graph summary.
Meta-Analysis of TCC for QOL in Breast Cancer Patients
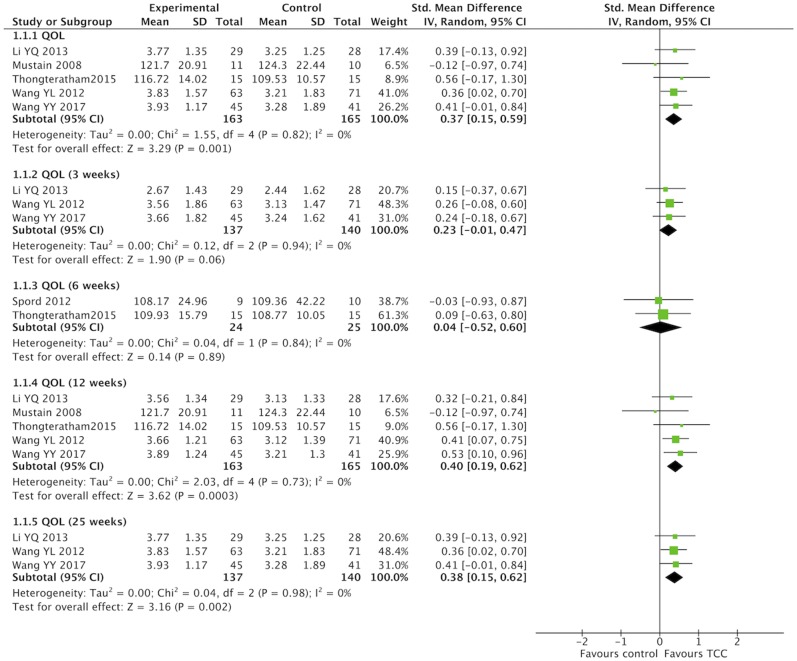
QOL were evaluated with different scales in studies that performed a meta-analysis. One study (65) used the MOS SF-36 to assess general QOL, and three studies (57, 63, 64) chosen the WHOQOL-BREF. One study (67) used the FACIT–F to assess QOL in chronic disease, while another study (62) measured cancer-special QOL with the FACT-B. In this meta-analysis, SMD were calculated for different measures of QOL. TCC had a positive effect on QOL in breast cancer patients compared with the non-exercised therapy (SMD = 0.37, 95% CI 0.15–0.59, p = 0.001, I2 = 0%) (Figure 3). Subgroup meta-analyses of 12 and 25 weeks durations showed that TCC improved QOL in breast cancer patients (12 weeks: SMD = 0.40, 95% CI 0.19–0.62, p = 0.0003, I2 = 0%; 25 weeks: SMD = 0.38, 95% CI 0.15–0.62, p = 0.002, I2 = 0%), but meta-analyses of 3 and 6 weeks both were no statistical significance (3 weeks: SMD = 0.23, 95% CI −0.01 to 0.47, p = 0.06, I2 = 0%; 6 weeks: SMD = 0.04, 95% CI −0.52 to 0.60, p = 0.89, I2 = 0%).
Figure 3.
Meta-analyses of Tai Chi Chun for quality of life in breast cancer patients compared with non-exercised therapy. Standardized mean difference and 95% confidence interval are calculated.
Meta-Analysis of TCC for Pain, Shoulder Function, Strength of Arm, Anxiety, and Fatigue in Breast Cancer Patients
For secondary outcomes, we did meta-analyses of pain, shoulder function, strength of arm, anxiety, and fatigue, respectively. The heterogeneity was high among the eligible studies in meta-analysis of TCC for shoulder function, even though we did sensitive analysis trying to delete one of the three included studies successively. In the meta-analyses of shoulder function and anxiety, the included studies were placed on one side of the no effect line, respectively. Therefore, we did meta-analyses of shoulder function and anxiety overlooking the heterogeneity. Meta-analyses showed that TCC was beneficial to alleviating pain (SMD = 0.30, 95% CI 0.08–0.51, p = 0.007, I2 = 0%) (Figure 4), recovering shoulder function (SMD = 1.34, 95% CI 0.43–2.25, p = 0.004, I2 = 92%) (Figure 5), increasing strength of arm (SMD = 0.44, 95% CI 0.20–0.68, p = 0.0004, I2 = 16%) (Figure 6), easing anxiety (MD = −4.25, 95% CI −5.87 to −2.63, p < 0.00001, I2 = 80%) (Figure 7), and relieving fatigue (SMD = −1.11, 95% CI −1.53 to −0.69, p < 0.00001, I2 = 30%) (Figure 8) compared with non-exercised therapy in breast cancer patients. TCC increased shoulder function in 3 weeks duration, but improved pain, strength of arm, and anxiety in 12 weeks in breast cancer patients.
Figure 4.
Meta-analyses of Tai Chi Chun for pain in breast cancer patients compared with non-exercised therapy. Standardized mean difference and 95% confidence interval are calculated.
Figure 5.
Meta-analyses of Tai Chi Chun for shoulder function in breast cancer patients compared with non-exercised therapy. Standardized mean difference and 95% confidence interval are calculated.
Figure 6.
Meta-analyses of Tai Chi Chun for strength of arm in breast cancer patients compared with non-exercised therapy. Standardized mean difference and 95% confidence interval are calculated.
Figure 7.
Meta-analyses of Tai Chi Chun for anxiety in breast cancer patients compared with non-exercised therapy. Mean difference and 95% confidence interval are calculated.
Figure 8.
Meta-analyses of Tai Chi Chun for fatigue in breast cancer patients compared with non-exercised therapy. Standardized mean difference and 95% confidence interval are calculated.
GRADE Evidence of Outcomes
GRADE system was used to assess the evidence quality of outcomes in this review. Risk of bias might exist because method of randomization, allocation concealment, and blinding were not definite. Outcomes measured by different methods might produce inconsistency. Although the heterogeneity in shoulder function and anxiety outcomes was high, inconsistency might not be downgraded because all the eligible studies in meta-analyses were on one side of the no effect line. The eligible studies and participants of each meta-analysis were small that imprecision and publication bias might exist. In general, the certainty of the six outcomes was very low (Table 2).
Table 2.
GRADE evidence profile of outcomes.
| Certainty assessment | No. of patients | Effect | Certainty | ||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|
| Outcomes | No. of studies | Study design | Risk of bias | Inconsis-tency | Indirect-ness | Impre-cision | Other considera-tions | TCC | CON | Absolute (95% CI) | |
| QOL | 5 | RT | Serious* | Serious† | Not serious | Serious‡ | Publication bias strongly suspected§ | 163 | 165 | SMD 0·37 higher (0·15 higher to 0·59 higher) | ⊕○○○○ VERY LOW |
| Pain | 4 | RT | Serious¶ | Not serious | Not serious | Serious‡ | Publication bias strongly suspected§ | 169 | 168 | SMD 0·3 higher (0·08 higher to 0·51 higher) | ⊕○○○○ VERY LOW |
| Shoulder function | 3 | RT | Serious || | Not serious | Not serious | Serious‡ | Publication bias strongly suspected§ | 157 | 166 | SMD 1·34 higher (0·43 higher to 2·25 higher) | ⊕○○○○ VERY LOW |
| Strength of arm | 4 | RT | Serious** | Serious† | Not serious | Serious‡ | Publication bias strongly suspected§ | 171 | 168 | SMD 0·44 higher (0·20 higher to 0·68 higher) | ⊕○○○○ VERY LOW |
| Fatigue | 3 | RT | Serious†† | Serious | Not serious | Serious‡ | Publication bias strongly suspected§ | 83 | 77 | SMD 1.11 lower (1.53 lower to 0.69 lower) | ⊕○○○○ VERY LOW |
| Anxiety | 2 | RT | Serious‡‡ | Not serious | Not serious | Serious‡ | Publication bias strongly suspected§ | 120 | 116 | MD 4·25 lower (5·87 lower to 2·63 lower) | ⊕○○○○ VERY LOW |
TCC, Tai Chi Chuan; CON, control; CI, confidence interval; QOL, quality of life; RT, randomized trials; SMD, standardized mean difference; MD, mean difference.
2/5 studies didn't mention the method of randomization, 4/5 studies didn't mention allocation concealment and blinding.
Measured by different scales.
The sample size was small.
Number of eligible studies was small.
1/4 studies didn't mention the method of randomization, 2/4 studies didn't mention allocation concealment, 3/4 studies didn't mention blinding.
1/3 studies didn't mention the method of randomization, 2/3 studies didn't mention allocation concealment and blinding.
2/4 study didn't mention the method of randomization, 2/4 studies didn't mention allocation concealment and blinding.
1/3 study didn't mention the method of randomization.
1/2 study didn't mention allocation concealment and blinding.
Discussion
This systematic review and meta-analysis concentrated on the QOL and psychosomatic symptoms in women breast cancer patients comparing TCC with non-exercise therapy.
Compared with the prior reviews (43, 44), this study searched more different language databases, included more RCTs, and made subgroup analysis on the basis of TCC duration. Lee et al. (43) and Pan et al. (44) made a systematic review of TCC for breast cancer in 2010 and 2015, respectively. Lee retrieved English, Chinese, and Korean databases, and Pan searched four English databases neglecting the different types of control intervention. In this review, we searched English, Chinese, Japanese, Korean, and Thai databases setting non-exercised therapy as the control treatment. Lee's review included seven studies, of which three were RCTs and four were non-RCTs, and only one meta-analysis of two RCTs was conducted; nine RCTs met the inclusion criteria for the Pan's review, and two to six studies were included in the meta-analyses; 15 RCTs were eligible in this review, but no more than five RCTs included in each meta-analysis. This review included 10 new RCTs (41, 56–64) which did not presented by Pan et al. (44) and might affect the results of meta-analysis. Pan did not search Chinese databases, while we searched four Chinese databases and found eight new Chinese articles (56–61, 63, 64) that met the inclusion criteria. The other two new studies (41, 62) were published in English from Thai and English databases, respectively. We made subgroup analysis according to the exercise time of TCC, and found that 12 weeks TCC was more effective than 3 or 6 weeks TCC in improving QOL, pain, and strength of arm in breast cancer patients. However, the two previous reviews did not conduct subgroup analysis according to the characteristics of TCC.
The findings of these two published reviews are quite different from those of our study. Lee et al. (43) found that TCC had no significant effect on QOL, physical outcomes (fatigue, body mass index, heart rate, and blood pressure) and psychological variables (self-esteem and depression) in patients with breast cancer. Pan et al. (44) discovered that TCC had a positive effect on handgrip dynamometer strength and limb elbow flexion, but had no significant difference in general health-related QOL, pain, and body mass index in breast cancer patients. In this review, TCC had an improvement on QOL in breast cancer patients. In the meta-analysis of TCC for QOL, Lee's review included two small sample trials, and Pan's review involved studies reporting the same trials, while we pooled data from five RCTs. Pan's review discovered that TCC failed to alleviate pain in breast cancer patients, whereas TCC had an inverse result in this systematic review. The meta-analysis of pain in this review included some new studies which influenced the result. Meta-analyses of fatigue and anxiety in this review were changed between the previous two reviews. Although this study discovered that TCC could reduce fatigue and anxiety in breast cancer patients compared with the non-exercised group, the result is uncertain because of a small number of eligible studies and high risk of selection bias, performance bias, detection bias, and publication bias. TCC was associated with positive effects on handgrip dynamometer strength and limb elbow flexion in Pan's review, and in this review also revealed that TCC improved strength of arm and shoulder function including range of motion.
QOL, a common gauge of cancer treatment effect, is widely used to measure the health of breast cancer patients (70) QOL is a multidimensional assessment with physical (including function/disability and symptoms/complications concepts), mental (including emotional distress, psychological well-being, perceived cognitive functioning, and spiritual/existential concerns concepts) and social (including socioeconomic challenges, role/relationship changes, perceived support/satisfaction, and social participation concepts) domains (70). Yan's and Tao's systematic reviews found TCC failed to improve QOL in breast cancer survivors (71, 72), but this study showed 12 weeks TCC had a positive influence on QOL. Three studies in Yan's systematic review were not included in this review because the intervention therapy of two studies (73, 74) was Tai Chi combining aerobics, and another study (75) was a prospective longitudinal study from a larger randomized controlled trial. We included some new studies that might produce the different pooled result. TCC improved the physical and mental health domains of QOL in cancer survivors at a low-level evidence in a recent review (76). Due to the limited number of included studies in the meta-analysis of QOL, we conducted a meta-analysis of overall QOL total score rather than a meta-analysis of each QOL domain in breast cancer patients, but obtained a very low-level evidence. Heather Greenlee and colleagues (12) recommends Qigong as C-graded therapy to improve QOL in breast cancer patients. TCC is one kind of Qigong and can be considered as a complementary health approach for QOL in breast cancer patients.
In this review, TCC also improved pain, shoulder function, strength of arm, anxiety, and fatigue in breast cancer patients. Pain is one of the most common symptoms in cancer patients and can be caused by tumors, surgery, chemotherapy, radiation therapy, targeted therapy, supportive care therapies, and diagnostic procedures (77). Half of breast cancer patients have mild pain and 16% have moderate to severe pain in 1 year after surgery (78). Pain has a psychological effect making patients product anxiety, nervousness, and anger (79). Control of surgery-related and non-operative pain in patients with breast cancer is essential. In addition to pain, side-effects of surgery include depressed shoulder function and arm strength (80). Decreased arm muscle strength in breast cancer patients after chemotherapy is also measured (81). Breast cancer patients have suffered psychological disorders as well as physical symptoms. About one-third of breast cancer survivors feel fatigue (82), and 40% patients are anxious in the year after diagnosis (83). Fatigue correlates with depression and sleep disturbance in breast cancer patients (84). Breast cancer patients experience persistent physical and mental disorders which influence QOL after or during cancer diagnosis and treatment. Three months exercise reduces fatigue, anxiety, and depression in breast cancer patients (85). American Society of Clinical Oncology Breast Cancer Survivorship Care Guideline recommends that primary care clinicians should counsel breast cancer patients to engage in regular physical activity to reduce cancer-related fatigue, musculoskeletal symptoms, pain, and obesity (86). Previous meta-analyses discovered that TCC improved psychological well-being including reduced stress, anxiety, depression and mood disturbance, and physical function in people with cancer (36, 87). In this review, TCC improved pain, shoulder function, strength of arm, and anxiety in breast cancer patients in 12 weeks, and also increased QOL in 12 and 25 weeks. We recommend breast cancer patients for more than 12 weeks TCC to manage symptoms and enhance QOL finally.
TCC is not only active in breast cancer, but also in other cancers. TCC improves vigor in patients with lung cancer undergoing chemotherapy (88). In addition, TCC can alleviate fatigue, enhance neck and shoulder joints mobility, and improves sleep in nasopharyngeal carcinoma patients (89, 90). For cancer survivors undergoing chemotherapy, TCC improves cancer-related fatigue, self-efficacy, and QOL (91).
As an exercise of body and mind, the mechanism of TCC is complex and evidence from basic research is lacking, thus we roughly discuss it. Resting-state functional magnetic resonance images of TCC experts found that TCC practitioners had greater functional homogeneity in the right post-central gyrus (PosCG), and less functional homogeneity in the left anterior cingulate cortex (ACC) and the right dorsal lateral prefrontal cortex (DLPFC) (92). The PosCG affects pain that its partial resection reliefs of severe limb pain (93). The posterior cingulate cortex is activated by visuospatial imagery (94). The caudal ACC is associated with the complex social interactions (95). The left ACC regulates the hypothalamic-pituitary-adrenal (HPA) axis (96). The prefrontal cortex is particularly important for cognitive control, and the DLPFC may reflect the expression of task goals (97, 98). In breast cancer patients, TCC may establish visuospatial imagery of the movements to posterior cingulate cortex, set task goals through the DLPFC, and reduce pain through the ACC. TCC may also mediate the HPA axis (99). The HPA axis plays a key role in suppressing and shaping immune responses (100, 101). Dysregulation of the HPA axis and the increased levels of pro-inflammatory cytokines [interleukin (IL)-6 and tumor necrosis factor (TNF-α)] could produce fatigue (102). TCC decreases cortisol, IL-6 and TNF-α in cancer survivors that might reduce cancer-related fatigue (35, 37, 103). TCC increases oxygen intake that might improve shoulder function and strength of in patients with breast cancer (104).
Limitations were also in this systematic review and meta-analyses. First, TCC interventions had Yang-style, Chen-style, simplified 24-action and some movements of TCC with different session length, weekly frequency and duration. The eligible studies had clinical heterogeneity because of different TCC interventions. Second, treatments such as chemotherapy, radiation therapy, hormone therapy, and surgery for breast cancer unlimited in this review might restrict the activity of patients to complete TCC. Third, the small quantity and low quality of eligible studies in each meta-analysis might produce risk of bias, inconsistency, imprecision, and publication bias. Finally, although main English, Chinese, Japanese, Korean, and Thai databases were searched, some published or gray literatures might have been missed and all the meta-analyses included <10 studies that publication bias might exist.
Conclusion
In this systematic review and meta-analysis, TCC had positive effects on QOL, pain, shoulder function, strength of arm, anxiety, and fatigue in breast cancer patients compared with the non-exercise therapy. TCC may have an improvement on QOL, physical function and psychological health in breast cancer patients. Due to the risk of bias in each study, further additional randomized controlled trials with a rigorous methodology and low risk of bias are needed to provide more reliable evidence.
Author Contributions
YT designed the study, interpreted data, and wrote the manuscript. X-CL, JL, JF, and H-YY contributed to literature search, figures, data collection, data analysis, data interpretation, and draft manuscript. LS, M-LL, LL, JY, X-LQ, and C-ZT contributed to data analysis and data interpretation.
Conflict of Interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Glossary
Abbreviations
- CAM
Complementary and alternative medicine
- CON
Control
- FACIT-F, Functional Assessment of Chronic Illness Therapy-Fatigue, FACT-B
Functional Assessment of Cancer Therapy–Breast
- GRADE, Grades of Recommendations Assessment
Development and Evaluation
- MOS SF-36
Medical Outcomes Study 36-Item Short-Form Health Survey
- QOL
Quality of life
- RRT
Routine rehabilitation training
- TCC
Tai Chi Chuan
- WHOQOL-BREF
World Health Organization Quality of Life Brief Questionnaire.
Footnotes
Funding. This work was financed by The Project First-Class Disciplines Development supported by Chengdu University of Traditional Chinese Medicine (CZYHW1901) and the Sichuan Science and Technology Program (2018HH0123, 2019YFH0108, and 2018SZ0257).
Supplementary Material
The Supplementary Material for this article can be found online at: https://www.frontiersin.org/articles/10.3389/fonc.2020.00607/full#supplementary-material
References
- 1.Bray F, Ferlay J, Soerjomataram I, Siegel RL, Torre LA, Jemal A. Global cancer statistics 2018: GLOBOCAN estimates of incidence and mortality worldwide for 36 cancers in 185 countries. CA Cancer J Clin. (2018) 6:394–424. 10.3322/caac.21492 [DOI] [PubMed] [Google Scholar]
- 2.Fidler MM, Gupta S, Soerjomataram I, Ferlay J, Steliarova-Foucher E, Bray F. Cancer incidence and mortality among young adults aged 20–39 years worldwide in 2012: a population-based study. Lancet Oncol. (2017) 12:1579–89. 10.1016/S1470-2045(17)30677-0 [DOI] [PubMed] [Google Scholar]
- 3.Siegel RL, Miller KD, Jemal A. Cancer statistics, 2019. CA Cancer J Clin. (2019) 1:7–34. 10.3322/caac.21551 [DOI] [PubMed] [Google Scholar]
- 4.Bodai BI, Tuso P. Breast cancer survivorship: a comprehensive review of long-term medical issues and lifestyle recommendations. Perm J. (2015) 2:48–79. 10.7812/TPP/14-241 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Allemani C, Matsuda T, Carlo VD, Harewood R, Matz M, Nikšić M, et al. Global surveillance of trends in cancer survival 2000-14 (CONCORD-3): analysis of individual records for 37513025 patients diagnosed with one of 18 cancers from 322 population-based registries in 71 countries. Lancet. (2018) 10125:1023–75. 10.1016/S0140-6736(17)33326-3 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Mollen EWJ, Ient J, Tjan-Heijnen VCG, Boersma LJ, Miele L, Smidt ML, et al. Moving breast cancer therapy up a notch. Front Oncol. (2018) 8:518. 10.3389/fonc.2018.00518 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Ganz PA, Petersen L, Bower JE, Crespi CM. Impact of adjuvant endocrine therapy on quality of life and symptoms: observational data over 12 months from the mind-body study. J Clin Oncol. (2016) 8:816–24. 10.1200/JCO.2015.64.3866 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Earl HM, Hiller L, Howard HC, Dunn JA, Young J, Bowden SJ, et al. Addition of gemcitabine to paclitaxel, epirubicin, and cyclophosphamide adjuvant chemotherapy for women with early-stage breast cancer (tAnGo): final 10-year follow-up of an open-label, randomised, phase 3 trial. Lancet Oncol. (2017) 6:755–69. 10.1016/s1470-2045(17)30319-4 [DOI] [PubMed] [Google Scholar]
- 9.Jensen RE, Potosky AL, Moinpour CM, Lobo T, Cella D, Hahn EA, et al. United states population-based estimates of patient-reported outcomes measurement information system symptom and functional satus feference values for individuals with cancer. J Clin Oncol. (2017) 17:1913–20. 10.1200/jco.2016.71.4410 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Grunfeld E, Dhesy-Thind S, Levine M. Clinical practice guidelines for the care and treatment of breast cancer: follow-up after treatment for breast cancer (summary of the 2005 update). CMAJ. (2005) 10:1319–20. 10.1503/cmaj.045062 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Ganz PA, Kwan L, Stanton AL, Krupnick JL, Rowland JH, Meyerowitz BE, et al. Quality of life at the end of primary treatment of breast cancer: first results from the moving beyond cancer randomized trial. J Natl Cancer Inst. (2004) 5:376–87. 10.1093/jnci/djh060 [DOI] [PubMed] [Google Scholar]
- 12.Greenlee H, DuPont-Reyes MJ, Balneaves LG, Carlson LE, Cohen MR, Deng G, et al. Clinical practice guidelines on the evidence-based use of integrative therapies during and following breast cancer treatment. CA Cancer J Clin. (2017) 3:194–232. 10.3322/caac.21397 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Goodwin PJ, Leszcz M, Ennis M, Koopmans J, Vincent L, Guther H, et al. The effect of group psychosocial support on survival in metastatic breast cancer. N Engl J Med. (2001) 24:1719–26. 10.1056/NEJMoa011871 [DOI] [PubMed] [Google Scholar]
- 14.Duijts SF, van Beurden M, Oldenburg HS, Hunter MS, Kieffer JM, Stuiver MM, et al. Efficacy of cognitive behavioral therapy and physical exercise in alleviating treatment-induced menopausal symptoms in patients with breast cancer: results of a randomized, controlled, multicenter trial. J Clin Oncol. (2012) 33:4124–33. 10.1200/JCO.2012.41.8525 [DOI] [PubMed] [Google Scholar]
- 15.van Waart H, Stuiver MM, van Harten WH, Geleijn E, Kieffer JM, Buffart LM, et al. Effect of low-Intensity physical activity and moderate- to high-Intensity physical exercise during adjuvant chemotherapy on physical fitness, fatigue, and chemotherapy completion rates: results of the pACES randomized clinical trial. J Clin Oncol. (2015) 17:1918–27. 10.1200/JCO.2014.59.1081 [DOI] [PubMed] [Google Scholar]
- 16.Borch KB, Braaten T, Lund E, Weiderpass E. Physical activity before and after breast cancer diagnosis and survival-the norwegian women and cancer cohort study. BMC Cancer. (2015) 15:967. 10.1186/s12885-015-1971-9 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Jones LW, Kwan ML, Weltzien E, Chandarlapaty S, Sternfeld B, Sweeney C, et al. Exercise and prognosis on the basis of clinicopathologic and molecular features in early stage breast cancer: the lACE and pathways studies. Cancer Ras. (2016) 18:5415–22. 10.1158/0008-5472.CAN-15-3307 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Holmes MD, Chen WY, Feskanich D, Kroenke CH, Colditz GA. Physical activity and survival after breast cancer diagnosis. JAMA. (2005) 20:2479–86. 10.1001/jama.293.20.2479 [DOI] [PubMed] [Google Scholar]
- 19.Carlson LE, Zelinski E, Toivonen K, Flynn M, Qureshi M, Piedalue KA, et al. Mind-body therapies in cancer: what is the latest evidence? Curr Oncol Rep. (2017) 10:67 10.1007/s11912-017-0626-1 [DOI] [PubMed] [Google Scholar]
- 20.Wayne PM, Kaptchuk TJ. Challenges inherent to t'ai chi research: part i–t'ai chi as a complex multicomponent intervention. J Altern Complement Med. (2008) 1:95–102. 10.1089/acm.2007.7170A [DOI] [PubMed] [Google Scholar]
- 21.National Center for Complementary and Integrative Health Tai Chi and Qi Gong: In Depth. Available online at: https://nccih.nih.gov/health/taichi/introduction.htm (accessed February 02, 2020)
- 22.Jahnke R, Larkey L, Rogers C, Etnier J, Lin F. A comprehensive review of health benefits of qigong and Tai Chi. Am J Health Promot. (2010) 6:e1–25. 10.4278/ajhp.081013-LIT-248 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Lauche R, Wayne PM, Dobos G, Cramer H. Prevalence, patterns, and predictors of t'ai chi and qigong use in the United States: results of a nationally representative survey. J Altern Complement Med. (2016) 4:336–42. 10.1089/acm.2015.0356 [DOI] [PubMed] [Google Scholar]
- 24.Yu AP, Tam BT, Lai CW, Yu DS, Woo J, Chung K-F, et al. Revealing the neural mechanisms underlying the beneficial effects of Tai Chi: a Neuroimaging perspective. Am J Chinese Med. (2018) 02:231–59. 10.1142/s0192415x18500131 [DOI] [PubMed] [Google Scholar]
- 25.Wolf SL, Coogler C, Xu T. Exploring the basis for Tai Chi chuan as a therapeutic exercise approach. Arch Phys Med Rehabil. (1997) 886-92. 10.1016/s0003-9993(97)90206-9 [DOI] [PubMed] [Google Scholar]
- 26.Klein PJ, Schneider R, Rhoads CJ. Qigong in cancer care: a systematic review and construct analysis of effective qigong therapy. Support Care Cancer. (2016) 7:3209–22. 10.1007/s00520-016-3201-7 [DOI] [PubMed] [Google Scholar]
- 27.Lan C, Lai JS, Chen SY. Tai Chi chuan an ancient wisdom on exercise and health promotion. Sports Med. (2002) 4:217–24. 10.2165/00007256-200232040-00001 [DOI] [PubMed] [Google Scholar]
- 28.Lan C, Chen SY, Lai JS, Wong AM. Tai Chi chuan in medicine and health promotion. Evid Based Complement Alternat Med. (2013) :502131. 10.1155/2013/502131 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Wang C, Schmid CH, Fielding RA, Harvey WF, Reid KF, Price LL, et al. Effect of Tai Chi versus aerobic exercise for fibromyalgia: comparative effectiveness randomized controlled trial. BMJ. (2018) 360:k851. 10.1136/bmj.k851 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Yeh GY, McCarthy EP, Wayne PM, Stevenson LW, Wood MJ, Forman D, et al. Tai Chi exercise in patients with chronic heart failure: a Randomized clinical trial. Arch Intern Med. (2011) 8:750–7. 10.1001/archinternmed.2011.150 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Wang C, Schmid CH, Iversen MD, Harvey WF, Fielding RA, Driban JB, et al. Comparative effectiveness of Tai Chi versus physical therapy for knee osteoarthritis: a randomized trial. Ann Intern Med. (2016) 2:77 10.7326/m15-2143 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Li F, Harmer P, Fitzgerald K, Eckstrom E, Stock R, Galver J, et al. Tai Chi and postural stability in patients with parkinson's disease. N Engl J Med. (2012) 6:511–9. 10.1056/NEJMoa1107911 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Song S, Yu J, Ruan Y, Liu X, Xiu L, Yue X. Ameliorative effects of Tai Chi on cancer-related fatigue: a meta-analysis of randomized controlled trials. Support Care Cancer. (2018) 7:2091–102. 10.1007/s00520-018-4136-y [DOI] [PubMed] [Google Scholar]
- 34.Hilfiker R, Meichtry A, Eicher M, Nilsson Balfe L, Knols RH, Verra ML, et al. Exercise and other non-pharmaceutical interventions for cancer-related fatigue in patients during or after cancer treatment: a systematic review incorporating an indirect-comparisons meta-analysis. Br J Sports Med. (2018) 10:651–8. 10.1136/bjsports-2016-096422 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Campo RA, Light KC, O'Connor K, Nakamura Y, Lipschitz D, LaStayo PC, et al. Blood pressure, salivary cortisol, and inflammatory cytokine outcomes in senior female cancer survivors enrolled in a Tai Chi chih randomized controlled trial. J Cancer Surviv. (2015) 1:115–25. 10.1007/s11764-014-0395-x [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Chen YW, Hunt MA, Campbell KL, Peill K, Reid WD. The effect of Tai Chi on four chronic conditions-cancer, osteoarthritis, heart failure and chronic obstructive pulmonary disease: a systematic review and meta-analyses. Br J Sports Med. (2016) 7:397–407. 10.1136/bjsports-2014-094388 [DOI] [PubMed] [Google Scholar]
- 37.Irwin MR, Olmstead R, Breen EC, Witarama T, Carrillo C, Sadeghi N, et al. Tai Chi, cellular inflammation, and transcriptome dynamics in breast cancer survivors with insomnia: a randomized controlled trial. J Natl Cancer Inst Monogr. (2014) 50:295–301. 10.1093/jncimonographs/lgu028 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Fong SSM, Liu KPY, Luk WS, Leung JCY, Chung JWY. Tai Chi qigong for survivors of breast cancer: a randomised controlled trial. Lancet. (2017) 360:S32 10.1016/s0140-6736(17)33170-7 [DOI] [Google Scholar]
- 39.Fong SS, Ng SS, Luk WS, Chung JW, Chung LM, Tsang WW, et al. Shoulder mobility, muscular strength, and quality of life in breast cancer survivors with and without Tai Chi qigong training. Evid Based Complement Alternat Med. (2013) 2013:787169. 10.1155/2013/787169 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Peppone LJ, Mustian KM, Janelsins MC, Palesh OG, Rosier RN, Piazza KM, et al. Effects of a structured weight-bearing exercise program on bone metabolism among breast cancer survivors: a feasibility trial. Clin Breast Cancer. (2010) 3:224–9. 10.3816/CBC.2010.n.030 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Irwin RM, Olmstead R, Carrillo C, Sadeghi N, Nicassio P, Ganz PA, et al. Tai Chi chih compared with cognitive behavioral therapy for the treatment of insomnia in survivors of breast cancer: a randomized, partially blinded, noninferiority trial. J Clin Oncol. (2017) 23:2656–65. 10.1200/JCO.2016 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Lee MS, Pittler MH, Ernst E. Is Tai Chi an effective adjunct in cancer care? a systematic review of controlled clinical trials. Support Care Cancer. (2007) 6:597–601. 10.1007/s00520-007-0221-3 [DOI] [PubMed] [Google Scholar]
- 43.Lee MS, Choi T-Y, Ernst E. Tai Chi for breast cancer patients: a systematic review. Breast Cancer Res Treat. (2010) 2:309–16. 10.1007/s10549-010-0741-2 [DOI] [PubMed] [Google Scholar]
- 44.Pan Y, Yang K, Shi X, Liang H, Zhang F, Lv Q. Tai Chi chuan exercise for patients with breast cancer: a systematic review and meta-analysis. Evid Based Complement Alternat Med. (2015) 2015:535237. 10.1155/2015/535237 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Moher D, Liberati A, Tetzlaff J, Altman DG, Group TP. Preferred reporting items for systematic reviews and meta analyses: the PRISMA statement. PLoS Med. (2009) 7:e1000097 10.1371/journal.pmed.1000097 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Monti DA, Kash KM, Kunkel EJ, Moss A, Mathews M, Brainard G, et al. Psychosocial benefits of a novel mindfulness intervention versus standard support in distressed women with breast cancer. Psychooncology. (2013) 11:2565–75. 10.1002/pon.3320 [DOI] [PubMed] [Google Scholar]
- 47.Prioli KM, Pizzi LT, Kash KM, Newberg AB, Morlino AM, Matthews MJ, et al. Costs and effectiveness of mindfulness-based art therapy versus standard breast cancer support group for women with cancer. Am Health Drug Benefits. (2017) 6:288–95. [PMC free article] [PubMed] [Google Scholar]
- 48.Higgins JPT, Altman DG, Gøtzsche PC, Jüni P, Moher D, Oxman AD, et al. The cochrane collaboration's tool for assessing risk of bias in randomised trials. BMJ. (2011) 343:d5928. 10.1136/bmj.d5928 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Group GW. Grading quality of evidence and strength of recommendations. BMJ. (2004) 7454:1490 10.1136/bmj.328.7454.1490 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Riley RD, Higgins JPT, Deeks JJ. Interpretation of random effects meta-analyses. BMJ. (2011) 342:d549. 10.1136/bmj.d549 [DOI] [PubMed] [Google Scholar]
- 51.Hedges LV. A random effects model for effect sizes. Psychol Bull. (1983) 2:388–395. [Google Scholar]
- 52.Barili F, Parolari A, Kappetein PA, Freemantle N. Statistical primer: heterogeneity, random- or fixed-effects model analyses? Interact Cardiovasc Thorac Surg. (2018) 3:317–21. 10.1093/icvts/ivy163 [DOI] [PubMed] [Google Scholar]
- 53.DerSimonian R, Laird N. Meta-analysis in clinical trials revisited. Contemp Clin Trials. (2015) 45:139–45. 10.1016/j.cct.2015.09.002 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Higgins JPT, Thompson SG, Deeks JJ, Altman DG. Measuring inconsistency in meta-analyses. BMJ. (2003) 7414:557–60. 10.1136/bmj.327.7414.557 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Higgins JPT, Green S. (editors.). Cochrane Handbook for Systematic Reviews of Interventions. Version 5.1.0. Oxford: The Cochrane Collaboration; (2011). [Google Scholar]
- 56.Han Q, Yang L, Huang S-Y, Zhen M-H, Huang S-M, Xei H. Effect of eight forms of taijiquan on cancer fatigue in breast cancer patients. J. Guangxi Univers. Chin. Med. (2019) 4:30–4. 10.1016/j.cct.2016.08.002 [DOI] [Google Scholar]
- 57.Wang YY. Effect of Tai Chi Chuan on Cancer-Related Fatigue And Quality Of Life in Postoperative Breast Cancer Patients Wirh Middle and Elderly Age. Wuhu: Anhui Normal University; (2017). p. 16-27. [Google Scholar]
- 58.Wang HY, Dai SJ, Hu M, Yu H, Liu SQ. Taiji exercise on patients with breast cancer after surgery impact of shoulder function and quality of life. J New Med. (2016) 3:231–3. [Google Scholar]
- 59.Zhu JY. The Effect Of Rehabilitation On Limb Function Of Patients With Breast Cancer And Analysis Of 24 Taijiquan. Wuhu: Anhui Normal University; (2016) 13-28. [Google Scholar]
- 60.Wang HY. Effects of taichi exercise pattern on anxiety among postoperative breast cancer patients. Chin J Mod Nurs. (2015) 28:3386–8. 10.3760/cma.j.isn.16742907.2015.28.010 [DOI] [Google Scholar]
- 61.Lv F, Yu Y, Liang D, Li Z-M, You W, Zhang B. Effect of baduanjin exercise and shadowboxing on quality of postoperati0n life for breast cancer patients. J Wuhan Inst Phy Educ. (2015) 7:80–3. [Google Scholar]
- 62.Thongteratham N, Pongthavornkamol K, Olson K, Ratanawichitrasin A, Nityasuddhi D, Wattanakitkrilert D. Effectiveness of Tai Chi qi qong program for thai women with breast cancer: a randomized control trial. Pacific Rim Int J Nurs Res. (2015) 4:280–94. [Google Scholar]
- 63.Li YQ, LI LL, Wei W. Influence of taichi yunshou on functional recovery of limbs in postoperative patients with breast cancer. Fujian J TCM. (2013) 5:57–8. [Google Scholar]
- 64.Wang YL, Sun XY, Wang YB, Niu F, Liu Y, Zhou LH, et al. Effects of different exercise patterns on upper limb function and quality of life in postoperative patients with breast cancer. Chin J Phy Med Rehabil. (2012) 1:64–6. 10.3760/cma.j.isn.0254-1424.2012.01.021 [DOI] [Google Scholar]
- 65.Spord LK, Janelsins MC, Palsh OG, Carroll JK, Heckler CE, Peppone JL. Health-related quality of life and biomarkers in breast cancer survivors participating in Tai Chi chuan. J Cancer Surviv. (2012) 2:146–54. 10.1007/s11764-011-0205-7 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Janelsins MC, Davis PG, Wideman L, Katula JA, Sprod LK, Peppone LJ, et al. Effects of Tai Chi chuan on insulin and cytokine levels in a randomized controlled pilot study on breast cancer survivors. Clin Breast Cancer. (2011) 3:161–70. 10.1016/j.clbc.2011.03.013 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Mustian KM, Palesh OG, Flecksteiner SA. Tai Chi chuan for breast cancer survivors. Med Sport Sci. (2008) 52:209-17. 10.1159/000134301 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Mustin KM, Katula JA, Zhao HW. A pilot study to assess the influence of Tai Chi chuan on functional capacity among breast cancer survivors. J Support Oncol. (2006) 3:139–45. [PubMed] [Google Scholar]
- 69.Morin CM, Bootzin RR, Buysse DJ, Edinger JD, Espie CA, Lichstein KL. Psychological and behavioral treatment of insomnia:update of the recent evidence (1998-2004). SLEEP. (2006) 11:1398–414. 10.1093/sleep/29.11.1398 [DOI] [PubMed] [Google Scholar]
- 70.Victorson D Cella D Wagner L Kramer Smith ML. Measuring quality of life in cancer survivors. In: Feuerstein M, editor. Handbook of Cancer Survivorship. Boston: Springer; (2007) p. 79–110. [Google Scholar]
- 71.Yan J-H, Pan L, Zhang X-M, Sun C-X, Cui G-H. Lack of efficacy of Tai Chi in improving quality of life in breast cancer survivors: a systematic review and meta-analysis. Asian Pac J Cancer Prev. (2014) 8:3715–20. 10.7314/apjcp.2014.15.8.3715 [DOI] [PubMed] [Google Scholar]
- 72.Tao WW, Jiang H, Tao XM, Jiang P, Sha LY, Sun XC. Effects of acupuncture, tuina, Tai Chi, qigong, and traditional chinese medicine five-Element music therapy on symptom management and quality of life for cancer patients: a meta-analysis. J Pain Symptom Manage. (2016) 4:728–47. 10.1016/j.jpainsymman.2015.11.027 [DOI] [PubMed] [Google Scholar]
- 73.He J-H, Yao L, Yao Z, Liu G-N. Rehabilitation effect of systematic exercise in adjuvant chemotherapy for breast cancer patients. Chin J Rehabil. (2011) 3:204–6. 10.3870/zgkf.2011.03.019 [DOI] [Google Scholar]
- 74.Qiang W-M, Dong F-Q, Yan L, Chen Y-H, Tang L. Comparison of two different exercise program in breast cancer patients after postoperative adjuvant chemotherapy. Chin J Nurs. (2011) 6:537–40. 10.3761/j.issn.0254-1769.2011.06.002 [DOI] [Google Scholar]
- 75.Rausch SM. Evaluating psychosocial effects of two intervention, Tai Chi and spiritual growth groups, in women with breast cancer. In College of Humanities and Sciences. Virginia: Virginia Commonwealth University; (2007). [Google Scholar]
- 76.Ni X, Javan R, Yates P, Hu W, Huang X. The effects of Tai Chi on quality of life of cancer survivors: a systematic review and meta-analysis. Support Care Cancer. (2019) 10:3701–16. 10.1007/s00520-019-04911-0 [DOI] [PubMed] [Google Scholar]
- 77.National Cancer Institute Cancer Pain (PDQ)-Health Professional Version (2020). Available online at: https://www.cancer.gov/about-cancer/treatment/side-effects/pain/pain-pdq (accessed February 02, 2020).
- 78.Huang J, Lim MY, Zhao B, Shao L. PM10 mass concentration and oxidative capacity of moxa smoke. QJM. (2015) 9:705–10. 10.1093/qjmed/hcv008 [DOI] [PubMed] [Google Scholar]
- 79.Butler DL, Koopman C, Neri E, Giese-Davis J, Palesh O. Effects of supportive-expressive group therapy on pain in women with metastatic breast cancer. Health Psychol. (2009) 5:579–87. 10.1037/a0016124 [DOI] [PubMed] [Google Scholar]
- 80.Ribeiro IL, Camargo PR, Alburquerque-Sendín F, Ferrari AV, Arrais CL, Salvini TF. Three-dimensional scapular kinematics, shoulder outcome measures and quality of life following treatment for breast cancer – a case control study. Musculoskelet Sci and Pract. (2019) 40:72-79. 10.1016/j.msksp.2019.01.012 [DOI] [PubMed] [Google Scholar]
- 81.Klassen O, Schmidt ME, Ulrich CM, Schneeweiss A, Potthoff K, Steindorf K, et al. Muscle strength in breast cancer patients receiving different treatment regimes. J Cachexia Sarcopenia Muscle. (2017) 2:305–16. 10.1002/jcsm.12165 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Bower JE, Ganz PA, Desmond KA, Rowland JH, Meyerowitz BE, Belin TR. Fatigue in breast cancer survivors occurrence, correlates, and impact on quality of life. J Clin Oncol. (2000) 4:743–53. 10.1200/JCO.2000.18.4.743 [DOI] [PubMed] [Google Scholar]
- 83.Hill J, Holcombe C, Clark L, Boothby MRK, Hincks A, Fisher J, et al. Predictors of onset of depression and anxiety in the year after diagnosis of breast cancer. Phychol Med. (2011) 7:1429–36. 10.1017/S0033291710001868 [DOI] [PubMed] [Google Scholar]
- 84.Bower JE, Ganz PA, Irwin MR, Kwan L, Breen EC, Cole SW. Inflammation and behavioral symptoms after breast cancer treatment: do fatigue, depression, and sleep disturbance share a common underlying mechanism? J Clin Oncol. (2011) 26:3517–22. 10.1200/JCO.2011.36.1154 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Rogers LQ, Courneya KS, Anton PM, Verhulst S, Vicari SK, Robbs RS, et al. Effects of a multicomponent physical activity behavior change intervention on fatigue, anxiety, and depressive symptomatology in breast cancer survivors: randomized trial. Psychooncology. (2017) 11:1901–6. 10.1002/pon.4254 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Runowicz CD, Leach CR, Henry NL, Henry KS, Mackey HT, Cowens-Alvarado RL, et al. American cancer society/American society of clinical oncology breast cancer survivorship care guideline. J Clin Oncol. (2016) 6:611–35. 10.1200/JCO.2015.64.3809 [DOI] [PubMed] [Google Scholar]
- 87.Wang C, Bannuru R, Ramel J, Kupelnick B, Scott T, Schmid CH. Tai Chi on psychological well-being: systematic review and meta-analysis. BMC Comlement ALten Med. (2010) 10:23. 10.1186/1472-6882-10-23 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Zhang LL, Wang SZ, Chen HL, Yuan AZ. Tai Chi exercise for cancer-Related fatigue in patients with lung cancer undergoing chemotherapy: a Randomized controlled trial. J Pain Symptom Manage. (2016) 3:504–11. 10.1016/j.jpainsymman.2015.11.020 [DOI] [PubMed] [Google Scholar]
- 89.Zhou W, Wan Y-H, Qiu Y-R, Luo X-M. Effects of Tai Chi exercise on cancer-related fatigue in patients with nasopharyngeal carcinoma undergoing chemoradiotherapy: a randomized controlled trial. J Pain Symptom Manage. (2017) 3:737–44. 10.1016/j.jpainsymman.2017.10.021 [DOI] [PubMed] [Google Scholar]
- 90.Fong SS, Ng SS, Lee HW, Pang MY, Luk WS, Chung JW, et al. The effects of a 6-month Tai Chi qigong training program on temporomandibular, cervical, and shoulder joint mobility and sleep problems in nasopharyngeal cancer survivors. Integr Cancer Ther. (2015) 1:16–25. 10.1177/1534735414556508 [DOI] [PubMed] [Google Scholar]
- 91.Murley B, Haas B, Hermanns M, Wang YT, Stocks E. Influence of Tai Chi on self-efficacy, quality of life, and fatigue among patients with cancer receiving chemotherapy: a pilot study brief. J Holist Nurs. (2019) 4:354–63. 10.1177/0898010119867557 [DOI] [PubMed] [Google Scholar]
- 92.Wei G-X, Dong H-M, Yang Z, Luo J, Zuo X-N. Tai Chi chuan optimizes the functional organization of the intrinsic human brain architecture in older adults. Front Aging Neurosci. (2014) 6:74. 10.3389/fnagi.2014.00074 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.Lewin W, Philips CG. Observations on partial removol of the post-central gyrus for pain. J neurol Neurosurg Psychiat. (1952) 15:143–7. 10.1136/jnnp.15.3.143 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94.Whittingstall K, Bernier M, Houde JC, Fortin D, Descoteaux M. Structural network underlying visuospatial imagery in humans. Cortex. (2014) 56:85–98. 10.1016/j.cortex.2013.02.004 [DOI] [PubMed] [Google Scholar]
- 95.Shinozaki J, Hanakawa T, Fukuyama H. Heterospecific and conspecific social cognition in the anterior cingulate cortex. Neuroreport. (2007) 10:993–7. 10.1097/WNR.0b013e3281ac2161 [DOI] [PubMed] [Google Scholar]
- 96.MacLullich AM, Ferguson KJ, Wardlaw JM, Starr JM, Deary IJ, Seckl JR. Smaller left anterior cingulate cortex volumes are associated with impaired hypothalamic-pituitary-adrenal axis regulation in healthy elderly men. J Clin Endocrinol Metab. (2006) 4:1591–4. 10.1210/jc.2005-2610 [DOI] [PubMed] [Google Scholar]
- 97.Miller EK, Cohen JD. An integrative theory of prefrontal cortex function. Annu Rev Neurosci. (2001) 24:167–202. 10.1146/annurev.neuro.24.1.167 [DOI] [PubMed] [Google Scholar]
- 98.Swann NC, Tandon N, Pieters TA, Aron AR. Intracranial electroencephalography reveals different temporal profiles for dorsal- and ventro-lateral prefrontal cortex in preparing to stop action. Cereb Cortex. (2013) 10:2479–88. 10.1093/cercor/bhs245 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99.Hamasaki H. Exercise and gut microbiota: clinical implications for the feasibility of Tai Chi. J Integr Med. (2017) 4:270–81. 10.1016/s2095-4964(17)60342-x [DOI] [PubMed] [Google Scholar]
- 100.Bodera P, Stankiewicz W, Kocik J. Interactions of orphanin fQ/nociceptin (OFQ/N) system with immune system factors and hypothalamic-pituitary-adrenal (HPA) axis. Pharmacol Rep. (2014) 2:288–91. 10.1016/j.pharep.2013.12.003 [DOI] [PubMed] [Google Scholar]
- 101.Yeh SH, Chuang H, Lin LW, Hsiao CY, Eng HL. Regular Tai Chi chuan exercise enhances functional mobility and cD4CD25 regulatory t cells. Br J Sports Med. (2006) 3:239–43. 10.1136/bjsm.2005.022095 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 102.Louati K, Berenbaum F. Fatigue in chronic inflammation - a link to pain pathways. Arthritis Res Ther. (2015) 17:254. 10.1186/s13075-015-0784-1 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 103.Jiang XP, Yang DC, Elliott RL, Head JF. Reduction in serum iL-6 after vacination of breast cancer patients with tumour-associated antigens is related to estrogen receptor status. Cytokine. (2000) 5:458–65. 10.1006/cyto.1999.0591 [DOI] [PubMed] [Google Scholar]
- 104.Lan C, Lai J-S, Wong M-K, Yu M-L. Cardiorespiratory function, flexibility, and body composition among geriatric Tai Chi chuan practitioners. Arch Phys Med Rehabil. (1996) 6:612–6. 10.1016/s0003-9993(96)90305-6 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.