Summary:
This comprehensive pooled analysis strongly supports a prevalence disparity in HPV+ oropharyngeal cancer by race. Furthermore, the pattern of HPV16,18 status and p16 expression in oropharyngeal cancer appears to differ by race and this may contribute to survival disparities.
Abstract
The landscape of human papillomavirus (HPV) infection in racial/ethnic subgroups of head and neck cancer (HNC) patients has not been evaluated carefully. In this study, a meta-analysis examined the prevalence of HPV in HNC patients of African ancestry. Additionally, a pooled analysis of subject-level data was also performed to investigate HPV prevalence and patterns of p16 (CDNK2A) expression amongst different racial groups. Eighteen publications (N = 798 Black HNC patients) were examined in the meta-analysis, and the pooled analysis included 29 datasets comprised of 3129 HNC patients of diverse racial/ethnic background. The meta-analysis revealed that the prevalence of HPV16 was higher among Blacks with oropharyngeal cancer than Blacks with non-oropharyngeal cancer. However, there was great heterogeneity observed among studies (Q test P < 0.0001). In the pooled analysis, after adjusting for each study, year of diagnosis, age, gender and smoking status, the prevalence of HPV16,18 in oropharyngeal cancer patients was highest in Whites (61.1%), followed by 58.0% in Blacks and 25.2% in Asians (P < 0.0001). There was no statistically significant difference in HPV16,18 prevalence in non-oropharyngeal cancer by race (P = 0.682). With regard to the pattern of HPV16,18 status and p16 expression, White patients had the highest proportion of HPV16,18+/p16+ oropharyngeal cancer (52.3%), while Asians and Blacks had significantly lower proportions (23.0 and 22.6%, respectively) [P < 0.0001]. Our findings suggest that the pattern of HPV16,18 status and p16 expression in oropharyngeal cancer appears to differ by race and this may contribute to survival disparities.
Introduction
Head and neck cancer (HNC) is the sixth most common cancer in the world, accounting for approximately 4% of all cancer cases (1). In 2012, there were an estimated 599 637 new cases of cancer of the oral cavity, larynx and oropharynx, and 324 794 deaths attributed to the disease worldwide (1). Although tobacco and alcohol use are the primary risk factors for developing HNC, human papillomavirus (HPV) is also an established risk factor for cancers arising in the oropharynx (2,3). Recently, HPV has also been reported to be associated with a subset of oral cavity cancers (4,5), but an etiological role has not been clearly demonstrated.
A recent review and meta-analysis from our group of HNC survival in relation to HPV demonstrated a survival advantage for all HPV-positive patients (6), but the survival advantage was only significant for patients with cancer of the oropharynx. Compared to patients with HPV-negative oropharyngeal cancer, the risk of death and risk of recurrence for patients with HPV-positive oropharyngeal cancer was reduced by ~28% and ~49%, respectively. In the USA, a clear disparity in HNC survival has been reported between Black and White patients, particularly for oropharyngeal cancers. Poor survival rates for Black Americans compared to White Americans have been observed (7), and some studies have suggested that this disparity may be explained at least partially by a difference in prevalence of HPV infection (8–10). Comparisons of HPV prevalence in cancer of the oral cavity and larynx between various racial/ethnic populations have been reported in a recent meta-analysis (11). However, a summary of HPV prevalence for Black patients was only reported for oral cavity cancer in this study (11). Furthermore, an assessment of attributed survival differences for oropharyngeal cancer between racial/ethnic populations was not conducted.
The goal of this study was to develop a more complete perspective of the landscape of HPV infection in ethnic subgroups of HNC patients by examining the published literature. We conducted a meta-analysis examining the prevalence of HPV in the Black population. We also performed a pooled analysis of cases reporting HNC and HPV status using subject-level data from the published literature to investigate HPV segregation and prevalence amongst different ethnic groups.
Materials and methods
This study was approved by the Fox Chase Cancer Center Institutional Review Committee.
Literature review and data collection
A PubMed search was conducted (from inception to December 2014) using the search terms, [‘human papillomavirus’ (All Fields) OR ‘HPV’ (All Fields)] AND [‘squamous cell carcinoma’ (All Fields) OR ‘cancer’ (All Fields)] AND [‘oropharyngeal’ (All Fields) OR ‘oropharynx’ (All Fields) OR ‘head and neck’ (All Fields) OR ‘tonsil’ (All Fields)]. All abstracts and full text of articles from the PubMed search were reviewed independently by two reviewers. When there was a discrepancy between reviewers, a third reviewer evaluated the article(s) to resolve the discrepancy. All studies that tested for the presence of HPV in HNC tissues from patients diagnosed with squamous cell carcinoma of the head and neck (oral cavity, oropharynx, larynx and hypopharynx) were eligible for inclusion in this analysis. The bibliographies of several review articles were also examined in order to identify additional publications that might have been missed by our PubMed search (11–15). This review identified 291 original articles that qualified conditionally for the analysis. Studies that used serology methods to detect HPV antibodies were excluded from the analysis, as this method does not identify which tissue is infected by HPV. Studies that primarily evaluated HPV in lip cancers were excluded from this analysis, with the exception of studies where it was impossible to distinguish lip cancer data from the other head and neck subsites. In addition, case reports and studies that included only HPV-positive HNC tumors/patients were excluded. Additional exclusion criteria includes studies of HNC patients who were co-infected with other diseases, such as HIV; studies in which the cancer tissues were sampled via cytobrushing and not biopsy or surgery; studies that classified HNC as HPV-related or HPV non-related tumors based on tumor site without directly testing that tissue for HPV; studies in which fewer than 80% of the eligible cases were tested for HPV; and studies that selected patient samples non-randomly, but applied pre-defined criteria for patient inclusion (e.g., patients with undifferentiated carcinoma only, metastasis only, positive lymph nodes only, advanced stage only, patients who underwent a specific treatment regimen, studies where smoking and drinking patient tissues were matched with nonsmoker and nondrinker patient tissues, etc.). For overlapping studies, the publication with the largest population and/or more complete information was included in this analysis. After accounting for these inclusion and exclusion criteria, 140 articles with data for all racial/ethnic populations were eligible for inclusion in this study. Of these, only 18 articles presented data that could be abstracted and were included in the meta-analysis of Black cancer patients. All 140 articles were eligible for inclusion in the pooled analysis. A flow diagram of study selection is illustrated in Figure 1.
Figure 1.
Flow diagram of study selection.
Meta-analysis of Black HNC patients
From each of the 18 articles that included data from Black HNC patients (818 cases), information on the number of patients, HPV prevalence, HPV genotype, tumor subsite, mean age, year of cancer diagnosis, geographic location of the study, tissue source, HPV test methodology and HPV-infected cancer site were extracted and tabulated. All data were abstracted independently by two reviewers and cross-referenced to confirm that there were no data entry errors. Three studies that included data for fewer than 10 Black patients (16–18) were therefore excluded from the meta-analysis, leaving 15 studies including 798 cases.
Pooled analysis
All investigators from the 140 studies were invited to submit their subject-level data for this pooled analysis; data from 22 studies were obtained. The remaining study investigators either did not respond or did not wish to participate. Common data elements included in the pooled analysis were HPV test method, HPV status, HPV genotype, DNA source, geographic location of the study, age at diagnosis, gender, race/ethnicity, p16 status, tobacco and alcohol use, clinical variables (such as tumor site, histology and stage) and survival variables (such as vital status and follow-up time). Seven additional articles reported demographic, clinical, HPV results, tobacco, alcohol and survival data in the publications, which enabled us to create pseudo-datasets for inclusion in the pooled analysis. All patients included in this analysis were diagnosed with cancers of the oral cavity, oropharynx or larynx. Patients with hypopharyngeal cancers were grouped with the patients with cancers of the larynx. Patients with metastases or unknown primaries were excluded from this analysis. In total, there were 29 datasets including a total of 3129 HNC cases.
Statistical analysis
The Meta-proportion of any HPV and HPV16 only was calculated for all HNC subsites combined as well as separately for oropharynx and non-oropharynx data. All statistical analyses were performed using Intercooled STATA SE (version 10) software (StataCorp. LP, College Station, TX). Meta-analyses of the proportion of HPV-positive HNC were performed using the metaprop command in STATA. HPV proportions were calculated for each individual study and the reported confidence intervals were based on Clopper–Pearson exact binomial procedures (19). Pooled proportions of the multiple studies were estimated using a random effects model. The Meta-prevalence estimates were calculated by multiplying the Meta-proportion and confidence interval values by 100. The Q-statistics were used to test for heterogeneity between the studies included in the meta-analyses. The I2 metric was also calculated to quantify variation between studies (20). Large between-study variation was observed when the I2 values were ≥50% while moderate between-study heterogeneity was denoted by I2 values between 25 and 50%. Evidence of publication bias or small study effects (P < 0.05) was assessed using the Egger’s test (21).
For the pooled analysis, unequal variance in age was observed between categories of race. Therefore, a square root transformation of age at diagnosis was performed. Adjusted HPV prevalence and 95% confidence intervals for each racial/ethnic group was calculated from logistic regression estimates for HPV-positive status, adjusting for study, year of diagnosis, square root of age, sex, history of alcohol drinking and smoking history. The adjusted prevalence refers to the average HPV prevalence while averaging the values of the covariates in the regression model. The logistic coefficients and standard errors are provided in Supplementary Materials, available at Carcinogenesis Online. A Likelihood Ratio chi-square test was performed to evaluate differences between the adjusted prevalence according to race and an analysis of variance (ANOVA) was used to compare the mean square root of age at diagnosis between racial groups (P values for pairwise comparisons were Bonferroni adjusted). P values < 0.05 were considered statistically significant. Mean age at diagnosis for each stratum was back transformed and reported. Follow-up time for overall survival refers to the interval between date of diagnosis and the date of last contact (if the patient was alive) or date of death. Hazard ratios (HR) were calculated and adjusted for each study and other confounders for risk of death or risk of disease progression (i.e., disease persistence, recurrence and/or metastasis). HR < 1.0 represents an overall survival benefit and HR > 1.0 represents poor overall survival.
Results
Description of studies: meta-analysis
Table 1 summarizes all published studies from which data were available to estimate HPV (any HPV or HPV16) prevalence in Black populations. Study size ranged from 13 to 161 patients. The majority (13/15, 87%) of studies included polymerase chain reaction (PCR)-based methods to test for the presence of HPV DNA. For all site strata (all head and neck, oropharynx and non-oropharynx), large heterogeneity was observed between the studies (Q test P-value range from 0.000 to 0.048; I2 values range from 62.1 to 94.6%). Nevertheless, as expected, the prevalence of any HPV or HPV16 was higher among oropharyngeal cancer patients (any HPV: 31.5%, 95% CI = 17.7–47.1; HPV16: 45.7%, 95% CI = 25.5–66.6) in comparison to non-oropharyngeal cancer patients (any HPV: 14.5%, 95% CI = 1.4–36.0; HPV16: 1.1%, 95% CI = 0.0–6.0). There was no evidence of publication bias or small study effect. The reasons for underlying heterogeneity were explored by stratifying the dataset according to geographic region (Sub-Saharan Africa versus USA) as well as HPV test methods (ISH versus PCR/RT-PCR). Large heterogeneity remained when stratified by HPV test method (data not shown). When stratified by geographic region (see Supplementary Table 1, available at Carcinogenesis Online), large heterogeneity was still observed except when data were limited to HPV16 infections only. For all head and neck subsites combined, the meta-prevalence of HPV16 in patients from Sub-Saharan Africa (N = 4 studies) was 1.0% (95% CI = 0.0–3.9), Q test P-value was 0.129, I2 was 47.0%. Large heterogeneity was still observed between the remaining eight studies that included patients from the USA (Q test P <0.0001, I2 = 89%). Further stratification of the Sub-Saharan Africa studies according to head and neck subsite resulted in a meta-prevalence of HPV16 in non-oropharyngeal cancers at 0.1% (95% CI = 0.0–1.8, Q test P value = 0.768, I2 = 0.0%). The only study in the USA that reported HPV16 data for non-oropharyngeal cancer showed a higher prevalence (13.6%, 95% CI = 1.9–31.7) than that of patients in Sub-Saharan Africa. There were no studies in Sub-Saharan Africa that reported data for HPV16 in oropharyngeal cancer patients and the large heterogeneity remained for the USA studies that reported HPV16 data in Black oropharyngeal cancer patients.
Table 1.
Meta-analysis of HPV prevalence in populations of African descent
| Study | HPV test method | HPV types detected | N | Any HPV N%, 95% CI |
HPV 16 N%, 95% CI |
|---|---|---|---|---|---|
| All head and neck | |||||
| Van Rensburg et al. (22) | ISH | 66 | 0.0% (0.0–5.4) | 0.0% (0.0–5.4) | |
| Gillison et al. (23) | PCR | 16, 18, 33, 31 | 48 | 20.8% (10.5–35.0) | |
| Boy et al. (24) | PCR | 16,18 | 21 | 9.5% (1.2–30.4) | 0.0% (0.0–16.1) |
| Agrawal et al. (25) | ISH | 16 | 13 | 0.0% (0.0–24.7) | 0.0% (0.0–24.7) |
| Lewis et al. (26) | ISH, PCR | 16, 33 | 26 | 11.5% (2.4–30.1) | |
| Jalouli et al. (27) | PCR | X | 20 | 65.0% (40.8–84.6) | |
| Jiron et al. (28) | PCR | 6,33,11,16,18,31,52,35,45,51,56 | 161 | 24.8% (18.4–32.3) | 20.5% (14.5–27.6) |
| Stephen et al. (29) | qRT-PCR | 16 | 31 | 16.1% (5.4–33.7) | 16.1% (5.4–33.7) |
| Babiker et al. (30) | PCR | 16, 18, 33, 31 | 100 | 8.0% (3.5–15.2) | 5.0% (1.6–11.3) |
| Isayeva et al. (31)a | qRT-PCR | 16, 18 | 30 | 60.0% (40.6–77.3) | 43.3% (25.5–62.6) |
| Ndiaye et al. (32) | PCR | 16, 35, 45 | 110 | 3.6% (1.0–9.0) | 0.9% (0.0–5.0) |
| Salazar et al. (33) | PCR, RT-PCR | 16 | 57 | 15.8% (7.5–27.9) | 15.8% (7.5–27.9) |
| Worsham et al. (10) | q-PCR | NR | 49 | 30.6% (18.2–45.4) | 30.6% (18.2–45.4) |
| Isayeva et al. (34)a | qRT-PCR | 16, 18 | 22 | 22.7% (7.8–45.4) | 13.6% (2.9–34.9) |
| Liu et al. (35)a | PCR | 16 | 44 | 72.7% (57.2–85.0) | |
| Total | 798 | 17% (8.8–27.0) | 13.7% (1.5–26.4) | ||
| P value, Q test | 0.000 | 0.000 | |||
| I 2 test | 89.8% | 93.8% | |||
| P value, Egger’s test | 0.419 | 0.643 | |||
| Oropharynx | |||||
| Lewis et al. (26) | ISH, PCR | 16, 33 | 26 | 11.5% (2.4–30.1) | |
| Jiron et al. (28) | PCR | 6,33,11,16,18,31,52,35,45,51,56 | 36 | 25.0% (12.1–42.2) | |
| Isayeva et al. (31)a | qRT-PCR | 16, 18 | 30 | 60.0% (40.6–77.3) | 43.3% (25.5–62.6) |
| Salazar et al. (33) | PCR, RT-PCR | 16 | 23 | 34.8 (16.4–57.3) | 34.8% (16.4–52.3) |
| Worsham et al. (10) | q-PCR | NR | 49 | 30.6% (18.2–45.4) | 30.6% (18.2–45.4) |
| Liu et al. (35)a | PCR | 16 | 44 | 72.7% (57.2–85.0) | |
| Total | 146 | 31.5% (17.7–47.1) | 45.7% (25.5–66.6) | ||
| P value, Q test | 0.003 | 0.000 | |||
| I 2 test | 75.5% | 84.1% | |||
| P value, Egger’s test | 0.997 | 0.807 | |||
| Non-oropharynx | |||||
| Van Rensburg et al. (22) | ISH | 66 | 0.0% (0.0–5.4) | 0.0% (0.0–5.4) | |
| Boy et al. (24) | PCR | 16, 18 | 21 | 9.5% (1.2–30.4) | 0.0% (0.0–16.1) |
| Jalouli et al. (27) | PCR | X | 20 | 65.0% (40.8–84.6) | |
| Jiron et al. (28) | PCR | 6,33,11,16,18,31,52,35,45,51,56 | 125 | 24.8% (17.5–33.3) | |
| Ndiaye et al. (32) | PCR | 16,35,45 | 105 | 3.8% (1.0–9.5) | 1.0% (0.0–5.2) |
| Isayeva et al. (34)a | qRT-PCR | 16,18 | 22 | 13.6% (2.9–34.9) | |
| Total | 337 | 14.5% (1.4–36.0) | 1.1% (0.0–6.0) | ||
| P value, Q test | 0.000 | 0.048 | |||
| I 2 test | 94.6% | 62.1% | |||
| P value, Egger’s test | 0.685 | 0.424 | |||
NR, not reported; X, HPV genotype unknown.
aStudies included in the pooled analysis.
Description of studies: pooled analysis
There were a total of 3129 patients included in this analysis (Table 2). Variations among the 29 studies were noted with regard to study size, the geographic region where the study was conducted, tumor site and the tissue source. Studies varied in size from 15 to 489 patients and were conducted mostly in Europe (48%, 14/29 studies), followed by the USA (31%, 9/29), Asia (17%, 5/29) and a single study in Australia. Most of the studies (65%, 19/29) involved patients diagnosed with cancers at both oropharyngeal and non-oropharyngeal sites (oral cavity, larynx, hypopharynx and non-oropharyngeal sites not otherwise specified). The remaining studies included patients diagnosed with oropharyngeal cancers only. Formalin-fixed paraffin-embedded (FFPE) tissues were examined in 66% of studies to test for the presence of HPV, rather than Fresh Frozen (FF) or Fresh Tissue (FT). All except for four studies used PCR methodology to detect HPV DNA, using either consensus or type-specific primers, and of these, five also evaluated HPV status using DNA in situ hybridization combined with PCR. Two studies detected HPV RNA using only RT-PCR and the other two detected both HPV RNA and DNA using RT-PCR and PCR. CDKN2A (p16) expression was evaluated in 16 studies using immunohistochemistry. With regard to race/ethnicity, the pooled dataset was diverse with patients representing African, African American, Asian and White populations. There was one study that included Aboriginal Australian patients. These patients were combined with the African and African American patients and classified as Black. There were 82 patients classified as other race (for 63 patients race was unknown and 19 patients included Pacific Islander, Middle Eastern, Indian, Hispanic or other not otherwise specified). These patients were grouped and classified as other race. Follow-up time was available for 19 studies and ranged from 0.03 to 244.5 months with a mean follow-up of 41.7 months and a median follow-up of 30.6 months.
Table 2.
Description of studies included in the pooled analysis
| Author (Ref) | Study size | Tissue source | HPV testing method | p16 expression | Geographic region | Race/ ethnicity | Tumor site | FU, months (median) |
|---|---|---|---|---|---|---|---|---|
| Cruz et al. (36)a | 35 | FF | PCR | — | Europe | W | NO | — |
| Tsuhako et al. (37)a | 88 | FFPE | PCR | — | Asia | AS | NO, O | — |
| Koskinen et al. (38)a | 61 | FF | PCR | — | Europe | W | NO, O | — |
| De Petrini et al. (39) | 70 | FF | PCR | — | Europe | W | NO, O | 30.4 |
| Ragin et al. (16) | 125 | FFPE | PCR | IHC | USA | W | NO, O | 48.4 |
| Armas et al. (40,41) | 280 | FFPE | PCR | IHC | Asia | AS | NO, O | 18.6 |
| Cohen et al. (42)a | 35 | FFPE | PCR | — | USA | UNK | O | — |
| Worden et al. (43) | 70 | FFPE | PCR | — | USA | AA, W | NO, O | 13.5 |
| Szarka et al. (44) | 33 | FF | PCR | — | Europe | W | NO, O | 25.4 |
| Szarka et al. (45) | 55 | FF | PCR | — | Europe | W | NO, O | 77.4 |
| Straetmans et al. (46) | 81 | FFPE | PCR/ISH | IHC | Europe | W | O | — |
| Tachezy et al. (47) | 135 | FFPE | PCR | — | Europe | W | NO, O | 47.7 |
| D’Souza et al. (48) | 246 | FFPE | PCR/ISH | — | USA | AA, AS, W | NO, O | 31.0 |
| Eng et al. (49) | 15 | FFPE | PCR | IHC | USA | W | NO, O | 69.8 |
| Chernock et al. (8) | 266 | FFPE | PCR/ISH | IHC | USA | AA, AS, W | O | — |
| Kabeya et al. (50)a | 31 | FF | PCR | IHC | Asia | AS | NO | — |
| Hoffman et al. (51)a | 78 | FF | PCR | IHC | Europe | W | NO, O | — |
| Park et al. (52) | 89 | FFPE | PCR | IHC | Asia | AS | O | 20.9 |
| Heusinkveld et al. (53)a | 41 | FFPE | PCR | — | Europe | W | NO, O | — |
| Bussu et al. (54,55) | 136 | FT | RT-PCR/HC2 | IHC | Europe | A, W | NO, O | 12.5 |
| Isayeva et al. (31,34) | 315 | FFPE | RT-PCR | IHC | USA | AA, AS, W | NO, O | 27.5 |
| Deng et al. (56,57) | 131 | FF | PCR | — | Asia | AS | NO, O | 25.1 |
| Morbini et al. (58) | 52 | FFPE | PCR/ISH | IHC | Europe | W | NO, O | 50.5 |
| Hong et al. (59) | 489 | FFPE | PCR | IHC | Australia | W, AB, AS | O | 49.0 |
| Kruger et al. (12) | 88 | FF | PCR | — | Europe | W | NO | — |
| Liu (35) | 44 | FFPE | PCR | IHC | USA | AA | O | 18.9 |
| Morbini et al. (60) | 41 | FFPE | PCR/ISH | IHC | Europe | W | O | 21.2 |
| Total | 3129 | 30.6 |
A, African; AA, African American; AB, Aboriginal Australian; AS, Asian; FU, follow-up; HC2, hybrid capture 2; ISH, in situ hybridization; IHC, immunohistochemistry; NO, non-oropharynx; O, Oropharynx; PCR, polymerase chain reaction; RT-PCR, real-time PCR (mRNA); UNK, unknown race; UNKP, unknown primary; W, White.
aPseudo datasets created from publication data.
Prevalence of HPV16 and HPV18 according to race and head and neck subsite
The prevalence of HPV16 and/or HPV18 (HPV16,18) stratified by race was calculated for all HNCs, oropharyngeal cancers only and non-oropharyngeal cancers only after adjusting for study, year of diagnosis, age, gender, alcohol drinking, and smoking status (Table 3, and Supplementary Table 2, available at Carcinogenesis Online, which summarizes the logistic coefficients and standard errors). As expected, the overall mean age for HPV-positive patients diagnosed with oropharyngeal cancers was lower than the mean age of HPV-positive patients diagnosed with non-oropharyngeal cancers irrespective of whether the patient carried HPV16 or HPV18 in their tumor. The mean age at diagnosis was 56.3 years for HPV16,18+ oropharyngeal cancer patients, and 60.1 years for HPV16,18+ non-oropharyngeal HNC patients (P < 0.0001). There was no statistically significant difference in the mean age at diagnosis of HPV16,18+ oropharyngeal cancer patients according to race. However, for non-oropharyngeal HNC patients, a Bonferroni post hoc test shows that Asians were statistically significantly older compared to Whites (HPV16,18: Asians, 64.1 years versus Whites, 54.9 years, P = 0.038).
Table 3.
Adjusted prevalence of HPV16 and HPV18 according to race stratified by head and neck subsite
| N | HPV16+ mean agea (years ± SD) | HPV16 prevalenceb % (95% CI) |
N | HPV18+ mean agea (years ± SD) | HPV18 prevalenceb | N | HPV16,18+ mean agea (years ± SD) | HPV16,18 prevalenceb | |
|---|---|---|---|---|---|---|---|---|---|
| All HNC | |||||||||
| Number of studies = 28 | Number of studies = 24 | Number of studies = 28 | |||||||
| Asian | 634 | 58.9 ± 0.64 | 28.4% (23.8–33.4) | 631 | 61.9 ± 0.90 | 1.6% (0.7–3.9) | 632 | 59.0 ± 0.68 | 26.0% (21.6–30.9) |
| Black | 158 | 56.6 ± 0.55 | 43.7% (34.2–53.8) | 85 | 56.7 ± 0.20 | 9.8% (4.0–21.9) | 131 | 56.7 ± 0.51 | 56.2% (45.1–66.7) |
| White | 2.123 | 56.5 ± 0.47 | 36.9% (34.0–40.0) | 1778 | 54.5 ± 0.50 | 1.6% (0.9–2.8) | 1915 | 56.6 ± 0.48 | 44.0% (40.8–47.3) |
| Otherc | 58 | 55.3 ± 0.52 | 34.3% (16.2–58.5) | 54 | — | 7.7% (1.0–39.6) | 58 | 55.3 ± 0.51 | 44.9% (23.1–68.8) |
| P = 0.0123¥ | P = 0.0077¥ | P < 0.0001¥ | |||||||
| Total | 2973 | 56.8 ± 0.50 | 35.0% (32.8–37.2) | 2548 | 58.2 ± 0.70 | 1.9% (1.2–2.8) | 2736 | 56.9 ± 0.51 | 39.3% (37.1–41.7) |
| Oropharynx | |||||||||
| Number of studies = 25 | Number of studies = 21 | Number of studies = 25 | |||||||
| Asian | 433 | 56.8 ± 0.63 | 25.9% (21.1–31.4) | 431 | 55.8 ± 1.02 | 1.6% (0.5–4.7) | 432 | 56.7 ± 0.63 | 25.2% (20.5–30.7) |
| Black | 120 | 56.7 ± 0.58 | 51.1% (39.0–63.0) | 65 | 57.0 ± 0.12 | 14.8% (5.6–33.7) | 110 | 56.9 ± 0.54 | 58.0% (45.0–70.0) |
| White | 1317 | 56.2 ± 0.46 | 57.3% (53.1–61.4) | 1100 | 56.5 ± 0.30 | 1.1% (0.4–2.5) | 1229 | 56.2 ± 0.46 | 61.1% (56.8–65.3) |
| Otherc | 44 | 55.9 ± 0.49 | 74.1% (35.4–93.7) | 42 | — | — | 44 | 55.9 ± 0.48 | 74.1% (35.5–93.7) |
| P < 0.0001¥ | P = 0.0025¥ | P < 0.0001¥ | |||||||
| Total | 1914 | 56.3 ± 0.49 | 46.6% (43.7–49.4) | 1638 | 56.4 ± 0.46 | 1.6% (1.0–2.7) | 1815 | 56.3 ± 0.48 | 48.7% (45.9–51.6) |
| Non-oropharynx | |||||||||
| Number of studies = 21 | Number of studies = 19 | Number of studies = 21 | |||||||
| Asian | 201 | 65.0 ± 0.50 | 27.1% (16.4–41.4) | 200 | 64.6 ± 0.80 | — | 200 | 64.1 ± 0.64 | 20.9% (11.9–34.0) |
| Black | 38 | 54.6 ± 0.22 | 13.3% (5.0–30.8) | 20 | 55.5 ± 0.90 | 3.2% (0.4–22.6)d | 21 | 54.9 ± 0.31 | 30.2% (12.9–55.7) |
| White | 806 | 59.2 ± 0.52 | 11.3% (8.7–14.6) | 678 | 51.9 ± 0.76 | 1.5% (0.6–3.8)d | 686 | 58.7 ± 0.59 | 17.2% (13.5–21.5) |
| Otherc | 14 | — | 6.7% (0.9–37.0) | 12 | — | 11.6% (1.3–56.3)d | 14 | — | 18.4% (4.2–53.3) |
| P = 0.0553¥ | P = 0.1434¥ | P = 0.6344¥ | |||||||
| Total | 1059 | 60.5 ± 0.55 | 13.4% (11.0–16.3) | 910 | 59.9 ± 0.88 | 1.4% (0.6–3.2)d | 921 | 60.1 ± 0.63 | 18.2% (15.2–21.8) |
aAge at diagnosis was back transformed after ANOVA using square root transformation.
bAdjusted for each study, year of diagnosis, square root age, gender, alcohol and smoking status.
cOther includes other race/ethnic groups and unknown race.
dSmoking status predicted HPV18 perfectly and was excluded as a covariate.
¥Chi-square P value for the differences between the four race/ethnic group categories.
As expected, the prevalence of HPV16,18 was higher in oropharyngeal cancer tissues compared to non-oropharyngeal cancer tissues (HPV16,18: 48.7% versus 18.2%). HPV16 was the predominant genotype carried in all patient tissues, 46.6% of oropharyngeal cancer patients and 13.4% of non-oropharyngeal HNC patients were positive for this genotype. In contrast, only approximately 1–2% of patients carried HPV18, irrespective of whether the cancer was diagnosed in the oropharynx or at a non-oropharyngeal head and neck site.
For oropharyngeal cancers, there was a statistically significant difference in the prevalence of HPV16,18 according to race. White patients had the highest prevalence of HPV16,18+ cancers followed by Blacks then Asians, however, only the prevalence in Asian patients was statistically significantly lower (61.1 versus 58.0% and 25.2%, respectively; P <0.0001). A similar pattern was observed for the prevalence of HPV16 infections. However, for HPV18, Black patients had the highest prevalence (14.8%) compared to Asians (1.6%) and Whites (1.1%) and this difference was statistically significant (P = 0.0025). For the non-oropharyngeal cancer patients, there was no statistically significant difference in HPV16 and/or 18 prevalence according to race.
Expression of p16 and HPV16,18 DNA according to race in oropharynx cancer patients
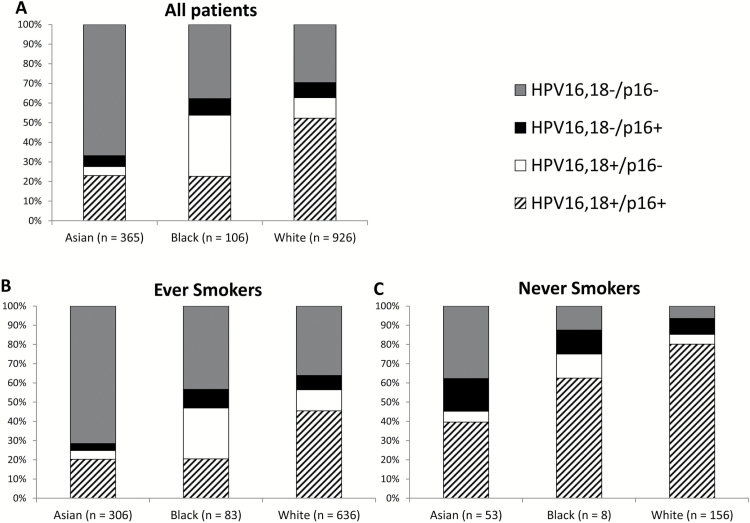
Twelve studies (1397 patients) presented with both HPV16,18 and p16 data. Among oropharyngeal cancer patients, the pattern of combined HPV16,18 and p16 status differed according to race, and this difference was statistically significant (Figure 2A, P < 0.0001). White patients had the highest proportion of cancers that were HPV16,18+/p16+ (52.3%). In contrast, Asian and Black patients had lower proportions of tumors with HPV16,18+/p16+ cancers (23.0 and 22.6%, respectively). In addition, Black patients had a higher proportion of cancers that were HPV16,18+, but p16− compared to Asian and White patients (31.1 versus 10.5% and 4.7%, respectively). The proportion of patients with HPV16,18−/p16− disease also differed significantly by race. Asian patients had the highest proportion of HPV16,18−/p16− cancers, in contrast to Black and White patients (66.8 versus 37.7% and 29.6%, respectively).
Figure 2.
Proportions of combined HPV16,18 and p16 status among all (A), ever smoker (B) and never smoker (C) oropharynx cancer patients stratified by race.
When the oropharyngeal cancer patients were stratified according to smoking history, the pattern of combined HPV16,18 and p16 status according to race co-segregated with the fraction of patients that were ever smokers (Figure 2B). Among never smokers (Figure 2C), as expected, patients with HPV16,18+/p16+ cancers comprised the predominant fraction among Asian, Black and White patients. However, White patients still had the highest proportion, and Asian patients had the lowest (White: 80.1%, Black: 62.5% Asian: 39.6%, P < 0.0001). Even among never smokers, Asians continued to have the largest proportion of patients with HPV16,18−/p16− cancers (37.4%), which was almost equal to the proportion of HPV16,18+/p16+ cancers (39.6%) observed in this subgroup.
Predictors of overall survival for oropharyngeal cancer patients according to race
Independent predictors of overall survival for oropharyngeal cancer patients were age at diagnosis, smoking history, late stage (III/IV) at diagnosis, and combined HPV16,18 and p16 status (Table 4). Patients with HPV16,18−/p16+ cancers had an increased risk of death compared to patients with HPV16,18+/p16+ oropharyngeal cancers. There was also an even greater increased risk of death for patients with p16− cancers irrespective of HPV status. When stratified according to smoking history, among never smokers, HPV16,18−/p16− patients were the only group with a statistically significantly increased risk of death compared to HPV16,18+/p16+ patients (Hazard Ratio[HR]: 2.70, 95% Confidence Interval [CI] 1.12–6.51). Patients with HPV16,18+/p16− or HPV16,18−/p16+ oropharyngeal cancers also had an increased risk of death compared to patients with HPV16,18+/p16+ oropharyngeal cancers, but the hazard ratios were not statistically significant. When stratified according to race, non-White patients differed in comparison to White patients regarding risk of death based on HPV16,18/p16 status. Table 4 shows that p16 status rather than HPV DNA status appeared to be a predictor of overall survival for non-White patients, but not for White patients. For non-Whites, the risk of death was statistically significantly increased for patients with p16-negative oropharyngeal cancers, irrespective of HPV16,18 status (HPV16,18+/p16−: HR = 2.95, 95% CI = 1.60–5.42, HPV16,18−/p16−: HR = 3.11, 95% CI = 1.97–4.92 versus HPV16,18−/p16+: HR = 0.69, 95% CI = 0.24–2.01). In contrast, the risk of death for White patients with p16+ cancers was dependent upon HPV16,18 status. White patients with HPV16,18-/p16+ oropharyngeal cancers had an increased risk of death (HR = 2.91, 95% CI = 1.72–4.92) in comparison to White patients with HPV16,18+/p16+ oropharyngeal cancers.
Table 4.
Predictors of overall survival (all-cause mortality) for oropharyngeal cancer patients according to smoking status and race
| All-cause mortality HR, 95% CIa | |||||||
|---|---|---|---|---|---|---|---|
| Oropharynx | All races (N = 880) | All races | All races | White (n = 475) | Non-White (n = 401) | Stages 0/I/II (n = 149) | Stages III/IV (n = 731) |
| Ever smokers (n = 746) | Never smokers (n = 134) | ||||||
| Race | |||||||
| White | Ref (1.00) | Ref (1.00) | Ref (1.00) | Ref (1.00) | Ref (1.00) | ||
| Asian | 0.87 (0.56–1.37) | 0.80 (0.50–1.28) | 1.27 (0.27–5.91) | 2.54 (0.73–8.78) | 0.67 (0.41–1.11) | ||
| Black | 0.85 (0.50–1.45) | 0.78 (0.45–1.37) | 2.14 (0.19–24.08) | 1.47 (0.27–7.82) | 0.71 (0.40–1.28) | ||
| Other | 0.57 (0.08–4.15) | — | 3.99 (0.46–34.18) | — | — | ||
| Age at diagnosis | 1.25 (1.09–1.44) | 1.23 (1.06–1.42) | 1.47 (0.90–2.39) | 1.75 (1.39–2.21) | 1.00 (0.89–1.26) | 1.45 (0.93–2.26) | 1.24 (1.07–1.43) |
| Smoking | |||||||
| Never smoker | Ref (1.00) | Ref (1.00) | Ref (1.00) | Ref (1.00) | Ref (1.00) | ||
| Ever smoker | 1.95 (1.34–2.83) | 1.70 (0.99–2.93) | 1.87 (1.11–3.17) | 2.58 (0.79–8.46) | 2.08 (1.39–3.11) | ||
| Alcohol | |||||||
| Never drinker | Ref (1.00) | Ref (1.00) | Ref (1.00) | Ref (1.00) | Ref (1.00) | Ref (1.00) | Ref (1.00) |
| Ever drinker | 1.00 (0.80–1.26) | 0.96 (0.76–1.22) | 1.45 (0.65–3.23) | 1.46 (0.92–2.34) | 0.89 (0.67–1.20) | 1.06 (0.51–2.22) | 1.00 (0.79–1.28) |
| Sex | |||||||
| Male | Ref (1.00) | Ref (1.00) | Ref (1.00) | Ref (1.00) | Ref (1.00) | Ref (1.00) | Ref (1.00) |
| Female | 0.74 (0.54–1.01) | 0.74 (0.53–1.05) | 0.69 (0.30–1.61) | 0.70 (0.49–1.01) | 0.87 (0.48–1.59) | 0.36 (0.16–0.81) | 0.87 (0.62–1.23) |
| Stage | |||||||
| 0/I/II | Ref (1.00) | Ref (1.00) | Ref (1.00) | Ref (1.00) | Ref (1.00) | ||
| III/IV | 2.08 (1.56–2.77) | 2.15 (1.59–2.90) | 1.83 (0.64–5.23) | 2.20 (1.53–3.16) | 2.13 (1.27–3.55) | ||
| HPV/p16 status | |||||||
| HPV16,18+/p16+ | Ref (1.00) | Ref (1.00) | Ref (1.00) | Ref (1.00) | Ref (1.00) | Ref (1.00) | Ref (1.00) |
| HPV16,18−/p16+ | 1.88 (1.19–2.97) | 1.82 (1.09–3.03) | 2.29 (0.74–7.08) | 2.91 (1.72–4.92) | 0.69 (0.24–2.01) | 7.96 (2.08–30.43) | 1.58 (0.95–2.63) |
| HPV16,18+/p16− | 3.24 (2.25–4.66) | 3.41 (2.32–5.01) | 2.19 (0.57–8.44) | 3.30 (2.09–5.21) | 2.95 (1.60–5.42) | 7.22 (2.26–23.11) | 2.85 (1.91–4.25) |
| HPV16,18−/p16− | 3.17 (2.39–4.20) | 3.30 (2.43–4.47) | 2.70 (1.12–6.51) | 2.82 (1.94–4.10) | 3.11 (1.97–4.92) | 4.36 (1.51–12.60) | 3.07 (2.28–4.13) |
aCovariates included: square root age, year of diagnosis, race, sex, smoking, alcohol, stage at diagnosis combined HPV16,18 and p16 status and study.
The risk of disease persistence, recurrence or metastasis based on HPV16,18/p16 status differed between White and non-White oropharyngeal cancer patients and is presented in Table 5. White patients that did not have HPV16,18+/p16+ disease had an increased risk of disease persistence and/or recurrence in comparison to patients diagnosed with HPV16,18+/p16+ disease. In contrast non-white patients with HPV16,18−/p16− were the only subgroup with a greater risk of disease persistence and/or recurrence in comparison to HPV16,18+/p16+ disease (HR = 2.70, 95% CI = 1.52–4.82). The risk of metastasis was only associated with non-White patients carrying HPV16,18−/p16− oropharyngeal cancers.
Table 5.
Risk of disease progression for oropharyngeal cancer patients according to HPV/p16 status and race
| Disease persistence and/or recurrence HR, 95% CIa | ||
|---|---|---|
| HPV/p16 status | White N = 475 |
Non-White N = 401 |
| HPV16,18+/p16+ | Ref (1.00) | Ref (1.00) |
| HPV16,18−/p16+ | 2.33 (1.22–4.45) | 0.52 (0.12–2.27) |
| HPV16,18+/p16− | 3.62 (2.21–5.95) | 1.44 (0.58–3.61) |
| HPV16,18−/p16− | 3.23 (2.14–4.88) | 2.70 (1.52–4.82) |
| Metastasis HR, 95% CIa | ||
| HPV16,18+/p16+ | Ref (1.00) | Ref (1.00) |
| HPV16,18−/p16+ | 1.84 (0.49–6.90) | 0.81 (0.35–1.88) |
| HPV16,18+/p16− | 2.61 (0.90–7.51) | 1.08 (0.48–2.42) |
| HPV16,18−/p16− | 2.08 (0.88–4.91) | 1.94 (1.26–2.99) |
aAdjusted for year of diagnosis, square root age, sex, race, stage, smoking, alcohol and study.
Discussion
This study expands on our prior reported meta-analysis of HPV and HNC (6). In that study, we showed that the presence of HPV infection, specifically in the oropharynx had a significant effect on disease-free survival and overall survival. Since the time of that publication, HPV-positive squamous cell carcinoma of the oropharynx has been well described and reported as a distinct clinical entity. Oropharyngeal cancer patients are often non-smokers, male, younger and White compared to traditional substance abuse-related (tobacco and alcohol) HNC. A dramatic increase in oropharyngeal cancer prevalence has been identified over the last decade (2,61,62). The number of cases of oropharyngeal cancer exceeded the number of cervical cancer cases in 2010 in the United States, and the number of HPV+ oropharyngeal cancer is expected to exceed the incidence of cervical cancer by 2020 (2). In addition, the more favorable outcome of HPV+ oropharyngeal cancer is well-documented and has been confirmed in multiple studies (63,64). These tumors appear to be HPV-related, and a hallmark of favorable tumors is p16 positivity.
For unclear reasons, the prevalence and favorable outcome of HPV+ oropharyngeal cancer is seen mostly in Whites. Variations in the prevalence of HPV have been noted previously in studies of Black patients with oropharyngeal cancer, where some report lower prevalence and others report a prevalence that is higher and/or comparable to White oropharyngeal cancer patients (9,10,35). In the first part of this study, the meta-analysis of published HPV prevalence and HNC in Black patients echoes these findings. Consistent with what is expected when comparing HPV prevalence in oropharyngeal and non-oropharyngeal cancer subsites, we show that for Black patients, cancers in the oropharynx have a higher prevalence of HPV16 (45.7%), than non-oropharyngeal sites (14.5%). There was large heterogeneity between the studies included in our meta-analysis. It is possible that differences in the HPV detection methods used in different studies may have influenced HPV positivity rates. For example, DNA ISH assays lack sensitivity and in general, PCR may lack specificity for transcriptionally active virus. Nevertheless, we observed that the meta-prevalence of HPV16 among Black patients is similar to the prevalence reported in our pooled analysis (i.e., higher in the oropharynx and lower in non-oropharyngeal sites).
We performed a pooled analysis of published HPV and HNC data in racial/ethnic subgroups in order to obtain a broader perspective. HPV status was obtained predominantly by PCR on FFPE tissues. Evaluation of HPV16, HPV18 and HPV16,18 prevalence by subsite and race yielded multiple findings. First, it is clear that HPV, specifically HPV16 or HPV18 within the oropharynx is most common in Whites (61%). There is a similar yet lower rate of HPV16,18+ disease in Blacks (58%) and a significant difference in the rate of HPV16,18+ disease in Asians (25%). This highlights the major HPV prevalence difference between Whites and Asians. This finding is curious, since the prevalence of HPV in Black patients has been reported to be statistically significantly lower than what has been reported for White patients in the literature (61,65). However, our pooled analysis reflects data from multiple institutions which is more reliable than a single study. The observed differences in HPV prevalence between Asians and Whites is also interesting and is not consistent with the previously reported meta-analysis (11). This inconsistency might be explained by differences in the type of Asian populations included in our study. This pooled analysis only included Asians from Taiwan (China) and Japan while the previously published meta-analysis included Asian populations from China and Korea. Significantly higher HPV prevalence was observed in Korean patients compared to Chinese patients and could explain the higher prevalence of HPV+ oropharyngeal cancer in Asians in that review (11).
An unexpected finding was the higher prevalence of HPV18 amongst Blacks. While HPV18 is rarely reported at either oropharyngeal (1.1%) or non-oropharyngeal cancer sites (1.5%) in Whites, HPV18 is nearly 15 times more frequently detected in Black oropharyngeal cancer patients. This major difference was unexpected. It is unclear if this is due to a higher rate of HPV18 infection in HNC in Blacks or a lower rate of HPV16+ oropharyngeal cancer in Blacks, thereby unmasking HPV18.
To better characterize oropharyngeal cancers, we evaluated by both HPV and p16 status. Canonical HPV oropharyngeal cancer is characterized by a HPV+/p16+ signature and p16 status has been reported previously as the best prognostic marker for this disease (63,66). Oropharyngeal cancer that develops in White nonsmokers is mostly likely to be HPV-associated. Our study confirmed this finding; nearly 80% of White nonsmokers were HPV+/p16+ (Figure 2C). As p16 loss is associated with smoking (67), amongst ever smokers, a much higher incidence of p16− disease was reported in all races. Although approximately 45% of ever smokers continue to be HPV+/p16+, only half that frequency of HPV+/p16+ is reported in non-Whites. Amongst Blacks and especially Asians, HPV−/p16+ disease comprises the majority of oropharyngeal disease, in distinction to Whites, where HPV+/p16+ disease is the predominant disease.
While it is not surprising that patients with HPV−/p16+ oropharyngeal cancer have a higher risk of death compared to patients with HPV+/p16+ oropharyngeal cancer, it was interesting to note that among non-Whites, the risk of death for patients with HPV−/p16+ oropharyngeal cancer was not different from patients diagnosed with HPV+/p16+ oropharyngeal cancer (HPV16,18−/p16+ HR: 0.69, 0.24–2.01). Unlike Whites (HPV16,18−/p16+ HR: 2.91, 1.72–4.92), the survival benefit among non-Whites appears to be attributed to p16 status rather than HPV. In Whites, the survival benefit appears to be attributed to HPV status rather than p16 status. However, it is possible that HPV16,18−/p16+ oropharyngeal cancers in non-Whites may be attributed to other high-risk HPV types. Further investigation of the possible role of high-risk HPV types other than HPV16,18 in non-White oropharyngeal cancer patients is needed. Overall, our findings suggest that the difference in HPV/p16 patterns according to race may impact survival differently. Given the multifactorial cause of racial survival disparities, such as poor socioeconomic status and poor access to care, the effect of HPV/p16 patterns on racial disparities in survival is not easily identified and further investigations are needed.
A limitation of this study is the use of publications as the source of patient data. Unlike database data, like SEER or The National Cancer Database, published data represent a sampling of the true population. A major assumption of our pooled analysis is that the landscape of the published literature is representative of the population as a whole. Given the dramatic differences noted in survival here between Whites and non-Whites, we feel it is highly unlikely that an error in sampling of the literature can explain these differences. A high fraction of cells with expression of p16 in both the nucleus and cytoplasm is the only good correlation with prognosis and with high-risk HPV mRNA. For each of the studies included in the pooled analysis, we did not have detailed information on the cutoffs used to define p16 status (i.e., fraction of p16 expression in nuclei versus cytoplasm). This is also a limitation of our study, as this detail may have provided more accurate correlations of p16 expression and outcome according to race.
The reasons for this difference in patterns of HPV/p16 in oropharyngeal cancer are unclear. While smoking status has predicted p16 status (67), even amongst never smokers in this study, the prevalence of HPV+/p16+ disease is lower in non-Whites. Possible explanations include genetic and environmental causes. The development of HPV+ oropharyngeal cancer has been associated with differences in sexual behavior patterns and marijuana use (68). Differential sexual and behavior patterns amongst Whites versus non-Whites have not been studied well. While the number of oral sex partners has been identified in the risk of developing HPV+ oropharyngeal cancer (68). The percentage difference in ever oral sex partners in individuals 45–60 years old between Whites and Blacks appears modest (about 15% difference in prevalence) from a few major studies (69,70), but this remains an area of active research. Other potential explanations are genetic differences between races and differences in the host response to HPV infection, which merit further investigation. Intratypic variation of HPV16 is associated with geographical distribution and may contribute to differences in outcome (71–76). For example, African and Asian-American intratypic variants of HPV16 show higher transforming potential in tumors of the anogenital tract. Therefore, in HNC, differential infection by HPV variants between races may also be an important area for investigation.
At this time, we do not have sufficient understanding to offer a clear recommendation as to how to reduce oropharyngeal HPV infection or the risk of developing HPV+ oropharyngeal cancer. This appears to be a problem of environment and biology, without a reversible modifiable factor to reduce risk. We hope that greater adoption of HPV vaccination will alter the incidence curve within about 20 years. Our study has examined HPV and HNC, with a focus on oropharyngeal cancer. This study demonstrates that while HPV-related oropharyngeal cancer (HPV+/p16+) represents the majority cause among White patients, Blacks and Asians have lower rates. Because HPV-related oropharyngeal cancer has a more favorable outcome regardless of race, the differential HPV prevalence amongst Blacks and Asians is expected to cause a significant outcome disparity in oropharyngeal cancer treatment. Further studies specifically examining racial differences in HPV+ oropharyngeal cancer are needed to corroborate these findings. However, this comprehensive pooled analysis of the published literature strongly supports a prevalence disparity in HPV+ oropharyngeal cancer that would predict an outcome/survival disparity.
Supplementary material
Supplementary data are available at Carcinogenesis online.
Funding
Supported by the American Cancer Society (RSG-14-033-01-CPPB to C.R.) and in part by National Cancer Institute (P30 CA006927) and Commonwealth of Pennsylvania. This work was also supported in part by The Lagrange Project – CRT Foundation/ISI Foundation, Turin, Italy to M.R.
Conflict of Interest Statement: None declared.
Supplementary Material
Abbreviations
- FF
fresh frozen
- FT
fresh tissue
- FFPE
formalin-fixed paraffin-embedded
- HPV
human papillomavirus
- HNC
head and neck cancer
- HR
hazard ratios
- PCR
polymerase chain reaction
References
- 1. Ferlay J., et al. (2013) GLOBOCAN 2012 v1.0, Cancer Incidence and Mortality Worldwide: IARC CancerBase No. 11 Lyon, France: International Agency for Research on Cancer. [Google Scholar]
- 2. Chaturvedi A.K., et al. (2011) Human papillomavirus and rising oropharyngeal cancer incidence in the United States. J. Clin. Oncol., 29, 4294–4301. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Gillison M.L., et al. (2012) Prevalence of oral HPV infection in the United States, 2009–2010. JAMA, 307, 693–703. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Hobbs C.G., et al. (2006) Human papillomavirus and head and neck cancer: a systematic review and meta-analysis. Clin. Otolaryngol., 31, 259–266. [DOI] [PubMed] [Google Scholar]
- 5. Syrjanen S., et al. (2011) Human papillomaviruses in oral carcinoma and oral potentially malignant disorders: a systematic review. Oral Dis., 17 (suppl. 1), 58–72. [DOI] [PubMed] [Google Scholar]
- 6. Ragin C.C., et al. (2007) Survival of squamous cell carcinoma of the head and neck in relation to human papillomavirus infection: review and meta-analysis. Int. J. Cancer, 121, 1813–1820. [DOI] [PubMed] [Google Scholar]
- 7. Zandberg D.P., et al. (2014) Oropharyngeal cancer is a driver of racial outcome disparities in squamous cell carcinoma of the head and neck: 10-year experience at the University of Maryland Greenebaum Cancer Center. Head Neck., 4, 564–572. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Chernock R.D., et al. (2011) Human papillomavirus-related squamous cell carcinoma of the oropharynx: a comparative study in whites and African Americans. Arch. Otolaryngol. Head Neck Surg., 137, 163–169. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Settle K., et al. (2009) Racial survival disparity in head and neck cancer results from low prevalence of human papillomavirus infection in black oropharyngeal cancer patients. Cancer Prev. Res. (Phila Pa), 2, 776–781. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Worsham M.J., et al. (2013) Improved survival with HPV among African Americans with oropharyngeal cancer. Clin. Cancer Res., 19, 2486–2492. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Ndiaye C., et al. (2014) HPV DNA, E6/E7 mRNA, and p16INK4a detection in head and neck cancers: a systematic review and meta-analysis. Lancet Oncol., 15, 1319–1331. [DOI] [PubMed] [Google Scholar]
- 12. Kruger M., et al. (2014) The prevalence of human papilloma virus (HPV) infections in oral squamous cell carcinomas: a retrospective analysis of 88 patients and literature overview. J. Craniomaxillofac.Surg., 42, 1506–1514. [DOI] [PubMed] [Google Scholar]
- 13. Mehanna H., et al. (2013) Prevalence of human papillomavirus in oropharyngeal and nonoropharyngeal head and neck cancer-systematic review and meta-analysis of trends by time and region. Head Neck, 35, 747–755. [DOI] [PubMed] [Google Scholar]
- 14. Dayyani F., et al. (2010) Meta-analysis of the impact of human papillomavirus (HPV) on cancer risk and overall survival in head and neck squamous cell carcinomas (HNSCC). Head Neck Oncol., 2, 15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Stein A.P., et al. (2014) Prevalence of human papillomavirus in oropharyngeal squamous cell carcinoma in the United States across time. Chem. Res. Toxicol., 27, 462–469. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Ragin C.C., et al. (2006) 11q13 amplification status and human papillomavirus in relation to p16 expression defines two distinct etiologies of head and neck tumours. Br. J. Cancer, 95, 1432–1438. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Ukpo O.C., et al. (2009) Human papillomavirus-associated oropharyngeal squamous cell carcinomas: primary tumor burden and survival in surgical patients. Ann. Otol. Rhinol. Laryngol., 118, 368–373. [DOI] [PubMed] [Google Scholar]
- 18. Wang X.I., et al. (2012) Changing trends in human papillomavirus-associated head and neck squamous cell carcinoma. Ann. Diagn. Pathol., 16, 7–12. [DOI] [PubMed] [Google Scholar]
- 19. Newcombe R.G. (1998) Two-sided confidence intervals for the single proportion: comparison of seven methods. Stat. Med., 17, 857–872. [DOI] [PubMed] [Google Scholar]
- 20. Higgins J.P.T., et al. (2003) Measuring inconsistency in meta-analyses. BMJ, 327, 557–560. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Egger M., et al. (1997) Bias in meta-analysis detected by a simple, graphical test. BMJ, 315, 629–634. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Van Rensburg E.J., et al. (1995) Detection of human papillomavirus DNA with in situ hybridisation in oral squamous carcinoma in a rural black population. S. Afr. Med. J., 85, 894–896. [PubMed] [Google Scholar]
- 23. Gillison M.L., et al. (2000) Evidence for a causal association between human papillomavirus and a subset of head and neck cancers. J. Natl. Cancer Inst., 92, 709–720. [DOI] [PubMed] [Google Scholar]
- 24. Boy S., et al. (2006) HPV detection in primary intra-oral squamous cell carcinomas--commensal, aetiological agent or contamination? J. Oral Pathol. Med., 35, 86–90. [DOI] [PubMed] [Google Scholar]
- 25. Agrawal Y., et al. (2008) Oral human papillomavirus infection before and after treatment for human papillomavirus 16-positive and human papillomavirus 16-negative head and neck squamous cell carcinoma. Clin. Cancer Res., 14, 7143–7150. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Lewis J.S., Jr., et al. (2010) p16 positive oropharyngeal squamous cell carcinoma:an entity with a favorable prognosis regardless of tumor HPV status. Am. J .Surg. Pathol., 34, 1088–1096. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Jalouli J., et al. (2012) Human papilloma virus, herpes simplex virus and epstein barr virus in oral squamous cell carcinoma from eight different countries. Anticancer Res., 32, 571–580. [PubMed] [Google Scholar]
- 28. Jiron J., et al. (2014) Racial disparities in human papillomavirus (HPV) associated head and neck cancer. Am. J. Otolaryngol., 35, 147–153. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Stephen J.K., et al. (2012) Human papillomavirus outcomes in an access-to-care laryngeal cancer cohort. Otolaryngol. Head Neck Surg., 146, 730–738. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Babiker A.Y., et al. (2013) Screening for high risk human papilloma virus (HR-HPV) subtypes, among Sudanese patients with oral lesions. Int. J. Clin. Exp. Med., 6, 275–281. [PMC free article] [PubMed] [Google Scholar]
- 31. Isayeva T., et al. (2014) African Americans with oropharyngeal carcinoma have significantly poorer outcomes despite similar rates of human papillomavirus-mediated carcinogenesis. Hum. Pathol., 45, 310–319. [DOI] [PubMed] [Google Scholar]
- 32. Ndiaye C., et al. (2013) The role of human papillomavirus in head and neck cancer in Senegal. Infect. Agent Cancer, 8, 14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Salazar C.R., et al. (2014) Human papillomavirus-associated head and neck squamous cell carcinoma survival: a comparison by tumor site and initial treatment. Head Neck Pathol., 8, 77–87. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Isayeva T., et al. (2015) The protective effect of p16(INK4a) in oral cavity carcinomas: p16(Ink4A) dampens tumor invasion-integrated analysis of expression and kinomics pathways. Mod. Pathol., 28, 631–653. [DOI] [PubMed] [Google Scholar]
- 35. Liu J.C., et al. (2015) High prevalence of discordant human papillomavirus and p16 oropharyngeal squamous cell carcinomas in an African American cohort. Head Neck. 38 (suppl. 1), E867–E872. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. Cruz I.B., et al. (1996) Age-dependence of human papillomavirus DNA presence in oral squamous cell carcinomas. Eur. J. Cancer B Oral Oncol., 32B, 55–62. [DOI] [PubMed] [Google Scholar]
- 37. Tsuhako K., et al. (2000) Comparative study of oral squamous cell carcinoma in Okinawa, Southern Japan and Sapporo in Hokkaido, Northern Japan; with special reference to human papillomavirus and Epstein-Barr virus infection. J. Oral Pathol. Med., 29, 70–79. [DOI] [PubMed] [Google Scholar]
- 38. Koskinen W.J., et al. (2003) Prevalence and physical status of human papillomavirus in squamous cell carcinomas of the head and neck. Int. J. Cancer, 107, 401–406. [DOI] [PubMed] [Google Scholar]
- 39. De Petrini M., et al. (2006) Head and neck squamous cell carcinoma: role of the human papillomavirus in tumour progression. New Microbiol., 29, 25–33. [PubMed] [Google Scholar]
- 40. Al-Swiahb J.N., et al. (2010) Prognostic impact of p16, p53, epidermal growth factor receptor, and human papillomavirus in oropharyngeal cancer in a betel nut-chewing area. Arch. Otolaryngol. Head Neck Surg., 136, 502–508. [DOI] [PubMed] [Google Scholar]
- 41. Armas G.L., et al. (2008) The impact of virus in N3 node dissection for head and neck cancer. Eur. Arch. Otorhinolaryngol., 265, 1379–1384. [DOI] [PubMed] [Google Scholar]
- 42. Cohen M.A., et al. (2008) Increased viral load correlates with improved survival in HPV-16-associated tonsil carcinoma patients. Acta Otolaryngol., 128, 583–589. [DOI] [PubMed] [Google Scholar]
- 43. Worden F.P., et al. (2008) Chemoselection as a strategy for organ preservation in advanced oropharynx cancer: response and survival positively associated with HPV16 copy number. J. Clin. Oncol., 26, 3138–3146. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44. Major T., et al. (2005) The characteristics of human papillomavirus DNA in head and neck cancers and papillomas. J. Clin. Pathol., 58, 51–55. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45. Feher E., et al. (2009) Investigation of the occurrence of torque tenovirus in malignant and potentially malignant disorders associated with human papillomavirus. J. Med. Virol., 81, 1975–1981. [DOI] [PubMed] [Google Scholar]
- 46. Straetmans J.M., et al. (2009) Human papillomavirus reduces the prognostic value of nodal involvement in tonsillar squamous cell carcinomas. Laryngoscope, 119, 1951–1957. [DOI] [PubMed] [Google Scholar]
- 47. Tachezy R., et al. (2009) Demographic and risk factors in patients with head and neck tumors. J. Med. Virol., 81, 878–887. [DOI] [PubMed] [Google Scholar]
- 48. D’Souza G., et al. (2010) Moderate predictive value of demographic and behavioral characteristics for a diagnosis of HPV16-positive and HPV16-negative head and neck cancer. Oral Oncol., 46, 100–104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49. Bennett K.L., et al. (2010) HPV status-independent association of alcohol and tobacco exposure or prior radiation therapy with promoter methylation of FUSSEL18, EBF3, IRX1, and SEPT9, but not SLC5A8, in head and neck squamous cell carcinomas. Genes Chromosomes Cancer, 49, 319–326. [DOI] [PubMed] [Google Scholar]
- 50. Kabeya M., et al. (2012) Prevalence of human papillomavirus in mobile tongue cancer with particular reference to young patients. Cancer Sci., 103, 161–168. [DOI] [PubMed] [Google Scholar]
- 51. Hoffmann M., et al. (2012) HPV DNA, E6*I-mRNA expression and p16INK4A immunohistochemistry in head and neck cancer - how valid is p16INK4A as surrogate marker? Cancer Lett., 323, 88–96. [DOI] [PubMed] [Google Scholar]
- 52. Park W.S., et al. (2012) Human papillomavirus in oropharyngeal squamous cell carcinomas in Korea: use of G1 cycle markers as new prognosticators. Head Neck, 34, 1408–1417. [DOI] [PubMed] [Google Scholar]
- 53. Heusinkveld M., et al. (2012) Systemic and local human papillomavirus 16-specific T-cell immunity in patients with head and neck cancer. Int. J. Cancer, 131, E74–E85. [DOI] [PubMed] [Google Scholar]
- 54. Bussu F., et al. (2013) HPV infection in squamous cell carcinomas arising from different mucosal sites of the head and neck region. Is p16 immunohistochemistry a reliable surrogate marker? Br. J. Cancer, 108, 1157–1162. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55. Bussu F., et al. (2014) Human papillomavirus (HPV) infection in squamous cell carcinomas arising from the oropharynx: detection of HPV DNA and p16 immunohistochemistry as diagnostic and prognostic indicators--a pilot study. Int. J. Radiat. Oncol. Biol. Phys., 89, 1115–1120. [DOI] [PubMed] [Google Scholar]
- 56. Deng Z., et al. (2011) Prevalence and clinical features of human papillomavirus in head and neck squamous cell carcinoma in Okinawa, southern Japan. Eur. Arch. Otorhinolaryngol., 268, 1625–1631. [DOI] [PubMed] [Google Scholar]
- 57. Deng Z., et al. (2013) Viral load, physical status, and E6/E7 mRNA expression of human papillomavirus in head and neck squamous cell carcinoma. Head Neck, 35, 800–808. [DOI] [PubMed] [Google Scholar]
- 58. Morbini P., et al. (2013) Oral HPV infection and persistence in patients with head and neck cancer. Oral Surg. Oral Med. Oral Pathol. Oral Radiol., 116, 474–484. [DOI] [PubMed] [Google Scholar]
- 59. Hong A., et al. (2013) HPV status of oropharyngeal cancer by combination HPV DNA/p16 testing: biological relevance of discordant results. Ann. Surg.Oncol., 20 (suppl. 3), S450–S458. [DOI] [PubMed] [Google Scholar]
- 60. Morbini P., et al. (2015) Identification of transcriptionally active HPV infection in formalin-fixed, paraffin-embedded biopsies of oropharyngeal carcinoma. Hum. Pathol., 46, 681–689. [DOI] [PubMed] [Google Scholar]
- 61. Fakhry C., et al. (2015) Oropharyngeal cancer survivorship in Denmark, 1977–2012. Oral Oncol., 51, 982–984. [DOI] [PubMed] [Google Scholar]
- 62. de Souza D.L., et al. (2012) Trends in the incidence of oral cavity and oropharyngeal cancers in Spain. Head Neck, 34, 649–654. [DOI] [PubMed] [Google Scholar]
- 63. Ang K.K., et al. (2010) Human papillomavirus and survival of patients with oropharyngeal cancer. N. Engl. J. Med., 363, 24–35. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64. Fakhry C., et al. (2008) Improved survival of patients with human papillomavirus-positive head and neck squamous cell carcinoma in a prospective clinical trial. J. Natl. Cancer Inst., 100, 261–269. [DOI] [PubMed] [Google Scholar]
- 65. Zandberg D.P., et al. (2015) Emergence of HPV16-positive oropharyngeal cancer in Black patients over time: University of Maryland 1992–2007. Cancer Prev. Res. (Phila), 8, 12–19. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66. Salazar C.R., et al. (2014) Combined P16 and human papillomavirus testing predicts head and neck cancer survival. Int. J. Cancer, 135, 2404–2412. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67. Junor E., et al. (2012) Benefit of chemotherapy as part of treatment for HPV DNA-positive but p16-negative squamous cell carcinoma of the oropharynx. Br. J. Cancer, 106, 358–365. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68. Gillison M.L., et al. (2008) Distinct risk factor profiles for human papillomavirus type 16-positive and human papillomavirus type 16-negative head and neck cancers. J. Natl. Cancer Inst., 100, 407–420. [DOI] [PubMed] [Google Scholar]
- 69. D’Souza G., et al. (2014) Differences in oral sexual behaviors by gender, age, and race explain observed differences in prevalence of oral human papillomavirus infection. PLoS One, 9, e86023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70. Rettig E.M., et al. (2016) Race is associated with sexual behaviors and modifies the effect of age on human papillomavirus serostatus among perimenopausal women. Sex Transm. Dis., 43, 231–237. [DOI] [PubMed] [Google Scholar]
- 71. Xi L.F., et al. (1997) Genomic variation of human papillomavirus type 16 and risk for high grade cervical intraepithelial neoplasia. J. Natl. Cancer Inst., 89, 796–802. [DOI] [PubMed] [Google Scholar]
- 72. Villa L.L., et al. (2000) Molecular variants of human papillomavirus types 16 and 18 preferentially associated with cervical neoplasia. J. Gen. Virol., 81, 2959–2968. [DOI] [PubMed] [Google Scholar]
- 73. Berumen J., et al. (2001) Asian-American variants of human papillomavirus 16 and risk for cervical cancer: a case-control study. J. Natl. Cancer Inst., 93, 1325–1330. [DOI] [PubMed] [Google Scholar]
- 74. Hildesheim A., et al. (2001) Human papillomavirus type 16 variants and risk of cervical cancer. J. Natl. Cancer Inst., 93, 315–318. [DOI] [PubMed] [Google Scholar]
- 75. Xi L.F., et al. (2002) Acquisition and natural history of human papillomavirus type 16 variant infection among a cohort of female university students. Cancer Epidemiol. Biomarkers Prev., 11, 343–351. [PubMed] [Google Scholar]
- 76. Tornesello M.L., et al. (2008) Human papillomavirus genotypes and HPV16 variants in penile carcinoma. Int. J. Cancer, 122, 132–137. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.