Abstract
Human Epidermal Growth Factor Receptor 2-positive breast cancer (HER2+ BC) is defined by increased amplification of the ERBB2/neu oncogene and/or overexpression of its associated HER2 transmembrane receptor protein. HER2+ BC represents approximately 15-20% of breast cancer, and it is independently associated with a higher grade, more aggressive phenotype, and worse prognosis. With the advent of trastuzumab, the prognostic landscape for HER2+ BC patients has considerably improved. However, both de novo and acquired resistance to trastuzumab remain a significant obstacle for many patients, requiring novel therapies for further clinical benefit. Over the last two decades, there has been extraordinary progress in the development of HER2+ BC treatment regimens, with extensions into HER2-amplified gastroesophageal junction cancer via the NCI-MATCH precision medicine trial program (NCT02465060). Trastuzumab, pertuzumab, T-DM1, and lapatinib are commonly recommended as a single agent (along with chemotherapy) or in combinations of anti-HER2 agents in neoadjuvant, adjuvant and metastatic settings according to National Comprehensive Cancer Network (NCCN) guidelines. Currently, the combination of trastuzumab, pertuzumab, and taxane chemotherapy are first-line for HER2+/HR- metastatic breast cancer with potential breakthrough therapies such as trastuzumab-deruxtecan (DS-8201a), margetuximab and tucatinib (ONT-380) on the horizon. Furthermore, recent clinical trials have demonstrated the potential utility of hormone receptor status, PAM-50 luminal intrinsic subtype, PD-L1, and TIL as predictive biomarkers for response to HER2+ therapies. We briefly introduce the origin of HER2, the invention of trastuzumab, and the classification of HER2+ BC. Each HER2-targeted therapy is then presented by indication, mechanism of action, and relevant clinical trials with subsequent elaboration and contextualization within clinical settings with an epilogue of potential future biomarkers for clinical use in HER2+ BC. We summarize the most significant and updated research in clinical practice relevant to HER2+ BC management and highlight the clinical status of upcoming anti-HER2 agents as well as immunotherapy drugs in combination with anti-HER2 agents.
Keywords: HER2+ BC, biomarkers, HER2+/HR+ BC, anti-HER therapy, clinical trials
Prologue
Origins of HER2 and the pre-trastuzumab era
The 2019 Lasker-DeBakey Clinical Medical Research Award honored Dennis J. Slamon, Axel Ullrich, and H. Michael Shepard for their paradigm-shifting invention of trastuzumab (Herceptin®), the first monoclonal antibody targeted against the neoplastic HER2 protein, and its development as a life-changing therapy for women with HER2+ breast cancer (BC). Eighty-five percent of HER2+ BC patients are now expected to survive for at least ten years, a spectacular improvement in the outcome of these patients [1]. Nevertheless, the journey continues, with new insights and innovations advancing the current Trastuzumab Era.
In 1978, Cohen et al. discovered the epidermal growth factor receptor (EGFR) through its ligand, the Epidermal Growth Factor (EGF), which had been shown to stimulate epithelial cell proliferation. EGFR was further found to have receptor tyrosine kinase (RTK) phosphorylating signal transduction properties, establishing the primary mechanism and relationship between ligand-receptor binding to cell proliferation [2]. In 1984, EGFR became the first receptor to be associated with an oncogene, v-ERBB, which was known to induce sarcomas and leukemias in chickens [3]. Soon after that, in 1985, Coussens et al. discovered another RTK highly similar (and likely related) to the human EGF receptor, naming it HER2 [4]. Interestingly, HER2 was later found to be synonymous with the human ERBB2 and mouse neu genes discovered by other researchers at this time [4-6]. Despite these fascinating discoveries, their clinical significance to breast cancer was not fully evident until 1987, when Slamon et al. reported HER2/neu to be amplified in 30% of invasive breast cancers and established a significant clinical correlation between HER2 amplification/overexpression and poor clinical outcome [6].
Before trastuzumab and other targeted therapies, surgery and chemotherapy were the mainstays of treatment for HER2+ BC. Chemotherapies of choice at this time were regimens that included cyclophosphamide (C), methotrexate (M), 5-fluorouracil (F) and anthracyclines (A). However, not only was HER2+ BC usually more aggressive and found in later stages, but it also had a higher incidence of resistance to hormone therapy (tamoxifen) and CMF chemotherapy [6-10]. CMF chemotherapy demonstrated increased dose-dependent response in HER2+ BC, suggesting the potential benefit of high-dose chemotherapy in HER2+ patients [11]. In the mid-1990s, taxanes demonstrated promise in both single-agent and combination regimens with other chemotherapy agents, particularly anthracyclines [12]. Additionally, Paik et al. reported in 1998 that the addition of anthracycline to a chemotherapy regimen of L-phenylalanine mustard and 5-FU led to superior disease-free survival (DFS) in HER2/ERBB2+ BC [13]. At this time, several different chemotherapy regimens demonstrated clinical benefit. Nonetheless, a cloud of uncertainty remained as to which regimen was the most superior in safety and efficacy. Before this could be ascertained, trastuzumab had arrived.
The advent of adjuvant trastuzumab and chemotherapy combination in the early 21st century led to a significant improvement in the poor prognosis for early HER2+ BC patients by reducing the risk of recurrence of early-stage HER2+ BC by 46% and mortality by 33% [14]. During this same period, the combination of trastuzumab and anthracycline chemotherapy was found to have synergistic cardiotoxicity, leading to the predominant use of taxanes in substitution of anthracyclines as the chemotherapy agent [15]. Despite these successes, however, approximately 15% of HER2+ BC patients relapse with adjuvant trastuzumab. Even more concerning is that the majority of those with the metastatic disease develop resistance to trastuzumab in the first year, requiring further improved therapies for the continued clinical benefit [16-18]. Since trastuzumab’s FDA approval in 1998, several molecular therapies have subsequently been approved, and promising novel agents are a possibility (Figure 1).
Figure 1.
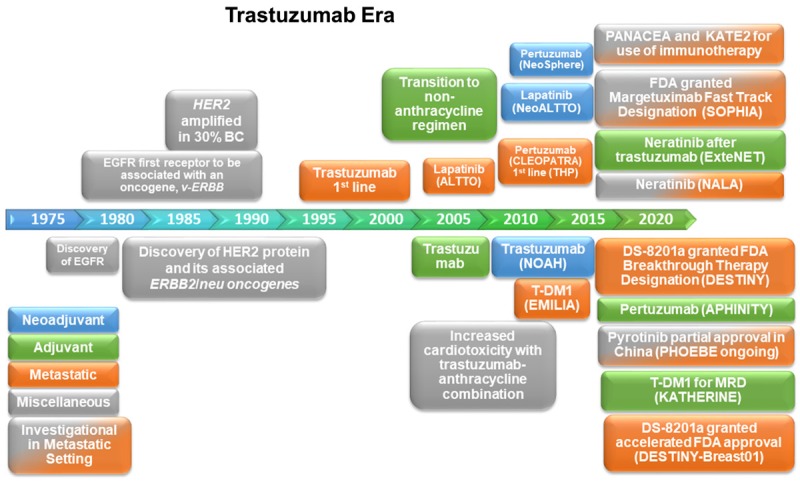
Timeline of Key Events, FDA-Approved Therapies, Therapies on the Horizon, and Their Clinical Settings for HER2+ BC.
The trastuzumab era
HER2+ BC and its classification
Breast cancer continues to be a significant cause of mortality among female cancer types, second only to lung cancer in the United States (Cancer Facts & Figures 2019. 2019, American Cancer Society: Atlanta, Georgia). Consequently, enormous efforts have been made to characterize breast cancer further and expand therapeutic decision-making to improve patient management in the clinic. Several classifications, including histologic and intrinsic subtyping, have been developed [19-21]. However, for this manuscript, the most practical classifications for molecular therapies include both TNM staging and biomarker subtyping. TNM staging classifies cancers clinically or pathologically using “T” for primary tumor size, “N” for number and location of lymph nodes containing cancer, and “M” for the presence of cancer in distant sites throughout the body to help predict prognosis and facilitate clinical decision-making. Biomarker subtyping, in contrast, distinguishes between 3 main clinical subtypes: Hormone Receptor-positive (HR+), Human Epidermal Growth Factor Receptor-positive (HER2+) and Triple Negative (TN).
Of these subtypes, HER2+ BC represents approximately 15-20% and is characterized by increased amplification/overexpression of ERBB2/neu [21-23]. Derived primarily from ductal breast tissue, HER2+ BC is associated with having a higher grade, more aggressive phenotype, and worse prognosis if untreated than its more prevalent HER2-/HR+ counterpart [24,25]. Furthermore, subsets of HER2+ patients may resemble differing prognostic paths when considering de novo versus recurrent metastatic disease or overlapping HER2+/HR+ tumor expressivity, which will be discussed later [26]. Lastly, data is lacking, and further analysis is necessary to elucidate the influence of gender, age, race/ethnicity and geographical differences in HER2+ BC.
HER2 is a member of the HER family (EGFR/HER1, HER2, HER3, HER4) of transmembrane receptor tyrosine kinases (RTK) involved in cell proliferation, motility, resistance to apoptosis, invasiveness, and angiogenesis [25]. A critical component and necessary distinction to its unique cell-signaling is the ligand-independent auto-dimerization and phosphorylation of HER2 and ligand-dependent heterodimerization and phosphorylation of HER2 with each of its other HER family members (Figure 2B). Of these dimerizations, three have demonstrated relevance to HER2+ BC: 1) EGFR/HER2 heterodimerization allows the EGFR to be recycled rather than degraded (typically occurs on activation), promoting sustained signaling capability, 2) HER2/HER2 homo-dimerization leads to downstream oncogenic RAS/MAPK and indirect PI3K/AKT pathway activation, and 3) HER2/HER3 heterodimerization promotes HER2 phosphorylation and activation of HER3 kinase domain, leading to extremely potent stimulation of the downstream oncogenic PI3K/AKT pathway beyond that of HER2 autophosphorylation (Figure 2B and 2C) [25,27].
Figure 2.
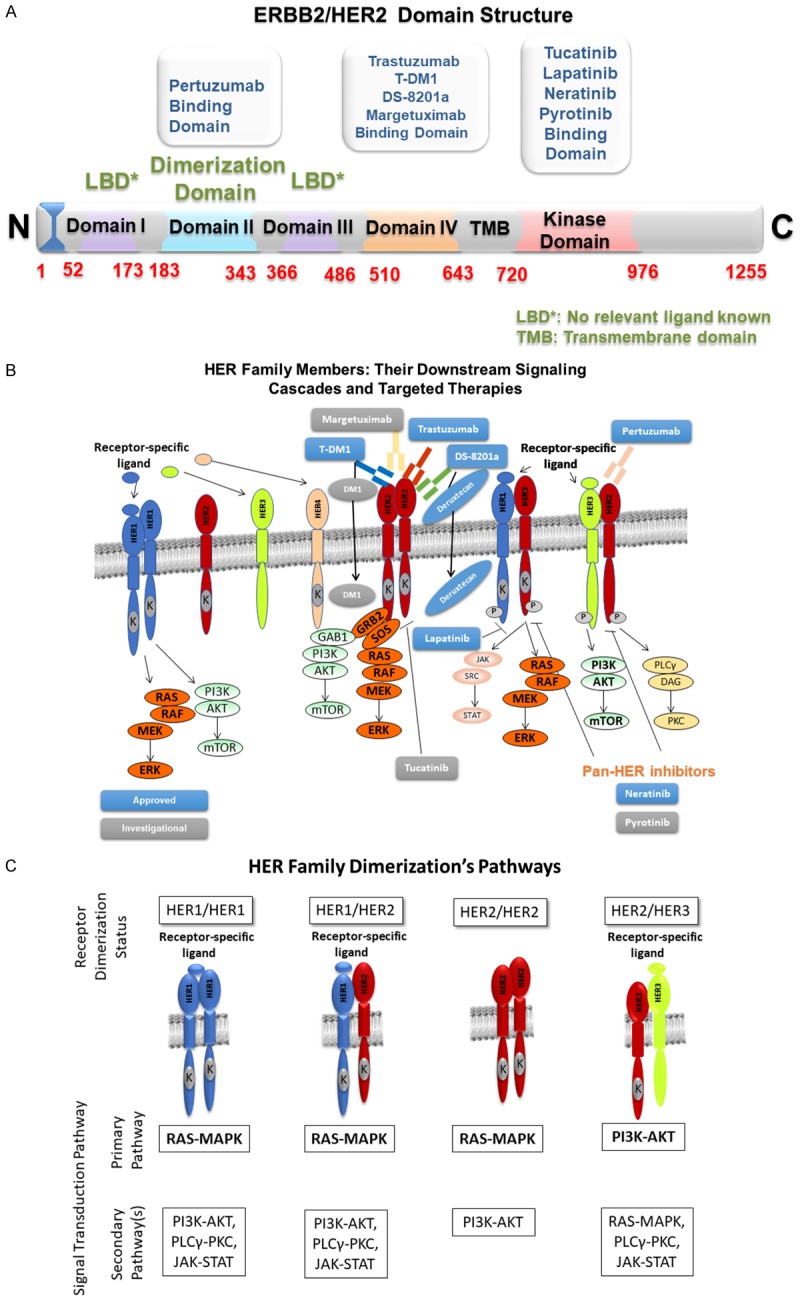
A. Binding Domains of the HER2 Receptor and Their Targeted Therapies. B. HER Family Members: Their Downstream Signaling Cascades and Targeted Therapies. C. HER Family Dimerization Combinations and Their Downstream Pathways.
It is well-known that breast cancer is a very heterogeneous disease, and the current clinical classifications of HR+, HER2+, and TN tumors help define important prognostic subsets of breast cancer patients and treatment options. Since its clinical distinction in the mid-1990s, HER2+ BC has been fervently researched and provided new therapeutic breakthroughs. However, it wasn’t until the last decade researchers and clinicians noted the prognostic differences and potential therapeutic significance to the HR status within HER2+ disease.
Multiple studies had observed a worse prognosis for HER2+/HR- patients compared to other, less aggressive forms of breast cancer. In 2010, Gomez et al. were the first to address and compare this HER2+/HR- subtype directly to its HER2+/HR+ counterpart. In their analysis, they discovered that the HER2+/HR- subtype demonstrated a poorer differentiation and greater extracapsular extension than HER2+/HR+ tumors, suggesting a more aggressive phenotype. Furthermore, the HER2+/HR- subtype demonstrated increased early recurrence rate, likelihood of relapse (hazard ratio [HR] 1.707; 95% CI, 1.079-2.699) and decreased 5-year DFS rates when compared to HER2+/HR+ disease (65.0% and 74.6%; P = 0.045) in the adjuvant setting. Five-year overall survival (OS) rates, however, were not statistically significant (75.5% and 82.4% for HER2+/HR- and HER2+/HR+ tumors, respectively (P = 0.140)) [28].
Clinical trials up to this point rarely distinguished the HR status when treating HER2+ BC patients. However, within the last decade, an increasing number of trials (ADAPT, ExteNET, ALTTO, SOLTI-PATRICIA, KATHERINE) are now making the distinction between these HR subtypes in various clinical settings. For example, some neoadjuvant trials have observed distinctly superior pathological complete response (pCR) rates in HER2+/HR- tumors treated with the combination of dual anti-HER2 and chemotherapy over HER2+/HR+ disease [29]. The data supports the initial observations by Gomez et al., demonstrating that the two populations have distinct predictive and prognostic values [28].
Currently-approved molecular therapies and their mechanism(s) of action
Trastuzumab (Herceptin®), FDA approved in 1998 for metastatic HER2+ BC, was the first targeted anti-HER2 therapy available for this population and led to significant improvement in prognosis over the previous standard of care (SOC) chemotherapy regimens. It was also the first humanized monoclonal antibody approved for HER2+ BC, functioning through multiple impressive molecular mechanisms. The first of these mechanisms occur via HER2 receptor degradation. By binding to the extracellular domain of the transmembrane HER2 receptor (Figure 2A and 2B), trastuzumab induces internalization and degradation of the HER2 receptor via a ubiquitin ligase, c-CBL (precise mechanism unknown) [18].
Secondly, trastuzumab’s binding to the HER2 receptor also functions to attract cytotoxic, innate immune cells to the tumor microenvironment. Such an event occurs via trastuzumab’s IgG1 Fc region activating the FcγRIII/CD16 found on natural killer (NK) cells, a concept commonly known as antibody-dependent cellular cytotoxicity (ADCC). Lastly, trastuzumab’s extracellular binding of HER2 leads to inhibition of its downstream signaling via the RAS/MAPK and PI3K/AKT pathways, ultimately suppressing cellular growth and proliferation signaling (Figure 2B and 2C). Despite significant prognostic improvement for HER2+ BC patients, a subset of patients possesses either de novo resistance (≈35%) or acquired after trastuzumab therapy (≈70%), suggesting the need for additional therapies to provide further clinical benefit for these patients [18].
Lapatinib (Tykerb®), a reversible, dual HER1/HER2 kinase inhibitor, was first FDA approved in 2007 in combination with capecitabine for metastatic breast cancer (mBC) that progressed on prior therapies. As a dual inhibitor of HER1/HER2 heterodimerization, it acts to decrease downstream cell-signaling of their associated growth and proliferative pathways of PI3K, MAPK, PLCγ, and STAT through decreased phosphorylation of proteins within each of these pathways (Figure 2B and 2C). Furthermore, lapatinib’s benefits appear to be independent of HER1 status in HER1+/HER2+ tumors (thus active primarily via HER2 inhibition), effective against a trastuzumab-resistant p95HER2 phenotype (truncated extracellular binding site prevents trastuzumab binding), inhibition of insulin-like growth factor 1 (IGF-1)-HER2 cross-talk, and may even decrease phosphorylation and cell-signaling of the HER3 receptor [30]. Unfortunately, its well-known diarrhea side effect limits some of its clinic use.
Pertuzumab (Perjeta®), a dual HER2/HER3 monoclonal antibody, was first FDA approved in 2012 in combination with trastuzumab and docetaxel for first-line therapy in HER2+ mBC following results of the CLEOPATRA trial. Subsequently, the NeoSphere trial provided further evidence in the neoadjuvant setting. After results of the APHINITY trial, pertuzumab achieved full approval in the early-stage HER2+ BC setting as well. Its synergistic efficacy, in combination with trastuzumab, is supported by evidence of pertuzumab binding to a different epitope within the extracellular domain of HER2 than trastuzumab (Figure 2A). Once bound, the precise mechanism(s) by which pertuzumab primarily acts are still under contention. However, many believe pertuzumab prevents potent ligand-dependent HER2/HER3 heterodimerization, suppressing downstream PI3K, and MAPK pathways (Figure 2B and 2C) [31,32]. Additionally, there is a very low chance of cross-reactivity since trastuzumab and pertuzumab bind at two different domains of the HER2 receptor (Figure 2A).
Ado-trastuzumab emtansine (Kadcyla® or T-DM1) is an antibody-drug conjugate (ADC) of trastuzumab covalently linked to the chemotherapy agent emtansine (DM1). In 2013, the FDA approved T-DM1 for metastatic HER2+ BC, becoming the first ADC approved for HER2+ mBC patients. Recently in 2019, T-DM1 was also approved in the adjuvant setting for patients with the residual invasive disease following the neoadjuvant trastuzumab and chemotherapy after the results KATHERINE Trial [33]. T-DM1 combines the same anti-HER2 properties of trastuzumab with the anti-tubulin properties of concentrated, high-dose DM1 chemotherapy. Once T-DM1 binds to HER2, it is internalized into the cancer cell and undergoes proteolytic cleavage, causing the release and activation of DM1 [34].
Neratinib (Nerlynx®) is an irreversible oral pan-TK (tyrosine kinase) inhibitor of several members of the HER family (EGFR/HER1, HER2, HER4). Following the results of the ExteNET trial, neratinib was FDA approved in 2017 for extended adjuvant treatment for early-stage HER2+ BC previously treated with adjuvant trastuzumab and is seeking approval for HER2+ mBC via the NALA trial. By decreasing phosphorylation of each HER’s intracellular TK domain, it inhibits downstream PI3K/AKT and RAS/MAPK pathways, which eventually decreases both cyclin D1 expression and retinoblastoma (RB) phosphorylation (Figure 2B and 2C) [30].
Trastuzumab-deruxtecan (Enhertu® or DS-8201a) is a novel ADC that connects the humanized monoclonal antibody, trastuzumab, via a tetrapeptide linkage to the topoisomerase 1 inhibitor, deruxtecan. The peptide linkage is stable throughout the bloodstream and cleaved selectively in HER2+ tumor cells that upregulate lysosomal proteases, leading to the targeted distribution of chemotherapy within cells. In comparison to the previously-approved ADC, T-DM1, DS-8201a offers a drug-to-antibody ratio of 8 vs. 3-4 and a payload that more easily crosses the cell membrane for greater cytotoxic effect to neighboring cells within the tumor microenvironment [35]. Since its inception, DS-8201a has been granted Fast Track Designation in 2016 and Breakthrough Therapy Designation in 2017 by the FDA for the treatment of patients with locally-advanced or metastatic HER2+ BC previously treated with either trastuzumab or pertuzumab and resistance to T-DM1. Most recently, DS-8201a received FDA accelerated approval in December 2019 for unresectable or metastatic HER2+ BC, who have received two or more prior anti-HER therapies.
Upcoming molecular therapies and their mechanism(s) of action
Margetuximab is a novel monoclonal antibody derivative of trastuzumab that binds to the same epitope of the HER2 receptor with similar affinity and antiproliferative effects as trastuzumab (Figure 2A). In comparison to trastuzumab, it has an IgG1 Fc region that is genetically engineered to have up to 6.6x greater affinity for the stimulatory CD16A FcγRIIIA on NK cells and 8.4x less affinity for inhibitory CD32B FcγRIIB found on immune effector cells (NK cells and macrophages) within the innate immune system’s ADCC process [36]. Therefore, it theoretically enhances the host’s immune recognition of cancer cells beyond that of trastuzumab. Margetuximab is currently under investigation in the SOPHIA trial, the first prospective study analyzing the significance of the FcγR genotype in HER2+ BC. Following preliminary results, it has achieved the FDA’s Fast Track designation for locally advanced or metastatic HER2+ BC patients previously treated with anti-HER2+ therapy.
Tucatinib (ONT-380) is a potent, selective, and reversible oral HER2 agent that binds to and inhibits the intracellular TK domain of the HER2 receptor (Figure 2A). Consequently, it inhibits pro-proliferative downstream cell-signaling pathways of HER2 receptor activation and demonstrates promise crossing the blood-brain-barrier as an option for trastuzumab-resistant HER2+ patients with brain metastases [37-39]. For a list of ongoing trials including DS-8201a, margetuximab, and tucatinib for HER2+ BC treatment, refer to Table 1.
Table 1.
Upcoming targeted therapies and their current clinical trials
| Compound | Phase | Title | ClinicalTrials.gov Identifier |
|---|---|---|---|
| Margetuximab | 2 | Phase 2 Study of the Monoclonal Antibody MGAH22 (Margetuximab) in Patients with Relapsed or Refractory Advanced Breast Cancer | NCT01828021 |
| Margetuximab | 3 | Margetuximab Plus Chemotherapy vs. Trastuzumab Plus Chemotherapy in the Treatment of HER2+ Metastatic Breast Cancer (SOPHIA) | NCT02492711 |
| Margetuximab | N/A | Margetuximab Expanded Access Program | NCT03133988 |
| DS-8201a | 2 | DS-8201a in HER2+ Breast Cancer (DESTINY-Breast01) | NCT03248492 |
| DS-8201a | 3 | DS-8201a in Pretreated HER2+ Breast Cancer That Cannot be Surgically Removed or Has Spread (DESTINY-Breast02) | NCT03523585 |
| DS-8201a | 3 | DS-8201a Versus T-DM1 for HER2+, Unresectable and/or Metastatic Breast Cancer Previously Treated with Trastuzumab and Taxane (DESTINY-Breast03) | NCT03529110 |
| DS-8201a | 3 | Trastuzumab Deruxtecan (DS-8201a) Versus Investigator’s Choice for HER-Low Breast Cancer That Has Spread or Cannot Be Surgically Removed (DESTINY-Breast04) | NCT03734029 |
| DS-8201a | 1 | Phase 1 Study to Evaluate the Effect of DS-8201a on the QT/QTc Interval in HER2-Expressive Breast Cancer | NCT03366428 |
| DS-8201a | 1 | Trastuzumab Deruxtecan (DS-8201a) with Nivolumab in Advanced Breast and Urothelial Cancer | NCT03523572 |
| DS-8201a | 1 | DS-8201a in Patients with Cancer That Tests Positive for HER2 Protein | NCT03368196 |
| DS-8201a | 1 | DS-8201a and Pembrolizumab in Participants with Locally Advanced/Metastatic Breast Cancer or Non-Small Cell Lung Cancer | NCT04042701 |
| Tucatinib | 1b/2 | Tucatinib, Palbociclib, and Letrozole in Metastatic Hormone Receptor-Positive and HER2-Positive Breast Cancer | NCT03054363 |
| Tucatinib | 1 | A Study of Tucatinib (ONT-380) Combined with Capecitabine and/or Trastuzumab in HER2+ Metastatic Breast Cancer | NCT02025192 |
| Tucatinib | 2 | A Study of Tucatinib vs. Placebo in Combination with Capecitabine & Trastuzumab in Patients with Advanced HER2+ Breast Cancer (HER2CLIMB) | NCT02614794 |
| Tucatinib | 3 | A Study of Tucatinib vs. Placebo in Combination with Ado-Trastuzumab Emtansine (T-DM1) for Patients with Advanced or Metastatic HER2+ Breast Cancer | NCT03975647 |
| Tucatinib | 1 | Tucatinib + Abemaciclib + Herceptin for HER2+ MBC | NCT03846583 |
| Tucatinib | 1 | A Study of Tucatinib (ONT-380) Combined with Ado-Trastuzumab Emtansine (T-DM1) in Patients with HER2+ Breast Cancer | NCT01983501 |
| Tucatinib | 2 | Tucatinib, Trastuzumab, and Capecitabine for the Treatment of HER2+ Leptomeningeal Disease (LMD) | NCT03501979 |
| Tucatinib | N/A | Expanded Access Use of Tucatinib for HER2+ Metastatic Breast Cancer | NCT03424473 |
Pyrotinib, an irreversible pan-TK inhibitor of the HER family (HER1, HER2, HER4) of transmembrane TK-receptors works by inhibiting downstream pro-proliferative signaling. It has not yet achieved FDA approval in the US but has received conditional approval in China for use in combination with capecitabine in the treatment of HER2+ mBC in patients previously treated with anthracycline or taxane chemotherapy regimens [40].
Cell cycle inhibitors (ribociclib, palbociclib, and abemaciclib), are small-molecule agents that prevent the interaction of cyclin D (D1,2,3) with their respective cyclin-dependent kinases (CDK4/6) in the G1-S checkpoint of the cell cycle (Figure 4) [41]. Without their inhibitory action, tumor cells can proceed through the cell cycle and maintain their proliferation. Furthermore, their importance to HER2+/HR+ BC is gaining appreciation within the scientific community, originating both from common upregulation of the Cyclin D1/CDK4/6/RB pathway in this population and their potential benefit beyond the previously FDA approved setting of HR+/HER2- mBC [42].
Figure 4.
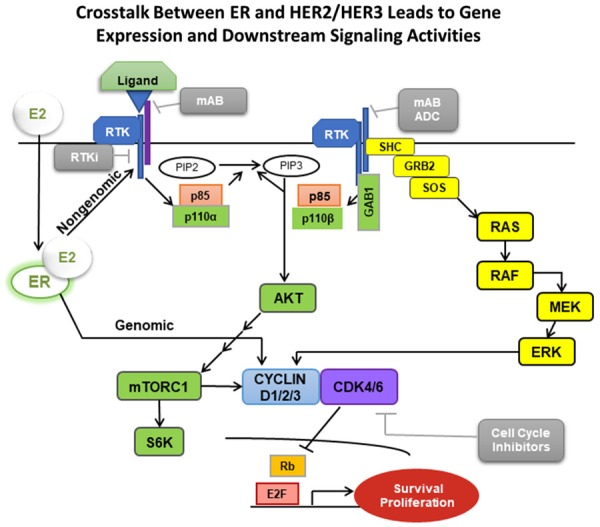
Crosstalk between ER and HER2/HER3 Cell-Signaling Pathways.
Atezolizumab is a monoclonal IgG1 antibody against PD-L1 (programmed death-ligand 1) with a genetically engineered FcγR that prevents normal IgG1-mediated ADCC by the tumor cell. It was first approved by the FDA in 2016 for urothelial carcinoma and is currently under early investigation for HER2+ mBC via the KATE2 trial. Its mechanism of action relies on inhibition of the immunosuppressive actions of PD-L1, an apoptosis-inducing ligand overexpressed on many tumor cells (including some HER2+ BC populations). Typically when PD-L1 binds to the PD-1 (programmed death) receptor found on cytotoxic CD8+ T cells, the T cell undergoes apoptosis, and the ultimate result is a suppression of overall adaptive immune surveillance in the tumor microenvironment and evasion of the cancer cell to immune recognition [43]. However, when this process is blocked with PD-1 or PD-L1 inhibitors (such as atezolizumab), the CD8+ T cells are more likely to recognize and destroy the tumor cell (Figure 3).
Figure 3.
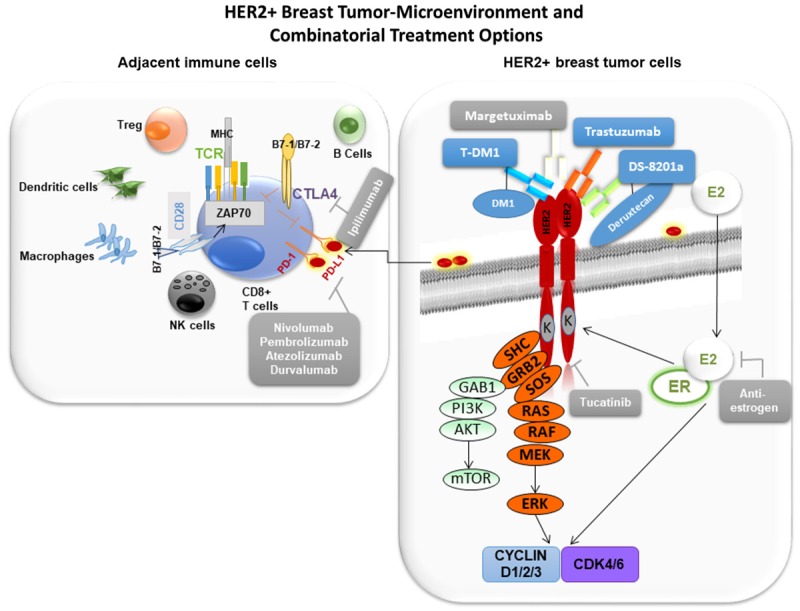
The HER2+ BC Tumor Microenvironment, Its Immune System Involvement, and Targeted Therapy Combinations.
Molecular therapies by clinical setting
Neoadjuvant setting
For several years, chemotherapy remained the SOC for neoadjuvant HER2+ BC treatment. However, in the early 2000s, trastuzumab transcended its use in the metastatic setting to demonstrate efficacy in the neoadjuvant setting through several small studies of early-stage HER2+ disease [44-46]. Eventually, the NOAH trial became the first large-scale trial to show trastuzumab’s clinical benefit in the neoadjuvant setting. The authors reported the addition of both neoadjuvant and adjuvant trastuzumab to neoadjuvant chemotherapy improved the preliminary 3-year DFS for the trastuzumab plus chemotherapy cohort over neoadjuvant chemotherapy only cohort (71% vs. 56%; HR 0.59; 95% CI, 0.38-0.90; P = 0.013). In their 5-year follow-up, the respective DFS rates were 58% vs. 43% (HR 0.64; 95% CI, 0.44-0.93; P = 0.016), suggesting continued clinical benefit [47-49].
With the FDA approval of lapatinib in 2007 for mBC, came the further study of its use in the neoadjuvant setting. In the NeoALTTO trial, Baselga et al. observed a near doubling of the pathological complete response (pCR) rate when adding lapatinib to trastuzumab and chemotherapy over the trastuzumab and chemotherapy combination alone (51.3% vs. 29.5%; P = 0.0001) and did so with a tolerable side effect profile absent of noteworthy cardiac events. Although, no statistical significance in DFS was observed between either drug alone (29.5% with trastuzumab vs. 24.7% with lapatinib; P = 0.34) [50].
The NeoSphere was the next landmark trial to demonstrate further clinical benefit in the neoadjuvant setting. In their primary analysis, Gianni et al. observed that patients given neoadjuvant trastuzumab and pertuzumab with docetaxel (PTD) had a superior pCR rate (45.8%) than other combinations of each anti-HER2 drug with docetaxel (TD 29.0%; P = 0.014; PD 24.0%) or the combination of pertuzumab and trastuzumab without docetaxel (PT, 16.8%) and similar tolerable side effect profiles between groups. On a 5-year follow-up, the progression-free survival (PFS) rates were PTD 86%, TD 81%, PD 73%, and PT 73%, with an overlap in confidence intervals between groups. The data suggested the potential benefit of added pertuzumab to trastuzumab and docetaxel despite no longer-term statistical significance [48,49].
WSG-ADAPT (NCT01745965) became the first trial to assess early HER2+/HR+ BC in the neoadjuvant setting by exploring T-DM1 with endocrine therapy. In their analysis, they observed that the arms of T-DM1 and endocrine therapy (ET; tamoxifen/AI) each demonstrated higher pCR rates (40.5% and 45.8%, respectively) than the trastuzumab plus ET control (6.7%; P < 0.001) after only 12 weeks. Furthermore, premenopausal patients had a distinctly more significant benefit from added ET to T-DM1 than the conjugate drug alone (pCR 47.6% vs. 28.6%). In contrast, the difference in post-menopausal women was a pCR of 64.3% vs. 50% in the T-DM1 plus ET and T-DM1-only group, respectively [51]. In addition to this study, there are two trials currently investigating the neoadjuvant HER2+/HR+ BC setting. Another ADAPT trial (NCT03272477) is studying the use of pertuzumab and trastuzumab with either taxane or endocrine therapy, and the HELEX trial (NCT00999804) is exploring the use of lapatinib plus trastuzumab with or without endocrine therapy based on HR status in 12- or 24-week regimens.
Another WSG-ADAPT trial (NCT01817452) explored the need for chemotherapy in addition to dual HER2-targeted therapy of pertuzumab plus trastuzumab in the neoadjuvant early HER2+/HR- BC setting. The authors observed a pCR of 89.2% for combination with paclitaxel vs. 36.3% for the dual HER2 therapy-only group, prompting early discontinuation of the trial for the superior clinical benefit [52]. Additionally, the KRISTINE trial explored the role of neoadjuvant systemic chemotherapy vs. targeted chemotherapy (T-DM1). Systemic chemotherapy demonstrated significant utility for optimizing efficacy at the cost of increased risk of both moderate and severe side effects. In the trial, the authors observed a pCR 55.7% in the trastuzumab, pertuzumab, docetaxel, and carboplatin combination vs. 44.4% in the T-DM1 plus pertuzumab combination (P = 0.016), although grade 3-4 adverse events were 64% vs. 13% between the two arms. Interestingly, in HER2+/HR- patients, those who received chemotherapy in their regimen had a pCR 73.2% vs. 54.2% in those who received the targeted therapy only (difference 19.0; 95% CI, 4.6-33.3) [53]. In summary, the inclusion of systemic chemotherapy appears necessary in the neoadjuvant setting of HER2+/HR- BC.
Adjuvant setting
The SOC adjuvant treatment for HER2+ BC had been systemic chemotherapy regimens, which were relatively adequate; however, their systemic toxicities (alopecia, myelosuppression, etc.) suggested the need for healthier, targeted, and more efficacious therapies. The development of trastuzumab and its success in the late-stage, metastatic setting led to its subsequent study in the early-stage, adjuvant setting.
Several different groups sought the utility of trastuzumab in different durations, sequences, and therapy combinations. One of these trials, the HERA trial, observed that one year of trastuzumab sequentially after adjuvant chemotherapy led to improved PFS (HR 0.54; 95% CI, 0.43-0.67; P < 0.0001) over adjuvant chemotherapy alone [54]. Next, an analysis of two trials (NCT00005970 and NCT00004067) by Romond and Perez, respectively, observed that the addition of trastuzumab concurrently with adjuvant chemotherapy in operable HER2+ BC resulted in a higher 3-yr DFS rate of 87.1% for the trastuzumab cohort vs. 75.4% in the non-trastuzumab chemotherapy cohort (HR 0.48; 95% CI, 0.39-0.59; P < 0.0001). They also observed that the relative risk of death was reduced by 33% in the trastuzumab group (P = 0.015). Regarding toxicity, however, the 3-year cumulative incidence of class III or IV congestive heart failure (CHF) or death from cardiac causes in the trastuzumab group was 4.1% in the B-31 trial and 2.9% in the N9831 trial, suggesting the importance of future studies in trastuzumab’s primary vs. synergistic cardiotoxicity when used with anthracyclines [55].
The BCRIG-006 trial addressed this cardiotoxicity by comparing the safety and efficacy of anthracycline (AT) vs. non-anthracycline based (NAT) chemotherapy concurrent with trastuzumab. Slamon et al. reported no significant difference between the AT and NAT in efficacy (5-yr DFS 84% vs. 81%; OS 92% vs. 91%); however, the rates of CHF were significantly higher in the AT group than the NAT group (2.0% vs. 0.4%; P = 0.001). The results of this trial suggest a non-inferiority of NAT efficacy alongside reduced cardiotoxicity, suggesting the NAT regimen to be superior in risk-benefit analysis for the treatment of adjuvant HER2+ BC [56]. Following this study, in 2008, the FDA approved the NAT regimen (docetaxel, carboplatin, and trastuzumab) for the adjuvant HER2+ BC setting [57].
Several trials evaluated the most efficacious duration of trastuzumab adjuvant therapy (6 weeks, 9 months, 1 year, 2 years). However, the HERA trial was the only study to investigate the duration of adjuvant trastuzumab beyond the arbitrary 1 year of adjuvant therapy by comparing 1 vs. 2 years. In their investigation, the authors found no difference in DFS rates and OS between the two durations of therapy (HR 0.99; 95% CI, 0.85-1.14; P = 0.86), closing the door on extended adjuvant trastuzumab beyond 1 year [58].
Despite the lack of extended adjuvant trastuzumab benefit, the question remained as to whether shortening adjuvant trastuzumab would be beneficial. Several studies (SOLD, ShortHER, PHARE, Hellenic Oncology study, PERSEPHONE) sought to answer this question [24,59-63]. All but the PERSEPHONE trial found that 1 year of trastuzumab had the most favorable outcomes. The PERSEPHONE reported 6 months being non-superior and non-inferior to 1 year of trastuzumab (4-yr DFS 89.8% for 12 months vs. 89.4% for 6 months; HR 1.07; 90% CI, 0.93-1.24; non-inferiority P = 0.011) [63]. Consequently, a shorter course may be considered for certain low-risk patients; however, 1 year of adjuvant trastuzumab remains the SOC for most early-stage HER2+ BC patients.
During this same period, lapatinib had been under investigation for potential benefit in the adjuvant setting as well. In the ALTTO trial, lapatinib and trastuzumab were each assessed alone and in combination with the goal of finding the optimal adjuvant treatment. In their analysis, the authors observed that combination adjuvant lapatinib and trastuzumab trended toward improvement; however, it did not reach statistical significance for improvement of DFS or OS at a 5-year follow-up compared to trastuzumab alone. Furthermore, the adjuvant lapatinib alone arm was closed early for poor efficacy alongside an increased number of adverse events (predominantly diarrhea, rash, etc.). Interestingly, they also observed a potential for hormone receptor status to predict response to dual anti-HER therapy (L+T vs. T), with HER2+/ER- patients trending toward greater benefit from the dual blockade than its HER2+/ER+ counterpart (6-yr DFS 84% vs. 80%; HR 0.80; 95% CI, 0.64-1.00) [64].
The next of these trials to assess dual anti-HER therapy was the APHINITY trial. In this study, the authors compared combination adjuvant pertuzumab and trastuzumab to SOC adjuvant trastuzumab in the early HER2+ BC setting. Von Minckwitz et al. observed borderline statistically significant improvement in 3-year DFS rates with the dual therapy for the overall study population (94.1% vs. 93.2%; HR 0.81; 95% CI, 0.66-1.00; P = 0.045) and clear benefit in a subset of node-positive patients (92.0% vs. 90.2%; HR 0.77; 95% CI, 0.62-0.96; P = 0.02). The results suggested a disproportionately greater benefit for higher-risk early HER2+ patients and may be a population to focus on for combination anti-HER therapy [65].
The phase III ExteNET trial became the first study to assess the utility of a pan-HER tyrosine kinase inhibitor, neratinib, in the adjuvant setting of HER2+ BC. In the trial, the authors reported an improved DFS of adjuvant trastuzumab SOC therapy with an additional 1 year of neratinib. At 5-year follow-up, the authors observed DFS rates of 90.2% and 87.7% for the neratinib-extension cohort and trastuzumab-only cohort, respectively (HR 0.73; 95% CI, 0.57-0.92; P = 0.0083) with the added benefit of a tolerable long-term safety profile [66,67]. Consequently, the FDA approved the use of extended adjuvant treatment of early HER2+ BC with neratinib following trastuzumab therapy in July 2017. Of note, retrospective data analyses of this trial demonstrated a distinct benefit of adjuvant neratinib in the HER2+/HR+ subpopulation as well [68,69] and are discussed later.
The KATHERINE trial is the most recent major trial in the adjuvant setting. In their study, the authors focused on patients with residual disease at the time of surgery despite neoadjuvant trastuzumab (or other anti-HER therapy) plus chemotherapy. They specifically analyzed patient response to different adjuvant therapies (T-DM1 vs. trastuzumab), finding that adjuvant T-DM1 led to decreased risk of invasive disease or death (12.2% vs. 22.2%), a significantly higher 3-year DFS rate (88.3% vs. 77.0%; HR 0.50; 95% CI, 0.39-0.64; P < 0.001), but greater toxicity profile when compared to trastuzumab [70]. This suggests the efficacy of T-DM1 for the treatment of subclinical micrometastases, leading to its FDA approval in the adjuvant setting. Of note, 93.8% of patients who had received dual anti-HER2 neoadjuvant therapy were given pertuzumab as the second anti-HER2 agent. Considering the previous report that prior treatment with pertuzumab was not clinically beneficial in T-DM1 therapy in the metastatic setting, this result of clinical benefit of T-DM1 with prior exposure to pertuzumab in the adjuvant setting is interesting and needs further exploration [71,72].
Metastatic setting
In 1998, trastuzumab became the first FDA approved molecular therapy in the setting of HER2+ mBC, after phase II trial results published by Baselga et al. in 1996 and Pegram et al. in 1998 demonstrated significant clinical response in patients who progressed on chemotherapy [73,74]. Of note, Baselga et al. only compared trastuzumab to chemotherapy control; whereas, Pegram et al. compared combination trastuzumab and chemotherapy to each alone. They found significantly improved clinical response rates than previous reports with the combination of either therapy alone. However, neither study appeared to cite distinct cardiotoxicity beyond that expected of chemotherapy explicitly. Instead, Cobleigh et al. were the first to publish the specific concern for trastuzumab to have potential cardiotoxicity side effects, noting dysfunction in 4.7% of patients [75].
In 2001, Slamon et al. published the first large phase III trial to confirm the numerous benefits of combination trastuzumab and chemotherapy in the setting of HER2+ mBC. In their study, they observed longer DFS (7.4 vs. 4.6 months; P < 0.001), improved objective response rate (ORR 50% vs. 32%; P < 0.001), and greater duration of response (9.1 vs. 6.1 months; P < 0.001), greater 1-year survival rates (78% vs. 67%; P = 0.008), longer OS (25.1 vs. 20.3 months; P = 0.046), and 20% risk reduction in death (HR 0.8; P = 0.046). Furthermore, 1.36% had cardiac dysfunction, with greatest representation by those receiving anthracycline, cyclophosphamide, and trastuzumab combination (27% of the subgroup) [76].
Despite the vast clinical benefits experienced by many HER2+ mBC patients, resistance to trastuzumab eventually occurs in the majority of patients, requiring the use of other novel therapies [16,17]. Initially, the resistance was thought to occur downstream or in parallel to the HER2 receptor via HER family heterodimerization of HER2 with HER1/3, PI3K, MAPK, IGF-IR, and/or other pathways [77,78]. However, the most important and clinically relevant to these pathways became the downstream PI3K cell-signaling pathway [79]. In their BOLERO-3 trial, Andre et al. reported an improved PFS benefit with added everolimus to trastuzumab and chemotherapy (median 7.0 vs. 5.8 months; HR 0.78; 95% CI, 0.65-0.95; P = 0.0067) in patients who had progressed on trastuzumab and chemotherapy, albeit with prevalent grade 3-4 bone marrow suppression [80]. Unfortunately, however, BOLERO-1 failed to demonstrate the improved PFS benefit with added everolimus in the first-line metastatic setting for the overall study population. Importantly, the HER2+/HR- subpopulation given everolimus demonstrated an improved PFS of 20.3 months vs. 13.1 months in the placebo cohort (HR 0.66; 95% CI, 0.48-0.91; P = 0.0049) [81].
Another treatment proposed to overcome trastuzumab resistance was the HER2/3 heterodimerization inhibitor, pertuzumab. However, the PHEREXA trial, which added pertuzumab to trastuzumab and capecitabine did not significantly improve PFS at 2-year follow up in comparison to either trastuzumab or capecitabine alone (PFS 9.0 vs. 11.1 months; HR 0.82; 95% CI, 0.65-1.02; P = .0731) for patients who had become resistant to trastuzumab. Interestingly, however, pertuzumab used alongside trastuzumab and docetaxel in the first-line setting did show significant benefit in the CLEOPATRA trial. In the trial, Baselga and group reported a significantly improved PFS over SOC trastuzumab and docetaxel (18.5 vs. 12.4 months; HR 0.62; 95% CI, 0.51-0.75; P < 0.001) [82]. These results led to the eventual FDA approval of pertuzumab, trastuzumab, and docetaxel combination as first-line therapy in HER2+ mBC in 2012.
The next therapeutic approach to make waves in HER2+ mBC came via a breakthrough in molecular biomanufacturing technology. The antibody-drug conjugate (ADC) of trastuzumab and chemotherapy agent DM1 (emtansine), T-DM1, demonstrated better PFS (9.6 vs. 6.4 months; HR 0.65; 95% CI, 0.55-0.77; P < 0.0001), OS (29.9 vs. 25.9 months; HR 0.75; 95% CI, 0.64-0.88), and fewer side effects than lapatinib and chemotherapy in trastuzumab-resistant patients via the EMILIA trial. These results led to its approval in 2013 for the HER2+ mBC setting [83]. The next step for T-DM1 was to show superiority in safety and efficacy over first-line therapy at the time, trastuzumab plus taxane. In the MARIANNE trial, Perez et al. observed no superiority but rather a non-inferiority in efficacy between each of the SOC trastuzumab plus taxane (T+T), T-DM1 (TD) alone, and T-DM1 plus pertuzumab (TD+P) arms (PFS 13.7, 14.1, 15.2 months in T+T, TD, TD+P, respectively). However, T-DM1 possessed a better safety profile than trastuzumab plus taxane therapy. Therefore, T-DM1 may be an optional first-line treatment approach for patients who may have a contraindication to taxane therapy [84].
Neratinib, a pan-HER tyrosine kinase inhibitor, became the latest of these molecular therapies to achieve FDA approval in 2017 for HER2+ mBC. In the NALA trial, the authors reported that patients who had received neratinib and capecitabine had significantly improved 12-month PFS over lapatinib and capecitabine (28.8% vs. 14.8%; P = 0.059) in heavily pretreated patients (> 2 prior anti-HER therapies). Additionally, tolerability was similar between arms, clinical benefit rate (CBR; P = 0.0328), and duration of response (DoR; P = 0.0004) were significantly improved. Of note, the 6 and 12-month OS trended toward improvement as well; however, they were not statistically significant [85]. Interestingly, Neratinib plus T-DM1 combination has shown positive preliminary results in a recent phase 1b trial of HER2+ mBC patients whose disease had progressed on the first-line trastuzumab and pertuzumab therapy. Of the 19 patients eligible for evaluation in the trial, 16% demonstrated complete response (CR), 47% partial response (PR), 11% stable disease (SD), and 26% progressed. Furthermore, a correlation between amplified ERBB2 in cell-free DNA (cfDNA) and clinical benefit was evident. Seventy-percent of ERBB2-amplified patients had CR or PR; whereas, only 24% of non-ERBB2-amplified patients had PR and none had CR [86].
Each of the current FDA approved therapies mentioned above have provided a unique contribution to HER2+ metastatic disease, whether it be through improved efficacy or fewer side effects. In spite of that, none have demonstrated a paradigm shift in treatment. Moreover, patients with HER2+ BC do not respond uniformly to HER2-based targeted therapies. Novel therapies are currently on the horizon, hoping to transform prognosis for HER2+ BC in a similar way that trastuzumab did in the late 20th century. Whether any of the following therapies fulfill this endeavor remains to be seen, although their preliminary results show promise as additional means to fight against HER2+ BC.
The next ADC to become relevant in HER2+ mBC was DS-8201a, a conjugate of trastuzumab and deruxtecan. This compound has recently achieved accelerated FDA approval in December 2019 for unresectable or metastatic HER2+ mBC patients who have received two or more prior anti-HER therapies in the metastatic setting after the results of phase II DESTINY-Breast01 trial. In their phase II analysis, Modi et al. observed an ORR of 60.3% (95% CI, 52.9-67.4), with a 4.3% CR and 56% PR, DoR of 14.8 months (95% CI, 13.8-16.9), and PFS of 16.4 months (95% CI, 12.7-not reached) which demonstrate validation of a previously-reported phase 1 ORR of 59.5% (95% CI, 49.7-68.7) in a similar patient population treated with DS-8201a [35,87]. Importantly, DS-8201a led to an incidence of interstitial lung disease (ILD) incidence in 13.6% and grade 3 or higher adverse events in 57.1% of the study population, leading to a Boxed Warning for ILD.
In a separate large phase 1 study of DS-8201a, Modi et al. reported an ORR of 50.0%, DCR of 85.3%, time to response (TTR) of 2.8 months, DoR of 11.0 months, and PFS of 12.9 months for the overall population, regardless of HR status in heavily-pretreated HER2-low (IHC 1+ or 2+/ISH-) expressing BC patients [88]. These results suggest a promising clinical benefit for the heavily-pretreated HER2+ mBC population overall and preliminary hope for a subset of HER2-low expressing population, which represents approximately 40% of BC and for which there is no present FDA approved HER2 therapies currently in existence. Consequently, the phase III DESTINY-Breast04 trial (NCT03734029) was initiated to determine the utility of DS-8201a in heavily pretreated and surgical unresectable/metastatic HER2-low expressing BC patients (Table 1).
Tucatinib (ONT-380), an oral and highly potent selective HER2 tyrosine kinase inhibitor, is the first of these hopeful therapies. In a recent phase 1b study of heavily pretreated HER2+ mBC patients, Hamilton et al. assessed tucatinib and capecitabine ± trastuzumab. They reported encouraging initial findings of improved PFS of 6.3 months, CBR 67%, ORR 58%, and a low toxicity profile for the triple therapy in comparison to other HER2 therapies [89]. Later results of that small phase 1b trial were difficult to interpret with ORR of 83% in the combination of tucatinib plus capecitabine (T+C), 40% in the combination of tucatinib plus trastuzumab (T+T), and 61% in the combination of tucatinib plus capecitabine plus trastuzumab (T+C+T). Although efficacy for the combinations appeared acceptable, the sample size was small in all arms of the trial [39].
Its subsequent phase II trial, HER2CLIMB (NCT02614794), Murthy et al. reported encouraging findings in heavily pretreated HER2+ mBC patients both with and without brain metastases randomized to either tucatinib or placebo, in combination with trastuzumab and capecitabine. They reported a 1-year PFS of 33.1% in the tucatinib-combination group vs. 12.3% in the placebo-combination group (HR 0.54; 95% CI, 0.42-0.71; P < 0.001) [90]. Furthermore, the 2-year OS was 44.9% vs. 26.6% in the tucatinib-combination and placebo-combination group, respectively (HR 0.66; 95% CI, 0.50-0.88; P = 0.005). Importantly, a subset population with brain metastases demonstrated a 1-year PFS of 24.9% in the tucatinib-combination group vs. 0% in the placebo-combination group (HR 0.48; 95% CI, 0.34-0.69; P < 0.001) [90].
Another study of tucatinib by Borges et al. demonstrated its combination with T-DM1 had a primary PFS benefit of 8.2 months for pretreated HER2+ mBC patients, ORR of 47% at the maximum-tolerated dose, a CBR of 58%, and DoR of 6.9 months. Of note, 60% of patients had been previously treated with trastuzumab and contained brain metastases. In this subpopulation (47% had the measurable disease), the combination treatment arm led to a PFS of 6.7-months, DoR of 6.9 months, and brain-specific ORR of 36% [91]. The importance of this result becomes more relevant when an estimated 25% of patients treated with trastuzumab develop symptomatic brain metastases, and little literature exists regarding proven treatment options to address this issue [92,93].
Pyrotinib, an irreversible pan-HER kinase inhibitor, became the next novel oral agent introduced to HER2+ mBC. In their phase II trial, Xu et al. assessed pyrotinib plus capecitabine (P+C) vs. lapatinib plus capecitabine (L+C) in heavily pretreated patients. At 15-month follow-up, the pyrotinib arm had a significantly prolonged PFS (18 vs. 7 months; HR 0.36; P < 0.0001), improved ORR of (78.5% vs. 57.1%; P = 0.01), and had a tolerable toxicity profile with no major increased toxicities [94]. A phase III PHOEBE (NCT03080805) trial is ongoing.
The phase III SOPHIA trial has recently provided evidence for a new monoclonal antibody to HER2, margetuximab that may transcend previous efficacy provided by trastuzumab. As mentioned previously, margetuximab possesses an engineered Fc to achieve higher immune response than presently approved HER2-targeted antibodies, achieving the FDA’s Fast Track designation for locally advanced or metastatic HER2+ BC patients previously treated with anti-HER2+ therapy. The authors have reported significantly improved PFS with margetuximab plus chemotherapy over trastuzumab plus chemotherapy (5.8 vs. 4.9 months; HR 0.76; P = 0.033), a potentially greater (but statistically insignificant) ORR of 22% vs. 16%, and a comparable safety profile. Of note, treatment effects were more pronounced in patients with the CD16A genotypes containing a lower-affinity 158F allele (PFS 6.9 vs. 5.1 months; HR 0.68; 95% CI, 0.52-0.90; P = 0.005) which is found in approximately 85% of the general population and is known to diminish clinical response to trastuzumab [95].
In 2019, published results from phase II KATE2 demonstrated the potential for integration of immunotherapy into the realm of HER2+ mBC. In the trial, Emens et al. investigated the combination of atezolizumab, a PD-L1 inhibitor, plus T-DM1 vs. placebo plus T-DM1. Unfortunately, the atezolizumab arm did not show evidence of improved PFS for the overall study population (HR 0.82; P = 0.3332). It did, however, show a benefit independently for the subsets of patients that were PD-L1+ and had high tumor-infiltrating lymphocytes (TIL+); PD-L1+ patients had PFS of 8.5 vs. 4.1 months comparing the atezolizumab vs. placebo arms, respectively, and the TIL+ patients had PFS 8.5 vs. 5.3 months comparing the atezolizumab vs. placebo arms, respectively [96]. The data suggest a potential use of TILs and PD-L1 status as predictive biomarkers in HER2+ mBC patients for response to the addition of PD-1 and PD-L1 inhibitors.
The phase Ib/II PANACEA trial reported a clearly superior clinical response and tolerable safety profile with combined pembrolizumab (PD-1 inhibitor) plus trastuzumab in trastuzumab-resistant HER2+ mBC patients when assessing for PD-L1 status. The median OS was 16.1 vs. 7 months for PD-L1+ and PD-L1- patients, respectively (P = 0.006). At 12-months, the OS rate was 65% vs. 12% and PFS were 12% vs. 0% in the PD-L1+ and PD-L1- cohorts, respectively [97,98]. In support of previous literature, these results suggest the potential for preferential benefit of immunotherapy in PD-L1+ and high TIL subgroups and the relevance of PD-L1 and TIL status in future HER2+ BC treatment algorithms [98].
Epilogue
Future biomarkers in HER2+ BC?
Several new biomarkers have the potential to be integrated into future treatment algorithms for HER2+ BC. As stated above, the KATE2 and PANACEA trials demonstrate that PD-L1 and TIL biomarkers may predict utility for immunotherapy agents such as pembrolizumab, nivolumab, atezolizumab, etc. While research is quickly gaining traction in this area, its use in early-phase clinical trials has only scratched the surface.
Another important discovery in HER2+ BC came via the adjuvant ExteNET trial. In their secondary analysis, the authors pointed out the potential for classifying and treating HER2+ BC based on HR status due to differences in patients’ responses to current therapies. Such a result was explained by the fact that the observation in the secondary analysis that HER2+/ER+ BC responded significantly better to extended adjuvant fulvestrant and neratinib compared to HER2+/ER- patients, suggesting its use as a potential biomarker [66]. A subsequent analysis by Sudhan et al. was undertaken to investigate the mechanisms behind this observation. Their data demonstrated significant findings of crosstalk in the HER2+/ER+ subpopulation (Figure 4). The crosstalk between HER2 and ER signaling has been postulated to be reciprocal, such that inhibition of either pathway may result in upregulation/activation of the other. Indeed, neratinib treatment induces ER-dependent gene transcription in HER2+ BC cells [99]. Furthermore, the combination of neratinib and fulvestrant was the only arm that reduced cyclin D1 mRNA and protein levels and induced cell cycle arrest. Ultimately, it appears dual HER and ER blockade is necessary mechanistically for durable clinical outcomes in patients with HER2+/ER+ BC [68].
Interestingly, crosstalk between the ER and the HER/GFR signaling was observed prior to the 20th century [100]. The cell signaling mechanisms of the ER pathway upregulation with HER inhibition and HER upregulation with ER inhibition were known for some time prior to the differential clinical responses observed in the ExteNET trial [100-102]. The work by Chan et al. and Sudhan et al. essentially revived its importance both preclinically and clinically, spurring clinical trials and successful treatment of HER2+/ER+ BC with dual anti-HER and anti-estrogen therapy [66,68,103,104].
One such clinical trial is the phase II SOLTI-1303 PATRICIA trial that is exploring the importance of HR status with the use of a cell cycle inhibitor, palbociclib, with trastuzumab ± letrozole in postmenopausal HER2+ mBC patients previously treated with anti-HER2 therapies. In their preliminary results, they observed that the PAM-50 luminal subtype might predict the response to combination palbociclib and trastuzumab therapy, leading to the addition of two treatment arms to their study [105].
Another is the ALTERNATIVE trial, which is an ongoing phase III trial taking this dual pathway inhibition (anti-HER and anti-estrogen) one step further. Johnston et al. have reported a superior PFS (11 vs. 5.7 months; HR 0.62; 95% CI, 0.45-0.88; P = 0.0064) with additional ORR, CBR, and OS benefit using triple therapy of lapatinib, trastuzumab, and letrozole over the dual trastuzumab and letrozole therapy in postmenopausal HER2+/HR+ mBC patients previously treated with endocrine and trastuzumab therapy in various settings [106]. Additionally, the phase II PERTAIN trial has also demonstrated a tolerable safety profile and significant improvement in the efficacy of this triple therapy combination over the dual therapy (PFS 18.89 vs. 15.80 months; 95% CI, 11.04-18.56; HR 0.65; P = 0.007), this time in the first-line setting of metastatic HER2+/HR+ BC [107]. Logically, the next step for this HER2+/HR+ population is quadruple therapy of a cell cycle inhibitor, anti-estrogen, and dual anti-HER2 therapy. A phase I trial, NCT03304080, is currently under recruitment to assess preliminary safety and efficacy of palbociclib, anastrozole, trastuzumab, and pertuzumab combination therapy [108].
Conclusion
The discovery of ERBB2/HER2 and the subsequent development of its transmembrane receptor inhibitor, trastuzumab, have drastically changed the landscape of HER2+ BC treatment and prognosis. Trastuzumab’s success has led to a paradigm shift toward the use of targeted molecular therapies beyond the standard, systemic chemotherapy. Notably, twenty years after trastuzumab’s approval, new insights continue to emerge, including immune-based biomarkers and therapies like margetuximab and trastuzumab-deruxtecan (DS-8201a). Only through these insights can we forge a new frontier, transcending our current Trastuzumab Era in HER2+ BC.
Acknowledgements
The authors acknowledge the Avera Cancer Institute, Sioux Falls, SD, for their financial support. We acknowledge the contribution of scientists whose articles we could not include in the review due to the limitation of space.
Disclosure of conflict of interest
None.
References
- 1.Perez EA, Romond EH, Suman VJ, Jeong JH, Sledge G, Geyer CE Jr, Martino S, Rastogi P, Gralow J, Swain SM, Winer EP, Colon-Otero G, Davidson NE, Mamounas E, Zujewski JA, Wolmark N. Trastuzumab plus adjuvant chemotherapy for human epidermal growth factor receptor 2-positive breast cancer: planned joint analysis of overall survival from NSABP B-31 and NCCTG N9831. J. Clin. Oncol. 2014;32:3744–52. doi: 10.1200/JCO.2014.55.5730. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Franklin MC, Carey KD, Vajdos FF, Leahy DJ, De Vos AM, Sliwkowski MX. Insights into ErbB signaling from the structure of the ErbB2-pertuzumab complex. Cancer Cell. 2004;5:317–328. doi: 10.1016/s1535-6108(04)00083-2. [DOI] [PubMed] [Google Scholar]
- 3.Downward J, Yarden Y, Mayes E, Scrace G, Totty N, Stockwell P, Ullrich A, Schlessinger J, Waterfield MD. Close similarity of epidermal growth factor receptor and v-erb-B oncogene protein sequences. Nature. 1984;307:521–527. doi: 10.1038/307521a0. [DOI] [PubMed] [Google Scholar]
- 4.Coussens L, Yang-Feng TL, Liao YC, Chen E, Gray A, McGrath J, Seeburg PH, Libermann TA, Schlessinger J, Francke U, et al. Tyrosine kinase receptor with extensive homology to EGF receptor shares chromosomal location with neu oncogene. Science. 1985;230:1132–1139. doi: 10.1126/science.2999974. [DOI] [PubMed] [Google Scholar]
- 5.Schechter AL, Stern DF, Vaidyanathan L, Decker SJ, Drebin JA, Greene MI, Weinberg RA. The neu oncogene: an erb-B-related gene encoding a 185,000-Mr tumour antigen. Nature. 1984;312:513–516. doi: 10.1038/312513a0. [DOI] [PubMed] [Google Scholar]
- 6.Slamon DJ, Clark GM, Wong SG, Levin WJ, Ullrich A, McGuire WL. Human breast cancer: correlation of relapse and survival with amplification of the HER-2/neu oncogene. Science. 1987;235:177–182. doi: 10.1126/science.3798106. [DOI] [PubMed] [Google Scholar]
- 7.Wright C, Nicholson S, Angus B, Sainsbury JR, Farndon J, Cairns J, Harris AL, Horne CH. Relationship between c-erbB-2 protein product expression and response to endocrine therapy in advanced breast cancer. Br J Cancer. 1992;65:118–121. doi: 10.1038/bjc.1992.22. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Allred DC, Clark GM, Tandon AK, Molina R, Tormey DC, Osborne CK, Gilchrist KW, Mansour EG, Abeloff M, Eudey L, et al. HER-2/neu in node-negative breast cancer: prognostic significance of overexpression influenced by the presence of in situ carcinoma. J. Clin. Oncol. 1992;10:599–605. doi: 10.1200/JCO.1992.10.4.599. [DOI] [PubMed] [Google Scholar]
- 9.Gusterson BA, Gelber RD, Goldhirsch A, Price KN, Säve-Söderborgh J, Anbazhagan R, Styles J, Rudenstam CM, Golouh R, Reed R, et al. Prognostic importance of c-erbB-2 expression in breast cancer. International (Ludwig) Breast Cancer Study Group. J. Clin. Oncol. 1992;10:1049–1056. doi: 10.1200/JCO.1992.10.7.1049. [DOI] [PubMed] [Google Scholar]
- 10.Carlomagno C, Perrone F, Gallo C, Laurentiis MD, Lauria R, Morabito A, Pettinato G, Panico L, D’Antonio A, Bianco AR, Placido SD. c-erb B2 overexpression decreases the benefit of adjuvant tamoxifen in early-stage breast cancer without axillary lymph node metastases. J. Clin. Oncol. 1996;14:2702–2708. doi: 10.1200/JCO.1996.14.10.2702. [DOI] [PubMed] [Google Scholar]
- 11.Muss HB, Thor AD, Berry DA, Kute T, Liu ET, Koerner F, Cirrincione CT, Budman DR, Wood WC, Barcos M, Henderson IC. c-erbB-2 expression and response to adjuvant therapy in women with node-positive early breast cancer. N Engl J Med. 1994;330:1260–1266. doi: 10.1056/NEJM199405053301802. [DOI] [PubMed] [Google Scholar]
- 12.Clemons M, Leahy M, Valle J, Jayson G, Ranson M, Howell A. Review of recent trials of chemotherapy for advanced breast cancer: the taxanes. Eur J Cancer. 1997;33:2183–2193. doi: 10.1016/s0959-8049(97)00260-8. [DOI] [PubMed] [Google Scholar]
- 13.Paik S, Bryant J, Park C, Fisher B, Tan-Chiu E, Hyams D, Fisher ER, Lippman ME, Wickerham DL, Wolmark N. erbB-2 and response to doxorubicin in patients with axillary lymph node-positive, hormone receptor-negative breast cancer. J Natl Cancer Inst. 1998;90:1361–1370. doi: 10.1093/jnci/90.18.1361. [DOI] [PubMed] [Google Scholar]
- 14.Dobson R. Trastuzumab halves risk of recurrence of breast cancer in some women. BMJ. 2005;331:986–986. [Google Scholar]
- 15.Gianni L, Salvatorelli E, Minotti G. Anthracycline cardiotoxicity in breast cancer patients: synergism with trastuzumab and taxanes. Cardiovasc Toxicol. 2007;7:67–71. doi: 10.1007/s12012-007-0013-5. [DOI] [PubMed] [Google Scholar]
- 16.Nahta R, Esteva FJ. HER2 therapy: molecular mechanisms of trastuzumab resistance. Breast Cancer Res. 2006;8:215. doi: 10.1186/bcr1612. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Nahta R, Yu D, Hung MC, Hortobagyi GN, Esteva FJ. Mechanisms of disease: understanding resistance to HER2-targeted therapy in human breast cancer. Nat Clin Pract Oncol. 2006;3:269–80. doi: 10.1038/ncponc0509. [DOI] [PubMed] [Google Scholar]
- 18.Vu T, Claret FX. Trastuzumab: updated mechanisms of action and resistance in breast cancer. Front Oncol. 2012;2:62. doi: 10.3389/fonc.2012.00062. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Moasser MM, Ai WZ. Neoplasia. In: Hammer GD, McPhee SJ, editors. Pathophysiology of Disease: An Introduction to Clinical Medicine. 8 edition. New York, NY: McGraw-Hill Education; 2019. [Google Scholar]
- 20.Dai X, Li T, Bai Z, Yang Y, Liu X, Zhan J, Shi B. Breast cancer intrinsic subtype classification, clinical use and future trends. Am J Cancer Res. 2015;5:2929–2943. [PMC free article] [PubMed] [Google Scholar]
- 21.Carey LA, Perou CM, Livasy CA, Dressler LG, Cowan D, Conway K, Karaca G, Troester MA, Tse CK, Edmiston S, Deming SL, Geradts J, Cheang MC, Nielsen TO, Moorman PG, Earp HS, Millikan RC. Race, breast cancer subtypes, and survival in the carolina breast cancer study. JAMA. 2006;295:2492. doi: 10.1001/jama.295.21.2492. [DOI] [PubMed] [Google Scholar]
- 22.Cancer Genome Atlas Network. Koboldt DC, Fulton RS, McLellan MD, Schmidt H, Kalicki-Veizer J, McMichael JF, Fulton LL, Dooling DJ, Ding L, Mardis ER, Wilson RK, Ally A, Balasundaram M, Butterfield YS, Carlsen R, Carter C, Chu A, Chuah E, Chun HJ, Coope RJ, Dhalla N, Guin R, Hirst C, Hirst M, Holt RA, Lee D, Li HI, Mayo M, Moore RA, Mungall AJ, Pleasance E, Robertson A, Schein JE, Shafiei A, Sipahimalani P, Slobodan JR, Stoll D, Tam A, Thiessen N, Varhol RJ, Wye N, Zeng T, Zhao Y, Birol I, Jones SJ, Marra MA, Cherniack AD, Saksena G, Onofrio RC, Pho NH, Carter SL, Schumacher SE, Tabak B, Hernandez B, Gentry J, Nguyen H, Crenshaw A, Ardlie K, Beroukhim R, Winckler W, Getz G, Gabriel SB, Meyerson M, Chin L, Park PJ, Kucherlapati R, Hoadley KA, Auman J, Fan C, Turman YJ, Shi Y, Li L, Topal MD, He X, Chao HH, Prat A, Silva GO, Iglesia MD, Zhao W, Usary J, Berg JS, Adams M, Booker J, Wu J, Gulabani A, Bodenheimer T, Hoyle AP, Simons JV, Soloway MG, Mose LE, Jefferys SR, Balu S, Parker JS, Hayes D, Perou CM, Malik S, Mahurkar S, Shen H, Weisenberger DJ, Triche T Jr, Lai PH, Bootwalla MS, Maglinte DT, Berman BP, Van Den Berg DJ, Baylin SB, Laird PW, Creighton CJ, Donehower LA, Getz G, Noble M, Voet D, Saksena G, Gehlenborg N, DiCara D, Zhang J, Zhang H, Wu CJ, Liu SY, Lawrence MS, Zou L, Sivachenko A, Lin P, Stojanov P, Jing R, Cho J, Sinha R, Park RW, Nazaire MD, Robinson J, Thorvaldsdottir H, Mesirov J, Park PJ, Chin L, Reynolds S, Kreisberg RB, Bernard B, Bressler R, Erkkila T, Lin J, Thorsson V, Zhang W, Shmulevich I, Ciriello G, Weinhold N, Schultz N, Gao J, Cerami E, Gross B, Jacobsen A, Sinha R, Aksoy B, Antipin Y, Reva B, Shen R, Taylor BS, Ladanyi M, Sander C, Anur P, Spellman PT, Lu Y, Liu W, Verhaak RR, Mills GB, Akbani R, Zhang N, Broom BM, Casasent TD, Wakefield C, Unruh AK, Baggerly K, Coombes K, Weinstein JN, Haussler D, Benz CC, Stuart JM, Benz SC, Zhu J, Szeto CC, Scott GK, Yau C, Paull EO, Carlin D, Wong C, Sokolov A, Thusberg J, Mooney S, Ng S, Goldstein TC, Ellrott K, Grifford M, Wilks C, Ma S, Craft B, Yan C, Hu Y, Meerzaman D, Gastier-Foster JM, Bowen J, Ramirez NC, Black AD, Pyatt RE, White P, Zmuda EJ, Frick J, Lichtenberg TM, Brookens R, George MM, Gerken MA, Harper HA, Leraas KM, Wise LJ, Tabler TR, McAllister C, Barr T, Hart-Kothari M, Tarvin K, Saller C, Sandusky G, Mitchell C, Iacocca MV, Brown J, Rabeno B, Czerwinski C, Petrelli N, Dolzhansky O, Abramov M, Voronina O, Potapova O, Marks JR, Suchorska WM, Murawa D, Kycler W, Ibbs M, Korski K, Spychała A, Murawa P, Brzeziński JJ, Perz H, Łaźniak R, Teresiak M, Tatka H, Leporowska E, Bogusz-Czerniewicz M, Malicki J, Mackiewicz A, Wiznerowicz M, Le XV, Kohl B, Nguyen VT, Thorp R, Nguyen VB, Sussman H, Bui DP, Hajek R, Nguyen PH, Tran VT, Huynh QT, Khan KZ, Penny R, Mallery D, Curley E, Shelton C, Yena P, Ingle JN, Couch FJ, Lingle WL, King TA, Gonzalez-Angulo AM, Mills GB, Dyer MD, Liu S, Meng X, Patangan M, Waldman F, Stöppler H, Rathmell W, Thorne L, Huang M, Boice L, Hill A, Morrison C, Gaudioso C, Bshara W, Daily K, Egea SC, Pegram M, Gomez-Fernandez C, Dhir R, Bhargava R, Brufsky A, Shriver CD, Hooke JA, Campbell JL, Mural RJ, Hu H, Somiari S, Larson C, Deyarmin B, Kvecher L, Kovatich AJ, Ellis MJ, King TA, Hu H, Couch FJ, Mural RJ, Stricker T, White K, Olopade O, Ingle JN, Luo C, Chen Y, Marks JR, Waldman F, Wiznerowicz M, Bose R, Chang LW, Beck AH, Gonzalez-Angulo AM, Pihl T, Jensen M, Sfeir R, Kahn A, Chu A, Kothiyal P, Wang Z, Snyder E, Pontius J, Ayala B, Backus M, Walton J, Baboud J, Berton D, Nicholls M, Srinivasan D, Raman R, Girshik S, Kigonya P, Alonso S, Sanbhadti R, Barletta S, Pot D, Sheth M, Demchok JA, Shaw KR, Yang L, Eley G, Ferguson ML, Tarnuzzer RW, Zhang J, Dillon LA, Buetow K, Fielding P, Ozenberger BA, Guyer MS, Sofia HJ, Palchik JD. Comprehensive molecular portraits of human breast tumours. Nature. 2012;490:61–70. doi: 10.1038/nature11412. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Cronin KA, Harlan LC, Dodd KW, Abrams JS, Ballard-Barbash R. Population-based estimate of the prevalence of HER-2 positive breast cancer tumors for early stage patients in the US. Cancer Invest. 2010;28:963–968. doi: 10.3109/07357907.2010.496759. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Giuliano AE, Hurvitz SA. Carcinoma of the female breast. In: Papadakis MA, McPhee SJ, Rabow MW, editors. Current medical diagnosis and treatment 2020. New York, NY: McGraw-Hill Education; 2020. [Google Scholar]
- 25.Ross JS, Slodkowska EA, Symmans WF, Pusztai L, Ravdin PM, Hortobagyi GN. The HER-2 receptor and breast cancer: ten years of targeted anti-HER-2 therapy and personalized medicine. Oncologist. 2009;14:320–368. doi: 10.1634/theoncologist.2008-0230. [DOI] [PubMed] [Google Scholar]
- 26.Tripathy D, Brufsky A, Cobleigh M, Jahanzeb M, Kaufman PA, Mason G, O’Shaughnessy J, Rugo HS, Swain SM, Yardley DA, Chu L, Li H, Antao V, Hurvitz SA. De Novo versus recurrent HER2-positive metastatic breast cancer: patient characteristics, treatment, and survival from the SystHERs registry. Oncologist. 2019 doi: 10.1634/theoncologist.2019-0446. [Epub ahead of print] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Waterman H, Sabanai I, Geiger B, Yarden Y. Alternative intracellular routing of ErbB receptors may determine signaling potency. J Biol Chem. 1998;273:13819–13827. doi: 10.1074/jbc.273.22.13819. [DOI] [PubMed] [Google Scholar]
- 28.Gómez HL, Castañeda CA, Vigil CE, Vidaurre T, Velarde RG, Cruz WR, Pinto JA, Suazo JF, Garces MR, Neciosup SP, Vallejos CS. Prognostic effect of hormone receptor status in early HER2 positive breast cancer patients. Hematol Oncol Stem Cell Ther. 2010;3:109–115. doi: 10.1016/s1658-3876(10)50020-7. [DOI] [PubMed] [Google Scholar]
- 29.Harbeck N. Neoadjuvant treatment of HER2-positive breast cancer: should therapy differ based on hormone receptor status? Ther Adv Med Oncol. 2018;10:1758835918782356. doi: 10.1177/1758835918782356. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Segovia-Mendoza M, González-González ME, Barrera D, Díaz L, García-Becerra R. Efficacy and mechanism of action of the tyrosine kinase inhibitors gefitinib, lapatinib and neratinib in the treatment of HER2-positive breast cancer: preclinical and clinical evidence. Am J Cancer Res. 2015;5:2531–2561. [PMC free article] [PubMed] [Google Scholar]
- 31.Yamashita-Kashima Y, Shu S, Yorozu K, Moriya Y, Harada N. Mode of action of pertuzumab in combination with trastuzumab plus docetaxel therapy in a HER2-positive breast cancer xenograft model. Oncol Lett. 2017;14:4197–4205. doi: 10.3892/ol.2017.6679. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Nami B, Maadi H, Wang Z. Mechanisms underlying the action and synergism of trastuzumab and pertuzumab in targeting HER2-positive breast cancer. Cancers (Basel) 2018;10 doi: 10.3390/cancers10100342. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.FDA approves ado-trastuzumab emtansine for early breast cancer. 2019 [Google Scholar]
- 34.Oostra D, Macrae E. Role of trastuzumab emtansine in the treatment of HER2-positive breast cancer. Breast Cancer (Dove Med Press) 2014;6:103–113. doi: 10.2147/BCTT.S67297. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Modi S, Saura C, Yamashita T, Park YH, Kim SB, Tamura K, Andre F, Iwata H, Ito Y, Tsurutani J, Sohn J, Denduluri N, Perrin C, Aogi K, Tokunaga E, Im SA, Lee KS, Hurvitz SA, Cortes J, Lee C, Chen S, Zhang L, Shahidi J, Yver A, Krop I DESTINY-Breast01 Investigators. Trastuzumab deruxtecan in previously treated HER2-positive breast cancer. N Engl J Med. 2020;382:610–621. doi: 10.1056/NEJMoa1914510. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Bang YJ, Giaccone G, Im SA, Oh DY, Bauer TM, Nordstrom JL, Li H, Chichili GR, Moore PA, Hong S, Stewart SJ, Baughman JE, Lechleider RJ, Burris HA. First-in-human phase 1 study of margetuximab (MGAH22), an Fc-modified chimeric monoclonal antibody, in patients with HER2-positive advanced solid tumors. Ann Oncol. 2017;28:855–861. doi: 10.1093/annonc/mdx002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Moulder SL, Borges VF, Baetz T, McSpadden T, Fernetich G, Murthy RK, Chavira R, Guthrie K, Barrett E, Chia SK. Phase I study of ONT-380, a HER2 inhibitor, in patients with HER2(+)-advanced solid tumors, with an expansion cohort in HER2(+) metastatic breast cancer (MBC) Clin Cancer Res. 2017;23:3529–3536. doi: 10.1158/1078-0432.CCR-16-1496. [DOI] [PubMed] [Google Scholar]
- 38.Murthy R, Borges VF, Conlin A, Chaves J, Chamberlain M, Gray T, Vo A, Hamilton E. Tucatinib with capecitabine and trastuzumab in advanced HER2-positive metastatic breast cancer with and without brain metastases: a non-randomised, open-label, phase 1b study. Lancet Oncol. 2018;19:880–888. doi: 10.1016/S1470-2045(18)30256-0. [DOI] [PubMed] [Google Scholar]
- 39.Murthy RK, Hamilton EP, Ferrario C, Aucoin N, Falkson CI, Chamberlain MC, Gray T, Borges VF. Clinical benefit of tucatinib after isolated brain progression: a retrospective pooled analysis of tucatinib phase 1b studies in HER2+ breast cancer. J. Clin. Oncol. 2018;36:1015–1015. [Google Scholar]
- 40.Blair HA. Pyrotinib: first global approval. Drugs. 2018;78:1751–1755. doi: 10.1007/s40265-018-0997-0. [DOI] [PubMed] [Google Scholar]
- 41.Cersosimo RJ. Cyclin-dependent kinase 4/6 inhibitors for the management of advanced or metastatic breast cancer in women. Am J Health Syst Pharm. 2019;76:1183–1202. doi: 10.1093/ajhp/zxz121. [DOI] [PubMed] [Google Scholar]
- 42.Spring L, Bardia A, Modi S. Targeting the cyclin D-cyclin-dependent kinase (CDK) 4/6-retinoblastoma pathway with selective CDK 4/6 inhibitors in hormone receptor-positive breast cancer: rationale, current status, and future directions. Discov Med. 2016;21:65–74. [PMC free article] [PubMed] [Google Scholar]
- 43.Lee HT, Lee JY, Lim H, Lee SH, Moon YJ, Pyo HJ, Ryu SE, Shin W, Heo YS. Molecular mechanism of PD-1/PD-L1 blockade via anti-PD-L1 antibodies atezolizumab and durvalumab. Sci Rep. 2017;7:5532. doi: 10.1038/s41598-017-06002-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Van Pelt AE, Mohsin S, Elledge RM, Hilsenbeck SG, Gutierrez MC, Lucci A, Kalidas M, Granchi T, Scott BG, Allred DC, Chang JC. Neoadjuvant trastuzumab and docetaxel in patients with breast cancer: preliminary results. Clin Breast Cancer. 2003;4:348–353. doi: 10.3816/cbc.2003.n.040. [DOI] [PubMed] [Google Scholar]
- 45.Coudert BP, Arnould L, Moreau L, Chollet P, Weber B, Vanlemmens L, Moluçon C, Tubiana N, Causeret S, Misset JL, Feutray S, Mery-Mignard D, Garnier J, Fumoleau P. Pre-operative systemic (neo-adjuvant) therapy with trastuzumab and docetaxel for HER2-overexpressing stage II or III breast cancer: results of a multicenter phase II trial. Ann Oncol. 2005;17:409–414. doi: 10.1093/annonc/mdj096. [DOI] [PubMed] [Google Scholar]
- 46.Buzdar AU, Ibrahim NK, Francis D, Booser DJ, Thomas ES, Theriault RL, Pusztai L, Green MC, Arun BK, Giordano SH. Significantly higher pathologic complete remission rate after neoadjuvant therapy with trastuzumab, paclitaxel, and epirubicin chemotherapy: results of a randomized trial in human epidermal growth factor receptor 2-positive operable breast cancer. J. Clin. Oncol. 2005;23:3676–3685. doi: 10.1200/JCO.2005.07.032. [DOI] [PubMed] [Google Scholar]
- 47.Gianni L, Eiermann W, Semiglazov V, Manikhas A, Lluch A, Tjulandin S, Zambetti M, Vazquez F, Byakhow M, Lichinitser M. Neoadjuvant chemotherapy with trastuzumab followed by adjuvant trastuzumab versus neoadjuvant chemotherapy alone, in patients with HER2-positive locally advanced breast cancer (the NOAH trial): a randomised controlled superiority trial with a parallel HER2-negative cohort. Lancet. 2010;375:377–384. doi: 10.1016/S0140-6736(09)61964-4. [DOI] [PubMed] [Google Scholar]
- 48.Gianni L, Pienkowski T, Im YH, Roman L, Tseng LM, Liu MC, Lluch A, Staroslawska E, de la Haba-Rodriguez J, Im SA, Pedrini JL, Poirier B, Morandi P, Semiglazov V, Srimuninnimit V, Bianchi G, Szado T, Ratnayake J, Ross G, Valagussa P. Efficacy and safety of neoadjuvant pertuzumab and trastuzumab in women with locally advanced, inflammatory, or early HER2-positive breast cancer (NeoSphere): a randomised multicentre, open-label, phase 2 trial. Lancet Oncol. 2012;13:25–32. doi: 10.1016/S1470-2045(11)70336-9. [DOI] [PubMed] [Google Scholar]
- 49.Gianni L, Pienkowski T, Im YH, Tseng LM, Liu MC, Lluch A, Starosławska E, de la Haba-Rodriguez J, Im SA, Pedrini JL. 5-year analysis of neoadjuvant pertuzumab and trastuzumab in patients with locally advanced, inflammatory, or early-stage HER2-positive breast cancer (NeoSphere): a multicentre, open-label, phase 2 randomised trial. Lancet Oncol. 2016;17:791–800. doi: 10.1016/S1470-2045(16)00163-7. [DOI] [PubMed] [Google Scholar]
- 50.Baselga J, Bradbury I, Eidtmann H, Di Cosimo S, De Azambuja E, Aura C, Gómez H, Dinh P, Fauria K, Van Dooren V. Lapatinib with trastuzumab for HER2-positive early breast cancer (NeoALTTO): a randomised, open-label, multicentre, phase 3 trial. Lancet. 2012;379:633–640. doi: 10.1016/S0140-6736(11)61847-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Harbeck N, Gluz O, Christgen M, Braun MW, Kummel S, Potenberg J, Aktas B, Schumacher C, Forstbauer H, Augustin D, Kraemer S, Just M, Tio J, Kleine-Tebbe A, Liedtke C, Kates RE, Hofmann D, Wuerstlein R, Kreipe HH, Nitz U WGSGAHH Investigators. Efficacy of 12-weeks of neoadjuvant TDM1 with or without endocrine therapy in HER2-positive hormone-receptor-positive early breast cancer: WSG-ADAPT HER2+/HR+ phase II trial. J. Clin. Oncol. 2015;33:506. [Google Scholar]
- 52.Nitz U, Gluz O, Christgen M, Grischke EM, Augustin D, Kümmel S, Braun MW, Potenberg J, Kohls A, Krauss K, Stefek A, Schumacher C, Forstbauer H, Reimer T, Fischer HH, Liedtke C, Wuerstlein R, Kreipe HH, Harbeck N. Final analysis of WSG-ADAPT HER2+/HR- trial: efficacy, safety, and predictive markers for 12-weeks of neoadjuvant dual blockade with trastuzumab + pertuzumab ± weekly paclitaxel in HER2+/HR- early breast cancer (EBC) J. Clin. Oncol. 2016;34:518. [Google Scholar]
- 53.Hurvitz SA, Martin M, Symmans WF, Jung KH, Huang CS, Thompson AM, Harbeck N, Valero V, Stroyakovskiy D, Wildiers H, Campone M, Boileau JF, Beckmann MW, Afenjar K, Fresco R, Helms HJ, Xu J, Lin YG, Sparano J, Slamon D. Neoadjuvant trastuzumab, pertuzumab, and chemotherapy versus trastuzumab emtansine plus pertuzumab in patients with HER2-positive breast cancer (KRISTINE): a randomised, open-label, multicentre, phase 3 trial. Lancet Oncol. 2018;19:115–126. doi: 10.1016/S1470-2045(17)30716-7. [DOI] [PubMed] [Google Scholar]
- 54.Piccart-Gebhart MJ, Procter M, Leyland-Jones B, Goldhirsch A, Untch M, Smith I, Gianni L, Baselga J, Bell R, Jackisch C, Cameron D, Dowsett M, Barrios CH, Steger G, Huang CS, Andersson M, Inbar M, Lichinitser M, Láng I, Nitz U, Iwata H, Thomssen C, Lohrisch C, Suter TM, Rüschoff J, Sütő T, Greatorex V, Ward C, Straehle C, McFadden E, Dolci MS, Gelber RD. Trastuzumab after adjuvant chemotherapy in HER2-positive breast cancer. N Engl J Med. 2005;353:1659–1672. doi: 10.1056/NEJMoa052306. [DOI] [PubMed] [Google Scholar]
- 55.Romond EH, Perez EA, Bryant J, Suman VJ, Geyer CE Jr, Davidson NE, Tan-Chiu E, Martino S, Paik S, Kaufman PA. Trastuzumab plus adjuvant chemotherapy for operable HER2-positive breast cancer. N Engl J Med. 2005;353:1673–1684. doi: 10.1056/NEJMoa052122. [DOI] [PubMed] [Google Scholar]
- 56.Slamon D, Eiermann W, Robert N, Pienkowski T, Martin M, Press M, Mackey J, Glaspy J, Chan A, Pawlicki M. Adjuvant trastuzumab in HER2-positive breast cancer. N Engl J Med. 2011;365:1273–1283. doi: 10.1056/NEJMoa0910383. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Burstein HJ, Piccart-Gebhart MJ, Perez EA, Hortobagyi GN, Wolmark N, Albain KS, Norton L, Winer EP, Hudis CA. Choosing the best trastuzumab-based adjuvant chemotherapy regimen: should we abandon anthracyclines? J. Clin. Oncol. 2012;30:2179–2182. doi: 10.1200/JCO.2012.42.0695. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Goldhirsch A, Gelber RD, Piccart-Gebhart MJ, De Azambuja E, Procter M, Suter TM, Jackisch C, Cameron D, Weber HA, Heinzmann D. 2 years versus 1 year of adjuvant trastuzumab for HER2-positive breast cancer (HERA): an open-label, randomised controlled trial. Lancet. 2013;382:1021–1028. doi: 10.1016/S0140-6736(13)61094-6. [DOI] [PubMed] [Google Scholar]
- 59.Pivot X, Romieu G, Debled M, Pierga JY, Kerbrat P, Bachelot T, Lortholary A, Espié M, Fumoleau P, Serin D, Jacquin JP, Jouannaud C, Rios M, Abadie-Lacourtoisie S, Tubiana-Mathieu N, Cany L, Catala S, Khayat D, Pauporté I, Kramar A. 6 months versus 12 months of adjuvant trastuzumab for patients with HER2-positive early breast cancer (PHARE): a randomised phase 3 trial. Lancet Oncol. 2013;14:741–748. doi: 10.1016/S1470-2045(13)70225-0. [DOI] [PubMed] [Google Scholar]
- 60.Mavroudis D, Saloustros E, Malamos N, Kakolyris S, Boukovinas I, Papakotoulas P, Kentepozidis N, Ziras N, Georgoulias V. Six versus 12 months of adjuvant trastuzumab in combination with dose-dense chemotherapy for women with HER2-positive breast cancer: a multicenter randomized study by the Hellenic Oncology Research Group (HORG) Ann Oncol. 2015;26:1333–1340. doi: 10.1093/annonc/mdv213. [DOI] [PubMed] [Google Scholar]
- 61.Joensuu H, Fraser J, Wildiers H, Huovinen R, Auvinen P, Utriainen M, Nyandoto P, Villman KK, Halonen P, Granstam-Bjorneklett H, Lundgren L, Sailas L, Turpeenniemi-Hujanen T, Tanner M, Yachnin J, Ritchie D, Johansson O, Huttunen T, Neven P, Canney P, Harvey VJ, Kellokumpu-Lehtinen PL, Lindman H. Effect of adjuvant trastuzumab for a duration of 9 weeks vs. 1 year with concomitant chemotherapy for early human epidermal growth factor receptor 2-positive breast cancer: the SOLD randomized clinical trial. JAMA Oncol. 2018;4:1199–1206. doi: 10.1001/jamaoncol.2018.1380. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Chen L, Zhou W, Hu X, Yi M, Ye C, Yao G. Short-duration versus 1-year adjuvant trastuzumab in early HER2 positive breast cancer: a meta-analysis of randomized controlled trials. Cancer Treat Rev. 2019;75:12–19. doi: 10.1016/j.ctrv.2019.02.003. [DOI] [PubMed] [Google Scholar]
- 63.Earl HM, Hiller L, Vallier AL, Loi S, McAdam K, Hughes-Davies L, Harnett AN, Ah-See ML, Simcock R, Rea D, Raj S, Woodings P, Harries M, Howe D, Raynes K, Higgins HB, Wilcox M, Plummer C, Mansi J, Gounaris I, Mahler-Araujo B, Provenzano E, Chhabra A, Abraham JE, Caldas C, Hall PS, McCabe C, Hulme C, Miles D, Wardley AM, Cameron DA, Dunn JA, Agarwal R, Algurafi H, Allerton R, Archer C, Armstrong A, Bale C, Barraclough L, Barthakur U, Bedi C, Benstead K, Bloomfield D, Bowen R, Bradley C, Brown J, Butt M, Churn M, Cleator S, Cliff J, Crellin P, Daly M, De Silva-Minor S, Dhadda A, Din O, Down S, Earl H, Eaton D, Eichholz A, Epurescu D, Goh C, Goodman A, Grieve R, Hadaki M, Harper-Wynne C, Harries M, Hayward L, Humphreys A, Innes H, Jafri M, Jegannathen A, Kelleher M, Kristeleit H, Lee D, Lupton S, MacGregor C, Malik Z, Mansi J, Marshall J, McAdam K, McGolick T, Mehra R, Miles D, Mithal N, Moss C, Moss A, Mukesh M, Neal A, Nelmes D, Neville-Webbe H, Newby J, O’Reilly S, Ostler P, Persic M, Pettit L, Raj S, Raja F, Rea D, Reed C, Rigg A, Roe H, Shah N, Simmonds P, Sims E, Smith S, Storey N, Taylor W, Thanvi N, Tipples K, Vaidya J, Varughese M, Vinayan A, Walji N, Waters S, Woodings P, Wright K, Yahya S. 6 versus 12 months of adjuvant trastuzumab for HER2-positive early breast cancer (PERSEPHONE): 4-year disease-free survival results of a randomised phase 3 non-inferiority trial. Lancet. 2019;393:2599–2612. doi: 10.1016/S0140-6736(19)30650-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Moreno-Aspitia A, Holmes EM, Jackisch C, Azambuja ED, Boyle FM, Hillman DW, Korde LA, Fumagalli D, Izquierdo MA, McCullough AE, Wolff AC, Pritchard KI, Untch M, Lang I, Xu B, Smith IE, Barrios CH, Gelber RD, Baselga J, Piccart-Gebhart MJ. Updated results from the phase III ALTTO trial (BIG 2-06; NCCTG (Alliance) N063D) comparing one year of anti-HER2 therapy with lapatinib alone (L), trastuzumab alone (T), their sequence (T→L) or their combination (L+T) in the adjuvant treatment of HER2-positive early breast cancer. J. Clin. Oncol. 2017;35:502–502. [Google Scholar]
- 65.von Minckwitz G, Procter M, de Azambuja E, Zardavas D, Benyunes M, Viale G, Suter T, Arahmani A, Rouchet N, Clark E, Knott A, Lang I, Levy C, Yardley DA, Bines J, Gelber RD, Piccart M, Baselga J. Adjuvant pertuzumab and trastuzumab in early her2-positive breast cancer. N Eng J Med. 2017;377:122–131. doi: 10.1056/NEJMoa1703643. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Chan A, Delaloge S, Holmes FA, Moy B, Iwata H, Harvey VJ, Robert NJ, Silovski T, Gokmen E, Von Minckwitz G, Ejlertsen B, Chia SKL, Mansi J, Barrios CH, Gnant M, Buyse M, Gore I, Smith J, Harker G, Masuda N, Petrakova K, Zotano AG, Iannotti N, Rodriguez G, Tassone P, Wong A, Bryce R, Ye Y, Yao B, Martin M. Neratinib after trastuzumab-based adjuvant therapy in patients with HER2-positive breast cancer (ExteNET): a multicentre, randomised, double-blind, placebo-controlled, phase 3 trial. Lancet Oncol. 2016;17:367–377. doi: 10.1016/S1470-2045(15)00551-3. [DOI] [PubMed] [Google Scholar]
- 67.Martin M, Holmes FA, Ejlertsen B, Delaloge S, Moy B, Iwata H, von Minckwitz G, Chia SK, Mansi J, Barrios CH. Neratinib after trastuzumab-based adjuvant therapy in HER2-positive breast cancer (ExteNET): 5-year analysis of a randomised, double-blind, placebo-controlled, phase 3 trial. Lancet Oncol. 2017;18:1688–1700. doi: 10.1016/S1470-2045(17)30717-9. [DOI] [PubMed] [Google Scholar]
- 68.Sudhan DR, Schwarz LJ, Guerrero-Zotano A, Formisano L, Nixon MJ, Croessmann S, González Ericsson PI, Sanders M, Balko JM, Avogadri-Connors F, Cutler RE, Lalani AS, Bryce R, Auerbach A, Arteaga CL. Extended adjuvant therapy with neratinib plus fulvestrant blocks ER/HER2 crosstalk and maintains complete responses of ER+/HER2+ breast cancers: implications to the ExteNET trial. Clin Cancer Res. 2019;25:771–783. doi: 10.1158/1078-0432.CCR-18-1131. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Unni N, Sudhan DR, Arteaga CL. Neratinib: inching up on the cure rate of HER2+ breast cancer? Clin Cancer Res. 2018;24:3483–3485. doi: 10.1158/1078-0432.CCR-18-1114. [DOI] [PubMed] [Google Scholar]
- 70.von Minckwitz G, Huang CS, Mano MS, Loibl S, Mamounas EP, Untch M, Wolmark N, Rastogi P, Schneeweiss A, Redondo A, Fischer HH, Jacot W, Conlin AK, Arce-Salinas C, Wapnir IL, Jackisch C, DiGiovanna MP, Fasching PA, Crown JP, Wülfing P, Shao Z, Rota Caremoli E, Wu H, Lam LH, Tesarowski D, Smitt M, Douthwaite H, Singel SM, Geyer CE. Trastuzumab emtansine for residual invasive HER2-positive breast cancer. N Eng J Med. 2018;380:617–628. doi: 10.1056/NEJMoa1814017. [DOI] [PubMed] [Google Scholar]
- 71.Dzimitrowicz H, Berger M, Vargo C, Hood A, Abdelghany O, Raghavendra AS, Tripathy D, Valero V, Hatzis C, Pusztai L, Murthy R. T-DM1 activity in metastatic human epidermal growth factor receptor 2-positive breast cancers that received prior therapy with trastuzumab and pertuzumab. J. Clin. Oncol. 2016;34:3511–3517. doi: 10.1200/JCO.2016.67.3624. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Fabi A, Giannarelli D, Moscetti L, Santini D, Zambelli A, Laurentiis MD, Caruso M, Generali D, Valle E, Leonardi V, Cannita K, Arpino G, Filippelli G, Ferretti G, Giampaglia M, Montemurro F, Nisticò C, Gasparro S, Cognetti F. Ado-trastuzumab emtansine (T-DM1) in HER2+ advanced breast cancer patients: does pretreatment with pertuzumab matter? Future Oncol. 2017;13:2791–2797. doi: 10.2217/fon-2017-0336. [DOI] [PubMed] [Google Scholar]
- 73.Baselga J, Tripathy D, Mendelsohn J, Baughman S, Benz CC, Dantis L, Sklarin NT, Seidman AD, Hudis CA, Moore J, Rosen PP, Twaddell T, Henderson IC, Norton L. Phase II study of weekly intravenous recombinant humanized anti-p185HER2 monoclonal antibody in patients with HER2/neu-overexpressing metastatic breast cancer. J. Clin. Oncol. 1996;14:737–744. doi: 10.1200/JCO.1996.14.3.737. [DOI] [PubMed] [Google Scholar]
- 74.Pegram MD, Lipton A, Hayes DF, Weber BL, Baselga JM, Tripathy D, Baly D, Baughman SA, Twaddell T, Glaspy JA, Slamon DJ. Phase II study of receptor-enhanced chemosensitivity using recombinant humanized anti-p185HER2/neu monoclonal antibody plus cisplatin in patients with HER2/neu-overexpressing metastatic breast cancer refractory to chemotherapy treatment. J. Clin. Oncol. 1998;16:2659–2671. doi: 10.1200/JCO.1998.16.8.2659. [DOI] [PubMed] [Google Scholar]
- 75.Cobleigh MA, Vogel CL, Tripathy D, Robert NJ, Scholl S, Fehrenbacher L, Wolter JM, Paton V, Shak S, Lieberman G, Slamon DJ. Multinational study of the efficacy and safety of humanized anti-HER2 monoclonal antibody in women who have HER2-overexpressing metastatic breast cancer that has progressed after chemotherapy for metastatic disease. J. Clin. Oncol. 1999;17:2639–2648. doi: 10.1200/JCO.1999.17.9.2639. [DOI] [PubMed] [Google Scholar]
- 76.Slamon DJ, Leyland-Jones B, Shak S, Fuchs H, Paton V, Bajamonde A, Fleming T, Eiermann W, Wolter J, Pegram M. Use of chemotherapy plus a monoclonal antibody against HER2 for metastatic breast cancer that overexpresses HER2. N Eng J Med. 2001;344:783–792. doi: 10.1056/NEJM200103153441101. [DOI] [PubMed] [Google Scholar]
- 77.Lu Y, Zi X, Zhao Y, Mascarenhas D, Pollak M. Insulin-like growth factor-I receptor signaling and resistance to trastuzumab (Herceptin) J Natl Cancer Inst. 2001;93:1852–1857. doi: 10.1093/jnci/93.24.1852. [DOI] [PubMed] [Google Scholar]
- 78.Cardoso F, Piccart MJ, Durbecq V, Di Leo A. Resistance to trastuzumab: a necessary evil or a temporary challenge? Clin Breast Cancer. 2002;3:247–257. doi: 10.3816/CBC.2002.n.028. [DOI] [PubMed] [Google Scholar]
- 79.Berns K, Horlings HM, Hennessy BT, Madiredjo M, Hijmans EM, Beelen K, Linn SC, Gonzalez-Angulo AM, Stemke-Hale K, Hauptmann M. A functional genetic approach identifies the PI3K pathway as a major determinant of trastuzumab resistance in breast cancer. Cancer Cell. 2007;12:395–402. doi: 10.1016/j.ccr.2007.08.030. [DOI] [PubMed] [Google Scholar]
- 80.André F, O’Regan R, Ozguroglu M, Toi M, Xu B, Jerusalem G, Masuda N, Wilks S, Arena F, Isaacs C, Yap YS, Papai Z, Lang I, Armstrong A, Lerzo G, White M, Shen K, Litton J, Chen D, Zhang Y, Ali S, Taran T, Gianni L. Everolimus for women with trastuzumab-resistant, HER2-positive, advanced breast cancer (BOLERO-3): a randomised, double-blind, placebo-controlled phase 3 trial. Lancet Oncol. 2014;15:580–591. doi: 10.1016/S1470-2045(14)70138-X. [DOI] [PubMed] [Google Scholar]
- 81.Hurvitz SA, Andre F, Jiang Z, Shao Z, Mano MS, Neciosup SP, Tseng LM, Zhang Q, Shen K, Liu D, Dreosti LM, Burris HA, Toi M, Buyse ME, Cabaribere D, Lindsay MA, Rao S, Pacaud LB, Taran T, Slamon D. Combination of everolimus with trastuzumab plus paclitaxel as first-line treatment for patients with HER2-positive advanced breast cancer (BOLERO-1): a phase 3, randomised, double-blind, multicentre trial. Lancet Oncol. 2015;16:816–829. doi: 10.1016/S1470-2045(15)00051-0. [DOI] [PubMed] [Google Scholar]
- 82.Baselga J, Cortés J, Kim SB, Im SA, Hegg R, Im YH, Roman L, Pedrini JL, Pienkowski T, Knott A, Clark E, Benyunes MC, Ross G, Swain SM. Pertuzumab plus trastuzumab plus docetaxel for metastatic breast cancer. N Engl J Med. 2011;366:109–119. doi: 10.1056/NEJMoa1113216. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Diéras V, Miles D, Verma S, Pegram M, Welslau M, Baselga J, Krop IE, Blackwell K, Hoersch S, Xu J, Green M, Gianni L. Trastuzumab emtansine versus capecitabine plus lapatinib in patients with previously treated HER2-positive advanced breast cancer (EMILIA): a descriptive analysis of final overall survival results from a randomised, open-label, phase 3 trial. Lancet Oncol. 2017;18:732–742. doi: 10.1016/S1470-2045(17)30312-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Perez EA, Barrios C, Eiermann W, Toi M, Im YH, Conte P, Martin M, Pienkowski T, Pivot X, Burris H 3rd, Petersen JA, Stanzel S, Strasak A, Patre M, Ellis P. Trastuzumab emtansine with or without pertuzumab versus trastuzumab plus taxane for human epidermal growth factor receptor 2-positive, advanced breast cancer: primary results from the phase III MARIANNE study. J. Clin. Oncol. 2017;35:141–148. doi: 10.1200/JCO.2016.67.4887. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Saura C, Oliveira M, Feng YH, Dai MS, Hurvitz SA, Kim SB, Moy B, Delaloge S, Gradishar WJ, Masuda N, Palacova M, Trudeau ME, Mattson J, Yap YS, Bryce R, Yao B, Bebchuk JD, Keyvanjah K, Brufsky A, Investigators N. Neratinib + capecitabine versus lapatinib + capecitabine in patients with HER2+ metastatic breast cancer previously treated with ≥ 2 HER2-directed regimens: findings from the multinational, randomized, phase III NALA trial. J. Clin. Oncol. 2019;37:1002–1002. [Google Scholar]
- 86.Abraham J, Montero AJ, Jankowitz RC, Salkeni MA, Beumer JH, Kiesel BF, Piette F, Adamson LM, Nagy RJ, Lanman RB, Sperinde J, Huang W, Allegra CJ, Srinivasan A, Wang Y, Pogue-Geile KL, Lucas PC, Jacobs SA. Safety and efficacy of T-DM1 plus neratinib in patients with metastatic HER2-positive breast cancer: NSABP foundation trial FB-10. J. Clin. Oncol. 2019;37:2601–2609. doi: 10.1200/JCO.19.00858. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Tamura K, Tsurutani J, Takahashi S, Iwata H, Krop IE, Redfern C, Sagara Y, Doi T, Park H, Murthy RK, Redman RA, Jikoh T, Lee C, Sugihara M, Shahidi J, Yver A, Modi S. Trastuzumab deruxtecan (DS-8201a) in patients with advanced HER2-positive breast cancer previously treated with trastuzumab emtansine: a dose-expansion, phase 1 study. Lancet Oncol. 2019;20:816–826. doi: 10.1016/S1470-2045(19)30097-X. [DOI] [PubMed] [Google Scholar]
- 88.Modi S, Tsurutani J, Tamura K, Park H, Sagara Y, Murthy R, Iwata H, Krop I, Doi T, Redfern C, Moreno-Aspitia A, Redman R, Lee C, Sugihara M, Fujisaki Y, Takahashi S. Trastuzumab deruxtecan (DS-8201a) in subjects with HER2-low expressing breast cancer: updated results of a large phase 1 study. Cancer Res. 2019;79 P6-17-02-P6-17-02. [Google Scholar]
- 89.Hamilton E, Borges V, Conlin A, Walker L, Moulder S. Abstract P4-21-01: efficacy results of a phase 1b study of ONT-380, an oral HER2-specific inhibitor, in combination with capecitabine (C) and trastuzumab (T) in HER2+ metastatic breast cancer (MBC), including patients (pts) with brain metastases (mets) Cancer Res. 2017;77 P4-21-01-P4-21-01. [Google Scholar]
- 90.Murthy RK, Loi S, Okines A, Paplomata E, Hamilton E, Hurvitz SA, Lin NU, Borges V, Abramson V, Anders C, Bedard PL, Oliveira M, Jakobsen E, Bachelot T, Shachar SS, Müller V, Braga S, Duhoux FP, Greil R, Cameron D, Carey LA, Curigliano G, Gelmon K, Hortobagyi G, Krop I, Loibl S, Pegram M, Slamon D, Palanca-Wessels MC, Walker L, Feng W, Winer EP. Tucatinib, trastuzumab, and capecitabine for her2-positive metastatic breast cancer. N Engl J Med. 2020;382:597–609. doi: 10.1056/NEJMoa1914609. [DOI] [PubMed] [Google Scholar]
- 91.Borges VF, Ferrario C, Aucoin N, Falkson C, Khan Q, Krop I, Welch S, Conlin A, Chaves J, Bedard PL, Chamberlain M, Gray T, Vo A, Hamilton E. Tucatinib combined with ado-trastuzumab emtansine in advanced ERBB2/HER2-positive metastatic breast cancer. JAMA Oncol. 2018;4:1214. doi: 10.1001/jamaoncol.2018.1812. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.Clayton AJ, Danson S, Jolly S, Ryder WD, Burt PA, Stewart AL, Wilkinson PM, Welch RS, Magee B, Wilson G, Howell A, Wardley AM. Incidence of cerebral metastases in patients treated with trastuzumab for metastatic breast cancer. Br J Cancer. 2004;91:639–643. doi: 10.1038/sj.bjc.6601970. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.Laakmann E, Müller V, Schmidt M, Witzel I. Systemic treatment options for HER2-positive breast cancer patients with brain metastases beyond trastuzumab: a literature review. Breast Care. 2017;12:168–171. doi: 10.1159/000467387. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94.Xu B, Ma F, Ouyang Q, Li W, Jiang Z, Tong Z, Liu Y, Li H, Yu S, Feng J, Wang S, Hu X, Zhu X, Zou J. Abstract PD3-08: a randomized phase II trial of pyrotinib plus capecitabine versus lapatinib plus capecitabine in patients with HER2-positive metastatic breast cancer previously treated with taxanes, anthracyclines and/or trastuzumab. Cancer Res. 2018;78 PD3-08-PD03-08. [Google Scholar]
- 95.Rugo HS, Im SA, Wright GLS, Escriva-de-Romani S, DeLaurentiis M, Cortes J, Bahadur SW, Haley BB, Oyola RH, Riseberg DA, Musolino A, Cardoso F, Curigliano G, Kaufman PA, Pegram MD, Edlich S, Hong S, Rock EP, Gradishar WJ. SOPHIA primary analysis: a phase 3 (P3) study of margetuximab (M) + chemotherapy (C) versus trastuzumab (T) + C in patients (pts) with HER2+ metastatic (met) breast cancer (MBC) after prior anti-HER2 therapies (Tx) J. Clin. Oncol. 2019;37:1000. [Google Scholar]
- 96.Emens L, Esteva F, Beresford M, Saura C, De Laurentiis M, Kim S, Im S, Patre M, Wang Y, Mani A. Abstract PD3-01: results from KATE2, a randomized phase 2 study of atezolizumab (atezo)+ trastuzumab emtansine (T-DM1) vs. placebo (pbo)+ T-DM1 in previously treated HER2+ advanced breast cancer (BC) Cancer Res. 2019:79. [Google Scholar]
- 97.Loi S, Giobbe-Hurder A, Gombos A, Bachelot T, Hui R, Curigliano G, Campone M, Biganzoli L, Bonnefoi H, Jerusalem G, Bartsch R, Rabaglio-Poretti M, Kammler R, Maibach R, Smyth M, Di Leo A, Colleoni M, Viale G, Regan M, Andre F. Abstract GS2-06: phase Ib/II study evaluating safety and efficacy of pembrolizumab and trastuzumab in patients with trastuzumab-resistant HER2-positive metastatic breast cancer: results from the PANACEA (IBCSG 45-13/BIG 4-13/KEYNOTE-014) study. Cancer Res. 2018;78 GS2-06-GS02-06. [Google Scholar]
- 98.Loi S, Giobbie-Hurder A, Gombos A, Bachelot T, Hui R, Curigliano G, Campone M, Biganzoli L, Bonnefoi H, Jerusalem G, Bartsch R, Rabaglio-Poretti M, Kammler R, Maibach R, Smyth MJ, Di Leo A, Colleoni M, Viale G, Regan MM, André F, Fumagalli D, Gelber RD, Goulioti T, Hiltbrunner A, Hui R, Roschitzki H, Ruepp B, Boyle F, Stahel R, Aebi S, Coates AS, Goldhirsch A, Karlsson P, Kössler I, Fournarakou S, Gasca A, Pfister R, Ribeli-Hofmann S, Weber M, Celotto D, Comune C, Frapolli M, Sánchez-Hohl M, Huang H, Mahoney C, Price K, Scott K, Shaw H, Fischer S, Greco M, King C, Andrighetto S, Piccart-Gebhart M, Findlay H, Jenkins M, Karantza V, Mejia J, Schneier P. Pembrolizumab plus trastuzumab in trastuzumab-resistant, advanced, HER2-positive breast cancer (PANACEA): a single-arm, multicentre, phase 1b-2 trial. Lancet Oncol. 2019;20:371–382. doi: 10.1016/S1470-2045(18)30812-X. [DOI] [PubMed] [Google Scholar]
- 99.Scaltriti M, Carmona FJ, Toska E, Cocco E, Hyman D, Cutler R, Avogadri-Connors F, Wick MJ, Papadopoulos KP, Lalani AS, Baselga J. Neratinib induces estrogen receptor function and sensitizes HER2-mutant breast cancer to anti-endocrine therapy. Eur J Cancer. 2016;69:S125. [Google Scholar]
- 100.Lee AV, Cui X, Oesterreich S. Cross-talk among estrogen receptor, epidermal growth factor, and insulin-like growth factor signaling in breast cancer. Clin Cancer Res. 2001;7:4429s–4435s. discussion 4411s-4412s. [PubMed] [Google Scholar]
- 101.Arpino G, Wiechmann L, Osborne CK, Schiff R. Crosstalk between the estrogen receptor and the HER tyrosine kinase receptor family: molecular mechanism and clinical implications for endocrine therapy resistance. Endocr Rev. 2008;29:217–233. doi: 10.1210/er.2006-0045. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 102.Xia W, Bacus S, Hegde P, Husain I, Strum J, Liu L, Paulazzo G, Lyass L, Trusk P, Hill J, Harris J, Spector NL. A model of acquired autoresistance to a potent ErbB2 tyrosine kinase inhibitor and a therapeutic strategy to prevent its onset in breast cancer. Proc Natl Acad Sci U S A. 2006;103:7795–7800. doi: 10.1073/pnas.0602468103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 103.Nayar U, Cohen O, Kapstad C, Cuoco MS, Waks AG, Wander SA, Painter C, Freeman S, Persky NS, Marini L, Helvie K, Oliver N, Rozenblatt-Rosen O, Ma CX, Regev A, Winer EP, Lin NU, Wagle N. Acquired HER2 mutations in ER+ metastatic breast cancer confer resistance to estrogen receptor-directed therapies. Nat Genet. 2019;51:207–216. doi: 10.1038/s41588-018-0287-5. [DOI] [PubMed] [Google Scholar]
- 104.Genuino AJ, Chaikledkaew U, The DO, Reungwetwattana T, Thakkinstian A. Adjuvant trastuzumab regimen for HER2-positive early-stage breast cancer: a systematic review and meta-analysis. Expert Rev Clin Pharmacol. 2019;12:815–824. doi: 10.1080/17512433.2019.1637252. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 105.Villagrasa P, Prat A, Oliveira M, de la Peña L, Pernas S, Gonzalez X, Cortés J, Simon SP, Jose R, Canes J, Ciruelos E. SOLTI-1303 PATRICIA: a phase II study of palbociclib and trastuzumab (HR+ with or without letrozole) in trastuzumab-pretreated, postmenopausal patients with HER2-positive metastatic breast cancer. JCO. 2018:36. [Google Scholar]
- 106.Johnston SRD, Hegg R, Im SA, Park IH, Burdaeva O, Kurteva G, Press MF, Tjulandin S, Iwata H, Simon SD, Kenny S, Sarp S, Izquierdo MA, Williams LS, Gradishar WJ. Phase III, randomized study of dual human epidermal growth factor receptor 2 (HER2) blockade with lapatinib plus trastuzumab in combination with an aromatase inhibitor in postmenopausal women with HER2-positive, hormone receptor-positive metastatic breast. J. Clin. Oncol. 2018;36:741–748. doi: 10.1200/JCO.2017.74.7824. [DOI] [PMC free article] [PubMed] [Google Scholar] [Retracted]
- 107.Rimawi M, Ferrero JM, de la Haba-Rodriguez J, Poole C, De Placido S, Osborne CK, Hegg R, Easton V, Wohlfarth C, Arpino G PERTAIN Study Group. First-line trastuzumab plus an aromatase inhibitor, with or without pertuzumab, in human epidermal growth factor receptor 2-positive and hormone receptor-positive metastatic or locally advanced breast cancer (PERTAIN): a randomized, open-label phase II trial. J. Clin. Oncol. 2018;36:2826–2835. doi: 10.1200/JCO.2017.76.7863. [DOI] [PubMed] [Google Scholar]
- 108.Cascetta KP, Poulikakos P, Shapiro CL, Bhardwaj AS, Fasano J, Irie H, Klein P, Goel A, Kalinsky K, Adams S, Andreopoulou E, Jaffer S, Ru M, Tiersten A. A multicenter, phase I/II trial of anastrozole, palbociclib, trastuzumab and pertuzumab in HR-positive, Her2-positive metastatic breast cancer. J. Clin. Oncol. 2018;36:TPS1103–TPS1103. [Google Scholar]


