Abstract
Pancreatic cancer (PC) is recognized as the most aggressive and deadliest malignancy because it has the highest mortality of all cancers in humans. Mutations in multiple tumor suppressors and oncogenes have been documented to be involved in pancreatic cancer progression and metastasis. The upregulation of tetraspanin 1 (TSPAN1), a transmembrane protein, has been reportedly observed in many human cancers. However, the role of TSPAN1 and its underlying molecular mechanisms in PC progression have not been fully elucidated. In this study, we validated the oncogenic role of TSPAN1 in PC, showing that TSPAN1 reinforces cell proliferation, migration, invasion and tumorigenesis. To investigate the upregulation of TSPAN1 in PC, we showed that miR-216a is the upstream negative regulator of TSPAN1 via direct binding to the TSPAN1 3’-untranslated region. Through RNA-Seq analysis, we for the first time revealed that TSPAN1 expression transcriptionally regulates ITGA2, which is involved in the actin cytoskeleton pathway. The stimulated cell proliferation and invasion initiated by TSPAN1 overexpression could be abolished by knockdown of ITGA2 in PC cells. Furthermore, TSPAN1 epigenetically regulates the expression of ITGA2 by modulating the levels of TET2 DNMT3B and DNMT1, resulting in hypomethylation of the CpG island of the ITGA2 promoter. In conclusion, the newly identified miR-216a/TSPAN1/ITGA2 axis is involved in the modulation of PC progression and represents a novel therapeutic strategy for future pancreatic cancer treatment.
Keywords: Pancreatic cancer, TSPAN1, miR-216a, ITGA2, DNA methylation
Introduction
Pancreatic cancer (PC) is a particularly aggressive type of cancer worldwide [1,2]. Despite recent advancements in the early detection and treatment of PC, its prognosis remains very poor due to multidrug resistance [3,4]. Therefore, exploring the molecular mechanism and developing new diagnostic and therapeutic strategies are prerequisites for the effective treatment of PC.
Tetraspanin, also known as the transmembrane 4 superfamily, is a family of small transmembrane proteins that structurally consist of four transmembrane domains [5]. Tetraspanin 1 (a member of the tetraspanin family) aggregates with other tetraspanins and various partner proteins to generate TSPAN-enriched microdomains (TEMs). TEMs are involved in a variety of biological activities such as cell adhesion, proliferation, differentiation, migration [6-9], and tumor progression [10,11]. The upregulation of tetraspanin 1 (TSPAN1) and its capacity to regulate tumor progression have been reported in many human cancers, including gastric, colon, prostate cancer and cholangiocarcinoma [12-15]. Currently, the upregulation of TSPAN1 in PC has also been reported [16-18]. However, the function of TSPAN1 in PC and its underlying molecular mechanisms have not yet been fully elucidated.
MicroRNAs (miRNAs) are a class of small noncoding RNA (~22 nucleotides) sequences that repress gene expression at the post-transcriptional level, mainly by binding to the 3’-untranslated region (3’-UTR) of target mRNAs [19]. Aberrant expression of miRNAs has been reported to be involved in cancer progression through multiple pathways in almost all types of cancers [20,21]. To date, several miRNAs have been shown to be implicated in pancreatic cancer development and progression, such as proliferation, apoptosis, metastasis and chemoresistance [22-24]. Restoring tumor-repressing miRNA expression in pancreatic cancer cells is a potential therapeutic strategy. In this study, we successfully investigated the upstream regulatory miRNAs of TSPAN1 in pancreatic cancer.
The integrin family includes 24 transmembrane heterodimers composed of an alpha (α) and beta (β) subunit [25]. Integrins are cell adhesion receptors that actively bind to different extracellular matrix (ECM) molecules and regulate various cellular pathological processes, including cell growth, differentiation, apoptosis and cell migration [26-28]. Aberrant expression of integrins is associated with tumorigenesis by disturbing the migration rate of cells [29-31]. ITGA2 (integrin alpha 2) functions as a transmembrane receptor to mediate cell adhesion to the ECM. The aberrant expression of ITGA2 and its ability to regulate invasion and migration have been confirmed in different type of cancers [32-34]; thus, it could be a candidate for cancer therapy [35,36]. However, the underlying mechanism of aberrant ITGA2 expression in PC is still unclear.
Aberrant DNA methylation is frequently observed in cancers [37,38]. Epigenetic regulation contributes to the development and progression of tumors by mediating the expression of either oncogenes or tumor suppressor genes [39,40]. A genomic-wide analysis of DNA-methylation in pancreatic carcinoma tissues identified differentially methylated candidate genes. Among these genes, ITGA2 hypomethylation was detected together with a high transcription level in PDAC, which was correlated with a poor survival rate [41]. A similar consequence was demonstrated in prostate cancer, where ITGA2 was epigenetically modified and resulted in an enhanced migration capacity [42]. However, the cause of aberrant methylation of the ITGA2 promoter in cancer is still inconclusive. In this study, we proposed a relationship between elevated TSPAN1 levels and hypomethylation of the ITGA2 promoter in pancreatic cancer.
In this research, we demonstrated that TSPAN1 functions as an oncogene and is subsequently regulated by miR-216a in PC. Moreover, the carcinogenic function of TSPAN1 is associated with downstream regulation of the ITGA2 gene. Furthermore, TSPAN1 was also shown to regulate ITGA2 expression via an epigenetic process. However, further elaboration is required to unveil the underlying mechanism. The study findings suggest that the newly identified miR-216a/TSPAN1/ITGA2 axis might be a novel therapeutic target in pancreatic cancer.
Material and methods
Cell culture
The human pancreatic cancer cell line (PANC-1) and Human pancreatic ductal epithelial cell line (HPDE6-C7) were purchased from the Cell Bank of the Chinese Academy of Sciences. PANC-1 and HPDE6-C7 cells were maintained in DMEM high glucose medium (Biological Industries: 01-052-1A) supplemented with 10% FBS (Biological Industries: 04-007-1A), 100 units/mL penicillin and 100 μg/mL streptomycin (Hyclone, Logan, UT, USA). Cells were cultured at 37°C in a humidified atmosphere at 5% CO2.
Generation of stable cell lines
To generate the TSPAN1-OV, TSPAN1-KD and TSPAN1-OV coupled with ITGA2-KD stable cell lines, we subcloned the CDS of TSPAN1 into the pCDH-EF1α-MCS-T2A-puro lentivirus vector (System Biosciences: CD527A-1) and cloned the shRNAs of TSPAN1 and ITGA2 into the pLVX-shRNA2-BSD lentivirus vector, which was modified by pLVX-shRNA2 (Clonetech: 632179). Each of the purified plasmids was packaged into a lentivirus and then transfected into PANC1 cells. After 36 hours of transfection, 2 μg/mL puromycin or 5 μg/mL blasticidin S was added to the medium to screen the positive overexpressed (OV) and knockdown (KD) cells for 10 days.
Quantitative real time-PCR (qPCR)
Cells were harvested, and total RNA was extracted with TRIzol (Invitrogen, Carlsbad, CA, USA) according to the manufacturer’s instructions. Reverse transcription was performed with the HiScript III 1st Strand cDNA Synthesis Kit (Vazyme, Nanjing, China), and qPCR was performed using the ChamQ SYBR Color qPCR Master Mix (Vazyme, Nanjing, China) on the ABI Prism 7900 system (Applied Biosystems, Foster City, CA, USA). GAPDH was used as an internal control. The primer sequences were as follows: Human TSPAN1, Forward: GTGGTCTTTGCTCTTGGTTTCC; Reverse: TTTCTTGATGGCAGGCACTA; Human ITGA2, Forward: TGTGGTGAGGACGGACTTTG; Reverse: CAGGGTAGCCTACATCGCAG; Human β-Actin, Forward: TGACGTGGACATCCGCAAAG; Reverse: CTGGAAGGTGGACAGCGAGG.
Cell proliferation assay
The proliferative potential of the cells was analyzed by seeding and culturing 4×103 cells per well in 96-well plates, and then detecting the signal every 24 hours. The signal was produced with the MTT Cell Proliferation and Cytotoxicity Assay Kit (Beyotime, Shanghai, China) according to the manufacturer’s instructions.
Western blotting
Proteins were extracted using cell lysis buffer (Beyotime, Shanghai, China) and a protease and phosphatase inhibitor cocktail (Beyotime, Shanghai, China). The protein was separated on 4-20% SDS-PAGE gels, and then subjected to immunoblotting with the appropriate primary antibody as follows: anti-TSPAN1 (ab96070, Abcam), anti-ITGA2 (ab133557, Abcam), or anti-β-Actin (ab8226, Abcam), which was used as a loading control. The luminescence was detected, and images were taken using the BeyoECL Moon Super Sensitivity Detection Kit (Beyotime, Shanghai, China).
Colony formation assay
Cells were seeded into 6-well plates (300 cells/well) and grown in humidified air containing 5% CO2 at 37°C for 10-14 days. The cells were washed with PBS, fixed with 4% paraformaldehyde and stained with crystal violet. After staining, photographs were taken and the numbers of colonies were counted.
Luciferase reporter assay
The 3’-UTR sequences of TSPAN1 containing the predicted complementary sites of miR-216a were cloned into the pmirGLO vector (Promega, Madison, WI) downstream of the luciferase2 gene. Cells were co-transfected with 50 nM of either the miR-216a mimic or scramble miR (NC) and 100 ng of the pmir-TSPAN1-3’UTR vector. Firefly luciferase activity and the renilla signal were measured 48 hours after transfection using the Dual-Luciferase Reporter Assay Kit (Promega, Madison, USA) according to the manufacturer’s instructions.
Promoter luciferase reporter assay
A total of 2117 bp of the promoter sequence, ranging from -2000 bases to +116 bases of the ITGA2 gene, were copied to the modified pGL4.11[luc2P] (Promega, Madison, WI) vector. The SV40 promoter, coupled with the Renilla luciferase gene coding sequence, was added to the pGL4.11[luc2P] vector. Cells were co-transfected with the pcDNA3.1-TSPAN1 plasmid or pcDNA3.1 empty vector and 50 ng of the pGL-ITGA2-promoter vector. Firefly luciferase activity and the renilla signal were measured 48 hours after transfection using the Dual-Luciferase Reporter Assay Kit (Promega, Madison, USA) according to the manufacturer’s instructions.
Wound healing assay
A total of 2×105 cells/well were seeded and cultured in 12-well plates to create a confluent monolayer. After the cells attached overnight, we scraped a straight line. After 48 h, microscopic images of the “scratch closure” were photographed under a microscope at an original magnification of 100×.
Transwell invasion assay
Cell invasion assays were performed in Transwell chambers coated with Matrigel (8 µm Transwell inserts, BD Biosciences). A total of 1×104 cells in serum-free DMEM medium were seeded in the upper chamber, and DMEM with 10% FBS was added to the bottom chamber as an attractant. After 48 hours of incubation, the penetrated cells on the filters were fixed in 20% methanol and stained with crystal violet. Stained cells were visualized under a light microscope (Leica, USA). For each insert, at least three selected fields were examined and counted.
In vivo tumorigenicity assays
Female nude mice (6-8 weeks old) were purchased from Weitonglihua Company (Beijing, China) and raised under specific pathogen-free conditions. All animal experiments in this study were approved by the Ethics Committee of the Academy of Military Medical Sciences. TSPAN1-OV or shTSPAN1 and the corresponding control cells (2×106) were subcutaneously injected into the right and left flanks of mice. Approximately 6 weeks after injection, mice were anesthetized with 1% pentobarbital sodium (75 mg/kg) and euthanized. The tumors were weighed, as previously reported (23). The tumor burden did not exceed the recommended dimensions.
Bisulphite sequencing
One CpG island of ITGA2, ranging from -309 bases to -216 bases, was selected for the bisulfite sequencing assay. The genomic DNA was purified from the TSPAN1-OV, OVCtrl, shCtrl, and shTSPAN1 cell lines with the EasyPure® Genomic DNA Kit (Transgene, Beijing, China). After bisulfite conversion using the EpiArt DNA Methylation Bisulfite Kit (Vazyme, Nanjing, China), bisulfite sequencing primers were used to amplify the selected CpG location sequence. The PCR product was cloned to the T vector and spread onto the LB-Agar plate with 100 μg/mL Ampicillin. Approximately 10 clones from each plate were randomly chosen for Sanger sequencing.
Statistical analysis
The data are presented as the means ± SD from at least three independent experiments. Quantitative results were analyzed using GraphPad Prism 5.0 software (GraphPad Software, Inc., La Jolla, CA, USA). A two-tailed Student’s paired t-test was used to test for significance between two groups. Multiple groups were compared by a one-way or two-way analysis of variance with Tukey’s post-test correction. Statistical significance was regarded as *P<0.05 or **P<0.01, ***P<0.001.
Results
TSPAN1 is preferentially upregulated in human pancreatic cancer cells
According to the TCGA database, the TSPAN1 expression level was higher in pancreatic adenocarcinoma (PAAD) tissues (n=179) compared to adjacent nontumor tissues (n=171) (Figure 1A). In addition, high expression of TSPAN1 was associated with a low survival rate (n=89) (Figure 1B). We verified the expression level of TSPAN1 in three pancreatic cancer cell lines (PANC-1, BxPC3, and ASPC1) and in one pancreatic ductal epithelial cell line (HPDE6-C7) by quantitative PCR. QPCR detected an increase in TSPAN1 mRNA in PC cells compared to that in HPDE6-C7 cells, indicating the oncogenic role of TSPAN1 in PC (Figure 1C).
Figure 1.
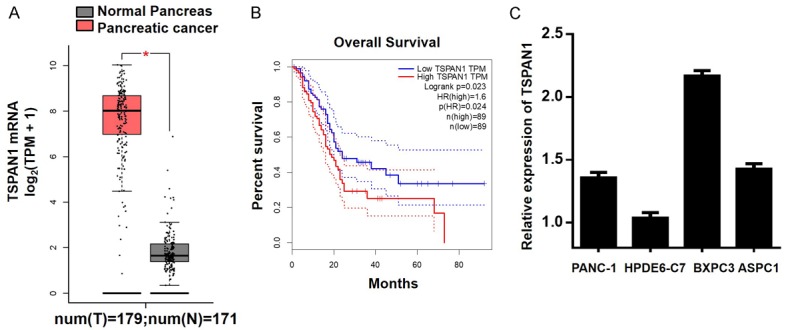
Upregulated expression of TSPAN1 in pancreatic cancer cell lines. A. TSPAN1 expression was higher in pancreatic cancer tissues than in healthy non-neoplastic tissues, according to the TCGA database. B. The expression level of TSPAN1 was positively associated with poor patient survival rates. C. The mRNA level of TSPAN1 was assessed by qPCR. Data are presented as the means ± SD. *P<0.05; **P<0.01.
TSPAN1 functions as an oncogene in PC
We established TSPAN1 overexpression (OV) and knockdown (KD) stable transfected cell lines in PANC-1 cells (PANC-1-TSPAN1-OV, PANC-1-shTSPAN1) and confirmed the relative mRNA and protein expression levels of TSPAN1 (Figure 2A). TSPAN1 overexpression significantly stimulated the proliferation and colony-forming capacity, while knockdown had the opposite effect (Figure 2B, 2C). Furthermore, a subcutaneous xenograft mouse model was established to observe the role of TSPAN1 in tumorigenesis in vivo (n=3/group). Both the tumor volumes and weights of the TSPAN1-OV group were significantly increased (P<0.01). Conversely, TSPAN1 knockdown suppressed tumor growth compared to its control (Figure 2D). Wound healing and Transwell assays demonstrated that the upregulation of TSPAN1 significantly enhanced the migration and invasion of PANC-1 cells, and vice versa (Figure 2E, 2F). Collectively, these results demonstrate that TSPAN1 promotes PANC-1 cell proliferation, migration, invasion and tumorigenesis.
Figure 2.
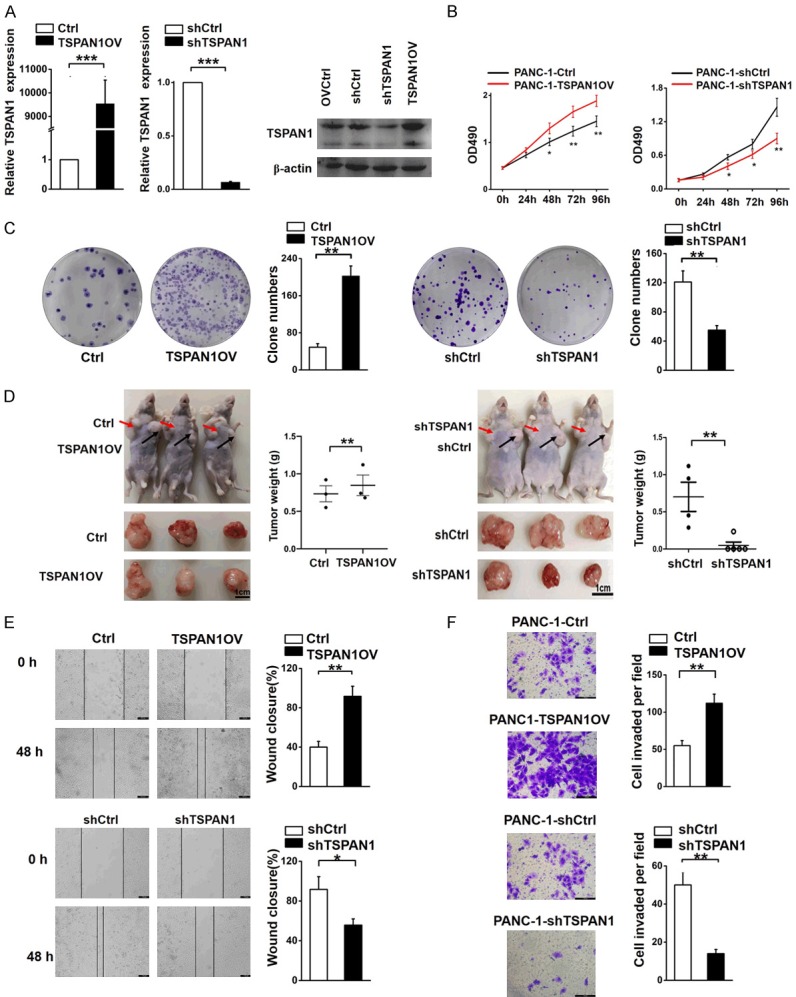
TSPAN1 promotes PC progression. (A) mRNA and protein levels of TSPAN1 in TSPAN1-OV, shTSPAN1, and homologous control stable cells. (B, C) Cell proliferation (B) and clone forming assays (C) were performed in TSPAN1-OV cells and shTSPAN1 cells and the corresponding control cells. (D) A xenograft nude mouse model was constructed to investigate the effects of TSPAN1 in vivo. The tumor size, morphology and tumor weight were monitored. (E) Wound healing and (F) Transwell assays were used to evaluate the migration and invasive potentials of TSPAN1 overexpression and knockdown cells and the corresponding control cells. Data are presented as the means ± SD (n=3). *P<0.05, **P<0.01, ***P<0.001.
TSPAN1 is a downstream target of miR-216a
To investigate the mechanism underlying the upregulation of TSPAN1 in PC, the miRNAs that potentially target TSPAN1 were identified. Both the TargetScan and miRanda databases predicted several possible miRNAs, but only the miRNAs with high-ranking target sites and low transcription in PC tissues were selected for further analysis. Three miRNAs, miR-133a-3p, miR-216a-3p, and miR-128-3p, met the requirements [43-45]. qPCR assays confirmed decreased transcription levels of miR-216a, miR-128 and miR-133a in PANC-1 cells compared with HPDE6-C7 (Figure 3A). In this study, miR-216a was confirmed as the upstream regulator of TSPAN1 due to its definite regulatory effect on TSPAN1 (Figure 3D).
Figure 3.
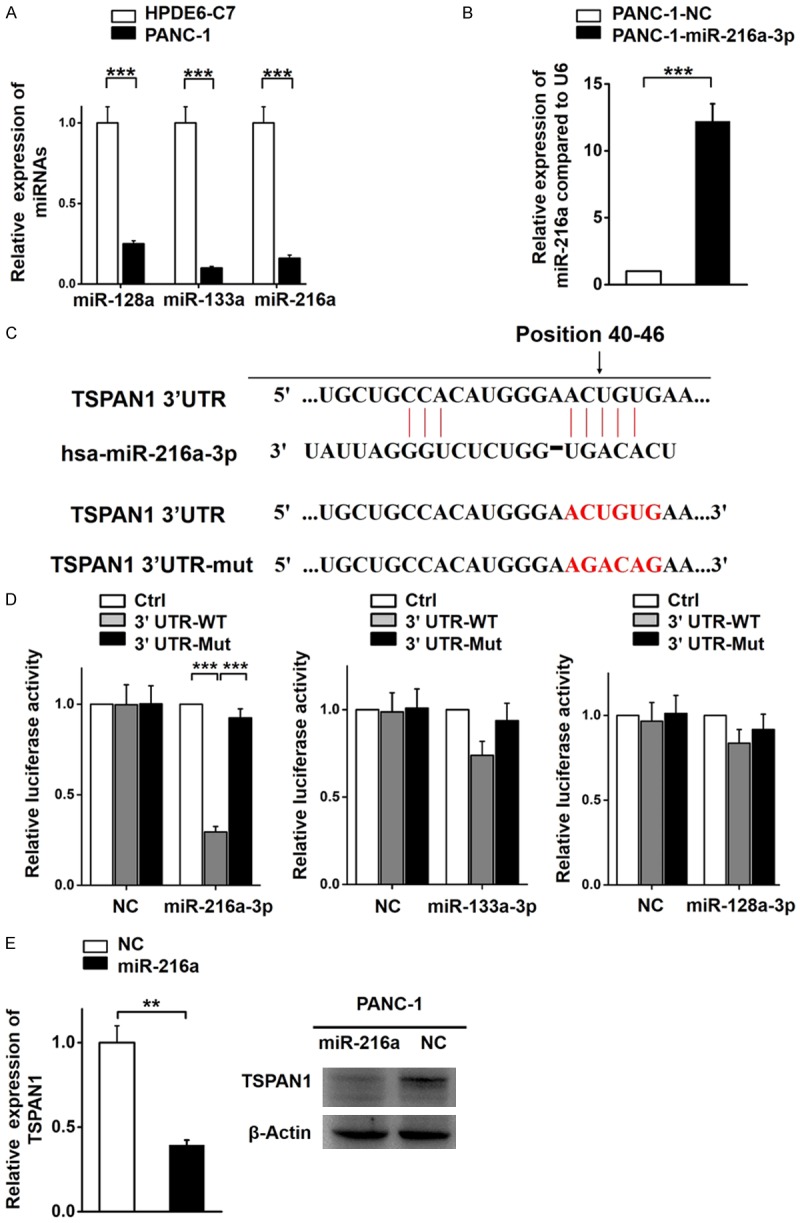
TSPAN1 is a target of miR-216a. (A) The relative expression levels of miR-128, miR-133a, and miR-216a in PANC-1 and HPDE6-C7 cells. (B) The relative miR-216a transcription level in PANC-1 cells stably transfected with the miR-216a overexpression vector or control vector. (C) The predicted binding sites for miR-216a in the 3’UTR of TSPAN1. (D) The relative luciferase activity of reporters containing the TSPAN1 3’UTR (wild-type or mutant) in PANC-1 cells co-transfected with miR-216a, miR-128-3p and miR-133a-3p overexpression and negative control vectors. Data are presented as the relative ratio of firefly luciferase and renilla luciferase activity. (E) The relative expression of TSPAN1 in stably transfected miR-216a overexpression PANC-1 cells and negative control cells, as determined by real-time PCR and western blot analyses. Data are shown as the means ± S.D., n=3. Statistical significance was assessed by Student’s t-test and *P<0.05, **P<0.01 and ***P<0.001 was considered significant.
Subsequently, we successfully established two stable transfected cell lines (PANC-1-miR-216a-3p and PANC-1-NC) (Figure 3B). To further investigate the interaction between miR-216a-3p and the TSPAN1 3’-UTR, we evaluated luciferase activity by inserting the wild-type (WT) and mutated TSPAN1 3’-UTR at the 3’ end of the firefly luciferase gene (Figure 3C). In PANC-1 cells, miR-216a significantly decreased the firefly luciferase activity of the TSPAN1-3’UTR-WT reporter but not that of the mutant edition (P<0.01; Figure 3D). This result indicates that TSPAN1 is a direct target of miR-216a. Moreover, we verified that TSPAN1 was suppressed by miR-216a at both the transcriptional and translational levels (Figure 3E). In summary, based on qPCR, western blotting and luciferase assays data, we proved that TSPAN1 is negatively regulated by miR-216a-3p in PC.
miR-216a inhibits PANC-1 cell proliferation, migration, and invasion
The functional regulatory role of miR-216a on TSPAN1 was investigated through various essential phenotypic experiments. We found that miR-216a-OV cells showed significantly slower proliferation rates (Figure 4A), reduced clone-forming potency (Figure 4B), and attenuated migration (Figure 4C) and invasion abilities (Figure 4D) compared with parallel stable cell lines containing the empty vector. To further verify the association between TSPAN1 and miR-216a, we ectopically overexpressed miR-216a in TSPAN1-OV cells and found that the promoted invasion ability of TSPAN1-OV cells could be partially eliminated with miR-216a transfection (Figure 4E). Therefore, we validated that downregulated miR-216a facilitates PC progression by targeting TSPAN1.
Figure 4.
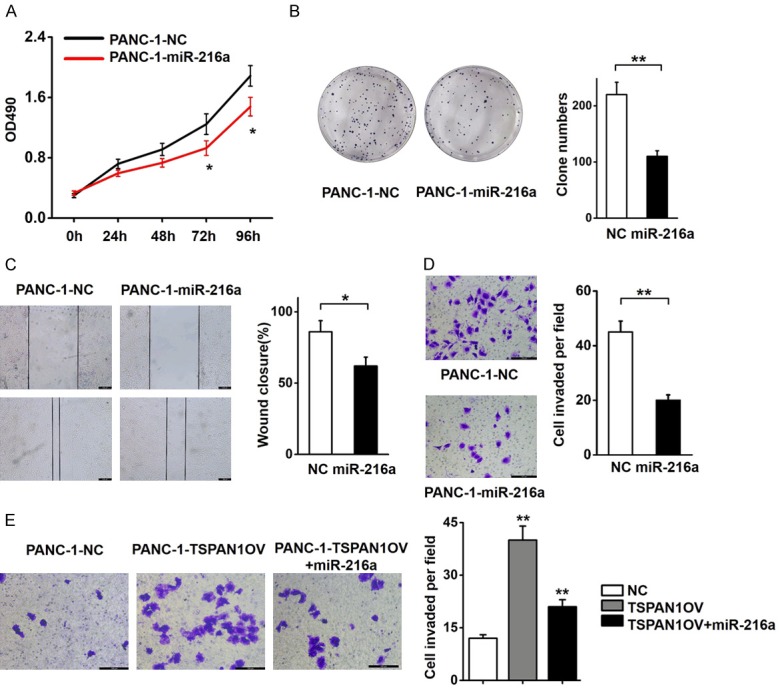
Effects of miR-216a on the proliferation, migration, and invasion of PC cells. (A) The effects of miR-216a on PANC-1 cell proliferation were determined using MTT assays. (B) PANC-1 cells stably transcribing miR-216a showed decreased colony formation rates compared to control cells. (C) Wound healing and (D) Transwell assays in PANC-1 cells transfected with the miR-216a overexpression vector or negative control vector. (E) Increased expression of miR-216a reduced the invasive potency of TSPAN1-OV PANC-1 cells. Data are shown as the means ± S.D., n=3. *P<0.05, **P<0.01 was considered significant.
TSPAN1 upregulates the expression of ITGA2 downstream
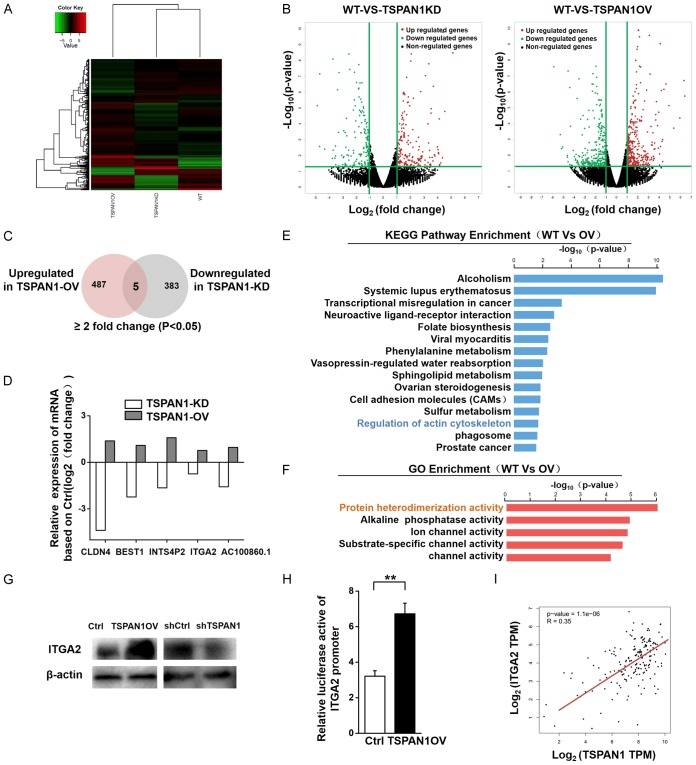
To explore the downstream pathways that may be regulated by TSPAN1, we performed RNA sequencing (RNA-Seq) using TSPAN1-OV and shTSPAN1 and their corresponding control cells (Figure 5A). We identified 518 differentially expressed genes (DEGs) in shTSPAN1 cells and 785 DEGs in TSPAN1-OV cells relative to the control group (fold change ≥2, P<0.05) (Figure 5B). To focus on the genes with potential oncogenic functions in PC, we further screened the DEGs upregulated in TSPAN1-OV cells and downregulated in TSPAN1-KD cells (Figure 5C). Among those DEGs, 5 genes, including CLDN4, BEST1, INTS4P2, ITGA2 and AC100860.1, presented identical trends as TSPAN1 in PANC-1 cells (Figure 5D). ITGA2, a crucial member of the integrin protein family, is particularly interesting since the functionality of TSPAN1 requires the formation of a complex with various integrins. The oncogenic functions of TSPAN1 in PC may partially be associated with its involvement with integrin-related pathways. Subsequently, we analyzed the biological pathways that were potentially altered due to TSPAN1 overexpression by KEGG pathway enrichment analysis. Regulation of the actin cytoskeleton pathway, which ITGA2 is involved in, was among the top 15 most enriched pathways (Figure 5E). The actin cytoskeleton is necessary for shaping cellular morphology and modulating several cellular processes, including cell movement, membrane protrusions, EMT processes, tumorigenesis and metastasis [46-48]. Gene ontology (GO) analysis (molecular functions) of the DEGs in cells overexpressing TSPAN1 showed that the most significant GO term was “protein heterodimerization activity”, in which ITGA2 plays an important role (Figure 5F). Considering all these elements, we selected ITGA2 for further study as the downstream target of TSPAN1 in PC. RNA-Seq (Figure 5D) results showed a remarkable increase in ITGA2 mRNA in TSPAN1-OV cells and decreased ITGA2 mRNA levels in shTSPAN1 cells, which was confirmed by Western blot analysis (Figure 5G).
Figure 5.
TSPAN1 regulates the downstream expression of ITGA2. (A, B) Hierarchical clustering plots (A) and volcano plots (B) were used to identify the DEGs (fold change >2 or 2.0, P<0.05) between WT, TSPAN1 overexpressing and TSPAN1 knockdown PANC-1 cells. (C) Venn diagram showing the overlap of genes downregulated following TSPAN1 KD and upregulated following TSPAN1 OV in PANC-1 cells based on the RNA-seq analysis. (D) The relative expression levels of DEGs based on the RNA-seq results of PANC-1 cells after TSPAN1 overexpression or knockdown compared with the expression in WT cells. (E) KEGG pathway enrichment analysis of DEGs in PANC-1 cells upon TSPAN1 overexpression was performed. The top 15 enriched signaling pathways are shown and are ranked on the basis of log10 (p-value) (F) GO analysis (molecular functions) of the DEGs in TSPAN1 overexpressing PANC-1 cells. (G) Western blot assay verified the protein levels of ITGA2 in TSPAN1 overexpressing, knockdown and control cells. (H) A luciferase reporter assay was performed to determine the effect of TSPAN1 overexpression on the transcriptional activity of ITGA2 in PANC-1 cells. Data are presented as the relative ratio of firefly luciferase activity and renilla luciferase activity. (I) Analysis of the possible correlation between TSPAN1 and ITGA2 mRNA levels in pancreatic cancer tissues based on the TCGA online dataset. Data are shown as the means ± S.D. (n=3). *P<0.05, **P<0.01.
To evaluate the transcriptional regulatory role of TSPAN1 on ITGA2, a luciferase promoter-reporter assay was subsequently performed with the promoter for ITGA2. TSPAN1 overexpression increased the activity of the ITGA2 promoter. Our results are consistent with the correlation analysis based on the online database (TCGA), which presented a positive correlation between TSPAN1 and ITGA2 mRNA levels in PC tissues (Figure 5I). These results indicate that TSPAN1 plays a pivotal role as an upstream regulator of ITGA2 in PC.
TSPAN1 promotes tumorigenesis in PC by regulating ITGA2
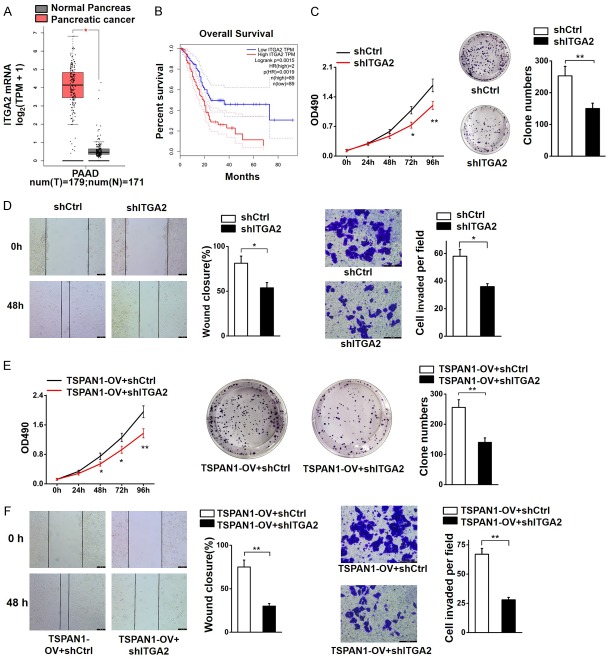
ITGA2 has been shown to be highly expressed in many types of malignancies, including the pancreas. According to the TCGA database, the expression level of ITGA2 was upregulated in human pancreatic cancer specimens (Figure 6A) and associated with a poor survival rate (Figure 6B). To determine the oncogenic function of ITGA2 in PC and further validate its association with TSPAN1, we stably knocked down ITGA2 in PANC-1 cells (PANC-1-shITGA2) and in PANC-1 cells overexpressing TSPAN1 (PANC-1-TSPAN1OV-shITGA2). The shITGA2 cells showed a significantly slower proliferation rate (Figure 6C) and attenuated migration and invasive abilities (Figure 6D) compared with the PANC-1-shCtrl cells. Simultaneously, we found that the tumor-promoting capacity of TSPAN1 could be markedly abolished by ITGA2 knockdown (Figure 6E, 6F). In summary, these results reveal that the oncogenic function of TSPAN1 in pancreatic cancer is closely related to its regulatory effect on ITGA2, which also plays a carcinogenic role in PC.
Figure 6.
ITGA2 knockdown abolished the oncogenic function of TSPAN1 in PC. (A) The expression level of ITGA2 in human pancreatic cancer specimens (n=179) and adjacent tissues (n=171) according to the TCGA database. (B) Association between the expression of ITGA2 and the patient survival rate (n=89). (C) Cell proliferation rate (left) and clone forming capacity (right) of shITGA2 and control cells. (D) Scratch motility assays (left) and Transwell assays (right) were used to evaluate the involvement of ITGA2 in the migration and invasion of PANC-1 cells. (E) ITGA2 knockdown abolished the promotion of cell proliferation (left) and clone formation capacity (right) caused by overexpression of TSPAN1. (F) ITGA2 knockdown abolished the migration (left) and invasion (right) caused by overexpression of TSPAN1. Scale bars=100 μm. Data are shown as the means ± SD., n=3. *P<0.05 and **P<0.01 was considered significant.
TSPAN1 regulates the expression of ITGA2 via an epigenetic mechanism
Many studies have shown the direct correlation between gene expression and epigenetic regulation [40]. According to the RNA-seq data, ITGA2 was increased in TSPAN1-OV cells and reduced in shTSPAN1 cells. Therefore, we hypothesized that TSPAN1 might regulate ITGA2 via an epigenetic mechanism. We performed bisulfite sequencing on the selected CpG island of the ITGA2 promoter (Figure 7D). The average abundance of 5-methylcytosine at this CpG island was statistically analyzed in each cell line (PANC-1, TSPAN1OV and shTSPAN1). A distinct promotion in methylation was observed across the ITGA2 promoter in shTSPAN1 cells compared with that in PANC-1 cells. Meanwhile, a lower level of 5-methylation in the ITGA2 promoter was observed in TSPAN1-OV cells (Figure 7A). These aberrant methylation levels of the ITGA2 promoter were inversely correlated with the expression of ITGA2 in each cell line (Figure 7B). In conclusion, upregulated TSPAN1 in PC could promote ITGA2 expression by regulating the methylation status of its promoter. Our results are consistent with data from the TCGA database indicating that the ITGA2 promoter was hypomethylated in pancreatic tumors compared with non-neoplastic tissues (Figure 7C).
Figure 7.
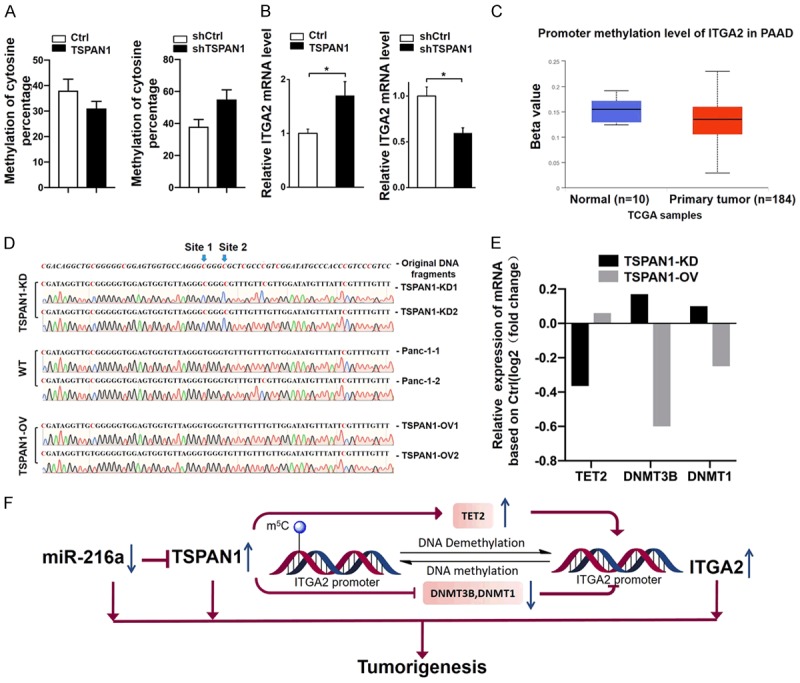
TSPAN1 regulates ITGA2 expression epigenetically. (A) Differential methylation of the ITGA2 promoter and (B) differential ITGA2 mRNA levels in TSPAN1-OV, shTSPAN1 and control cells. (C) Comparison of ITGA2 promoter methylation levels in PC (n=184) and non-neoplastic tissues (n=10) based on the TCGA dataset. (D) Methylation status at the selected CpG site of representative sequences of the ITGA2 promoter in different cell lines. The methylation of site 1 and site 2 were markedly regulated by TSPAN1. (E) Relative expression levels of TET2, DNMT3B and DNMT1 in TSPAN1-OV or shTSPAN1 PANC-1 cells compared with that in WT cells. (F) Schematic representation of how downregulated miR-216a in pancreatic cancer results in increased expression of TSPAN1, which can accelerate tumorigenesis by epigenetically upregulating the expression of ITGA2 via mediating the demethylase and methyltransferase levels. Data are shown as the means ± S.D., n=3. *P<0.05 was considered significant.
In addition, based on the bisulfite sequencing results, the selected CpG island of the ITGA2 promoter from each cell line could be categorized into two representative sequences (Figure 7D). As shown in these 6 typical sequences, two major methylation sites were identified to be affected by TSPAN1 (site 1 and site 2). The cytosines on sites 1 and 2 were methylated only in TSPAN1-KD cells, while they were unmethylated or showed a low probability of being methylated in TSPAN1-OV cells and PANC-1 cells. However, the mechanism by which TSPAN1 regulates the methylation level of ITGA2 remains unclear. According to our previous RNA-seq results, overexpression of TSPAN1 resulted in an increase of TET2 and a decrease in the methyltransferases DNMT3B and DNMT1, and the opposite pattern was observed in TSPAN1 knockdown cells (Figure 7E). The combined action of increased TET2 and decreased DNMT3B and DNMT1 might work together to reduce the methylation level of the ITGA2 promoter. Thus, we suggest that TSPAN1 might indirectly regulate the methylation of the ITGA2 promoter by mediating DNA methyltransferases and demethylases.
Discussion
The present study focused on the oncogenic role and underlying mechanisms of TSPAN1 in PC progression. TSPAN1 overexpression significantly promoted cell proliferation, migration, invasion and tumorigenesis, indicating its essential role in tumor growth and its potential as a therapeutic target for treatment. TSPAN1 expression may be driven by an upstream regulator miR-216a, and the effects of TSPAN1 may be involved in transcriptional regulation of the downstream gene ITGA2. This leads us to propose an oncogenic miR-216a/TSPAN1/ITGA2 axis in pancreatic cancer.
The present study identifies several important discoveries about the functions and mechanisms of TSPAN1 in PC. We confirmed the abnormally elevated level of TSPAN1 in PC cells. We verified that the overexpression of TSPAN1 leads to increased proliferation, migration, invasion and tumorigenesis. To verify the mechanism of TSPAN1 upregulation in PC, we screened the downregulated miRNAs in PC cells. We demonstrated complementary binding between miR-216a and the TSPAN1-3’-UTR. miR-216a functions as a tumor suppressor that partially abolishes the functions of TSPAN1. Our data were consistent with several previous studies, which suggested that the expression of miR-216a negatively regulates the proliferation and metastasis of pancreatic carcinoma [43]. Collectively, the regulatory relationship between the miR-216a and TSPAN1 in PC was clarified by this study.
Aberrant expression of integrins is associated with the development of tumorigenesis by distressing the cellular migration rate. ITGA2 encodes the alpha 2 integrin that frequently forms the heterodimer a2b1 integrin, functioning as a transmembrane receptor to mediate cell adhesion to the ECM. Accumulating evidence has shown aberrant expression of ITGA2 and its potency for regulating the invasion and migration in multiple cancers [33,34,49,50]. In PC, ITGA2 has been identified as the hub gene [34]. This is consistent with our findings in the current study that ITGA2 was upregulated in PC cells. In addition, we performed RNA sequencing using TSPAN1-OV, shTSPAN1 and control PC cells. According to the KEGG pathway enrichment analysis, the actin cytoskeleton activity pathway was among the top 15 most enriched pathways. It is well established that the actin cytoskeleton is necessary for shaping cell morphology, cell migration, membrane protrusions, and EMT processes. ITGA2, a critical member of this pathway, could facilitate the migration of cancer cells and promote cancer aggressiveness. In this study, ITGA2 was shown to be transcriptionally regulated by TSPAN1. Therefore, we hypothesize that TSPAN1 reinforces metastasis in PC by regulating the expression of ITGA2. The phenotype results of ITGA2 knockdown in TSPAN1-OV cells seemed to confirm our hypothesis. Finally, we confirmed the oncogenic role of ITGA2 in PC and demonstrated that the oncogenic function of TSPAN1 in PC is closely related to its regulatory effect on ITGA2.
ITGA2 has been reported to be upregulated in PC partly due to aberrant hypomethylation of its promoter, and this phenomenon has also be found in other cancers [42]. However, the underlying mechanism that leads to this hypomethylation of the ITGA2 promoter is still unclear. In this study, we propose a perspective that TSPAN1 can regulate methylation-related enzymes and, in turn, affect the methylation level of the ITGA2 promoter.
The RNA-seq results revealed that overexpression of TSPAN1 resulted in the elevation of TET2 enzymes, but a decrease in the levels of the methyltransferases DNMT3B and DNMT1. Meanwhile, we performed bisulfite sequencing on the selected fragments of the ITGA2 promoter in TSPAN1 overexpression, knockdown, and control cells. We found epigenetic regulation of ITGA2 expression via alterations in the methylation status of ITGA2, which was regulated by TSPAN1. In addition, we identified two predicted significant methylation sites affected by TSPAN1. Our results reveal the mechanism by which TSPAN1 regulates ITGA2 expression, and also verified that the regulation of methyltransferases by TSPAN1 is one of the reasons for the hypomethylation of ITGA2 in PC. However, the other mechanisms responsible for the hypomethylation of ITGA2 in PC still need further elaboration.
Conclusion
In summary, we reported a regulatory network for TSPAN1 in PC. We verified the oncogenic role of TSPAN1 in pancreatic cancer whereby TSPAN1 reinforced cell proliferation, migration, invasion and tumorigenesis both in vitro and in vivo. We for the first time demonstrated that miR-216a is an upstream regulator of TSPAN1. The upregulation of TSPAN1 in PC is the consequence of low expression of miR-216a. In addition, TSPAN1 regulates the downstream transcriptional activity of ITGA2, which could abolish the functions of TSPAN1 in pancreatic cancer. Moreover, we demonstrated that TSPAN1 epigenetically regulates the expression of ITGA2 by affecting methyltransferase and demethylases levels. Collectively, we propose a newly identified oncogenic miR-216a/TSPAN1/ITGA2 axis (Figure 7F), which is involved in the modulation of PC progression and represents a novel therapeutic approach for pancreatic cancer treatment.
Acknowledgements
This study was supported by the National Natural Science Foundation of China (Grant# 81702802) and the programs of the Beijing Municipal Education Commission (Grant# KM201810005032 and KM201910005005).
Disclosure of conflict of interest
None.
References
- 1.Siegel RL, Miller KD, Jemal A. Cancer statistics, 2018. CA Cancer J Clin. 2018;68:7–30. doi: 10.3322/caac.21442. [DOI] [PubMed] [Google Scholar]
- 2.Vincent A, Herman J, Schulick R, Hruban RH, Goggins M. Pancreatic cancer. Lancet. 2011;378:607–620. doi: 10.1016/S0140-6736(10)62307-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Li HY, Cui ZM, Chen J, Guo XZ, Li YY. Pancreatic cancer: diagnosis and treatments. Tumour Biol. 2015;36:1375–1384. doi: 10.1007/s13277-015-3223-7. [DOI] [PubMed] [Google Scholar]
- 4.Stan SD, Singh SV, Brand RE. Chemoprevention strategies for pancreatic cancer. Nat Rev Gastroenterol Hepatol. 2010;7:347–356. doi: 10.1038/nrgastro.2010.61. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Hemler ME. Tetraspanin functions and associated microdomains. Nat Rev Mol Cell Biol. 2005;6:801–811. doi: 10.1038/nrm1736. [DOI] [PubMed] [Google Scholar]
- 6.Bailey RL, Herbert JM, Khan K, Heath VL, Bicknell R, Tomlinson MG. The emerging role of tetraspanin microdomains on endothelial cells. Biochem Soc Trans. 2011;39:1667–1673. doi: 10.1042/BST20110745. [DOI] [PubMed] [Google Scholar]
- 7.Charrin S, le Naour F, Silvie O, Milhiet PE, Boucheix C, Rubinstein E. Lateral organization of membrane proteins: tetraspanins spin their web. Biochem J. 2009;420:133–154. doi: 10.1042/BJ20082422. [DOI] [PubMed] [Google Scholar]
- 8.Jiang X, Zhang J, Huang Y. Tetraspanins in cell migration. Cell Adh Migr. 2015;9:406–415. doi: 10.1080/19336918.2015.1005465. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Zoller M. Tetraspanins: push and pull in suppressing and promoting metastasis. Nat Rev Cancer. 2009;9:40–55. doi: 10.1038/nrc2543. [DOI] [PubMed] [Google Scholar]
- 10.Richardson MM, Jennings LK, Zhang XA. Tetraspanins and tumor progression. Clin Exp Metastasis. 2011;28:261–270. doi: 10.1007/s10585-010-9365-5. [DOI] [PubMed] [Google Scholar]
- 11.Lazo PA. Functional implications of tetraspanin proteins in cancer biology. Cancer Sci. 2007;98:1666–1677. doi: 10.1111/j.1349-7006.2007.00584.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Wang Y, Liang Y, Yang G, Lan Y, Han J, Wang J, Yin D, Song R, Zheng T, Zhang S, Pan S, Liu X, Zhu M, Liu Y, Cui Y, Meng F, Zhang B, Liang S, Guo H, Liu Y, Hassan MK, Liu L. Tetraspanin 1 promotes epithelial-to-mesenchymal transition and metastasis of cholangiocarcinoma via PI3K/AKT signaling. J Exp Clin Cancer Res. 2018;37:300. doi: 10.1186/s13046-018-0969-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Chen L, Zhu YY, Zhang XJ, Wang GL, Li XY, He S, Zhang JB, Zhu JW. TSPAN1 protein expression: a significant prognostic indicator for patients with colorectal adenocarcinoma. World J Gastroenterol. 2009;15:2270–2276. doi: 10.3748/wjg.15.2270. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Lu Z, Luo T, Nie M, Pang T, Zhang X, Shen X, Ma L, Bi J, Wei G, Fang G, Xue X. TSPAN1 functions as an oncogene in gastric cancer and is downregulated by miR-573. FEBS Lett. 2015;589:1988–1994. doi: 10.1016/j.febslet.2015.05.044. [DOI] [PubMed] [Google Scholar]
- 15.Munkley J, McClurg UL, Livermore KE, Ehrmann I, Knight B, McCullagh P, McGrath J, Crundwell M, Harries LW, Leung HY, Mills IG, Robson CN, Rajan P, Elliott DJ. The cancer-associated cell migration protein TSPAN1 is under control of androgens and its upregulation increases prostate cancer cell migration. Sci Rep. 2017;7:5249. doi: 10.1038/s41598-017-05489-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Tian J, Zhang R, Piao H, Li X, Sheng W, Zhou J, Dong M, Zhang X, Yan X, Shang W, Zhao J, Xu L, Liu F, Shi G. Silencing Tspan1 inhibits migration and invasion, and induces the apoptosis of human pancreatic cancer cells. Mol Med Rep. 2018;18:3280–3288. doi: 10.3892/mmr.2018.9331. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Zhang X, Shi G, Gao F, Liu P, Wang H, Tan X. TSPAN1 upregulates MMP2 to promote pancreatic cancer cell migration and invasion via PLCgamma. Oncol Rep. 2019;41:2117–2125. doi: 10.3892/or.2019.6989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Hou FQ, Lei XF, Yao JL, Wang YJ, Zhang W. Tetraspanin 1 is involved in survival, proliferation and carcinogenesis of pancreatic cancer. Oncol Rep. 2015;34:3068–3076. doi: 10.3892/or.2015.4272. [DOI] [PubMed] [Google Scholar]
- 19.Bagga S, Bracht J, Hunter S, Massirer K, Holtz J, Eachus R, Pasquinelli AE. Regulation by let-7 and lin-4 miRNAs results in target mRNA degradation. Cell. 2005;122:553–563. doi: 10.1016/j.cell.2005.07.031. [DOI] [PubMed] [Google Scholar]
- 20.Bitarte N, Bandres E, Boni V, Zarate R, Rodriguez J, Gonzalez-Huarriz M, Lopez I, Javier Sola J, Alonso MM, Fortes P, Garcia-Foncillas J. MicroRNA-451 is involved in the self-renewal, tumorigenicity, and chemoresistance of colorectal cancer stem cells. Stem Cells. 2011;29:1661–1671. doi: 10.1002/stem.741. [DOI] [PubMed] [Google Scholar]
- 21.Zhou JN, Zeng Q, Wang HY, Zhang B, Li ST, Nan X, Cao N, Fu CJ, Yan XL, Jia YL, Wang JX, Zhao AH, Li ZW, Li YH, Xie XY, Zhang XM, Dong Y, Xu YC, He LJ, Yue W, Pei XT. MicroRNA-125b attenuates epithelial-mesenchymal transitions and targets stem-like liver cancer cells through small mothers against decapentaplegic 2 and 4. Hepatology. 2015;62:801–815. doi: 10.1002/hep.27887. [DOI] [PubMed] [Google Scholar]
- 22.Zhu Y, Gu J, Li Y, Peng C, Shi M, Wang X, Wei G, Ge O, Wang D, Zhang B, Wu J, Zhong Y, Shen B, Chen H. MiR-17-5p enhances pancreatic cancer proliferation by altering cell cycle profiles via disruption of RBL2/E2F4-repressing complexes. Cancer Lett. 2018;412:59–68. doi: 10.1016/j.canlet.2017.09.044. [DOI] [PubMed] [Google Scholar]
- 23.Yan H, Li Q, Wu J, Hu W, Jiang J, Shi L, Yang X, Zhu D, Ji M, Wu C. MiR-629 promotes human pancreatic cancer progression by targeting FOXO3. Cell Death Dis. 2017;8:e3154. doi: 10.1038/cddis.2017.525. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.He Z, Guo X, Tian S, Zhu C, Chen S, Yu C, Jiang J, Sun C. MicroRNA-137 reduces stemness features of pancreatic cancer cells by targeting KLF12. J Exp Clin Cancer Res. 2019;38:126. doi: 10.1186/s13046-019-1105-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Takada Y, Ye X, Simon S. The integrins. Genome Biol. 2007;8:215. doi: 10.1186/gb-2007-8-5-215. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Plow EF, Haas TA, Zhang L, Loftus J, Smith JW. Ligand binding to integrins. J Biol Chem. 2000;275:21785–21788. doi: 10.1074/jbc.R000003200. [DOI] [PubMed] [Google Scholar]
- 27.Barczyk M, Carracedo S, Gullberg D. Integrins. Cell Tissue Res. 2010;339:269–280. doi: 10.1007/s00441-009-0834-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Karimi F, O’Connor AJ, Qiao GG, Heath DE. Integrin clustering matters: a review of biomaterials functionalized with multivalent integrin-binding ligands to improve cell adhesion, migration, differentiation, angiogenesis, and biomedical device integration. Adv Healthc Mater. 2018;7:e1701324. doi: 10.1002/adhm.201701324. [DOI] [PubMed] [Google Scholar]
- 29.Sun Z, Schwenzer A, Rupp T, Murdamoothoo D, Vegliante R, Lefebvre O, Klein A, Hussenet T, Orend G. Tenascin-C promotes tumor cell migration and metastasis through integrin alpha9beta1-mediated YAP inhibition. Cancer Res. 2018;78:950–961. doi: 10.1158/0008-5472.CAN-17-1597. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Taherian A, Li X, Liu Y, Haas TA. Differences in integrin expression and signaling within human breast cancer cells. BMC Cancer. 2011;11:293. doi: 10.1186/1471-2407-11-293. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Bai X, Gao C, Zhang L, Yang S. Integrin alpha7 high expression correlates with deteriorative tumor features and worse overall survival, and its knockdown inhibits cell proliferation and invasion but increases apoptosis in breast cancer. J Clin Lab Anal. 2019;33:e22979. doi: 10.1002/jcla.22979. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Ding W, Fan XL, Xu X, Huang JZ, Xu SH, Geng Q, Li R, Chen D, Yan GR. Epigenetic silencing of ITGA2 by MiR-373 promotes cell migration in breast cancer. PLoS One. 2015;10:e0135128. doi: 10.1371/journal.pone.0135128. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Gong J, Lu X, Xu J, Xiong W, Zhang H, Yu X. Coexpression of UCA1 and ITGA2 in pancreatic cancer cells target the expression of miR-107 through focal adhesion pathway. J Cell Physiol. 2019;234:12884–12896. doi: 10.1002/jcp.27953. [DOI] [PubMed] [Google Scholar]
- 34.Li C, Zeng X, Yu H, Gu Y, Zhang W. Identification of hub genes with diagnostic values in pancreatic cancer by bioinformatics analyses and supervised learning methods. World J Surg Oncol. 2018;16:223. doi: 10.1186/s12957-018-1519-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Funahashi Y, Sugi NH, Semba T, Yamamoto Y, Hamaoka S, Tsukahara-Tamai N, Ozawa Y, Tsuruoka A, Nara K, Takahashi K, Okabe T, Kamata J, Owa T, Ueda N, Haneda T, Yonaga M, Yoshimatsu K, Wakabayashi T. Sulfonamide derivative, E7820, is a unique angiogenesis inhibitor suppressing an expression of integrin alpha2 subunit on endothelium. Cancer Res. 2002;62:6116–6123. [PubMed] [Google Scholar]
- 36.Guo P, Moses-Gardner A, Huang J, Smith ER, Moses MA. ITGA2 as a potential nanotherapeutic target for glioblastoma. Sci Rep. 2019;9:6195. doi: 10.1038/s41598-019-42643-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Olkhov-Mitsel E, Savio AJ, Kron KJ, Pethe VV, Hermanns T, Fleshner NE, van Rhijn BW, van der Kwast TH, Zlotta AR, Bapat B. Epigenome-wide DNA methylation profiling identifies differential methylation biomarkers in high-grade bladder cancer. Transl Oncol. 2017;10:168–177. doi: 10.1016/j.tranon.2017.01.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Zeng XQ, Wang J, Chen SY. Methylation modification in gastric cancer and approaches to targeted epigenetic therapy (review) Int J Oncol. 2017;50:1921–1933. doi: 10.3892/ijo.2017.3981. [DOI] [PubMed] [Google Scholar]
- 39.Ocker M, Bitar SA, Monteiro AC, Gali-Muhtasib H, Schneider-Stock R. Epigenetic regulation of p21(cip1/waf1) in human cancer. Cancers (Basel) 2019;11 doi: 10.3390/cancers11091343. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Tao YF, Xu LX, Lu J, Hu SY, Fang F, Cao L, Xiao PF, Du XJ, Sun LC, Li ZH, Wang NN, Su GH, Li YH, Li G, Zhao H, Li YP, Xu YY, Zhou HT, Wu Y, Jin MF, Liu L, Zhu XM, Ni J, Wang J, Xing F, Zhao WL, Pan J. Early B-cell factor 3 (EBF3) is a novel tumor suppressor gene with promoter hypermethylation in pediatric acute myeloid leukemia. J Exp Clin Cancer Res. 2015;34:4. doi: 10.1186/s13046-014-0118-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Nones K, Waddell N, Song S, Patch AM, Miller D, Johns A, Wu J, Kassahn KS, Wood D, Bailey P, Fink L, Manning S, Christ AN, Nourse C, Kazakoff S, Taylor D, Leonard C, Chang DK, Jones MD, Thomas M, Watson C, Pinese M, Cowley M, Rooman I, Pajic M APGI. Butturini G, Malpaga A, Corbo V, Crippa S, Falconi M, Zamboni G, Castelli P, Lawlor RT, Gill AJ, Scarpa A, Pearson JV, Biankin AV, Grimmond SM. Genome-wide DNA methylation patterns in pancreatic ductal adenocarcinoma reveal epigenetic deregulation of SLIT-ROBO, ITGA2 and MET signaling. Int J Cancer. 2014;135:1110–1118. doi: 10.1002/ijc.28765. [DOI] [PubMed] [Google Scholar]
- 42.Chin SP, Marthick JR, West AC, Short AK, Chuckowree J, Polanowski AM, Thomson RJ, Holloway AF, Dickinson JL. Regulation of the ITGA2 gene by epigenetic mechanisms in prostate cancer. Prostate. 2015;75:723–734. doi: 10.1002/pros.22954. [DOI] [PubMed] [Google Scholar]
- 43.Hou BH, Jian ZX, Cui P, Li SJ, Tian RQ, Ou JR. miR-216a may inhibit pancreatic tumor growth by targeting JAK2. FEBS Lett. 2015;589:2224–2232. doi: 10.1016/j.febslet.2015.06.036. [DOI] [PubMed] [Google Scholar]
- 44.Qin Y, Dang X, Li W, Ma Q. miR-133a functions as a tumor suppressor and directly targets FSCN1 in pancreatic cancer. Oncol Res. 2013;21:353–363. doi: 10.3727/096504014X14024160459122. [DOI] [PubMed] [Google Scholar]
- 45.Han H, Wang L, Xu J, Wang A. miR-128 induces pancreas cancer cell apoptosis by targeting MDM4. Exp Ther Med. 2018;15:5017–5022. doi: 10.3892/etm.2018.6047. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Pollard TD, Cooper JA. Actin, a central player in cell shape and movement. Science. 2009;326:1208–1212. doi: 10.1126/science.1175862. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Hall A. The cytoskeleton and cancer. Cancer Metastasis Rev. 2009;28:5–14. doi: 10.1007/s10555-008-9166-3. [DOI] [PubMed] [Google Scholar]
- 48.Pollard TD, Borisy GG. Cellular motility driven by assembly and disassembly of actin filaments. Cell. 2003;112:453–465. doi: 10.1016/s0092-8674(03)00120-x. [DOI] [PubMed] [Google Scholar]
- 49.Chuang YC, Wu HY, Lin YL, Tzou SC, Chuang CH, Jian TY, Chen PR, Chang YC, Lin CH, Huang TH, Wang CC, Chan YL, Liao KW. Blockade of ITGA2 induces apoptosis and inhibits cell migration in gastric cancer. Biol Proced Online. 2018;20:10. doi: 10.1186/s12575-018-0073-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Ferraro A, Boni T, Pintzas A. EZH2 regulates cofilin activity and colon cancer cell migration by targeting ITGA2 gene. PLoS One. 2014;9:e115276. doi: 10.1371/journal.pone.0115276. [DOI] [PMC free article] [PubMed] [Google Scholar]