Abstract
Circular RNAs (circRNAs) have been shown to regulate the development and progression of various cancers. However, the expression and function of circRNAs in papillary thyroid cancer (PTC) remain largely unknown. This study is aimed to investigate the potential roles of circEIF3I in PTC and elucidate the functional mechanism. We found that the expression of circEIF3I was significantly upregulated in PTC tissues compared to adjacent normal tissues. CircEIF3I expression was positively associated with tumor size, TNM stage and metastasis. CircEIF3I knockdown remarkably suppressed the proliferation, migration and invasion of PTC cells. Mechanistically, circEIF3I promoted KIF2A expression through competitively interacting with miR-149. In conclusion, circEIF3I upregulation in PTC tissues facilitates KIF2A expression by inhibiting miR-149, leading to malignant progression of PTC. This study suggested circEIF3I/miR-149/KIF2A axis might be a potential therapeutic target.
Keywords: Papillary thyroid cancer, circular RNA, circEIF3I, miR-149, KIF2A
Introduction
As the most common malignancy in the endocrine system, the incidence of thyroid cancer (TC) is increasing at a high rate [1]. It is estimated that there are about 2000 new cases diagnosed with TC every year in the USA [2]. Papillary thyroid carcinoma (PTC) accounts for ~80% of all thyroid cancer cases [3]. Thyroidectomy combined with radioiodine and levothyroxine improves the prognosis of PTC patients [4]. Yet, many cases would recur within 10 years, which leads to death [5]. Thus, it is critical to have a better understanding of the mechanisms involved in PTC progression.
Circular RNAs (circRNAs) are a group of non-coding RNAs, which have closed loop structures and no 5’ cap or 3’ Poly A tail [6]. They were first discovered in the early 1990s, and were considered as splicing errors [7]. Thus, they did not draw too much attention in the beginning. However, with the development of high-throughput sequencing technology, researchers find that circRNAs are widely expressed in a cell-type specific manner [8], and participate in many biology processes [9-12]. Recently, studies have shown that dysregulation of circRNA expression is involved in human cancer [13,14]. circRNAs may regulate the proliferation, migration, differentiation of tumor cells [13,14]. For example, overexpression of circTCF25 inhibits miR-103a-3p and miR-107 to promote CDK6 expression, leading to bladder cancer proliferation and migration [15]. In addition, circ002059 low expression is associated with the metastasis and malignant stage of gastric cancer [16]. Due to the important function of circRNAs in different cancers, we sought to explore the function of circEIF3I in PTC progression.
Through unbiased circRNA analysis, we found that circEIF3I was highly expressed in PTC tissues, and was associated with the malignant stage and survival of PTC patients. Further studies showed that circEIF3I promoted PTC progression both in vitro and in vivo. Mechanistically, circEIF3I upregulation in PTC tissues inhibited miR-149 function, which facilitated KIF2A expression, and thus led to malignant progression of PTC. In all, our findings proved that circEIF3I/miR-149/KIF2A axis plays an essential role in PTC progression, and might be a potential therapeutic target.
Materials and methods
Human tissues
All PTC tumor tissue and adjacent normal tissues were obtained from PTC patients who underwent surgical resection at the First Affiliated Hospital of Zhengzhou University. All samples were frozen in liquid nitrogen and stored at -80°C until use. All patients were provided with written informed consent for their tissue usage, and the use of human samples was approved by the Ethics Committee of the First Affiliated Hospital of Zhengzhou University.
Cell culture and transfection
Papillary thyroid normal and cancer cell lines Nthy-ori 3-1, K1, TPC-1, IHH4 and BCPAP were grown in RPMI1640 media containing 10% heat-inactivated FBS (Thermo Fisher Scientific, USA), 100 units/ml penicillin, and 100 μg/ml streptomycin at 37°C with 5% CO2. For transfection, siRNAs, miR-149 mimics, miR-149 inhibitors and negative controls were obtained from GenePharma Co., Ltd (Shanghai, China) and transfected into cell lines using Lipofectamine 2000 (Invitrogen; Thermo Fisher Scientific, Inc.), according to the manufacturer’s protocol. 48 h later, the transfection efficiency was measured by qRT-PCR as described below.
Total RNA extraction and qRT-PCR
We extracted total RNAs with TRIzol Reagent (Thermo Fisher Scientific, USA), and resuspended in RNase-free H2O. 1.5 μg of total RNA was reverse-transcripted with PrimeScriptTM RT reagent kit (Takara, Japan). qRT-PCR was performed with SYBR Premix Ex TaqTM kit (Takara, Japan) according to the manufacturer’s instructions. The house keeping gene U6 and β-actin were used as internal control. The primer sequences were as follows: circEIF3I (Forward, 5’-ATGAGAGTGGAGAGCTCAACC-3’ and reverse, 5’-CTGGTAGCCCATCTGCTTGTCCG-3’), miR-149 (Forward, 5’-GGCTCTGGCTCCGTGTCTT-3’ and reverse, 5’-CAGTGCAGGGT CCGAGGTATT-3’), KIF2A (Forward: 5’-CCTGACCTTGTTCCTGATGAAG-3’ and reverse, 5’-TGCTGAACCAACCACTCTATTATC-3’), U6 (Forward, 5’-CAAATTCGTGAAGCGTTCCATA-3’ and reverse, 5’-AGTGCAGGGTCCGAGGTATTC-3’) and β-actin (Forward, 5’-GCGGACTATGACTTAGTTGCGTTACA-3’ and reverse, 5’-TGCTGTCACCTTCACCGTTCCA-3’).
Western blot
20 µg protein lysates were separated by 12% SDS-PAGE, and blocked with 5% nonfat milk, incubated with primary antibody at 4°C overnight; Then the membranes were incubated with secondary antibodies at room temperature for 1 h, and detected with enhanced chemiluminescence reagents (Thermo Fisher Scientific, USA).
CCK8 proliferation assays
Cell viability was examined with the Cell Counting Kit-8 (CCK8; Beyotime, China). In brief, TPC-1 and IHH4 cells were seeded into 96-well plates at a density of 4×103 cells/well. At different time points, 10 μl CCK-8 solution was added the wells, and incubated for another 4 h at 37°C, and the absorbance at 450 nm was measured using ELX-800 spectrometer reader (Bio-Tek Instruments, USA).
Tumor xenograft assay
1×107 cells were injected into five weeks old BALB/c nude mice (4 mice/group). The size of tumors was recorded with vernier caliper once a week. After 5 weeks, the mice were sacrificed, and tumor tissues were collected, photographed weighted and prepared for western blot. All animal experiments have been approved by the First Affiliated Hospital of Zhengzhou University.
Colony formation assay
1000 transfected cells were seeded into a 6-well plate in RPMI-1640 medium. Two weeks after incubation, the cells were washed with PBS and fixed with 4% paraformaldehyde, and stained with 0.1% crystal violet. The number of colonies was counted under microscope (Olympus, Japan).
Luciferase assays
miR-149 was predicted as the target miRNA of circEIF3I using Circinteractome tool (https://circinteractome.nia.nih.gov) and KIF2A was predicted as miR-149 target gene using TargetScan7 (http://www.targetscan.org/vert_71/). Then sequences containing putative binding sites for miR-149 were inserted into the pGL3 vector (Promega Corporation, Madison, WI, USA). For the luciferase assays, 1×105 transfected cells were seeded into 24-well plates and cultured for 48 h, and then firefly luciferase activity and renilla luciferase activity were measured with Dual-Luciferase Reporter Assay System (Promega, USA).
Cell migration and invasion assays
Transwell migration and invasion assays were performed with 8.0 mm pore filters. For invasion assays, 2×104 transfected cells in serum-free medium were seeded into the upper chambers pre-coated with Matrigel (BD Biosciences, USA), and complete culture medium was added to the lower chamber as the chemoattractant. Then, the cells were incubated for 24 h, and the invaded cells were fixed, stained with 0.1% crystal violet, and photographed under a microscope at a magnification of ×200. Five randomly selected fields were used to count the invaded cells. All the experimental procedure for migration assays were the same except that migration assays used non-treated chambers.
Statistical analyses
Statistical analysis was performed with SPSS 19.0 software. For comparison among the groups, Student’s t test, one-way ANOVA and two-way ANOVA were performed, and P<0.05 was defined as statistically significant. All data were shown as mean ± SD, and all the experiments were repeated at least three times.
Results
CircEIF3I was upregulated in PTC tissues
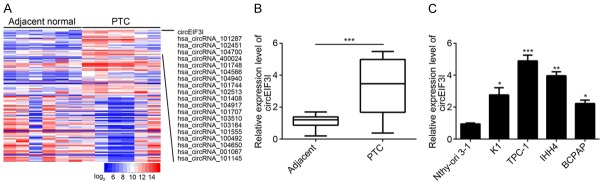
Circular RNAs (circRNAs) have been shown to regulate the development and progression of various cancers. However, the expression and function of circRNAs in papillary thyroid cancer (PTC) remain largely unknown. To explore the functions of circRNAs in PTC, we analyzed circRNA expression in one public database GSE93522. Among the 100 most differentially expressed circRNAs, circEIF3I was the highest overexpressed (Figure 1A). Later, we confirmed that circEIF3I was upregulated in PTC tissues compared with adjacent normal tissues (Figure 1B). In the meanwhile, circEIF3I was also upregulated in PTC cell lines (K1, TPC-1, IHH4 and BCPAPA) compared with normal PTC cell line Nthy-ori 3-1 (Figure 1C). All these data indicated that circEIF3I was upregulated in PTC tissues.
Figure 1.
CircEIF3I was upregulated in PTC tissues. A. Heatmap for 50 most upregulated or downregulated circRNAs in PTC tissues according to a GEO dataset (GSE93522). B. CircEIF3I level was increased in PTC tissues (n=48) compared to adjacent normal tissues (n=48). C. CircEIF3I level was upregulated in PTC cell lines compared to Nthy-ori 3-1 cells. *P<0.05, **P<0.01 and ***P<0.001.
Regulation of circEIF3I on PTC cell proliferation, migration and invasion
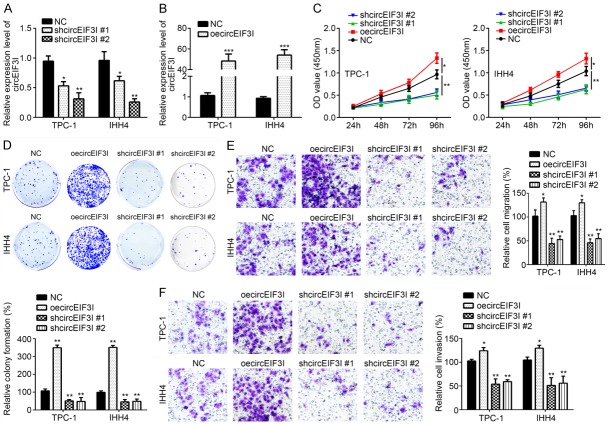
Then, we sought to know whether circEIF3I affects in PTC progression. To research it, we analyzed the effects of circEIF3I on PTC cell proliferation, migration and invasion. We knocked down and overexpressed circEIF3I in TPC-1 and IHH4 cells respectively (Figure 2A and 2B). CCK-8 assays showed that circEIF3I knockdown significantly decreased TPC-1 and IHH4 proliferation, while circEIF3I overexpression increased TPC-1 and IHH4 proliferation (Figure 2C). Colony formation assays also showed similar results (Figure 2D). These results told us that circEIF3I promoted the proliferation of PTC cells. Then, transwell assays showed that overexpression of circEIF3I could increase the migration and invasion of PTC cells, while circEIF3I knockdown suppressed the migration and invasion of PTC cells (Figure 2E and 2F). These showed that circEIF3I promoted the migration and invasion of PTC cells. Thus, circEIF3I promotes PTC cell proliferation, migration and invasion, and functions as an oncogene in PTC.
Figure 2.
Regulation of circEIF3I on PTC cell proliferation, migration and invasion. A and B. circEIF3I expression was significantly decreased or increased by transfection with sh-circEIF3I or oe-circEIF3I plasmids compared to negative control (NC) group. C. CCK8 assays showed that proliferation of PTC-1 and IHH4 cells was inhibited by circEIF3I depletion, and vice versa. D. Colony formation assay showed that circEIF3I depletion reduced the colony formation number, and vice versa. E and F. Transwell assay indicated that circEIF3I depletion suppressed migration and invasion, and vice versa. *P<0.05, **P<0.01 and ***P<0.001
MiR-149 expression and correlation with circEIF3I in PTC tissues
How circEIF3 promoted PTC progression was investigated. We found that miR-149 was significantly downregulated in PTC tissues (Figure 3A). Besides, we found that there was a negative correlation between miR-149 and circEIF3I in PTC tissues (Figure 3B). So we wondered whether circEIF3I could regulate miR-149 expression. Through binding partner predication, we found that a GAGCCAGA motif on circEIF3I matches that of miR-149 (Figure 3C). Luciferase results showed that miR-149 transfection inhibited circEIF3I luciferase activity (Figure 3D), indicating that miR-149 interacted with circEIF3I. To further confirm whether miR-149 interacted with circEIF3I through this motif, the motif was mutated to CUCGGUCU and luciferase assay was conducted. Results showed that binding motif mutation abolished the inhibitory effect of miR-149 transfection on circEIF3I luciferase activity (Figure 3D), suggesting that circEIF3I interacted with miR-149 through GAGCCAGA motif. From previous studies, we know that circRNAs usually interact with miRNA to regulate miRNA expression [17,18]. From above data, we know that circEIF3I and miR-149 expression was negatively correlated. We wonder whether circEIF3I could sponge miR-149. From loss of function experiments, we found that circEIF3I knockdown led to upregulation of miR-149 while circEIF3I overexpression resulted in miR-149 downregulation (Figure 3E). Thus, circEIF3I served as a miR-149 sponge.
Figure 3.
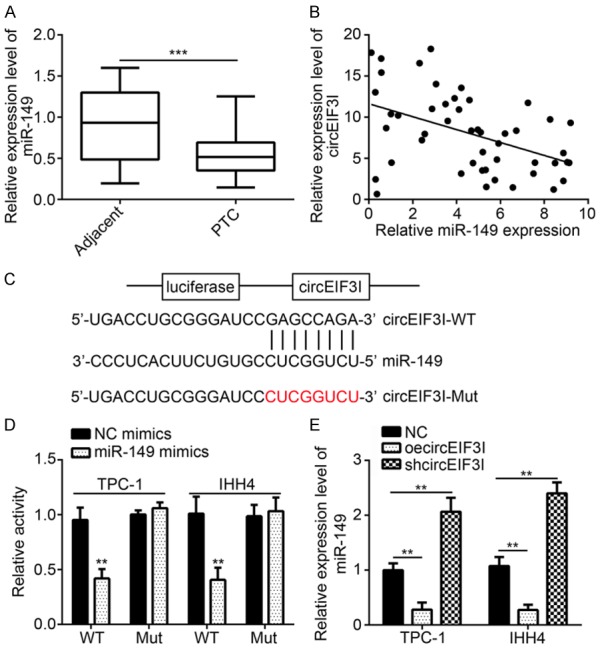
MiR-149 expression and correlation with circEIF3I in PTC tissues. A. qRT-PCR showed that miR-149 was downregulated in PTC tissues compared to adjacent normal tissues. B. There was a reverse correlation between miR-149 and circEIF3I in PTC tissues. C. The predicted binding site with miR-149 and circEIF3I-Mut sequence was shown. D. Luciferase reporter assay showed that miR-149 overexpression suppressed the activity of circEIF3I-WT reporter. E. qRT-PCR analysis showed that circEIF3I overexpression inhibited miR-149 expression, and vice versa. **P<0.01 and ***P<0.001.
CircEIF3I regulated PTC progression through sponging miR-149
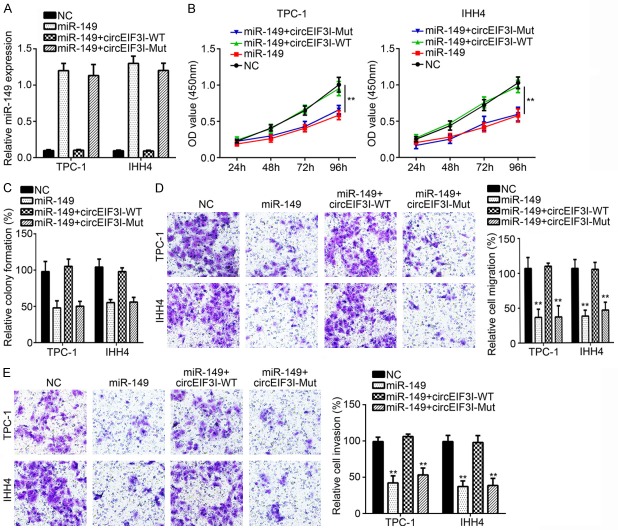
Firstly, we analyzed the function of miR-149 in PTC progression. We found that miR-149 overexpression in TPC-1 and IHH4 cells inhibited the cell proliferation, colony formation, invasion and migration (Figure 4A-E), indicating that miR-149 suppressed PTC progression. We then overexpressed wild-type (WT) or mutant (Mut) circEIF3I in miR-149 overexpressing cells (Figure 4A). We found that WT-circEIF3I overexpression significantly reversed the inhibitory effects of miR-149 on proliferation, colony formation, migration and invasion (Figure 4B-E). However, Mut-circEIF3I overexpression had no these roles (Figure 4B-E). These results indicated that circEIF3I promoted PTC progression through miR-149.
Figure 4.
circEIF3I regulated PTC progression through sponging miR-149. A. qRT-PCR was used to analyze miR-149 expression. B and C. CCK8 assay and colony formation assay showed that cellular proliferation was inhibited by miR-149 overexpression and further reversed by circEIF3I-WT transfection in the meantime. D and E. Transwell assay was utilized to detect the ability of migration and invasion. **P<0.01.
KIF2A was targeted by miR-149
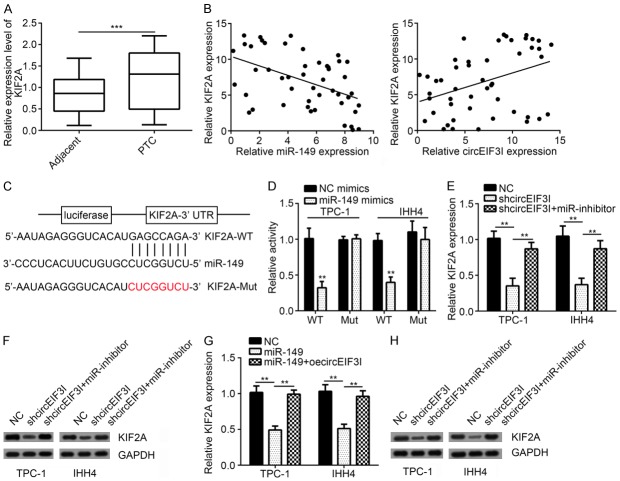
According to previous studies, miRNA binds to the 3’ UTR of mRNA and regulates mRNA expression [19]. Thus, we wonder whether miR-149 binds to and regulates mRNA expression in PTC tissues. We found that KIF2A gene was significantly overexpressed in PTC tissues (Figure 5A). Besides, its expression was negatively correlated with miR-149 and positively correlated with circEIF3I (Figure 5B). Through binding motif predication, we found that miR-149 may bind to KIF2A through GAGCCAGA motif (Figure 5C). Luciferase assays showed that miR-149 overexpression inhibited KIF2A luciferase activity, while had no impact on the binding motif mutated KIF2A (Figure 5D). All these results showed that miR-149 could bind to the 3’ UTR of KIF2A mRNA.
Figure 5.
KIF2A was targeted by miR-149. A. qRT-PCR showed that KIF2A was upregulated in PTC tissues compared to adjacent normal tissues. B. There was a reverse correlation between miR-149 and KIF2A, and a positive correlation between KIF2A and circEIF3I in PTC tissues. C. The predicted binding site with miR-149 and KIF2A-3’UTR-Mut sequence was shown. D. Luciferase reporter assay showed that miR-149 overexpression suppressed the activity of KIF2A-3’UTR-WT reporter. E and F. qRT-PCR and WB analysis showed that circEIF3I knockdown inhibited KIF2A expression. G and H. qRT-PCR and WB analysis showed that miR-149 overexpression inhibited KIF2A expression. **P<0.01 and ***P<0.001.
As we knew that circEIF3I sponged miR-149, and circEIF3I expression was positively correlated with KIF2A expression. We analyzed KIF2A expression in circEIF3I knockdown cells. Results showed that circEIF3I knockdown led to downregulation of KIF2A expression (Figure 5E and 5F). And this downregulation is mainly through miR-149 because miRNA inhibition resulted in increased KIF2A expression in circEIF3-silenced cells (Figure 5E and 5F). In the meanwhile, overexpression of circEIF3I in miR-149 overexpressing cells led to KIF2A upregulation in PTC cells (Figure 5G and 5H). All these results showed that circEIF3I and miR-149 formed an axis to regulate KIF2A expression.
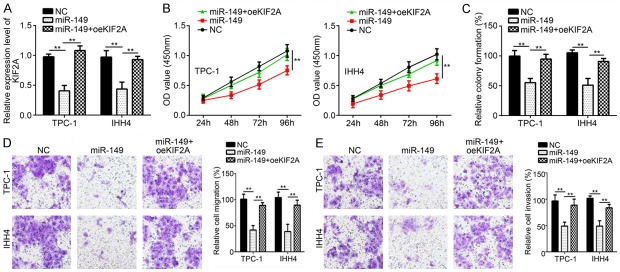
KIF2A reversed the roles of miR-149 in PTC
Next, we wondered whether KIF2A would reverse the function of miR-149 in PTC, and whether miR-149 suppressed PTC progression through KIF2A. We overexpressed KIF2A expression in miR-149 overexpressing cells (Figure 6A). We found that KIF2A overexpression rescued the proliferation, colony formation, invasion and migration defects resulted from miR-149 overexpression (Figure 6B-E). These results showed that miR-149 inhibited PTC progression through KIF2A.
Figure 6.
KIF2A reverses the roles of miR-149 in PTC. A. Relative expression of KIF2A was measured by qRT-PCR. B and C. CCK8 and colony formation assay was used to detect cellular proliferation. D and E. Transwell assay was utilized to analyze cell migration and invasion. **P<0.01.
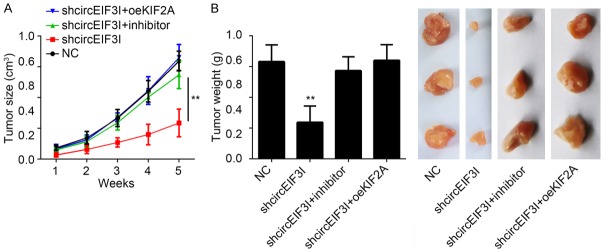
In vivo effect of circEIF3I knockdown on PTC growth
All the above results told us that circEIF3I promoted PTC progression through miR-149/KIF2A axis in vitro. We wanted to know whether circEIF3I promoted PTC progression in vivo. We knocked down circEIF3I and inhibited miR-149 or overexpressed KIF2A in PTC cells. Then these cells were injected into nude mice and tumor growth was monitored. The results showed that circEIF3I silencing led to decreased tumor growth rate (Figure 7A) and smaller tumors (Figure 7B). However, miR-149 inhibition or KIF2A overexpression rescued the tumor volumes and weights (Figure 7A and 7B). Besides, in clinical patient samples, we also found that circEIF3I expression was positively correlated with the tumor size and clinical stage (Table 1). These results indicated that circEIF3I promoted PTC progression in vivo through KIF2A.
Figure 7.
In vivo effect of circEIF3I knockdown on PTC growth. A. Tumor volume was determined every one week. NC: negative control. Inhibitor: miR-149 inhibitors. oeKIF2A: overexpression of KIF2A. B. Tumor weight was measured 5 week post injection. **P<0.01.
Table 1.
Correlation between circEIF3I expression and clinicopathological characteristics in PTC samples
| Clinicopathological charateristics | circEIF3I expression | p-value | |
|---|---|---|---|
|
| |||
| low (n=22) | high (n=26) | ||
| Age (Years) | 0.771 | ||
| <45 | 8 | 11 | |
| ≥45 | 14 | 15 | |
| Gender | 0.401 | ||
| Male | 9 | 14 | |
| Female | 13 | 12 | |
| Tumor size | 0.041* | ||
| ≤2 cm | 14 | 8 | |
| >2 cm | 8 | 18 | |
| TNM staging | 0.020* | ||
| I+II | 17 | 11 | |
| III+IV | 5 | 15 | |
| Lymph node metastasis | 0.042* | ||
| Yes | 15 | 9 | |
| No | 7 | 17 | |
P<0.05 was considered as statistically significant.
Chi-square test was used.
Discussion
The past decades have witnessed the growing list of non-coding RNAs [20]. Among the non-coding RNAs, circRNAs are discovered to be widespread and abundant [8]. With the development of next-generation sequencing, people have found that circRNAs are expressed in a tissue-dependent manner [21]. Accumulating evidences indicate that circRNAs participate in many biological processes, including tumorigenesis [22-24]. For example, circ0001649 is downregulated in liver cancer, and regulates liver cancer metastasis [25]. CircCCDC66 is overexpressed in colon cancer, and enhances colon cancer development [26]. Additionally, circ000284 was overexpressed in cervical cancer and promotes cervical progression [27]. These studies showed the important role of circRNAs in tumorigenesis. In this study, we wanted to explore the function of circRNAs in PTC progression, and found that circEIF3I was upregulated in PTC, and promoted the progression of PTC both in vitro and in vivo.
Studies have shown that circRNAs display tissue-specific expression patterns, and promote cancer progression. Yet, little is known about circRNAs function in PTC. With the purpose of studying circRNA functions in PTC. We conducted an unbiased analysis of circRNAs in PTC, and found that circEIF3I was overexpressed. In the meanwhile, circEIF3I expression was also correlated with the tumor size, metastasis and the malignant stages. All these data indicated that circEIF3I may serve as an oncogene to promote PTC progression. Then, overexpression experiments showed that circEIF3I overexpression significantly promoted the proliferation, migration and invasion of PTC cells. In vivo experiments also showed that circEIF3I deficiency suppressed PTC tumorigenesis. All these data demonstrated that circEIF3I functioned as an oncogene in PTC.
Abnormal expression of miRNAs has been reported to contribute to PTC progression [28]. For example, miR-199a-3p could down regulate MET expression to inhibit PTC cell migration and proliferation [29]. MiR-146b could promote PTC cell migration, invasion and contribute to chemotherapy resistance [30]. Studies have shown that circRNA can interact with miRNAs [31]. In this study, we found that circEIF3I bound to miR-149. CircEIF3I overexpression led to miR-149 downregulation. Further function studies proved that circEIF3I promoted PTC progression through miR-149. Thus, circEIF3I served as a miR-149 sponge to promote PTC progression.
KIF2A was reported to function in microtube formation and brain development [32,33]. Previous studies have shown that KIF2A regulates the development of cancers, such as glioma [34] and breast cancer progression [35]. Yet, no study has shown that KIF2A would function in PTC. In this study, we first demonstrated that KIF2A promoted PTC progression. We found that that miR-149 inhibited KIF2A expression, and inhibited PTC progression through KIF2A. As we know that circEIF3I promoted PTC progression through miR-149, while miR-149 inhibited PTC progression through KIF2A. Thus, circEIF3I promoted PTC progression through KIF2A, forming a critical circEIF3I/miR-149/KIF2A axis in the regulation of PTC progression.
Overall, we discovered for the first time that circEIF3I was an oncogene, and promoted PTC progression. Besides, we also found that circEIF3I sponged miR-149 to promote KIF2A expression, leading to PTC progression. Besides, we demonstrated a critical circEIF3I/miR-149/KIF2A axis in the regulation of PTC progression.
Acknowledgements
We thank all patients involved in this study.
Disclosure of conflict of interest
None.
References
- 1.Brown RL, de Souza JA, Cohen EE. Thyroid cancer: burden of illness and management of disease. J Cancer. 2011;2:193–9. doi: 10.7150/jca.2.193. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Siegel RL, Miller KD, Jemal A. Cancer statistics, 2017. CA Cancer J Clin. 2017;67:7–30. doi: 10.3322/caac.21387. [DOI] [PubMed] [Google Scholar]
- 3.Pitoia F, Bueno F, Urciuoli C, Abelleira E, Cross G, Tuttle RM. Outcomes of patients with differentiated thyroid cancer risk-stratified according to the American thyroid association and Latin American thyroid society risk of recurrence classification systems. Thyroid. 2013;23:1401–7. doi: 10.1089/thy.2013.0011. [DOI] [PubMed] [Google Scholar]
- 4.Nikiforova MN, Nikiforov YE. Molecular genetics of thyroid cancer: implications for diagnosis, treatment and prognosis. Expert Rev Mol Diagn. 2008;8:83–95. doi: 10.1586/14737159.8.1.83. [DOI] [PubMed] [Google Scholar]
- 5.Lang BH, Wong KP, Wan KY, Lo CY. Significance of metastatic lymph node ratio on stimulated thyroglobulin levels in papillary thyroid carcinoma after prophylactic unilateral central neck dissection. Ann Surg Oncol. 2012;19:1257–63. doi: 10.1245/s10434-011-2105-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Meng S, Zhou H, Feng Z, Xu Z, Tang Y, Li P, Wu M. CircRNA: functions and properties of a novel potential biomarker for cancer. Mol Cancer. 2017;16:94. doi: 10.1186/s12943-017-0663-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Nigro JM, Cho KR, Fearon ER, Kern SE, Ruppert JM, Oliner JD, Kinzler KW, Vogelstein B. Scrambled exons. Cell. 1991;64:607–13. doi: 10.1016/0092-8674(91)90244-s. [DOI] [PubMed] [Google Scholar]
- 8.Salzman J, Gawad C, Wang PL, Lacayo N, Brown PO. Circular RNAs are the predominant transcript isoform from hundreds of human genes in diverse cell types. PLoS One. 2012;7:e30733. doi: 10.1371/journal.pone.0030733. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Rybak-Wolf A, Stottmeister C, Glazar P, Jens M, Pino N, Giusti S, Hanan M, Behm M, Bartok O, Ashwal-Fluss R, Herzog M, Schreyer L, Papavasileiou P, Ivanov A, Ohman M, Refojo D, Kadener S, Rajewsky N. Circular RNAs in the mammalian brain are highly abundant, conserved, and dynamically expressed. Mol Cell. 2015;58:870–85. doi: 10.1016/j.molcel.2015.03.027. [DOI] [PubMed] [Google Scholar]
- 10.Huang S, Yang B, Chen BJ, Bliim N, Ueberham U, Arendt T, Janitz M. The emerging role of circular RNAs in transcriptome regulation. Genomics. 2017;109:401–407. doi: 10.1016/j.ygeno.2017.06.005. [DOI] [PubMed] [Google Scholar]
- 11.Chen LL. The biogenesis and emerging roles of circular RNAs. Nat Rev Mol Cell Biol. 2016;17:205–11. doi: 10.1038/nrm.2015.32. [DOI] [PubMed] [Google Scholar]
- 12.Chen JY, Hour TC, Yang SF, Chien CY, Chen HR, Tsai KL, Ko JY, Wang LF. Autophagy is deficient in nasal polyps: implications for the pathogenesis of the disease. Int Forum Allergy Rhinol. 2015;5:119–23. doi: 10.1002/alr.21456. [DOI] [PubMed] [Google Scholar]
- 13.Chen J, Li Y, Zheng QP, Bao CY, He J, Chen B, Lyu DB, Zheng BQ, Xu Y, Long ZW, Zhou Y, Zhu HY, Wang YN, He XH, Shi YQ, Huang SL. Circular RNA profile identifies circPVT1 as a proliferative factor and prognostic marker in gastric cancer. Cancer Lett. 2017;388:208–219. doi: 10.1016/j.canlet.2016.12.006. [DOI] [PubMed] [Google Scholar]
- 14.Kristensen LS, Hansen TB, Veno MT, Kjems J. Circular RNAs in cancer: opportunities and challenges in the field. Oncogene. 2018;37:555–565. doi: 10.1038/onc.2017.361. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Zhong Z, Lv M, Chen J. Screening differential circular RNA expression profiles reveals the regulatory role of circTCF25-miR-103a-3p/miR-107-CDK6 pathway in bladder carcinoma. Sci Rep. 2016;6:30919. doi: 10.1038/srep30919. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Li P, Chen S, Chen H, Mo X, Li T, Shao Y, Xiao B, Guo J. Using circular RNA as a novel type of biomarker in the screening of gastric cancer. Clin Chim Acta. 2015;444:132–6. doi: 10.1016/j.cca.2015.02.018. [DOI] [PubMed] [Google Scholar]
- 17.Memczak S, Jens M, Elefsinioti A, Torti F, Krueger J, Rybak A, Maier L, Mackowiak SD, Gregersen LH, Munschauer M, Loewer A, Ziebold U, Landthaler M, Kocks C, le Noble F, Rajewsky N. Circular RNAs are a large class of animal RNAs with regulatory potency. Nature. 2013;495:333–8. doi: 10.1038/nature11928. [DOI] [PubMed] [Google Scholar]
- 18.Hansen TB, Jensen TI, Clausen BH, Bramsen JB, Finsen B, Damgaard CK, Kjems J. Natural RNA circles function as efficient microRNA sponges. Nature. 2013;495:384–8. doi: 10.1038/nature11993. [DOI] [PubMed] [Google Scholar]
- 19.Fang Z, Rajewsky N. The impact of miRNA target sites in coding sequences and in 3’UTRs. PLoS One. 2011;6:e18067. doi: 10.1371/journal.pone.0018067. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Morris KV, Mattick JS. The rise of regulatory RNA. Nat Rev Genet. 2014;15:423–37. doi: 10.1038/nrg3722. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Barrett SP, Salzman J. Circular RNAs: analysis, expression and potential functions. Development. 2016;143:1838–47. doi: 10.1242/dev.128074. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Wang X, Zhang Y, Huang L, Zhang J, Pan F, Li B, Yan Y, Jia B, Liu H, Li S, Zheng W. Decreased expression of hsa_circ_001988 in colorectal cancer and its clinical significances. Int J Clin Exp Pathol. 2015;8:16020–5. [PMC free article] [PubMed] [Google Scholar]
- 23.Hansen TB, Kjems J, Damgaard CK. Circular RNA and miR-7 in cancer. Cancer Res. 2013;73:5609–12. doi: 10.1158/0008-5472.CAN-13-1568. [DOI] [PubMed] [Google Scholar]
- 24.Peng L, Yuan XQ, Li GC. The emerging landscape of circular RNA ciRS-7 in cancer (Review) Oncol Rep. 2015;33:2669–74. doi: 10.3892/or.2015.3904. [DOI] [PubMed] [Google Scholar]
- 25.Qin M, Liu G, Huo X, Tao X, Sun X, Ge Z, Yang J, Fan J, Liu L, Qin W. Hsa_circ_0001649: a circular RNA and potential novel biomarker for hepatocellular carcinoma. Cancer Biomark. 2016;16:161–9. doi: 10.3233/CBM-150552. [DOI] [PubMed] [Google Scholar]
- 26.Hsiao KY, Lin YC, Gupta SK, Chang N, Yen L, Sun HS, Tsai SJ. Noncoding effects of circular RNA CCDC66 promote colon cancer growth and metastasis. Cancer Res. 2017;77:2339–2350. doi: 10.1158/0008-5472.CAN-16-1883. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Ma HB, Yao YN, Yu JJ, Chen XX, Li HF. Extensive profiling of circular RNAs and the potential regulatory role of circRNA-000284 in cell proliferation and invasion of cervical cancer via sponging miR-506. Am J Transl Res. 2018;10:592–604. [PMC free article] [PubMed] [Google Scholar]
- 28.Aragon Han P, Weng CH, Khawaja HT, Nagarajan N, Schneider EB, Umbricht CB, Witwer KW, Zeiger MA. MicroRNA expression and association with clinicopathologic features in papillary thyroid cancer: a systematic review. Thyroid. 2015;25:1322–9. doi: 10.1089/thy.2015.0193. [DOI] [PubMed] [Google Scholar]
- 29.Minna E, Romeo P, De Cecco L, Dugo M, Cassinelli G, Pilotti S, Degl’Innocenti D, Lanzi C, Casalini P, Pierotti MA, Greco A, Borrello MG. miR-199a-3p displays tumor suppressor functions in papillary thyroid carcinoma. Oncotarget. 2014;5:2513–28. doi: 10.18632/oncotarget.1830. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Chou CK, Yang KD, Chou FF, Huang CC, Lan YW, Lee YF, Kang HY, Liu RT. Prognostic implications of miR-146b expression and its functional role in papillary thyroid carcinoma. J Clin Endocrinol Metab. 2013;98:E196–205. doi: 10.1210/jc.2012-2666. [DOI] [PubMed] [Google Scholar]
- 31.Dong Y, He D, Peng Z, Peng W, Shi W, Wang J, Li B, Zhang C, Duan C. Circular RNAs in cancer: an emerging key player. J Hematol Oncol. 2017;10:2. doi: 10.1186/s13045-016-0370-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Homma N, Takei Y, Tanaka Y, Nakata T, Terada S, Kikkawa M, Noda Y, Hirokawa N. Kinesin superfamily protein 2A (KIF2A) functions in suppression of collateral branch extension. Cell. 2003;114:229–39. doi: 10.1016/s0092-8674(03)00522-1. [DOI] [PubMed] [Google Scholar]
- 33.Jaillard S, Andrieux J, Plessis G, Krepischi AC, Lucas J, David V, Le Brun M, Bertola DR, David A, Belaud-Rotureau MA, Mosser J, Lazaro L, Treguier C, Rosenberg C, Odent S, Dubourg C. 5q12.1 deletion: delineation of a phenotype including mental retardation and ocular defects. Am J Med Genet A. 2011;155A:725–31. doi: 10.1002/ajmg.a.33758. [DOI] [PubMed] [Google Scholar]
- 34.Zhang X, Ma C, Wang Q, Liu J, Tian M, Yuan Y, Li X, Qu X. Role of KIF2A in the progression and metastasis of human glioma. Mol Med Rep. 2016;13:1781–7. doi: 10.3892/mmr.2015.4700. [DOI] [PubMed] [Google Scholar]
- 35.Wang J, Ma S, Ma R, Qu X, Liu W, Lv C, Zhao S, Gong Y. KIF2A silencing inhibits the proliferation and migration of breast cancer cells and correlates with unfavorable prognosis in breast cancer. BMC Cancer. 2014;14:461. doi: 10.1186/1471-2407-14-461. [DOI] [PMC free article] [PubMed] [Google Scholar]