Abstract
Evolutionarily conserved YT521-B homology (YTH) domain-containing proteins, including YTHDF1-3 and YTHDC1-2, are known to confer m6A-dependent RNA-binding activity. The YTH domain-containing proteins participate in numerous RNA processes, such as mRNA splicing, nuclear export, translation and decay in post-transcriptional regulation. Most recently, it has been found that YTH domain-containing proteins play important roles in post-transcriptional modification process hence modulate the expression of genes involved in cancer and other processes including cell cycle progression, cell proliferation, migration and invasion, inflammatory, immunity and autophagy. In this review, we summarize the roles and molecular mechanisms of YTH domain-containing proteins in cancer development and progression. In addition, we discuss the prospect of using YTH domain-containing proteins as a new diagnostic biomarkers and therapeutic targets for cancers.
Keywords: YTH domain-containing proteins, m6A modification, tumorigenesis and development, biomarker
Introduction
The N6-methyladenosine (m6A) modification is the most abundant conserved post-translational modification found in a wide range of cellular RNAs [1]. The dynamic and reversible modification of m6A is facilitated by methyltransferases (‘writers’), demethylases (‘erasers’) and m6A-binding proteins (‘readers’) [2]. Methyltransferases promotes methylation of m6A in RNA, including methyltransferase-like protein 3 (METTL3), METTL5 [3], METTL14, METTL16 [4], RBM15 [5], Wilms’ tumor 1-associating protein (WTAP), KIAA1429, Hakai, VIRMA [5], and ZCCHC4 [6]. Demethylases, which mainly include fat mass and obesity-associated protein (FTO), as well as AlkB homolog 5 (ALKBH5), removes the m6A methylation group of RNA. The final m6A-binding protein is an enzyme that binds to the m6A methylation site and plays a specific role on gene expression, including YT521-B homology (YTH) domain family proteins (YTHDFs), YTH domain-containing protein (YTHDCs), IGF2BPs, and eIF3 [7-9].
The YTH domain-containing proteins were identified using the RNA affinity chromatography approach and mass spectrometry [10]. The domain is found in 174 different proteins of eukaryotes and is characterized by 14 invariant residues within an α-helix/β-sheet structure. Also, the YTH domain bind to a short degenerated single-stranded RNA sequence motif [11]. In eukaryotes, the YTH domain-containing proteins comprise five functional genes: YTHDF1, YTHDF2, YTHDF3, YTHDC1, and YTHDC2, distributed in the nucleus and cytoplasm. YTH domain-containing proteins first recognize the m6A modification of target RNAs, then direct different complexes to regulate RNA signaling pathways, including RNA folding, RNA splicing, protein translation, and RNA metabolism [8]. YTHDF2 was the first read protein to be identified and has been mostly studied for its bio-function of influencing mRNA stability [12,13]. YTHDF2 binds to m6A of target mRNA through its C-terminal YTD domain and accelerates the degradation of the targeted mRNA through its N-terminal domain [12]. YTHDF1 facilitates the translation of m6A-modified mRNAs in the cytoplasm, whereas YTHDF3 facilitates mRNA translation in synergy with YTHDF1 and accelerates the decay of m6A-modified transcripts mediated through YTHDF2 [14].
Recent studies have revealed the bio-functions of m6A modification and its molecular machinery in human cancers. Independent of its catalytic activity and m6A readers, methyltransferase METTL3 promotes lung cancer cell growth, survival, and invasion by increasing the translation process of a set of target oncogenes, such as EGFR and the Hippo pathway effector TAZ [15]. The first identified m6A demethylase FTO is highly expressed and plays a critical oncogenic role in acute myeloid leukemia (AML) by targeting a cohort of critical transcripts, such as ASB2 and RARA [16]. Yang et al. reported that the crosstalk between YTHDF2 and miR-145 is closely correlated with the malignancy of hepatocellular carcinoma (HCC) [17]. In pancreatic cancer, YTHDF2 promotes cell proliferation but inhibits cell migration and invasion by regulating EMT possibly via YAP signaling [18]. Overexpression of YTHDF1 is associated with poor prognosis in patients with HCC [19]. YTHDF3 is downregulated in colorectal cancer (CRC), whereas YTHDC1 is abundantly expressed in colon adenocarcinoma [20]. Many studies have shown that the YTH domain protein plays a significant role in human cancer progression. However, review articles regarding the regulatory role of YTH domain-containing proteins are limited. Besides, the function of YTHDC1 in alternative splicing and other RNA metabolic processes [21], as well as the mechanism of YTH proteins in m6A recognition, has not been reviewed [8,22]. Herein, we summarized the most recent important progress of YTH domain-containing proteins in cancer development and progression, including cell cycle progression, cell proliferation, migration and invasion, inflammatory, immunity, and autophagy. Also, we covered the mechanisms of the YTH domain-containing proteins in cancer-associated RNA splicing, nuclear export, translation, and decay.
The YTH domain-containing proteins (i.e., YTHDFs and YTHDCs) belong to a protein family that is highly conserved in eukaryotes. They bind single-stranded RNA with the conserved YTH domain (>60% identity) located at the C terminus and influence the fate of their transcript target [11,12]. The YTH family proteins participate in various biological processes, including RNA splicing, nuclear export, translation, and decay. Also, they may play different biological functions because of their different cellular localization. YTHDF2 promotes the m6A dependent RNA decay by recruiting m6A modified mRNA to processing bodies (P-bodies). Also, YTHDF1 and YTHDF3 promote the translation efficiency of m6A-modified mRNAs by interacting with translation initiation factors [14,23]. Among the YTH domain-containing proteins, YTHDC1 is the only one constitutively enriched in the nucleus. It regulates mRNA splicing by bridging the interactions between the trans- and cis-regulatory elements to bind targeted mRNAs [24]. YTHDC2 also plays multiple roles in mRNA translation and decay due to its multiple RNA-binding domains [25].
Recent findings show that YTH domain-containing proteins play important roles in carcinogenesis and development. The roles and regulatory mechanisms of the YTH domain-containing proteins on cancer-associated RNA splicing, nuclear export, translation, and decay are summarized in Table 1.
Table 1.
Roles and target RNAs of YTH domain-containing proteins regulating RNA splicing, nuclear export, translation and decay
| YTH family | Target RNAs | Cis-element on RNA | Regulation | The roles of target RNAs | Ref |
|---|---|---|---|---|---|
| YTHDF1 | C-MYC | 5’-UTR | Stabilizing the transcript | Promoting tumor cell proliferation | [63] |
| YTHDF1 | β-catenin | NA | Upregulating the Wnt/β-catenin pathway | Promoting tumorigenicity and stem cell-like activity of CRC cells | [64] |
| YTHDF1 | HINT2 | 3’-UTR | Promoting mRNA translation | Restraining cell growth and migration in ocular melanoma | [42] |
| YTHDF1 | EIF3C | 3’-UTR | Promoting mRNA translation | Facilitating tumorigenesis and metastasis | [41] |
| YTHDF2 | ORF50 | NA | Potentiating the KSHV lytic cycle | Anti-viral effects | [86] |
| YTHDF2 | EGFR | 3’-UTR | Promoting the degradation of mRNA | Inhibiting tumor growth | [52] |
| YTHDF2 | CircFOREIGN | CDS | Inducing RNA decay or translation | Dampening innate immunity | [79] |
| YTHDF2 | MiR-493-3p | 3’-UTR | Suppressing the translation of mRNA | Suppressing PCa cell proliferation and migration | [55] |
| YTHDF2 | YAP | NA | Decreasing the stability of mRNA | Promoting cell migration and invsion | [18] |
| YTHDF2 | ATG5 | NA | Inducing mRNA decay | Promoting autophagy and adipogenesis | [87] |
| ATG7 | |||||
| YTHDF2 | PETN | NA | Promoting mRNA degradation | Promoting GC cell proliferation and metastasis | [53] |
| YTHDF2 | Smad7 | NA | Stabilizing the mRNA transcripts | Inhibiting osteoblast differentiation | [88] |
| Smurf1 | |||||
| YTHDF2 | PRDM2 | NA | Promoting mRNA decay | The positive p53 regulator | [54] |
| YTHDF3 | FOXO3 | NA | Promoting its translation | Regulation of antiviral innate immunity | [43] |
| YTHDC1 | SRSF3 | 3’-UTR | Export of methylated mRNA from the nucleus to the cytoplasm | Selective processing and metabolism of mammalian mRNAs | [30] |
| YTHDC1 | CPSF6 | 3’-UTR | Mediating nuclear export | Development of both embryo and germline | [89] |
| SRSF3 | |||||
| SRSF7 | |||||
| YTHDC2 | HIF-1α | 5’-UTR | Promoting translation | Promoting cell metastasis | [47] |
| Twist1 | |||||
| YTHDC2 | c-Jun | 5’-UTR | Promoting translation | Promoting cell proliferation | [48] |
| ATF-2 |
YTH domain-containing proteins regulate RNA splicing and nuclear export
Alternative splicing is the process by which identical pre-mRNAs are variably spliced into unique mature transcripts. And the process contributes to proteomic diversity in normal development [26]. Among the YTH domain-containing proteins, YTHDC1 was identified as a splicing factor that promotes exon inclusion [24] (Figure 1). The protein interacts with components of the splicing machinery by modulating alternative splicing in a concentration-dependent manner [27]. It is noteworthy that many aberrant alternative splicing events have been recently reported to occur in cancer cells. The unbalanced alternative splicing has been implicated in several cancers. And may promote the proliferation and invasion of tumor cells by the formation of certain pro-tumorigenic isoforms and the reduction of anti-tumorigenic isoforms [28,29].
Figure 1.
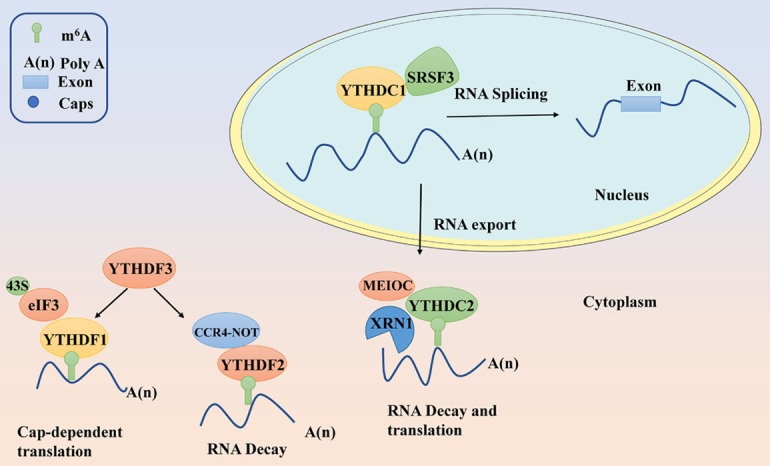
Summary of YTH domain-containing proteins regulating RNA splicing, nuclear export, translation and decay. YTH domain-containing proteins determine the fate of target m6A-modified RNA transcripts in cytoplasm and nucleus.
One of the most dramatic effects of YTHDC1 is RNA nuclear export because of its unique YTH family protein constitutively enriched in the nucleus [24]. In eukaryotes, the export of mature mRNA is regulated by the dynamics of protein-RNA interactions (Figure 1). YTHDC1 mediates the export of m6A modified mRNA from the nucleus to the cytoplasm in HeLa cells by interacting with the splicing factor and nuclear export adaptor protein SRSF3 (Table 1) [30]. YTHDC1 might promote exon inclusion, splicing, and mRNA export from the nucleus to the cytoplasm by recruiting splicing factor and nuclear export adaptor protein SRSF3, while blocking SRSF10 binding to m6A-modified pre-mRNAs to nuclear speckles [24] (Figure 1). SRSF3 is an essential factor which drives the tumorigenic process in various types of cancers, including breast cancer, colon cancer, ovarian cancer, osteosarcoma, and glioblastoma [31]. Moreover, evidence indicates that YTHDC1 is associated with prostate cancer [32]. Likewise, low mRNA levels of YTHDC1 were directly linked to poor prognosis in endometrial cancer [33]. All these findings reveal the close correlation between cancers and YTHDC1, owing to its alternative pre-mRNA splicing ability. And suggest that YTH domain-containing proteins might play vital roles in various cancers through their alternative splicing. However, there is still a need to conduct further studies to explore their bio-function and corresponding molecular mechanism in various types of cancer.
YTH domain-containing proteins regulate mRNA translation
The control of mRNA translation in eukaryotic cells is critical for gene regulation during various biological processes [34]. In the cytoplasmic compartment, YTHDF1 [23,35], YTHDF3 [14,36], and YTHDC2 [25,37] have all been identified as translation regulators, but are recruited to different regions and interact with different mRNA targets (Table 1). YTHDF1 enhances the translation of its target transcripts to ensure effective protein production from dynamic transcripts by interacting with initiation factors and facilitating ribosome loading in an m6A-dependent manner [23]. YTHDF3 as a partner of YTHDF1 significantly promotes the translation of its target mRNAs by interacting with YTHDF1. While YTHDF1 functions in translation regulation, YTHDF3 serves as a hub for fine-tuning the RNA accessibility of YTHDF1 and modulating the turnover rate of m6A-modified transcripts [14] (Figure 1).
For most cellular mRNAs, initiation of mRNA translation involves the recognition of 7-methyl-guanylic (m7G) cap structure at the 5’ terminus by cap-binding complex, eukaryotic initiation factor 4F (eIF4F). The eIF4F combines with 50 caps and recruits the 40S ribosomal subunit to the mRNA by interacting with the cap structure and associating with the translation initiation factor eIF3 [38] (Figure 1). However, contrary to the mechanism of cap-dependent translation, there is a cap-independent/m6A-dependent translation that directly recruits eIF3 and assembles translation initiation complexes by engaging the 40S ribosomal subunit without cap-binding proteins [39]. Interestingly, YTHDF3 has been shown to play a role in cap-independent translation to facilitate mRNA translation, including m6A-mediated circular RNA translation through a YTHDF1-independent mechanism [39,40] (Figure 1). Because of their role in regulating translation of target mRNA, YTH translation regulators play a critical role in tumorigenesis and development. For instance, YTHDF1 facilitates the tumorigenesis and metastasis of ovarian cancer by promoting the translation of EIF3C mRNA in an m6A-dependent manner [41]. Also, YTHDF1 promotes the translation of HINT2 mRNA, a tumor suppressor in ocular melanoma [42]. Under homeostatic condition, YTHDF3 acts as a negative regulator of antiviral immunity by promoting the translation of FOXO3 [43] which promotes cholangiocarcinoma migration and invasion through the Akt-FOXO3-NF-κβ signaling pathway [44].
YTHDC2 is essential for maintaining a transcriptome in the male germline [45]. Also, it directly recognizes m6A-containing transcripts to facilitate the translation efficiency of its targets, which are involved in the progression of meiotic prophase I by destabilizing the transcripts after translation is completed [25]. Recent studies have shown that YTHDC2 might facilitate translation associated with the ribosome by remodeling of the head and region of the 40S subunit and promoting disassembly of the ribosome-mRNA complex [37]. Likewise, dysregulation of the mRNA translation in the regulation of gene expression can be used as a biomarker for cancer development and progression [46]. For instance, YTHDC2 knockdown inhibits metastasis of colon tumor cells by attenuating the expression of metastasis-related genes, such as hypoxia-inducible factor-1alpha (HIF-1α) and twist1 [47]. Conversely, YTHDC2 can also be upregulated by drug treatment or its upstream regulators. Besides, YTHDC2 can be induced in tumor cell lines and hepatocytes after the administration of tumor necrosis factor (TNF)-α. And this reveals that activation/recruitment of c-Jun and ATF-2 to the cAMP response element of the YTHDC2 promoter is necessary for the transcription of YTHDC2, which plays an essential role in HCC progression [48].
YTH domain-containing proteins regulate mRNA decay
Given that the accuracy of genetic information encoded by DNA is important for the survival of cells and organisms, various surveillance pathways exist to control the quality of proteins involved in critical functions [49]. Ribonucleic acid (RNA) decay is a crucial step in the regulation of the RNA life cycle. YTHDF2 is composed of a C-terminal RNA-binding domain (C-YTHDF2) and a P/Q/N-rich N terminus (N-YTHDF2). YTHDF2 enhances its ability to mediate RNA deadenylation and RNA degradations through the recruitment of CCR4-NOT complex and by interacting with the SH domain of CNOT1 via its N-terminal region [50] (Figure 1). Interestingly, both N-YTHDF2 [50] and C-YTHDF2 could lead to a significantly reduced the total mRNA level, suggesting YTHDF2 is the primary RNA binding protein to facilitate RNA decay among the YTH domain-containing family of proteins [12]. Notably, the latest study indicates YTHDF2 can also interact with HRSP12 RBP and recruit RNase P/MRP endonuclease to promote mRNA decay [51]. In HCC cells, YTHDF2 promotes degradation of its target mRNA EGFR, which consequently inhibits cell proliferation and growth [52]. Also, YTHDF2 is involved in LINC00470-induced PTEN mRNA degradation in gastric cancer cells. PTEN has been characterized as a classical tumor suppressor [53]. YTHDF2 can downregulate the expression of the positive p53 regulator PRDM2 by promoting the decay of PRDM2 mRNAs in arsenite -transformed keratinocyte model cells, suggesting that arsenite could alter p53 expression in cell transformation and carcinogenesis through YTHDF2 [54]. In contrast, YTHDF2 is also regulated by none coding RNAs. As such, YTHDF2 translation can be suppressed by the overexpression of miR-493-3p. And this has been shown to inhibit the progression of prostate cancer (PCa) [55].
Other than YTHDF2, recent findings indicate that other YTH domain-containing proteins are also involved in the mRNA decay machinery [14,50]. Both YTHDF1 and YTHDF3 jointly promote deadenylation that affects the partitioning of methylated transcripts to YTHDF2 for accelerated decay [14] (Figure 1). In addition to YTHDF1-3, YTHDC2 may interact with the RNA transcript Cyclin A2 action in wild-type spermatocytes through the regulation of RNA stability [56]. Besides, YTHDC2 is a putative ATP-dependent RNA helicase that regulates RNA levels during meiosis by interacting with the meiosis-specific protein MEIOC in an RNA-independent manner to destabilize the stability of target transcripts during meiosis prophase I [25] (Figure 1). These findings suggest that YTH domain-containing proteins might regulate many aspects of mRNA decay via multiple mechanisms.
In summary, YTH domain-containing proteins play various roles in m6A-mediated gene expression through various mechanisms. In the cytoplasm, YTHDF1, YTHDF2 and, YTHDC2 regulates the translation of target mRNA in cap-independent/m6A-dependent translation manner. Also, YTHDF2 and YTHDC2 play crucial roles in RNA stability by regulating the RNA decay process. In the nucleus, YTHDC1 mediates the RNA splicing and export by interacting with the splicing factor and nuclear export adaptor protein. Given that YTH domain-containing proteins influence the expression of several genes, they are widely involved in cancer development and progression via various molecular mechanisms.
The role of YTH domain-containing protein signaling pathway in cancer development
Many studies have shown that YTH domain-containing protein expression is significantly correlated with cancer pathogenesis (Table 2). They are frequently upregulated and recognized as oncogenes in various cancers. Specifically, YTHDF1 is significantly up-regulated in the high grade of HCC [19] and has also been identified as a key mediator of tumor immune evasion [57]. YTHDF2 has been closely associated with pancreatic cancer, in which it promotes proliferation while inhibiting the migration and invasion of tumor cells [18]. On the other hand, YTHDF3 plays a role in seminoma phenotype maintenance [58]. Meanwhile, altered YTHDC1 alternative splicing is functionally coupled with RNA decay and transcription with consecutive impact on the processing of specific cancer-associated genes, including BRCA2 and PGR [59]. Studies have also reported that YTHDC2 contributes to the metastatic ability of colon tumor cells in vivo [48]. Numerous studies have demonstrated that the YTH domain-containing proteins are associated with an increased risk of various types of cancers, including HCC [19,48,52], prostate cancer [32,55], pancreatic cancers [18,60], gastric cancer [61], lung cancer [62], testicular germ cancer [58] and colon cancer [47] (Table 1).
Table 2.
Emerging YTH domain-containing proteins acting as potential diagnostic biomarkers or therapeutic targets in patients with cancer
| Genes | Cancers | Detectedmethods | Tissue expression | Roles in clinic | Ref |
|---|---|---|---|---|---|
| YTHDF1 | Colorectal cancer | IHC/TCGA | Overexpression | Poor prognosis | [63,64] |
| YTHDF1 | Hepatocellular carcinoma | TCGA | Overexpression | Poor prognosis | [19] |
| YTHDF1 | Breast cancer | IHC | Upregulated | Poor prognosis | [65] |
| YTHDF1 | Merkel cell carcinoma | IHC/WB | Highly amplified and expressed | Worse prognosis | [9] |
| YTHDF1 | Non-small cell lung cancer | IHC/TCGA | Significantly higher | Good prognosis | [66] |
| YTHDF1 | Ovarian cancer | GEO/qRT-PCR | Overexpression | Poor prognosis | [41] |
| YTHDF2 | Hepatocellular carcinoma | IHC | Downregulated | Tumor suppressor | [52] |
| YTHDF2 | Prostate cancer | IHC | Upregulated | Potential therapeutic target | [55] |
| YTHDF2 | Gastric cancer | TCGA/WB/qRT-PCR | Upregulated | Oncogene | [61] |
| YTHDF2 | Pancreatic cancer | IHC/TCGA | Upregulated | Predictive biomarker and therapeutic target | [18] |
| YTHDF2 | Lung cancer | IHC | Significantly higher | Oncogene | [62] |
| YTHDF2 | Acute myeloid leukemia | WB | Overexpressed | Therapeutic target | [72] |
| YTHDF3 | Testicular germ cancer | TCGA/IHC/RT-qPCR | Significantly higher | Novel biomarkers | [58] |
| YTHDF3 | Breast cancer | IHC | Upregulated | Poor prognosis | [65] |
| YTHDC1 | Prostate cancer | WB | Upregulated | Oncogene | [32] |
| YTHDC2 | Colon cancer | qRT-PCR | Upregulated | Diagnostic marker and therapeutic target | [47] |
| YTHDC2 | Hepatocellular carcinoma | IHC/WB/qRT-PCR | Significantly higher | Oncogene | [48] |
| YTHDC2 | Pancreatic adenocarcinoma | MLPA assay | Upregulated | Potential biomarker and therapeutic target | [60] |
| YTHDC2 | Head and neck squamous cell carcinoma | TCGA | Downdregulated | Prognosis marker | [90] |
Role of YTHDF1 in cancer development
Recent evidence suggests that YTHDF1 acts as an oncogene by regulating different signaling pathways in various cancers, such as CRC [63,64], HCC [19], breast cancer [65], merkel cell carcinoma [9], and non-small cell lung cancer [66]. Bai et al. reported that YTHDF1 mediated the Wnt/β-catenin pathway to affect tumorigenicity and stem cell-like activity of CRC cells by interacting with its downstream targets WNT6 and FZD9 mRNA [64] (Figure 2). Wnt/β-catenin signaling pathway plays a critical role in the regulation of cellular development and stemness and is strongly associated with the progression of various cancers, including CRC, HCC, and pancreatic ductal adenocarcinoma [67].
Figure 2.
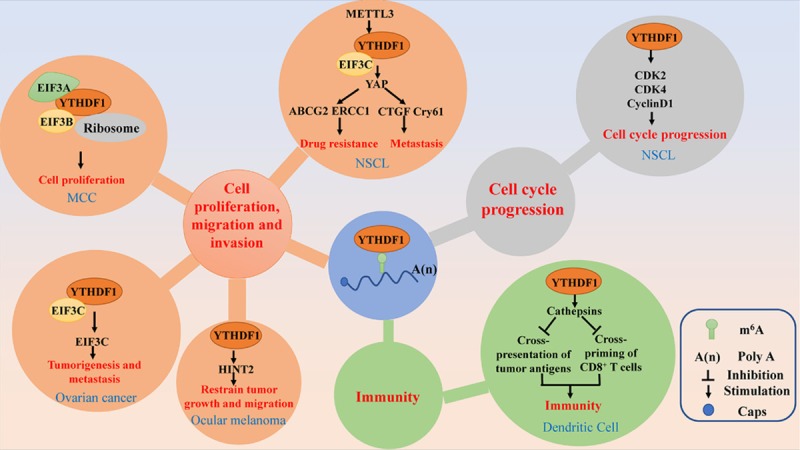
The main signal pathways of YTHDF1 involved in human cancers. YTHDF1 plays important roles in cancer related cell cycle progression, cell proliferation, migration and invasion, drug resistance and immunity through different signal pathways.
The JAK2-STAT3 pathway plays vital functions in oncogenesis and progression and is reported to contribute to tumor invasion in glioblastoma cancer [68]. It has been reported that the downregulation of METTL3 reduces the m6A level of JAK2, thus leading to the inhibition of YTHDF1-mediated JAK2 translation [69]. The non-receptor tyrosine kinase JAK2 phosphorylates STAT3 and activate the JAK2-STAT3 pathway [70]. In addition, YTHDF1, which also plays an essential role in regulating cell cycle progression and metabolism, is significantly upregulated and positively correlated with the pathology stage of HCC patients [19]. Moreover, YTHDF1 expression is significantly associated with various CRC characteristics, such as depth, lymph node metastasis, advanced cancer stages, and poor prognosis, indicating that its expression might act as a useful independent prognostic factor for CRC. Mechanistically, YTHDF1 can be directly upregulated by oncogenic transcription factor c-Myc [63].
Cell-mediated immunity plays a vital role in the pathogenesis of various cancers. Han et al. reported that YTHDF1 mediates tumor immune evasion. In detail, YTHDF1 recognizes and binds the transcripts encoding lysosomal proteases marked by m6A, thus promoting the translation of lysosomal cathepsins, which then reduces the cross-presentation of tumor antigens and the antigen-specific CD8+ T cell antitumor response [57] (Figure 2).
Role of YTHDF2 in cancer development and progression
At present, YTHDF2 is the most extensively studied YTH domain-containing protein in cancer among the YTHDFs and YTHDCs. Significant sequence homology among YTHDF1 and YTHDF2 indicates that they may play a similar role in oncogenesis and progression. Generally, YTHDF2 plays a pro-tumorigenic role in various cancers. As one of the RUNX1 translocation partner genes, it regulates the expression of proteins with unique leukemogenic potential [71]. YTHDF2 is overexpressed in a broad spectrum of human AML and is required for disease initiation and propagation of mouse and human AML [72]. In prostate cancer, downregulation of YTHDF2, which is usually upregulated in both cancer cells and tissues, significantly inhibits the cell proliferation and migration by raising m6A levels of target RNAs [55]. A study found that both the mRNA and protein levels of YTHDF2 were higher in pancreatic cancer tissues than normal tissues of patients, indicating that it might play an oncogene role in pancreatic cancer [18]. Also, it has been reported that METTL3 depletion downregulates the m6A level of SOCS3, thereby inhibiting the YTHDF2-dependent mRNA decay of SOCS3 [69]. Moreover, SOCS3 is a key negative regulator of the JAK2-STAT3 signaling pathway (Figure 3) and contributes to tumor invasion of glioblastoma cancer [73].
Figure 3.
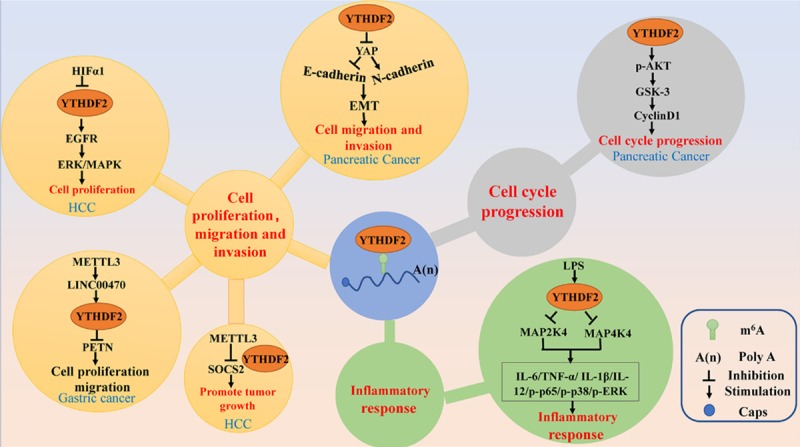
The main signal pathways of YTHDF2 in human cancers. YTHDF2 regulates cancer cell cycle progression, cell proliferation, migration and invasion, and inflammatory response through different signal pathway.
Hippo pathway is an emerging signaling pathway that regulates cell proliferation, apoptosis, and stem cell function. As such, it promotes cell metastasis by regulating the cell polarity and cell adhesion proteins. Chen et al. observed a higher mRNA and protein levels of YTHDF2 in the pancreatic cancer tissues than normal tissues. Furthermore, the YTHDF2 levels were higher in patients with later stages of the disease, suggesting that it might act as a marker for clinical diagnosis and prognosis of pancreatic cancers (Table 2). Besides, it promotes cell proliferation and inhibits the epithelial-mesenchymal transition (EMT) in pancreatic cancer cells. Mechanistically, YTHDF2 inhibits EMT by suppressing upstream YAP molecules, thereby inhibiting E-cadherin, which is a negative EMT regulator. Thus, resulting in the suppression of the invasion and adhesion ability pancreatic cancer cells [18] (Figure 3). Yes-associated protein (YAP) as the core components of the Hippo pathway promotes the proliferation and survival of epithelial cells. Also, activated YAP is involved in the EMT, which contributes to tumor growth and metastasis in response to the TGFβ/BMP (transforming growth factor-beta/bone morphogenetic protein) signaling [73,74].
The generic mitogen-activated protein kinases (MAPK) signaling pathway consists of four distinct cascades, including the extracellular signal-related kinases (ERK1/2), Jun amino-terminal kinases (JNK1/2/3), p38-MAPK, and ERK5. These signaling pathways play a critical role in linking extracellular transcription factor stimulation to cell proliferation, differentiation, migration, senescence, and apoptosis [75]. In HCC cells, YTHDF2 promotes the degradation of EGFR mRNA by directly binding the m6A modification site of its 3’-UTR, which in turn suppresses ERK/MAPK signaling, thus inhibiting cell proliferation and growth [52] (Figure 3).
In addition to the above listed anti-tumorigenic roles of YTHDF2 ascribed to the MAPK/ERK pathway, YTHDF2 has been shown to play the opposite role in different tumor cells. For example, YTHDF2 knockdown increases the proportion of G1/G0-phase cells with a corresponding decrease in the fraction of S-phase cells. Mechanistically, YTHDF2 knockdown down-regulates the expression of p-GSK3β, Cyclin D1, and p-Akt, indicating that YTHDF2 enhances the ability of cell proliferation possibly via Akt/GSK3b/Cyclin D1 pathway in pancreatic cancer cells [18]. The activation of Cyclin D1 through the Akt/GSK3β pathway leads to aberrant cell cycle re-entry via the activation of the Cyclin D1-Rb-E2F1 axis [76] (Figure 3). Studies have demonstrated the potential role of Akt/GSK3b/Cyclin D1 signaling in tumor initiation and progression. The AKT-mediated phosphorylation of GSK3β is a key signaling cascade involved in the regulation of the nuclear export and the cytoplasmic proteasomal degradation of cyclin D1 and is strongly associated with the growth, hypoxia, and metabolism of tumor cells. Furthermore, as the most important AKT target and the downstream of the AKT pathway, cyclin D1/Cdk4 regulates radio and chemo-sensitivity of tumor cells [77].
Recently, a similar causal link between YTHDF2 and inflammatory response was established, which shows that YTHDF2 might play a negative regulatory role in LPS-induced inflammatory responses of macrophages. It stabilizes the mRNA transcripts, thus increasing the mRNA expression levels of MAP2K4 and MAP4K4. Therefore, YTHDF2 knockdown activates MAPK and NF-κB signaling pathways, thereby promoting proinflammatory cytokine expression and thus aggravates the inflammatory response. In detail, YTHDF2 knockdown increases the expression of the LPS-induced IL-6, TNF-α, IL-1β, and IL-12 and the phosphorylation of NF-κB and MAPK signaling proteins, such as p65, p38, and ERK1/2 [78] (Figure 3). Grace et al. demonstrated that YTHDF2 is essential for suppression of innate immunity by sequestering m6A modified circRNAs which are potent adjuvants to induce antigen-specific T cell activation, antibody production, and anti-tumor immunity [79] (Figure 3). It is widely accepted that inflammation, innate immunity, and cancer have a causal tumor-associated relationship [80]. Another study indicates that the reduction of YTHDF2 leads to inflammation and vascular abnormalization by processing inflammatory cytokines IL11 and SERPINE2 mRNAs to decay in HCC [81].
Role of YTHDF3 in the development and progression of various cancers
The expression of YTHDF1 has been studied using different methods, including immunohistochemistry (IHC), RT-PCR, and TCGA [19,63,64], whereas YTHDF3 expression has been analyzed mainly by IHC [58]. This shows that several approaches can be employed to determine the expression of YTH family proteins in cancer cells or tissues. However, further studies are needed to explore the potential molecular mechanism of YTHDF3 in cancer development and progression.
Recent reports show that YTHDF3 is correlated with various types of cancers, although the roles and molecular mechanisms of YTHDF3 in tumorigenesis have not been fully elucidated. Similar to YTHDF1, YTHDF3 is upregulated in acute myeloid leukemia clinical samples [82,83]. In addition, sporadic data indicate significantly higher levels of YTHDF3 mRNA in seminomas (SEs) than in non-seminomatous tumors (NSTs) [58].
Activation of the Hippo/YAP signaling pathway is crucial for cancer progression, such as CRC by promoting cell proliferation, invasion, and metastasis. In normal cells, by directly binding the WW domain of YAP, long none coding RNA (lnc RNA) GAS5 can promote the translocation of endogenous YAP from the nucleus to the cytoplasm, and then phosphorylation and subsequently ubiquitination, resulting in ubiquitin-mediated degradation of YAP to inhibit CRC development (Figure 4). Whist in CRC cells, translocation of YAP from the cytoplasm to the nucleus can activate YTHDF3 expression, thereby facilitating m6A-modified GAS5 degradation, and eventually increasing the expression of YAP to promote CRC progression (Figure 4). All these results indicate a negative functional loop of the GAS5-YAP-YTHDF3 axis. And identifies the molecular mechanism for YTHDF3 mediated m6A-induced decay of GAS5 on YAP signaling during the CRC progression (Figure 4). Also, these findings provide new insight into the development of a promising approach for CRC treatment [84].
Figure 4.
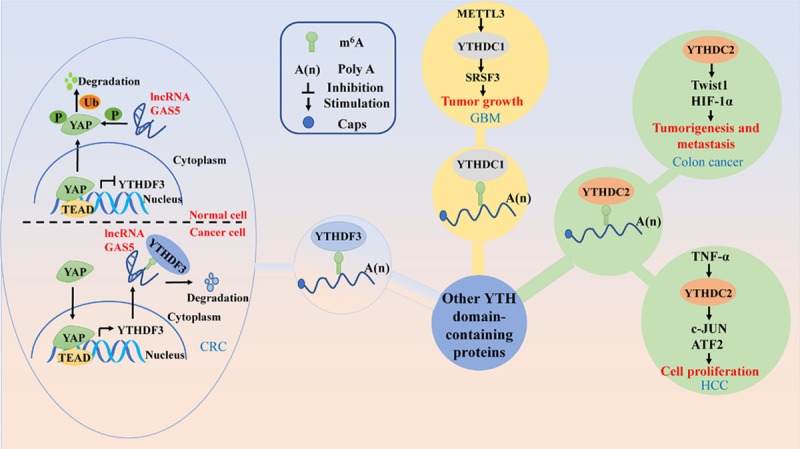
The main signal pathways of other YTH domain-containing proteins in human cancers. YTHDF3 plays different roles in normal cells and cancer cells by different mechanisms. YTHDC1 induces tumor growth, and YTHDC2 regulates cancer cell proliferation, tumorigenesis and metastasis in human cancers.
Forkhead box protein O3 (FOXO3) plays a crucial role in various cancers and promotes cholangiocarcinoma migration and invasion through the Akt-FOXO3-NF-κB signal pathway [44]. Under the homeostatic condition, YTHDF3 acts as a negative regulator of antiviral immunity by promoting the translation of FOXO3. In detail, YTHDF3, which cooperates with PABP1 and eIF4G2, promotes FOXO3 translation by directly binding its translation initiation region, thus suppress IFN-stimulated genes, which are essential effectors of the IFN-dependent antiviral immune response under basal conditions [43].
Role of YTHDCs in cancer development and progression
The role of YTHDCs in tumorigenesis is exemplified by the function of YTHDC1 as an mRNA splicing protein. Recent studies have revealed the different expression patterns of YTHDC1 in human cancers. The expression of YTHDC1, particularly the alternative splicing component has been detected in a panel of prostate cell lines and not in the benign prostate cell lines, indicating that YTHDC1 may act as an oncogene in prostate cancer [32]. Besides, YTHDC1 is abundantly expressed in colon adenocarcinoma but not in rectal adenocarcinoma [20], suggesting that YTHDC1 might be differentially expressed in various cancers during to tumor heterogeneity. YTHDC1 can also mediate the expresison of target mRNAs by interacting with methyltransferases. For instance, downregulation of METTL3 inhibits the m6A modification levels of SRSFs, which leads to YTHDC1-dependent nonsense-mediated mRNA decay of SRSF transcripts and decreases SRSF protein expression. Downregulated expression of SRSFs leads to larger changes in alternative splicing isoform switches, which affects glioblastoma tumor growth and progression [85] (Figure 4). However, further studies should be conducted to understand how YTHDC1 drives tumorigenesis.
Concerning YTHDC2, growing evidence shows that YTHDC2 also induces tumorigenic effects. Besides, it plays an essential role in the growth and proliferation of HCC cells and its expression in hepatocytes could be induced by tumor necrosis factor (TNF)-α [48] (Figure 4). Moreover, YTHDC2 is frequently involved in pancreatic adenocarcinoma susceptibility [60]. Also, YTHDC2 is associated with colon cancer progression and contributes to colon tumor metastasis by promoting the translation of HIF-1α [47] (Figure 4).
Emerging YTH domain-containing proteins acts as a diagnostic biomarker/therapeutic target for cancer patients
Almost all the reported YTH family members, which are closely associated with the progression of various types of cancer, regulates some specific downstream RNA targets to mediate various RNA processes, such as RNA splicing, nuclear export, translation, and decay. For instance, C-MYC, β-catenin, HINT2 [42], EIF3C [41], PETN [53], EGFR, YAP, c-Jun, and ATF-2 are the targets of YTH domain-containing proteins and play important roles in tumorigenesis [18,52,63,64]. Photoactivatable-ribonucleoside-enhanced crosslinking and immunoprecipitation (PAR-CLIP) and RNA binding protein immunoprecipitation (RIP) studies have shown that more than 2000 target mRNAs are targets for YTHDF2 [51].
Nearly all the YTH proteins, including YTHDF1-3 and YTHDC1-2, are upregulated in most types of cancer (Table 2). A higher level of YTHDF1 expression predicts poor disease outcome in CRC [63,64], HCC [19], and breast cancer [65]. Also, YTHDF2 acts as prognostic marker and therapeutic target or oncogene in prostate cancer [55], gastric cancer [61], pancreatic cancer [18], Lung cancer [62], acute myeloid leukemia [72]. However, YTHDF2 is reported to be downregulated and acts as a tumor suppressor in HCC [52] (Table 2). Similarly, the upregulation of YTHDC1 acts as a diagnostic marker, therapeutic target or oncogene in colon cancer [47], HCC [48], pancreatic adenocarcinoma [60]. However, its downregulation indicates a good prognosis in head and neck squamous cell carcinoma [89] (Table 2). The difference in expressions and roles played by a particular YTH protein in the development and progression of different cancers can be attributed to tumor heterogeneity. Notably, there are limited reports on YTHDF3 and YTHDC1 relative to other YTH proteins. The former is upregulated and acts as a diagnostic and prognostic marker for testicular germ cancer [58] and breast cancer [65], whereas the latter is overexpressed and acts as an oncogene in prostate cancer [32] (Table 2).
Conclusions and perspective
Several studies have investigated the roles and molecular mechanisms of YTH domain-containing proteins in various cancers, especially in last the three years. YTH domain-containing proteins are known to regulate the process of target RNAs in RNA splicing, nuclear export, translation, and decay (Table 1). Also, it has been shown that the regulatory mechanisms of YTH domain-containing proteins are not uniform, many signaling pathways are involved in its regulation. For instance, the main binding regions of YTHDF2 and its targets are predominantly the stop codon region, the 3’ untranslated region (3’-UTR), and the coding region (CDS), which indicates that YTHDF2 may play a role in mRNA stability and/or translation [12]. Indeed, the main functions of YTHDF2-mediated RNA processing are mRNA stability and translation, as well as cell growth and proliferation. At the same time, increasing number of evidence shows the upstream regulation mechanism of YTH domain-containing proteins and their roles in cancer. For example, circFOREIGN, HIF1α, and miR-493-3p dysregulate the expression of the YTHDF2-downstream signal pathway by modulating the expression of YTHDF2 [52,55,79]. MiRNAs target the 3’-UTR, 5’-UTR, and CDS of YTH domain-containing proteins to regulate the expression of YTH domain-containing protein downstream targets and affect tumor cell proliferation, migration, and invasion. In addition, the role of YTH family proteins in driving expression and production of downstream pro-tumorigenic cytokines appears to play a key role in cancer development.
Interestingly, METTL3 enhances YAP translation by recruiting YTHDF1/3 and eIF3b to the translation initiation factors and this has been shown to promote EMT in lung cancer [91]. On the other hand, YTHDF2 directly binds to YAP mRNA to decrease its stability, thereby inhibiting EMT in pancreatic cancer cells [18]. This phenomenon suggests that different YTH domain-containing proteins on the same mRNA transcript (e.g., YAP) may result in distinct fates probably because of multiple mechanisms.
Almost all the YTH family proteins act as oncogenes in various types of cancer, except that YTHDF2 is downregulated in HCC and YTHDC2 is downregulated in head and neck squamous cell carcinoma (Table 2). For example, YTHDF2 not only promotes cancer cell proliferation [18,55], metastasis [55] but also acts as a tumor suppressor to inhibit cell proliferation [52]. Furthermore, YTHDF2 represses tumor cell proliferation and growth by directly binding with the m6A modification site of EGFR 3’-UTR to promote EGFR mRNA decay in HCC cells [52] (Figure 3).
Moreover, YTH domain-containing proteins play an important role in autophagy, adipogenesis, inflammatory response, and immunity, which are crucial processes in cancer development and progression. Studies have shown that Atg5 and Atg7 transcripts with higher m6A levels caused by FTO silencing are captured by YTHDF2, thereby leading to their degradation and translation, thus alleviating autophagy and adipogenesis (Table 1) [86]. Concerning the role of YTHDF2 in inflammatory response, it has been established that YTHDF2 inhibits LPS-induced inflammatory responses by downregulating inflammatory cytokines [78] (Figure 3). Moreover, it has been demonstrated that YTHDF2 suppresses innate immunity by sequestering m6A modified circRNAs [79].
In conclusion, YTH domain-containing proteins are closely associated with various types of cancer. However, much more investigation is still required to explore the comprehensive and profound features of YTH domain-containing proteins or other new m6A readers in tumorigenesis and development. Based on these findings, some of the YTH domain-containing proteins and their critical targets could offer new insights into the identification of early cancer diagnosis and therapeutic targets for the treatment of human cancers.
Acknowledgements
This study was supported by the National Natural Science Foundation of China (81802371); Key Projects of National Natural Science Foundation of China (81730108); Key Project of Zhejiang Province Ministry of Science and Technology (2015C03055); Zhejiang Provincial Natural Science Foundation (LQ17H160009); Key Project of Hangzhou Ministry of Science and Technology (20162013A07); Zhejiang Province Medical Science and Technology Project (2018KY108), Hangzhou Agricultural and Social Development Scientific Research Independent Application Project (20191203B22); and Opening Project of Zhejiang Provincial Preponderant and Characteristic Subject of Key University (Chinese Traditional Medicine), Zhejiang Chinese Medical University (ZYX2018005).
Disclosure of conflict of interest
None.
References
- 1.Fu Y, Dominissini D, Rechavi G, He C. Gene expression regulation mediated through reversible m6A RNA methylation. Nat Rev Genet. 2014;15:293–306. doi: 10.1038/nrg3724. [DOI] [PubMed] [Google Scholar]
- 2.Du K, Zhang L, Lee T, Sun T. m6A RNA methylation controls neural development and is involved in human diseases. Mol Neurobiol. 2019;56:1596–1606. doi: 10.1007/s12035-018-1138-1. [DOI] [PubMed] [Google Scholar]
- 3.van Tran N, Ernst FGM, Hawley BR, Zorbas C, Ulryck N, Hackert P, Bohnsack KE, Bohnsack MT, Jaffrey SR, Graille M, Lafontaine DLJ. The human 18S rRNA m6A methyltransferase METTL5 is stabilized by TRMT112. Nucleic Acids Res. 2019;47:7719–7733. doi: 10.1093/nar/gkz619. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Warda AS, Kretschmer J, Hackert P, Lenz C, Urlaub H, Hobartner C, Sloan KE, Bohnsack MT. Human METTL16 is a N6-methyladenosine (m6A) methyltransferase that targets pre-mRNAs and various non-coding RNAs. EMBO Rep. 2017;18:2004–2014. doi: 10.15252/embr.201744940. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Melstrom L, Chen J. RNA N6-methyladenosine modification in solid tumors: new therapeutic frontiers. Cancer Gene Ther. 2020 doi: 10.1038/s41417-020-0160-4. [Epub ahead of print] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Ma H, Wang X, Cai J, Dai Q, Natchiar SK, Lv R, Chen K, Lu Z, Chen H, Shi YG, Lan F, Fan J, Klaholz BP, Pan T, Shi Y, He C. N6-methyladenosine methyltransferase ZCCHC4 mediates ribosomal RNA methylation. Nat Chem Biol. 2019;15:88–94. doi: 10.1038/s41589-018-0184-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Patil DP, Pickering BF, Jaffrey SR. Reading m6A in the transcriptome: m6A-binding proteins. Trends Cell Biol. 2018;28:113–127. doi: 10.1016/j.tcb.2017.10.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Liao S, Sun H, Xu C. YTH domain: a family of N6-methyladenosine (m6A) readers. Genomics Proteomics Bioinformatics. 2018;16:99–107. doi: 10.1016/j.gpb.2018.04.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Orouji E, Peitsch WK, Orouji A, Houben R, Utikal J. Oncogenic role of an epigenetic reader of m6A RNA modification: YTHDF1 in merkel cell carcinoma. Cancers (Basel) 2020;12 doi: 10.3390/cancers12010202. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Dominissini D, Moshitch-Moshkovitz S, Schwartz S, Salmon-Divon M, Ungar L, Osenberg S, Cesarkas K, Jacob-Hirsch J, Amariglio N, Kupiec M, Sorek R, Rechavi G. Topology of the human and mouse m6A RNA methylomes revealed by m6A-seq. Nature. 2012;485:201–206. doi: 10.1038/nature11112. [DOI] [PubMed] [Google Scholar]
- 11.Zhang Z, Theler D, Kaminska KH, Hiller M, de la Grange P, Pudimat R, Rafalska I, Heinrich B, Bujnicki JM, Allain FH, Stamm S. The YTH domain is a novel RNA binding domain. J Biol Chem. 2010;285:14701–14710. doi: 10.1074/jbc.M110.104711. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Wang X, Lu Z, Gomez A, Hon GC, Yue Y, Han D, Fu Y, Parisien M, Dai Q, Jia G, Ren B, Pan T, He C. N6-methyladenosine-dependent regulation of messenger RNA stability. Nature. 2014;505:117–120. doi: 10.1038/nature12730. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Chen M, Wei L, Law CT, Tsang FH, Shen J, Cheng CL, Tsang LH, Ho DW, Chiu DK, Lee JM, Wong CC, Ng IO, Wong CM. RNA N6-methyladenosine methyltransferase-like 3 promotes liver cancer progression through YTHDF2-dependent posttranscriptional silencing of SOCS2. Hepatology. 2018;67:2254–2270. doi: 10.1002/hep.29683. [DOI] [PubMed] [Google Scholar]
- 14.Shi H, Wang X, Lu Z, Zhao BS, Ma H, Hsu PJ, Liu C, He C. YTHDF3 facilitates translation and decay of N6-methyladenosine-modified RNA. Cell Res. 2017;27:315–328. doi: 10.1038/cr.2017.15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Lin S, Choe J, Du P, Triboulet R, Gregory RI. The m6A methyltransferase METTL3 promotes translation in human cancer cells. Mol Cell. 2016;62:335–345. doi: 10.1016/j.molcel.2016.03.021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Li Z, Weng H, Su R, Weng X, Zuo Z, Li C, Huang H, Nachtergaele S, Dong L, Hu C, Qin X, Tang L, Wang Y, Hong GM, Wang X, Chen P, Gurbuxani S, Arnovitz S, Li Y, Li S, Strong J, Neilly MB, Larson RA, Jiang X, Zhang P, Jin J, He C, Chen J. FTO plays an oncogenic role in acute myeloid leukemia as a N6-methyladenosine RNA demethylase. Cancer Cell. 2017;31:127–141. doi: 10.1016/j.ccell.2016.11.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Yang Z, Li J, Feng G, Gao S, Wang Y, Zhang S, Liu Y, Ye L, Li Y, Zhang X. MicroRNA-145 modulates N6-methyladenosine levels by targeting the 3’-Untranslated mRNA region of the N6-methyladenosine binding YTH domain family 2 protein. J Biol Chem. 2017;292:3614–3623. doi: 10.1074/jbc.M116.749689. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Chen J, Sun Y, Xu X, Wang D, He J, Zhou H, Lu Y, Zeng J, Du F, Gong A, Xu M. YTH domain family 2 orchestrates epithelial-mesenchymal transition/proliferation dichotomy in pancreatic cancer cells. Cell Cycle. 2017;16:2259–2271. doi: 10.1080/15384101.2017.1380125. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Zhao X, Chen Y, Mao Q, Jiang X, Jiang W, Chen J, Xu W, Zhong L, Sun X. Overexpression of YTHDF1 is associated with poor prognosis in patients with hepatocellular carcinoma. Cancer Biomark. 2018;21:859–868. doi: 10.3233/CBM-170791. [DOI] [PubMed] [Google Scholar]
- 20.Liu X, Liu L, Dong Z, Li J, Yu Y, Chen X, Ren F, Cui G, Sun R. Expression patterns and prognostic value of m6A-related genes in colorectal cancer. Am J Transl Res. 2019;11:3972–3991. [PMC free article] [PubMed] [Google Scholar]
- 21.Adhikari S, Xiao W, Zhao YL, Yang YG. m6A: signaling for mRNA splicing. RNA Biol. 2016;13:756–759. doi: 10.1080/15476286.2016.1201628. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Zhao YL, Liu YH, Wu RF, Bi Z, Yao YX, Liu Q, Wang YZ, Wang XX. Understanding m6A function through uncovering the diversity roles of YTH domain-containing proteins. Mol Biotechnol. 2019;61:355–364. doi: 10.1007/s12033-018-00149-z. [DOI] [PubMed] [Google Scholar]
- 23.Wang X, Zhao BS, Roundtree IA, Lu Z, Han D, Ma H, Weng X, Chen K, Shi H, He C. N6-methyladenosine modulates messenger RNA translation efficiency. Cell. 2015;161:1388–1399. doi: 10.1016/j.cell.2015.05.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Xiao W, Adhikari S, Dahal U, Chen YS, Hao YJ, Sun BF, Sun HY, Li A, Ping XL, Lai WY, Wang X, Ma HL, Huang CM, Yang Y, Huang N, Jiang GB, Wang HL, Zhou Q, Wang XJ, Zhao YL, Yang YG. Nuclear m6A reader YTHDC1 regulates mRNA splicing. Mol Cell. 2016;61:507–519. doi: 10.1016/j.molcel.2016.01.012. [DOI] [PubMed] [Google Scholar]
- 25.Hsu PJ, Zhu Y, Ma H, Guo Y, Shi X, Liu Y, Qi M, Lu Z, Shi H, Wang J, Cheng Y, Luo G, Dai Q, Liu M, Guo X, Sha J, Shen B, He C. Ythdc2 is an N6-methyladenosine binding protein that regulates mammalian spermatogenesis. Cell Res. 2017;27:1115–1127. doi: 10.1038/cr.2017.99. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Venables JP. Aberrant and alternative splicing in cancer. Cancer Res. 2004;64:7647–7654. doi: 10.1158/0008-5472.CAN-04-1910. [DOI] [PubMed] [Google Scholar]
- 27.Nayler O, Hartmann AM, Stamm S. The ER repeat protein YT521-B localizes to a novel subnuclear compartment. J Cell Biol. 2000;150:949–962. doi: 10.1083/jcb.150.5.949. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Kozlovski I, Siegfried Z, Amar-Schwartz A, Karni R. The role of RNA alternative splicing in regulating cancer metabolism. Hum Genet. 2017;136:1113–1127. doi: 10.1007/s00439-017-1803-x. [DOI] [PubMed] [Google Scholar]
- 29.Frankiw L, Baltimore D, Li G. Alternative mRNA splicing in cancer immunotherapy. Nat Rev Immunol. 2019;19:675–687. doi: 10.1038/s41577-019-0195-7. [DOI] [PubMed] [Google Scholar]
- 30.Roundtree IA, Luo GZ, Zhang Z, Wang X, Zhou T, Cui Y, Sha J, Huang X, Guerrero L, Xie P, He E, Shen B, He C. YTHDC1 mediates nuclear export of N6-methyladenosine methylated mRNAs. Elife. 2017:6. doi: 10.7554/eLife.31311. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Song X, Wan X, Huang T, Zeng C, Sastry N, Wu B, James CD, Horbinski C, Nakano I, Zhang W, Hu B, Cheng SY. SRSF3-regulated RNA alternative splicing promotes glioblastoma tumorigenicity by affecting multiple cellular processes. Cancer Res. 2019;79:5288–5301. doi: 10.1158/0008-5472.CAN-19-1504. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Luxton HJ, Simpson BS, Mills IG, Brindle NR, Ahmed Z, Stavrinides V, Heavey S, Stamm S, Whitaker HC. The oncogene metadherin interacts with the known splicing proteins YTHDC1, Sam68 and T-STAR and plays a novel role in alternative mRNA splicing. Cancers (Basel) 2019;11 doi: 10.3390/cancers11091233. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Zhang B, zur Hausen A, Orlowska-Volk M, Jager M, Bettendorf H, Stamm S, Hirschfeld M, Yiqin O, Tong X, Gitsch G, Stickeler E. Alternative splicing-related factor YT521: an independent prognostic factor in endometrial cancer. Int J Gynecol Cancer. 2010;20:492–499. doi: 10.1111/IGC.0b013e3181d66ffe. [DOI] [PubMed] [Google Scholar]
- 34.Sonenberg N, Hinnebusch AG. Regulation of translation initiation in eukaryotes: mechanisms and biological targets. Cell. 2009;136:731–745. doi: 10.1016/j.cell.2009.01.042. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Shi H, Zhang X, Weng YL, Lu Z, Liu Y, Lu Z, Li J, Hao P, Zhang Y, Zhang F, Wu Y, Delgado JY, Su Y, Patel MJ, Cao X, Shen B, Huang X, Ming GL, Zhuang X, Song H, He C, Zhou T. m6A facilitates hippocampus-dependent learning and memory through YTHDF1. Nature. 2018;563:249–253. doi: 10.1038/s41586-018-0666-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Li A, Chen YS, Ping XL, Yang X, Xiao W, Yang Y, Sun HY, Zhu Q, Baidya P, Wang X, Bhattarai DP, Zhao YL, Sun BF, Yang YG. Cytoplasmic m6A reader YTHDF3 promotes mRNA translation. Cell Res. 2017;27:444–447. doi: 10.1038/cr.2017.10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Kretschmer J, Rao H, Hackert P, Sloan KE, Hobartner C, Bohnsack MT. The m6A reader protein YTHDC2 interacts with the small ribosomal subunit and the 5’-3’ exoribonuclease XRN1. RNA. 2018;24:1339–1350. doi: 10.1261/rna.064238.117. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Stoneley M, Willis AE. Cellular internal ribosome entry segments: structures, trans-acting factors and regulation of gene expression. Oncogene. 2004;23:3200–3207. doi: 10.1038/sj.onc.1207551. [DOI] [PubMed] [Google Scholar]
- 39.Meyer KD, Patil DP, Zhou J, Zinoviev A, Skabkin MA, Elemento O, Pestova TV, Qian SB, Jaffrey SR. 5’ UTR m(6)A promotes cap-independent translation. Cell. 2015;163:999–1010. doi: 10.1016/j.cell.2015.10.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Coots RA, Liu XM, Mao Y, Dong L, Zhou J, Wan J, Zhang X, Qian SB. m6A facilitates eIF4F-independent mRNA translation. Mol Cell. 2017;68:504–514. e7. doi: 10.1016/j.molcel.2017.10.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Liu T, Wei Q, Jin J, Luo Q, Liu Y, Yang Y, Cheng C, Li L, Pi J, Si Y, Xiao H, Rao S, Wang F, Yu J, Zou D, Yi P. The m6A reader YTHDF1 promotes ovarian cancer progression via augmenting EIF3C translation. Nucleic Acids Res. 2020 doi: 10.1093/nar/gkaa048. [Epub ahead of print] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Jia R, Chai P, Wang S, Sun B, Xu Y, Yang Y, Ge S, Yang YG, Fan X. m6A modification suppresses ocular melanoma through modulating HINT2 mRNA translation. Mol Cancer. 2019;18:161. doi: 10.1186/s12943-019-1088-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Zhang Y, Wang X, Zhang X, Wang J, Ma Y, Zhang L, Cao X. RNA-binding protein YTHDF3 suppresses interferon-dependent antiviral responses by promoting FOXO3 translation. Proc Natl Acad Sci U S A. 2019;116:976–981. doi: 10.1073/pnas.1812536116. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Dana P, Saisomboon S, Kariya R, Okada S, Obchoei S, Sawanyawisuth K, Wongkham C, Pairojkul C, Wongkham S, Vaeteewoottacharn K. CD147 augmented monocarboxylate transporter-1/4 expression through modulation of the Akt-FoxO3-NF-kappaB pathway promotes cholangiocarcinoma migration and invasion. Cell Oncol (Dordr) 2020;43:211–222. doi: 10.1007/s13402-019-00479-3. [DOI] [PubMed] [Google Scholar]
- 45.Wojtas MN, Pandey RR, Mendel M, Homolka D, Sachidanandam R, Pillai RS. Regulation of m6A transcripts by the 3’→5’ RNA helicase YTHDC2 is essential for a successful meiotic program in the mammalian germline. Mol Cell. 2017;68:374–387. e12. doi: 10.1016/j.molcel.2017.09.021. [DOI] [PubMed] [Google Scholar]
- 46.Bhat M, Robichaud N, Hulea L, Sonenberg N, Pelletier J, Topisirovic I. Targeting the translation machinery in cancer. Nat Rev Drug Discov. 2015;14:261–278. doi: 10.1038/nrd4505. [DOI] [PubMed] [Google Scholar]
- 47.Tanabe A, Tanikawa K, Tsunetomi M, Takai K, Ikeda H, Konno J, Torigoe T, Maeda H, Kutomi G, Okita K, Mori M, Sahara H. RNA helicase YTHDC2 promotes cancer metastasis via the enhancement of the efficiency by which HIF-1alpha mRNA is translated. Cancer Lett. 2016;376:34–42. doi: 10.1016/j.canlet.2016.02.022. [DOI] [PubMed] [Google Scholar]
- 48.Tanabe A, Konno J, Tanikawa K, Sahara H. Transcriptional machinery of TNF-alpha-inducible YTH domain containing 2 (YTHDC2) gene. Gene. 2014;535:24–32. doi: 10.1016/j.gene.2013.11.005. [DOI] [PubMed] [Google Scholar]
- 49.Popp MW, Maquat LE. Nonsense-mediated mRNA decay and cancer. Curr Opin Genet Dev. 2018;48:44–50. doi: 10.1016/j.gde.2017.10.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Du H, Zhao Y, He J, Zhang Y, Xi H, Liu M, Ma J, Wu L. YTHDF2 destabilizes m6A-containing RNA through direct recruitment of the CCR4-NOT deadenylase complex. Nat Commun. 2016;7:12626. doi: 10.1038/ncomms12626. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Park OH, Ha H, Lee Y, Boo SH, Kwon DH, Song HK, Kim YK. Endoribonucleolytic cleavage of m6A-containing RNAs by RNase P/MRP complex. Mol Cell. 2019;74:494–507. e8. doi: 10.1016/j.molcel.2019.02.034. [DOI] [PubMed] [Google Scholar]
- 52.Zhong L, Liao D, Zhang M, Zeng C, Li X, Zhang R, Ma H, Kang T. YTHDF2 suppresses cell proliferation and growth via destabilizing the EGFR mRNA in hepatocellular carcinoma. Cancer Lett. 2019;442:252–261. doi: 10.1016/j.canlet.2018.11.006. [DOI] [PubMed] [Google Scholar]
- 53.Yan J, Huang X, Zhang X, Chen Z, Ye C, Xiang W, Huang Z. LncRNA LINC00470 promotes the degradation of PTEN mRNA to facilitate malignant behavior in gastric cancer cells. Biochem Biophys Res Commun. 2020;521:887–893. doi: 10.1016/j.bbrc.2019.11.016. [DOI] [PubMed] [Google Scholar]
- 54.Zhao T, Sun D, Zhao M, Lai Y, Liu Y, Zhang Z. N6-methyladenosine mediates arsenite-induced human keratinocyte transformation by suppressing p53 activation. Environ Pollut. 2020;259:113908. doi: 10.1016/j.envpol.2019.113908. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Li J, Meng S, Xu M, Wang S, He L, Xu X, Wang X, Xie L. Downregulation of N6-methyladenosine binding YTHDF2 protein mediated by miR-493-3p suppresses prostate cancer by elevating N6-methyladenosine levels. Oncotarget. 2018;9:3752–3764. doi: 10.18632/oncotarget.23365. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Bailey AS, Batista PJ, Gold RS, Chen YG, de Rooij DG, Chang HY, Fuller MT. The conserved RNA helicase YTHDC2 regulates the transition from proliferation to differentiation in the germline. Elife. 2017:6. doi: 10.7554/eLife.26116. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Han D, Liu J, Chen C, Dong L, Liu Y, Chang R, Huang X, Wang J, Dougherty U, Bissonnette MB, Shen B, Weichselbaum RR, Xu MM, He C. Anti-tumour immunity controlled through mRNA m6A methylation and YTHDF1 in dendritic cells. Nature. 2019;566:270–274. doi: 10.1038/s41586-019-0916-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Lobo J, Costa AL, Cantante M, Guimaraes R, Lopes P, Antunes L, Braga I, Oliveira J, Pelizzola M, Henrique R, Jeronimo C. m6A RNA modification and its writer/reader VIRMA/YTHDF3 in testicular germ cell tumors: a role in seminoma phenotype maintenance. J Transl Med. 2019;17:79. doi: 10.1186/s12967-019-1837-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Hirschfeld M, Zhang B, Jaeger M, Stamm S, Erbes T, Mayer S, Tong X, Stickeler E. Hypoxia-dependent mRNA expression pattern of splicing factor YT521 and its impact on oncological important target gene expression. Mol Carcinog. 2014;53:883–892. doi: 10.1002/mc.22045. [DOI] [PubMed] [Google Scholar]
- 60.Fanale D, Iovanna JL, Calvo EL, Berthezene P, Belleau P, Dagorn JC, Bronte G, Cicero G, Bazan V, Rolfo C, Santini D, Russo A. Germline copy number variation in the YTHDC2 gene: does it have a role in finding a novel potential molecular target involved in pancreatic adenocarcinoma susceptibility? Expert Opin Ther Targets. 2014;18:841–850. doi: 10.1517/14728222.2014.920324. [DOI] [PubMed] [Google Scholar]
- 61.Zhang J, Pi J, Liu Y, Yu J, Feng T. Knockdown of YTH N6-methyladenosine RNA binding protein 2 (YTHDF2) inhibits proliferation and promotes apoptosis in MGC-803 gastric cancer cells. Xi Bao Yu Fen Zi Mian Yi Xue Za Zhi. 2017;33:1628–1634. [PubMed] [Google Scholar]
- 62.Sheng H, Li Z, Su S, Sun W, Zhang X, Li L, Li J, Liu S, Lu B, Zhang S, Shan C. YTH domain family 2 promotes lung cancer cell growth by facilitating 6-phosphogluconate dehydrogenase mRNA translation. Carcinogenesis. 2019 doi: 10.1093/carcin/bgz152. [Epub ahead of print] [DOI] [PubMed] [Google Scholar]
- 63.Nishizawa Y, Konno M, Asai A, Koseki J, Kawamoto K, Miyoshi N, Takahashi H, Nishida N, Haraguchi N, Sakai D, Kudo T, Hata T, Matsuda C, Mizushima T, Satoh T, Doki Y, Mori M, Ishii H. Oncogene c-Myc promotes epitranscriptome m(6)A reader YTHDF1 expression in colorectal cancer. Oncotarget. 2018;9:7476–7486. doi: 10.18632/oncotarget.23554. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Bai Y, Yang C, Wu R, Huang L, Song S, Li W, Yan P, Lin C, Li D, Zhang Y. YTHDF1 regulates tumorigenicity and cancer stem cell-like activity in human colorectal carcinoma. Front Oncol. 2019;9:332. doi: 10.3389/fonc.2019.00332. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Liu L, Liu X, Dong Z, Li J, Yu Y, Chen X, Ren F, Cui G, Sun R. N6-methyladenosine-related genomic targets are altered in breast cancer tissue and associated with poor survival. J Cancer. 2019;10:5447–5459. doi: 10.7150/jca.35053. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Shi Y, Fan S, Wu M, Zuo Z, Li X, Jiang L, Shen Q, Xu P, Zeng L, Zhou Y, Huang Y, Yang Z, Zhou J, Gao J, Zhou H, Xu S, Ji H, Shi P, Wu DD, Yang C, Chen Y. YTHDF1 links hypoxia adaptation and non-small cell lung cancer progression. Nat Commun. 2019;10:4892. doi: 10.1038/s41467-019-12801-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Polakis P. Wnt signaling in cancer. Cold Spring Harb Perspect Biol. 2012;4 doi: 10.1101/cshperspect.a008052. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Zheng Q, Han L, Dong Y, Tian J, Huang W, Liu Z, Jia X, Jiang T, Zhang J, Li X, Kang C, Ren H. JAK2/STAT3 targeted therapy suppresses tumor invasion via disruption of the EGFRvIII/JAK2/STAT3 axis and associated focal adhesion in EGFRvIII-expressing glioblastoma. Neuro Oncol. 2014;16:1229–1243. doi: 10.1093/neuonc/nou046. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Wu R, Liu Y, Zhao Y, Bi Z, Yao Y, Liu Q, Wang F, Wang Y, Wang X. m6A methylation controls pluripotency of porcine induced pluripotent stem cells by targeting SOCS3/JAK2/STAT3 pathway in a YTHDF1/YTHDF2-orchestrated manner. Cell Death Dis. 2019;10:171. doi: 10.1038/s41419-019-1417-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Schindler C, Levy DE, Decker T. JAK-STAT signaling: from interferons to cytokines. J Biol Chem. 2007;282:20059–20063. doi: 10.1074/jbc.R700016200. [DOI] [PubMed] [Google Scholar]
- 71.Nguyen TT, Ma LN, Slovak ML, Bangs CD, Cherry AM, Arber DA. Identification of novel Runx1 (AML1) translocation partner genes SH3D19, YTHDf2, and ZNF687 in acute myeloid leukemia. Genes Chromosomes Cancer. 2006;45:918–932. doi: 10.1002/gcc.20355. [DOI] [PubMed] [Google Scholar]
- 72.Paris J, Morgan M, Campos J, Spencer GJ, Shmakova A, Ivanova I, Mapperley C, Lawson H, Wotherspoon DA, Sepulveda C, Vukovic M, Allen L, Sarapuu A, Tavosanis A, Guitart AV, Villacreces A, Much C, Choe J, Azar A, van de Lagemaat LN, Vernimmen D, Nehme A, Mazurier F, Somervaille TCP, Gregory RI, O’Carroll D, Kranc KR. Targeting the RNA m6A reader YTHDF2 selectively compromises cancer stem cells in acute myeloid leukemia. Cell Stem Cell. 2019;25:137–148. e6. doi: 10.1016/j.stem.2019.03.021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Zhao B, Tumaneng K, Guan KL. The Hippo pathway in organ size control, tissue regeneration and stem cell self-renewal. Nat Cell Biol. 2011;13:877–883. doi: 10.1038/ncb2303. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Xu S, Zhang H, Chong Y, Guan B, Guo P. YAP promotes VEGFA expression and tumor angiogenesis though Gli2 in human renal cell carcinoma. Arch Med Res. 2019;50:225–233. doi: 10.1016/j.arcmed.2019.08.010. [DOI] [PubMed] [Google Scholar]
- 75.Sun Y, Liu WZ, Liu T, Feng X, Yang N, Zhou HF. Signaling pathway of MAPK/ERK in cell proliferation, differentiation, migration, senescence and apoptosis. J Recept Signal Transduct Res. 2015;35:600–604. doi: 10.3109/10799893.2015.1030412. [DOI] [PubMed] [Google Scholar]
- 76.Marathe S, Liu S, Brai E, Kaczarowski M, Alberi L. Notch signaling in response to excitotoxicity induces neurodegeneration via erroneous cell cycle reentry. Cell Death Differ. 2015;22:1775–1784. doi: 10.1038/cdd.2015.23. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Shimura T. Acquired radioresistance of cancer and the AKT/GSK3beta/cyclin D1 overexpression cycle. J Radiat Res. 2011;52:539–544. doi: 10.1269/jrr.11098. [DOI] [PubMed] [Google Scholar]
- 78.Yu R, Li Q, Feng Z, Cai L, Xu Q. m6A reader YTHDF2 regulates LPS-induced inflammatory response. Int J Mol Sci. 2019;20 doi: 10.3390/ijms20061323. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Chen YG, Chen R, Ahmad S, Verma R, Kasturi SP, Amaya L, Broughton JP, Kim J, Cadena C, Pulendran B, Hur S, Chang HY. N6-methyladenosine modification controls circular RNA immunity. Mol Cell. 2019;76:96–109. e9. doi: 10.1016/j.molcel.2019.07.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Coussens LM, Werb Z. Inflammation and cancer. Nature. 2002;420:860–867. doi: 10.1038/nature01322. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Hou J, Zhang H, Liu J, Zhao Z, Wang J, Lu Z, Hu B, Zhou J, Feng M, Shen B, Huang X, Sun B, He C, Xia Q. YTHDF2 reduction fuels inflammation and vascular abnormalization in hepatocellular carcinoma. Mol Cancer. 2019;18:163. doi: 10.1186/s12943-019-1082-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Gao J, Aksoy BA, Dogrusoz U, Dresdner G, Gross B, Sumer SO, Sun Y, Jacobsen A, Sinha R, Larsson E, Cerami E, Sander C, Schultz N. Integrative analysis of complex cancer genomics and clinical profiles using the cBioPortal. Sci Signal. 2013;6:pl1. doi: 10.1126/scisignal.2004088. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Cerami E, Gao J, Dogrusoz U, Gross BE, Sumer SO, Aksoy BA, Jacobsen A, Byrne CJ, Heuer ML, Larsson E, Antipin Y, Reva B, Goldberg AP, Sander C, Schultz N. The cBio cancer genomics portal: an open platform for exploring multidimensional cancer genomics data. Cancer Discov. 2012;2:401–404. doi: 10.1158/2159-8290.CD-12-0095. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Ni W, Yao S, Zhou Y, Liu Y, Huang P, Zhou A, Liu J, Che L, Li J. Long noncoding RNA GAS5 inhibits progression of colorectal cancer by interacting with and triggering YAP phosphorylation and degradation and is negatively regulated by the m6A reader YTHDF3. Mol Cancer. 2019;18:143. doi: 10.1186/s12943-019-1079-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Li F, Yi Y, Miao Y, Long W, Long T, Chen S, Cheng W, Zou C, Zheng Y, Wu X, Ding J, Zhu K, Chen D, Xu Q, Wang J, Liu Q, Zhi F, Ren J, Cao Q, Zhao W. N6-methyladenosine modulates nonsense-mediated mRNA decay in human glioblastoma. Cancer Res. 2019;79:5785–5798. doi: 10.1158/0008-5472.CAN-18-2868. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Hesser CR, Karijolich J, Dominissini D, He C, Glaunsinger BA. N6-methyladenosine modification and the YTHDF2 reader protein play cell type specific roles in lytic viral gene expression during Kaposi’s sarcoma-associated herpesvirus infection. PLoS Pathog. 2018;14:e1006995. doi: 10.1371/journal.ppat.1006995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Wang X, Wu R, Liu Y, Zhao Y, Bi Z, Yao Y, Liu Q, Shi H, Wang F, Wang Y. m6A mRNA methylation controls autophagy and adipogenesis by targeting Atg5 and Atg7. Autophagy. 2019:1–15. doi: 10.1080/15548627.2019.1659617. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Zhang Y, Gu X, Li D, Cai L, Xu Q. METTL3 regulates osteoblast differentiation and inflammatory response via Smad signaling and MAPK signaling. Int J Mol Sci. 2019;21 doi: 10.3390/ijms21010199. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Kasowitz SD, Ma J, Anderson SJ, Leu NA, Xu Y, Gregory BD, Schultz RM, Wang PJ. Nuclear m6A reader YTHDC1 regulates alternative polyadenylation and splicing during mouse oocyte development. PLoS Genet. 2018;14:e1007412. doi: 10.1371/journal.pgen.1007412. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Zhao X, Cui L. Development and validation of a m6A RNA methylation regulators-based signature for predicting the prognosis of head and neck squamous cell carcinoma. Am J Cancer Res. 2019;9:2156–2169. [PMC free article] [PubMed] [Google Scholar]
- 91.Jin D, Guo J, Wu Y, Du J, Yang L, Wang X, Di W, Hu B, An J, Kong L, Pan L, Su G. m6A mRNA methylation initiated by METTL3 directly promotes YAP translation and increases YAP activity by regulating the MALAT1-miR-1914-3p-YAP axis to induce NSCLC drug resistance and metastasis. J Hematol Oncol. 2019;12:135. doi: 10.1186/s13045-019-0830-6. [DOI] [PMC free article] [PubMed] [Google Scholar] [Retracted]


