Abstract
To evaluate whether cytoreductive therapy is needed for myelodysplastic syndromes (MDS) patients with excess blasts type 2 (MDS-EB2) and acute myeloid leukemia derived from MDS (MDS-AML) before HLA-matched sibling donor peripheral blood stem cell transplantation (MSD-PBSCT), we retrospectively analyzed 80 cases of MDS-EB2 and MDS-AML patients who received MSD-PBSCT between February 2006 and December 2019 in our hospital. The 3-years overall survival (OS) rate and disease-free survival (DFS) rate were (59.1±5.8)% and (52.5±5.7)%, respectively. The 3-years non-relapse mortality (NRM) rate and relapse rate (RR) were (22.4±0.2)% and (25.4±0.2)%, respectively. Univariate analysis showed that, hematopoietic cell transplantation comorbidity index (HCT-CI) ≥ 2, poor/very poor karyotype and occurrence of grade III-IV acute graft-versus-host disease (aGVHD) are risk factors for OS. Patients received pre-transplant cytoreductive therapy (PCT) and obtained complete remission (CR) had significantly higher OS rate than those who failed to achieve CR (non-CR group) and those who did not receive PCT (non-PCT group) [(80.0±8.3)% versus (38.1±10.6)% versus (56.1±9.3)%, P=0.010]. PCT significantly increased the OS rate [(62.2±10.0)% versus (20.0±17.9)%, P=0.013] for MDS-AML patients but not for MDS-EB2 patients [(59.2±11.1)% versus (62.9±10.1)%, P=0.991]. Our findings suggest reducing tumor burden by cytoreductive therapy to obtain CR before transplant improves OS. For MDS-AML patients, PCT is beneficial, while for MDS-EB2 patients, PCT is not necessary.
Keywords: Peripheral blood stem cell transplantation, myelodysplastic syndrome, pre-transplant cytoreductive therapy
Introduction
Myelodysplastic syndromes (MDS) comprise a heterogeneous group of hematopoietic stem cell disorders of ineffective hematopoiesis and cytopenia with a high risk of developing into acute myeloid leukemia (AML) [1]. Allogeneic hematopoietic stem cell transplantation (allo-HSCT) is the only curative treatment for patients with advanced MDS and AML derived from MDS (MDS-AML), because of the graft-versus-leukemia (GVL) mediated anti-tumor effect [2-4]. Despite the recent advances in allo-HSCT, 3-year survival rate is only 38% to 45% for advanced MDS and there’s still unmet needs for patients with high blast proportion at the point of transplant [5]. High blast proportion at the point of transplant is a risk factor of allo-HSCT, especially in patients receiving reduced intensity conditioning (RIC) regimen [6]. Included in numerous integrated prognostic scoring systems, high bone marrow (BM) blast proportion is associated with unfavorable disease outcome in MDS [7]. Whether patients with higher BM blast benefit from pre-transplant cytoreductive therapy (PCT) remains unknown.
PCT includes traditional AML-like chemotherapy and hypomethylating agents (HMAs) therapy, such as azacitidine or decitabine [8-10]. PCT is given in advanced MDS patients to control disease progression before transplantation, reduce disease burden and prevent relapse after transplantation [3,4,11]. Nevertheless, PCT may impair patient’s body function and delay the transplant procedure due to early mortality, long-lasting myelosuppression and organ toxicities [12]. In addition, although HMAs are well tolerated in elderly frail patients, they are usually not sufficient to achieve favorable remission rate [13]. Therefore, further studies are needed to evaluate and improve the existing PCT strategies.
In addition to high blast proportion, several prognostic models have been developed to assess the risks, predict outcomes and assist decision-making and prognostic counseling before transplant [14-17]. Among these models, the hematopoietic stem cell transplantation comorbidity index (HCT-CI) is the most used to predict transplant-related mortality [14]. It is also used to determine the optimal conditioning intensity according to a comorbidity and disease status-based risk stratification, for example in AML or MDS patients [18]. Therefore, the HCT-CI provides a valuable risk assessment for outcomes after allo-HSCT [19].
To answer these questions and guide clinical care of advanced MDS patients, we retrospectively analyzed the outcomes of 80 MDS patients with excess blasts type 2 (MDS-EB2) and MDS-AML patients who received HLA-matched sibling donor peripheral blood stem cell transplantation (MSD-PBSCT) in our hospital, clarified the risk factors affecting overall survival (OS) and explored whether PCT contributes to long term survival in these patients.
Subjects and methods
A total of 80 MDS-EB2 and MDS-AML patients who received MSD-PBSCT between February 2006 and December 2019 in our hospital were enrolled in this study. 51 patients were diagnosed as MDS-EB2 and 29 patients were diagnosed as MDS-AML before transplant. Data were collected until December 31st 2019 with a median follow-up of 888 days (range 7 to 4970 days). All time-to-event outcomes were counted from the date of transplantation to the date of event or the last follow-up. We used HCT-CI to evaluate the physical condition of patients at transplant. The karyotypes were determined according to revised International Prognostic Scoring System (IPSS-R) risk stratification at diagnosis and its relationship to the outcomes after MSD-PBSCT were investigated. Acute and chronic graft-versus-host disease (GVHD) were diagnosed and graded according to recent National Institutes of Health (NIH) consensus criteria. This study was approved by our center’s Institutional Review Board. All patients or their legal representative provided written informed consent before participation.
Statistical analysis
Outcomes of primary interest were OS and disease-free survival (DFS) which defined as survival in the absence of new relapse or progression after transplantation. Univariate survival analyses were performed using the Kaplan-Meier method; outcomes of groups were compared using the log-rank test. Cumulative incidences of relapse and non-relapse mortality (NRM) were calculated as each other’s competing risks. Differences in relapse and NRM between groups were compared by the Gray-test. All data were measured from the day of transplantation to death or the last follow-up and were artificially censored at three years after transplantation. P-value < 0.05 was considered statistically significant. All statistical analyses were conducted using R.3.3.3 and SPSS 22.0 statistical software.
Result
Patients and clinical characteristics
The clinical characteristics of the patients were summarized in Table 1. The median age was 42 years old (range 4 to 63). More than half of the patients are male (50, 62.5%). Except for 29 MDS-AML patients, 3 (5.9%) patients were classified as very low/low/intermediate risk group, 22 (43.1%) as high risk group and 26 (51.0%) as very high risk group according to the IPSS-R risk stratification. Based on the IPSS-R risk stratification, 49 (61.3%) patients were classified into good karyotype group, 20 (25.0%) patients were intermediate karyotype group, 4 (5.0%) patients were poor karyotype and 7 (8.8%) patients were very poor karyotype. All patients received busulfan (Bu) plus cyclophosphamide (Cy)-based modified myeloablative conditioning (MAC). For prophylaxis of acute GVHD (aGVHD), 53.8% patients were given tacrolimus and 45.0% patients were given cyclosporine A, with an additional short course of methotrexate treatment. Rapamycin and Mycophenolate Mofetil (MMF) were used in one case due to renal dysfunction.
Table 1.
Clinical and transplantation characteristics of the patients undergoing MSD-PBSCT
| N (%) | |
|---|---|
| Median age | |
| < 42 years | 38 (47.5%) |
| ≥ 42 years | 42 (52.5%) |
| Sex of patient | |
| Male | 50 (62.5%) |
| Female | 30 (37.5%) |
| WHO classification | |
| MDS-EB2 | 51 (63.7%) |
| MDS-AML | 29 (36.3%) |
| IPSS-R stratification | |
| Very Low/Low/Intermediate risk | 3 (3.8%) |
| High risk | 22 (27.5%) |
| Very high risk | 26 (32.5%) |
| MDS-AML | 29 (36.3%) |
| Karyotype | |
| Good | 49 (61.3%) |
| Intermediate | 20 (25.0%) |
| Poor/Very Poor | 11 (13.8%) |
| HCT-CI | |
| < 2 scores | 53 (66.3%) |
| ≥ 2 scores | 27 (33.8%) |
| Age of donor | |
| < 42 years | 38 (47.5%) |
| ≥ 42 years | 42 (52.5%) |
| Match sex donor-recipient | |
| Male-Male | 25 (31.3%) |
| Female-Female | 11 (13.8%) |
| Male-Female | 20 (25.0%) |
| Female-Male | 24 (30.0%) |
| Pre-transplant BM blasts | |
| < 10% | 39 (48.8%) |
| ≥ 10% | 41 (51.2%) |
| PCT | |
| Yes | 49 (61.3%) |
| No | 31 (38.8%) |
| Pre-transplant status | |
| PCT and CR | 28 (35.0%) |
| PCT and non-CR | 21 (26.3%) |
| Non-PCT | 31 (38.8%) |
| cGVHD | |
| Limited | 17 (23.0%) |
| Extensive | 19 (25.7%) |
| No | 38 (51.4%) |
| aGVHD | |
| No | 41 (52.6%) |
| Grade I-II | 26 (33.3%) |
| Grade III-IV | 11 (14.1%) |
Risk factors that affect OS
In the median follow-up of 888 days (range 7 to 4970 days), 49 patients were alive and 43 patients were diseases free. The OS rate and DFS rate at 3 years were (59.1±5.8)% and (52.5±5.7)% respectively (Figure 1). The 3-year NRM rate and relapse rate (RR) were (22.4±0.2)% and (25.4±0.2)%, respectively. Out of the 31 death cases, 12 cases died from relapse and 19 cases died from NRM. The reasons of NRM were aGVHD (7 cases), multiple organ failure (3 cases), chronic GVHD (cGVHD) (2 cases), pulmonary infection (4 cases), infectious shock (2 cases) and cerebral hemorrhage (1 case). 37 patients developed aGVHD and 11 patients were classified as grade III-IV aGVHD. The cumulative incidence of grade III-IV aGVHD was (14.1±0.2)%. 36 patients developed cGVHD, including limited cGVHD in 17 patients (23.0%) and extensive cGVHD in 19 patients (25.7%). The cumulative incidence of cGVHD was (48.8±0.4)% for patients who survived more than 100 days after transplantation. Univariate analysis was performed to figure out the risk factors affecting OS and DFS after PBSCT. The detail results were summarized in Table 2.
Figure 1.
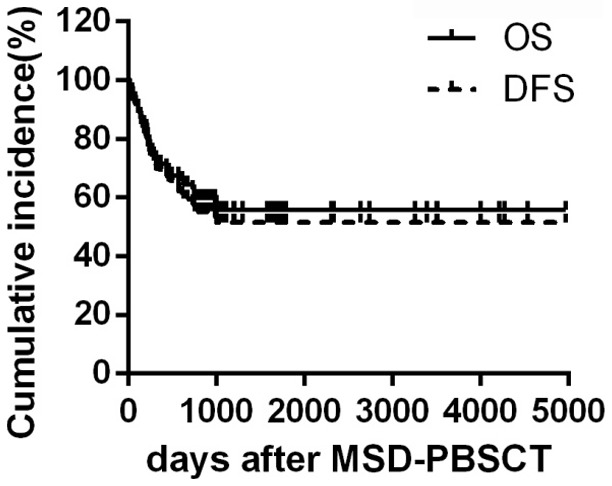
Overall survival and disease-free survival rate in total patients. The cumulative incidence of overall survival and disease-free survival in 80 MDS-EB2 and MDS-AML patients that received MSD-PBSCT.
Table 2.
Univariate analysis of the risk factors influencing OS and DFS for patients undergoing MSD-PBSCT
| N | 3y-OS (%) | X2 value | P value | 3y-DFS (%) | X2 value | P value | ||
|---|---|---|---|---|---|---|---|---|
| Age | < 40 years | 33 | 66.9±8.8 | 1.916 | 0.166 | 59.7±8.7 | 0.883 | 0.348 |
| ≥ 40 years | 47 | 53.8±7.5 | 47.8±7.5 | |||||
| Diagnosis | MDS-EB2 | 51 | 61.3±7.4 | 0.958 | 0.328 | 61.0±7.2 | 5.480 | 0.019 |
| MDS-AML | 29 | 55.0±9.3 | 37.4±9.1 | |||||
| IPSS-R stratification | Very Low/Low/ Intermediate | 3 | 66.7±27.2 | 2.405 | 0.493 | 66.7±27.2 | 6.932 | 0.074 |
| High | 22 | 70.4±10.4 | 72.2±9.7 | |||||
| Very high risk | 26 | 52.0±10.3 | 49.0±11.3 | |||||
| MDS-AML | 29 | 54.5±10.6 | 37.4±9.1 | |||||
| Karyotype | Good | 49 | 68.3±6.8 | 6.536 | 0.038 | 60.4±7.1 | 5.822 | 0.054 |
| Intermediate | 20 | 38.5±17.5 | 48.1±11.5 | |||||
| Poor/Very Poor | 11 | 36.4±14.5 | 27.3±13.4 | |||||
| HCT-CI | < 2 scores | 53 | 64.6±7.2 | 4.119 | 0.042 | 60.7±7.0 | 5.561 | 0.018 |
| ≥ 2 scores | 27 | 47.5±9.7 | 35.9±9.5 | |||||
| Pre-transplant BM blasts | < 10% | 39 | 72.2±7.7 | 5.144 | 0.023 | 63.5±7.8 | 2.949 | 0.086 |
| ≥ 10% | 41 | 47.3±8.1 | 42.7±8.0 | |||||
| PCT | Yes | 49 | 61.0±7.4 | 0.398 | 0.528 | 50.1±7.3 | 0.121 | 0.728 |
| No | 31 | 56.1±9.3 | 56.6±9.3 | |||||
| Pre-transplant status | PCT and CR | 28 | 80.0±8.3 | 9.252 | 0.010 | 67.7±8.9 | 7.244 | 0.027 |
| PCT and non-CR | 21 | 38.1±10.6 | 28.6±9.9 | |||||
| Non-PCT | 31 | 56.1±9.3 | 56.6±9.3 | |||||
| Treatment options | HMAs | 10 | 80.0±12.6 | 1.995 | 0.369 | 70.0±14.5 | 2.293 | 0.318 |
| chemotherapy | 29 | 59.4±9.7 | 47.1±9.5 | |||||
| HMAs+chemotherapy | 10 | 50.0±15.8 | 40.0±15.5 | |||||
| Age of donor | < 40 years | 32 | 65.2±9.3 | 1.748 | 0.186 | 58.2±8.9 | 0.863 | 0.353 |
| ≥ 40 years | 48 | 54.9±7.4 | 49.0±7.4 | |||||
| Match sex donor-recipient | Male-Male | 25 | 52.7±10.8 | 0.460 | 0.928 | 47.4±10.1 | 1.799 | 0.615 |
| Female-Female | 11 | 63.6±14.5 | 45.5±15.0 | |||||
| Male-Female | 20 | 63.5±11.1 | 58.7±11.3 | |||||
| Female-Male | 24 | 58.6±11.1 | 54.9±11.0 | |||||
| GVHD prophylaxis | Based on CsA | 36 | 52.7±9.5 | 0.186 | 0.666 | 49.3±9.0 | 0.007 | 0.943 |
| Based on FK506 | 43 | 62.2±7.5 | 53.3±7.6 | |||||
| Grade III-IV aGVHD | Yes | 11 | 24.2±13.8 | 11.784 | 0.001 | 13.6±11.7 | 10.812 | 0.001 |
| No | 67 | 66.3±6.1 | 60.0±6.2 | |||||
| cGVHD | Yes | 36 | 72.9±7.8 | 3.504 | 0.061 | 61.9±8.5 | 1.602 | 0.206 |
| No | 38 | 55.9±8.6 | 52.5±8.1 |
HCT-CI score predict the OS, NRM and occurrence of severe aGVHD
To examine the efficacy of HCT-CI in predicting the disease outcome, we compared the outcome of patients whose HCT-CI scores before transplant were < 2 or ≥ 2. Patients with HCT-CI < 2 scores had a significantly higher 3-year OS rate than patients with higher HCT-CI scores [(64.6±7.2)% versus (47.5±9.7)%, P=0.042] (Figure 2), mainly due to higher NRM rate in patients with HCT-CI ≥ 2. The 3-year NRM rate was significantly lower in patients with HCT-CI < 2 (14.5±0.3)% compared to (37.8±1.0)% in patients with HCT-CI ≥ 2 (P=0.013). Moreover, higher HCT-CI is closely related to the development of severe aGVHD. 4 out of 53 patients developed grade III-IV aGVHD in HCT-CI < 2 group [(7.5±0.1)%] while 7 out 25 patients developed grade III-IV aGVHD in HCT-CI ≥ 2 group [(28.0±0.8)%] (P=0.017).
Figure 2.
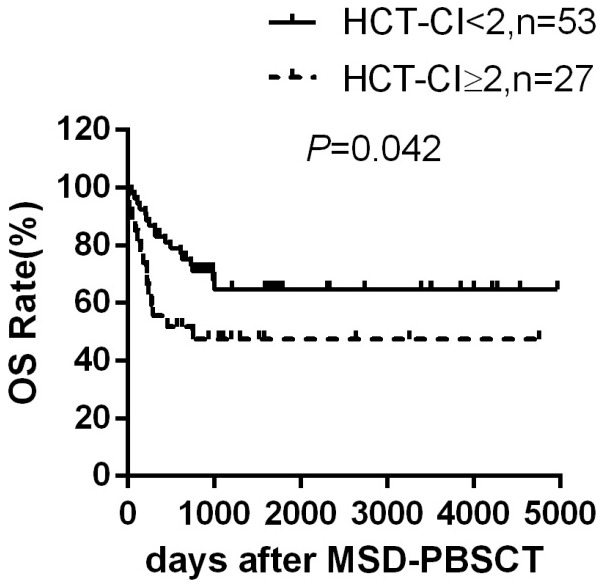
Overall survival according to HCT-CI. Overall survival rate in patients with HCT-CI < 2 scores (n=53) and patients with HCT-CI ≥ 2 scores (n=27) after MSD-PBSCT.
Acute GVHD and abnormal karyotype significantly compromise OS after MSD-PBSCT
Among all the patients analyzed, 11 patients developed grade III-IV aGVHD. These patients had dramatically lower OS rate (24.2±13.8)% compared to those without grade III-IV aGVHD (66.3±6.1)% (P=0.001) (Figure 3), which is mainly resulted from the higher grade III-IV aGVHD-related mortality. On the contrary, patients suffered from cGVHD had higher OS rate (72.9±7.8)% compared to those free of cGVHD (55.9±8.6)% (P=0.061) possibly due to cGVHD-mediated GVL effect, although the difference is no statistically significant. Besides, abnormal karyotype has been reported as another factor that significantly affected OS rate [20]. Patients with normal karyotype at diagnosis had superior OS than patients with intermediate and poor/very poor karyotype [(68.3±6.8)% versus (38.5±17.5)% versus (36.4±14.5)%, P=0.038].
Figure 3.
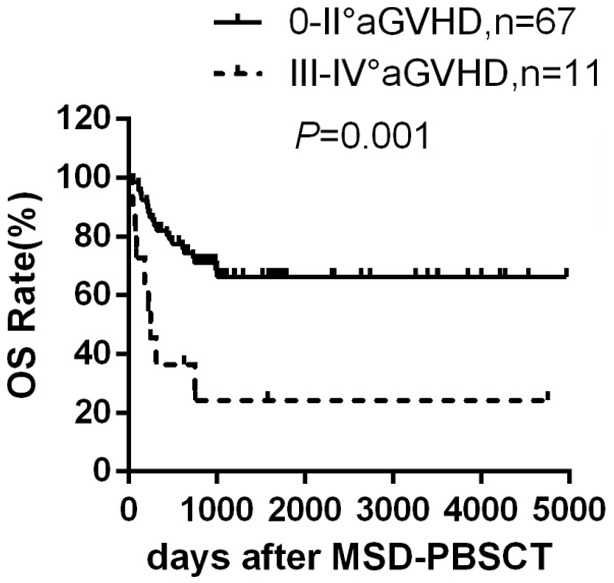
Overall survival according to aGVHD grade. Overall survival rate in evaluable patients developed grade 0-II aGVHD (n=67) and patients developed grade III-IV aGVHD (n=11) after MSD-PBSCT.
The effect of PCT on OS
Whether PCT is beneficial for patients with advanced MDS and MDS-AML remains unknown. Therefore, we analyzed MDS-EB2 and MDS-AML patients receiving MSD-PBSCT to explore the relationship between PCT and disease outcome in these patients.
PCT could not affect OS for total patients
In our study, we found that patients whose pre-transplant BM blasts < 10% had higher 3-year OS rate than those whose pre-transplant BM blasts ≥ 10% [(72.2±7.7)% versus (47.3±8.1)%, P=0.023], suggesting that the higher tumor burden before transplant significantly affect OS. We then assessed the impact of PCT (including HMAs therapy, chemotherapy and HMAs combined with chemotherapy) on the disease outcome. Surprisingly, the 3-year OS rate and 3-year DFS rate had no significant difference between the two groups [(61.0±7.4)% versus (56.1±9.3)%, P=0.528 and (50.1±7.3)% versus (56.6±9.3)%, P=0.728]. However, since patients respond differently to cytoreductive treatment, we suspect that could also affect the outcome of disease after transplant. Among the 49 patients who received PCT, 28 cases (57.1%) achieved complete remission (CR) including 16 MDS-AML patients and 12 MDS-EB2 patients, and 21 cases (42.9%) obtained non-remission (NR) or partial remission (PR), which are combined as non-CR group. 31 patients did not receive PCT (non-PCT group). We next explored the disease outcome in these subgroups separately.
Reducing tumor burden using PCT to obtain CR could improve OS in general
Patients in CR group had significantly higher OS rate and DFS rate than that of the non-CR group and non-PCT group [(80.0±8.3)% versus (38.1±10.6)% versus (56.1±9.3)%, P=0.010 and (67.7±8.9)% versus (28.6±9.9)% versus (56.6±9.3)%, P=0.027] (Figure 4). Moreover, decreased RR was observed in the CR group and non-PCT group compared to non-CR group [(21.6±0.6)% versus (12.9±0.4)% versus (47.6±1.3)%, P=0.024]. Patients in CR group tend to have reduced NRM rate compared to the other two groups [(10.7±0.4)% versus (23.8±0.9)% versus (31.4±0.9)%, P=0.200]. These data strongly demonstrated that the response to PCT dramatically affects the prognosis in patients.
Figure 4.
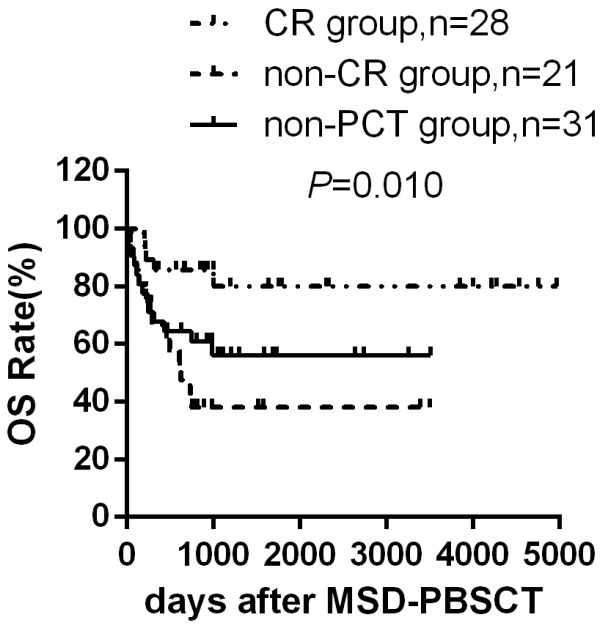
Overall survival according to PCT and disease status. Overall survival rate of patients in CR group (n=28), non-CR group (n=21) and non-PCT group (n=31) after MSD-PBSCT.
To further assess the optimal PCT regime, we compared the HMAs therapy (10 cases, 20.4%), chemotherapy (29 cases, 59.2%) and HMAs combined with chemotherapy (10 cases, 20.4%). Patients who received HMAs therapy showed the best 3-year OS rate, suggesting that HMAs is ideal for PCT, though statistical significance is not achieved due to the sample volumes [(80.0±12.6)% versus (59.4±9.7)% versus (50.0±15.8)%, P=0.369].
PCT could improve OS of patients with MDS-AML but not patients with MDS-EB2
To avoid the influence of disease subtypes, we next analyzed the prognosis in MDS-EB2 patients and MDS-AML patients separately. For the 51 MDS-EB2 patients, 25 patients received PCT and 12 patients obtained CR (48.0%). Overall, in MDS-EB2 patients, neither the application of PCT (Figure 5A) [(59.2±11.1)% versus (62.9±10.1)%, P=0.991] nor the status of CR is associated with improved OS [(73.3±17.6)% versus (46.2±13.8)% versus (62.9±10.1)%, P=0.213]. However, for the 29 MDS-AML patients, the CR rate was 66.7% (16 out of 24), and the application of PCT significantly enhanced the OS rate [(62.2±10.0)% versus (20.0±17.9)%, P=0.013] (Figure 5B). In addition, these patients who achieved CR before transplantation obtained survival advantage over the other two groups [(81.3±9.8)% versus (25.0±15.3)% versus (20.0±17.9)%, P=0.003].
Figure 5.
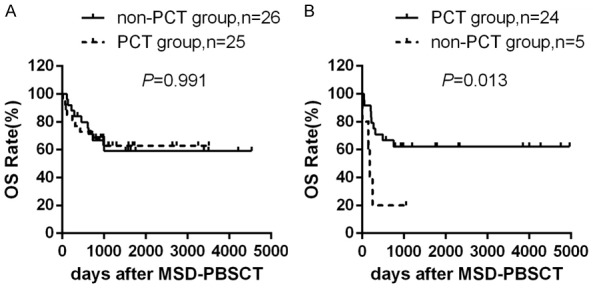
Overall survival according to PCT. A. Overall survival rate for MDS-EB2 patients in PCT group (n=25) and in non-PCT group (n=26) after MSD-PBSCT. B. Overall survival rate for MDS-AML patients in PCT group (n=24) and in non-PCT group (n=5) after MSD-PBSCT.
To avoid the influence of other risk factors on OS, we further compared the age of donor and recipient, sex, HCT-CI, karyotype and match sex donor-recipient in the PCT group and the non-PCT group in MDS-EB2 and MDS-AML patients, respectively (Tables 3 and 4). No bias is found in the MDS-EB2 patients; for the MDS-AML patients, more patients with HCT-CI ≥ 2 [(4 out of 5) versus (8 out of 24)] or poor/very poor karyotype [(2 out of 5) versus (1 out of 24)] were enrolled in non-PCT group, which may be due to the limited cases numbers.
Table 3.
Clinical and transplantation characteristics of the MDS-EB2 patients undergoing MSD-PBSCT
| PCT Group (N/%) | Non-PCT Group (N/%) | X2 value | P value | ||
|---|---|---|---|---|---|
| Age | < 40 years | 8 (32.0%) | 12 (46.2%) | 1.071 | 0.301 |
| ≥ 40 years | 17 (68.0%) | 14 (53.8%) | |||
| Sex | Male | 17 (68.0%) | 17 (65.4%) | 0.039 | 0.843 |
| Female | 8 (32.0%) | 9 (34.6%) | |||
| HCT-CI | < 2 scores | 19 (76.0%) | 17 (65.4%) | 0.692 | 0.406 |
| ≥ 2 scores | 6 (24.0%) | 9 (34.6%) | |||
| Karyotype | Good | 16 (64.0%) | 15 (57.7%) | 0.346 | 0.841 |
| Intermediate | 5 (20.0%) | 7 (26.9%) | |||
| Poor/Very Poor | 4 (16.0%) | 4 (15.4%) | |||
| Age of donor | < 40 years | 9 (36.0%) | 9 (34.6%) | 0.011 | 0.918 |
| ≥ 40 years | 16 (64.0%) | 17 (65.4%) | |||
| Match sex donor-recipient | Male-Male | 6 (24.0%) | 10 (38.5%) | 1.510 | 0.680 |
| Female-Female | 3 (12.0%) | 3 (11.5%) | |||
| Male-Female | 6 (24.0%) | 6 (23.1%) | |||
| Female-Male | 10 (40.0%) | 7 (26.9%) |
Table 4.
Clinical and transplantation characteristics of the MDS-AML patients undergoing MSD-PBSCT
| PCT Group (N/%) | Non-PCT Group (N/%) | X2 value | P value | ||
|---|---|---|---|---|---|
| Age | < 40 years | 12 (50.0%) | 1 (20.0%) | 1.506 | 0.220 |
| ≥ 40 years | 12 (50.0%) | 4 (80.0%) | |||
| Sex | Male | 12 (50.0%) | 4 (80.0%) | 1.506 | 0.220 |
| Female | 12 (50.0%) | 1 (20.0%) | |||
| HCT-CI | < 2 scores | 16 (66.7%) | 1 (20.0%) | 3.715 | 0.054 |
| ≥ 2 scores | 8 (33.3%) | 4 (80.0%) | |||
| Karyotype | Good | 17 (70.8%) | 1 (20.0%) | 7.196 | 0.027 |
| Intermediate | 6 (25.0%) | 2 (40.0%) | |||
| Poor/Very Poor | 1 (4.2%) | 2 (40.0%) | |||
| Age of donor | < 40 years | 13 (54.2%) | 1 (20.0%) | 1.934 | 0.164 |
| ≥ 40 years | 11 (45.8%) | 4 (80.0%) | |||
| Match sex donor-recipient | Male-Male | 8 (33.3%) | 1 (20.0%) | 4.624 | 0.202 |
| Female-Female | 5 (20.8%) | 0 (0%) | |||
| Male-Female | 7 (29.2%) | 1 (20.0%) | |||
| Female-Male | 4 (16.7%) | 3 (60.0%) |
In summary, we found that PCT, especially using the HMAs regime, is beneficial to MDS-AML patients who achieved CR before transplant in terms of OS and DFS, however, it did not improve the prognosis of MDS-EB2 patients. Due to the limited number of cases enrolled, clinical studies on larger group of patients with proper trial design are needed to verify the conclusions.
Discussion
In this study, we explored the risk factors that affect the prognosis of MDS-AML and MDS-EB2 patients after MSD-PBSCT. OF note, in this study, the 3 years OS rate and DFS rate were (59.1±5.8)% and (52.5±5.7)% for these patients, higher than that of 38% to 45% for advanced MDS receiving allo-HSCT after MAC or reduced-intensity conditioning according to the Center for International Blood and Marrow Transplantation Research (CIBMTR) [5], possibly due to the lower average age of the patients included in our study (median age 42). We found that higher blast portion or HCT-CI score and the incidence of severe aGVHD are closely associated with poor prognosis. Importantly, we found that patients who achieved CR after PCT had significantly higher survival rate, especially in the MDS-AML patients. These findings indicate that PCT contributes to improved disease outcome in the MDS-AML patients and the response to PCT could be used as a critical prognostic factor to guide the clinical treatment before allo-HSCT.
Large retrospective multicenter studies clearly demonstrated that higher blast burden at the time of HSCT increases the risk of subsequent relapse in MDS patients [21-23]. Therefore, some studies suggested reducing the disease burden before transplantation as adjuvant therapy to allo-HSCT [3,6]. Our study showed that the benefit of PCT relies on the patient’s response to the treatment and the sensitive patients who achieved CR had superior OS, especially in MDS-AML patients. Considering the overall CR rate was 57.1% in all patients, it is necessary to carefully identify the patients sensitive to PCT and further optimize the therapy to obtain higher CR rate.
On the other hand, a few studies suggested that pre-transplant therapy to reduce disease burden did not improve OS [21,24]. Similar post-transplant relapse and survival rates were observed in advanced MDS or MDS-AML patients who received induction chemotherapy before or after HSCT followed by MAC [12]. Delays in HLA-identical siblings HSCT and MAC regimen are also associated with shortened survival for patients with intermediate-2 and high-risk disease [25], especially in patients under the age of 40 and took quality of life into consideration [26]. Furthermore, there’s concern if induction chemotherapy induces treatment-related morbidity and mortality. For example, intensive induction therapy leads to 20% mortality in patients with high-risk MDS and complex karyotype [27]. Additionally, chemotherapy might impair patient’s body function and delay HSCT, which results in disease progression and inferior posttransplant outcomes [28]. Since it is not clear whether the risk of relapse reflects the tumor biology or inadequacy treatment before HSCT, these controversial results indicate that precise subcategorization of patient is critical for the choice of optimal therapeutic scheme.
In addition to high blast portion, we also explored other risk factors that affecting patient survival after allo-HSCT including the occurrence of GVHD and HCT-CI score. Of note, development of cGVHD has been reported as one of the most powerful antineoplastic mechanisms after HSCT, which reduces relapse and improves patient survival [29,30]. Our data showed that patients with cGVHD tend to have superior OS (P=0.061). On the other hand, aGVHD directly contributed to 36.8% of NRM (7 cases in 19). Therefore, effective strategies to decrease aGVHD-related adverse events are important to improve the HSCT outcomes. The regime of full myeloablative dose Bu and Flu had the advantage of lower non-hematologic toxicity in AML and MDS adults and is well tolerated in advanced patients receiving HLA-identical sibling HSCT [31]. The new conditioning regimen of 5-day decitabine administration contributed to low RR, incidence and severity of GVHD, and enhanced survival in allo-HSCT recipients with MDS and MDS/MPN [32].
HCT-CI is a tool that sensitively captures the burdens of influential comorbidities prior to allo-HSCT [10]. Comorbidities evaluated at the time of HSCT are powerful prognostic markers of NRM independent of age [33,34]. Recently, a strong association between higher HCT-CI score and development of grade III-IV aGVHD was demonstrated. It was shown that grade III-IV aGVHD patients whose HCT-CI scores ≥ 3 had 2.63-fold higher mortality risk than those who had scores of 0-2 or did not develop aGVHD [35]. In our study, patients with HCT-CI scores lower than 2 had significantly higher OS rate, lower NRM rate and less occurrence of grade III-IV aGVHD. Consistent with other studies [32,36], HCT-CI, rather than age alone, can be used as a predictor for OS after transplantation. Moreover, the HCT-CI could be useful in designing trials for GVHD prevention and could inform expectations for GVHD treatment trials [35].
Despite of the important findings, our study also had limitations, such as the relatively small sample size and the bias due to patient selection for cytoreductive therapy before transplantation. Therefore, large sample and prospective cohort studies are needed to validate the conclusion in the future.
In conclusion, MSD-PBSCT is an effective treatment for MDS-EB2 and MDS-AML patients. HCT-CI is an important prognostic assessment to predict the OS, NRM and the occurrence of grade III-IV aGVHD. Obtaining CR by PCT improves OS for these patients. For MDS-AML patients, PCT is recommended, however for MDS-EB2 patients, PCT is not necessary.
Acknowledgements
We thank Professor Han for his valuable assistance and all of the physicians and staff members of the Institute of Hematology & Blood Diseases Hospital, Chinese Academy of Medical Sciences & Peking Uion Medical College. This work was supported by the National Natural Science Foundation of China (81670171), the National Science Foundation of Tianjin (18JCYBJC25300) and the Fundamental Research Funds for the Central Universities of PUMC (3332018114).
Disclosure of conflict of interest
None.
References
- 1.Greenberg PL, Stone RM, Al-Kali A, Barta SK, Bejar R, Bennett JM, Carraway H, De Castro CM, Deeg HJ, DeZern AE, Fathi AT, Frankfurt O, Gaensler K, Garcia-Manero G, Griffiths EA, Head D, Horsfall R, Johnson RA, Juckett M, Klimek VM, Komrokji R, Kujawski LA, Maness LJ, O’Donnell MR, Pollyea DA, Shami PJ, Stein BL, Walker AR, Westervelt P, Zeidan A, Shead DA, Smith C. Myelodysplastic syndromes, version 2.2017, NCCN clinical practice guidelines in oncology. J Natl Compr Canc Netw. 2017;15:60–87. doi: 10.6004/jnccn.2017.0007. [DOI] [PubMed] [Google Scholar]
- 2.Koreth J, Pidala J, Perez WS, Deeg HJ, Garcia-Manero G, Malcovati L, Cazzola M, Park S, Itzykson R, Ades L, Fenaux P, Jadersten M, Hellstrom-Lindberg E, Gale RP, Beach CL, Lee SJ, Horowitz MM, Greenberg PL, Tallman MS, DiPersio JF, Bunjes D, Weisdorf DJ, Cutler C. Role of reduced-intensity conditioning allogeneic hematopoietic stem-cell transplantation in older patients with de novo myelodysplastic syndromes: an international collaborative decision analysis. J. Clin. Oncol. 2013;31:2662–70. doi: 10.1200/JCO.2012.46.8652. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.de Witte T, Bowen D, Robin M, Malcovati L, Niederwieser D, Yakoub-Agha I, Mufti GJ, Fenaux P, Sanz G, Martino R, Alessandrino EP, Onida F, Symeonidis A, Passweg J, Kobbe G, Ganser A, Platzbecker U, Finke J, van Gelder M, van de Loosdrecht AA, Ljungman P, Stauder R, Volin L, Deeg HJ, Cutler C, Saber W, Champlin R, Giralt S, Anasetti C, Kroger N. Allogeneic hematopoietic stem cell transplantation for MDS and CMML: recommendations from an international expert panel. Blood. 2017;129:1753–1762. doi: 10.1182/blood-2016-06-724500. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Kobbe G, Schroeder T, Haas R, Germing U. The current and future role of stem cells in myelodysplastic syndrome therapies. Expert Rev Hematol. 2018;11:411–422. doi: 10.1080/17474086.2018.1452611. [DOI] [PubMed] [Google Scholar]
- 5.Pasquini M, Wang Z, Horowitz MM, Gale RP. 2013 report from the Center for international blood and marrow transplant research (CIBMTR): current uses and outcomes of hematopoietic cell transplants for blood and bone marrow disorders. Clin Transpl. 2013:187–97. [PubMed] [Google Scholar]
- 6.Brierley CK, Steensma DP. Allogeneic stem cell transplantation in myelodysplastic syndromes: does pretransplant clonal burden matter? Curr Opin Hematol. 2016;23:167–74. doi: 10.1097/MOH.0000000000000217. [DOI] [PubMed] [Google Scholar]
- 7.Kantarjian H, O’Brien S, Ravandi F, Cortes J, Shan J, Bennett JM, List A, Fenaux P, Sanz G, Issa JP, Freireich EJ, Garcia-Manero G. Proposal for a new risk model in myelodysplastic syndrome that accounts for events not considered in the original international prognostic scoring system. Cancer. 2008;113:1351–61. doi: 10.1002/cncr.23697. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.de Witte T, Hagemeijer A, Suciu S, Belhabri A, Delforge M, Kobbe G, Selleslag D, Schouten HC, Ferrant A, Biersack H, Amadori S, Muus P, Jansen JH, Hellstrom-Lindberg E, Kovacsovics T, Wijermans P, Ossenkoppele G, Gratwohl A, Marie JP, Willemze R. Value of allogeneic versus autologous stem cell transplantation and chemotherapy in patients with myelodysplastic syndromes and secondary acute myeloid leukemia. Final results of a prospective randomized European intergroup trial. Haematologica. 2010;95:1754–61. doi: 10.3324/haematol.2009.019182. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Fenaux P, Mufti GJ, Hellstrom-Lindberg E, Santini V, Gattermann N, Germing U, Sanz G, List AF, Gore S, Seymour JF, Dombret H, Backstrom J, Zimmerman L, McKenzie D, Beach CL, Silverman LR. Azacitidine prolongs overall survival compared with conventional care regimens in elderly patients with low bone marrow blast count acute myeloid leukemia. J. Clin. Oncol. 2010;28:562–9. doi: 10.1200/JCO.2009.23.8329. [DOI] [PubMed] [Google Scholar]
- 10.Kantarjian HM, Thomas XG, Dmoszynska A, Wierzbowska A, Mazur G, Mayer J, Gau JP, Chou WC, Buckstein R, Cermak J, Kuo CY, Oriol A, Ravandi F, Faderl S, Delaunay J, Lysak D, Minden M, Arthur C. Multicenter, randomized, open-label, phase III trial of decitabine versus patient choice, with physician advice, of either supportive care or low-dose cytarabine for the treatment of older patients with newly diagnosed acute myeloid leukemia. J. Clin. Oncol. 2012;30:2670–7. doi: 10.1200/JCO.2011.38.9429. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.de Witte TM, Bowen D, Robin M, Malcovati L, Mufti G, Niederwieser D, Yakoubagha I, Kroger N. Should patients with high-risk or transformed myelodysplastic syndrome proceed directly to allogeneic transplant without prior cytoreduction by remission-induction chemotherapy or hypomethylating agent therapy? Clin Lymphoma Myeloma Leuk. 2014;14(Suppl):S42–5. doi: 10.1016/j.clml.2014.06.015. [DOI] [PubMed] [Google Scholar]
- 12.Alessandrino EP, Della Porta MG, Pascutto C, Bacigalupo A, Rambaldi A. Should cytoreductive treatment be performed before transplantation in patients with high-risk myelodysplastic syndrome? J. Clin. Oncol. 2013;31:2761–2. doi: 10.1200/JCO.2012.48.0525. [DOI] [PubMed] [Google Scholar]
- 13.Dombret H, Seymour JF, Butrym A, Wierzbowska A, Selleslag D, Jang JH, Kumar R, Cavenagh J, Schuh AC, Candoni A, Recher C, Sandhu I, Bernal del Castillo T, Al-Ali HK, Martinelli G, Falantes J, Noppeney R, Stone RM, Minden MD, McIntyre H, Songer S, Lucy LM, Beach CL, Dohner H. International phase 3 study of azacitidine vs conventional care regimens in older patients with newly diagnosed AML with >30% blasts. Blood. 2015;126:291–9. doi: 10.1182/blood-2015-01-621664. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Sorror ML, Maris MB, Storb R, Baron F, Sandmaier BM, Maloney DG, Storer B. Hematopoietic cell transplantation (HCT)-specific comorbidity index: a new tool for risk assessment before allogeneic HCT. Blood. 2005;106:2912–9. doi: 10.1182/blood-2005-05-2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Gratwohl A, Stern M, Brand R, Apperley J, Baldomero H, de Witte T, Dini G, Rocha V, Passweg J, Sureda A, Tichelli A, Niederwieser D. Risk score for outcome after allogeneic hematopoietic stem cell transplantation: a retrospective analysis. Cancer. 2009;115:4715–26. doi: 10.1002/cncr.24531. [DOI] [PubMed] [Google Scholar]
- 16.Todisco E, Ciceri F, Oldani E, Boschini C, Mico C, Vanlint MT, Donnini I, Patriarca F, Alessandrino PE, Bonifazi F, Arcese W, Barberi W, Marenco P, Terruzzi E, Cortelazzo S, Santarone S, Proia A, Corradini P, Tagliaferri E, Falcioni S, Irrera G, Dallanegra L, Castagna L, Santoro A, Camboni A, Sacchi N, Bosi A, Bacigalupo A, Rambaldi A. The CIBMTR score predicts survival of AML patients undergoing allogeneic transplantation with active disease after a myeloablative or reduced intensity conditioning: a retrospective analysis of the gruppo Italiano trapianto di midollo osseo. Leukemia. 2013;27:2086–91. doi: 10.1038/leu.2013.208. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Sorror ML, Storb RF, Sandmaier BM, Maziarz RT, Pulsipher MA, Maris MB, Bhatia S, Ostronoff F, Deeg HJ, Syrjala KL, Estey E, Maloney DG, Appelbaum FR, Martin PJ, Storer BE. Comorbidity-age index: a clinical measure of biologic age before allogeneic hematopoietic cell transplantation. J. Clin. Oncol. 2014;32:3249–56. doi: 10.1200/JCO.2013.53.8157. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Sorror ML, Sandmaier BM, Storer BE, Maris MB, Baron F, Maloney DG, Scott BL, Deeg HJ, Appelbaum FR, Storb R. Comorbidity and disease status based risk stratification of outcomes among patients with acute myeloid leukemia or myelodysplasia receiving allogeneic hematopoietic cell transplantation. J. Clin. Oncol. 2007;25:4246–54. doi: 10.1200/JCO.2006.09.7865. [DOI] [PubMed] [Google Scholar]
- 19.Sorror ML. How i assess comorbidities before hematopoietic cell transplantation. Blood. 2013;121:2854–63. doi: 10.1182/blood-2012-09-455063. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.van Gelder M, de Wreede LC, Schetelig J, van Biezen A, Volin L, Maertens J, Robin M, Petersen E, de Witte T, Kroger N. Monosomal karyotype predicts poor survival after allogeneic stem cell transplantation in chromosome 7 abnormal myelodysplastic syndrome and secondary acute myeloid leukemia. Leukemia. 2013;27:879–88. doi: 10.1038/leu.2012.297. [DOI] [PubMed] [Google Scholar]
- 21.Saber W, Horowitz MM. Transplantation for myelodysplastic syndromes: who, when, and which conditioning regimens. Hematology Am Soc Hematol Educ Program. 2016;2016:478–484. doi: 10.1182/asheducation-2016.1.478. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Symeonidis A, van Biezen A, de Wreede L, Piciocchi A, Finke J, Beelen D, Bornhauser M, Cornelissen J, Volin L, Mufti G, Chalandon Y, Ganser A, Bruno B, Niederwieser D, Kobbe G, Schwerdtfeger R, de Witte T, Robin M, Kroger N. Achievement of complete remission predicts outcome of allogeneic haematopoietic stem cell transplantation in patients with chronic myelomonocytic leukaemia. A study of the chronic malignancies working party of the European group for blood and marrow transplantation. Br J Haematol. 2015;171:239–246. doi: 10.1111/bjh.13576. [DOI] [PubMed] [Google Scholar]
- 23.Sierra J, Perez WS, Rozman C, Carreras E, Klein JP, Rizzo JD, Davies SM, Lazarus HM, Bredeson CN, Marks DI, Canals C, Boogaerts MA, Goldman J, Champlin RE, Keating A, Weisdorf DJ, de Witte TM, Horowitz MM. Bone marrow transplantation from HLA-identical siblings as treatment for myelodysplasia. Blood. 2002;100:1997–2004. [PubMed] [Google Scholar]
- 24.Nakai K, Kanda Y, Fukuhara S, Sakamaki H, Okamoto S, Kodera Y, Tanosaki R, Takahashi S, Matsushima T, Atsuta Y, Hamajima N, Kasai M, Kato S. Value of chemotherapy before allogeneic hematopoietic stem cell transplantation from an HLA-identical sibling donor for myelodysplastic syndrome. Leukemia. 2005;19:396–401. doi: 10.1038/sj.leu.2403640. [DOI] [PubMed] [Google Scholar]
- 25.Cutler CS, Lee SJ, Greenberg P, Deeg HJ, Perez WS, Anasetti C, Bolwell BJ, Cairo MS, Gale RP, Klein JP, Lazarus HM, Liesveld JL, McCarthy PL, Milone GA, Rizzo JD, Schultz KR, Trigg ME, Keating A, Weisdorf DJ, Antin JH, Horowitz MM. A decision analysis of allogeneic bone marrow transplantation for the myelodysplastic syndromes: delayed transplantation for low-risk myelodysplasia is associated with improved outcome. Blood. 2004;104:579–85. doi: 10.1182/blood-2004-01-0338. [DOI] [PubMed] [Google Scholar]
- 26.Cutler C. Timing of allogeneic stem cell transplantation for myelodysplastic syndromes and aplastic anemia. Hematology Am Soc Hematol Educ Program. 2014;2014:77–81. doi: 10.1182/asheducation-2014.1.77. [DOI] [PubMed] [Google Scholar]
- 27.Beran M, Shen Y, Kantarjian H, O’Brien S, Koller CA, Giles FJ, Cortes J, Thomas DA, Faderl S, Despa S, Estey EH. High-dose chemotherapy in high-risk myelodysplastic syndrome: covariate-adjusted comparison of five regimens. Cancer. 2001;92:1999–2015. doi: 10.1002/1097-0142(20011015)92:8<1999::aid-cncr1538>3.0.co;2-b. [DOI] [PubMed] [Google Scholar]
- 28.Bhatt VR, Loberiza FR Jr, Schmit-Pokorny K, Lee SJ. Time to insurance approval in private and public payers does not influence survival in patients who undergo hematopoietic cell transplantation. Biol Blood Marrow Transplant. 2016;22:1117–1124. doi: 10.1016/j.bbmt.2016.03.008. [DOI] [PubMed] [Google Scholar]
- 29.Weisdorf D, Zhang MJ, Arora M, Horowitz MM, Rizzo JD, Eapen M. Graft-versus-host disease induced graft-versus-leukemia effect: greater impact on relapse and disease-free survival after reduced intensity conditioning. Biol Blood Marrow Transplant. 2012;18:1727–33. doi: 10.1016/j.bbmt.2012.06.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Caballero JC, Sanchez Barba M, Hernandez Sanchez JM, Such E, Janusz K, Sanz G, Cabrero M, Chillon C, Cervera J, Hurtado AM, Jerez A, Calderon Cabrera C, Valcarcel D, Lumbreras E, Abaigar M, Lopez Cadenas F, Hernandez Rivas JM, Del Canizo MC, Diez Campelo M. Chronic graft-versus-host disease could ameliorate the impact of adverse somatic mutations in patients with myelodysplastic syndromes and hematopoietic stem cell transplantation. Ann Hematol. 2019;98:2151–2162. doi: 10.1007/s00277-019-03751-6. [DOI] [PubMed] [Google Scholar]
- 31.De La Serna J, Sanz J, Bermudez A, Cabrero M, Serrano D, Vallejo C, Gomez V, Moraleda JM, Perez SG, Caballero MD, Conde E, Lahuerta JJ, Sanz G. Toxicity and efficacy of busulfan and fludarabine myeloablative conditioning for HLA-identical sibling allogeneic hematopoietic cell transplantation in AML and MDS. Bone Marrow Transplant. 2016;51:961–6. doi: 10.1038/bmt.2016.42. [DOI] [PubMed] [Google Scholar]
- 32.Cao YG, He Y, Zhang SD, Liu ZX, Zhai WH, Ma QL, Pang AM, Wei JL, Yang DL, Huang Y, Feng SZ, Jiang EL, Han MZ. Conditioning regimen of 5-day decitabine administration for allogeneic stem cell transplantation in patients with myelodysplastic syndrome and myeloproliferative neoplasms. Biol Blood Marrow Transplant. 2020;26:285–291. doi: 10.1016/j.bbmt.2019.09.001. [DOI] [PubMed] [Google Scholar]
- 33.Cutler C, Logan B, Nakamura R, Johnston L, Choi S, Porter D, Hogan WJ, Pasquini M, MacMillan ML, Hsu JW, Waller EK, Grupp S, McCarthy P, Wu J, Hu ZH, Carter SL, Horowitz MM, Antin JH. Tacrolimus/sirolimus vs tacrolimus/methotrexate as GVHD prophylaxis after matched, related donor allogeneic HCT. Blood. 2014;124:1372–7. doi: 10.1182/blood-2014-04-567164. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Kanakry CG, O’Donnell PV, Furlong T, de Lima MJ, Wei W, Medeot M, Mielcarek M, Champlin RE, Jones RJ, Thall PF, Andersson BS, Luznik L. Multi-institutional study of post-transplantation cyclophosphamide as single-agent graft-versus-host disease prophylaxis after allogeneic bone marrow transplantation using myeloablative busulfan and fludarabine conditioning. J. Clin. Oncol. 2014;32:3497–505. doi: 10.1200/JCO.2013.54.0625. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Sorror ML, Martin PJ, Storb RF, Bhatia S, Maziarz RT, Pulsipher MA, Maris MB, Davis C, Deeg HJ, Lee SJ, Maloney DG, Sandmaier BM, Appelbaum FR, Gooley TA. Pretransplant comorbidities predict severity of acute graft-versus-host disease and subsequent mortality. Blood. 2014;124:287–295. doi: 10.1182/blood-2014-01-550566. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Fraccaroli A, Prevalsek D, Fritsch S, Haebe S, Bucklein V, Schulz C, Hubmann M, Stemmler HJ, Ledderose G, Hausmann A, Schmid C, Tischer J. Sequential HLA-haploidentical transplantation utilizing post-transplantation cyclophosphamide for GvHD prophylaxis in high-risk and relapsed/refractory AML/MDS. Am J Hematol. 2018;93:1524–1531. doi: 10.1002/ajh.25281. [DOI] [PubMed] [Google Scholar]


