Abstract
Treatment of patients with hepatocellular carcinoma (HCC) remains a serious challenge due to high heterogeneity and limited treatment options. In the past few decades, immune therapy, especially immune checkpoint therapy, has become an alternative option for the treatment of malignancies including HCC. Immune checkpoint inhibitors (ICIs) have raised attention because of their significant antitumor effect and low toxicity. However, such immunotherapy fails to be responsive in a major proportion of patients with HCC. Recent studies suggest that failures in antigen presentation, an impaired immune microenvironment, alterations in immune checkpoint molecules and immune-suppressive cells are responsible for the heterogeneous responses and resistance. Based on the specific characteristics above, we proposed a model stratifying patients with HCC into two subtypes that could predict response or resistance to ICI. Furthermore, supplementing ICIs with agents targeting the microenvironment could achieve an increased response rate, which is a step forward in precision treatment for HCC. In addition, emerging studies have revealed that liver transplantation, epigenetic drugs and other novel strategies also provide synergistic effects with ICIs in the treatment of HCC.
Keywords: Hepatocellular carcinoma, immune checkpoint inhibitors, resistance, combination therapy
Background
Liver cancer was the sixth most commonly diagnosed cancer and the fourth leading cause of cancer-related deaths worldwide in 2018 [1]. Among all liver cancer cases, hepatocellular carcinoma (HCC) constitutes 75-85%. The main risk factors for HCC have been well demonstrated, including chronic viral hepatitis, heavy alcohol intake and obesity. Due to differences in etiology and high mortality, HCC is regarded as a heterogeneous and refractory disease [2]. Therefore, it is a focus of research to explore strategies to control HCC. Liver transplantation (LT) and hepatectomy are curative treatments for HCC, and the indications have been safely expanded [3,4]. However, some tumors are still too advanced to be cured by surgical resection and orthotopic liver transplantation at diagnosis. Therefore, it is of great importance to administer palliative treatments to achieve downstaging for surgical therapy or delay the progression of tumors.
In the past few decades, cancer immunotherapy has experienced a paradigm shift from “novelty” to “common clinical practice”, and it has become one of the most effective treatments and has been validated in various tumors [5,6]. In the tumor microenvironment, tumor cells interact with the host immune response to promote or inhibit tumor progression. The immune system can recognize cancer cells and kill them via the immune response. In the early stages of research, most researchers spared no efforts to enhance the antitumor immune responses directly or indirectly via effector cells, cytokines and antibodies. Cytokines are one of the most important components of the immune system and contribute to the growth, differentiation and activation of immune cells. Most cytokines are produced by immune cells, including interleukins (ILs, e.g., IL-1α, IL-1β, IL-2, IL-5, etc.) and other cytokines [e.g., tumor necrosis factor (TNF) and interferon (IFN)] [7]. Several studies have revealed that an alteration in cytokine levels is correlated with carcinogenesis and progression in different tumors, including liver cancer [7,8]. T cell receptor (TCR)-engineered T cell therapy and chimeric antigen receptor (CAR) T cell therapy are two types of adoptive T cell therapy that use genetically modified T cells to treat cancers [9]. By genetic engineering, T cells can be endowed with the capacity to react against tumors, generating an intracellular signaling cascade causing the release of cytokines and enhancement of cytotoxic activity [10,11]. However, the unsatisfactory effect and frequent immune-related adverse events of these immune enhancement strategies due to immune escape and immune suppression have been discouraging [12,13].
Since the advent of ICIs, the concept of normalizing the tumor immune microenvironment by correcting dysfunctions of the immune response has drawn attention again to immunotherapy. Immune checkpoint therapy, which is at the forefront of immunotherapy, has demonstrated clinical activity in several malignances, including HCC, although the response rate to ICIs varies in patients [14,15]. In this review, we present a description of the current state of immune checkpoint therapy for HCC and attempt to provide insight into the resistance mechanisms. However, there are still a number of unanswered questions remaining; thus, we give our suggestions carefully and raise some future possible solutions based on current research.
Current state of immune checkpoint therapy-an acceptable strategy for advanced HCC
In the tumor microenvironment, a group of cell surface molecules, named immune checkpoints, determine T cell activation and the intensity of the immune response. They can be either stimulatory or inhibitory and participate in various stages of the T cell response [16]. The most studied immune checkpoint molecules include cytotoxic T lymphocyte-associated antigen 4 (CTLA-4), programmed cell death protein-1 (PD-1), programmed cell death protein ligand-1 (PD-L1), T cell immunoglobulin- and mucin-domain-containing molecule-3 (TIM-3), lymphocyte activation gene-3 (LAG-3) and TNF receptor superfamily member 4 (TNFRSF4). Zhou et al reported that the expression levels of immune checkpoint inhibitory molecules were significantly upregulated on tumor-associated antigen (TAA)-specific T cells isolated from human HCC tissues compared with the levels on T cells isolated from normal liver tissues or blood [17]. The responses of HCC-derived T cells to tumor antigens could be further restored by PD-L1, TIM3, or LAG3. Inhibitors of CTLA-4 and PD-1 have already been FDA-approved for the treatment of melanoma and are also currently being developed for HCC. Especially for patients with Barcelona clinical liver cancer (BCLC) stage B or C HCC that is not amenable to curative treatment, immune checkpoint therapy has become a promising approach.
CTLA-4 negatively regulates the T cell response by binding to B7 and delivering an inhibitory signal directly. In addition, it can also interfere with the binding between B7 and CD28 and result in the suppression of T cell activation [18]. It has been demonstrated that blockage of CTLA-4 can lead to the enhancement of antitumor effects by changing cytokine and chemokine profiles [19]. Tremelimumab is a monoclonal antibody (mAb) that blocks CTLA-4 and further inhibits CTLA-4-mediated downregulation of T cell activation. A clinical trial showed that tremelimumab had effective antitumor and antiviral activity in patients with advanced HCC resulting from HCV-induced liver cirrhosis [20].
Success in blocking CTLA-4 has led to progress in targeting other immune checkpoints, namely, PD-1/PD-L1. Binding of PD-1 to its ligand can suppress T cell migration, proliferation, and secretion of cytotoxic mediators and thus limits the activity of T cells in various stages of the immune response [14]. In HCC, the expression levels of PD-1 and PD-L1 had a significant correlation with CD8+ T cell infiltration, and high PD-1 expression was demonstrated as an independent poor prognostic factor for disease-free survival [21]. Nivolumab, a human IgG4 mAb against PD-1, received FDA approval in 2017 for advanced HCC patients who previously received sorafenib [22]. In the phase 1/2 trial, 262 patients with advanced-stage HCC were treated with nivolumab in a dose-escalation cohort (n=48) and a dose-expansion cohort (n=214); the objective response rates (ORR) were 15% and 20%, respectively, with a 9-month survival up to 66% [23]. The feasibility and safety of nivolumab were validated in many other centers as well, and most studies showed promising efficacy with acceptable adverse effects [24-27]. A clinical study based on an Asian cohort demonstrated a shorter duration of response in Asian patients than in the intention-to-treat (ITT) cohort, although overall survival was similar and unaffected by etiology in the Asian cohort [26]. Pembrolizumab, another humanized IgG4 antibody to PD-1, has also been assessed in a phase 2 study of advanced HCC patients who were sorafenib-refractory, in which the ORR was 16.3% among 104 treated patients [28].
In addition to the studies mentioned above, other agents targeting immune checkpoint molecules, such as durvalumab (NCT03899428), avelumab (NCT03389126) and XmAb®22841 (NCT03849469), are all in ongoing clinical trials for HCC (Table 1). Owing to the diverse expression levels of immune checkpoint molecules in different patients, the utilization of multiple ICIs might enhance antitumor activity, albeit with additional toxicity. The combination therapy of nivolumab and CTLA-4 blockade has achieved a significantly higher response rate than monotherapy in patients with melanoma, although most of those patients experienced treatment-related adverse events [29,30]. In the field of HCC, Zhou et al reported that combining anti-PD-L1 antibodies with antibodies against TIM3, LAG3, or CTLA4 further increased tumor-infiltrating lymphocyte functions [17]. Kim et al demonstrated that 4-1BB costimulation with agonistic antibodies could enhance anti-PD-1 antibody-mediated CD8+ TIL reinvigoration [31]. Other agonists targeting stimulatory molecules such as CD137 and TNFRSF4 also achieved satisfactory efficacy in HCC models [32,33]. Recently, strategies such as dual utilization of tremelimumab plus durvalumab (NCT03298451) and nivolumab plus ipilimumab (NCT03202204 and NCT03222076) have been validated in HCC-specific cohorts. Agonists of glucocorticoid-induced TNF receptor (GITR) or TNFRSF4, combined with classical ICIs, are also being evaluated in phase 1/2 studies (NCT03126110 and NCT03241173).
Table 1.
Ongoing clinical trials on immune checkpoint therapy in hepatocellular carcinoma
| Conditions | Strategies | Phases | Enrollment | Study Designs | Start Year | Completion year | NCT Number | Status |
|---|---|---|---|---|---|---|---|---|
| HCC | Nivolumab | Phase 3 | 1723 | Randomized | 2015 | 2020 | NCT02576509 | Active, not recruiting |
| HCC | Pembrolizumab | Phase 2 | 29 | Single Group Assignment | 2016 | 2020 | NCT02658019 | Active, not recruiting |
| HCC | Pembrolizumab | Phase 3 | 414 | Randomized | 2016 | 2020 | NCT02702401 | Active, not recruiting |
| HCC | Pembrolizumab | Phase 2 | 150 | Non-Randomized | 2016 | 2021 | NCT02702414 | Active, not recruiting |
| Selected cancers (including HCC) | INCAGN01876 (GITR stimulant) + nivolumab + ipilimumab | Phase 1/2 | 285 | Non-Randomized | 2017 | 2020 | NCT03126110 | Recruiting |
| HCC | Pembrolizumab | Phase 3 | 450 | Randomized | 2017 | 2022 | NCT03062358 | Recruiting |
| HCC | Nivolumab + ipilimumab | Phase 1 | 50 | Randomized | 2017 | 2020 | NCT03203304 | Recruiting |
| HCC | Nivolumab + ipilimumab | Phase 2 | 45 | Randomized | 2017 | 2022 | NCT03222076 | Recruiting |
| Selected cancers (including HCC) | INCAGN01949 (TNFRSF4 agonist) + nivolumab + ipilimumab | Phase 1/2 | 52 | Non-Randomized | 2017 | 2020 | NCT03241173 | Active, not recruiting |
| HCC | Tremelimumab + durvalumab | Phase 3 | 1310 | Randomized | 2017 | 2021 | NCT03298451 | Recruiting |
| HCC | Pembrolizumab | Phase 2 | 50 | Single Group Assignment | 2017 | 2020 | NCT03337841 | Not yet recruiting |
| HCC | Avelumab | Phase 2 | 30 | Single Group Assignment | 2017 | 2020 | NCT03389126 | Active, not recruiting |
| HCC | Nivolumab | Phase 3 | 530 | Randomized | 2017 | 2025 | NCT03383458 | Recruiting |
| HCC | Pembrolizumab | Phase 2 | 60 | Single Group Assignment | 2017 | 2020 | NCT03163992 | Recruiting |
| HCC | Pembrolizumab | Phase 2 | 30 | Single Group Assignment | 2018 | 2020 | NCT03419481 | Recruiting |
| HCC | Nivolumab + ipilimumab | Phase 2 | 40 | Single Group Assignment | 2018 | 2022 | NCT03510871 | Not yet recruiting |
| HCC | Nivolumab | Phase 2 | 50 | Single Group Assignment | 2018 | 2020 | NCT03630640 | Recruiting |
| HCC | Pembrolizumab | Not Applicable | 200 | Randomized | 2018 | 2021 | NCT03755739 | Recruiting |
| HCC | Ipilimumab + nivolumab | Phase 1/2 | 32 | Single Group Assignment | 2019 | 2022 | NCT03682276 | Recruiting |
| HCC | Pembrolizumab | Phase 3 | 950 | Randomized | 2019 | 2025 | NCT03867084 | Recruiting |
| Solid tumors (including HCC) | Pembrolizumab + XmAb®22841 (CTLA-4 x LAG-3 dual inhibitor) | Phase 1 | 242 | Non-Randomized | 2019 | 2027 | NCT03849469 | Recruiting |
| HCC | Pembrolizumab/nivolumab/JS001 (PD-1 inhibitor) | Phase 2 | 50 | Single Group Assignment | 2019 | 2019 | NCT03939975 | Completed |
| HCC | Durvalumab + tremelimumab | Phase 2 | 30 | Non-Randomized | 2019 | 2020 | NCT03638141 | Recruiting |
| HCC | Durvalumab | Phase 2 | 30 | Non-Randomized | 2019 | 2020 | NCT03899428 | Not yet recruiting |
| HCC | Nivolumab + ipilimumab | Phase 3 | 1084 | Randomized | 2019 | 2023 | NCT04039607 | Recruiting |
CTLA-4: Cytotoxic T lymphocyte-associated antigen 4; GITR: Glucocorticoid-induced TNF receptor; HCC: Hepatocellular carcinoma; LAG-3: Lymphocyte activation gene-3; PD-1: Programmed cell death protein-1; TNF: Tumor necrosis factor; TNFRSF4: TNF receptor superfamily member 4.
The dilemma in immune checkpoint therapy-heterogeneous response rates in HCC
The encouraging results from clinical trials of immune checkpoint therapy have resulted in increased clinical implementation in various types of cancer, including HCC. However, only approximately 20% of advanced HCC patients benefit from ICIs, and most of them have disease progression after 3-9 months [34]. These results indicate that a substantial proportion of patients treated with ICIs suffer primary or acquired resistance. Therefore, studying the underlying mechanism and maximizing the curative effect of immune checkpoint therapy have become a focus in the field of HCC treatment.
The immune response in the tumor microenvironment is a multistep process. The generation and activation of tumor-specific CD8+ T cells are the basis of the immune response, which requires successful presentation of TAAs by antigen-presenting cells (APCs) and immunorecognition of these antigenic peptides displayed by major histocompatibility complex (MHC) I/II molecules [35]. Naïve CD8+ T cells subsequently differentiate into effector T cells and kill tumor cells with a cascade of cytolytic molecules (e.g., IFN-γ, granzyme and perforin). The amplitude and quality of the response result from the regulation of costimulatory molecules, immune checkpoint molecules and immune-modulating cells. Thus, abnormal conduct in any step would contribute to resistance to immune checkpoint therapy (Figure 1).
Figure 1.
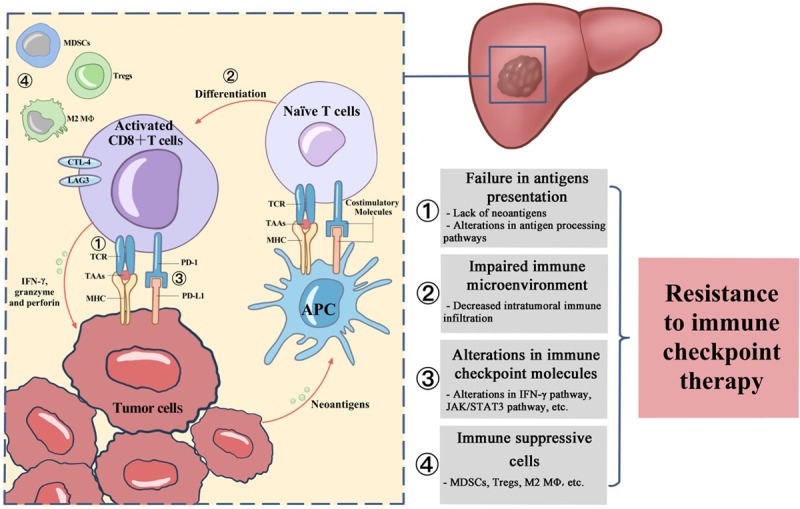
The putative mechanism of resistance to immune checkpoint inhibitors. The schematic shows the detailed steps involved in the generation and activation of tumor-specific T cells. The boxes on the right list the possible abnormities contributing to resistance to immune checkpoint therapy.
Failures in antigen presentation
A lack of neoantigens and alterations in antigen-processing pathways are associated with an impaired antitumor immune response, since neoantigens are essential for immune response reactivation in immune therapy. Some researchers have performed comparative analyses of various TAA-specific T cell responses in HCC patients and identified useful antigens for immunotherapy, such as GPC3, P53, multidrug resistance-associated protein 3 (MRP3), α-fetoprotein (AFP), and human telomerase reverse transcriptase (hTERT) [19,36]. TAA-specific immunotherapy combined with systemic treatment or immune checkpoint inhibitors has the possibility to produce stronger immune responses than monotherapies. Anagnostou et al demonstrated that the loss of 7-18 putative neoantigens could be observed in resistant clones by comparing the neoantigen landscape of matched biopsy samples from patients with non-small-cell lung cancer (NSCLC) treated with ICIs [37]. In the field of HCC, high-throughput sequencing might also reveal the differences in neoantigens between resistant clusters and responsive clusters. Mutated human leukocyte antigen (HLA) ligands are regarded as promising targets for tumor-specific immunotherapy [38,39]. A multiomics study pointed out that exome-derived mutated HLA ligands remained elusive in HCC, which results from HCC having a lower mutational burden than other malignancies such as melanoma and lung cancer [40]. The results were consistent with the higher response rate to immune checkpoint therapy in tumors harboring high levels of mutations, such as melanoma.
Antigen presentation apparatuses such as MHC class I molecules and β2-microglobulin (B2M) are also necessary for immune therapy. B2M is essential for proper MHC class I folding and further transport to cell surface. Zaretsky et al found that a 4-bp homozygous frameshift deletion in B2M was closely associated with acquired resistance to a PD-1 inhibitor in a late-relapse patient with melanoma [41]. Moreover, copy number alterations in B2M were observed in nonresponders to CTLA-4 blockade [42]. The expression of HLA class I molecules and transporters associated with antigen processing genes (TAP1 and TAP2) varies in different HCC cell lines [43], which may contribute to resistance to immune therapy. Umemoto et al indicated that HLA class I expression on HCC cells was correlated with CD3-positive cell infiltration, and patients with high HLA class I expression showed an improved prognosis compared with those with low HLA class I expression [44]. These findings revealed that proficient antigen presentation is essential for immunotherapy.
Impaired immune microenvironment
In the past few years, the success of immune therapy has drawn attention towards the tumor immune microenvironment. The immune microenvironment, consisting of intratumoral infiltrating immune cells, cytokines and chemokines, is the functional foundation of immune response. Heterogeneity in the immune microenvironment contributes to the different response rates to immune checkpoint therapy. Sia et al found that approximately 25% of HCCs, making up the “immune class”, had significantly higher immune infiltration and more tumor-infiltrated lymphocytes (TILs) than the rest of HCCs [45]. Patients with tumors belonging to this particular class had markers of inflammatory response and cytolytic activity with high expression levels of PD-1/PD-L1. The immune class contained 2 subtypes, characterized by adaptive immune response elements [e.g., T cell receptor G, CD8A, granzyme B (GZMB), and IFN-γ signaling] or immunesuppressive components [e.g., transforming growth factor-β (TGF-β) signaling and M2 macrophages], though there was no significant difference in PD-1/PD-L1 expression. Furthermore, they discovered another group of patients characterized by exclusion of TILs and enrichment in catenin beta 1 (CTNNB1) mutations, named the “HCC exclusion class”, and those within the group might exhibit innate resistance to immune therapy [46,47]. This might be because activation of β-catenin could promote immune escape as a result of defective recruitment of dendritic cells and impaired T cell activity [48]. In addition, Kurebayashi et al classified the immune microenvironment of HCC into three distinct immune subtypes based on immune cell infiltration: immune-high, immune-mid, and immune-low, and the expression of PD-1/PD-L1 was associated with the immune-high subtype [49]. Recently, Zhang et al identified three distinctive HCC subtypes with immunocompetent, immunodeficient, and immunosuppressive features [50]. The immunocompetent subtype showed relatively higher T cell infiltration levels than the other two subtypes, while the immunosuppressive subtype was characterized by high frequencies of immunosuppressive cells with upregulated immune checkpoint molecules. The immunocompetent and immunosuppressive subtypes had significantly increased immune cell infiltration and better prognosis than the immunodeficient subtype. Therefore, the studies above revealed that microenvironments with robust immune infiltration were closely associated with high response rates to immunotherapy.
Alterations in immune checkpoint molecules
As is widely reported, an elevated level of immune checkpoint molecules indicates a poor prognosis in HCC patients and an aggressive phenotype in tumors [51,52]. Apparently, the existence of immune checkpoint molecules is the basis for immune checkpoint therapy, which could help to select a subgroup of HCC patients who are most likely to respond to ICIs. However, the expression pattern of immune checkpoint molecules differs from patient to patient due to the high heterogeneity in HCC, and alternations of these essential molecules may mediate resistance. For instance, the expression level of PD-L1 in multiple kinds of tumor cells is considered to be closely associated with intratumoral immune cell infiltration, reflecting an immune-reactive milieu with an effective response to PD-1 inhibitors [53]. The expression of peroxisome proliferator-activated receptor α (PPARα) was significantly suppressed in HCC tissues compared with para-cancerous tissues, and PPARα overexpression significantly inhibited PD-L1 expression in HCC with increased release of inflammatory cytokines by T cells [54]. Cell cycle-related kinase (CCRK) plays an important role in tumor immunity and hepatocarcinogenesis. The silencing of tumorous Ccrk could upregulate PD-L1 expression and increase intratumoral CD8+ T cells in transgenic mice, which enhanced the efficacy of a PD-L1 inhibitor in HCC treatment [55]. In addition, low expression of phosphorylated extracellular signaling-regulated kinase (pERK) in mouse and human HCC samples was associated with significant enrichment of infiltrating inflammatory cells and intratumoral CD8+ cytotoxic T lymphocytes expressing PD-1 [56]. Thymocyte selection-associated high mobility group box protein (TOX), which is a part of T cell exhaustion signatures, could maintain robust PD-1 expression and promote CD8+ T cell exhaustion by regulating endocytic recycling of PD-1 [57]. The IFN-γ pathway is essential for the surface expression of PD-L1 and MHC molecules. Activated T cells release IFN-γ to bind to IFN-γ receptors (IFNGR) on tumor cells, and subsequently, Janus kinase 1 (JAK1)/JAK2 and signal transducer and activator of transcription (STAT) signaling is triggered to activate IFN-related genes, such as interferon regulatory factor 1 (IRF1). The activation of IRF1 regulates the transcription of genes, resulting in increased expression of PD-L1 and MHC molecules [58]. Mutations in JAK1 and JAK2 were discovered in patients with metastatic melanoma who had acquired resistance to anti-PD-1 therapy [41]. Xu et al reported that the activation of the JAK/STAT3 pathway could promote the expression of PD-L2 in HCC [59]. The expression of immune checkpoint molecules is regulated by diverse pathways, and modulating the immune microenvironment by targeting these pathways might be a promising approach against resistance. In addition, some studies pointed out that alternative immune checkpoints could be upregulated in those with adaptive resistance to certain ICIs. Koyama et al reported that upregulation of TIM-3 and other immune checkpoints was observed in PD-1 inhibitor-resistant mouse models and patients with lung cancer [60]. Similarly, a trend of LAG3 and TIM3 upregulation on circulating T cells was observed in patients resistant to PD-1/PD-L1 blockade, which suggested that those patients could benefit from dual use of ICIs [61].
Immune-suppressive cells
However, the expression of immune checkpoint molecules and robust immune infiltration do not guarantee a high response to the treatment. More specifically, other components of the immune microenvironment may contribute to T cell dysfunction and exhaustion or immune checkpoint molecule dysregulation, which further develop the resistance to ICIs. Immune-suppressive cells, including regulatory T cells (Tregs), myeloid-derived suppressor cells (MDSCs), and M2-polarized tumor-associated macrophages (TAMs), have been shown to influence the efficacy of immune checkpoint inhibitors. Ngjow et al constructed PD-1 inhibitor-sensitive and PD-1 inhibitor-resistant tumor mouse models and further reported that the abrogation of Tregs could recover sensitivity to PD-1 inhibitors in resistant tumors, which demonstrated that Tregs are partly responsible for resistance to immune checkpoint inhibitors [62]. Tumor-infiltrating MDSCs from patients with HCC could effectively inhibit autologous CD8+ T cell proliferation, which helped to shape the tumor immunosuppressive microenvironment and induce immune checkpoint therapy resistance [55]. Indoleamine 2,3-dioxygenase (IDO) is an enzyme associated with an aggressive tumor phenotype as well as resistance to immunotherapy [63]. A study performed by Holmgaard et al found that IDO-overexpressing cells could recruit and activate MDSCs through Tregs and further reduce the tumor response to immunotherapy, which was validated in melanoma cell lines [64]. ICIs could promote the induction of IDO and provide adaptive resistance in insensitive HCC tumors [65], although the infiltration of MDSCs in those resistant tumors remains to be further investigated. M2-polarized TAMs are able to downregulate the antitumor activity of T cells induced by ICIs, since they can recruit regulatory T cells and release anti-inflammatory cytokines, resulting in an immunosuppressive microenvironment. Osteopontin (OPN) is a tumor-specific inflammatory biomarker associated with tumor progression and immunosuppression. Zhu et al demonstrated that OPN-high HCC featured M2-like polarization of macrophages and upregulated PD-L1 expression via the activation of the colony-stimulating factor-1 (CSF1)/CSF1 receptor (CSF1R) pathway [66]. Blocking CSF1/CSF1R signaling prevented the recruitment of M2-polarized TAMs and thereby sensitized HCC to anti-PD-L1 blockade. Therefore, these specific types of cells could induce an immune-suppressive microenvironment to prevent antitumor cytotoxic activity and ultimately result in resistance.
Based on the rationale above, we accordingly established a model stratifying patients with HCC into two clusters, named the responsive cluster and the resistant cluster (Figure 2). The responsive cluster is characterized by robust immune infiltration and enrichment of immune checkpoint molecules, while the resistant cluster is characterized by exclusion of tumor-infiltrating lymphocytes and molecular signatures of resistance. Hopefully, our resistance-related classification may provide an overview of patients’ immune landscapes and help to precisely select candidates for immune checkpoint therapy.
Figure 2.
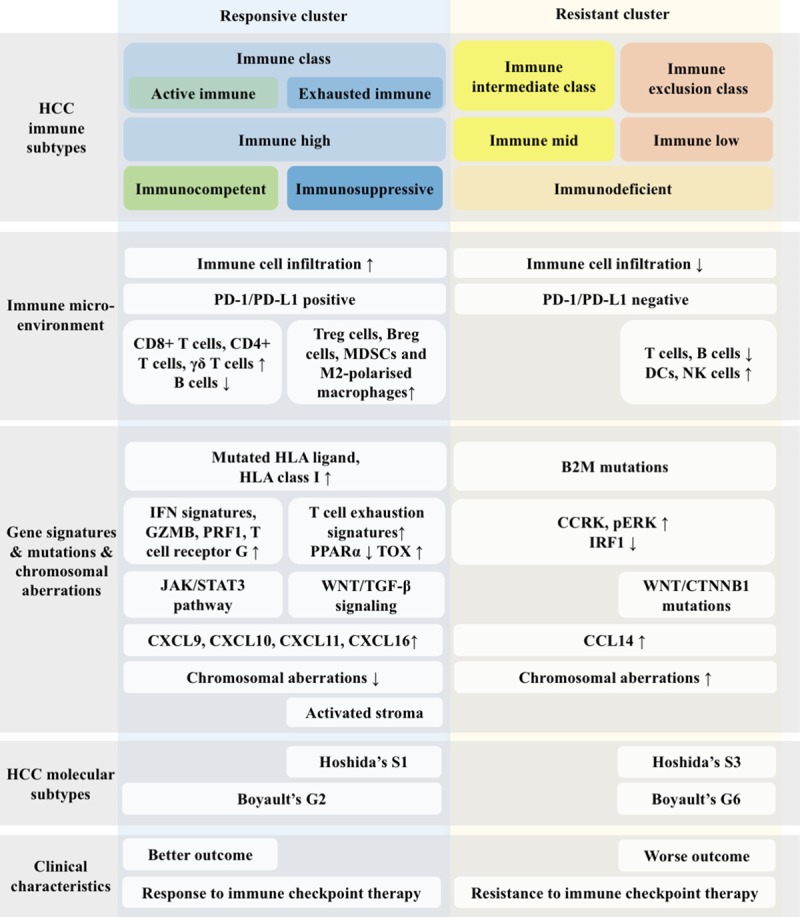
Schematic summary of HCC classification according to the immune landscape in the tumor microenvironment. This classification defines two clusters based on the immune landscape and clinical characteristics, the responsive cluster and the resistant cluster, each of which is characterized by distinct immune-related parameters and might present a different response rate to immune checkpoint therapy.
The future of immune checkpoint therapy-a step forward for precision treatment
Immune checkpoint therapy has become a promising treatment for advanced HCC, although the low response rates in HCC patients are concerning. Recently, some researchers pointed out that combination with locoregional treatment presented great potential to enhance the antitumor activity of ICIs in HCC patients [67,68]. Eradicating tumors directly can lead to the activation of the immune system and a decrease in immunosuppression, which results from changes in cytokine profiles and T cell subset populations [69-71]. Agents targeting the tumor microenvironment could also provide a profound effect on improving responsiveness to immune checkpoint therapy [72]. For instance, strategies combining antiangiogenetic drugs or other targeted therapies (e.g., therapies targeting Wnt/β-catenin signaling, the mTOR pathway, etc.) have already shown encouraging results in vitro and in mouse models [73-76], and related clinical trials are underway (Table 2). In addition, emerging studies have suggested that liver transplantation, epigenetic drugs and other novel strategies might also result in synergistic effects when they are combined with ICIs in the treatment of advanced HCC (Table 3).
Table 2.
Ongoing clinical trials of immune checkpoint therapy combined with targeted therapy in hepatocellular carcinoma
| Conditions | Strategies | Phases | Enrollment | Study Designs | NCT Number | Status |
|---|---|---|---|---|---|---|
| HCC | Nivolumab + ipilimumab + cabozantinib | Phase 1/2 | 620 | Non-Randomized | NCT01658878 | Active, not recruiting |
| HCC | Durvalumab + tremelimumab, durvalumab + bevacizumab | Phase 2 | 545 | Randomized | NCT02519348 | Recruiting |
| Solid tumors (including HCC) | Mogamulizumab (CCR4 antagonist) + nivolumab | Phase 1/2 | 114 | Single Group Assignment | NCT02705105 | Completed |
| Gastrointestinal or thoracic malignancies (including HCC) | Ramucirumab + MEDI4736 (PD-L1 inhibitor) | Phase 1 | 114 | Non-Randomized | NCT02572687 | Active, not recruiting |
| HCC | Pembrolizumab + lenvatinib | Phase 1 | 97 | Single Group Assignment | NCT03006926 | Active, not recruiting |
| Solid tumors (including HCC) | Atezolizumab + cabozantinib | Phase 1/2 | 1732 | Non-Randomized | NCT03170960 | Recruiting |
| HCC | Avelumab + axitinib | Phase 1 | 22 | Non-Randomized | NCT03289533 | Active, not recruiting |
| HCC | Pembrolizumab + sorafenib tosylate | Phase 1/2 | 27 | Single Group Assignment | NCT03211416 | Recruiting |
| HCC | Nivolumab + cabozantinib | Phase 1 | 15 | Single Group Assignment | NCT03299946 | Recruiting |
| HCC | Nivolumab + lenvatinib | Phase 1 | 30 | Non-Randomized | NCT03418922 | Active, not recruiting |
| HCC | Nivolumab + sorafenib | Phase 2 | 40 | Non-Randomized | NCT03439891 | Recruiting |
| HCC | Atezolizumab + bevacizumab | Phase 3 | 480 | Randomized | NCT03434379 | Recruiting |
| HCC | Nivolumab + bevacizumab | Phase 1 | 12 | Single Group Assignment | NCT03382886 | Active, not recruiting |
| HCC | Pembrolizumab + bavituximab | Phase 2 | 34 | Single Group Assignment | NCT03519997 | Recruiting |
| Solid tumors (including HCC) | Avelumab + regorafenib | Phase 1/2 | 362 | Non-Randomized | NCT03475953 | Recruiting |
| HCC | Pembrolizumab + regorafenib | Phase 1 | 40 | Non-Randomized | NCT03347292 | Recruiting |
| HCC | APL-501 (PD-1 inhibitor) + APL-101 (c-MET inhibitor) | Phase 1/2 | 119 | Non-Randomized | NCT03655613 | Recruiting |
| Solid tumors (including HCC) | Nivolumab + vorolanib, pembrolizumab + vorolanib | Phase 1 | 56 | Non-Randomized | NCT03511222 | Recruiting |
| Gastrointestinal malignancies (including HCC) | Durvalumab + cabozantinib | Phase 1 | 30 | Single Group Assignment | NCT03539822 | Recruiting |
| HCC | Atezolizumab + cabozantinib | Phase 3 | 740 | Randomized | NCT03755791 | Recruiting |
| HCC | Pembrolizumab + lenvatinib | Phase 3 | 750 | Randomized | NCT03713593 | Recruiting |
| HCC | Durvalumab + bevacizumab | Phase 3 | 888 | Randomized | NCT03847428 | Recruiting |
| HCC | Nivolumab + lenvatinib | Phase 2 | 50 | Single Group Assignment | NCT03841201 | Recruiting |
| HCC | Nivolumab + GT90001 (angiogenesis inhibitor) | Phase 1/2 | 20 | Single Group Assignment | NCT03893695 | Recruiting |
| HCC | Durvalumab + tivozanib | Phase 1/2 | 42 | Sequential Assignment | NCT03970616 | Not yet recruiting |
| HCC | Nivolumab + lenvatinib | Phase 2/3 | 216 | Randomized | NCT04044651 | Not yet recruiting |
| HCC | Atezolizumab + bevacizumab | Phase 3 | 662 | Randomized | NCT04102098 | Not yet recruiting |
| Selected cancers (including HCC) | MK-3475 (PD-1 inhibitor) + INCB024360 (IDO inhibitor) | Phase 1/2 | 444 | Non-Randomized | NCT02178722 | Active, not recruiting |
| Solid tumors (including HCC) | Nivolumab + galunisertib (TGF-β inhibitor) | Phase 1/2 | 75 | Non-Randomized | NCT02423343 | Active, not recruiting |
| HCC | Nivolumab + CC-122 (CRBN protein modulator) | Phase 1/2 | 21 | Single Group Assignment | NCT02859324 | Active, not recruiting |
| HCC | Nivolumab + SF1126 (PI3K inhibitor) | Phase 1 | 14 | Single Group Assignment | NCT03059147 | Active, not recruiting |
| Selected cancers (including HCC) | Pembrolizumab + INCAGN01876 (GITR stimulant) + Epacadostat(IDO inhibitor) + pembrolizumab | Phase 1/2 | 10 | Single Group Assignment | NCT03277352 | Active, not recruiting |
| HCC | Nivolumab + BMS-986205 (IDO1 inhibitor) | Phase 1/2 | 23 | Single Group Assignment | NCT03695250 | Recruiting |
| Solid tumors (including HCC) | Nivolumab + copanlisib (PI3K inhibitor) | Phase 1/2 | 160 | Non-Randomized | NCT03735628 | Recruiting |
| Selected cancers (including HCC) | Atezolizumab + KY1044 (ICOS agonist) | Phase 1/2 | 412 | Non-Randomized | NCT03829501 | Recruiting |
| HCC | Nivolumab + abemaciclib (CDK4 inhibitor) | Phase 2 | 27 | Single Group Assignment | NCT03781960 | Not yet recruiting |
| HCC | Nivolumab + cabiralizumab (CSF1R antagonist), nivolumab + BMS-986253 (IL-8 inhibitor) | Phase 2 | 74 | Randomized | NCT04050462 | Not yet recruiting |
| Advanced cancer (including HCC) | Pembrolizumab/nivolumab/atezolizumab/avelumab + ALT-803 (IL-15R agonist) | Phase 2 | 611 | Non-Randomized | NCT03228667 | Recruiting |
| Liver cancer | TSR-022 (HAVCR2 protein inhibitor) + TSR-042 (PD-1 inhibitor) | Phase 2 | 42 | Single Group Assignment | NCT03680508 | Not yet recruiting |
| HCC or NSCLC | Nivolumab + BMS-813160 (CCR2 antagonist), nivolumab + BMS-986253 (IL-8 inhibitor) | Phase 2 | 50 | Randomized | NCT04123379 | Not yet recruiting |
BTC: Bile tract carcinoma; CCR: C-C motif chemokine receptor; CDK4: Cyclin dependent kinase 4; CRBN: Cereblon; CSF1R: Colony stimulating factor 1 receptor; CTLA-4: Cytotoxic T lymphocyte-associated antigen 4; GITR: Glucocorticoid-induced TNF receptor; HAVCR2: Hepatitis A virus cellular receptor 2; HCC: Hepatocellular carcinoma; ICOS: Inducible T cell co-stimulator; IDO: Indoleamine 2,3-dioxygenase; IL: Interleukin; LAG-3: Lymphocyte activation gene-3; NSCLC: Non-small-cell lung cancer; PD-1: Programmed cell death protein-1; PD-L1: programmed cell death protein ligand-1; PI3K: Phosphatidylinositol 3-kinase; TGF-β: Transforming growth factor-β.
Table 3.
Ongoing clinical trials of immune checkpoint therapy combined with novel strategies in hepatocellular carcinoma
| Conditions | Strategies | Phases | Enrollment | Study Designs | NCT Number | Status |
|---|---|---|---|---|---|---|
| Solid tumors (including HCC) | Pembrolizumab + p53MVA vaccine (vaccine therapy) | Phase 1 | 19 | Single Group Assignment | NCT02432963 | Active, not recruiting |
| HCC | Pembrolizumab + Talimogene Laherparepvec (gene therapy) | Phase 1 | 244 | Non-Randomized | NCT02509507 | Recruiting |
| HCC | Pembrolizumab + elbasvir/grazoprevir (antiviral therapy) + ribavirin (antiviral therapy) | Phase 1/2 | 30 | Non-Randomized | NCT02940496 | Active, not recruiting |
| HCC | Nivolumab + Pexastimogene Devacirepvec (vaccinia virus-based therapy) | Phase 1/2 | 30 | Single Group Assignment | NCT03071094 | Active, not recruiting |
| Liver, pancreatic, bile duct, or gallbladder cancer | Durvalumab + guadecitabine (DNMTi) | Phase 1 | 90 | Single Group Assignment | NCT03257761 | Recruiting |
| Solid tumors (including HCC) | FT500 (NK cell replacement) + nivolumab/pembrolizumab/atezolizumab | Phase 1 | 76 | Non-Randomized | NCT03841110 | Recruiting |
| HCC or Liver dominant metastatic cancer | Nivolumab + tadalafil + vancomycin | Phase 2 | 27 | Single Group Assignment | NCT03785210 | Recruiting |
| Solid tumors (including HCC) | Nivolumab/pembrolizumab + metformin (metabolic modulator), nivolumab/pembrolizumab + rosiglitazone (metabolic modulator) | Phase 2 | 108 | Randomized | NCT04114136 | Not yet recruiting |
DNMTi: DNA methyltransferase inhibitors; HCC: Hepatocellular carcinoma; p53MVA vaccine: Modified vaccinia virus Ankara vaccine expressing p53.
LT is a curative treatment for HCC, although the high recurrence rate after LT has limited its effect. It has been reported that the recurrence of HCC might be partly attributable to the immunosuppressive microenvironment due to the use of immunosuppressants post-LT. Thus, immune checkpoint therapy might achieve favorable response rates as an adjuvant therapy in recipients undergoing LT. However, the utilization of ICIs is still controversial in liver transplant recipients due to the increased possibility of rejection and liver failure. T cells can recognize and kill both tumor cells and donor cells, which means that the activation of T cells would be a “double-edged sword”. Three studies reviewed 33 liver transplant recipients (mainly with HCC) treated with ICIs with a relatively low response rate of 15.2%, and subsequent graft rejection was observed in 10 patients [77-79]. Rejection after immune checkpoint therapy seems inevitable, so using this method to prevent or treat tumor recurrence still has a long way to go. To acquire a durable response while avoiding rejection, we should adjust the dose of immunosuppressants and immune checkpoint inhibitors to reach a balance and further develop biomarkers for tumor response and transplant rejection (Figure 3).
Figure 3.
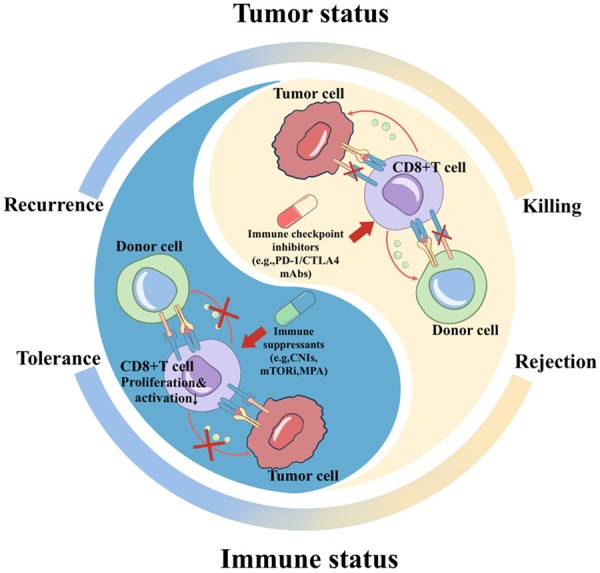
The tumor microenvironment under the treatment of immunosuppressants and immune checkpoint inhibitors. Tolerance of donor graft cells is achieved when T cells are inhibited due to immune suppressants, although the risk of tumor recurrence increases. Tumor cell killing can be induced by immune checkpoint therapy, although donor cells will be recognized and killed as well. CNIs: calcineurin inhibitors; MPA: mycophenolic acid; mTORi, mammalian target of rapamycin inhibitors.
Epigenetic drugs, such as bromodomain and extraterminal domain inhibitors (iBET), histone deacetylase inhibitors (HDACi), histone methyltransferase inhibitors (HMTi) and DNA methyltransferase inhibitors (DNMTi), targeting modifications at the DNA and histone levels are a novel group of antitumor agents [80]. They can both exert influences at the tumor cell and the immune system level, resulting in tumor cell killing and immunosuppressive microenvironment remodeling. It has been reported that tumors surrounding fibrotic livers were markedly enriched with monocytic MDSCs, which was significantly correlated with reduced tumor-infiltrating lymphocytes. Inhibiting monocytic MDSCs by a combination of iBET762 and anti-PD-L1 therapy could recruit tumor-infiltrating lymphocytes and further lead to tumor eradication and prolonged survival in the fibrotic-HCC mouse model [81]. Llopiz et al tested HDACi in combination with ICIs for their ability to enhance tumoricidal effects in a murine model of HCC, showing a satisfactory result with enhanced IFN-γ production and a decrease in regulatory T cells [82]. Hong et al demonstrated that epigenetic modulation with an enhancer of Zeste homolog 2 (EZH2) inhibitor and a DNMT1 inhibitor could be a novel potential strategy to augment immunotherapy for HCC by stimulating T cell trafficking into the tumor microenvironment [83]. Guadecitabine, which is a second-generation DNMTi, also showed potential for combination treatment with immune checkpoint therapy [84], and related clinical trials are currently ongoing (NCT03257761). Targeting the cancer epigenome has provided a feasible approach for individualized therapy, and combining epigenetic treatment with ICIs will achieve increasing success.
Tumor vaccines, oncolytic viruses and adoptive cellular therapies are also the focus of treatment for HCC and might also provide a synergistic effect in combination treatments for HCC. Chung et al genetically engineered a modified vaccinia Ankara (MVA) viral vector expressing the human p53 transgene (p53MVA), which has achieved satisfactory results in several preclinical studies [85,86]. A phase 1 trial evaluated the safety and tolerability of a combination of the p53MVA vaccine and pembrolizumab in patients with solid tumors (NCT02432963). The results showed that 3 of 11 patients retained stable disease for more than 30 weeks with few side effects [87]. FT500 is an induced pluripotent stem cell (iPSC)-derived NK cell replacement product, and the clinical investigation of FT500 in combination with ICIs is now ongoing (NCT03841110). Pexastimogene devacirepvec is an oncolytic vaccinia virus, and a randomized, phase 2b trial showed that it presented a tolerable safety profile and induced T cell responses in HCC patients, though it did not improve overall survival [88]. Its preliminary activity with nivolumab is currently being tested (NCT03071094). Clinical trials combining other strategies, such as metabolic modulators (NCT04114136) and gene therapy (NCT02509507), are also currently being carried out, and more clinical data remain to be collected.
Conclusions
With the emergence of immune checkpoint therapy in the last decade, we are now entering a new era of immune therapy, following the era of cytotoxic agents and targeted therapy. Immune checkpoint therapy has become a promising treatment for various tumors, including NSCLC, melanoma and renal cell carcinoma. In the field of liver cancer, ICI-based strategies will be an important component of anticancer treatment in the near future, albeit with high cost and immune-related adverse events. However, there is still a proportion of patients who do not benefit from immune checkpoint therapy. Thus, it is of great importance to stratify patients into different subtypes based on genomic and/or transcriptomic landscapes and further identify predictive biomarkers to precisely select patients with high response rates. Combined treatment consisting of conventional therapy and immune checkpoint therapy has partly solved the problem, while combinations with targeted therapy, epigenetic drugs and other novel strategies still require more validation in clinical trials. Therefore, we need to spare no efforts in developing new strategies and minimizing adverse events in immune checkpoint therapy for patients with HCC.
Acknowledgements
We thank Ms. Cen for technical assistance and secretarial work. This work was supported by National Major Scientific and Technological Special Project (No. 2017ZX10203205), Key Research & Development Plan of Zhejiang Province (No. 2019C3050), Zhejiang Provincial Natural Science Foundation of China (No. LQ19H160030) and Cheung Kong Scholar Program of China (No. T2014146).
Disclosure of conflict of interest
None.
Abbreviations
- AFP
α-fetoprotein
- APCs
Antigen-presenting cells
- BCLC
Barcelona Clinical Liver Cancer
- B2M
β2-microglobulin
- BTC
Bile tract carcinoma
- CA
Cryoablation
- CAR
Chimeric antigen receptor
- CCR
C-C motif chemokine receptor
- CCRK
Cell cycle-related kinase
- CDK4
Cyclin dependent kinase 4
- CNIs
calcineurin inhibitors
- CRBN
Cereblon
- CSF1
Colony stimulating factor-1
- CTLA-4
Cytotoxic T lymphocyte-associated antigen 4
- CTNNB1
Catenin beta 1
- DNMTi
DNA methyltransferase inhibitors
- ECOG
Eastern Cooperative Oncology Group
- EZH2
Enhancer of Zeste Homolog 2
- FDA
Food and Drug Administration
- GITR
Glucocorticoid-induced TNF receptor
- GZMB
Granzyme B
- HAVCR2
Hepatitis A virus cellular receptor 2
- HBV
Hepatitis B virus
- HCC
Hepatocellular carcinoma
- HCV
Hepatitis C virus
- HDACi
Histone deacetylase inhibitors
- HLA
Human leukocyte antigen
- HMTi
Histone methyltransferase inhibitors
- hTERT
Human telomerase reverse transcriptase
- iBET
Bromodomain and extraterminal domain inhibitors
- ICI
immune checkpoint inhibitors
- ICOS
Inducible T cell co-stimulator
- IDO
Indoleamine 2,3-dioxygenase
- IFN
Interferon
- IFNGR
IFN-γ receptors
- IL
Interleukin
- iPSC
Induced pluripotent stem cells
- ITT
Intent-to-treat
- JAK1
Janus kinase 1
- LAG-3
Lymphocyte activation gene-3
- LT
Liver transplantation
- ORR
Objective response rate
- mAb
Monoclonal antibody
- MDSC
Myeloid-derived suppressor cell
- MHC
Major histocompatibility complex
- MPA
Mycophenolic acid
- mTOR
Mammalian target of rapamycin
- mTTP
Median time to progression
- NSCLC
Non-small-cell lung cancer
- OPN
Osteopontin
- pERK
Phosphorylated extracellular signaling-regulated kinase
- PD-1
Programmed cell death protein-1
- PD-L1
programmed cell death protein ligand-1
- p53MVA vaccine
Modified vaccinia virus Ankara vaccine expressing p53
- PPARα
Peroxisome proliferator-activated receptor α
- PVTT
Portal vein tumor thrombosis
- STAT
Signal transducers and activators of transcription
- TAA
Tumor-associated antigen
- TAM
Tumor-associated macrophage
- TCR
T-cell receptor
- TGF-β
Transforming growth factor-β
- TIL
Tumor-infiltrated lymphocyte
- TIM-3
T-cell immunoglobulin- and mucin-domain-containing molecule-3
- TNF
Tumor necrosis factor
- TNFRSF4
TNF receptor superfamily member 4
- TOX
Thymocyte selection-associated high mobility group box protein
- Treg
Regulatory T cells
References
- 1.Bray F, Ferlay J, Soerjomataram I, Siegel RL, Torre LA, Jemal A. Global cancer statistics 2018: globocan estimates of incidence and mortality worldwide for 36 cancers in 185 countries. CA Cancer J Clin. 2018;68:394–424. doi: 10.3322/caac.21492. [DOI] [PubMed] [Google Scholar]
- 2.Llovet JM, Zucman-Rossi J, Pikarsky E, Sangro B, Schwartz M, Sherman M, Gores G. Hepatocellular carcinoma. Nat Rev Dis Primers. 2016;2:16018. doi: 10.1038/nrdp.2016.18. [DOI] [PubMed] [Google Scholar]
- 3.Ma KW, Cheung TT. Surgical resection of localized hepatocellular carcinoma: patient selection and special consideration. J Hepatocell Carcinoma. 2017;4:1–9. doi: 10.2147/JHC.S96085. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Xu X, Lu D, Ling Q, Wei X, Wu J, Zhou L, Yan S, Wu L, Geng L, Ke Q, Gao F, Tu Z, Wang W, Zhang M, Shen Y, Xie H, Jiang W, Wang H, Zheng S. Liver transplantation for hepatocellular carcinoma beyond the Milan criteria. Gut. 2016;65:1035–1041. doi: 10.1136/gutjnl-2014-308513. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Sanmamed MF, Chen L. A paradigm shift in cancer immunotherapy: from enhancement to normalization. Cell. 2018;175:313–326. doi: 10.1016/j.cell.2018.09.035. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Sprinzl MF, Galle PR. Current progress in immunotherapy of hepatocellular carcinoma. J Hepatol. 2017;66:482–484. doi: 10.1016/j.jhep.2016.12.009. [DOI] [PubMed] [Google Scholar]
- 7.Lippitz BE. Cytokine patterns in patients with cancer: a systematic review. Lancet Oncol. 2013;14:e218–e228. doi: 10.1016/S1470-2045(12)70582-X. [DOI] [PubMed] [Google Scholar]
- 8.Budhu A, Wang XW. The role of cytokines in hepatocellular carcinoma. J Leukoc Biol. 2006;80:1197–1213. doi: 10.1189/jlb.0506297. [DOI] [PubMed] [Google Scholar]
- 9.Mizukoshi E, Kaneko S. Immune cell therapy for hepatocellular carcinoma. J Haematologica Oncol. 2019;12:52. doi: 10.1186/s13045-019-0742-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Kershaw MH, Westwood JA, Darcy PK. Gene-engineered T cells for cancer therapy. Nat Rev Cancer. 2013;13:525–541. doi: 10.1038/nrc3565. [DOI] [PubMed] [Google Scholar]
- 11.Chen Y, E CY, Gong ZW, Liu S, Wang ZX, Yang YS, Zhang XW. Chimeric antigen receptor-engineered T-cell therapy for liver cancer. Hepatobiliary Pancreat Dis Int. 2018;17:301–309. doi: 10.1016/j.hbpd.2018.05.005. [DOI] [PubMed] [Google Scholar]
- 12.Mandal R, Chan TA. Personalized oncology meets immunology: the path toward precision immunotherapy. Cancer Discov. 2016;6:703–713. doi: 10.1158/2159-8290.CD-16-0146. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Sharma P, Hu-Lieskovan S, Wargo JA, Ribas A. Primary, adaptive, and acquired resistance to cancer immunotherapy. Cell. 2017;168:707–723. doi: 10.1016/j.cell.2017.01.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Postow MA, Callahan MK, Wolchok JD. Immune checkpoint blockade in cancer therapy. J. Clin. Oncol. 2015;33:1974–1982. doi: 10.1200/JCO.2014.59.4358. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Sharma P, Allison JP. Immune checkpoint targeting in cancer therapy: toward combination strategies with curative potential. Cell. 2015;161:205–214. doi: 10.1016/j.cell.2015.03.030. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Pardoll DM. The blockade of immune checkpoints in cancer immunotherapy. Nat Rev Cancer. 2012;12:252–264. doi: 10.1038/nrc3239. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Zhou G, Sprengers D, Boor PPC, Doukas M, Schutz H, Mancham S, Pedroza-Gonzalez A, Polak WG, de Jonge J, Gaspersz M, Dong H, Thielemans K, Pan Q, Ijzermans JNM, Bruno MJ, Kwekkeboom J. Antibodies against immune checkpoint molecules restore functions of tumor-infiltrating t cells in hepatocellular carcinomas. Gastroenterology. 2017;153:1107–1119. e1110. doi: 10.1053/j.gastro.2017.06.017. [DOI] [PubMed] [Google Scholar]
- 18.Ramagopal UA, Liu W, Garrett-Thomson SC, Bonanno JB, Yan Q, Srinivasan M, Wong SC, Bell A, Mankikar S, Rangan VS, Deshpande S, Korman AJ, Almo SC. Structural basis for cancer immunotherapy by the first-in-class checkpoint inhibitor ipilimumab. Proc Natl Acad Sci U S A. 2017;114:E4223–E4232. doi: 10.1073/pnas.1617941114. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Mizukoshi E, Nakamoto Y, Arai K, Yamashita T, Sakai A, Sakai Y, Kagaya T, Yamashita T, Honda M, Kaneko S. Comparative analysis of various tumor-associated antigen-specific t-cell responses in patients with hepatocellular carcinoma. Hepatology. 2011;53:1206–1216. doi: 10.1002/hep.24149. [DOI] [PubMed] [Google Scholar]
- 20.Sangro B, Gomez-Martin C, de la Mata M, Inarrairaegui M, Garralda E, Barrera P, Riezu-Boj JI, Larrea E, Alfaro C, Sarobe P, Lasarte JJ, Perez-Gracia JL, Melero I, Prieto J. A clinical trial of CTLA-4 blockade with tremelimumab in patients with hepatocellular carcinoma and chronic hepatitis C. J Hepatol. 2013;59:81–88. doi: 10.1016/j.jhep.2013.02.022. [DOI] [PubMed] [Google Scholar]
- 21.Chang H, Jung W, Kim A, Kim HK, Kim WB, Kim JH, Kim BH. Expression and prognostic significance of programmed death protein 1 and programmed death ligand-1, and cytotoxic T lymphocyte-associated molecule-4 in hepatocellular carcinoma. Apmis. 2017;125:690–698. doi: 10.1111/apm.12703. [DOI] [PubMed] [Google Scholar]
- 22.Finkelmeier F, Waidmann O, Trojan J. Nivolumab for the treatment of hepatocellular carcinoma. Expert Rev Anticancer Ther. 2018;18:1169–1175. doi: 10.1080/14737140.2018.1535315. [DOI] [PubMed] [Google Scholar]
- 23.El-Khoueiry AB, Sangro B, Yau T, Crocenzi TS, Kudo M, Hsu C, Kim TY, Choo SP, Trojan J, Welling TH Rd, Meyer T, Kang YK, Yeo W, Chopra A, Anderson J, Dela Cruz C, Lang L, Neely J, Tang H, Dastani HB, Melero I. Nivolumab in patients with advanced hepatocellular carcinoma (checkmate 040): an open-label, non-comparative, phase 1/2 dose escalation and expansion trial. Lancet. 2017;389:2492–2502. doi: 10.1016/S0140-6736(17)31046-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Finkelmeier F, Czauderna C, Perkhofer L, Ettrich TJ, Trojan J, Weinmann A, Marquardt JU, Vermehren J, Waidmann O. Feasibility and safety of nivolumab in advanced hepatocellular carcinoma: real-life experience from three German centers. J Cancer Res Clin Oncol. 2018;145:253–259. doi: 10.1007/s00432-018-2780-8. [DOI] [PubMed] [Google Scholar]
- 25.Kambhampati S, Bauer KE, Bracci PM, Keenan BP, Behr SC, Gordan JD, Kelley RK. Nivolumab in patients with advanced hepatocellular carcinoma and child-pugh class b cirrhosis: safety and clinical outcomes in a retrospective case series. Cancer. 2019;125:3234–3241. doi: 10.1002/cncr.32206. [DOI] [PubMed] [Google Scholar]
- 26.Yau T, Hsu C, Kim TY, Choo SP, Kang YK, Hou MM, Numata K, Yeo W, Chopra A, Ikeda M, Kuromatsu R, Moriguchi M, Chao Y, Zhao H, Anderson J, Dela Cruz C, Kudo M. Nivolumab in advanced hepatocellular carcinoma: sorafenib-experienced asian cohort analysis. J Hepatol. 2019;71:543–552. doi: 10.1016/j.jhep.2019.05.014. [DOI] [PubMed] [Google Scholar]
- 27.Scheiner B, Kirstein MM, Hucke F, Finkelmeier F, Schulze K, von Felden J, Koch S, Schwabl P, Hinrichs JB, Waneck F, Waidmann O, Reiberger T, Müller C, Sieghart W, Trauner M, Weinmann A, Wege H, Trojan J, Peck-Radosavljevic M, Vogel A, Pinter M. Programmed cell death protein-1 (PD-1)-targeted immunotherapy in advanced hepatocellular carcinoma: efficacy and safety data from an international multicentre real-world cohort. Aliment Pharmacol Ther. 2019;49:1323–1333. doi: 10.1111/apt.15245. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Zhu AX, Finn RS, Edeline J, Cattan S, Ogasawara S, Palmer D, Verslype C, Zagonel V, Fartoux L, Vogel A, Sarker D, Verset G, Chan SL, Knox J, Daniele B, Webber AL, Ebbinghaus SW, Ma J, Siegel AB, Cheng AL, Kudo M, Alistar A, Asselah J, Blanc JF, Borbath I, Cannon T, Chung K, Cohn A, Cosgrove DP, Damjanov N, Gupta M, Karino Y, Karwal M, Kaubisch A, Kelley R, Van Laethem JL, Larson T, Lee J, Li D, Manhas A, Manji GA, Numata K, Parsons B, Paulson AS, Pinto C, Ramirez R, Ratnam S, Rizell M, Rosmorduc O, Sada Y, Sasaki Y, Stal PI, Strasser S, Trojan J, Vaccaro G, Van Vlierberghe H, Weiss A, Weiss KH, Yamashita T. Pembrolizumab in patients with advanced hepatocellular carcinoma previously treated with sorafenib (KEYNOTE-224): a non-randomised, open-label phase 2 trial. Lancet Oncol. 2018;19:940–952. doi: 10.1016/S1470-2045(18)30351-6. [DOI] [PubMed] [Google Scholar]
- 29.Larkin J, Chiarion-Sileni V, Gonzalez R, Grob JJ, Cowey CL, Lao CD, Schadendorf D, Dummer R, Smylie M, Rutkowski P, Ferrucci PF, Hill A, Wagstaff J, Carlino MS, Haanen JB, Maio M, Marquez-Rodas I, McArthur GA, Ascierto PA, Long GV, Callahan MK, Postow MA, Grossmann K, Sznol M, Dreno B, Bastholt L, Yang A, Rollin LM, Horak C, Hodi FS, Wolchok JD. Combined nivolumab and ipilimumab or monotherapy in untreated melanoma. N Engl J Med. 2015;373:23–34. doi: 10.1056/NEJMoa1504030. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Long GV, Atkinson V, Lo S, Sandhu S, Guminski AD, Brown MP, Wilmott JS, Edwards J, Gonzalez M, Scolyer RA, Menzies AM, McArthur GA. Combination nivolumab and ipilimumab or nivolumab alone in melanoma brain metastases: a multicentre randomised phase 2 study. Lancet Oncol. 2018;19:672–681. doi: 10.1016/S1470-2045(18)30139-6. [DOI] [PubMed] [Google Scholar]
- 31.Kim HD, Park S, Jeong S, Lee YJ, Lee H, Kim CG, Kim KH, Hong SM, Lee JY, Kim S, Kim HK, Min BS, Chang JH, Ju YS, Shin EC, Song GW, Hwang S, Park SH. 4-1BB delineates distinct activation status of exhausted tumor-infiltrating CD8(+) T cells in hepatocellular carcinoma. Hepatology. 2020;71:955–971. doi: 10.1002/hep.30881. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Gauttier V, Judor JP, Le Guen V, Cany J, Ferry N, Conchon S. Agonistic anti-CD137 antibody treatment leads to antitumor response in mice with liver cancer. Int J Cancer. 2014;135:2857–2867. doi: 10.1002/ijc.28943. [DOI] [PubMed] [Google Scholar]
- 33.Morales-Kastresana A, Sanmamed MF, Rodriguez I, Palazon A, Martinez-Forero I, Labiano S, Hervas-Stubbs S, Sangro B, Ochoa C, Rouzaut A, Azpilikueta A, Bolanos E, Jure-Kunkel M, Gutgemann I, Melero I. Combined immunostimulatory monoclonal antibodies extend survival in an aggressive transgenic hepatocellular carcinoma mouse model. Clin Cancer Res. 2013;19:6151–6162. doi: 10.1158/1078-0432.CCR-13-1189. [DOI] [PubMed] [Google Scholar]
- 34.Harding JJ. Immune checkpoint blockade in advanced hepatocellular carcinoma: an update and critical review of ongoing clinical trials. Future Oncol. 2018;14:2293–2302. doi: 10.2217/fon-2018-0008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Jenkins RW, Barbie DA, Flaherty KT. Mechanisms of resistance to immune checkpoint inhibitors. Br J Cancer. 2018;118:9–16. doi: 10.1038/bjc.2017.434. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Flecken T, Schmidt N, Hild S, Gostick E, Drognitz O, Zeiser R, Schemmer P, Bruns H, Eiermann T, Price DA, Blum HE, Neumann-Haefelin C, Thimme R. Immunodominance and functional alterations of tumor-associated antigen-specific CD8+ T-cell responses in hepatocellular carcinoma. Hepatology. 2014;59:1415–1426. doi: 10.1002/hep.26731. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Anagnostou V, Smith KN, Forde PM, Niknafs N, Bhattacharya R, White J, Zhang T, Adleff V, Phallen J, Wali N, Hruban C, Guthrie VB, Rodgers K, Naidoo J, Kang H, Sharfman W, Georgiades C, Verde F, Illei P, Li QK, Gabrielson E, Brock MV, Zahnow CA, Baylin SB, Scharpf RB, Brahmer JR, Karchin R, Pardoll DM, Velculescu VE. Evolution of neoantigen landscape during immune checkpoint blockade in non-small cell lung cancer. Cancer Discov. 2017;7:264–276. doi: 10.1158/2159-8290.CD-16-0828. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Rammensee HG, Singh-Jasuja H. HLA ligandome tumor antigen discovery for personalized vaccine approach. Expert Rev Vaccines. 2013;12:1211–1217. doi: 10.1586/14760584.2013.836911. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Vonderheide RH, Nathanson KL. Immunotherapy at large: the road to personalized cancer vaccines. Nat Med. 2013;19:1098–1100. doi: 10.1038/nm.3317. [DOI] [PubMed] [Google Scholar]
- 40.Löffler MW, Mohr C, Bichmann L, Freudenmann LK, Walzer M, Schroeder CM, Trautwein N, Hilke FJ, Zinser RS, Mühlenbruch L, Kowalewski DJ, Schuster H, Sturm M, Matthes J, Riess O, Czemmel S, Nahnsen S, Königsrainer I, Thiel K, Nadalin S, Beckert S, Bösmüller H, Fend F, Velic A, Maček B, Haen SP, Buonaguro L, Kohlbacher O, Stevanović S, Königsrainer A, Rammensee HG. Multi-omics discovery of exome-derived neoantigens in hepatocellular carcinoma. Genome Med. 2019;11:28. doi: 10.1186/s13073-019-0636-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Zaretsky JM, Garcia-Diaz A, Shin DS, Escuin-Ordinas H, Hugo W, Hu-Lieskovan S, Torrejon DY, Abril-Rodriguez G, Sandoval S, Barthly L, Saco J, Homet Moreno B, Mezzadra R, Chmielowski B, Ruchalski K, Shintaku IP, Sanchez PJ, Puig-Saus C, Cherry G, Seja E, Kong X, Pang J, Berent-Maoz B, Comin-Anduix B, Graeber TG, Tumeh PC, Schumacher TN, Lo RS, Ribas A. Mutations associated with acquired resistance to PD-1 blockade in melanoma. N Engl J Med. 2016;375:819–829. doi: 10.1056/NEJMoa1604958. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Roh W, Chen PL, Reuben A, Spencer CN, Prieto PA, Miller JP, Gopalakrishnan V, Wang F, Cooper ZA, Reddy SM, Gumbs C, Little L, Chang Q, Chen WS, Wani K, De Macedo MP, Chen E, Austin-Breneman JL, Jiang H, Roszik J, Tetzlaff MT, Davies MA, Gershenwald JE, Tawbi H, Lazar AJ, Hwu P, Hwu WJ, Diab A, Glitza IC, Patel SP, Woodman SE, Amaria RN, Prieto VG, Hu J, Sharma P, Allison JP, Chin L, Zhang J, Wargo JA, Futreal PA. Integrated molecular analysis of tumor biopsies on sequential CTLA-4 and PD-1 blockade reveals markers of response and resistance. Sci Transl Med. 2017;9 doi: 10.1126/scitranslmed.aah3560. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Kurokohchi K, Carrington M, Mann DL, Simonis TB, Alexander-Miller MA, Feinstone SM, Akatsuka T, Berzofsky JA. Expression of HLA class I molecules and the transporter associated with antigen processing in hepatocellular carcinoma. Hepatology. 1996;23:1181–1188. doi: 10.1002/hep.510230537. [DOI] [PubMed] [Google Scholar]
- 44.Umemoto Y, Okano S, Matsumoto Y, Nakagawara H, Matono R, Yoshiya S, Yamashita Y, Yoshizumi T, Ikegami T, Soejima Y, Harada M, Aishima S, Oda Y, Shirabe K, Maehara Y. Prognostic impact of programmed cell death 1 ligand 1 expression in human leukocyte antigen class I-positive hepatocellular carcinoma after curative hepatectomy. J Gastroenterol. 2014;50:65–75. doi: 10.1007/s00535-014-0933-3. [DOI] [PubMed] [Google Scholar]
- 45.Sia D, Jiao Y, Martinez-Quetglas I, Kuchuk O, Villacorta-Martin C, Castro de Moura M, Putra J, Camprecios G, Bassaganyas L, Akers N, Losic B, Waxman S, Thung SN, Mazzaferro V, Esteller M, Friedman SL, Schwartz M, Villanueva A, Llovet JM. Identification of an immune-specific class of hepatocellular carcinoma, based on molecular features. Gastroenterology. 2017;153:812–826. doi: 10.1053/j.gastro.2017.06.007. [DOI] [PubMed] [Google Scholar]
- 46.Harding JJ, Nandakumar S, Armenia J, Khalil DN, Albano M, Ly M, Shia J, Hechtman JF, Kundra R, El Dika I, Do RK, Sun Y, Kingham TP, D’Angelica MI, Berger MF, Hyman DM, Jarnagin W, Klimstra DS, Janjigian YY, Solit DB, Schultz N, Abou-Alfa GK. Prospective genotyping of hepatocellular carcinoma: clinical implications of next-generation sequencing for matching patients to targeted and immune therapies. Clin Cancer Res. 2019;25:2116–2126. doi: 10.1158/1078-0432.CCR-18-2293. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Pinyol R, Sia D, Llovet JM. Immune exclusion-Wnt/CTNNB1 class predicts resistance to immunotherapies in HCC. Clin Cancer Res. 2019;25:2021–2023. doi: 10.1158/1078-0432.CCR-18-3778. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Ruiz de Galarreta M, Bresnahan E, Molina-Sanchez P, Lindblad KE, Maier B, Sia D, Puigvehi M, Miguela V, Casanova-Acebes M, Dhainaut M, Villacorta-Martin C, Singhi AD, Moghe A, von Felden J, Tal Grinspan L, Wang S, Kamphorst AO, Monga SP, Brown BD, Villanueva A, Llovet JM, Merad M, Lujambio A. β-catenin activation promotes immune escape and resistance to anti-PD-1 therapy in hepatocellular carcinoma. Cancer Discov. 2019;9:1124–1141. doi: 10.1158/2159-8290.CD-19-0074. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Kurebayashi Y, Ojima H, Tsujikawa H, Kubota N, Maehara J, Abe Y, Kitago M, Shinoda M, Kitagawa Y, Sakamoto M. Landscape of immune microenvironment in hepatocellular carcinoma and its additional impact on histological and molecular classification. Hepatology. 2018;68:1025–1041. doi: 10.1002/hep.29904. [DOI] [PubMed] [Google Scholar]
- 50.Zhang Q, Lou Y, Yang J, Wang J, Feng J, Zhao Y, Wang L, Huang X, Fu Q, Ye M, Zhang X, Chen Y, Ma C, Ge H, Wang J, Wu J, Wei T, Chen Q, Wu J, Yu C, Xiao Y, Feng X, Guo G, Liang T, Bai X. Integrated multiomic analysis reveals comprehensive tumour heterogeneity and novel immunophenotypic classification in hepatocellular carcinomas. Gut. 2019;68:2019–2031. doi: 10.1136/gutjnl-2019-318912. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Calderaro J, Rousseau B, Amaddeo G, Mercey M, Charpy C, Costentin C, Luciani A, Zafrani ES, Laurent A, Azoulay D, Lafdil F, Pawlotsky JM. Programmed death ligand 1 expression in hepatocellular carcinoma: relationship with clinical and pathological features. Hepatology. 2016;64:2038–2046. doi: 10.1002/hep.28710. [DOI] [PubMed] [Google Scholar]
- 52.Kim HD, Song GW, Park S, Jung MK, Kim MH, Kang HJ, Yoo C, Yi K, Kim KH, Eo S, Moon DB, Hong SM, Ju YS, Shin EC, Hwang S, Park SH. Association between expression level of PD1 by tumor-infiltrating CD8(+) T cells and features of hepatocellular carcinoma. Gastroenterology. 2018;155:1936–1950. e1917. doi: 10.1053/j.gastro.2018.08.030. [DOI] [PubMed] [Google Scholar]
- 53.Taube JM, Klein A, Brahmer JR, Xu H, Pan X, Kim JH, Chen L, Pardoll DM, Topalian SL, Anders RA. Association of PD-1, PD-1 ligands, and other features of the tumor immune microenvironment with response to anti-PD-1 therapy. Clin Cancer Res. 2014;20:5064–5074. doi: 10.1158/1078-0432.CCR-13-3271. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Wang G, Liu Y, Huang J, Liu J, Wang J, Yang J. PPARα suppresses PD-L1-mediated immune escape by down-regulating SPP1 in human hepatocellular carcinoma. Cancer Res Treat. 2019 doi: 10.4143/crt.2019.111. [Epub ahead of print] [DOI] [PubMed] [Google Scholar]
- 55.Zhou J, Liu M, Sun H, Feng Y, Xu L, Chan AWH, Tong JH, Wong J, Chong CCN, Lai PBS, Wang HK, Tsang SW, Goodwin T, Liu R, Huang L, Chen Z, Sung JJ, Chow KL, To KF, Cheng AS. Hepatoma-intrinsic CCRK inhibition diminishes myeloid-derived suppressor cell immunosuppression and enhances immune-checkpoint blockade efficacy. Gut. 2018;67:931–944. doi: 10.1136/gutjnl-2017-314032. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Chen J, Ji T, Zhao J, Li G, Zhang J, Jin R, Liu J, Liu X, Liang X, Huang D, Xie A, Lin H, Cang Y, Cai X. Sorafenib-resistant hepatocellular carcinoma stratified by phosphorylated ERK activates PD-1 immune checkpoint. Oncotarget. 2016;7:41274–41284. doi: 10.18632/oncotarget.8978. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Wang X, He Q, Shen H, Xia A, Tian W, Yu W, Sun B. TOX promotes the exhaustion of antitumor CD8+ T cells by preventing PD1 degradation in hepatocellular carcinoma. J Hepatol. 2019;71:731–741. doi: 10.1016/j.jhep.2019.05.015. [DOI] [PubMed] [Google Scholar]
- 58.Keenan TE, Burke KP, Van Allen EM. Genomic correlates of response to immune check point blockade. Nat Med. 2019;25:389–402. doi: 10.1038/s41591-019-0382-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Xu P, Sun Z, Wang Y, Miao C. Long-term use of indomethacin leads to poor prognoses through promoting the expression of PD-1 and PD-L2 via TRIF/NF-κB pathway and JAK/STAT3 pathway to inhibit TNF-α and IFN-γ in hepatocellular carcinoma. Exp Cell Res. 2015;337:53–60. doi: 10.1016/j.yexcr.2015.07.007. [DOI] [PubMed] [Google Scholar]
- 60.Koyama S, Akbay EA, Li YY, Herter-Sprie GS, Buczkowski KA, Richards WG, Gandhi L, Redig AJ, Rodig SJ, Asahina H, Jones RE, Kulkarni MM, Kuraguchi M, Palakurthi S, Fecci PE, Johnson BE, Janne PA, Engelman JA, Gangadharan SP, Costa DB, Freeman GJ, Bueno R, Hodi FS, Dranoff G, Wong KK, Hammerman PS. Adaptive resistance to therapeutic PD-1 blockade is associated with upregulation of alternative immune checkpoints. Nat Commun. 2016;7:10501. doi: 10.1038/ncomms10501. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Macek Jilkova Z, Aspord C, Kurma K, Granon A, Sengel C, Sturm N, Marche PN, Decaens T. Immunologic features of patients with advanced hepatocellular carcinoma before and during sorafenib or anti-programmed death-1/programmed death-L1 treatment. Clin Transl Gastroenterol. 2019;10:e00058. doi: 10.14309/ctg.0000000000000058. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Ngiow SF, Young A, Jacquelot N, Yamazaki T, Enot D, Zitvogel L, Smyth MJ. A threshold level of intratumor CD8+ T-cell PD1 expression dictates therapeutic response to anti-PD1. Cancer Res. 2015;75:3800–3811. doi: 10.1158/0008-5472.CAN-15-1082. [DOI] [PubMed] [Google Scholar]
- 63.Uyttenhove C, Pilotte L, Théate I, Stroobant V, Colau D, Parmentier N, Boon T, Van den Eynde BJ. Evidence for a tumoral immune resistance mechanism based on tryptophan degradation by indoleamine 2,3-dioxygenase. Nat Med. 2003;9:1269–1274. doi: 10.1038/nm934. [DOI] [PubMed] [Google Scholar]
- 64.Holmgaard Rikke B, Zamarin D, Li Y, Gasmi B, Munn David H, Allison James P, Merghoub T, Wolchok Jedd D. Tumor-expressed IDO recruits and activates MDSCs in a treg-dependent manner. Cell Rep. 2015;13:412–424. doi: 10.1016/j.celrep.2015.08.077. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Brown ZJ, Yu SJ, Heinrich B, Ma C, Fu Q, Sandhu M, Agdashian D, Zhang Q, Korangy F, Greten TF. Indoleamine 2,3-dioxygenase provides adaptive resistance to immune checkpoint inhibitors in hepatocellular carcinoma. Cancer Immunol Immunother. 2018;67:1305–1315. doi: 10.1007/s00262-018-2190-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Zhu Y, Yang J, Xu D, Gao XM, Zhang Z, Hsu JL, Li CW, Lim SO, Sheng YY, Zhang Y, Li JH, Luo Q, Zheng Y, Zhao Y, Lu L, Jia HL, Hung MC, Dong QZ, Qin LX. Disruption of tumour-associated macrophage trafficking by the osteopontin-induced colony-stimulating factor-1 signalling sensitises hepatocellular carcinoma to anti-PD-L1 blockade. Gut. 2019;68:1653–1666. doi: 10.1136/gutjnl-2019-318419. [DOI] [PubMed] [Google Scholar]
- 67.Duffy AG, Ulahannan SV, Makorova-Rusher O, Rahma O, Wedemeyer H, Pratt D, Davis JL, Hughes MS, Heller T, ElGindi M, Uppala A, Korangy F, Kleiner DE, Figg WD, Venzon D, Steinberg SM, Venkatesan AM, Krishnasamy V, Abi-Jaoudeh N, Levy E, Wood BJ, Greten TF. Tremelimumab in combination with ablation in patients with advanced hepatocellular carcinoma. J Hepatol. 2017;66:545–551. doi: 10.1016/j.jhep.2016.10.029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Wehrenberg-Klee E, Goyal L, Dugan M, Zhu AX, Ganguli S. Y-90 radioembolization combined with a PD-1 inhibitor for advanced hepatocellular carcinoma. Cardiovasc Intervent Radiol. 2018;41:1799–1802. doi: 10.1007/s00270-018-1993-1. [DOI] [PubMed] [Google Scholar]
- 69.Giardino A, Innamorati G, Ugel S, Perbellini O, Girelli R, Frigerio I, Regi P, Scopelliti F, Butturini G, Paiella S, Bacchion M, Bassi C. Immunomodulation after radiofrequency ablation of locally advanced pancreatic cancer by monitoring the immune response in 10 patients. Pancreatology. 2017;17:962–966. doi: 10.1016/j.pan.2017.09.008. [DOI] [PubMed] [Google Scholar]
- 70.Minami Y, Nishida N, Kudo M. Radiofrequency ablation of liver metastasis: potential impact on immune checkpoint inhibitor therapy. Eur Radiol. 2019;29:5045–5051. doi: 10.1007/s00330-019-06189-6. [DOI] [PubMed] [Google Scholar]
- 71.Zhang H, Hou X, Cai H, Zhuang X. Effects of microwave ablation on T-cell subsets and cytokines of patients with hepatocellular carcinoma. Minim Invasive Ther Allied Technol. 2017;26:207–211. doi: 10.1080/13645706.2017.1286356. [DOI] [PubMed] [Google Scholar]
- 72.Cheng H, Sun G, Chen H, Li Y, Han Z, Li Y, Zhang P, Yang L, Li Y. Trends in the treatment of advanced hepatocellular carcinoma: immune checkpoint blockade immunotherapy and related combination therapies. Am J Cancer Res. 2019;9:1536–1545. [PMC free article] [PubMed] [Google Scholar]
- 73.Kudo M. Pembrolizumab for the treatment of hepatocellular carcinoma. Liver Cancer. 2019;8:143–154. doi: 10.1159/000500143. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Li H, Li X, Liu S, Guo L, Zhang B, Zhang J, Ye Q. Programmed cell death-1 (PD-1) checkpoint blockade in combination with a mammalian target of rapamycin inhibitor restrains hepatocellular carcinoma growth induced by hepatoma cell-intrinsic PD-1. Hepatology. 2017;66:1920–1933. doi: 10.1002/hep.29360. [DOI] [PubMed] [Google Scholar]
- 75.Matsuda A, Ishiguro K, Yan IK, Patel T. Extracellular vesicle-based therapeutic targeting of β-catenin to modulate anticancer immune responses in hepatocellular cancer. Hepatol Commun. 2019;3:525–541. doi: 10.1002/hep4.1311. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Li G, Liu D, Cooper TK, Kimchi ET, Qi X, Avella DM, Li N, Yang QX, Kester M, Rountree CB, Kaifi JT, Cole DJ, Rockey DC, Schell TD, Staveley-O’Carroll KF. Successful chemoimmunotherapy against hepatocellular cancer in a novel murine model. J Hepatol. 2017;66:75–85. doi: 10.1016/j.jhep.2016.07.044. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Munker S, De Toni EN. Use of checkpoint inhibitors in liver transplant recipients. United European Gastroenterol J. 2018;6:970–973. doi: 10.1177/2050640618774631. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.DeLeon TT, Salomao MA, Aqel BA, Sonbol MB, Yokoda RT, Ali AH, Moss AA, Mathur AK, Chascsa DM, Rakela J, Bryce AH, Borad MJ. Pilot evaluation of PD-1 inhibition in metastatic cancer patients with a history of liver transplantation: the Mayo Clinic experience. J Gastrointest Oncol. 2018;9:1054–1062. doi: 10.21037/jgo.2018.07.05. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.De Bruyn P, Van Gestel D, Ost P, Kruse V, Brochez L, Van Vlierberghe H, Devresse A, del Marmol V, Le Moine A, Aspeslagh S. Immune checkpoint blockade for organ transplant patients with advanced cancer. Curr Opin Oncol. 2019;31:54–64. doi: 10.1097/CCO.0000000000000505. [DOI] [PubMed] [Google Scholar]
- 80.Jones PA, Issa JP, Baylin S. Targeting the cancer epigenome for therapy. Nat Rev Genet. 2016;17:630–641. doi: 10.1038/nrg.2016.93. [DOI] [PubMed] [Google Scholar]
- 81.Liu M, Zhou J, Liu X, Feng Y, Yang W, Wu F, Cheung OK, Sun H, Zeng X, Tang W, Mok MTS, Wong J, Yeung PC, Lai PBS, Chen Z, Jin H, Chen J, Chan SL, Chan AWH, To KF, Sung JJY, Chen M, Cheng AS. Targeting monocyte-intrinsic enhancer reprogramming improves immunotherapy efficacy in hepatocellular carcinoma. Gut. 2020;69:365–379. doi: 10.1136/gutjnl-2018-317257. [DOI] [PubMed] [Google Scholar]
- 82.Llopiz D, Ruiz M, Villanueva L, Iglesias T, Silva L, Egea J, Lasarte JJ, Pivette P, Trochon-Joseph V, Vasseur B, Dixon G, Sangro B, Sarobe P. Enhanced anti-tumor efficacy of checkpoint inhibitors in combination with the histone deacetylase inhibitor Belinostat in a murine hepatocellular carcinoma model. Cancer Immunol Immunother. 2019;68:379–393. doi: 10.1007/s00262-018-2283-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Hong YK, Li Y, Pandit H, Li S, Pulliam Z, Zheng Q, Yu Y, Martin RCG. Epigenetic modulation enhances immunotherapy for hepatocellular carcinoma. Cell Immunol. 2019;336:66–74. doi: 10.1016/j.cellimm.2018.12.010. [DOI] [PubMed] [Google Scholar]
- 84.Liu M, Zhang L, Li H, Hinoue T, Zhou W, Ohtani H, El-Khoueiry A, Daniels J, O’Connell C, Dorff TB, Lu Q, Weisenberger DJ, Liang G. Integrative epigenetic analysis reveals therapeutic targets to the DNA methyltransferase inhibitor guadecitabine (SGI-110) in hepatocellular carcinoma. Hepatology. 2018;68:1412–1428. doi: 10.1002/hep.30091. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Daftarian P, Song GY, Ali S, Faynsod M, Longmate J, Diamond DJ, Ellenhorn JD. Two distinct pathways of immuno-modulation improve potency of p53 immunization in rejecting established tumors. Cancer Res. 2004;64:5407–5414. doi: 10.1158/0008-5472.CAN-04-0169. [DOI] [PubMed] [Google Scholar]
- 86.Song GY, Srivastava T, Ishizaki H, Lacey SF, Diamond DJ, Ellenhorn JD. Recombinant modified vaccinia virus ankara (MVA) expressing wild-type human p53 induces specific antitumor CTL expansion. Cancer Invest. 2011;29:501–510. doi: 10.3109/07357907.2011.606248. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Chung V, Kos FJ, Hardwick N, Yuan Y, Chao J, Li D, Waisman J, Li M, Zurcher K, Frankel P, Diamond DJ. Evaluation of safety and efficacy of p53MVA vaccine combined with pembrolizumab in patients with advanced solid cancers. Clin Transl Oncol. 2019;21:363–372. doi: 10.1007/s12094-018-1932-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Moehler M, Heo J, Lee HC, Tak WY, Chao Y, Paik SW, Yim HJ, Byun KS, Baron A, Ungerechts G, Jonker D, Ruo L, Cho M, Kaubisch A, Wege H, Merle P, Ebert O, Habersetzer F, Blanc JF, Rosmorduc O, Lencioni R, Patt R, Leen AM, Foerster F, Homerin M, Stojkowitz N, Lusky M, Limacher JM, Hennequi M, Gaspar N, McFadden B, De Silva N, Shen D, Pelusio A, Kirn DH, Breitbach CJ, Burke JM. Vaccinia-based oncolytic immunotherapy pexastimogene devacirepvec in patients with advanced hepatocellular carcinoma after sorafenib failure: a randomized multicenter phase IIb trial (traverse) Oncoimmunology. 2019;8:1615817. doi: 10.1080/2162402X.2019.1615817. [DOI] [PMC free article] [PubMed] [Google Scholar]


