Abstract
Background
The use of e-cigarettes is on the rise around the world. Many case reports of acute lung injury due to e-cigarette use have been published in recent months in the USA, but no comparable cases have emerged in Germany up to the present report. The use of e-cigarettes has risen very rapidly in the USA in recent years, simultaneously with the legalization of marijuana sale in many American states. Most of the cases described there involved the use, not only of nicotine, but of tetrahydrocannabinol (THC, the psychoactive ingredient in marijuana) as well, though some of the patients had indeed not used additives (e.g. THC).
Methods
We report three cases in Germany of acute pulmonary illness that we consider to have been caused by the use of e-cigarettes.
Results
All three patients were hospitalized for acute shortness of breath. Two displayed partial respiratory insufficiency and bilateral pulmonary infiltrates. All three stated that they had used ordinary, commercially available e-cigarettes every day for at least the past three months. In the first patient, a 48-year-old man, the complete blood count and bronchial lavage findings indicated eosinophilic inflammation. The second patient, a 22-year-old man, developed multiple episodes of hemoptysis, with computed tomography (CT) showing diffuse alveolar bleeding; his complete blood count also revealed eosinophilic inflammation. The third patient, a 34-year-old man, displayed acute ground-glass lung opacities as well as fibrosing changes on CT corresponding to pulmonary sarcoidosis. All three recovered on high-dose systemic corticosteroid treatment and were discharged from the hospital in 2 to 12 days.
Conclusion
In the first two cases, acute pulmonary injury was very likely due to e-cigarette consumption, as all other possible causes were ruled out. A possible link to e-cigarette use was present in the third case. We thus describe the first three suspected cases of acute lung disease due to e-cigarette use in Germany. These patients do not share any common, typical clinical picture; rather, their symptoms represent different components of the wide spectrum of interstitial lung disease. A uniform national registry should be established to improve our understanding of the adverse effects of e-cigarettes and the resulting acute and chronic changes in the lungs.
E-cigarettes are battery-powered devices in which liquids (“vape juices”) are heated up. They constitute the most commonly used tobacco product among young people in the USA (1). Heating the liquid produces an aerosol that users inhale. Inhaling is known as “vaping,” from the English verb “to vape.” The liquid/vapor contains nicotine (not always present) and flavors, as well as formaldehyde, acetaldehyde, acrolein, reactive oxygen compounds, and metals such as nickel, chrome, and lead, all of which are potentially harmful to health (2, 3). According to the industry association of the e-cigarette industry in Germany (Verband des E-Zigarettenhandels [VdeH) e.V.), there were some 3.5 million users nationwide in 2017 (4). Originally, the products were advertised as alternatives to traditional cigarettes, and later they were also recommended as a smoking cessation aid. Especially the UK National Health Service (NHS) is recommending this kind of smoking cessation method (5), because it has shown a greater effectiveness than other nicotine replacement products (6).
E-cigarettes had been generally assumed to be less harmful to health than traditional cigarettes, even though because of their being marketed as a lifestyle product rather than a medicinal product requring license approval, no longer-term studies have been conducted regarding adverse effects. In 2019, however, a multitude of reports appeared in the USA about patients who had had sustained lung injury associated with the use of e-cigarettes; this became known as “E-cigarette, or vaping, associated lung injury (EVALI)” (box) (7– 12). According to the US Centers for Disease Control and Prevention (CSC), 2711 persons received inpatient treatment, and 60 died (as per 21 January 2020) (13).
BOX. Definition of e-cigarette, or vaping, associated lung injury (EVALI)*.
Use of an e-cigarette (vaping) or dabbing in the 90 days before symptom onset
Pulmonary infiltrate, densities, or ground-glass opacities on chest CT
Pulmonary infection clinically ruled out
Other disease ruled out as explanation of the radiological changes
*According to criteria of the NEJM (11)
One of the largest case series describes 53 patients from Wisconsin and Illinois seen between January 2018 and August 2019 (11). The young patients (median age 19 years) mostly had to be treated in _hospital (94%). Bilateral pulmonary infiltrates were described in all patients. 84% had consumed liquids containing tetrahydrocannabinol (THC). Because of severe respiratory failure, 32% of patients had to be ventilated temporarily; one patient died. Different types of lung injury were described with the pattern of lipoid pneumonia, toxic pneumonitis, or alveolar hemorrhage (11).
In the currently largest case series, from Utah/USA, 60 patients (median age 27 years) were described who had contracted acute lung injury in association with using e-cigarettes. 33/60 (55%) required treatment in intensive care and two died. In this group, 51/60 (83%) vaped liquids containing THC only or in combination with nicotine (12). 14/60 (23%) of the patients had asthma, which may have been a predisposing factor.
For Germany, the German Federal Institute for Risk Assessment (BfR) envisaged in a press release in November 2019 “no increased risk for consumers of e-cigarettes in Germany, provided they use products that meet European and German rules and regulations” (14).
The following is a report of three cases of patients with acute lung injury, which has to be considered as associated with the use of e-cigarettes.
Methods
We present the cases of three patients who were treated at Hannover Medical School (MHH) and at the Hospital of Braunschweig City between June and September 2019. These patients were suspected of having EVALI—defined as acute lung injury associated with the use of e-cigarettes (box) (11).
Results
Case 1
A male patient aged 48 was transported by ambulance to the Hospital of Braunschweig City’s emergency department because of subacute dyspnea accompanied by a non-productive cough and fatigue. The patient complained of increasing symptoms over the previous seven days. For three years he had been using e-cigarettes on a daily basis (before that, he had smoked tobacco cigarettes); he credibly denied mixing the vape with other substances. He had no history of previous lung disease nor had he had contact with pneumotoxic substances. At admission the patient had a temperature of 38.5 °C and arterial hypoxemia (with an oxygen saturation level of 91% on room air). Clinical examination showed that he was in good general health, respiratory auscultation sounds were normal.
A lung function test found a moderate diffusing capacity for carbon monoxide (DLCO) at 49% of the desired value, with respiratory mechanics almost normal. High-resolution computed tomography (HRCT) of the lung showed bilateral infiltrates (CT images in the table). Of note in terms of laboratory chemistry was leukocytosis with an increase in eosinophil granulocytes (960/µL, up to 500/µL is considered normal). Immunoserology testing (rheumatoid factors, antinuclear antibodies [ANA], anti-neutrophil cytoplasmic antibodies [ANCA], ENA screening [ENA, extractable nuclear antigens]) yielded normal results. Bronchoscopy was macroscopically normal, bronchoalveolar lavage (BAL) confirmed eosinophilia (10%, 0–5% is considered normal). Because of respiratory failure, no lung biopsy was undertaken.
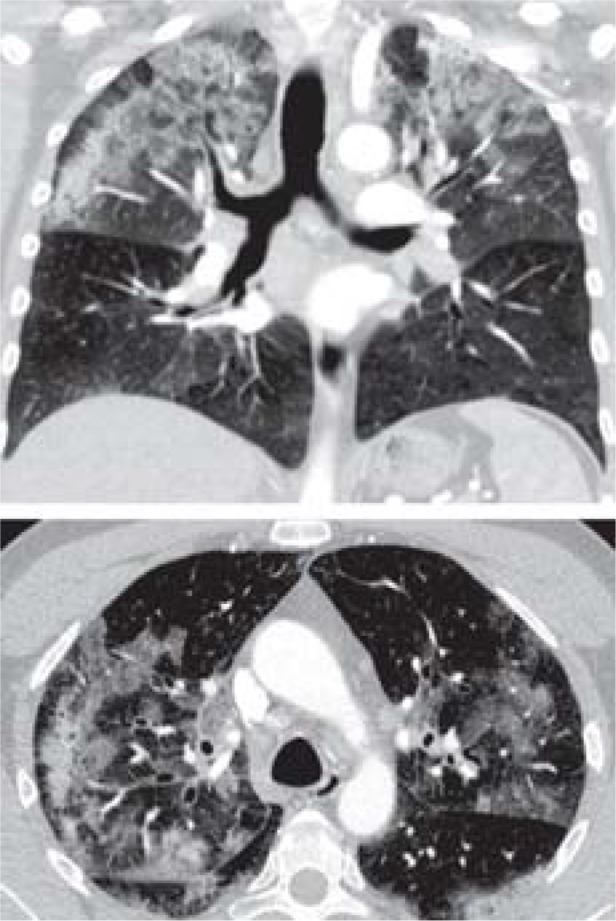
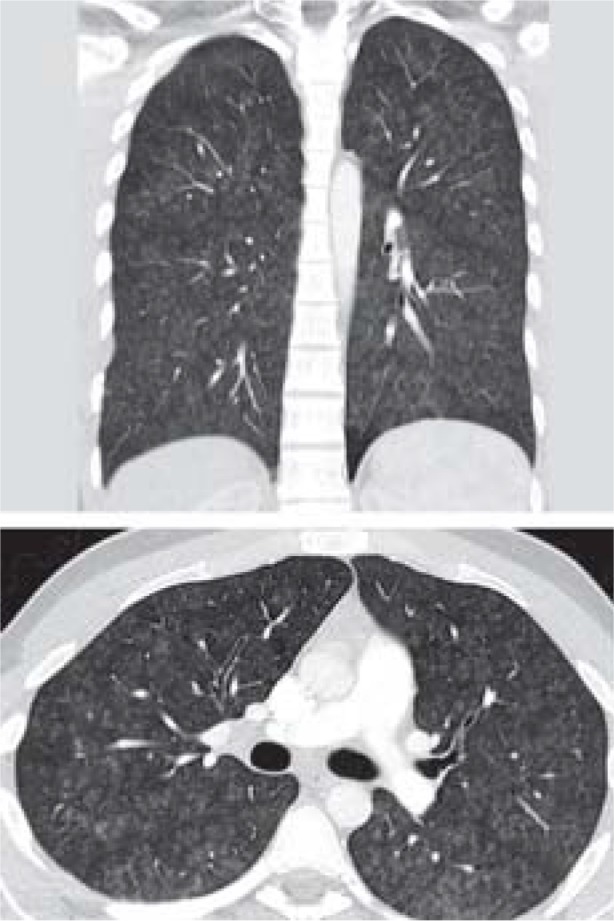
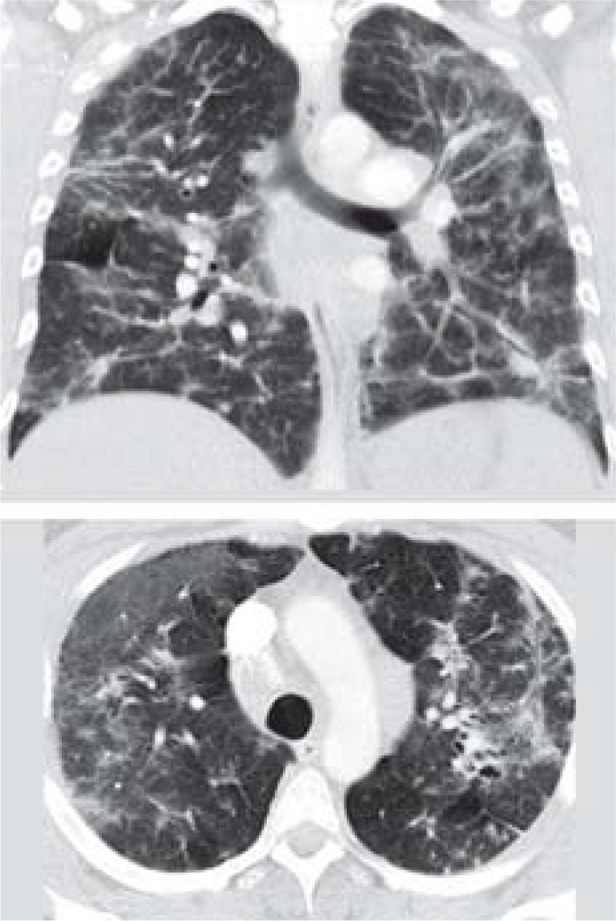
Table. CT findings of acute lung injury in the coronal (top) and transverse planes, and demographic data and findings.
| Patient 1 | Patient 2 | Patient 3 | |
 |
 |
 |
|
| Age, in years | 43 | 21 | 34 |
| Sex | Male | Male | Male |
| Eosinophils per µL (up to 500/µL) | 960 | 690 | 160 |
| C-reactive protein (<5.0) mg/l | 40.4 | 25 | 9.7 |
| Immunology (laboratory)* | Normal | Normal | Interleukin-2 receptor raised |
| CT result | Patchy opacities in both upper fields | Diffuse ground-glass opacities consistent with diffuse alveolar hemorrhage | Hilar lymphadenopathy, fibrotic areas, diffuse ground-glass opacities |
| Bronchial lavage | M: 65%, N: 15%, E: 10%, L: 2%, epithelial cells: 8% | Bloody, M: 40%, N: 50% | M: 39%, N: 3%, L: 57% |
| Histology, lung | Not done | Mildly fibrosed bronchial wall | Multifocal granulomatous inflammation, pneumonitis, organizing pneumonia |
| Treatment | Sultamicillin for 5 days, Prednisolone 1 mg/kg body weight | Sultamicillin for 5 days, Prednisolone 1 mg/kg body weight | Prednisolone 0.5 mg/kg body weight |
| Inpatient treatment, days | 15 | 12 | 2 |
| Intermittent need for oxygen | Yes, without ventilation | Yes, without ventilation | No |
| Oxygen at discharge | No | No | No |
| Intensive care stay/ Intermediate care station | Yes | No | No |
* Immunology lab: ENA-/ANA screening, rheumatoid factor, c- and p-ANCA and immunglobulin E;
ANA, antinuclear antibodies; ANCA, anti-neutrophil cytoplasmic antibodies; CT, computed tomography; ENA, extractable nuclear antigens; E, eosinophil granulocytes;
L, lymphocytes; M, makrophages; N, neutrophil granulocytes
Steroid treatment with prednisolone 1 mg/kg body weight was initiated for suspected acute eosinophilic pneumonia. The patient’s clinical condition stabilized over the following 48 hours. After 15 days he was discharged without the need for oxygen administration. After two more weeks, he was readmitted as an inpatient with severe hypoxemia, while still taking prednisolone 20 mg/day. He reported no further exposure to the presumed toxic substance. A differential blood count showed eosinophils 5500/µL. The steroid dose was increased (starting with 100 mg intravenous prednisolone for three days, followed by 1 mg/kg body weight) and the patient stabilized after seven days, so that oxygen administration could be stopped. He was discharged with the recommendation to taper off the steroid treatment slowly (starting with 50 mg/day and reducing by 10 mg every seven days, from 20 mg by 5 mg every two weeks).
Case 2
A 22-year-old patient with hemoptysis was referred from another hospital. Two weeks previously, he had found increasing amounts of blood in his expectorate. He reported having had a dry cough for a year. He had no history of lung disease, drug misuse, or contact with pneumotoxic substances. The patient had initially received antibiotic therapy with amoxicillin on an outpatient basis. His condition had not notably improved and he was admitted to hospital where he received 80 mg/day intravenous prednisolone for four days. He was transferred to our institution when he did not improve. At this point he had partial respiratory failure and needed 2 L/mon oxygen. On auscultation, basal crackles (rales) were heard.
The patient had been using e-cigarettes every day for two years. Before that, he had not smoked tobacco. He denied mixing in substances other than the commercially available vaping liquids. In another hospital, drug screening at admission had yielded negative results (tests for cannabis, amphetamines, opiates, cocaine).
All laboratory tests showed low inflammatory markers when the patient was admitted to our hospital (table). Of note was his raised eosinophil count of 690/µL (table). Computed tomography showed diffuse ground-glass opacities in both lungs (CT images are shown in the Table). Immunoserology (rheumatoid factor, ANA, ENA screening, ANCA) was normal. The bronchoscopic findings were normal, the BAL was hemorrhagic, and granulocytic inflammation was present (50% neutrophilic granulocytes). No pathogens were confirmed microbiologically nor virologically. Transbronchial biopsy showed normal lung architecture in a poor quality specimen. The patient’s condition stabilized with steroid therapy (he did not require any more oxygen), the hemoptysis ceased. The patient was treated with prednisolone at a dose of 0.5 mg/kg body weight in the end and was able to be discharged from hospital after 12 days.
Case 3
A 34-year-old patient was referred to the MHH with subacute effort dyspnea by a pneumonologist in private practice. The patient had been an active smoker for 17 years and had switched to e-cigarettes 12 months previously. After the switch his exercise capacity had successively decreased. He had no history of lung disease, drug misuse, or contact with pneumotoxic substances. The thoracic CT image he had brought with him (externally produced) showed a mixed picture of consolidations, alveolitic changes, and bilateral hilar lymph node enlargement (CT images are shown in the Table). Lung function tests yielded normal results (total lung capacity [TLC] 83% of the desired value FEV1/FVC 86%), only the diffusion capacity was notably reduced, to 56% of the desired value. Partial oxygen pressure (pO2) was lowered to 64 mm Hg. The patient was in good general health, and respiratory auscultation sounds were normal (vesicular breath sounds).
Endobronchially, no abnormalities were found, 7% of lymphocytes and 92% alveolar macrophages were confirmed in the BAL. No pathogens were found on microbiology and virology testing. Histologically the picture was mixed, with multifocal granulomatous inflammation that was consistent with sarcoidosis, but additionally, changes were seen that resembled toxic pneumonitis and areas with organizing pneumonia. Treatment with oral steroids (0.5 mg/kg body weight) was initiated, and the patient notably clinically improved within a few days. Subsequently the patient had no symptoms and was not limited in terms of exercise capacity.
Discussion
In the first two cases, known causes of acute lung injury can be ruled out with a high probability. In the third patient, radiology and histology found changes that are consistent with sarcoidosis, but he also had signs of toxic pneumonitis and areas of cryptogenic associated pneumonia, which are not typical of sarcoidosis. EVALI was therefore suspected. This suspicion was supported by the patient’s rapid clinical recovery on steroid treatment. No indications of an association between sarcoidosis and e-cigarette consumption exist to date, and we therefore think that an association between the patient’s self reported e-cigarette use and the acute symptoms is possible. In the cases described in this article, interstitial changes were particularly notable. In 10% of patients with interstitial lung changes, the disease cannot be precisely classified (unclassified interstitial lung disease [ILD]) (15). The estimated prevalence and incidence of interstitial lung disease is 60–80/100 000 population (16). A direct association between e-cigarette use and lung disease seems clinically suggestive, but we cannot prove it.
In addition to the liquids intended for this purpose by the manufacturers, it is possible to vaporize other substances in e-cigarettes—for example, THC-containing oils. In the largest and most recently published case series from the US, 80% of the young patients had vaped THC-containing liquids (with or without nicotine), 17% had used exclusively nicotine containing liquids, and the remaining ones had used other additives (11). THC-containing liquids are under suspicion of causing acute lung injury. They had been suspected as the cause of pneumonitis or organizing pneumonia in earlier case reports (17, 18). In the meantime, vitamin E acetate has been identified as the trigger for most of the changes (19). But there were also patients who had developed lung injury without this additive (11). In two of the cases described in this article, patients were not tested for THC because of the clinical assessment. One patient had been tested externally, but the result was negative. We therefore assume that the lung injury developed without intake of THC.
The liquids contained in e-cigarettes are a mixture of different chemical substances, which contain for example volatile substances such as propylene glycol and glycerin. These can also cause lung injury (20, 21). Added flavorings such as menthol or linalool are also considered dangerous; diacetyl and 2,3-pentanedione, which have been confirmed in vapor in substantial concentrations, are pneumotoxic (22– 25). Several case reports exist of acute lung injury in association with nicotine-containing liquids—for example, the occurrence of diffuse alveolar hemorrhage (cf Case 1), lipoid pneumonia, and interstitial pneumonia or acute eosinophil pneumonia (cf Case 2), bronchiolitis, exogenous allergic alveolitis, or cobalt-associated giant cell pneumonitis (7, 26– 30). Potentially the heating elements in e-cigarettes could constitute an additional burden, owing to manganese and zinc (31). Individual manufacturers do not provide any details about the precise composition of the aerosol that forms when the recommended liquids are vaped. The extent to which toxic lung injury develops or to which existing hypersensitivity plays a part remains unknown, and the same goes for a possible dose–response effect. Today we cannot gauge what the long-term sequelae of e-cigarette use might be (32).
It has been assumed that e-cigarettes are less harmful than tobacco smoke (the “harm reduction” hypothesis) and are being advertised as a smoking cessation aid by the manufacturers (industry association of the e-cigarette industry in Germany, VdeH) (6, 33). The importance of e-cigarettes is being discussed in smoking cessation programs, such as the UK Stop Smoking Service (SSS). In one study of the SSS, the proportion of nicotine abstaining patients was 8% after one year (34). A randomized controlled trial of smoking cessation using e-cigarettes compared with traditional nicotine substitutes showed that the cessation rate after one year was higher, at 18% vs 9.9%, if tobacco cigarettes were swapped for e-cigarettes (6). Overall, the assumption that e-cigarettes provide a good option for smoking cessation seems more than questionable though (35).
Even though in the patients described in this article, the suspicion of EVALI seems probable, because the changes identified are similar to those described in the US cases and no other causes for the changes were found, we cannot prove that the cases of lung injury were associated with e-cigarette use. The clustering of cases in the US should, however, prompt increased attention and awareness in Germany too. It is not clear to date whether a comparable problem does not exist in Germany or whether it remains unnoticed. The assumption that only certain liquids that aren’t commercially available in Europe are responsible for the acute lung injury has not been confirmed; furthermore there is a sizeable black market in Germany for e-cigarettes and similar products. This is highlighted by an example from Bremerhaven, where eight cases were reported as requiring hospital admission after consuming illegal additives to e-cigarettes or e-shisha (36). The Centers for Disease Control and Prevention (CDC) recommended on 31 August 2019 that all patients who had used e-cigarettes in the preceding 90 days should be questioned for respiratory complaints (37). A relevant medical history should be taken and documented in every medical consultation in Germany too.
As a matter of course, extensive differential diagnostic evaluation should be undertaken in suspected EVALI, and all patients should be advised to completely stop using e-cigarettes or traditional cigarettes.
In most of the cases, steroids were used for treatment. In our patients these yielded rapid improvement. In the largest case series, of patients from Utah/USA, 57/60 patients (95%) were given cortisone treatment (12). In 10% (6/60) of cases, patients relapsed, 3/6 had again had exposure to the assumed toxic substance. In our Case 1, the patient assured us that he hadn’t used the e-cigarette again. Supportive therapy depends on circumstances (bacterial superinfection or similar) (11).
Effectively, the described cases should be considered suspected cases of acute lung injury in the context of e-cigarette use. In the context of the widespread use of these products, it is currently to be considered a rare adverse effect. In Germany, cases of poisoning are notifiable by law. The provision is for physicians to report poisonings—including suspected cases—to the poisons documentation and assessment unit in the BfR. In our view, it would make sense as a next step to initiate a national registry, which collects all suspected cases, in order to analyze information on the frequency, associations with the toxic substance (dose–response effect), predisposing factors (for example, pre-existing disorders such as asthma [12]), possible causes and the kind of injury, as well as preventive and therapeutic measures in an expert manner. This could conceivably be done by structured reports to the poisons emergency call center in the first instance and further supervision of cases by the BfR, if needed in cooperation with the local public health authorities.
Summary
We described the cases of three patients whose acute lung injury with signs of respiratory failure may have been associated with the use of e-cigarettes. We did not find any other causes for the changes. All patients improved after treatment with high-dose prednisolone and after giving up vaping.
Key messages.
We presented two probable suspected cases and one possible suspected case of acute lung injury after e-cigarette use.
The described cases had greatly heterogeneous clinical findings; there was no pathognomonic injury pattern.
The fact that the products are not standardized and a multitude of toxic substances develops during vaping could explain different patterns of injury.
In order to uncover associations between e-cigarette use and (acute) lung injury, a national registry should be initiated.
E-cigarette use should become part of standard medical history taking.
Acknowledgments
Translated from the original German by Birte Twisselmann, PhD.
Footnotes
Conflict of interest statement The authors declare that no conflict of interest exists.
References
- 1.King BA, Jones CM, Baldwin GT, Briss PA. The EVALI and Youth Vaping Epidemics - Implications for Public Health. N Engl J Med. 2020;382:689–691. doi: 10.1056/NEJMp1916171. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Bundesinstitut für Risikobewertung. Vergiftungsfälle: Cannabidiolhaltige Liquids für E-Zigaretten können manipuliert sein. Stellungnahme Nr. 005/2020 vom 23. Januar 2020. www.bfr.bund.de/cm/343/vergiftungsfaelle-cannabidiolhaltige-liquids-fuer-e-zigaretten-koennen-mani puliert-sein.pdf (last accessed on 25 February 2020) [Google Scholar]
- 3.Deutsches Krebsforschungsinstitut. Krebsprävention/Inormation zur Tabakkontroll/E-Zigarette. www.dkfz.de/de/tabakkontrolle/E-Zigaretten (last accessed on 25 February 2020) [Google Scholar]
- 4.Verband des eZigarettenhandels. Verbraucher - Startseite/Material & Downloads/Archiv: Daten & Fakten zur E-Zigarette (September 2017) https://vd-eh.de/material/daten-fakten-zur-e-zigarette/verbraucher/ (last accessed on 25 February 2020) [Google Scholar]
- 5.UK Nationals Health Service (NHS) Using e-cigarettes to stop smoking. www.nhs.uk/live-well/quit-smoking/using-e-cigarettes-to-stop-smoking/ (last accessed on 29 March 2019) [Google Scholar]
- 6.Hajek P, Phillips-Waller A, Przulj D, et al. A randomized trial of e-cigarettes versus nicotine-replacement therapy. N Engl J Med. 2019;14:380–629-37. doi: 10.1056/NEJMoa1808779. [DOI] [PubMed] [Google Scholar]
- 7.Agustin M, Yamamoto M, Cabrera F, Eusebio Diffuse alveolar hemorrhage induced by vaping. Case Rep Pulmonol. 2018;7 doi: 10.1155/2018/9724530. 9724530. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Viswam D, Trotter S, Burge PS, Walters GI. Respiratory failure caused by lipoid pneumonia from vaping e-cigarettes. BMJ Case Rep. 2018 doi: 10.1136/bcr-2018-224350. pii: bcr-2018-224350. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Sommerfeld CG, Weiner DJ, Nowalk A, Larkin A. Hypersensitivity pneumonitis and acute respiratory distress syndrome from e-cigarette use. Pediatrics. 2018;141 doi: 10.1542/peds.2016-3927. pii: e20163927. [DOI] [PubMed] [Google Scholar]
- 10.Center of Diseases and Control (CDC) Health advisory: severe pulmonary disease associated with use of e-cigarette products. Atlanta, GA: US Department of Health and Human Services, CDC 2019. https://emergency.cdc.gov/han/han00421.asp (last accessed on 25 February 2020) [Google Scholar]
- 11.Layden JE, Ghinai I, Pray I, et al. Pulmonary illness related to e-cigarette use in Illinois and Wisconsin—preliminary report. N Engl J Med. 2019 doi: 10.1056/NEJMoa1911614. doi: 10.1056/NEJMoa1911614. [DOI] [PubMed] [Google Scholar]
- 12.Blagev DP, Harris D, Dunn AC, et al. Clinical presentation, treatment, and short-term outcomes of lung injury associated with e-cigarettes or vaping: a prospective observational cohort study. Lancet. 2019 doi: 10.1016/S0140-6736(19)32679-0. doi: 10.1016/S0140-6736(19)32679-0. [DOI] [PubMed] [Google Scholar]
- 13.Center of Diseases Control (CDC) Outbreak of lung injury associated with the use of e-Cigarette, or vaping, products. www.cdc.gov/tobacco/basic_information/e-cigarettes/severe-lung-disease.html (last accessed on 25 February 2020) [Google Scholar]
- 14.Bundesinstitut für Risikobewertung „Dampfen“. BfR rät vom Selbstmischen von E-Liquids ab. 43/2019, 15.11.2019. www.bfr.bund.de/de/presseinformation/2019/43/dampfen__bfr_raet_vom_selbstmischen_ von_e_liquids_ab-243082.html (last accessed on 25 February 2020) [Google Scholar]
- 15.Ryerson CJ, Urbania TH, Richeldi L, et al. Prevalence and prognosis of unclassifiable interstitial lung disease. Eur Respir J. 2013;42:750–757. doi: 10.1183/09031936.00131912. [DOI] [PubMed] [Google Scholar]
- 16.McCarthy C, Lara Gallego B, Trapnell BC, Mc-Cormack FX. Epidemiology of rare lung diseases: the challenges and opportunities to improve research and knowledge. Adv Exp Med Biol. 2017;1031:419–442. doi: 10.1007/978-3-319-67144-4_24. [DOI] [PubMed] [Google Scholar]
- 17.Anderson RP, Zechar K. Lung injury from inhaling butane hash oil mimics pneumonia. Respir Med Case Rep. 20192;6:171–17313. doi: 10.1016/j.rmcr.2019.01.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.He T, Oks M, Esposito M, Steinberg H, Makaryus M. “Tree-in-bloom”: severe acute lung injury induced by vaping cannabis oil. Ann Am Thorac Soc. 2017;14:468–470. doi: 10.1513/AnnalsATS.201612-974LE. [DOI] [PubMed] [Google Scholar]
- 19.Blount BC, Karwowski MP, Shields PG, et al. Vitamin E acetate in bronchoalveolar-lavage fluid associated with EVALI. N Engl J Med. 2020;382:697–705. doi: 10.1056/NEJMoa1916433. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Umweltbundesamt Christoph Hutzler, Frank Henkler, Andreas Luch. Inhaltsstoffe und Emissionen von E-Zigaretten. Umid 1, 2016. www.umweltbundesamt.de/sites/default/files/medien/2218/publikationen/umid_1_2016_bfr_e-zigarette.pdf (last accessed on 25 February 2020) [Google Scholar]
- 21.Lee MS, Allen JG, Christiani DC. Endotoxin and and (1- 3)-ß-D-glucan contamination in electronic cigarette products sold in the United States. Environ Health Perspect. 2019;127 doi: 10.1289/EHP3469. 47008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Floyd EL, Queimado L, Wang J, Regens JL, Johnson DL. Electronic cigarette power affects count concentration and particle size distribution of vaping aerosol. PLoS One. 2018;13(12) doi: 10.1371/journal.pone.0210147. e0210147. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Burstyn I. Peering through the mist: systematic review of what the chemistry of contaminants in electronic cigarettes tells us about health risks. BMC Public Health. 2014;14 doi: 10.1186/1471-2458-14-18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Allen JG, Flanigan SS, LeBlanc M, et al. Flavoring chemicals in e-cigarettes: diacetyl, 2,3-pentanedione, and acetoin in a sample of 51 products, including fruit-, candy-, and cocktail-flavored e-cigarettes. Environ Health Perspect. 2016;124:733–739. doi: 10.1289/ehp.1510185. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Uchiyama S, Inaba Y, Kunugita N. Determination of acrolein and other carbonyls in cigarette smoke using coupled silica cartridges impregnated with hydroquinone and 2,4-dinitrophenylhydrazine. J Chromatogr A. 2010:;1217:4383–4388. doi: 10.1016/j.chroma.2010.04.056. [DOI] [PubMed] [Google Scholar]
- 26.Sommerfeld CG, Weiner DJ, Nowalk A, Larkin A. Hypersensitivity pneumonitis and acute respiratory distress syndrome from e-cigarette use. Pediatrics. 2018;141 doi: 10.1542/peds.2016-3927. e20163927. [DOI] [PubMed] [Google Scholar]
- 27.Arter ZL, Wiggins A, Hudspath C, Kisling A, Hostler DC, Hostler JM. Acute eosinophilic pneumonia following electronic cigarette use. Respir Med Case Rep. 2019;27 doi: 10.1016/j.rmcr.2019.100825. 100825. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.McCauley L, Markin C, Hosmer D. An unexpected consequence of electronic cigarette use. Chest. 2012;141:1110–1113. doi: 10.1378/chest.11-1334. [DOI] [PubMed] [Google Scholar]
- 29.Flower M, Nandakumar L, Singh M, Wyld D, Windsor M, Fielding D. Respiratory bronchiolitis-associated interstitial lung disease secondary to electronic nicotine delivery system use confirmed with open lung biopsy. Respirol Case Rep. 2017;5 doi: 10.1002/rcr2.230. e00230. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Fels Elliott DR, Shah R, Hess CA, et al. Giant cell interstitial pneumonia secondary to cobalt exposure from e-cigarette use. Eur Respir J. 2019;54 doi: 10.1183/13993003.01922-2019. pii: 1901922. [DOI] [PubMed] [Google Scholar]
- 31.Olmedo P, Goessler W, Tanda S, et al. Metal concentrations in e-cigarette liquid and aerosol samples: the contribution of metallic coils. Environ Health Perspect. 2018;126 doi: 10.1289/EHP2175. 027010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Fonseca Fuentes X, Kashyap R, Hays JT, et al. VpALI—vaping-related acute lung injury: a new killer around the block. Mayo Clinic Proceedings. 2019;94:2534–2545. doi: 10.1016/j.mayocp.2019.10.010. [DOI] [PubMed] [Google Scholar]
- 33.Stephens WE. Comparing the cancer potencies of emissions from vapourised nicotine products including e-cigarettes with those of tobacco smoke. Tobacco Control. 2018;27:10–17. doi: 10.1136/tobaccocontrol-2017-053808. [DOI] [PubMed] [Google Scholar]
- 34.Bauld L, Hiscock R, Dobbie F, et al. English stop-smoking services: one-year outcomes. Int J Environ Res Public Health. 2016;13 doi: 10.3390/ijerph13121175. pii: E1175. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Pisinger C, Dagli E, Filippidis FT, et al. on behalf of the ERS Tobacco Control Committee: ERS and tobacco harm reduction Eur Respir J. 2019;54 doi: 10.1183/13993003.02009-2019. 1902009. [DOI] [PubMed] [Google Scholar]
- 36.Bremerhaven.de. E-Zigaretten und E-Shisha - gefährliche Zusätze? 25. 10. 2019. www.bremerhaven.de/de/aktuelles/e-zigaretten-und-e-shisha-gefaehrliche-zusaetze.95813.html (last accessed on 25 February 2020) [Google Scholar]
- 37.Centers for Disease Control and Prevention. Health Alert Network: severe pulmonary disease associated with using e-cigarette products. August 30 2019. https://emergency.cdc.gov/han/han00421.asp. (last accessed on 25 February 2020) [Google Scholar]


