Abstract
Background
The conservative treatment of peripheral arterial disease (PAD), as recommended in current guidelines, encompasses measures such as lifestyle modification and risk-factor management. In addition, in patients with vasogenic intermittent claudication (IC), it is recommended that patients first be given drugs to improve perfusion and undergo supervised gait training. Revascularization is not recommended for asymptomatic persons, but it is considered mandatory for patients with critical ischemia. In this article on conservative and revascularizing treatment strategies for IC, we address the following questions: whether all treatment options are available, how effective they are, and whether the reality of treatment for IC in Germany corresponds to what is recommended in the guidelines.
Methods
In 2014, the German Society for Angiology carried out a comprehensive literature search in order to prepare a new version of the S3 guideline on PAD. This literature search was updated up to 2018, with identical methods, for the present review.
Results
The benefit of lifestyle modification and risk factor treatment is supported by high-level evidence (evidence level I, recommendation grade A). The distance patients are able to walk without pain is increased by drug therapy as well (evidence level IIb), but the therapeutic effect is only moderate. Supervised exercise training (SET), though supported by high-level evidence (I, A), is of limited efficacy, availability, and applicability, and patient compliance with it is also limited. In patients with IC, revascularization leads to complete relief of symptoms more rapidly than gait training, and its long-term benefit is steadily improving owing to advances in medical technology. A combination of arterial revascularization and gait training yields the best results. In a clinical trial, patients with IC who underwent combined therapy increased the distance they could walk without pain by 954 m in six months, compared to 407 m in a group that underwent gait training alone.
Conclusion
In the treatment of vasogenic IC, SET and drugs to increase perfusion are now giving way to revascularization, which is more effective. As far as can be determined, SET is not currently implemented at all in the German health care system. It would be desirable for SET to be more available and more widely used, both to sustain the benefit of revascularization over the long term and to lower the general cardiovascular risk.
Peripheral arterial disease (PAD) is caused by atherosclerotic lesions in the pelvic and leg arteries. The incidence of atherogenic risk factors is rising worldwide (1), mainly due to the increasing age of the population. PAD is the third most common sequelae of arteriosclerosis after coronary heart disease and cerebrovascular diseases (1, 2). As a marker disease, it points to a high cardiovascular risk (3). It advances in stages (table), initially causing a reduction in pain-free walking distance (intermittent claudication, IC), which impairs quality of life and ability to work. At the stage of critical limb ischemia (CLI), pain at rest occurs, followed ultimately by tissue necrosis and amputation. The majority of symptomatic PAD patients suffer from IC (4).
Table. Classification of peripheral arterial disease according to Fontaine stages and Rutherford categories.
| Fontaine | Rutherford | ||||
| Stage | Clinical picture | Grade | Category | Clinical picture | |
| I | Asymptomatic | 0 | 0 | Asymptomatic | |
| II a | Walking distance >200 m | I | 1 | Mild intermittent claudication | |
| II b | Walking distance <200 m | 2 | Moderate intermittent claudication | ||
| 3 | Severe intermittent claudication | ||||
| III | Ischemic rest pain | II | 4 | Ischemic rest pain | |
| IV | Ulcers, gangrene | III | 5 | Minor necrosis | |
| 6 | Major necrosis | ||||
Critical limb ischemia covers stages III and IV or categories 4–6.
Current guidelines (3– 8) recommend lifestyle changes and risk factor management for all stages of PAD. In IC, vasoactive drugs and supervised exercise training (SET) are considered standard to achieve risk-free improvement in walking distance. Revascularization (percutaneous transluminal angioplasty [PTA]/stenting, vascular surgery) is not recommended at the asymptomatic stage, is recommended only in exceptional cases in IC (e.g., if conservative treatment fails), but is urgently recommended in CLI. Angioplasty for IC is not known to achieve better medium- and long-term outcomes compared to SET (3, 9). Unfortunately, in Germany as well as other countries (9), SET remains a largely theoretical option that is unachievable in a practical sense. Therefore, SET cannot be used as a yardstick for the results of arterial revascularization. Moreover, although revascularized patients are relieved of their symptoms in the immediate and longer term, most clinical studies have compared only the medium- and long-term benefit to SET.
The primary objective of this review article is to investigate whether guideline-compliant conservative treatment for IC can be recommended as an evidence-based approach in view of its limited efficacy, availability, practicability, and acceptance among patients. In light of the treatment reality in Germany, the benefit of arterial revascularization in IC is evaluated, particularly in combination with SET.
Methods
In 2014, the German Society for Angiology (Deutsche Gesellschaft für Angiologie) performed an extensive literature search for the new edition of its S3 guidelines on PAD. For the present article, that particular literature search has been extended to 2018 using MEDLINE, PubMed, and the Cochrane Database of Systematic Reviews. Search terms included: “intermittent claudication,” “claudication,” “periphere arterielle Verschlusskrankheit,” “PAVK,” “peripheral arterial disease,” “PAD,” “exercise therapy,” “supervised exercise training,” “angioplasty,” “endovascular therapy,” “endovascular revascularization,” “surgical revascularization,” “stent,” “pharmacotherapy.” The reference lists of individual publications were checked for previously unidentified studies. Particular importance was assigned to randomized controlled trials (RCTs) and their meta-analyses.
Epidemiology and concomitant diseases
More than 200 million people worldwide suffer from PAD (1). Its prevalence rises with age and stands at 20% of all primary care patients aged over 65 years in Germany (10). A third of PAD patients have IC (1, 10), while only 1–3% have CLI (4). IC remains stable over 5 years in 70–80%, worsens in 10–20%, and develops into CLI in 5–10% of patients (4). The number of undetected cases of symptomatic PAD is high (1, 11, 12).
Risk factors include age, sex, cigarette smoking, arterial hypertension, hyperlipidemia, diabetes, obesity, lack of exercise, and previous cardiovascular events in other vascular territories. Risk factors such as cigarette smoking are avoidable, while others can be modified, thereby affecting the age at which PAD manifests and its severity.
PAD signalizes generalized atherosclerosis with the risk of cardiac and cerebral complications and reduced life expectancy, which is generally underestimated (1, 11, 12). The following factors may explain the lack of awareness. PAD does not take priority in multimorbid patients, it is considered to be self-inflicted, and its detection is delayed due to the fragmentation of medicine into sub-disciplines. Patients are also treated late, insufficiently, and all too often as inpatients (12). Furthermore, the public is poorly informed about PAD (3, 12, 13).
Conservative treatment of PAD—basic measures
The goal of basic measures is to avoid the progression of asymptomatic PAD, improve walking distance and quality of life in IC, preserve limbs in CLI, and reduce cardiovascular morbidity and mortality. There is a high level of evidence (I, A) for the following measures (3– 8): lifestyle changes, tobacco abstinence, adequate exercise, weight reduction, treatment of diabetes, arterial hypertension, and hyperlipidemia, as well as the administration of platelet aggregation inhibitors in symptomatic patients.
According to the guidelines, revascularization is not indicated in asymptomatic PAD (3– 8). Exceptions include, for instance, endovascular or open surgical correction of stenosis in the setting of impending bypass occlusion (2). The extent of ischemia in diabetics is underestimated due to polyneuropathy (14). In objectively proven CLI (toe–brachial index, Doppler frequency spectrum), revascularization is considered despite freedom from symptoms (3, 5).
Conservative treatment of intermittent claudication
In addition to basic measures, a solid foundation also comprises pharmacological improvement of arterial perfusion and supervised exercise training (SET; Box). The following are not recommended (3– 8): intermittent pneumatic compression, transcutaneous electrical nerve stimulation, extracorporeal shock wave therapy, and sympathicolysis.
BOX. Supervised exercise training.
Supervised exercise training (SET) under medical supervision and with instruction from physiotherapists should be offered to all PAD patients with intermittent claudication as an integral part of basic treatment. SET is carried out in PAD sports groups. In a first step, the patient‘s individual capacity is determined, following which an appropriate training plan is drawn up. Training should take place a least 3 × per week in training sessions of 30–60 min for a minimum period of 3–6 months (3).
Vasoactive drugs
Although the improvement in pain-free walking distance with cilostazol or naftidrofuryl is significant (IIb), it does not exceed 50–100 m at 1 year (3– 8). For this reason, there is only a consensus recommendation on these drugs (3). Angiotensin-converting enzyme (ACE) inhibitors, statins, and acetylsalicylic acid (ASA) confer similar improvements. These effects do not appear to be additive. There is no proof of efficacy for pentoxyfylline, buflomedil, ginkgo, and prostanoids.
Exercise training
Prior to the introduction of endovascular revascularization, SET was the most effective method to treat IC, since the alternatives were all higher-risk vascular surgical procedures (15). SET is considered a natural, effective, safe, cost-effective, and guideline-compliant treatment for IC with a high level of evidence (16, 17), assuming symptoms are tolerable (3, 5). At 1 year, SET was significantly more effective in terms of maximum walking distance (+ 210 m) compared to home-based exercise training (+ 120 m) or ineffective walking advice (18). Most meta-analyses show that SET is able to increase pain-free walking distance by more than 100% of the baseline value (16, 17). There are reports on an improvement of more than 500 m at 1 year with SET (19), as well as others on its ineffectiveness (20). As early on as 2006, a Cochrane review (21) was deemed unable to demonstrate the efficacy of SET in IC due to the paucity of evidence (15).
Quality of life improves with SET, for instance, in terms of physical function at 3–6 months (18). SET‘s mode of action is unclear. It improves, for example, gait mechanics, bioenergetics of muscles and collaterals, neoangiogenesis, as well as microcirculation (22). SET has no effect on mortality and amputation rates (16), it increases neither the ankle–brachial index (ABI) nor maximum blood flow in the calf muscles (16, 18, 20, 22), and is not more effective than cycling, strength training, and arm and shoulder muscle training (23).
SET is not offered or reimbursed sufficiently in Germany. There are approximately 6000 heart sports groups (www.dgpr.de), but only 240 vascular sports groups (www.deutsche-gefaessliga.de) in Germany. Less than a third of all vascular surgeons are able to provide access to SET for their patients (24). Due to concomitant diseases (for example cardiac disorders) or lack of adherence, SET, if available at all, is used by only a third of IC patients (25). The discontinuation rate at 1 year is as high as 70% (19, 24). SET is contraindicated in individuals with diabetes, since the warning signal of “calf pain” is lacking due to polyneuropathy (14).
The efficacy of SET depends on whether donor and recipient arteries are able to develop collateral circulation (figure 1). If the inflow into collateral vessels is impaired by occlusive processes upstream (figure 2), the vascular circulation needs to be prepared for SET using either endovascular or vascular surgical techniques (3, 5). Failure to do so can result in severe calf muscle injury (26).
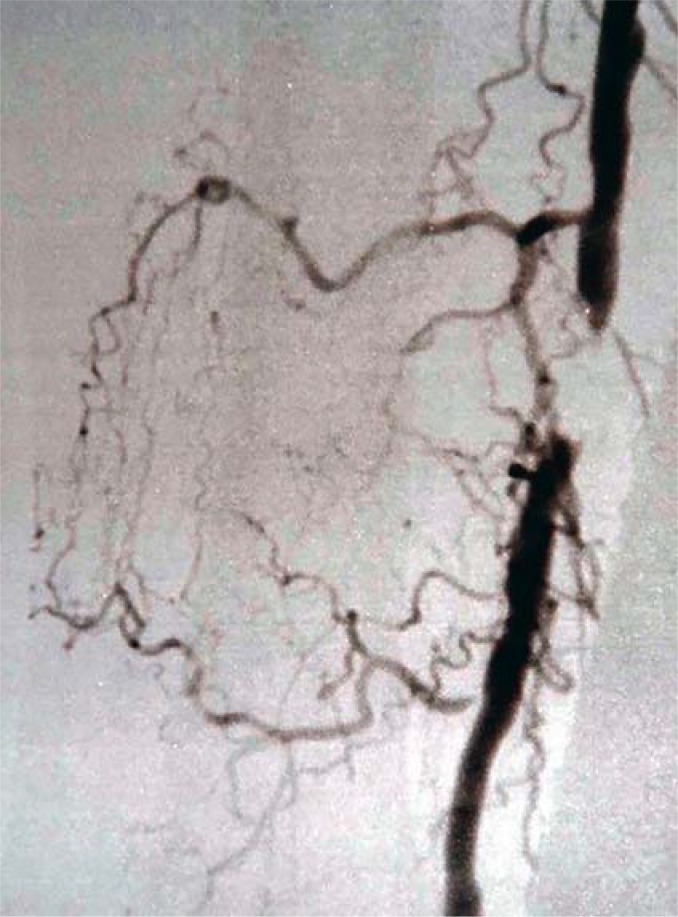
Figure 1.
Short-segment occlusion of the right superficial femoral artery. Structured supervised exercise training can improve collateral circulation.
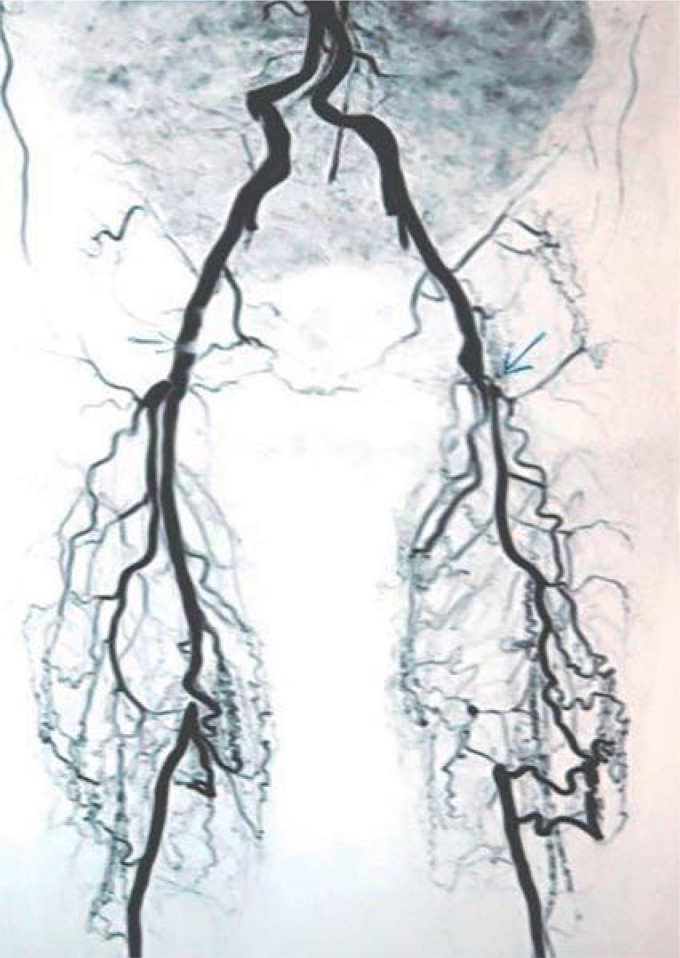
Figure 2.
Strong collateral circulation between the deep femoral artery and the popliteal artery is hindered by upstream high-grade stenosis of the common femoral artery, meaning that exercise training is unable to have a sufficient effect.
Revascularization for intermittent claudication
Contrary to recommendations (3), the majority of inpatient PAD patients in Germany receive treatment for IC. In 2009, intermittent claudication was identified in at least 49%, and CLI in at least a third of patients admitted on an inpatient basis with PAD as the main diagnosis. IC patients underwent 65% of endovascular procedures, 55% of all bypass surgeries, and 78% of all inguinal patch plasty procedures (27). A total of 41% of patients in the German statutory Federal Miners‘ Insurance (Bundesknappschaft) that were treated between 2010 and 2012 in an inpatient setting with PAD as a main diagnosis had IC. Of these, approximately 27% were treated by means of vascular surgical intervention and around 50% using endovascular methods (28). Many IC patients are revascularized in the outpatient setting (29), and the overall number of angioplasty procedures for IC is likely to be underestimated. According to the German PTA registry (30), 77% of the PAD patients treated (as outpatients or inpatients) had IC.
Due to the perioperative (2–3%) or interventional risk of complications (1%), revascularization was hitherto considered justified only in significantly limiting CI (3, 5). Warnings were even made that endovascular measures could increase the risk of amputation compared to conservative treatment (5). However, most treatment studies have not investigated IC and CLI patients separately, despite the fact that the risk is higher and the outcome worse in CLI (31), meaning that the risk in IC was overestimated. For example, an RCT (60% CLI, 40% IC) on short-segment vascular lesions yielded a periprocedural mortality rate of 0%. However, 4% and 5% of CLI patients died at 1 year following surgery and percutaneous transluminal angioplasty, respectively, compared to 0% and only 1% of IC patients, respectively (32). The amputation risk in IC patients revascularized for PAD is only 0.4% per year compared to 12% in CLI patients at 6 months (33). Modern treatment materials and strategies, as well as growing experience, have led to better and safer outcomes with endovascular treatment (34). For this reason, PTA is indicated more liberally today (7). Nitinol stenting following femoral artery PTA is able to reduce the restenosis rate of around 60% to 35% at 1 year (1). Iliac artery dilatation with primary stent implantation has a patency rate of around 90% at 1 year (3). Modern studies show rates for periinterventional mortality, morbidity, and amputation risk in IC to be virtually 0% (19, 33– 36). Due to its favorable benefit/risk ratio, an endovascular-first strategy is recommended in short-segment aortoiliac (<5 cm) as well as femoropopliteal (<25 cm) lesions (7). This is associated with a significantly lower complication rate and lower 30-day mortality in aortoiliac (37) and femoropopliteal (38) disease compared to bypass surgery. Even at 24 months following angioplasty, quality of life remains significantly increased in terms of mobility, self-care, and pain (30).
Data from the US (29) demonstrate that the major amputation rate as well as periprocedural morbidity and mortality in inpatient IC patients declined significantly between 1998 and 2007 despite an increase in comorbidities. This is attributed to the two-thirds drop in surgical revascularizations and the 50% rise in early endovascular interventions (IC and CLI), as a result of the safety of these methods. Outpatient interventions went up by 80%, while the amputation rate in IC was 0.3% (29).
Vascular surgical procedures such as thromboendarterectomy for occlusive processes of the inguinal artery, bypass (evidence level I for IC and CLI, 7) for long-segment femoropopliteal occlusion, or combinations with endovascular interventions (hybrids) are available. Above-knee bypass for IC normalizes walking distance (32, 39) in the long term (3, 4), with a primary patency of approximately 80% at 5 years if veins are used (3, 39). The fact that this treatment option is used so frequently (27, 28) is an indication of its efficacy and the high demand. An interdisciplinary discussion should be held prior to all revascularization procedures.
Exercise training versus revascularization for IC
Following revascularization, the patient immediately has a better walking distance, in contrast to SET (39, 40). However, meta-analyses of RCTs (9, e1– e3) showed that endovascular revascularization alone conferred no advantage at 1 year in terms of walking distance and the future risk of revascularization or amputation compared to SET. Ultimately, the studies are limited by the fact that SET is the wrong evaluation benchmark for comparison, since it is no more than a theoretical treatment alternative for revascularization that is virtually non-existent in practice. Furthermore, all the above-mentioned RCTs excluded the total period between intervention and time of observation (1–2 years) from their comparisons, meaning that the integrated overall benefit in terms of walking distance and patient quality of life was negated. Also, none of the trials compared revascularization with the inferior alternatives, such as unsupervised exercise training or walking advice, which would have more accurately reflected the treatment reality.
Modern RCTs show an advantage for endovascular therapy at all vascular levels in IC even at 1 year compared to SET (19). The MIMIC study already demonstrated this in 2008 for femoral dilatation (36). The improvement in walking distance and quality of life following primary stenting of the superficial femoral artery compared to best medical treatment is also evident at 2 years, thereby highlighting the value of endovascular procedures for the “real-world” situation without SET (34).
According to the most recent RCT on this topic published in 2015, PTA combined with selective stenting and SET is the most effective approach (19). For example, the pain-free walking distance in IC patients increased with SET or PTA/stenting combined with SET (baseline walking distance 135 and 117 m, respectively) to 181 and 724 m after 1 month, respectively, and to 542 and 1071 m at 6 months, respectively. Patients were symptom-free following PTA/stenting, whereas walking distance rose significantly more slowly with SET. Several meta-analyses also concluded that SET and angioplasty together have a more far-reaching effect on functional outcome and quality of life than does SET alone (e3, e4). The combination of angioplasty and unsupervised exercise training was not superior to SET alone (e1).
Successful revascularization encourages patients to be mobile and enables them to benefit from the long-term advantages of SET. At 1 year, a third of patients that had undergone PTA/stenting combined with SET had relevant restenosis of the target lesion; however, only 17% of these required repeat angioplasty (19). The 2017 ESC guidelines recommend (evidence level IIa) combining revascularization with SET if activity in everyday life is restricted (7). The same applies to bypass surgery. Whereas SET for IC increased the pain-free walking distance by 120 m within 1 year, bypass surgery normalized walking distance immediately (32, e5); the combination of vascular surgery and SET had the strongest effect (31, e5, e6).
The comparison of a one-off endovascular intervention with long-term SET fails to recognize the fact that numerous follow-up interventions are needed to maintain treatment success. Although this puts a burden on patients and cost-bearers, this has not been called into question as yet. Precise data on this topic for IC are lacking. Therefore, patients should always be strongly encouraged to increase their level of physical activity and, where available, participate in SET following both angioplasty and vascular surgery in order to ensure the long-term success of these costly treatments and reduce their cardiovascular risk.
Key messages.
Patients with any stage of PAD should be advised to make lifestyle changes (exercise, nicotine abstinence, weight reduction) and receive pharmacological treatment for concomitant diseases (antihypertensive drugs, acetylsalicylic acid [ASA], statins, etc.).
Asymptomatic PAD does not require revascularization. Exceptions include service interventions in the case of impending bypass occlusion due to PAD or individuals with diabetic neuropathy and proven critical leg ischemia.
The value of SET in IC patients is highly evident, in contrast to unsupervised exercise training or walking advice. However, availability, effectiveness, practicability, and patient adherence are low. This makes comparisons between revascularization and SET a poor reflection of reality, while on the other hand highlighting structural problems in the health system.
Endovascular or vascular surgical interventions in IC result in freedom from symptoms more rapidly compared to SET. Thanks to technical advances, these procedures are now lower-risk, more effective, and more sustainable in the long term. Arterial revascularization followed by SET achieves the best results.
Patients that have undergone revascularization should be strongly advised to increase their physical activity and—where available—participate in SET. In this way, it is possible to ensure the medium- and long-term success of this expensive treatment (compared to SET), prevent reintervention, and reduce the cardiovascular risk.
Acknowledgments
Translated from the original German by Christine Rye.
Footnotes
Conflict of interests PD Dr. Schmidt received honoraria for consultancy activities from Cook and Bard. He was reimbursed for congress fees and travel expenses and received honoraria for speaking from Cordis, Abbott, Cook, and Bard. He received study support (third-party funding) from Abbott, Bard, Cook, Medtronic, and Intervascular.
The remaining authors state that they have no conflicts of interest.
References
- 1.Fowkes FG, Rudan D, Rudan I, et al. Comparison of global estimators of prevalence and risk factors for peripheral arterial disease in 2000 and 2010: a systematic review and analysis. Lancet. 2013;382:1329–1340. doi: 10.1016/S0140-6736(13)61249-0. [DOI] [PubMed] [Google Scholar]
- 2.Alberts MJ, Bhatt DL, Mas JL, et al. Three-year follow-up and event rates in the international reductuion of atherothrombosis for continued health registry. Eur Heart J. 2009;30:2318–2326. doi: 10.1093/eurheartj/ehp355. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Lawall H, Huppert P, Espinola-Klein C, Rümenapf G. The diagnosis and treatment of peripheral arterial vascular disease. Dtsch Arztebl Int. 2016;113:729–736. doi: 10.3238/arztebl.2016.0729. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Norgren L, Hiatt WR, Dormandy JA, et al. Inter-society consensus for the management of peripheral arterial disease (TASC II) J Vasc Surg. 2007;45:S5–S67. doi: 10.1016/j.jvs.2006.12.037. [DOI] [PubMed] [Google Scholar]
- 5.Conte MS, Pomposelli FB, Clair DG, et al. Societey for Vascular Surgery Lower Extremity Guidelines Writing Group, Society for Vascular Surgery practice guidelines for atherosclerotic occlusive disease of the lower extremities: management of asymptomatic disease and claudication. J Vasc Surg. 2015;61:2S–41S. doi: 10.1016/j.jvs.2014.12.009. [DOI] [PubMed] [Google Scholar]
- 6.NICE Clinical Guideline 147. Lower limb periphral arterial disease: diagnosis and management. www.nice.org.uk/cg147/evidence/lower-limb-periphral-arterial-disease-full-guideline-186865021(last accessed on 4 January 2020) [Google Scholar]
- 7.Aboyans V, Ricco JB, Bartelink MEL, et al. 2017 ESC guidelines on the diagnosis and treatment of peripheral arterial diseases, in collaboration with the European Society for Vascular Surgery (ESVS) Eur J Vasc Endovasc Surg. 2018;55:305–368. doi: 10.1016/j.ejvs.2017.07.018. [DOI] [PubMed] [Google Scholar]
- 8.Gerhard-Herman MD, Gornik HL, Barrett C, et al. 2016 AHA/ACC guideline on the management of patients with lower extremity peripheral artery disease: a report of the American College of Cardiology/American Heart Association task force on clinical practice guidelines. J Am Coll Cardiol. 2017;69:1465–1508. doi: 10.1016/j.jacc.2016.11.008. [DOI] [PubMed] [Google Scholar]
- 9.Murphy TP, Cutlip DE, Regensteiner JG, et al. Supervised exercise, stent revascularization, or medical therapy for claudication due to aortoiliac peripheral artery disease The CLEVER study. J Am Coll Cardiol. 2015;65:999–1009. doi: 10.1016/j.jacc.2014.12.043. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Diehm C, Schuster A, Allenberg JH, et al. High prevalence of peripheral arterial disease and co-morbidity in 6880 primary care patients: cross-sectional study. Atherosclerosis. 2004;172:95–105. doi: 10.1016/s0021-9150(03)00204-1. [DOI] [PubMed] [Google Scholar]
- 11.Lange S, Diehm C, Darius H, et al. High prevalence of peripheral arterial disease and low treatment rates in elderly primary care patients with diabetes. Exp Clin Endocrinol Diabetes. 2004;112:566–573. doi: 10.1055/s-2004-830408. [DOI] [PubMed] [Google Scholar]
- 12.Hockley T. Peripheral patients? a call to action on peripheral arterial disease. LSE Enterprise. 2017 [Google Scholar]
- 13.Hirsch AT, Murphy TP, Lovell MB, et al. Gaps in public knowledge of peripheral arterial disease: the first national PAD public awareness survey. Circulation. 2007;116:2086–2094. doi: 10.1161/CIRCULATIONAHA.107.725101. [DOI] [PubMed] [Google Scholar]
- 14.Chantelau E. The fate of the ischaemic limb in diabetes: it is neuropathy that makes the difference. Vasa. 2001;30:15–20. [Google Scholar]
- 15.Amendt K. [Ist der allgemein akzeptierte therapeutische Nutzen des Gehtrainings bei Patienten mit arterieller Verschlusskrankheit evidenzbasiert? ] Hämostaseologie. 2006;26:224–228. [PubMed] [Google Scholar]
- 16.Lane R, Harwood A, Watson L, Leng GC. Exercise for intermittent claudication. Cochrane Database Syst Rev. 2017;12 doi: 10.1002/14651858.CD000990.pub4. CD000990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Watson L, Ellis B, Leng GC. Exercise for intermittent claudication. Cochrane Database Syst Rev. 2008;4 doi: 10.1002/14651858.CD000990.pub2. CD000990. [DOI] [PubMed] [Google Scholar]
- 18.Hageman D, Fokkenrood HJ, Gommans LN, van den Houten MM, Teijink JA. Supervised exercise therapy versus home-based exercise therapy versus walking advice for intermittent claudication. Cochrane Database Syst Rev. 2018;4 doi: 10.1002/14651858.CD005263.pub4. CD005263. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Fakhry F, Spronk S, van der Laan L, et al. Endovascular revascularization and supervised exercise for peripheral artery disease and intermittent claudication. A randomized clinical trial. JAMA. 2015;314:1936–1944. doi: 10.1001/jama.2015.14851. [DOI] [PubMed] [Google Scholar]
- 20.Gelin J, Jivegård L, Taft C, et al. Treatment efficacy of intermittent claudication by surgical intervention, supervised physical exercise training compared to no treatment in unselected randomised patients I: one year results of functional and physiological improvements. Eur J Vasc Endovasc Surg. 2001;22:107–113. doi: 10.1053/ejvs.2001.1413. [DOI] [PubMed] [Google Scholar]
- 21.Leng GC, Fowler B, Ernst E. Exercise for intermittent claudication. Cochrane Database Syst Rev. 2000;2 doi: 10.1002/14651858.CD000990. CD000990. [DOI] [PubMed] [Google Scholar]
- 22.Gardner AW, Forrester L, Smith GV. Altered gait profile in subjects with peripheral arterial disease. Vasc Med. 2001;6:31–34. [PubMed] [Google Scholar]
- 23.Lauret GJ, Fakhry F, Fokkenrood HJP, Huninck MGM, Tijink JAW, Spronk S. Modes of exercise training for intermittent claudication. Cochrane Database Syst Rev. 2014,;7 doi: 10.1002/14651858.CD009638.pub2. CD009638. [DOI] [PubMed] [Google Scholar]
- 24.Makris GC, Lattimer CR, Lavida A, Geroulakos G. Availability of supervised exercise programs and the role of structured home-based exercise in peripheral arterial disease. Eur J Vasc Endovasc Surg. 2012;44:569–557. doi: 10.1016/j.ejvs.2012.09.009. [DOI] [PubMed] [Google Scholar]
- 25.Diehm C. Rehabilitation gefäßkranker Patienten Interventionelle Kardiologie, Angiologie und Kardiovaskularchirurgie. In: Hombach V, editor. Schattauer. Stuttgart: 2001. 749 pp. [Google Scholar]
- 26.Li S, Lackner T, Willcockson G, et al. Histopathological study of calf muscle in claudicating patients with periphera artery disease after supervised exercise therapy. Arter Throm Vasc Biol; 2019;(1) A631. [Google Scholar]
- 27.Malyar N, Fürstenberg T, Wellmann J, et al. Recent trends in morbidity and in-hospital outcomes of in-patients with peripheral arterial disease: a nationwide population-based analysis. Eur Heart J. 2013;34:2706–2714. doi: 10.1093/eurheartj/eht288. [DOI] [PubMed] [Google Scholar]
- 28.Niemöller K, May D, Freisinger E, et al. Prognosis improvement by specialized vascular medicine in patients with peripheral artery disease. Vasa. 2018;47(99):10–11. (FV-2.2) [Google Scholar]
- 29.Egerova NN, Guillerme S, Gelijns A, et al. An analysis of the outcomes of a decade of experience with lower extremity revascularization including limb salvage, lengths of stay, and safety. J Vasc Surg. 2010;51:878–885. doi: 10.1016/j.jvs.2009.10.102. [DOI] [PubMed] [Google Scholar]
- 30.Schulte KL, Hardung D, Tiefenbacher C, et al. Real-world outcomes of endovascular treatment in a non-selected population with peripheral artery disease—prospective study with 2-year follow-up. Vasa. 2019;48:433–441. doi: 10.1024/0301-1526/a000798. [DOI] [PubMed] [Google Scholar]
- 31.Taylor SM, Cull DL, Kalbaugh CA, et al. Comparison of interventional outcomes according to preoperative indication: a single center analysis of 2,240 limb revascularizations. J Am Coll Surg. 2009;208:770–778. doi: 10.1016/j.jamcollsurg.2009.01.025. [DOI] [PubMed] [Google Scholar]
- 32.Holm J, Arfvidsson B, Jivegard L, et al. Chronic lower limb ischaemia A prospective randomized controlled study comparing the 1-year results of vascular surgery and percutaneous transluminal angioplasty (PTA) Eur J Vasc Surg. 1991;5:517–522. doi: 10.1016/s0950-821x(05)80338-x. [DOI] [PubMed] [Google Scholar]
- 33.Fridh EB, Andersson M, Thuresson M, et al. Amputation rates, mortality, and pre-operative comorbidities in patients revascularised for intermittent claudication or critical limb ischemia: a population based study. Eur J Vasc Endovasc Surg. 2017;54:480–486. doi: 10.1016/j.ejvs.2017.07.005. [DOI] [PubMed] [Google Scholar]
- 34.Lindgren H, Qvarfordt P, Bergman S, et al. Primary stenting of the superficial femoral artery in patients with intermittent claudication has durable effects on health related quality of life at 24 months: results of a randomized controlled trial. Cardiovasc Intervent Radiol. 2018;41:872–881. doi: 10.1007/s00270-018-1925-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Mazari FA, Khan JA, Carradice D, et al. Randomized clinical trial of percutaneous transluminal angioplasty, supervised exercise and combined treatment for intermittent claudication due to femoropopliteal arterial disease. Br J Surg. 2012;99:39–48. doi: 10.1002/bjs.7710. [DOI] [PubMed] [Google Scholar]
- 36.Greenhalgh RM, Belch JJ, Brown LC, et al. The adjuvant benefit of angioplasty in patients with mild to moderate intermittent claudication(MIMIC) managed by supervised exercise, smoking cessation advise and best medical therapy: results from two randomized trials for stenotic femoropopliteal and aortoiliac artery disease. Eur J Vasc Endovasc Surg. 2008;36:680–688. doi: 10.1016/j.ejvs.2008.10.007. [DOI] [PubMed] [Google Scholar]
- 37.Indes JE, Pfaff MJ, Farrokhyar F, et al. Clinical outcomes of 5358 patients undergoing direct open bypass or endovascular treatment for aortoiliac occlusive disease: a systematic review and meta-analysis. J Endovasc Ther. 2013;20:443–455. doi: 10.1583/13-4242.1. [DOI] [PubMed] [Google Scholar]
- 38.Aihara H, Soga Y, Mii S, et al. Comparison of long-term outcome after endovascular therapy versus bypass surgery in claudication patients with Trans-Atlantic Inter-Society Consensus-II C and D femoropopliteal disease. Circ J. 2014;78:457–464. doi: 10.1253/circj.cj-13-1147. [DOI] [PubMed] [Google Scholar]
- 39.Eugster T, Marti R, Gurke L, Stierli P. Ten years after arterial bypass surgery for claudication: venous bypass is the primary procedure for TASC C and D lesions. World J Surg. 2011;35:2328–2331. doi: 10.1007/s00268-011-1237-x. [DOI] [PubMed] [Google Scholar]
- 40.Spronk S, Bosch JL, den Hoed PT, Veen HF, Pattynama PM, Hunink MG. Intermittent claudication: clinical effectiveness of endovascular revascularization versus supervised hospital-based exercise training - randomized controlled study. Radiology. 2009;250:586–595. doi: 10.1148/radiol.2501080607. [DOI] [PubMed] [Google Scholar]
- E1.Ahimastos AA, Pappas EP, Buttner PG, Walker PJ, Kingwell BA, Golledge J. A meta-analysis of the outcome of endovascular and noninvasive therapies in the treatment of intermittent claudication. J Vasc Surg. 2011;54:1511–1521. doi: 10.1016/j.jvs.2011.06.106. [DOI] [PubMed] [Google Scholar]
- E2.Pandey A, Banerjee S, Ngo C, et al. Comparative efficacy of endovascular revascularization versus supervised exercise training in patients with intermittent claudication. JACC: Cardiovasc Interv. 2017;10:712–724. doi: 10.1016/j.jcin.2017.01.027. [DOI] [PubMed] [Google Scholar]
- E3.Fakhry F, Fokkenrood HJP, Spronk S, Teijink JAW, Rouwet EV, Hunink MGM. Endovascular revascularisation versus conservative management for intermittent claudication. Cochrane Database Syst Rev. 2018;3 doi: 10.1002/14651858.CD010512.pub2. CD010512. [DOI] [PMC free article] [PubMed] [Google Scholar]
- E4.Frans FA, Bipat S, Reekers JA, Legemate DA, Koelemay MJW. Systematic review of exercise training or percutaneous transluminal angioplasty for intermittent claudication. Brit J Surg. 2012;99:16–28. doi: 10.1002/bjs.7656. [DOI] [PubMed] [Google Scholar]
- E5.Lundgren F, Dahllöf AG, Lundholm K, Scherstén T, Volkmann R. Intermittent claudication—surgical reconstruction or physical training? A prospective randomized trial of treatment efficiency. Ann Surg. 1989;209:346–355. doi: 10.1097/00000658-198903000-00016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- E6.Jakubsevičienė E, Vasiliauskas D, Velička L, Kubilius R, Milinavičienė E, Venclovienė J. Effectiveness of a new exercise program after lower limb arterial blood flow surgery in patients with peripheral arterial disease: a randomized clinical trial. Int J Environ Res Public Health. 2014;11:7961–7976. doi: 10.3390/ijerph110807961. [DOI] [PMC free article] [PubMed] [Google Scholar]