Abstract
Purpose
To analyse the impact of different optimization strategies on the compatibility between planned and delivered doses during radiotherapy of cervical cancer.
Material/methods
Four treatment plans differing in optimisation strategies were prepared for ten cervical cancer cases. These were: volumetric modulated arc therapy with (_OPT) and without optimization of the doses in the bone marrow and for two sets of margins applied to the clinical target volume that arose from image guidance based on the bones (IG(B)) and soft tissues (IG(ST)). The plans were subjected to dosimetric verification by using the ArcCHECK system and 3DVH software. The planned dose distributions were compared with the corresponding measured dose distributions in the light of complexity of the plans and its deliverability.
Results
The clinically significant impact of the plans complexity on their deliverability is visible only for the gamma passing rates analysis performed in a local mode and directly in the organs. While more general analyses show statistically significant differences, the clinical relevance of them has not been confirmed. The analysis showed that IG(ST)_OPT and IG(B)_OPT significantly differ from IG(ST) and IG(B). The clinical acceptance of IG(ST)_OPT obtained for hard combinations of gamma acceptance criteria (2%/2 mm) confirm its satisfactory deliverability. In turn, for IG(B)_OPT in the case of the rectum, the combination of 2%/2 mm did not meet the criteria of acceptance.
Conclusion
Despite the complexity of the IG(ST)_OPT, the results of analysis confirm the acceptance of its deliverability when 2%/2 mm gamma acceptance criteria are used during the analysis.
Keywords: Cervical cancer, VMAT, gamma analysis, Treatment plan complexity, 3DVH software.
1. Introduction
The recent advances in radiation therapy techniques have removed some of the limitations of gynaecologic radiation therapy. Compared to the three-dimensional conformal radiation therapy, the intensity-modulated techniques, such as intensity modulated radiation therapy (IMRT), volumetric modulated arc therapy (VMAT) and tomotherapy (HT), can significantly reduce the dose to the bowels, rectum, bladder or bone marrow (BM) that implies reduction of gastrointestinal, urinary and haematological toxicities.1, 2, 3, 4 As a result of the above observations, the European Society of Gynaecological Oncology (ESGO), European Society for Radiotherapy and Oncology (ESTRO) and European Society of Pathology (ESP) recommend intensity-modulated techniques as most appropriate for external beam radiation therapy during the management of cervical cancer.5 Nevertheless, there are still open questions focused on the practical usage of dynamic techniques during radiation therapy of cervical cancer.
First of them concerns the strategy of dose optimization while preparing a plan intended to reduce dose in BM. The reduction of doses in BM were observed when the planning strategy assumed the usage of BM as an organ at risk (OAR) during treatment plan optimization and when BM was not used as an optimization structure.6, 7, 8, 9 One of the last studies published by Murakami et al. showed the superiority of the strategy when BM is used as OAR during optimization.10 This observation is in line with our previous study where we show additionally that for conventional (C-arm) accelerators, the best results of dose reduction in BM are observed for VMAT.11
The second question focuses on a proper selection of an image guidance (IG) procedure during radiation therapy.12,13 Selection of IG procedure directly implies the size of margins that should be applied to the clinical target volume (CTV) to create the planning target volume (PTV).14 With OARs located close to the CTV, the size of CTV-to-PTV margins can impact on the dose distribution in the OARs obtained from the optimization process. In our most recent study,15 we showed that IG based on the bony anatomy (IG(B)) allows to establish smaller margins for lymph nodes (CTV2) than those for the vagina and paravaginal tissues (CTV1) and the IG based on soft tissues (IG(ST)) indicates the opposite: margins for CTV1 are smaller than for CTV2. While decreasing the margins for CTV1 reduces the doses in the bladder and rectum, doses cumulated in BM are independent of the size of the margin resulting from different IG types.15 We showed also the superiority of VMAT for dose reduction in BM in comparison to IMRT. Finally, we recommended the VMAT plans supported by IG(ST) protocols for radiation therapy of cervical cancer.15
The third question concerns the compatibility between planned and delivered doses during radiation therapy of cervical cancer. Correctly added CTV-to-PTV margin should compensate intra-fraction movement.16 Properly implemented IG protocols allow us to appropriately react to inter-fraction changes in patient anatomy.17,18 Nevertheless, it is not possible to compensate by the above-mentioned solutions a potential incompatibility between planned and delivered dose that depends on technical uncertainties of dose delivery on the accelerator.19 The VMAT plans with dose optimization in BM11 and supported by IG(ST) or IG(B)15 are more complex than other evaluated plans. Therefore, it is rational to check whether the compatibility between planned and delivered doses for these proposals is comparable to that between doses obtained for other plans that are technically easier to implement.
2. Aim
Based on a randomly selected group of ten patients with cervical cancer, a dosimetric study was performed to estimate a potential impact of the different strategies of dose optimization on the compatibility between planned and delivered doses during radiation therapy of cervical cancer.
3. Material and methods
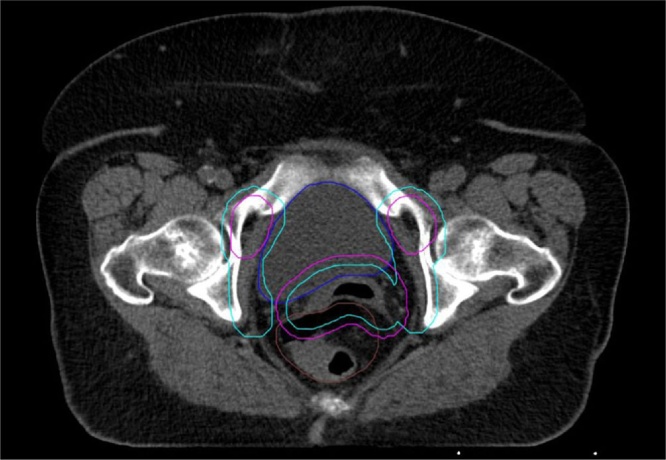
We used anonymised computed tomography (CT) images with the outlines of CTV and OARs that were created for ten randomly selected patients with cervical cancer undergoing radiation therapy in our centre. The CT images (Definition AS, Siemens, Germany) were performed in a supine position (2 mm slice thickness) with a knee and feet support (Combifix; CIVCO Radiotherapy, Coralville, IA, USA). The clinical target volume (CTV) was defined according to the guidelines presented by Lim et al. and Small et al. and included the upper vagina, parametrial/paravaginal tissues, common, external and internal iliac lymph nodes.20,21 Due to the different nature of potential movements for the treated volume, the CTV was split to CTV1 (vagina and paravaginal tissues) and to CTV2 (lymph nodes). Taking into account our previous observation15 that the size of CTV-to-PTV margins depends on the type of IG being used during radiation therapy, we created two sets of CTV-to-PTV margins. Margins computed according to the IG based on soft tissues (IG(ST)) were smaller for CTV1 than for CTV2: 8 mm (for CTV1) and 12 mm (for CTV2). A reverse relationship was applied for the IG based on the bones (IG(B)) where margins were 13 mm (for CTV1) and 7 mm (for CTV2). Fig. 1 shows an example of the CTV-to-PTV margin applied according to the IG(ST) protocol (cyan), and according to the IG(B) protocol (magenta) for the representative patient from the group analysed in this study.
Fig. 1.
Two sets of the PTV-to-CTV margin applied according to IG(ST) protocol – outline in a cyan colour, and according to IG(B) protocol - magenta colour. Size of the PTV-to-CTV margins established according with the study of Jodda et al 15.
Additionally, the following OARs were delineated: rectum, bladder, bowels and total BM. The rectum was contoured from the anus to the sigmoid flexure. The bowels were contoured from the L4-5 interspace to its lowest extent in the pelvis as an entire bag. The BM was contoured from the L4 vertebral body to the ischial tuberosities, including the pelvis, L4-5, and sacrum.
The treatment plans were prepared for Varian TrueBeam™ accelerator (Varian Medical Systems, Palo Alto, CA, USA) using Eclipse™ treatment planning system ver. 15.6 (Varian Medical Systems, Palo Alto, CA, USA). The analytic anisotropic algorithm with the spatial resolution of 2.5 mm was used for computing dose to the irradiated region. We prepared, for every patient, four X6 MV 2-arc VMAT treatment plans for two sets of margins calculated on the basis of two different schemes of IG-protocols (IG(ST) and IG(B)) and for two optimization scenarios: with and without optimization of the doses in the bone marrow (BM). External beam dose was 50.4 Gy delivered in 28 daily fractions.
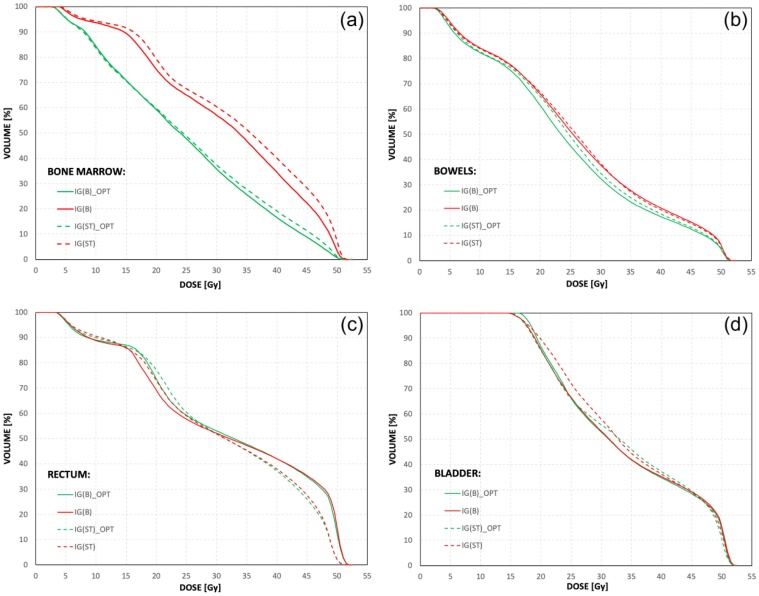
The plans were optimized using constraints similar to the RTOG 0418 trial, the volume of small bowel receiving >40 Gy was limited to <30%, <60% of the rectum was to receive >30 Gy, and <35% of the bladder was to receive >45 Gy.22 For plans with optimization of doses in BM, two-step optimization was used. Firstly, according to the study presented by Albuquerque et al.,23 we tried to reduce the volume of BM receiving doses >20 Gy to the volume of <80% of the whole pelvic BM and, secondly, we tried to reduce the dose in this structure as much as possible. For each plan, the ICRU-83 plan normalization criteria were followed, with prescription to the median dose on PTV.24 As a result, each prepared plan met the constraints used during the optimization process. The doses in PTV were clinically acceptable for each plan. Fig. 2 shows an example of dose-volume histograms for each OAR included in the analysis and for each plan prepared for the representative patient from the group analysed in this study.
Fig. 2.
The dose volume histograms for (a) bone marrow, (b) bowels, (c) rectum and (d) bladder computed for each prepared plan for the representative patient from the analysed group. The abbreviations mean respectively: IG(B)_OPT – VMAT plan with dose optimisation in the bone marrow, prepared for the PTV-to-CTV margins according to image guidance based on the bony anatomy; IG(B) - VMAT plan without dose optimisation in the bone marrow, prepared for the PTV-to-CTV margins according to image guidance based on the bony anatomy; IG(ST)_OPT - VMAT plan with dose optimisation in the bone marrow, prepared for the PTV-to-CTV margins according to image guidance based on the soft tissues; IG(ST) - VMAT plan without dose optimisation in the bone marrow, prepared for the PTV-to-CTV margins according to image guidance based on the soft tissues.
The dose distributions for every planning scenario were clinically similar in the bowels and bladder (e.g. Figs. 2b and 2d) and, were different in the BM and the rectum (e.g. Figs. 2a and 2c). The main stratificator for dose distribution in the BM was the scenario of optimization – better results were observed for plans where BM was used as an optimizing structure (IG(B)_OPT and IG(ST)_OPT). The impact of the size of the CTV-to-PTV margin on the doses in BM was negligible. For the rectum, better dose distribution was observed when the CTV-to-PTV margins based on IG(ST) protocol were used. The optimization scenarios have no effect on the stratification of the plans by the dose in the rectum. The complexity25, 26, 27 of the treatment plans was higher for the scenarios with BM optimization (IG(ST)_OPT and IG(B)_OPT). Table 1 shows three complexity indices gathered after plan acceptance, directly from the plan properties window and used in this study. These are: relative monitor units per arc (RMU [MU/Gy]), mean monitor units per control point (MMU [MU]) and mean dose rate per control point (MDR [MU/min]). The RMU was defined as the total monitor units (from all arcs) divided by the number of arcs and the prescribed fraction dose, the MMU was the sum of monitor units from control points divided by the number of control points, and the MDR was the sum of dose rates from control points divided by the number of control points. While the RMU was described by the mean value calculated from values of RMU for every patient, the MMU and MDR were characterised by two parameters, i.e. average value and variability. The average value was calculated from the mean values obtained for the patients from the analysed group. The variability has defined as the averaged value of the standard deviations obtained for these patients.
Table 1.
The indices used to describe complexity of the treatment plans.
| PLAN | Treatment Plan Complexity Indices |
||||
|---|---|---|---|---|---|
| Relative Monitor Units (RMU [MU/Gy]) | Mean Monitor Units (MMU [MU]) |
Mean Dose Rate (MDR [MU/min]) |
|||
| Mean | Average | Variability | Average | Variability | |
| IG(ST)_OPT | 170 | 0.804 | 0.253 | 312 | 83 |
| IG(ST) | 145 | 0.677 | 0.202 | 276 | 66 |
| IG(B)_OPT | 174 | 0.821 | 0.274 | 318 | 81 |
| IG(B) | 160 | 0.722 | 0.213 | 293 | 68 |
| Values fixed for dose optimization | <180 | Not set | Not set | 600 | Not set |
All prepared plans underwent pre-treatment dosimetrical verification. In particular, we compared the planned dose to the measured doses by using the ArcCHECK diode array detector and to the predicted doses by using the 3DVH software (both from: SunNuclear, Melbourne, FL, USA).
First method of dose comparison, using the ArcCHECK device, was based on the analysis of 2D gamma passing rates (GPR) at the diode level. The treatment plans were recalculated to the geometry of the ArcCHECK device, which is a cylindrical water-equivalent phantom with 1386 diode detectors arranged in a 3D helical fashion at 10 mm intervals within a cylinder with a diameter and length of 21 cm (the angles between the diodes are 5.45 degrees and each diode in the active detector is 0.8 × 0.8 mm2 and is positioned 2.9 cm below the surface).28 In the next step, the doses were delivered to the ArcCHECK according to the plan guidelines for its geometry. Then, the DICOM file of treatment plan (recalculated for ArcCHECK geometry) as well as the data measured by the ArcCHECK diodes were imported to SNC Patient v.8.2.0 (SunNuclear, Melbourne, FL, USA) software, and a dose grid corresponding to diode detector locations was extracted to compare the calculated and measured doses. As a result, the analysis of 2D GPR at the diode level (on the cylindrical plane) was possible (Fig. 3a).
Fig. 3.
Schematic view of the tasks workflow before the analysis of (a) 2D and (b) 3D gamma passing rates.
In contrast to the SNC Patient software, the 3DVH software allows the analysis of 3D GPR directly on patients' CT images for the entire irradiated volume. The data measured by the ArcCHECK diodes was imported into the 3DVH software along with the following four DICOM files from the treatment planning station: the treatment plan, CT images, structures, and calculated dose. The discrepancies between the planned dose and the measured planar dose were calculated. The calculated errors were back-projected into the original treatment plan. Through this process, a newly perturbed 3D dose distribution, which reflects any errors detected by the per-beam planar QA was obtained.29,30 In addition, interpolation was applied as the detector pitch between the ArcCHECK diodes was not as dense as those of the planned dose grids. Through this process, the 3DVH software calculated the 3D gamma indices for the entire plan as well as the DVH differences for each structure and the 3D gamma indices between the planned and perturbed matrices (Fig. 3b).29, 30, 31
The analyses of 2D and 3D GPR were performed in the global as well as the local mode and for different combinations of criteria of the dose differences (DD: 1%, 2% and 3%) and the distances-to-agreement (DTA: 1 mm, 2 mm and 3 mm).32,33 For global analysis, the per cent of the dose differences (DD) was normalised to the maximum planned dose and for local, was normalised to the planned dose in evaluated point. For both methods the threshold was 5% and was normalised to the maximum planned dose.34 The GPR value is the rate that satisfies the gamma criterion.28 The agreement between planned and measured doses was acceptable when the GPR was 95% or higher. The Mantel test was used to analyse similarity between the GPR matrices obtained from 2D and 3D analyses as well as GPRs matrices from the global and local mode. To check the similarity between GPRs matrices obtained for different planning scenarios, the Friedman test supported by multiple pairwise comparisons (Nemenyi's procedure) was used. Additionally, with the aid of 3DVH software, the differences between delivered and planned doses were calculated and visualised. All tests were performed at the 0.05 significance level.
4. Results
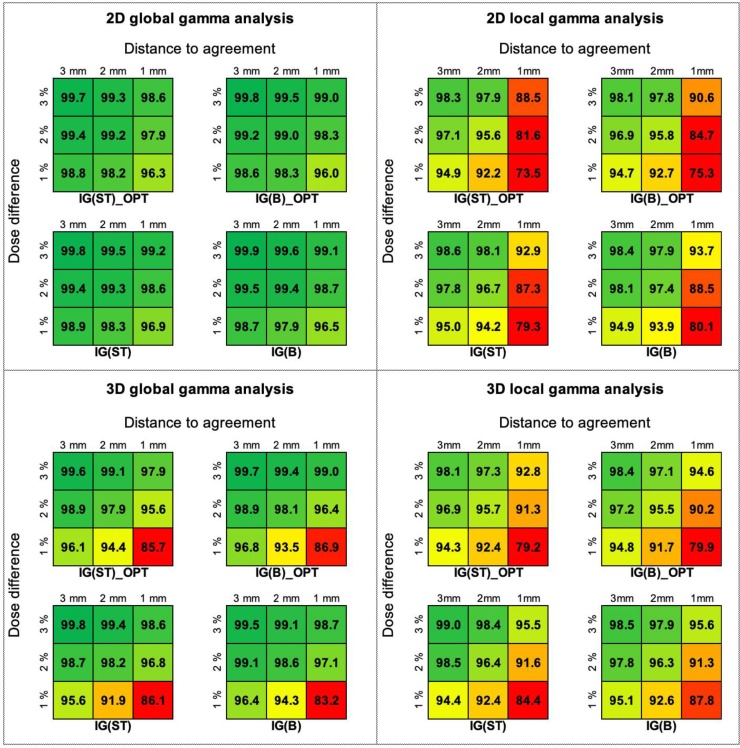
For each treatment plan used in this study, the 2D and 3D GPR analyses were performed in both the global and local regime. Fig. 4 shows the mean values of GPRs, from ten patients, obtained during the analyses performed on the cylindrical planes (the case of 2D analysis) and in the whole irradiated volume (the case of 3D analysis). The statistical analysis showed that the GPRs for different planning scenarios obtained in the global mode for 2D and 3D analysis were comparable (p = 0.120 for 2D analysis and p = 0.281 for 3D analysis). In the local mode, the GPRs for IG(ST)_OPT and IG(B)_OPT significantly differed from the GPRs for IG(ST) and IG(B) (p = 0.011 for 2D analysis and p = 0.026 for 3D analysis). Nevertheless, from the clinical point of view, all GPRs obtained from the 2D global analysis were acceptable (>95%). The results from the 3D global analysis were almost as good as for the 2D analysis. Only for the combinations of gamma criteria, such as the 1%/1 mm and 1%/2 mm, the GPRs were lower than 95%. The results of GPR analysis obtained for the local mode were worse than for the global mode. Nevertheless, the GPRs were acceptable for each combination of 3% and 2% of the DD and 3 mm and 2 mm of the DTA.
Fig. 4.
The results of general GPR analysis performed on the cylindrical planes (the case of 2D analysis) and in the whole irradiated volume (the case of 3D analysis) obtained from a global and local modes.
Despite the differences between the GPRs obtained from the 2D and 3D analysis, the corresponding matrices of GPRs characterised a high, statistically significant, correlation. There were no statistically significant correlations between the GPRs matrices obtained from the global and local mode. Table 2 shows the correlation coefficients (with the corresponding p-values) obtained as a result of comparisons of GPRs matrices received during the 2D and 3D analysis, for the global and local regimes.
Table 2.
The correlation coefficients (with the corresponding p-values) obtained as a result of comparisons of GPRs matrices received during the 2D and 3D analysis, for the global and local regimes. Mantel testing performed at 0.05 significance level.
| Correlation coefficient (p-values) |
||||
|---|---|---|---|---|
| 2D vs. 3D analysis |
Global vs. Local mode |
|||
| PLAN | Global mode | Local mode | 2D analysis | 3D analysis |
| IG(ST)_OPT | 0.850 (0.046) | 0.881 (0.035) | 0.774 (0.061) | 0.804 (0.076) |
| IG(ST) | 0.908 (0.024) | 0.835 (0.049) | 0.625 (0.201) | 0.731 (0.093) |
| IG(B)_OPT | 0.877 (0.010) | 0.875 (0.046) | 0.705 (0.121) | 0.763 (0.079) |
| IG(B) | 0.974 (0.021) | 0.915 (0.048) | 0.554 (0.289) | 0.739 (0.124) |
While the analysis of 2D GPRs provides general information about the overall compliance of planned and measured doses, the analysis of 3D GPRs, in addition to the above possibilities, allows to focus on the specified sub-volumes of the irradiated region (e.g. PTV and OARs).
Fig. 5 shows the results of analysis for PTV: (a) the differences between the volumes determined for the same level of the measured doses and the calculated doses and the 3D GPR analysis performed in (b) the global and (c) the local mode. The differences between volumes measured for the same level of the calculated and measured doses have got random nature. The highest mean differences gathered from averaged dose-volume histograms from calculated and measured plans were about 1.5% (Fig. 5a). Friedman testing supported by multiple pairwise comparisons (Nemenyi's procedure) show statistically significant differences between the scenarios of image guidance ( IG(ST) and IG(ST)_OPT vs. IG(B) and IG(B)_OPT) in the global mode (p = 0.033) as well as in the local mode (p = 0.020). Nevertheless, from the clinical point of view, the differences between matrices were insignificant because, for each matrix, the range of acceptable GPRs was similar and applied to the combinations of 3% and 2% of the DD and 3 mm, 2 mm and 1 mm of the DTA in the local and global mode.
Fig. 5.
The results of analysis for PTV: (a) the average differences in the volumes counted for the same level of the measured doses and the calculated doses and the 3D GPR analysis performed in (b) the global and (c) the local mode.
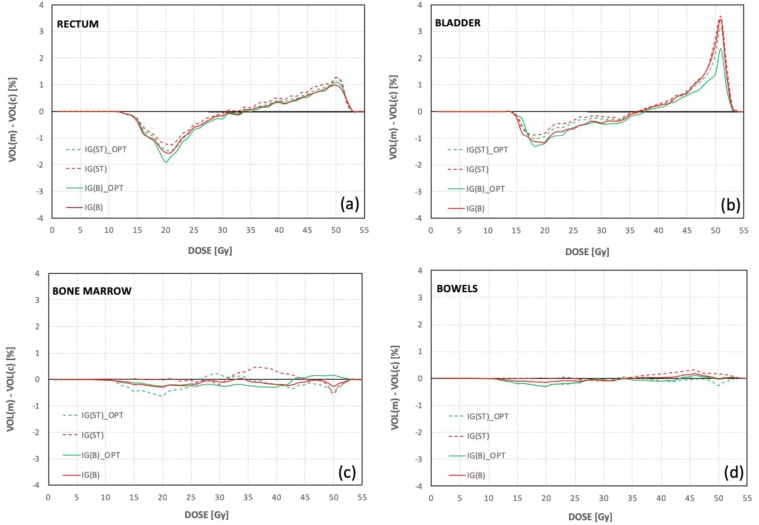
While for BM and the bowels the differences in the volumes calculated for the same level of measured and planned doses were small and constant, respectively, around -0.5 % and 0.5 % (Figs. 6c and 6d), the differences in the volumes for the rectum and bladder were characterised by greater variability (Figs. 6a and 6b). In general, for the level of low and intermediate doses (from 15 Gy to 35 Gy) the calculated doses cover bigger volumes of the bladder and rectum than measured doses and for the level of high doses (from 45 Gy to 55 Gy) the opposite relation was observed. Analysing the averaged data from the calculated and measured dose-volume histograms, the highest differences in volumes were about -2% and 1.1% for the rectum and about −1.3% and 3.5% for the bladder (Figs. 6c and 6d).
Fig. 6.
The average differences between the volumes counted for the same level of the measured doses and the calculated doses for (a) bone marrow, (b) bowels, (c) rectum and (d) bladder.
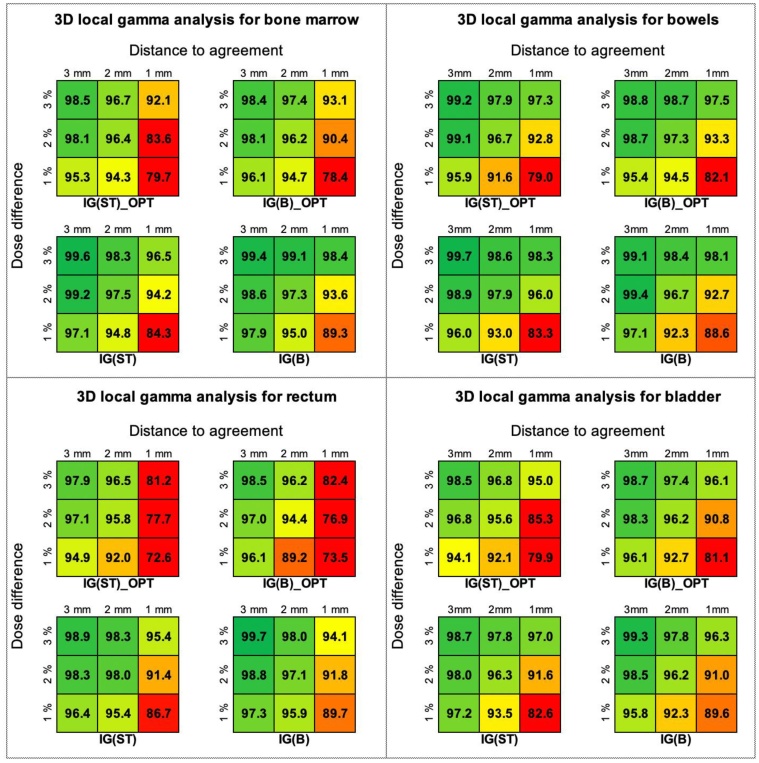
The results of the 3D GPR global analysis performed for BM, bowels, rectum and bladder are similar as for PTV, i.e. the range of acceptable GPRs was for the combinations of 3% and 2% of the DD and 3 mm, 2 mm and 1 mm of the DTA. Friedman testing performed for the 3D GPRs results obtained in the local mode showed statistically significant differences between scenarios differentiated by optimization in BM (IG(ST)_OPT and IG(B)_OPT vs. IG(ST) and IG(B)) for BM and rectum. The p-values were 0.015 for BM and 0.022 for the rectum. Different observation was noted for the bladder where IG(ST)_OPT was significantly different from other planning scenarios (p = 0.044). For the bowels, no statistically significant differences were observed (p = 0.081). From the clinical point of view, almost all planning scenarios successfully passed the gamma criterion for combinations of 3% and 2% of the DD and 3 mm and 2 mm of the DTA. The exception is the IG(B)_OPT, for which the analysis of GPRs in the rectum showed that the results for the combination of 2% of the DD and 2 mm of the DTA did not meet the criteria of acceptation. Fig. 7 shows the results of 3D gamma passing rates analysis performed in the local mode for the bone marrow, bowels, rectum and bladder.
Fig. 7.
The results of 3D gamma passing rates analysis performed in local mode for bone marrow, bowels, rectum and bladder.
5. Discussion
Our previous studies showed that VMAT is the best intensity-modulated technique intended for cervical cancer radiation therapy on conventional accelerators.11,15 While the planned dose in PTV is comparable for all VMAT plans, the dose in OARs depends on a specific scenario of VMAT plans preparation and delivery. Dose in BM is effectively reduced when BM is used as the optimization structure,11 and the doses in the rectum and bladder are better when the size of PTV-to-CTV margin is based on IG(ST) protocols.15 Therefore, in this study, we prepared four VMAT plans with differing in optimization strategies for ten randomly selected patient with cervical cancer. The dose distributions obtained in this study agree with our previous studies, where we set that the IG(ST)_OPT is the best solution for radiation therapy of cervical cancer (Fig. 2).35, 36, 37, 38 There are a lot of indices describing the treatment plan complexity.27 In this study, we use three of them - relative monitor units per arc (RMU [MU/Gy]), mean monitor units per control point (MMU [MU]), and mean dose rate per control point (MDR [MU/min]). The selection of these indices was dictated by the ease of their determination based on data available in Eclipse treatment planning system, and their being routinely used in our clinical practice. Determining other indices often requires in-house software or scripts that are currently not available in our centre.
The planning scenarios covering the optimization of the doses in BM (IG(ST)_OPT and IG(B)_OPT) were more complex than plans without BM optimization (IG(ST) and IG(B)) (Table 2). Therefore, a rational hypothesis was made that for these plans the accuracy between the dose calculations and its delivery can be worse than for IG(ST) and IG(B) plans. Moreover, taking into account the differences between geometrical conditions of the PTV in the IG(ST)_OPT and IG(B)_OPT (Fig. 1), we set an additional hypothesis that for the IG(ST)_OPT the potential uncertainty of dose delivery could be the highest for BM, while for IG(B)_OPT the uncertainty of dose delivery can be the highest in the rectum and bladder. It follows from the fact that the IG(ST)_OPT assumes dose optimization in BM when a larger PTV-to-CTV margin is added to the lymph nodes (CTV2) and not when PTV-to-CTV margin is based on IG(B). In turn, the PTV used for IG(B)_OPT covers bigger areas of the rectum and bladder than PTV in IG(ST)_OPT which makes optimization of the doses in these structures harder than in scenarios based on soft tissues (Fig. 1).
To check the impact of the plan complexity on its deliverability, the pre-treatment verification was performed for each prepared plan. The general GPR analyses performed on the cylindrical planes (the case of 2D analysis) and in the whole irradiated volume (the case of 3D analysis) showed that the results only for the local mode are statistically stratified by the usage the optimization of the BM during plan preparation. The IG(ST)_OPT and IG(B)_OPT plans are more complex than IG(ST) and IG(B) (Table 1), which makes them more difficult to deliver. Nevertheless, from the clinical point of view, all GPRs obtained from 2D and 3D analysis were always acceptable for each combination of 3% and 2% of the DD and 3 mm and 2 mm of the DTA (Fig. 4), which makes statistical considerations only theoretical. To check clinical importance of the plans complexity for their deliverability, a more in-depth analysis is needed of the GPRs results in the OARs and in the PTV. Despite the more frequent use of 2D analysis in hospitals, only 3D analysis (in this study through the usage of 3DVH software) allows to focus on the GPRs results related to the OARs and the PTV. Due to the different methodology and measurement workflow for 2D and 3D solutions (Fig. 3), the results obtained for these solutions are obviously different and cannot be mixed. The correlations found by us (Table 2) show only general relations between GPRs results from 2D and 3D analyses.
For the PTV we did not confirm the impact of the plan complexity on its deliverability. The statistical difference was observed only between the scenarios of image guidance (IG(ST) and IG(ST)_OPT vs. IG(B) and IG(B)_OPT). This difference was caused by the different shapes of PTVs for soft tissue and bone-based scenarios (Fig. 1) and was insignificant clinically (i.e. similar range of acceptable GPRs; Figs. 5b and 5c). The analyses performed for the OARs showed that in a local mode the IG(ST)_OPT and IG(B)_OPT significantly differ from IG(ST) and IG(B). For BM, the worst GPRs were obtained for IG(ST)_OPT. Nevertheless, the clinical acceptance for this plan was obtained for hard combinations of gamma acceptance criteria (GPR = 96.4 % for DD/DTA = 2%/2 mm) that confirm its satisfactory deliverability (Fig. 7). A similar finding was made for the bladder where GPR at the 2%/2 mm for IG(ST)_OPT was 95.6%. In turn, for the rectum, the worst results were observed for IG(B)_OPT, for which the combination of DD/DTA set as 2%/2 mm did not meet the criteria of acceptance; the GPR was 94.4 (Fig. 7). Given the above observations, the IG(ST)_OPT plan seems to be better than the IG(B)_OPT plan.
6. Conclusion
The clinically significant impact of the plans complexity on their deliverability during radiation therapy of cervical cancer is visible only for the gamma passing rates analysis performed in a local mode and directly in the organs trough the 3DVH software. While more general analyses show statistically significant differences, their clinical relevance was not confirmed.
The planning scenario including the bone marrow during optimization of the doses followed by the dose delivery management based on a soft tissues image guidance (IG(ST)_OPT) is one of the most complex plans of all analysed in this study. Nevertheless, the results of the gamma passing rates analysis confirm acceptance of plan deliverability when relatively hard gamma acceptance criteria are used during the analysis. Therefore, we recommend IG(ST)_OPT for radiation therapy of cervical cancer.
Conflict of interest
None.
Financial disclosure
None.
Acknowledgement
We would like to thank Astra Concept Ltd (Sun Nuclear regional distributor) for sharing their own version of the 3DVH software that was used in this study.
References
- 1.Mundt A.J., Meli L.K., Roeske J.C. Preliminary analysis of chronic gastrointestinal toxicity in gynecology patients treated with intensity-modulated whole pelvic radiation therapy. Int J Radiat Oncol Biol Phys. 2003;56:1354–1360. doi: 10.1016/s0360-3016(03)00325-0. [DOI] [PubMed] [Google Scholar]
- 2.Fernandez-Ots A., Crook J. The role of intensity modulated radiotherapy in gynecological radiotherapy: Present and future. Rep Pract Oncol Radiother. 2013;6:363–370. doi: 10.1016/j.rpor.2013.08.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Jodda A., Urbanski B., Piotrowski T., Roszak A., Malicki J. Zależność pomiędzy zastosowaniem technik napromieniania w radioterapii a uzyskanym rozkładem dawek w raku szyjki lub trzonu macicy ‒ przeglad literatury. Zesz Nauk Wco Lett Oncol Sci. 2013;10:88–92. [Google Scholar]
- 4.Souto-Del Bosque M.A., Cervantes-Bonilla M.A., Palacios-Saucedo G.C. Clinical and dosimetric factors associated with the development of hematologic toxicity in locally advanced cervical cancer treated with chemotherapy and 3D conformal radiotherapy. Rep Pract Oncol Radiother. 2018;23:392–397. doi: 10.1016/j.rpor.2018.07.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Cibula D., Pötter R., Planchamp F. The European Society of Gynaecological Oncology/European Society for Radiotherapy and Oncology/European Society of Pathology: Guidelines for the management of patients with cervical cancer. Int J Gynecol Cancer. 2018;28:641–655. doi: 10.1097/IGC.0000000000001216. [DOI] [PubMed] [Google Scholar]
- 6.Mell L.K., Tiryaki H., Ahn K.H., Mundt A.J., Roeske J.C., Aydogan B. Dosimetric comparison of bone marrow-sparing intensity-modulated radiotherapy versus conventional techniques for treatment of cervical cancer. Int J Radiat Oncol Biol Phys. 2008;71:1504–1510. doi: 10.1016/j.ijrobp.2008.04.046. [DOI] [PubMed] [Google Scholar]
- 7.Kim Y.B., Kim J.H., Jeong K.K., Seong J., Suh C.O., Kim G.E. Dosimetric comparisons of three-dimensional conformal radiotherapy, intensity-modulated radiotherapy, and helical tomotherapy in whole abdominopelvic radiotherapy for gynecologic malignancy. Technol Cancer Res Treat. 2009;8:369–377. doi: 10.1177/153303460900800507. [DOI] [PubMed] [Google Scholar]
- 8.Renard-Oldrini S., Brunaud C., Huger S. Dosimetric comparison between the intensity modulated radiotherapy with fixed field and Rapid Arc of cervix cancer. Cancer Radiother. 2012;16:209–214. doi: 10.1016/j.canrad.2012.02.002. [DOI] [PubMed] [Google Scholar]
- 9.Park J.M., Park S.Y., Kim J.I., Kang H.C., Choi C.H. A comparison of treatment plan quality between Tri-Co-60 intensity modulated radiation therapy and volumetric modulated arc therapy for cervical cancer. Phys Med. 2017;40:11–16. doi: 10.1016/j.ejmp.2017.06.018. [DOI] [PubMed] [Google Scholar]
- 10.Murakami N., Okamoto H., Kasamatsu T. A Dosimetric Analysis of Intensity-modulated Radiation Therapy with Bone Marrow Sparing for Cervical Cancer. Anticancer Res. 2014;34:5091–5098. [PubMed] [Google Scholar]
- 11.Jodda A., Urbański B., Piotrowski T., Malicki J. Relations between doses cumulated in bone marrow and dose delivery techniques during radiation therapy of cervical and endometrial cancer. Phys Med. 2017;36:54–59. doi: 10.1016/j.ejmp.2017.03.006. [DOI] [PubMed] [Google Scholar]
- 12.Ahamad A., D’Souza W., Salehpour M. Intensity modulated radiation therapy after hysterectomy: Comparison with conventional treatment and sensitivity of the normal-tissue-sparing effect to margin size. Int J Radidt Oncol Biol Phys. 2005;62:1117–1124. doi: 10.1016/j.ijrobp.2004.12.029. [DOI] [PubMed] [Google Scholar]
- 13.Ríos I., Vásquez I., Cuervo E., Garzón O., Burbano J. Problems and solutions in IGRT for cervical cancer. Rep Pract Oncol Radiother. 2018;23:517–527. doi: 10.1016/j.rpor.2018.05.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Jadon R., Pembroke C.A., Hanna C.L. A systematic review of organ motion and image-guided strategies in external beam radiotherapy for cervical cancer. Clin Oncol (R Coll Radiol) 2014;26:185–196. doi: 10.1016/j.clon.2013.11.031. [DOI] [PubMed] [Google Scholar]
- 15.Jodda A., Piotrowski T., Urbański B., Malicki J. Relations between dose cumulated in organs at risk and treatment based on different image-guidance strategies of cervical cancer. Phys Med. 2019;57:183–190. doi: 10.1016/j.ejmp.2019.01.008. [DOI] [PubMed] [Google Scholar]
- 16.Sun R., Mazeron R., Chargari C., Barillot I. CTV to PTV in cervical cancer: From static margins to adaptive radiotherapy. Cancer Radiother. 2016;20:622–628. doi: 10.1016/j.canrad.2016.07.088. [DOI] [PubMed] [Google Scholar]
- 17.Tan L.T., Tanderup K., Kirisits C., Leeuw A., Nout R. Image-guided adaptive radiotherapy in cervical cancer. Sem Rad Oncol. 2019;29:284–298. doi: 10.1016/j.semradonc.2019.02.010. [DOI] [PubMed] [Google Scholar]
- 18.Piotrowski T., Kaczmarek K., Bajon T., Ryczkowski A., Jodda A., Kaźmierska J. Evaluation of image-guidance strategies for prostate cancer. Technol Cancer Res Treat. 2014;13:583–591. doi: 10.7785/tcrtexpress.2013.600258. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Van der Merwe D., Van Dyk J. Accuracy requirements and uncertainties in radiotherapy: A report of the International Atomic Energy Agency. Acta Oncol (Madr) 2017;56:1–6. doi: 10.1080/0284186X.2016.1246801. [DOI] [PubMed] [Google Scholar]
- 20.Lim K., Small W., Portelance L. Consensus guidelines for delineation of clinical target volume for intensity-modulated pelvic radiotherapy for the definitive treatment of cervix cancer. Int J Radiat Oncol Biol Phys. 2011;79:348–355. doi: 10.1016/j.ijrobp.2009.10.075. [DOI] [PubMed] [Google Scholar]
- 21.Small W., Mell L.K., Anderson P. Consensus guidelines for the delineation of the clinical target volume for intensity modulated pelvic radiotherapy in the postoperative treatment of endometrial and cervical cancer. Int J Radiat Oncol Biol Phys. 2008;71:428–434. doi: 10.1016/j.ijrobp.2007.09.042. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Klopp A.H., Moughan J., Portelance L. Hematologic toxicity in RTOG 0418: A phase 2 study of postoperative IMRT for gynecologic cancer. Int J Radiat Oncol Biol Phys. 2013;86:83–90. doi: 10.1016/j.ijrobp.2013.01.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Albuquerque K., Giangreco D., Morrison C. Radiation-related predictors of hematologic toxicity after concurrent chemoradiation for cervical cancer and implications for bone marrow sparing pelvic IMRT. Int J Radiat Oncol Biol Phys. 2011;79:1043–1047. doi: 10.1016/j.ijrobp.2009.12.025. [DOI] [PubMed] [Google Scholar]
- 24.International Commission on Radiation Units and Measurements ICRU report 83: Prescribing, recording, and reporting photon beam intensity-modulated radiation therapy (IMRT) J ICRU. 2010;10(1) [Google Scholar]
- 25.Jurado-Bruggeman D., Victor Hernández V., Sáez J. Multi-centre audit of VMAT planning and pre-treatment verification. Radiother Oncol. 2017;124:302–310. doi: 10.1016/j.radonc.2017.05.019. [DOI] [PubMed] [Google Scholar]
- 26.Hernandez V., Saez J., Pasler M., Jurado-Bruggeman D., Jornet N. Comparison of complexity metrics for multi-institutional evaluations of treatment plans in radiotherapy. Phys Im Radiat Oncol. 2018;5:37–43. doi: 10.1016/j.phro.2018.02.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Glenn M.C., Hernandez V., Saez J. Treatment plan complexity does not predict IROC Houston anthropomorphic head and neck phantom performance. Phys Med Biol. 2019;63(20) doi: 10.1088/1361-6560/aae29e. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Song J.H., Shin H.J., Kay C.S., Son S.H. Dosimetric verification by using the ArcCHECK system and 3DVH software for various target sizes. PLoS One. 2015;10(3) doi: 10.1371/journal.pone.0119937. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Zhen H., Nelms B.E., Tome W.A. Moving from gamma passing rates to patient DVH-based QA metrics in pretreatment dose QA. Med Phys. 2011;38:5477–5489. doi: 10.1118/1.3633904. [DOI] [PubMed] [Google Scholar]
- 30.Nelms B.E., Zhen H., Tome W.A. Per-beam, planar IMRT QA passing rates do not predict clinically relevant patient dose errors. Med Phys. 2011;38:1037–1044. doi: 10.1118/1.3544657. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Bresciani S., Poli M., Miranti A. Comparison of two different EPID-based solutions performing pretreatment quality assurance: 2D portal dosimetry versus 3D forward projection method. Phys Med. 2018;52:65–71. doi: 10.1016/j.ejmp.2018.06.005. [DOI] [PubMed] [Google Scholar]
- 32.Low D.A., Harms W.B., Mutic S., Purdy J.A. A technique for the quantitative evaluation of dose distributions. Med Phys. 1998;25:656–661. doi: 10.1118/1.598248. [DOI] [PubMed] [Google Scholar]
- 33.Sunjic S., Ceberg C., Bokulic T. Statistical analysis of the gamma evaluation acceptance criteria: A simulation study of 2D dose distributions under error free conditions. Phys Med. 2018;52:42–47. doi: 10.1016/j.ejmp.2018.06.633. [DOI] [PubMed] [Google Scholar]
- 34.Depuydt T., Van Esch A., Huyskens D.P. A quantitative evaluation of IMRT dose distributions: Refinement and clinical assessment of the gamma evaluation. Radiother Oncol. 2002;62(3):309–319. doi: 10.1016/s0167-8140(01)00497-2. [DOI] [PubMed] [Google Scholar]
- 35.Crowe S.B., Kairn T., Kenny J. Treatment plan complexity metrics for predicting IMRT pre-treatment quality assurance results. Australas Phys Eng Sci Med. 2014;37(3):475–482. doi: 10.1007/s13246-014-0274-9. [DOI] [PubMed] [Google Scholar]
- 36.Park J.M., Park S., Kim H., Kim J.H., Carlson J., Ye S.J. Modulation indices for volumetric modulated arc therapy. Phys Med Biol. 2014;59(23):7315–7340. doi: 10.1088/0031-9155/59/23/7315. [DOI] [PubMed] [Google Scholar]
- 37.Masi L., Doro R., Favuzza V., Cipressi S., Livi L. Impact of plan parameters on the dosimetric accuracy of volumetric modulated arc therapy. Med Phys. 2013;40(7) doi: 10.1118/1.4810969. [DOI] [PubMed] [Google Scholar]
- 38.Du W., Cho S.H., Zhang X., Hoffman K.E., Kudchadker R.J. Quantification of beam complexity in intensity-modulated radiation therapy treatment plans. Med Phys. 2014;41(2):21716. doi: 10.1118/1.4861821. [DOI] [PubMed] [Google Scholar]