Abstract
Glial cell line-derived neurotrophic factor (GDNF) supports function and survival of dopamine neurons that degenerate in Parkinson’s disease (PD). Ectopic delivery of GDNF in clinical trials to treat PD is safe but lacks significant therapeutic effect. In pre-clinical models, ectopic GDNF is effective but causes adverse effects, including downregulation of tyrosine hydroxylase, only a transient boost in dopamine metabolism, aberrant neuronal sprouting, and hyperactivity. Hindering development of GDNF mimetic increased signaling via GDNF receptor RET by activating mutations results in cancer. Safe and effective mode of action must be defined first in animal models to develop successful GDNF-based therapies. Previously we showed that about a 2-fold increase in endogenous GDNF expression is safe and results in increased motor and dopaminergic function and protection in a PD model in young animals. Recently, similar results were reported using a novel Gdnf mRNA-targeting strategy. Next, it is important to establish the safety of a long-term increase in endogenous GDNF expression. We report behavioral, dopamine system, and cancer analysis of five cohorts of aged mice with a 2-fold increase in endogenous GDNF. We found a sustained increase in dopamine levels, improvement in motor learning, and no side effects or cancer. These results support the rationale for further development of endogenous GDNF-based treatments and GDNF mimetic.
Keywords: GDNF, safety evaluation, motor function, dopamine system, long-term side effects
Graphical Abstract
Ectopic GDNF delivery raises concerns about efficacy in clinical trials of Parkinson’s disease and safety issues in preclinical studies. Andressoo and colleagues showed that a long-term, 2-fold increase in endogenous GDNF levels in mice is safe and improves motor and dopaminergic system function, supporting further development of endogenous GDNF-based therapies.
Introduction
Glial cell line-derived neurotrophic factor (GDNF) protects and promotes the survival and function of midbrain dopaminergic neurons in cell culture1 and in animal models of Parkinson’s disease (PD).2 Intracranial ectopic delivery of GDNF has been tested in clinical trials for PD. Most of the studies conducted on PD patients reported an increased putamenal 18Fluoro-dopamine uptake, suggesting a neurotrophic effect.3, 4, 5, 6, 7, 8 However, of major concern is the lack of clinical benefit upon GDNF delivery into the brain.3, 4, 5, 6, 7, 8, 9, 10, 11 Results from the most recent double-blind, placebo-controlled phase II trial showed no significant improvements in PD patients where GDNF was delivered into the putamina once per month for 40 weeks compared with the placebo group.3 Notably, however, a post hoc analysis revealed that 9 (43%) patients who were treated with GDNF, but no placebo patients, demonstrated a significant motor improvement in the OFF state,3 providing hope for late-onset efficacy. The same patients who participated in the study also took part in a second open-label phase of the trial where all received monthly infusions of GDNF for another 40 weeks.4 Results showed significant improvements in both groups, again suggesting that GDNF may offer hopes for PD patients, although any conclusions derived from the second part of the trial must take into account that the trial was not placebo controlled.4 At the time of this writing, a trial of adeno-associated virus, serotype 2 (AAV2) vector delivering GDNF in the putamina of subjects with advanced PD is ongoing. However, although results from the phase I study reported no side effects, AAV2-GDNF delivery also had no effect on the progression of PD evaluated until 18 months post-infusion.5
Contrary to the clinical trials, in PD preclinical models, ectopically delivered GDNF is effective.2,12, 13, 14, 15 However, efficacy comes with side effects. Nigrostriatal delivery of ectopic GDNF results in a decrease in striatal and nigral levels of tyrosine hydroxylase (TH), the rate-limiting enzyme of catecholamine biosynthesis;16, 17, 18 aberrant arborization of striatal dopaminergic fibers toward the GDNF injection site due to non-physiological chemoattraction;18, 19, 20 hyperactivity;19,21, 22, 23 loss of body weight;24 and only a transient elevation in dopamine turnover for a few months upon viral delivery.16,19,23 All of these, alone or in combination, may emerge once efficacy in humans is achieved, and they are likely to present a clinical concern. Therefore, it is important to first establish a safe mode of action in animal models. This objective formed the first rationale of our study.
The second rationale of our study relates to the safety concern related to the development of GDNF mimetic that activates GDNF receptor RET (REarranged during Transfection).25 Activating mutations in RET result in early cancer syndrome named multiple endocrine neoplasia type 2 (MEN2) with tumors in thyroid, adrenal glands, and tongue,26,27 and GDNF overexpression in testes results in testicular cancer in mice.28 Systemic application of GDNF mimetic to treat PD for years may therefore result in cancer. This concern has substantially reduced enthusiasm in developing GDNF mimetic. Our second objective was to analyze the long-term outcome of elevated endogenous GDNF expression on tumor formation.
Previously, we generated a mouse model where a constitutive, about 2-fold elevation in endogenous GDNF levels was achieved by replacing the 3′ untranslated region (3′ UTR) of Gdnf with a 3′ UTR that is less responsive to inhibitory molecules such as microRNAs (GDNF hypermorphic mice, Gdnfwt/hyper).29 Because the 3′ UTR-mediated regulation occurs at the post-transcriptional level, increase of endogenous GDNF levels in Gdnfwt/hyper mice is limited to the cells that naturally transcribe Gdnf. We found that young 10-week-old Gdnfwt/hyper mice have improved motor coordination, enhanced dopamine system function, are protected in a chemically induced model of PD, and do not display adverse outcomes related to ectopic GDNF overexpression.29,30 Very recently, others used long non-coding RNAs to enhance endogenous GDNF protein translation and reached a similar conclusion in young animals.31 In order to proceed with endogenous GDNF-based drug development and GDNF mimetic research, a preclinical safety evaluation of long-term elevation of endogenous GDNF is important. Chronic elevation in endogenous GDNF may trigger side effects similar to ectopic GDNF during a longer time period, trigger new side effects such as neuropsychiatric conditions that relate to chronically elevated striatal dopamine,32, 33, 34, 35 or result in cancer. Here we report the results of analysis of five cohorts of aged Gdnfwt/hyper mice and wild-type gender-matched littermate controls for the dopamine system, multiple behavioral endpoints covering dopamine-related brain functions, and cancer formation.
Results
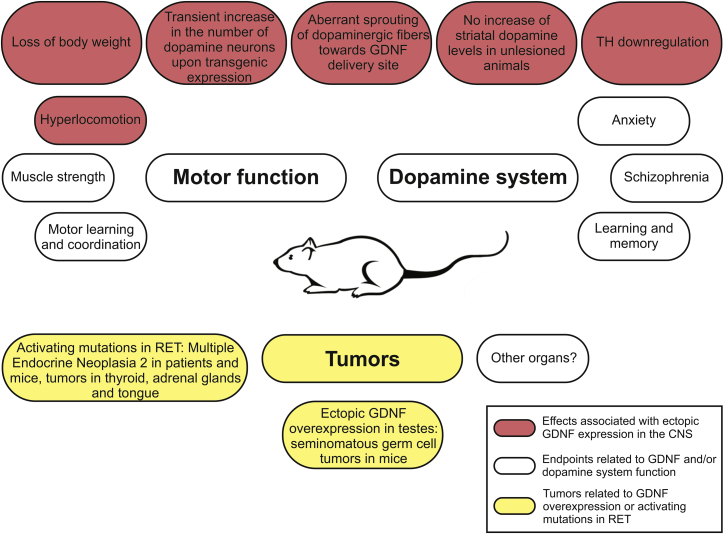
This study was designed to investigate the outcome of a long-term, about 2-fold increase in endogenous GDNF levels by analyzing aged Gdnfwt/hyper mice and wild-type littermate controls. We used a set of behavioral tests to evaluate motor function and other endpoints that associate with changes in GDNF levels and the brain dopamine system. At the endpoint of about 19 months of age, mice were analyzed for various brain dopamine system parameters and for tumor formation. An overview of the side effects and physiological functions that associate with the dopamine system and with GDNF/RET signaling analyzed in this study is depicted in Figure 1.
Figure 1.
An Overview of Side Effects and Physiological Functions That Associate with the Dopamine System Function and/or with GDNF/RET Signaling Analyzed in This Study
Analysis of Motor Function and Learning in Aged Gdnfwt/hyper Mice
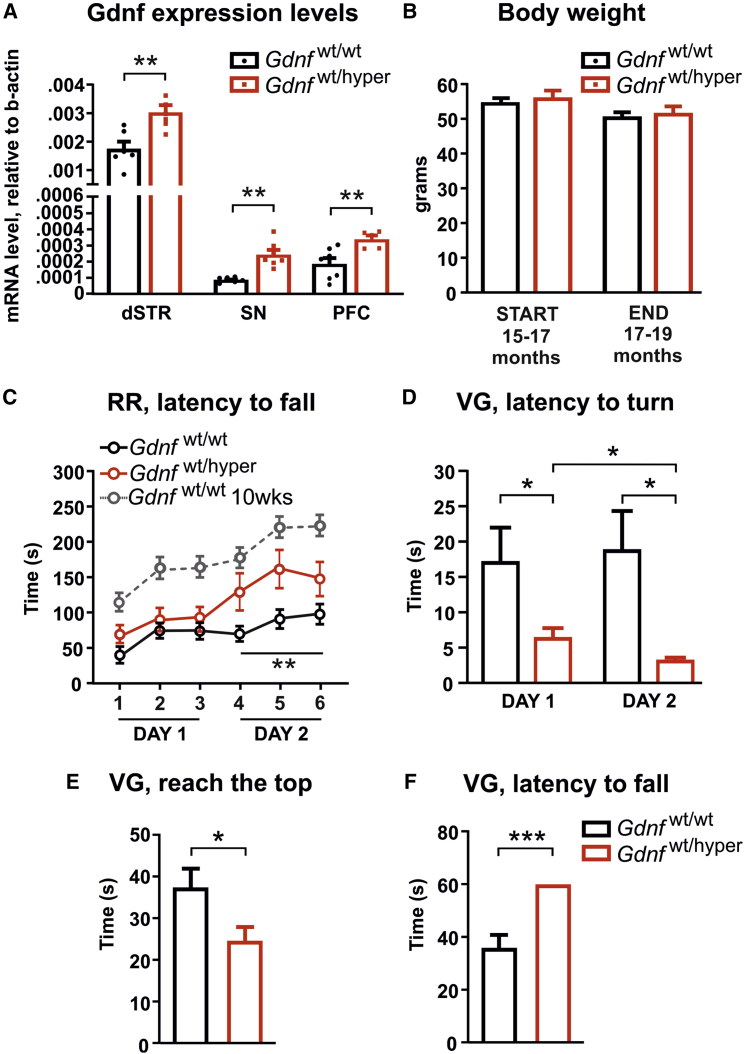
Behavioral analysis of Gdnfwt/hyper mice using gender-matched littermates as controls was initiated at 15–17 months of age and concluded by 17–19 months of age. Quantitative PCR (qPCR) analysis revealed a similar about 2-fold increase in Gdnf mRNA levels in old Gdnfwt/hyper mice, as reported previously in young Gdnfwt/hyper animals (Figure 2A).29 Ectopic GDNF overexpression in the nigrostriatal tract has been reported to cause a loss in body weight,24 which may influence outcome of motor tests. We observed no difference in body weight between Gdnfwt/wt and Gdnfwt/hyper mice (Figure 2B). First, old Gdnfwt/wt and Gdnfwt/hyper mice were analyzed with a variety of tests that evaluate motor coordination and learning under forced movement conditions. We performed an accelerating rotarod test, on 2 consecutive days, with three trials per day. The performance of Gdnfwt/hyper mice improved significantly on day 2 (Figure 2C). Notably, on day 2, aged Gdnfwt/hyper animals performed as well as untrained (day 1) young wild-type mice at 10 weeks of age in a comparable genetic background (Figure 2C, dotted line).30 Next, we performed the vertical grid test, which evaluates motor function and the righting-reflex. Time to turn upward, to climb to the upper edge, and to fall off the grid were measured with one trial per day for 2 consecutive days. We found that Gdnfwt/hyper animals turn faster on day 1 and have a faster learning curve compared with controls at day 2 (Figure 2D). This is consistent with the observed increase in motor learning in the rotarod experiment. In addition, Gdnfwt/hyper mice reached the upper edge of the grid faster (Figure 2E), and none of the Gdnfwt/hyper mice fell off the grid during the experiment (Figure 2F). Thus, constitutively increased endogenous GDNF levels do not cause changes in body weight but improve motor learning and the righting-reflex in 15- to 17-month-old mice.
Figure 2.
Increased Endogenous GDNF Level Improves Motor Learning in Old Gdnfwt/hyper Mice
(A) Levels of Gdnf mRNA in the dorsal striatum (dSTR), substantia nigra (SN), and prefrontal cortex (PFC) of Gdnfwt/wt and Gdnfwt/hyper mice quantified by quantitative PCR and normalized to Actb expression level. Welch’s t test: p = 0.0039 (dSTR), p = 0.0015 (SN), and p = 0.0060 (PFC). (B) Animal body weight before (START) and after (END) experiments. Welch’s t test: p = 0.561 (START) and p = 0.666 (END). (C) Latency to fall in accelerating rotarod test. ANOVA comparing 15- to 17-month-old Gdnfwt/wt and Gdnfwt/hyper animals (black and red lines, respectively) revealed significant genotype effect (day 1: p = 0.295, day 2: p = 0.009). ANOVA comparing the performance between days 1 and 2 within the same group showed significant improvement in Gdnfwt/hyper animals (p = 0.017), but not in wild-type littermates (p = 0.081). The gray dotted line represents the rotarod performance of Gdnfwt/hyper mice tested in Mätlik et al.30 at 10 weeks of age. (D) Latency to turn in the vertical grid test at days 1 and 2 of the experiment. Welch’s t test comparing Gdnfwt/wt and Gdnfwt/hyper mice at day 1 (p = 0.041) and day 2 (p = 0.01). Paired Student’s t test comparing the latency to turn of Gdnfwt/wt (p = 0.758) and Gdnfwt/hyper mice (p = 0.049) at day 1 versus day 2. (E) Latency to reach the top of the grid in the vertical grid test. Welch’s t test: p = 0.037. (F) Latency to fall off the grid in the vertical grid test. Welch’s t test: p < 0.001. (A) dSTR and SN: n = 6 Gdnfwt/wt and n = 7 Gdnfwt/hyper; PFC: n = 7 Gdnfwt/wt and n = 5 Gdnfwt/hyper. (B–F) n = 25 Gdnfwt/wt and n = 20 Gdnfwt/hyper. Data are presented as mean ± SEM. ∗p < 0.05, ∗∗p < 0.01, ∗∗∗p < 0.001. RR, rotarod; VG, vertical grid.
Analysis of Voluntary Motor Behavior and Muscle Strength in Aged Gdnfwt/hyper Mice
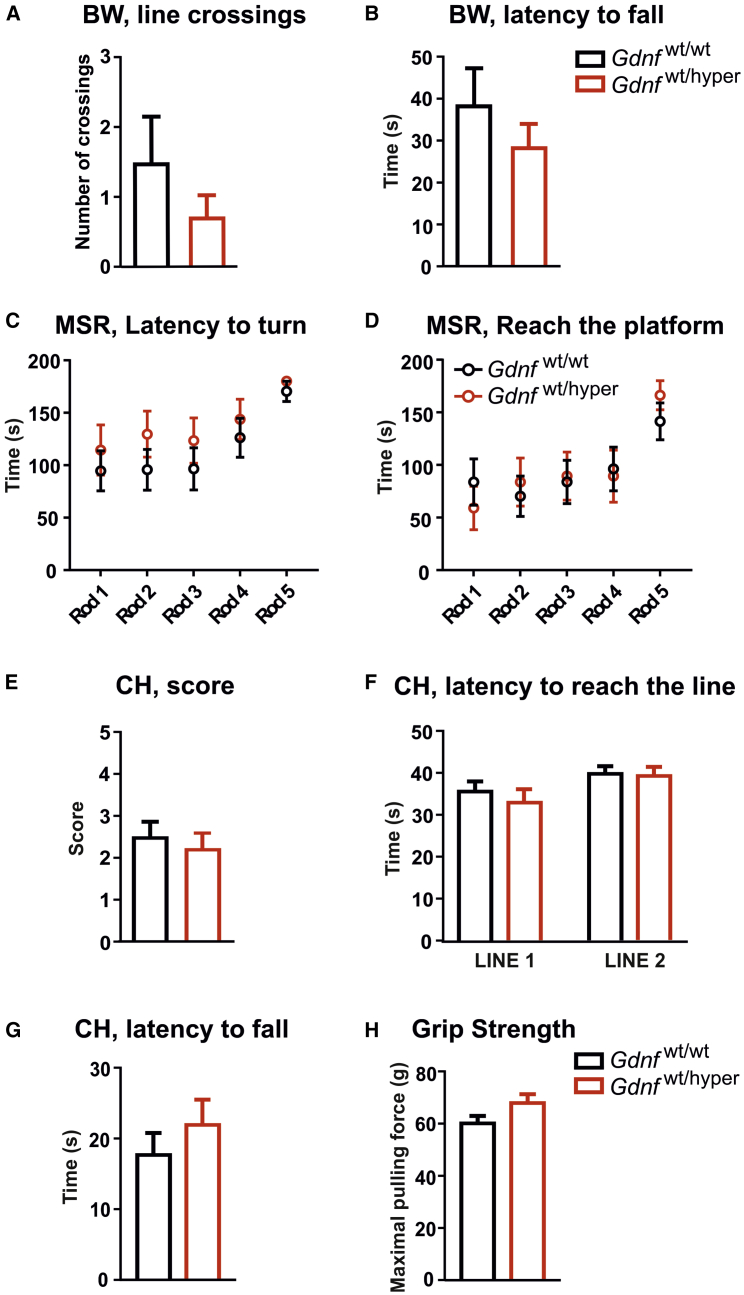
Next, we performed tests to investigate voluntary motor behavior of Gdnfwt/wt and Gdnfwt/hyper mice. First, we performed the beam walking test. No differences were recorded between Gdnfwt/wt and Gdnfwt/hyper mice (Figures 3A and 3B). We then investigated motor coordination and balance in the multiple static rods (MSRs) test, which consists of a series of five wooden rods of decreasing diameters. The latencies to turn to face the fixed end of the rod and then to travel to the supporting beam were recorded, and no differences were detected between genotypes (Figures 3C and 3D). Because muscle strength may influence the outcome of motor tests, we measured this parameter. In the coat hanger test, we observed no difference between genotypes in the coat hanger score, latency to reach the lines, and latency to fall (Figures 3E–3G; see Materials and Methods for details). Similar results were obtained in the forelimb grip strength test (Figure 3H). Our data suggest that motor balance under voluntary movement conditions and muscle strength in aged Gdnfwt/hyper mice are comparable with their wild-type littermates.
Figure 3.
Voluntary Motor Behavior and Muscular Strength Are Not Affected in Aged Gdnfwt/hyper Mice
(A) Number of crossed lines during beam walking test. Welch’s t test: p = 0.319. (B) Latency to fall from the beam in the beam walking test. Mann-Whitney U test: p = 0.721. (C) Latency to turn on each rod in the multiple static rods test. Multiple t test: rod 1 (p = 0.428), rod 2 (p = 0.652), rod 3 (p = 0.859), rod 4 (p = 0.833), and rod 5 (p = 0.304). (D) Time to reach the platform (escape and travel to the supporting beam) on each rod in the multiple static rods test. Multiple t test: rod 1 (p = 0.517), rod 2 (p = 0.26), rod 3 (p = 0.374), rod 4 (p = 0.526), and rod 5 (p = 0.404). (E) Score given in the coat hanger test. Welch’s t test: p = 0.652. (F) Latency to reach the lines in the coat hanger test. Welch’s t test: line 1 (end of horizontal part), p = 0.458; line 2 (diagonal part of the coat hanger), p = 0.823. (G) Latency to fall off in the coat hanger test. Mann-Whitney U test: p = 0.375. (H) Maximal forepaw pulling force measured in the grip strength test. Welch’s t test: p = 0.06. (A, B, and E–H) n = 25 Gdnfwt/wt and n = 20 Gdnfwt/hyper. (C and D) n = 18 Gdnfwt/wt and n = 13 Gdnfwt/hyper. Data are presented as mean ± SEM. BW, beam walking; CH, coat hanger; MSR, multiple static rod.
Analysis of the Dopamine System and Related Functions in Aged Gdnfwt/hyper Mice
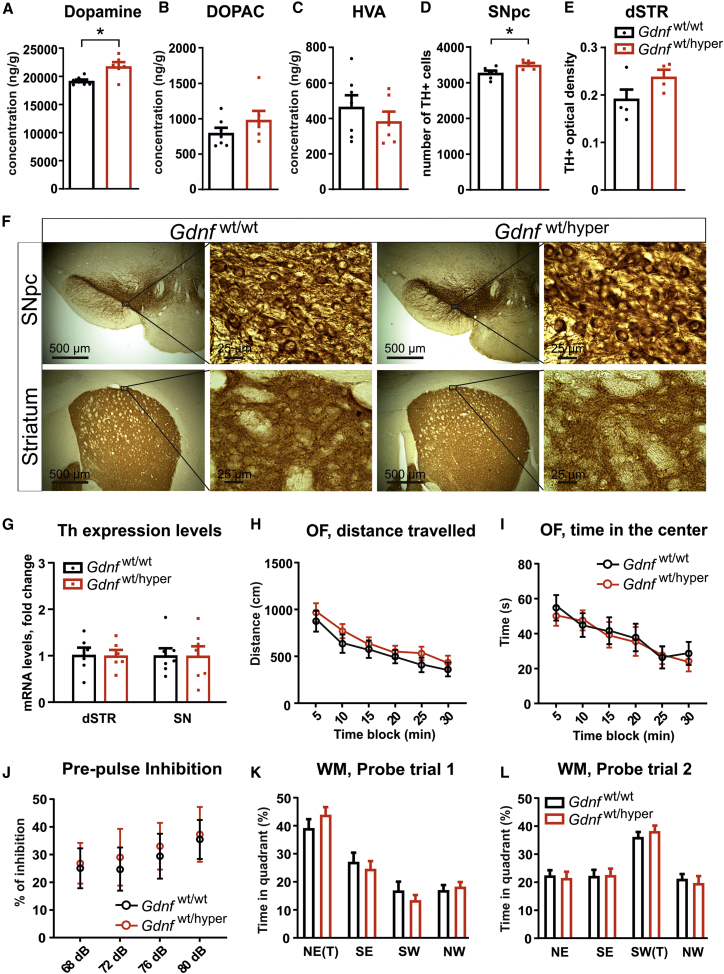
We measured total tissue dopamine levels in the striatum of Gdnfwt/wt and Gdnfwt/hyper animals using high-performance liquid chromatography (HPLC). We detected increased striatal dopamine levels in Gdnfwt/hyper mice (Figure 4A), whereas the levels of the main dopamine metabolites, 3,4-dihydroxyphenylacetic acid (DOPAC) and homovanillic acid (HVA), were unaltered (Figures 4B and 4C). Next, we counted the number of dopamine cells in the substantia nigra (SN) pars compacta (SNpc) and analyzed dopaminergic fibers in the dorsal striatum (dSTR) by TH immunostaining in Gdnfwt/wt and Gdnfwt/hyper mice (Figure 4F). We found a small but significant increase in the number of dopamine neurons in the SNpc of Gdnfwt/hyper animals (Figure 4D). The intensity and pattern of TH immunostaining in the dSTR did not significantly differ between the genotypes (Figure 4E). Th mRNA levels in the dSTR and SN did not differ between Gdnfwt/wt and Gdnfwt/hyper mice (Figure 4G). Our results indicate that about a 2-fold lifelong increase of endogenous GDNF increases striatal dopamine levels and the number of dopamine neurons in the SNpc without affecting the level of Th mRNA in the dSTR and SN, and without causing aberrant sprouting of TH-positive fibers.
Figure 4.
Sustained Increase in GDNF and Dopamine Cell Numbers and Striatal Levels in Old Gdnfwt/hyper Mice Does Not Cause Adverse Effects
(A–C) HPLC analysis of striatal (A) dopamine and its metabolites (B) DOPAC and (C) HVA. Welch’s t test: p = 0.016 (dopamine), p = 0.249 (DOPAC), and p = 0.359 (HVA). (D) TH-positive cell counts indicating the number of dopaminergic neurons in the SNpc of Gdnfwt/wt and Gdnfwt/hyper mice. Welch’s t test: p = 0.03. (E) Striatal optical density measurement of TH-positive fibers in Gdnfwt/wt and Gdnfwt/hyper mice. Welch’s t test: p = 0.095. (F) Representative coronal brain slices showing TH-immunoreactive cell bodies in the SNpc (upper panel, low and higher magnification) and TH-immunoreactive fibers in the striatum (lower panel, low and higher magnification of the dSTR) of Gdnfwt/wt and Gdnfwt/hyper mice. (G) Levels of Th mRNA in the dSTR and SN of Gdnfwt/wt and Gdnfwt/hyper mice quantified by quantitative PCR and normalized to the geometric mean of Actb, Gapdh, and Pgk1 expression levels. Welch’s t test: p = 0.646 (dSTR) and p = 0.898 (SNpc). (H) Total distance traveled in the open field test calculated in 5-min blocks. Multiple t test: 5 min (p = 0.546), 10 min (p = 0.300), 15 min (p = 0.635), 20 min (p = 0.593), 25 min (p = 0.261), and 30 min (p = 0.491). (I) Time spent in the center of the open field arena during the open field test calculated in 5-min blocks. Multiple t tests: 5 min (p = 0.632), 10 min (p = 0.767), 15 min (p = 0.834), 20 min (p = 0.846), 25 min (p = 0.875), and 30 min (p = 0.626). (J) Response to startle stimulus with different prepulse stimuli, relative to response to startle stimulus alone. Two-way repeated-measures ANOVA test, genotype effect p = 0.792. (K and L) Percentage of time spent in quadrants of the water maze during the probe (K) trial 1 and (L) trial 2. Welch’s t test, genotype effect probe 1: NE p = 0.268, SE p = 0.564, SW p = 0.356, NW p = 0.633; probe 2: NE p = 0.763, SE p = 0.925, SW p = 0.56, NW p = 0.635. “(T)” indicates the quadrant that contained the escape platform during training. (A–C) n = 7 Gdnfwt/wt and n = 6 Gdnfwt/hyper. (D) n = 6 Gdnfwt/wt and n = 5 Gdnfwt/hyper. (E) n = 5 Gdnfwt/wt and n = 4 Gdnfwt/hyper. (G) dSTR: n = 6 Gdnfwt/wt and n = 6 Gdnfwt/hyper; SN: n = 7 Gdnfwt/wt and n = 7 Gdnfwt/hyper. (H–L) n = 25 Gdnfwt/wt and n = 20 Gdnfwt/hyper. Data are presented as mean ± SEM. ∗p < 0.05, ∗∗p < 0.01. NE, northeast; NW, northwest; SE, southeast; SW southwest.
Nigrostriatal recombinant GDNF injection results in hyperlocomotion19,21, 22, 23 and genetic modifications, or drugs that increase dopamine and dopamine transmission cause hyperactivity36, 37, 38, 39 and induce anxiety.34,35 We did not observe differences in the distance traveled or time spent in the center of the arena in open field tests, suggesting a lack of hyperlocomotion and anxiety-like behaviors in Gdnfwt/hyper animals, respectively (Figures 4H and 4I). Enhanced striatal dopamine function is associated with schizophrenia.32,33 We measured sensorimotor gating using the prepulse inhibition (PPI) test, a parameter altered in schizophrenic patients and mice with a schizophrenia-like phenotype,40,41 and found no difference between genotypes (Figure 4J). Ectopic overexpression of GDNF in the hippocampus of aged rats improves cognitive function,42 and reduced levels of endogenous GDNF in Gdnf knockout (KO) heterozygous mice affect learning performance in the Morris water maze (MWM) test.43 Analysis of animals using the MWM test revealed no difference in spatial learning and memory between genotypes (Figures 4K and 4L). Thus, despite the sustained increase in striatal dopamine levels, an about 2-fold constitutive elevation of endogenous GDNF does not result in enhanced spontaneous locomotion or anxiety, impaired sensorimotor gating, or alteration in spatial learning or memory in aged Gdnfwt/hyper mice.
Analysis of Cancer in Aged Gdnfwt/hyper Mice
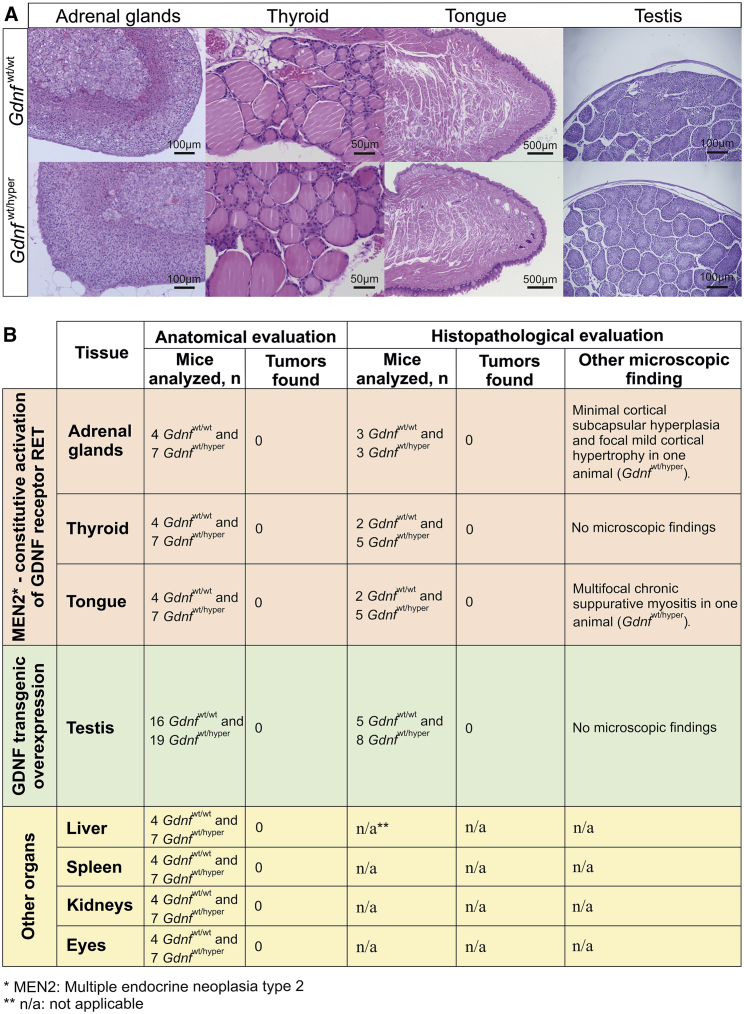
Overexpression of GDNF in the testes causes seminomatous germ cell tumors in 100% of mice by 12 months of age.28 Mutations resulting in constitutive activation of GDNF receptor RET convert the RET gene into an oncogene.44 The resulting condition, MEN2B, triggers tumors in endocrine organs, most prominently in the thyroid (C-cell hyperplasia) and adrenal glands (chromaffin cells hyperplasia), as well as in the tongue.27 In a mouse model genocopying the MEN2B Met918Thr mutation, tumors appear between 2–5 and 8–12 months of age in MEN2B homozygous and heterozygous mice, respectively.26 Anatomical and histopathological evaluation of the adrenal glands, thyroid, tongue, and testes of 17- to 19-month-old Gdnfwt/wt and Gdnfwt/hyper mice revealed no cancer, neoplasia, or abnormal cell masses (Figures 5A and 5B). Anatomical evaluation of other organs, including liver, spleen, kidneys, and eyes, detected no tumors (Figure 5B). The observed histological alterations were sporadic and characteristic of old age (Figure 5B).
Figure 5.
Gdnfwt/hyper Mice Do Not Develop Multiple Endocrine Neoplasia or Testicular Tumors
(A) Representative 4-μm-thick slices from adrenal glands, thyroid, tongue, and testes. All slides were stained with hematoxylin and eosin. Scale bars are indicated in each image. (B) Main findings from anatomical and histopathological evaluation of different organs in 17- to 19-month-old Gdnfwt/wt and Gdnfwt/hyper mice.
Discussion
Previously we reported that about a 2-fold elevation of endogenous GDNF levels increases motor coordination and brain dopamine function without side effects in young mice.29,30 This conclusion was recently confirmed by others,31 suggesting that means to elevate endogenous GDNF expression may constitute a safe and efficient treatment route for PD. One possible way to stimulate GDNF signaling is GDNF mimetic, i.e., small-molecule GDNF receptor RET agonists that penetrate the blood-brain barrier (BBB) and can be delivered systemically. However, constitutively activating mutations in RET result in MEN,26,27 posing a serious safety concern for potential future chronic application of GDNF mimetic. Before making a substantial investment into developing GDNF mimetic, it would therefore be important to analyze the long-term outcome of a chronic increase in endogenous GDNF. If, similar to mutations in RET with consequent constitutive activation of RET signaling, a chronic increase in GDNF results in MEN, investment into GDNF mimetic would hardly be rational. Likewise, another critical organ are the testes, where ectopic GDNF overexpression results in cancer in mice.28 Very recently, hematopoietic stem cell transplantation-based macrophage-mediated GDNF delivery was shown to be effective and safe in a mouse model of PD.45 Chen et al.45 reported that about 5 months after GDNF delivery, no tumors were found, although a need for long-term evaluation with a focus on MEN2 organs was noted by the authors.45 Increased endogenous GDNF may also result in new side effects that manifest only upon chronic increase in endogenous GDNF expression upon aging. We hypothesized that upon aging some adverse effects reported for ectopic GDNF will possibly appear in Gdnfwt/hyper mice, and that we will likely detect new side effects or MEN2-like neoplastic changes or cancer. To our surprise, we found no adverse effects. Instead, aged Gdnfwt/hyper mice showed increased motor learning and performance in accelerating rotarod and vertical grid tests. After 1 day of training, the performance level of aged Gdnfwt/hyper mice was comparable with that of young 10-week-old wild-type mice. How a constitutive increase in endogenous GDNF expression increases motor learning and function until high age remains currently unknown, but is an interesting subject of future inquiry. Endogenous GDNF is expressed in several central nervous system (CNS) regions and cell types that regulate motor function, including striatum,29,46 motor cortex, cerebellum, and spinal cord.47, 48, 49, 50 Future investigation, including generation and analysis of conditional GDNF hypermorphic mice, may reveal in which of the above-mentioned CNS regions and how an elevation of endogenous GDNF is responsible for increased motor learning and function in aged animals.
We also found that aged Gdnfwt/hyper mice display sustained elevation in striatal tissue dopamine levels and a sustained increase in the number of dopamine cells in the SNpc with no evidence for Th mRNA downregulation or aberrant sprouting of TH-positive dopamine fibers in the striatum. We noticed variability in Th mRNA expression levels, especially in the SN. However, analysis of striatal dopamine level and number of TH-positive cells in the SN followed by measurement of striatal TH-positive fibers suggest that TH protein function and level remain in the physiological range despite the observed alterations at the mRNA level. A small but non-significant trend for an increase in TH-positive fibers in Gdnfwt/hyper animals likely reflects a small but significant increase in dopamine cell numbers in the SN. We also found no decrease in body mass or hyperlocomotion in Gdnfwt/hyper mice. Because dopamine neurons are the main RET-expressing neurons in the CNS, and dopamine neurons in mice lacking RET do not respond to GDNF in vitro and in vivo,51,52 we hypothesize that GDNF mediates those effects via RET receptor as opposed to two other reported receptors for GDNF in the CNS: neural cell adhesion molecule (NCAM) and Syndecan-3.53,54 Unfortunately, quantitative analysis of RET phosphorylation in vivo in the brain is very challenging, and many laboratories, including ours, have failed to generate reproducible results, leaving quantitation of RET activation in Gdnfwt/hyper animals a future challenge.
The effects of long-term ectopic and endogenous GDNF elevation are summarized in Table 1. Our previous29,30 and current results suggest that constitutive about 2-fold elevation of endogenous GDNF is safe and enhances dopamine system function, improves motor performance, and may overcome the adverse effects associated with ectopic overexpression. Our results encourage future studies on increased endogenous GDNF using various means,29, 30, 31,55 support further development of recently created GDNF mimetic,25,56 and encourage other means of moderate GDNF application including macrophage-mediated GDNF delivery.45
Table 1.
Main Outcomes of Elevated Endogenous GDNF in Aged Gdnfwt/hyper Mice
| Phenotypes Reported in Previous Studies | Method | Reference | Long-Term Elevation of Endogenous GDNF (This Study) |
|---|---|---|---|
| Increase in dopamine turnover, but no changes in striatal dopamine tissue level | striatal or nigral recombinant lentiviral vector delivery of GDNF in unlesioned animals | 16 | elevated and sustained striatal dopamine levels |
| Downregulation of TH levels in the STR and SN | striatal recombinant lentiviral vector delivery of GDNF | 16, 17, 18 | no changes in TH levels in the STR and SN |
| Striatal dopaminergic fibers sprouting toward GDNF injection site | nigrostriatal recombinant GDNF injection and viral gene delivery | 18, 19, 20 | no increase of striatal TH-positive dopaminergic fibers density |
| Hyperactivity | nigrostriatal recombinant GDNF injection | 19,21, 22, 23 | no difference in spontaneous locomotor activity |
| Loss in body weight | nigrostriatal virus-mediated GDNF delivery | 24 | no change in body weight |
| Tumors in testes in all mice used by 12 months of age | transgenic overexpression of GDNF in undifferentiated spermatogonia | 28 | no tumors found in testes |
| Thyroid and adrenal neoplasia | GDNF receptor RET constitutive activation due to point mutation (MEN2B mice) | 26 | no tumors found in thyroid and adrenal glands |
Materials and Methods
Animals
Mice were 15–17 months old when the behavioral experiments started and 17–19 months old by the end of the experiments. Because the oestrus cycle is believed to enhance experimental variation and because of the considerable costs of aging studies, we analyzed only male mice. However, in our previous study,30 we observed improved motor coordination in young Gdnfwt/hyper male mice and a trend for improved motor coordination in young female mice.30 In the future it would be of great interest to analyze female mice for the same parameters. Wild-type littermates were used as controls in all experiments. Animals were maintained in 129Ola/ICR/C57bl6 mixed genetic background and maintained at temperature-controlled conditions at 20°C–22°C under a 12-h/12-h light/dark cycle at relative humidity of 50%–60%. Cages and bedding material (aspen chips; Tapvei Oy, Finland) were changed every week, and wooden tube and aspen shavings were provided as enrichment. Mice received food and water ad libitum. Altogether, five cohorts of mice were used in this study for a total of 80 animals (41 Gdnfwt/wt and 39 Gdnfwt/hyper mice). The experiments performed in each cohort are as follows: cohort 1 (7 Gdnfwt/wt + 7 Gdnfwt/hyper), all of the behavioral tests listed below (except MSRs), HPLC, TH immunohistochemistry, and stereological analysis of TH-positive cells in the SNpc; cohort 2 (6 Gdnf wt/wt + 4 Gdnf wt/hyper), all of the behavioral tests listed below and the striatal optical density (OD) measurement of TH-positive fibers; cohort 3 (12 Gdnfwt/wt + 9 Gdnfwt/hyper), all of the behavioral tests listed below and gene expression analyses via qPCR (7 Gdnfwt/wt + 7 Gdnfwt/hyper); cohort 4 (12 Gdnfwt/wt + 12 Gdnfwt/hyper), post mortem anatomical and histopathological evaluation of the testes; and cohort 5 (4 Gdnfwt/wt + 7 Gdnfwt/hyper), post mortem anatomical analysis of liver, spleen, kidneys, and eyes and histopathologic evaluation of adrenal glands, thyroid, tongue, and testes.
All experiments were conducted following the 3R principles of the European Union (EU) directive 2010/63/EU governing the care and use of experimental animals, and were approved by the County Administrative Board of Southern Finland (license numbers ESAVI-2010-09011/Ym-23 and ESAVI/11198/04.10.07/2014). The protocols were authorized by the national Animal Experiment Board of Finland.
Behavioral Tests
Behavioral tests, including rotarod, vertical grid, beam walking, coat hanger, grip strength, open field, and PPI, were performed as described previously by Mätlik et al.30
MSRs
The MSRs test was performed to investigate motor coordination and balance. The MSRs are a series of five 60-cm-long wooden rods of decreasing diameters (rod 1: 27 mm; rod 2: 21 mm; rod 3: 15 mm; rod 4: 11 mm; and rod 5: 8 mm), each perpendicularly screwed at one end to a supporting beam. The apparatus was elevated 60 cm above a soft surface. The animal was placed 2 cm from the distal end of the rod, facing away from the supporting beam. The latencies to turn to face the fixed end of the rod and then to travel to the supporting beam were recorded in a period of a maximum of 3 min.
MWM
The MWM was used to evaluate spatial learning and memory. The system consisted of a black circular swimming pool (Ø 120 cm) and an escape platform (Ø 10 cm) submerged 0.5 cm under the water surface in the center of one of four imaginary quadrants. The animals were released to swim in random positions facing the wall, and the time to reach the escape platform (maximum time 60 s) and the swimming distance were measured in every trial. In addition, thigmotaxis, the time spent swimming within the outermost ring of the pool (10 cm from the wall), was measured. Two training blocks consisting of three trials each were conducted daily. The interval between trials was 4–5 min and between training blocks about 5 h. The hidden platform remained in a constant location for 3 days (six initial training sessions) and was thereafter moved to the opposite quadrant for 2 days (four reverse training sessions). Probe trials were conducted approximately 18 h after the last initial and reverse training sessions. Mice were allowed to swim in the maze for 60 s without the platform available. Spatial memory in the probe trials was estimated by preference of swimming in the trained region (imaginary circular area of Ø 30 cm, around the previous platform location) over swimming in corresponding regions in the three other quadrants. After the second probe trial, the mice were tested for one block of three trials, with the platform made visible in the quadrant not employed previously.
Tissue Isolation
After deep anesthesia with CO2, mice were euthanized by cervical dislocation followed by decapitation. The brain was quickly removed from the skull, immersed in ice-cold saline, and placed in an ice-cooled brain block (Stoelting, Wood Dale, IL, USA). Brain regions of interest were collected as described by Kumar et al.29 using a puncher (inner diameter, 2 mm), snap frozen, and stored at −80°C until processed.
RNA Isolation and qPCR
Total RNA was isolated from frozen tissues using Trizol Reagent (Thermo Fisher Scientific) according to the manufacturer’s protocol, and RNA quantity and quality (absorbance 260/280 nm > 1.8) were assessed using a NanoDrop (NanoDrop Technologies, Wilmington, DE, USA). A total of 200 ng DNase I (Thermo Fisher Scientific, MA, USA)-treated total RNA was reverse transcribed to complementary DNA using random hexamer primers and RevertAid Reverse Transcriptase (Thermo Fisher Scientific, MA, USA). Complementary DNA was diluted 1:10 and stored at −20°C until analysis. qPCR was performed with BioRad C1000 Touch Thermal Cycler upgraded to CFX384 System (Bio-Rad), supplied with SYBR Green I Master (Roche) and 250 pmol primers, in 10 μL total volume in 384-well plates. The following primer pairs were used: Gdnf (forward [F]: 5′-CGCTGACCAGTGACTCCAATATGC-3′, reverse [R]: 5′-TGCCGCTTGTTTATCTGGTGACC-3′), Th (F: 5′-CCCAAGGGCTTCAGAAGAG-3′, R: 5′-GGGCATCCTCGATGAGACT-3′), Actb (F: 5′-CTGTCGAGTCGCGTCCA-3′, R: 5′-ACGATGGAGGGGAATACAGC-3′), Gapdh (F: 5′-CCTCGTCCCGTAGACAAAA-3′, R: 5′-ATGAAGGGGTCGTTGATGGC-3′), and Pgk1 (F: 5′-TTGGACAAGCTGGACGTGAA-3′, R: 5′-AACGGACTTGGCTCCATTGT-3′). Each sample was run in triplicate. Expression level of Gdnf was normalized to Actb housekeeping gene expression level, and expression level of Th was normalized to Actb and to the geometric mean of Actb, Gapdh, and Pgk1 housekeeping genes expression level. Both reference systems for Th revealed a similar result, and data using the geometric mean of Actb, Gapdh, and Pgk1 as a reference are presented in Figure 4G. Results for a biological repeat were discarded when the Cq value for one or more of the replicates was 40 or 0, or when the Cq difference between replicates was >1.
HPLC
Monoamines and their respective metabolites were analyzed from the dissected brain samples as described previously57 using HPLC with electrochemical detection. The values are presented as nanograms per gram of wet tissue weight.
TH Immunohistochemistry
For striatal OD measurement of TH-positive fibers (cohort 2), mice were anesthetized with sodium pentobarbital (100 mg/kg intraperitoneally [i.p.]) and intracardially perfused with PBS followed by 4% paraformaldehyde (PFA) in 0.1 M phosphate buffer (pH 7.4). The brains were then fixed in 4% PFA overnight and stored in phosphate buffer containing 20% sucrose at 4°C. For striatal dopamine level measurement and TH immunohistochemistry (cohort 1), an alternative “light” perfusion method was used. In brief, the brains were cooled after PBS perfusion, and dSTR was dissected from the rostral part of the brain for HPLC analysis, whereas the posterior part containing the midbrain was fixed overnight in 4% PFA for stereological analysis of TH-positive cells in the SNpc. Coronal striatal (30 μm) and nigral (40 μm) serial sections were cut using a freezing microtome and stored at −20°C until processed for TH immunostaining. Staining of freely floating brain sections was performed using standard immunohistochemical procedures, and the following antibodies were used: rabbit anti-TH (1:2,000; AB 152; Millipore) and biotinylated goat anti-rabbit (1:200; BA1000; Vector Laboratories). Vectastain Elite ABC peroxidase kit (Vector Laboratories) was used for visualization. Further details are provided by Mijatovic et al.58
Stereological Analysis of TH-Positive Cells
The number of TH-positive neurons in the SNpc was assessed by a person blinded to the identity of the samples, as described by Kumar et al.29 In brief, cells positive for TH were counted at the medial region of the SNpc, around the medial terminal nucleus. From each animal, every third section between −3.08 and −3.28 mm caudally from bregma was selected (three sections per animal). StereoInvestigator (MBF Bioscience) was used to outline the SNpc, and positively stained cells were counted within the defined outlines according to the optical dissector rules. Cells were counted at regular predetermined intervals (x = 100 μm; y = 80 μm) within the counting frame (60 μm × 60 μm) superimposed on the image using a 60 × oil objective (Olympus BX51 [Olympus Optical] equipped with an Optronics camera). The counting frame positions within the SNpc were randomized by the software. The coefficient of error (CE) was calculated as an estimate of precision, and values <0.1 were accepted. Failure in staining or perfusion resulting in spoiled sections was an exclusion criterion.
Striatal OD Measurement
A person who was blinded to the identity of the genotypes performed the OD analysis of the striata of Gdnfwt/wt and Gdnfwt/hyper mice. Images were generated using 3DHISTECH Pannoramic 250 FLASH II digital slide scanner at Genome Biology Unit supported by HiLIFE and the Faculty of Medicine, University of Helsinki and Biocenter Finland. Measurements of striatal TH-positive fibers were performed using Fiji Is Just ImageJ (ImageJ, Version 1.52).59 Image calibration was done by using a calibrated OD step tablet containing 21 steps with a density range of 0.05–3.05 OD. Analysis was done from five to six striatal sections from each animal, and the final reading was calculated as an average. Dorsal and ventral striatum were analyzed separately, and the nonspecific background correction in each section was done by subtracting the OD value of the corpus callosum from the striatal OD value of the same section. Failure in staining or perfusion resulting in spoiled sections was an exclusion criterion.
Histopathology
Mice were autopsied at 17–19 months of age. Anatomical evaluation of mice upon autopsy and histopathological assessment of tissues associated with GDNF/RET signaling-induced cancer (thyroid, adrenal glands, tongue, and testes) and four control organs selected at random for detailed examination (liver, spleen, eyes, and kidneys) were performed by a professional pathologist at the Finnish Centre for Laboratory Animal Pathology (FCLAP), Faculty of Veterinary Medicine, University of Helsinki. The tissue samples were fixed in phosphate-buffered 4% PFA or 4% formaldehyde and routinely processed into histological slides: dehydrated, embedded in paraffin, and sectioned at 4 μm thickness. All slides were stained with hematoxylin and eosin. Microscopic findings were classified with standard pathological nomenclature, and severities of findings were graded as minimal, mild, moderate, marked, or severe. Grades of severity for microscopic findings are subjective. No tumors were found in the samples examined.
Statistical Analysis
Comparisons between two groups were analyzed with a Welch’s t test or Mann-Whitney U test. Comparisons between the same subjects were performed with a paired Student’s t test. Analysis of rotarod test and PPI test was performed with a two-way analysis of variance (ANOVA). Assessments with p < 0.05 were considered significant.
Author Contributions
G.T. performed qPCR experiments, measured striatal OD, dissected mice for histopathological evaluation, and prepared the figures. J.K. dissected the brain tissues and performed stereology and HPLC. V.V., C.V., and N.K. performed behavioral experiments. G.T., J.K., V.V., T.P.P., and J.-O.A. analyzed and interpreted the data. J.-O.A. designed the experiments and provided funding. G.T. and J.-O.A. wrote the manuscript. All authors reviewed and approved the manuscript.
Conflicts of Interest
The authors declare no competing interests.
Acknowledgments
G.T. was supported by the Finnish Parkinson Foundation. J.-O.A. was supported by the Academy of Finland (grant no. 297727); Sigrid Jusélius Foundation; Faculty of Medicine at the University of Helsinki; Helsinki Institute of Life Science (HiLIFE) Fellow grant; European Research Council (ERC) (grant no. 724922); and Alzheimerfonden. The authors thank Prof. Mart Saarma for his support. Prof. Mart Saarma initiated the in vivo GDNF studies and provided funding support from Sigrid Jusélius Foundation and Academy of Finland. The authors also thank Daniel R. Garton for language editing.
References
- 1.Lin L.F., Doherty D.H., Lile J.D., Bektesh S., Collins F. GDNF: a glial cell line-derived neurotrophic factor for midbrain dopaminergic neurons. Science. 1993;260:1130–1132. doi: 10.1126/science.8493557. [DOI] [PubMed] [Google Scholar]
- 2.Hoffer B.J., Hoffman A., Bowenkamp K., Huettl P., Hudson J., Martin D., Lin L.F., Gerhardt G.A. Glial cell line-derived neurotrophic factor reverses toxin-induced injury to midbrain dopaminergic neurons in vivo. Neurosci. Lett. 1994;182:107–111. doi: 10.1016/0304-3940(94)90218-6. [DOI] [PubMed] [Google Scholar]
- 3.Whone A., Luz M., Boca M., Woolley M., Mooney L., Dharia S., Broadfoot J., Cronin D., Schroers C., Barua N.U. Randomized trial of intermittent intraputamenal glial cell line-derived neurotrophic factor in Parkinson’s disease. Brain. 2019;142:512–525. doi: 10.1093/brain/awz023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Whone A.L., Boca M., Luz M., Woolley M., Mooney L., Dharia S., Broadfoot J., Cronin D., Schroers C., Barua N.U. Extended Treatment with Glial Cell Line-Derived Neurotrophic Factor in Parkinson’s Disease. J. Parkinsons Dis. 2019;9:301–313. doi: 10.3233/JPD-191576. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Heiss J.D., Lungu C., Hammoud D.A., Herscovitch P., Ehrlich D.J., Argersinger D.P., Sinharay S., Scott G., Wu T., Federoff H.J. Trial of magnetic resonance-guided putaminal gene therapy for advanced Parkinson’s disease. Mov. Disord. 2019;34:1073–1078. doi: 10.1002/mds.27724. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Gill S.S., Patel N.K., Hotton G.R., O’Sullivan K., McCarter R., Bunnage M., Brooks D.J., Svendsen C.N., Heywood P. Direct brain infusion of glial cell line-derived neurotrophic factor in Parkinson disease. Nat. Med. 2003;9:589–595. doi: 10.1038/nm850. [DOI] [PubMed] [Google Scholar]
- 7.Slevin J.T., Gerhardt G.A., Smith C.D., Gash D.M., Kryscio R., Young B. Improvement of bilateral motor functions in patients with Parkinson disease through the unilateral intraputaminal infusion of glial cell line-derived neurotrophic factor. J. Neurosurg. 2005;102:216–222. doi: 10.3171/jns.2005.102.2.0216. [DOI] [PubMed] [Google Scholar]
- 8.Lang A.E., Gill S., Patel N.K., Lozano A., Nutt J.G., Penn R., Brooks D.J., Hotton G., Moro E., Heywood P. Randomized controlled trial of intraputamenal glial cell line-derived neurotrophic factor infusion in Parkinson disease. Ann. Neurol. 2006;59:459–466. doi: 10.1002/ana.20737. [DOI] [PubMed] [Google Scholar]
- 9.Ferreira R.N., de Miranda A.S., Rocha N.P., Simoes E Silva A.C., Teixeira A.L., da Silva Camargos E.R. Neurotrophic Factors in Parkinson’s Disease: What Have we Learned from Pre-Clinical and Clinical Studies? Curr. Med. Chem. 2018;25:3682–3702. doi: 10.2174/0929867325666180313101536. [DOI] [PubMed] [Google Scholar]
- 10.Kirkeby A., Barker R.A. Parkinson disease and growth factors—is GDNF good enough? Nat. Rev. Neurol. 2019;15:312–314. doi: 10.1038/s41582-019-0180-6. [DOI] [PubMed] [Google Scholar]
- 11.Nutt J.G., Burchiel K.J., Comella C.L., Jankovic J., Lang A.E., Laws E.R., Jr., Lozano A.M., Penn R.D., Simpson R.K., Jr., Stacy M., Wooten G.F., ICV GDNF Study Group. Implanted intracerebroventricular. Glial cell line-derived neurotrophic factor Randomized, double-blind trial of glial cell line-derived neurotrophic factor (GDNF) in PD. Neurology. 2003;60:69–73. doi: 10.1212/wnl.60.1.69. [DOI] [PubMed] [Google Scholar]
- 12.d’Anglemont de Tassigny X., Pascual A., López-Barneo J. GDNF-based therapies, GDNF-producing interneurons, and trophic support of the dopaminergic nigrostriatal pathway. Implications for Parkinson’s disease. Front. Neuroanat. 2015;9:10. doi: 10.3389/fnana.2015.00010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Kearns C.M., Gash D.M. GDNF protects nigral dopamine neurons against 6-hydroxydopamine in vivo. Brain Res. 1995;672:104–111. doi: 10.1016/0006-8993(94)01366-p. [DOI] [PubMed] [Google Scholar]
- 14.Sauer H., Rosenblad C., Björklund A. Glial cell line-derived neurotrophic factor but not transforming growth factor beta 3 prevents delayed degeneration of nigral dopaminergic neurons following striatal 6-hydroxydopamine lesion. Proc. Natl. Acad. Sci. USA. 1995;92:8935–8939. doi: 10.1073/pnas.92.19.8935. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Kordower J.H., Emborg M.E., Bloch J., Ma S.Y., Chu Y., Leventhal L., McBride J., Chen E.Y., Palfi S., Roitberg B.Z. Neurodegeneration prevented by lentiviral vector delivery of GDNF in primate models of Parkinson’s disease. Science. 2000;290:767–773. doi: 10.1126/science.290.5492.767. [DOI] [PubMed] [Google Scholar]
- 16.Georgievska B., Kirik D., Björklund A. Overexpression of glial cell line-derived neurotrophic factor using a lentiviral vector induces time- and dose-dependent downregulation of tyrosine hydroxylase in the intact nigrostriatal dopamine system. J. Neurosci. 2004;24:6437–6445. doi: 10.1523/JNEUROSCI.1122-04.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Rosenblad C., Georgievska B., Kirik D. Long-term striatal overexpression of GDNF selectively downregulates tyrosine hydroxylase in the intact nigrostriatal dopamine system. Eur. J. Neurosci. 2003;17:260–270. doi: 10.1046/j.1460-9568.2003.02456.x. [DOI] [PubMed] [Google Scholar]
- 18.Georgievska B., Kirik D., Björklund A. Aberrant sprouting and downregulation of tyrosine hydroxylase in lesioned nigrostriatal dopamine neurons induced by long-lasting overexpression of glial cell line derived neurotrophic factor in the striatum by lentiviral gene transfer. Exp. Neurol. 2002;177:461–474. doi: 10.1006/exnr.2002.8006. [DOI] [PubMed] [Google Scholar]
- 19.Hudson J., Granholm A.C., Gerhardt G.A., Henry M.A., Hoffman A., Biddle P., Leela N.S., Mackerlova L., Lile J.D., Collins F. Glial cell line-derived neurotrophic factor augments midbrain dopaminergic circuits in vivo. Brain Res. Bull. 1995;36:425–432. doi: 10.1016/0361-9230(94)00224-o. [DOI] [PubMed] [Google Scholar]
- 20.Love S., Plaha P., Patel N.K., Hotton G.R., Brooks D.J., Gill S.S. Glial cell line-derived neurotrophic factor induces neuronal sprouting in human brain. Nat. Med. 2005;11:703–704. doi: 10.1038/nm0705-703. [DOI] [PubMed] [Google Scholar]
- 21.Emerich D.F., Plone M., Francis J., Frydel B.R., Winn S.R., Lindner M.D. Alleviation of behavioral deficits in aged rodents following implantation of encapsulated GDNF-producing fibroblasts. Brain Res. 1996;736:99–110. [PubMed] [Google Scholar]
- 22.Hebert M.A., Gerhardt G.A. Behavioral and neurochemical effects of intranigral administration of glial cell line-derived neurotrophic factor on aged Fischer 344 rats. J. Pharmacol. Exp. Ther. 1997;282:760–768. [PubMed] [Google Scholar]
- 23.Hebert M.A., Van Horne C.G., Hoffer B.J., Gerhardt G.A. Functional effects of GDNF in normal rat striatum: presynaptic studies using in vivo electrochemistry and microdialysis. J. Pharmacol. Exp. Ther. 1996;279:1181–1190. [PubMed] [Google Scholar]
- 24.Manfredsson F.P., Tumer N., Erdos B., Landa T., Broxson C.S., Sullivan L.F., Rising A.C., Foust K.D., Zhang Y., Muzyczka N. Nigrostriatal rAAV-mediated GDNF overexpression induces robust weight loss in a rat model of age-related obesity. Mol. Ther. 2009;17:980–991. doi: 10.1038/mt.2009.45. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Mahato A.K., Kopra J., Renko J.M., Visnapuu T., Korhonen I., Pulkkinen N., Bespalov M.M., Domanskyi A., Ronken E., Piepponen T.P. Glial cell line-derived neurotrophic factor receptor REarranged during transfection agonist supports dopamine neurons in Vitro and enhances dopamine release In Vivo. Mov. Disord. 2020;35:245–255. doi: 10.1002/mds.27943. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Smith-Hicks C.L., Sizer K.C., Powers J.F., Tischler A.S., Costantini F. C-cell hyperplasia, pheochromocytoma and sympathoadrenal malformation in a mouse model of multiple endocrine neoplasia type 2B. EMBO J. 2000;19:612–622. doi: 10.1093/emboj/19.4.612. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Moline J., Eng C. Multiple endocrine neoplasia type 2: an overview. Genet. Med. 2011;13:755–764. doi: 10.1097/GIM.0b013e318216cc6d. [DOI] [PubMed] [Google Scholar]
- 28.Meng X., de Rooij D.G., Westerdahl K., Saarma M., Sariola H. Promotion of seminomatous tumors by targeted overexpression of glial cell line-derived neurotrophic factor in mouse testis. Cancer Res. 2001;61:3267–3271. [PubMed] [Google Scholar]
- 29.Kumar A., Kopra J., Varendi K., Porokuokka L.L., Panhelainen A., Kuure S., Marshall P., Karalija N., Härma M.A., Vilenius C. GDNF Overexpression from the Native Locus Reveals its Role in the Nigrostriatal Dopaminergic System Function. PLoS Genet. 2015;11:e1005710. doi: 10.1371/journal.pgen.1005710. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Mätlik K., Võikar V., Vilenius C., Kulesskaya N., Andressoo J.O. Two-fold elevation of endogenous GDNF levels in mice improves motor coordination without causing side-effects. Sci. Rep. 2018;8:11861. doi: 10.1038/s41598-018-29988-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Espinoza S., Scarpato M., Damiani D., Managò F., Mereu M., Contestabile A., Peruzzo O., Carninci P., Santoro C., Papaleo F. SINEUP Non-coding RNA Targeting GDNF Rescues Motor Deficits and Neurodegeneration in a Mouse Model of Parkinson’s Disease. Mol. Ther. 2020;28:642–652. doi: 10.1016/j.ymthe.2019.08.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Howes O.D., Kambeitz J., Kim E., Stahl D., Slifstein M., Abi-Dargham A., Kapur S. The nature of dopamine dysfunction in schizophrenia and what this means for treatment. Arch. Gen. Psychiatry. 2012;69:776–786. doi: 10.1001/archgenpsychiatry.2012.169. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Howes O.D., Kapur S. The dopamine hypothesis of schizophrenia: version III—the final common pathway. Schizophr. Bull. 2009;35:549–562. doi: 10.1093/schbul/sbp006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Labarca C., Schwarz J., Deshpande P., Schwarz S., Nowak M.W., Fonck C., Nashmi R., Kofuji P., Dang H., Shi W. Point mutant mice with hypersensitive alpha 4 nicotinic receptors show dopaminergic deficits and increased anxiety. Proc. Natl. Acad. Sci. USA. 2001;98:2786–2791. doi: 10.1073/pnas.041582598. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Zarrindast M.R., Khakpai F. The Modulatory Role of Dopamine in Anxiety-like Behavior. Arch. Iran Med. 2015;18:591–603. [PubMed] [Google Scholar]
- 36.Miyamoto Y., Yamada K., Noda Y., Mori H., Mishina M., Nabeshima T. Hyperfunction of dopaminergic and serotonergic neuronal systems in mice lacking the NMDA receptor epsilon1 subunit. J. Neurosci. 2001;21:750–757. doi: 10.1523/JNEUROSCI.21-02-00750.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Vacher C.M., Gassmann M., Desrayaud S., Challet E., Bradaia A., Hoyer D., Waldmeier P., Kaupmann K., Pévet P., Bettler B. Hyperdopaminergia and altered locomotor activity in GABAB1-deficient mice. J. Neurochem. 2006;97:979–991. doi: 10.1111/j.1471-4159.2006.03806.x. [DOI] [PubMed] [Google Scholar]
- 38.Schmidt L.S., Miller A.D., Lester D.B., Bay-Richter C., Schülein C., Frikke-Schmidt H., Wess J., Blaha C.D., Woldbye D.P., Fink-Jensen A., Wortwein G. Increased amphetamine-induced locomotor activity, sensitization, and accumbal dopamine release in M5 muscarinic receptor knockout mice. Psychopharmacology (Berl.) 2010;207:547–558. doi: 10.1007/s00213-009-1685-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Tilley M.R., Cagniard B., Zhuang X., Han D.D., Tiao N., Gu H.H. Cocaine reward and locomotion stimulation in mice with reduced dopamine transporter expression. BMC Neurosci. 2007;8:42. doi: 10.1186/1471-2202-8-42. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Bolkan S.S., Carvalho Poyraz F., Kellendonk C. Using human brain imaging studies as a guide toward animal models of schizophrenia. Neuroscience. 2016;321:77–98. doi: 10.1016/j.neuroscience.2015.05.055. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Mena A., Ruiz-Salas J.C., Puentes A., Dorado I., Ruiz-Veguilla M., De la Casa L.G. Reduced Prepulse Inhibition as a Biomarker of Schizophrenia. Front. Behav. Neurosci. 2016;10:202. doi: 10.3389/fnbeh.2016.00202. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Pertusa M., García-Matas S., Mammeri H., Adell A., Rodrigo T., Mallet J., Cristòfol R., Sarkis C., Sanfeliu C. Expression of GDNF transgene in astrocytes improves cognitive deficits in aged rats. Neurobiol. Aging. 2008;29:1366–1379. doi: 10.1016/j.neurobiolaging.2007.02.026. [DOI] [PubMed] [Google Scholar]
- 43.Gerlai R., McNamara A., Choi-Lundberg D.L., Armanini M., Ross J., Powell-Braxton L., Phillips H.S. Impaired water maze learning performance without altered dopaminergic function in mice heterozygous for the GDNF mutation. Eur. J. Neurosci. 2001;14:1153–1163. doi: 10.1046/j.0953-816x.2001.01724.x. [DOI] [PubMed] [Google Scholar]
- 44.Lodish M.B., Stratakis C.A. RET oncogene in MEN2, MEN2B, MTC and other forms of thyroid cancer. Expert Rev. Anticancer Ther. 2008;8:625–632. doi: 10.1586/14737140.8.4.625. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Chen C., Guderyon M.J., Li Y., Ge G., Bhattacharjee A., Ballard C., He Z., Masliah E., Clark R.A., O’Connor J.C., Li S. Non-toxic HSC Transplantation-Based Macrophage/Microglia-Mediated GDNF Delivery for Parkinson’s Disease. Mol. Ther. Methods Clin. Dev. 2019;17:83–98. doi: 10.1016/j.omtm.2019.11.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Hidalgo-Figueroa M., Bonilla S., Gutiérrez F., Pascual A., López-Barneo J. GDNF is predominantly expressed in the PV+ neostriatal interneuronal ensemble in normal mouse and after injury of the nigrostriatal pathway. J. Neurosci. 2012;32:864–872. doi: 10.1523/JNEUROSCI.2693-11.2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Pochon N.A., Menoud A., Tseng J.L., Zurn A.D., Aebischer P. Neuronal GDNF expression in the adult rat nervous system identified by in situ hybridization. Eur. J. Neurosci. 1997;9:463–471. doi: 10.1111/j.1460-9568.1997.tb01623.x. [DOI] [PubMed] [Google Scholar]
- 48.Trupp M., Belluardo N., Funakoshi H., Ibáñez C.F. Complementary and overlapping expression of glial cell line-derived neurotrophic factor (GDNF), c-ret proto-oncogene, and GDNF receptor-alpha indicates multiple mechanisms of trophic actions in the adult rat CNS. J. Neurosci. 1997;17:3554–3567. doi: 10.1523/JNEUROSCI.17-10-03554.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Henderson C.E., Phillips H.S., Pollock R.A., Davies A.M., Lemeulle C., Armanini M., Simmons L., Moffet B., Vandlen R.A., Simpson LC corrected to Simmons L. GDNF: a potent survival factor for motoneurons present in peripheral nerve and muscle. Science. 1994;266:1062–1064. doi: 10.1126/science.7973664. [DOI] [PubMed] [Google Scholar]
- 50.Hantman A.W., Jessell T.M. Clarke’s column neurons as the focus of a corticospinal corollary circuit. Nat. Neurosci. 2010;13:1233–1239. doi: 10.1038/nn.2637. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Drinkut A., Tillack K., Meka D.P., Schulz J.B., Kügler S., Kramer E.R. Correction to: Ret is essential to mediate GDNF’s neuroprotective and neuroregenerative effect in a Parkinson disease mouse model. Cell Death Dis. 2018;9:634. doi: 10.1038/s41419-018-0636-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Taraviras S., Marcos-Gutierrez C.V., Durbec P., Jani H., Grigoriou M., Sukumaran M., Wang L.C., Hynes M., Raisman G., Pachnis V. Signalling by the RET receptor tyrosine kinase and its role in the development of the mammalian enteric nervous system. Development. 1999;126:2785–2797. doi: 10.1242/dev.126.12.2785. [DOI] [PubMed] [Google Scholar]
- 53.Bespalov M.M., Sidorova Y.A., Tumova S., Ahonen-Bishopp A., Magalhães A.C., Kulesskiy E., Paveliev M., Rivera C., Rauvala H., Saarma M. Heparan sulfate proteoglycan syndecan-3 is a novel receptor for GDNF, neurturin, and artemin. J. Cell Biol. 2011;192:153–169. doi: 10.1083/jcb.201009136. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Paratcha G., Ledda F., Ibáñez C.F. The neural cell adhesion molecule NCAM is an alternative signaling receptor for GDNF family ligands. Cell. 2003;113:867–879. doi: 10.1016/s0092-8674(03)00435-5. [DOI] [PubMed] [Google Scholar]
- 55.Mätlik K., Olfat S., Garton D.R., Montaño-Rodriguez A., Turconi G., Porokuokka L.L., Panhelainen A., Schweizer N., Kopra J., Cowlishaw M.C. Gene Knock Up via 3′UTR editing to study gene function in vivo. bioRxiv. 2019 doi: 10.1101/775031. [DOI] [Google Scholar]
- 56.Sidorova Y.A., Bespalov M.M., Wong A.W., Kambur O., Jokinen V., Lilius T.O., Suleymanova I., Karelson G., Rauhala P.V., Karelson M. A Novel Small Molecule GDNF Receptor RET Agonist, BT13, Promotes Neurite Growth from Sensory Neurons in Vitro and Attenuates Experimental Neuropathy in the Rat. Front. Pharmacol. 2017;8:365. doi: 10.3389/fphar.2017.00365. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Valros A., Palander P., Heinonen M., Munsterhjelm C., Brunberg E., Keeling L., Piepponen P. Evidence for a link between tail biting and central monoamine metabolism in pigs (Sus scrofa domestica) Physiol. Behav. 2015;143:151–157. doi: 10.1016/j.physbeh.2015.02.049. [DOI] [PubMed] [Google Scholar]
- 58.Mijatovic J., Airavaara M., Planken A., Auvinen P., Raasmaja A., Piepponen T.P., Costantini F., Ahtee L., Saarma M. Constitutive Ret activity in knock-in multiple endocrine neoplasia type B mice induces profound elevation of brain dopamine concentration via enhanced synthesis and increases the number of TH-positive cells in the substantia nigra. J. Neurosci. 2007;27:4799–4809. doi: 10.1523/JNEUROSCI.5647-06.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Schindelin J., Arganda-Carreras I., Frise E., Kaynig V., Longair M., Pietzsch T., Preibisch S., Rueden C., Saalfeld S., Schmid B. Fiji: an open-source platform for biological-image analysis. Nat. Methods. 2012;9:676–682. doi: 10.1038/nmeth.2019. [DOI] [PMC free article] [PubMed] [Google Scholar]