Abstract
This study aimed to investigate vitamin D receptor (VDR) expression levels and evaluate their clinical significance in patients with colorectal cancer (CRC). VDR protein expression was validated by immunohistochemistry in 188 CRC tissues and 134 normal colorectal tissues. The associations between VDR expression and clinicopathologic characteristics, including prognostic outcomes, were analyzed. VDR expression in normal colorectal tissue was higher than that in CRC (83.6% versus 34.6%, P = 4.489 × 10-20) and generated moderate diagnostic performance for CRC detection (AUC = 0.88, sensitivity = 0.87, specificity = 0.84). Low VDR expression was associated with invasion depth (P = 0.001) and poor survival in CRC (P = 0.031). Univariate Cox analysis demonstrated VDR expression (P = 0.036) was a significant prognostic predictor for survival in patients with CRC. Low VDR expression could be a valuable diagnostic and prognostic biomarker for CRC patients. Targeting VDR may offer a potential therapeutic strategy for blocking CRC.
Keywords: Colorectal cancer, vitamin D receptor, progression, prognosis
Introduction
Colorectal cancer (CRC) is the 3th most frequently diagnosed malignancy and the 4th leading cause of cancer-related death in the world in 2012, and there has been an increasing trend in incidence and mortality of this cancer in developing countries, including China [1,2]. Since CRC is largely asymptomatic until it develops to advanced stages, the prognosis worsens with advancing stage, and merely 5% of patients diagnosed with distant metastases survive 5 years [3,4]. Hence, it is still particularly urgent to establish the molecular mechanism of CRC, as well as the biomarkers for tumor growth and development as new prognostic and therapeutic targets that feature high sensitivity, specificity, and accuracy.
The active form of vitamin D, calcitriol, is a steroid hormone that plays an important role of regulation of cell proliferation, apoptosis, differentiation, inflammation, invasion, metastasis, angiogenesis, miRNA expression, and cancer-related signaling pathways [5,6]. These diverse effects are largely mediated by the vitamin D receptor (VDR), which is a member of the nuclear receptor superfamily present in many types of epithelial and mesenchymal cells, including those of the colorectum [7,8]. Increasing evidence shows that VDR expression plays an essential role in some cancer types, such as melanoma, breast cancer, cholangiocarcinoma, glioblastoma multiforme, bladder cancer, pancreatic carcinoma, and gastric cancer [8-12]. With regard to colorectal cancer, there is one meta-analysis that showed a higher risk of colorectal cancer associated with the TaqI (rs731236) polymorphism which belongs to one of the VDR single-nucleotide polymorphisms (SNPs) [13]. There are two studies by Evans and Ferrer-Mayorga indicating that high VDR expression is associated with better clinical outcome in CRC [14,15]. Nevertheless, to our knowledge, there are no studies of VDR expression in a Chinese CRC cohort and no information concerning the role of VDR in the prognosis of CRC. Therefore, in this study we used immunohistochemistry (IHC) to investigate the expression of VDR in CRC tissue microarrays, assessed its associations with clinicopathologic data and survival, and further analyzed the relevance of receptor expression for CRC progression.
Materials and methods
Patients and tumor cases
A total of 188 patients with CRC who underwent surgical resection without any preoperative treatment and 134 normal colorectal tissues were recruited from 2009 to 2012 at the Affiliated Hospital of Shihezi University. There were no restrictions regarding age, sex, or stage of disease. At the same time, data on clinicopathologic measures were obtained, such as age, gender, tumor size, tumor site, degree of differentiation, depth of invasion, lymph node metastasis, and clinical stage (TNM stage) (Table 1). Two pathologists independently carried out the diagnosis of CRC according to the 7th Edition of the American Joint Committee on Cancer TNM classification. All procedures were conducted in accordance with the ethical standards of the hospital. We followed the 98 CRC patients after radical resection of colorectal cancer up to December 10, 2015. Informed consent on the use of clinical specimens was obtained from all patients.
Table 1.
Clinicopathologic demographics for the 188 patients with CRC
| Total Cases (n = 188) | |
|---|---|
| Characteristic | NO (%) |
| Gender | |
| Male | 111 (59.0) |
| Female | 77 (41.0) |
| Age (yrs) | |
| ≤ 60 | 54 (28.7) |
| > 60 | 134 (71.3) |
| Tumor location | |
| Colon | 91 (48.4) |
| Rectum | 97 (51.6) |
| Size (cm) | |
| ≤ 5 cm | 97 (51.6) |
| > 5 cm | 91 (48.4) |
| General type | |
| Bulge type | 61 (32.4) |
| Ulcer type | 121 (64.4) |
| Mushroom type | 6 (3.2) |
| Differentiationa | |
| High | 8 (4.3) |
| Middle | 156 (83.0) |
| Low | 24 (12.8) |
| Invasion depth | |
| T1-T2 | 46 (24.5) |
| T3-T4 | 142 (75.5) |
| Lymph node metastasis | |
| N0 | 112 (59.6) |
| N1-N3 | 76 (40.4) |
| TNM Stageb | |
| I+II | 110 (58.5) |
| III+IV | 78 (41.5) |
Histologic grade was based on WHO classification published in 2010.
TNM stage was assessed according to the 7th Edition of the AJCC Cancer Staging Manual.
Tissue microarray and immunohistochemistry
Tissue microarrays consisted of a patient’s colorectal cancer, and colorectal normal tissue. VDR protein expression was detected using the Envision system (Dako, Carpinteria, CA). After being fixed in formalin and embedded in paraffin, each 4 μm section was prepared on microslides and baked at 65°C for 2 h and deparaffinized using xylene and rehydrated using alcohol. Antigen was repaired using pressure cooker heating for 10 min with boiling citric acid buffer (pH = 6.0), cooling to room temperature for 30 min. Then the slices were immersed in hydrogen peroxide (3%) for 10 min to block endogenous peroxidase. Each slice was incubated with a diluted 1:800 mouse monoclonal anti-VDR antibody (Santa Cruz, sc-13133) at 4°C overnight in a humid box. Then, the slices were washed with PBS and incubated in Envision Two antibody at 37°C for 30 min. Finally, 3,3’-diaminobenzidine and hematoxylin were used to visualize immunoreactivity and stain structures.
Brown granules in the cytoplasm or nucleus were considered positive for VDR protein staining. A semi-quantitative score was applied according to the percentage of VDR staining intensity and positive cells. Scores were as follows: 0 (0%-5%), 1 (6%-25%), 2 (26%-50%), 3 (51%-75%), and 4 (> 76%). The scoring for staining intensity were: 0 points (no color); 1 point (buff); 2 points (yellow); and 3 points (brown). Hence, the range of IS was from 0 to 12 points. The optimal cut-off values for the evaluation system were as follows: scores of 0 to 3 were defined as “low expression levels”, and 4 to 12 points indicate “high expression levels”. All immunostained results were assessed by two independent pathologists who were blinded to all clinical and pathologic information and finally assigned a consistent score. Variations in the enumeration, within a range of 5%, were reevaluated, and a consensus decision was made.
Statistical analysis
Statistical analysis was implemented using SPSS 20.0 and GraphPad Prism 5.01. Receiver operating characteristic (ROC) curves were constructed and the area under the curve (AUC) was calculated to assess the feasibility of using VDR as a diagnostic tool to discriminate CRC tissues and normal colorectal tissues. Categorical data were compared using a χ2 test to analyze the differences in VDR expression and clinicopathologic factors. The results are shown with standard deviation. Survival curves were analyzed by Kaplan-Meier method and compared by log-rank test. Cox proportional hazard test was used to evaluate multivariate hazard ratios for the variables. All statistical tests were two-tailed, and differences were considered significant at p < 0.05.
Results
VDR was expressed higher in normal colorectal tissue compared to CRC
The expression of VDR was examined in normal colorectal tissues and CRC tissues by immunohistochemical staining. Interestingly, VDR expression in normal colorectal cells was predominantly present in the cell membrane and the cytoplasm. Similarly, VDR was also mainly localized in the cytoplasm and the membrane of cancer cells. We evaluated VDR expression in 134 normal colorectal tissues and 188 CRC samples (Table 1). As a result, box-plots showed that the trend in VDR immunoreactivity score decreased in a stepwise manner from normal cells, to stage I+II, to stage III+IV CRC tissues, as shown by t-test. More importantly, the differences between every pair of groups were statistically significant (all P < 0.001, Figure 1C). In addition, as shown in Table 2 and Figure 1D, a striking difference exists in the comparison of VDR expression levels in normal, stage I+II, and stage III+IV CRC tissues by Chi-square test. We conducted an in-depth analysis on VDR expression during cancer progression. VDR expression was significantly decreased in stage I+II, and stage III+IV CRC tissues compared to normal colorectal tissue (both P < 0.001).The decrease in frequency from stage I+II to stage III+IV (46.36% versus 37.18%, P = 0.196) was not significant, but the marginal differences in VDR expression between the two tissues remained visible (Table 2; Figure 1). Furthermore, the four-level score (0-2, 3, 4, 5-8, and 9-12) distributions of VDR protein expression in normal colorectal tissues and stage I+II, or stage III+IV CRC were significantly distinct (Figure 1E). The results above indicated that VDR expression had potential value as a biomarker for CRC detection. To further evaluate the diagnostic value of the VDR, we constructed ROC curves and calculated AUC values. As shown in Figure 2, VDR generated moderate diagnostic performance (AUC = 0.88, sensitivity = 0.87, specificity = 0.84).
Figure 1.
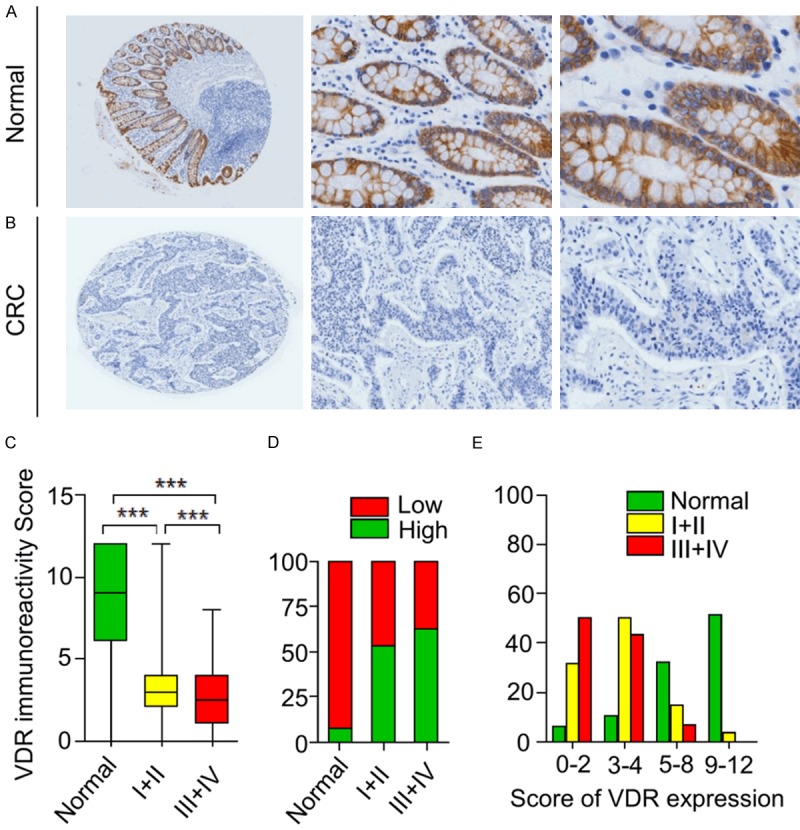
Immunohistochemical analysis of the VDR protein in normal tissues and CRC tissues. Typical VDR staining in normal tissues (A) and CRC (B) (left image magnification, × 40; middle image magnification, × 200; right image magnification, × 400). VDR staining was localized to the cytoplasm and the cell membrane. (C) (Box plot) Range of VDR expression score in normal, stage I-II, and stage III-IV CRC tissues (***P < 0.001). (D) VDR low was expression associated with progression of CRC (CRC:I-II, P = 1.55 × 10-13; CRC:III-IV, P = 1.23 × 10-17; I-II:III-IV, P = 0.196). (E) (Frequency distribution histogram) frequency distribution of normal tissues, I-II, and III-IV CRC tissues in four-level scores (0-2, 3, 4, 5-8, and 9-12) of VDR expression.
Table 2.
VDR protein expression during cancer progression by IHC analysis in colorectal tissue
| Cancer progression | Immunostaining | P-value | |
|---|---|---|---|
|
| |||
| low (%) | high (%) | ||
| NormalA | 10 (7.46) | 124 (92.54) | A:B, P = 1.55 × 10-13 *** |
| I+IIB | 59 (53.64) | 51 (46.36) | B:C, P = 0.196 |
| III+IVC | 49 (62.82) | 29 (37.18) | A:C, P = 1.23 × 10-17 *** |
P < 0.001 as calculated by Pearson’s χ2 test.
A: Normal; B: I+II; C: III+IV.
Figure 2.
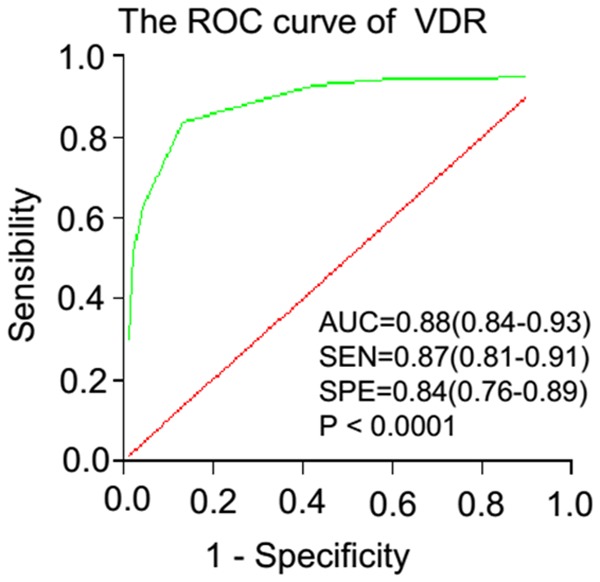
ROC curve analysis of VDR in differentiating CRC tissues and normal colorectal tissues.
Low VDR expression had an association with invasion depth in CRC
To determine whether the level of VDR protein expression is related to the development of CRC, we further explored the relationship between VDR expression and clinicopathologic characteristics of 188 CRC patients (Table 3). Among the variables, VDR expression was significantly correlated with invasion depth (P = 0.001). However, no significant correlation was found between VDR expression and other clinicopathologic variables, such as gender, age, and tumor location, size, general type, differentiation, lymph node metastasis, or clinical stage. This result may be attributable to tumor heterogeneity or limited samples.
Table 3.
Correlations between VDR expression of CRC and clinicopathologic factors
| Variable | VDR expression | P-value | ||
|---|---|---|---|---|
|
| ||||
| Total Cases (%) (n = 188) | Low No. (%) | High No. (%) | ||
| Gender | 0.944 | |||
| Male | 111 (59.0) | 64 (57.66) | 47 (42.34) | |
| Female | 77 (41.0) | 44 (57.14) | 33 (42.86) | |
| Age (yrs) | 0.064 | |||
| ≤ 60 | 54 (28.7) | 31 (57.41) | 23 (42.59) | |
| > 60 | 134 (71.3) | 57 (42.54) | 77 (57.46) | |
| Tumor location | 0.421 | |||
| Colon | 91 (48.4) | 55 (60.44) | 36 (39.56) | |
| Rectum | 97 (51.6) | 53 (54.64) | 44 (45.36) | |
| Size (cm) | 0.272 | |||
| ≤ 5 cm | 97 (51.6) | 52 (53.61) | 45 (46.39) | |
| > 5 cm | 91 (48.4) | 56 (61.54) | 35 (38.46) | |
| General type | 0.239 | |||
| Bulging type | 61 (32.4) | 30 (49.18) | 31 (50.82) | |
| Ulcer type | 121 (64.4) | 75 (61.98) | 46 (38.02) | |
| Mushroom type | 6 (3.2) | 3 (50.00) | 3 (50.00) | |
| Differentiation | 0.117 | |||
| High | 8 (4.3) | 2 (25.00) | 6 (75.00) | |
| Middle | 156 (83.0) | 90 (57.69) | 66 (42.31) | |
| Low | 24 (12.8) | 16 (66.67) | 8 (33.33) | |
| Invasion depth | 0.001 | |||
| T1-T2 | 46 (24.5) | 17 (36.96) | 29 (63.04) | |
| T3-T4 | 142 (75.5) | 91 (64.08) | 51 (35.92) | |
| Lymph node metastasis | 0.315 | |||
| N0 | 112 (59.6) | 61 (54.46) | 51 (45.54) | |
| N1-N3 | 76 (40.4) | 47 (61.84) | 29 (38.16) | |
| TNM Stage | 0.210 | |||
| I+II | 110 (58.5) | 59 (53.64) | 51 (46.36) | |
| III+IV | 78 (41.5) | 49 (62.82) | 29 (37.18) | |
P < 0.05 was considered significant.
Low VDR expression marks poor prognosis in CRC patients
To show whether new or less-known biomarkers, such as VDR, play a role in survival prognosis, we needed to validate this CRC patient cohort first. We accordingly detected overall survival of these CRC patients against several established conventional risk factors that affected survival using a Kaplan-Meier method and Log-rank test. The cohort included 188 CRC patients with 67 alive, 31 dead, and 90 lost to follow-up. Apparently, TNM staging greatly correlated with overall survival among these CRC patients in a severity-dependent manner (Figure 3A and 3B, log-rank P < 0.0001). Having validated the patient cohort in terms of conventional risk factors for survival, we had great confidence and analyzed this less-known risk factor for survival as described below.
Figure 3.
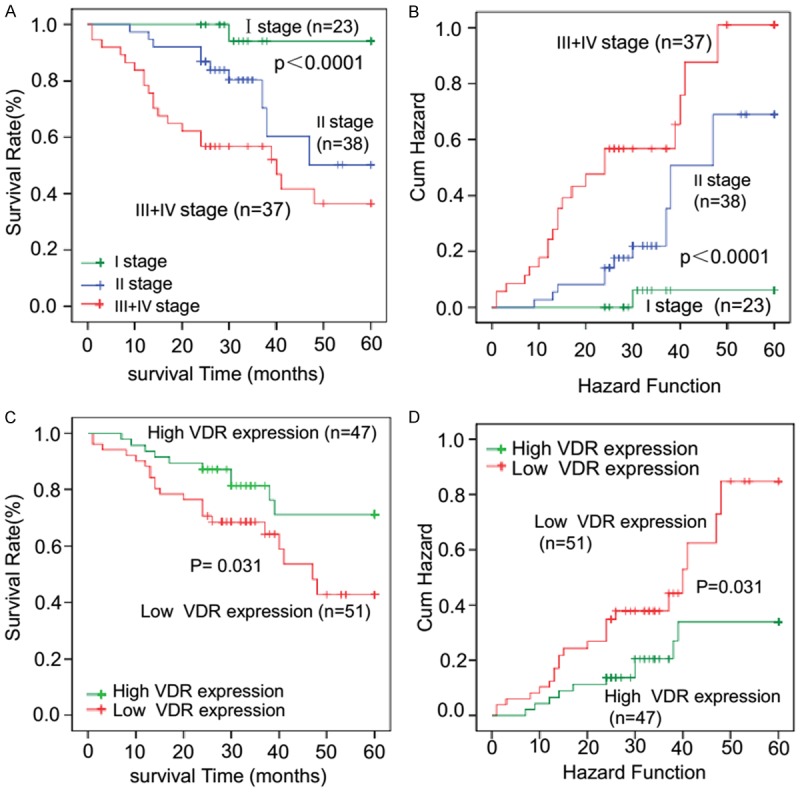
Survival curves against the established traditional prognostic risk factors affecting survival validate the CRC patient cohort for analyzing promising new biomarkers. The cohort consisted of 180 CRC patients, of whom 67 were alive, 31 were dead, and 90 were lost to follow-up. To validate the cohort for survival analysis, overall survival is analyzed against several established factors affecting survival in CRC patients using Kaplan-Meier method. A. Panel TNM staging describes a typical survival hierarchy that patients with the earliest stage I have the best survival while patients with the latest stages III+IV show the poorest survival. B. Panel Hazard Function shows that patients with the latest stage III+IV show highest risk of death while earliest stage I has the lowest risk of death. TNM staging was determined according to the AJCC TNM staging system. C. Kaplan-Meier survival analyses of patients with low VDR expression and those with high expression. CRC patients with low VDR expression (IS < 4) show a significantly lower survival rate after surgery compared to those with high VDR expression (IS ≥ 4) (P < 0.05). D. Patients with high VDR expression present lower risk of death compared to those with low VDR expression (P < 0.05).
Next, to assess the value of VDR in the prognosis of CRC patients, the relationship between VDR expression and the overall survival (OS) of 188 CRC patients was evaluated by Kaplan-Meier method. At the last follow-up, the survival rate of VDR high expression patients was 71.2%, but the rate of the VDR low expression group was 42.8%. As shown in Figure 3C and 3D, it was also obvious that VDR expression nicely associated with survival among these CRC patients in a dose-dependent way. CRC patients with high VDR expression presented longer OS rates and lower risk of death compared to those with low VDR expression (log-rank P = 0.031).
To identify independent prognostic factors for CRC survival, we also conducted univariate and multivariate Cox analysis (Table 4). Differentiation (P = 4.164 × 10-4), T stage (P = 0.008), N stage (P = 0.001), M stage (P = 6.165 × 10-5), TNM stage (P = 0.001) and VDR expression (P = 0.036) were significant prognostic predictors for survival in patients with CRC. However, multivariate Cox proportional hazard regression analysis indicated that T stage (P = 0.019) was an independent risk factor for CRC among all clinicopathologic factors, and VDR expression (P = 0.436, Table 4) was not. Future study should increase the frequency of follow-up or prolong the follow-up time. These data still suggest that VDR can be viewed as a promising biomarker that predicts poor prognosis in CRC.
Table 4.
Univariate and multivariate Cox regression analyses of prognostic variables in CRC patients
| Variables | Univariate analysis | Multivariate analysis | ||||||
|---|---|---|---|---|---|---|---|---|
|
|
|
|||||||
| HRa | 95% CIb | P value | HRa | 95% CIb | P value | |||
| Gender | ||||||||
| Male vs. female | 1.336 | 0.659 | 2.707 | 0.422 | 1.427 | 0.660 | 3.084 | 0.366 |
| Age (years) | ||||||||
| > 60 vs. ≤ 60 | 1.147 | 0.436 | 3.021 | 0.781 | 1.001 | 0.354 | 2.828 | 0.999 |
| Tumor site | ||||||||
| Rectum vs. colon | 0.738 | 0.362 | 1.507 | 0.405 | 0.480 | 0.218 | 1.061 | 0.070 |
| Tumor size (cm) | ||||||||
| > 5 cm vs. ≤ 5 cm | 0.541 | 0.254 | 1.153 | 0.112 | 0.447 | 0.193 | 1.035 | 0.060 |
| Differentiation | ||||||||
| High-medium vs. poor | 4.780 | 2.005 | 11.396 | 4.164 × 10-4 *** | 2.249 | 0.844 | 5.995 | 0.105 |
| T stage | ||||||||
| T1-T2 vs. T3-T4 | 14.897 | 2.026 | 109.526 | 0.008* | 11.555 | 1.506 | 88.626 | 0.019* |
| N stage | ||||||||
| Present vs. absent | 3.651 | 1.745 | 7.639 | 0.001*** | 1.881 | 0.823 | 4.298 | 0.134 |
| M stage | ||||||||
| Present vs. absent | 0.099 | 0.032 | 0.307 | 6.165 × 10-5 *** | 0.362 | 0.105 | 1.253 | 0.109 |
| TNM stage | ||||||||
| I-II vs. III-IV | 3.651 | 1.745 | 7.639 | 0.001*** | ||||
| VDR expression | ||||||||
| High vs. low | 0.447 | 0.210 | 0.949 | 0.036* | 0.720 | 0.314 | 1.647 | 0.436 |
HR = hazard ratio;
CI = confidence interval;
P < 0.05;
P < 0.001.
Discussion
Colorectal cancer (CRC) is the fifth leading cause of cancer death among both men and women, with few treatment options especially for advanced and metastatic patients in China [16,17]. Existing diagnostic methods, such as TNM classification systems, endoscopy, CT, and carcinoembryonic antigen (CEA), are limited for this lethal disease [18,19]. Therefore, new insights into promising biomarkers of this cancer are urgently needed. It has been reported that the function of VDR is related to patterns of gene expression and the outcome between proliferation, differentiation, or apoptosis [20]. Although recent epidemiologic and etiologic studies have suggested that the carcinogenesis of CRC involves multiple factors, stages, and alterations in gene expression [21,22], the precise mechanism(s) responsible for the development of CRC are largely unknown. The role of VDR in colorectal cancer has drawn more attention recently. However, there is limited information on VDR expression studies, and several documented reports have shown conflicting results in this regard [15,23,24]. Conclusive results are needed to demonstrate associations of VDR with CRC at the expression level.
VDR is closely related to the regulation of genes that control cell proliferation, differentiation, and apoptosis and modulation of the immune response [20]. There is evidence that VDR may be linked to inhibition of tumor development. For example, the interaction between vitamin D and vitamin D receptor can inhibit primary cultures of patient-derived colon cancer-associated fibroblasts [15]. Mutations in the VDR might correlate with therapeutic response for multiple myeloma patients [25]. Using IHC analyses on tissue microarrays, we demonstrated that the VDR expression in the CRC tissues was significantly reduced compared with normal colorectal tissues. Low VDR expression was associated with invasion depth. Moreover, VDR deficiency is a poor prognostic factor for short overall survival rate in CRC which indicates that down-regulation of VDR predicts adverse prognosis.
These findings suggest that VDR may play an important role in the pathogenesis of CRC, and may be a biomarker for diagnosis and treatment of CRC.
Our observation of down-regulated protein expression of VDR in CRC tissues is in agreement with the findings by Ferrer-Mayorga et al. and Kure et al. [15,23], and another study also supports the conclusion at the RNA level [14]. But this result differs from that of Matusiak et al. [24], who found VDR levels are low in normal colonic epithelial cells and enhanced in precancerous lesions and early stages of colonic tumorigenesis, whereas they decline in advanced stages. The following factor may result from this discrepancy, of which sample size may be one, as Matusiak et al. used a randomly selected smaller sample size (n = 10) [26] than those of Ferrer-Mayorga et al. (n = 658) [15] and Kure et al. (n = 619) [23] and compared to ours in this study (n = 188). The possible factors resulting in decreased protein expression of VDR in CRC compared with normal colorectal tissue may be due to the following reasons. Kure et al. [23] reported that VDR protein expression level is independently associated with PIK3CA and KRAS mutations in colorectal cancer, and the findings support potential interactions between the VDR, RAS-MAPK, and PI3K-AKT pathways. In addition, several studies have demonstrated that Snail2 by cooperating with Snail1, specifically represses VDR gene promoter to inhibit vitamin D receptor expression in colon cancer [26-28]. Interestingly, a study has shown that it is the VDR promoter that regulates the expression of VDR by affecting transcription [29]. Moreover, Pilon et al. [30] has found that VDR gene promoter methylation may reduce VDR gene expression in malignant adrenal tumors. For those reasons, whether the alterations in VDR play a vital role in regulation of the abnormal expression model between CRC and normal tissues urgently should be determined.
The detailed biologic significance of altered VDR expression in cancers remains poorly understood. Our results indicate that low VDR expression is associated with invasion depth and poor prognosis in CRC. Invasion of cancer cells into blood and lymphatic vessels is a crucial point for cancer metastasis and prognosis. VDR is the pivotal point of the cancer-related and most of the non-cancer-related actions of vitamin D, and evidence is provided by reports showing that VDR has anti-cancer effects in CRC [5,31]. When VD binds to VDR, high circulating levels of vitamin D decrease susceptibility to pre-cancerous conditions such as IBD and also decelerate the progression of colonic neoplasms preventing an APC or β-catenin mutation [20]. In addition, low VDR expression may change patterns of gene expression such as p21, p27, Bcl-2, and Bax, to influence the outcome between proliferation, differentiation, or apoptosis [32]. One study shows that the presence of VDR can target the wnt/β-catenin pathway by up regulating key tumor suppressor genes such as E-cadherin, which promotes an epithelial phenotype and vice versa [20,32]. Another study shows that VDR deletion enhances EGFR and β-catenin signals and up-regulates the colonic RAS to promote tumor cell migration [33]. The finding that low expression of VDR may alter the motility of CRC cells through the above signaling pathways, appears to provide a reasonable explanation for our observation that low-expressed VDR may be involved in aggressiveness and poor prognosis, which warrants detailed investigations in vitro. Furthermore, we found a negative correlation between VDR expression level and OS. The findings by Ferrer-Mayorga et al. [15] and Evans et al. [14] at both protein and RNA levels are consistent with our point of view, but the findings by Kure et al. [23] do not support this result. Possible factors leading to the difference may be population heterogeneity and genetic backgrounds of different ethnicities, which need to be further clarified in studies using uniform ethnic groups with a larger sample size.
In conclusion, this is the first study showing that the protein of VDR is low-expressed in Chinese CRC patients, and correlates with invasion depth and poor prognosis. These findings may potentiate the gene as a candidate biomarker for cancer aggressiveness and poor prognosis in Chinese patients with CRC. In future research, we need to further explore the mechanism of the interaction between VDR and CRC in colorectal cancer cell lines and animal models. Although the mechanisms on why the VDR has low expression and how it contributes to invasion depth and poor prognosis of Chinese CRC are unknown, the findings of our study provide a prerequisite for a further study of the mechanism related to carcinogenesis.
Acknowledgements
This study was supported by Grants from the National Natural Science Foundation of China (No. 81760436, U1903305, 81773116), the Medical and Health Science and Technology Project of the Suzhou High Tech Zone (No. 2017Z009, SYSD2018071, SS2019047, SGY2019D01, SGY2018A01, sys2018008, Y9510101), the Young and Middle-Aged Leading Talents in Scientific and Technological Innovation of XPCC (No. 2019CB022), and the International Science and Technology Cooperation Project of Shihezi University (No. GJHZ201702). The funders were not involved in the study design, data collection and analysis, decision to publish, or preparation of the manuscript.
Disclosure of conflict of interest
None.
References
- 1.Torre LA, Bray F, Siegel RL, Ferlay J, Lortet-Tieulent J, Jemal A. Global cancer statistics, 2012. CA Cancer J Clin. 2015;65:87–108. doi: 10.3322/caac.21262. [DOI] [PubMed] [Google Scholar]
- 2.Arnold M, Sierra MS, Laversanne M, Soerjomataram I, Jemal A, Bray F. Global patterns and trends in colorectal cancer incidence and mortality. Gut. 2017;66:683–691. doi: 10.1136/gutjnl-2015-310912. [DOI] [PubMed] [Google Scholar]
- 3.Das V, Kalita J, Pal M. Predictive and prognostic biomarkers in colorectal cancer: a systematic review of recent advances and challenges. Biomed Pharmacother. 2017;87:8–19. doi: 10.1016/j.biopha.2016.12.064. [DOI] [PubMed] [Google Scholar]
- 4.Booth RA. Minimally invasive biomarkers for detection and staging of colorectal cancer. Cancer Lett. 2007;249:87–96. doi: 10.1016/j.canlet.2006.12.021. [DOI] [PubMed] [Google Scholar]
- 5.Bandera Merchan B, Morcillo S, Martin-Nuñez G, Tinahones FJ, Macías-González M. The role of vitamin D and VDR in carcinogenesis: through epidemiology and basic sciences. J Steroid Biochem Mol Biol. 2017;167:203–218. doi: 10.1016/j.jsbmb.2016.11.020. [DOI] [PubMed] [Google Scholar]
- 6.Zeljic K, Supic G, Magic Z. New insights into vitamin D anticancer properties: focus on miRNA modulation. Mol Genet Genomics. 2017;292:511–524. doi: 10.1007/s00438-017-1301-9. [DOI] [PubMed] [Google Scholar]
- 7.Pandolfi F, Franza L, Mandolini C, Conti P. Immune modulation by vitamin D: special emphasis on its role in prevention and treatment of cancer. Clin Ther. 2017;39:884–893. doi: 10.1016/j.clinthera.2017.03.012. [DOI] [PubMed] [Google Scholar]
- 8.Slominski AT, Brozyna AA, Zmijewski MA, Jóźwicki W, Jetten AM, Mason RS, Tuckey RC, Elmets CA. Vitamin D signaling and melanoma: role of vitamin D and its receptors in melanoma progression and management. Lab Invest. 2017;97:706–724. doi: 10.1038/labinvest.2017.3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Al-Azhri J, Zhang Y, Bshara W, Zirpoli G, McCann SE, Khoury T, Morrison CD, Edge SB, Ambrosone CB, Yao S. Tumor expression of vitamin D receptor and breast cancer histopathological characteristics and prognosis. Clin Cancer Res. 2017;23:97–103. doi: 10.1158/1078-0432.CCR-16-0075. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Chiang KC, Yeh TS, Huang CC, Chang YC, Juang HH, Cheng CT, Pang JS, Hsu JT, Takano M, Chen TC, Kittaka A, Hsiao M, Yeh CN. MART-10 represses cholangiocarcinoma cell growth and high vitamin D receptor expression indicates better prognosis for cholangiocarcinoma. Sci Rep. 2017;7:43773. doi: 10.1038/srep43773. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Jóźwicki W, Brożyna AA, Siekiera J, Slominski AT. Expression of vitamin D receptor (VDR) positively correlates with survival of urothelial bladder cancer patients. Int J Mol Sci. 2015;16:24369–86. doi: 10.3390/ijms161024369. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Wang K, Dong M, Sheng W, Liu Q, Yu D, Dong Q, Li Q, Wang J. Expression of vitamin D receptor as a potential prognostic factor and therapeutic target in pancreatic cancer. Histopathology. 2015;67:386–397. doi: 10.1111/his.12663. [DOI] [PubMed] [Google Scholar]
- 13.Serrano D, Gnagnarella P, Raimondi S, Gandini S. Meta-analysis on vitamin D receptor and cancer risk: focus on the role of TaqI, ApaI, and Cdx2 polymorphisms. Eur J Cancer Prev. 2016;25:85–96. doi: 10.1097/CEJ.0000000000000132. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Evans SR, Nolla J, Hanfelt J, Shabahang M, Nauta RJ, Shchepotin IB. Vitamin D receptor expression as a predictive marker of biological behavior in human colorectal cancer. Clin Cancer Res. 1998;4:1591–5. [PubMed] [Google Scholar]
- 15.Ferrer-Mayorga G, Gómez-López G, Barbáchano A, Fernández-Barral A, Peña C, Pisano DG, Cantero R, Rojo F, Muñoz A, Larriba MJ. Vitamin D receptor expression and associated gene signature in tumour stromal fibroblasts predict clinical outcome in colorectal cancer. Gut. 2017;66:1449–1462. doi: 10.1136/gutjnl-2015-310977. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Chen W, Zheng R, Baade PD, Zhang S, Zeng H, Bray F, Jemal A, Yu XQ, He J. Cancer statistics in China, 2015. CA Cancer J Clin. 2016;66:115–32. doi: 10.3322/caac.21338. [DOI] [PubMed] [Google Scholar]
- 17.National Collaborating Centre for Cancer (UK) Colorectal Cancer: The Diagnosis and Management of Colorectal Cancer. National Collaborating Centre for Cancer. 2011 [PubMed] [Google Scholar]
- 18.Locker GY, Hamilton S, Harris J, Jessup JM, Kemeny N, Macdonald JS, Somerfield MR, Hayes DF, Bast RC Jr ASCO. ASCO 2006 update of recommendations for the use of tumor markers in gastrointestinal cancer. J. Clin. Oncol. 2006;24:5313–27. doi: 10.1200/JCO.2006.08.2644. [DOI] [PubMed] [Google Scholar]
- 19.Swiderska M, Choromańska B, Dąbrowska E, Konarzewska-Duchnowska E, Choromańska K, Szczurko G, Myśliwiec P, Dadan J, Ladny JR, Zwierz K. The diagnostics of colorectal cancer. Contemp Oncol (Pozn) 2014;18:1–6. doi: 10.5114/wo.2013.39995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Byers SW, Rowlands T, Beildeck M, Bong YS. Mechanism of action of vitamin D and the vitamin D receptor in colorectal cancer prevention and treatment. Rev Endocr Metab Disord. 2012;13:31–38. doi: 10.1007/s11154-011-9196-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Lochhead P, Chan AT, Giovannucci E, Fuchs CS, Wu K, Nishihara R, O’Brien M, Ogino S. Progress and opportunities in molecular pathological epidemiology of colorectal premalignant lesions. Am J Gastroenterol. 2014;109:1205–14. doi: 10.1038/ajg.2014.153. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Zhang K, Civan J, Mukherjee S, Patel F, Yang H. Genetic variations in colorectal cancer risk and clinical outcome. World J Gastroenterol. 2014;20:4167–4177. doi: 10.3748/wjg.v20.i15.4167. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Kure S, Nosho K, Baba Y, Irahara N, Shima K, Ng K, Meyerhardt JA, Giovannucci EL, Fuchs CS, Ogino S. Vitamin D receptor expression is associated with PIK3CA and KRAS mutations in colorectal cancer. Cancer Epidemiol Biomarkers Prev. 2009;18:2765–72. doi: 10.1158/1055-9965.EPI-09-0490. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Matusiak D, Murillo G, Carroll RE, Mehta RG, Benya RV. Expression of vitamin D receptor and 25-hydroxyvitamin D3-1{alpha}-hydroxylase in normal and malignant human colon. Cancer Epidemiol Biomarkers Prev. 2005;14:2370–6. doi: 10.1158/1055-9965.EPI-05-0257. [DOI] [PubMed] [Google Scholar]
- 25.Liu Y, Rui HB. Mutations of vitamin D receptor gene found in patients with multiple myeloma. Zhonghua Zhong Liu Za Zhi. 2017;39:121–126. doi: 10.3760/cma.j.issn.0253-3766.2017.02.009. [DOI] [PubMed] [Google Scholar]
- 26.Larriba MJ, Martín-Villar E, García JM, Pereira F, Peña C, de Herreros AG, Bonilla F, Munoz A. Snail2 cooperates with Snail1 in the repression of vitamin D receptor in colon cancer. Carcinogenesis. 2009;30:1459–1468. doi: 10.1093/carcin/bgp140. [DOI] [PubMed] [Google Scholar]
- 27.Larriba MJ, Bonilla F, Muñoz A. The transcription factors Snail1 and Snail2 repress vitamin D receptor during colon cancer progression. J Steroid Biochem Mol Biol. 2010;121:106–109. doi: 10.1016/j.jsbmb.2010.01.014. [DOI] [PubMed] [Google Scholar]
- 28.Pálmer HG, Larriba MJ, García JM, Ordóñez-Morán P, Peña C, Peiró S, Puig I, Rodríguez R, de la Fuente R, Bernad A, Pollán M, Bonilla F, Gamallo C, de Herreros AG, Muñoz A. The transcription factor SNAIL represses vitamin D receptor expression and responsiveness in human colon cancer. Nat Med. 2004;10:917–9. doi: 10.1038/nm1095. [DOI] [PubMed] [Google Scholar]
- 29.Esteban LM, Eisman JA, Gardiner EM. Chapter 12-vitamin D receptor promoter and regulation of receptor expression. Vitamin D. 2005;95:193–217. [Google Scholar]
- 30.Pilon C, Rebellato A, Urbanet R, Guzzardo V, Cappellesso R, Sasano H, Fassina A, Fallo F. Methylation status of vitamin D receptor gene promoter in benign and malignant adrenal tumors. Int J Endocrinol. 2015;2015:375349. doi: 10.1155/2015/375349. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Pandolfi F, Franza L, Mandolini C, Conti P. Immune modulation by vitamin D: special emphasis on its role in prevention and treatment of cancer. Clin Ther. 2017;39:884–893. doi: 10.1016/j.clinthera.2017.03.012. [DOI] [PubMed] [Google Scholar]
- 32.Stubbins RE, Hakeem A, Núñez NP. Using components of the vitamin D pathway to prevent and treat colon cancer. Nutr Rev. 2012;70:721–729. doi: 10.1111/j.1753-4887.2012.00522.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Dougherty U, Mustafi R, Sadiq F, Almoghrabi A, Mustafi D, Kreisheh M, Sundaramurthy S, Liu W, Konda VJ, Khare S, Hart J, Joseph L, Wyrwicz A, Karczmar GS, Li YC, Bissonnette M. The renin-angiotensin system mediates EGF receptor-vitamin D receptor cross-talk in colitis-associated colon cancer. Clin Cancer Res. 2014;20:5848–5859. doi: 10.1158/1078-0432.CCR-14-0209. [DOI] [PMC free article] [PubMed] [Google Scholar]


