Abstract
Background: Since December 2019, there had been an outbreak of COVID-19 in Wuhan, China. At present, diagnosis COVID-19 were based on real-time RT-PCR, which have to be performed in biosafe laboratory and is unsatisfactory for suspect case screening. Therefore, there is an urgent need for rapid diagnostic test for COVID-19. Objective: To evaluate the diagnostic performance and clinical utility of the colloidal gold immunochromatography assay for SARS-Cov-2 specific IgM/IgG anti-body detection in suspected COVID-19 cases. Methods: In the prospective cohort, 150 patients with fever or respiratory symptoms were enrolled in Taizhou Public Health Medical Center, Taizhou Hospital, Zhejiang province, China, between January 20 to February 2, 2020. All patients were tested by the colloidal gold immunochromatography assay for COVID-19. At least two samples of each patient were collected for RT-PCR assay analysis, and the PCR results were performed as the reference standard of diagnosis. Meanwhile 26 heathy blood donor were recruited. The sensitivity and specificity of the immunochromatography assay test were evaluated. Subgroup analysis were performed with respect to age, sex, period from symptom onset and clinical severity. Results: The immunochromatography assay test had 69 positive result in the 97 PCR-positive cases, achieving sensitivity 71.1% [95% CI 0.609-0.797], and had 2 positive result in the 53 PCR-negative cases, achieving specificity 96.2% [95% CI 0.859-0.993]. In 26 healthy donor blood samples, the immunochromatography assay had 0 positive result. In subgroup analysis, the sensitivity was significantly higher in patients with symptoms more than 14 days 95.2% [95% CI 0.741-0.998] and patients with severe clinical condition 86.0% [95% CI 0.640-0.970]. Conclusions: The colloidal gold immunochromatography assay for SARS-Cov-2 specific IgM/IgG anti-body had 71.1% sensitivity and 96.2% specificity in this population, showing the potential for a useful rapid diagnosis test for COVID-19. Further investigations should be done to evaluate this assay in variety of clinical settings and populations.
Keywords: COVID-19, immunoglobulin M/immunoglobulin G, rapid diagnosis
Introduction
In December 2019, there were reports of a number of atypical pneumonia cases in Wuhan, Hubei province of China, and the cause was later determined to be a novel coronavirus now named SARS-Cov-2 [1]. Since then, it had become a pandemic and spread nationally as well as to other countries [2].
The SARS-Cov-2 is a new type of coronavirus and its nucleic acid sequence is different from those of SARS-CoV and MERS-CoV [1,3]. Most patients with SARS-Cov-2 infection develop symptoms of fever, dry cough, dyspnea, myalgia, fatigue, headache, and pneumonia. In severe cases, the disease can rapidly progress into acute respiratory distress syndrome, septic shock, bleeding, and coagulation dysfunction, metabolic acidosis, and death [4]. Laboratory tests have shown that the total number of white blood cells in patients’ peripheral blood was normal or slightly decreased, the lymphocyte count was decreased, C-reactive protein (CRP) and erythrocyte sedimentation rate are increased, and procalcitonin was normal. Chest CT examination reveals multiple small plaques and stromal changes in both lungs of patients, and the plaques and stromal changes can further develop into multiple ground glass opacity [4-6].
The current standard laboratory test for diagnosing COVID-19 is the reverse transcription-polymerase chain reaction (RT-PCR) to detect viral RNAs [7]. However, false negative results of RT-PCR may be obtained if sampling was not carried out properly. RT-PCR also requires sophisticated equipment and high standards of laboratory quality-assurance. Given the fast pandemic of COVID-19 which has already infected tens of thousands of people, it is urgent to develop a quick and easy-to-use method for patients screening. For this purpose, colloidal gold immunochromatography test of IgM/IgG offers the advantages of widely used, (whole blood, serum and plasma can be detected), simple operation (no professional technical personnel required for equipment), fast detection (10-15 minutes for the whole process) and low cost.
In this study, 150 suspected case of COVID-19 were enrolled in Taizhou Public Health Medical Center, Taizhou Hospital of Zhejiang Province, China. The clinical record for each patient was complete. The SARS-Cov-2 specific IgM/IgG antibodies in the peripheral blood of the patients were examined by colloidal gold immunochromatography assay to evaluate their sensitivity, specificity, and seroconversion. The aim of the study was to evaluate the potential of these antibodies to be used for COVID-19 patients screening.
Methods
Study design and participants
We performed this prospective cohort study in Taizhou Public Health Medical Center, Taizhou Hospital, Zhejiang province, China. Between January 20, 2020 to February 2, 2020, we identified 150 patients who met the suspected COVID-19 case definition according to the Diagnosis and Treatment Guideline (trial version 5) of China (Figure 1) [8]. A suspected COVID-19 case was defined as a pneumonia that had related epidemiological history and fulfilled two of the three criteria: fever and/or respiratory symptoms; imaging manifestations of pneumonia; low or normal white-cell count or low lymphocyte count. All patient took isolation for at least 2 weeks. Besides, 26 healthy blood donors from Taizhou Blood Center were also recruited.
Figure 1.
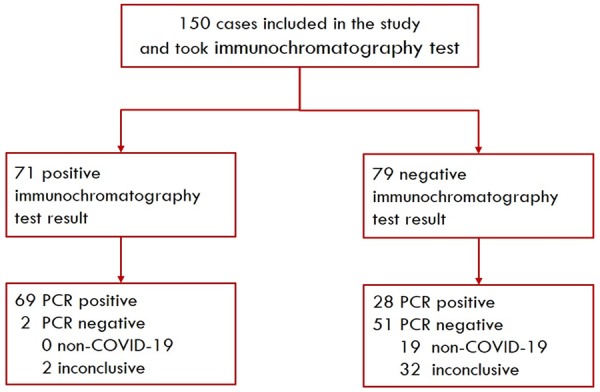
Flow of enrolled suspected COVID-19 patients. Abbreviation: COVID-19 = Coronavirus disease-19.
Reference standard of diagnosis
We took the analysis of real-time reverse-transcription-polymerase chain-reaction (RT-PCR) assay for COVID-19 as the reference standard of diagnosis. At least two different samples were obtained from each patient. If the PCR result was inconclusive, then repeated sample collection was required.
A patient with at least one positive RT-PCR result (CT threshold <37) was defined as PCR positive, and was clinically confirmed COVID-19 diagnosis. Patient with two consecutive negative RT-PCR results (CT threshold >40) was defined as PCR negative, but exclusion of COVID-19 could not be made merely based on PCR results as the sensitivity of current RT-PCR analysis for COVID-19 is still unsatisfactory. In this study, a PCR negative patient would be further diagnosed as non-COVID-19 if the symptom could be well explained by identified condition or infection of the patient and recover after corresponding treatments. Other PCR negative patients were considered inconclusive.
The severity of patients with confirmed COVID-19 were categorized into ordinary and severe. A severe case was defined as a case met any of the following: 1. Respiratory distress, RR≥30/min; 2. In resting state, oxygen saturation ≤93%; 3. Partial arterial oxygen pressure (PaO2)/oxygen absorption concentration (FiO2) ≤300 mmHg.
This study obtained approval from the Medical Ethics Committee of Taizhou Hospital, Zhejiang province of China, and informed consent was obtained from each enrolled subject.
Procedure
For each participant, exposure history, clinical symptoms (including fever, cough, fatigue, diarrhea, dizziness, chest tightness), chest computed tomographic (CT), and comorbidities were collected. Disease onset date was defined as the day when symptoms were noticed.
For each participant, 3 mL of peripheral venous blood was collected; serum samples were collected from the blood. Nasopharyngeal and oropharyngeal swab samples were collected and sent to referral laboratory for RT-PCR test. If the RT-PCR result was negative or indetermined, a repeated sample collection and PCR test would be performed by CDC, Taizhou. All patients would be quarantined for at least 2 weeks according to the clinical guideline of China.
Blood samples of participants were tested for IgM/IgG antibodies against COVID-19 using the colloidal gold immunochromatography antibody detection kit (Shanghai Outdo Biotech Co. Ltd, China, LOT: 20200101). The results of interpretation was showed in Figure 2. The sera were incubated at 56°C for 30 minutes to heat-inactivate viruses before serological analysis.
Figure 2.
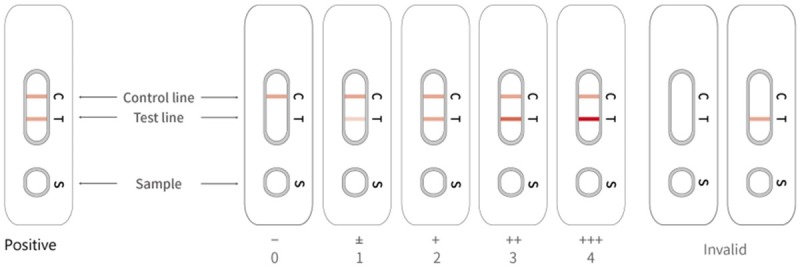
The colloidal gold immunochromatography results and interpretation. The appearance of two lines indicates a positive result, whereas a valid negative test produces only the control line. Intensity of antibody: 0=IgM/IgG negative, 1=IgM/IgG weakly positive(±), 2=IgM/IgG +, 3=IgM/IgG ++, 4=IgM/IgG +++.
Statistical analysis
Sample size estimates were based on the number of positive (confirmed COVID-19) and negative (exclude COVID-19) cases to achieve a 95% CI of the sensitivity and specificity estimates up to 10%. Categorical variables were described as frequency rates and percentages, continuous variables were described using mean standard deviation or median and interquartile range (IQR) values as appropriate. The continuous variable data were compared using independent t-test when the data were normally distributed; otherwise, the Mann-Whitney test was conducted. The categorical variables were compared using a χ2 test or fisher’s exact test. 95% CI for proportions were calculated using the Clopper-Pearson exact binomial method. Subgroup analysis of the sensitivity and specificity were performed with respect to age, sex, period from symptom onset and clinical severity. Statistical analyses were performed using the SPSS (version 22.0), GraPhpad Prism 7.0 software and R 3.5.1. P values ≤0.05 were considered statistically significant.
Results
Clinical characteristics
The clinical characteristics of the 150 suspected COVID-19 cases, grouped into 97 PCR positive cases (COVID-19 confirmed) and 53 PCR negative cases (COVID-19 not confirmed), were demonstrated in Table 1.
Table 1.
Clinical characteristics of patients
| Characteristics | PCR Positive group (n=97) | PCR Negative group (n=53) | P Valuea |
|---|---|---|---|
| Sex - n (%) | 0.615 | ||
| Male | 59 (60.8) | 30 (56.6) | |
| Female | 38 (39.2) | 23 (43.4) | |
| Median age (IQR) - years | 38.0 (46.0-56.0) | 32.0 (20.0-42.5) | 0.000 |
| Age group - n (%) | 0.055 | ||
| ≤35 | 17 (17.5) | 31 (58.5) | |
| 36-55 | 54 (55.7) | 15 (28.3) | |
| 56-65 | 17 (17.5) | 4 (7.5) | |
| >65 | 9 (9.3) | 3 (5.7) | |
| Period from symptom onset - n (%) | 0.004 | ||
| 0-7 days | 40 (41.2) | 50 (94.3) | |
| 8-14 days | 33 (34.0) | 3 (5.7) | |
| ≥15 days | 24 (24.7) | 0 (0.0) | |
| Clinical severity - n (%) | - | ||
| Ordinary | 76 (78.4) | - | |
| Severe | 21 (21.6) | - | |
| Wuhan exposure - n (%) | 75 (77.3) | 25 (47.2) | 0.000 |
| Chest CT - n (%) | 0.000 | ||
| Ground glass opacity | 95 (97.9) | 27 (50.9) | |
| Normal | 2 (2.1) | 26 (49.1) | |
| Clinical symptoms - n (%) | |||
| Fever | 71 (73.2) | 30 (56.6) | 0.038 |
| Cough | 19 (19.6) | 23 (43.4) | 0.002 |
| Fatigue | 3 (3.1) | 3 (5.7) | 0.740 |
| Dizziness | 3 (3.1) | 2 (3.8) | - |
| Chest tightness | 3 (3.1) | 6 (11.3) | 0.095 |
| Diarrhea | 2 (2.1) | 1 (1.9) | - |
| Underlying comorbidities - n (%) | |||
| Hypertension | 16 (16.5) | 9 (17.0) | 0.939 |
| Diabetes | 7 (7.2) | 1 (1.9) | 0.313 |
| Tuberculosis | 2 (2.1) | 0 (0.0) | 0.540 |
| Malignancy | 2 (2.1) | 0 (0.0) | 0.540 |
| Chronic liver disease | 3 (3.1) | 0 (0.0) | 0.494 |
| Other chronic disease | 17 (17.5) | 8 (15.1) | 0.702 |
| No comorbidity | 53 (54.6) | 35 (66.0) | 0.175 |
P values indicate differences between PCR Positive group and PCR Negative group.
P<0.05 was considered statistically significant.
Among the 97 COVID-19 patients, there were 59 males (60.8%) and 38 females (39.2%); The median of age was 46 (IQR, 38-56); 75 (77.3%) patients had histories of exposure in Wuhan; 21 (21.6%) had severe clinical condition; Chest CT of 95 patients (97.9%) showed ground glass opacity; Fever (73.2%) and cough (19.6%) were the most common symptoms; hypertension (16.5%) and diabetes (7.2%) were the most common comorbidity conditions.
Comparing the PCR positive and PCR negative groups, some characteristics seemed to associate with the presence of COVID-19, including age ≥35, related exposure history, ground glass opacity in chest CT, fever, comorbidities like diabetes. However none of these characteristics showed high enough specificity, which illustrated the difficulty of differential diagnosis in the suspect COVID-19 cases.
Diagnostic performance of immunochromatography assay for SARS-Cov-2 specific IgM/IgG
In 150 suspect COVID-19 cases, the colloidal gold immunochromatography assay showed (as in Table 2) the sensitivity 71.1% [95% CI 0.609-0.797], specificity 96.2% [95% CI 0.859-0.993], positive predictive value 97.2% [95% CI 0.893-0.995] and negative predictive value 64.6% [95% CI 0.529-0.748] taking the RT-PCR results as the reference standard. In the 26 blood samples of healthy donors, the immunochromatography assay gave 0 positive result, achieving specificity 100% [95% CI 0.868-1.000].
Table 2.
Diagnostic performance of the immunochromatography assay test versus RT-PCR reference standard
| RT-PCR | Sensitivity (95% CI) | Specificity (95% CI) | Positive predictive value (95% CI) | Negative predictive value (95% CI) | |||
|---|---|---|---|---|---|---|---|
|
| |||||||
| Positive | Negative | ||||||
| Specific IgM/IgG | Positive | 69 | 2 | 0.711 (0.609-0.797) | 0.962 (0.859-0.993) | 0.972 (0.893-0.995) | 0.646 (0.529-0.748) |
| Negative | 28 | 51 | |||||
| Total | 97 | 53 | |||||
Abbreviation: RT-PCR = reverse transcription-polymerase chain reaction RT-PCR; CI = confidence interval.
We also compared the sensitivity of immunochromatography assay in different subgroups. The subgroup analysis results were shown in Figure 3. The sensitivity of assay increased significantly along with the symptom duration, namely 55% in 0-7 days, 73% in 8-14 days and 96% after 14 days. Besides, the sensitivity of assay was also higher in the male (76.3%) than in the female (63.2%), higher in patients older than 65 (100%) than in other age groups, higher in patients with severe clinical condition (86%). Limited by the sample size, these association was not statistically significant, which required further investigation.
Figure 3.
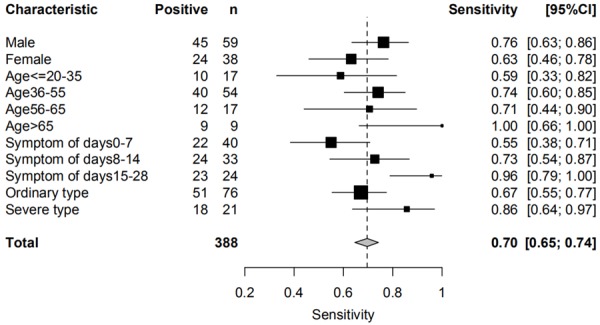
Sensitivities of immunochromatography assay in different subgroups. Abbreviation: CI = confidence interval.
Seroconversion time of specific IgM/IgG in COVID-19 patients
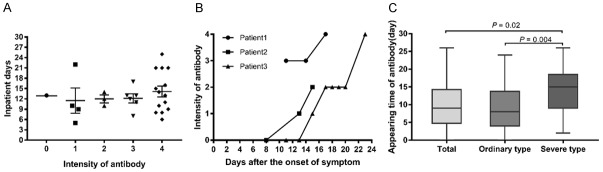
To better understand the antibody response against COVID-19, we also performed immunochromatography assay test for the 97 confirmed COVID-19 patients during the follow-up. Among the 28 COVID-19 cases with antibody negative at the enrollment, 27 turned to be antibody positive before discharge. The immunochromatography assay results and inpatient days of these 28 patients were shown in Figure 4A. There was no significant difference in the days after the onset of symptom in antibody intensity (P>0.05). Their antibody intensity between this finding suggested the immunochromatography assay a useful tool in epidemiological survey and surveillance.
Figure 4.
Time of seroconversion from onset of symptoms in patients infected with COVID-19. A. Scatter diagram of antibody level distribution in 28 patients one day before discharge. B. Seroconversion in patients 1, 2, 3. C. Appearing time of IgM/IgG. Intensity of antibody: 0=IgM/IgG negative, 1=IgM/IgG weakly positive(±), 2=IgM/IgG +, 3=IgM/IgG ++, 4=IgM/IgG +++. P<0.05 was considered statistically significant. Abbreviation: COVID-19 = Coronavirus disease-19.
Three patients underwent the serosurveillance during the quarantine. Their antibody intensity assessed by the semi-quantitative reading of immunochromatography assay was shown in Figure 4B. It implied that both the seroprevalence and intensity of SARS-Cov-2 specific IgM/IgG grew in the course of disease.
Among the 97 COVID-19 patients, seroconversion was observed on day median 9 (5-14.5) from the onset of symptom. In the 21 patient with severe clinical conditions, the seroconversion was observed on average later than those without severe clinical conditions (15 (9-18.5) days vs 8 (4-13.7) days) (P=0.004) (Figure 4C). Therefore the antibody detection may serve as an potential prognositic factor of the COVID-19.
Discussion
We used and evaluated a convenient colloidal gold immunochromatography assay to detect IgM/IgG specific to COVID-19 employing synthetic antigens of the S, M, and N proteins of COVID-19. Serous diagnostic tests such as rapid detection of antiviral antibodies or viral antigens had been widely used in many clinical laboratories [9-11]. The current standard laboratory test for diagnosing COVID-19 infection is to use reverse transcription-polymerase chain reaction (RT-PCR) for viral genomic RNA [7,12]. However, RT-PCR is relatively time-consuming, expensive and has high requirements for laboratory. Current clinical observations indicate that the sensitivity and reliability of RT-PCR are unsatisfactory for COVID-19. These defects of RT-PCR may hinder the infection control efforts. Thus, a rapid and accurate assay for COVID-19 screening or diagnosis which can be used in local hospital is highly desired. The Colloidal gold immunochromatography assay has advantages of time saving (10-15 min), relatively cheap and easy to use, making it a suitable candidate of rapid screening assay for the currently widespread COVID-19 infection which threating the world. To our knowledge, this was the first study to evaluate the diagnosis performance and clinical utility of IgM/IgG test for COVID-19 in hospital setting.
In this study, we found that the IgM/IgG test assay demonstrated high sensitivity 71.1% [95% CI 0.609-0.797] and specificity 96.2% [95% CI 0.859-0.993] in 150 suspect COVID-19 cases taking RT-PCR result as reference standard. In addition, considering the imperfect sensitivity of the reference standard (RT-PCR), it was probable that the two inconclusive cases with positive antibody result and negative PCR result were actual COVID-19 cases omitted by current diagnosis protocol.
Our findings also provided insights to the anti-body response in COVID-19 patients. We found that the sensitivity of antibody assays as well as the antibody density both increased along with the course of disease. The sensitivity for patients within 7 days from onset of symptom was 55.0% and for patients after 14 days after onset of symptom was 95.8% which was informative for the application of antibody dection in epedimiolgical survey and surveillance. Besides, seroconversion time and antibody density may also serve as a prognositic factor of COVID-19.
In general, the colloidal gold immunochromatography assay for COVID-19 IgM/IgG had high sentivity and specificity. It provided a fast and accurate diagnostic method that can be used as a triage test before nucleic acid analysis in several important scenarios for COVID-19 infection control: diagnosing the patients with fever of unknown origin in outpatient and emergency department in area of COVID-19 spreading; managing suspect cases or close contacts of confirmed cases under quarantine; and screening patients with asymptomatic infection which may be of particularly importance for COVID-19. This study was a single center hospital based study and therefore mainly represent the local situation. Multi-center research was required to furher evaluate the diagnostic performance in variety of population, prevalence and clinical setting, and to understand the anti-body response in COVID-19.
Acknowledgements
This work was financially supported by National Natural Science Foundation of China (Grant NO. 81672086 and 81903417). Yi Shen, Department of Epidemiology and Health Statistics School of Public Health, Zhejiang University.
Disclosure of conflict of interest
None.
References
- 1.Zhu N, Zhang D, Wang W, Li X, Yang B, Song J, Zhao X, Huang B, Shi W, Lu R, Niu P, Zhan F, Ma X, Wang D, Xu W, Wu G, Gao GF, Tan W China Novel Coronavirus Investigating and Research Team. A novel coronavirus from patients with pneumonia in China, 2019. N Engl J Med. 2020;382:727–733. doi: 10.1056/NEJMoa2001017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.COVID-19 National Incident Room Surveillance Team. COVID-19, Australia: Epidemiology Report 5 (Reporting week ending 19:00 AEDT 29 February 2020) Commun Dis Intell (2018) 2020:44. doi: 10.33321/cdi.2020.44.20. [DOI] [PubMed] [Google Scholar]
- 3.Chan JF, Kok KH, Zhu Z, Chu H, To KK, Yuan S, Yuen KY. Genomic characterization of the 2019 novel human-pathogenic coronavirus isolated from a patient with atypical pneumonia after visiting Wuhan. Emerg Microbes Infect. 2020;9:221–236. doi: 10.1080/22221751.2020.1719902. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Huang C, Wang Y, Li X, Ren L, Zhao J, Hu Y, Zhang L, Fan G, Xu J, Gu X, Cheng Z, Yu T, Xia J, Wei Y, Wu W, Xie X, Yin W, Li H, Liu M, Xiao Y, Gao H, Guo L, Xie J, Wang G, Jiang R, Gao Z, Jin Q, Wang J, Cao B. Clinical features of patients infected with 2019 novel coronavirus in Wuhan, China. Lancet. 2020;395:497–506. doi: 10.1016/S0140-6736(20)30183-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Kanne JP. Chest CT findings in 2019 novel coronavirus (2019-nCoV) infections from Wuhan, China: key points for the radiologist. Radiology. 2020;295:16–17. doi: 10.1148/radiol.2020200241. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Wang D, Hu B, Hu C, Zhu F, Liu X, Zhang J, Wang B, Xiang H, Cheng Z, Xiong Y, Zhao Y, Li Y, Wang X, Peng Z. Clinical characteristics of 138 hospitalized patients with 2019 novel coronavirus-infected pneumonia in Wuhan, China. JAMA. 2020 doi: 10.1001/jama.2020.1585. [Epub ahead of print] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Corman VM, Landt O, Kaiser M, Molenkamp R, Meijer A, Chu DKW, Bleicker T, Brünink S, Schneider J, Schmidt ML, Mulders DGJC, Haagmans BL, van der Veer B, van den Brink S, Wijsman L, Goderski G, Romette JL, Ellis J, Zambon M, Peiris M, Goossens H, Reusken C, Koopmans MPG, Drosten C. Detection of 2019 novel coronavirus (2019-nCoV) by real-time RT-PCR. Euro Surveill. 2020;25 doi: 10.2807/1560-7917.ES.2020.25.3.2000045. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.<Pneumonia diagnosis and treatment for novel coronavirus infection - trial version 5.pdf>. http://kns.cnki.net/KCMS/detail/11.2787.R.20200208.1034.002.html.
- 9.Yang F, Feng S, Li Y, He Y, Jin X, Wang X, Zhou Z, Xiao Y, Bi D. Development of immunochromatographic test strips for rapid, quantitative detection of H9AIV antibodies. J Chromatogr B Analyt Technol Biomed Life Sci. 2018;1095:59–64. doi: 10.1016/j.jchromb.2018.07.024. [DOI] [PubMed] [Google Scholar]
- 10.Li X, Chen H, Wei J, Lv N, You L. The evaluation of colloidal gold immunochromatographic assay (GICA) for rapid diagnosis of influenza A disease. Clin Chem Lab Med. 2011;49:1533–1537. doi: 10.1515/CCLM.2011.235. [DOI] [PubMed] [Google Scholar]
- 11.Paulini I, Siqueira-Silva J, Thomaz L, Rocha L, Harsi C, Bellei N, Granato C. Development of a prototype immunochromatographic test for rapid diagnosis of respiratory adenovirus infection. Braz J Infect Dis. 2017;21:500–506. doi: 10.1016/j.bjid.2017.03.023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Chu DKW, Pan Y, Cheng SMS, Hui KPY, Krishnan P, Liu Y, Ng DYM, Wan CKC, Yang P, Wang Q, Peiris M, Poon LLM. Molecular diagnosis of a novel coronavirus (2019-nCoV) causing an outbreak of pneumonia. Clin Chem. 2020 doi: 10.1093/clinchem/hvaa029. [Epub ahead of print] [DOI] [PMC free article] [PubMed] [Google Scholar]