Abstract
Brain tumors include those that originate within the brain (primary tumors) as well as those that arise from other cancers (metastatic tumors). The fragile nature of the brain poses a major challenge to access focal malignancies, which certainly limits both diagnostics and therapeutic approaches. This limitation has been alleviated with the advent of liquid biopsy technologies. Liquid biopsy represents a highly convenient, fast and non-invasive method, which allows multiple sampling and dynamic pathological detection. Biomarkers derived from liquid biopsies can promptly reflect changes on the gene expression profiling of tumors. Biomarkers derived from tumor cells contain abundant genetic information, which may provide a strong basis for the diagnosis and the individualized treatment of brain tumor patients. A series of body fluids can be assessed for liquid biopsy, including peripheral blood, cerebrospinal fluid (CSF), urine or saliva. Interestingly, the sensitivity and specificity of biomarkers from the CSF of patients with brain tumors is typically higher than those detected in the peripheral blood and other sources. Hence, here we describe and properly discuss the clinical roles of distinct classes of CSF biomarkers, isolated from patients with brain tumors, such as circulating tumor DNA (ctDNA), microRNA (miRNA), proteins, and extracellular vesicles (EVs).
Keywords: Liquid biopsy, cerebrospinal fluid, brain tumor, biomarkers
Introduction
CSF is an important source of potential molecular biomarkers, mostly collected by lumbar puncture or surgery around the brain area. For instance, CSF contains various biomarkers, such as ctDNA, miRNA, proteins, and EVs, which are typically derived from brain tumor cells [1]. Usually, tumor cells co-exist with their microenvironment. Therefore, tumor-related markers can be more prominent in fluids nearby the site of the disease. CSF is usually considered an extension of the extracellular compartment within the central nervous system (CNS) and, as such, a major route for brain tumors [2]. The biomarker content of patients with brain tumors is mostly low or even undetectable, since the blood-brain barrier has a major impact on the release of putative biomarkers into the systemic circulation. Nevertheless, CSF is a suitable repository of clinical biomarkers, and an increasing number of studies have reported that CSF-derived biomarkers are more abundant than those in the peripheral blood and other sources. For instance, ctDNAs derived from brain tumor cells are more abundantly present in the CSF than in the plasma [3]. In addition, CSF is a better source of circulating nucleic acids than the plasma from brain tumor patients [4]. One particular clinical study, composed by eight brain tumor patients, indicated that the detection of tumor-specific mutations in CSF ctDNA has higher sensitivity when comparing with plasma ctDNA (100% vs. 37.5%, respectively) [5]. Although plasma is still a more common source for the quantitative isolation and detection of nucleic acids (possibly due to the negative impact of the blood brain barrier), CSF has been a more qualitative source of nucleic acids [5,6]. Hence, CSF may provide a less invasive diagnosis and treatment monitoring of brain tumors patients [7]. Currently, liquid biopsy techniques including Enzyme-linked Immunosorbent Assay (ELISA), Polymerase Chain Reaction (PCR), and Next-Generation Sequencing (NGS) have been standardized for the detection of potential CSF biomarkers. Based on these techniques, changes on the expression of ctDNA, miRNA, proteins, and EVs (Figure 1) from brain tumor cells can be examined in the CSF and, more precisely, translated into the diagnosis and treatment, as well as monitoring recurrence and treatment response of brain tumors.
Figure 1.
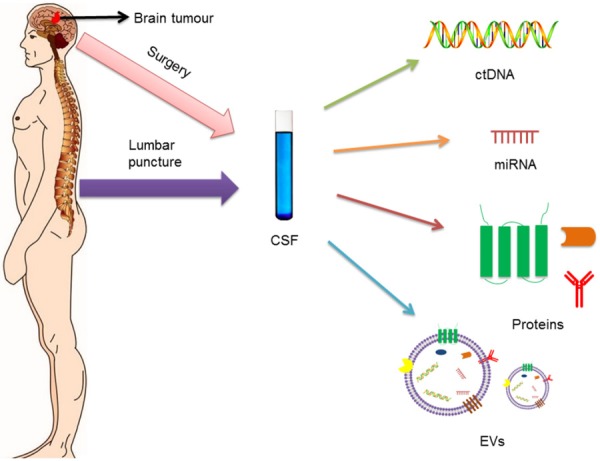
Molecular biomarkers, secreted by brain tumor cells, can enter into the cerebrospinal fluid (CSF). Therefore, CSF may contain increased levels of ctDNA, miRNA, proteins and EVs. Fortunately, CSF is easily obtained through lumbar puncture or surgery. Using liquid biopsy techniques, changes on the expression of ctDNA, miRNA, proteins and EVs from brain tumor cells can be examined in the CSF.
Cerebrospinal fluid biomarkers related to brain tumors
CtDNAs
CtDNA is referred to the DNA that comes from tumor cells and stably circulates in body fluids. Tumor-derived ctDNAs have been extracted from CSF samples of patients with brain tumors, and a series of genes mutations have been assessed [8]. Interestingly, one particular study has been demonstrated that CSF-derived ctDNAs can better reflect sequence mutations in driving genes when compared to plasma ctDNAs [9]. Two new ways have been utilized to detect genetic mutations: droplet-digital PCR (ddPCR) and NGS [10-12]. One particular study has performed ddPCR with targeted amplicon sequencing to search for mutations in CSF ctDNA of primary and metastatic brain tumor patients [13]. A number of tumor gene mutations were detected in CSF-derived ctDNAs from 7 patients with solid brain tumors, where 6 had detectable tumor mutations in at least one of the following genes: NF2, AKT1, BRAF-V600, NRAS, KRAS, TP53, and EGFR (Table 1) [14]. Interestingly, gene mutations in RGS12, CASR, AQR, MTMR4, and KDM6A were detected in CSF-derived ctDNA from medulloblastoma patients [8]. Moreover, CSF-derived ctDNAs were extracted from 53 patients to study alterations in 341 cancer-associated genes by NGS, and somatic alterations were detected in more than half of the patients with primary and metastatic brain tumors, but not detected in patients without brain tumors [11].
Table 1.
CtDNAs characterized in CSF samples derived from patients with brain tumors
| Brain Tumor Types | Example | Findings | Clinical Roles | Reference |
|---|---|---|---|---|
| primary tumors | ||||
| Gliomas | IDH1, TP53, EGFR, PTEN, FGFR2, ERBB2 | mutations | diagnosis/therapy | [3] |
| EGFR | amplification | diagnosis/therapy | [15] | |
| CDKN2A/B | deletions | diagnosis/therapy | [15] | |
| IDH1/2, TP53, ATRX, TERT, H3F3A, HIST1H3B | mutations | diagnosis/therapy | [6] | |
| H3F3A, HIST1H3B, TP53, ATRX, PDGFRA, FAT1, PPM1D, IDH1, NF1, PIK3CA, ACVR1 | mutations | diagnosis/therapy | [5] | |
| H3F3A, HIST1H3B | mutations | diagnosis/therapy | [16] | |
| PCNSL | MYD88 | mutations | diagnosis/therapy | [17-19] |
| Medulloblastoma | RGS12, CASR, AQR, MTMR4, KDM6A | mutations | diagnosis/therapy | [8] |
| VS | NF2 | mutations | diagnosis/therapy | [14] |
| Meningioma | AKT1 | mutations | diagnosis/therapy | [14] |
| Metastatic tumors | ||||
| SCNSL | MYD88 | mutations | diagnosis/therapy | [20] |
| Melanoma | BRAF-V600, NRAS | mutations | diagnosis/therapy | [11,14] |
| Lung cancer | EGFR | mutations | diagnosis/therapy | [11,14,26] |
| KRAS | mutations | diagnosis/therapy | [11] | |
| Breast cancer | TP53, PIK3CA | mutations | diagnosis/therapy | [27] |
| Colon cancer | KRAS, TP53 | mutations | diagnosis/therapy | [14] |
| Bladder cancer | TP53, AKT2 | mutations | diagnosis/therapy | [11] |
| Ovarian cancer | BRCA1, CDKN2B | mutations | diagnosis/therapy | [11] |
Gene mutations in IDH1, TP53, EGFR, PTEN, FGFR2, and ERBB2 (Table 1) have been detected in CSF-derived ctDNA of patients with glioblastoma (GBM) [3]. The genetic alterations including amplification of EGFR and deletions of CDKN2A/B have been also observed in CSF-derived ctDNA from GBM patients by NGS (Table 1) [15]. Gene mutation analyses of other genes, such as IDH1/2, TP53, ATRX, TERT, H3F3A, and HIST1H3B, have been detected in CSF ctDNAs and equally contributed to the diagnosis and treatment of diffuse gliomas patients (Table 1) [6]. Similarly, the most frequently genes mutations, such as H3F3A, HIST1H3B, TP53, ATRX, PDGFRA, FAT1, PPM1D, IDH1, NF1, PIK3CA, and ACVR1 (Table 1), have been detected in CSF ctDNA of brainstem glioma patients [5]. Of note, it is appropriate to point out that H3F3A and HIST1H3B mutations were also detected in CSF-derived ctDNAs of diffuse midline glioma patients (Table 1) [16].
Similarly, MYD88 mutation (Table 1) has been detected in CSF extracted from patients with primary central nervous system lymphoma (PCNSL) [17-19]. Gene mutation in MYD88 has also been detected in CSF-derived ctDNA of one patient with secondary central nervous system lymphoma (SCNSL) [20]. Another genes whose mutations have diagnostic potential, such as CD79B, were found in PCNSL patients [21]. However, no CD79B mutation has been detected in CSF so far. Interestingly, several studies have also shown that neither CD79B nor MYD88 mutations have been found in glioma patients [22,23]. Still, CD79B and MYD88 mutations, which were detected in CSF-derived ctDNAs, may play an important role distinguishing PCNSL from other brain tumors [24]. Therefore, MYD88 and CD79B could be potentially used as molecular signatures for lymphomas.
In patients with brain metastases derived from melanoma, BRAF-V600 and NRAS mutations (Table 1) have been monitored in CSF-derived ctDNAs [11,14]. Since ctDNA is not suitable to track tumor evolution in the brain, it is unable to monitor brain metastasis due to melanoma [25]. Similarly, we may also find other genetic mutations in CSF-derived ctDNAs from patients with other types of brain metastases. For example, EGFR [11,26] and KRAS [11] mutations (Table 1) might be detected in CSF-derived ctDNAs in cases of brain metastasis due to primary lung cancer. TP53 and PIK3CA mutations (Table 1) were also detected in CSF-derived ctDNAs from HER2-positive brain metastasis originated from breast cancer [27]. In patients with brain metastases due to primary bladder cancer, TP53 and AKT2 mutations have been detected in CSF-derived ctDNAs. Moreover, BRCA1 and CDKN2B mutations (Table 1) were linked to brain metastases due to primary ovarian cancer [11].
MiRNAs
MiRNAs are small non-coding RNAs (~22 nucleotides in length) that can be released from brain tumor cells [28]. Free miRNAs possibly result from tumor cell death or secretion of tumor cells, leading to the release of nucleic acids in the extracellular matrix. The main function of miRNAs includes the modulation of gene expression by mRNA silencing and/or degradation. Interestingly, a single miRNA may be able to target several mRNAs simultaneously (pleiotropic effects) [28,29]. The association between miRNAs and brain tumorigenesis was first introduced in 2005. Three years later, the presence of miRNAs in circulating body fluids from patients with brain tumor was finally detected [30]. In fact, miRNAs can be released into biological fluids such as plasma or CSF [31]. However, due to the existence of the blood-brain barrier, it has been hypothesized that miRNAs present in the CSF can better reflect the brain physiology and related pathologies more accurately than plasma miRNAs [32]. Several studies have demonstrated the causes and significance of extracting miRNAs from CSF [14,32-34]. Still, due to the presence of RNA-degrading enzymes in the blood [35], the expression/secretion of miRNAs in the CSF appears to define, more accurately, the malignant process of brain tumors [36].
Differences in brain miRNA profiles may depend on the source of the brain region [37], suggesting that different types of brain tumors correspond to distinct types and levels of miRNAs [38,39]. Extracellular vesicles (EVs) are nanometer size membrane-closed particles that can contain a variety of miRNAs [40,41]. The incorporation of miRNAs into EVs results in protection from degradation in the biofluid environment [42]. EVs can be isolated from CSF [43,44] and, apparently, this procedure can be more feasible that isolating and sequencing exosomal miRNAs from CSF [32,33,37]. Multiple CSF-related miRNAs have been found to be significantly associated with primary and metastatic brain tumors. Intriguingly, certain miRNAs may be upregulated in some brain tumors while downregulated in others, indicating that combinations of miRNA signatures can be useful to distinguish brain tumors. For instance, meningiomas and brain metastasis show elevated expression of miR-935, while miR-935 expression is absent in lymphomas and gliomas (Table 2) [45]. Similarly, miR-451 and miR-711 are upregulated in meningiomas, gliomas, and medulloblastoma while downregulated in lymphomas (Table 2) [45]. In particular, miR-125b and miR-223 are important diagnostic biomarkers for GBM, medulloblastoma, and brain metastasis (Table 2) [45]. Therefore, differential miRNA expression can be used as an unique approach for the minimally invasive diagnosis of GBM [33]. CSF-related miRNAs have also been extracted from 118 patients diagnosed with different types of brain tumors and non-neoplastic neuropathologies [32]. As a result, miR-10b and miR-21 levels in the CSF were noticeably increased in GBM and brain metastasis patients when compared with tumors in remission and other non-neoplastic conditions (Table 2) [32]. In addition, miR-200 levels (Table 2) were solely elevated in brain metastases, but not under other pathological conditions, which allows the discrimination between GBM and metastatic brain tumors. Comparative analysis of these particular miRNAs allowed the distinction of GBM and metastatic brain tumors from healthy controls, with an accuracy of 91-99% [32]. The levels of miR-15b were also significantly elevated in gliomas, suggesting that the combined measurement of miR-15b and miR-21 levels could permit a more comprehensive diagnosis of gliomas than solely analyzing miR-21 yields (Table 2) [33]. In addition, a number of miRNAs, including miR-21, miR-218, miR-193b, miR-331, miR-374a, miR-548c, miR-520f, miR-27b, and miR-30b were also detected in the CSF and closely related to GBM differentiation (Table 2) [43].
Table 2.
MiRNAs characterized in CSF samples derived from patients with brain tumors
| Brain Tumor Types | Example | Findings | Clinical Roles | Reference |
|---|---|---|---|---|
| primary tumors | ||||
| Gliomas | miR-451, miR-711 | upregulated | diagnosis/therapy response | [45] |
| miR-125b, miR-223 | upregulated | diagnosis/therapy response | [45] | |
| miR-10b | Upregulated | diagnosis/therapy response/tumor relapse | [32] | |
| miR-21 | upregulated | diagnosis/therapy response/tumor relapse | [32,44,90,91] | |
| miR-15b | upregulated | diagnosis/therapy response | [33] | |
| miR-21, miR-218, miR-193b, miR-331, miR-374a, miR-548c, miR-520f, miR-27b, miR-30b | upregulated | diagnosis/therapy response | [43] | |
| miR-151a | upregulated | therapy response | [104] | |
| PCNSL | miR-21, miR-19b, miR-92 | upregulated | diagnosis/therapy response | [38,46] |
| miR-451, miR-711 | upregulated | diagnosis/therapy response | [45] | |
| Medulloblastoma | miR-451, miR-711 | upregulated | diagnosis/therapy response | [45] |
| miR-125b, miR-223 | upregulated | diagnosis/therapy response | [45] | |
| Meningioma | miR-451, miR-711, miR-935 | upregulated | diagnosis/therapy response | [45] |
| Metastatic tumors | ||||
| SCNSL | miR-30c | upregulated | diagnosis | [47] |
| Lung cancer | miR-10b, miR-21, miR-200 | upregulated | diagnosis/therapy response/tumor relapse | [32] |
| miR-125b, miR-223, miR-935 | upregulated | diagnosis/therapy response | [45] | |
| Breast cancer | miR-10b, miR-21, miR-200 | upregulated | diagnosis/therapy response/tumor relapse | [32] |
| miR-125b, miR-223, miR-935 | upregulated | diagnosis/therapy response | [45] |
The levels of miR-21 are largely overexpressed in gliomas but, importantly, they can be also overexpressed in PCNSL. Except for miR-21, other miRNAs like miR-19b and miR-92a can also be helpful in the diagnosis and monitoring of PCNSL [38]. One clinical study, composed by thirty-nine PCNSL patients, indicated that ~97% sensitivity could be achieved in the diagnosis of PCNSL by combining with the CSF analyses of miR-21, miR-19b, and miR-92 levels (Table 2) [46]. Interestingly, one particular study has indicated that miR-30c levels in CSF noticeably increased in SCNSL patients when compared with PCNSL (Table 2) [47].
Melanoma has a strong tendency to metastasize to the brain. CSF cytology is often used to search for melanoma-derived brain metastases. However, this procedure is not sensitive enough to diagnose this metastatic subtype [30]. Fortunately, it has been observed that the presence of three mRNA markers in the CSF-MAGE-3, MART-1 and tyrosinase-may diagnose melanoma brain metastasis. The correlation between the detection of these melanoma-associated RNAs in the CSF and the development of CNS metastases, after 3 months, is significant [48]. Nevertheless, the clinical utility of miRNAs as CSF biomarkers has not been validated yet. Further research aiming the detection of miRNAs in the CSF of patients with melanoma-derived brain metastases is still warranted.
Proteins
Similar to nucleic acids detected in the CSF, certain protein biomarkers also appear to be more concentrated in this compartment than in the plasma. For instance, the levels of glial fibrillary acidic protein (GFAP) were quantitatively determined in the CSF of brain tumor patients. Hence, it was observed that GFAP levels from GBM patients surpassed those from other brain tumors and cerebral lesions of distinct etiology (Table 3) [49]. Other proteins detected in the CSF, including Tenascin, Osteopontin (OPN), and Matrix metalloproteinases (MMPs), have also been elevated in glioma patients. Extracellular matrix (ECM) is a significant component of this environment. Tenascin is present in the ECM and it is highly expressed during development in migratory cells. The levels of tenascin in CSF are reported to increase in astrocytic tumors when compared to non-astrocytic primary CNS tumors, metastases and non-malignant controls (Table 3) [50]. Tenascin levels appear to increase proportionally to the grade of astrocytic tumors [50]. OPN is a crucial chemokine for macrophages, mediates the interaction between GBM tumor cells and the innate immune system [51]. In the CSF, the levels of OPN and its derivatives are markedly increased in glioma patients when compared to healthy patients and control patients affected by other brain tumors (Table 3) [52]. MMPs exist in both membrane-bound and secreted forms. Analysis of MMP-2 and MMP-9 levels in the CSF may be conducive to diagnose gliomas and even estimate tumor recurrence (Table 3) [53]. Due to its role in the metastasis and invasion of brain tumors, extensive work has been pursued to develop MMP inhibitors for their treatment.
Table 3.
Proteins characterized in CSF samples derived from patients with brain tumors
| Brain Tumor Types | Example | Findings | Clinical Roles | Reference |
|---|---|---|---|---|
| primary tumors | ||||
| Gliomas | GFAP | high levels | diagnosis/therapy response | [49] |
| Tenascin | high levels | diagnosis/therapy response | [50] | |
| OPN | high levels | diagnosis/therapy response/tumor relapse | [52] | |
| MMP-2, MMP-9 | high levels | diagnosis/therapy/tumor relapse | [53] | |
| VEGF | high levels | diagnosis/therapy/therapy response | [54-56] | |
| CCL4 | high levels | diagnosis/therapy | [55] | |
| FGF | high levels | diagnosis | [56] | |
| NGF | high levels | diagnosis | [57] | |
| IL-6 | high levels | diagnosis/therapy/therapy response | [58] | |
| IL-8 | high levels | diagnosis/therapy | [59] | |
| PCNSL | CXCL13 | high levels | diagnosis | [60] |
| IL-10 | high levels | diagnosis | [60,61] | |
| IL-6, B2M | high levels | diagnosis | [61] | |
| sIL-2R | high levels | diagnosis | [61,62] | |
| sCD27 | high levels | diagnosis | [64] | |
| ATIII | high levels | diagnosis/therapy response | [65] | |
| OPN | high levels | diagnosis | [66] | |
| Neopterin | high levels | diagnosis | [67] | |
| sTACI, sBCMA | high levels | diagnosis/therapy response | [68] | |
| APRIL, BAFF | high levels | diagnosis/therapy response/tumor relapse | [69] | |
| BAFF, TACI | high levels | diagnosis/therapy | [70] | |
| haemopexin, apolipoprotein A1, transferrin | high levels | diagnosis | [71] | |
| Medulloblastoma | PGD2S | low levels | diagnosis/therapy response/tumor relapse | [106] |
| VS | ABCA3, KLF11 | high levels | tumor relapse | [105] |
| BASP1, PRDX2 | low levels | tumor relapse | [105] | |
| Meningioma | EFEMP1 | high levels | diagnosis | [74] |
| Metastatic tumors | ||||
| SCNSL | CXCL13 | high levels | diagnosis | [60] |
| IL-10 | high levels | diagnosis | [60,61] | |
| IL-6, B2M, sIL-2R | high levels | diagnosis | [61] | |
| OPN | high levels | diagnosis | [66] | |
| haemopexin, apolipoprotein A1, transferrin | high levels | diagnosis | [71] | |
| Melanoma | CXCL10, IL-8 | high levels | diagnosis/therapy | [72] |
| Lung cancer | CEA | high levels | diagnosis/therapy | [73] |
| Breast cancer | CEA | high levels | diagnosis/therapy | [73] |
Growth factors and cytokines have also been identified as potential biomarkers present in the CSF of GBM patients. About 90% of patients with malignant gliomas present elevated vascular endothelial growth factor (VEGF) levels in the CSF (Table 3) [54]. Interestingly, related to CSF levels of cleaved OPN, VEGF and C-C motif chemokine ligand (CCL) 4 levels in the CSF were significantly increased in glioma patients when compared with non-tumors (Table 3) [55]. In addition, one particular study has shown that CSF fibroblast growth factor (FGF) and VEGF levels in patients with high-grade glioma were apparently higher than patients with low-grade glioma (Table 3) [56]. A further study has indicated that nerve growth factor (NGF) levels in the CSF elevate proportionally to the glioma grade (Table 3) [57]. The levels of 19 tumor-related CSF proteins have been examined, and results demonstrated that GBM patients have significant increases in interleukin (IL)-6 levels compared with patients with low grade gliomas and normal subjects (Table 3) [58]. Similarly, CSF IL-8 levels were also markedly increased in astrocytic tumors patients when compared with healthy patients (Table 3) [59]. Altogether, these studies suggest that CSF proteins have a potential use as glioma biomarkers.
Several CSF-related protein biomarkers, such as CXCL13, IL-10, IL-6, B2M, sIL-2R, sCD27, ATIII, OPN, Neopterin, sTACI, sBCMA, APRIL, and BAFF, have a putative diagnostic value in lymphomas. The elevated CXC chemokine ligand (CXCL) 13 plus IL-10 was 99.3% specific for PCNSL and SCNSL, with a sensitivity significantly greater than standard CSF tests (Table 3) [60]. IL-10, IL-6, beta-2 microglobulin (B2M), and soluble IL-2 receptor (sIL-2R) levels in CSF from patients with CNS lymphoma were apparently higher than non-lymphoma patients (Table 3) [61]. A specificity of 90% was also found for increased sIL-2R in the CSF, which correlated with the proper diagnosis of patients initially suspected of having PCNSL (Table 3) [62]. CD27 is a receptor molecule that integrates the tumor necrosis factor receptor (TNFR) superfamily and, as such, it may regulate the activation of B cell and synthesis of immunoglobulin [63]. A total of 42 CSF samples were collected from various types of brain tumor patients, and results indicated that the levels of Soluble CD27 (sCD27) were significantly higher in the PCNSL group when compared to controls with unrelated brain tumors (Table 3) [64]. Antithrombin III (ATIII) has been reported at significantly different levels in patients with CNS lymphoma and non-neoplastic patients. Indeed, ATIII concentrations in patients with CNS lymphoma were significantly higher than in control patients, and the elevation of ATIII was 75% sensitive and 98% specific along this population study (Table 3) [65]. Osteopontin (OPN), a pro-inflammatory cytokine, is frequently associated with the progression, metastatic spread and poor prognosis of various malignancies. One study has shown that the sensitivity and specificity of CSF OPN in a combined patient group of PCNSL and SCNSL was 87% and 86% higher than the control group, respectively (Table 3) [66]. Neopterin is part of the pteridin class of molecules, and it is considered a marker for the activation of the immune system. CSF neopterin levels have been significantly higher in the patients with PCNSL than in those with other brain tumors. In this context, the sensitivity of this approach was 96% and the specificity was 93% for the diagnosis of PCNSL (Table 3) [67]. CSF soluble transmembrane activator and CAML-interactor (sTACI) and soluble B-cell maturation antigen (sBCMA) levels were significantly increased in PCNSL patients when compared with control groups (Table 3) [68]. Similarly, a proliferation-inducing ligand (APRIL) and B cell activating factor (BAFF) levels in CSF from patients with PCNSL were also apparently higher than other brain tumors (Table 3) [69]. In addition, one particularly study has shown that the sensitivity and specificity of CSF-derived BAFF and TACI as diagnostic markers for PCNSL were 100% (Table 3) [70]. Another three proteins - haemopexin, apolipoprotein A1, and transferrin - were also detected at significantly increased levels in the CSF of CNS lymphoma patients (Table 3) [71].
CXCL10 and IL-8 chemokines are usually increased in the CSF of patients with melanoma-related brain metastasis (Table 3) [72]. Likely, carcino-embryonic antigen (CEA) has been measured in the CSF of patients with primary and metastatic brain tumors, suggesting that CEA levels in metastatic brain tumors are apparently higher than those in primary brain tumors (Table 3) [73]. EGF containing fibulin-like extracellular matrix protein 1 (EFEMP1) was also identified in the CSF of patients with meningioma and some healthy controls. Surprisingly, EFEMP1 levels were significantly higher in the CSF samples of meningioma patients when compared to the controls (Table 3) [74]. Prospective studies, including larger patient cohorts, will be further necessary to validate the diagnostic value of some attractive CSF protein biomarkers, for different types of brain tumors.
Extracellular vesicles
Extracellular vesicles (EVs) are membrane-bound nanoparticles, released by various types of cells [75-77]. Most of them range in size from 30 nm to 1000 nm [78,79]. Based on the size, biogenesis, and biophysical characteristics, EVs can be classified as exosomes, microvesicles and apoptotic bodies [80,81]. These vesicles are seminal to multiple biologic processes and, at the same time, capable of promoting tumor progression [82-84]. EVs, sometimes referred to as ‘exosomes’, carry an abundant array of lipids, DNAs, miRNAs, and proteins (Figure 2A), which can reflect their identity for analysis in liquid biopsy for brain tumors [85-88]. As shown in Figure 2B, the secretion of extracellular vesicles from brain tumor cell is quite complicated. A number of studies have shown that EVs can be found and isolated from the CSF of brain tumor patients [43,44,75,89]. CSF-derived EVs provide a platform for detection of tumor specific biomarkers in the brain. For instance, the analysis of mutated IDH1 in CSF-derived EVs of patients with glioma may play a new role for the diagnosis [89]. Moreover, the levels of miR-21 in CSF-derived EVs of GBM patients were, in average, 10-fold higher than the levels in control subjects, and miR-21 in CSF-derived EVs yielded a diagnostic sensitivity and specificity of 87% and 93% for GBM, respectively [44]. Another study also indicated that the miR-21 signature from CSF-derived EVs have a diagnostic significance for GBM patients [90]. Indeed, it plays an important role not only in diagnosis but also in the prognosis and over the course of metastasis. The levels of exosomal miR-21 in the CSF from glioma patients were found significantly higher than in the controls. Furthermore, the levels of miR-21 may affect the expression of target genes. Therefore, miR-21 levels may have a predictive value in glioma recurrence and/or metastasis [91]. Epidermal growth factor receptor variant III (EGFRvIII) was also detected in CSF-derived EVs from EGFRvIII tissue-positive and tissue-negative GBM patients. In this case, EGFRvIII in CSF-derived EVs yielded a diagnostic sensitivity and specificity of 61% and 98% for EGFRvIII-positive GBM. Therefore, it looks feasible to direct mutation-specific therapies for GBM [92].
Figure 2.

Extracellular vesicles (EVs) are small membrane-bound nanoparticles, released by all human cell types and tissues. A. Exosomes (nano-sized EVs) carry an abundant array of lipids, DNA, miRNA and proteins which reflect their identity for further analysis after liquid biopsy. B. Illustrative mechanism of EV secretion from brain tumor cells.
Despite recent advances, in-depth validation of CSF-derived EVs as biomarkers is still expected. For this, acquiring CSF-derived EV samples from brain tumor patients, in a larger large-scale, is warranted but will certainly require a coordinated multi-institutional effort.
Clinical roles of CSF biomarkers in brain tumors
Diagnosis
Some patients affected by brain tumors are eventually diagnosed at advanced stages and, therefore, lack the best timing for a more effective treatment. Therefore, early diagnosis of brain tumors and a timely treatment are of great importance to improve the survival rate of the patients. Although brain biopsy is still the gold standard for diagnosing brain tumors, biomarkers obtained from the CSF of brain tumor patients have unique advantages including easy access and less trauma. CSF-derived biomarkers, such as ctDNA, miRNA, proteins, and EVs, could be used for early diagnosis of brain tumors and, importantly, they could also indicate the type of brain tumor involved as well as the severity of the disease.
CSF-derived ctDNA has been a suitable tool for identifying genomic alterations of patients with primary and metastatic brain tumors. Many researchers have analyzed single and multiple genes present in CSF ctDNA, and they have discovered that most of identified genetic alterations included point mutations, amplifications, and small deletions (Table 1) [3,5,6,8,11,14-20,26,27,93-96]. More than 50%-75% of the brain tumor patients had somatic alterations in the CSF ctDNA [3,8,11,14]. Interestingly, brain tumors may be diagnosed by studying mutations in CSF ctDNA gene and, in addition, the respective tumor size can be correlated with level of mutation involved. In fact, one particular study has shown that the mutation levels detected by ddPCR analysis, can be closely associated with the tumor size [3]. In conclusion, the study of gene mutations (and their levels) in CSF ctDNA from patients with brain tumors have a great significance for the diagnosis. Multiple miRNAs identified in CSF have also been found to be significantly associated with primary and metastatic brain tumors. Similarly, several studies have also suggested that the miRNA yields in the CSF could help distinguish different types of brain tumors and other CNS-related diseases (Table 2) [30,32,33,38,43-47]. Protein markers can also play an important role in the diagnosis of brain tumors. A representative number of studies have demonstrated that the levels of CSF proteins have a diagnostic value for brain tumor patients (Table 3) [49,50,52-62,64-74]. In this context, EVs might also play an indirect but supportive diagnostic role, due to its abundance of DNA, miRNA, and proteins.
Treatment
After diagnosing a brain tumor type, a treatment plan should be followed according to the tumor characteristics. Currently, the strategies used for brain tumor therapy include neurosurgery, radiation and/or chemotherapy after surgery. In the current era of molecular classification of brain tumors, it sounds feasible that a preoperative knowledge of biomarkers with prognostic significance could help the surgical planning, intraoperative decision-making and dosage regimen.
CSF ctDNA provides a minimally invasive method to assess the genomic alterations of a brain tumor, in such a way that personalized treatment(s) might be established according to its molecular characteristics (Table 1) [3,5,6,8,11,14-20,26,27,97,98]. Analyses of CSF-derived ctDNA from some lymphoma patients have indicated that the detection of tumor-specific mutations is conducive to the adoption of a targeted therapy [99]. The use of CSF miRNAs as biomarkers can also be extremely helpful to determine whether surgery should be performed in patients with brain tumor [32]. In fact, even after a complete surgical resection under optimal conditions, a number of patients can still relapse and be refractory to re-treatment. The relapsed tumor tends to evolve under treatment and may present different genetic changes from the original primary tumor [100]. Unfortunately, there is no standard treatment for the recurrent brain tumors. Still, some of treatment options may include temozolomide (TMZ) rechallenge, lomustine, and antiangiogenic therapy, with the addition of re-irradiation and/or re-resection depending on the tumor location and general condition of the patient [101]. The prognosis of brain metastases is poor, and the development of effective treatment plans is constantly challenging. Still, CSF ctDNA has the potential to identify specific genomic alterations during brain metastasis that might facilitate the design of personalized treatments to target this advanced condition. For example, brain metastases originated from lung adenocarcinoma, containing EGFR mutations detectable in the CSF, have responded well to treatment with EGFR-tyrosine kinase inhibitors [102]. Similarly, many researchers elaborated that CSF-derived proteins may be helpful in the management of brain tumors (Table 3) [53-55,58,59,70,72,73]. Since CSF-derived EVs contain a large amount of genetic information related to brain tumor cells, they also have a potential value in targeted therapy [79].
Taken together, it has been assumed that the content of tumor biomarkers (i.e., ctDNA, miRNA, and proteins) in the CSF might correlate with brain tumor burden. Hence, after surgery or chemoradiotherapy, we could anticipate whether a selected regimen is correct or should be updated according to the biomarker profiling.
Monitoring recurrence and treatment response
Post-treatment monitoring of patients with primary or metastatic brain tumors is a standard medical procedure. Shortly after chemo and/or radiotherapy, the appearance of an enlarged or newly enhanced lesion, called pseudoprogression, is frequently found [103]. Indeed, the tumor itself does not necessarily grow, and might subside or stabilize without a modified treatment [103]. Due to the potential ocurrence of pseudoprogression, we cannot solely rely on imaging data to confirm or not tumor recurrence [38]. It is reasonable to consider that tumor recurrence may lead to an increase in the levels of tumor-derived biomarkers in the CSF, which could also distinguish recurrence from pseudoprogression. Afterwards, we may reduce unnecessary surgical procedures with imaging alterations according to the treatment outcome. MiRNA profiling could be used for monitoring recurrence and/or examining the efficacy of treatment (Table 2) [32,38,40,43-46,90,91,104]. Upon tumor removal, miRNA levels decrease below the levels as previously established but, if tumor recurrence appears, miRNA levels may re-adjust. As an example, levels of exosome-derived miR-21 significantly correlate with the recurrence or metastasis of gliomas [91]. By testing whether CSF levels of specific miRNAs can reflect disease activity and/or treatment response, Teplyuk and colleagues figured out that both miR-10b and miR-200 levels in CSF increase during relapse and, contrarily, they can be reduced by improving erlotinib dosage [32]. Similarly, the levels of CSF-derived exosomal miR-151a might predict the response to TMZ treatment in GBM patients [104]. A number of studies have shown that changes in levels of CSF-derived proteins may also reflect whether the tumor has recurred and/or treatment is effective (Table 3) [49,50,52-55,58,65,68,69]. Remarkably, increased ATP-binding cassette subfamily A member 3 (ABCA3) and Krueppel-like factor 11 (KLF11) levels and decreased brain acid soluble protein 1 (BASP1) and peroxiredoxin-2 (PRDX2) levels in CSF acquired during vestibular schwannoma (VS) surgery typically correlate with vestibular schwannoma growth at early phases or upon recurrence (Table 3) [105]. Furthermore, levels of CSF prostaglandin D2 synthase (PGD2S) were obviously decreased in medulloblastoma patients and, therefore, could also be used to monitor response to treatment and tumor recurrence (Table 3) [106].
In summary, tumor biomarkers in the CSF can be used to distinguish pseudoprogression from recurrence. In that way, some patients without true tumor recurrence will refrain from unnecessary surgical procedures, such as re-resections or brain biopsies to confirm recurrence. Meanwhile, the levels of tumor biomarkers in CSF can vary according to the dosage and the type of drug applied, so that a better drug regimen can be established according to the yields of distinct tumor markers (Figure 3).
Figure 3.
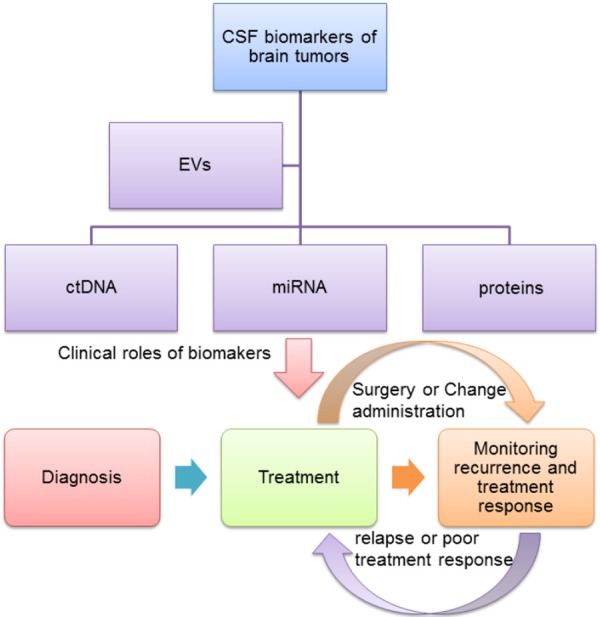
Changes on the expression of CSF-derived molecular biomarkers (i.e., ctDNA, miRNA, proteins and EVs) can be translated into the diagnosis, treatment, monitoring recurrence and treatment response of brain tumors. EVs play indirect clinical roles, due to its abundance of DNA, miRNA, and proteins. After diagnosing a brain tumor type, a treatment plan should be followed up according to the tumor characteristics. If the tumor recurs or responds poorly, we can re-operate on the patients or change the medication regimen.
Conclusion
Since brain tumors cannot be readily accessed by biopsy for frequent analysis, liquid biopsy techniques have been increasingly considered as a safer and more accessible approach to monitor disease progression. As sequencing-based technologies improve and related costs tend to be more manageable, the identification of “actionable” mutations have become even more important and accessible for medical assessment and therapeutics.
Biomarkers in the CSF of brain tumor patients can provide information on diagnosis, treatment, monitoring recurrence and treatment response. Distinguishing different types of brain tumors as well as accessing the severity of these tumors are major milestones that the analysis of tumor biomarkers in CSF have been provided. As the need for tailored therapies increase, biomarker analyses are required to predict response to specific treatments and, therefore, support the development of more individualized therapies. In fact, patient-specific tumor biomarkers are the basis of individualized treatment. Monitoring recurrence and treatment response is another important potential of the tumor biomarkers. The use of the tumor biomarkers in the CSF to distinguish pseudoprogression from recurrence will avoid unnecessary reoperations and biopsies. Moreover, changes on the levels of certain brain tumor biomarkers can reflect the efficacy of a certain treatment, whether drug resistance reaction occurs and/or when to stop treatment, especially when side effects outweigh therapeutic benefits.
Nevertheless, the acquisition of tumor biomarkers in CSF still has some shortcomings. The detection of tumor biomarkers in the CSF of brain tumor patients might be still challenging, possibly due limited concentration, sample size, sensitivity of gene mutation detection, improper extraction methods, lack of proper procedures for handling and storing samples, or even lack of standardized isolation procedures. In fact, one particular study has shown that CSF ctDNA cannot be detected in every patient with brain tumor, and ctDNA was only detected in 74% of the CSF samples [8]. Due to technological difficulties in their detection and lack of standardization, CSF miRNAs are still challenging to precisely measure [107].
We can certainly identify that the biological value of CSF-derived miRNAs, proteins, and EVs from certain conditions, including vestibular schwannoma and metastatic brain tumors, still require further validation. But, most importantly, we believe that continuous efforts to explore and test relevant biomarkers in the CSF of brain tumors patients will be translated, in the near future, into clinically relevant tools to support the diagnosis, treatment, and monitoring recurrence and treatment response of brain tumor and other CNS-related diseases.
Acknowledgements
The work was supported by grants from The National Natural Sciences Foundations of China (No. 81960457 and 81960456). We are very grateful for the language editing service provided by editsprings.
Disclosure of conflict of interest
None.
Abbreviations
- CSF
cerebrospinal fluid
- ctDNA
circulating tumor DNA
- miRNA
microRNA
- EVs
extracellular vesicles
- CNS
central nervous system
- ELISA
Enzyme-linked Immunosorbent Assay
- PCR
Polymerase Chain Reaction
- NGS
Next-Generation Sequencing
- ddPCR
droplet-digital PCR
- GBM
glioblastoma
- PCNSL
primary central nervous system lymphoma
- SCNSL
secondary central nervous system lymphoma
- GFAP
glial fibrillary acidic protein
- OPN
osteopontin
- MMP
matrix metalloproteinase
- ECM
extracellular matrix
- VEGF
vascular endothelial growth factor
- CCL
C-C motif chemokine ligand
- FGF
fibroblast growth factor
- NGF
nerve growth factor
- IL
interleukin
- CXCL
CXC chemokine ligand
- B2M
beta-2 microglobulin
- sIL-2R
soluble IL-2 receptor
- TNFR
tumor necrosis factor receptor
- sCD27
soluble CD27
- ATIII
Antithrombin III
- Staci
soluble transmembrane activator and CAML-interactor
- sBCMA
soluble B-cell maturation antigen
- APRIL
a proliferation-inducing ligand
- BAFF
B cell activating factor
- CEA
carcino-embryonic antigen
- EFEMP1
EGF containing fibulin-like extracellular matrix protein 1
- EGFRvIII
epidermal growth factor receptor variant III
- TMZ
temozolomide
- ABCA3
ATP-binding cassette subfamily A member 3
- KLF11
krueppel-like factor 11
- BASP1
brain acid soluble protein 1
- PRDX2
peroxiredoxin-2
- VS
vestibular schwannoma
- PGD2S
prostaglandin D2 synthase
References
- 1.Bertero L, Siravegna G, Ruda R, Soffietti R, Bardelli A, Cassoni P. Review: peering through a keyhole: liquid biopsy in primary and metastatic central nervous system tumours. Neuropathol Appl Neurobiol. 2019;45:655–670. doi: 10.1111/nan.12553. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Samuel N, Remke M, Rutka JT, Raught B, Malkin D. Proteomic analyses of CSF aimed at biomarker development for pediatric brain tumors. J Neurooncol. 2014;118:225–238. doi: 10.1007/s11060-014-1432-3. [DOI] [PubMed] [Google Scholar]
- 3.De Mattos-Arruda L, Mayor R, Ng CKY, Weigelt B, Martinez-Ricarte F, Torrejon D, Oliveira M, Arias A, Raventos C, Tang J, Guerini-Rocco E, Martinez-Saez E, Lois S, Marin O, de la Cruz X, Piscuoglio S, Towers R, Vivancos A, Peg V, Ramon y Cajal S, Carles J, Rodon J, Gonzalez-Cao M, Tabernero J, Felip E, Sahuquillo J, Berger MF, Cortes J, Reis-Filho JS, Seoane J. Cerebrospinal fluid-derived circulating tumour DNA better represents the genomic alterations of brain tumours than plasma. Nat Commun. 2015;6:8839. doi: 10.1038/ncomms9839. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Bettegowda C, Sausen M, Leary R, Kinde I, Agrawal N, Bartlett B, Wang H, Luber B, Kinzler K, Vogelstein B, Papadopoulos N. Detection of circulating tumor DNA in early and late stage human malignancies. Neuro Oncol. 2014;16:iii7–iii7. doi: 10.1126/scitranslmed.3007094. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Pan C, Diplas BH, Chen X, Wu Y, Xiao X, Jiang L, Geng Y, Xu C, Sun Y, Zhang P, Wu W, Wang Y, Wu Z, Zhang J, Jiao Y, Yan H, Zhang L. Molecular profiling of tumors of the brainstem by sequencing of CSF-derived circulating tumor DNA. Acta Neuropathol. 2019;137:297–306. doi: 10.1007/s00401-018-1936-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Martinez-Ricarte F, Mayor R, Martinez-Saez E, Rubio-Perez C, Pineda E, Cordero E, Cicuendez M, Poca MA, Lopez-Bigas N, Ramon YCS, Vieito M, Carles J, Tabernero J, Vivancos A, Gallego S, Graus F, Sahuquillo J, Seoane J. Molecular diagnosis of diffuse gliomas through sequencing of cell-free circulating tumor DNA from cerebrospinal fluid. Clin Cancer Res. 2018;24:2812–2819. doi: 10.1158/1078-0432.CCR-17-3800. [DOI] [PubMed] [Google Scholar]
- 7.Mattox AK, Yan H, Bettegowda C. The potential of cerebrospinal fluid-based liquid biopsy approaches in CNS tumors. Neuro Oncol. 2019;21:1509–1518. doi: 10.1093/neuonc/noz156. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Wang Y, Springer S, Zhang M, McMahon KW, Kinde I, Dobbyn L, Ptak J, Brem H, Chaichana K, Gallia GL, Gokaslan ZL, Groves ML, Jallo GI, Lim M, Olivi A, Quinones-Hinojosa A, Rigamonti D, Riggins GJ, Sciubba DM, Weingart JD, Wolinsky JP, Ye X, Oba-Shinjo SM, Marie SK, Holdhoff M, Agrawal N, Diaz LA Jr, Papadopoulos N, Kinzler KW, Vogelstein B, Bettegowda C. Detection of tumor-derived DNA in cerebrospinal fluid of patients with primary tumors of the brain and spinal cord. Proc Natl Acad Sci U S A. 2015;112:9704–9709. doi: 10.1073/pnas.1511694112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Li JH, He ZQ, Lin FH, Chen ZH, Yang SY, Duan H, Jiang XB, Al-Nahari F, Zhang XH, Wang JH, Zhang GH, Zhang ZF, Li C, Mou YG. Assessment of ctDNA in CSF may be a more rapid means of assessing surgical outcomes than plasma ctDNA in glioblastoma. Mol Cell Probes. 2019;46:101411. doi: 10.1016/j.mcp.2019.06.001. [DOI] [PubMed] [Google Scholar]
- 10.Olmedillas-Lopez S, Garcia-Arranz M, Garcia-Olmo D. Current and emerging applications of droplet digital PCR in oncology. Mol Diagn Ther. 2017;21:493–510. doi: 10.1007/s40291-017-0278-8. [DOI] [PubMed] [Google Scholar]
- 11.Pentsova EI, Shah RH, Tang J, Boire A, You D, Briggs S, Omuro A, Lin X, Fleisher M, Grommes C, Panageas KS, Meng F, Selcuklu SD, Ogilvie S, Distefano N, Shagabayeva L, Rosenblum M, DeAngelis LM, Viale A, Mellinghoff IK, Berger MF. Evaluating cancer of the central nervous system through next-generation sequencing of cerebrospinal fluid. J. Clin. Oncol. 2016;34:2404–2415. doi: 10.1200/JCO.2016.66.6487. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Firme MR, Marra MA. The molecular landscape of pediatric brain tumors in the next-generation sequencing era. Curr Neurol Neurosci Rep. 2014;14:474. doi: 10.1007/s11910-014-0474-4. [DOI] [PubMed] [Google Scholar]
- 13.Connolly ID, Li Y, Gephart MH, Nagpal S. The “liquid biopsy”: the role of circulating DNA and RNA in central nervous system tumors. Curr Neurol Neurosci Rep. 2016;16:25. doi: 10.1007/s11910-016-0629-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Pan W, Gu W, Nagpal S, Gephart MH, Quake SR. Brain tumor mutations detected in cerebral spinal fluid. Clin Chem. 2015;61:514–522. doi: 10.1373/clinchem.2014.235457. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Miller AM, Shah RH, Pentsova EI, Pourmaleki M, Briggs S, Distefano N, Zheng Y, Skakodub A, Mehta SA, Campos C, Hsieh WY, Selcuklu SD, Ling L, Meng F, Jing X, Samoila A, Bale TA, Tsui DWY, Grommes C, Viale A, Souweidane MM, Tabar V, Brennan CW, Reiner AS, Rosenblum M, Panageas KS, DeAngelis LM, Young RJ, Berger MF, Mellinghoff IK. Tracking tumour evolution in glioma through liquid biopsies of cerebrospinal fluid. Nature. 2019;565:654–658. doi: 10.1038/s41586-019-0882-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Huang TY, Piunti A, Lulla RR, Qi J, Horbinski CM, Tomita T, James CD, Shilatifard A, Saratsis AM. Detection of Histone H3 mutations in cerebrospinal fluid-derived tumor DNA from children with diffuse midline glioma. Acta Neuropathol Commun. 2017;5:28. doi: 10.1186/s40478-017-0436-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Hiemcke-Jiwa LS, Minnema MC, Radersma-van Loon JH, Jiwa NM, de Boer M, Leguit RJ, de Weger RA, Huibers MMH. The use of droplet digital PCR in liquid biopsies: a highly sensitive technique for MYD88 p. (L265P) detection in cerebrospinal fluid. Hematol Oncol. 2018;36:429–435. doi: 10.1002/hon.2489. [DOI] [PubMed] [Google Scholar]
- 18.Hiemcke-Jiwa LS, Leguit RJ, Radersma-van Loon JH, Westerweel PE, Rood JJM, Doorduijn JK, Huibers MMH, Minnema MC. Efficacy of ibrutinib in a patient with transformed lymphoplasmacytic lymphoma and central nervous system involvement. Leuk Lymphoma. 2018;59:1256–1259. doi: 10.1080/10428194.2017.1369074. [DOI] [PubMed] [Google Scholar]
- 19.Rimelen V, Ahle G, Pencreach E, Zinniger N, Debliquis A, Zalmai L, Harzallah I, Hurstel R, Alamome I, Lamy F, Voirin J, Drenou B. Tumor cell-free DNA detection in CSF for primary CNS lymphoma diagnosis. Acta Neuropathol Commun. 2019;7:43. doi: 10.1186/s40478-019-0692-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Zorofchian S, Lu G, Zhu JJ, Duose DY, Windham J, Esquenazi Y, Ballester LY. Detection of the MYD88 p.L265P mutation in the CSF of a patient with secondary central nervous system lymphoma. Front Oncol. 2018;8:382. doi: 10.3389/fonc.2018.00382. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Akhter A, Masir N, Elyamany G, Phang KC, Mahe E, Al-Zahrani AM, Shabani-Rad MT, Stewart DA, Mansoor A. Differential expression of Toll-like receptor (TLR) and B cell receptor (BCR) signaling molecules in primary diffuse large B-cell lymphoma of the central nervous system. J Neurooncol. 2015;121:289–296. doi: 10.1007/s11060-014-1655-3. [DOI] [PubMed] [Google Scholar]
- 22.Nakamura T, Tateishi K, Niwa T, Matsushita Y, Tamura K, Kinoshita M, Tanaka K, Fukushima S, Takami H, Arita H, Kubo A, Shuto T, Ohno M, Miyakita Y, Kocialkowski S, Sasayama T, Hashimoto N, Maehara T, Shibui S, Ushijima T, Kawahara N, Narita Y, Ichimura K. Recurrent mutations of CD79B and MYD88 are the hallmark of primary central nervous system lymphomas. Neuropathol Appl Neurobiol. 2016;42:279–290. doi: 10.1111/nan.12259. [DOI] [PubMed] [Google Scholar]
- 23.Peng S, Dhruv H, Armstrong B, Salhia B, Legendre C, Kiefer J, Parks J, Virk S, Sloan AE, Ostrom QT, Barnholtz-Sloan JS, Tran NL, Berens ME. Integrated genomic analysis of survival outliers in glioblastoma. Neuro Oncol. 2017;19:833–844. doi: 10.1093/neuonc/now269. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Hiemcke-Jiwa LS, Leguit RJ, Snijders TJ, Jiwa NM, Kuiper JJW, de Weger RA, Minnema MC, Huibers MMH. Molecular analysis in liquid biopsies for diagnostics of primary central nervous system lymphoma: review of literature and future opportunities. Crit Rev Oncol Hematol. 2018;127:56–65. doi: 10.1016/j.critrevonc.2018.05.010. [DOI] [PubMed] [Google Scholar]
- 25.Calapre L, Warburton L, Millward M, Ziman M, Gray ES. Circulating tumour DNA (ctDNA) as a liquid biopsy for melanoma. Cancer Lett. 2017;404:62–69. doi: 10.1016/j.canlet.2017.06.030. [DOI] [PubMed] [Google Scholar]
- 26.Huang R, Xu X, Li D, Chen K, Zhan Q, Ge M, Zhou X, Liang X, Guan M. Digital PCR-based detection of EGFR mutations in paired plasma and CSF samples of lung adenocarcinoma patients with central nervous system metastases. Target Oncol. 2019;14:343–350. doi: 10.1007/s11523-019-00645-5. [DOI] [PubMed] [Google Scholar]
- 27.Siravegna G, Geuna E, Mussolin B, Crisafulli G, Bartolini A, Galizia D, Casorzo L, Sarotto I, Scaltriti M, Sapino A, Bardelli A, Montemurro F. Genotyping tumour DNA in cerebrospinal fluid and plasma of a HER2-positive breast cancer patient with brain metastases. ESMO Open. 2017;2:e000253. doi: 10.1136/esmoopen-2017-000253. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Acunzo M, Romano G, Wernicke D, Croce CM. MicroRNA and cancer--a brief overview. Adv Biol Regul. 2015;57:1–9. doi: 10.1016/j.jbior.2014.09.013. [DOI] [PubMed] [Google Scholar]
- 29.Hayes J, Peruzzi PP, Lawler S. MicroRNAs in cancer: biomarkers, functions and therapy. Trends Mol Med. 2014;20:460–469. doi: 10.1016/j.molmed.2014.06.005. [DOI] [PubMed] [Google Scholar]
- 30.Verheul C, Kleijn A, Lamfers MLM. Cerebrospinal fluid biomarkers of malignancies located in the central nervous system. Handb Clin Neurol. 2017;146:139–169. doi: 10.1016/B978-0-12-804279-3.00010-1. [DOI] [PubMed] [Google Scholar]
- 31.Petrescu GED, Sabo AA, Torsin LI, Calin GA, Dragomir MP. MicroRNA based theranostics for brain cancer: basic principles. J Exp Clin Cancer Res. 2019;38:231. doi: 10.1186/s13046-019-1180-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Teplyuk NM, Mollenhauer B, Gabriely G, Giese A, Kim E, Smolsky M, Kim RY, Saria MG, Pastorino S, Kesari S, Krichevsky AM. MicroRNAs in cerebrospinal fluid identify glioblastoma and metastatic brain cancers and reflect disease activity. Neuro Oncol. 2012;14:689–700. doi: 10.1093/neuonc/nos074. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Baraniskin A, Kuhnhenn J, Schlegel U, Maghnouj A, Zollner H, Schmiegel W, Hahn S, Schroers R. Identification of microRNAs in the cerebrospinal fluid as biomarker for the diagnosis of glioma. Neuro Oncol. 2012;14:29–33. doi: 10.1093/neuonc/nor169. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Tumilson CA, Lea RW, Alder JE, Shaw L. Circulating microRNA biomarkers for glioma and predicting response to therapy. Mol Neurobiol. 2014;50:545–558. doi: 10.1007/s12035-014-8679-8. [DOI] [PubMed] [Google Scholar]
- 35.Touat M, Duran-Pena A, Alentorn A, Lacroix L, Massard C, Idbaih A. Emerging circulating biomarkers in glioblastoma: promises and challenges. Expert Rev Mol Diagn. 2015;15:1311–1323. doi: 10.1586/14737159.2015.1087315. [DOI] [PubMed] [Google Scholar]
- 36.Figueroa JM, Carter BS. Detection of glioblastoma in biofluids. J Neurosurg. 2018;129:334–340. doi: 10.3171/2017.3.JNS162280. [DOI] [PubMed] [Google Scholar]
- 37.Gallego JA, Gordon ML, Claycomb K, Bhatt M, Lencz T, Malhotra AK. In vivo microRNA detection and quantitation in cerebrospinal fluid. J Mol Neurosci. 2012;47:243–248. doi: 10.1007/s12031-012-9731-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Zorofchian S, Iqbal F, Rao M, Aung PP, Esquenazi Y, Ballester LY. Circulating tumour DNA, microRNA and metabolites in cerebrospinal fluid as biomarkers for central nervous system malignancies. J Clin Pathol. 2019;72:271–280. doi: 10.1136/jclinpath-2018-205414. [DOI] [PubMed] [Google Scholar]
- 39.Kopkova A, Sana J, Vecera M, Fadrus P, Lipina R, Smrcka M, Lojova M, Slaby O. MicroRNAs in cerebrospinal fluid as biomarkers in brain tumor patients. Klin Onkol. 2019;32:181–186. doi: 10.14735/amko2019181. [DOI] [PubMed] [Google Scholar]
- 40.Saenz-Antonanzas A, Auzmendi-Iriarte J, Carrasco-Garcia E, Moreno-Cugnon L, Ruiz I, Villanua J, Egana L, Otaegui D, Sampron N, Matheu A. Liquid biopsy in glioblastoma: opportunities, applications and challenges. Cancers (Basel) 2019;11 doi: 10.3390/cancers11070950. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Morad G, Moses MA. Brainwashed by extracellular vesicles: the role of extracellular vesicles in primary and metastatic brain tumour microenvironment. J Extracell Vesicles. 2019;8:1627164. doi: 10.1080/20013078.2019.1627164. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Ricklefs FL, Maire CL, Reimer R, Duhrsen L, Kolbe K, Holz M, Schneider E, Rissiek A, Babayan A, Hille C, Pantel K, Krasemann S, Glatzel M, Heiland DH, Flitsch J, Martens T, Schmidt NO, Peine S, Breakefield XO, Lawler S, Chiocca EA, Fehse B, Giebel B, Gorgens A, Westphal M, Lamszus K. Imaging flow cytometry facilitates multiparametric characterization of extracellular vesicles in malignant brain tumours. J Extracell Vesicles. 2019;8:1588555. doi: 10.1080/20013078.2019.1588555. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Akers JC, Hua W, Li H, Ramakrishnan V, Yang Z, Quan K, Zhu W, Li J, Figueroa J, Hirshman BR, Miller B, Piccioni D, Ringel F, Komotar R, Messer K, Galasko DR, Hochberg F, Mao Y, Carter BS, Chen CC. A cerebrospinal fluid microRNA signature as biomarker for glioblastoma. Oncotarget. 2017;8:68769–68779. doi: 10.18632/oncotarget.18332. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Akers JC, Ramakrishnan V, Kim R, Skog J, Nakano I, Pingle S, Kalinina J, Hua W, Kesari S, Mao Y, Breakefield XO, Hochberg FH, Van Meir EG, Carter BS, Chen CC. MiR-21 in the extracellular vesicles (EVs) of cerebrospinal fluid (CSF): a platform for glioblastoma biomarker development. PLoS One. 2013;8:e78115. doi: 10.1371/journal.pone.0078115. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Drusco A, Bottoni A, Lagana A, Acunzo M, Fassan M, Cascione L, Antenucci A, Kumchala P, Vicentini C, Gardiman MP, Alder H, Carosi MA, Ammirati M, Gherardi S, Luscri M, Carapella C, Zanesi N, Croce CM. A differentially expressed set of microRNAs in cerebro-spinal fluid (CSF) can diagnose CNS malignancies. Oncotarget. 2015;6:20829–20839. doi: 10.18632/oncotarget.4096. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Baraniskin A, Kuhnhenn J, Schlegel U, Schmiegel W, Hahn S, Schroers R. MicroRNAs in cerebrospinal fluid as biomarker for disease course monitoring in primary central nervous system lymphoma. J Neurooncol. 2012;109:239–244. doi: 10.1007/s11060-012-0908-2. [DOI] [PubMed] [Google Scholar]
- 47.Baraniskin A, Chomiak M, Ahle G, Gress T, Buchholz M, Turewicz M, Eisenacher M, Margold M, Schlegel U, Schmiegel W, Hahn S, Schroers R. MicroRNA-30c as a novel diagnostic biomarker for primary and secondary B-cell lymphoma of the CNS. J Neurooncol. 2018;137:463–468. doi: 10.1007/s11060-018-2749-0. [DOI] [PubMed] [Google Scholar]
- 48.Hoon DS, Kuo CT, Wascher RA, Fournier P, Wang HJ, O’Day SJ. Molecular detection of metastatic melanoma cells in cerebrospinal fluid in melanoma patients. J Invest Dermatol. 2001;117:375–378. doi: 10.1046/j.0022-202x.2001.01417.x. [DOI] [PubMed] [Google Scholar]
- 49.Szymas J, Morkowski S, Tokarz F. Determination of the glial fibrillary acidic protein in human cerebrospinal fluid and in cyst fluid of brain tumors. Acta Neurochir (Wien) 1986;83:144–150. doi: 10.1007/BF01402394. [DOI] [PubMed] [Google Scholar]
- 50.Yoshida J, Wakabayashi T, Okamoto S, Kimura S, Washizu K, Kiyosawa K, Mokuno K. Tenascin in cerebrospinal fluid is a useful biomarker for the diagnosis of brain tumour. J Neurol Neurosurg Psychiatry. 1994;57:1212–1215. doi: 10.1136/jnnp.57.10.1212. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Wei J, Marisetty A, Schrand B, Gabrusiewicz K, Hashimoto Y, Ott M, Grami Z, Kong LY, Ling X, Caruso H, Zhou S, Wang YA, Fuller GN, Huse J, Gilboa E, Kang N, Huang X, Verhaak R, Li S, Heimberger AB. Osteopontin mediates glioblastoma-associated macrophage infiltration and is a potential therapeutic target. J Clin Invest. 2019;129:137–149. doi: 10.1172/JCI121266. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Schuhmann MU, Zucht HD, Nassimi R, Heine G, Schneekloth CG, Stuerenburg HJ, Selle H. Peptide screening of cerebrospinal fluid in patients with glioblastoma multiforme. Eur J Surg Oncol. 2010;36:201–207. doi: 10.1016/j.ejso.2009.07.010. [DOI] [PubMed] [Google Scholar]
- 53.Friedberg MH, Glantz MJ, Klempner MS, Cole BF, Perides G. Specific matrix metalloproteinase profiles in the cerebrospinal fluid correlated with the presence of malignant astrocytomas, brain metastases, and carcinomatous meningitis. Cancer. 1998;82:923–930. doi: 10.1002/(sici)1097-0142(19980301)82:5<923::aid-cncr18>3.0.co;2-2. [DOI] [PubMed] [Google Scholar]
- 54.Sampath P, Weaver CE, Sungarian A, Cortez S, Alderson L, Stopa EG. Cerebrospinal fluid (vascular endothelial growth factor) and serologic (recoverin) tumor markers for malignant glioma. Cancer Control. 2004;11:174–180. doi: 10.1177/107327480401100305. [DOI] [PubMed] [Google Scholar]
- 55.Yamaguchi Y, Shao Z, Sharif S, Du XY, Myles T, Merchant M, Harsh G, Glantz M, Recht L, Morser J, Leung LL. Thrombin-cleaved fragments of osteopontin are overexpressed in malignant glial tumors and provide a molecular niche with survival advantage. J Biol Chem. 2013;288:3097–3111. doi: 10.1074/jbc.M112.362954. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Peles E, Lidar Z, Simon AJ, Grossman R, Nass D, Ram Z. Angiogenic factors in the cerebrospinal fluid of patients with astrocytic brain tumors. Neurosurgery. 2004;55:562–567. doi: 10.1227/01.neu.0000134383.27713.9a. discussion 567-568. [DOI] [PubMed] [Google Scholar]
- 57.Li QY, Yang Y, Zhang Y, Zhang ZJ, Gong AH, Yuan ZC, Lu PS, Zhan LP, Wang P, Feng Y, Xu WL. Nerve growth factor expression in astrocytoma and cerebrospinal fluid: a new biomarker for prognosis of astrocytoma. Chin Med J (Engl) 2011;124:2222–2227. [PubMed] [Google Scholar]
- 58.Shen F, Zhang Y, Yao Y, Hua W, Zhang HS, Wu JS, Zhong P, Zhou LF. Proteomic analysis of cerebrospinal fluid: toward the identification of biomarkers for gliomas. Neurosurg Rev. 2014;37:367–380. doi: 10.1007/s10143-014-0539-5. [DOI] [PubMed] [Google Scholar]
- 59.Koper OM, Kaminska J, Sawicki K, Reszec J, Rutkowski R, Jadeszko M, Mariak Z, Dymicka-Piekarska V, Kemona H. Cerebrospinal fluid and serum IL-8, CCL2, and ICAM-1 concentrations in astrocytic brain tumor patients. Ir J Med Sci. 2018;187:767–775. doi: 10.1007/s11845-017-1695-8. [DOI] [PubMed] [Google Scholar]
- 60.Rubenstein JL, Wong VS, Kadoch C, Gao HX, Barajas R, Chen L, Josephson SA, Scott B, Douglas V, Maiti M, Kaplan LD, Treseler PA, Cha S, Hwang JH, Cinque P, Cyster JG, Lowell C. CXCL13 plus interleukin 10 is highly specific for the diagnosis of CNS lymphoma. Blood. 2013;121:4740–4748. doi: 10.1182/blood-2013-01-476333. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Sasagawa Y, Akai T, Tachibana O, Iizuka H. Diagnostic value of interleukin-10 in cerebrospinal fluid for diffuse large B-cell lymphoma of the central nervous system. J Neurooncol. 2015;121:177–183. doi: 10.1007/s11060-014-1622-z. [DOI] [PubMed] [Google Scholar]
- 62.Ikeguchi R, Shimizu Y, Shimizu S, Kitagawa K. CSF and clinical data are useful in differentiating CNS inflammatory demyelinating disease from CNS lymphoma. Mult Scler. 2018;24:1212–1223. doi: 10.1177/1352458517717804. [DOI] [PubMed] [Google Scholar]
- 63.van Westrhenen A, Smidt LCA, Seute T, Nierkens S, Stork ACJ, Minnema MC, Snijders TJ. Diagnostic markers for CNS lymphoma in blood and cerebrospinal fluid: a systematic review. Br J Haematol. 2018;182:384–403. doi: 10.1111/bjh.15410. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Murase S, Saio M, Takenaka K, Shinoda J, Nishimura Y, Sakai N, Takami T. Increased levels of CSF soluble CD27 in patients with primary central nervous system lymphoma. Cancer Lett. 1998;132:181–186. doi: 10.1016/s0304-3835(98)00181-5. [DOI] [PubMed] [Google Scholar]
- 65.Roy S, Josephson SA, Fridlyand J, Karch J, Kadoch C, Karrim J, Damon L, Treseler P, Kunwar S, Shuman MA, Jones T, Becker CH, Schulman H, Rubenstein JL. Protein biomarker identification in the CSF of patients with CNS lymphoma. J. Clin. Oncol. 2008;26:96–105. doi: 10.1200/JCO.2007.12.1053. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Strehlow F, Bauer S, Martus P, Weller M, Roth P, Schlegel U, Seidel S, Scheibenbogen C, Korfel A, Kreher S. Osteopontin in cerebrospinal fluid as diagnostic biomarker for central nervous system lymphoma. J Neurooncol. 2016;129:165–171. doi: 10.1007/s11060-016-2162-5. [DOI] [PubMed] [Google Scholar]
- 67.Viaccoz A, Ducray F, Tholance Y, Barcelos GK, Thomas-Maisonneuve L, Ghesquieres H, Meyronet D, Quadrio I, Cartalat-Carel S, Louis-Tisserand G, Jouanneau E, Guyotat J, Honnorat J, Perret-Liaudet A. CSF neopterin level as a diagnostic marker in primary central nervous system lymphoma. Neuro Oncol. 2015;17:1497–1503. doi: 10.1093/neuonc/nov092. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Thaler FS, Laurent SA, Huber M, Mulazzani M, Dreyling M, Kodel U, Kumpfel T, Straube A, Meinl E, von Baumgarten L. Soluble TACI and soluble BCMA as biomarkers in primary central nervous system lymphoma. Neuro Oncol. 2017;19:1618–1627. doi: 10.1093/neuonc/nox097. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Mulazzani M, Huber M, Borchard S, Langer S, Angele B, Schuh E, Meinl E, Dreyling M, Birnbaum T, Straube A, Koedel U, von Baumgarten L. APRIL and BAFF: novel biomarkers for central nervous system lymphoma. J Hematol Oncol. 2019;12:102. doi: 10.1186/s13045-019-0796-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Mizutani H, Nakane S, Ikeda T, Nakamura H, Takamatsu K, Makino K, Tawara N, Mukaino A, Watari M, Matsui H, Mukasa A, Ando Y. CSF TACI and BAFF levels in patients with primary CNS lymphoma as novel diagnostic biomarkers. Ann Clin Transl Neurol. 2018;5:1611–1616. doi: 10.1002/acn3.668. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Zheng W, Song Y, Xie Y, Lin N, Tu M, Liu W, Ping L, Ying Z, Zhang C, Deng L, Wang X, Lu Y, Zhu J. Cerebrospinal fluid proteins identification facilitates the differential diagnosis of central nervous system diffuse large B cell lymphoma. J Cancer. 2017;8:3631–3640. doi: 10.7150/jca.20267. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Lok E, Chung AS, Swanson KD, Wong ET. Melanoma brain metastasis globally reconfigures chemokine and cytokine profiles in patient cerebrospinal fluid. Melanoma Res. 2014;24:120–130. doi: 10.1097/CMR.0000000000000045. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Batabyal SK, Ghosh B, Sengupta S, Ghosh SN, Chatterjee R. Cerebrospinal fluid and serum carcinoembryonic antigen in brain tumors. Neoplasma. 2003;50:377–379. [PubMed] [Google Scholar]
- 74.Jia L, Liu C, Xin Y, Zhang A, Zhou Y, Dong D, Ren L. Evaluating EFEMP1 in cerebrospinal fluid and serum as a potential diagnosis biomarker for meningiomas. Clin Lab. 2017;63:1717–1722. doi: 10.7754/Clin.Lab.2017.170602. [DOI] [PubMed] [Google Scholar]
- 75.Saugstad JA, Lusardi TA, Van Keuren-Jensen KR, Phillips JI, Lind B, Harrington CA, McFarland TJ, Courtright AL, Reiman RA, Yeri AS, Kalani MYS, Adelson PD, Arango J, Nolan JP, Duggan E, Messer K, Akers JC, Galasko DR, Quinn JF, Carter BS, Hochberg FH. Analysis of extracellular RNA in cerebrospinal fluid. J Extracell Vesicles. 2017;6:1317577. doi: 10.1080/20013078.2017.1317577. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Woith E, Fuhrmann G, Melzig MF. Extracellular vesicles-connecting kingdoms. Int J Mol Sci. 2019;20 doi: 10.3390/ijms20225695. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Zheng J, Tan J, Miao YY, Zhang Q. Extracellular vesicles degradation pathway based autophagy lysosome pathway. Am J Transl Res. 2019;11:1170–1183. [PMC free article] [PubMed] [Google Scholar]
- 78.Spinelli C, Adnani L, Choi D, Rak J. Extracellular vesicles as conduits of non-coding RNA emission and intercellular transfer in brain tumors. Noncoding RNA. 2018;5 doi: 10.3390/ncrna5010001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Pan Y, Long W, Liu Q. Current advances and future perspectives of cerebrospinal fluid biopsy in midline brain malignancies. Curr Treat Options Oncol. 2019;20:88. doi: 10.1007/s11864-019-0689-3. [DOI] [PubMed] [Google Scholar]
- 80.Shao H, Im H, Castro CM, Breakefield X, Weissleder R, Lee H. New technologies for analysis of extracellular vesicles. Chem Rev. 2018;118:1917–1950. doi: 10.1021/acs.chemrev.7b00534. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Yamamoto T, Kosaka N, Ochiya T. Latest advances in extracellular vesicles: from bench to bedside. Sci Technol Adv Mater. 2019;20:746–757. doi: 10.1080/14686996.2019.1629835. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Hochberg FH, Atai NA, Gonda D, Hughes MS, Mawejje B, Balaj L, Carter RS. Glioma diagnostics and biomarkers: an ongoing challenge in the field of medicine and science. Expert Rev Mol Diagn. 2014;14:439–452. doi: 10.1586/14737159.2014.905202. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Giusti I, Di Francesco M, Dolo V. Extracellular vesicles in glioblastoma: role in biological processes and in therapeutic applications. Curr Cancer Drug Targets. 2017;17:221–235. doi: 10.2174/1568009616666160813182959. [DOI] [PubMed] [Google Scholar]
- 84.Cufaro MC, Pieragostino D, Lanuti P, Rossi C, Cicalini I, Federici L, De Laurenzi V, Del Boccio P. Extracellular vesicles and their potential use in monitoring cancer progression and therapy: the contribution of proteomics. J Oncol. 2019;2019:1639854. doi: 10.1155/2019/1639854. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Hallal S, Ebrahimkhani S, Shivalingam B, Graeber MB, Kaufman KL, Buckland ME. The emerging clinical potential of circulating extracellular vesicles for non-invasive glioma diagnosis and disease monitoring. Brain Tumor Pathol. 2019;36:29–39. doi: 10.1007/s10014-019-00335-0. [DOI] [PubMed] [Google Scholar]
- 86.Han L, Lam EW, Sun Y. Extracellular vesicles in the tumor microenvironment: old stories, but new tales. Mol Cancer. 2019;18:59. doi: 10.1186/s12943-019-0980-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Wang H, Jiang D, Li W, Xiang X, Zhao J, Yu B, Wang C, He Z, Zhu L, Yang Y. Evaluation of serum extracellular vesicles as noninvasive diagnostic markers of glioma. Theranostics. 2019;9:5347–5358. doi: 10.7150/thno.33114. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Fontanilles M, Duran-Pena A, Idbaih A. Liquid biopsy in primary brain tumors: looking for stardust! Curr Neurol Neurosci Rep. 2018;18:13. doi: 10.1007/s11910-018-0820-z. [DOI] [PubMed] [Google Scholar]
- 89.Chen WW, Balaj L, Liau LM, Samuels ML, Kotsopoulos SK, Maguire CA, Loguidice L, Soto H, Garrett M, Zhu LD, Sivaraman S, Chen C, Wong ET, Carter BS, Hochberg FH, Breakefield XO, Skog J. BEAMing and droplet digital PCR analysis of mutant IDH1 mRNA in glioma patient serum and cerebrospinal fluid extracellular vesicles. Mol Ther Nucleic Acids. 2013;2:e109. doi: 10.1038/mtna.2013.28. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Rennert RC, Hochberg FH, Carter BS. ExRNA in biofluids as biomarkers for brain tumors. Cell Mol Neurobiol. 2016;36:353–360. doi: 10.1007/s10571-015-0284-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Shi R, Wang PY, Li XY, Chen JX, Li Y, Zhang XZ, Zhang CG, Jiang T, Li WB, Ding W, Cheng SJ. Exosomal levels of miRNA-21 from cerebrospinal fluids associated with poor prognosis and tumor recurrence of glioma patients. Oncotarget. 2015;6:26971–26981. doi: 10.18632/oncotarget.4699. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.Figueroa JM, Skog J, Akers J, Li H, Komotar R, Jensen R, Ringel F, Yang I, Kalkanis S, Thompson R, LoGuidice L, Berghoff E, Parsa A, Liau L, Curry W, Cahill D, Bettegowda C, Lang FF, Chiocca EA, Henson J, Kim R, Breakefield X, Chen C, Messer K, Hochberg F, Carter BS. Detection of wild-type EGFR amplification and EGFRvIII mutation in CSF-derived extracellular vesicles of glioblastoma patients. Neuro Oncol. 2017;19:1494–1502. doi: 10.1093/neuonc/nox085. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.Verduin M, Compter I, Steijvers D, Postma AA, Eekers DBP, Anten MM, Ackermans L, Ter Laan M, Leijenaar RTH, van de Weijer T, Tjan-Heijnen VCG, Hoeben A, Vooijs M. Noninvasive glioblastoma testing: multimodal approach to monitoring and predicting treatment response. Dis Markers. 2018;2018:2908609. doi: 10.1155/2018/2908609. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94.Kurian AW, Hare EE, Mills MA, Kingham KE, McPherson L, Whittemore AS, McGuire V, Ladabaum U, Kobayashi Y, Lincoln SE, Cargill M, Ford JM. Clinical evaluation of a multiple-gene sequencing panel for hereditary cancer risk assessment. J. Clin. Oncol. 2014;32:2001–2009. doi: 10.1200/JCO.2013.53.6607. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95.Ballester LY, Glitza Oliva IC, Douse DY, Chen MM, Lan C, Haydu LE, Huse JT, Roy-Chowdhuri S, Luthra R, Wistuba II, Davies MA. Evaluating circulating tumor DNA from the cerebrospinal fluid of patients with melanoma and leptomeningeal disease. J Neuropathol Exp Neurol. 2018;77:628–635. doi: 10.1093/jnen/nly046. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96.Stallard S, Savelieff MG, Wierzbicki K, Mullan B, Miklja Z, Bruzek A, Garcia T, Siada R, Anderson B, Singer BH, Hashizume R, Carcaboso AM, McMurray KQ, Heth J, Muraszko K, Robertson PL, Mody R, Venneti S, Garton H, Koschmann C. CSF H3F3A K27M circulating tumor DNA copy number quantifies tumor growth and in vitro treatment response. Acta Neuropathol Commun. 2018;6:80. doi: 10.1186/s40478-018-0580-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97.Huang WT, Lu NM, Hsu WY, Chang SE, Atkins A, Mei R, Javey M. CSF-ctDNA SMSEQ analysis to tailor the treatment of a patient with brain metastases: a case report. Case Rep Oncol. 2018;11:68–74. doi: 10.1159/000486568. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98.Sindeeva OA, Verkhovskii RA, Sarimollaoglu M, Afanaseva GA, Fedonnikov AS, Osintsev EY, Kurochkina EN, Gorin DA, Deyev SM, Zharov VP, Galanzha EI. New frontiers in diagnosis and therapy of circulating tumor markers in cerebrospinal fluid in vitro and in vivo. Cells. 2019;8 doi: 10.3390/cells8101195. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99.Hickmann AK, Frick M, Hadaschik D, Battke F, Bittl M, Ganslandt O, Biskup S, Docker D. Molecular tumor analysis and liquid biopsy: a feasibility investigation analyzing circulating tumor DNA in patients with central nervous system lymphomas. BMC Cancer. 2019;19:192. doi: 10.1186/s12885-019-5394-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100.Zhang RR, Pointer KB, Kuo JS, Dempsey RJ. Mutational analysis reveals the origin and therapy-driven evolution of recurrent glioma. Neurosurgery. 2014;75:N9–10. doi: 10.1227/NEU.0000000000000580. [DOI] [PubMed] [Google Scholar]
- 101.van Linde ME, Brahm CG, de Witt Hamer PC, Reijneveld JC, Bruynzeel AME, Vandertop WP, van de Ven PM, Wagemakers M, van der Weide HL, Enting RH, Walenkamp AME, Verheul HMW. Treatment outcome of patients with recurrent glioblastoma multiforme: a retrospective multicenter analysis. J Neurooncol. 2017;135:183–192. doi: 10.1007/s11060-017-2564-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 102.Yang H, Cai L, Zhang Y, Tan H, Deng Q, Zhao M, Xu X. Sensitive detection of EGFR mutations in cerebrospinal fluid from lung adenocarcinoma patients with brain metastases. J Mol Diagn. 2014;16:558–563. doi: 10.1016/j.jmoldx.2014.04.008. [DOI] [PubMed] [Google Scholar]
- 103.Thust SC, van den Bent MJ, Smits M. Pseudoprogression of brain tumors. J Magn Reson Imaging. 2018 doi: 10.1002/jmri.26171. [Epub ahead of print] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 104.Zeng A, Wei Z, Yan W, Yin J, Huang X, Zhou X, Li R, Shen F, Wu W, Wang X, You Y. Exosomal transfer of miR-151a enhances chemosensitivity to temozolomide in drug-resistant glioblastoma. Cancer Lett. 2018;436:10–21. doi: 10.1016/j.canlet.2018.08.004. [DOI] [PubMed] [Google Scholar]
- 105.Huang X, Xu J, Shen Y, Zhang L, Xu M, Chen M, Ren J, Zhou L, Gong H, Zhong P. Protein profiling of cerebrospinal fluid from patients undergoing vestibular schwannoma surgery and clinical significance. Biomed Pharmacother. 2019;116:108985. doi: 10.1016/j.biopha.2019.108985. [DOI] [PubMed] [Google Scholar]
- 106.Rajagopal MU, Hathout Y, MacDonald TJ, Kieran MW, Gururangan S, Blaney SM, Phillips P, Packer R, Gordish-Dressman H, Rood BR. Proteomic profiling of cerebrospinal fluid identifies prostaglandin D2 synthase as a putative biomarker for pediatric medulloblastoma: a pediatric brain tumor consortium study. Proteomics. 2011;11:935–943. doi: 10.1002/pmic.201000198. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 107.Kopkova A, Sana J, Fadrus P, Slaby O. Cerebrospinal fluid microRNAs as diagnostic biomarkers in brain tumors. Clin Chem Lab Med. 2018;56:869–879. doi: 10.1515/cclm-2017-0958. [DOI] [PubMed] [Google Scholar]


