Abstract
ARNTL2 is a transcriptional activator implicated in the molecular clock feedback system and is overexpressed in some malignant tumors. This study aimed to detect the effects of ARNTL2 knockdown by siRNA on the proliferation and invasion of colon carcinoma and clarify the molecular mechanisms of ARNTL2 in the development of colon carcinoma (CC). The CC microarray dataset GSE50760 was downloaded from the Gene Expression Omnibus (GEO) database. The expression levels of ARNTL2 in CC tissues and cancer cells were analyzed by immunohistochemistry and western blot, respectively. The knockdown of ARNTL2 expression was induced by RNA interference in colon cancer cells. The proliferation was detected by Cell Counting Kit-8 and clonal formation assays. The invasion and migration in vitro were detected by wound healing and transwell assays. Besides, a tumorigenicity test in the nude mice was performed to confirm whether ARNTL2 expression promoted the proliferation and invasion of CC cells. Furthermore, the expression of epithelial-mesenchymal transition (EMT) and PI3K/AKT signaling pathway-related factors were analyzed by western blot. Results showed that bioinformatics analysis found that ARNTL2 was upregulated in CC tissues. ARNTL2 was highly expressed in tissues and CC cells. Knockdown of ARNTL2 inhibited CC cells viability, colony formation, migration activity and reduced the size of tumors in the nude mice. Moreover, knockdown of ARNTL2 suppressed the expression of SMOC2, which may be the target gene of ARNTL2, and simultaneously inhibited the expression of EMT and PI3K/AKT signaling pathway-related factors. Taken together, downregulation of ARNTL2 could suppress CC cell proliferation and migration via SMOC2-EMT through inactivation of PI3K/AKT signaling pathway.
Keywords: ARNTL2, SMOC2, colon carcinoma, PI3K/Akt signaling pathway, epithelial mesenchymal transition
Introduction
According to the global cancer statistics in 2018, colon carcinoma (CC) is the fourth leading cause of cancer-related death [1]. Till now, therapeutic management for patients with CC is mainly depending on the surgery, chemotherapy, radiation therapy, immunotherapy, and targeted therapy. Recurrence and metastasis of CC are important factors that are associated with poor prognosis and high mortality. To study the mechanisms underlying the metastasis and recurrence of CC, new approaches may be uncovered to inhibit the proliferation and metastasis of tumor cells. Genomics of tumor molecular profiling enables the validation of novel therapeutic strategies [2]. However, the molecular mechanisms underlying the tumorigenesis and progression of CC are still not clear.
Most physiology and behavior of mammalian organisms rely on the circadian rhythm regulation, which is called the circadian clock system. It consists of a central pacemaker in the brain’s suprachiasmatic nuclei (SCN) and subsidiary clocks throughout the body [3,4]. At the molecular level, the circadian rhythms are operated by auto-regulatory transcriptional-translational feedback loops. The circadian genes mainly contain ARNTL (BMAL1) and its paralog ARNTL2 (BMAL2), CLOCK, PERIOD (PER1-3), and CRYPTOCHROME (CRY1 and 2). ARNTL or ARNTL2 and CLOCK encode the basic Helix-Loop-Helix-PAS transcription factors that heterodimerize with E-boxes to induce downstream clock genes PER and CRY expression. PER and CRY encode transcription factors that act as the negative regulators in the feedback loops. When PER and CRY are overexpressed at the protein level, they may transport into the nucleus and then form a complex with casein Kinase Iε (CKIε) by direct interaction, inhibiting the transcriptional activity of CLOCK-BMAL1 with a transcription-translation negative feedback loops [5-7]. Both BMAL1 and BMAL2 have a similar ornament in dimerizing with CLOCK to activate the E-box-dependent transcription. It has been revealed that PER2 shows a stronger inhibitory effect on BMAL2-CLOCK than that on BMAL1-CLOCK [8]. Bmal1 and Bmal2 form a circadian paralog pair that is functionally redundant. In mouse, Bmal2 is regulated by Bmal1, and knockout of Bmal1 alone results in a functionally double knockout of Bmal1 and Bmal2 [9]. ARNTL2 (Aryl hydrocarbon receptor nuclear translocator like 2) plays a vital role in the regulation of the circadian transcription.
Epithelial-to-mesenchymal transition (EMT) has been increasingly recognized to promote carcinoma invasion and metastasis. Upregulation of EMT regulators can promote the invasion and metastasis of colorectal carcinoma (CRC), as is shown by downregulation of E-cadherin expression and upregulation of N-cadherin and vimentin (VIM) expression [10]. It has been reported that EMT and matrix metalloproteinases (MMP) expression have been involved in the migration and intravasation in HCT-116 human colorectal cells [11]. Secreted modular calcium-binding protein2 (SMOC2) belongs to secreted protein acidic and rich in cysteine (SPARC) family of matricellular proteins. SMOC2 regulates the expression of ECM and MMPs, and modulates cell-matrix interactions, focal adhesions and actin stress fiber organization via activating cellular integrins [12]. SMOC2 also marks intestinal stem cells, and its induction in colorectal cancer cells has been shown to promote EMT activity [13]. SMOC2 is widely expressed during embryonic development, enhances responses to angiogenic growth factors, and mediates cell adhesion [14]. Moreover, ARNTL2 may directly regulate the expression of SMOC2 in lung adenocarcinoma [15]. The activity of EMT is regulated by many different signaling pathways. However it is still not clear whether ARNTL2 can regulate SMOC2-EMT expression and induce the proliferation and metastasis of CC cells.
PI3K/AKT/mTOR signaling pathway plays an important role in the growth and migration, resulting in the tumorigenesis and progression of HT-29 colorectal cancer cells [16]. AKT is activated in many human carcinomas, and the AKT-driven EMT may confer the motility that is required for invasion and metastasis. The invasive cancer cells show significantly increased expression of vimentin, decreased expression of E-cadherin, and activated AKT [17]. Here, we hypothesized that activation of the PI3K/AKT/mTOR signaling pathway promoted the activity of EMT and induced the proliferation and metastasis in CC cells.
This study aimed to explore the roles of ARNTL2 in modulating CC cell proliferation and migration. The expression of ARNTL2 in 60 pairs of CC and the matched adjacent tissues was detected by immunohistochemistry (IHC), and ARNTL2 in five CC cell lines and one normal colon cell line was determined by western blot. siRNA was designed and synthesised against ARNTL2, and the carcinogenic effect of ARNTL2 was investigated in CC cell lines, HCT-15 and HCT-116. The results showed that the expression of ARNTL2 was suppressed by RNAi in human CC cell lines. The effects of ARNTL2 on cell proliferation and migration were sequentially assessed in vitro and vivo. We also analyzed the possible mechanisms of ARNTL2 in regulating the proliferation and migration of HCT-15 and HCT-116 cells. This study may add to our perception of the pro-oncogenic activities of ARNTL2 in CC cell progression and metastasis.
Materials and methods
Data studies and analysis from GEO, oncomine, and TCGA database
Firstly, data was downloaded and processed from GEO, Oncomine, and TCGA. ARNTL2-related mRNA microarray datasets were collected from GEO and Oncomine. Moreover, ARNTL2-related mRNA datasets and clinical survival data from TCGA were analyzed to evaluate the survival of ARNTL2 in CC. The applied criteria for selecting eligible datasets were as follows: (1) human research; (2) both CC samples and normal controls were included in the dataset; (3) All CC samples were without any treatment by surgery.
Human CC specimens
60 paired CC and the matched adjacent tissues (5 cm away from the tumor tissues) were collected from CC patients undergoing surgery between January 2017 and December 2018 in Zhujiang Hospital. Of the patients recruited, 31 were men, and 29 were women. None of the patients received chemotherapy or radiotherapy before surgery. Tumor stages were assessed according to the methods recommended by the American Joint Committee on Cancer (AJCC) staging system. This study was approved by the Ethics Committee of Zhujiang Hospital and performed following the ethical standards. Written informed consent was obtained from each patient for the use of their tissue samples.
Immunohistochemisty staining
The tissue specimens were fixed and embedded in paraffin to make 4 um-thick slices. Then, paraffin was dewaxed and hydrated. IHC staining of ARNTL2 was carried out according to the instructions of the streptavidin-peroxidase staining kit, which was purchased from Youningwei Biotechnology Co., Ltd. (Shanghai, China). Rabbit anti-human ARNTL2 polyclonal antibody was purchased from Bioss Biotechnology Co., Ltd. (Beijing, China). The tissues were incubated overnight at 4°C with the primary antibodies that were diluted to the recommended concentrations. Then, 3,3’-diaminobenzidine (DAB) staining was performed, and the results were observed under a microscope. Tumors were considered positive, if they present only nuclear staining, with or without cytoplasmic staining.
Cell culture
Human CC cell lines (HCT-15, SW-48, HT-29, HCT-116, and HT-55) and human normal colon cell line (NCM-460) were provided by the Institute of Digestive Disease in Southern Medical University. Cells were cultured in Dulbecco’s modified Eagle’s medium (DMEM; Gibco, NY, USA) supplemented with 10% (v/v) heat-inactivated fetal bovine serum (FBS) (Hyclone, Thermo Scientific, Waltham, Mass) at 37°C with 5% CO2.
Analysis of ARNTL2 expression by immunofluorescence microscopy
SW48, HCT-15, HCT-116, and NCM-460 cells were seeded on the glass coverslips in 24-well plates overnight, and then fixed in 4% paraformaldehyde, followed by treatment with the permeabilizing agent of 0.5% Triton X-100. After being washed, coverslips were blocked by 5% bovine serum albumin for 1 hr. Next, cells were incubated with anti-ARNTL2 antibody (1:100) overnight at 4°C and then immunostained with the fluorescent-labeled secondary antibodies. The nuclei were stained by DAPI (1:5,000). Finally, the coverslips were examined under a fluorescent microscope.
Transfection of small interfering RNA (siRNA)
Two pairs of siRNA targeting ARNTL2 mRNA (si-ARNTL2) were synthesized by the Shanghai Jima Pharmaceutical Technology Company Limited (Guangzhou, China). The sequence of Si-1 was 5’TACCTATCTTCTTTCAACAAGGATA3’, and Si-2 was 5’ CAGAGAAGCTCATAGCCAAACTGAA 3’. Cells were trypsinized and seeded onto 24-well plates (105 cells/well) in 500 µl of growth medium without antibiotics until the cells reached 70%-80% confluency at the time of transfection. Lipofectamine 2000 (LF2000; Invitrogen, San Diego, CA, USA) was used for all transfections according to the protocol provided by the company. Fluorescently labeled FAM-siRNA was used to assess transfection efficiency.
Quantitative real-time PCR assay
48 hours after transfection, RNA was extracted from cells with Trizol, followed by reverse transcription and real-time PCR (RT-qPCR). The ARNTL2 mRNA expression was detected, and the most potency of si-ARNTL2 in suppressing gene expression was determined. qPCR was carried out by using SYBR® Premix Ex Taq TM (Takara) on the LightCycler 480 (Roche Applied Science, Indianapolis, IN). The sequences of qPCR primers of ARNTL2 gene used in this study were shown as follows: sense: 5’ACTTGGTGCTGGTAGTATTGGA3’, anti-sense: 3’TGTTGGACTCGAATCATCAAGG5’. qPCR reaction system contained SYBR premix ex Taq 12.5 μl, upstream or downstream primer (10 μM) 1.0 μl, cDNA 2 μl, and water 8.5 μl. The procedures for PCR cycles were 95°C for 30 seconds, 39 cycles of 95°C for 5 seconds, and 60°C for 30 seconds. Each reaction was performed in triplicate and analyzed individually. β-actin was used as an internal control. The mapping software, monitor, was used to calculate the expression levels by using the 2-ΔΔCt method. The most effective siRNA sequence was used for the following experiments.
CCK-8 proliferation assay
Firstly, HCT-15 and HCT-116 cells were transfected with the specific target ARNTL2 gene siRNA as the experimental group (si-ARNTL2), and the non-specific target gene siRNA as the negative control group (NC). And then 100 μl of cell suspension was plated in 96-well plates and incubated in an incubation box with 5% CO2 at 37°C. After 12 h, 24 h, 36 h, 48 h, and 60 h of incubation, 10 µl of CCK-8 (Beyotime Biotechnology Co., Ltd. Shanghai, China) was added to each well, followed by incubation at 37°C for approximately 2 h. The absorption (OD value) was measured at 450 nm using a spectrophotometer.
Colony formation assay
Transfected HCT-15 and HCT-116 cells were seeded into six-well plates (200-300 cells/well) and incubated in an incubator with 5% CO2 at 37°C. Due to the unstable silencing influence of siRNA, transient transfection needed to be performed repeatedly during the experiment. After 2 weeks, the medium was removed, and the cells were fixed with 75% ethanol for 30 min and stained with 0.2% crystal violet. The number of clones was counted. Each experiment was repeated three times.
Wound healing and transwell assays
HCT-15 and HT-116 cells were plated into a 6-well plate, respectively. After the confluency approached 80%, cells were transfected with the specific target ARNTL2 gene siRNA as the experimental group (si-ARNTL2) and the non-specific target gene siRNA as the negative control group (NC), respectively. Then, a scratch was made by a micropipette tip in a monolayer of cells. The detached cells were washed away with phosphate-buffered saline (PBS). Cells were incubated in DMEM medium containing 10% FBS at 37°C. The distance of wound healing was calculated at 0 h, 24 h, and 48 h, respectively. Images were captured from 5 random fields by using an inverted microscope. Triplicate wells for each condition were examined.
Transwell migration assay
The invasion of HCT-15 and HCT-116 cells was assessed by using a transwell invasion assay. The transwell chambers coated with 5 µl Matrigel basement membrane matrix were placed into 24-well culture plates. Cells were divided into the si-ARNTL2 group and the negative control (NC) group. Cells were collected after transfection for 24 h or 48 h and inoculated into the transwell chamber at a density of 5 × 105 cells/ml. The transwell chamber was removed after 48 h. Cells were stained and counted under a microscope. The cells were stained blue, while the polycarbonate membrane pore appeared as black dots. Three random wells in each group were selected for scoring, and the experiment was repeated three times.
In vivo tumor xenograft assays
To verify the influence of ARNTL2 knockdown on tumor growth, a xenograft model of nude mice in vivo was established. Six-week-old female athymic nude mice were purchased from the Model Animal Research Center of Southern Medical University. A lentiviral shRNA vector targeting ARNTL2, generated by Shanghai Jima Pharmaceutical Technology Company Limited (Guangzhou, China), was produced by inserting the specific oligonucleotides into the lentiviral vector plasmid. Two groups of mice (five mice for each group) were examined. One group was injected with cells transfected with ARNTL2 shRNA vectors (ARNTL2-shRNA). The other group was injected with cells transfected with empty vector control shRNA (NC). All the cells were selected with puromycin (5 μg/mL). Every mouse was injected subcutaneously with 5 × 106 cells in 0.2 ml of PBS into the right scapular region. The tumor volumes were measured.
Western blot
Proteins were purified from HCT-15 and HCT-116 cells transfected with si-ARNTL2 and scramble siRNA as a negative control (NC), respectively. Briefly, cells from each group were centrifuged, the lysates were prepared using RIPA buffer (Beyotime Biotechnology Co., Ltd. Shanghai, China). After centrifugation for 10 min at 4°C, the proteins were separated by 12% SDS-PAGE and transferred to PVDF membranes (Millipore, Billerica, MA, USA). The membranes were moved to the blocking buffer containing 5% non-fat milk (diluted in TBST) for 1 h at room temperature, followed by incubation with the primary antibodies at 4°C overnight. Next, the membranes were washed with TBST and incubated with a secondary antibody. Antibody-antigen complexes on the membranes were detected by ECL western blotting reagent (Millipore; Merck KGaA). Rabbit anti-human antibodies of ARNTL2 and SMOC2 was purchased from Bioss Biotechnology Co., Ltd. (Beijing, China), other antibodies including E-cadherin, N-cadherin, Vimentin, MMP-2, MMP-9, AKT, phosphorylated-AKT, PI3K, phosphorylated-PI3K, mTOR and phosphorylated-mTOR were purchased from Abcam Corporation (America). The rabbit antibodies against human GAPDH were obtained from Cell Signaling Corporation (America).
Statistical analysis
The statistical analysis was performed with SPSS 22.0 (IBM, Armonk, NY) and GraphPad Prism, version 6 (GraphPad Software Inc. San Diego, CA, USA). The t-test was used to analyze the difference between colon cancer and normal tissues. The Kaplan-Meier curve was analyzed by the log-rank test. The relationship between the ARNTL2 expression and clinical-pathological parameters of CC patients was analyzed by x2 tests. Student’s t-test or one-way analysis of variance (ANOVA) was used to calculate the statistical significance between the groups. All data were expressed as mean ± S.D. n=3/group. *P<0.05, **P<0.01, and ***P<0.001.
Results
Expression level of ARNTL2 in colon carcinoma by data studies
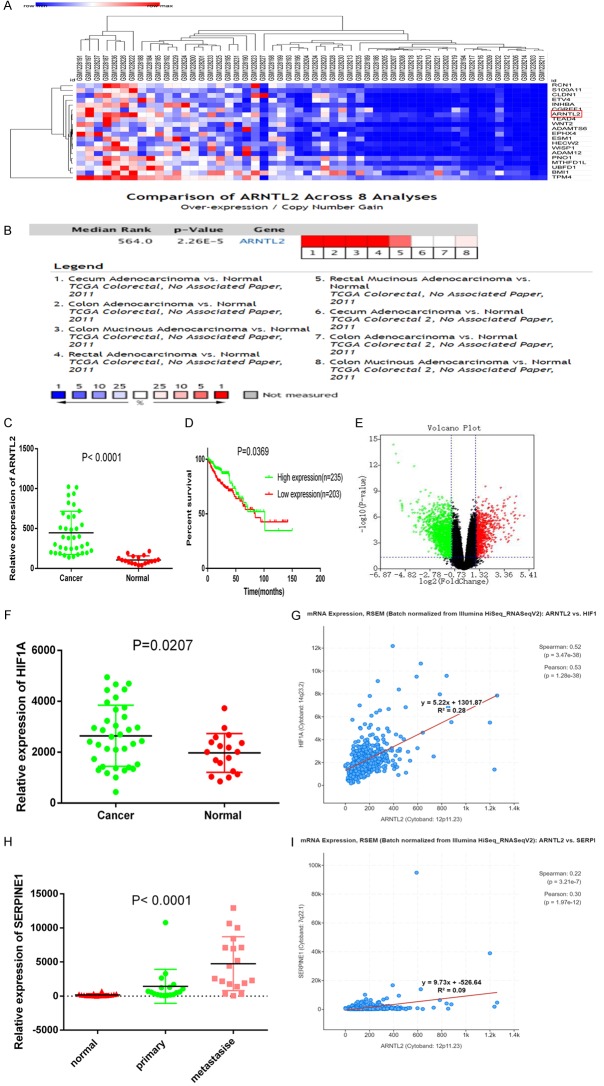
The mRNA expression profiles of the GSE50760 database contained 54 samples (the normal colon, the primary CC, and liver metastasis). The difference in gene expression from the GSE50760 database was presented by heatmap (Figure 1A). The ARNTL2 expression difference in the Oncomine database was presented in Figure 1B. The expression levels of ARNTL2 were also displayed as scatter plots and Volcanic Map (Figure 1C, 1E). The cut-off criteria were fold change (FC) > 2.0 and P<0.05. The survival time for patients with CC characterized by high expression of ARNTL2 was significantly shorter, which was obtained by survival analysis in the TCGA database (Figure 1D). Furthermore, the expression of ARNTL2 in colorectal cancer samples was more than that in normal group.
Figure 1.
Expression profiles of enriched genes in CC and their relationship with tumors. ARNTL2 was highly expressed in CC, and was associated with the poor survival. A. A heatmap revealed the different pathological stages for the expression patterns of CC oncogenes in non-tumorous and CC tissues. Red meant high expression, and blue meant low expression. B. Oncomine database searched for TCGA-derived CC dataset and comparison of ARNTL2 expression, it was highly expressed in CC. C. The expression of ARNTL2 was upregulated in CC tissues in GSE50760 dataset. P<0.05. D. Kaplan-Meier survival curves according to the median expression of ARNTL2 in CC patients. E. Differentially expressed genes in GSE50760 dataset shown in a volcano plot. The green and red spots represent low and high expression genes, respectively (cutoff criteria are P<0.05 and |FC| > 2.0). FC: fold change. F, H. Relative expression of HIF1A and SERPINE1 associated with ARNTL2 was highly expressed in CC in GSE50760 dataset. G, I. The correlation between HIF1A, SERPINE1 and ARNTL2 was analyzed in TCGA database.
Hypoxia-inducible factor-1 (HIF-1), a heterodimeric transcription factor, can be activated by hypoxia. HIF-1α accumulation has been detected in various cancer cells, such as liver, prostate, colon, and breast cancer [18]. The prognosis of CC is correlated with the stage at the primary diagnosis. SERPINE1 induction occurs in the progression of many cancers. SERPINE1 is the second most commonly upregulated gene, indicating its important roles in the tumor biology of rectal cancer [19]. The expression of HIF1A and SERPINE1, the ARNTL2 co-expression genes, was analyzed in the GSE50760 database (Figure 1F, 1H). The correlation between HIF1A or SERPINE1 and ARNTL2 was analyzed in the TCGA database (Figure 1G, 1I). It was showed that ARNTL2 was closely related to the survival time, formation, and progression of colorectal carcinoma.
ARNTL2 was frequently highly expressed in CC
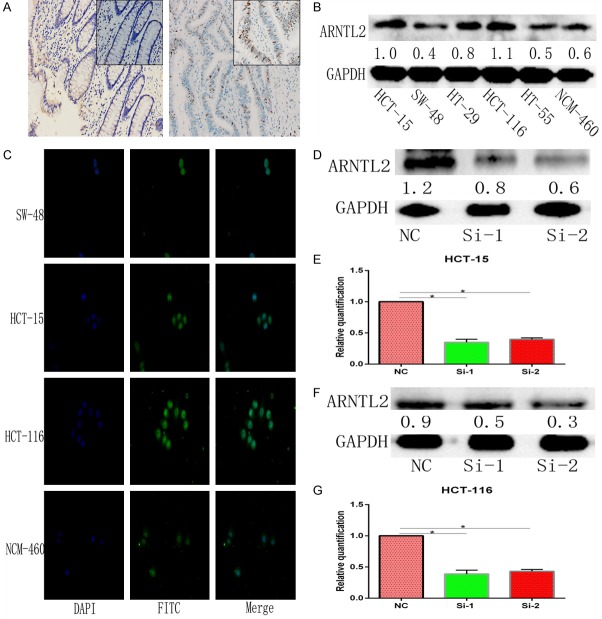
The expression of ARNTL2 in 60 pairs of CC and the adjacent tissues were detected by immunohistochemistry staining analysis. It revealed that ARNTL2 was strongly expressed in the nucleus of the tumor cells, which was differentially higher than that in the normal cells (Figure 2A). The relationship between ARNTL2 and clinicopathology parameters showed that ARNTL2 was significantly associated with T-stage (P=0.01) and M-stage (P<0.05) in CC. No difference in statistics between ARNTL2 expression and gender, age, and N-stage was observed (Table 1).
Figure 2.
Expression of ARNTL2 in clinical samples, screening of cell lines, and successful transfection of si-ARNTL2. A. ARNTL2 expression was upregulated in patients with CC. Representative IHC staining of ARNTL2 protein in the paired adjacent normal tissues (left) and CC tissues (right). ARNTL2 was highly expressed in the CC tissues. B. ARNTL2 was upregulated in CC cells. Detection of ARNTL2 expression in CC cells and normal colon cells by western blot. C. The expression of ARNTL2 in CC cells and normal colon cells was detected by cellular immunofluorescence, and the localization of ARNTL2 in the nucleus was determined. D, F. The protein expression of ARNTL2 for 48 h was decreased by western blot after transfection with si-ARNTL2 (si-1, si-2), compared with that transfected with the negative control siRNA in HCT-15 and HCT-116 cells. E, G. The mRNA expression of ARNTL2 for 24 h was decreased by qRT-PCR after transfection with si-ARNTL2 (si-1, si-2), compared with that transfection with the negative control siRNA in HCT-15 and HCT-116 cells (*P<0.05).
Table 1.
The relationship between ARNTL2 expression and clinicopathological features in patients with CC
| Clinical data | Number | ARNTL2 expression | Percentage (%) | x2 | P | |
|---|---|---|---|---|---|---|
|
| ||||||
| Low | High | |||||
| Gender | ||||||
| Male | 31 | 16 | 15 | 0.517 | 4.933 | 0.177 |
| Female | 29 | 12 | 17 | 0.483 | ||
| Age (year) | ||||||
| <60 | 22 | 9 | 13 | 0.367 | 0.933 | 0.817 |
| ≥60 | 38 | 18 | 20 | 0.633 | ||
| T-stage | ||||||
| T1-2 | 20 | 9 | 11 | 0.333 | 6.667 | 0.010* |
| T3 | 40 | 18 | 22 | 0.667 | ||
| N-stage | ||||||
| N0-1 | 28 | 13 | 15 | 0.467 | 0.067 | 0.796 |
| N2 | 32 | 16 | 16 | 0.533 | ||
| M-stage | ||||||
| M0 | 19 | 13 | 6 | 0.317 | 9.733 | 0.021* |
| M1 | 41 | 21 | 20 | 0.683 | ||
significant difference.
The expression of ARNTL2 in several CC cell lines was monitored by western blot analysis. It showed that the expression of ARNTL2 in HCT-15 and HCT-116 cells was much higher than that in other cell lines (Figure 2B, P<0.05). Furthermore, ARNTL2 was predominantly detected in the nucleus of CC cell lines. Stronger fluorescent anti-ARNTL2 signals were detected in the nucleus of HCT-15 and HCT-116 cells, compared with weaker signals in SW48 and NCM-460 cells lines (Figure 2C). To study the functional impact of ARNTL2 knockdown, HCT-15 and HCT-116 cell lines were chosen to carry out the subsequent experiments.
Knockdown of ARNTL2 inhibited the mRNA and protein expression of ARNTL2 in CC cells
As the results of RT-PCR and Western Blot showed, both mRNA and protein expression levels of ARNTL2 in HCT-15 cells were successfully decreased by transient transfection of si-1 and si-2, respectively, compared with those in the NC group (Figure 2D, 2E). Furthermore, similar results were also obtained in HCT-116 cells (Figure 2F, 2G). These results showed that the expression of ARNTL2 in HCT-15 and HCT-116 cells was successfully knocked down by siRNA.
Knockdown of ARNTL2 suppressed cell proliferation in vitro
To further explore the effects of ARNTL2 on CC cell proliferation, CCK-8 and colony formation assays were performed to detect the exact relationship between ARNTL2 and tumor growth. In CCK-8 assay, after 12 h, 24 h, 36 h, 48 h, and 60 h of transfection with si-1 and si-2, respectively, the cellular proliferation activity in both HCT-15 and HCT-116 cells was significantly decreased than that in the NC group with significant difference statistically (Figure 3A, 3B). Moreover, colony formation assays revealed that the number of cell communities in both HCT-15 and HCT-116 cells with ARNTL2 knockdown was significantly decreased than that in the NC group (Figure 3C-E). These results revealed that ARNTL2 knockdown inhibited the proliferation and colony formation of CC in vitro.
Figure 3.
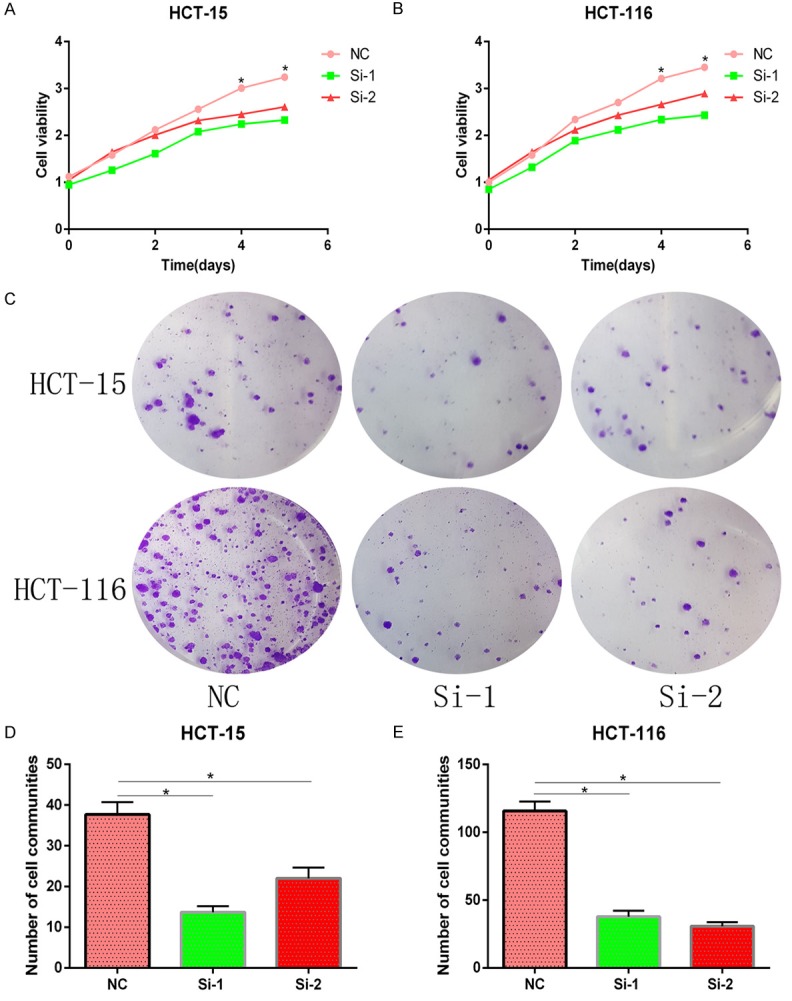
ARNTL2 knockdown inhibited the proliferation of HCT-15 and HCT-116 cells. A, B. In the CCK-8 assay, both HCT-15 and HCT-116 cells transfected with si-ARNTL2 (si-1, si-2) showed a lower level of cellular proliferation activity, compared with the negative control (*P<0.05). C-E. Colony formation study showed that both HCT-15 and HCT-116 cells transfected with si-ARNTL2 (si-1, si-2) had a 3-fold decrease in colony formation rate, compared with the NC group (*P<0.05).
Knockdown of ARNTL2 inhibited cell migration and invasion in vitro
To further investigate the migration and invasion activity of CC cells with knockdown of ARNTL2, wound-healing, transwell cell migration/invasion assays were performed in HCT-15 and HCT-116 cells. Wound-healing assay demonstrated that knockdown of ARNTL2 reduced cell healing ability after scratching at 0 h and 24 h, as showed that the healing width in both the si-1-transfected group and si-2-transfected group was significantly narrower than that in the NC group (Figure 4A-D). Furthermore, the migration (Figure 4E, 4F) and invasion (Figure 4G, 4H) activity were significantly attenuated after the ARNTL2 knockdown. These results suggested that cell migration and invasion activity were significantly decreased in CC cells with ARNTL2 knockdown.
Figure 4.
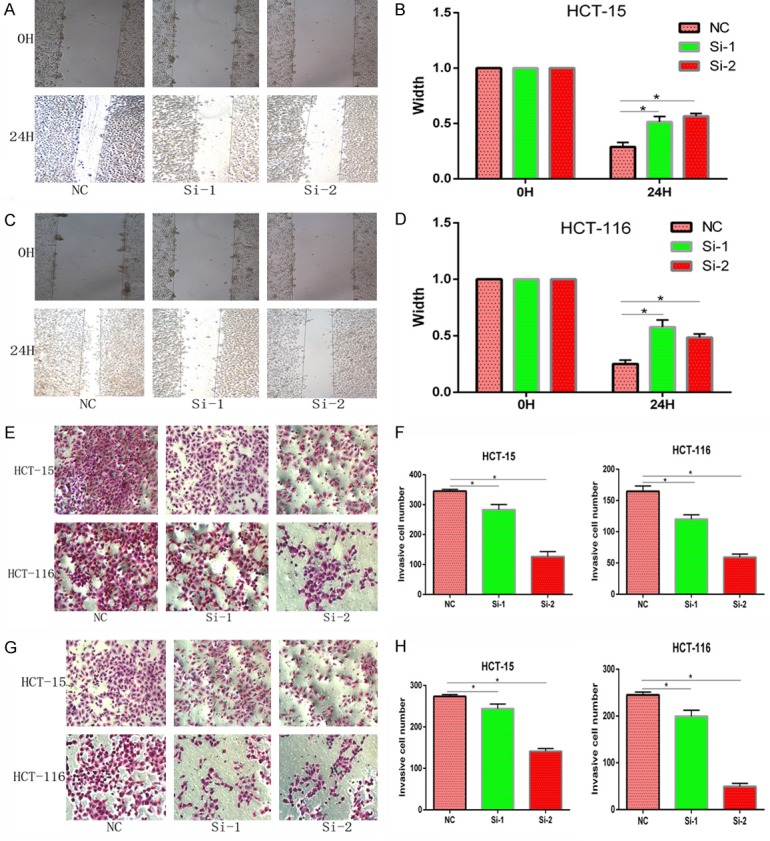
Knockdown of ARNTL2 suppressed the migration and invasion activity of CC. A-D. Wound-healing assay showed that migration activity was regulated by ARNTL2. The healing activity of HCT-15 and HCT-116 cells with ARNTL2 knockdown by si-ARNTL2 (si-1, si-2) was decreased than that of the negative control. E-H. Transwell migration and invasion assay showed that ARNTL2 knockdown significantly inhibited cell migration and invasion activity (*P<0.05).
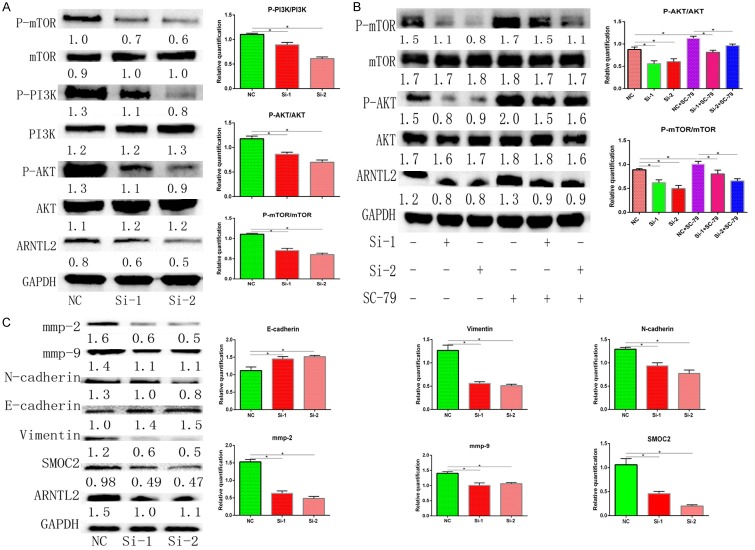
Knockdown of ARNTL2 deactivates PI3K/AKT signaling pathway in CC cells
PI3K/AKT signaling pathway has been involved in tumor development, invasion, and metastasis. To clarify the regulatory mechanism of ARNTL2 and PI3K/AKT in CC development, the expression of p-AKT, AKT, p-PI3K, PI3K, mTOR, and p-mTOR was detected by western blot in HCT-15 cells transfected with si-ARNTL2. The result showed that ARNTL2 knockdown notably inhibited the expression of p-AKT, p-PI3K, and p-mTOR. However, the expression of AKT, PI3K, and mTOR was not significantly changed (Figure 5A). Furthermore, we examined the effects of ARNTL2 knockdown on HCT-15 and HCT-116 cells treated with SC-79, which is a PI3K/AKT activator. The results revealed that SC-79 promoted the expression of p-AKT, p-PI3K, and p-mTOR, which were abrogated by ARNTL2 knockdown (Figure 5B). These results suggested that inhibition of the PI3K/AKT signaling pathway might be responsible for ARNTL2 knockdown in the modulation of CC development.
Figure 5.
A. ARNTL2 knockdown inactivated PI3K/AKT/mTOR signaling pathway in HCT-15 cells. The protein expression of the related factors in PI3K/AKT/mTOR signaling pathway was detected by western blot. ARNTL2 knockdown suppressed P-PI3K, P-AKT, and P-mTOR expression, while the expression of PI3K, AKT and mTOR were not affected. B. HCT-15 cells with ARNTL2-knockdown by si-ARNTL2 (si-1, si-2) were pretreated with P-AKT agonist SC-79 for 48 h before harvesting. The detection of AKT, p-AKT, mTOR, p-mTOR expression was performed by western blot. The expression of ARNTL2, p-AKT, p-mTOR was upregulated with the SC-79 after ARNTL2 knockdown. C. Knockdown of ARNTL2 suppressed EMT in HCT-15 cells. ARNTL2 knockdown markedly decreased the protein expression of N-cadherin, Vimentin and increased E-cadherin. The expression of SMOC2, MMP-2 and MMP-9 were suppressed with the inhibition of EMT (*P<0.05).
Knockdown of ARNTL2 inhibited the activity of EMT in CC cells
Epithelial-mesenchymal transition (EMT) is related to the proliferation and invasion of tumor cells. Since ARNTL2 could regulate the migration and invasion of CC cells, we next investigated the effects of ARNTL2 on EMT in HCT-15 cells. Western Blot analysis showed that ARNTL2 knockdown decreased the expression of SMOC2, MMP-2, MMP-9, N-cadherin, and Vimentin and increased the expression of E-cadherin (Figure 5C). These findings indicated that knockdown of ARNTL2 suppressed the expression of SMOC2, and simultaneously inhibited the invasion activity through upregulation of E-cadherin expression and downregulation of N-cadherin and Vimentin expression, inhibiting the activity of EMT.
Furthermore, we examined the effects of ARNTL2 knockdown on the growth of HCT-15 and HCT-116 cells treated with SC-79. The results revealed that SC-79 promoted the growth and proliferation of the cells. The colony formation assay was also performed. SC-79 significantly increased the colony formation rate in HCT-15 and HCT-116 cells with ARNTL2 knockdown (Supplementary Figure 1).
Knockdown of ARNTL2 inactivated PI3K/AKT signaling pathway and inhibited the activity of EMT in HCT-15 cells. Similar results were also obtained in HCT 116 cells (Supplementary Figure 2).
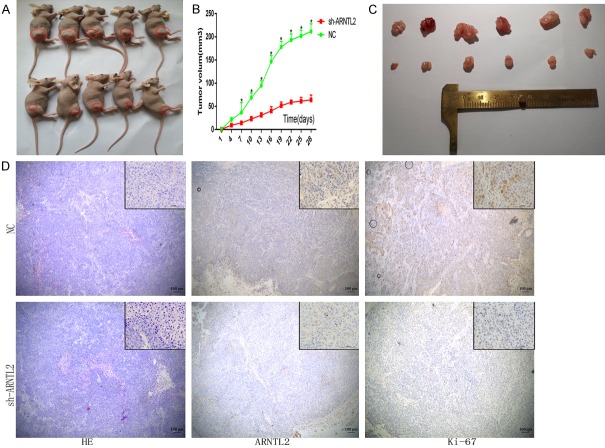
Knockdown of ARNTL2 suppressed tumor growth in the xenograft model in nude mice
To further evaluate the effects of ARNTL2 on colon cancer in vivo, xenograft models were constructed by using HCT-15 and HCT-116 cells. Cells were transfected with ARNTL2-shRNA and NC, respectively. The results showed that the tumor volume in the ARNTL2-shRNA group was relatively smaller, compared with that in the NC group. This implied that knockdown of ARNTL2 suppressed the tumor formation and growth of colon cancer (Figure 6A-C). Furthermore, the expression of ARNTL2 and Ki-67 in the nude mice was detected by immunohistochemistry and decreased significantly after transfection with ARNTL2-shRNA (Figure 6D).
Figure 6.
Knockdown of ARNTL2 suppressed CC invasion in vivo. A. ARNTL2 knockdown significantly inhibited CC invasion in vivo. At the indicated times, subcutaneous tumor volumes were measured with calipers (mean ± SD, n=5). B. HCT-15 cells stably infected with ARNTL2 shRNA or control shRNA were injected into the nude mice. C. Tumors were removed from mice after post-cells injection for 28 days. D. The tumors were analyzed by HE staining, and the expression of ARNTL2 and growth index Ki-67 was detected by immunohistochemistry.
Discussion
Circadian rhythms disorder is associated with metabolism, obesity, depression and cancers, which seriously affects human health [20-22]. Of note, the circadian desynchrony is closely related to the tumorigenesis of many cancers, including breast cancer [23], prostate cancer [24], oral cancer [25], colorectal cancer [26], non-Hodgkin lymphoma [27], lung cancer, ovarian cancer, endometrial cancers, and hepatocellular carcinoma [22]. It was found that the expression of circadian clock genes Per1, Per2, Cry1, and Cry2 in colorectal carcinoma was significantly increased, compared with that in human colorectal adenoma (P<0.05) [26]. Some research investigated the mRNA expression of Per1, Per2, Per3, Bmal1, and Clock in colorectal cancer tissues and the adjacent paired normal tissues from 16 patients. The expression of Per1 and Per3 was significantly decreased in tumor tissues (P<0.0001). However, no difference in the expression of Per2, Bmal1, and Clock was observed (P > 0.05) [28]. It has been also reported that the mRNA expression of ARNTL2 and SERPINE1 is upregulated in the primary colorectal cancer tissues from surgery. Meanwhile, increased mRNA expression of ARNTL2 and SERPINE1 are also found in colon cancer cell lines. They also found that ARNTL2 was associated with lymph node involvement. The co-expression of SERPINE1 and ARNTL2 is associated with microsatellite instability [29]. It has been demonstrated that ARNTL2 is related to tumor invasion and progression. However, the regulatory mechanisms of ARNTL2 in cell proliferation and metastasis of colorectal carcinoma are still unclear.
In this study, we firstly analyzed a microarray mRNA chip GSE50760, and the bioinformatics results indicated that ARNTL2 was upregulated in CC. Then, the survival time for patients with CC characterized by high expression of ARNTL2 was significantly shorter, which is obtained by survival analysis in the TCGA database. Then, the immunohistochemistry staining assay of 60 paired colon carcinoma tissue and the adjacent normal tissue demonstrated that high expression of ARNTL2 was correlated with the advanced clinicopathological stage of CC. Moreover, the protein expression of ARNTL2 was significantly upregulated in 5 CC cell lines by western blot. These results showed that increased expression of ARNTL2 in CC was validated by bioinformatics analysis, immunohistochemistry staining assay, and western blot. However, the mechanism of ARNTL2 in the regulation of cell proliferation and metastasis in CC was still not clear. So this study aimed to explore the effects of ARNTL2 on CC metastasis by RNA interfering in vivo and in vitro.
In this study, it was revealed that ARNTL2 knockdown suppressed the expression of SMOC2 and the activity of EMT, as showed by decreased expression of SMOC2, MMP-9, MMP-2, N-cadherin, Vimentin and increased expression of E-cadherin. In vitro study, knockdown of ARNTL2 significantly inhibited cell proliferation, invasion, and metastasis. In the nude mice, the tumor volume after the ARNTL2 knockdown was relatively smaller. These results suggested that knockdown of ARNTL2 suppressed the tumor formation and growth of CC. It indicated that the transcription factor ARNTL2 played a key role in colon carcinoma metastasis. Our analysis also showed that SMOC2 may be a potential regulator associated with matricellular protein and ARNTL2 may regulate the SMOC2 via EMT to promote the tumor metastasis in colon carcinoma. It was reported that the ability of SMOC2 to link structural extracellular matrix proteins to cancer cell integrins may provide critical molecular signals for survival, cell cycle progression, and other cancer stem cell properties during lung adenocarcinoma metastasis [15]. Our research was consistent with the research. But the mechanism of how ARNTL2 acts on SMOC2 to promote metastasis still necessitates further research.
PI3K/AKT signaling pathway plays an important role in regulating tumorigenesis and metastasis. Activation of PI3K/AKT signaling pathway is associated with CRC progression, and overexpression of AKT is related to colon carcinogenesis [30]. Activation of AKT signaling, a central feature of EMT, is the initial step for metastasis in CRC. EMT-related transcription factors and genes contain Snail, Slug, β-catenin, vimentin, MMP-2, and MMP-9, which are correlated with increased migration and invasion mediated in AKT/HCT-116 cells. It has been shown that the expression of E-cadherin is decreased with concomitantly increased formation of microvessel in AKT/HCT-116 tumors [31]. The mTOR kinase is a downstream effector of PI3K/AKT signaling and regulates EMT, motility, and metastasis of CRC via RhoA and Rac1 signaling pathways [32]. It has been reported that pharmacological inhibition or sh RNA-mediated knockdown of PI3K blocks upregulation of the Dbp mRNA expression. The inhibition of PI3K significantly reduced the promoter activity of the Dbp gene and decreased the recruitment of BMAL1/CLOCK to the E-box in the Dbp promoter. Moreover, inhibition of PI3K blocks the heterodimerization of BMAL1 and CLOCK. It has been suggested that PI3K signaling plays a modulatory role in the regulation of the transcriptional rhythm of Dbp gene by modulating the activity of BMAL1 and CLOCK [33]. PTEN is a key molecular controller of the PI3K signaling, and loss of PTEN function is often observed in a variety of cancers. It has been found that oxidation-driven loss of PTEN function results in the activation of mTOR signaling and the expression of BMAL1 [34]. Thus, we speculated that ARNTL2 might play an important role in tumorigenesis through the PI3K/AKT pathway. Consistently, our study showed that the expression of p-AKT, p-pI3K, and p-mTOR was decreased in HCT-116 and HCT-15 cells with ARNTL2 knockdown by western blot analysis. Our study also showed that knockdown of ARNTL2 inhibited the phosphorylation of AKT and suppressed the activity of EMT in CC cells. Furthermore, treatment with SC-79, a PI3K/AKT agonists, might recover the reduced expression of p-AKT induced by ARNTL2. These suggested that ARNTL2 activated PI3K/AKT signaling pathway and promoted the progression and metastasis of CC. However, the molecular mechanism of ARNTL2 in regulating the tumorigenesis of CC via the PI3K/AKT signaling pathway needs to be further explored.
Conclusions
Our study demonstrated that the expression of ARNTL2 was increased in CC. Knockdown of ARNTL2 inhibited CC cell proliferation and invasion through regulation of the activity of EMT and mToR/PI3K/AKT signaling pathway. These results suggested that ARNTL2 played an important role in CC development and might become a therapeutic target for managing patients who suffered from CC.
Disclosure of conflict of interest
None.
Supporting Information
References
- 1.Bray F, Ferlay J, Soerjomataram I, Siegel RL, Torre LA, Jemal A. Global cancer statistics 2018: globocan estimates of incidence and mortality worldwide for 36 cancers in 185 countries. CA Cancer J Clin. 2018;68:394–424. doi: 10.3322/caac.21492. [DOI] [PubMed] [Google Scholar]
- 2.Tsimberidou AM. Targeted therapy in cancer. Cancer Chemother Pharmacol. 2015;76:1113–1132. doi: 10.1007/s00280-015-2861-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Dibner C, Schibler U, Albrecht U. The mammalian circadian timing system: organization and coordination of central and peripheral clocks. Annu Rev Physiol. 2010;72:517–549. doi: 10.1146/annurev-physiol-021909-135821. [DOI] [PubMed] [Google Scholar]
- 4.Mohawk JA, Green CB, Takahashi JS. Central and peripheral circadian clocks in mammals. Annu Rev Neurosci. 2012;35:445–62. doi: 10.1146/annurev-neuro-060909-153128. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Okano T, Yamamoto K, Okano K, Hirota T, Kasahara T, Sasaki M, Takanaka Y, Fukada Y. Chicken pineal clock genes: implication of BMAL2 as a bidirectional regulator in circadian clock oscillation. Genes Cells. 2001;6:825–836. doi: 10.1046/j.1365-2443.2001.00462.x. [DOI] [PubMed] [Google Scholar]
- 6.Schibler U, Gotic I, Saini C, Gos P, Curie T, Emmenegger Y, Sinturel F, Gosselin P, Gerber A, Fleury-Olela F, Rando G, Demarque M, Franken P. Clock-talk: interactions between central and peripheral circadian oscillators in mammals. Cold Spring Harb Symp Quant Biol. 2015;80:223–32. doi: 10.1101/sqb.2015.80.027490. [DOI] [PubMed] [Google Scholar]
- 7.Schoenhard JA, Eren M, Johnson CH, Vaughan DE. Alternative splicing yields novel BMAL2 variants: tissue distribution and functional characterization. Am J Physiol Cell Physiol. 2002;283:C103–14. doi: 10.1152/ajpcell.00541.2001. [DOI] [PubMed] [Google Scholar]
- 8.Sasaki M, Yoshitane H, Du NH, Okano T, Fukada Y. Preferential inhibition of BMAL2-CLOCK activity by PER2 reemphasizes its negative role and a positive role of BMAL2 in the circadian transcription. J Biol Chem. 2009;284:25149–25159. doi: 10.1074/jbc.M109.040758. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Shi S, Hida A, McGuinness OP, Wasserman DH, Yamazaki S, Johnson CH. Circadian clock gene Bmal1 is not essential; functional replacement with its paralog, Bmal2. Curr Biol. 2010;20:316–321. doi: 10.1016/j.cub.2009.12.034. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Trung V, Datta PK. Regulation of EMT in colorectal cancer: a culprit in metastasis. Cancers. 2017;9 doi: 10.3390/cancers9120171. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Hsieh SL, Hsieh S, Lai PY, Wang JJ, Li CC, Wu CC. Carnosine suppresses human colorectal cell migration and intravasation by regulating EMT and MMP expression. Am J Chin Med. 2019;47:477–494. doi: 10.1142/S0192415X19500241. [DOI] [PubMed] [Google Scholar]
- 12.De S, Chen J, Narizhneva NV, Heston W, Brainard J, Sage EH, Byzova TV. Molecular pathway for cancer metastasis to bone. J Biol Chem. 2003;278:39044–50. doi: 10.1074/jbc.M304494200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Shvab A, Haase G, Ben-Shmuel A, Gavert N, Brabletz T, Dedhar S, Ben-Ze’ev A. Induction of the intestinal stem cell signature gene SMOC-2 is required for L1-mediated colon cancer progression. Oncogene. 2016;35:549–557. doi: 10.1038/onc.2015.127. [DOI] [PubMed] [Google Scholar]
- 14.Maier S, Paulsson M, Hartmann U. The widely expressed extracellular matrix protein SMOC-2 promotes keratinocyte attachment and migration. Exp Cell Res. 2008;314:2477–2487. doi: 10.1016/j.yexcr.2008.05.020. [DOI] [PubMed] [Google Scholar]
- 15.Brady JJ, Chuang CH, Greenside PG, Rogers ZN, Murray CW, Caswell DR, Hartmann U, Connolly AJ, Sweet-Cordero EA, Kundaje A, Winslow MM. An ARNTL2-driven secretome enables lung adenocarcinoma metastatic self-sufficiency. Cancer Cell. 2016;29:697–710. doi: 10.1016/j.ccell.2016.03.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Hu X, Zheng W, Luo Y, Ou X, Song L, Zhang S, He T, Guo Z, Zhu J, Shi H, Huang W, Yu R. Arca subcrenata polypeptides inhibit human colorectal cancer HT-29 cells growth via suppression of IGF-1R/Akt/mTOR signaling and ATP production. Nutr Cancer. 2020;72:260–272. doi: 10.1080/01635581.2019.1625935. [DOI] [PubMed] [Google Scholar]
- 17.Barrette K, Van Kelst S, Wouters J, Marasigan V, Fieuws S, Agostinis P, van den Oord J, Garmyn M. Epithelial-mesenchymal transition during invasion of cutaneous squamous cell carcinoma is paralleled by AKT activation. Br J Dermatol. 2014;171:1014–21. doi: 10.1111/bjd.12967. [DOI] [PubMed] [Google Scholar]
- 18.Lee SY, Kim HJ, Oh SC, Lee DH. Genipin inhibits the invasion and migration of colon cancer cells by the suppression of HIF-1 alpha accumulation and VEGF expression. Food Chem Toxicol. 2018;116:70–76. doi: 10.1016/j.fct.2018.04.005. [DOI] [PubMed] [Google Scholar]
- 19.Hughes R, Parry J, Beynon J, Jenkins G. Molecular changes consistent with increased proliferation and invasion are common in rectal cancer. Clin Transl Oncol. 2011;13:753–759. doi: 10.1007/s12094-011-0728-4. [DOI] [PubMed] [Google Scholar]
- 20.Albrecht U. The circadian clock, metabolism and obesity. Obes Rev. 2017;18(Suppl 1):25–33. doi: 10.1111/obr.12502. [DOI] [PubMed] [Google Scholar]
- 21.Gery S, Koeffler HP. Circadian rhythms and cancer. Cell Cycle. 2010;9:1097–1103. doi: 10.4161/cc.9.6.11046. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Pinho M, Sehmbi M, Cudney LE, Kauer-Sant’anna M, Magalhaes PV, Reinares M, Bonnin CM, Sassi RB, Kapczinski F, Colom F, Vieta E, Frey BN, Rosa AR. The association between biological rhythms, depression, and functioning in bipolar disorder: a large multi-center study. Acta Psychiatrica Scandinavica. 2016;133:102–108. doi: 10.1111/acps.12442. [DOI] [PubMed] [Google Scholar]
- 23.Stevens RG, Brainard GC, Blask DE, Lockley SW, Motta ME. Breast cancer and circadian disruption from electric lighting in the modern world. CA Cancer J Clin. 2014;64:207–18. doi: 10.3322/caac.21218. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Cao Q, Gery S, Dashti A, Yin D, Zhou Y, Gu J, Koeffler HP. A role for the clock gene Per1 in prostate cancer. Cancer Res. 2009;69:7619–7625. doi: 10.1158/0008-5472.CAN-08-4199. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Yuan H, Li H, Ma H, Niu Y, Wu Y, Zhang S, Hu Z, Shen H, Chen N. Genetic polymorphisms in key DNA repair genes and risk of head and neck cancer in a Chinese population. Exp Ther Med. 2012;3:719–724. doi: 10.3892/etm.2012.476. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Momma T, Okayama H, Saitou M, Sugeno H, Yoshimoto N, Takebayashi Y, Ohki S, Takenoshita S. Expression of circadian clock genes in human colorectal adenoma and carcinoma. Oncol Lett. 2017;14:5319–5325. doi: 10.3892/ol.2017.6876. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Lahti TA, Partonen T, Kyyroenen P, Kauppinen T, Pukkala E. Night-time work predisposes to non-hodgkin lymphoma. Int J Cancer. 2008;123:2148–51. doi: 10.1002/ijc.23566. [DOI] [PubMed] [Google Scholar]
- 28.Orhan T, Nielsen PB, Hviid TVF, Rosen AW, Gogenur I. Expression of circadian clock genes in human colorectal cancer tissues using droplet digital PCR. Cancer Invest. 2019;37:90–98. doi: 10.1080/07357907.2019.1571079. [DOI] [PubMed] [Google Scholar]
- 29.Mazzoccoli G, Pazienza V, Panza A, Valvano MR, Benegiamo G, Vinciguerra M, Andriulli A, Piepoli A. ARNTL2 and SERPINE1: potential biomarkers for tumor aggressiveness in colorectal cancer. J Cancer Res Clin Oncol. 2012;138:501–511. doi: 10.1007/s00432-011-1126-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Roy HK, Olusola BF, Clemens DL, Karolski WJ, Ratashak A, Lynch HT, Smyrk TC. AKT proto-oncogene overexpression is an early event during sporadic colon carcinogenesis. Carcinogenesis. 2002;23:201–205. doi: 10.1093/carcin/23.1.201. [DOI] [PubMed] [Google Scholar]
- 31.Suman S, Kurisetty V, Das TP, Vadodkar A, Ramos G, Lakshmanaswamy R, Damodaran C. Activation of AKT signaling promotes epithelial-mesenchymal transition and tumor growth in colorectal cancer cells. Mol Carcinog. 2014;53(Suppl 1):E151–60. doi: 10.1002/mc.22076. [DOI] [PubMed] [Google Scholar]
- 32.Gulhati P, Bowen KA, Liu J, Stevens PD, Rychahou PG, Chen M, Lee EY, Weiss HL, O’Connor KL, Gao T, Evers BM. mTORC1 and mTORC2 regulate EMT, motility, and metastasis of colorectal cancer via RhoA and Rac1 signaling pathways. Cancer Res. 2011;71:3246–3256. doi: 10.1158/0008-5472.CAN-10-4058. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Morishita Y, Miura D, Kida S. PI3K regulates BMAL1/CLOCK-mediated circadian transcription from the Dbp promoter. Biosci Biotechnol Biochem. 2016;80:1131–1140. doi: 10.1080/09168451.2015.1136885. [DOI] [PubMed] [Google Scholar]
- 34.Matsumoto CS, Almeida LO, Guimaraes DM, Martins MD, Papagerakis P, Papagerakis S, Leopoldino AM, Castilho RM, Squarize CH. PI3K-PTEN dysregulation leads to mTOR-driven upregulation of the core clock gene BMAL1 in normal and malignant epithelial cells. Oncotarget. 2016;7:42393–42407. doi: 10.18632/oncotarget.9877. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.