Abstract
This study investigates the inhibitory effect and potential mechanism of ligustrazine combined with paeonol on hepatic fibrosis, as to provide a new therapeutic strategy for clinical hepatic fibrosis. The degree of liver injury collagen deposition and inflammation was assessed by hematoxylin and eosin staining, Masson, Sirius red staining and biochemically serum analysis. ATP and ROS levels in each group were detected by chemical fluorescence method. The apoptotic rate was measured by Tunel assay. Mito-Tacker fluorescence staining and mitochondrial DNA copy number were measured to observe the effect of ligustrazine or/and paeonol on mitochondrial function of hepatic stellate cell (HSC). The expression of relevant proteins and genes were evaluated by using immunofluorescence RT-PCR and western blot. Ligustrazine or/and paeonol significantly improve the pathological changes in liver tissue induced by CCl4, however, they reduced the levels of liver and fibrosis markers in tissue and serum. ROS, NOX1 and NOX2 were significantly increased and GSH was decreased in HSC, with the intervention of Ligustrazine or/and paeonol. We further found that Ligustrazine or/and paeonol can effectively inhibit liver inflammation in vivo. The expression of TNF-α, IL-6 and IL-8 was upregulated in HSC. Moreover, Ligustrazine or/and paeonol promotes apoptosis and inhibit proliferation of HSC. Additionally, the inhibiting effects of the drug on collagen deposition was due to the interference with the expression of signaling pathway related proteins and genes such as, MMPS, TGF-β, PDGF and BMP-2 in HSC. Mitochondrial activity of HSC was inhibited by Ligustrazine or/and paeonol. The inhibitory effects of ligustrazine or/and Paeonol on mitochondrial function is partially balanced by mitochondrial protective agent SS-31. Ligustrazine combined with paeonol exerts significant anti-hepatic fibrosis effect in vivo and in vitro. This may due to the disruption of HSC mitochondrial function, thereby induced promoting oxidative stress, apoptosis, inflammation and inhibiting the formation and deposition of extracellular matrix.
Keywords: Hepatic fibrosis, hepatic stellate cells, ligustrazine, paeonol, combination therapy, mitochondria
Introduction
Liver disease is a major health problem worldwide, which may develop into cirrhosis, portal hypertension, hepatic encephalopathy and even lead to liver failure and death if not treated on time. Hepatic fibrosis is characterized by excessive deposition of extra cellular matrix (ECM) which is a compensatory response to tissue repair of various chronic liver injuries [1]. It has been suggested that, the activation of hepatic stellate cells (HSC) plays an important role in the formation of hepatic fibrosis [2]. If there is no effective treatment, fibrous nodules will form that will destroy the normal structure and function of liver. The liver injuries develop into cirrhosis eventually and some even into liver cancer. Liver disease attacks many people in China and various chronic hepatopathy seriously effects human health and life worldwide, therefore it is more pressing to clarify the mechanism of various hepatopathy and seek possible treatment strategies.
Hepatic fibrosis is a complex pathological process involving multiple cells and pathways rather than dominated and regulated by a single cell or signaling pathway. Modern medicine believes that by reducing the production of collagen fibers and enhancing their degradation, liver fibrosis can be reversed, with the possibility of reversal of cirrhosis [3]. However, there are few anti-fibrosis drugs with definite curative effect and few side effects at present, most of which are still in the experimental research phase. At this time, traditional Chinese medicine shows its unique advantages of integrate regulation. Some traditional Chinese medicine (TCM) such as salvia miltiorrhiza (dang-shen), Ligusticum wallichii (chuan-xiong), Artemisia apiacea (qing-hao) and their extracts, derivatives and some compound preparations under the guidance of TCM theory has been proved in experimental study that shows obvious anti-hepatic fibrosis effect [4-6]. Combined with TCM characteristic or holistic therapy, treatment based on syndrome differentiation, clinical efficacy can be improved by work out basic principles of treatment at all stages and adopt multiple methods.
Considering the complexity of the formation and development of hepatic fibrosis, the effective anti-hepatic fibrosis activity of the active ingredients in traditional Chinese medicine and its active ingredients, and the lack of clinical effective chemical drugs, we propose a strategy to combine the active ingredients of traditional Chinese medicine for the treatment of hepatic fibrosis [7,8]. Our previous studies have confirmed that Ligustrazine and Paeonol have definite anti-hepatic fibrosis effects [9,10]. The purpose of our research was to investigate whether Ligustrazine combined with paeonol have synergistic anti-fibrotic effect in inhibiting collagen production in liver, as well as their working mechanism.
Materials and methods
Animals and treatment
All experimental animals received appropriate humane care in accordance with guidelines of National Institutes of Health (USA), and the experimental procedures of animals were approved by the institutional and local committee on the care and use of animals of Nanjing University of Chinese Medicine (Nanjing, China). Male Sprague-Dawley rats weigh between 200 and 250 g, were obtained from Shanghai Slac Laboratory Animal Co., Ltd. (Shanghai, China), and divided into 5 groups i-e (control group, model group, ligustrazine group (100 mg/kg), paeonol group (100 mg/kg) and combination group (combined therapy with ligustrazine 100 mg/kg and paeonol 100 mg/kg) according to the method of randomized allocation. 50% olive oil CCl4 reagent was intraperitoneally injected to four groups of rats, except for control group, which were injected with pure olive oil 1 ml/kg per rat, twice a week. The treatment group was given corresponding drugs by gavage once every other day. The rats were fed in the quiet environment under room temperature, and were ad libitum to eat and drink. After treatment and modeling, serum indexes of liver fibrosis and liver tissue injury were detected by semi-automatic biochemical instrument and enzyme-linked immunosorbent assay (ELISA).
Cell experiment
Cells were divided into model group, ligustrazine group (20 μM/L), paeonol group (25 μM/L) and combined intervention group (Combined intervention with ligustrazine 20 μM/L and paeonol 25 μM/L). HSC-T6 (Shanghai Cell Bank of Chinese Academy of Sciences) cells were selected as experimental cells (HSC-T6 cells were activated automatically after culturing in specific medium) and were treated for 24 hours.
Histopathological observation
Liver tissue was fixed with 10% neutral formaldehyde solution and embedded in paraffin. The sections cuts were 4~5 μm thick. After routine H&E staining, optical microscope was used to observe the pathological variation of liver tissue. After Masson and Sirius red staining, the collagen fiber deposition in liver tissue was observed. Internationally accepted ISHAK pathological score of liver biopsy and fibrosis staging criteria were used to evaluate inflammatory response score and fibrosis stage of all specimens.
Serological index detection
The contents of ALT, AST, ALP, LDH, LN, Collagen IV, HA, PC III, Hydroxyproline, IL-6 and IL-8 in serum were measured by automatic biochemical analyzer.
Determination of ATP contents in mitochondria
The ATP contents in HSC mitochondria were determined by ATP detection kit. Supernatant fluid was taken after mitochondria was centrifuged, and standard curve of ATP content of mitochondria in each group was obtained according to the instructions. After that the ATP content of mitochondria in each group was calculated according to the manufacturer’s instructions.
DNA copy number
DNA was extracted and quantified. The measurement of amount of mtDNA (NADH dehydrogenase, subunit 1 [ND1]) relative to the previously described (27) nuclear gene HGB was used to calculate mtDNAcn. The Real-Time PCR System was used to implement experiment of qPCR, with SYBR Green Master Mix. The forward and reverse primer sequences of ND1 gene were 5’-AAC ATA CCC ATG GCC AAC CT and 5’-AGC GAA GGG TTG TAGTAG CCC, respectively. The forward and reverse primer sequences of HGB gene were 5’-GCT TCT GAC ACA ACT GTG TTC ACT AGC and 5’-CAC CAA CTT CAT CCA CGT TCA CC, respectively. Additionally, the cycling conditions for ND1 and HGB were initial heating step of 95°C for 10 min, followed by 40 cycles of 95°C for 15 s, 58°C for 20 s, and 72°C for 20 s. 10 ng DNA was used in each sample, which was amplified in triplicate. The same reference sample was used to amplify ND1 and the single-copy gene HGB in separate runs. The standard curve of five-point serial-dilution series with reference DNA was established.
Mitotracker
Based on Kit instructions, the staining of mitotracker was carried out. In short, NRCs were coated on the cover slips, which were subsequently stained with 0.01 μM MitoTracker® Red FM for half h after treatment, and mounted with DAPI with PBS cleaning. The laser scanning confocal microscope was used to image the NRCs. Based on the data of six fields observed and counted randomly; the percentage of fragmented mitochondria was calculated by using the following formula:
Percentage of fragmented mitochondria (%) = cells with fragmented mitochondria/the total number of cells.
ROS assay
The ROS levels were measured in HSC in vitro and in the mouse lung in vivo. By using the ROS Assay Kit, ROS levels on cells were measured according to the manufacturer’s guidelines. Briefly, after incubation for 24 h in 6-well plates, HSCs were treated with different reagents at specified concentrations for 24 h. In our experiment, after PBS cleaning and incubation with DCFH-DA solutions (10 μM) for 20 min, HSCs were imaged by using fluorescence microscope (excitation: 488 nm, emission wavelengths: 525 nm). In addition, HSCs was detached by trypsinization and then suspended in DCFH-DA solution (10 μM) for 20 min. The fluorescence intensity was detected under the condition of flow cytometry.
Tunel assay
Based on TUNEL peroxidase apoptosis detection kit instructions, the apoptosis of cells was implemented. The TUNEL-positive cells were checked by light microscopy. Apoptotic HSC was identified by positive TUNEL staining and morphology (cell shrinkage, chromatin condensation or/and imagination, and formation of apoptotic bodies).
Western blot anaylsis
Precooled RIPA lysate was added at 1:5 mass/volume ratio to 100 mg liver tissue, instantly followed by PMSF and phosphatase inhibitor. After incubation on ice for 30 minutes, the centrifugation was performed for liver tissue with the condition of 4°C, 15000 rpm/min for 15 min. The supernatant was taken as cell lysate. HSC was decomposed with cold RIPA pyrolysis buffer to get cell lysates. The cell lysates were stored overnight at -20°C. BCA method was used to determine the protein concentration. The rest were added with 6 × electrophoretic buffer of equal volume and boiled water bath for 10 minutes. Before experiment, the protein was centrifuged 4500 rpm/min-1 for 10 min, sampled with prepared SDS-PAGE gel (sample size 50 μg), electrophoresed 40 mA for 1.5 h, and transferred to PVDF membrane under 100 V/1.5 h, sealed with skimmed milk powder then incubated with primary and secondary antibodies. Electro-chemiluminescence (ECL) was used to color and image analysis system to practice semi-quantitative analysis.
Real-time PCR
According to the instruction, extraction of total RNA of liver tissue and HSC was carried out with Trizol reagent, and RNA content was determined by enzyme labeling instrument. Reverse transcript mRNA into cDNA and the reverse transcription products were obtained for real-time quantitative PCR. Using GAPDH as internal reference, the specific primers of PCR were listed as follows. Real-time fluorescence quantitative PCR based on comparative cycle threshold value was used to detect the relative expression of mRNA. The content of mRNA in each sample was standardized by the content of GAPDH in each internal reference.
Statistical analysis
The measurement data were described with mean ± SD and the comparison between groups was analyzed by one-way ANOVA. The SPSS 17.0 software was used for statistical analysis. Comparisons between the two groups were performed using Student’s unpaired t-test. P value less than 0.05 was considered statistically significant.
Results
Ligustrazine or/and paeonol mitigates liver injury and fibrosis in rats
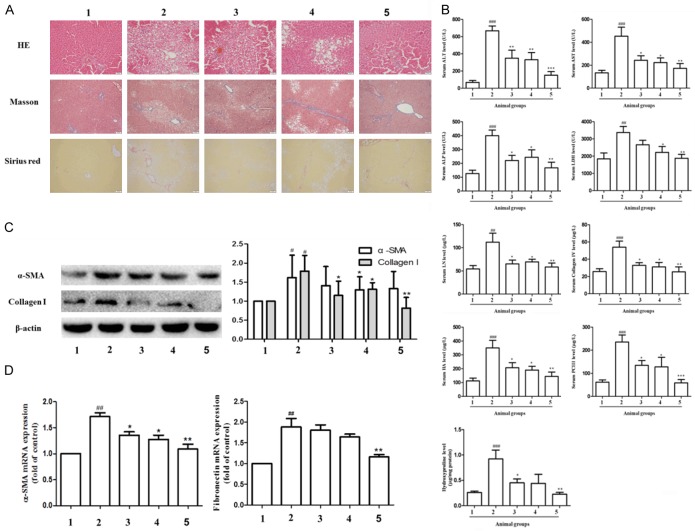
We initially investigated the effects of Ligustrazine or/and paeonol on liver injury and fibrosis in vivo. After H&E staining, Masson and Sirius red staining, the hepatic cord of the control group were arranged neatly. On the contrary, the structure of hepatic lobule was destroyed in model group. In hepatic cord arranged mussily, inflammatory cell were infiltrated, and hepatic cell edema was observed. Moreover, a large number of fibrous tissue was proliferated, and some pseudo lobules were formed. In ligustrazine or/and paeonol group, the degeneration and necrosis of hepatocytes were significantly reduced, so did the connective tissue and hepatocyte edema, with occasionally pseudo lobular formation and inflammatory cell infiltration (Figure 1A). Compared with model group, the levels of ALT, AST, ALP and LDH in ligustrazine and paeonol group were significantly lowered, and the effect of combination group was more obvious. Compared with model group, the levels of LN, Collagen IV, HA, PC III, and Hydroxyproline in ligustrazine and paeonol group were significantly lower (Figure 1B). Western blot further showed that the protein and gene expression of α-SMA, Collagen I and fibronectin was highly expressed in the model group, while the expression was inhibited in paeonol and ligustrazine group, and it was significantly downregulated in the combination group (Figure 1C and 1D).
Figure 1.
Effects of ligustrazine or/and Paeonol on liver tissue, serum liver injury indices and hepatic fibrosis markers in rats. A. HE (original magnification ×200), Masson (×100) and Sirius red (×100) staining in control model, ligustrazine or/and Paeonol treatment group liver tissue. B. Serum ALT, AST, ALP, LDH, LN, Collagen IV, HA, PC III, and Hydroxyproline activities were measured by automatic biochemical analyzer, respectively. C. Western blot analyses of protein expression of α-SMA and Collagen I. D. RT-PCR analysis of Fibronectin and α-SMA gene. 1: control group, 2: model group, 3: ligustrazine group (100 mg/kg), 4: paeonol group (100 mg/kg), 5: combination group (combined therapy with ligustrazine 100 mg/kg and paeonol 100 mg/kg). #, P<0.05, ##, P<0.01 and ###, P<0.001 vs. control group. *, P<0.05, **, P<0.01 and ***, P<0.001 vs. model group.
Ligustrazine or/and paeonol induced oxidative stress of HSC
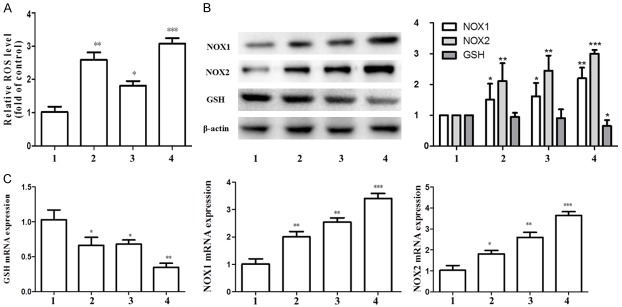
Next we explored the molecular events underlying Ligustrazine or/and paeonol inhibition of proliferation and activation of HSC. We all know that ROS play an important role in various cellular regulatory processes, so the effect of drugs on oxidative stress of HSC has attracted much attention for the first time. Compared with the control group, the expression level of ROS gene in HSC was significantly increased in the combination group (Figure 2A). The combination group increased the expression level of NOX1 and NOX2 gene and protein, and decreased the oxidation of GSH (Figure 2B, 2C). These results suggest that the combination of drugs can increase the level of oxidative stress in HSC, and may disrupted HSC mitochondrial function by oxidative stress.
Figure 2.
Effect of ligustrazine or/and Paeonol on oxidative stress index of HSC. A. The ROS Assay Kit was used to detect the levels of ROS. B, C. Western blot and RT-PCR analyses of protein and gene expression of NOX1, NOX2 and GSH. β-Actin was used as internal control. All the results presented were from three independent experiments. 1: model group, 2: ligustrazine group (20 μM/L), 3: paeonol group (25 μM/L), 4: combined intervention group (Combined intervention with ligustrazine 20 μM/L and paeonol 25 μM/L). *, P<0.05, **, P<0.01 and ***, P<0.001 vs. model group.
Ligustrazine or/and paeonol enhance inflammation of HSC
In previous studies, we found that Ligustrazine and paeonol had good anti-inflammatory effects. In this study, we further explored the anti-inflammatory effects of the combination of two drugs. Immunofluorescence staining showed that, the expression of α-SMA and TNF-α increased significantly in model group, while the expression decreased after paeonol and ligustrazine intervention. The effect of combination of Paeonol and ligustrazine was more evident (Figure 3A). By assessing the gene expression level of IL-6 and IL-8 in serum, we found that the combination group had potential anti-inflammatory effects (Figure 3B). Western blot results showed that, the combined treatment group significantly increased the expression of TNF-α and IL-6 protein in HSC (Figure 3C). Paeonol or ligustrazine can increase the gene expression level of TNF-α, and the combined treatment is more effective (Figure 3D).
Figure 3.
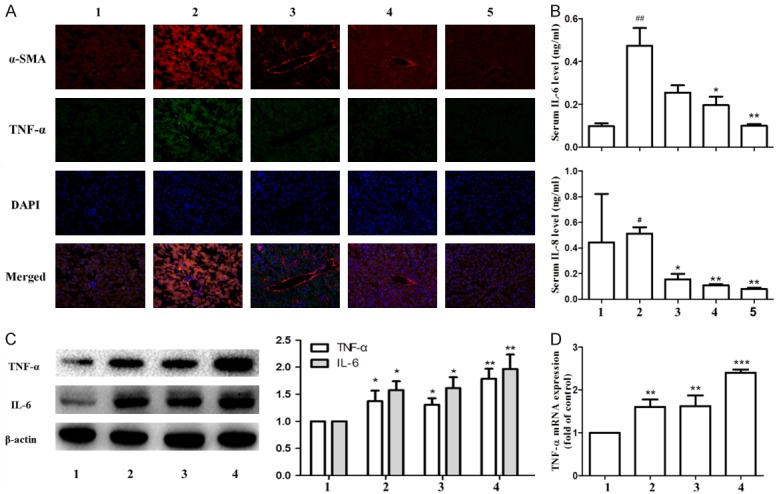
Effects of ligustrazine or/and Paeonol on liver fibrosis indices and inflammation indices. A. α-SMA and TNF-α in livers was visualized by immunofluorescence staining. Each panel represents a separate experiment. B. Determination of IL-6 and IL-8 in serum. A, B. 1: control group, 2: model group, 3: ligustrazine group (100 mg/kg), 4: paeonol group (100 mg/kg), 5: combination group (combined therapy with ligustrazine 100 mg/kg and paeonol 100 mg/kg). C. Western blot analyses of protein expression of TNF-α and IL-6. D. RT-PCR analyses of gene expression of TNF-α. C, D. 1: model group, 2: ligustrazine group (20 μM/L), 3: paeonol group (25 μM/L), 4: combined intervention group (Combined intervention with ligustrazine 20 μM/L and paeonol 25 μM/L). #, P<0.05 and ##, P<0.01 vs. control group. *, P<0.05, **, P<0.01 and ***, P<0.001 vs. model group.
Ligustrazine or/and paeonol on HSC cycle and expression of apoptosis-related protein
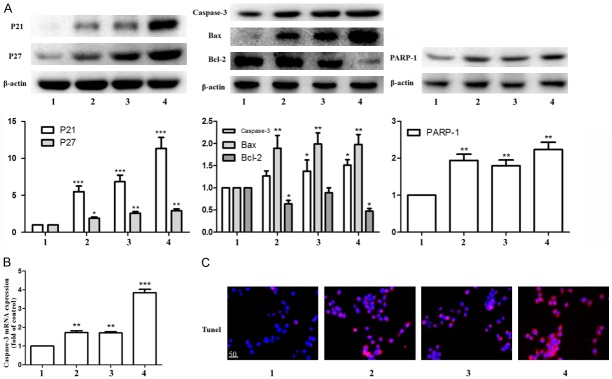
Inhibition of cell proliferation ability is the key to inhibit HSC activation. We then investigated the effect of Ligustrazine and paeonol on HSC proliferation and apoptosis. Western blot results showed that paeonol group, ligustrazine group and combination group promotes cyclin-dependent kinase negatively regulate the expression of protein P21 and P27, and then inhibit cell proliferation. At the same time, they could promote the expression of apoptotic regulatory protein Bax, executive protein Caspase-3 and its activation index PARP-1; inhibit the expression of Bcl-2, so to promote cell apoptosis (Figure 4A). The combination group also significantly promoted Caspase-3 gene expression (Figure 4B). Tunnel method results showed that the apoptotic status of HSC was more obvious in the combination group (Figure 4C).
Figure 4.
Effects of ligustrazine or/and Paeonol on HSC cycle and expression of apoptosis-related protein. A. Western blot analyses of P21, P27, Caspase-3, Bax, Bcl-2 and PARP-1. B. RT-PCR analysis of Caspase-3 gene. C. Tunel analyses apoptotic in HSC. 1: model group, 2: ligustrazine group (20 μM/L), 3: paeonol group (25 μM/L), 4: combined intervention group (Combined intervention with ligustrazine 20 μM/L and paeonol 25 μM/L). *, P<0.05, **, P<0.01 and ***, P<0.001 vs. model group.
Ligustrazine or/and paeonol on liver fibrosis markers, ECM major components and MMPs related protein and gene expression in HSC
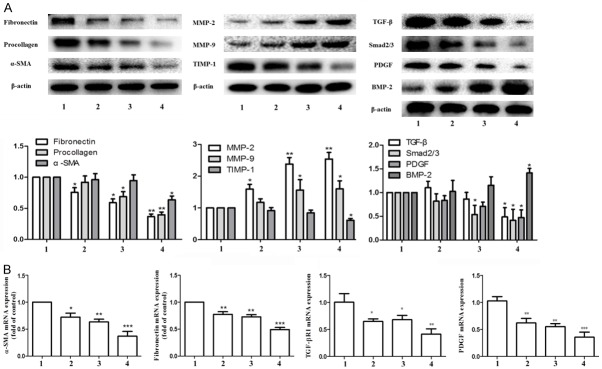
Multiple signaling pathways regulate HSC cell activation and extracellular matrix deposition. Therefore, our study focused on these pathways that regulate extracellular matrix deposition. Cytological studies showed that the expression of α-SMA, and fibronectin and Procollagen, was higher in activated HSC, while the expression of matrix metalloproteinase (MMP-2, MMP-9) was lower. Paeonol, ligustrazine and their combination can inhibit the expression of HSC activation-related proteins and mRNA expression, and increase the expression of MMP-2 and MMP-9, as well as inhibit the expression of TIMP-1, thus inhibiting the deposition of ECM and accelerating its degradation. The corresponding pharmacological effects of the combination group were better with significant differences. Compared with the model group, the combination group significantly reduced the expression of TGF-β, Smad2/3, PDGF, increased the expression of BMP-2 in HSC, and inhibit the occurrence of collagen deposition (Figure 5A, 5B).
Figure 5.
Effects of ligustrazine or/and Paeonol on liver fibrosis markers, ECM major components and MMPs related protein and gene expression in HSC. A. Western blot analyses of α-SMA, Fibronectin, Procollagen, MMP-2, MMP-9, TIMP-1, TGF-β, Smad2/3, PDGF and BMP-2. B. RT-PCR analysis of α-SMA, Fibronectin, TGF-β and PDGF gene. 1: model group, 2: ligustrazine group (20 μM/L), 3: paeonol group (25 μM/L), 4: combined intervention group (Combined intervention with ligustrazine 20 μM/L and paeonol 25 μM/L). *, P<0.05, **, P<0.01 and ***, P<0.001 vs. model group.
Anti-hepatic fibrosis effect of ligustrazine or/and paeonol depend on interfering with HSC mitochondrial function
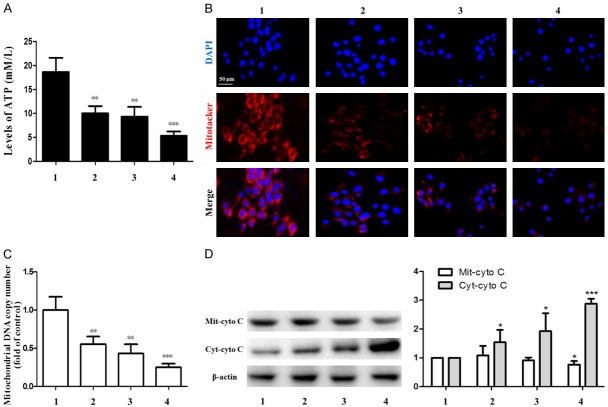
There are many ways for combined therapy to prevent liver fibrosis, but what is its internal mechanism? Our results found that the ATP level of mitochondria in HSC was significantly decreased in the combination group compared to the control group (Figure 6A). Immunofluorescence staining demonstrated that the activity of mitochondria in HSC was inhibited in the combination group (Figure 6B). The combined drug group had a significant effect on reducing the number of DNA replication in mitochondria (Figure 6C). In combination group, cytochrome C (Cyto C) in mitochondrial membrane space was released into cytoplasm and caused damage to mitochondrial function in HSC (Figure 6D). These results suggested that combination therapy can reduce ATP level by inhibiting HSC mitochondrial function.
Figure 6.
Effects of ligustrazine or/and Paeonol on mitochondria in HSC. A. ATP content in HSC mitochondria was determined by ATP detection kit. B. Illustrating the Immunofluorescence microscopic finding the number of mitochondria in each group. Blue color indicates nuclei stained by DAPI. Red color indicates mitotracker-positively stained cells. C. Mitochondrial DNA copy number was tested. D. Western blot analyses of protein expression of Mit-cyto C and enhances protein expression of Cyt-cyto C. 1: model group, 2: ligustrazine group (20 μM/L), 3: paeonol group (25 μM/L), 4: combined intervention group (Combined intervention with ligustrazine 20 μM/L and paeonol 25 μM/L). *, P<0.05, **, P<0.01 and ***, P<0.001 vs. model group.
Compared with the combination group, the protein and gene expression of GSH and α-SMA increased significantly in the combined drug+SS-31 group, while the protein and gene expression of TNF-α and Caspase-3 decreased. From the above data, it can be seen that the Ligustrazine and paeonol got anti-hepatic fibrosis effect by inhibiting the mitochondrial activity of HSC (Figure 7A, 7B). Our study suggests that the mechanism of inhibiting the proliferation and activation of HSC by Ligustrazine and paeonol may be related to the disruption of cell mitochondrial function.
Figure 7.
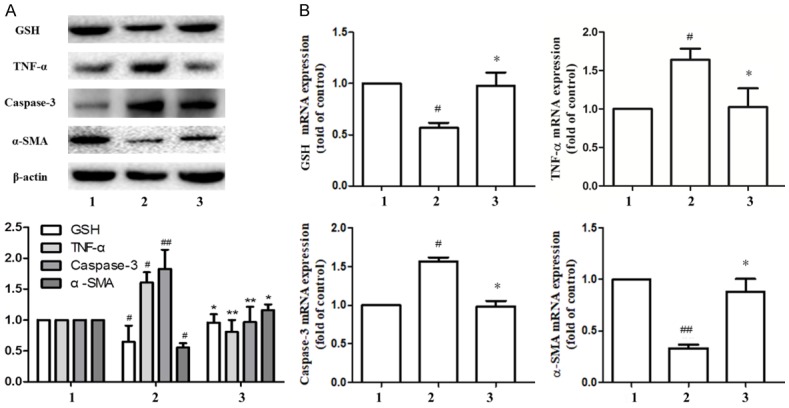
Anti-hepatic fibrosis effect of ligustrazine or/and Paeonol was required interfering with HSC mitochondrial function. A, B. Western blot and PCR analyses of protein or gene of GSH, α-SMA, TNF-α and Caspase-3. 1: model group, 2: combined intervention group (Combined intervention with ligustrazine 20 μM/L and paeonol 25 μM/L), combined intervention group at the same time to add mitochondrial protective agent SS-31. #, P<0.05 and ##, P<0.01 vs. model group. *, P<0.05, **, P<0.01 and ***, P<0.001 vs. combined group.
Discussion
Liver fibrosis refers to the pathological changes characterized by persist existence of liver injury inducement, continuous necrosis of hepatocytes, secondary inflammation of the liver or persistent inflammation during repair process, abnormal proliferation of fibrous connective tissue and excessive deposition of extracellular matrix in the liver. Therefore, theoretically, if we can eliminate pathogenic factors and stimulants by numerous ways, inhibit inflammatory reactions and extracellular matrix deposition and promote its degradation, it is expected to reverse or prevent the occurrence and progress of liver fibrosis, and ultimately prevent the occurrence of cirrhosis.
As active products of traditional Chinese herbal, ligustrazine and paeonol have been used as a folk remedy with heat-clearing, anti-inflammatory and detoxification effects for a long time. Our results exhibited the effects of ligustrazine and paeonol on the preventing hepatic fibrosis in a CCl4-induced hepatic fibrosis model of rats. The mechanisms of combination therapy were investigated through the evaluation of relevant cytokines and analyzed the expression of protein in HSC. Finally, we confirmed that the ligustrazine and paeonol got anti-hepatic fibrosis effect by suppressing the mitochondrial activity of HSC.
Compared with untreated rats, high number of inflammatory cell infiltrates, extensive necrosis of hepatocytes around the lobule, and increased deposition of collagen fibers in hepatic lobules were found in CCl4-treated rats through histopathological examination. However, these pathological changes could be alleviated in the combined treatment group. In addition, the serum levels of ALT, AST, ALP and LDH were significantly downregulated in the combination group, which are important markers of hepatocellular damage. The levels of LN, Collagen IV, HA, PC III, and Hydroxyproline were upregulated in the model group, whereas downregulated in the combination group. HSCs do not express α-SMA under static conditions. However, in response to inflammatory and cytokines, HSCs are activated with high expressed α-SMA, fibronectin and collagen I, and a large number of ECM components rapidly proliferated and synthesized [8]. The combination therapy could reduce the gene or protein expression of the HSC activation markers mentioned above.
The formation of ROS can be affected by mitochondria dysfunction or diminishing or/and inhibiting antioxidant system, and the overproduction of ROS may lead to apoptosis and cell death [11]. NOXs expressed in HSCs play a key role in liver fibrosis [12]. The present study revealed that ligustrazine and paeonol elevated ROS, NOX1 and NOX2 levels, reduced the expression of GSH as well, which suggested that combined therapy had an effect on lipid peroxidation injury of HSC.
It is reported the inflammatory plays a crucial role in the process leading to organ damage under various stresses [13]. As mentioned previously, CCl4 treatment leads to liver injury, and its mechanism may be closely related with inflammation through elevating the secretion of pro-inflammatory cytokine [14]. Our results confirmed that the expression of TNF-α, IL-18 and IL-6 were upregulated in CCl4-treated mice in vivo, which may be related with mechanism of liver injury. But in HSC, it was found that ligustrazine and paeonol reduced the expression of TNF-α and IL-6, which suggested that combination therapy induced inflammatory response in HSC.
In the process of hepatic fibrosis, the transformation of quiescent HSCs to activated myofibroblasts is the central step for development. Besides, the elevated expression of fibrotic ECM proteins also plays an important role in the hepatic fibrosis [15]. Thus, the suppression of activated HSCs is considered as an important strategy for antifibrosis therapy. Our results showed ligustrazine and paeonol had protective effects on hepatic fibrosis in vitro hepatic fibrosis through inducing apoptosis of HSC. Then the effects of ligustrazine and paeonol on the expression of different proteins regulating apoptosis, including p21, p27, Bax, Bcl-2, caspase-3 and PARP-1, were explored in subsequent experiments. With the elevated expression of p21 and p27 in HSC-T6 cells, the activities of caspase-3, Bax, PARP-1 and the expression of Bcl-2 were inhibited.
TGF-β1 is a pleiotropic cytokine, which plays an important role in HSCs transdifferentiation to myofibroblasts, and induction of transcription of collagen and ECM components [16]. Fibronectin is a member of ECM protein, which is a multifunctional glycoprotein induced by HSCs. Fibronectin is essential for collagen matrix assembly in vitro, and can be stimulated by TGF-β1. Fibronectin binds ECM components with integrin, a transmembrane receptor, and acts as a scaffold for collagen deposition [17]. In our study, the expression of TGF-β1, fibronectin, α-SMA and procollagen deposition were inhibited after the intervention of ligustrazine and paeonol. These results suggested the expression of α-SMA could be induced by activated HSCs, which further stimulated the expression of TGF-β1, fibronectin and procollagen. It is reported that MMPS/TIMPS system is directly related to the regulation of liver fibrosis, and MMP-2 and MMP-9 play the most effective role by destroying the normal basolateral matrix [18]. However, TIMP-1 exerts its physiological function by inhibiting MMP. In our study, the downregulated expressions of MMP-2 and MMP-9 in fibrotic rats indicated that there might be early response in liver injury. The expression of MMP-2 and MMP-9 were upregulated in treatment of ligustrazine and paeonol treatment, thereby promoting the degradation of ECM and futher alleviating liver fibrosis. Besides, the expressions of TIMP-1 was significantly increased by CCl4 injection, which were significantly downregulated by treatment of ligustrazine and paeonol.
Mitochondria, the center of cell metabolism. Mechanistically, cell survival is an ATP-dependent process that requires well-organized mitochondria for sufficient energy production [7]. Our results demonstrated the depleted ATP synthesis and suppressed Mitochondrial DNA copy number were induced by incubation of activated mitochondria in HSC with ligustrazine and paeonol. Moreover, compared with control group, an abrupt release of cyto-c in HSC was induced by treatment of ligustrazine and paeonol. In order to verify that the combined treatment is destroying the mitochondria of HSC, we added the mitochondrial protectant SS-31. SS-31 protects mitochondrial structure by interacting with cardiolipin on the inner mitochondrial membrane and protecting cristae structure [19]. SS-31 abolished the reduced in TNF-α and Caspase-3, at the same time increased expression of GSH and α-SMA.
This study suggested that ligustrazine and paeonol had potential protective effects on CCl4-induced liver injury, which is dependent on their ability to inhibit the expression of α-SMA, Collagen I and Fibronectin, as well as impair of mitochondrial function in HSC to against hepatic fibrosis (Figure 8). However, there are still many shortcomings in this study, which will be further solved in the follow-up study. It is necessary to select suitable compatibility schemes of ligustrazine and paeonol, and to optimize the related formulation in order to further improve the anti-hepatic fibrosis effect. To study the effects of ligustrazine combined with paeonol intervention in vitro on activation and inflammatory factor release of Kupffer cell. The components of traditional Chinese medicine are complex. To further identify the active ingredients of ligustrazine combined with paeonol, relevant pharmacological experiments and pharmaco-chemical analysis need to be carried out.
Figure 8.
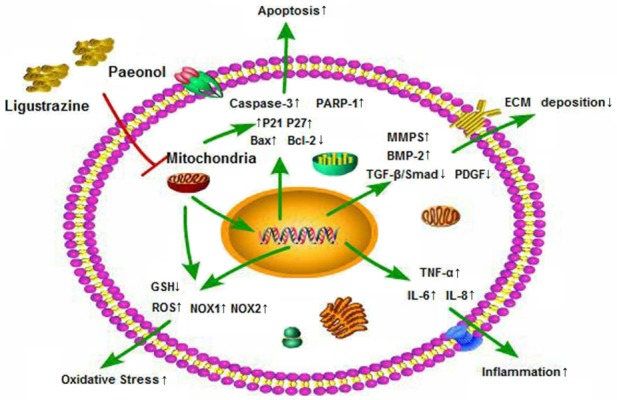
Proposed mechanisms underlying the positive therapeutic effects of combined ligustrazine and paeonol preserved hepatic function in a setting of liver injury. The protective effects of combined ligustrazine and paeonol on liver function are completed by inhibiting oxidative stress, inflammation, cellular apoptosis, ecxtracellular matrix deposition, through destroying mitochondrial function of HSC.
Acknowledgements
This work was supported by the National Natural Science Foundation of China (81600483, 31571455, 31401210, and 31600653), the Six Talent Peaks Project in Jiangsu Province (WSN-102), the Natural Science Foundation of Jiangsu Province (BK20140955), the Nanjing Medical Science and technique Development Foundation (QRX17190, YKK17153, YKK14143), Chinese Foundation for Hepatitis Prevention and Control Tianqing Hepatology Research Foundation (TQGB20170027, TQGB20140235), the Open Project Program of Jiangsu Key Laboratory for Pharmacology and Safety Evaluation of Chinese Materia Medica (No. JKLPSE201804) and the Project of the Priority Academic Program Development of Jiangsu Higher Education Institutions (PAPD), and the Postgraduate Research & Practice Innovation Program of Jiangsu Province (KYCX19_1257).
Disclosure of conflict of interest
None.
References
- 1.Cai XG, Xia JR, Li WD, Lu FL, Liu J, Lu Q, Zhi H. Anti-fibrotic effects of specific-siRNA targeting of the receptor for advanced glycation end products in a rat model of experimental hepatic fibrosis. Mol Med Rep. 2014;10:306–14. doi: 10.3892/mmr.2014.2207. [DOI] [PubMed] [Google Scholar]
- 2.Zheng H, Wang X, Zhang Y, Chen L, Hua L, Xu W. Pien-Tze-Huang ameliorates hepatic fibrosis via suppressing NF-kappaB pathway and promoting HSC apoptosis. J Ethnopharmacol. 2019;244:111856. doi: 10.1016/j.jep.2019.111856. [DOI] [PubMed] [Google Scholar]
- 3.Huang Y, Deng X, Liang J. Modulation of hepatic stellate cells and reversibility of hepatic fibrosis. Exp Cell Res. 2017;352:420–426. doi: 10.1016/j.yexcr.2017.02.038. [DOI] [PubMed] [Google Scholar]
- 4.Chen Q, Chen L, Kong D, Shao J, Wu L, Zheng S. Dihydroartemisinin alleviates bile duct ligation-induced liver fibrosis and hepatic stellate cell activation by interfering with the PDGF-betaR/ERK signaling pathway. Int Immunopharmacol. 2016;34:250–258. doi: 10.1016/j.intimp.2016.03.011. [DOI] [PubMed] [Google Scholar]
- 5.Hui J, Gao J, Wang Y, Zhang J, Han Y, Wei L, Liu X, Wu J. Panax notoginseng saponins ameliorate experimental hepatic fibrosis and hepatic stellate cell proliferation by inhibiting the Jak2/Stat3 pathways. J Tradit Chin Med. 2016;36:217–24. doi: 10.1016/s0254-6272(16)30030-9. [DOI] [PubMed] [Google Scholar]
- 6.Peng R, Wang S, Wang R, Wang Y, Wu Y, Yuan Y. Antifibrotic effects of tanshinol in experimental hepatic fibrosis by targeting PI3K/AKT/mTOR/p70S6K1 signaling pathways. Discov Med. 2017;23:81–94. [PubMed] [Google Scholar]
- 7.Ji K, Lin K, Wang Y, Du L, Xu C, He N, Wang J, Liu Y, Liu Q. TAZ inhibition promotes IL-2-induced apoptosis of hepatocellular carcinoma cells by activating the JNK/F-actin/mitochondrial fission pathway. Cancer Cell Int. 2018;18:117. doi: 10.1186/s12935-018-0615-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Li D, Li W, Chen Y, Liu L, Ma D, Wang H, Zhang L, Zhao S, Peng Q. Anti-fibrotic role and mechanism of Periplaneta Americana extracts in CCl4-induced hepatic fibrosis in rats. Acta Biochim Biophys Sin (Shanghai) 2018;50:491–498. doi: 10.1093/abbs/gmy024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Kong D, Zhang F, Wei D, Zhu X, Zhang X, Chen L, Lu Y, Zheng S. Paeonol inhibits hepatic fibrogenesis via disrupting nuclear factor-kappaB pathway in activated stellate cells: in vivo and in vitro studies. J Gastroenterol Hepatol. 2013;28:1223–33. doi: 10.1111/jgh.12147. [DOI] [PubMed] [Google Scholar]
- 10.Zhang F, Lu S, He J, Jin H, Wang F, Wu L, Shao J, Chen A, Zheng S. Ligand activation of PPARgamma by ligustrazine suppresses pericyte functions of hepatic stellate cells via SMRT-mediated transrepression of HIF-1alpha. Theranostics. 2018;8:610–626. doi: 10.7150/thno.22237. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Shi S, Yao L, Guo K, Wang X, Wang Q, Li W. Hepatocellular toxicity of oxalicumone a via oxidative stress injury and mitochondrial dysfunction in healthy human liver cells. Mol Med Rep. 2018;17:743–752. doi: 10.3892/mmr.2017.7979. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Gan D, Zhang W, Huang C, Chen J, He W, Wang A, Li B, Zhu X. Ursolic acid ameliorates CCl4-induced liver fibrosis through the NOXs/ROS pathway. J Cell Physiol. 2018;233:6799–6813. doi: 10.1002/jcp.26541. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Liu Y, Wen PH, Zhang XX, Dai Y, He Q. Breviscapine ameliorates CCl4-induced liver injury in mice through inhibiting inflammatory apoptotic response and ROS generation. Int J Mol Med. 2018;42:755–768. doi: 10.3892/ijmm.2018.3651. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Shi H, Dong L, Jiang J, Zhao J, Zhao G, Dang X, Lu X, Jia M. Chlorogenic acid reduces liver inflammation and fibrosis through inhibition of toll-like receptor 4 signaling pathway. Toxicology. 2013;303:107–14. doi: 10.1016/j.tox.2012.10.025. [DOI] [PubMed] [Google Scholar]
- 15.Cheng CF, Pan TM. Ankaflavin and monascin induce apoptosis in activated hepatic stellate cells through suppression of the Akt/NF-kappaB/p38 signaling pathway. J Agric Food Chem. 2016;64:9326–9334. doi: 10.1021/acs.jafc.6b03700. [DOI] [PubMed] [Google Scholar]
- 16.Eissa LA, Kenawy HI, El-Karef A, Elsherbiny NM, El-Mihi KA. Antioxidant and anti-inflammatory activities of berberine attenuate hepatic fibrosis induced by thioacetamide injection in rats. Chem Biol Interact. 2018;294:91–100. doi: 10.1016/j.cbi.2018.08.016. [DOI] [PubMed] [Google Scholar]
- 17.Liu XY, Liu RX, Hou F, Cui LJ, Li CY, Chi C, Yi E, Wen Y, Yin CH. Fibronectin expression is critical for liver fibrogenesis in vivo and in vitro. Mol Med Rep. 2016;14:3669–75. doi: 10.3892/mmr.2016.5673. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Zhang J, Yang A, Wu Y, Guan W, Xiong B, Peng X, Wei X, Chen C, Liu Z. Stachydrine ameliorates carbon tetrachloride-induced hepatic fibrosis by inhibiting inflammation, oxidative stress and regulating MMPs/TIMPs system in rats. Biomed Pharmacother. 2018;97:1586–1594. doi: 10.1016/j.biopha.2017.11.117. [DOI] [PubMed] [Google Scholar]
- 19.Szeto HH, Liu S, Soong Y, Seshan SV, Cohen-Gould L, Manichev V, Feldman LC, Gustafsson T. Mitochondria protection after acute ischemia prevents prolonged upregulation of IL-1beta and IL-18 and arrests CKD. J Am Soc Nephrol. 2017;28:1437–1449. doi: 10.1681/ASN.2016070761. [DOI] [PMC free article] [PubMed] [Google Scholar]