Abstract
Purpose
To describe the presentation, clinical course and management of a patient with posterior hypopyon secondary to atypical (fungal) endogenous endophthalmitis.
Observations
A 55-year-old Asian Indian female presented with decreased vision in the left eye (OS). The best-corrected visual acuity was 20/20 in the right eye (OD) and counting fingers (CF) in the left eye (OS) at the time of initial presentation. Slit-lamp examination revealed 1+ cells and 1+ flare in the anterior chamber of OS. Clinical examination and imaging assessment with fundus photography revealed vitritis, a posterior hypopyon and retinal exudates. The patient had an episode of fever one month before presentation for which an intravenous dextrose infusion was administered. After carefully evaluating the patient, ocular images, detailed history and necessary laboratory tests, a working diagnosis of endogenous endophthalmitis was reached. Empirical treatment with topical and systemic antibiotics, and topical cycloplegics and steroids was initiated. Therapeutic and diagnostic pars plana vitrectomy (PPV) was subsequently performed; microbiology and cytology analyses revealed evidence of fungal elements. Therefore, systemic anti-fungal treatment was initiated; the patient demonstrated significant clinical improvement with good visual outcome.
Conclusion and importance
Posterior hypopyon in endophthalmitis is a rarely observed entity and is typically obscured due to dense vitritis. Such clinical manifestation may suggest a possible infectious etiology as described in this case.
Keywords: Fungal, Endogenous endophthalmitis, Immunocompetent, Posterior hypopyon, Aspergillus, Cytology, Contaminated infusions, Dextrose
1. Introduction
Endophthalmitis is an acute, vision threatening condition resulting from a bacterial or fungal infection of ocular fluids (aqueous and vitreous) and may often lead to irreversible vision loss if not managed timely and adequately. It can broadly be classified into exogenous and endogenous types depending on the primary locus or the pathogenesis of the underlying condition.1 Exogenous endophthalmitis is acquired from an external source. Most common cause of exogenous endophthalmitis is surgery, trauma, or other ocular interventions such as intraocular injection. Acute post-cataract endophthalmitis accounts for 40–80% of all the cases encountered,2 though increased incidence of post-injection endophthalmitis has been reported recently.3 On the other hand, endogenous endophthalmitis, in most cases, is a sequela of an already existing systemic infection, intravenous drug abuse, immunosuppression for systemic indication and/or an underlying concomitant chronic disease.4 Endogenous endophthalmitis can be bacterial or fungal, with or without systemic manifestations of the disease process.5 Exposure from an external source via the ocular surface or hematogenous seeding of the eye with the pathogenic organism from an internal locus initiates the pathological events. Underlying etiology of the endophthalmitis is an important determinant of disease presentation, severity, progression and outcome. Decreased vision is the most common complaint for nearly all cases, accompanied sometimes by fever (predominantly with endogenous endophthalmitis), eye pain, or hyperemia. On examination, anterior chamber hypopyon is a common manifestation. Retinal structures are often obscured on fundoscopic examination due to dense vitritis, very often presenting as yellow glow. Choroid is usually the first structure to get infected due to high vascularity; hence, the disease typically starts in the posterior segment with retinal involvement followed by posterior hypopyon or vitreous seeding. Soon thereafter, one can see frank picture of fulminant endophthalmitis, with findings of hypopyon, dense vitritis and yellow glow.
2. Case report
A 56-year-old Asian Indian female presented with painless progressive loss of vision in the left eye (OS) for a duration of 15 days. The onset of ocular symptoms was gradual with no prior treatment for the complaint. There was no history of any chronic systemic illness and family history was non-contributory. Patient denied previous history of similar complaints, ocular trauma or surgery. One month prior to the onset of ocular symptoms, patient had developed fever for which she was admitted and treated with intravenous infusions at a local health care facility. There was no history or evidence of an exogenous source of infection (e.g. surgery, intravenous catheters, dental procedures, intravenous drug abuse) or an internal infective locus/ongoing infectious disease process (e.g. chest, urinary tract or abdominal infection).
On ophthalmic examination, the Early Treatment Diabetic Retinopathy Study (ETDRS) best-corrected visual acuity (BCVA) was 20/20 in the right eye (OD) and counting fingers (CF) close to face in OS. Pupillary reactions, intraocular pressure (IOP), and anterior segment structures were normal in OD. Slit-lamp examination revealed presence of 1+ cells, 1+ flare, and pigment deposition on corneal endothelium in OS. A quiet anterior chamber was observed in OD.
Dilated fundus examination of OS revealed clear media with 2+ vitreous cells and presence of creamy – fluffy exudates on the surface of retina in the posterior pole. Adjacent to the inferotemporal arcade, a – boat/crescent – shaped hypopyon was observed, which obscured the underlying vasculature (Fig. 1). Remaining visible retinal vasculature appeared normal. Careful examination of peripheral fundus with indirect ophthalmoscopy did not reveal any lesion. Examination of OD was within normal limits.
Fig. 1.
Fundus photograph of the left eye showing significant vitritis, posterior hypopyon (blue arrow) and superficial retinal exudates.
Laboratory evaluation including erythrocyte sedimentation rate (ESR), C-reactive protein (CRP) levels, complete blood counts (CBC), renal function tests, liver function tests, peripheral blood smears, blood and urine cultures were unremarkable. Serology for HIV and Hepatitis B surface antigen was negative. Chest radiography was performed and revealed no abnormalities.
Due to dense vitritis and posterior hypopyon, which suggested an infectious etiology, topical and systemic therapy was considered. The patient was started initially on topical antibiotic (moxifloxacin 0.5% q1h), topical corticosteroids (betamethasone 0.1% q1h), topical cycloplegic (atropine 1% tid) in OS and systemic antibiotics (intravenous ciprofloxacin 200 mg bid). However, since the initial laboratory and systemic investigations were non-contributory, a diagnostic and therapeutic pars plana vitrectomy (PPV) was performed with posterior hyaloid removal in OS on the next day with subsequent administration of empirical intravitreal antibiotics (vancomycin 1mg/0.1 ml + ceftazidime 2.25 mg/0.1 ml). Vitreous samples obtained were sent for potassium hydroxide (KOH) mount, gram stain, fungal and bacterial culture and sensitivity, pan-fungal Polymerase Chain Reaction (PCR) testing, Toxoplasma PCR and cytology examination. The investigations did not reveal any positive finding. However, definitive diagnosis was obtained by cytology performed on the vitreous sample smear which showed evidence of fungal profile besides polymorphs, macrophages, lymphocytes, and spindle shaped eosinophilic cells (Fig. 2). Ribbon shaped, sparsely septate to aseptate hyphae branching at acute angles (most consistent with aspergillus) were seen, and hence confirming the diagnosis of fungal (Aspergillus) endophthalmitis. Additionally, Galactomannan assay for Aspergillus antigen test was also performed and revealed a negative serum 0.15 value (cut off index of below 0.5) which helped to rule out invasive aspergillosis.
Fig. 2.
Cytology of vitreous samples using H&E stain (A) showing ribbon-like, septate fungal (Aspergillus) hyphae. Similar findings are seen on Giemsa Stain (B).Branching hyphae at acute angles (marked by red arrow) with scattered inflammatory cells are seen on Methylene Blue stain (C) suggestive of Aspergillus sp. Frequent septae are seen more prominently (marked by red arrows) on Lactophenol Cotton Blue staining (D). Oral Itraconazole 200 mg twice daily was started and continued for six weeks with monitoring of liver function tests. (For interpretation of the references to colour in this figure legend, the reader is referred to the Web version of this article.)
On post-operative day 1, clear media and presence of mild retinal whitening inferior to the optic disc were noted in OS (Fig. 4A). This lesion appeared to be the posterior focal point of entry of the infection (Aspergillus) into the vitreous cavity from the choroid via retina.
Fig. 4.
Fundus photos showing significant clinical improvement with clear media on post-operative day 1 (A) and visual acuity of CF at 1 meter, RPE atrophy (blue arrow) is visible at 6 weeks (B) and pigmentary changes are visible (yellow arrow) at 18 months (C), with best-corrected visual acuity of 20/60. Media haze (C) is attributable to the development of posterior sub-capsular cataract.
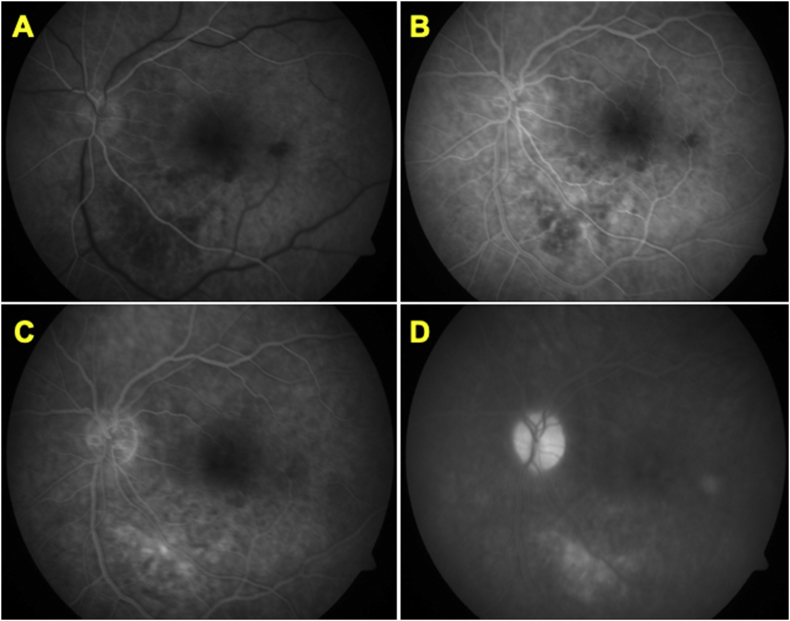
Fluorescein angiography (Fig. 3. ) revealed hypofluorescent area in the location of retinal whitening which turned hyperfluorescent in late stages. Optic disc staining was seen in the recirculation phase of angiogram. Vessels and macula appeared normal. At the time of last follow-up visit, 18 months after surgery, BCVA was 20/60. Fig. 4 shows a comparison of fundus findings and improvement in clinical picture from first post-operative day to the most recent visit with subsequent improvement in vision.
Fig. 3.
Fluorescein angiography of the left eye, conducted on third post-operative day. Early arterio-venous phase (A) showing choroidal hypofluorescence which evolves into stippled hyperfluorescence (B) during laminar flow; further increasing in intensity in (C). Recirculation phase (D) shows disc staining and persistent choroidal hyperfluorescence of the lesion. These features on FA are suggestive of choroiditis.
3. Discussion
Endogenous endophthalmitis varies between 2 and 15% of all cases of endophthalmitis.6,7 Bacteria and fungi are the main causative agents that infect ocular structures following dissemination and seeding from a remote source of infection. Both anterior and posterior segment structures are affected secondary to either direct colonization by the agents or as a sequela of the infection in adjacent ocular structures. Fungal endophthalmitis is a rarer presentation with potentially worse visual outcomes. Endogenous fungal endophthalmitis (FE) is encountered much less frequently compared to exogenous FE. Endogenous FE, when encountered, is usually seen in immunocompromised individuals and can be associated with chronic systemic diseases, e.g. diabetes, long term steroid use, chemotherapy, malignancy or intravenous drug abuse. Candida is the most common causative agent of endogenous FE (80%), followed by Aspergillus species (20%).8,9 Aspergillus gains access to the human body via inhalation and are combated by alveolar macrophages and neutrophils. In immunocompromised individuals, the organism invades and disseminates via hematogenous seeding. As a rare occurrence, direct hematogenous access via contaminated needles (as seen in intravenous drug abusers), prolonged indwelling catheters, or contaminated intravenous infusions is emerging as an etiology. Endogenous FE in immunocompetent patients still remains an uncommon presentation. Disease can present either as unilateral (75%) or bilateral (25%) ocular involvement.10
Our patient did not have any underlying disease condition or compromised immune status to account for higher predisposition. However, as indicated, the patient was administered intravenous dextrose infusions as a part of fever management at a local health care facility. Patient developed ocular symptoms one month thereafter.
Aspergillus endophthalmitis in immunocompetent subjects has been reported by Sachdev et al.11 and Gupta et al12,13 all of whom had received intravenous infusion as part of the management of minor systemic ailments. In all of these cases, common observation was the absence of any other predisposing factor and a shared history of recent intravenous infusion. This common observation raised the suspicion of contaminated dextrose/infusion solutions, pointing towards a possible common infectious etiology. In the context of our case report, it is important to note similar findings observed by Dogra et al..14 They reported the presence of crescentic, creamy-white exudate below the inferior vascular arcade suggestive of posterior hypopyon as a sequala of fungal endophthalmitis. However, media haze was significant due to dense vitritis; hence, posterior hypopyon was not appreciated in detail.
In typical cases of fungal endophthalmitis, posterior hypopyon is difficult to document because dense vitritis often obscures fundus details at presentation. Gupta et al.12 reported 12 cases, wherein culture microbiology of the infusion fluids was performed; such analysis further validates findings in our case regarding contaminated infusions being the source of fungal endophthalmitis. It was confirmed that sealed and sterile solutions and needles were employed for all the infusions. Due to the non-availability of originally used infusion sets or fluid, as a next step, the quality of the samples obtained from the sealed bottles of intravenous dextrose solution was tested. Eleven of 72 samples showed the growth of different types of fungi, thus providing evidence for the suspected underlying etiology.
Our case may have presented early because of macular involvement and significant vision loss; hence, the demonstration and documentation of this rare fundus finding was possible. The presence of exudates was limited to the posterior pole; the exudates appear to arise from the surface of the retina and have sedimented in the sub-hyaloid space. Gravitational settling in the potential sub-hyaloid space imparts a characteristic boat-shaped appearance to exudates, which in most cases is obscured by dense vitritis at presentation.
As mentioned above, chorioretinitis is the initial manifestation of endogenous FE, since choroid is the first layer to get infected due to high vascularity followed by retina. Greyish-white chorio-retinal lesions are observed with overlying fluffy, creamy-white exudates. Aspergillus species tend to reside longer in the retinal and choroidal layers, before breaking through inner limiting membrane/posterior cortical hyaloid; eventually developing vitreous exudates and yellow fundal glow. This often results from an ongoing disease process or delayed intervention.3 The lesions typically involve the macula. Disease may vary from silent chorio-retinitis to highly symptomatic and fulminant disease course with lesions that are confluent with indistinct margins in the macular area. Retinal involvement may manifest as subretinal infiltrates/abscess to full thickness retinal necrosis. Takebayashi et al.13 classified the endogenous endophthalmitis into various stages: (1) stage I: chorio-retinal changes without extension into the vitreous cavity; (2) stage II: fungal mass penetrating through the inner limiting membrane and budding into the vitreous cavity; (3) stage III: vitreous opacity resulting in a blurred fundus; (4) stage IV: the findings in stage III plus complications of retinal detachment.
In our patient, the fungal elements were detected on cytology of the vitreous; both retina and choroid revealed focal involvement. We have reported a rare presentation of fungal endogenous endophthalmitis where a characteristic ‘boat shaped’ posterior hypopyon was observed, owing to a predominantly clear anterior vitreous in stage II disease. Our case is an atypical presentation of the disease process following an unusual etiology.
According to the guidelines provided by the Infectious Disease Society of America (IDSA),15 it is recommended that Aspergillus endophthalmitis be treated with systemic oral or intravenous voriconazole plus intravitreal voriconazole or intravitreal amphotericin B deoxycholate. Intravitreal steroids may also be used in combination with anti-microbial agents. In case of vitreous seeding, vitrectomy is recommended followed by intravitreal injections for persistent disease. Topical therapy is recommended in case of associated fungal keratitis. We did not need to use intravitreal anti-fungal because of the rapid response after pars plana vitrectomy and systemic anti-fungal therapy. Our case had a good visual outcome because of high initial index of suspicion based on previous experiences and prompt intervention.
4. Conclusion
Fungal endogenous endophthalmitis is a rare condition that warrants immediate intervention and aggressive management to prevent the risk of irreversible vision loss secondary to macular involvement. The index case demonstrates the rare presentation of a posterior hypopyon in an immunocompetent individual with fungal endogenous endophthalmitis due to Aspergillus. A high index of suspicion and early intervention with vitrectomy and systemic therapy resulted in favorable visual outcome.
The case also highlights the importance of suspecting fungal etiology in endogenous endophthalmitis in cases of immunocompetent individuals without any contributory history except administration of contaminated intravenous dextrose solution. This unique history of exposure can be a clue for fungal endogenous endophthalmitis in healthy adults in developing countries.
Patient consent
Consent to publish the case report was not obtained. This report does not contain any personal information that could lead to the identification of the patient.
Authorship
All authors attest that they meet the current ICMJE criteria for Authorship.
Funding
No funding or grant support.
Declaration of competing of interest
None for all authors.
References
- 1.Romero C.F. Endogenous endophthalmitis: case report and brief review. Am Fam Physician. 1999;60(2):510–514. [PubMed] [Google Scholar]
- 2.Durand M.L. Bacterial and fungal endophthalmitis. Clin Microbiol Rev. 2017;30(3):597–613. doi: 10.1128/CMR.00113-16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Samiy N., D'Amico D.J. Endogenous fungal endophthalmitis. Int Ophthalmol Clin. 1996;36(3):147–162. doi: 10.1097/00004397-199603630-00014. [DOI] [PubMed] [Google Scholar]
- 4.Simunovic M.P. Endophthalmitis following intravitreal injection versus endophthalmitis following cataract surgery: clinical features, causative organisms and post-treatment outcomes. Br J Ophthalmol. 2012;96(6):862–866. doi: 10.1136/bjophthalmol-2011-301439. [DOI] [PubMed] [Google Scholar]
- 5.Okada A.A. Endogenous bacterial endophthalmitis. Report of a ten-year retrospective study. Ophthalmology. 1994;101(5):832–838. [PubMed] [Google Scholar]
- 6.Kresloff M.S., Castellarin A.A., Zarbin M.A. Endophthalmitis. Surv Ophthalmol. 1998;43(3):193–224. doi: 10.1016/s0039-6257(98)00036-8. [DOI] [PubMed] [Google Scholar]
- 7.Lingappan A. Endogenous fungal endophthalmitis: causative organisms, management strategies, and visual acuity outcomes. Am J Ophthalmol. 2012;153(1):162–166 e1. doi: 10.1016/j.ajo.2011.06.020. [DOI] [PubMed] [Google Scholar]
- 8.Rao N.A., Hidayat A.A. Endogenous mycotic endophthalmitis: variations in clinical and histopathologic changes in candidiasis compared with aspergillosis. Am J Ophthalmol. 2001;132(2):244–251. doi: 10.1016/s0002-9394(01)00968-0. [DOI] [PubMed] [Google Scholar]
- 9.Arevalo J.F. Endogenous endophthalmitis in the developing world. Int Ophthalmol Clin. 2010;50(2):173–187. doi: 10.1097/IIO.0b013e3181d26dfc. [DOI] [PubMed] [Google Scholar]
- 10.Gupta P. Endogenous mycotic endophthalmitis in an immunocompetent patient. Int Ophthalmol. 2009;29(4):315–318. doi: 10.1007/s10792-008-9235-1. [DOI] [PubMed] [Google Scholar]
- 11.Sachdev N. Bilateral simultaneous endogenous Aspergillus endophthalmitis in an immunocompetent patient. Retin Cases Brief Rep. 2010;4(1):14–17. doi: 10.1097/ICB.0b013e318196b26c. [DOI] [PubMed] [Google Scholar]
- 12.Gupta A. Fungal endophthalmitis after a single intravenous administration of presumably contaminated dextrose infusion fluid. Retina. 2000;20(3):262–268. [PubMed] [Google Scholar]
- 13.Takebayashi H., Mizota A., Tanaka M. Relation between stage of endogenous fungal endophthalmitis and prognosis. Graefes Arch Clin Exp Ophthalmol. 2006;244(7):816–820. doi: 10.1007/s00417-005-0182-5. [DOI] [PubMed] [Google Scholar]
- 14.Dogra M. Presumably contaminated intravenous infusion-induced Aspergillus terreus endogenous endophthalmitis presenting with posterior hypopyon. Indian J Ophthalmol. 2018;66(4):593–595. doi: 10.4103/ijo.IJO_695_17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Patterson T.F. Practice guidelines for the diagnosis and management of aspergillosis: 2016 update by the infectious diseases society of America. Clin Infect Dis. 2016;63(4):e1–e60. doi: 10.1093/cid/ciw326. [DOI] [PMC free article] [PubMed] [Google Scholar]