Highlights
-
•
Passiflora setacea: important source of antioxidants and bioactive compounds.
-
•
The effect of pasteurization on the quality of passiflora setacea was evaluated.
-
•
31 attributes were obtained in the CATA method, of which 7 showed a difference.
-
•
Binomial 82 °C/20 s is suitable for maintaining nutritional and functional quality.
Chemical compounds studied in this article: DPPH: 2,2-Diphenyl-1-picrylhydrazyl (PubChem CID: 74358); TPTZ: 2,4,6-Tris(2-pyridyl)-s-triazine (PubChem CID: 77258); Ascorbic acid (PubChem CID: 54670067); Putrescine (PubChem CID: 1045); Spermidine (PubChem CID: 1102); Agtamine (PubChem CID: 199); Orientin (PubChem CID: 5281675); Isoorientin (PubChem CID: 114776); Vitexin (PubChem CID: 5280441); Isovitexin (PubChem CID: 162350); Hesperetin (PubChem CID: 72281); Epicatechin (Pubchem CID: 72276)
Keywords: Bioactive compounds, Thermal process, Food quality, Sensory analysis, Passiflora setacea
Abstract
Passiflora setacea is a wild species of passion fruit with interesting functional properties. Fruit seasonality demands conservation methods to enable its consumption throughout the year. We evaluated High Temperature Short Time (HTST) and Low Temperature Long Time (LTLT) binomials on physical, chemical, antioxidant and sensory characteristics of Passiflora setacea pulps. In natura (IN) and pasteurized pulps were analysed for DPPH, FRAP, ORAC, total phenolic content (TPC), vitamin C, bioactive amines, flavonoids, color, remaining enzymatic activity (REA), microbiological analyzes, sensory evaluation and physical stability. All binomials reached microbiological standards. Binomials 82 °C/20 s and 82 °C/40 s were selected for providing higher total antioxidant activity (TAA), TPC and lower REA. The highest levels of antioxidant activity, flavonoids, vitamin C were kept by 82 °C/20 s, without difference from IN pulp. LTLT binomial showed higher retention of bioactive amines, but also higher REA. Sensory acceptance was not affected by the binomials but pasteurized-cooked flavor was more checked for 82 °C 40 s than IN pulp.
1. Introduction
The study of ecosystems and their species contribute to food and nutritional security. Among the native plants of the Brazilian savannah, the botanical genus Passiflora stands out by presenting great diversity of species and varieties. It is believed that there are more than 400 species found naturally in tropical America, of which approximately 150 occur in Brazil and 70 produce edible fruits (Faleiro, Junqueira, & Braga, 2005).
The native and wild species of Passiflora have great potential for in natura (IN) consumption, as ornamental plant, medicinal, functional food and use by the cosmetic and food industries thanks to the variety of compounds found (Perdomo, 2016). Despite the richness of diversity and benefits, only the species Passiflora edulis Sims, the regular passion fruit, is produced on a commercial scale, as processed juice. The species Passiflora setacea, from the Brazilian savannah, presents great potential for consumption due to the pleasant and sweet aroma of the fruits. According to popular knowledge, leaves and fruits of this plant would also present tranquilizing properties, which gave rise to the common name of the species “sleep passion fruit” (Vieira, 2010).
Passiflora setacea had its first variety, BRS Pérola do Cerrado, launched in 2013 by Embrapa Cerrados (Brazilian Agricultural Research Corporation, Brazilian Savannah) an ecoregional research center focused on the sustainable development of agriculture in the Cerrado Biome. It is considered an important source of antioxidants and bioactive compounds, such as phenolic compounds and bioactive amines, according to the research by Embrapa Cerrados and Passitec-Technological development for functional use of wild passifloras Network (Costa et al., 2008, Bomtempo, 2011, Gadioli, 2017).
Some studies from Passitec Network have elucidated the functional properties of Passiflora setacea. The immunomodulatory effects of the consumption of Passiflora setacea juice by overweight volunteers were evaluated by Duarte (2015). Higher phagocytosis of pathogens and tumor cells has been reported in the presence of phenolic compounds, suggesting that the immune system is more efficient due to the acute consumption of Passiflora setacea juice. However, seasonality impairs fruit availability along the year and processing is necessary to enhance the consumption by urban population. Processing promotes the prolongation of the shelf life and food safety. However, it can alter the composition of foods and interfere in the interactions between food constituents. Pasteurization, particularly, has a positive impact by inactivating undesirable enzymes and antinutritional factors, and promoting microbiological safety. The destabilization of pectin by the pectinesterase enzyme found in the pulp causes viscosity decrease and phase separation, impairing juice quality (Kimball, 1991, Corrêa Neto and Faria, 1999). For this reason, the inactivation of that enzyme is target to design the thermal process. However, the negative impact with respect to nutrient losses (Correia, Faraoni, & Pinheiro-Santana, 2008) must be evaluated as a side effect, as the functional properties of Passiflora setacea pulp depend on the retention of bioactive compounds after pasteurization.
On the other hand the systematic review by the authors Al-Suhaimi et al. (2018) concluded that several conventional processing methods have significant effects on natural antioxidants in fruits and vegetables. Thermal processing procedures such as roasting, blanching, drying and pasteurization can be harmful to bioactive compounds. However, some studies have found that thermal damage to bioactive compounds does not reduce the overall antioxidant characteristics of food products. Non-thermal procedures (UV, pulsed electric field, high hydrostatic pressure, irradiation and combined non-thermal methods) do not significantly deteriorate phytochemicals important for health promotion and in some cases may improve their activity and availability.
Therefore, the goal of this work was to evaluate the effect of HTST and LTLT binomials on physical, chemical, antioxidant and sensory characteristics of Passiflora setacea pulps. This work will provide information on viability to consume this passiflora, as an important source of bioactive compounds along the year, thus improving its contribution to a healthy and functional diet.
2. Material and methods
2.1. Obtention of Passiflora setacea pulp
Passiflora setacea BRS Pérola do Cerrado was harvested in Embrapa Cerrados experimental field, Planaltina, Brasília, DF, with approximate altitude of 1050 m. The fruits were obtained from the dry season crop of 2016. The process of obtaining Passiflora setacea pulp was carried out in seven stages, the first was the reception of the raw material, later washing and disinfection, selection, cutting and removal of pulp, pulp packaging, finally cold storage at −18 °C.
2.2. Pasteurization of Passiflora setacea pulp
The pulp was submitted to thermal treatment in the HTST pasteurization system (Fig. 1 of the Supplementary Material) of the Embrapa Cerrados Food Science and Technology Laboratory (Celestino & Sanchez, 2018). The system consisted of three stages: heating, temperature maintenance and cooling. The pasteurization system was thoroughly sanitized with 3% peracetic acid before and after the operations. Water at 55 °C was pumped to stabilize the system flow with the expulsion of air from the pipeline. Then the pulp was pumped and entered the heating stage, where it was circulated in a cylindrical chiller of 9 m in form of serpentine and 0.6 cm of internal diameter, immersed in a hot bath of vegetable oil. The chiller output had a flow rate of 36.0 mL/s, with the residence time being 7.0 s.
The temperature of the oil bath was 215, 220, 230 °C and the respective pulp temperature was 72, 77, 82 °C at the exit from the heating stage.
After leaving the heating stage, the pulp immediately entered the temperature maintenance stage, consisting of a water bath and cylindrical chillers in the form of a serpentine of 0.6 cm internal diameter. Binomials formed by combining temperatures of 72 and 82° C and equivalent times 20, 40, 75 and 300 s were tested. The residence time (pasteurization time) of the pulp in the bath varied according to the length of the chillers used (Table 1 of the Supplementary Material). After pasteurizing, the pulp was immediately cooled in the third stage (cooling). The pasteurized pulp was pumped into a 15 m serpentine cylindrical chiller, which was dipped in a 40% alcohol solution at −5 °C. The flow rate at the chiller output was 9 mL/s with the cooling time being at 47 s. The pulp exit temperature of this stage ranged from 6 to 8 °C. However, for the longer pasteurization time, the use of the HTST pasteurization system was not feasible. Thus, the pulp was placed in a vessel and heated rapidly to the desired temperature which was held for 300 s and immediately cooled in the third stage of the pasteurizing system as described above. The pulp was also subjected to a LTLT pasteurization at 63 °C/30 min, and was also immediately cooled in the third stage of the pasteurizing system as described above.
2.3. Evaluation of the quality of Passiflora setacea pulp submitted to pasteurization
The quality evaluation of Passiflora setacea pulp submitted to pasteurization was divided into two stages. In the first stage, we determined the time/temperature binomial that maintained the antioxidant properties and promoted enzymatic inactivation. In the second stage, we evaluated antioxidant properties, flavonoids profile, microbial load with the binomials chosen from the first stage (Fig. 2, supplementary material).
In the first stage, the different HTST pasteurization binomials formed by the combination of the temperatures of 72 and 82 °C and equivalent times 20, 40, 75 and 300 s, besides the binomial 63° C/30 min (LTLT pasteurization) were compared. The total antioxidant activity (TAA) variables expressed in EC50, total phenolic content (TPC), remaining enzymatic activity (REA) and physical stability were considered.
The binomials selected in the second stage were evaluated for microbiological quality (mold and yeast counts, thermotolerant coliforms and aerobic mesophiles, presence of Salmonella spp.), coloration, vitamin C and TPC, flavonoids and bioactive amines contents, TAA, DPPH, ferric reducing antioxidant power, (FRAP) and oxigen radical absorbence capacity (ORAC), besides sensory acceptance and descriptive profile. IN pulp was analyzed in the two stages of the experiment.
2.3.1. Total antioxidant activity (TAA)
TAA of Passiflora setacea pulp was evaluated in the first and second stages of the experiment. In the first stage, it was determined by the oxidant activity reduction of DPPH radical (Brand-Wiliams, Cuvelier, & Berset, 1995) in 50% (EC50). In the second stage, TAA was evaluated by DPPH, FRAP and ORAC, expressed as equivalent trolox per gram fresh weight (μmol TE g−1). The extracts were obtained from 10 g of pulp with 10 g of the sample, 40 mL of 50% methanol (methanol: distilled water, 50:50, v/v) were added, homogenized and allowed to stand for 60 min at room temperature and out of the light and finally 40 mL of 70% acetone (acetone: distilled water, 70:30, v/v) were added (Larrauri, Rupérez, & Saura-Calixto, 1997).
2.3.1.1. DPPH method
TAA of the bioactive compounds presented in Passiflora setacea pulp was determined by the oxidant activity reduction of DPPH radical (Brand-Wiliams et al., 1995) in 50% (EC50). From the extract, five different dilutions were prepared 50; 100; 200; 300 and 400 mg of extract/L. In a dark environment, a 1.0 mL aliquot of each dilution of the extract, in triplicate, was charged with 3.0 mL of 0.06 mM DPPH root solution and homogenized. Absorbance readings at 515 nm of the dilutions were monitored every minute, with absorbance reduction being observed until stabilization. The final value after stabilization was noted. The absorbance of a control solution consisting of 1.0 mL of methanol and 3.0 mL of DPPH solution was also determined. The % reduction between dilution and control was expressed according to Eq. (1).
| (1) |
in which:
R = % DPPH reduction;
AbsC: absorbance control;
AbsD: absorbance dilution.
From the values obtained, a graph of % DPPH reduction x concentration in mg extract/L was constructed. For the calculation of the EC50 the straight equation was used, replacing the value % reduction by 50 to obtain the concentration of the extract with capacity to reduce in 50% the activity of DPPH radicals. Lower values of EC50 represented higher values of antioxidant capacity of the pasteurized pulp.
The analysis of antioxidant activity by FRAP was determined by the method proposed by Benzie and Strain (1996) and with some modifications made by Pulido, Bravo, and Saura-Calixto (2000), the absorbance being measured at 595 nm.
The analysis of antioxidant activity by the ORAC method was conducted by the method proposed by Wang, Cao & Prior (1997), with some modifications made by Prior, Wu, and Schaich (2005), by using as working solution the fluorescein, left at 37 °C for 5 min and finely the addition of 2,2′-Azobis(2-amidinopropane) dihydrochloride (AAPH). The absorbance readings were between 450 and 500 nm and the emission readings were between 510 and 550 nm.
2.3.2. Total phenolic content (TPC)
The pulp extracts were obtained according to the method proposed by Larrauri et al. (1997) as performed for the determination of antioxidant activity. The analyzes were performed in triplicate. The volumes of 1 mL of extract, 1 mL of Folin Ciocalteau (1:3), 2 mL of 20% sodium carbonate, and 2 mL of distilled water were homogenized in a tube shaker and then allowed to stand for 30 min in the dark. The absorbance readings were done at 700 nm. The standard curve was obtained from six dilutions with 0, 10, 20, 30, 40 and 50 μg of gallic acid. The value of TPC was determined by mg gallic acid eq/100 g fresh weight.
2.3.3. Remaining enzymatic activity
According to Versteeg, Rombouts, Spaansen, and Pilnik (1980), pectin esterase from acid fruit pulps is more thermally resistant than deteriorating microorganisms and the peroxidase enzyme, which is responsible for changes in taste and aroma. For this reason, the inactivation of pectin esterase is used as a parameter to define time and temperature of the pasteurization process at which pulp or juice should be submitted (Badolato, 2000). The enzymatic activity analysis was performed in triplicate, according to the method proposed by Rouse and Atkins (1955, chap. 2) with modification proposed by Collet, Shigeoka, Badolato, and Tadini (2005), using Thermo Fisher Scientific Star pH-orion pot A211. Four mL of pulp were added to 40 mL of 0.1% aqueous solution of apple pectin (Sigma-P8471, 72% methoxylation degree), pH 7 and temperature of 33 °C. The pH of the mixture was recorded after 40 min, time needed for the stabilization. The pectin esterase activity was obtained by the difference between the initial pH (7,0) and pH after 40 min, considering the difference of 0.01 equivalent to 1 U. The value of the enzymatic activity was determined as U/mL of pulp. The remaining enzymatic activity of pectinesterase (REA) was obtained by Eq. (2).
| (2) |
in which:
REA = Remaining enzymatic activity, %;
EANP = Enzymatic activity of in natura pulp, U/mL;
EAPP = Enzymatic activity of pasteurized pulp, U/mL.
2.3.4. Physical stability
Samples of juice prepared with fresh and pasteurized pulp in the proportion 33:67 of pulp and water (De Carvalho, de Oliveira, & Costa, 2018), were submitted to sedimentation tests. For the measurement of the physical stability, a method similar to that of Godoy, Antunes, and Zonta (1998) was applied. Samples of 25 mL of juice were placed in graduated plastic tubes with a 50 mL cap and illuminated by a cold lamp and allowed to stand for 24 h at room temperature. The clarified volume was observed. The stability tests were done in triplicate and the results expressed in mL/100 mL.
2.3.5. Microbiological analyzes
Microbiological analyzes were performed for IN pulps and pasteurized pulps in the selected binomials to ensure a safe food (ANVISA, 2001, MAPA, 2003). Molds and yeasts, aerobic mesophylls, total and thermotolerant coliforms and Salmonella spp. were evaluated.
2.3.6. Color
The pulp color of Passiflora setacea was measured using the MiniScan® EZ Spectrophotometer (HunterLab, Reston, United States), obtaining the values of the coordinates L, a and b of the Hunter system. From the values of the coordinates L, a and b, the parameters related to hue angle (h°, Eq. (3)), chroma (C, Eq. (4)) and browning index (BI, Eqs. (5) and (6)) (Castañón et al., 1999, Francis, 1975, Maskan, 2001) were calculated.
| (3) |
| (4) |
| (5) |
in which:
| (6) |
2.3.7. Vitamin C
Vitamin C was quantified by liquid chromatography analysis on the Shimadzu® HPLC equipment with a model LC-10AT VP high-pressure pump, a model SIL-10AF autosampler, and a SPD-M10A UV visible diode matrix detector (Shimadzu®, Kyoto, Japan). A Lichospher 100 RP18, 250 mm, 4 mm, 5 mm column (Merck, Germany) was used. Ascorbic acid (AA) content was determined before conversion of dehydroascorbic acid (DHA) and then total Vitamin C after conversion of DHA. The samples were kept protected from light at room temperature. The mobile phase was composed of 1 mM monobasic sodium phosphate (NaH2PO4) and 1 mM EDTA, pH adjusted to 3.0 with phosphoric acid (H3PO4), in isocratic elution at a flow rate of 1 mL/min. Detection was performed at 245 nm and ascorbic acid was used as a standard, with a curve ranging from 0 to 100 µL/mL (De Carvalho et al., 2018).
2.3.8. Bioactive amines
All extracts were analyzed according to the methodology described by Fiechter, Sivec, and Mayer (2013) for the determination of bioactive amines, which were quantified by liquid chromatography. Initially, extracts were derived by the reaction of 6-aminoquinolyl-N-hydroxysuccinimidyl carbamate (AQC) using the Waters AccQ.Fluor® kit (Waters Corporation, 1993). A pre-column was used for the derivation and then filtered through a 0.20 μm pore filter (Whatman®, GE Healthcare, UK). Five μL of the extract were neutralized with 35 μL of AccQ.Fluor® borate buffer and 10 μL of AQC reagent, finally the samples were heated at 55 °C for 10 min in a water bath to complete the bypass reaction. The results were expressed in mg/kg. Amines were identified and quantified in a Waters Acquity Ultra Performance LC (UPLC®) liquid chromatograph (Waters, Milford, MA, USA) equipped with an Acquity® tunable ultraviolet detector (TUV) (Waters, Milford, MA, USA) at 249 nm. For separation, an Acquity UPLC® BEH C18 reverse phase column (2.1 × 50 mm, 1.7 μm) was applied. Mobile phases were (A) 0.1 mol/L sodium acetate buffer in ultrapure water with pH adjusted to 4.8 with acetic acid and (B) acetonitrile. The sample injection volume in the column was 2 μL, and flow rate was set at 0.8 mL/min and the gradient was: 13 min at 11% B, 19 min at 30% B, 24 min at 11% B and 45 min at 11% B.
2.3.9. Flavonoids quantification
Flavonoids were quantified according to the method applied by De Carvalho et al. (2018). Briefly, extracts were obtained by mixing 5 g of pulp and 10 mL of ethanol: water (1:1) solution. One-mL of the extract was mixed to 0.2 mL of methanol 50% with HCl (1.2 N) and tert-butylhydroquinone (TBHQ, 0.4 g/L) to get flavonoid aglycones, sonicated and filtered through a HV 0.45 mm millex filter. a Shimadzu® HPLC system, equipped with a high-pressure pump model LC-10AT VP, an autosampler model SIL-10AF and UV visible diode array detector model SPD-M10A (Shimadzu®, Kyoto, Japan) was used to separation, identification and quantification. Chromatograms were obtained by using 270, 340 and 380 nm wavelengths. Mobile phases were (A) Water: Tetrahydrofuran: Trifluoroacetic Acid (99.79:0.2:0.01) and (B) Acetonitrile. For vitexine, isovitexine, orientin and isoorientine, running was carried out with 80% A 20% B, with a flow rate of 0.5 mL/minute for 25 min. For hesperitin, the flow of 0.7 mL/min for 25 min was used with 70% A, 30% B.
2.4. Sensory analysis
One-hundred and forty evaluators were recruited by filling out a questionnaire related to demographic data (age, sex, educational level) and consumption patterns of fresh or industrialized passion fruit nectars. Only consumers with a minimum consumption of once a month were included in the study.
Acceptance and Check all that Apply (CATA) tests were performed at the laboratory of Dietetic Technique at University of Brasília, College of Health Sciences. After signing the informed consent form and completing the enrollment form, consumers carried out the sensory tests. Participants received approximately 30 mL of each of the four samples (IN and pasteurized in the binomials of 82 °C/20 s, 82 °C/40 s and 63 °C/30 min), at a temperature of 10 ± 1 °C, served in a plastic cup marked with a three-digit random code numbers. Samples were presented to consumers in a monadic sequence, in randomized complete blocks. Water and unsalted crackers were served along with the samples to clean the taste buds. Acceptance test of Passiflora setacea pulp was performed by using the 9-point structured hedonic scale after which the taster was instructed to complete the CATA form. CATA test was used for descriptive characterization of Passiflora setacea pulps. The CATA method consists in presenting samples to be evaluated accompanied by a list of predefined descriptors. Descriptors considered adequate to describe the product must be checked by the taster (Varela & Ares, 2012).
The descriptors were determined in a previous session, in which 15 evaluators received 3 pairs of samples, comprising IN and a pasteurized pulp. Evaluators were asked to compare the samples concerning their differences and similarities and come up with attributes to describe the samples.
2.5. Experimental design and statistical analysis
A completely randomized design was used, with three replicates. In the first step, techniques of analysis of variance (ANOVA) and regression analysis was used to estimate statistical parameters for calculating the dependent variables EC50, TPC and REA in the conditions of temperature and time of the factorial design 2 × 5, being 2 temperatures (72 and e 82 °C) and 5 times (zero, 20, 40, 75 e 300 s).
For the second step, the data of quality analyzes were completely randomized, totaling 4 treatments, IN pulp, LTLT pasteurization (63 °C/30 min) and HTST (82/20 and 82/40). Analysis of variance (ANOVA) was carried out (p < 0.05), followed by the Tukey averages comparison test, when significant. At this step, Principal Component Analysis (PCA) was performed.
ANOVA was also applied to acceptance data. The frequency of use of each descriptor by CATA method was determined by counting the number of tasters that used that descriptor to characterize each sample, thus obtaining the global frequency table of attributes per sample. The Cochran Q test followed by correspondence analysis were used. The Cochran's Q test is a non-parametric statistical test used for randomized block analysis with binary variables (Varela & Ares, 2012), which was used to assess whether consumers perceived significant differences between samples.
3. Results and discussion
The definition of the pasteurization binomials was based primarily on higher TAA, TPC and lower REA. IN pulp of Passiflora setacea presented total antioxidant activity in EC50, TPC and REA of 404.74 mg/L, 52.12 mg/100 g and 100%, respectively. LTLT pasteurization presented 465.00 mg/L, 49.91 mg/100 g and 70% for TAA, TPC and REA, respectively.
Table 1 shows the adjusted regression equations and respective determination coefficients for TAA (EC50 in mg/L), TPC (mg/100 g) and REA (%) in Passiflora setacea pulp subjected to HTST at temperatures of 72 and 82 °C, for 20, 40, 75 and 300 s.
Table 1.
Adjusted regression equations and respective determination coefficients for TAA (EC50, mg/L), total phenolic content (mg/100 g) and REA (%) in Passiflora setacea pulp subjected to HTST at temperatures of 72 and 82 °C, for 0, 20, 40, 75 and 300 s.
| Temperature (°C) | Adjusted equation | R2 | SEE |
|---|---|---|---|
| Total antioxidant activity (EC50, mg/L) | |||
| 72 | 0.99 | 17.259 | |
| 82 | 0.98 | 34.823 | |
| Total phenolic content (mg/100 g) | |||
| 72 | 0.98 | 1.768 | |
| 82 | 0.98 | 1.863 | |
| Remaining enzymatic activity (REA, %) | |||
| 72 | 0.98 | 4.273 | |
| 82 | 0.98 | 4.779 | |
SEE = Standard error of estimate.
It is worth noting that lower values of EC50 represented higher values of antioxidant capacity of the pasteurized pulp. Regardless of temperature, reductions in TAA, TPC and REA were observed as heat treatment time was increased.
A more pronounced reduction in the TAA and TPC was observed when the times equivalent to 75 and 300 s were adopted, in the temperatures of 72 and 82 °C. EC50, TPC and REA values were estimated from the regression equations presented in Table 1. When the different binomials resulting from the combinations between the temperatures of 72 and 82 °C and the times of 75 and 300 s were adopted, estimated values of EC50 and TPC, between 609.00 and 1.006.00 mg/L and between 30.00 and 39.00 mg/100 g were obtained, respectively (Table 2 of the Supplementary Material). On the other hand, when the times equivalent to 20 and 40 s associated with the different temperatures were adopted, TAA and TPC content were estimated between 459.00 and 637.00 mg/L and between 41.00 and 49.00 mg/100 g, respectively. Thus, the adoption of the 20 and 40 s times allowed higher TAA and higher TPC, which are variables that represent the functional quality of the pulp. Regarding REA, the highest estimated value was obtained when the 72 °C/20 s binomial was adopted, being equivalent to 76.03%. On the other hand, when the temperature of 82 °C associated with the times of 20 and 40 s was adopted, REA was estimated in 68.91 and 55.31%, respectively.
Next, the physical stability of Passiflora setacea pulps submitted to the binomials 72 °C/20 s, 72 °C/40 s, 82 °C/20 s and 82 °C/40 s, besides the binomial referring to the slow pasteurization (63 °C/30 min), was evaluated (Fig. 3, Supplementary Material). The pasteurized pulps adopting the times of 20 and 40 s and temperature of 82 °C were those that presented higher physical stability, and average values equivalent to 48.7 and 41.3 mL of the clarified phase per 100 mL, respectively, were obtained. However, for the binomials 72 °C/20 s and 72 °C/40 s, mean values were equivalent to 72.0 and 60.0 mL/100 mL, respectively. When the binomial 63 °C/30 min (LTLT) was analyzed, 59.3 mL/100 mL was obtained. The treatments 82 °C/20 s, 82 °C/40 s were selected to continue with further tests together with 63 °C/30 min, for maintaining a high content of TAA, TPC and a reduction in the REA. Although the pasteurization 63 °C/30 min showed a more intense separation of phases, the selection of this binomial is justified for it is a non-costly process for the producer, in terms of non-acquisition of tubular pasteurizer, necessary equipment for the application of the other two binomials.
Microbiological analysis was performed to verify the effect of binomials 82 °C/20 s, 82 °C/40 s and 63 °C/30 min on the reduction of the microbial load of the pulp (Table 2). The pasteurization of the pulp in the three binomials provided a reduction of counts of molds, yeasts and aerobic mesophiles below the minimum limit of detection (ANVISA, 2001). No total coliforms, thermotolerant coliforms and Salmonella spp. were detected in Passiflora setacea pulp, whether pasteurized or not.
Table 2.
Molds and yeast counts, aerobic mesophylls, total coliforms, thermotolerant coliforms, and Salmonella spp. for in natura (IN) and pasteurized Passiflora setacea pulp in the three selected time/temperature binomials.
| Treatments | Molds and yeasts (log UFC/g) | Aerobic mesophylls (log UFC/g) | Total coliforms (NMP/g) | Thermotolerant coliforms (NMP/g) | Salmonella spp. |
|---|---|---|---|---|---|
| In natura | 2,2 | 2.8 | <3* | <3* | Absence |
| 63 °C/30 min | <1.0* | <1.0* | <3* | <3* | Absence |
| 82 °C/20 s | <1.0* | <1.0* | <3* | <3* | Absence |
| 82 °C/40 s | <1.0* | <1.0* | <3* | <3* | Absence |
Estimated data.
The effect of pasteurization binomials on color, vitamin C, antioxidant analysis, TPC, flavonoids and bioactive amines is presented in Table 3. The saturation of color decreased with all thermal treatments, indicating the likely formation of pigments that interfere with the original color. LTLT also resulted in a significant increase in the browning index, emphasizing the effect of the time on non-enzymatic browning reactions (Ling, Tang, Kong, Mitcham, & Wang, 2015).
Table 3.
Color, vitamin C content, total phenolic content (TPC), antioxidant activity, flavonoids and bioactive amine for in nature (IN) and pasteurized Passiflora setacea pulp in the three selected time/temperature binomials.
| Variables | Treatments |
p-value | |||
|---|---|---|---|---|---|
| In natura | 82 °C/20 s | 82 °C/40 s | 63 °C/30 min | ||
| Color | |||||
| Chroma | 32.11 ± 0.10a | 26.57 ± 0.87b | 23.56 ± 0.84b | 24.28 ± 0.90b | 0.0001 |
| h° (hue angle) | 77.36 ± 0.13a | 78.10 ± 0.68ab | 77.10 ± 0.21ab | 77.52 ± 0.48b | 0.002 |
| Browning index | 90.92 ± 6.72a | 102.84 ± 10.55ab | 106.11 ± 11.31ab | 116.72 ± 10.95b | <0.0001 |
| Vitamin C (mg/100 g) | 37.66 ± 0.83ª | 35.120. ± 0.40b | 30.77 ± 0.77c | 33.04 ± 0.79d | <0.0001 |
| Total phenolic content | |||||
| (mg GAE/mL) | 0.108 ± 0.01a | 0.090 ± 0.01b | 0.072 ± 0.01c | 0.094 ± 0.01b | <0.0001 |
| Antioxidant activity | |||||
| DPPH | |||||
| (μmol TE g−1) | 777.36 ± 0.29a | 715.71 ± 0.63b | 591.39 ± 0.45c | 763.20 ± 0.49a | 0.0001 |
| FRAP | |||||
| (μmol TE g−1) | 69.09 ± 0.81a | 57.12 ± 0.23b | 48.18 ± 0.55c | 61.63 ± 0.65ab | 0.0001 |
| ORAC (μmol TE g−1) | 136.42 ± 0.42a | 121.46 ± 0.53b | 30.11 ± 0.84c | 41.12 ± 0.63c | <0,0001 |
| Flavonoids (mg/100 g) | |||||
| Epicatechin | 848.11 ± 0.84a | 710.5 ± 0.90b | 596.46 ± 0.91c | 693.13 ± 0.79b | <0.0001 |
| Orientin | 19.83 ± 0.30a | 7.669 ± 0.12b | 0.00 ± 0.00c | 0.00 ± 0.00c | <0.0001 |
| Isoorientin | 211.60 ± 0.29a | 106.79 ± 0.78b | 54.09 ± 0.73c | 76.70 ± 0.37c | <0.0001 |
| Vitexin | 5.45 ± 0.08a | 5.40 ± 0.61a | 5.16 ± 0.15a | 5.38 ± 0.28a | <0.0001 |
| Isovitexin | 57.80 ± 0.49a | 22.02 ± 0.31b | 12.90 ± 0.22c | 14.10 ± 0.57c | <0.0001 |
| Hespernitin | 14.55 ± 0.12a | 10.01 ± 0.50c | 9.19 ± 0.11c | 11.49 ± 0.48c | <0.0001 |
| Bioactive amines (mg/kg) | |||||
| Spermidine | 20.03 ± 2.37ab | 16.51 ± 1.33b | 16.58 ± 0.72b | 21.14 ± 1.86a | 0.005 |
| Putrescine | 77.42 ± 9.15ab | 63.52 ± 5.49b | 74.85 ± 2.20ab | 85.43 ± 7.01a | 0.006 |
| Agmatine | 8.37 ± 1.1a | 6.91 ± 0.62ab | 6.21 ± 0.42b | 8.20 ± 0.75a | 0.007 |
Averages followed by the same lowercase letter in the line did not differ statistically by the Tukey test at 5% probability.
Regarding vitamin C retention, there was a significant difference in all treatments compared to IN pulp and also between all binomials. HTST 82 °C/20 s treatment showed retention of 93.25%, followed by the LTLT treatment, with 87.7%, and HTST 82 °C/40 s treatment, which presented retention of 81.7%. In spite of the significant differences, the retention of vitamin C represented at least 100% of Estimated Average Requirement (EAR) of vitamin C which is 90 mg/day for adult men and 75 mg/day for adult women (Institute of Medicine, 2000).
Significant differences between treatments and IN pulp were shown regarding TPC. The HTST treatment at 82 °C/20 s and LTLT presented the highest retention values (about 83%), whereas the HTST treatment at 82 °C/40 s showed retention of 66.6% for TPC. Regarding flavonoids, none of the thermal treatments significantly affected vitexin content. On the other hand, no retention was observed for orientin in LTLT treatment or HTST treatment 82 °C/40 s, whereas the retention of HTST treatment 82 °C/20 s was about 39%.
HTST 82 °C/20 s treatment was the best regarding retention of most flavonoids as it kept the same or higher amounts of certain flavonoids in comparison to the other binomials. The remaining amounts of epicatechin, isoorientin, isovitexin and hespernitin were 83.7%, 50.5%, 38.0% and 69.4%, respectively. With concerns to epicatechin, LTLT pasteurization differed from 82 °C/40 s treatment, with retention of 82% against 70% for this flavonoid (Table 3). Pacheco-Palencia, Duncan, and Talcott (2009) used a 80 °C for 1, 5, 10, 30 and 60 min thermal processing in pulp of Euterpe oleracea and Euterpe precatoria, popularly known as açai, and analyzed polyphenolic compounds, including anthocyanins and non-anthocyanin polyphenols, as isovitexin, epicatequin, orientin and isoorientin. Non-anthocyanin polyphenols remained constant during heat treatment at 80 °C for up to 60 min, thus differing from our results. On the other hand, the authors verified the anthocyanin degradation in the tested binomials, being directly related to the exposure time. Longer exposure times caused more intense anthocyanin degradation. It is possible that the degradation of polyphenolic compounds in Passiflora setacea pulp, different from that observed in açaí pulp, is related to the chemical composition. The main non-aqueous components of açai pulp are lipids, with 48.24 g/100 g dry basis (Tonon, Alexandre, Hubinger, & Cunha, 2009). On the other hand, the main non-aqueous components of Passiflora setacea pulp are carbohydrates, totaling 81.95 g/100 g in the dry basis (Viana, Costa, & Celestino, 2016).
Three different methods to assess in vitro antioxidant activity were applied with slightly different results. According to ORAC test, TAA decreased about 11.0% in HTST 82 °C/20 s treatment. However, there were reductions in TAA of 77.9 and 69.9% for binomials 82 °C/40 s (HTST) and 63 °C/30 min (LTLT), respectively. DPPH test showed a different result, as TAA of LTLT treatment did not differ from TAA of IN pulp. HTST 82 °C/20 s treatment showed TAA 8.0% lower than IN pulp and it was significantly higher than 82 °C/40 s treatment, which decreased 24.0% in relation to IN pulp. FRAP was closer to DPPH result, with the difference that LTLT and 82 °C/20 s treatments did not differ.
Results similar to ours were obtained by Saeeduddin et al. (2015) in pasteurized pear (Pyrus bretschneideri) juice at 65 °C for 10 min and 95 °C for 2 min, being the reduction in TAA associated with the decrease in the concentration of antioxidant compounds present in the samples, such as phenolic compounds and ascorbic acid. Different results were obtained in pomegranate (Punica granatum) juice and orange passion fruit (Passiflora caerulea) juice. Vegara, Mena, Martí, Saura, and Valero (2013) found no reduction in ATT in pomegranate (Punica granatum) juice when the binomials 65 °C/30 s and 90 °C/5 s were adopted. Dos Reis, Facco, Flôres, and Rios (2018) verified an increase in TAA in pasteurized orange passion fruit juice (88 °C/15 s). Increased TAA in some products has been associated with better extraction of bioactive compounds after heat treatment, as observed by Mena, Vegara, Martin, García-Viguera, Saura, and Valero (2013) when analyzing anthocyanins in pomegranate juice subjected to heat treatments at 65, 80 and 95 °C for 30 and 60 s.
Regarding bioactive amines, one can observe that best retention was found after LTLT treatment, as none of the contents differed from those of IN pulps. For agmatine contents, 82 °C/40 s treatment differed from IN pulp, with a retention of 74%.
The amines present in foods are rapidly metabolized in the body by means of aminoxidases (Shalaby, 1996). The absorbed amines may have functional properties, as in the case of polyamines spermine and spermidine which contribute to cell division, stress response and inhibition of lipid oxidation. The biogenic amines histamine, tyramine, tryptamine, prutescine, play an important role as neuroactive or vasoactive. In foods, the presence of amines can also be used as indicator of quality or hygienic conditions of the products (Costa, Rodrigues, Frasao, & Conte-Junior, 2018). However, they may cause adverse health effects such as nausea, migraine, among others when consumed in large quantities (Bardócz, 1995).
Sperimidine, putrescine, agmatine were detected in our Passiflora setacea pulps, being putrescine, the major bioactive amine found (Table 3). Studies by Gadioli (2017) determined the presence of five amines in the Passiflora setacea pulp: histamine, agmatine, tyramine, prutescine and spermidine, and the pulp had a content of prutescin of 2.1 mg/100 g. This amine can cause a putrid taste to food, so it is considered a factor that interferes with the acceptability of passionflower juice. Other authors determined amines in five tropical fruits, among them, pineapple, guava, papaya, mango and P. alata. Santiago-Silva, Labanca, and Gloria (2011) five amines were detected in the fruit pulps (spermidine, spermine, putrescine, serotonin and agmatine). Total amine levels ranged from 0.77 mg/100 g mango to 7.53 mg/100 g Passiflora alata, putrescine and spermine were found in most of them. In the Passiflora alata, the spermidine, spermine, putrescine, agmatine and serotonin amines were found with values ranging from 3.05 mg/100 g and 0.18 mg/100 g for spermidine and serotonin, respectively.
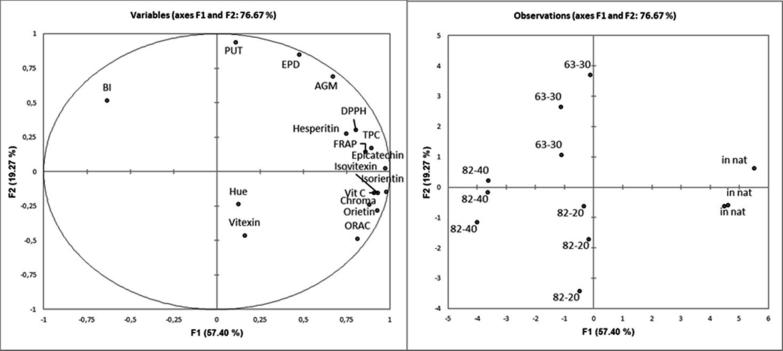
Principal Component Analysis (PCA) was carried out to explore how the different treatments affected Passiflora setacea pulps characteristic (Fig. 1). It was possible to clearly discriminate the samples submitted to the heat treatment of the untreated sample (IN pulp), based on the analyzed variables. The first dimension, which explains 57.4% of total variation, shows that pulps pasteurized at 82 °C/40 s differed most from IN pulp. In the same dimension, pulps submitted to 82 °C/20 s or to LTLT were similar. IN pulp was positively associated to vitamin C, TAA, TPC and color saturation. Hue and vitexin were close to the axis origin and therefore, are neutral variables, not associated to any treatment. Browning index was more associated to pulps pasteurized at 82 °C/40 s in the first dimension, and to pulps at slow pasteurization in the second one, indicating that among the treatments, pasteurization at 82 °C/20 s led to lower non enzymatic browning. The second dimension was useful to discriminate 60 °C/30 min and 82 °C/40 s treatments. LTLT pasteurization was more associated to bioactive amines retention, whereas HTST pasteurization was closer to hue and vitexin variables and also in the lower half of the map, to orientin, isorientin, chroma, vitamin C and ORAC variables.
Fig. 1.
Principal Component Analysis of Passiflora setacea pulps at different processing conditions. Dependent variables are: Hue, Chroma, BI–browning index, epicatechin, hesperitin, orientin, isorientin, vitexin, isovitexin, VIT C-vitamin C, TPC-total phenolic content, PUT–putrescin, AGM – Agmatine, EPD – Spermidine, and in vitro antioxidant activity by DPPH, FRAP and ORAC methods. Independent variables are: IN pulp, and pasteurized pulps at 60–30 (60 °C/30 min), 82–20 (82 °C/20 s) and 82–40 (82 °C/40 s).
From the PCA results it was possible to verify that at 82 °C, the increase of the exposure time from 20 s to 40 s intensified the changes in the quality of Passiflora setacea pulp. Thus, the binomial 82 °C/20 s enabled results closer to those obtained when LTLT (63 °C/30 min) was adopted. It is important to highlight that all tested binomials differed from IN pulp, which is in agreement with the results obtained when using Tukey's test analysis (Table 3). Significant changes in quality due to increased exposure time have also been observed in other products such as pomegranate juice (Mena et al., 2013) and pear juice (Saeeduddin et al., 2015).
In recent years, CATA has been gaining a lot of strength in sensory food analysis (Grasso, Monahan, Hutchings, & Brunton, 2017). This method provides information on the attributes perceived by consumers with concerns to color, flavor, aroma, texture and appearance and can also be used in conjunction with hedonic scales. A total of 30 attributes were obtained (Table 4). There was a difference in 7 out of the 30 attributes among the samples. IN and LTLT (63 °C/30 min) pulps were checked more often than HTST pulps for homogeneous attribute. IN and LTLT pulps were both checked more often for cloudy attribute, but LTLT pulp did not differ from 82 °C/20 s pulp, which in turn did not differ from 82 °C/40 s pulp indicating a gradual reduction in cloudiness with the intensity of heat treatment. The 82 °C/20 s pulp was more checked than IN pulp for dark yellow attribute, which can be associated to non-enzymatic browning in heat treatments. No difference was observed among pasteurization treatments for this attribute. With concerns to flavor, 82 °C/40 s pulp was checked more often for pasteurized/cooked descriptor in comparison to IN pulp, suggesting that 40 s could be a limit to heat treatment at this temperature considering flavor. For the attribute very acid, IN pulp differed from 82 °C/40 s pulp, which was less often selected. Possibly, the emergence of other flavors associated with heat treatment in this pulp could have interfered with the perception of acidity. Regarding the texture, the attribute velvety was more frequently checked for IN pulp than for 82 °C/20 s pulp. For attribute diluted-fluid-thinned 82 °C/20 s pulp was selected significantly less often than IN and LTLT pulps. This attribute is possibly associated to lower homogeneity and to the loss of viscosity associated to the pectin degradation in IN pulps.
Table 4.
Cochran’s Q tests for each attribute and acceptance in pasteurized and in natura (IN) Passiflora setacea pulp.
| Check-all-that-apply | |||||
|---|---|---|---|---|---|
| Attributes | In natura | 82 °C/20 s | 82 °C/40 s | 63 °C/30 min | p-valor |
| Appearance | |||||
| Homogeneous* | 0.170b | 0.319a | 0.376a | 0.255b | 0.000 |
| Brightnessns | 0.191a | 0.184a | 0.177a | 0.227a | 0.691 |
| Cloudy* | 0.489a | 0.291 cb | 0.284c | 0.433 ba | 0.000 |
| Particles in suspensionns | 0.440a | 0.475a | 0.475a | 0.504a | 0.649 |
| Whitishns | 0.191a | 0.149a | 0.177a | 0.213a | 0.408 |
| Aroma | |||||
| Floralns | 0.248a | 0.241a | 0.191a | 0.255a | 0.317 |
| Weak passion fruit aromans | 0.355a | 0.348a | 0.397a | 0.404a | 0.599 |
| Sourns | 0.291a | 0.234a | 0.291a | 0.262a | 0.510 |
| Sweetns | 0.135a | 0.156a | 0.149a | 0.184a | 0.598 |
| Strong passion fruit aromans | 0.078a | 0.121a | 0.071a | 0.092a | 0.341 |
| Insipidns | 0.106a | 0.099a | 0.092a | 0.057a | 0.350 |
| Passion fruit aromans | 0.227a | 0.220a | 0.199a | 0.213a | 0.940 |
| Color | |||||
| Yellowns | 0.390a | 0.270a | 0.284a | 0.312a | 0.091 |
| Dark yellow* | 0.028b | 0.163a | 0.071ab | 0.092ab | 0.000 |
| Brown yellowns | 0.184a | 0.248a | 0.206a | 0.241a | 0.400 |
| Light yellowns | 0.362a | 0.270a | 0.383a | 0.305a | 0.085 |
| Flavor | |||||
| Bitterns | 0.128a | 0.128a | 0.106a | 0.078a | 0.300 |
| Artificial tastens | 0.121a | 0.128a | 0.142a | 0.128a | 0.930 |
| Ripe fruitns | 0.170a | 0.099a | 0.170a | 0.128a | 0.162 |
| Pasteurized-cooked* | 0.035b | 0.085ab | 0.135a | 0.113ab | 0.009 |
| Unpleasantns | 0.177a | 0.099a | 0.106a | 0.121a | 0.103 |
| Astringentns | 0.106a | 0.106a | 0.135a | 0.121a | 0.794 |
| Pleasantns | 0.298a | 0.262a | 0.262a | 0.298a | 0.744 |
| Acidns | 0.319a | 0.348a | 0.376a | 0.369a | 0.674 |
| Damaged fruit flavorns | 0.163a | 0.156a | 0.142a | 0.199a | 0.557 |
| Passion fruit flavorns | 0.291a | 0.340a | 0.312a | 0.270a | 0.436 |
| Veryacid* | 0.199a | 0.142ab | 0.085b | 0.135ab | 0.030 |
| Texture | |||||
| Velvety* | 0.213b | 0.348a | 0.312ab | 0.298ab | 0.035 |
| Grainyns | 0.085a | 0.085a | 0.071a | 0.085a | 0.957 |
| Viscous-densens | 0.199a | 0.298a | 0.262a | 0.312a | 0.064 |
| Diluted-fluid-thinned* | 0.489a | 0.248b | 0.355ab | 0.305a | 0.000 |
| Acceptance | |||||
| Apperance | 6.93 ± 1.62a | 7.11 ± 1.48a | 6.87 ± 1.72a | 6.97 ± 1.69a | 0,646 |
| Flavor | 5.95 ± 2.22a | 6.33 ± 1.99a | 6.14 ± 2.16a | 6.21 ± 1.92a | 0,489 |
| Aroma | 6.32 ± 1.92a | 6.46 ± 1.83a | 6.41 ± 1.81a | 6.48 ± 1.74a | 0,888 |
| Texture | 7.11 ± 1.175a | 7.17 ± 1.46a | 6.92 ± 1.70a | 7.10 ± 1.63a | 0,618 |
| Overall Impression | 6.23 ± 1.98a | 6.59 ± 1.74a | 6.39 ± 1.83a | 6.52 ± 1.73a | 0,369 |
CATA: (*) Indicates significant differences (p < 0.05), whereas (ns) indicates no significant differences according to Cochran's Q test for each attribute. Different letters in a row indicate significant difference according to multiple pairwise comparisons: McNemar (Bonferroni) test (p < 0.05).
Acceptance: Means followed by the same lowercase letter in a column do not differ significantly by the Tukey test (p < 0.05). n: 140 consumers.
In the case of the acceptability of Passiflora setacea pulp submitted to pasteurization, no significant variation (p > 0.05) was observed in each of the time/temperature and IN pulp variables as a result of pasteurization time and temperature increase. In literature, no studies were found related to the assessment of attributes and acceptability of Passiflora setacea pulp submitted to pasteurization processing. Janzantti, Santo, and Monteiro, 2014 studied the shelf life of fresh and pasteurized Passiflora edulis pulp, submitted to pasteurization at 70 and 90 °C. Higher acceptance for all the attributes were achieved for pulps pasteurized at 70 °C.
4. Conclusion
Pasteurization is presented as an effective conservation method to keep the supply of Passiflora setacea pulp out of the harvest periods, with little loss of bioactive compounds of interest and few sensory alterations. The consumption of Passiflora setacea in natura or in some culinary preparations throughout the year could give consumers greater effectiveness of the functional effects already studied. HTST treatments were more effective for physical stability than LTLT. Indeed, LTLT an IN pulps showed similarity in sensory attributes related to variables such as cloudy and diluted-fluid-thinned. Regarding bioactive compounds, LTLT was more associated to the retention of bioactive amines and 82 °C/20 s HTST treatment to the retention of phenolic compounds. Future studies should evaluate the changes along refrigerated and frozen storages. The feasibility of LTLT treatment should be also evaluated considering its lower cost, as a batch process, and its advantage to process lower amounts of raw material, despite its higher risk of recontamination as a batch process and the lower physical stability in comparison to HTST methods.
CRediT authorship contribution statement
Beatriz Alejandra Ortega Sanchez: Conceptualization, Methodology, Formal analysis, Investigation, Writing - original draft, Writing - review & editing. Sonia Maria Costa Celestino: Conceptualization, Methodology, Investigation, Formal analysis. Maria Beatriz de Abreu Gloria: Investigation. Isadora Costa Celestino: Investigation. María Isabel Ordóñez Lozada: Investigation. Samuel Dias Araújo Júnior: Investigation. Ernandes Rodrigues de Alencar: Formal analysis, Writing - review & editing. Lívia de Lacerda de Oliveira: Supervision, Conceptualization, Methodology, Formal analysis, Validation, Writing - original draft, Writing - review & editing.
Declaration of Competing Interest
The authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.
Acknowledgment
The funder was the National Council fo Scientific and Technological Development- CNP-q (IDs 404847/2012-9).
Footnotes
Supplementary data to this article can be found online at https://doi.org/10.1016/j.fochx.2020.100084.
Contributor Information
Beatriz Alejandra Ortega Sanchez, Email: ortegaleja30@gmail.com, alejaor_19@hotmail.com.
Lívia de Lacerda de Oliveira, Email: ortegaleja30@gmail.com.
Appendix A. Supplementary data
The following are the Supplementary data to this article:
References
- ANVISA, Agência Nacional de Vigilância Sanitária (2001). Regulamento Técnico sobre padrões microbiológicos para alimentos, RDC n° 12. https://33880/2568070/rdc_12_2001.pdf/15ffddf6-3767-4527-bfac-740a0400829b.
- Badolato, G. G. (2000). Tratamento térmico mínimo do suco de laranja natural: cinética da inativação da pectinesterase (Dissertação de mestrado). Universidade de São Paulo, São Paulo, SP. 10.11606/D.3.2000.tde-25102001-172837.
- Bardócz S. Polyamines in food and their consequences for food quality and human health. Trends in Food Science & Technology. 1995;6:341–346. doi: 10.1016/S0924-2244(00)89169-4. [DOI] [Google Scholar]
- Benzie I.F., Strain J.J. The ferric reducing ability of plasma (FRAP) as a measure of “antioxidant power”: The FRAP assay. Analytical Biochemistry. 1996;239:70–76. doi: 10.1006/abio.1996.0292. [DOI] [PubMed] [Google Scholar]
- Bomtempo L. Universidade Federal de Minas Gerais; Belo Horizonte, MG: 2011. Aminas bioativas em maracujá: Influência da espécie, das condições climáticas e do amadurecimento. Dissertação de mestrado. [Google Scholar]
- Brand-Wiliams W., Cuvelier M.E., Berset C. Use of a free radical method to evaluate antioxidant activity. Food Science and Technology. 1995;28:25–30. [Google Scholar]
- Castañón X., López-Malo A., Argaiz A. Effect of storage temperature on the microbial and color stability of banana purees prepared with the addition of vanillin or potassium sorbate. Food Science and Technology International. 1999;05:51–58. doi: 10.1177/108201329900500105. [DOI] [Google Scholar]
- Celestino, S. M. C., & Sanchez, B. A. O. (2018). Construção e operação de um sistema de pasteurização de bancada para alimentos líquidos. https://ainfo.cnptia.embrapa.br/digital/bitstream/item/178881/1/CT-180-Sonia-Maria.pdf/ Accessed 20 November 2018.
- Collet L.S.F.C.A., Shigeoka D.S., Badolato G.G., Tadini C.C. A kinetic study on pectinesterase inactivation during continuous pasteurization of orange juice. Journal of Food Engineering. 2005;69:125–129. doi: 10.1016/j.jfoodeng.2004.08.002. [DOI] [Google Scholar]
- Corrêa Neto R.S., Faria J.A.F. Fatores que influem na qualidade do suco de laranja. Ciência e Tecnologia de Alimentos. 1999;19:153–160. doi: 10.1590/S0101-20611999000100028. [DOI] [Google Scholar]
- Correia F.L., Faraoni A.S., Pinheiro-Santana H.M. Efeitos do processamento industrial de alimentos sobre a estabilidade de vitaminas. Alimentos e Nutrição. 2008;19:83–95. [Google Scholar]
- Costa, A. M., Campos, A. V. S., Cohen, K. O., Tupinambá, D. D., Paes, N. S., Sousa, H. N., Santos, A. L. B., Silva, K. N., Faria, D. A., Junqueira, N. T. V., & Faleiro, F. G. (2008, Outubro). Características físico-química-funcional da polpa de Passiflora setacea recém processada e congelada. II Simpósio Internacional Savanas Tropicais, Brasília, DF.
- Costa, M.P., Rodrigues, B.L., Frasao, B.S., Conte-Junior, C.A. (2018). Biogenic Amines as Food Quality Index and Chemical Risk for Human Consumption, Chapter 2. In Handbook of Food Bioengineering, Food Quality: Balancing Health and Disease, Academic Press, A.M. Holban & A.M. Grumezescu (Eds.), Food Quality: Balancing Health and Disease, Academic Press (pp. 75–108). https://doi.org/10.1016/B978-0-12-811442-1.00002-X.
- De Carvalho M.V.O., de Oliveira L.L., Costa A.M. Effect of training system and climate conditions on phytochemicals of Passiflora setacea, a wild Passiflora from Brazilian savannah. Food Chemistry. 2018;266:350–358. doi: 10.1016/j.foodchem.2018.05.097. [DOI] [PubMed] [Google Scholar]
- Dos Reis L.C.R., Facco E.M.P., Flôres S.H., Rios A.O. Stability of functional compounds and antioxidant activity of fresh and pasteurized orange passion fruit (Passiflora caerulea) during cold storage. Food Research International. 2018;106:481–486. doi: 10.1016/j.foodres.2018.01.019. [DOI] [PubMed] [Google Scholar]
- Duarte I.A.E. Universidade de Brasília; Brasília, DF: 2015. Efeito agudo do consumo de suco de Passiflora setacea na capacidade fagocitária e na produção de corpúsculos lipídicos e radicais livres por monócitos de voluntários com sobrepeso (Dissertação de mestrado) http://dx.doi.org/10.26512/2015.12.D.19924. [Google Scholar]
- Faleiro, F. G., Junqueira, N. T. V., & Braga, M. F. (2005). Maracujá: germoplasma e melhoramento genético. (1ª ed.). Brasília: Embrapa Cerrados, (Capítulo 20).
- Fiechter G., Sivec G., Mayer H.K. Application of UHPLC for the simultaneous analysis of free amino acids and biogenic amines in ripened acid-curd cheeses. Journal of Chromatography B. 2013;927:191–200. doi: 10.1016/j.jchromb.2012.12.006. [DOI] [PubMed] [Google Scholar]
- Francis F.J. The origin of tan-1 a/b. Journal of Food Science. 1975;40:412. doi: 10.1111/j.1365-2621.1975.tb02214.x. [DOI] [Google Scholar]
- Gadioli I.L. Universidade de Brasília; Brasilia, DF: 2017. Obtenção, caracterização química, propriedades antioxidantes e antimicrobianas de extratos de polpas, sementes e folhas de passifloras silvestres para formulação de nanoemulsões (Tese de doutorado) [Google Scholar]
- Godoy R.C.B., Antunes P.L., Zonta E.P. Estabilização de néctar de goiaba (Psidium guayava L.) com gomas xantana, carragena e amido ceroso. Revista Brasileira de Agrociência. 1998;4:105–110. [Google Scholar]
- Grasso S., Monahan F.J., Hutchings S.C., Brunton N.P. The effect of health claim information disclosure on the sensory characteristics of plant sterol-enriched turkey as assessed using the Check-All-That-Apply (CATA) methodology. Food Quality and Preference. 2017;57:69–78. doi: 10.1016/j.foodqual.2016.11.013. [DOI] [Google Scholar]
- Institute of Medicine (2000). Dietary reference intakes: applications in dietary assessment. https://www.nap.edu/catalog/9956/dietary-reference-intakes-applications-in-dietary-assessment/ Accessed 10 February 2018.
- Janzantti N., Santo C.G., Monteiro M. Shelf life of fresh and pasteurized organic passion fruit (Passiflora edulis F. flavicarpa Deg.) pulp. Journal of Food Processing & Preservation. 2014;38:260–270. doi: 10.1111/j.1745-4549.2012.00772.x. [DOI] [Google Scholar]
- Kimball D.A. Chapman & Hall ITP; New York: 1991. Citrus processing quality control and technology. [Google Scholar]
- Larrauri J.A., Rupérez P., Saura-Calixto F. Effect of drying temperature on the stabilitity of polyphenols and antioxidant activity of red grape pomace peels. Journal Agriculture and Food Chemistry. 1997;45:1390–1393. doi: 10.1021/jf960282f. [DOI] [Google Scholar]
- Ling B., Tang J., Kong F., Mitcham E.J., Wang S. Kinetics of food quality changes during thermal processing: A review. Food and Bioprocess Technology. 2015;8:343–358. doi: 10.1007/s11947-014-1398-3. [DOI] [Google Scholar]
- MAPA, Ministério da Agricultura, Pecuária e Abastecimento (2003). Instrução normativa n° 62. http://www.lex.com.br/doc_598283_instrucao_normativa_n_62_de_26_de_agosto_de_2003.aspx/ Accessed 10 March 2018.
- Maskan M. Kinetics of colour change of kiwifruits during hot air and microwave drying. Journal of Food Engineering. 2001;48:169–175. doi: 10.1016/S0260-8774(00)00154-0. [DOI] [Google Scholar]
- Mena P., Vegara S., Martí N., García-Viguera C., Saura D., Valero M. Changes on indigenous microbiota, colour, bioactive compounds and antioxidant activity of pasteurised pomegranate juice. Food Chemistry. 2013;141:2122–2129. doi: 10.1016/j.foodchem.2013.04.118. [DOI] [PubMed] [Google Scholar]
- Pacheco-Palencia L.A., Duncan C.E., Talcott S.T. Phytochemical composition and thermal stability of two commercial acai species, Euterpe oleracea and Euterpe precatoria. Food Chemistry. 2009;115:1199–1205. doi: 10.1016/j.foodchem.2009.02.032. [DOI] [Google Scholar]
- Perdomo I.C. Universidade Federal de Santa Catarina; Florianópolis, SC: 2016. Produção de fenólicos, flavonoides e potencial antioxidante de extrato de calos de Passiflora setacea e Passiflora tenuifila (Passifloraceae) cultivados in vitro (Dissertação de mestrado) [Google Scholar]
- Prior R.L., Wu X., Schaich K. Standardized methods for the determination of antioxidant capacity and phenolics in foods and dietary supplements. Journal of Agricultural and Food Chemistry. 2005;53:4290–4302. doi: 10.1021/jf0502698. [DOI] [PubMed] [Google Scholar]
- Pulido R., Bravo L., Saura-Calixto F. Antioxidant activity of dietary as determined by a modified ferric reducing/antioxidant power assay. Journal of Agricultural and Food Chemistry. 2000;48:3396–3402. doi: 10.1021/jf9913458. [DOI] [PubMed] [Google Scholar]
- Rouse A.H., Atkins C.D. University of Florida; Gainesville: 1955. Pectinesterase and pectin in commercial citrus juice as determined by methods used at the citrus experiment station. [Google Scholar]
- Saeeduddin M., Abid M., Jabbar S., Wu T., Hashim M.M., Awad F.N.…Zeng X. Quality assessment of pear juice under ultrasound and commercial pasteurization processing conditions. LWT – Food Science and Technology. 2015;64:452–458. doi: 10.1016/j.lwt.2015.05.005. [DOI] [Google Scholar]
- Santiago-Silva P., Labanca R.A., Gloria M.B.A. Functional potential of tropical fruits with respect to free bioactive amines. Food Research International. 2011;44:1264–1268. doi: 10.1016/j.foodres.2010.11.026. [DOI] [Google Scholar]
- Shalaby A.R. Significance of biogenic amines to food safety and human health. Food Research International. 1996;29:675–690. doi: 10.1016/S0963-9969(96)00066-X. [DOI] [Google Scholar]
- Tonon R.V., Alexandre D., Hubinger M.D., Cunha R.L. Steady and dynamic 466 shear rheological properties of açai pulp (Euterpe oleraceae Mart.) Journal of Food Engineering. 2009;467(92):425–431. doi: 10.1016/j.jfoodeng.2008.12.014. [DOI] [Google Scholar]
- Varela P., Ares G. Sensory profiling, the blurred line between sensory and consumer science. A review of novel methods for product characterization. Food Research International. 2012;48:893–908. doi: 10.1016/j.foodres.2012.06.037. [DOI] [Google Scholar]
- Vegara S., Mena P., Martí N., Saura D., Valero M. Approaches to understanding the contribution of anthocyanins to the antioxidant capacity of pasteurized pomegranate juices. Food Chemistry. 2013;141:1630–1636. doi: 10.1016/j.foodchem.2013.05.015. [DOI] [PubMed] [Google Scholar]
- Versteeg C., Rombouts F.M., Spaansen C.H., Pilnik W. Thermostability and orange juice cloud destabilizing properties of multiple pectinesterases from orange. Journal of Food Science. 1980;45:969–971. doi: 10.1111/j.1365-2621.1980.tb07489.x. [DOI] [Google Scholar]
- Viana, M., Costa, A., Celestino, S. (2016). Informações para a composição de tabela nutricional da polpa do maracujá BRS pérola do cerrado. https://ainfo.cnptia.embrapa.br/digital/bitstream/item/159343/1/Bolpd-335.pdf/ Accessed 26 December 2019.
- Vieira A.S. Universidade de Brasília; 2010. Características físico-químicas e composição mineral da polpa de Passiflora setacea. Dissertação de mestrado. [Google Scholar]
- Wang H., Cao G., Prior R.L. Oxygen radical absorbing capacity of anthocyanins. Journal of Agricultural and Food Chemistry. 1997;45:304–309. doi: 10.1021/jf960421t. [DOI] [Google Scholar]
- Waters Corporation (1993). Waters AccQ-Tag chemistry package (instruction manual). https://www.waters.com/webassets/cms/support/docs/wat0052881.pdf/ Accessed 10 May 20180.
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.