Abstract
Background
The selective occurrence of hepatotoxicity observed with use of pazopanib may be attributed to its high level of plasma protein binding and low hepatic extraction ratio. The primary objective was to investigate changes in free drug concentration amongst patients with varying albumin concentrations.
Methods
A HPLC-MS/MS method using C18 column (4.6 × 150 mm, 5 μm) with ESI source in positive mode had been developed and validated for the quantitative determination of free pazaopanib concentration in human plasma. Prior to sample preparation, patient samples were subjected to 6-hour equilibrium dialysis with molecular weight cut-off set at 8000 Da.
Results
The calibration curves were linear over the range of 5–1000 ng/mL, with a lower limit of quantification of 5 ng/mL. The intra-day and inter-day precisions and accuracies were all within ± 15 %, at 3 different quality controls. Higher median fraction unbound of pazopanib were observed in patients (n = 17) with lower than normal albumin concentrations.
Conclusion
With the developed assay, monitoring of plasma free concentrations may be evaluated as an indicator of pazopanib exposure in patients.
Keywords: Pazopanib, Plasma free drug concentration, Fraction unbound, Liquid chromatography-tandem mass spectrometry, Equilibrium dialysis, Pharmacology, Clinical toxicology, Oncology, Evidence-based medicine, Clinical research
Pazopanib; Plasma free drug concentration; Fraction unbound; Liquid chromatography-tandem mass spectrometry; Equilibrium dialysis; Pharmacology; Clinical toxicology; Oncology; Evidence-based medicine; Clinical research.
1. Introduction
Pazopanib (Votrient ®) is a drug indicated for the treatment of advanced renal cell carcinoma. It belongs to the drug class of tyrosine-kinase inhibitors that targets vascular endothelial growth factor receptors (VEGFR-1, VEGFR-2, VEGFR-3), platelet derived growth factor receptor and stem cell growth factor receptor (c-Kit) [1]. The current dosing guidelines recommend a starting dosage of 800 mg once daily for treatment [2]. In particular, there have been concerns of hepatotoxicity with the use of pazopanib [3, 4, 5, 6] because a high proportion of renal cancer patients (up to 40–50%) had experienced increases in serum transaminases and bilirubin in clinical trials, leading to a black box warning mandated by US Food and Drug Administration (FDA) in 2012 [7].
To investigate the effect of pazopanib-induced hepatotoxicity, a quantification method for plasma free drug concentration of pazopanib will be useful to correlate with drug exposure. Since free drug molecules traverse cell membranes and diffuse more efficiently to exert therapeutic effects, plasma free drug concentration may be a more appropriate indicator of the related toxicological response. This is especially pertinent for drugs such as pazopanib that are highly bound to plasma proteins because free drug concentrations may fluctuate even without obvious change to total drug concentration. Therefore, free drug quantification is more likely to correlate better with target tissue exposure and toxicity than total drug concentration [8].
To date, most other studies had correlated drug exposures with measures that depend on total drug concentrations such as trough concentrations (Cmin) and Area under the curve (AUC), mainly due to its convenience of sampling and ease of preparation [9, 10, 11, 12]. While a quantitative method for free drug concentration of pazopanib with a lower limit of quantification at 0.5 mcg/mL had been developed by Escudero et al, the reported use of a UV detector may face challenges of sensitivity when measuring analyte at low abundance [13]. The use of unique mass transitions to identify compounds would be more reliable as compared to the use of retention times because compounds of similar chemical structures may co-elute together. Hence, the primary aim of our study was to develop a sensitive analytical method using high performance liquid chromatography-tandem mass spectrometry (HPLC-MS/MS) coupled with equilibrium dialysis to quantify plasma free drug concentration of pazopanib, for use in patient samples.
The unique pharmacokinetic profile of pazopanib presents additional challenge for the management of its related hepatotoxicity problem. Pazopanib exhibits very high level of plasma protein binding (>99.9%), mainly bound to albumin [8]. Although pazopanib is extensively metabolized in the liver by CYP3A4 enzymes, it exhibits a low calculated hepatic extraction ratio of 0.00346 (<0.3). The implication lies in that clearance of pazopanib is independent from blood flow and depends mainly on intrinsic clearance and plasma protein binding [2]. Mathematically, this increases the risk that a small change in the percentage of plasma protein binding would lead to a disproportionate change in plasma free drug concentration. In the clinical context, inter-patient variability in the level of plasma proteins is likely to accentuate the variation in free drug concentration [14, 15, 16].
For this reason, we also hypothesized that a lower albumin level will implicate a higher fraction unbound (fu%). This could be explained by the fact that this patient group will be having fewer plasma proteins made available for binding to drugs. Also, hypoalbuminemia underscores poor liver function which could lead to decrease in intrinsic clearance and potentially, an elevation of free drug concentration. Thus, our secondary goal was to apply our developed method to investigate the relationship between albumin levels and plasma free drug concentrations of pazopanib using equilibrium dialysis as a non-disruptive approach to preserve its intrinsic binding affinity. Using actual patient plasma samples, we then seeked to evaluate the correlation between the two aforementioned variables of interest for clinical application.
2. Materials and methods
2.1. Chemicals and reagents
Pazopanib was purchased from LC Laboratories ® (Woburn, MA, USA) while erlotinib (internal standard, IS) was obtained from BioVision ® (South Milpitas, CA, USA). Acetonitrile (ACN) [High performance liquid chromatography (HPLC) grade] was obtained from Tedia ® (Fairfield, OH, USA) while formic acid (reagent grade) was from Sigma-Aldrich (St Louis, MO, USA). Pooled human plasma was obtained from Biowest ® (Kansas City, MO, USA) and 10 × Phosphate buffered saline (PBS) of ultra-pure grade was purchased from Vivantis ® (Selangor, Malaysia). Rapid Equilibrium Dialysis (RED) devices with a molecular weight cut-off of 8000 Da were purchased from Thermo Fisher Scientific (Rockford, IL, USA).
2.2. Instruments and conditions
The LC-MS/MS setup consisted of high-performance liquid chromatography (HPLC) system (Agilent Technologies HPLC 1200 Series, California, USA) coupled to a triple quadruple mass spectrometer (QTRAP ® 3200, AB SCIEX, Framingham, MA, USA). Chromatographic separation of pazopanib and IS were carried out using an Agilent C18 Column (4.6 × 150 mm, 5 μm, Santa Clara, California, USA). The injection volume used was 10 μL and total chromatographic run time was 10.0 min. The mobile phase used consisted of 0.1% v/v formic acid in water (A) and 0.1% (v/v) formic acid in ACN (B). At a flow rate of 1 mL/min, the gradient conditions were set as follows: 80A:20B from 0-3 min, 50A:50B from 3-7 min and 80A:20B from 8-10 min.
Quantification was subsequently carried out via positive electrospray ionization (ESI) multiple reaction monitoring (MRM). The chosen precursor to product ions (Q1/Q3) selected for pazopanib and erlotinib were set at the m/z (mass: charge ratio) of 438.3/357.2 and 394.5/278.1 respectively. For mass spectrometer parameters, ion spray voltage was 5000 V and temperature was 550 °C. Curtain gas, nebulizer gas and the heater gas were ultrahigh purity (UHP) nitrogen gas and their pressures were adjusted to 25, 50 and 55 psi respectively (Table 1). Following the LC-MS/MS run, the acquired data was processed with Analyst® software version 1.4.2 (AB SCIEX, Framingham, MA, USA).
Table 1.
Analyte specific parameters of pazopanib and erlotinib (Internal Standard).
| ANALYTE | ANALYTE SPECIFIC PARAMETERS |
||||||
|---|---|---|---|---|---|---|---|
| Parent mass (m/z) | Product mass (m/z) | Collision energy (V) | Declustering potential (V) | Entrance potential (V) | Exit potential (V) | Typical retention time (min) | |
| PAZOPANIB | 438.3 | 357.2 | 39 | 53 | 6 | 4 | 5.00 |
| ERLOTINIB (IS) | 394.5 | 278.1 | 41 | 42 | 4 | 3 | 5.30 |
2.3. Preparation of stock solutions, calibration standards and quality controls
Stock solutions containing pazopanib (5.0 mg/mL) and erlotinib (2.5 mg/mL) were prepared in dimethyl sulfoxide (DMSO) and stored at -20 °C. Working solutions were diluted down from stock solutions using ACN: H2O (1:1) using serial dilution. A set of eight non-zero calibration standards (5, 10, 25, 50, 100, 250, 500, 1000 ng/mL) were prepared by adding the appropriate pazopanib working solutions into pooled human plasma. QC samples of 12 ng/mL (QC low), 120 ng/mL (QC medium), 900 ng/mL (QC high) and Lower Limit of Quantification (LLOQ) sample of 5 ng/mL were prepared in the same manner and in triplicates.
2.4. Pre-treatment of calibration standards and quality controls
Firstly, 95 μL of pooled human plasma was pipetted to a 2 mL Eppendorf tube, followed by 5 μL of the respective pazopanib working solution (concentrations described in Section 2.3), 100 μL of PBS and 20 μL of internal standard (500 ng/mL). 1.4 mL of diethyl ether was added for liquid-liquid extraction, using positive-displacement pipettes (Gilson, Middleton, USA). The tube was vortex-mixed for 3 min, followed by centrifugation at 10,000 rpm for 10 min. The upper layer was then extracted into a 1.5 mL tube and evaporated to dryness under a stream of nitrogen gas at 37 °C. The samples were then reconstituted in 200 μL of the mobile phase consisting of equal volumes of 0.1% v/v formic acid in water (A) and 0.1% v/v formic acid in ACN (B) for the chromatographic runs.
2.5. Preparation of patient plasma samples
With written informed consent, 17 patient samples were recruited from the National Cancer Centre, Singapore (NCCS) between January 2014 to January 2016. The inclusion criteria extend to patients who were already receiving standardized oral pazopanib 800 mg daily for the past week. Patients who were receiving treatment that involves a combination of tyrosine kinase inhibitors were excluded. The blood sample was then centrifuged at 3000 rpm to separate the plasma from the buffy coat. 1 mL of plasma was collected and stored in cryotubes at -20 °C until analysis. This study had been approved by the SingHealth Institutional Review Board (IRB) approval (CIRB Ref 2012/077/F).
The collected plasma samples were subsequently analysed for its total and free drug concentration using the following method: Single-Use Plate Rapid Equilibrium Dialysis (RED) Device (Thermo Fisher Scientific, Rockford, USA) with a molecular weight cut-off of 8000 Da was used. A total of 300 μL of the patient plasma and 500 μL of Phosphate Buffered Saline (PBS) were loaded into the sample chamber and buffer chamber respectively, according to manufacturer's instructions. The samples were then incubated at 37 °C on an orbital shaker (LM-570D, Yihder Technology, Xinbei, China) at 250 rpm for 6 h to establish equilibrium. Content from both sample and buffer chambers was collected after equilibrium dialysis. Liquid-liquid extraction was performed on the sample using diethyl ether where the upper layer was then collected and evaporated to dryness under a stream of nitrogen gas. The samples were then reconstituted in the 200 μL of mobile phase consisting of equal volumes of 0.1% v/v formic acid in water (A) and 0.1% v/v formic acid in ACN (B). This was done in technical duplicates for each patient sample.
The fraction unbound (fu%) was determined using the following equation: fu% = (concentration of analyte in buffer chamber/concentration of analyte in plasma chamber) × 100%
2.6. Data analysis
Each sample used for calibration standards (concentrations described in Section 2.3) was quantified in triplicates and the mean peak area ratio of pazopanib: IS was quantified against pazopanib: IS concentration (ng/mL) to determine the reliability of the LC-MS/MS method. The least-squares linear regression analysis was employed to plot the calibration curves, using a weighting factor of 1/x2. The validation of the method was carried out following guidelines for Bioanalytical Method Validation published by the FDA for precision, accuracy, selectivity, sensitivity, carry-over effect, recovery and stability [7]. Statistical analysis was conducted using Statistical Package for the Social Sciences (SPSS, IBM), and p-values less than 0.05 were considered statistically significant.
3. Results and discussion
3.1. Linearity and limit of quantification
The linearity of calibration curves for pazopanib expressed as correlation coefficients (R2) was at least 0.99. The average least square linear regression parameters were as follows: y = 0.2200 x (±0.018) + 0.0560 (±0.0192). The final calibration curves ranged from 5-1000 ng/mL. The upper limit was imposed because it was found that at higher concentrations, there might be ion suppression effect in which simultaneous processing of more than one component in the ion source could result in competition and reduction in mass spectrometry signal, leading to a plateau.
3.2. Validation
3.2.1. Precision and accuracy
Precision was calculated using the formula: (standard deviation of measured concentration/mean measured concentration) × 100%; while accuracy was determined using the formula of (measured concentration/nominal concentration) × 100%.
To investigate intra-day precision and accuracy, three replicates of each QC samples (QC low, medium and high) were prepared and analysed in one analytical run. To investigate inter-day precision and accuracy, a total of three analytical runs were performed on three separate days.
For plasma samples, intra-day precision ranged from 1.16-12.31% and inter-day precision ranged from 5.29-10.80%. Intra-day and inter-day accuracies of QC samples ranged from 91.80-109.56% and 95.22–103.14% respectively. For LLOQ, the intra-day and inter-day precisions were 8.23–15.58% and 10.57% while the intra-day and inter-day accuracies were 97.06–111.98% and 109.64% accordingly (Table 2). Overall, these values were within the allowance set by FDA of 15% for QC samples and within 20% for LLOQ samples.
Table 2.
Intra-day and inter-day precision and accuracy expressed as percentages (%) from nominal controls obtained from Quality Control (QC) samples in three analytical runs.
| Nominal concentration (ng/mL) | Intra-day precision (%) | Intra-day accuracy (%) | Inter-day precision (%) | Inter-day accuracy (%) |
|---|---|---|---|---|
| LLOQ (5 ppba) | 8.23 | 97.06 |
10.57 |
109.64 |
| 12.13 | 119.89 | |||
| 15.58 | 111.98 | |||
| QC Low (12 ppb) | 1.67 | 91.80 |
5.29 |
95.22 |
| 1.10 | 102.06 | |||
| 11.62 | 96.98 | |||
| QC Medium (120 ppb) | 1.16 | 109.33 |
5.44 |
103.14 |
| 12.31 | 101.71 | |||
| 4.44 | 98.38 | |||
| QC High (900 ppb) | 1.60 | 109.56 |
10.80 |
98.30 |
| 1.73 | 96.52 | |||
| 12.24 | 88.83 |
units for Quality Controls measured in parts per billion.
3.2.2. Selectivity, sensitivity and carry-over
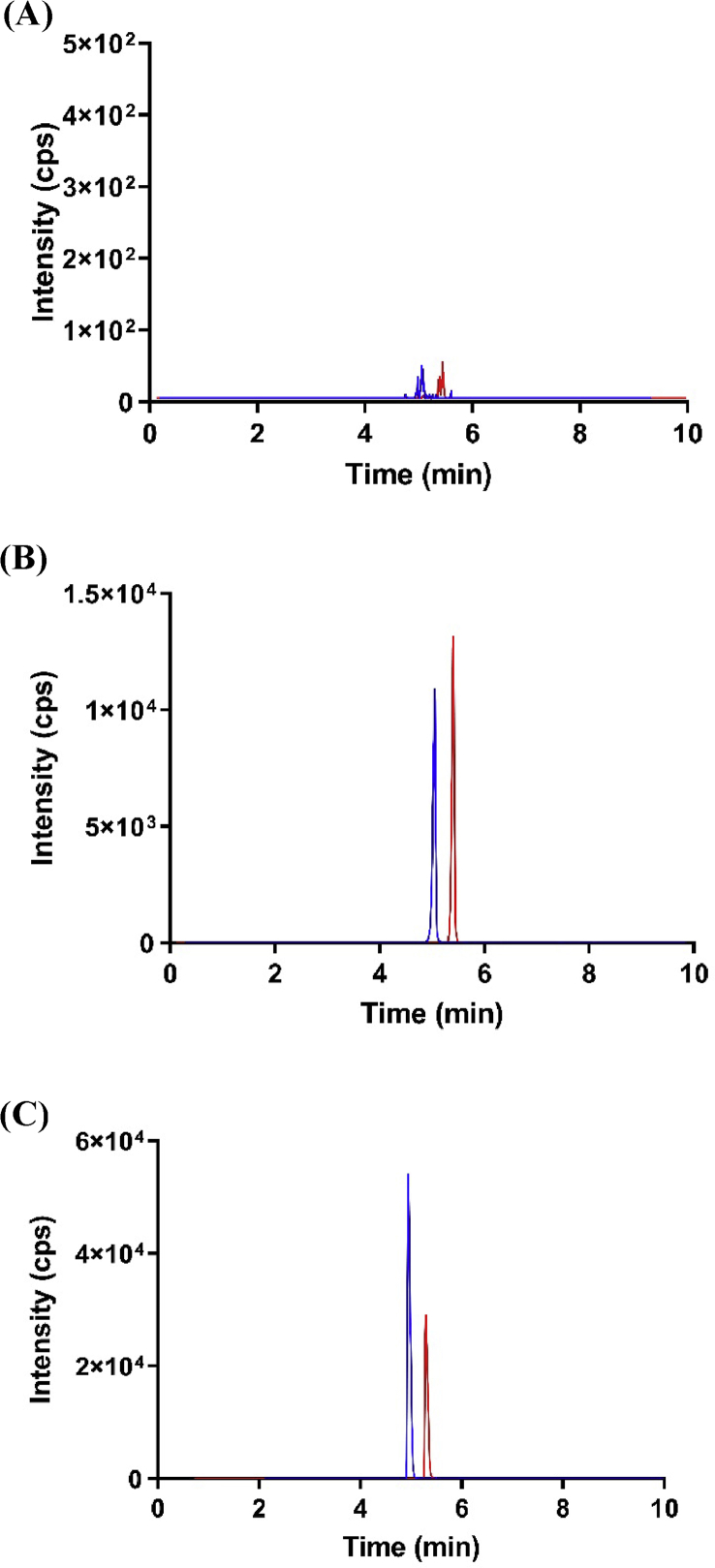
Our method is selective since the chromatograms showed clear separation between analyte and IS with little interference. Our method is also sensitive as the signal at the LLOQ was found to be at least five times of the blank sample. Figure 1A depicted a representative chromatogram for a blank response. There was no significant carry-over effect found, with minimal difference in the readings of blanks before and after a run of QC high (900 ng/mL). Figure 1B and C depicted a representative chromatogram of a calibration standard and patient plasma sample spiked with internal standard respectively.
Figure 1.
Chromotograms of pazopanib and internal standard for blank response (A), calibration standard (B) and actual patient plasma sample spiked with internal standard (C). Blue color denotes pazopanib (m/z of 438.3/357.2) while red line denotes internal standard of erlotinib (394.5/278.1).
3.2.3. Recovery
For the preparation of neat samples, 195 μL of mobile phase, 5 μL of pazopanib working solution and 20 μL of internal standard (500 ng/mL) were pipetted into an Eppendorf tube. The extraction recovery was calculated with the following formula: recovery = (mean peak area of drug extracted from plasma/mean peak area of non-extracted neat samples) × 100%. At concentration 12, 120 and 900 ng/mL, the mean recoveries were found to be 123.67%, 113.48%, 114.56% respectively.
3.2.4. Stability
Stability tests of pazopanib were done using the QC samples (low, medium and high) as summarised in (Table 3). No significant degradation of the QC samples of pazopanib was detected after storing the samples at bench top conditions, auto sampler conditions or after 1 month of storage at -80 °C. Three freeze-thaw cycles also did not result in significant degradation in the samples. The precision and accuracy for the various stability tests were found to be within the acceptable allowance of 15% [7].
Table 3.
Stability data of pazopanib at various conditions expressed as precision and accuracy in percentages from nominal concentration.
| Stability Test | Conditions | Concentrations | Precision (%) | Accuracy (%) |
|---|---|---|---|---|
| Autosampler | Extracted samples stored for 14 h at room temperature | 12 ppba | 10.43 | 96.45 |
| 120 ppb | 5.78 | 96.56 | ||
| 900 ppb | 5.28 | 96.02 | ||
| Freeze Thaw | Three freeze thaw cycles | 12 ppb | 1.40 | 93.79 |
| 120 ppb | 12.39 | 93.50 | ||
| 900 ppb | 3.51 | 100.49 | ||
| Bench Top | Plasma samples stored at room temperature for 8h | 12 ppb | 4.10 | 113.11 |
| 120 ppb | 7.80 | 93.21 | ||
| 900 ppb | 7.31 | 97.02 | ||
| Long Term | Stored at -80 °C for 1 month | 12 ppb | 4.35 | 100.58 |
| 120 ppb | 4.36 | 113.89 | ||
| 900 ppb | 10.33 | 114.23 |
units for Quality Controls measured in parts per billion.
3.3. Equilibrium dialysis of actual patient samples
A total of 17 patients' plasma samples were tested with our developed method. Their mean serum albumin level was 38.3 ± 6.3 g/L, with 3 of the patients presented with hypoalbuminemia (defined as when albumin concentration falls below 35 g/L). The optimal time to reach equilibrium dialysis was chosen to be 6 h according to a previously validated method [15]. The average steady state concentration of our measured patients' samples was 30.9 ± 11.2 μg/mL, consistent with what had been reported in studies [2, 16] while free drug concentration of pazopanib in our measured patients’ samples was found to be 5.84 ± 3.02 ng/mL (expressed in mean ± standard deviations). There were no statistically significant differences between free drug concentration of pazopanib in our range of plasma albumin levels of 29–44 g/L.
3.4. Effect of variable albumin levels in human plasma on fu% of pazopanib
Patients' plasma samples were subsequently categorized into 3 groups of varying levels of albumin, with very low albumin level and low albumin level being defined as less than 30 g/L and less than 40 g/L respectively (Table 4). The median fraction unbound was observed to be higher in patients’ samples with lower than normal albumin levels (0.0173 ± 0.0060 and 0.0227 ± 0.0122 in very low and low albumin levels respectively) compared to patients with normal albumin level (0.0129 ± 0.0061). Comparing plasma free drug concentrations, higher median plasma free drug concentration was trended for patient group with low albumin level when compared to patient samples with very low and normal levels (6.49 ± 3.65 ng/mL vs 3.44 ± 1.32 ng/mL and 4.88 ± 2.71 ng/mL). It was noteworthy that the limited number of patients belonging to very low albumin levels (n = 2) may have made it difficult to draw robust inferences for basis of comparison. The effect of varying albumin levels on fu% of pazopanib in actual patient plasma samples would need to be further demonstrated using a patient cohort of larger sample size with wider albumin range for it to be meaningful.
Table 4.
fu% and plasma free drug concentration of pazopanib at different albumin levels in actual patient plasma samples.
| Albumin levels (g/L) (n = no. of patients) |
fu%b (Median ± Interquartile range) | Plasma free drug concentrationc (ng/mL) (Median ± Interquartile range) |
|---|---|---|
| Normal (n = 8) | 0.0129 ± 0.0061 | 4.88 ± 2.71 |
| Low (<40 g/L) (n = 7) |
0.0227 ± 0.0122 | 6.49 ± 3.65 |
| Very Low (<30 g/L) (n = 2) |
0.0173 ± 0.0060 | 3.44 ± 1.32 |
p-value for Kruskal-Wallis test = 0.43. There were no statistically significant differences between normal, low and very low albumin levels.
p-value for Kruskal-Wallis test = 0.37. There were no statistically significant differences between normal, low and very low albumin levels.
When interpreting this data to understand the reason behind toxicity, fu% serves as a surrogate measure for the changes to free drug concentration of pazopanib. In patients with severe hypoalbuminemia, we would be concerned if the total pazopanib concentration may be misinterpreted as a falsely lower exposure to free active pazopanib than what was reflected in the patient. Thus, our findings had provided greater depth to interpret free drug concentration of pazopanib in the event of therapeutic drug monitoring [17, 18, 19, 20] and could allow physiologically based pharmacokinetic models to be built. There could be potential use of albumin levels to complement laboratory liver function tests to guide clinicians in dosage adjustments such as using lower doses to minimise drug-use related adverse events. Given how pazopanib is currently used with a standard fixed dose despite it showing wide inter-patient variability in terms of treatment outcome, the measure of free drug concentration in relation to albumin levels could be adopted to refine clinical practice in the future. The use of serum albumin levels is currently being employed in clinical practice for anti-epileptic drugs such as phenytoin, in which albumin level is considered as a correction factor to measure for free phenytoin [21].
There are a few method limitations worth mentioning: our method had been validated based on total drug concentration of pazopanib spiked in pooled human plasma because quantitative determination of plasma free drug concentration in the buffer compartment had been challenging, given how their absolute values were near the LLOQ range. Also, the accuracy of the measured buffer concentration would be especially prone to influence of non-specific binding and/or change in volume [22]. The use of RED device also relied on the assumption that equilibrium had been achieved within the predetermined incubation time and limits assay usage in setting that requires fast turnovers.
While several methods had been documented in literature for simultaneous quantification of tyrosine-kinase inhibitors [23, 24, 25], related drugs of interest [26] and/or its metabolites [27, 28], our study had allowed for the additional determination of unbound drug concentration. Before clinical application, our findings still need to be further validated in a larger independent patient cohort and consider patient-related factors such as the concurrent medications and the presence of underlying comorbidities. Future work could utilise the approach for quantification of drugs with narrow therapeutic window that has a similar pharmacokinetic profile of highly protein-bound and low hepatic extraction ratio.
4. Conclusion
In summary, we had developed an HPLC-MS/MS coupled with equilibrium dialysis method for the quantitative determination of plasma drug concentration of pazopanib and validated the method in accordance with FDA's guidelines. Applying this assay on actual patient samples, preliminary data suggested that albumin levels had an influence on plasma free drug concentration of pazopanib, with higher free pazopanib concentration and fu% being observed in samples with lower albumin levels. Future clinical studies may consider analyzing plasma free drug concentration of pazopanib as an indicator of drug exposure.
Declarations
Author contribution statement
Y.L. Toh: Conceived and designed the experiments; Performed the experiments; Analyzed and interpreted the data; Contributed reagents, materials, analysis tools or data; Wrote the paper.
Y.Y. Pang: Conceived and designed the experiments; Performed the experiments; Analyzed and interpreted the data; Contributed reagents, materials, analysis tools or data.
C.K. Toh, M. Shwe and R. Kanesvaran: Contributed reagents, materials, analysis tools or data.
A. Chan and H.K. Ho: Conceived and designed the experiments; Analyzed and interpreted the data; Contributed reagents, materials, analysis tools or data; Wrote the paper.
Funding statement
We would like to acknowledge the National University of Singapore for funding this study (R148-000-272-114 and R148-000-295-114).
Competing interest statement
The authors declare no conflict of interest.
Additional information
No additional information is available for this paper.
References
- 1.Harrison M., Lang J. Pazopanib for the treatment of patients with advanced renal cell carcinoma. Clin. Med. Insights Oncol. 2010;4:95–105. doi: 10.4137/CMO.S4088. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.GSK . 2015. Prescribing Information for Votrient® (Pazopanib), Medication Guide. [Google Scholar]
- 3.Kapadia S., Hapani S., Choueiri T.K. Risk of liver toxicity with the angiogenesis inhibitor pazopanib in cancer patients. Acta Oncol. (Stockh.) 2013;52:1202–1212. doi: 10.3109/0284186X.2013.782103. [DOI] [PubMed] [Google Scholar]
- 4.Shah R.R., Morganroth J., Shah D.R. Hepatotoxicity of tyrosine kinase inhibitors: clinical and regulatory perspectives. Drug Saf. 2013;7:491–503. doi: 10.1007/s40264-013-0048-4. [DOI] [PubMed] [Google Scholar]
- 5.Shah C., Saiyed M.M. Value Health; 2015. Hematological Toxicities Associated with Pazopanib Use in Cancer Patients: a Meta-Analysis. [Google Scholar]
- 6.Gupta S., Spiess P.E. The prospects of pazopanib in advanced renal cell carcinoma. Ther Adv Urol. 2013;5:223–232. doi: 10.1177/1756287213495099. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Guidelines for Bioanalytical Method Validation. FDA; 2013. [Google Scholar]
- 8.Hurtwitz H.I., Dowlati A., Saini S. Phase 1 trial of pazopanib in patients with advanced cancer. Clin. Canc. Res. 2009;15:4220–4227. doi: 10.1158/1078-0432.CCR-08-2740. [DOI] [PubMed] [Google Scholar]
- 9.Verheijen R., Bins S., Mathijssen R., Lolkema M. Individualized pazopanib dosing: a prospective feasibility study in cancer patient. Clin. Canc. Res. 2016;22(23) doi: 10.1158/1078-0432.CCR-16-1255. [DOI] [PubMed] [Google Scholar]
- 10.Nienke A.G. Optimizing the dose in cancer patients treated with imatinib, sunitinib and pazopanib. Br. J. Clin. Pharmacol. 2017;83:2195–2204. doi: 10.1111/bcp.13327. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Suttle A.B., Ball H., Molimard M. Relationships between pazopanib exposure and clinical safety and efficacy in patients with advanced renal cell carcinoma. Br. J. Canc. 2014;111:1–8. doi: 10.1038/bjc.2014.503. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Minocha M., Khurana V., Mitra A.K. Determination of pazopanib in mouse plasma and brain tissue by liquid chromatography-tandem mass spectrometry. J Chromatography B. 2012;901:85–92. doi: 10.1016/j.jchromb.2012.06.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Escudero V., Perez J.J., Valenzuela B. Development and validation of an HPLC-UV method for pazopanib quantification in human plasma and application to patients with cancer in routine clinical practice. Ther. Drug Monit. 2015;37:172–179. doi: 10.1097/FTD.0000000000000121. [DOI] [PubMed] [Google Scholar]
- 14.Gurevich K. Effect of blood protein concentrations on drug-dosing regimes: practical guidance. Theor. Biol. Med. Model. 2013;10:20. doi: 10.1186/1742-4682-10-20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Serlemitsos Day M., Ellington K., Akalu A. Should Medications be co-administered with albumin in hypoalbuminemic patients. J Pharma Care Health Sys. 2017;4:167. [Google Scholar]
- 16.Imbs D.C., Paludetto M.N., Negrier S. Determination of unbound fraction of pazopanib in vitro and in cancer patients reveals albumin as the main binding site. Invest. N. Drugs. 2015;34:41–48. doi: 10.1007/s10637-015-0304-9. [DOI] [PubMed] [Google Scholar]
- 17.Verheijen R., Beijnen J., Schellens J. Clinical pharmacokinetics and pharmacodynamics of pazopanib: towards optimized dosing. Clin. Pharmacokinet. 2017;56:987–997. doi: 10.1007/s40262-017-0510-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Grassi P., Verzoni E., Ratta R. Drugs R D; 2017. Does Dose Modification Affect Efficacy of First-Line Pazopanib in Metastatic Renal Cell Carcinoma. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Ryan C.W. Dosing strategies and optimization of targeted therapy in advanced renal cell carcinoma. J. Oncol. Pharm. Pract. 2015 doi: 10.1177/1078155215618769. [DOI] [PubMed] [Google Scholar]
- 20.Sparidans R.W., Ahmed T.T., Muiwijk E.W. Liquid chromatography-tandem mass spectrometric assay for therapeutic drug monitoring of tyrosine kinase inhibitor pazopanib in human plasma. J Chromatography B. 2012;905:137–140. doi: 10.1016/j.jchromb.2012.08.004. [DOI] [PubMed] [Google Scholar]
- 21.Hong J.M., Choi Y.C., Kim W.J. Differences between the measured and calculated free serum phenytoin concentrations in Epileptic Patients. Yonsei Med. J. 2009;50:517–520. doi: 10.3349/ymj.2009.50.4.517. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Isbell J., Yuan D., Torrao L. Plasma protein binding of highly bound drugs determined with equilibrium gel filtration of nonradiolabeled compounds and LC-MS/MS Detection. J. Pharmacol. Sci. 2019;108:1053–1060. doi: 10.1016/j.xphs.2018.10.004. [DOI] [PubMed] [Google Scholar]
- 23.Andriamanana I., Gana I., Duretz B. Simultaneous analysis of anticancer agents bortezomib, imatinib, nilotinib, dasatinib, erlotinib, lapatinib, sorafenib, sunitinib and vandetanib in human plasma using LC/MS/MS. J Chromatography B. 2013;1:83–91. doi: 10.1016/j.jchromb.2013.01.037. [DOI] [PubMed] [Google Scholar]
- 24.Gotze L., Hegele A., Metzelder S.K. Development and clinical application of a LC-MS/MS method for simultaneous determination of various tyrosine kinase inhibitors in human plasma. Clin. Chim. Acta. 2012;413:143–149. doi: 10.1016/j.cca.2011.09.012. [DOI] [PubMed] [Google Scholar]
- 25.Van Erp N.P., de Wit D., Guchelaar H.J. A validated assay for the simultaneous quantification of six tyrosine kinase inhibitors and two active metabolites in human serum using liquid chromatography coupled with tandem mass spectrometry. J Chromatography B. 2013;937:23–43. doi: 10.1016/j.jchromb.2013.08.013. [DOI] [PubMed] [Google Scholar]
- 26.Pang Y.Y., Tan Y.L., Ho H.K. Investigation of the effect of plasma albumin levels on regorafenib-induced hepatotoxicity using a validated liquid chromatography-tandem mass spectrometry method. J Chromatography B. 2017;1061–1062:220–224. doi: 10.1016/j.jchromb.2017.07.023. [DOI] [PubMed] [Google Scholar]
- 27.Paludetto M.N., Puisset F., Louedec F.L. Simultaneous monitoring of pazopanib and its metabolites by UPLC-MS/MS. J. Pharmaceut. Biomed. Anal. 2018;I54:373–383. doi: 10.1016/j.jpba.2018.03.013. [DOI] [PubMed] [Google Scholar]
- 28.Paludetto M.N., Stigliani J.L., Robert A. Involvement of pazopanib and sunitinib aldehyde reactive metabolites in toxicity and drug-drug interactiosn in vitro and in patient samples. Chem. Res. Toxicol. 2020;33:181–190. doi: 10.1021/acs.chemrestox.9b00205. [DOI] [PubMed] [Google Scholar]