Abstract
Cardiac amyloidosis (CA) has emerged as a previously underestimated cause of heart failure and mortality. Underdiagnosis resulted mainly from unawareness of the true disease prevalence and the non-specific symptoms of the disease. CA results from extracellular deposition of misfolded protein fibrils, commonly derived from transthyretin (ATTR) or immunoglobulin light chains (AL). A significant proportion of older patients with heart failure and other extracardiac manifestations suffer from ATTR-CA, whereas AL-CA is still considered a rare disease. This article provides an overview of CA with a special focus on current and emerging diagnostic modalities. Furthermore, we provide a diagnostic algorithm for the evaluation of patients with suspected CA in every-day practice.
Keywords: Amyloidosis, Cardiomyopathy, Heart failure, Transthyretin, Light chains
Abbreviations: AA, amyloid A amyloidosis; AApoA-1, apolipoprotein A-1 amyloidosis; AL, light chain amyloidosis; ATTR, transthyretin amyloidosis; ATTRv, variant transthyretin amyloidosis; ATTRwt, wild type transthyretin amyloidosis; CA, cardiac amyloidosis; ECV, Extracellular volume; EMB, endomyocardial biopsy; LGE, late gadolinium enhancement; LV, left ventricular/ left ventricular; MGUS, monoclonal gammopathy of undetermined significance; MRI, magnetic resonance imaging; NT-proBNP, N-terminal pro B-type natriuretic peptide; PET, positron-emission tomography; SPECT, single photon emission computed tomography; 99mTc-DPD, 99mTc-3,3-diphosphono-1,2-propanodicarboxylic acid
1. Introduction
Amyloidosis is a disorder in which proteins with unstable tertiary structures misfold, aggregate and form insoluble fibrils that infiltrate the extracellular space of organs and soft tissues [1]. The term amyloid (from the ancient Greek word ἄμυλοv = starch) was adopted by Virchow in 1854 due to the starch-like affinity of amyloid deposits for iodine. More than 30 different precursor proteins with amyloidogenic potential have been identified. Each one is associated with distinct clinical features and organ tropisms. Two of them, the liver-synthesized transport protein transthyretin and amyloidogenic monoclonal immunoglobulin light chains, which may be oversecreted in plasma cell dyscrasias, account for the vast majority of all cases of cardiac amyloidosis (CA). Other rare types of amyloidosis involving the heart include serum amyloid A amyloidosis (AA) and apolipoprotein A-1 amyloidosis (AApoA-1). Myocardial involvement in systemic amyloidosis is the main predictor of outcome and is associated with high mortality if not treated appropriately at an early disease stage [2], [3].
Until recently CA was considered a rare entity. Advances in cardiac imaging and the fact that specific therapies became available for transthyretin amyloidosis (ATTR) in recent years led to increased recognition of this entity as an underestimated cause of heart failure (HF) [4]. The rise of amyloidosis incidence and the growing availability of disease modifying drugs have increased the interest for the disease among cardiologists. This article summarizes current knowledge on optimal diagnostic management of patients with suspected CA with a special focus on the most common types of ATTR-CA and light chain CA (AL-CA). Furthermore, we address some controversial issues and unanswered questions which are of potential interest for future research.
2. Epidemiology
There are two distinct forms of ATTR amyloidosis. The hereditary or variant form (ATTRv) is linked to one of >120 identified amyloidogenic TTR gene mutations that predispose to instability of the tetrameric structure of TTR and dissociation in monomers or oligomers which form amyloid. ATTRv is characterized by great epidemiologic variability. It is a generally rare autosomal dominant disease, but some genetic variants are endemic in specific geographic regions and ethnic groups [5], [6], [7], [8], [9], [10], [11], [12]. The Val30Met (currently named Val50Met) mutation is the most frequent TTR mutation in the world. It is endemic in Portugal, Sweden, Japan and Majorca [5]. Some mutations are linked to neuropathic phenotype, others to isolated cardiac phenotype, whereas mixed neuropathic and cardiac phenotype is common in others. Four mutations (Val122Ile, Leu111Met, Thr60Ala, and Ile68Leu) were associated with a mainly cardiac phenotype in the West-European patient cohort of the recently published THAOS registry [6]. Val122Ile is observed in patients from West Africa or of West African descent, with 3.4% of African Americans aged <65 years carrying at least one copy of the mutation [7], [8]. The penetrance of this mutation is uncertain and likely relatively low. The Leu111Met variant has been identified only in Danish families and is associated with an exclusively cardiac phenotype [9]. The Thr60Ala mutation affects 1% of the population of Donegal in northwest Ireland [10]. The Ile68Leu mutation is endemic in central-northern Italy and is associated with greater penetrance in men and with older patient age [11], [12].
The non-hereditary or wild type form (ATTRwt) is characterized by a normal transthyretin gene sequence and still unclear disease etiology. The prevalence of ATTRwt increases with age and nearly all affected patients are older than 60 years. An autopsy study reported that among adults aged >80 years 25% have myocardial ATTR amyloid deposits [13]. Recent studies reported a prevalence of 13% among patients with HF and preserved ejection fraction [14], [15], 16% in patients with aortic valve stenosis undergoing transcatheter valve replacement [16], [17], [18], [19] with as high as 30% in the subgroup of low-flow, low gradient stenosis and 5% of patients with hypertrophic cardiomyopathy [20]. Despite initially considered a disease of older males, later studies demonstrated that women represent up to 20% of ATTRwt patients [21].
In contrast, AL amyloidosis which is the consequence of plasma cell dyscrasia with production of amyloidogenic monoclonal immunoglobulin light chain, is a rare condition with prevalence of 8–12/million [22], [23], [24], [25] and incidence of approximately 1/100,000/year in the USA. The disease becomes more prevalent with increasing age and males are more frequently affected. The underlying pathology is monoclonal gammopathy or smoldering myeloma in 90%. Multiple myeloma or B-cell lymphoma are identified as a cause in 10% of all AL cases. Symptomatic cardiac involvement is present at diagnosis in 60–80% of patients [24].
Other forms of CA that are considered very rare include serum amyloid A amyloidosis and apolipoprotein A-1 amyloidosis. AA amyloidosis occurs in the setting of chronic inflammatory diseases (e.g. tuberculosis, inflammatory bowel disease, rheumatoid arthritis, periodic fever syndromes) and the amyloid fibrils are composed of the degradation products of the non-specific, acute phase reactant protein serum amyloid A. In contrast to other forms of systemic amyloidosis, cardiac involvement in AA amyloidosis is uncommon, ranging between 10 and 15% depending on the population studied [26], [27]. AA amyloidosis is likely underdiagnosed in developing countries, where chronic infections are more prevalent and undertreated. Current estimates report an incidence of 1 to 2 cases per million person-years for AA amyloidosis [27].
ApoA-1 amyloidosis is a rare form of amyloidosis that may cause cardiomyopathy [28]. ApoA-1 is produced by the liver and small intestine and is involved in the transportation and metabolism of high-density lipoprotein. Like in ATTR amyloidosis a wild-type, age-related phenotype and a hereditary phenotype exist. More than 20 mutations have been reported to be associated with this form of amyloidosis, which is associated with an autosomal dominant inheritance and variable penetrance [28], [29], [30]. The hereditary form is associated with involvement of various organs (heart, liver, kidney, skin, gonades and peripheral nervous system). The exact incidence of the disease in the general population is not known.
The following sections of this article refer to the two common types of AL- and ATTR-CA.
3. Clinical manifestations and natural history
General symptoms such as fatigue, weight loss, cachexia and muscle weakness are reported in all age groups and types of amyloidosis. The most common initial presentation of CA is HF with preserved or mid-range left ventricular (LV) systolic function and LV hypertrophy. Peripheral edema and progressive exertional dyspnea are cardinal manifestations of CA. Syncope, falls and symptomatic hypotension as a consequence of autonomic dysfunction are frequently reported and the combination of hypotension with LV hypertrophy should be a warning sign. High-grade atrioventricular block with the need for permanent pacing is a hallmark of the disease [31], whereas malignant ventricular arrhythmias are probably of less clinical importance even in advanced stages of CA. This has yet to be studied in current patient cohorts with adequate representation of ATTR-CA. Atrial fibrillation is the most common arrhythmia and is associated with higher risk of atrial thrombi [32], [33] and frequent relapses after repeated catheter ablation or cardioversion [34].
Extracardiac manifestations are often present and serve as red flags for early diagnosis. In AL amyloidosis these may include severe proteinuria, renal failure and generalized edema, hepatomegaly, ascites, pleural effusion, peripheral neuropathy, autonomic dysfunction with severe hypotension, diarrhea, carpal tunnel syndrome and soft tissue manifestations (e.g. macroglossia, periorbital purpura, subcutaneous amyloidomas). History of bilateral carpal tunnel syndrome, lumbal stenosis, presbyacusis and spontaneous biceps tendon rupture are common in ATTRwt [35]. The latter is a common finding in ATTR-CA [36]. ATTRv is typically characterized by peripheral sensorimotor polyneuropathy, cardiomyopathy or mixed phenotype. Autonomic neuropathy is common and often severe diarrhea, hypotension and erectile dysfunction are the leading symptoms. Renal or lung involvement have been rarely reported. Carpal tunnel syndrome is also seen and may precede cardiac or nerve involvement by several years in both ATTRv and ATTRwt. Family history with three-generation pedigree is essential in the evaluation of patients with ATTRv, as symptoms cluster in families and provide significant clues for the diagnosis.
Each type of CA (ATTRv, ATTRwt, AL) is linked to a distinct natural history, which is of relevance for the differential diagnosis. In AL amyloidosis a steep functional decline is observed from the initial presentation. Consequently, suspicion of AL amyloidosis should alert physicians to perform a rapid and stringent diagnostic work-up, since further progression of the disease significantly worsens prognosis without implementation of effective anti-plasma cell therapies that have been available for years.
ATTR amyloidosis is characterized by a more insidious and slowly progressive disease with severe symptoms developing years after initial manifestations. A recent report from the national amyloidosis center in the UK reported 17 medical contacts including 3 inpatient hospital admissions from first cardiac symptoms to diagnosis of ATTRv or ATTRwt-CA [37]. These differences in the disease course are possibly due to distinct underlying pathophysiologic mechanisms of cardiac damage in AL-CA vs ATTR-CA. In vitro studies demonstrated that circulating amyloidogenic light chains have a direct toxic effect in the myocardium leading to increased apoptosis, oxidative stress, altered calcium handling and activation of specific signal transduction pathways [38], [39], [40].
4. Diagnosis
Diagnosis of CA requires a high degree of clinical suspicion as the disease masquerades a plethora of cardiac and systemic diseases. Endomyocardial biopsy (EMB) has been used to diagnose CA, if a definite diagnosis could not be established via extracardiac biopsy. However, non-biopsy diagnosis of ATTR-CA with bone-tracer scintigraphy was proven feasible and highly accurate compared to EMB and may therefore substitute EMB in certain cases [41]. In the following sections, we describe the role of different diagnostic modalities and present a recommended algorithm for the systematic approach of patients with suspected CA.
4.1. ECG
Although ECG may be normal even at advanced stages of CA, it can provide clues for amyloid infiltration and further support the diagnosis in conjunction with imaging findings. The typical finding is normal or low QRS voltage (<1 mV in the precordial and <0,5 mV in the extremity leads) in patients with LV hypertrophy on echocardiography. This is more common in AL-CA (45%) but less frequent in ATTR-CA (23–31%) [42]. Other features include pseudo-infarct pattern with Q waves or slow R-wave progression in the precordial leads, atrioventricular block or bundle branch block. Atrial fibrillation is the most common arrhythmia with a high relapse rate [43], [44]. Given its low sensitivity, ECG should not be used alone as a screening tool for patients with suspected CA or at risk for CA e.g. TTR mutation carriers.
4.2. Laboratory testing
As no single parameter exists for diagnosing ATTR-CA, the goal of laboratory testing in patients with suspected CA is primarily to search for markers of plasma cell disease causing AL-amyloidosis. These include elevated serum free light chain immunoglobulins, pathologic κ to λ free light chain ratio and monoclonal gammopathy in serum and urine immunofixation with reported sensitivity of >95% for the detection of AL amyloidosis [45], [46]. Caution is required in the interpretation of positive findings as up to 5% of the general population aged >65 years have monoclonal gammopathy of undetermined significance (MGUS) [47]. ATTR-CA may coexist with MGUS and should be differentiated from AL amyloidosis by seeking further evidence of amyloid infiltration in affected organs with identification of the precursor protein. Renal dysfunction also leads to higher serum concentration of free light chains [48]. Approximately 2–3% of patients with AL amyloidosis have no laboratory evidence of monoclonal disorders. Despite this being extremely rare, rule out of AL amyloidosis should not be merely based on laboratory testing if clinical suspicion is high.
Natriuretic peptides are markedly and disproportionally elevated in CA and mildly elevated troponin on repeated occasions is common. However, ischemic heart disease may coexist with amyloidosis and thus, evaluation by coronary angiography should be performed in selected patients. Amyloidogenic light chains modulate p38 mitogen-activated protein kinase, which can directly promote NT-proBNP expression. Thus, for the same range of hemodynamic abnormalities plasma levels of NT-proBNP are higher in AL- than in ATTRwt- and ATTRv-CA [49].
Furthermore, biomarkers play an important role in the follow-up of patients with known MGUS. In case of MGUS with an abnormal free light chain ratio with elevation of the involved light chain, N-terminal pro B-type natriuretic peptide (NT-proBNP) and urinary albumin should be monitored during follow up to detect progression of MGUS to systemic amyloidosis [50], [51]. After establishment of diagnosis of CA, serum NT-proBNP and cardiac troponin T combined with the difference in serum free light chain values facilitate staging of AL amyloidosis, as the severity of cardiac involvement is a powerful predictor of outcome [52]. According to a contemporary staging system, patients with AL amyloidosis are assigned a score of 1 for each one of the following: difference in free light chains ≥18 mg/dL, cardiac Troponin T ≥0.025 ng/mL and NT-proBNP ≥1800 pg/mL, creating stages I to IV with additive scores of 0 to 3 points, respectively. The respective median overall survival is 94.1, 40.3, 14 and 5.8 months, respectively [52]. Biomarker-based staging systems were recently proposed for ATTR-CA. Thresholds of Troponin and NT-proBNP or estimated glomerular filtration rate and NT-proBNP were used to risk stratify ATTR patients. In a scoring system in wtATTR-CA and ATTRv-CA, stage I was defined as NT-proBNP ≤ 3000 ng/L and eGFR ≥ 45 ml/min, stage III was defined as NT-proBNP > 3000 ng/L and eGFR < 45 ml/min, and the remainder were stage II. Median survival of stage I patients was 69.2 months, stage II patients 46.7 months and stage III patients 24.1 months [53]. The Mayo Clinic staging system for wtATTR-CA used thresholds of troponin T and NT-proBNP (>0.05 ng/ml and >3000 pg/ml, respectively). The 3 stages were defined as stage I: both biomarker values below threshold, stage II: 1 biomarker above the threshold and stage III: both biomarkers above the threshold. These 3 stages had a median survival of 66, 42, and 20 months, respectively in this study [3].
Additional laboratory testing should be ordered according to suspected organ involvement (e.g. liver function tests, urine protein analysis).
4.3. Echocardiography
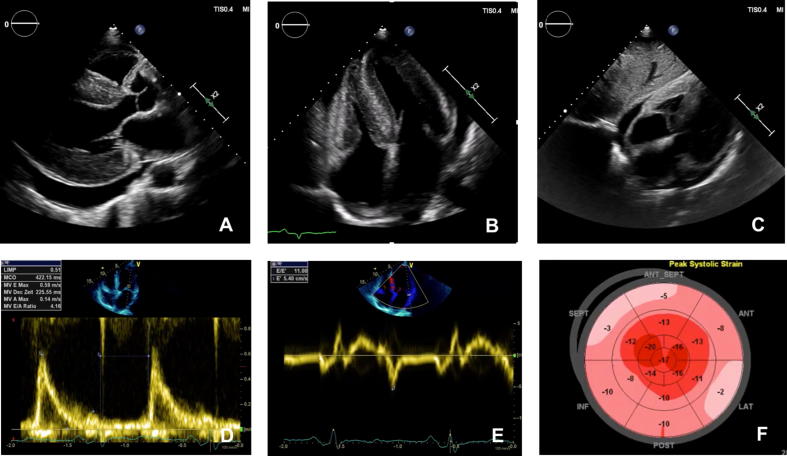
Transthoracic echocardiography is essential for the initial evaluation of patients with suspected CA. Although subtle abnormalities at early stages are non-specific, some findings are highly suggestive of CA in the appropriate clinical context. LV hypertrophy without dilation is an almost ubiquitous finding, whereas outflow tract obstruction is very rare. Increased LV wall thickness of ≥12 mm was reported to be an early sign of cardiac amyloidosis and therefore caution should be taken when examining patients with possible amyloidosis in order not to miss the diagnosis before the establishment of significant LV hypertrophy [54]. Right ventricular hypertrophy, thickening of cardiac valves and interatrial septum, granular appearance of the myocardium and pericardial effusions are common (Fig. 1A-C). The echocardiographic hallmark of CA is LV diastolic dysfunction (Fig. 1D-E), which gradually progresses in restrictive filling with consecutive biatrial dilation and often secondary insufficiency of the atrioventricular valves. LV systolic function is almost invariably abnormal despite preservation of a normal or mildly reduced ejection fraction until late disease stages. Global longitudinal strain of the LV is profoundly reduced with relative sparing of the apical region, giving a characteristic appearance in the bull’s eye plot, called “cherry on the top” or “cupcake” appearance (Fig. 1F). The latter has a high sensitivity (93%) and specificity (82%) for CA and helps differentiate between various phenocopies of LV hypertrophy such as hypertensive heart disease, hypertrophic cardiomyopathy and storage diseases [55], [56], [57], [58]. Longitudinal systolic and diastolic deformation parameters derived from speckle tracking imaging and doppler-derived left ventricular (LV) Tei index were shown to predict mortality of patients with CA [59]. ATTR-CA has been associated with more prominent left ventricular hypertrophy than AL-CA but significant overlap has been shown so that the echocardiographic features of ATTR- and AL-CA are indistinguishable. In our experience, echocardiography combined with a careful physical examination and history taking reveals sufficient clues and red flags for CA. The most relevant for screening patients at risk in every-day practice are summarized in Table 1.
Fig. 1.
Echocardiographic presentation of patients with cardiac amyloidosis: Parasternal long axis, apical four chamber and subcostal view from a patient with ATTRv-CA Marked left and right ventricular hypertrophy with speckled myocardial appearance are present, as well as pericardial effusion. (A-C). Pulsed wave doppler of the mitral valve inflow reveals restrictive filling pattern with marked reduction in mitral annular early diastolic velocity (e′ 5.4 cm/sec) in a patient with ATTRwt-CA (D, E). F: Global longitudinal strain of the patient with ATTRwt-CA is significantly reduced (−10.4%) with characteristic sparing of the strain values in apical segments (F).
Table 1.
Common clinical and echocardiographic “red flags” of cardiac amyloidosis.
| Clinical |
|---|
| History of spontaneous (biceps) tendon rupture |
| History of bilateral carpal tunnel syndrome |
| Peripheral polyneuropathy (tingling, numbness, pain, loss of temperature discrimination) |
| Autonomic neuropathy (orthostatic hypotension, erectile dysfunction, diarrhea/constipation) |
| Low-flow, low-gradient aortic valve stenosis |
| Mild troponin elevation on repeated occasions |
| Elevated serum free light chains and free light chain ratio, monoclonal gammopathy in serum and urine, Bence-Jones proteins in the urine |
| Macroglossia, periorbital purpura |
| Proteinuria, nephrotic syndrome |
| Echocardiographic |
| LV wall thickness ≥ 12 mm and heart failure in men ≥ 60 y/ women > 70 y |
| LV wall thickness ≥ 12 mm and AV-Block or pacemaker |
| LV wall thickness ≥ 12 mm and diastolic dysfunction |
| LV wall thickness ≥ 12 mm and reduced GLS with apical sparing |
| Infiltrative phenotype (biventricular hypertrophy, thickening of cardiac valves, pericardial effusion, thickening of the interatrial septum) |
4.4. Magnetic resonance imaging (MRI)
Cardiac MRI with a combination of native and contrast enhanced imaging provides accurate anatomical and functional assessment of the myocardium as well as tissue-characterization, which is of incremental value in the evaluation of cardiomyopathies. CA has a characteristic appearance of diffuse, subendocardial or transmural late gadolinium enhancement (LGE) of non-ischemic pattern, which is of prognostic value [60]. T1 mapping is a newer technique that provides quantitative analysis of myocardial relaxation time. Native T1 increases with amyloid infiltration and correlates with markers of systolic and diastolic dysfunction [61]. It is an early disease marker with high diagnostic accuracy for CA in case of a high pretest probability [62]. As it does not require contrast administration, it is of interest for patients with severely impaired renal function, in whom gadolinium-based contrast agents are contraindicated. Extracellular volume (ECV) measurement using gadolinium-based contrast agent is an ancillary method for the assessment of myocardial amyloid burden, however it requires the application of contrast media. ECV is globally elevated in CA, often with values >40% and higher in ATTR- than AL-CA. ECV elevation may be detected early before the development of LV hypertrophy, LGE or elevation in serum biomarkers [63]. Like echocardiography, cardiac MRI cannot differentiate between AL- and ATTR-CA. Currently, cardiac MRI is complementary to echocardiography rather than routine application but has great potential for prognosis and serial assessment of response to treatment [64].
4.5. Bone-tracer scintigraphy
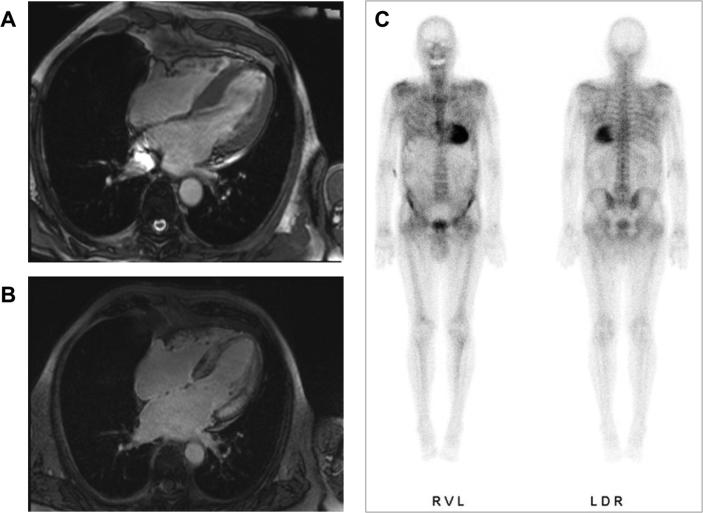
Cardiac uptake of 99mTc-phosphate derivatives was first demonstrated in the 1980s as an accidental finding in patients with ATTR-CA undergoing scintigraphy for metastatic bone disease. In 2005 a small study demonstrated the diagnostic value of 99mTc-3,3-diphosphono-1,2-propanodicarboxylic acid (99mTc-DPD) for ATTR-CA [65]. Since then, several studies reported on the high sensitivity of bone-tracer scintigraphy for ATTR-CA with only mild or absent tracer uptake in AL-CA, attributed possibly to higher calcium content in ATTR amyloid deposits [66], [67], [68]. These results were recently validated in a large-scale multicenter trial, which showed high diagnostic accuracy of 99mTc-phosphate scintigraphy for non-invasive detection of ATTR-CA [41]. In summary, a scan demonstrating grade 2 or 3 uptake according to the visual Perugini scale combined with negative serum and urine analysis for elevated free light chains and monoclonal gammopathy demonstrated 99% sensitivity, 100% positive predictive value and 100% specificity for ATTR-CA. The technique met wide clinical adoption following the publication of the study and is now integrated into the recommended diagnostic algorithm for CA. Fig. 2 demonstrates the typical finding of 99mTc-DPD scintigraphy in a patient with ATTR-CA.
Fig. 2.
Cardiac MRI depicting concentric LV hypertrophy and diffuse transmural LGE in a patient with ATTRwt-CA. (A, B). 99mTc-DPD planar scintigraphy with grade 3 myocardial tracer uptake (C).
Despite this paradigm shift, bone-tracer scintigraphy does not provide quantitative analysis of tracer uptake and the diagnostic yield of scintigraphy at an early disease stage of ATTR-CA has not been sufficiently studied. False-negative scans have been regarded as extremely rare but a recently published study reported very low sensitivity (10%) of 99mTc-DPD scintigraphy in 55 patients with ATTRv-CA carrying the Phe64Leu TTR mutation [69]. This finding needs confirmation in further studies, as it is of great importance for the subset of patients with ATTRv.
4.6. Positron-emission tomography (PET)
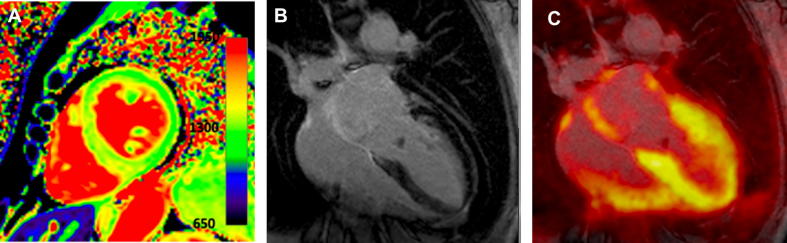
Targeted molecular imaging of amyloid deposits in the heart using 18F- and 11C-labelled PET radiotracers with amyloid-specific affinity has been shown feasible for both AL and ATTR amyloidosis. These are benzothiazole and stilbene derivatives with very similar chemical structure and already received market approval in 2012 for β-amyloid brain imaging to differentiate Alzheimer’s disease from other dementias. Following the fundamental work from Dorbala et al [70], a series of investigations revealed a very high accuracy of 18F- florbetapir, 18F- florbetapen and 11C-Pittsburgh compound B for the detection of amyloid in the heart [71], [72], [73], [74], [75], [76]. Notably, AL patients exhibited higher myocardial tracer activity than ATTR, indicating a different binding mechanism and distinct biologic properties of ATTR and AL amyloid. All studies included small study samples, however, according to a meta-analysis of six studies including 98 patients, 95% sensitivity and 98% specificity were calculated [77]. Furthermore, whole-body 18F-Florbetapir PET/CT demonstrated high accuracy for the detection of subclinical organ involvement in AL amyloidosis, which is of paramount clinical importance considering the devastating nature of the disease [78], [79]. Fig. 3 demonstrates integrated 18F-Flutemetamol PET/MRI in a patient with ATTRv-CA. PET imaging benefits from a superior spatial resolution compared to single photon emission computed tomography (SPECT), provides quantitative analysis of tracer uptake and has the potential to accurately quantify amyloid burden, facilitate prognosis and assess disease progression and response to therapy. These issues are of special interest for future research and may improve our understanding of the underlying molecular mechanisms of amyloidosis.
Fig. 3.
Integrated 18F-Flutemetamol PET/MRI in a patient with ATTRv-CA. The patient had known ATTRv with polyneuropathy under treatment with tafamidis and presented with new onset dyspnea. T1 mapping revealed prolongation of relaxation time (A). Subendocardial late gadolinium enhancement was present (B). PET images showed 18F-Flutemetamol uptake of the left and right ventricle, indicative of advanced stage myocardial amyloid infiltration (C).
4.7. Endomyocardial and tissue biopsy
As presented above, EMB can be omitted in case of unequivocal positive scintigraphy for ATTR-CA (grade 2 or 3 cardiac uptake) without laboratory evidence of plasma cell proliferation or clinical suspicion of AL amyloidosis. These findings are diagnostic for ATTR-CA [41]. Histological confirmation of amyloid in one of the involved organs or a surrogate site (e.g. abdominal fat, bone marrow, rectum, salivary glands) is needed for the diagnosis of systemic AL amyloidosis. The presence of typical cardiac MRI findings in a patient with already established diagnosis of AL amyloidosis via histologic confirmation of amyloid in extracardiac organs should also be considered diagnostic for AL-CA. In such cases, endomyocardial biopsy is not necessary and treatment should not be delayed in order to obtain cardiac biopsies. If extracardiac biopsies are not feasible or non-diagnostic, EMB is the method of choice to diagnose AL-CA.
Since the integration of bone tracer scintigraphy in the diagnostic pathway of CA, the need for EMB is reduced and should be abolished in case of Perugini-grade 2 or 3 myocardial tracer uptake in bone-tracer scintigraphy and no laboratory evidence of monoclonal gammopathy in serum and urine or abnormal free light chain ratio or clinical suspicion of AL-CA. Likewise, biopsies of abdominal fat or gingiva, rectum, upper gastrointestinal tract and salivary glands are now less frequently performed for ATTR amyloidosis but are still of value in patients with suspected AL amyloidosis due to their ease, convenience and high yield. The yield of a fat pad biopsy in AL amyloidosis is >70% but much lower in ATTRv (45–67%) and ATTRwt (15%) and thus, a negative result is insufficient to exclude diagnosis of AL and ATTR [80], [81].
4.8. Histopathological examination
The presence of amyloid fibrils can be confirmed by their characteristic appearance on electron microscopy and their ability to bind Congo red, leading to the characteristic apple-green birefringence under polarized light, or thioflavin-T producing an intense yellow-green fluorescence [82]. Once histologic diagnosis of amyloid is made, the type of amyloid must be identified. Light microscopy immunohistochemistry with the use of commercial antibodies lacks specificity and caution is warranted because of the observed rates of false positive transthyretin-antibody staining of amyloid deposits in patients with AL amylodosis and a low-sensitivity for light chain staining, which may be misleading and result to malpractice [83]. The cause of the strong nonspecific transthyretin staining is not known. Especially a strong and diffuse transthyretin staining needs confirmation with other methodology. This limitation can be overcome when amyloid typing is performed with custom-made antibodies at specialized centers [84]. Immunoelectron microscopy can achieve 100% specificity and can correctly classify more than 99% of patients with systemic amyloidosis [85]. Mass spectrometry-based proteomics can overcome those limitations greatly improving the diagnostic accuracy, as the method is antibody-independent [86]. Mass spectrometry diagnostics can be performed after laser capture microdissection of Congo red positive areas from slides obtained from paraffin-embedded tissue or on protein extracted from the whole sample [86], [87]. This method is not widely available, and in case of discrepancies in clinical and pathologic diagnoses, tissue samples need to be sent to referral centers for such testing in order to convincingly rule out AL amyloidosis.
4.9. Genetic testing
TTR gene sequencing of the exons 1–4 and genetic counseling are recommended in all patients with ATTR amyloidosis. Due to incomplete or late penetrance as well as reports of ATTRv even in older individuals >80 years, differentiation between the hereditary and wild type form of the disease is not feasible based only on family history or clinical criteria. Genetic counseling and testing should also be offered in family members, especially if they become symptomatic. Since gene silencing therapies are currently approved only for ATTRv, it is important to offer genetic testing in all patients diagnosed with ATTR. Furthermore, detection of a pathogenic gene variant enables cascade screening and early diagnosis of relatives.
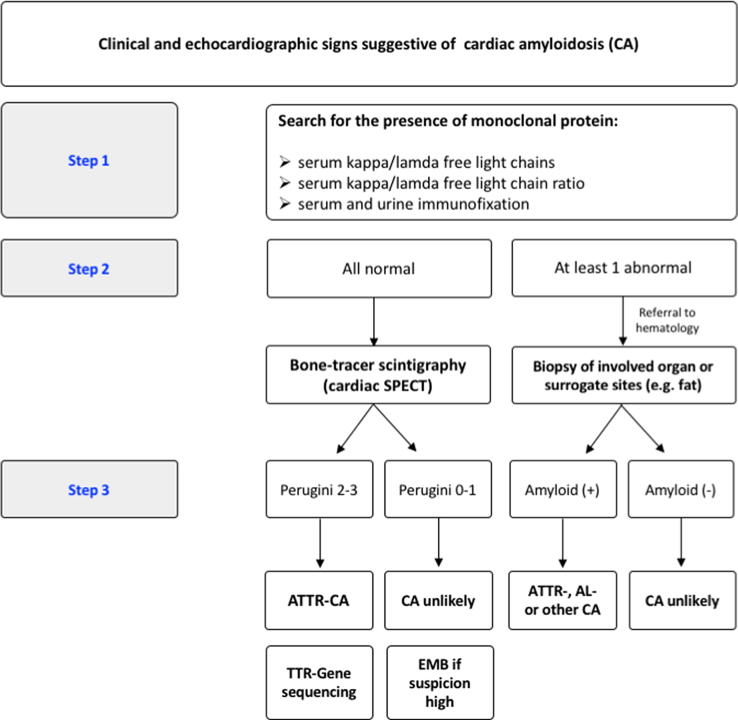
The accumulating evidence on the different diagnostic modalities has led to the widespread adoption of standardized diagnostic algorithms for suspected CA. A common approach is presented in Fig. 4. Overall, amyloidosis remains a multifaceted systemic disease with enigmatic pathophysiology. In our experience, a systematic approach to diagnosis and management of this growing patient population warrants a close collaboration of experienced specialists in the context of interdisciplinary amyloidosis programs. In our point of view, cardiology, hematology, neurology and nuclear medicine constitute the fundamental core of amyloidosis programs.
Fig. 4.
Proposed algorithm for the diagnostic evaluation of patients with suspected cardiac amyloidosis.
5. Future outlook
There is an ongoing need to raise awareness for CA, especially of the ATTR type which is still underrecognized out of specialized clinics and academic centers. For this purpose, it is essential to identify cost-effective and straightforward tools and algorithms for large-scale screening of populations at risk. Multimodality imaging is anticipated to play a pivotal role in all aspects of CA: screening of individuals at risk, early and accurate diagnosis, prognostication and assessment of response to treatment. As no single imaging method was proven perfect so far, complementary use of the available modalities is currently adopted but further refinements are currently on the spotlight of research and hold promise for precision diagnostics in the near future.
6. Conclusion
CA is a devastating disease with an underestimated prevalence and high mortality. Early diagnosis is impeded by suboptimal awareness of the disease and the non-specific clinical presentation. Increased LV wall thickness in males >65 years or females >70 years in addition to signs and symptoms of HF or other red flags (e.g. carpal tunnel syndrome or peripheral neuropathy) should trigger evaluation for CA [88]. Repurposed bone scintigraphy and laboratory testing may exclude or confirm the diagnosis in most cases of suspected ATTR-CA, whereas tissue biopsy for histopathologic examination is still the test of choice for AL amyloidosis and certain clinical scenarios. Myocardial imaging is steadily evolving and will possibly expand its applications in the near future in key aspects of diagnosis, risk assessment and response to treatment. Due to the systemic nature of the disease collaboration of the caring specialists in the context of interdisciplinary amyloidosis programs is essential for accurate diagnosis and optimal long-term care.
Funding
This work was supported by the German Research Foundation (Deutsche Forschungsgesellschaft, DFG) grants to PL (LU2139/2-1) and MP (Clinician Scientist Program UMEA of the University Duisburg-Essen).
Declaration of Competing Interest
The authors have no conflicts of interest.
References
- 1.Benson M.D., Buxbaum J.N., Eisenberg D.S. Amyloid nomenclature 2018: recommendations by the International Society of Amyloidosis (ISA) nomenclature committee. Amyloid. 2018;25(4):215–219. doi: 10.1080/13506129.2018.1549825. [DOI] [PubMed] [Google Scholar]
- 2.Falk R.H., Alexander K.M., Liao R., Dorbala S. AL (Light-Chain) cardiac amyloidosis: a review of diagnosis and therapy. J. Am. Coll. Cardiol. 2016 doi: 10.1016/j.jacc.2016.06.053. [DOI] [PubMed] [Google Scholar]
- 3.Grogan M., Scott C.G., Kyle R.A. Natural history of wild-type transthyretin cardiac amyloidosis and risk stratification using a novel staging system. J. Am. Coll. Cardiol. 2016;68(10):1014–1020. doi: 10.1016/J.JACC.2016.06.033. [DOI] [PubMed] [Google Scholar]
- 4.Wechalekar A.D., Gillmore J.D., Hawkins P.N. Systemic amyloidosis. Lancet. 2016;387(10038):2641–2654. doi: 10.1016/S0140-6736(15)01274-X. [DOI] [PubMed] [Google Scholar]
- 5.Ando Y., Coelho T., Berk J.L. Guideline of transthyretin-related hereditary amyloidosis for clinicians. Orphanet. J. Rare Dis. 2013;8(1):31. doi: 10.1186/1750-1172-8-31. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.T. Damy, A.V. Kristen, O.B. Suhr et al., Transthyretin cardiac amyloidosis in continental Western Europe: an insight through the Transthyretin Amyloidosis Outcomes Survey (THAOS). doi: 10.1093/eurheartj/ehz173. [DOI] [PMC free article] [PubMed]
- 7.Jacobson D.R., Alexander A.A., Tagoe C., Buxbaum J.N. Prevalence of the amyloidogenic transthyretin (TTR) V122I allele in 14 333 African-Americans. Amyloid. 2015;22(3):171–174. doi: 10.3109/13506129.2015.1051219. [DOI] [PubMed] [Google Scholar]
- 8.Maurer M.S., Hanna M., Grogan M. Genotype and phenotype of transthyretin cardiac amyloidosis. J. Am. Coll. Cardiol. 2016;68(2):161–172. doi: 10.1016/j.jacc.2016.03.596. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Suhr O.B., Svendsen I.H., Andersson R., Danielsson A., Holmgren G., Ranlov P.J. Hereditary transthyretin amyloidosis from a Scandinavian perspective. J. Intern. Med. 2003;254(3):225–235. doi: 10.1046/j.1365-2796.2003.01173.x. [DOI] [PubMed] [Google Scholar]
- 10.Reilly M.M., Staunton H., Harding A.E. Familial amyloid polyneuropathy (TTR ala 60) in north west Ireland: a clinical, genetic, and epidemiological study. J. Neurol. Neurosurg. Psychiatry. 1995;59(1):45–49. doi: 10.1136/jnnp.59.1.45. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Rapezzi C., Quarta C.C., Obici L. Disease profile and differential diagnosis of hereditary transthyretin-related amyloidosis with exclusively cardiac phenotype: an Italian perspective. Eur. Heart J. 2013;34(7):520–528. doi: 10.1093/eurheartj/ehs123. [DOI] [PubMed] [Google Scholar]
- 12.Gagliardi C., Perfetto F., Lorenzini M. Phenotypic profile of Ile68Leu transthyretin amyloidosis: an underdiagnosed cause of heart failure. Eur. J. Heart Fail. 2018;20(10):1417–1425. doi: 10.1002/ejhf.1285. [DOI] [PubMed] [Google Scholar]
- 13.Tanskanen M., Peuralinna T., Polvikoski T. Senile systemic amyloidosis affects 25% of the very aged and associates with genetic variation in alpha2-macroglobulin and tau: A population-based autopsy study. Ann. Med. 2008 doi: 10.1080/07853890701842988. [DOI] [PubMed] [Google Scholar]
- 14.Mohammed S.F., Mirzoyev S.A., Edwards W.D. Left ventricular amyloid deposition inpatientswith heart failure and preserved ejection fraction. JACC Hear Fail. 2014;2(2):113–122. doi: 10.1016/j.jchf.2013.11.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.González-López E., Gallego-Delgado M., Guzzo-Merello G. Wild-type transthyretin amyloidosis as a cause of heart failure with preserved ejection fraction. Eur. Heart J. 2015;36(38):2585–2594. doi: 10.1093/eurheartj/ehv338. [DOI] [PubMed] [Google Scholar]
- 16.Treibel T.A., Fontana M., Gilbertson J.A. Occult transthyretin cardiac amyloid in severe calcific aortic stenosis. Circ Cardiovasc Imaging. 2016;9(8):1–10. doi: 10.1161/CIRCIMAGING.116.005066. [DOI] [PubMed] [Google Scholar]
- 17.Castano A., Narotsky D.L., Hamid N. Unveiling transthyretin cardiac amyloidosis and its predictors among elderly patients with severe aortic stenosis undergoing transcatheter aortic valve replacement. Eur. Heart J. 2017;38(38):2879–2887. doi: 10.1093/eurheartj/ehx350. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Longhi S., Lorenzini M., Gagliardi C. Coexistence of degenerative aortic stenosis and wild-type transthyretin-related cardiac amyloidosis. JACC Cardiovasc. Imaging. 2016;9(3):325–327. doi: 10.1016/j.jcmg.2015.04.012. [DOI] [PubMed] [Google Scholar]
- 19.Cavalcante J.L., Rijal S., Abdelkarim I. Cardiac amyloidosis is prevalent in older patients with aortic stenosis and carries worse prognosis. J. Cardiovasc. Magn. Reson. 2017;19(1):98. doi: 10.1186/s12968-017-0415-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Damy T., Costes B., Hagège A.A. Prevalence and clinical phenotype of hereditary transthyretin amyloid cardiomyopathy in patients with increased left ventricular wall thickness. Eur. Heart J. 2016;37(23):1826–1834. doi: 10.1093/eurheartj/ehv583. [DOI] [PubMed] [Google Scholar]
- 21.González-López E., Gagliardi C., Dominguez F. Clinical characteristics of wild-type transthyretin cardiac amyloidosis: disproving myths. Eur. Heart J. 2017;38(24):1895–1904. doi: 10.1093/eurheartj/ehx043. [DOI] [PubMed] [Google Scholar]
- 22.R.A. Kyle, A. Linos, C.M. Beard et al., Incidence and natural history of primary systemic amyloidosis in Olmsted County, Minnesota, 1950 through 1989, Blood, 1992. doi: 10.1182/blood.v79.7.1817.bloodjournal7971817. [PubMed]
- 23.Pinney J.H., Smith C.J., Taube J.B. Systemic Amyloidosis in England: an epidemiological study. Br. J. Haematol. 2013;161(4):525–532. doi: 10.1111/bjh.12286. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Muchtar E., Buadi F.K., Dispenzieri A., Gertz M.A. Immunoglobulin light-chain amyloidosis: from basics to new developments in diagnosis, prognosis and therapy. Acta Haematol. 2016;135(3):172–190. doi: 10.1159/000443200. [DOI] [PubMed] [Google Scholar]
- 25.Hemminki K., Li X., Försti A., Sundquist J., Sundquist K. Incidence and survival in non-hereditary amyloidosis in Sweden. BMC Public Health. 2012;12(1):974. doi: 10.1186/1471-2458-12-974. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Girnius S., Dember L., Doros G., Skinner M. The changing face of AA amyloidosis: a single center experience. Amyloid. 2011;18(sup1):226–228. doi: 10.3109/13506129.2011.574354085. [DOI] [PubMed] [Google Scholar]
- 27.Okuda Y., Yamada T., Ueda M., Ando Y. First nationwide survey of 199 patients with amyloid A amyloidosis in Japan. Intern. Med. 2018;57(23):3351–3355. doi: 10.2169/internalmedicine.1099-18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Arciello A., Piccoli R., Monti D.M. Apolipoprotein A-I: the dual face of a protein. FEBS Lett. 2016;590(23):4171–4179. doi: 10.1002/1873-3468.12468. [DOI] [PubMed] [Google Scholar]
- 29.Eriksson M., Schönland S., Yumlu S. Hereditary apolipoprotein AI-associated amyloidosis in surgical pathology specimens: identification of three novel mutations in the APOA1 gene. J. Mol. Diagn. 2009;11(3):257–262. doi: 10.2353/jmoldx.2009.080161. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Rowczenio D., Dogan A., Theis J.D. Amyloidogenicity and clinical phenotype associated with five novel mutations in apolipoprotein A-I. Am. J. Pathol. 2011;179(4):1978–1987. doi: 10.1016/j.ajpath.2011.06.024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Mathew V., Olson L.J., Gertz M.A., Hayes D.L. Symptomatic conduction system disease in cardiac amyloidosis. Am. J. Cardiol. 1997;80(11):1491–1492. doi: 10.1016/S0002-9149(97)82785-3. [DOI] [PubMed] [Google Scholar]
- 32.Longhi S., Quarta C.C., Milandri A. Atrial fibrillation in amyloidotic cardiomyopathy: prevalence, incidence, risk factors and prognostic role. Amyloid. 2015;22(3):147–155. doi: 10.3109/13506129.2015.1028616. [DOI] [PubMed] [Google Scholar]
- 33.Martinez-Naharro A., Gonzalez-Lopez E., Corovic A. High prevalence of intracardiac thrombi in cardiac amyloidosis. J. Am. Coll. Cardiol. 2019;73(13):1733–1734. doi: 10.1016/j.jacc.2019.01.035. [DOI] [PubMed] [Google Scholar]
- 34.Barbhaiya C.R., Kumar S., Baldinger S.H. Electrophysiologic assessment of conduction abnormalities and atrial arrhythmias associated with amyloid cardiomyopathy. Hear Rhythm. 2016;13(2):383–390. doi: 10.1016/j.hrthm.2015.09.016. [DOI] [PubMed] [Google Scholar]
- 35.Maurer M.S., Elliott P., Comenzo R., Semigran M., Rapezzi C. Addressing common questions encountered in the diagnosis and management of cardiac amyloidosis. Circulation. 2017;135(14):1357–1377. doi: 10.1161/CIRCULATIONAHA.116.024438. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Geller H.I., Singh A., Alexander K.M., Mirto T.M., Falk R.H. Association between ruptured distal biceps tendon and wild-type transthyretin cardiac amyloidosis. JAMA. 2017;318(10):962. doi: 10.1001/jama.2017.9236. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Lane T., Fontana M., Martinez-Naharro A. Natural history, quality of life, and outcome in cardiac transthyretin amyloidosis. Circulation. 2019;140(1):16–26. doi: 10.1161/CIRCULATIONAHA.118.038169. [DOI] [PubMed] [Google Scholar]
- 38.Liao R., Jain M., Teller P. Infusion of light chains from patients with cardiac amyloidosis causes diastolic dysfunction in isolated mouse hearts. Circulation. 2001;104(14):1594–1597. doi: 10.1161/circ.104.14.1594. [DOI] [PubMed] [Google Scholar]
- 39.Brenner D.A., Jain M., Pimentel D.R. Human amyloidogenic light chains directly impair cardiomyocyte function through an increase in cellular oxidant stress. Circ. Res. 2004;94(8):1008–1010. doi: 10.1161/01.RES.0000126569.75419.74. [DOI] [PubMed] [Google Scholar]
- 40.Shi J., Guan J., Jiang B. Amyloidogenic light chains induce cardiomyocyte contractile dysfunction and apoptosis via a non-canonical p38alpha MAPK pathway. Proc. Natl. Acad. Sci. USA. 2010;107(9):4188–4193. doi: 10.1073/pnas.0912263107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Gillmore J.D., Maurer M.S., Falk R.H. Nonbiopsy diagnosis of cardiac transthyretin amyloidosis. Circulation. 2016;133(24):2404–2412. doi: 10.1161/CIRCULATIONAHA.116.021612. [DOI] [PubMed] [Google Scholar]
- 42.Quarta C.C., Solomon S.D., Uraizee I. Left ventricular structure and function in transthyretin-related versus light-chain cardiac amyloidosis. Circulation. 2014;129(18):1840–1849. doi: 10.1161/CIRCULATIONAHA.113.006242. [DOI] [PubMed] [Google Scholar]
- 43.Van Den Berg M.P., Mulder B.A., Klaassen S.H.C. Heart failure with preserved ejection fraction, atrial fibrillation, and the role of senile amyloidosis. Eur. Heart J. 2019;40(16):1287–1293. doi: 10.1093/eurheartj/ehz057. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Tan N.Y., Mohsin Y., Hodge D.O. Catheter ablation for atrial arrhythmias in patients with cardiac amyloidosis. J. Cardiovasc. Electrophysiol. 2016;27(10):1167–1173. doi: 10.1111/jce.13046. [DOI] [PubMed] [Google Scholar]
- 45.Palladini G., Russo P., Bosoni T. Identification of amyloidogenic light chains requires the combination of serum-free light chain assay with immunofixation of serum and urine. Clin. Chem. 2009;55(3):499–504. doi: 10.1373/clinchem.2008.117143. [DOI] [PubMed] [Google Scholar]
- 46.Palladini G., Jaccard A., Milani P. Circulating free light chain measurement in the diagnosis, prognostic assessment and evaluation of response of AL amyloidosis: comparison of Freelite and N latex FLC assays. Clin. Chem. Lab. Med. 2017;55(11):1734–1743. doi: 10.1515/cclm-2016-1024. [DOI] [PubMed] [Google Scholar]
- 47.Dispenzieri A., Katzmann J.A., Kyle R.A. Prevalence and risk of progression of light-chain monoclonal gammopathy of undetermined significance: a retrospective population-based cohort study. Lancet. 2010;375(9727):1721–1728. doi: 10.1016/S0140-6736(10)60482-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Hutchison C.A., Harding S., Hewins P. Quantitative assessment of serum and urinary polyclonal free light chains in patients with chronic kidney disease. Clin. J. Am. Soc. Nephrol. 2008;3(6):1684–1690. doi: 10.2215/CJN.02290508. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Perfetto F., Bergesio F., Grifoni E. Different NT-proBNP circulating levels for different types of cardiac amyloidosis. J. Cardiovasc. Med. (Hagerstown) 2016;17(11):810–817. doi: 10.2459/JCM.0000000000000349. [DOI] [PubMed] [Google Scholar]
- 50.van de Donk N.W.C.J., Palumbo A., Johnsen H.E. The clinical relevance and management of monoclonal gammopathy of undetermined significance and related disorders: recommendations from the European Myeloma Network. Haematologica. 2014;99(6):984–996. doi: 10.3324/haematol.2013.100552. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Bianchi G., Kyle R.A., Colby C.L. Impact of optimal follow-up of monoclonal gammopathy of undetermined significance on early diagnosis and prevention of myeloma-related complications. Blood. 2010;116(12):2019–2025. doi: 10.1182/blood-2010-04-277566. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Kumar S., Dispenzieri A., Lacy M.Q. Revised prognostic staging system for light chain amyloidosis incorporating cardiac biomarkers and serum free light chain measurements. J. Clin. Oncol. 2012;30(9):989–995. doi: 10.1200/JCO.2011.38.5724. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Gillmore J.D., Damy T., Fontana M. A new staging system for cardiac transthyretin amyloidosis. Eur. Heart J. 2018;39(30):2799–2806. doi: 10.1093/eurheartj/ehx589. [DOI] [PubMed] [Google Scholar]
- 54.D. Di Nunzio, A. Recupero, C. de Gregorio, C. Zito, S. Carerj, G. Di Bella, Echocardiographic Findings in Cardiac Amyloidosis: Inside Two-Dimensional, Doppler, and Strain Imaging, 2019. doi: 10.1007/s11886-019-1094-z. [DOI] [PubMed]
- 55.Baccouche H., Maunz M., Beck T. Differentiating cardiac amyloidosis and hypertrophic cardiomyopathy by use of three-dimensional speckle tracking echocardiography. Echocardiography. 2012;29(6):668–677. doi: 10.1111/j.1540-8175.2012.01680.x. [DOI] [PubMed] [Google Scholar]
- 56.Koyama J., Ikeda S., Ikeda U. Echocardiographic assessment of the cardiac amyloidoses. Circ. J. 2015;79(4):721–734. doi: 10.1253/circj.CJ-14-1425. [DOI] [PubMed] [Google Scholar]
- 57.Phelan D., Collier P., Thavendiranathan P. Relative apical sparing of longitudinal strain using two-dimensional speckle-tracking echocardiography is both sensitive and specific for the diagnosis of cardiac amyloidosis. Heart. 2012;98(19):1442–1448. doi: 10.1136/heartjnl-2012-302353. [DOI] [PubMed] [Google Scholar]
- 58.Phelan D., Thavendiranathan P., Popovic Z. Application of a parametric display of two-dimensional speckle-tracking longitudinal strain to improve the etiologic diagnosis of mild to moderate left ventricular hypertrophy. J. Am. Soc. Echocardiogr. 2014;27(8):888–895. doi: 10.1016/j.echo.2014.04.015. [DOI] [PubMed] [Google Scholar]
- 59.Liu D., Hu K., Herrmann S. Value of tissue Doppler-derived Tei index and two-dimensional speckle tracking imaging derived longitudinal strain on predicting outcome of patients with light-chain cardiac amyloidosis. 2017;33:837–845. doi: 10.1007/s10554-017-1075-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Fontana M., Pica S., Reant P. Prognostic value of late gadolinium enhancement cardiovascular magnetic resonance in cardiac amyloidosis. Circulation. 2015;132(16):1570–1579. doi: 10.1161/CIRCULATIONAHA.115.016567. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Karamitsos T.D., Piechnik S.K., Banypersad S.M. Noncontrast T1 mapping for the diagnosis of cardiac amyloidosis. JACC Cardiovasc Imaging. 2013;6(4):488–497. doi: 10.1016/j.jcmg.2012.11.013. [DOI] [PubMed] [Google Scholar]
- 62.Fontana M., Banypersad S.M., Treibel T.A. Native T1 mapping in transthyretin amyloidosis. JACC Cardiovasc Imaging. 2014;7(2):157–165. doi: 10.1016/j.jcmg.2013.10.008. [DOI] [PubMed] [Google Scholar]
- 63.Banypersad S.M., Sado D.M., Flett A.S. Quantification of myocardial extracellular volume fraction in systemic AL amyloidosis: an equilibrium contrast cardiovascular magnetic resonance study. Circ. Cardiovasc. Imaging. 2013;6(1):34–39. doi: 10.1161/CIRCIMAGING.112.978627. [DOI] [PubMed] [Google Scholar]
- 64.Martinez-Naharro A., Treibel T.A., Abdel-Gadir A. Magnetic resonance in transthyretin cardiac amyloidosis. J. Am. Coll. Cardiol. 2017;70(4):466–477. doi: 10.1016/j.jacc.2017.05.053. [DOI] [PubMed] [Google Scholar]
- 65.Perugini E., Guidalotti P.L., Salvi F. Noninvasive etiologic diagnosis of cardiac amyloidosis using 99m Tc-3,3-diphosphono-1,2-propanodicarboxylic acid scintigraphy. J. Am. Coll. Cardiol. 2005;46(6):1076–1084. doi: 10.1016/j.jacc.2005.05.073. [DOI] [PubMed] [Google Scholar]
- 66.Rapezzi C., Guidalotti P., Salvi F., Riva L., Perugini E. Usefulness of 99mTc-DPD scintigraphy in cardiac amyloidosis. J. Am. Coll. Cardiol. 2008;51(15):1509–1510. doi: 10.1016/j.jacc.2007.12.038. [DOI] [PubMed] [Google Scholar]
- 67.Rapezzi C., Quarta C.C., Guidalotti P.L. Usefulness and limitations of 99mTc-3,3-diphosphono-1,2-propanodicarboxylic acid scintigraphy in the aetiological diagnosis of amyloidotic cardiomyopathy. Eur. J. Nucl. Med. Mol. Imaging. 2011;38(3):470–478. doi: 10.1007/s00259-010-1642-7. [DOI] [PubMed] [Google Scholar]
- 68.Castano A., Haq M., Narotsky D.L. Multicenter study of planar technetium 99m pyrophosphate cardiac imaging. JAMA Cardiol. 2016;1(8):880. doi: 10.1001/jamacardio.2016.2839. [DOI] [PubMed] [Google Scholar]
- 69.Musumeci M.B., Cappelli F., Russo D. Low sensitivity of bone scintigraphy in detecting Phe64Leu mutation-related transthyretin cardiac amyloidosis. JACC Cardiovasc. Imaging. December 2019 doi: 10.1016/j.jcmg.2019.10.015. [DOI] [PubMed] [Google Scholar]
- 70.Dorbala S., Vangala D., Semer J. Imaging cardiac amyloidosis: A pilot study using 18 F-florbetapir positron emission tomography. Eur. J. Nucl. Med. Mol. Imaging. 2014;41(9):1652–1662. doi: 10.1007/s00259-014-2787-6. [DOI] [PubMed] [Google Scholar]
- 71.Osborne D.R., Acuff S.N., Stuckey A., Wall J.S. A routine PET/CT protocol with streamlined calculations for assessing cardiac amyloidosis using 18F-florbetapir. Front. Cardiovasc. Med. 2015:2. doi: 10.3389/fcvm.2015.00023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Lee S.P., Lee E.S., Choi H. 11C-Pittsburgh B PET imaging in cardiac amyloidosis. JACC Cardiovasc. Imaging. 2015;8(1):50–59. doi: 10.1016/j.jcmg.2014.09.018. [DOI] [PubMed] [Google Scholar]
- 73.Law W.P., Wang W.Y.S., Moore P.T., Mollee P.N., Ng A.C.T. Cardiac amyloid imaging with 18F-florbetaben PET: a pilot study. J. Nucl. Med. 2016;57(11):1733–1739. doi: 10.2967/jnumed.115.169870. [DOI] [PubMed] [Google Scholar]
- 74.Pilebro B., Arvidsson S., Lindqvist P. Positron emission tomography (PET) utilizing Pittsburgh compound B (PIB) for detection of amyloid heart deposits in hereditary transthyretin amyloidosis (ATTR) J Nucl Cardiol. 2018;25(1):240–248. doi: 10.1007/s12350-016-0638-5. [DOI] [PubMed] [Google Scholar]
- 75.Ezawa N., Katoh N., Oguchi K., Yoshinaga T., Yazaki M., Sekijima Y. Visualization of multiple organ amyloid involvement in systemic amyloidosis using 11C-PiB PET imaging. Eur. J. Nucl. Med. Mol. Imaging. 2018;45(3):452–461. doi: 10.1007/s00259-017-3814-1. [DOI] [PubMed] [Google Scholar]
- 76.Dietemann S., Nkoulou R. Amyloid PET imaging in cardiac amyloidosis: a pilot study using 18F-flutemetamol positron emission tomography. Ann. Nucl. Med. 2019 doi: 10.1007/s12149-019-01372-7. [DOI] [PubMed] [Google Scholar]
- 77.Kim Y.J., Ha S., Kim Y.il. Cardiac amyloidosis imaging with amyloid positron emission tomography: A systematic review and meta-analysis. J. Nucl. Cardiol. 2018 doi: 10.1007/s12350-018-1365-x. [DOI] [PubMed] [Google Scholar]
- 78.Ehman E.C., El-Sady M.S., Kijewski M.F. Early detection of multiorgan light-chain amyloidosis by whole-body 18 F-florbetapir PET/CT. J. Nucl. Med. 2019;60(9):1234–1239. doi: 10.2967/jnumed.118.221770. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Khor Y.M., Cuddy S., Harms H.J. Quantitative [18F]florbetapir PET/CT may identify lung involvement in patients with systemic AL amyloidosis. Eur. J. Nucl. Med. Mol. Imaging. December 2019 doi: 10.1007/s00259-019-04627-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Van Gameren II, Hazenberg B.P.C., Bijzet J., Van Rijswijk M.H. Diagnostic accuracy of subcutaneous abdominal fat tissue aspiration for detecting systemic amyloidosis and its utility in clinical practice. Arthritis Rheum. 2006;54(6):2015–2021. doi: 10.1002/art.21902. [DOI] [PubMed] [Google Scholar]
- 81.Quarta C.C., Gonzalez-Lopez E., Gilbertson J.A. Diagnostic sensitivity of abdominal fat aspiration in cardiac amyloidosis. Eur. Heart J. 2017;38(24):1905–1908. doi: 10.1093/eurheartj/ehx047. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Glenner G.G. Amyloid deposits and amyloidosis. The beta-fibrilloses (first of two parts) N. Engl. J. Med. 1980;302(23):1283–1292. doi: 10.1056/NEJM198006053022305. [DOI] [PubMed] [Google Scholar]
- 83.Satoskar A.A., Efebera Y., Hasan A. Strong tranthyretin immunostaining, potential pitfall in cardiac amyloid typing. Am. J. Surg. Pathol. 2011;35(11):1685–1690. doi: 10.1097/PAS.0b013e3182263d74. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Schönland S.O., Hegenbart U., Bochtler T. Immunohistochemistry in the classification of systemic forms of amyloidosis: a systematic investigation of 117 patients. Blood. 2012;119(2):488–493. doi: 10.1182/blood-2011-06-358507. [DOI] [PubMed] [Google Scholar]
- 85.Gilbertson J.A., Theis J.D., Vrana J.A. A comparison of immunohistochemistry and mass spectrometry for determining the amyloid fibril protein from formalin-fixed biopsy tissue. J. Clin. Pathol. 2015;68(4):314–317. doi: 10.1136/jclinpath-2014-202722. [DOI] [PubMed] [Google Scholar]
- 86.Vrana J.A., Gamez J.D., Madden B.J., Theis J.D., Bergen H.R., Dogan A. Classification of amyloidosis by laser microdissection and mass spectrometry-based proteomic analysis in clinical biopsy specimens. Blood. 2009;114(24):4957–4959. doi: 10.1182/blood-2009-07-230722. [DOI] [PubMed] [Google Scholar]
- 87.Fernández de Larrea C., Verga L., Morbini P. A practical approach to the diagnosis of systemic amyloidoses. Blood. 2015;125(14):2239–2244. doi: 10.1182/blood-2014-11-609883. [DOI] [PubMed] [Google Scholar]
- 88.Witteles R.M., Bokhari S., Damy T. Screening for transthyretin amyloid cardiomyopathy in everyday practice. JACC Hear Fail. 2019;7(8):709–716. doi: 10.1016/j.jchf.2019.04.010. [DOI] [PubMed] [Google Scholar]