The Carbohydrate-Insulin Model (CIM) of obesity posits that the high insulin-to-glucagon ratio elicited by a high-glycemic load diet (i.e., with large amounts of fast-digesting, high-glycemic index sources of carbohydrate) shifts substrate partitioning from oxidation in lean tissues to storage in adipose tissue, predisposing to a positive energy balance [1]. Several specific predictions distinguish the CIM from the standard ‘calories in, calories out’ view of obesity, wherein hyperinsulinemia arises secondary to weight gain. According to these predictions, isocaloric high vs. low-glycemic load diets (i.e., those diets that raise postprandial glycemia and insulinemia) will increase anabolic stimulation of adipose compared to muscle, thereby promoting fat storage. In response to this change in metabolic fuel distribution, energy expenditure will decrease and hunger and voluntary food intake will increase. Importantly, the CIM holds that a positive energy balance results, over the long term, from increasing adiposity – not the converse, as premised in conventional thinking. Thus, the CIM may inform a biologically based approach to target the metabolic dysfunction driving weight gain.
In support of a key CIM prediction, energy expenditure and energy intake during weight-loss maintenance were approximately 200–300 kcal/d greater between 10 and 20 weeks on low-vs high-carbohydrate diets controlled for protein [2]. However, long-term feeding studies in humans such as this have inherent limitations, including high cost, logistical challenges, and potential for nonadherence.
Animal research offers an opportunity to test the CIM largely free of these limitations, although translation to humans can be problematic. The meticulous study by Hu et al. [3] in a recent issue of Molecular Metabolism provides interesting data on macronutrients and obesity in mice. The article and section titles notwithstanding, it does not comprise a meaningful test of the CIM.
Comparing diets ranging widely in carbohydrates, fats, and proteins, Hu et al. reported no metabolic advantage of reduced carbohydrate intake. Unfortunately, the composition of the diets precludes inferences related to the CIM because of muddled effects on fasting and postprandial insulin concentrations. The CIM specifically recognizes that “high insulin levels in blood may arise from primary hypersecretion (postulated to cause weight gain) or as a compensatory response to insulin resistance” [1]. However, the low-carbohydrate diets contained predominantly saturated fat (cocoa butter, coconut oil, palm oil) and sugar (sucrose) or glucose equivalents (maltodextrin, which has a glycemic index equal to glucose [4]). Such high combined amounts of saturated fat and sugar as a proportion of total energy would rarely be consumed by humans and virtually never by rodents in nature.
In rodents, saturated fat induces insulin resistance in liver and muscle, leading to compensatory (not primary) hyperinsulinemia. High intake of saturated (but not unsaturated) fat also causes severe hypothalamic inflammation and insulin resistance [5], especially when consumed with sugar. This metabolic dysfunction – exacerbated by the additional, independent effects of sugar and maltodextrin – would drive weight gain through numerous well-described central and peripheral mechanisms.
Even as the low-carbohydrate diets were strongly biased to produce metabolic dysfunction, the high-carbohydrate diets were biased in the opposite direction, with the use of cornstarch as the leading energy source. Depending to some extent on the amylose to amylopectin ratio, raw cornstarch (the type used by the manufacturer, according to personal communication) produces a low insulin response [6], a potential reason for the minimal postprandial glycemic and insulinemic differences among the diets.
Thus, the low-carbohydrate diets elicited neuroinflammation, insulin resistance, and compensatory hyperinsulinemia, whereas the high-carbohydrate diets barely elicited primary hyperinsulinemia – a combination that fatally confounds tests of the CIM. Consider the opposite scenario. One might construct a high-carbohydrate diet primarily with sugar and saturated fat, causing obesity and metabolic dysfunction in comparison to a higher fat diet with a balanced distribution of mono- and polyunsaturated fat and low-glycemic index sources of carbohydrate. But advocates of a high-carbohydrate diet would probably not consider this a fair test of macronutrients and obesity.
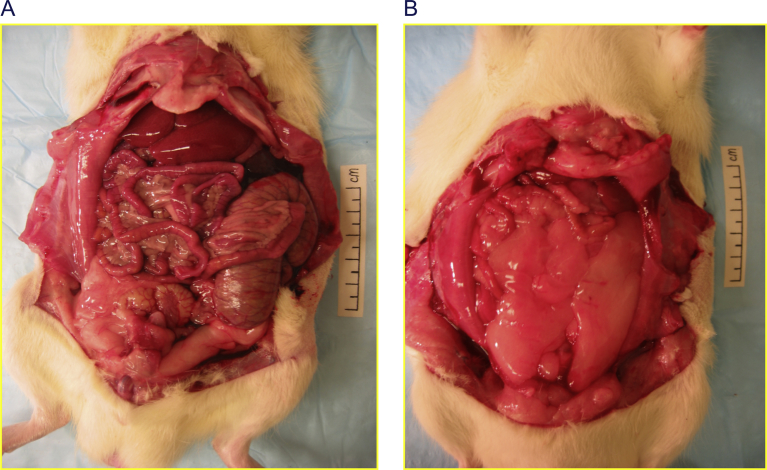
To circumvent these design limitations, several groups of investigators have compared diets varying in glycemic index in rodents, controlling for fat amount and type, sugar and other potentially confounding factors. An implicit advantage of this design is greater clinical relevance, as rodents and humans respond to high intakes of fat in demonstrably different ways. In those studies, the high-glycemic index diets resulted in marked primary hyperinsulinemia before changes in systemic insulin resistance or body composition were observed. Increased body fat mass (Figure 1), lower energy expenditure, and greater food intake developed subsequently [1,7].
Figure 1.
Greater adiposity among rats fed high-vs low-glycemic index diets. Representative weight-matched animals consumed diets that had either a low-glycemic index (Panel A) or high-glycemic index (Panel B), as described in Pawlak et al. [7]. This study demonstrates four key predictions of the CIM, namely raising glycemic load would: 1) initially produce primary hyperinsulinemia; and subsequently 2) reduce energy expenditure, 3) increase hunger, and 4) increase adiposity when controlling for body weight. In the high-glycemic index diet group, mean body fat mass was 71% greater and lean mass was reduced commensurately. With a primary focus on glycemic load, the CIM can be tested by varying carbohydrate amount or glycemic index. Studies in rodents targeting the latter, such as this, are arguably more relevant and interpretable due to species-specific differences in metabolic response to macronutrients and potential confounding by fatty acid type. Other studies in mice or rats with consistent findings are summarized in Ludwig and Ebbeling [1].
The evolutionarily conserved anabolic biochemical effects of insulin provide a unique opportunity to test the CIM in experimental animals. Mice genetically incapable of sustained hyperinsulinemia or those lacking insulin receptors in adipose tissue are protected from diet-induced obesity [8]. Peripheral injection of insulin in rodents, even when calorie-restricted to prevent excessive weight gain, increases adiposity [9]. In humans, genetically determined insulin secretion strongly predicts body weight, but body weight does not predict insulin secretion according to a recent bi-directional Mendelian randomization study [10]. Moreover, drugs that decrease insulin secretion cause weight loss [8], whereas those that increase insulin secretion (or insulin itself, in the treatment of type 2 diabetes) cause weight gain in humans.
As has been explicitly stated, the CIM is not a single nutrient, single hormone model—many dietary and environmental influences may impact body weight regulation through pancreatic hormone secretion, adipocyte anabolic state, or other mechanisms [1]. The CIM, like any multicomponent conceptual framework of complex phenomena, is at best a rough approximation of biology, subject to revision as new knowledge accrues. However, in its present iterations, the CIM makes specific and testable predictions, many of which have received support from appropriately designed rodent research and clinical studies. In light of the striking failure of conventional obesity prevention and treatment on a population basis, all sides of this debate would do well to avoid categorical conclusions about the validity of the CIM in any species at this time.
Conflicts of Interest
DSL received royalties for books on obesity and nutrition that recommend a carbohydrate-modified diet and grants from the NIH and philanthropic organizations unaffiliated with the food industry to study the Carbohydrate-Insulin Model of obesity. CBE received grants from the NIH and philanthropic organizations unaffiliated with the food industry to study the Carbohydrate-Insulin Model of obesity. BTB JDJ report no conflicts of interest.
Footnotes
This commentary refers to “The carbohydrate-insulin model does not explain the impact of varying dietary macronutrients on the body weight and adiposity of mice by Sumei Hu et al.”, https://doi.org/10.1016/j.molmet.2019.11.010.
References
- 1.Ludwig D.S., Ebbeling C.B. The carbohydrate-insulin model of obesity: beyond “calories in, calories out”. JAMA Internal Medicine. 2018;178(8):1098–1103. doi: 10.1001/jamainternmed.2018.2933. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Ludwig D.S., Lakin P.R., Wong W.W., Ebbeling C.B. Scientific discourse in the era of open science: a response to Hall et al. regarding the Carbohydrate-Insulin Model. International Journal of Obesity. 2019 doi: 10.1038/s41366-019-0466-1. [Epub ahead of print] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Hu S., Wang L., Togo J., Yang D., Xu Y., Wu Y. The carbohydrate-insulin model does not explain the impact of varying dietary macronutrients on body weight and adiposity of mice. Molecular Metabolism. 2019 doi: 10.1016/j.molmet.2019.11.010. [Preprint] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Hofman D.L., van Buul V.J., Brouns F.J. Nutrition, health, and regulatory aspects of digestible maltodextrins. Critical Reviews in Food Science and Nutrition. 2016;56(12):2091–2100. doi: 10.1080/10408398.2014.940415. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Milanski M., Degasperi G., Coope A., Morari J., Denis R., Citra D.E. Saturated fatty acids produce an inflammatory response predominantly through the activation of TLR4 signaling in hypothalamus: implications for the pathogenesis of obesity. Journal of Neuroscience. 2009;29(2):359–370. doi: 10.1523/JNEUROSCI.2760-08.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Brown M.A., Storlien L.H., Brown I.L., Higgins J.A. Cooking attenuates the ability of high-amylose meals to reduce plasma insulin concentrations in rats. British Journal of Nutrition. 2003;90(4):823–827. doi: 10.1079/bjn2003958. [DOI] [PubMed] [Google Scholar]
- 7.Pawlak D.B., Kushner J.A., Ludwig D.S. Effects of dietary glycaemic index on adiposity, glucose homoeostasis, and plasma lipids in animals. Lancet. 2004;364(9436):778–785. doi: 10.1016/S0140-6736(04)16937-7. [DOI] [PubMed] [Google Scholar]
- 8.Page M.M., Johnson J.D. Mild suppression of hyperinsulinemia to treat obesity and insulin resistance. Trends in Endocrinology and Metabolism. 2018;29(6):389–399. doi: 10.1016/j.tem.2018.03.018. [DOI] [PubMed] [Google Scholar]
- 9.Astley C.M., Todd J.N., Salem R.M., Vedantam S., Ebbeling C.B., Huang P.L. Genetic evidence that carbohydrate-stimulated insulin secretion leads to obesity. Clinical Chemistry. 2018;64(1):192–200. doi: 10.1373/clinchem.2017.280727. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Torbay N., Bracco E.F., Geliebter A., Stewart I.M., Hashim S.A. Insulin increases body fat despite control of food intake and physical activity. American Journal of Physiology. 1985;248(1 Pt 2):R120–R124. doi: 10.1152/ajpregu.1985.248.1.R120. [DOI] [PubMed] [Google Scholar]