Abstract
Objective
To analyze the diagnosis and treatment of patients with chronic renal failure complicated with novel coronavirus pneumonia, and to evaluate the effect of blood purification technology on the treatment and prognosis of such patients.
Methods
Two COVID-19 cases undergoing hemodialysis with chronic renal failure were retrospectively analysed in our hospital.
Results
Two COVID-19 patients were admitted to hospital due to cough, with or without fever. Laboratory tests showed decreased lymphocyte count, elevated PCT, IL-10, IL-6, TNF-α, IL-2R, high-sensitivity cardiac troponin I, NT-proBNP, creatinine, and urea nitrogen. Chest CT scan showed multiple blurred plaques and patchy shadows in both patients. Two patients received continuous venovenous hemodiafiltration (CVVHDF) every other day for 4–6 h everytime, in addition to the standard treatment. After CVVHDF, not only cytokines were reduced, but also liver function and cardiac function significantly improved. Both patients did not develop severe pneumonia. They were discharged on March 1, 2020 when meeting the discharge criteria.
Conclusion
Two COVID-19 patients on maintenance hemodialysis discharged after a month of hospitalization. The removal of cytokines through blood purification technology may be beneficial for the recovery of COVID-19 patients.
Keywords: Chronic renal failure, SARS-CoV-2, COVID-19, Hemodialysis, CVVHDF, Cytokine storm
1. Introduction
Since December 2019, multiple cases of unexplained viral pneumonia have been detected in Wuhan, Hubei Province [1], [2]. On January 8, 2020, the Chinese Center for Disease Control and Prevention officially announced the pneumonia was caused by a new type of coronavirus, severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2) [2]. As of March 3, 2020, SARS-CoV-2 has infected a total of 80,422 people in China, with a mortality rate of 3.71%. More than 12,000 people were diagnosed outside China, showing a global pandemic trend. At present, the treatment of COVID-19 has become a research hotspot. However, relevant information about clinical characteristics, treatment options, prognosis of chronic renal failure patients infected by SARS-CoV-2 has not been reported. Two COVID-19 patients with chronic renal failure in Tongji Hospital, Tongji Medical College, Huazhong University of Science and Technology were retrospectively analysed.
2. Case presentation
2.1. Medical history
Case 1, male, 79 years old, was admitted to the community hospital on January 25, 2020 because of “fever and cough for 2 days”. After receiving antibiotic, he felt no relief and came to our hospital for further treatment. Physical examination: T 37.7 °C, P 88 bpm, R 20 times/min, BP 120/57 mmHg, clear consciousness, rough breathing sounds of both lungs. Medical history: hypertension for more than 30 years, hyperlipidemia for 2 years, gout for more than 2 years, chronic renal insufficiency for 2 years, right nephrectomy for hydronephrosis in 1986, coronary stent implantation on December 19, 2019. Arteriovenous fistula of left upper limb for hemodialysis was performed on December 25, 2019. No history of diabetes, no history of hepatitis, tuberculosis, etc.
Case 2, female, 40 years old, was admitted to our hospital on January 27, 2020 for “cough and wheezing for a week”. In the outpatient department of respiratory medicine, supportive treatment and antibiotics (anseima, ceftin, et al) were given. Physical examination on admission: T 36.2 °C, P 102 bpm, BP 142/94mmhg, R 23 times/min, clear consciousness, severe anemia, rough breathing sound in both lungs. Past history: glomerulonephritis and chronic renal failure for 10 years, hypertension for seven years. No history of diabetes, no history of hepatitis, tuberculosis, etc.
2.2. Laboratory test
After admission, the blood routine, serum biochemistry, procalcitonin (PCT), cytokines and other test indicators are shown in Table 1 . Laboratory test of case 1 showed normal white blood cells, decreased lymphocyte count and hemoglobin, increased levels of PCT, IL-10, TNF-α, IL-2R, high-sensitivity cardiac troposin I, NT-proBNP, creatinine and other indicators. In case 2, laboratory examination showed increased white blood cells, decreased lymphocyte count and hemoglobin, increased PCT, IL6, TNF-α, IL-2R, high-sensitivity cardiac troponin I, NT-proBNP, creatinine and other indicators. After CVVHDF, serum cytokines were mostly reduced, meanwhile creatinine and amino terminal brain natriuretic peptide precursor were significantly improved (Table 1). Case 1 tested positive for new coronavirus nucleic acid from pharynx swab (Magnetic viral RNA extraction Kit on PAN9600 Automated Nucleic Acid Extraction System supplied by Tianlong Tech Co. LTD), and case 2 tested positive for serum IgM antibody with rapid detection kit for new coronavirus (Serum IgM antibodies against SARS-CoV-2 detected by chemiluminescence kit supplied by Yhlo Biotech Co. LTD).
Table 1.
Laboratory data of chronic renal failure patients with COVID-19 before and after dialysis.
| Laboratory test | Case1 |
Case2 |
||
|---|---|---|---|---|
| Before CVVHDF |
After CVVHDF | Before CVVHDF | After CVVHDF | |
| Total white cell count (x109 /L NR 3.5–9.5) |
8.59 | 7.90 | 15.49 | 5.68 |
| Lymphocyte count (x109/L NR 1.10–3.20) |
0.92 | 0.74 | 1.01 | 0.58 |
| Hemoglobin (g/L NR 130–175) |
85 | 92 | 35 | 83 |
| PCT (ng/mL NR 0.02–0.05) |
0.8 | 0.58 | 1.59 | 1.40 |
| ALT (U/L NR ≤ 41) | 57 | 6 | 11 | 15 |
| AST (U/L NR ≤ 40) | 51 | 23 | 16 | 16 |
| Creatinine (umol/L NR 59–104) |
776 | 245 | 1175 | 558 |
| Urea (mmol/L NR 3.6–9.5) |
26.93 | 20.70 | 33.71 | 24.3 |
| eGFR (ml/min/1.73 m2 NR <90) |
5.2 | 20.8 | 3.1 | 7.6 |
| Hypersensitive cardiac troponin (pg/ml NR <34.2) |
85.4 | 67.8 | 24.3 | 30.4 |
| NT-proBNP (pg/ml NR (486) |
>70000 | 22,490 | 11,218 | 2481 |
| IL-6 (pg/ml NR <7) | 88 | 41.06 | 124.9 | 15.07 |
| IL-10 (pg/ml NR <9.1) | 36.7 | 6.3 | <5.0 | 10.8 |
| IL-2R (pg/ml NR 223–710) |
2575 | 2091 | 1622 | 1200 |
| TNF-α(pg/ml NR <8.1) | 28.2 | 28.6 | 12.7 | 16.7 |
Note: CVVHDF, continuous venovenous hemodiafiltration; PCT, procalcitonin; ALT, alanine aminotransferase; AST, aspartate aminotransferase; NT-proBNP, Amino terminal brain natriuretic peptide precursor; IL, Interleukin; TNF-α,Tumor necrosis factor-α.
2.3. Imageological diagnosis
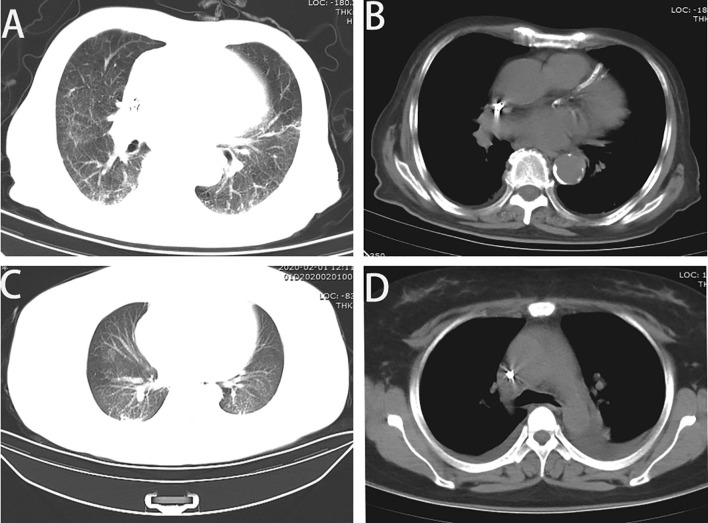
Case 1: Chest CT scan showed strong dual lung texture, and multiple fuzzy plaques in both lungs. Bilateral pleural effusion, bilateral pleural thickening, adhesion. Left ventricular enlargement, pericardial effusion, multiple calcifications in aortic and coronary artery walls. (Fig. 1 A, B). Case 2: Chest CT scan showed scattered ground glass patches and smut shadows in both lungs. Pleural effusion on both sides, poor lung dilation (Fig. 1C, D).
Fig. 1.
Characterized chest CT scan of COVID-19 patients with chronic renal failure. A: Case 1: Multiple blurred plaques and patchy shadows were seen in both lungs. B: Left ventricular enlargement, pericardial effusion, and multiple calcification of the aorta and coronary artery wall. C: Case 2 with scattered ground glass patches and smut shadows on both lungs, left upper lobe cord shadow, D: Bilateral pleural effusion, small heart, pericardial effusion.
2.4. Clinical diagnosis and treatment
Case 1 was diagnosed as: 1. New coronavirus pneumonia; 2. Heart failure, level IV; 3. Chronic renal failure; 4. Ischemic coronary heart disease; 5. Hypertension, grade III; 6. Paroxysmal atrial tachycardia; 7. Hyperlipidemia. Case 2 was diagnosed as: 1. New coronavirus pneumonia; 2. Chronic renal failure, 3. Hypertension, grade II; 4. Severe anemia.
In accordance with the “Trial Version 6“ issued by the National Health and Health Commission, they received supportive treatment, water and electrolyte balance, closely monitoring on vital signs and oxygen saturation. Besides, we provided effective oxygen therapy, antiviral therapy (arbidol in both patients) and antibacterial therapy (cefoperazone/sulbactam intravenousinfusion in case 1, oral cefdinir dispersible tablet in case 2) in time. Both of them received continuous venovenous hemodiafiltration (CVVHDF), which was performed every other day for 4–6 h. Case 2 received blood transfusion because of severe anemia. During hospitalization, none of the two patients progressed to severe pneumonia. Both of whom were discharged on March 1, 2020 after disappearance of symptoms for three days and negative pharynx swab test for twice.
3. Discussion
SARS-CoV-2 is an enveloped, single-stranded RNA beta coronavirus [3], [4], [5]. COVID-19 is not only harmful to respiratory system characterized by viral pneumonia, but also combined with damage to kidney, heart, blood, nervous system and other organs.
Hyperviremia and cytokine storm are important causes for COVID-19′s evolution to severe pneumonia, even to multiple organ dysfunction in a few cases [6]. In addition, comorbidities such as diabetes, heart failure and acute renal failure indicate poor prognosis6. According to the research on SARS-CoV and MERS-CoV, human coronavirus can replicate to a high titer at early stage. This high replication may lead to enhanced cytopathic effects and higher levels of pro-inflammatory cytokines produced by infected epithelial cells. These cytokines, in turn, induce large numbers of inflammatory cells penetrating into the lung [7], [8], [9], [10]. Disrupting the antiviral response by antagonizing IFN can achieve innate immune delay or immune evasion [10], [11], [12], [13], [14]. Excessive release of inflammatory cytokines and the formation of a series of self-amplifying cytokines activate a cascade of reactions, causing damage to diffuse alveolar, transparent membranes formation and fibrotic protein exudation. In severe cases, cytokine storm can induce hemodynamic instability, DIC, and multiple organ dysfunction. Increased levels of IL-6, IL-10, TNF-α and other inflammatory cytokines in COVID-19 patients are related to poor prognosis [15]. This is consistent with the phenomenon that two cases suffered from multiple organ dysfunction, with serum cytokines significantly increased.
To date, there are currently no effective antiviral drugs against SARS-CoV-2. Although glucocorticoids can inhibit the excessive activation of inflammatory factors, large doses of glucocorticoids will delay the elimination of new coronavirus due to immunosuppressive effects [16]. Compared with other COVID-19 patients, these 2 cases receive continuous venovenous hemodiafiltration, every other day for 4–6 h due to chronic renal failure. CVVHDF is suitable for patients with acute and chronic renal failure, especially those with the following conditions: Routine dialysis is prone to hypotension, refractory hypertension, excessive body fluids and heart failure that conventional hemodialysis cannot control, severe secondary hyperparathyroidism, uremia neuropathy, unstable cardiovascular function, and multiple organ failure. Moreover, blood purification can be applied more widely, such as persistent inflammatory fever, acute respiratory distress syndrome, volume overload, combined with extracorporeal membrane oxygenation (ECMO) treatment, et al. [17]. Both patients met the above indications. CVVHDF can remove small and medium molecules such as chemical toxins, endotoxins, bilirubin, urea nitrogen, creatinine, cytokines, inflammatory factors and water. Thus the reduction of excessive cytokine may relieve cytokine storm and multi-organ function failure for COVID-19 patient. So it is speculated that blood purification technology can protect COVID-19 patients from severe pneumonia via cytokine purification and organ function support. Furthermore, COVID-19 patients with over-activated inflammatory reactions may benefit from blood purification, such as plasma exchange, adsorption, perfusion, and blood/plasma filtration if available, with or without renal failure. These speculations require more clinical trials to verify.
COVID-19 patients with abnormal chest CT imaging, even in asymptomatic patients, characterized by unilateral focal or bilateral diffuse scrub-like changes within 1–3 weeks [18].When the new coronavirus nucleic acid test was positive, chest CT showed a sensitivity of 97% for COVID-19 [19]. Chest CT has a high diagnostic sensitivity for COVID-19, which can be used as the main tool for COVID-19 detection in epidemic areas. Chest CT scan of these two cases showed multiple blurred plaques and patchy shadows in both lungs, similar with COVID-19 patients without chronic renal failure.
4. Conclusions
In summary, two COVID-19 patients on maintenance hemodialysis were cured after a month of hospitalization. Blood purification technology seems to be helpful for preventing COVID-19 patients with chronic renal failure from severe pneumonia or even multiple organ dysfunction.
CRediT authorship contribution statement
Chunjin Ke: Conceptualization, Writing - original draft. Yufeng Wang: Data curation, Formal analysis. Xing Zeng: Software, Supervision. Chunguang Yang: Funding acquisition, Writing - review & editing. Zhiquan Hu: Project administration, Resources.
Declaration of Competing Interest
The authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.
Acknowledgments
Acknowledgment
This work was supported by National Natural Science Foundation of China (No.81702989).
Compliance with ethical standards
All procedures performed in studies involving human participants were in accordance with the ethical standards of the Lifespan institutional research committee and with the 1964 Helsinki declaration and its later amendments or comparable ethical standards.
Footnotes
Supplementary data to this article can be found online at https://doi.org/10.1016/j.clinbiochem.2020.04.008.
Contributor Information
Chunguang Yang, Email: cgyang-hust@hotmail.com.
Zhiquan Hu, Email: huzhiquan2000@163.com.
Appendix A. Supplementary data
The following are the Supplementary data to this article:
References
- 1.Zhu N., Zhang D., Wang W. A Novel Coronavirus from Patients with Pneumonia in China, 2019. N. Engl. J. Med. 2020 doi: 10.1056/NEJMoa2001017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Li Q., Guan X., Wu P. Early transmission dynamics in wuhan, china, of novel coronavirus-infected pneumonia. N. Engl. J. Med. 2020 doi: 10.1056/NEJMoa2001316. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Habibzadeh P., Stoneman E.K. The novel coronavirus: a bird's eye view. Int. J. Occup Environ. Med. 2020;11:65–71. doi: 10.15171/ijoem.2020.1921. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Chen Y., Liu Q., Guo D. Emerging coronaviruses: Genome structure, replication, and pathogenesis. J. Med. Virol. 2020 doi: 10.1002/jmv.26234. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Chan J.F., Kok K.H., Zhu Z. Genomic characterization of the 2019 novel human-pathogenic coronavirus isolated from a patient with atypical pneumonia after visiting Wuhan. Emerg. Microbes Infect. 2020;9:221–236. doi: 10.1080/22221751.2020.1719902. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Channappanavar R., Perlman S. Pathogenic human coronavirus infections: causes and consequences of cytokine storm and immunopathology. Semin. Immunopathol. 2017;39:529–539. doi: 10.1007/s00281-017-0629-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Channappanavar R., Fehr A.R., Vijay R. Dysregulated Type I Interferon and Inflammatory Monocyte-Macrophage Responses Cause Lethal Pneumonia in SARS-CoV-Infected Mice. Cell Host Microbe. 2016;19:181–193. doi: 10.1016/j.chom.2016.01.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Totura A.L., Whitmore A., Agnihothram S. Toll-Like Receptor 3 Signaling via TRIF Contributes to a Protective Innate Immune Response to Severe Acute Respiratory Syndrome Coronavirus Infection. mBio. 2015;6:e00638–e715. doi: 10.1128/mBio.00638-15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Zaki A.M., van Boheemen S., Bestebroer T.M., Osterhaus A.D., Fouchier R.A. Isolation of a novel coronavirus from a man with pneumonia in Saudi Arabia. N. Engl. J. Med. 2012;367:1814–1820. doi: 10.1056/NEJMoa1211721. [DOI] [PubMed] [Google Scholar]
- 10.Ng M.L., Tan S.H., See E.E., Ooi E.E., Ling A.E. Proliferative growth of SARS coronavirus in Vero E6 cells. J. Gen. Virol. 2003;84:3291–3303. doi: 10.1099/vir.0.19505-0. [DOI] [PubMed] [Google Scholar]
- 11.Kindler E., Thiel V., Weber F. Interaction of SARS and MERS Coronaviruses with the Antiviral Interferon Response. Adv. Virus Res. 2016;96:219–243. doi: 10.1016/bs.aivir.2016.08.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Totura A.L., Baric R.S. SARS coronavirus pathogenesis: host innate immune responses and viral antagonism of interferon. Curr. Opin. Virol. 2012;2:264–275. doi: 10.1016/j.coviro.2012.04.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Sun L., Xing Y., Chen X. Coronavirus papain-like proteases negatively regulate antiviral innate immune response through disruption of STING-mediated signaling. PLoS ONE. 2012;7 doi: 10.1371/journal.pone.0030802. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Thiel V., Weber F. Interferon and cytokine responses to SARS-coronavirus infection. Cytokine Growth Factor Rev. 2008;19:121–132. doi: 10.1016/j.cytogfr.2008.01.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Gong J., Dong H., Xia Q. Correlation Analysis Between disease severity and inflammation-related parameters in patients with COVID-19 pneumonia. MedRxiv. 2020 doi: 10.1186/s12879-020-05681-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Auyeung T.W., Lee J.S.W., Lai W.K. The use of corticosteroid as treatment in SARS was associated with adverse outcomes: a retrospective cohort study. J. Infection. 2005;51:98–102. doi: 10.1016/j.jinf.2004.09.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Rangaswami J., Bhalla V., Blair J.E.A. Cardiorenal syndrome: classification, pathophysiology, diagnosis, and treatment strategies a scientific statement from the American heart association. Circulation. 2019;139:E840–E878. doi: 10.1161/CIR.0000000000000664. [DOI] [PubMed] [Google Scholar]
- 18.Shi H., Han X., Jiang N. Radiological findings from 81 patients with COVID-19 pneumonia in Wuhan, China: a descriptive study. Lancet Infect. 2020;Feb:24. doi: 10.1016/S1473-3099(20)30086-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Ai T., Yang Z., Hou H. Correlation of Chest CT and RT-PCR Testing in Coronavirus Disease 2019 (COVID-19) in China: A Report of 1014 Cases. Radiology. 2020 doi: 10.1148/radiol.2020200642. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.