See Article, pages 26–39
When planes crash or experience a near miss, the United States Federal Aviation Authority has a longstanding policy that mandates a review that “will ensure that all of the facts, conditions, and circumstances leading to an event are recorded and evaluated, and that action is taken to prevent similar events to the extent practical and feasible.” Safety and efficacy of air travel is immeasurably better for it.
Development pathways for pharmacological therapeutic agents are long and fraught with unpredictable expense and outcome. We are, as a society, fortunate that there are individuals and organizations with the skills, knowledge and resources needed to achieve the spectacular advances in the diagnosis and treatment of many diseases, such as the advent of direct-acting antiviral agents for hepatitis C or checkpoint inhibitors for a host of neoplasia. COVID-19 will, hopefully, soon be added to the list of tamed scourges. For medicine to advance at the pace and cost we desire, it is important, however, to give as much consideration to the facts, conditions, and circumstances that lead to failed phase III studies as we do to successes.
There can be few more pertinent examples of this than the growing number of advanced (pivotal phase II or III) clinical trials in NASH that have failed or nearly failed to meet their primary endpoint(s).[1], [2], [3], [4] The overarching issue is that, as a field, by enrolling large numbers of patients into trials likely to fail, we are disadvantaging our patients and incurring tremendous cost that will be borne not by just the pharmaceutical industry but, ultimately, by patients.
In this issue of the Journal, Harrison et al. 4 report the results of 2 large phase III trials in patients with NASH and advanced fibrosis. Here, the ASK-1 inhibitor, selonsertib, was compared to placebo in ~1,700 patients with NASH and bridging fibrosis (F3, STELLAR-3) or compensated cirrhosis (F4, STELLAR-4). The primary efficacy endpoint was ≥1 stage improvement in fibrosis on liver biopsy without worsening of NASH. Additional endpoints included changes in non-invasive tests (NITs), progression to cirrhosis (in STELLAR-3), and the development of liver-related events. Unfortunately, although selonsertib was well tolerated, neither trial met or had a trend for meeting the primary endpoint. Unfortunately, although selonsertib led to dose-dependent reductions in hepatic phospho-p38 expression, suggestive of pharmacodynamic activity, dose-dependent activity was not seen, nor did it significantly impact any of the endpoints.
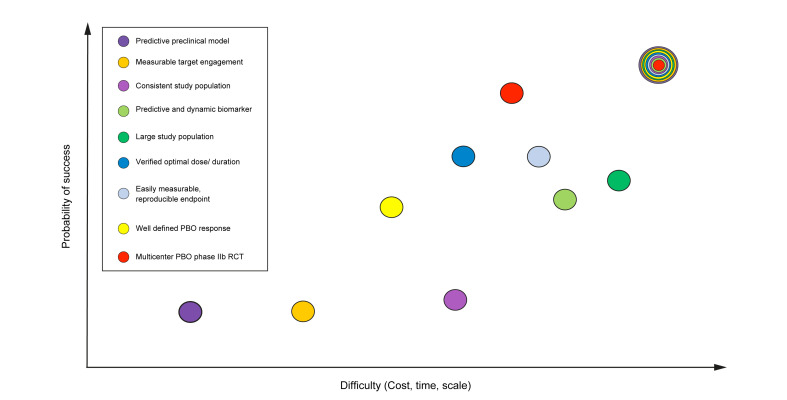
Two phase III studies totally failing to achieve their efficacy endpoints is the therapeutic equivalent of a plane crash and merits detailed consideration. There are several factors that likely contributed to the failure of these large trials. While one can never be sure that a trial will succeed in phase III, chances of success can be maximized by broadly following the design of a successful placebo-controlled phase IIb study, earnest interpretation of preliminary data and studying a similar population in phase II as in phase III (Fig. 1 ). STELLAR 3 and 4 were designed and conducted without such data, greatly adding to the risk of failure to meet endpoints.
Fig. 1.
Mitigating risk of failure in phase III.
Various elements culminating in the design and execution of a phase III trial can heavily influence the probability of success. Each of these aspects, depicted by colored circles, are associated with varying degrees of difficulty, measured by cost, time and scale. Each also differentially contributes to the predictivity of success in a phase III program. While one can never be certain that the primary endpoint will be met, the more of these critical elements are satisfied, the more predictable an outcome will be (multicolored circle).
PBO, placebo; RCT, randomized controlled trial.
In the absence of a placebo-controlled phase IIb study, STELLAR 3 and 4 were based on a phase II study,5 in which patients with NASH and F2/F3 were recruited in a multicenter trial and randomized in a 2:2:1:1:1 ratio to receive 24 weeks of treatment with 6 mg or 18 mg of selonsertib alone; 6 mg or 18 mg of selonsertib with 125 mg of simtuzumab; or 125 mg of simtuzumab alone. The proportion of patients with ≥1 stage reduction in fibrosis in both selonsertib arms (n = 30 and 27, respectively) were numerically higher but not statistically different from that observed in the simtuzumab arm (n = 20). Simtuzumab, because of a lack of efficacy in the 105/106 studies, was, perhaps fatefully ignoring the possibility of synergy with selonsertib, equated with placebo.2 It is difficult to know if the lack of statistical superiority of selonsertib in phase II was a function of inadequate power, because of underdosing or due to true lack of efficacy. Furthermore, placebo should not be supplanted by a pharmacologic intervention irrespective of lack of efficacy in a prior trial. It is difficult to know if additional elements such as the exclusion of stage 2 patients in STELLAR 3 would have positively impacted the outcome. Preliminary data from the Atlas study raise the theoretical potential for selonsertib, perhaps at higher doses, to be used in synergy with other agents.6
The design of STELLAR-3 and 44 had several attributes and addressed critical issues that plague the NASH therapeutic landscape, such as challenges with histological interpretation of fibrosis and directly linking NITs to outcomes independent of histologic change. Of particular interest was the demonstration that enhanced liver fibrosis (ELF) had the ability to identify those most likely to progress from F3 to cirrhosis in addition to predicting which patients with cirrhosis at baseline were most likely to have a liver-related event. The authors defined a change in ELF of 0.5 points, as that value correlated with clinical liver events in a previous study,2 which correlated in the current study with other NITs, liver biochemistry tests, glycemic parameters, CK-18 values, serum bile acid values, and body weight, but not histologic features. These findings give hope that at some point reliance on histologic endpoints will be a historical concept for NASH clinical trials.
A successful therapeutic in the NASH cirrhosis space has been particularly elusive, and selonsertib was not the first, nor will it be the last to fail. Irrespective of the endpoint — histological, hepatic venous pressure gradient (HVPG) or outcome driven, no drug has thus far been successful.1 , 3 , 4 , 7 Cirrhosis (F4) represents arguably a broader spectrum of disease than F1-3 and thus should be carefully subclassified accordingly.
One could argue that statements on natural history derived from a clinical trial may not reflect true natural history, however such controlled settings provide insight into factors that may influence outcomes. In comparing available data from NASH cirrhosis trials, the significant difference in liver-related events ranges from 3% (selonsertib) to 21% (emricasan) per annum.2 , 4 These seemingly disparate estimates in rates of decompensation can be explained by differences in various factors such as model for end-stage liver disease scores, HVPG, hepatic collagen content and to what extent clinical events were adjudicated. The STELLAR studies provided additional support for ELF to identify those at highest risk of decompensation. Second, and surprising, cardiac outcomes, measured via major adverse cardiac events, were nearly 6-fold higher among patients with compensated cirrhosis than among those with bridging fibrosis. This difference is unexplained, although it may reflect a higher prevalence of structural abnormalities, such as cardiomyopathy, in patients with cirrhosis. These lessons will inform more careful stratification and therefore more accurate prediction of outcomes for the design of future trials.
It is now widely known that the placebo response in NASH trials is substantial and varies widely across studies and endpoints. Data from the STELLAR and REGENERATE studies illustrate that an expected placebo response for ≥1-stage improvement in fibrosis without NASH worsening is ~10–14%.4 , 8 Industry, academia and regulatory agencies should expect the efficacy of future compounds to be compared to this benchmark.
The successes and failures of the pioneers of the NASH therapeutic landscape have, unequivocally advanced the field. Bruegel's masterful depiction of Icarus falling into the sea is captivating in that the tragedy of this heroic failure lies in its unobserved nature. Phase III studies that fail to achieve their endpoints should, in contrast, be carefully studied, with the knowledge gained applied to the development of minimum criteria required for progression to advanced clinical trial stages that impact thousands of patients. The onus is now on Pharma, academia and regulatory bodies to ensure that the process leading to the design of a phase III program do not fly too close to the sun, or at least not with feathers bound by wax.
The STELLAR studies did not meet their primary outcomes, and selonsertib was ineffective in reversing fibrosis in patients with NASH and stage F3/F4 fibrosis – those most in need of treatment. Nonetheless, important lessons were learned from this and similar trials about the extent of confidence needed to progress to a phase III program.
Financial support
The authors received no financial support to produce this manuscript.
Authors' contributions
MR: Study concept, writing the draft and approving the final version. MN: Study concept, writing the draft and approving the final version.
Conflict of interest
Mary E. Rinella, MD: Consulting: Intercept Pharmaceuticals, Gilead Sciences, NGM Biopharmaceuticals, Enanta, Immuron, Fractyl, Prociento, Gelesis, Merck, Bristol-Myers Squibb, Metacrine, Viking Therapeutics, Allergan, Cymabay, Boehringer Ingelheim, Genentech, Sagimet Bio, Terns, Siemens and Novartis. Independent research grant funding from Novartis. Support in kind from Owl. Mazen Noureddin, MD; MHSc: MN has been on the advisory board for Gilead, Intercept, Pfizer, Novartis, Allergan, Blade, EchoSens North America, OWL, Terns, Simenes, Zydus, and Abbott; MN has received research support from Allergan, BMS, Gilead, Galmed, Galectin, Genfit, Conatus, Enanta, Novartis, Shire and Zydus; MN is a minor shareholder or has stocks in Anaetos and Viking.
Please refer to the accompanying ICMJE disclosure forms for further details.
Footnotes
Author names in bold designate shared co-first authorship
Contributor Information
Mary E. Rinella, Email: mrinella@nm.org.
Mazen Noureddin, Email: mazen.noureddin@cshs.org.
Supplementary data
References
- 1.Garcia-Tsao G., Bosch J., Kayali Z., Harrison S.A., Abdelmalek M.F., Lawitz E. Randomized placebo-controlled trial of emricasan for non-alcoholic steatohepatitis-related cirrhosis with severe portal hypertension. J Hepatol. 2020;72(5):885–895. doi: 10.1016/j.jhep.2019.12.010. [DOI] [PubMed] [Google Scholar]
- 2.Harrison S.A., Abdelmalek M.F., Caldwell S., Shiffman M.L., Diehl A.M., Ghalib R. Simtuzumab is ineffective for patients with bridging fibrosis or compensated cirrhosis caused by nonalcoholic steatohepatitis. Gastroenterology. 2018;155:1140–1153. doi: 10.1053/j.gastro.2018.07.006. [DOI] [PubMed] [Google Scholar]
- 3.Harrison S.A., Goodman Z., Jabbar A., Vemulapalli R., Younes Z.H., Freilich B. A randomized, placebo-controlled trial of emricasan in patients with NASH and F1-F3 fibrosis. J Hepatol. 2020;72(5):816–827. doi: 10.1016/j.jhep.2019.11.024. [DOI] [PubMed] [Google Scholar]
- 4.Harrison S.A., Wai-Sun Wong V., Okanoue T., Bzowej N., Vuppalanchi R., Younes Z. Selonsertib for patients with bridging fibrosis or compensated cirrhosis due to NASH: Results from randomized phase III STELLAR trials. J Hepatol. 2020;73(1):26–39. doi: 10.1016/j.jhep.2020.02.027. [DOI] [PubMed] [Google Scholar]
- 5.Loomba R., Lawitz E., Mantry P.S., Jayakumar S., Caldwell S.H., Arnold H. The ASK1 inhibitor selonsertib in patients with nonalcoholic steatohepatitis: a randomized, phase 2 trial. Hepatology. 2018;67(2):549–559. doi: 10.1002/hep.29514. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Sciences G. https://www.gilead.com/news-and-press/press-room/press-releases/2019/12/gilead-announces-topline-results-from-phase-2-atlas-study-in-patients-with-bridging-fibrosis-f3-and-compensated-cirrhosis-f4-due-to-nonalcoholic-s [cited 2020]. Available at.
- 7.Chalasani N., Abdelmalek M.F., Garcia-Tsao G., Vuppalanchi R., Alkhouri N., Rinella M. Effects of belapectin, an inhibitor of galectin-3, in patients with nonalcoholic steatohepatitis with cirrhosis and portal hypertension. Gastroenterology. 2020;158:1334–1345.e5. doi: 10.1053/j.gastro.2019.11.296. [DOI] [PubMed] [Google Scholar]
- 8.Younossi Z.M., Ratziu V., Loomba R., Rinella M., Anstee Q.M., Goodman Z. Obeticholic acid for the treatment of non-alcoholic steatohepatitis: interim analysis from a multicentre, randomised, placebo-controlled phase 3 trial. Lancet. 2019;394(10215):2184–2196. doi: 10.1016/S0140-6736(19)33041-7. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.