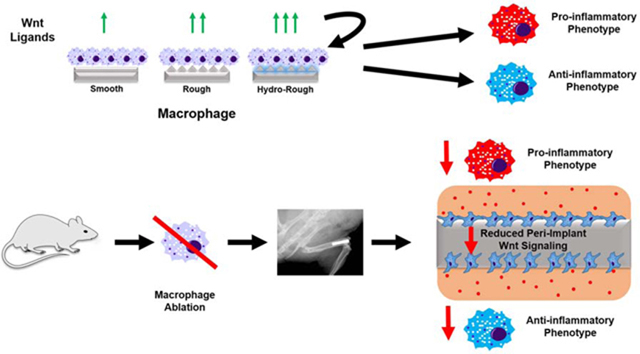
Abstract
Macrophages are among the first cells to interact with biomaterials and ultimately determine their integrative fate. Biomaterial surface characteristics like roughness and hydrophilicity can activate macrophages to an anti-inflammatory phenotype. Wnt signaling, a key cell proliferation and differentiation pathway, has been associated with dysregulated macrophage activity in disease. However, the role Wnt signaling plays in macrophage activation and response to biomaterials is unknown. The aim of this study was to characterize the regulation of Wnt signaling in macrophages during classical pro-and anti-inflammatory polarization and in their response to smooth, rough, and rough-hydrophilic titanium (Ti) surfaces. Peri-implant Wnt signaling in macrophage-ablated (MaFIA) mice instrumented with intramedullary Ti rods was significantly attenuated compared to untreated controls. Wnt ligand mRNA were upregulated in a surface modification-dependent manner in macrophages isolated from the surface of Ti implanted in C57Bl/6 mice. In vitro, Wnt mRNAs were regulated in primary murine bone-marrow-derived macrophages cultured on Ti in a surface modification-dependent manner. When macrophageal Wnt secretion was inhibited, macrophage sensitivity to both physical and biological stimuli was abrogated. Loss of macrophage-derived Wnts also impaired recruitment of mesenchymal stem cells and T-cells to Ti implants in vivo. Finally, inhibition of integrin signaling decreased surface-dependent upregulation of Wnt genes. These results suggest that Wnt signaling regulates macrophage response to biomaterials and that macrophages are an important source of Wnt ligands during inflammation and healing.
Keywords: Macrophage, Wnt signaling, Microstructured Titanium
Graphical abstract
1. Introduction
Successful integration of implanted biomaterials with the surrounding tissue is dependent on prompt resolution of inflammation and recruitment of stem cells that generate new tissue at the biomaterial-tissue interface. Important steps in this process include debridement, angiogenesis, differentiation of progenitor cells, generation of new tissue, and remodeling [1]. Macrophages, highly plastic members of the innate immune system, play a longitudinal role in the injury and healing response of many tissues, including bone [2,3]. Local tissue injury at the biomaterial implantation site spurs the recruitment of neutrophils and macrophages via damage-associated molecular patterns, cytokines, and chemokines. Once at the site, these cells clear cellular debris by phagocytosis [4]. Following removal of these pro-inflammatory signals, the immunological response can either subside as healing and regeneration begin or intensify as chronic inflammation [5]. Macrophages orchestrate this process by controlling cytokines in the injury microenvironment [3,5,6], recruiting progenitor cells and adaptive immune cells [7–10], acting as antigen-presenting cells in the presence of danger stimuli [11], and by remodeling extracellular matrix [12–14].
We and others have shown that biomaterial surface characteristics affect cellular response. We have demonstrated that roughened titanium (Ti) induces osteogenic differentiation of mesenchymal stem cells (MSCs) in vitro and leads to more rapid osseointegration in vivo than smooth implants [15–18]. Our group has also found that macrophages play a key role in enhancing osseointegration of roughened Ti implants. Roughened Ti causes anti-inflammatory macrophage polarization and increased production of transforming growth factor beta 1 (TGF-β1), interleukin (IL) 10, and arginase; this phenotypic change may be further enhanced by increasing the hydrophilicity of these roughened surfaces [19,20]. However, the majority of the work investigating macrophage response to biomaterials in bone-dwelling implants has focused exclusively on M1/M2 polarization and the release of pro-and anti-inflammatory cytokines [4,21–24]; other classes of secreted factors have not been well-explored.
Wnt ligands are a novel class of secreted signaling molecules that are essential to osseointegration [25–27], but whose source during bone healing is unclear. Wnt ligands are lipid-modified glycoproteins traditionally associated with differentiation and proliferation processes during morphogenesis and cancer [28–34]. Recently, Wnts have also been shown to be present in the microenvironment at early stages after injury and wound healing [35–38]. These molecules are first palmitoleate modified by Porcupine (Porcn) in the endoplasmic reticulum (ER), allowing them to be bound to Wntless (Wls, GPR177) and subsequently trafficked to the Golgi apparatus [39]. Wnt signaling has been traditionally classified as canonical or non-canonical signaling. Canonical, β-catenin-dependent signaling leads to activation of T-cell factor/lymphoid enhancer factor (TCF/LEF), while non-canonical signaling is β-catenin-independent and can result in cytoskeletal rearrangement (planar cell polarity pathway) or release of calcium as a second messenger (Wnt-Ca2+ pathway). Early study of Wnts in macrophages suggested that macrophages possess the molecular machinery for Wnt signaling and that Wnt signaling alters macrophage activity, including cytokine production and phagocytosis [40]. In addition, canonical Wnt signaling can limit excessive pro-inflammatory activation due to pathogen-associated molecular patterns [41]. In the biomaterial context, the likely mechanism of response to physical cues is through integrin signaling, and a number of studies have linked integrin activation to Wnt signaling. For example, Du et al demonstrated that ECM stiffness enhanced integrin/focal adhesion kinase activation, which induced Wnt1 expression in MSCs [42]. Similarly, integrin α5β1 binding to high affinity ECM ligands induced MSC osteoblastic differentiation [43]. Conversely, in T cells, activation of canonical Wnt signaling was found to induce integrin α4β1 and a more pro-inflammatory cellular phenotype [44]. These findings suggest that crosstalk between integrin/focal adhesion kinase and Wnt signaling occurs in various cell types and activation of these pathways regulates phenotypic change.
Studies of Wnt signaling in macrophages suggest that ligands from innate immune cells are critical regulators of normal tissue development [31,32], and also appear to play a role in regulating healing and regeneration in a variety of tissues. Inhibition of Wnt signaling can improve cardiac remodeling in infarct models [45–48]. Conversely, in a mouse hepatic fibrosis model, loss of macrophage Wnt signaling exacerbated the fibrotic response by consequent loss of matrix metalloproteinase activity [36]. These studies underscore the importance of macrophage Wnt signaling in tissue healing. While the characterization of the immunological response to implanted biomaterials has expanded in recent years, a paucity of useful measurements has hampered the field’s ability to determine or predict the efficacy of new materials, especially during the transition from in vitro to in vivo studies. Newly discovered cues in physiologic healing, such as measuring Wnt secretion by macrophages, may serve as novel and more predictive markers of the inflammatory response to biomaterials. Furthermore, exploration the role of macrophage Wnt signaling in healing may reveal this signaling pathway to be a novel target for implants.
In this study, we sought to characterize Wnt signaling in macrophages in the response to biomaterial surface properties during osseointegration. We explored the contribution of macrophages to Wnt signaling in peri-implant tissue, how secretion of Wnt ligands is regulated in response to Ti surface cues in vitro and in vivo, how macrophages may serve as an autocrine target of Wnt signaling, and the importance of Wnt ligands to macrophages in their recruitment of other cell types.
2. Materials and Methods
2.1. Mouse Femoral Implant
Animal experiments were carried out in accordance with a protocol approved the Virginia Commonwealth University Institutional Animal Care and Use Committee (AD10001108). For in vivo studies, 10–12-week-old male mice C57BL/6 (Jackson Strain #000664) were anesthetized using isoflurane/O2 gas and monitored for unconsciousness by pedal reflex. The skin overlaying the knee was opened and the patellar tendon was bisected longitudinally using a scalpel and retracted to reveal the femoral condyles. A dental drill was used to access the medullary canal and a cylindrical Ti implant (Ø= 1mm) with a rough or rough-hydrophilic (rough-hydro) surface was then press fit, with placement confirmed by x-ray (n=6 mice per sham, rough implant, or rough-hydro implant condition). Mice were administered 1mg/kg buprenorphine SR LAB prior to recovery from anesthesia to relieve post-operative pain for 72 hours. Animals were monitored until initial ambulation and every 24 hours afterwards. All animals had access to food and water ad libitum for the duration of the study. No signs of infection were present in this study.
Mice were euthanized by CO2 asphyxiation at 1, 3, or 7 days post-operatively, and femoral bones were harvested. To isolate peri-implant tissue, femurs were cut mid shaft, and the implant and surrounding marrow were flushed using Accutase (Innovative Cell Technologies, San Diego, CA). Following 20-minute incubation in Accutase at 37°C, cells were pelleted and resuspended either in TRIzol™ Reagent (Thermo Fisher Scientific, Waltham, MA, USA) for quantitative real-time polymerase chain reaction (qPCR), in MojoSort Buffer (BioLegend, San Diego, CA) for magnetic sorting, or in 1% bovine serum albumin in PBS (staining buffer) for flow cytometry analysis as described below.
2.2. Macrophage Ablation
Macrophages were ablated in male C57BL/6-Tg(Csf1r-EGFP-NGFR/FKBP1A/TNFRSF6)2Bck/J mice, also known as macrophage Fas Ligand-induced apoptosis (MaFIA) mice (Jackson Strain #005070). MaFIA mice (10–12 weeks old) were treated with 5mg/kg AP20187 (Takara Bio USA) in 1% DMSO + 10% PEG-400 + 2% Tween-80 in sterile water daily for 3 days, then every other day through the end of the study to induce dimerization of Fas and subsequent apoptosis in colony stimulating factor 1 receptor promoter (Csf1r)-expressing cells [49]. Untreated age-and sex-matched MaFIA mice were used as controls.
2.3. Csf1r-iCre; Wls−/− Mouse
FVB-Tg(Csf1r-cre/Esr1*)1Jwp/J (Jackson Strain # 019098) mice containing a tamoxifen-inducible Csf1r-iCre allele were crossed with 129S-Wlstm1.1Lan/J (Jackson Strain # 012888) mice to produce Csf1r-iCre+; Wlsfl/fl mice. Prior to experimental use, animals were injected for five consecutive days with 100 μL of 20 mg/mL tamoxifen in corn oil to induce Cre-mediated knockout of Wls. For in vitro experiments, macrophages were grown from harvested marrow as described in section 2.6 after five days of tamoxifen exposure; macrophages for control groups were isolated from Csf1r-iCre+; Wlsfl/fl mice that were not injected with tamoxifen. For in vivo experiments, mice underwent instrumentation as described in 2.1 after five days of tamoxifen exposure; Csf1r-iCre-; Wlsfl/fl littermates injected concommitantly with tamoxifen were used as controls.
2.4. Gene Expression
mRNA was extracted from tissue or cells using TriZol, and 1ug RNA was converted to cDNA using iScript cDNA synthesis kit (BioRad, Hercules, CA). qPCR using pre-designed PrimePCR™ primers and SsoAdvanced Universal SYBR green supermix (BioRad) was performed to assess gene expression (Supplemental Table 1). Differences were determined by 2-ΔΔCT analysis calculated using endogenous housekeeping gene (Gapdh) and respective controls.
2.5. Magnetic Cell Separation
Macrophages were isolated using MojoSort mouse anti-allophycocyanin (APC) magnetic nanobeads (BioLegend). Single-cell suspensions from peri-implant tissue were incubated with rat anti-mouse CD11b-APC (BioLegend) for 15 minutes on ice. Following washing, CD11b-APC-stained macrophages were incubated with anti-APC magnetic nanobeads for 15 minutes on ice and washed again. Magnetic bead-conjugated cells were isolated by positive selection using the provided MojoSort magnet. Finally, cells were suspended in TRIzol for mRNA extraction and subsequent qPCR.
2.6. Cell Isolation
For in vitro studies, primary murine macrophages were differentiated from bone marrow harvested from 10–12-week-old male C57Bl/6 or Csf1r-iCre+; Wlsfl/fl mice (Jackson). Mice were euthanized by CO2 asphyxiation, and femoral and tibial bones were then opened with scissors and flushed with PBS. The resultant marrow was treated with ACK lysis buffer (Thermo Fisher Scientific) and plated in tissue culture flasks. Naïve macrophages were generated by culturing cells in DMEM (Thermo Fisher Scientific) supplemented with 10% fetal bovine serum (Thermo Fisher Scientific), 50 U/mL penicillin-50 μg/mL streptomycin (Thermo Fisher Scientific), and 30 ng/mL murine recombinant macrophage colony stimulating factor (M-CSF, PeproTech, Rocky Hill, NJ) for seven days. Cells were then detached with Accutase and plated onto modified Ti surfaces or tissue culture polystyrene (TCPS) in 24-well plates for subsequent experiments.
2.7. In Vitro Surface Studies
Naïve macrophages were seeded at a density of 50,000 cells/cm2 on TCPS or 15mm-diameter smooth, rough, or rough-hydro grade 2 unalloyed Ti disks (Institut Straumann AG, Basel, Switzerland) in 24-well plates as previously described [20,50] (n=6 per surface condition). Cells were cultured for 12 hours, and then mRNA levels of canonical and non-canonical Wnt ligands, receptors, and related proteins measured by qPCR.
2.8. Recombinant Wnt Treatment
Naïve macrophages were plated on tissue culture polystyrene (TCPS) and treated with 100 ng/mL of recombinant Wnt3a, −5a, −5b, −7a, −9b, and −11 (R & D Systems, Minneapolis, MN) for 24 hours. Cells were harvested using TriZOL for subesquent qPCR as described above. Conditioned media was collected and secretion of Il-1b, Il-4, Il-6, Il-10, Il-12, Tnf-α, and CXCL10 was measured by enzyme-linked immunosorbent assay (ELISA) (PeproTech, Rocky Hill, NJ). Protein secretion was normalized to DNA content measured in cell lysate (Quant-IT™ PicoGreen dsDNA Assay, Thermo Fisher Scientific).
2.9. Inhibition of Macrophage Wnt Signaling
Wnt signaling was inhibited pharmacologically using Wnt-C59 (Cayman Chemical, Ann Arbor, MI), a small molecule inhibitor of Porcn activity that blocks the palmitoleation of Wnt proteins and their transport from the endoplasmic reticulum. Following 7 days of culture with MCSF, macrophages were treated with 500 μM Wnt-C59 or vehicle (dimethyl sulfoxide, DMSO) while being plated on surfaces. This concentration was chosen based on inhibition of Wnt3a and −5a secretion by macrophages as measured by direct ELISA (Supplemental figure 1). In a second experiment, Wnt ligand secretion was inhibited genetically in macrophages by knockout of Wls using Csf1r-iCre+; Wlsfl/fl mice (described in section 2.3). Following treatment with tamoxifen, marrow from Csf1r-iCre+; Wlsfl/fl mice was cultured with M-CSF for seven days before being sub-passaged onto surfaces. Wnt-inhibited macrophages were exposed to M1polarizing stimulus (100 ng/mL Escherichia coli lipopolysaccharide, Sigma-Aldrich, St Louis, MO) [51–53], M2-polarizing stimulus (20ng/mL IL-4 and IL-13, PeproTech) [53,54], or modified surface stimuli (smooth, rough, rough-hydro) for 24 hours and subsequently harvested for qPCR or ELISA.
2.10. Rescue of Surface Polarization by recombinant Wnt Ligands
To assess the capability of Wnt ligands to rescue surface-mediated macrophage polarization, macrophages were first treated with 500uM Wnt-C59 and subsequently cultured on TCPS, smooth, rough-hydrophilic surfaces for 2 hours. Next, macrophages on smooth surfaces were treated with 100ng/mL recombinant Wnt3a, −5a, −7a, or −9b while macrophages on rough-hydrophilic surfaces were treated with 100ng/mL recombinant Wnt5b, −7a, −9b, or −11. After 24 hours, conditioned media were collected, and IL-1β, IL-6, IL-12(p40), CXCL-10, TNF-α, IL-4, and IL-10 were measured by ELISA.
2.11. Loss of Macrophage Wnt Signaling In Vivo
To inhibit global Wnt secretion, mice were injected intraperitoneally with 50 mg/kg Wnt-C59 dissolved in a 5% DMSO, 30% PEG300, 1% Tween80, 64% PBS solution for 3 consecutive days prior to implantation, and every other day following surgery. To inhibit macrophageal Wnt secretion, Csf1r-iCre+; Wlsfl/fl mice were injected with tamoxifen for 5 consecutive days prior to implantation. Seven days after surgery, mice were euthanized. Cells were detached from the implant surface into single cell suspension using Accutase and washed with staining buffer for staining and flow cytometric analysis.
Prior to fluorescent staining, Fc receptors were blocked by incubation with anti-CD16/32 (BioLegend) to prevent non-specific binding of subsequent fluorescent antibodies (BioLegend). After incubation and washing, cell suspensions were incubated with fluorescent antibodies to identify macrophages (CD45+/CD68+/CD11b+), pro-inflammatory macrophages (CD45+/CD68+/CD80+), anti-inflammatory macrophages (CD45+/CD68+/CD206+), MSCs (CD11b-/Sca-1+/CD105+), CD4 T-cells (CD3+/CD4+), and CD8 T-cells (CD3+/CD8a+). Stained cell suspensions were analyzed using a Guava® easyCyte 6–2L Benchtop Flow Cytometer (MilliporeSigma, Burlington, MA) using a total of 5000 events per sample (n=6/group). Results were analyzed using FlowJo software.
2.12. Regulation of Wnt Genes by Focal Adhesion Kinase
To identify the mechanism by which surface cues induce Wnt-dependent differentiation, macrophages were pre-treated with PF-573228 (Cayman Chemical), a small molecule ATP-competitive FAK inhibitor, at a dose of 5uM [44]. They were then cultured on surfaces with fresh media and inhibitor for 24 hours prior to harvest and RNA extraction for qPCR.
2.13. Statistical Analysis
Data for in vivo studies are presented as mean ± SD of n=6 mice per surface, while in vitro studies are presented as the mean ± SD of n=6 culture samples per exposure. Gene expression is presented as “fold-change” (2-ΔΔCt) compared to Gapdh housekeeping gene (Δ1) and to untreated TCPS controls (Δ2), while protein production is normalized to DNA quantity in each sample. Statistical analysis was performed in Prism Graphpad V7 software. Single-factor, equal variance ANOVA was used to ensure samples within groups were not significantly different (α=0.05). Once the resulting p-value within sample groups was found to be insignificant, multiple comparisons were made between groups using Tukey’s HSD test.
3. Results
3.1. Ablation of macrophages in vivo attenuates Wnt ligand expression in peri-implant tissue
To measure the contribution of macrophages to Wnt signaling in peri-implant tissues, MaFIA mice underwent macrophage ablation by treatment with AP20187 and received rough or rough-hydro femoral Ti implants. In vivo, Wnt ligand mRNA was higher in peri-implant tissues in mice instrumented with rough-hydro implants than rough-hydrophobic Ti implants at 1d and 3d (Figure 1). Ablation of macrophages in MaFIA mice yielded dramatically lower levels of Wnt ligand mRNA in peri-implant tissue compared at 1d, 3d, and 7d compared to control mice. These results demonstrate that macrophages contribute significantly to the milieu of Wnt ligands at the implant site, and loss of macrophage Wnt ligands affects Wnt signaling within peri-implant tissue during both immune cell-predominant (1–3d) and early osteogenic (7d) time points, which were determined by peri-implant tissue gene expression of hematopoietic markers and MSC markers (Supplemental figure 2). These findings suggest that the loss of macrophages alters microenvironmental Wnt signaling during the transition from inflammation to osteogenesis.
Figure 1.
Macrophage ablation reduces peri-implant Wnt signaling in bone. Macrophages were ablated in MaFIA mice by treatment with AP20187 daily for 3d prior to surgery and every other day post-operatively. Control groups only underwent the 3d ablation process, while sham, rough, and rough-hydro groups underwent treatment until sacrifice at 1, 3 or 7d post-surgery (n=6 per group). Expression of Wnt ligand genes in peri-implant tissue was measured using qPCR. Results are normalized to expression of the housekeeping gene Gapdh and presented as fold-change (2-ΔΔCt). A) Heat map demonstrating the change in Wnt gene expression for with macrophage ablation in Sham, Rough, and Rough-Hydro peri-implant tissue. B) Comparison of Wnt gene expression on Rough-Hydro surfaces in Control (green) and Ablated (orange) animals. p<0.05: A vs. respective Control.
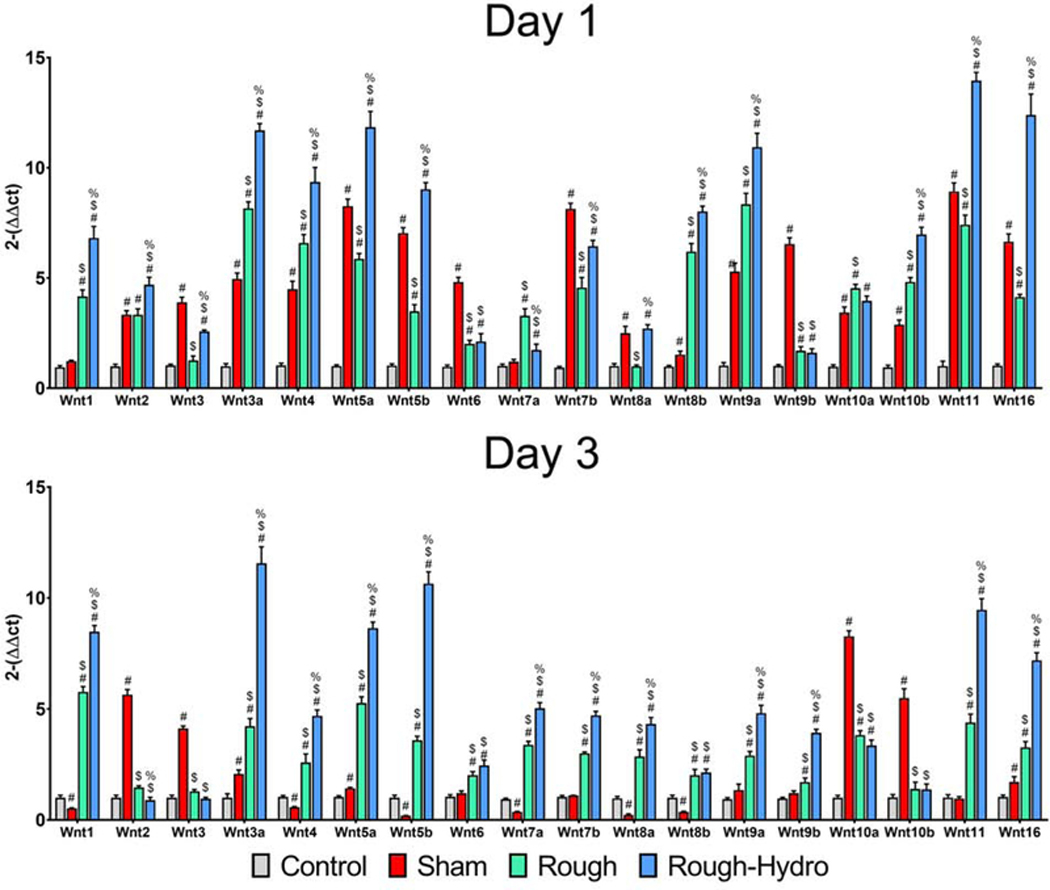
3.2. Surface properties induce differential Wnt signaling in macrophages in vivo
To understand how surface properties alter macrophage Wnt expression in vivo, C57Bl/6 mice were instrumented with rough or rough-hydro Ti intramedullary femoral implants, and macrophages were isolated from harvested implants 1d or 3d post-operatively. Quantification of mRNA for several Wnt genes revealed surface modification-dependent upregulation (Figure 2). Nearly all Wnt genes were upregulated from rough to rough-hydro surfaces, but the highest were Wnt3a. −4, −5a, −5b, −9a, −11, −16. These findings suggest that Wnt genes and their products serve an early inflammatory purpose in the context of bone-dwelling implants and are regulated by physical cues.
Figure 2.
Macrophages upregulate Wnt ligand expression in response to modified Ti surfaces in vivo. C57Bl/6 mice were instrumented with rough or rough-hydro Ti implants, and peri-implant tissue was collected at 1, 3, or 7d (n=6 per group). Macrophages were isolated by cell sorting using CD11b-APC antibody and subsequent anti-APC magnetic nanobeads. Expression of Wnt ligand genes in peri-implant tissue was measured using qPCR. Results are normalized to expression of the housekeeping gene Gapdh and presented as fold-change (2-ΔΔCt). p<0.05: # vs. respective Control, $ vs. respective Sham, % vs. respective Rough.
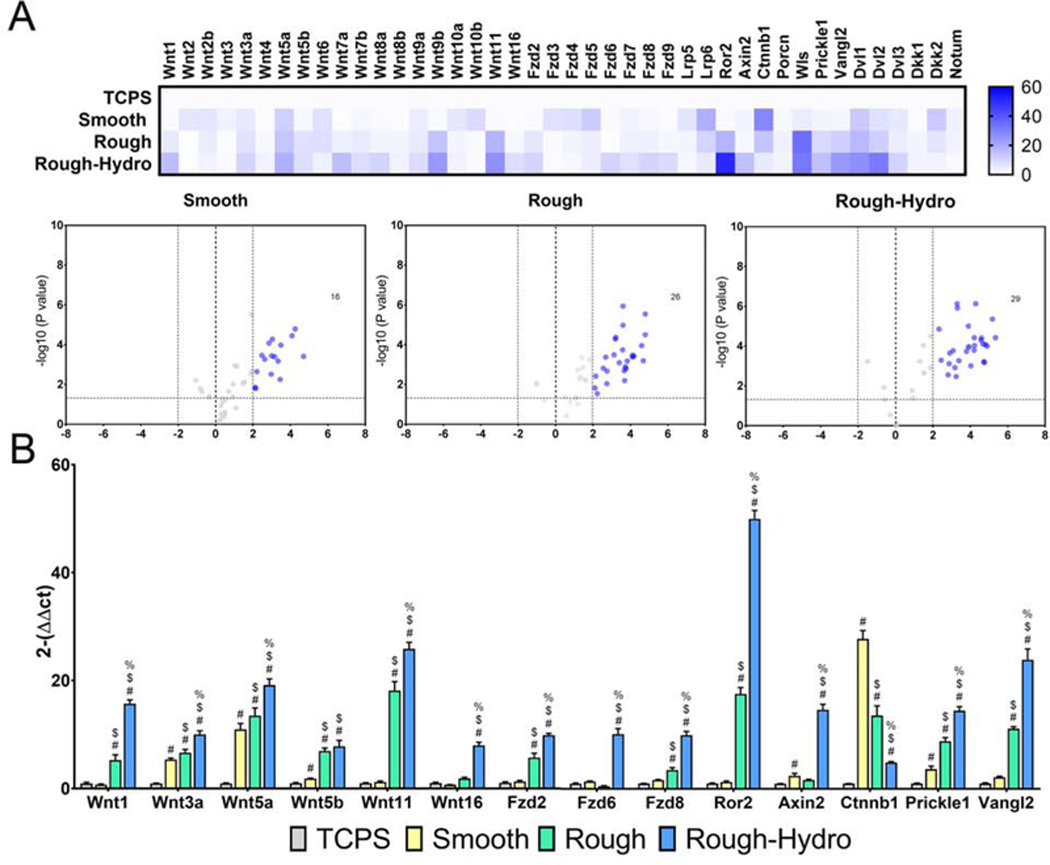
3.3. Surface modifications alter macrophage Wnt signaling-related gene expression
To characterize the effect of smooth, rough, and rough-hydro surface modification on macrophage Wnt signaling in vitro, mRNA for Wnt ligands, production of ligands, receptors, antagonists, and downstream mediators of Wnt signaling in macrophages was quantified. The presence of surface modifications significantly altered several genes related to Wnt signaling: 16 genes were upregulated on smooth, 26 on rough, and 29 on rough-hydro surfaces (Figure 3A). Wnt1, −3a, −5a, −5b, −11, and −16 were upregulated in a surface-dependent manner like that found in vivo (Figure 3B). Wnt signaling-related genes Fzd2, −6, −7, −8, −9, Ror2, Axin2, Prickle, Vangl2, Dvl1, −2, −3, and Dkk1 were regulated in a similar fashion. Conversely, expression of Fzd4, −5, Lrp5, Lrp6, Ctnnb1, Dkk2, and Notum decreased in a surface modification-dependent fashion from smooth to rough to rough-hydro. Ti surface modifications significantly altered Wnt-related gene expression in macrophages, suggesting a role for both canonical and noncanonical Wnt signaling during macrophage polarization on modified Ti surfaces.
Figure 3.
Macrophages modulate Wnt-related gene expression in response to modified Ti surfaces in vitro. Primary macrophages were grown from whole marrow isolated from C57Bl/6 mice using macrophage colony stimulating factor. Following 7d of culture, macrophages were plated on smooth, rough, or rough-hydro Ti disks, with tissue culture polystyrene (TCPS) serving as control (n=6 per group). Expression of Wnt-related genes was measured using qPCR. Results are normalized to expression of the housekeeping gene Gapdh and presented as fold-change (2-ΔΔCt). (A) Heat map and volcano plot summarizing changes in macrophage gene expression on smooth, rough, or roughhydro surfaces. (B) Changes in gene expression of Wnt ligands, Wnt receptors, Wnt signaling downstream mediators, and Wnt signaling inhibitors. p<0.05: # vs. respective Control, $ vs. respective Smooth, % vs. respective Rough.
3.4. Treatment with recombinant Wnt ligands alters macrophage phenotype
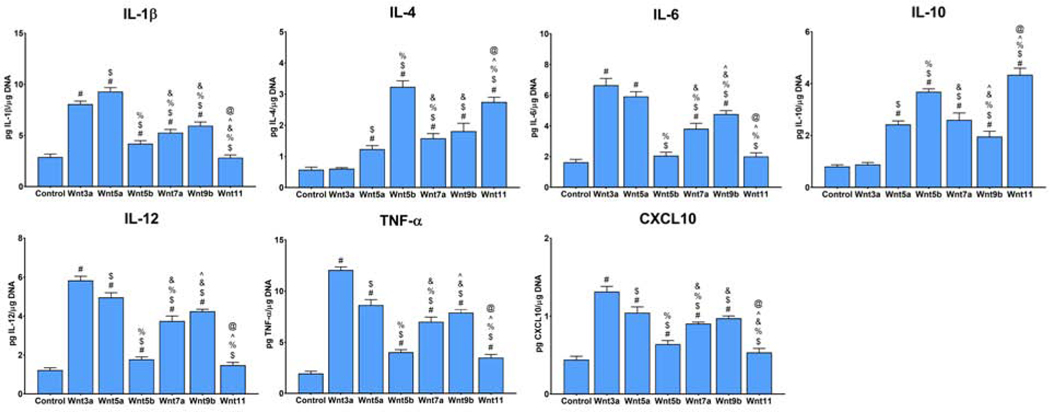
To understand the significance of Wnts to the macrophageal polarization response to surface modifications, macrophages were treated with recombinant ligands found to be upregulated on modified Ti surfaces: Wnt3a, Wnt5a, Wnt5b, Wnt7a, Wnt9b, and Wnt11. First, secretion of IL-1β, IL-12, TNF-α, CXCL-10, IL-4, and IL-12 were measured by ELISA (Figure 4).
Figure 4.
Treatment with recombinant Wnt ligands alters expression of polarization gene markers. Primary macrophages cultured on TCPS were treated with recombinant Wnt3a, −5a, −5b, −7a, −9b, or −11 for 1d prior to harvest. ELISA was performed to measure pro-(IL-1β, IL-12, TNF-α, CXCL-10) and anti-inflammatory (IL-4, IL-10) protein secretion. Results are normalized to DNA quantity in each sample. p<0.05: # vs. Control, $ vs. Wnt3a, % vs. Wnt5a, & vs. Wnt5b, ^ vs. Wnt7a, and @ vs. Wnt9b.
Treatment with Wnt3a and −5a primarily enhanced production of pro-inflammatory IL-1β, IL-12, TNF-α, CXCL-10, while treatment with Wnt5b and −11 predominantly enhanced anti-inflammatory IL-4 and IL-10 secretion. Despite their upregulation in surfaces in previous experiments, recombinant Wnt7a and Wnt9b weakly increased macrophage polarization, suggesting a role in regulation of other surface-dependent behaviors. Next, gene expression of anti-inflammatory or M2-associated markers Il13, Arg1, Chil3, Mrc1, Retnla, and Tgfb1 were measured in Wnt3a, −5a, −5b, or −11-treated cells, the Wnt ligands with the highest polarizing effect on macrophages, to confirm the effects on polarization (Supplemental figure 3). These findings suggest that some Wnt ligands are capable of driving macrophage polarization in the absence of other stimuli, suggesting a role for this ligand class as an intermediate step in regulation of macrophage phenotype. Additionally, these data suggest that Wnt3a and −5a preferentially induce pro-inflammatory polarization, while Wnt5b and Wnt11 predominantly induce an anti-inflammatory phenotype.
3.5. Inhibition of Wnt signaling disrupts classical and surface-mediated macrophage polarization
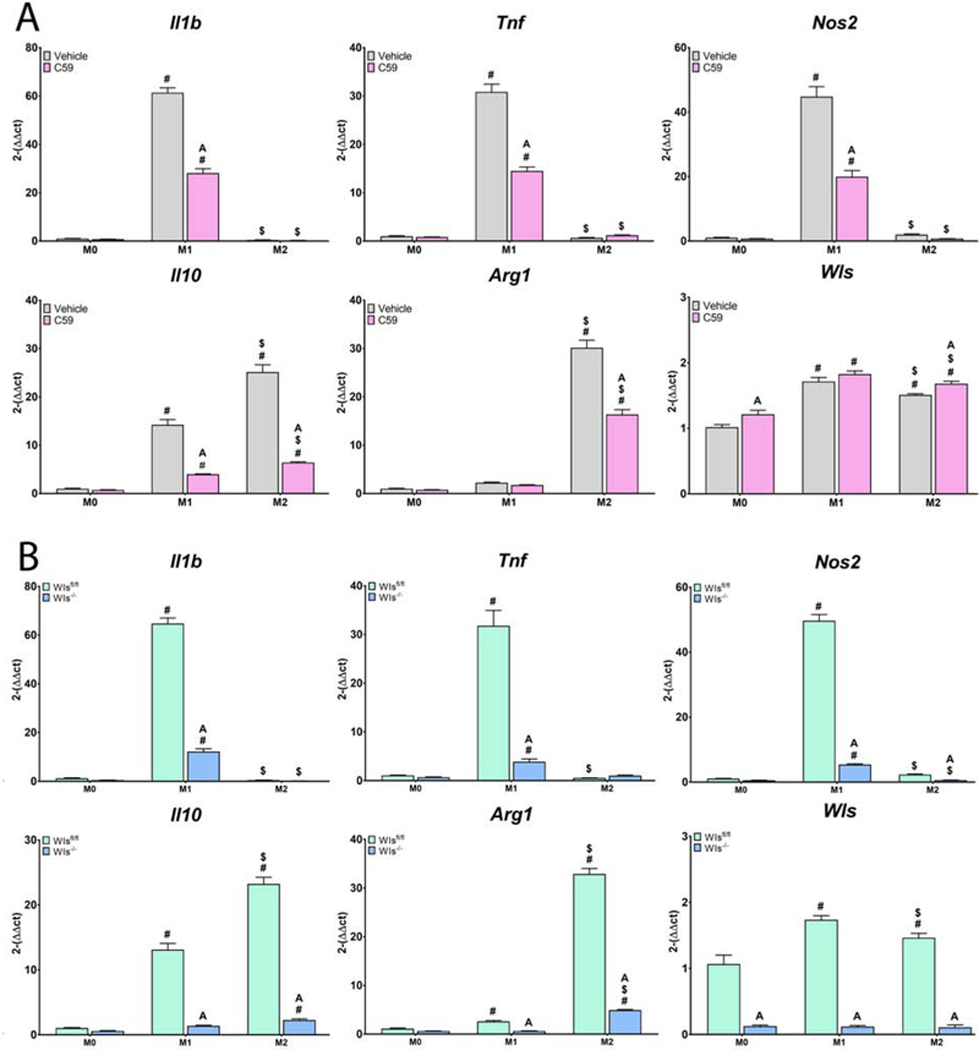
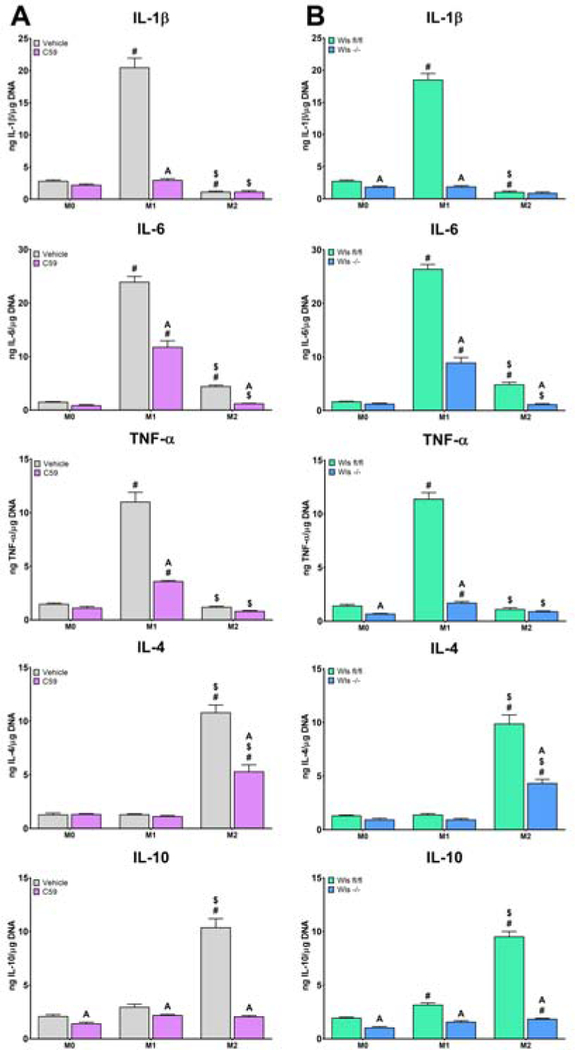
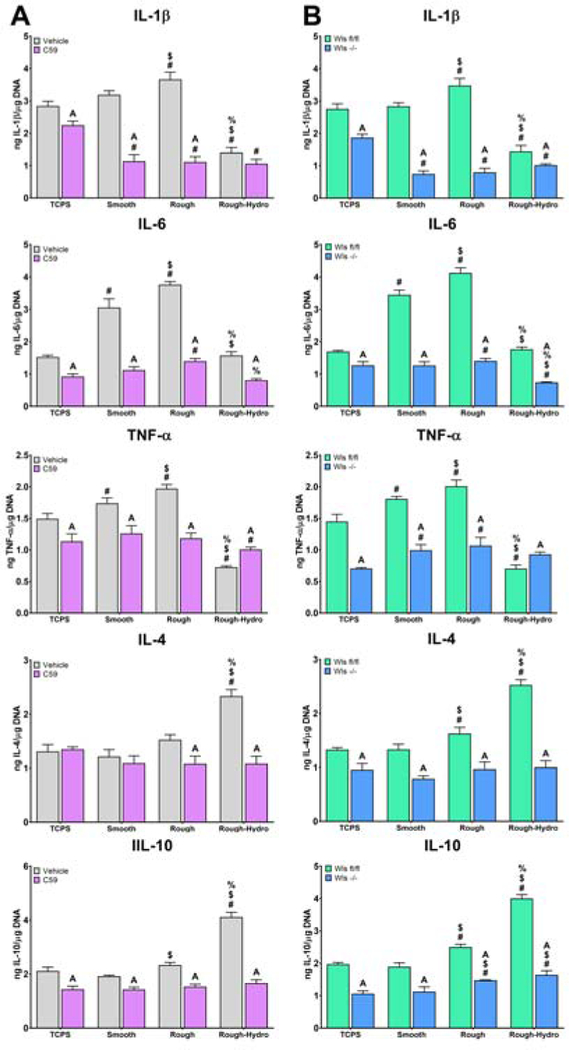
We next explored the effects of Wnt inhibition on macrophage response to both classical M1/M2 and surface-mediated polarization stimuli using Wnt-C59 or by knockout of Wls in Csf1-rexpresing cells. Classical M1 or M2 polarization was achieved by adding LPS or IL-4/IL-13, respectively, to the media of cells cultured on TCPS. Inhibition of macrophage secretion attenuated both M1 and M2 polarization, as evidenced by decreased expression of Il1b, Tnf, Nos2, and Il10 in LPS-exposed macrophages, and decreased expression of Il10 and Arg1 in IL-4/13-exposed macrophages (Figure 5). This attenuation of classical polarization was further evidenced by decreased IL-1β, IL-6, and TNF-α protein secretion in M1-polarized macrophages, as well as decreased IL-4 and IL-10 production in Wnt-inhibited M2-polarized macrophages (Figure 6).
Figure 5.
Loss of Wnt signaling attenuates inflammatory gene expression in response to classical polarizing stimuli. Macrophageal Wnt secretion was inhibited A) pharmacologically using Wnt-C59 or B) genetically by treating Csf1riCre+; Wlsfl/fl mice with tamoxifen prior to culture of macrophages. Wls expression was used to verify pharmacological inhibition of Wnt secretion, as evidenced by its upregulation upon treatment with Wnt-C59, as well as genetic knockout of Wls, as evidenced by nearly absent expression. Pro-(Il1b, Tnf, Nos2) or anti-inflammatory (Il10, Arg1) genes in response to M1 (lipopolysaccharide) or M2 (Interleukin-4 and −13) stimuli. p<0.05: # vs. M0, $ vs. M1, % vs. M2, A vs. Control of corresponding group.
Figure 6.
Loss of Wnt signaling attenuates inflammatory cytokine production in response to classical polarizing stimuli. Macrophageal Wnt secretion was inhibited A) pharmacologically using Wnt-C59 or B) genetically by treating Csf1r-iCre+; Wlsfl/fl mice with tamoxifen prior to culture of macrophages. Pro-(IL-1β, IL-6, TNF-α) and anti-inflammatory (IL-4, IL-10) cytokines were measured by ELISA and normalized to DNA quantity in each sample (n=6 per group). p<0.05: # vs. M0, $ vs. M1, % vs. M2, A vs. Control of corresponding group.
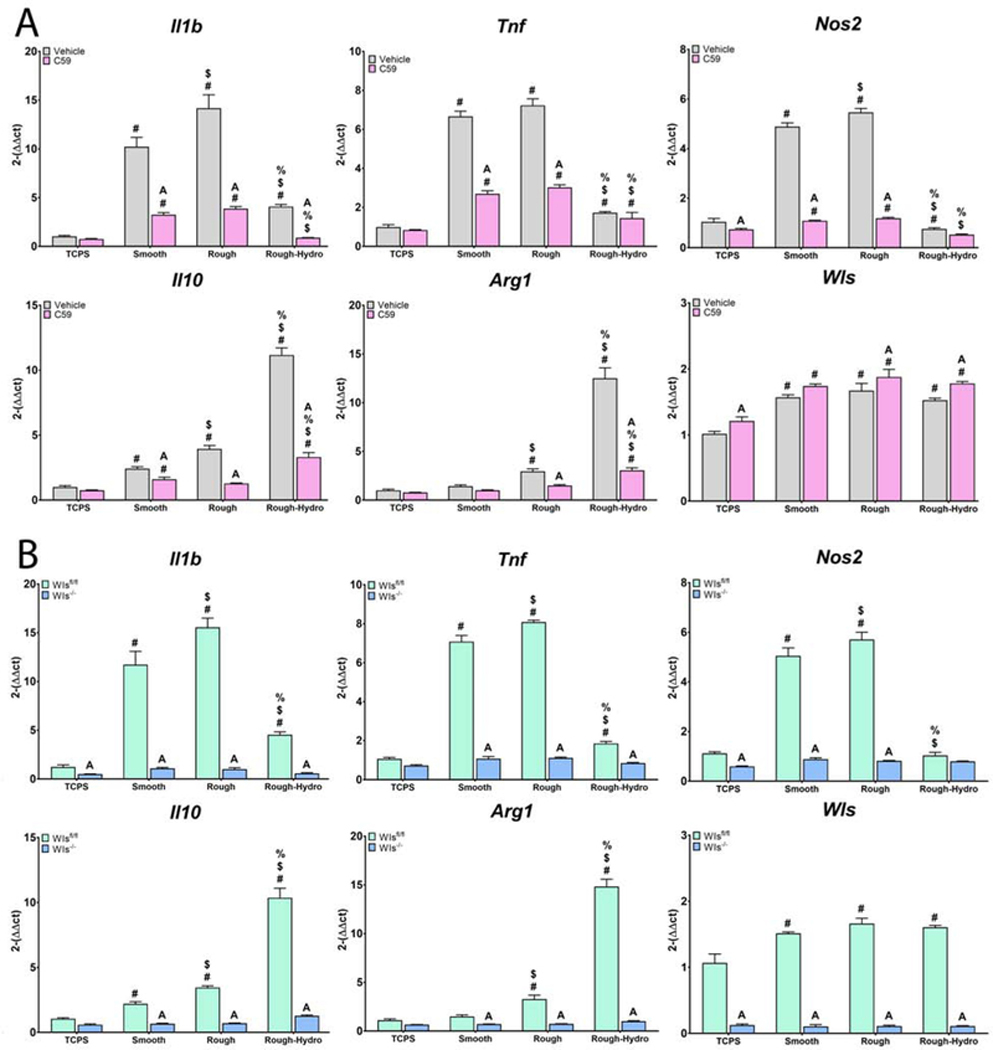
Next, surface-mediated polarization was achieved by culturing cells on smooth, rough, or rough-hydro Ti surfaces. Inhibition of macrophage Wnt secretion prior to exposure to surface stimuli attenuated the resultant polarization response compared to untreated cells, as demonstrated by attenuated upregulation of pro-inflammatory (Il1b, Tnf, Nos2) genes on smooth and rough Ti as well as anti-inflammatory (Il10, Arg1) genes on rough and rough-hydro (Figure 7). This attenuation of surface-mediated polarization was further evidenced by decreased IL-1β, IL-6, and TNF-α protein secretion by macrophages on smooth and rough surfaces and decreased IL-4 and IL-10 production on rough and rough-hydro surfaces (Figure 8). These results suggest that autocrine Wnt signaling serves as a common regulator of macrophage polarization to both chemical and physical stimuli and that loss of Wnt signaling impairs macrophages’ responsiveness to cytokine and surface cues.
Figure 7.
Loss of Wnt signaling attenuates inflammatory gene expression in response to Ti surface cues. Macrophageal Wnt secretion was inhibited A) pharmacologically using Wnt-C59 or B) genetically by treating Csf1riCre+; Wlsfl/fl mice with tamoxifen prior to culture of macrophages. Wls expression was used to verify pharmacological inhibition of Wnt secretion, as evidenced by its upregulation upon treatment with Wnt-C59, as well as genetic knockout of Wls, as evidenced by nearly absent expression. Pro-(Il1b, Tnf, Nos2) or anti-inflammatory (Il10, Arg1) genes in response to smooth, rough, or rough-hydro Ti surface modifications. p<0.05: # vs. TCPS, $ vs. Smooth, % vs. Rough, A vs. Control of corresponding group.
Figure 8.
Loss of Wnt signaling attenuates inflammatory cytokine production in response to Ti surface cues. Macrophageal Wnt secretion was inhibited A) pharmacologically using Wnt-C59 or B) genetically by treating Csf1riCre+; Wlsfl/fl mice with tamoxifen prior to culture of macrophages. Pro-(IL-1β, IL-6, TNF-α) and anti-inflammatory (IL4, IL-10) cytokines were measured by ELISA and normalized to DNA quantity in each sample (n=6 per group). p<0.05: # vs. TCPS, $ vs. Smooth, % vs. Rough, A vs. Control of corresponding group.
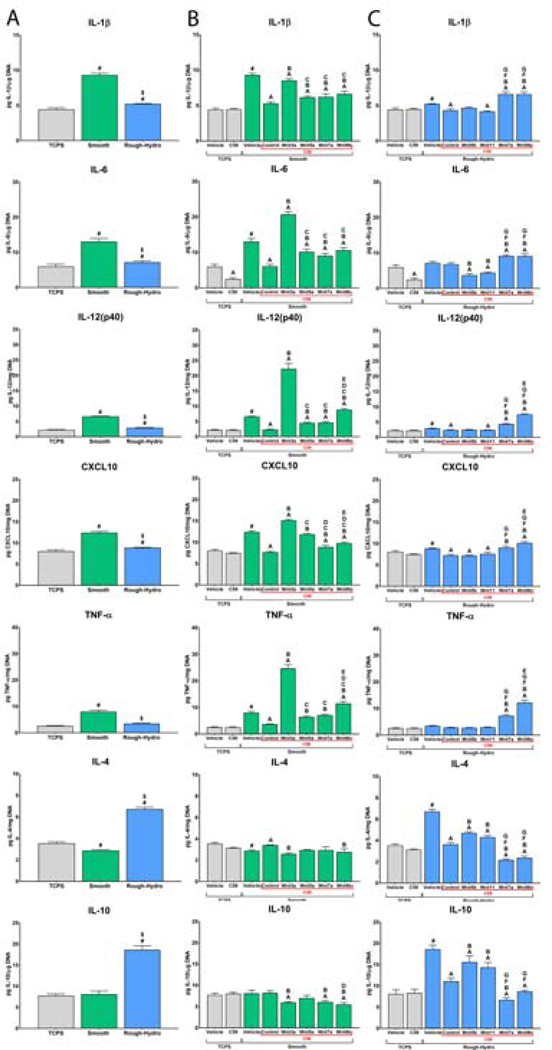
3.6. Recombinant Wnts rescue impaired polarization of Wnt-inhibited macrophages
To determine if impaired macrophage polarization by inhibited Wnt secretion was rescuable, Wnt-C59-treated macrophages were cultured on modified Ti surfaces in media supplemented with recombinant Wnt ligands. First, culture on smooth Ti enhanced macrophage pro-inflammatory activation, as evidenced by increased IL-1β, IL-6, IL-12(p40), CXCL-10, and TNF-α, while culture on rough-hydro Ti enhanced IL-4 and IL-10 production (Figure 9A). Next, based on findings presented in Figure 4, macrophages on smooth Ti were treated with either Wnt3a or Wnt5a, while macrophages on rough-hydro surfaces were treated with Wnt5b or Wnt11. Treatment of Wnt-inhibited macrophages with Wnt3a while on smooth Ti nearly entirely rescued pro-inflammatory activation; Wnt5a also rescued pro-inflammatory polarization, albeit to a lesser extent (Figure 9B). Similarly, Wnt-inhibited macrophages recovered their anti-inflammatory phenotype when treated with recombinant Wnt5b or Wnt11. In contrast, treatment with Wnt7a or −9b did little to rescue polarization to either phenotype on their respective surface. These data support that certain Wnts serve a mediatory role in Ti surface-stimulated macrophage polarization. However, rescue was not fully achieved by any single Wnt ligand treatment, suggesting a tandem of Wnts controls the phenotypic response of macrophages to these physical cues.
Figure 9.
Recombinant Wnt ligands rescue impaired Wnt-inhibited macrophage polarization. Macrophages were treated with 500nM Wnt-C59 or equivalent volume of DMSO vehicle and plated on smooth or rough-hydro surfaces. After 2 hours, macrophages were (A) left untreated to observe only surface effects, treated with (B) Wnt3a, −5a, −7a, or −9b on smooth surfaces, or (C) Wnt5b, −7a, −9b, or −11 on rough-hydro surfaces for 24 hours. Pro-(IL-1β, IL-6, IL12(p40), CXCL-10, TNF-α) and anti-inflammatory (IL-4, IL-10) chemokines and cytokines were measured by ELISA and normalized to DNA quantity in each sample (n=6 per group). p<0.05: # vs. TCPS-Vehicle, A vs. vehicle of corresponding vehicle group, B vs. control corresponding Wnt-C59-treated group, C vs. Wnt-C59 + Wnt3a group, D vs. Wnt-C59 + Wnt5a group, E vs. Wnt-C59 + Wnt7a group, F vs. Wnt-C59 + Wnt5b group, G vs. Wnt-C59 + Wnt11 group.
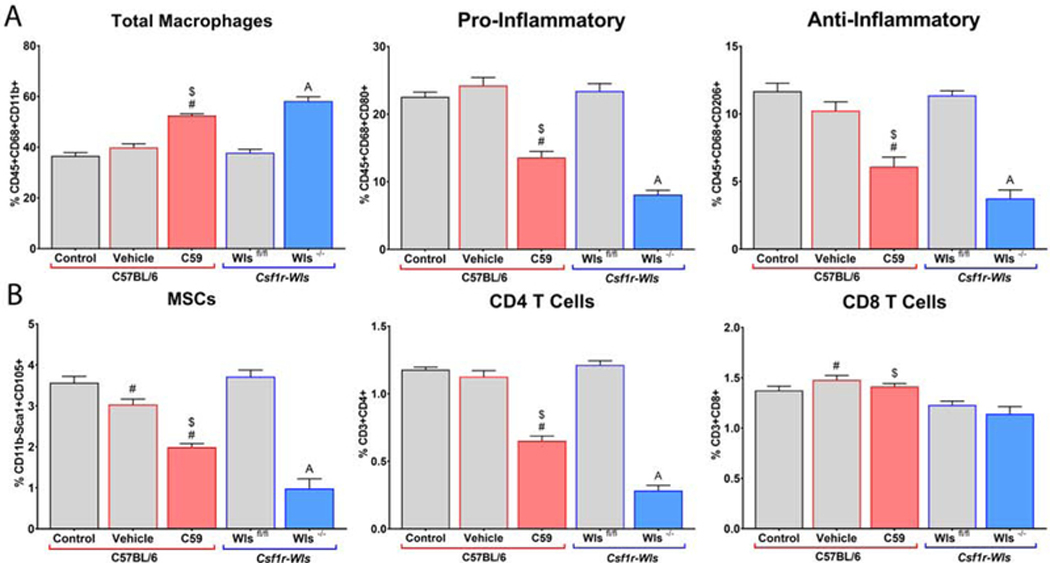
3.7. Loss of macrophage Wnts impairs polarization and cell recruitment in vivo
To characterize the functional consequences of loss of Wnt ligands on the response to implants in vivo, global Wnt signaling was blocked pharmacologically by intraperitoneal injection with Wnt-C59 or specifically from macrophages in tamoxifen-treated Csf1r-iCre+; Wlsfl/fl mice instrumented with rough Ti implants. Rough implants were elected as they are the most common class of modified Ti used clinically. Flow cytometric analysis of cells isolated from the implant surface 7d post-operatively revealed an increased proportion of total macrophages, but decreased proportions of both pro-and anti-inflammatory polarized macrophages when Wnt signaling was inhibited by both methods (Figure 10A). Furthermore, flow cytometry revealed decreased proportions of mesenchymal stem cells and CD4 T-cells in Wnt-inhibited groups (Figure 10B). We have shown previously that macrophages are necessary for the proper recruitment of these cell types to the implant site regardless of the surface modification, and together with previous data, these findings suggest that Wnt ligands are responsible for modifying implant-associated macrophage phenotypes to ensure the proper recruitment of cells and progression of the integrative process. Specifically, the decrease in peri-implant Wnt signaling at 7d in macrophage-ablated animals in Figure 1 is likely due to decreased recruitment of MSCs, which use Wnt signaling to differentiate into bone-forming osteoblasts.
Figure 10.
Wnt signaling regulates macrophage polarization and cell recruitment to rough Ti implants. C57Bl/6 mice were treated with Wnt-C59 or DMSO vehicle for 3d prior to and every other day following implantation. Csf1r-iCre+; Wlsfl/fl experimental mice and Csf1r-iCre-; Wlsfl/fl littermate control mice were injected with tamoxifen for 5d prior to implantation (n=6 per group). Cells were isolated from implant surfaces 7d post-operatively and subjected to flow cytometry. A) Total Macrophages were identified as CD68+, Pro-Inflammatory as CD68+/CD80+, and AntiInflammatory as CD68+/CD206+. B) MSCs were identified as CD11b-/Sca-1+/CD105+, CD4 T-cells as CD3+/CD4+, and CD8 T-cells as CD3+/CD8a+. p<0.05: # vs. Control, $ vs. Vehicle, A vs. Wlsfl/fl.
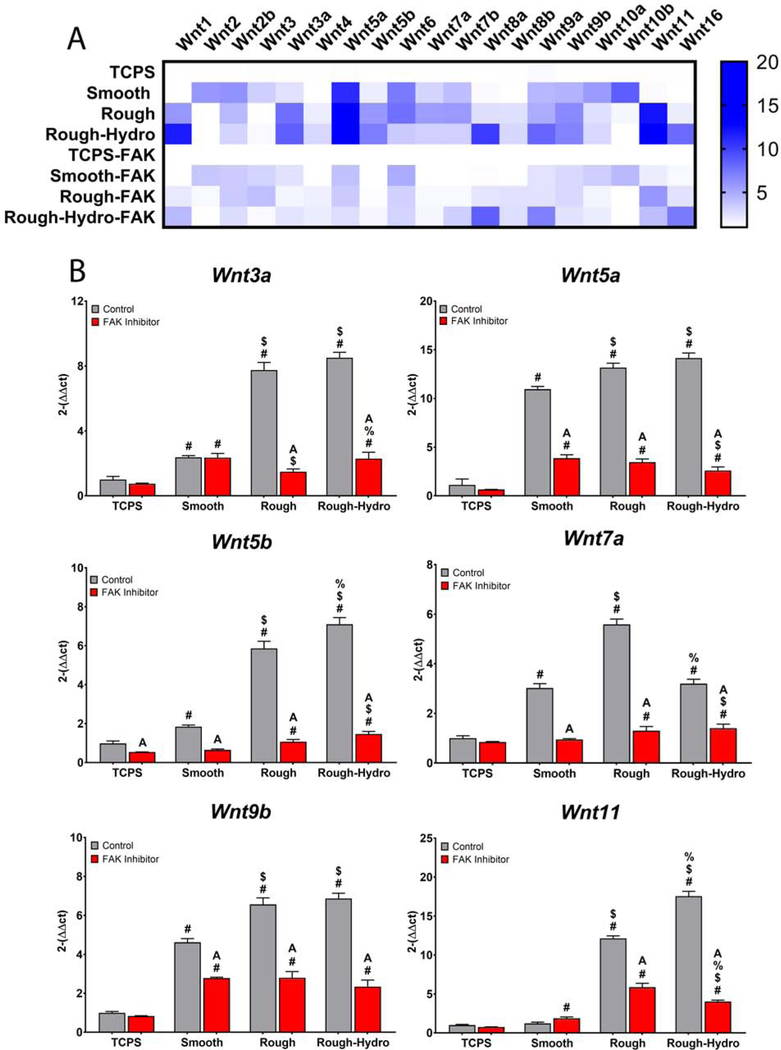
3.8. Inhibition of Focal Adhesion Kinase Decreases Surface-Dependent Wnt Regulation
Finally, to identify a mechanism by which macrophage upregulate Wnt production in response to biomaterial surface characteristics, macrophages were treated with PF-573228, a small molecule FAK inhibitor, and plated on Ti surfaces. Compared to untreated cells on all surfaces, treatment with FAK inhibitor decreased expression of several Wnt genes (Figure 11); interestingly, genes for all the previously explored Wnt ligands, Wnt3a, −5a, −5b, −7a, −9b, and 11 were less upregulated with FAK inhibitor treatment (Figure 11B). However, other Wnt ligands such as Wnt2a, Wnt2b, Wnt, and Wnt10b were barely affected by FAK inhibition, indicating that these Wnt ligands do not contribute to the macrophage polarization observed in response to biomaterial surface properties. These results reveal that integrin/FAK signaling plays a role in the surface-dependent induction of Wnt expression in macrophages.
Figure 11.
Focal adhesion kinase inhibition impairs surface-dependent Wnt upregulation. Macrophages were treated with 5uM PF-573228 and plated on surfaces for 24 hours prior to harvest and qPCR (n=6 per group). A) Heat map summarizing changes in expression of 19 Wnt-encoding genes in control and FAK-inhibited macrophages. B) Comparison of 6 Wnt ligands previously shown to be involved in macrophage surface-mediated polarization. p<0.05: # vs. TCPS, $ vs. Smooth, % vs. Rough, A vs. Control of corresponding group.
4. Discussion
While macrophages and Wnt ligands have long been known to play separate, critical roles in osseointegration, no studies have yet explored how macrophages might contribute Wnts to bone healing. Although the loss of macrophage Wnt signaling has been tied to phenotypic changes in macrophages in other disease models, the importance of altered macrophage polarization on modified Ti surfaces in the context of Wnt signaling during osseointegration is also unknown. Here, we demonstrate that macrophages significantly contribute to Wnt signaling in the peri-implant microenvironment in vivo, that biomaterial surface properties modulate Wnt signaling gene expression in macrophages, and that Wnt ligands can induce the production of pro-and anti-inflammatory cytokines characteristic of polarized macrophages. We also show that Wnt signaling plays a vital role in both classical and surface-mediated macrophage activation, and that the loss of polarization by Wnt inhibition may be partially rescued by recombinant Wnt ligands. We then show in vivo that the loss of macrophage-derived Wnt ligands impairs the recruitment of MSCs and CD4 T-cells. Finally, we demonstrate that integrin signaling, a mediating factor in other cell responses to topographical cues, also regulates surface-dependent Wnt expression in macrophages. Our data suggest that Wnt signaling is used by macrophages to modulate pro-or anti-inflammatory activation on Ti biomaterials and that Wnt ligands are key signaling molecules used by macrophages to regulate inflammatory, wound healing, and regenerative microenvironments.
We previously demonstrated that macrophages alter their secretory contributions to the implant microenvironment in response to varied biomaterial surface characteristics [19,20]. Macrophages grown on smooth Ti surfaces produced a pro-inflammatory microenvironment and that rough surfaces resulted in an increase in anti-inflammatory cytokine production. We also showed that surface wettability had the greatest effect on macrophage activation, with hydrophobic Ti surfaces yielding a predominantly pro-inflammatory environment and hydrophilic Ti surfaces activating macrophages into an anti-inflammatory phenotype. In the present study, we found that biomaterial surface characteristics like roughness and hydrophilicity modulate the expression of Wnt ligands, receptors, and associated molecules. Interestingly, the expression of Wnt signaling molecules was affected by material composition (TCPS vs. Ti), suggesting that changes in Wnt expression may be associated with protein adsorption onto the biomaterial. In addition, macrophages grown on rough-hydro surfaces upregulate many non-canonical Wnt ligands, suggesting that their mechanistic sensitivity may be Wnt-dependent, as non-canonical ligands are more related to mechanotransduction via Rock and Jnk1. This finding appears to agree with other studies demonstrating that mechanical cues modulate the phenotype of various other cell types through non-canonical Wnt signaling [55–57]. Indeed, pro-and anti-inflammatory macrophage phenotypes have been shown to have morphologies as characteristically distinct as their cytokine secretions and surface markers [58–60], suggesting a similar role here.
In this study, we sought to characterize the effects of Wnt ligands on macrophages. This is particularly important in osseous healing and regeneration because anti-inflammatory macrophage phenotypes have been shown to promote MSC-to-osteoblast differentiation [61]. We see that Wnt ligands activating both canonical and non-canonical pathways alter the phenotype of bone marrow-derived macrophages and that surface modifications alter macrophage Wnt signaling, suggesting that differential regulation of Wnt signaling drives aspects of macrophage activation on Ti surfaces. While we have characterized the importance of Wnt ligands to autocrine regulation of macrophage phenotype here, future studies should explore the importance of macrophage-derived Wnts on other cell types. While Figure 1 reveals that loss of macrophages is associated with significantly attenuated Wnt signaling during the inflammatory response to implants on days 1 and 3, the sustained decrease in Wnt expression through day 7 certainly suggests that macrophages serve to enhance Wnt signaling during osteogenesis; this remains to be explored. Additionally, recent literature has suggested the possibility of TLR4-agonizing contaminants in commercially available recombinant Wnts affecting studies of immune cell polarization in response to this family of ligands [62]. However, our data reveal that some—but not all—induce pro-inflammatory activation, while recombinant Wnt5b and Wnt11 elicit a strong anti-inflammatory polarization, suggesting the polarizing effect is ligand-dependent and not via endotoxin.
The role of Wnt signaling on macrophages in inflammatory diseases or homeostasis is unclear and the few reports available have seemingly contradictory results [36–38,45–47,63]. Studies using mice with phagocytic cell-specific genetic knockouts for Wls and Porcn have yielded conflicting results regarding the effect of loss of Wnt ligands on macrophage activation. During the response to myocardial infarct, macrophages isolated from macrophage-Wls−/− mice exhibited a more anti-inflammatory phenotype, although they also secreted higher levels of IL-6 [38]. Cardiac remodeling was enhanced in these mice, with tissue from the previously injured area exhibiting enhanced vascularity compared to control mice. However, in a murine liver injury model, hepatic tissue from macrophage-Wls−/− mice did not exhibit differential pro-inflammatory cytokine gene expression from control, and the resultant tissue was predominantly ductular instead of hepatocyte-laden, indicating a loss of regenerative capability without macrophage Wnts [36]. These conflicting results may be due to variation in Wnt signaling by tissue resident macrophages, whose phenotypes are uniquely adapted to the tissue they inhabit and differ from those of similarly-polarized monocyte-derived macrophages [64–66]. Conflicting results in these studies and others may also be due to both the experimental mechanism of Wnt ablation and the cell in which secretion is ablated; dysfunction of Wls has been shown to lead to ER stress in embryonic Drosophila cells [39], but not pharmacologic inhibition of Porcn in carcinoma cells [67]. These discrepancies underscore the oversimplification of the role of Wnt-secreting proteins and the need for further characterization of these models. Nevertheless, our data support that macrophage-derived Wnt signaling contributes to tissue healing and regeneration by the autocrine and paracrine effects of these ligands, demonstrated through both genetic and pharmacologic knockdown of Wnt secretion.
Our results reveal that loss of Wnt signaling by Porcn inhibition alters macrophage behavior in response to various stimuli in vitro and impaired behavior in vivo. Ablation of Wnt secretion by Porcn inhibition and macrophage Wls knockout attenuated indicators of classical M1/M2 macrophage activation as well our paradigm of surface-dependent anti-inflammatory polarization. These results suggest that Wnt ligands play a fundamental role in macrophage activation and production of an anti-inflammatory microenvironment in response to the chemical and physical characteristics of implanted biomaterials. Furthermore, our in vivo results showed that systemic ablation of macrophages resulted in decreased Wnt molecule expression in the injury and implantation site, indicating that Wnt signaling in the initial stages after implantation is heavily modulated by macrophages. We also found that cells isolated from biomaterials with rough-hydrophilic modifications exhibit a very similar pattern of Wnt expression compared to cells isolated from the injury site in sham animals, suggesting that macrophage-derived Wnt ligands are important to bone healing generally. The biomimetic nature of rough-hydrophilic biomaterials appears to preserve appropriate macrophage Wnt ligand production, producing a phenotype amenable to osseointegration.
Mechanistically, we hypothesize that integrin-based interactions with modified Ti surfaces drives Wnt-mediated macrophage polarization, as inhibition of FAK decreases expression of a number of Wnt genes. Our findings presented here appear to agree with the role of Wnt in phenotypic changes by MSCs in response to surface cues [43]; Wnt ligands, while not necessarily needed to initiate physical cue-dependent responses, serve to amplify existing signals and produce a mature cellular phenotype. This intermediary role for Wnts may also explain why Wnt3a and Wnt5a are upregulated greatly by macrophages on rough-hydro Ti, but in isolation appear to promote pro-inflammatory macrophage phenotypes, as we have shown here. Our results suggest that Wnt ligands serve to fine-tune polarization in response to physical cues—not drive it exclusively. Future studies will attempt to better elucidate the mechanistic role of macrophage Wnt to surface-mediated polarization by selectively knocking out canonical and non-canonical Frizzled, LRP, or tyrosine kinase receptors associated with various Wnt ligands.
In the context of bone-dwelling materials, our data demonstrate that macrophage Wnt secretion serves as a useful metric for evaluating the inflammatory response to implants in a more mechanistic nature than measuring anti-inflammatory cytokines, as Wnts directly serve to modulate macrophage polarity. Additionally, considering its established role in regulating stem and progenitor cells in various tissues, studying the role of macrophage Wnt signaling in healing may elucidate a novel means by which macrophages orchestrate biomaterial integrative fate. Macrophage Wnt ligands have been shown to regulate wound healing, chronic inflammation, and fibrosis by activating fibroblasts, modulating angiogenesis, and inducing stem cell differentiation [35,68,69], all relevant topics to the field of biomaterials and tissue engineering.
5. Conclusion
Our data demonstrate that macrophages serve as a major driver of Wnt signaling during the osteoimmunological response to Ti implants in vivo and upregulate Wnt gene expression in response to modified Ti both in vitro and in vivo. Furthermore, we show here that loss of Wnt signaling attenuates macrophage polarization, and that Wnt ligands can modify macrophage phenotype in response to both biochemical and physical cues. We additionally demonstrate that loss of macrophageal Wnt secretion not only attenuates the polarization of macrophages on the implant surface but decreased recruitment of other cell types critical to osseous healing. These findings suggest a role for autocrine Wnt signaling in macrophages during the immune response to implanted biomaterials.
Supplementary Material
Supplemental figure 1. Dose-dependent inhibition of macrophage Wnt secretion by Wnt-C59. Primary macrophages were treated with various concentrations of Wnt-C59 dissolved in DMSO (vehicle). Wnt secretion was characterized by direct ELISA for Wnt3a and Wnt5a (n=3 per dose). Data were normalized by DNA quantity in each sample. p<0.05: # vs. Control.
Supplemental figure 2. Identification of immune cell-and osteogenic cell-predominant time points during the response to Ti implants. RNA was extracted from peri-implant tissue in MaFIA mice to measure general markers of immune cells (Cd14, Cd45, Cd34) and mesenchymal stem cells (Dlx5, Runx2, Sp7) (n=6 per group). Results are relative to Gapdh expression and presented as fold-change (2-ΔΔCt). p<0.05: # vs. respective Control, $ vs. respective Sham, % vs. respective Rough.
Supplemental figure 3. Treatment with recombinant Wnt ligands alters expression of polarization gene markers. Primary macrophages cultured on TCPS were treated with recombinant Wnt3a, −5a, −5b, or −11 for 1d prior to harvest. qPCR was performed to measure gene expression for various pro-and anti-inflammatory markers (n=6 per exposure group). Results are presented as fold-change (2-ΔΔCt) compared to Gapdh expression. p<0.05: # vs. Control, $ vs. Wnt3a, % vs. Wnt5a, & vs. Wnt5b.
Supplemental table 1. Unique assay identification codes for qPCR Primers.
6. Acknowledgment
Research reported in this publication was supported by the National Institute of Dental and Craniofacial Research of the National Institutes of Health under award number R01DE028919. Ti implants and surfaces were supplied by Institut Straumann AG, Basel, Switzerland.
Footnotes
The raw/processed data required to reproduce these findings cannot be shared at this time as the data also forms part of an ongoing study.
Declaration of interests
☒ The authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
ADDIN Mendeley Bibliography CSL_BIBLIOGRAPHY
- [1].Gruber R, Bosshardt DD, Dental Implantology and Implants -Tissue Interface, in: Stem Cell Biol. Tissue Eng. Dent. Sci., Elsevier, 2015: pp. 735–747. doi: 10.1016/B978-0-12-397157-9.00078-3. [DOI] [Google Scholar]
- [2].Sinder BP, Pettit AR, McCauley LK, Macrophages: Their Emerging Roles in Bone, J. Bone Miner. Res 30 (2015) 2140–2149. doi: 10.1002/jbmr.2735. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [3].Batoon L, Millard SM, Raggatt LJ, Pettit AR, Osteomacs and Bone Regeneration, Curr. Osteoporos. Rep 15 (2017) 385–395. doi: 10.1007/s11914-017-0384-x. [DOI] [PubMed] [Google Scholar]
- [4].Sheikh Z, Brooks PJ, Barzilay O, Fine N, Glogauer M, Macrophages, foreign body giant cells and their response to implantable biomaterials, Materials (Basel). 8 (2015) 5671–5701. doi: 10.3390/ma8095269. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [5].Ariel A, Maridonneau-Parini I, Rovere-Querini P, Levine JS, Mühl H, Macrophages in inflammation and its resolution, Front. Immunol 3 (2012) 2–3. doi: 10.3389/fimmu.2012.00324. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [6].Brancato SK, Albina JE, Wound macrophages as key regulators of repair: Origin, phenotype, and function, Am. J. Pathol 178 (2011) 19–25. doi: 10.1016/j.ajpath.2010.08.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [7].Baht GS, Vi L, Alman BA, The Role of the Immune Cells in Fracture Healing, Curr. Osteoporos. Rep 16 (2018) 138–145. doi: 10.1007/s11914-018-0423-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [8].Van Linthout S, Miteva K, Tschöpe C, Crosstalk between fibroblasts and inflammatory cells, Cardiovasc. Res 102 (2014) 258–269. doi: 10.1093/cvr/cvu062. [DOI] [PubMed] [Google Scholar]
- [9].Wynn T, Barron L, Macrophages: Master Regulators of Inflammation and Fibrosis, Semin. Liver Dis 30 (2010) 245–257. doi: 10.1055/s-0030-1255354. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [10].Wu AC, Raggatt LJ, Alexander KA, Pettit AR, Kon T, Aizawa T, Tsay A, Fitch J, Barnes GL, Graves DT, Einhorn TA, Xing Z, Lu C, Hu D, Yu Y. -y, Wang X, Colnot C, Nakamura M, Wu Y, Miclau T, Marcucio RS, Unraveling macrophage contributions to bone repair, Dis. Model. Mech 3 (2013) 1–7. doi: 10.1242/dmm.003186. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [11].Zhang X, Mosser D, Macrophage activation by endogenous danger signals, J. Pathol 214 (2008) 161–178. doi: 10.1002/path.2284. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [12].Gurtner GC, Werner S, Barrandon Y, Longaker MT, Wound repair and regeneration, Nature. 453 (2008) 314–321. doi: 10.1038/nature07039. [DOI] [PubMed] [Google Scholar]
- [13].Hesketh M, Sahin KB, West ZE, Murray RZ, Macrophage phenotypes regulate scar formation and chronic wound healing, Int. J. Mol. Sci 18 (2017) 1–10. doi: 10.3390/ijms18071545. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [14].Valentin JE, Stewart-Akers AM, Gilbert TW, Badylak SF, Macrophage Participation in the Degradation and Remodeling of Extracellular Matrix Scaffolds, Tissue Eng. Part A 15 (2009) 1687–1694. doi: 10.1089/ten.tea.2008.0419. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [15].Khan MR, Donos N, Salih V, Brett PM, The enhanced modulation of key bone matrix components by modified Titanium implant surfaces, Bone. 50 (2012) 1–8. doi: 10.1016/j.bone.2011.07.040. [DOI] [PubMed] [Google Scholar]
- [16].Jemat A, Ghazali MJ, Razali M, Otsuka Y, Surface modifications and their effects on titanium dental implants, Biomed Res. Int 2015 (2015). doi: 10.1155/2015/791725. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [17].Chrcanovic BR, Albrektsson T, Wennerberg A, Reasons for failures of oral implants, J. Oral Rehabil 41 (2014) 443–476. doi: 10.1111/joor.12157. [DOI] [PubMed] [Google Scholar]
- [18].Olivares-Navarrete R, Hyzy SL, Hutton DL, Erdman CP, Wieland M, Boyan BD, Schwartz Z, Direct and indirect effects of microstructured titanium substrates on the induction of mesenchymal stem cell differentiation towards the osteoblast lineage., Biomaterials. 31 (2010) 2728–35. doi: 10.1016/j.biomaterials.2009.12.029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [19].Hotchkiss KM, Ayad NB, Hyzy SL, Boyan BD, Olivares-Navarrete R, Dental implant surface chemistry and energy alter macrophage activation in vitro, Clin. Oral Implants Res 28 (2017) 414–423. doi: 10.1111/clr.12814. [DOI] [PubMed] [Google Scholar]
- [20].Hotchkiss KM, Reddy GB, Hyzy SL, Schwartz Z, Boyan BD, Olivares-Navarrete R, Titanium surface characteristics, including topography and wettability, alter macrophage activation, Acta Biomater. 31 (2016) 425–434. doi: 10.1016/j.actbio.2015.12.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [21].Alfarsi MA, Hamlet SM, Ivanovski S, The Effect of Platelet Proteins Released in Response to Titanium Implant Surfaces on Macrophage Pro-Inflammatory Cytokine Gene Expression, Clin. Implant Dent. Relat. Res 17 (2015) 1036–1047. doi: 10.1111/cid.12231. [DOI] [PubMed] [Google Scholar]
- [22].Chen X, Li HS, Yin Y, Feng Y, Tan XW, Macrophage proinflammatory response to the titanium alloy equipment in dental implantation, Genet. Mol. Res 14 (2015) 9155–9162. doi: 10.4238/2015.August.7.25. [DOI] [PubMed] [Google Scholar]
- [23].Kzhyshkowska J, Gudima A, Riabov V, Dollinger C, Lavalle P, Vrana NE, Macrophage responses to implants: prospects for personalized medicine, J. Leukoc. Biol 98 (2015) 953–962. doi: 10.1189/jlb.5VMR0415-166R. [DOI] [PubMed] [Google Scholar]
- [24].Landgraeber S, Jäger M, Jacobs JJ, Hallab NJ, The pathology of orthopedic implant failure is mediated by innate immune system cytokines, Mediators Inflamm. 2014 (2014). doi: 10.1155/2014/185150. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [25].Krishnan OA, Venkatesh; Byrant HU; MacDougald, Regulation of bone mass by Wnt signaling, J. Clin …. 116 (2006) 1202–1209. doi: 10.1172/JCI28551.1202. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [26].He X, Wang H, Jin T, Xu Y, Mei L, Yang J, TLR4 activation promotes bone marrow MSC proliferation and osteogenic differentiation via wnt3a and wnt5a signaling, PLoS One. 11 (2016) 1–20. doi: 10.1371/journal.pone.0149876. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [27].Okamoto M, Udagawa N, Uehara S, Maeda K, Yamashita T, Nakamichi Y, Kato H, Saito N, Minami Y, Takahashi N, Kobayashi Y, Noncanonical Wnt5a enhances Wnt/β-catenin signaling during osteoblastogenesis, Sci. Rep 4 (2014) 1–8. doi: 10.1038/srep04493. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [28].Austin TW, Solar GP, Ziegler FC, Liem L, Matthews W, A role for the Wnt gene family in hematopoiesis: expansion of multilineage progenitor cells., Blood. 89 (1997) 3624–35. [PubMed] [Google Scholar]
- [29].Chakrabarti R, Celià-Terrassa T, Kumar S, Hang X, Wei Y, Choudhury A, Hwang J, Peng J, Nixon B, Grady JJ, DeCoste C, Gao J, van Es JH, Li MO, Aifantis I, Clevers H, Kang Y, Notch ligand Dll1 mediates cross-talk between mammary stem cells and the macrophageal niche, Science (80-. ). 360 (2018) eaan4153. doi: 10.1126/science.aan4153. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [30].Kobayashi Y, Maeda K, Takahashi N, Roles of Wnt signaling in bone formation and resorption, Jpn. Dent. Sci. Rev 44 (2008) 76–82. doi: 10.1016/j.jdsr.2007.11.002. [DOI] [Google Scholar]
- [31].Lobov IB, Rao S, Carroll TJ, Vallance JE, Ito M, Ondr JK, Kurup S, Glass DA, Patel MS, Shu W, Morrisey EE, McMahon AP, Karsenty G, Lang RA, WNT7b mediates macrophage-induced programmed cell death in patterning of the vasculature, Nature. 437 (2005) 417–421. doi: 10.1038/nature03928. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [32].Muley A, Odaka Y, Lewkowich IP, Vemaraju S, Yamaguchi TP, Shawber C, Dickie BH, Lang RA, Myeloid Wnt ligands are required for normal development of dermal lymphatic vasculature, PLoS One. 12 (2017) 1–19. doi: 10.1371/journal.pone.0181549. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [33].Newman AC, Hughes CCW, Macrophages and angiogenesis: A role for Wnt signaling, Vasc. Cell 4 (2012) 1. doi: 10.1186/2045-824X-4-13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [34].Yu B, Chang J, Liu Y, Li J, Kevork K, Al-Hezaimi K, Graves DT, Park NH, Wang CY, Wnt4 signaling prevents skeletal aging and inflammation by inhibiting nuclear factor-κB, Nat. Med 20 (2014) 1009–1017. doi: 10.1038/nm.3586. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [35].Burgy O, Königshoff M, The WNT signaling pathways in wound healing and fibrosis, Matrix Biol. (2018) #pagerange#. doi: 10.1016/j.matbio.2018.03.017. [DOI] [PubMed] [Google Scholar]
- [36].Irvine KM, Clouston AD, Gadd VL, Miller GC, Wong W-Y, Melino M, Maradana MR, MacDonald K, Lang RA, Sweet MJ, Blumenthal A, Powell EE, Deletion of Wntless in myeloid cells exacerbates liver fibrosis and the ductular reaction in chronic liver injury, Fibrogenesis Tissue Repair. 8 (2015) 19. doi: 10.1186/s13069-015-0036-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [37].Lin S-L, Li B, Rao S, Yeo E-J, Hudson TE, Nowlin BT, Pei H, Chen L, Zheng JJ, Carroll TJ, Pollard JW, McMahon AP, Lang RA, Duffield JS, Macrophage Wnt7b is critical for kidney repair and regeneration, Proc. Natl. Acad. Sci 107 (2010) 4194–4199. doi: 10.1073/pnas.0912228107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [38].Palevski D, Levin-Kotler LP, Kain D, Naftali-Shani N, Landa N, Ben-Mordechai T, Konfino T, Holbova R, Molotski N, Rosin-Arbesfeld R, Lang RA, Leor J, Loss of macrophage Wnt secretion improves remodeling and function after myocardial infarction in mice, J. Am. Heart Assoc 6 (2017) 1–18. doi: 10.1161/JAHA.116.004387. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [39].Zhang P, Zhou L, Pei C, Lin X, Yuan Z, Dysfunction of Wntless triggers the retrograde Golgi-to-ER transport of Wingless and induces ER stress, Sci. Rep 6 (2016) 19418. doi: 10.1038/srep19418. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [40].Brandenburg J, Reiling N, The Wnt Blows: On the Functional Role of Wnt Signaling in Mycobacterium tuberculosis Infection and Beyond., Front. Immunol. 7 (2016) 635. doi: 10.3389/fimmu.2016.00635. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [41].Schaale K, Neumann J, Schneider D, Ehlers S, Reiling N, Wnt signaling in macrophages: Augmenting and inhibiting mycobacteria-induced inflammatory responses, Eur. J. Cell Biol 90 (2011) 553–559. doi: 10.1016/j.ejcb.2010.11.004. [DOI] [PubMed] [Google Scholar]
- [42].Du J, Zu Y, Li J, Du S, Xu Y, Zhang L, Jiang L, Wang Z, Chien S, Yang C, Extracellular matrix stiffness dictates Wnt expression through integrin pathway, Sci. Rep 6 (2016) 20395. doi: 10.1038/srep20395. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [43].Saidak Z, Le Henaff C, Azzi S, Marty C, Da Nascimento S, Sonnet P, Marie PJ, Wnt/β-Catenin Signaling Mediates Osteoblast Differentiation Triggered by Peptide-induced α5β1 Integrin Priming in Mesenchymal Skeletal Cells, J. Biol. Chem 290 (2015) 6903–6912. doi: 10.1074/jbc.M114.621219. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [44].Sorcini D, Bruscoli S, Frammartino T, Cimino M, Mazzon E, Galuppo M, Bramanti P, Al-Banchaabouchi M, Farley D, Ermakova O, Britanova O, Izraelson M, Chudakov D, Biagioli M, Sportoletti P, Flamini S, Raspa M, Scavizzi F, Nerlov C, Migliorati G, Riccardi C, Bereshchenko O, Wnt/β-Catenin Signaling Induces Integrin α4β1 in T Cells and Promotes a Progressive Neuroinflammatory Disease in Mice, J. Immunol 199 (2017) 3031–3041. doi: 10.4049/jimmunol.1700247. [DOI] [PubMed] [Google Scholar]
- [45].Yang D, Fu W, Li L, Xia X, Liao Q, Yue R, Chen H, Chen X, An S, Zeng C, Wang WE, Therapeutic effect of a novel Wnt pathway inhibitor on cardiac regeneration after myocardial infarction, Clin. Sci 131 (2017) 2919–2932. doi: 10.1042/CS20171256. [DOI] [PubMed] [Google Scholar]
- [46].Physiology C, Downregulation of S100A4 Alleviates Cardiac Fibrosis via Wnt / β -Catenin Pathway in Mice, 210029 (2018) 2551–2560. doi: 10.1159/000489683. [DOI] [PubMed] [Google Scholar]
- [47].He W, Zhang L, Ni A, Zhang Z, Mirotsou M, Mao L, Pratt RE, Dzau VJ, Exogenously administered secreted frizzled related protein 2 ( Sfrp2 ) reduces fi brosis and improves cardiac function in a rat model of myocardial infarction, Proc. Natl. Acad. Sci 107 (2010) 21110–21115. doi: 10.1073/pnas.1004708107/-/DCSupplemental.www.pnas.org/cgi/doi/10.1073/pnas.1004708107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [48].Akhmetshina A, Palumbo K, Dees C, Bergmann C, Venalis P, Zerr P, Horn A, Kireva T, Beyer C, Zwerina J, Schneider H, Sadowski A, Riener MO, MacDougald OA, Distler O, Schett G, Distler JHW, Activation of canonical Wnt signalling is required for TGF-β-mediated fibrosis, Nat. Commun 3 (2012). doi: 10.1038/ncomms1734. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [49].Burnett SH, Conditional macrophage ablation in transgenic mice expressing a Fas-based suicide gene, J. Leukoc. Biol 75 (2004) 612–623. doi: 10.1189/jlb.0903442. [DOI] [PubMed] [Google Scholar]
- [50].Cyprus GN, Overlin JW, Hotchkiss KM, Kandalam S, Olivares-Navarrete R, Cigarette smoke increases pro-inflammatory markers and inhibits osteogenic differentiation in experimental exposure model, Acta Biomater. (2018). doi: 10.1016/J.ACTBIO.2018.06.018. [DOI] [PubMed] [Google Scholar]
- [51].Tan Z, Xie N, Cui H, Moellering DR, Abraham E, Thannickal VJ, Liu G, Pyruvate Dehydrogenase Kinase 1 Participates in Macrophage Polarization via Regulating Glucose Metabolism, J. Immunol 194 (2015) 6082–6089. doi: 10.4049/jimmunol.1402469. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [52].O’Carroll C, Fagan A, Shanahan F, Carmody RJ, Identification of a Unique Hybrid Macrophage-Polarization State following Recovery from Lipopolysaccharide Tolerance, J. Immunol 192 (2014) 427–436. doi: 10.4049/jimmunol.1301722. [DOI] [PubMed] [Google Scholar]
- [53].Tsitsilashvili E, Sepashvili M, Chikviladze M, Shanshiashvili L, Mikeladze D, Myelin basic protein charge isomers change macrophage polarization., J. Inflamm. Res 12 (2019) 25–33. doi: 10.2147/JIR.S189570. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [54].Pajarinen J, Tamaki Y, Antonios JK, Lin T-H, Sato T, Yao Z, Takagi M, Konttinen YT, Goodman SB, Modulation of mouse macrophage polarization in vitro using IL-4 delivery by osmotic pumps., J. Biomed. Mater. Res. A 103 (2015) 1339–45. doi: 10.1002/jbm.a.35278. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [55].Li J, Hu C, Han L, Liu L, Jing W, Tang W, Tian W, Long J, MiR-154–5p regulates osteogenic differentiation of adipose-derived mesenchymal stem cells under tensile stress through the Wnt/PCP pathway by targeting Wnt11, Bone. 78 (2015) 130–141. doi: 10.1016/j.bone.2015.05.003. [DOI] [PubMed] [Google Scholar]
- [56].Kaneuji T, Ariyoshi W, Okinaga T, Toshinaga A, Takahashi T, Nishihara T, Mechanisms involved in regulation of osteoclastic differentiation by mechanical stress-loaded osteoblasts, Biochem. Biophys. Res. Commun 408 (2011) 103–109. doi: 10.1016/j.bbrc.2011.03.128. [DOI] [PubMed] [Google Scholar]
- [57].Uehara S, Udagawa N, Kobayashi Y, Non-canonical Wnt signals regulate cytoskeletal remodeling in osteoclasts, Cell. Mol. Life Sci 75 (2018) 3683–3692. doi: 10.1007/s00018-018-2881-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [58].Leavy O, The shape of things to come, Nat. Rev. Immunol 13 (2013) 775 10.1038/nri3559. [DOI] [Google Scholar]
- [59].McWhorter FY, Wang T, Nguyen P, Chung T, Liu WF, Modulation of macrophage phenotype by cell shape, Proc. Natl. Acad. Sci 110 (2013) 17253–17258. doi: 10.1073/pnas.1308887110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [60].Bertani FR, Mozetic P, Fioramonti M, Iuliani M, Ribelli G, Pantano F, Santini D, Tonini G, Trombetta M, Businaro L, Selci S, Rainer A, Classification of M1/M2-polarized human macrophages by label-free hyperspectral reflectance confocal microscopy and multivariate analysis, Sci. Rep 7 (2017) 8965. doi: 10.1038/s41598-017-08121-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [61].Gong L, Zhao Y, Zhang Y, Ruan Z, The Macrophage Polarization Regulates MSC Osteoblast Differentiation in vitro., Ann. Clin. Lab. Sci 46 (2016) 65–71. http://www.ncbi.nlm.nih.gov/pubmed/26927345 (accessed April 11, 2019). [PubMed] [Google Scholar]
- [62].Yu CH, Nguyen TTK, Irvine KM, Sweet MJ, Frazer IH, Blumenthal A, Recombinant Wnt3a and Wnt5a elicit macrophage cytokine production and tolerization to microbial stimulation via Toll-like receptor 4, Eur. J. Immunol 44 (2014) 1480–1490. doi: 10.1002/eji.201343959. [DOI] [PubMed] [Google Scholar]
- [63].Sessa R, Yuen D, Wan S, Rosner M, Padmanaban P, Ge S, Smith A, Fletcher R, Baudhuin-Kessel A, Yamaguchi TP, Lang RA, Chen L, Monocyte-derived Wnt5a regulates inflammatory lymphangiogenesis, Cell Res. 26 (2016) 262–265. doi: 10.1038/cr.2015.105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [64].Piersma B, Bank RA, Boersema M, Signaling in Fibrosis: TGF-β, WNT, and YAP/TAZ Converge, Front. Med 2 (2015) 1–14. doi: 10.3389/fmed.2015.00059. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [65].Amiya T, Nakamoto N, Chu P, Teratani T, Nakajima H, Fukuchi Y, Taniki N, Yamaguchi A, Shiba S, Miyake R, Katayama T, Ebinuma H, Kanai T, Bone marrow-derived macrophages distinct from tissue-resident macrophages play a pivotal role in Concanavalin A-induced murine liver injury via CCR9 axis, Sci. Rep 6 (2016) 35146. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [66].Italiani P, Boraschi D, Development and Functional Differentiation of Tissue-Resident Versus Monocyte-Derived Macrophages in Inflammatory Reactions, in: 2017: pp. 23–43. doi: 10.1007/978-3-319-54090-0_2. [DOI] [PubMed] [Google Scholar]
- [67].Wang W, Xu L, Liu P, Jairam K, Yin Y, Chen K, Sprengers D, Peppelenbosch MP, Pan Q, Smits R, Blocking Wnt Secretion Reduces Growth of Hepatocellular Carcinoma Cell Lines Mostly Independent of β-Catenin Signaling, Neoplasia. 18 (2016) 711–723. doi: 10.1016/j.neo.2016.10.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [68].Cosin-Roger J, Ortiz-Masià MD, Barrachina MD, Macrophages as an Emerging Source of Wnt Ligands: Relevance in Mucosal Integrity, Front. Immunol 10 (2019). doi: 10.3389/fimmu.2019.02297. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [69].Malsin ES, Kim S, Lam AP, Gottardi CJ, Macrophages as a Source and Recipient of Wnt Signals, Front. Immunol 10 (2019). doi: 10.3389/fimmu.2019.01813. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Supplemental figure 1. Dose-dependent inhibition of macrophage Wnt secretion by Wnt-C59. Primary macrophages were treated with various concentrations of Wnt-C59 dissolved in DMSO (vehicle). Wnt secretion was characterized by direct ELISA for Wnt3a and Wnt5a (n=3 per dose). Data were normalized by DNA quantity in each sample. p<0.05: # vs. Control.
Supplemental figure 2. Identification of immune cell-and osteogenic cell-predominant time points during the response to Ti implants. RNA was extracted from peri-implant tissue in MaFIA mice to measure general markers of immune cells (Cd14, Cd45, Cd34) and mesenchymal stem cells (Dlx5, Runx2, Sp7) (n=6 per group). Results are relative to Gapdh expression and presented as fold-change (2-ΔΔCt). p<0.05: # vs. respective Control, $ vs. respective Sham, % vs. respective Rough.
Supplemental figure 3. Treatment with recombinant Wnt ligands alters expression of polarization gene markers. Primary macrophages cultured on TCPS were treated with recombinant Wnt3a, −5a, −5b, or −11 for 1d prior to harvest. qPCR was performed to measure gene expression for various pro-and anti-inflammatory markers (n=6 per exposure group). Results are presented as fold-change (2-ΔΔCt) compared to Gapdh expression. p<0.05: # vs. Control, $ vs. Wnt3a, % vs. Wnt5a, & vs. Wnt5b.
Supplemental table 1. Unique assay identification codes for qPCR Primers.