Abstract
BACKGROUND
The reliability of preoperative nodal diagnosis of advanced gastric cancer by multi-detector spiral computed tomography (MDCT) is still unclear.
AIM
To examine the diagnostic ability of MDCT more precisely by using data on intranodal pathological metastatic patterns.
METHODS
A total of 108 patients with advanced gastric cancer who underwent MDCT and curative gastrectomy at Kanazawa Medical University Hospital were enrolled in this study. The nodal sizes measured on computed tomography (CT) images were compared with the pathology results. A receiver-operating characteristic curve was constructed, from which the critical value (CV) was calculated by using the data of the first 69 patients retrospectively. By using the CV, sensitivity and specificity were calculated with prospectively collected data from 39 consecutive patients. This enabled a more precise one-to-one correspondence of lymph nodes between CT and pathological examination by using the size data of lymph node mapping. The intranodal pathological metastatic patterns were classified into the following four types: Small nodular, peripheral, large nodular, and diffuse.
RESULTS
Although all the cases were clinically suspected as having metastasis, 81 had lymph node metastasis and 27 had no metastasis. The number of dissected, detected on CT, and metastatic nodes were, 4241, 897, and 801, respectively. The CV obtained from the receiver-operating characteristic was 7.6 mm for the long axis. The sensitivity was 91.4% and the specificity was 47.3% in the prospective phase. The large nodular and diffuse metastases were easy to diagnose because metastatic nodes with a large axis often exhibit these forms.
CONCLUSION
The ability of MDCT to contribute to a nodal diagnosis of advanced gastric cancer was examined prospectively with precise size data from node mapping, using a CV of 7.6 mm for the long axis that was calculated from the retrospectively collected data. The sensitivity was as high as 91%, and would be improved when referring to the enhanced patterns. However, its specificity was as low as 47%, because most of metastatic nodes in gastric cancer being small in size. The small nodular or peripheral type metastatic nodes were often small and considered difficult to diagnose.
Keywords: Advanced gastric cancer, Lymph node metastasis, Multi-detector spiral computed tomography, Pathological diagnosis
Core tip: The preoperative nodal diagnostic ability of multi-detector spiral computed tomography for advanced gastric cancer was examined more precisely using data from patients for whom precise one-to-one correspondence of lymph nodes could be performed between computed tomography and intranodal pathological metastatic patterns by using lymph node mapping. The number of dissected, metastatic, and detected nodes on computed tomography were 4241, 801, and 897, respectively. The sensitivity of multi-detector spiral computed tomography for nodal diagnosis was as good as 91% with the critical value of 7.6 mm for the long axis. The large nodular or diffuse metastases were easy to diagnose. However, the specificity was as low as 47%, because most of the metastatic nodes in gastric cancer were small nodes.
INTRODUCTION
Gastric cancer is the most common cancer and one of the leading causes of cancer-related deaths worldwide[1,2]. Surgical resection is essential for treating gastric cancer; therefore, the status of lymph node metastasis is an important factor of long-term survival[3-6]. Therefore, accurate preoperative diagnosis of lymph node metastasis is important for guiding lymph node dissection or applying preoperative chemotherapy, which improves prognosis. The Japanese classification of gastric carcinoma third English edition[7] and the TNM staging system[8] stipulate that the N-factor is determined by the number of metastatic nodes. However, the number of metastatic nodes is difficult to assess, because it requires the ability to judge the nature of each lymph node.
Multi-detector computed tomography (MDCT) is widely used for preoperative lymph node detection, because of its main advantages, which are its ability to objectively image the anatomy and superior spatial resolution. In a previous study, the normal upper limit of lymph node size on abdominal computed tomography (CT) scans ranged from 6 to 11 mm[9]. With the development of medical device technology, smaller lymph nodes can now be detected. At present, with the popularization of three-dimensional imaging and multi-planar reconstruction (MPR) post-processing technology, we can observe the precise position and shape of lymph nodes in all directions through different sections, which is of great significance for accurate preoperative diagnosis of lymph node metastasis, Relevant research confirmed that isotropic MDCT with multi-planar reconstruction images can improve the accuracies of preoperative N staging of advanced gastric cancer[10].
However, the reliability of preoperative diagnosis of nodal metastasis by MDCT is still unclear. A preoperative nodal diagnosis by MDCT is made on the assumption that the size of the metastatic lymph node is large[11,12]. However, pathological metastatic lymph nodes may not be necessarily large in size[13,14]. Several patterns of the morphological distribution of metastatic foci have been identified, and the sizes of metastatic nodes vary according to the pathological metastatic pattern[15,16]. In addition, a one-to-one correspondence between lymph nodes confirmed on preoperative images and those assessed pathologically have not been reported to date.
In our hospital, all dissected nodes are usually harvested by experienced surgeons and mapped individually. By measuring the size of the lymph node during harvest, pathological metastasis lymph nodes could be detected precisely by using preoperative MDCT. The purpose of this study was to examine the preoperative nodal diagnostic ability of MDCT more precisely by using postoperative lymph node mapping and data on pathological metastatic patterns.
MATERIALS AND METHODS
Patients
One hundred eight consecutive patients who underwent surgical treatment at Kanazawa Medical University Hospital between January 2014 and October 2018 were enrolled in this study. All the patients were diagnosed as having advanced gastric cancer by gastroscopy, diagnosed as having adenocarcinoma by endoscopic biopsy, and suspected as having clinical metastatic lymph nodes preoperatively on the basis of clinicopathological features or CT images.
Multidetector spiral computed tomography
All the patients were routinely examined using MDCT before surgery. Basically, enhanced CT was performed. For patients with a history of renal dysfunction, a contrast media allergy, and an advanced age, image contrast enhancement was not performed. The MDCT equipment used was SOMATOM Definition Flash (Siemens Healthineers, Germany), SOMATON Sensation 16 (Siemens Healthineers, Germany), or Aquilion 64 (Canon Medical Systems, Japan). The contrast medium used was iohexol (Omnipaque, Daiichi-Sankyo, Japan) or iomeprol (Iomeron, Bracco, Switzerland).
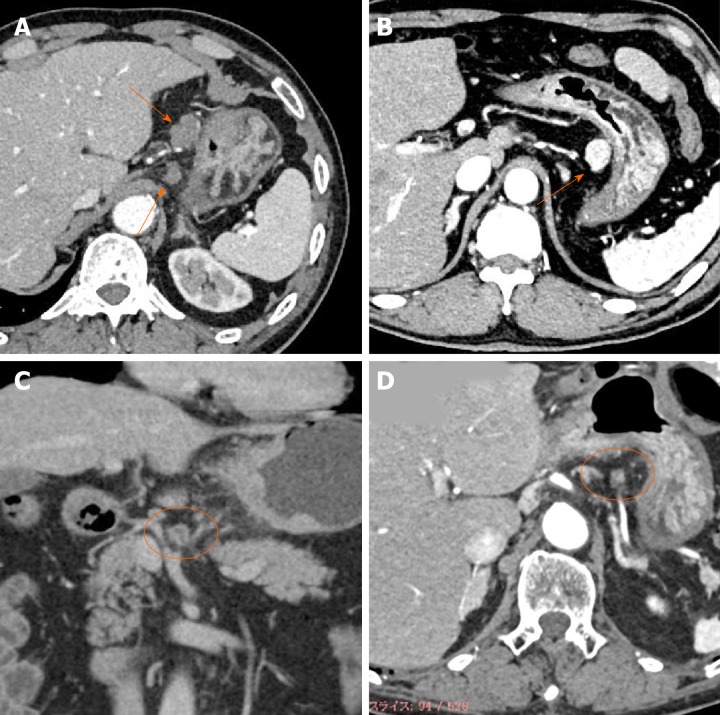
The radiologist (Z.J.) re-checked all the CT images for this study. The lymph nodes corresponding to Nos. 1 to 16 according to the Japanese classification of gastric carcinoma[17] were identified, and their locations, sizes, and enhancement patterns were recorded. The enhancement patterns were classified into the following four types: weak enhancement (WE), obvious enhancement (OB), ring enhancement (RI), and partial enhancement (PA) (Figure 1).
Figure 1.
Typical images of four types of enhancement of metastatic lymph nodes. A: Weak enhancement; B: Obvious enhancement; C: Ring enhancement; D: Partial enhancement.
Surgery, harvest, mapping procedure, and pathological investigation
All the patients underwent curative gastrectomy with lymph node dissection. The standard extent of nodal dissection for advanced gastric cancer in our hospital was D2, although, for some cases, D1 + was selected because of the tumor location (e.g., omission of No. 12a nodal dissection for upper tumor or omission of No. 10 nodal dissection in total gastrectomy for lesser curvature cancer). For high-risk patients, limited surgery was selected to reduce the extent of nodal dissection within the range where R0 could be achieved. All the dissected nodes were harvested by the operator after surgery, the anatomical location of each node was labeled with a serial number, and lymph node mapping was performed for all the cases (Figure 2). All nodes were fixed in formaldehyde and made on a plane of maximal dimension that contained the hilus of the node. Pathological nodal metastasis was defined as the presence of tumor cells, tumor cell clusters or tumor cell nests within the lymph node, as detected in hematoxylin eosin staining. The size of the metastatic foci was not included as a criterion for nodal metastasis; therefore, nodal metastasis included micrometastases and isolated tumor cells. The regional lymph nodes of the stomach were classified into stations numbered according to the Japanese classification of gastric carcinoma.
Figure 2.
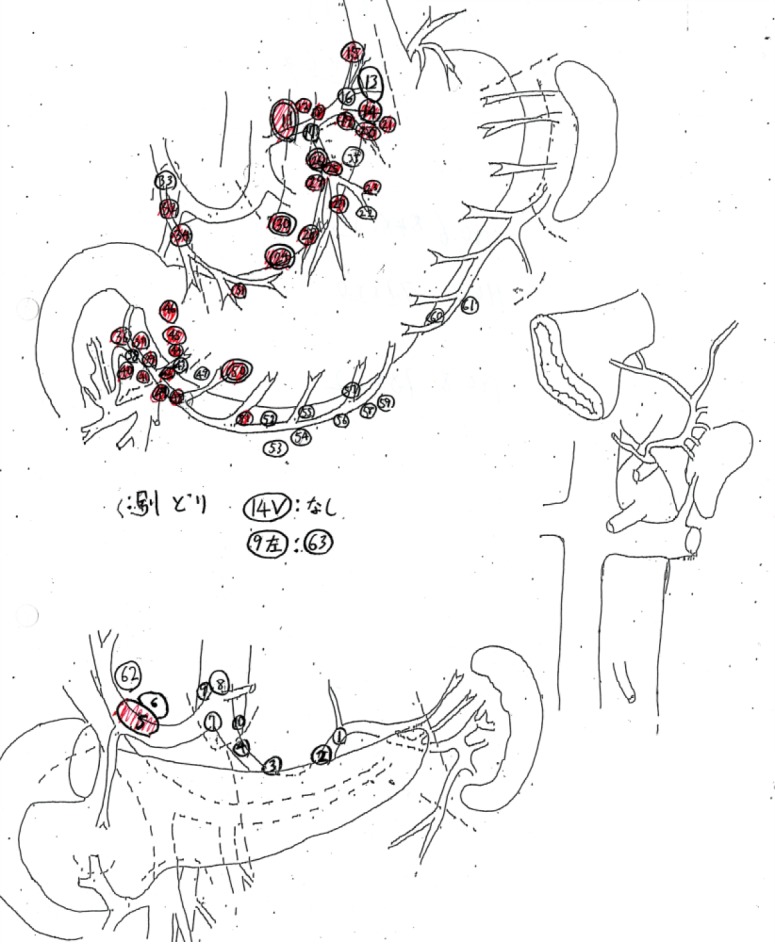
Sample of a lymph node map routinely created in our hospital. The dissected lymph nodes were harvested, separated individually, and the location of the nodes recorded with serial numbers on a map. In the prospective study, sizes of each lymph node were also measured. Red markers on maps are pathologically confirmed metastatic lymph nodes.
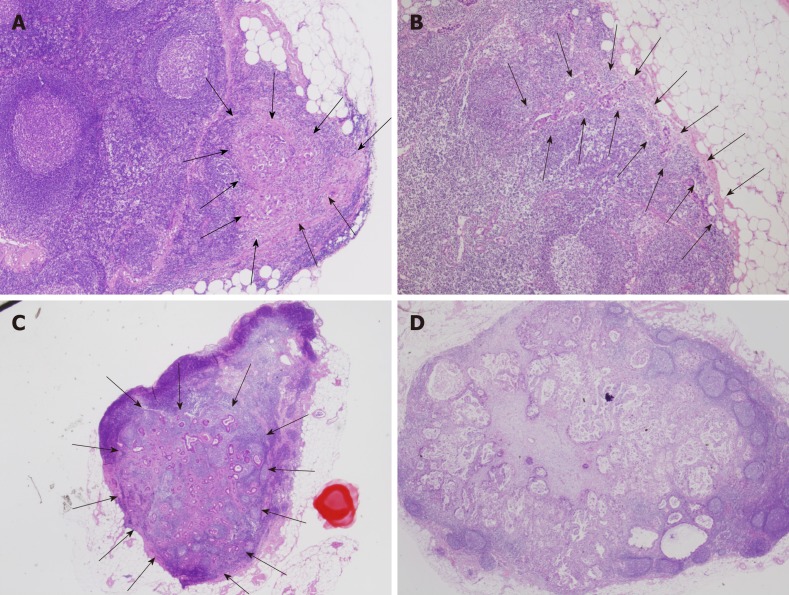
The intranodal pathological metastatic patterns were classified into the following four types: Small nodular (SN), peripheral (PP), large nodular (LN), and diffuse (DF) (Figure 3).
Figure 3.
Typical images of intranodal morphological metastatic patterns. A: Small nodular type; B: Peripheral type; C: Large nodular type; D: Diffuse type.
Study design
The study was divided into two parts, a retrospective study and a prospective study.
Sixty-nine patients who underwent surgical treatment between September 2014 and April 2017 were examined in March 2017 for the retrospective study style. In this series, CT images, lymph node maps, and pathological results were available before the study analysis. The lymph node map did not contain the sizes of the nodes in this series. First, the CT images were re-checked and data were recorded by the radiologist, who was blinded to the map and pathological results. Next, CT imaging data were compared with the pathology results according to the map.
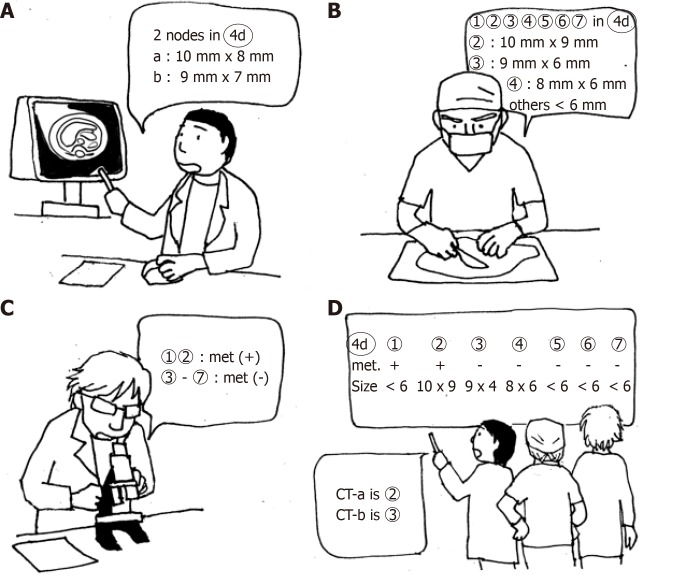
From May 2017 to October 2018, 39 patients were enrolled for the prospective study stile. CT images were analyzed before surgery. At the mapping after surgery, the surgeon measured the size of lymph nodes to be > 7 mm. Postoperative CT imaging data were compared with the pathology results according to the map. In this series, unlike the retrospective study, a more precise one-to-one correspondence of lymph nodes between the CT images and pathological results was possible by using the size data of the map (Figure 4).
Figure 4.
A schema for precise one-to-one correspondence of lymph nodes. A: CT examination before surgery. B: Lymph node mapping. All dissected nodes were mapped and measured when the size was > 7 mm. C: Pathological examination. D: One-to-one correspondence between CT images and pathological results by using the size data of the map.
Data analyses
The numbers of lymph nodes detected by CT and those actually obtained by surgery were compared. Next, by using the sizes of the nodes measured on CT images in the retrospective study, a receiver-operating characteristic curve was constructed, from which the critical value was calculated for the diagnosis of nodal metastasis. Furthermore, the validity of the critical values was assessed using the sensitivity and specificity of the prospective data. Finally, the points to improve the diagnostic ability for nodal metastasis and the diagnostic limitations were examined with consideration of the CT enhancement pattern and pathological metastatic pattern.
In addition, the sizes of all pathological metastatic nodes were measured using the pathologic slides, and for all of these nodes the intranodal pathological metastatic patterns were classified.
Ethical considerations and statistical analyses
This study was approved by the ethics committee of Kanazawa Medical University and conducted in accordance with the Good Clinical Practice guidelines and Declaration of Helsinki. All the patients provided written informed consent for CT, surgery, and the use of their data. Regarding data use in the retrospective study, the patients were given the opportunity to optout of the study at any time.
Statistical analysis was performed using SPSS statistics 22 (IBM, United States).
RESULTS
The characteristics of the patients are summarized in Table 1. Although all the patients were suspected as having metastasis clinically in the preoperative staging, 81 had lymph node metastasis and 27 had no metastasis. The main procedure of lymph node dissection in our hospital was standard nodal dissection (D1 + or D2), although, some patients selected R0 limited surgery (D0 or D1) because of the risk hedging. Metastatic lymph nodes pathologically diagnosed after operation accounted for approximately 18.9% of the total number of lymph nodes removed. Moreover, the number of lymph nodes observed on CT before operation accounted for approximately 21.2% of the total number of lymph nodes dissected.
Table 1.
Patient characteristics
| Retrospective | Prospective | ||
| n | 69 | 39 | |
| Age (yr), median (range) | 71.3 (54-88) | 72 (42-89) | |
| Sex | Male | 48 | 26 |
| Female | 21 | 13 | |
| Depth of invasion | pT2 (MP) | 12 | 8 |
| pT3 (SS) | 14 | 14 | |
| pT4 (SE·SI) | 43 | 17 | |
| Type of gastrectomy | Distal | 42 | 25 |
| Total | 18 | 11 | |
| Others | 9 | 3 | |
| Lymph node dissection | Limited (D0·D1) | 23 | 12 |
| Standard (D1+·D2) | 42 | 25 | |
| Extended (D2+) | 4 | 2 | |
| No. of lymph nodes | Detected on CT | 596 | 301 |
| Harvested | 2913 | 1328 | |
| Cases with a pathological metastasis | 53 | 28 | |
| No. of nodes involved in the pathological metastasis | 630 | 171 | |
Figure 5 shows the receiver-operating characteristic curve created from the data of the retrospective study. The critical values obtained from the curve was 7.55 mm for the long axis and 6.55 mm for the short axis. The sensitivity and specificity of the diagnostic ability of MDCT for nodal metastasis were respectively 86.8% and 80.1% for the long axis and 80.8% and 72.9% for the short axis (Table 2). Furthermore, the lymph nodes were divided into group 1 nodes (Nos. 1-7) and group 2-3 nodes (Nos. 8-16), and by using the critical values the sensitivity and specificity were examined separately. The sensitivity and specificity of the nodes belonging to group 1 were comparable with those in the overall examination; by contrast, the results of the nodes belonging to group 2 were slightly inferior.
Figure 5.
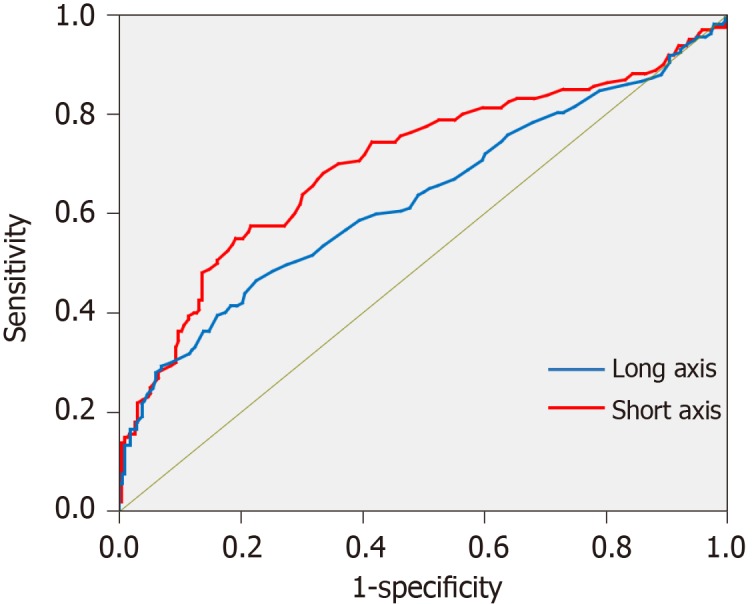
Receiver-operating characteristic curve for diagnosis of nodal metastasis using multi-detector computed tomography created from the data of the retrospective study.
Table 2.
Sensitivity and specificity of the diagnostic ability of multi-detector computed tomography in the retrospective and prospective stages based on the critical values
| Critical values: 7.6 mm × 6.6 mm | Retrospective | Prospective | |
| All locations | |||
| Long axis | Sensitivity | 86.8 | 91.4 |
| Specificity | 80.1 | 47.3 | |
| AUC | 0.706 | 0.849 | |
| Short axis | Sensitivity | 80.8 | 88.9 |
| Specificity | 72.9 | 52.1 | |
| AUC | 0.634 | 0.813 | |
| Group 1 (Nos.1-7) | |||
| Long axis | Sensitivity | 89.6 | 97 |
| Specificity | 80.4 | 49.7 | |
| AUC | 0.74 | 0.864 | |
| Short axis | Sensitivity | 86.4 | 90.9 |
| Specificity | 67.9 | 52.9 | |
| AUC | 0.686 | 0.826 | |
| Group 2 and 3 (Nos.8-16) | |||
| Long axis | Sensitivity | 73.3 | |
| Specificity | 88.2 | ||
| AUC | 0.638 | ||
| Short axis | Sensitivity | 80 | |
| Specificity | 73.5 | ||
| AUC | 0.702 | ||
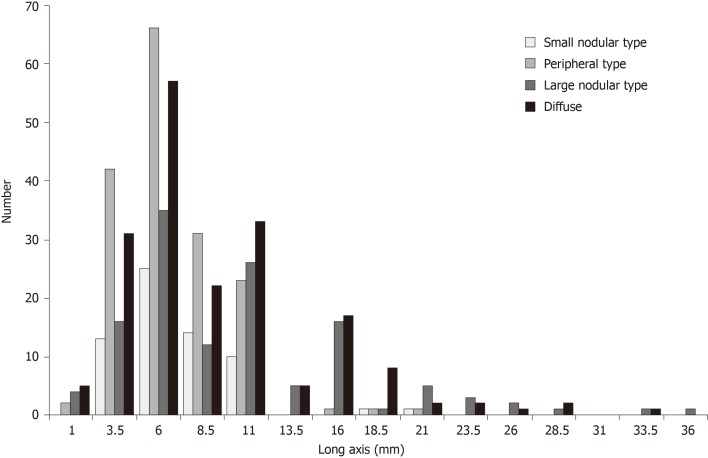
To verify the accuracy of the critical values, diagnostic abilities were calculated using the data from prospective study phase. By referring to the sizes and serial numbers of the lymph nodes measured in the map, a one-to-one correspondence becomes possible between lymph nodes detected by MDCT and the pathological results in the prospective study. As shown in Table 2, when the critical values were used in the diagnosis of nodal metastasis, the sensitivity was high, but the specificity was low. Unfortunately, the number of group 2 nodes in the prospective study was too small, and the effective statistical data could not be obtained. The relationship between the size of the long axis and the intranodal pathological metastatic patterns of all metastatic lymph nodes is shown in Figure 6. The size of the long axis was tabulated at 2.5 mm intervals to create a histogram. In all the intranodal pathological metastatic patterns, the mode of the long axis was approximately 6 mm. The peripheral and small nodular types had many small metastatic lymph nodes; however, most large metastatic lymph nodes were of the large nodular or diffuse type. Table 3 shows the results of counting the number of all metastatic lymph nodes larger than the long axis of the critical values, according to the four intranodal pathological metastatic patterns. Only 43.8% of the metastatic lymph nodes were larger than the critical values. The larger nodes were only 28.1% of all the PP-type nodes and 52.7% of the LN- or DF-type nodes.
Figure 6.
Distribution of lymph node metastasis in four types based on the long axis.
Table 3.
Comparative distribution of lymph nodes due to the intranodal pathological metastatic pattern based on the critical value of 7.6 mm in 77 cases observed under microscopy
| SN | PP | LN | DF | |
| > 7.6 mm | 26 | 47 | 73 | 92 |
| < 7.6 mm | 38 | 120 | 55 | 93 |
SN: Small nodular type; PP: Peripheral type; LN: Large nodular type; DF: Diffuse type.
Table 4 shows the distributions of the CT enhanced morphologies in the prospective study according to lymph node station. For example, as for lymph nodes located at station 3, MDCT revealed 124 nodes in total, of which 103 showed weak enhancement. Of the 103 nodes, 8 were metastatic nodes. Of the lymph nodes detected by MDCT, 93% and 7.3% were No. 1-7 nodes belonging to groups 1 and 2-3, respectively. On the other hand, 20.1% and 45.5% of the metastatic lymph nodes belonged to groups 1 and 2, respectively. The metastatic rate of the lymph nodes of WE was 10.8%, whereas those of the lymph nodes of OB, RI, and PA were 44.7%, 83.3%, and 33.3%, respectively. All group 2 nodes that presented RI had metastasis. Table 5 represents the relationship between enhanced morphology and intranodal pathological metastatic patterns. The ratios of the metastatic lymph nodes with enhanced types other than WE were 56% for SN, 42% for PP, 82% for LN, and 71% for DF.
Table 4.
Number of the nodes and distribution of morphologies on contrast-enhanced computed tomography in the prospective study
|
WE |
OB |
RI |
PA |
||||||
| n | met (-) | met (+) | met (-) | met (+) | met (-) | met (+) | met (-) | met (+) | |
| No.1 | 20 | 11 | 2 | 1 | 4 | 2 | |||
| No.2 | 14 | 5 | 1 | 5 | 1 | 2 | |||
| No.3 | 124 | 95 | 8 | 10 | 9 | 2 | |||
| No.4 | 57 | 28 | 5 | 6 | 3 | 2 | 1 | 7 | 5 |
| No.5 | 13 | 8 | 1 | 3 | 1 | ||||
| No.6 | 43 | 20 | 4 | 3 | 4 | 2 | 6 | 4 | |
| No.7 | 8 | 3 | 2 | 2 | 1 | ||||
| No.8 | 16 | 7 | 1 | 1 | 5 | 1 | 1 | ||
| No.9 | 4 | 3 | 1 | ||||||
| No.10 | 1 | 1 | |||||||
| No.16 | 1 | 1 | |||||||
“No.1” to “No.16” are station numbers of regional lymph nodes of stomach, classified according to the Japanese classification of gastric carcinoma. “n” represents the sum of lymph nodes of each station visualized on CT. WE: Weak enhancement; OB: Obvious enhancement; RI: Ring enhancement; PA: Partial enhancement; met (-): No matastatic nodes; met (+): Metastatic nodes.
Table 5.
Relationship between the enhanced morphology of the metastatic lymph nodes detected on multi-detector computed tomography and the intranodal pathological metastatic patterns in the prospective study
| Small nodular type | Peripheral type | Large nodular type | Diffuse type | |
| Weak enhancement | 4 | 7 | 3 | 8 |
| Obvious enhancement | 3 | 1 | 7 | 10 |
| Ring enhancement | 2 | 4 | 4 | |
| Partial enhancement | 2 | 2 | 3 | 6 |
DISCUSSION
We examined the preoperative nodal diagnostic ability of MDCT by using lymph node mapping and data of pathological metastatic patterns. The ability of MDCT to visualize lymph nodes was high. MDCT could visualize even small lymph nodes but could detect only 27% of all regional lymph nodes. The critical value obtained from the receiver-operating characteristic curve of the retrospective study was 7.6 mm for the long axis. The sensitivity and specificity of the diagnostic ability of MDCT for nodal metastasis were 91.4% and 47.3%, respectively, in the prospective study. The effect on the area under the curve was excellent at 0.85. The reason for the high sensitivity is that large gastric lymph nodes are often metastatic, while the low specificity is due to most metastatic nodes in gastric cancer being small in size. Lymph nodes that show OB, RI, and PA patterns are more likely to metastasize than those that show the WE pattern, and are considered useful in making a metastatic diagnosis. When the diagnostic ability of MDCT was examined for each intranodal pathological metastatic pattern, diagnoses were made easily if the metastatic patterns were LN or DF, because metastatic lymph nodes with a long axis often exhibit these forms. LN or DF patterns also made for easy diagnoses because these nodes usually did not exhibit a contrast pattern of WE. In contrast, SN or PP metastatic nodes were often small and considered difficult to diagnose.
The novelty of this paper is that we performed an exact one-to-one correspondence with the lymph nodes visualized on preoperative CT and the pathological specimens using the lymph node map created during the postoperative harvesting of the dissected nodes, to examine the diagnostic ability of MDCT for lymph node metastasis. Estimating the diagnostic ability of CT for nodal metastasis has a certain degree of difficulty and error, because of the similar lymph nodes in the same location in both the pathological specimens and CT images, and a relative comparison is difficult to achieve. In our hospital, the preparation of pathological specimens from lymph nodes is based on the Japanese classification of gastric carcinoma, and lymph nodes are prepared in the maximum plane including the hilus. Nevertheless, the specimens are not necessarily prepared in the largest plane, and the nodes are sometimes reduced when fixed. Then, in retrospective studies, a one-to-one correspondence of lymph nodes was still difficult to attain, even if the lymph node maps were referenced. Therefore, we measured the size of the lymph nodes during mapping after surgery. This enabled a precise one-to-one correspondence of lymph nodes between the CT images and pathological results in the prospective study phase.
Although multi-slice spiral CT scanning is the most widely used standard diagnostic tool[10-12], it is not considered as the gold standard technique for N-staging and its accuracy for preoperative diagnosis of metastatic lymph nodes in advanced gastric cancer is not high[18-20]. According to the recently revised RECIST standard 1.1[21], metastatic lymph nodes must meet the criteria of at least 15 mm in diameter of the short axis on CT, while lymph nodes < 10 mm are considered normal. However, metastatic lymph nodes observed on CT are not only found in larger lymph nodes but also in lymph nodes with small diameters[14], and some large diameter lymph nodes are not necessarily metastases. To consider this issue more precisely, we also examined intranodal pathological metastatic patterns of metastatic lymph nodes. It is also a unique point of this work. The intranodal metastatic patterns were classified into four types in accordance with the simple and useful classification described article by Fujimura et al[15]. In our study, the most common metastatic pattern was the DF type, 50% of which were nodes larger than the critical values, followed by the PP type nodes (31%), of which 72% were smaller than the critical values. If all lymph nodes can be visualized by MDCT and the size can be measured, only 43.8% of metastatic lymph nodes can be diagnosed, and only 28.1% with the PP type can be detected. Therefore, the diagnostic ability of MDCT for nodal metastasis seemed to have a certain limit in sensitivity. We excluded the patients with early gastric cancer in this study. The detection sensitivity would be reduced when early gastric cancer was included, because the nodal metastasis rate of early gastric cancer was low, and many metastases were microscopic[13].
Through this study, we speculate that the accuracy of diagnosis of lymph node metastasis by MDCT can be improved to some extent. The reference to CT enhanced morphology may be effective as one of the improvements. The nodes that showed the OB, RI, and PA patterns are more likely to metastasize than the WE type. The advantage of MDCT in processing three-dimensional and multi-planar reconstruction images may also be effective for measurement of both the long and short diameters of nodes.
The purpose of preoperative nodal diagnosis in advanced gastric cancer will focus on extracting appropriate cases of preoperative neoadjuvant chemotherapy (NAC)[22,23] and considering the appropriate surgery. Reducing the disadvantages of applying NAC to metastasis-negative cases is important[24]. Although node-negative cases are difficult to distinguish using MDCT, lymph nodes diagnosed as metastasis by MDCT are highly pathological metastases, and obvious node-positive cases can be extracted. MDCT is suitable for identifying NAC indications. On the other hand, as the specificity is low, a strategy for omitting lymph node dissection by referring to MDCT results should not be adopted.
The limitation of this study is the few cases of positive group 2 nodal metastasis, and the critical values could not be calculated separately for group 1 and 2 nodes. The mean size of the lymph nodes observed on CT differed owing to the location of the nodes; for example, the nodes that belonged to station 8 were larger than the group 1 nodes[18]. Therefore, appropriately adjusting the threshold size according to different lymph node location would be helpful in the diagnosis of metastatic nodes. In our study, especially in the prospective study, the metastatic group 2 nodes were fewer, and the analysis of the appropriate critical value failed. However, because the number of group 2 lymph nodes was smaller than that of group 1 lymph nodes and a one-to-one correspondence is possible without using the lymph node map, the critical values calculated in the other studies will probably be useful[12,18].
In conclusion, the sensitivity of MDCT for the nodal diagnosis of advanced gastric cancer was as high as 90% with the critical value of 7.6 mm for the long axis and would be improved when referring to the enhanced OB, RI, and PA patterns. The nodal diagnosis of cases with the LN or DF pattern was slightly easier. Obvious node-positive cases suitable for NAC can be identified using MDCT. However, the specificity was as low as 47%, so it seemed difficult to decide the omission of nodal dissection on the basis of the MDCT finding.
ARTICLE HIGHLIGHTS
Research background
The reliability of preoperative diagnosis of nodal metastasis of advanced gastric cancer by multi-detector spiral computed tomography (MDCT) is still unclear. A preoperative nodal diagnosis by MDCT is made on the assumption that the size of the metastatic lymph node is large. However, pathological metastatic lymph nodes may not be necessarily large in size.
Research motivation
A one-to-one correspondence between lymph nodes confirmed on preoperative images and those assessed pathologically have not been reported to date. In our hospital, all dissected nodes are usually harvested by experienced surgeons and mapped individually. By measuring the size of the lymph node during harvest, pathological metastasis lymph nodes could be detected precisely by using preoperative MDCT.
Research objectives
The purpose of this study was to examine the preoperative nodal diagnostic ability of MDCT more precisely by using postoperative lymph node mapping and data on pathological metastatic patterns.
Research methods
A total of 108 patients with advanced gastric cancer who underwent MDCT and curative gastrectomy were enrolled in this study. The nodal sizes measured on computed tomography (CT) images were compared with the pathology results. A receiver-operating characteristic curve was constructed, from which the critical value (CV) was calculated by using the data of the first 69 patients retrospectively. By using the CV, sensitivity and specificity were calculated with prospectively collected data from 39 consecutive patients. This enabled a more precise one-to-one correspondence of lymph nodes between CT and pathological examination by using the size data of lymph node mapping. The intranodal pathological metastatic patterns were classified into the following four types: Small nodular, peripheral, large nodular, and diffuse.
Research results
Although all the cases were clinically suspected as having metastasis, 81 had lymph node metastasis and 27 had no metastasis. The number of dissected, detected on CT, and metastatic nodes were, 4241, 897, and 801, respectively. The CV obtained from the receiver-operating characteristic was 7.6 mm for the long axis. The sensitivity and specificity of the diagnostic ability of MDCT for nodal metastasis were respectively 86.8% and 80.1% in the retrospective phase. To verify the accuracy of the CV, diagnostic abilities were calculated using the data from prospective study phase. By referring to the sizes and serial numbers of the lymph nodes measured in the map, a one-to-one correspondence becomes possible between lymph nodes detected by MDCT and the pathological results in the prospective study. The sensitivity was 91.4% and the specificity was 47.3% in the prospective phase. Only 43.8% of the metastatic lymph nodes were larger than the critical values. The larger nodes were only 28.1% of all the peripheral type nodes and 52.7% of the large nodular or diffuse type nodes.
Research conclusions
The ability of MDCT to contribute to a nodal diagnosis of advanced gastric cancer was examined prospectively with precise size data from node mapping, using a CV of 7.6 mm for the long axis that was calculated from the retrospectively collected data. The sensitivity was as high as 91%, and would be improved when referring to the enhanced patterns. The nodal diagnosis of cases with the large nodular or diffuse pattern was slightly easier. However, its specificity was as low as 47%, because most of metastatic nodes in gastric cancer being small in size. The small nodular or peripheral type metastatic nodes were often small and considered difficult to diagnose.
Research perspectives
Obvious node-positive cases of advanced gastric cancer suitable for neoadjuvant chemotherapy can be identified using MDCT because the sensitivity for the nodal diagnosis was as high as 90% with the critical value of 7.6 mm for the long axis. However, the specificity was as low as 47%, so it seemed difficult to decide the omission of nodal dissection on the basis of the MDCT finding.
Footnotes
Institutional review board statement: This study was reviewed and approved by the Ethics Committee of the Kanazawa Medical University Hospital.
Informed consent statement: Patients were not required to give informed consent to the study because the analysis used anonymous clinical data that were obtained after each patient agreed to treatment by written consent. Regarding data use in the retrospective study, the patients were given the opportunity to optout of the study at any time.
Conflict-of-interest statement: The authors have no conflicts of interest to declare.
Manuscript source: Unsolicited manuscript
Peer-review started: November 21, 2019
First decision: December 12, 2019
Article in press: February 23, 2020
Specialty type: Oncology
Country/Territory of origin: Japan
Peer-review report’s scientific quality classification
Grade A (Excellent): 0
Grade B (Very good): B, B
Grade C (Good): C
Grade D (Fair): 0
Grade E (Poor): 0
P-Reviewer: Tanabe S, Barreto SG S-Editor: Zhou JJ L-Editor: A E-Editor: Qi LL
Contributor Information
Zhi-Yong Jiang, Department of Surgical Oncology, Kanazawa Medical University, 1-1 Daigaku, Uchinada-machi, Kahoku-gun, Ishikawa 920-0293, Japan.
Shinichi Kinami, Department of Surgical Oncology, Kanazawa Medical University, 1-1 Daigaku, Uchinada-machi, Kahoku-gun, Ishikawa 920-0293, Japan. kinami@kanazawa-med.ac.jp.
Naohiko Nakamura, Department of Surgical Oncology, Kanazawa Medical University, 1-1 Daigaku, Uchinada-machi, Kahoku-gun, Ishikawa 920-0293, Japan.
Takashi Miyata, Department of Surgical Oncology, Kanazawa Medical University, 1-1 Daigaku, Uchinada-machi, Kahoku-gun, Ishikawa 920-0293, Japan.
Hideto Fujita, Department of Surgical Oncology, Kanazawa Medical University, 1-1 Daigaku, Uchinada-machi, Kahoku-gun, Ishikawa 920-0293, Japan.
Hiroyuki Takamura, Department of Surgical Oncology, Kanazawa Medical University, 1-1 Daigaku, Uchinada-machi, Kahoku-gun, Ishikawa 920-0293, Japan.
Nobuhiko Ueda, Department of Surgical Oncology, Kanazawa Medical University, 1-1 Daigaku, Uchinada-machi, Kahoku-gun, Ishikawa 920-0293, Japan.
Takeo Kosaka, Department of Surgical Oncology, Kanazawa Medical University, 1-1 Daigaku, Uchinada-machi, Kahoku-gun, Ishikawa 920-0293, Japan.
Data sharing statement
No additional data are available.
References
- 1.Tunaci M. Carcinoma of stomach and duodenum: radiologic diagnosis and staging. Eur J Radiol. 2002;42:181–192. doi: 10.1016/s0720-048x(02)00035-9. [DOI] [PubMed] [Google Scholar]
- 2.Torre LA, Bray F, Siegel RL, Ferlay J, Lortet-Tieulent J, Jemal A. Global cancer statistics, 2012. CA Cancer J Clin. 2015;65:87–108. doi: 10.3322/caac.21262. [DOI] [PubMed] [Google Scholar]
- 3.Kwee RM, Kwee TC. Imaging in assessing lymph node status in gastric cancer. Gastric Cancer. 2009;12:6–22. doi: 10.1007/s10120-008-0492-5. [DOI] [PubMed] [Google Scholar]
- 4.Msika S, Chastang C, Houry S, Lacaine F, Huguier M. Lymph node involvement as the only prognostic factor in curative resected gastric carcinoma: a multivariate analysis. World J Surg. 1989;13:118–23; discussion 123. doi: 10.1007/BF01671171. [DOI] [PubMed] [Google Scholar]
- 5.Roder JD, Böttcher K, Busch R, Wittekind C, Hermanek P, Siewert JR. Classification of regional lymph node metastasis from gastric carcinoma. German Gastric Cancer Study Group. Cancer. 1998;82:621–631. [PubMed] [Google Scholar]
- 6.Kodama Y, Sugimachi K, Soejima K, Matsusaka T, Inokuchi K. Evaluation of extensive lymph node dissection for carcinoma of the stomach. World J Surg. 1981;5:241–248. doi: 10.1007/BF01658301. [DOI] [PubMed] [Google Scholar]
- 7.Japanese Gastric Cancer Association. Japanese classification of gastric carcinoma: 3rd English ed. Gastric Cancer. 2011;14:101–112. doi: 10.1007/s10120-011-0041-5. [DOI] [PubMed] [Google Scholar]
- 8.Sobin LH, Gospodarowics MK, Wittekind C. TNM Classification of Malignant Tumours 7th ed. New York: Wiley-Blackwell Sci Pub, 2009. [Google Scholar]
- 9.Dorfman RE, Alpern MB, Gross BH, Sandler MA. Upper abdominal lymph nodes: criteria for normal size determined with CT. Radiology. 1991;180:319–322. doi: 10.1148/radiology.180.2.2068292. [DOI] [PubMed] [Google Scholar]
- 10.Kim YN, Choi D, Kim SH, Kim MJ, Lee SJ, Lee WJ, Kim S, Kim JJ. Gastric cancer staging at isotropic MDCT including coronal and sagittal MPR images: endoscopically diagnosed early vs. advanced gastric cancer. Abdom Imaging. 2009;34:26–34. doi: 10.1007/s00261-008-9380-z. [DOI] [PubMed] [Google Scholar]
- 11.Dai CL, Yang ZG, Xue LP, Li YM. Application value of multi-slice spiral computed tomography for imaging determination of metastatic lymph nodes of gastric cancer. World J Gastroenterol. 2013;19:5732–5737. doi: 10.3748/wjg.v19.i34.5732. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Yan C, Zhu ZG, Yan M, Zhang H, Pan ZL, Chen J, Xiang M, Chen MM, Liu BY, Yin HR, Lin YZ. Value of multidetector-row computed tomography in the preoperative T and N staging of gastric carcinoma: a large-scale Chinese study. J Surg Oncol. 2009;100:205–214. doi: 10.1002/jso.21316. [DOI] [PubMed] [Google Scholar]
- 13.Kim DJ, Kim W. Is Lymph Node Size a Reliable Factor for Estimating Lymph Node Metastasis in Early Gastric Cancer? J Gastric Cancer. 2018;18:20–29. doi: 10.5230/jgc.2018.18.e1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Noda N, Sasako M, Yamaguchi N, Nakanishi Y. Ignoring small lymph nodes can be a major cause of staging error in gastric cancer. Br J Surg. 1998;85:831–834. doi: 10.1046/j.1365-2168.1998.00691.x. [DOI] [PubMed] [Google Scholar]
- 15.Fujimura T, Yonemura Y, Taniguchi K. Preoperative diagnosis by computed tomography for lymph node metastasis in gastric cancer (Japanese) Hokuriku Geka Gakkaishi. 1997;16:23–26. [Google Scholar]
- 16.Yanagita S, Natsugoe S, Uenosono Y, Arima H, Kozono T, Ehi K, Arigami T, Higashi H, Aikou T. Morphological distribution of metastatic foci in sentinel lymph nodes with gastric cancer. Ann Surg Oncol. 2008;15:770–776. doi: 10.1245/s10434-007-9713-0. [DOI] [PubMed] [Google Scholar]
- 17.Japanese Gastric Cancer Association. Japanese classification of gastric carcinoma 15th ed. Tokyo: Kanehara Sci Pub, 2017: 20-23. [DOI] [PubMed] [Google Scholar]
- 18.Saito T, Kurokawa Y, Takiguchi S, Miyazaki Y, Takahashi T, Yamasaki M, Miyata H, Nakajima K, Mori M, Doki Y. Accuracy of multidetector-row CT in diagnosing lymph node metastasis in patients with gastric cancer. Eur Radiol. 2015;25:368–374. doi: 10.1007/s00330-014-3373-9. [DOI] [PubMed] [Google Scholar]
- 19.Kubota K, Suzuki A, Shiozaki H, Wada T, Kyosaka T, Kishida A. Accuracy of Multidetector-Row Computed Tomography in the Preoperative Diagnosis of Lymph Node Metastasis in Patients with Gastric Cancer. Gastrointest Tumors. 2017;3:163–170. doi: 10.1159/000454923. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Ohashi M, Morita S, Fukagawa T, Wada T, Kushima R, Onaya H, Katai H. Evaluation of 64-Channel Contrast-Enhanced Multi-detector Row Computed Tomography for Preoperative N Staging in cT2-4 Gastric Carcinoma. World J Surg. 2016;40:165–171. doi: 10.1007/s00268-015-3318-8. [DOI] [PubMed] [Google Scholar]
- 21.Eisenhauer EA, Therasse P, Bogaerts J, Schwartz LH, Sargent D, Ford R, Dancey J, Arbuck S, Gwyther S, Mooney M, Rubinstein L, Shankar L, Dodd L, Kaplan R, Lacombe D, Verweij J. New response evaluation criteria in solid tumours: revised RECIST guideline (version 1.1) Eur J Cancer. 2009;45:228–247. doi: 10.1016/j.ejca.2008.10.026. [DOI] [PubMed] [Google Scholar]
- 22.Yoshikawa T, Rino Y, Yukawa N, Oshima T, Tsuburaya A, Masuda M. Neoadjuvant chemotherapy for gastric cancer in Japan: a standing position by comparing with adjuvant chemotherapy. Surg Today. 2014;44:11–21. doi: 10.1007/s00595-013-0529-1. [DOI] [PubMed] [Google Scholar]
- 23.Kodera Y. Neoadjuvant chemotherapy for gastric adenocarcinoma in Japan. Surg Today. 2017;47:899–907. doi: 10.1007/s00595-017-1473-2. [DOI] [PubMed] [Google Scholar]
- 24.Fukagawa T, Katai H, Mizusawa J, Nakamura K, Sano T, Terashima M, Ito S, Yoshikawa T, Fukushima N, Kawachi Y, Kinoshita T, Kimura Y, Yabusaki H, Nishida Y, Iwasaki Y, Lee SW, Yasuda T, Sasako M Stomach Cancer Study Group of the Japan Clinical Oncology Group. A prospective multi-institutional validity study to evaluate the accuracy of clinical diagnosis of pathological stage III gastric cancer (JCOG1302A) Gastric Cancer. 2018;21:68–73. doi: 10.1007/s10120-017-0701-1. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
No additional data are available.