Abstract
Background
Nearly half of the patients with breast cancer experience clinically significant mental distress within the first year of receiving their cancer diagnosis. There is an urgent need to identify scalable and cost-efficient ways of delivering empirically supported mental health interventions to patients with breast cancer.
Objective
The aim of this study was to evaluate the feasibility of in-clinic recruitment for a mobile phone app study and to evaluate the usability and preliminary impact of a suite of mental health apps (IntelliCare) with phone coaching on psychosocial distress symptoms in patients recently diagnosed with breast cancer.
Methods
This pilot study adopted a within-subject, 7-week pre-post study design. A total of 40 patients with breast cancer were recruited at a US National Cancer Institute–designated clinical cancer center. Self-reported distress (Patient Health Questionnaire-4) and mood symptoms (Patient-Reported Outcomes Measurement Information System depression and anxiety scales) were assessed at baseline and postintervention. App usability was assessed at postintervention.
Results
The minimum recruitment threshold was met. There was a significant decrease in general distress symptoms, as well as symptoms of depression and anxiety, from baseline to postintervention. Overall, participants reported high levels of ease of app use and learning. Scores for app usefulness and satisfaction were reinforced by some qualitative feedback suggesting that tailoring the apps more for patients with breast cancer could enhance engagement.
Conclusions
There is a dire need for scalable, supportive interventions in cancer. The results from this study inform how scalable mobile phone–delivered programs with additional phone support can be used to support patients with breast cancer.
International Registered Report Identifier (IRRID)
RR2-10.2196/11452
Keywords: breast cancer, mental health, mHealth
Introduction
Background
Nearly 50% of the women diagnosed with breast cancer report clinically significant levels of distress (ie, elevated symptoms of depression or anxiety) within the first year of receiving their cancer diagnosis [1-4]. Untreated symptoms of depression and anxiety in patients with breast cancer lead to poor quality of life [5], increased mortality [6,7], and high economic costs [8]. Although therapies that emphasize skills acquisition, such as cognitive behavioral therapy and acceptance-based therapy, have demonstrated efficacy in reducing distress in patients with breast cancer [9-12], almost half of the patients with breast cancer report unmet supportive care needs [13-16]. One reason is the reliance on in-person delivery of mental health services, which poses numerous barriers, such as high financial cost [17], high time investment [18,19], social stigma [20], and a severe shortage of trained therapists [21-23]. Despite increased efforts by clinicians and researchers to assess for distress during the cancer treatment process, distress management through mobile technology remains an overlooked component of care [14,24,25].
Mobile Phone Apps for Patients With Cancer
Mobile phone apps are frequently cited as a potential method of extending effective care in a cost-effective manner [26-28]. Given that 81% of American adults own a mobile phone [29], it is an ideal platform from which to deliver brief, empirically supported interventions to anyone who needs them. Models of internet interventions [30] and behavioral intervention technologies [31] highlight key strengths of mobile health (mHealth) interventions: portability, accessibility, and the ability to program an automated intervention to adapt to a user’s input. Numerous randomized controlled trials demonstrate the efficacy of app-based interventions in reducing the symptoms of depression and anxiety [32-36], including those that are coupled with support from a coordinator [32,37,38]. However, empirical reviews of apps for patients with cancer [25,39] fail to identify any publicly available mental health intervention that target patients with breast cancer. Thus, despite the potential scalability and impact of an app-based intervention that teaches distress management skills to patients with breast cancer, more work is needed.
App Design and Coaching to Promote Engagement
An app-based mental health intervention can be deployed where and when a patient needs it most, guiding users through brief and practical skills training to manage their distress. However, there are some weaknesses to app interventions, including software bugs, ownership of a compatible mobile device, and poor engagement and usage. Specifically, many apps suffer from poor engagement for a variety of reasons, such as requiring lengthy engagement times that do not match user preferences [40]. In reality, people use apps in short, frequent bursts and tend to prefer apps that support a limited set of tasks [41]. Thus, an app intervention that is designed to provide quick and targeted interventions can potentially fit well with patients with breast cancer who are receiving active cancer treatment and who must deal with the inevitable sequelae of anticancer care, including time constraints and conflicts with work and outside activities [35].
Studies suggest that pairing an app with human support (eg, coaching via phone, SMS text messaging) can further increase engagement and usage, thereby promoting outcomes [37,38]. On the basis of the Efficiency Model of Support [38], a human coach can support participants in using and benefiting from an app intervention. Coaches work with users to set goals and target potential points at which users may fail to benefit from the app (ie, addressing obstacles to effective use), which increases accountability and promotes engagement [38].
The aim of this study was to conduct a pilot study that evaluated a set of brief, targeted app interventions that promote mental health. The IntelliCare platform is a collection of apps that utilize an elemental, skills-based approach to improving mental health [32,42]. Table 1 contains descriptions of the IntelliCare apps and their purposes. Many of the exercises contained in the apps can be completed in less than a minute. Exercises are meant to be intuitive, requiring few instructions to complete, and most of these exercises can be found on the first screen that is presented by the app. Each app has a Help feature that contains educational and technical content regarding the specific app in question. A total of two trials of 8-week interventions showed significant and substantial reductions in depression and anxiety symptom severity among noncancer patients with average app use of 195 to 216 times, with a median use of less than 1 min [32,42]. However, these results may not be generalizable to patients with breast cancer who face unique challenges and life circumstances, which makes them potentially unique from other populations.
Table 1.
Description of IntelliCare apps, their objectives, and which apps were available for each type of mobile phone platform at the time of the study.
| App name | Objective | Mobile phone platform |
| Aspire | Promotes awareness of and striving toward personal goals and values. Helps users identify their values and keep track of their progress. | Android |
| Day to Day | Promotes knowledge about ways to bolster mood. Users receive a daily stream of knowledge tidbits and are prompted to build on a theme every day (eg, cultivate gratitude and problem solve). | Android and iOS |
| Daily Feats | Promotes goal setting and attainment. An in-app calendar allows users to track their successes and identify new tasks to complete. | Android and iOS |
| Worry Knot | Promotes knowledge about worry and provides an interactive exercise to decrease worry. The app also tracks the user’s progress and provides tailored feedback on ways to distract oneself from worrying thoughts. | Android and iOS |
| Social Force | Encourages users to identify supportive individuals in their life. The app prompts users to reach out to these people for encouragement. | Android |
| My Mantra | Increases self-efficacy and a positive perspective of oneself. The app prompts users to come up with personal mantras and to construct personalized photo albums that serve as reminders of these mantras. | Android and iOS |
| Thought Challenger | Increases the ability to identify and challenge negative thinking patterns. Guides users through a cognitive restructuring exercise and tracks the output of past exercises. | Android and iOS |
| iCope | Promotes coping and positive reinforcement by having users write and send themselves messages when encouragement is most needed. | Android |
| Purple Chill | Increases relaxation skills by providing a library of mindfulness and guided meditation audio files. | Android |
| MoveMe | Promotes mood through physical activity. The app prompts users to schedule exercises and provides instructional videos and lessons to increase motivation to exercise. | Android |
| Slumber Time | Promotes healthy sleeping by prompting users to keep an active sleep diary. The app also provides a checklist of things to do before bedtime to promote healthy sleep habits. | Android |
| Boost Me | Promotes positive mood by having users schedule positive activities throughout the day. A mood tracker allows users to see their progress and the impact of different activities on their mood. | Android |
There were two broad aims of this study. The first was to examine, in a single-group pre-post design, the feasibility and usability of the IntelliCare apps in patients recently diagnosed with breast cancer to inform a larger trial. We examined recruitment and retention rates to inform a potential future randomized trial. On the basis of the considerations of the size of the clinic from which participants were recruited, as well as the decision to recruit patients early in the breast cancer diagnostic pathway (at a time when it may not be appropriate to participate in a study that requires an immediate face-to-face consent and app download process), a threshold of 1 to 2 participants per week was the threshold to determine feasibility of in-clinic recruitment for a larger study [43]. The second aim was to examine the usability and preliminary impact of the IntelliCare apps in reducing distress in patients recently diagnosed with breast cancer. Note, this study initially sought to recruit the caregivers of patients with breast cancer; however, because of low enrollment, we decided to exclude the caregivers from the analyses. It was hypothesized that patients newly diagnosed with breast cancer would have decreases in general distress symptoms, as well as depression and anxiety symptoms, over a 7-week intervention period [43]. Quantitative and open-ended feedback was collected at the end of the study to evaluate usability and satisfaction of using the apps and coaching.
Methods
Overview
This was a single-group, 7-week pre-post study of patients with breast cancer in the United States. The decision to use a 7-week duration was based on the duration of brief face-to-face psychotherapy (typically 6-8 weeks) and previous reviews finding that the duration of app interventions usually range between 6 days and 8 weeks [44]. All participants received the IntelliCare apps and coaching. Self-report measures were obtained at baseline and postintervention to examine mental health outcomes. Additional measures were administered at the end of the study to evaluate user satisfaction and ways to improve the intervention for a future trial.
Participants
A total of 40 female patients with breast cancer (age: mean 56.8 years, SD 11.6 years) actively receiving cancer treatment were enrolled over a course of 29 weeks. Among those that indicated their race, most participants self-identified as white (31/38, 82%), followed by black (4/38, 11%), Hispanic (1/38, 3%), American Indian or Alaska Native (1/38, 3%), and multiracial (1/38, 3%). The median number of days from cancer diagnosis to study enrollment was 21 days. Among those who reported their breast cancer stage, 11% (3/28) of the patients were diagnosed with stage 0, 25% (7/28) of the patients were diagnosed with stage 1, 39% (11/28) of the patients were diagnosed with stage 2, and 25% (7/28) of the patients were diagnosed with stage 3. Rural-urban commuting area (RUCA) codes V3.0 from the United States Department of Agriculture were determined using participant zip codes. RUCA codes range from 1 (most metropolitan) to 10 (most rural) and are based on US Census tract data of population density, urbanization, and daily commuting. In this study, 47% (17/36) of the participants resided in an area characterized as most urban or metropolitan (RUCA=1), 42% (15/36) of the participants resided in an area characterized as metropolitan or micropolitan (RUCA=2-6), and 11% (4/36) of the participants resided in an area characterized as small town or rural (RUCA=7-10).
To limit barriers to entry, inclusion criteria were limited to the following: (1) patient diagnosed with breast cancer within the last 2 months, (2) age at least 18 years, (3) proficient in English at a sixth-grade level, and (4) has a mobile phone or is willing to carry one around if provided. Participants were not required to have a minimum level of familiarity with mobile devices or technology. Note, a total of 12 caregivers were also enrolled and were provided the same apps. Owing to the low number of caregivers enrolled, in this study, we focused on data obtained from patients with breast cancer.
Procedure
Patients with breast cancer were recruited from a breast care clinic in a US National Cancer Institute–designated clinical cancer center. Surgical oncologists and nurses handed out a study flyer to patients with breast cancer during a normal scheduled visit. Patients had an opportunity to speak to a research staff member, who provided more details about the study and answered questions. If an eligible patient expressed interest in participating, the patient was led through the consenting process by a research staff member. Research staff described the aims of the study, introduced the IntelliCare apps, and reviewed the study timeline. After providing written consent, participants scheduled a 30-min coaching call (see description below) that took place sometime within the next 10 days with a research staff member, which marked the initiation of their treatment in the study. Participants were guided to download the apps in the consent session, but they were told not to open them until the coaching call. Participants then completed a battery of measures that took approximately 10 to 15 min to complete. Following the initial coaching call, participants received an SMS text message (via Qualtrics’ SMS tool) every week to remind them to try two new exercise modules in the app. After 7 weeks (postintervention), participants completed another battery of self-report measures on the Web. They also provided feedback about their experiences of using the app and coaching. See Figure 1 for information on patient recruitment and flow. Participants were compensated with a US $50 gift card for providing feedback. Informed consent was obtained from all individual participants included in the study. All procedures performed were in accordance with the ethical standards of the University of Virginia Institutional Review Board (IRB-HSR# 20648) and with the 1964 Helsinki declaration.
Figure 1.
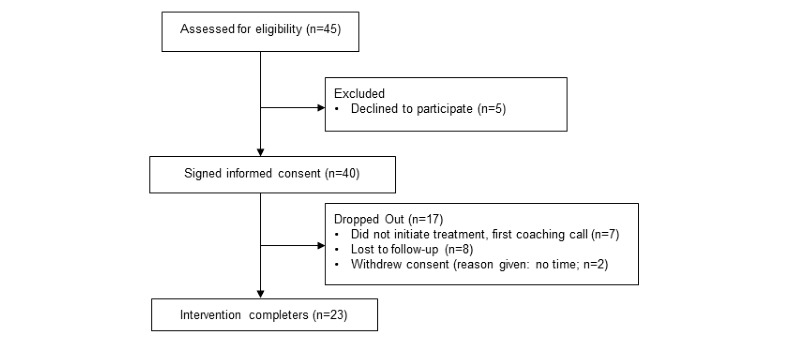
Study flow.
Materials
Participants used their own personal mobile phone. A concerted effort was made to include both Android and iOS users into the study, given the differences between users of these platforms in previous work [26]. A total of 3 participants did not own a mobile phone or have an appropriate mobile phone plan that enables downloading and using a native mobile phone app, and they were provided with a Samsung S7 Android phone with an unlimited data plan. These individuals were able to use the phones for nonstudy purposes. All IntelliCare apps were available for Android users, and a total of five apps (ie, Thought Challenger, Worry Knot, Daily Feats, My Mantra, and Day To Day) were available to iOS users at the time of the study. Android users were instructed to try two new apps every week for the first 6 weeks and to use any combination of apps for the seventh week. iOS users were instructed to try one new app every week for the first 5 weeks and to use any combination of apps for the sixth and seventh weeks. Among the initially enrolled 40 female patients with breast cancer, 31 had an Android phone and 9 had an iOS phone.
Phone Coaching
A manualized coaching protocol was adopted from a previous IntelliCare study [32], based on the Efficiency Model of Support [38]. The goals of coaching are to address usability issues, increase engagement with the app, promote fit of the intervention by assessing the needs of patients with cancer, promote knowledge of the skills found in the app, and encourage implementation of the skills in daily life [38]. Usability concerns include issues related to the usability of the intervention, fit of the intervention tool to one’s needs, knowledge of how to use the intervention, and implementation failures. Coaches were instructed to focus on app-related issues and to refrain from engaging in traditional counseling with participants. An initial coaching call (designed to last 30 min) focused on orienting participants to downloading and using the app, setting expectations of the coach’s role, assessing how the apps may meet participants’ needs, and building rapport. Participants were told that they could contact coaches at any time with any app-related questions. A total of 2 coaches with a bachelor’s degree were trained and closely monitored by the lead author (PC). Finally, an unstructured 10-min phone call 3 weeks after the initial coaching call served as a check-in to make sure that participants did not have any lingering concerns or questions.
Measures
General Psychological Distress
The Patient Health Questionnaire-4 (PHQ-4) [31] is widely used in cancer settings as a brief screener of general distress and symptom burden, and it is well validated in both general and clinical samples [31,32]. Individuals are asked to rate (0=not at all and 3=nearly every day) the degree to which they experienced different states (eg, “Little interest or please in doing things”) over the past 2 weeks. Scores range from 0 to 12; a score of 6 to 8 indicates moderate mood symptoms, whereas a score of 9 and higher indicates severe mood symptoms. The PHQ-4 was administered at baseline and postintervention.
Symptoms of Depression and Anxiety
Depression symptoms were assessed with the 4-item scale from the Patient-Reported Outcomes Measurement Information System (PROMIS) [30] 29-item profile version 2.0 (PROMIS-29 Profile v2.0). PROMIS, a US National Institutes of Health Roadmap program, provides sensitive and reliable measures of patient-reported outcomes. Participants are asked to report (1=never and 5=always) the degree to which they experienced various depressed states (eg, “I felt worthless” and “I felt hopeless”) over the past 7 days. Continuous anxiety symptoms were assessed with the 4-item scale from the PROMIS-29 Profile v2.0. Participants are asked to report (1=never and 5=always) the degree to which they have experienced different anxious states (eg, “My worries overwhelmed me” and “I felt fearful”) over the past 7 days. The PROMIS scales were administered at baseline and postintervention. Consistent with PROMIS scoring recommendations, raw summed scores were converted into T-scores for analyses, with higher scores indicating greater symptom levels.
User Feedback
User feedback was assessed at postintervention. The USE-short form [45] was used to examine usability and satisfaction of the IntelliCare app suite as a whole. It is composed of 21 items that assess user experience (eg, “I would recommend it to a friend,” “It is easy to learn to use it,” and “It is simply to use”), which comprises the domains of usefulness, ease of use, ease of learning, and satisfaction. Items are scored on a 7-point Likert scale (1=strongly disagree and 7=strongly agree). The USE measure is a well-validated scale that is commonly used to evaluate the user experience of mHealth interventions [46,47].
Participants also provided open-ended feedback during telephone interviews with the research staff. The interviews covered the following topics related to the apps: general impressions, design quality, technical needs, and design suggestions to promote app implementation and usage. In addition, participants were asked to provide feedback on the following aspects of phone coaching: general experience with coaches, usefulness of coaching, additional or unmet coaching needs, suggestions to improve the coaching experience.
Data Analysis
Outcome data were stored in a secured Qualtrics server for highly sensitive data. Analyses were done in SPSS Statistics for Windows, version 25.0 (IBM Corp, Armonk, NY).
Quantitative user data were analyzed descriptively by obtaining means and SDs. Qualitative feedback data were reviewed for emerging themes. Specifically, responses were coded on the domains of the following: (1) ways to improve the design and user interface of the apps, (2) the specific apps that were most helpful (and why), (3) the specific apps that were least helpful (and why), (4) obstacles and barriers to using the apps, and (5) ways to improve the usefulness of coaching calls [43].
Paired t tests were used to analyze self-reported outcome data among patients with breast cancer [43] and to examine whether the use of the IntelliCare apps was associated with changes in distress and symptoms of depression and anxiety before vs after the 7-week intervention.
Results
Feasibility of In-Clinic Recruitment
See Figure 1 for information on study flow. A total of 45 patients with breast cancer were assessed for eligibility, of which 40 signed the informed consent form. A total of 23 patients with breast cancer completed the 7-week intervention, and 17 individuals prematurely dropped out because of noninitiation of treatment (ie, failure to complete the first coaching call), lost contact, and withdrawal of consent because of the perceived time burden of being in the study.
Patients with breast cancer were recruited over a span of 29 weeks, from March 2018 to September 2018. Thus, the minimum recruitment threshold of in-clinic recruitment of 1 to 2 participants was met (note, the recruitment rate is higher if the 12 caregivers who provided informed consent are included in the total count). Incremental adjustments were made during the trial to increase the efficiency of the patient recruiting process. Specifically, we were able to identify key personnel (ie, nurses and patient navigators) and clinic procedures to more easily identify eligible patients. These changes did not have an impact on the study procedures after the informed consent form was signed. A paper discussing the challenges and potential solutions of in-clinic recruitment for mHealth pilot studies, based on our experience of conducting this study, is forthcoming.
Distress and Mood Symptoms
Table 2 contains the descriptive statistics of psychosocial outcomes. On the basis of the PROMIS T-scores, there were significant reductions in symptoms of depression (t22=2.35; P=.03; 95% CI 0.32 to 5.03; Cohen d=0.52) over the 7-week intervention period. Although there was also a reduction in symptoms of anxiety (t22=2.05; P=.05; 95% CI −0.05 to 7.52; Cohen d=0.45), this did not reach significance.
Table 2.
Means and SDs of psychosocial outcomes at each time point, along with the results of paired t tests.
| Outcomes | Baseline, mean (SD) | Postintervention, mean (SD) | P value |
| Depression symptoms | 53.77 (9.60) | 51.09 (10.45) | .03 |
| Anxiety symptoms | 60.26 (8.84) | 56.53 (9.67) | .05 |
| General distress | 3.96 (2.65) | 2.83 (2.48) | .02 |
Consistent with the previous findings, patients with breast cancer reported significant reductions in general psychological distress (PHQ-4) [48] over the 7-week intervention period (t22=2.61; P=.02; 95% CI 0.23 to 2.03; Cohen d=0.55). At baseline, among those who completed the 7-week study, 22% (6/28) of patients reported at least a moderate level of distress, whereas 8% patients (3/38) reported at least a moderate level of distress at postintervention.
App Usage
The median number of total IntelliCare app launches was 97, roughly equal to two app launches per day over the course of the trial. Table 3 contains additional app usage statistics for the individual apps.
Table 3.
Median number of app launches and median duration of app launches of individual IntelliCare apps.
| App name | App launches (number) | Duration (in seconds) |
| Aspire | 10.5 | 20 |
| Day to Day | 20 | 48 |
| Daily Feats | 33 | 38 |
| Worry Knot | 11.5 | 32.5 |
| Social Force | 2 | 27.5 |
| My Mantra | 5 | 21 |
| Thought Challenger | 7 | 19 |
| iCope | 7 | 27 |
| Purple Chill | 24 | 17 |
| MoveMe | 6.5 | 20 |
| Slumber Time | 9 | 35 |
| Boost Me | 10 | 78 |
Feedback
Patients with breast cancer rated the apps highly in terms of ease of use (mean 5.62, SD 1.3) and ease of learning (mean 5.67, SD 1.6) on the USE-short form. In general, participants had favorable yet relatively lower ratings for the domains of usefulness (mean 4.26, SD 1.8) and satisfaction (mean 4.05, SD 1.9).
A closer examination of the qualitative feedback of the patients with breast cancer supported the quantitative findings. Thematic analyses revealed that many participants found the apps very easy to use. A common theme was that despite not being computer or technologically savvy, participants found the apps to be fairly easy to use. Participants also reported that they generally liked the simple, straightforward design, which helped them to navigate the apps. Another theme that emerged was the utility of phone coaching. Participants reported that their interactions with coaches were pleasant and helpful in using the apps. There was general agreement that coaches helped patients with breast cancer feel supported while in the study, and the frequency and duration of phone calls were not viewed as overly burdensome, although none wanted more phone calls with coaches. It is worth noting that the sentiment of phone coaching as useful was not unanimous, as a minority of participants felt that phone coaching was unnecessary.
Additional themes hinted at ways to improve the IntelliCare apps for patients with breast cancer. A common theme was that participants reported that the look and feel of the apps, including the content (eg, examples of distressing thoughts), were not relevant to someone with breast cancer (eg, “the apps are not relevant to someone going through cancer...some questions or things don’t pertain to cancer” and “you should tailor [the apps] to situational cancer”). Another recurring theme was related to the timing of app use in relation to cancer stage and treatment progress. Many patients with breast cancer reported that the apps may be most useful for patients diagnosed with a more severe stage of cancer (eg, stage 3 or 4) or those undergoing chemotherapy.
Discussion
Principal Findings
Overall, patients with breast cancer found the apps easy to use and navigate. Feedback obtained at the end of the study highlighted several areas for potential improvement, all of which entail making the apps more relevant for patients with breast cancer and their experiences.
This study established the feasibility of recruiting patients newly diagnosed with breast cancer to engage in an mHealth intervention from a relatively small breast surgery oncology clinic. Receiving a cancer diagnosis is a life-changing moment for many individuals. Psychosocial distress is known to peak around the time of breast cancer diagnosis and the early stages of cancer treatment [49,50]. Thus, recruiting individuals around the time of diagnosis is a significant challenge to evaluating mobile app interventions. To meet the minimum threshold of feasibility (1 to 2 participants per week) [43], our team needed to adjust to the structure and flow of the clinic. For example, a significant amount of time was devoted to introducing the study to nurses and patient navigators. Research assistants had to coordinate with the clinic staff to present the study to eligible patients. Researchers who are interested in conducting an mHealth pilot study in patients newly diagnosed with cancer are encouraged to factor in clinic space, staff, and patient flow when designing their study and calculating enrollment figures.
Patients with breast cancer identified several areas of improvement for a future trial. As the IntelliCare apps were designed for use in the general population, many patients reported wanting the appearance and content of the apps to reflect their experiences. A wealth of studies demonstrate the importance of tailoring digital interventions for end users [51,52]. In recent years, there has been a notable rise in the awareness of breast cancer through media and social campaigns [53,54], leading many patients with breast cancer to strongly identify with their diagnosis [55,56]. The most prominent theories of behavior change [57-59] stress that interventions that are perceived as personally relevant are most likely to succeed in changing people’s behavior. Thus, to increase engagement with an app-delivered intervention for patients with breast cancer, it is important to tailor it in ways that are meaningful to those end users. For example, adding examples of cancer-related worrying thoughts (eg, “My cancer will never go away” and “I’m not strong enough to go through chemotherapy”) to the Thought Challenger app may improve engagement with the app. Future work should also consider tailoring the app based on cancer stage and timing of treatment. For example, introducing the apps to patients right before starting chemotherapy may provide them with the needed coping skills during cancer treatment. Finally, although the patients with breast cancer generally found the coaching to be useful, none reported wanting more coaching calls, and a few participants found the coaching to be unnecessary. On the basis of this feedback, future studies may consider only providing coaching to a subset of patients with breast cancer who are in greatest need. For example, by leveraging a Sequential, Multiple Assignment, Randomized Trial [60], individuals who struggle to engage with the apps could be identified and provided with coaching. Providing support on an individual basis maximizes the scalability of app-based interventions by providing a more efficient use of resources.
Pilot studies are often conducted to obtain an effect size estimate to power a larger trial; therefore, this study’s findings should not be overinterpreted in light of the relatively small sample. However, the results suggest that the IntelliCare apps have a moderate effect (based on Cohen d) in reducing mood and anxiety symptoms in patients recently diagnosed with breast cancer. Although the effect sizes obtained in this study are smaller than those reported in previous IntelliCare trials among the general population [32,42], they are comparable with the effect sizes of other mental health interventions (eg, mindfulness and in-person therapy) that have been tested among patients with breast cancer [61,62]. Achieving even a modest reduction of mental health symptoms may justify the expanded use of digital mental health interventions in patients with breast cancer, given their scalability, cost, and accessibility.
Limitations and Future Directions
The findings from this study should be interpreted in light of several limitations. Given the size and characteristics of the sample in this study, these findings may not be generalizable to other cancer populations (eg, pancreatic and lung). The findings related to the potential impact of IntelliCare on distress symptoms should be replicated in a larger sample of patients with breast cancer. As this was a single-arm trial, we cannot rule out the possibility that the observed improvements were because of factors other than IntelliCare, such as the natural course of the problems. It will be important to evaluate the IntelliCare apps in a randomized controlled trial among patients with breast cancer. Thus, it is important to not overinterpret the study’s findings because of the absence of a control condition. As this study was conducted in a US National Cancer Institute–designated clinical cancer center, the findings regarding in-clinic recruitment feasibility have limited generalizability to other settings that may not possess as many resources. In addition, although the participants in this study were guided to download the apps during the informed consent session, future work should examine the benefits of providing more structure in teaching users how to navigate treatment apps. Finally, as there were more apps available to Android users than iOS users, it is hard to determine which apps were most efficacious in reducing distress symptoms. Future work should consider standardizing the order in which apps are tried, to allow for a better understanding of the effect of each app on psychosocial outcomes.
Finally, although the dropout rate of individuals in this study was generally at par with other app interventions, it was noticeably higher than that reported in previous IntelliCare studies in the general population. This may be attributed to the fact that individuals in this study were dealing with the stress of a recent breast cancer diagnosis. Similarly, the app usage rates were considerably lower than those reported in previous IntelliCare trials. This is consistent with research indicating that intervention impact and engagement generally decrease when moving from general to clinical samples [63]. Future studies should continue to explore the ways to address dropout in populations at high risk of dropout, such as providing added human support or connecting patients with additional resources in their community. Despite a higher dropout rate and a decrease in app usage in this study than those reported in previous IntelliCare trials in the general population, findings suggest that patients with breast cancer are still able to use, and benefit from, an app-delivered mental health program.
Conclusions
Mobile phone apps hold significant promise to overcome barriers in providing psychosocial care for patients with breast cancer [64-67]. However, relatively few publicly available apps have been empirically validated for treating mood symptoms [33,44], and those that have been validated have not been tested among patients with breast cancer [25]. IntelliCare, which has been rigorously studied in the general population [32,68,69], has the potential to make a significant public health impact by providing support to a large population of patients with breast cancer.
Acknowledgments
This project is funded by a seed grant from the University of Virginia Center for Engineering in Medicine. EL is supported by a research grant K08 MH112878 from the National Institute of Mental Health.
Abbreviations
- mHealth
mobile health
- PHQ-4
Patient Health Questionnaire-4
- PROMIS
Patient-Reported Outcomes Measurement Information System
- RUCA
rural-urban commuting area
Footnotes
Conflicts of Interest: DM has equity ownership in and EL has received consulting fees from “Actualize Therapy,” a company developing and making available mobile technology products related to the research reported in this paper. DM and EL will not have direct access to the final raw dataset.
References
- 1.Burgess C, Cornelius V, Love S, Graham J, Richards M, Ramirez A. Depression and anxiety in women with early breast cancer: five year observational cohort study. Br Med J. 2005;330(7493):702. doi: 10.1136/bmj.38343.670868.d3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Zabora J, BrintzenhofeSzoc K, Curbow B, Hooker C, Piantadosi S. The prevalence of psychological distress by cancer site. Psychooncology. 2001;10(1):19–28. doi: 10.1002/1099-1611(200101/02)10:1<19::aid-pon501>3.0.co;2-6. [DOI] [PubMed] [Google Scholar]
- 3.Grabsch B, Clarke DM, Love A, McKenzie DP, Snyder RD, Bloch S, Smith G, Kissane DW. Psychological morbidity and quality of life in women with advanced breast cancer: a cross-sectional survey. Palliat Support Care. 2006 Mar;4(1):47–56. doi: 10.1017/s1478951506060068. [DOI] [PubMed] [Google Scholar]
- 4.Henselmans I, Helgeson VS, Seltman H, de Vries J, Sanderman R, Ranchor AV. Identification and prediction of distress trajectories in the first year after a breast cancer diagnosis. Health Psychol. 2010 Mar;29(2):160–8. doi: 10.1037/a0017806. [DOI] [PubMed] [Google Scholar]
- 5.Reich M, Lesur A, Perdrizet-Chevallier C. Depression, quality of life and breast cancer: a review of the literature. Breast Cancer Res Treat. 2008 Jul;110(1):9–17. doi: 10.1007/s10549-007-9706-5. [DOI] [PubMed] [Google Scholar]
- 6.Watson M, Haviland J, Greer S, Davidson J, Bliss J. Influence of psychological response on survival in breast cancer: a population-based cohort study. Lancet. 1999 Oct 16;354(9187):1331–6. doi: 10.1016/s0140-6736(98)11392-2. [DOI] [PubMed] [Google Scholar]
- 7.Onitilo AA, Nietert PJ, Egede LE. Effect of depression on all-cause mortality in adults with cancer and differential effects by cancer site. Gen Hosp Psychiatry. 2006;28(5):396–402. doi: 10.1016/j.genhosppsych.2006.05.006. [DOI] [PubMed] [Google Scholar]
- 8.Carlson LE, Bultz BD. Efficacy and medical cost offset of psychosocial interventions in cancer care: making the case for economic analyses. Psychooncology. 2004 Dec;13(12):837–49; discussion 850. doi: 10.1002/pon.832. [DOI] [PubMed] [Google Scholar]
- 9.Tatrow K, Montgomery GH. Cognitive behavioral therapy techniques for distress and pain in breast cancer patients: a meta-analysis. J Behav Med. 2006 Feb;29(1):17–27. doi: 10.1007/s10865-005-9036-1. [DOI] [PubMed] [Google Scholar]
- 10.Gudenkauf LM, Antoni MH, Stagl JM, Lechner SC, Jutagir DR, Bouchard LC, Blomberg BB, Glück S, Derhagopian RP, Giron GL, Avisar E, Torres-Salichs MA, Carver CS. Brief cognitive-behavioral and relaxation training interventions for breast cancer: a randomized controlled trial. J Consult Clin Psychol. 2015 Aug;83(4):677–88. doi: 10.1037/ccp0000020. http://europepmc.org/abstract/MED/25939017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Johnson JA, Rash JA, Campbell TS, Savard J, Gehrman PR, Perlis M, Carlson LE, Garland SN. A systematic review and meta-analysis of randomized controlled trials of cognitive behavior therapy for insomnia (CBT-I) in cancer survivors. Sleep Med Rev. 2016 Jun;27:20–8. doi: 10.1016/j.smrv.2015.07.001. [DOI] [PubMed] [Google Scholar]
- 12.Zhang M, Huang L, Feng Z, Shao L, Chen L. Effects of cognitive behavioral therapy on quality of life and stress for breast cancer survivors: a meta-analysis. Minerva Med. 2017 Feb;108(1):84–93. doi: 10.23736/S0026-4806.16.04528-6. [DOI] [PubMed] [Google Scholar]
- 13.Aranda S, Schofield P, Weih L, Yates P, Milne D, Faulkner R, Voudouris N. Mapping the quality of life and unmet needs of urban women with metastatic breast cancer. Eur J Cancer Care (Engl) 2005 Jul;14(3):211–22. doi: 10.1111/j.1365-2354.2005.00541.x. [DOI] [PubMed] [Google Scholar]
- 14.Harrison JD, Young JM, Price MA, Butow PN, Solomon MJ. What are the unmet supportive care needs of people with cancer? A systematic review. Support Care Cancer. 2009 Aug;17(8):1117–28. doi: 10.1007/s00520-009-0615-5. [DOI] [PubMed] [Google Scholar]
- 15.Cawley M, Kostic J, Cappello C. Informational and psychosocial needs of women choosing conservative surgery/primary radiation for early stage breast cancer. Cancer Nurs. 1990 Apr;13(2):90–4. doi: 10.1097/00002820-199004000-00003. [DOI] [PubMed] [Google Scholar]
- 16.Girgis A, Boyes A, Sanson-Fisher R, Burrows S. Perceived needs of women diagnosed with breast cancer: rural versus urban location. Aust N Z J Public Health. 2000 Apr;24(2):166–73. doi: 10.1111/j.1467-842x.2000.tb00137.x. [DOI] [PubMed] [Google Scholar]
- 17.Chi M. The hidden cost of cancer: helping clients cope with financial toxicity. Clin Soc Work J. 2019;47(3):249–57. doi: 10.1007/s10615-017-0640-7. [DOI] [Google Scholar]
- 18.Yabroff KR, Davis WW, Lamont EB, Fahey A, Topor M, Brown ML, Warren JL. Patient time costs associated with cancer care. J Natl Cancer Inst. 2007 Jan 3;99(1):14–23. doi: 10.1093/jnci/djk001. [DOI] [PubMed] [Google Scholar]
- 19.Sun W, Chen K, Terhaar A, Wiegmann DA, Heidrich SM, Tevaarwerk AJ, Sesto ME. Work-related barriers, facilitators, and strategies of breast cancer survivors working during curative treatment. Work. 2016;55(4):783–95. doi: 10.3233/WOR-162449. http://europepmc.org/abstract/MED/28059814. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Holland JC, Kelly BJ, Weinberger MI. Why psychosocial care is difficult to integrate into routine cancer care: stigma is the elephant in the room. J Natl Compr Canc Netw. 2010 Apr;8(4):362–6. doi: 10.6004/jnccn.2010.0028. [DOI] [PubMed] [Google Scholar]
- 21.Davis AS, McIntosh DE, Phelps L, Kehle TJ. Addressing the shortage of school psychologists: a summative overview. Psychol Sch. 2004 Apr;41(4):489–95. doi: 10.1002/pits.10192. [DOI] [Google Scholar]
- 22.Shafran R, Clark D, Fairburn C, Arntz A, Barlow D, Ehlers A, Freeston M, Garety P, Hollon S, Ost L, Salkovskis P, Williams J, Wilson G. Mind the gap: improving the dissemination of CBT. Behav Res Ther. 2009 Nov;47(11):902–9. doi: 10.1016/j.brat.2009.07.003. [DOI] [PubMed] [Google Scholar]
- 23.Kaltenthaler E, Sutcliffe P, Parry G, Beverley C, Rees A, Ferriter M. The acceptability to patients of computerized cognitive behaviour therapy for depression: a systematic review. Psychol Med. 2008 Nov;38(11):1521–30. doi: 10.1017/S0033291707002607. [DOI] [PubMed] [Google Scholar]
- 24.Bultz BD, Carlson LE. Emotional distress: the sixth vital sign--future directions in cancer care. Psychooncology. 2006 Feb;15(2):93–5. doi: 10.1002/pon.1022. [DOI] [PubMed] [Google Scholar]
- 25.Bender JL, Yue RY, To MJ, Deacken L, Jadad AR. A lot of action, but not in the right direction: systematic review and content analysis of smartphone applications for the prevention, detection, and management of cancer. J Med Internet Res. 2013 Dec 23;15(12):e287. doi: 10.2196/jmir.2661. https://www.jmir.org/2013/12/e287/ [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Luxton DD, McCann RA, Bush NE, Mishkind MC, Reger GM. mHealth for mental health: integrating smartphone technology in behavioral healthcare. Prof Psychol Res Pr. 2011;42(6):505–12. doi: 10.1037/a0024485. [DOI] [Google Scholar]
- 27.Muñoz RF, Chavira DA, Himle JA, Koerner K, Muroff J, Reynolds J, Rose RD, Ruzek JI, Teachman BA, Schueller SM. Digital apothecaries: a vision for making health care interventions accessible worldwide. Mhealth. 2018;4:18. doi: 10.21037/mhealth.2018.05.04. doi: 10.21037/mhealth.2018.05.04. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Muñoz RF, Bunge EL, Chen K, Schueller SM, Bravin JI, Shaughnessy EA, Pérez-Stable EJ. Massive open online interventions: a novel model for delivering behavioral-health services worldwide. Clin Psychol Sci. 2016;4(2):194–205. doi: 10.1177/2167702615583840. [DOI] [Google Scholar]
- 29.Pew Research Center. 2019. Jun 12, [2019-10-01]. Mobile Fact Sheet http://www.pewinternet.org/fact-sheet/mobile/#.
- 30.Ritterband LM, Thorndike FP, Cox DJ, Kovatchev BP, Gonder-Frederick LA. A behavior change model for internet interventions. Ann Behav Med. 2009 Aug;38(1):18–27. doi: 10.1007/s12160-009-9133-4. http://europepmc.org/abstract/MED/19802647. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Mohr DC, Schueller SM, Montague E, Burns MN, Rashidi P. The behavioral intervention technology model: an integrated conceptual and technological framework for eHealth and mHealth interventions. J Med Internet Res. 2014 Jun 5;16(6):e146. doi: 10.2196/jmir.3077. https://www.jmir.org/2014/6/e146/ [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Mohr DC, Tomasino KN, Lattie EG, Palac HL, Kwasny MJ, Weingardt K, Karr CJ, Kaiser SM, Rossom RC, Bardsley LR, Caccamo L, Stiles-Shields C, Schueller SM. IntelliCare: an eclectic, skills-based app suite for the treatment of depression and anxiety. J Med Internet Res. 2017 Jan 5;19(1):e10. doi: 10.2196/jmir.6645. https://www.jmir.org/2017/1/e10/ [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Bakker D, Kazantzis N, Rickwood D, Rickard N. Mental health smartphone apps: review and evidence-based recommendations for future developments. JMIR Ment Health. 2016 Mar 1;3(1):e7. doi: 10.2196/mental.4984. https://mental.jmir.org/2016/1/e7/ [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Bakker D, Kazantzis N, Rickwood D, Rickard N. A randomized controlled trial of three smartphone apps for enhancing public mental health. Behav Res Ther. 2018 Oct;109:75–83. doi: 10.1016/j.brat.2018.08.003. [DOI] [PubMed] [Google Scholar]
- 35.Firth J, Torous J, Nicholas J, Carney R, Pratap A, Rosenbaum S, Sarris J. The efficacy of smartphone-based mental health interventions for depressive symptoms: a meta-analysis of randomized controlled trials. World Psychiatry. 2017 Oct;16(3):287–98. doi: 10.1002/wps.20472. doi: 10.1002/wps.20472. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Firth J, Torous J, Nicholas J, Carney R, Rosenbaum S, Sarris J. Can smartphone mental health interventions reduce symptoms of anxiety? A meta-analysis of randomized controlled trials. J Affect Disord. 2017 Aug 15;218:15–22. doi: 10.1016/j.jad.2017.04.046. https://linkinghub.elsevier.com/retrieve/pii/S0165-0327(17)30015-0. [DOI] [PubMed] [Google Scholar]
- 37.Mohr DC, Cuijpers P, Lehman K. Supportive accountability: a model for providing human support to enhance adherence to eHealth interventions. J Med Internet Res. 2011 Mar 10;13(1):e30. doi: 10.2196/jmir.1602. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Schueller SM, Tomasino KN, Mohr DC. Integrating human support into behavioral intervention technologies: the efficiency model of support. Clin Psychol Sci Pract. 2017 Mar;24(1):27–45. doi: 10.1111/cpsp.12173. [DOI] [Google Scholar]
- 39.Davis SW, Oakley-Girvan I. mHealth education applications along the cancer continuum. J Cancer Educ. 2015 Jun;30(2):388–94. doi: 10.1007/s13187-014-0761-4. [DOI] [PubMed] [Google Scholar]
- 40.Torous J, Nicholas J, Larsen ME, Firth J, Christensen H. Clinical review of user engagement with mental health smartphone apps: evidence, theory and improvements. Evid Based Ment Health. 2018 Aug;21(3):116–9. doi: 10.1136/eb-2018-102891. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Oulasvirta A, Tamminen S, Roto V, Kuorelahti J. Interaction in 4-second Bursts: The Fragmented Nature of Attentional Resources in Mobile HCI. Proceedings of the SIGCHI Conference on Human Factors in Computing Systems; CHI'05; April 2 - 7, 2005; Oregon, Portland, USA. New York, NY, United States: Association for Computing Machinery (ACM); 2005. pp. 919–28. [DOI] [Google Scholar]
- 42.Mohr DC, Schueller SM, Tomasino KN, Kaiser SM, Alam N, Karr C, Vergara JL, Gray EL, Kwasny MJ, Lattie EG. Comparison of the effects of coaching and receipt of app recommendations on depression, anxiety, and engagement in the IntelliCare platform: Factorial randomized controlled trial. J Med Internet Res. 2019 Aug 28;21(8):e13609. doi: 10.2196/13609. https://www.jmir.org/2019/8/e13609/ [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Chow PI, Showalter SL, Gerber MS, Kennedy E, Brenin DR, Schroen AT, Mohr DC, Lattie EG, Cohn WF. Use of mental health apps by breast cancer patients and their caregivers in the United States: protocol for a pilot pre-post study. JMIR Res Protoc. 2019 Jan 14;8(1):e11452. doi: 10.2196/11452. https://www.researchprotocols.org/2019/1/e11452/ [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Donker T, Petrie K, Proudfoot J, Clarke J, Birch M, Christensen H. Smartphones for smarter delivery of mental health programs: a systematic review. J Med Internet Res. 2013 Nov 15;15(11):e247. doi: 10.2196/jmir.2791. https://www.jmir.org/2013/11/e247/ [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Lund AM. Measuring usability with the USE questionnaire. Usability Interface. 2001;8(2):3–6. https://www.researchgate.net/publication/230786746_Measuring_Usability_with_the_USE_Questionnaire. [Google Scholar]
- 46.Ben-Zeev D, Brenner CJ, Begale M, Duffecy J, Mohr DC, Mueser KT. Feasibility, acceptability, and preliminary efficacy of a smartphone intervention for schizophrenia. Schizophr Bull. 2014 Nov;40(6):1244–53. doi: 10.1093/schbul/sbu033. http://europepmc.org/abstract/MED/24609454. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Corden ME, Koucky EM, Brenner C, Palac HL, Soren A, Begale M, Ruo B, Kaiser SM, Duffecy J, Mohr DC. MedLink: a mobile intervention to improve medication adherence and processes of care for treatment of depression in general medicine. Digit Health. 2016;2:2055207616663069. doi: 10.1177/2055207616663069. http://europepmc.org/abstract/MED/29942564. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Kroenke K, Spitzer RL, Williams JB, Löwe B. An ultra-brief screening scale for anxiety and depression: the PHQ-4. Psychosomatics. 2009;50(6):613–21. doi: 10.1176/appi.psy.50.6.613. [DOI] [PubMed] [Google Scholar]
- 49.Hegel MT, Moore CP, Collins ED, Kearing S, Gillock KL, Riggs RL, Clay KF, Ahles TA. Distress, psychiatric syndromes, and impairment of function in women with newly diagnosed breast cancer. Cancer. 2006 Dec 15;107(12):2924–31. doi: 10.1002/cncr.22335. doi: 10.1002/cncr.22335. [DOI] [PubMed] [Google Scholar]
- 50.Reece JC, Chan Y, Herbert J, Gralow J, Fann JR. Course of depression, mental health service utilization and treatment preferences in women receiving chemotherapy for breast cancer. Gen Hosp Psychiatry. 2013;35(4):376–81. doi: 10.1016/j.genhosppsych.2013.03.017. [DOI] [PubMed] [Google Scholar]
- 51.Yardley L, Morrison L, Bradbury K, Muller I. The person-based approach to intervention development: application to digital health-related behavior change interventions. J Med Internet Res. 2015 Jan 30;17(1):e30. doi: 10.2196/jmir.4055. https://www.jmir.org/2015/1/e30/ [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Yardley L, Spring BJ, Riper H, Morrison LG, Crane DH, Curtis K, Merchant GC, Naughton F, Blandford A. Understanding and promoting effective engagement with digital behavior change interventions. Am J Prev Med. 2016 Nov;51(5):833–42. doi: 10.1016/j.amepre.2016.06.015. [DOI] [PubMed] [Google Scholar]
- 53.Moffett J. Moving beyond the ribbon: an examination of breast cancer advocacy and activism in the US and Canada. Cult Dyn. 2003;15(3):287–306. doi: 10.1177/09213740030153004. [DOI] [Google Scholar]
- 54.Vraga EK, Stefanidis A, Lamprianidis G, Croitoru A, Crooks AT, Delamater PL, Pfoser D, Radzikowski JR, Jacobsen KH. Cancer and social media: a comparison of traffic about breast cancer, prostate cancer, and other reproductive cancers on Twitter and Instagram. J Health Commun. 2018;23(2):181–9. doi: 10.1080/10810730.2017.1421730. [DOI] [PubMed] [Google Scholar]
- 55.Twombly R. What's in a name: who is a cancer survivor? J Natl Cancer Inst. 2004 Oct 6;96(19):1414–5. doi: 10.1093/jnci/96.19.1414. [DOI] [PubMed] [Google Scholar]
- 56.Park CL, Zlateva I, Blank TO. Self-identity after cancer: 'survivor', 'victim', 'patient', and 'person with cancer'. J Gen Intern Med. 2009 Nov;24(Suppl 2):S430–5. doi: 10.1007/s11606-009-0993-x. http://europepmc.org/abstract/MED/19838845. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Bandura A. Human agency in social cognitive theory. Am Psychol. 1989 Sep;44(9):1175–84. doi: 10.1037/0003-066x.44.9.1175. [DOI] [PubMed] [Google Scholar]
- 58.Ajzen I. From intentions to actions: a theory of planned behavior. In: Kuhl J, Beckman J, editors. Action Control. Berlin, Heidelberg: Springer; 1985. pp. 11–39. [Google Scholar]
- 59.Bandura A. Self-efficacy: toward a unifying theory of behavioral change. Psychol Rev. 1977 Mar;84(2):191–215. doi: 10.1037//0033-295x.84.2.191. [DOI] [PubMed] [Google Scholar]
- 60.Almirall D, Nahum-Shani I, Sherwood NE, Murphy SA. Introduction to SMART designs for the development of adaptive interventions: with application to weight loss research. Transl Behav Med. 2014 Sep;4(3):260–74. doi: 10.1007/s13142-014-0265-0. http://europepmc.org/abstract/MED/25264466. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Zainal NZ, Booth S, Huppert FA. The efficacy of mindfulness-based stress reduction on mental health of breast cancer patients: a meta-analysis. Psychooncology. 2013 Jul;22(7):1457–65. doi: 10.1002/pon.3171. [DOI] [PubMed] [Google Scholar]
- 62.Duijts SF, Faber MM, Oldenburg HS, van Beurden M, Aaronson NK. Effectiveness of behavioral techniques and physical exercise on psychosocial functioning and health-related quality of life in breast cancer patients and survivors--a meta-analysis. Psychooncology. 2011 Feb;20(2):115–26. doi: 10.1002/pon.1728. [DOI] [PubMed] [Google Scholar]
- 63.Chambers DA, Glasgow RE, Stange KC. The dynamic sustainability framework: addressing the paradox of sustainment amid ongoing change. Implement Sci. 2013 Oct 2;8:117. doi: 10.1186/1748-5908-8-117. https://implementationscience.biomedcentral.com/articles/10.1186/1748-5908-8-117. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Abrol E, Groszmann M, Pitman A, Hough R, Taylor RM, Aref-Adib G. Exploring the digital technology preferences of teenagers and young adults (TYA) with cancer and survivors: a cross-sectional service evaluation questionnaire. J Cancer Surviv. 2017 Dec;11(6):670–82. doi: 10.1007/s11764-017-0618-z. http://europepmc.org/abstract/MED/28634734. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Stubbins R, He T, Yu X, Puppala M, Ezeana CF, Chen S, y Alvarado MV, Ensor J, Rodriguez A, Niravath P, Chang J, Wong ST, Patel T. A behavior-modification, clinical-grade mobile application to improve breast cancer survivors' accountability and health outcomes. JCO Clin Cancer Inform. 2018;(2):1–11. doi: 10.1200/cci.18.00054. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Lubberding S, van Uden-Kraan CF, Te Velde EA, Cuijpers P, Leemans CR, Verdonck-de Leeuw IM. Improving access to supportive cancer care through an eHealth application: a qualitative needs assessment among cancer survivors. J Clin Nurs. 2015 May;24(9-10):1367–79. doi: 10.1111/jocn.12753. [DOI] [PubMed] [Google Scholar]
- 67.Ringwald J, Marwedel L, Junne F, Ziser K, Schäffeler N, Gerstner L, Wallwiener M, Brucker SY, Hautzinger M, Zipfel S, Teufel M. Demands and needs for psycho-oncological ehealth interventions in women with cancer: cross-sectional study. JMIR Cancer. 2017 Nov 24;3(2):e19. doi: 10.2196/cancer.7973. https://cancer.jmir.org/2017/2/e19/ [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Lattie EG, Schueller SM, Sargent E, Stiles-Shields C, Tomasino KN, Corden ME, Begale M, Karr CJ, Mohr DC. Uptake and usage of IntelliCare: a publicly available suite of mental health and well-being apps. Internet Interv. 2016 May;4(2):152–8. doi: 10.1016/j.invent.2016.06.003. https://linkinghub.elsevier.com/retrieve/pii/S2214-7829(15)30029-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Rubanovich CK, Mohr DC, Schueller SM. Health app use among individuals with symptoms of depression and anxiety: a survey study with thematic coding. JMIR Ment Health. 2017 Jun 23;4(2):e22. doi: 10.2196/mental.7603. https://mental.jmir.org/2017/2/e22/ [DOI] [PMC free article] [PubMed] [Google Scholar]


