Abstract
Background
Immune parameters (IP) have been extensively studied to distinguish between latent tuberculosis (LTBI) and active tuberculosis (TB).
Objective
To determine the IP associated with LTBI, compared to active TB and individuals not infected by M. tuberculosis published in literature.
Methods
We conducted a systematic search using Google Scholar and PubMed databases, combining the MeSH terms latent tuberculosis, Mycobacterium tuberculosis, cytokines, and biological markers, with the free terms, biomarkers and cytokines. Spanish, English, and Portuguese articles comparing the concentration of IP associated with LTBI, either in plasma/serum or in vitro, in adults and nonimmunocompromised versus individuals with TB or without M. tuberculosis infection between 2006 July and 2018 July were included. Two blinded reviewers carried out the searches, read the abstracts, and selected the articles for analysis. Participants' information, diagnostic criteria, IP, detection methods, and biases were collected.
Results
We analyzed 36 articles (of 637 abstracts) with 93 different biomarkers in different samples. We found 24 parameters that were increased only in active TB (TGF-α, CSF3, CSF2, CCL1 [I-309], IL-7, TGF-β1, CCL3 [MIP-1α], sIL-2R, TNF-β, CCL7 [MCP-3], IFN-α, fractalkine, I-TAG, CCL8 [MCP-2], CCL21 [6Ckine], PDGF, IL-22, VEGF-A, LXA4, PGE2, PGF2α, sCD163, sCD14, and 15-Epi-LXA4), five were elevated in LTBI (IL-5, IL-17F, IL-1, CCL20 [MIP-3α], and ICAM-1), and two substances were increased among uninfected individuals (IL-23 and basic FGF). We found high heterogeneity between studies including failure to account for the time/illness of the individuals studied; varied samples and protocols; different clinical classification of TB; different laboratory methods for IP detection, which in turn leads to variable units of measurement and assay sensitivities; and selection bias regarding TST and booster effect. None of the studies adjusted the analysis for the effect of ethnicity.
Conclusions
It is mandatory to harmonize the study of immune parameters for LTBI diagnosis. This systematic review is registered with PROSPERO CRD42017073289.
1. Background
Latent tuberculosis (LTBI) is defined as the presence of a positive tuberculin skin test (TST) or interferon-γ (IFNγ) release assays (IGRAs) in the absence of clinical or radiographic signs of disease. These accepted tests are imperfect for LTBI diagnosis for several reasons: (1) the sensitivity and specificity are between 71 and 82% for TST and 81 and 86% for IGRAs [1], (2) the sensitivity is reduced in immunocompromised patients, (3) there is inability to differentiate between LTBI and active tuberculosis (TB), (4) a positive TST or IGRA result does not automatically imply LTBI, as individuals who eliminate the infection successfully might still be TST- or IGRA-positive because of memory T cell responses, which partly explains the low predictive value of TST and IGRAs [1], and (5) genetic factors may impact test sensitivity as well as the susceptibility for acquisition of mycobacterial infection [2–4]. To date, there is no available diagnostic tool that allows diagnosis of LTBI and differentiates clearly between LTBI and active TB.
For the above-mentioned reasons, the World Health Organization, governments and nongovernmental organization, and private sector established as one of the priorities the identification of “what biomarkers or combinations of markers will help distinguish the various stages of the spectrum of LTBI (from sterilizing immunity to subclinical active disease)” [5].
The improvement in high-throughput cytokine measurement platforms has sparked enthusiasm for identification of novel pathways involved in the pathogenesis of TB that can inform development of assays for LTBI determination. In tuberculous infection, some important immune molecules are known to play a pivotal role in the protective response against the bacteria. Among the main ones described are IFN-γ, produced by T CD4+, CD8+, and NK cells, and IL-1 and TNF-α, secreted by macrophages and lymphocytes, known to prevent the growth and multiplication of mycobacteria in host cells [6, 7]. However, additional biomarkers such as IL-2, IL-5, IL-10, IL-1RA, and MCP have been studied for their ability to differentiate between the LTBI and active TB [8], and it is believed that the cellular and immune profile expressed during tuberculous infection depends to a great extent on the stage of disease, i.e., LTBI or active, where immune biomarkers present in blood could have the ability to differentiate with greater precision between both stages [9].
Despite advances in the study of immune parameters, there are pervasive limitations in the analysis and conclusions of many of these studies. Cytokine/chemokine expression is affected by ethnicity [2, 10], cell simulation protocols (or no stimulation) [11–13], time of LTBI (which in most cases is impossible to quantify), and if the comparison group is people with TB, the clinical manifestations of disease (pulmonary vs. extrapulmonary TB) [14].
In order to identify which immune parameters are increased exclusively in LTBI, in addition to finding gaps in knowledge and study design of previous published papers, we performed a systematic review. The question posed is the following: what are the cytokines associated with LTBI, compared to cytokines expressed among individuals with active TB and those not infected by M. tuberculosis?
2. Methods
According to the Preferred Reporting Items for Systematic reviews and Meta-Analysis protocols (PRISMA-P), this systematic review was registered with the International Prospective Register of Systematic Reviews (PROSPERO) on August 31, 2017 (registration number CRD42017073289).
2.1. Eligibility Criteria
Studies were selected according to the following criteria.
2.1.1. Study Designs
We included clinical trials, prospective and retrospective comparative cohorts, and case-control and cross-sectional studies. We excluded descriptive studies, case reports and series, and reviews.
2.1.2. Participants
The participants are those from articles published between January 2006 and July 2018, which compared people with LTBI with 18 years or older, or adults and children, without any immunocompromising medical conditions, versus individuals with active TB or without M. tuberculosis infection under the same conditions. We excluded manuscripts assessing the production of IFN-γ as part of the evaluation of IGRAs, which were performed in animal models, immunocompromised individuals, and studies exclusively conducted in children.
2.1.3. Exposure
Articles that evaluated the expression of cytokines associated with LTBI, either in plasma or in vitro, with or without stimulation of mycobacterial antigens were included. The antigens used to perform cell stimulation were not restricted.
2.1.4. Comparators
The comparators are the expression of cytokines associated with active TB confirmed by clinical and epidemiological contact, X-rays, and/or laboratory and/or subjects with no evidence of M. tuberculosis infection, evidenced by negative results of the tuberculin skin test or interferon-gamma release assays.
2.1.5. Outcome
People with LTBI were compared to those with active TB or with no evidence of M. tuberculosis.
2.1.6. Timing
There was no restriction on the length of follow-up for clinical trials or cohort studies.
2.1.7. Setting
There was no restriction on the type of setting.
2.1.8. Language
Articles in English, Spanish, or Portuguese were included.
2.2. Information Sources
Search for original articles utilized two electronic databases: Google Scholar and PubMed.
To identify additional literature, the reference list of all papers was reviewed, and we followed the same process for abstract reviewing and data extraction as we did for papers identified by electronic search. Articles suggested by the reviewers, not detected in the previous searches, were also included.
2.3. Search Strategy
Papers published between July 2006 and July 2018 were included. We used the following MeSH terms in English, Spanish, and Portuguese languages: latent tuberculosis, Mycobacterium tuberculosis, cytokines, and biological markers. In addition, we used the free terms biomarkers and cytokines. Additional file 1 contains the search strategies used.
2.4. Study Selection, Data Collection Process, and Data Items
Once the articles were identified using each of the search strategies, we proceeded with the elimination of duplicate items. Subsequently, the titles and abstracts of all manuscripts identified by two independent evaluators were reviewed according to the selection criteria. All disagreements between the two reviewers were resolved with a third evaluator by consensus. Articles that met the selection criteria were read completely by the same reviewers, blinded and independently.
The data extracted and typed in an Excel file from the selected articles were the following: consecutive number of the article (whole number assigned by investigators), article title, year, first author, journal, study country of origin, outcome or result reported in the article, type of study population (special feature), number of patients in the intervention or comparison group, follow-up in each group, type of control or unexposed population, number of patients in the control or unexposed group, follow-up in the control or nonexposed group (months), age, sex (female percentage), active TB diagnostic method, LTBI diagnostic method, LTBI time, immune parameters studied, increased IP (with and without statistical differences) (the group in which the IP was increased is reported first), IP that remained normal, decreased IP (with or without statistical differences), IP concentration values, the level of confidence they used in their statistical analyses (90%, 95%, and 99%), method of detection of IP, if ethnicity was reported, the study populations, type of study, quality of the study (see below), bias (types of bias), proportion of BCG vaccine, conflict of interest statement, and other important findings such as the cell stimulation used (times and antigens used).
We conducted a pilot study for the search strategies, abstract reviewing, and data extraction of full-text articles to standardize all process and concepts before starting each step. A third reviewer was in charge of comparing the files to identify disagreements at each step of the process. A fourth reviewer participated in the validation of the biological findings, only at the end of the full-data extraction for included papers to avoid investigator bias.
2.5. Risk of Bias in Individual Studies
Selection bias was controlled through the application of inclusion and exclusion criteria to eligible titles and/or summaries; likewise, possible information biases were controlled by the independent revision of two observers, where at the end of the review, a third reviewer compared their findings. The risk of bias of the studies was assessed using the Newcastle-Ottawa scales for case-control and cohort studies [15] and the National Institutes of Health (NIH) evaluation scale for observational studies [16]. The Jadad scale was applied to evaluation of clinical trials [17] (Additional files 3 and 4).
The Newcastle-Ottawa scale evaluates four main points: population, that is, the choice of cases or exposed people, and controls or not exposed; the measurement of the outcome and exposure; and the comparability between groups [15]. Similarly, the NIH scale is based on 14 questions that include the clear definition of the objective, the population (including the sample size), the measurement of dependent and independent variables, and the control of the confounders [16].
For both scales, one or two points are given when a study complies with the evaluated requirements (comparability for Newcastle-Ottawa). This final score determines the risk of bias: high risk (0-2 points), moderate (between 3 and 6 points), and low risk of bias (≥7 points).
2.6. Summary Measures
Due to the clinical heterogeneity of the population, the samples and the stimulation protocol used, the multiple techniques used for immune parameter detection, the different units reported for the substances, and the differences in the diagnosis of LTBI and active TB, it is was deemed inadequate to perform a meta-analysis [18, 19]. Therefore, we report the systematic review with a qualitative synthesis of the papers.
3. Results
3.1. Articles
Upon searching according to the keywords, 637 relevant articles were retrieved; among them, 58 met the selection criteria and were read in full text. At the end, 36 met all criteria and were included in the systematic review (Figure 1). The excluded articles and the reasons for exclusion are provided in Additional file 2.
Figure 1.
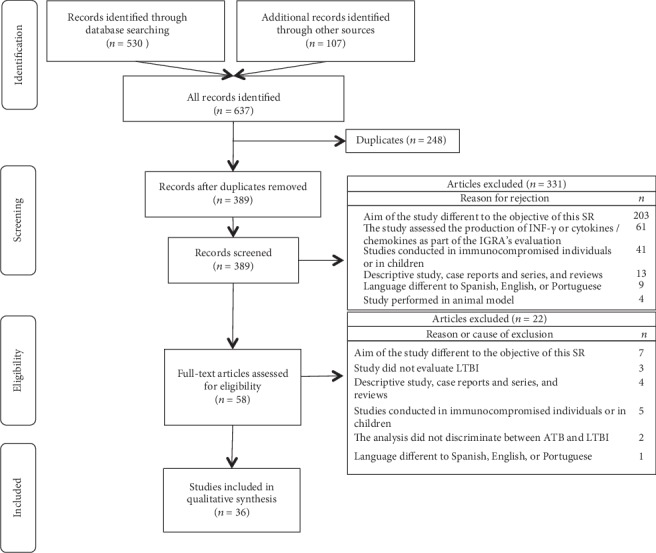
PRISMA diagram showing the results of systematic searches and articles analyzed. Legend: ATB: active tuberculosis; LTBI: latent tuberculosis infection.
Publications included 34 cross-sectional and 2 cohort studies, the latter with follow-up for 6 and 24 months after baseline sampling.
3.2. Participants
Most of the studies evaluated individuals with active TB treatment or within hospital programs. Their community or family contacts or voluntary hospital or community-based controls with or without TB infection served as controls. Five studies were conducted in healthcare workers, four in places endemic for TB, and one from a region with a high rate of malnutrition. The minimum and maximum numbers of subjects included in the studies were 7 and 148 in the LTBI group, 10 and 147 in the active TB group, and 8 and 168 in the noninfected group. Table 1 describes the characteristics of the population included in each study.
Table 1.
Characteristics of studies included in the systematic review.
| First author, year of publication | Country where the study was conducted | Outcome of interest | Special feature of the population under study | Number of people with LTBI | Number of people in the control group | Age | Sex (% women) | Proportion with BCG vaccination (%) | Active TB diagnosis method | LTBI diagnosis method | Immune parameters evaluated | Increased immune parameters (with statistical differences)∗ | Samples | Antigen and times used for stimulation | Method of detection of cytokines, commercial kit |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Zeev T. Handzel, 2007 [23] | East European, Ethiopian and Israel | LTBI | Immigrant patients from Eastern Europe and Ethiopia and their contacts in Israel | 39 | PTB = 39 NI = 21 |
Not reported | Not reported | Not reported | Culture, clinical diagnosis, X-ray, TST > 15 | TST | INF-γ, IL-2R, IL-10, IL-6, IL-12p70 | Unstimulated LTBI vs. NI: IL-10 and IL-6 Stimulated LTBI vs. NI: INF-γ TB vs. LTBI: sIL-2R, INF-γ, and IL-10 Serum TB vs. LTBI: sIL-2R, IL-10 |
Plasma samples from whole blood (unstimulated, antigen-stimulated, or mitogen-stimulated) and serum | PPD Time: 48 hours |
ELISA (R&D Systems, Minneapolis, MN, USA) |
|
| |||||||||||||||
| Novel N. Chegou, 2009 [24] | South Africa | LTBI | Contacts of people with TB and patients with TB from an endemic area | 34 | PTB = 23 | Mean ± SD TB: 30.3 ± 13.6 LTBI/NI: 31.8 ± 14.2 |
TB: 26 LTBI/NI: 58.8 |
Not reported | Smear (ZN) | QuantiFERON-TB Gold In-Tube Test, TST | (IL)-1α, IL-1β, IL-2, IL-4, IL-5, IL-6, IL-7, IL-10, IL-12(p40), IL-12(p70), IL-13, IL-15, IL-17, CXCL8, IL-1ra, sCD40L, CCL11, fractalkine, G-CSF, GM-CSF, IFN-γ, CXCL10, CCL2, CCL3, CCL4, TGF-α, TNF-α, VEGF | Unstimulated TB vs. LTBI: EGF, TGF-α, TNF-α, and sCD40L Stimulated LTBI vs. TB: sCD40L, VEGF. TB vs. LTBI: IL-1α |
Plasma samples from whole blood (unstimulated, antigen-stimulated, or mitogen-stimulated) | ESAT-6, CFP-10, and TB7.7 Time: not specifically referred |
Microbead-based method, LINCO-plex® kits (Millipore, St. Charles, Missouri, USA) |
|
| |||||||||||||||
| R. Biselli, 2010 [25] | Italy | LTBI | Laboratory personnel without M. tuberculosis infection and TB cases of infectious diseases L. Spallanzani, and the Infectious Diseases Department of Sapienza Universita di Roma | 20 | PTB = 20 NI = 20 |
Median LTBI: 42.3 TB: 35.7 NI: 31.4 |
LTBI: 40 TB: 45 NI: 30 |
LTBI: 0 TB: 35 NI: 0 |
Culture | QuantiFERON-TB Gold In-Tube Test, TST | INF-γ, IL-2 | Stimulated LTBI and TB vs. NI: INF-γ LTBI vs. TB and NI: IL-2 |
Plasma samples from whole blood (unstimulated, antigen-stimulated, or mitogen-stimulated) | ESAT-6, CFP-10, and TB7.7 Time: 18 and 72 hours |
ELISA, ELISA assay (DRG GmbH, Germany) |
|
| |||||||||||||||
| Jayne S. Sutherland, 2010 [26] | South Africa | LTBI | TB case contacts and TB cases | 20 | TB/NRCF = 36 NI = 19 |
Median (IQR) LTBI: 27 (19–39) TB: 25 (20–37) NI: 22 (18–31) |
TB: 27 LTBI: 74 NI: 65 |
Not reported | Smear (ZN, auramine-rodhamine), culture | TST | TNF-α, IFN-γ, IL-10, IL-12(p40), IL-13, IL-17, IL-18 | Stimulated LTBI vs. NI: IFN-γ, IL-13, and IL-17 TB vs. NI: IL-10, IL-12(p40), IL-13, IL-17, IFN-γ, and TNF-α. TB vs. LTBI: TNF-α and IL-12(p40) |
Blood culture supernatant unstimulated or antigen-stimulated | ESAT-6/CFP-10, PPD, or TB10.4 Time: 7 days |
Microbead-based method, 7-plex kit, BioRad |
|
| |||||||||||||||
| Subash Babu, 2010 [27] | India | LTBI | Adult population with and without M. tuberculosis exposure | 25 | NI = 25 | Median (range) LTBI: 32 (19-50) NI: 30 (15-48) |
LTBI: 40 NI: 40 |
All participants | N/A | TST | IL-2, IFN-γ, TNF-α, IL-12, IL-4, IL-5, IL-10, IL-13, IL-17, IL-23, IL-6, IL-1β, IL-23 | Stimulated NI vs. LTBI: IL-17, IL-23 |
Nonstimulated and antigen-stimulated PBMC culture supernatants | PPD or Mtb CFA Time: 24 hours |
Microbead-based method and ELISA for IL-23, BioRad |
|
| |||||||||||||||
| Marc Frahm, 2011 [28] | Not reported | LTBI | Adult population with and without TB from two previous cohorts | 32 | PTB = 9 EPTB: 3 NI = 26 |
Median (range) LTBI: 50 (2–66) TB: 43.5 (4–93) NI: 46.5 (26–62) |
LTBI: 47 TB: 33 NI: 27 |
LTBI: 31 TB: 33 NI: 0 |
Culture from a clinical specimen or clinical diagnosis | QuantiFERON-TB Gold In-Tube Test, TST | IL-1β, IL-1RA, IL-2, IL-2R, IL-4, IL-5, IL-6, IL-7, IL-8, IL-10, IL-12 p40/70, IL-13, IL-15, IL-17, TNF-α, IFN-α, IFN-γ, GM-CSF, MIP-1α, MIP-1β, IP-10, MIG, eotaxin, RANTES, MCP | Stimulated LTBI and TB vs. NI: INF-γ, IP-10, MIG, IL-2, MCP-1, IL-15, IL-RA. TB vs. LTBI: IL-15. With a more flexible cut-off point: MCP-1, IL-1RA, IFN-α and IL-4 |
Plasma samples from whole blood (unstimulated, antigen-stimulated, or mitogen-stimulated) | ESAT-6, CFP-10, and TB7.7 Time: 16–24 hours |
Microbead-based method, Human Cytokine 25-plex (Biosource, Camarillo, CA) |
|
| |||||||||||||||
| Ji Young Hong, 2012 [46] | Korea | LTBI | Contacts of patients with confirmed TB. Cases of TB were hospitalized patients with comorbidities | 22 | PTB = 46 NI = 32 Combined EPTB lesion: 2 (4.3%) |
Median (range) LTBI: 37.5 (22–53) TB: 30 (22–74) NI: 28 (22–57) |
LTBI: 18 TB: 25 NI: 18 |
LTBI: 90.9 TB: 54.3 NI: 75.0 |
Culture | QuantiFERON-TB Gold In-Tube Test, TST | IP-10, INF-γ | Unstimulated plasma TB vs. LTBI and NI: IP-10 Stimulated plasma LTBI and TB vs. NI: IP-10, INF-γ Serum TB vs. LTBI and NI: IP-10 |
Plasma samples from whole blood (unstimulated, antigen-stimulated, or mitogen-stimulated) and serum | ESAT-6, CFP-10, and TB7.7 Time: 20 hours |
ELISA (R&D Systems, Minneapolis, MN, USA) |
|
| |||||||||||||||
| S.Y. Kim, 2012 [45] | Not reported | LTBI | TB case partners with and without LTBI | 19 | PTB = 32 NI = 30 |
Median (range) LTBI: 47 (23-60) TB: 31 (20-77) NI: 28 (22-57) |
LTBI: 68.4 TB: 46.8 NI: 53.3 |
LTBI: 94.7 TB: 64.5 NI: 76.7 |
Smear (ZN), culture, and/or pathology | QuantiFERON-TB Gold In-Tube Test, TST | IFN-γ, IL-2, IL-10, IL-13, IL-17, TNF-α | Stimulated LTBI and TB vs. NI: IFN-γ, IL-2, IL-10, and IL-13 |
Plasma samples from whole blood (unstimulated, antigen-stimulated, or mitogen-stimulated) | ESAT-6, CFP-10, and TB7.7 Time: 20 hours |
Microbead-based method, MILLIPLEX® MAP human cytokine/chemokine kit (Millipore, Billerica, MA, USA) |
|
| |||||||||||||||
| Pierre-Alain Rubbo, 2012 [29] | France | LTBI | Healthcare workers with high risk of M. tuberculosis exposure | 41 | NI = 29 | Median (IQ): 44 (36–50) | All participants: 84.3 | All participants | N/A | QuantiFERON-TB Gold In-Tube Test | IL-1RA, IL-2, IL-2R, IL-4, IL-5, IL-6, IL-7, IL-10, IL-12p40/70, IL-13, IL-15, IL-17, TNF-α, GM-CSF, MIP-1α, MIP-1β, IP-10, MIG, eotaxin, RANTES, MCP, IFN-γ | Stimulated LTBI vs. NI: IL-2, IL-15, IP-10, and CXCL9 |
Plasma samples from whole blood (unstimulated, antigen-stimulated, or mitogen-stimulated) | ESAT-6, CFP-10, and TB7.7 Time: 24 hours |
Microbead-based method, cytokine human panel (Invitrogen, Villebon sur Yvette, France) |
|
| |||||||||||||||
| Sen Wang, 2012 [30] | China | LTBI | Adults living in an endemic area to TB | 73 | PTB = 66 NI = 76 |
Median (range) LTBI: 41 (18–83) TB: 45 (16–86) NI: 38 (18–50) |
LTBI: 52.1 TB: 40.9 NI: 45.2 |
LTBI: 74.0 TB: 78.9 NI: 89.5 |
TB contact history, smear (ZN), culture, clinical diagnosis, and R-rays | QuantiFERON-TB Gold In-Tube Test, TST | IP-10, IL-2, TNF-α, INF-γ | Unstimulated LTBI vs. TB: IP-10 LTBI and TB vs. NI: IP-10, IL-2, TNF-α, INF-γ TB vs. LTBI: TNF-α Stimulated TB vs. LTBI: IFN-γ, IP-10, and IL-2 |
Plasma samples from whole blood (unstimulated, antigen-stimulated, or mitogen-stimulated) | ESAT-6, CFP-10, and TB7.7 Time: 20 hours |
DuoSet ELISA, the DuoSet ELISA development kit (R&D Systems Inc, MN, USA) |
|
| |||||||||||||||
| Yang Yu, 2012 [31] | China | LTBI | Individuals exposed to M. tuberculosis, healthy volunteers without infection, and hospitalized patients with TB | 20 | PTB = 12 NI = 12 |
Mean LTBI 1: 40.7 LTBI 2: 46.1 TB: 38.5 NI: 30.7 |
LTBI 1: 60 LTBI 2: 50 TB: 58.3 NI: 41.6 |
Not reported | Culture, clinical diagnosis, X-ray, and/or HRCT | T–SPOT®, TST | CCL1, CCL2, CCL3, CCL4, CCL5, CCL7, CCL8, CCL11, CCL13, CCL15, CCL17, CCL20, CCL21, CCL24, CCL26, CCL27, CXCL5, CXCL, CXCL8, CXCL9, CXCL10, CXCL11, CXCL12, CXCL1, IL-2, IL-15, IL-4, IL-13, IL-7, IL-9, IL-5, GM-CSF, IL-6, IL-12, G-CSF, TNF-α, IL-10, IFN-γ, IL-1RA, IL-1β, IL-17 | Stimulated PBMCs LTBI 1 vs. NI: IP-10, CXCL11, and CXCL12 LTBI 2 vs. LTBI 1: IL-2, CXCL10, CXCL11, and CXCL12 TB vs. NI: IL-2, IP-10, CXCL11, IL-6, IL-9, IL-10, CCL-8, CXCL13, CXCL12, CCL1, CCL21 Plasma: TB vs. NI: IL-6, CCL1, IL-9, and CXCL9 |
Nonstimulated and antigen-stimulated PBMC culture supernatants and plasma | Lysed bacteria proteins and ESAT-6 Time: 72 hours |
Microbead-based method, human cytokine/chemokine panel (MPXHCYTO-60K, MPXHCYP2-62K, and MPXHCYP3-63K, Millipore, USA) |
|
| |||||||||||||||
| Novel N. Chegou, 2012 [32] | South Africa | LTBI | TB case contacts and TB cases from a high TB-endemic community | 23 | PTB: 15 | Mean (SD) 31.5 (15.9) | All participants: 39.5 | Not reported | ZN | TST | EGF, fractalkine, IFN-a2, IFN-c, IL-4, IL-10, IL-12(p40), TGF-a, TNF-a, VEGF, IP-10, RANTES | Unstimulated TB vs. contact: EGF, IFN-a2, and IL-4. Stimulated ESAT-6/CFP-10 TB vs. contacts: EGF, TGF-a, and TNF-a. Stimulated Rv0081 Contacs vs. TB: IFN-g, IFN-a2, IL-12(p40), IP-10, TNF-a, VEGF, IL-10, and RANTES. Stimulated Rv2032 TB vs. contacts: fractalkine, IL-12(p40), TGF-a, TNF-a, VEGF, IL-10, RANTES. Stimulated Rv1737c TB vs. contacts: IL-10, TGF-a, TNF-a, IL-12(p40), and EGF |
Plasma samples from whole blood (unstimulated or antigen-stimulated) | Resuscitation-promoting factors (Rv0867c, Rv2389c) and DosR regulon-encoded antigens (Rv2032, Rv0081, Rv1737c) Time: 7 days |
Microbead-based method, Milliplex kits (Merck Millipore, St. Charles, Missouri, USA) |
|
| |||||||||||||||
| D. Anbarasu, 2013 [33] | India | LTBI | Family of TB cases from an endemic area to M. tuberculosis | 7 | PTB = 10 | Range LTBI: 28-55 TB: 26-52 |
LTBI: 28.6 TB: 30 |
Not reported | Smear (ZN) and culture | TST | IL-1β, IL-1RA, IL-2, IL-5, IL-6, IL-7, IL-8, IL-9, IL-10, IL-12 (p70), IL-13, IL-15, IL-17, eotaxin, FGF basic, G-CSF, GM-CSF, IP-10, MCP-1, MIP-1α, MIP-1β, PDGF, RANTES, and VEGF | Stimulated CFP-10, Rv3716c, and TrxC LTBI vs. TB: IL-6 Stimulated FbpB/Rv2626c TB vs. LTBI: G-CSF, IL-7, IL-8, IL-9, and PDGF. LTBI vs. TB: IL-6 |
Blood culture supernatant unstimulated or antigen-stimulated | Protein fraction 11_24 (Rv2626c and FbpB) Time: 6 days |
Microbead-based method, Bio-Plex multiplex cytokine assay system (Bio-Rad Laboratories, Hercules, CA, USA) |
|
| |||||||||||||||
| Yun-Gyoung Hur, 2013 [47] | Malawi | LTBI | TB cases from a cohort with their contacts | It is not clear (143 in LTBI and NI) | PTB = 15 | Mean TB: 41 LTBI: 40 |
LTBI: 67 TB: 60 |
Not reported | Smear (ZN) | TST | IL-10, IL-13, IL-17, CXCL10, TNF-α. | Stimulated LTBI and TB vs. NI: IFN-γ, CXCL10, IL-10, TNF-α, and IL-17. LTBI vs. TB and NI: IL-10 LTBI vs. TB: IL-17 TB vs. LTBI: IL-17 and IL-10, in the following |
Blood culture supernatant unstimulated or antigen-stimulated | PPD or ESAT-6 Time: 6 days |
DuoSet ELISA, R&D Systems |
|
| |||||||||||||||
| Mayer-Barber, 2014 [34] | China and India (cohort reported by Andrade BB, 2013) | LTBI | TB cases from a Chinese cohort and healthy community controls India TB cases (pulmonary and extrapulmonary), LTBI, and healthy donors recruited as part of a TB cohort study |
China: 14 India: 39 |
PTB = 94 Healthy controls = 11 India: PTB: 97 EPTB: 35 Healthy controls: 40 |
Median (IQR) PTB: 27 (23-44.7) Healthy controls: 33 (23-40) LTBI: 38.5 (34.2-43.5) India Median (IQR) Healthy control: 29 (21-59) LTBI: 25 (21-49) EPTB: 33 (18-65) PTB: 40 (19-70) |
PTB: 38.3 Healthy controls: 54.5 LTBI: 85.7 India PTB: 33 EPTB: 84 Healthy controls: 75 LTBI: 77 |
Not reported | Smear (ZN) India Smear and culture |
QuantiFERON-TB Gold In-Tube Test India QuantiFERON-TB Gold In-Tube Test and TST, absence of chest radiograph or pulmonary symptoms |
IL-1α, IL-1β, IL-10, IL-1Ra, IL1R1, IL1R2, IFN-γ, IFN-α, IFN-β, TNF-α, PGF2α, PGE2, LXA4, 15-Epi-LXA4 India IL-1α, IL-1β, IL-10, IL-1Ra, IFN-γ, IFN-α, IFN-β, TNF-α, PGF2α, PGE2, LXA4, 15-Epi-LXA4, IL-1R1, IL-1R2 |
LTBI vs. NI and TB IFN-α NI vs. LTBI and TB: IL-1α, IL-1β, TNF-α, IL1Ra TB vs. LTBI and NI: IL-10, IL-1RI, IFN-γ, PGF2α, PGE2 India LTBI vs. NI and TB: IL1Ra, PGF2α NI vs. LTBI and TB: IL-1α, sIL-1R1 TB vs. LTBI and NI: IL-1 β, PGE2, TNF-α, IFN-γ, IFN-α, IL-10, LXA4, 15-Epi-LXA4 |
Plasma samples | Not apply | ELISA kits (R&D Systems) and FlowCytomix Multiplex Arrays (eBioscience, San Diego, CA) and Oxford Biomedical Research (Oxford, MI) India ELISA kits (R&D Systems) and enzyme immunoassay (EIA) kits (Cayman Chemical, Ann Harbour, MI) and Oxford Biomedical Research (Oxford, MI) |
|
| |||||||||||||||
| Ikaria Sauzullo, 2014 [35] | Italy | LTBI | Healthcare workers studied for LTBI | TST+/QFT− = 34 TST+/QFT+ = 29 Total 63 |
PNI = 126 | Mean (range) 43 (25–60) |
All participants: 50.5 | All participants: 3.1 | N/A | QuantiFERON-TB Gold In-Tube Test or TST and had one of the following risk factors: chest X-ray suggestive of prior TB infection, a history of exposure to a case of active TB, or coming from an area with a high prevalence of TB infection | IFN-γ, IL-2 | Stimulated LTBI vs. NI: IL-2, INF-γ |
Plasma samples from whole blood (unstimulated, antigen-stimulated, or mitogen-stimulated) | ESAT-6, CFP-10, and TB7.7 Time: 72 hours |
ELISA (DRG GmbH, Germany) |
|
| |||||||||||||||
| K. Kim, 2014 [36] | Australia | LTBI | Patients of the Western Australian Tuberculosis Control Program | 30 | PTB = 23 EPTB: ~8 (25%) |
Median (IQR) LTBI: 32 (25-39) TB: 35 (29-42.5) |
LTBI: 50 TB: 32.3 |
Not reported | Culture | IGRAs, TST | IFN-γ, TNF-α, IL-10 | Stimulated LTBI vs. TB: IFN-γ TB vs. LTBI: TNF-α |
Nonstimulated and antigen-stimulated PBMC culture supernatants | PPD, ESAT-6, or CFP-10 Time: 6 hours |
ELISA, BD OptEIA™ Sets (BD Biosciences, USA) |
|
| |||||||||||||||
| Yun Hee Jeong, 2015 [37] | South Korea | LTBI | Patients with active TB and contacts with LTBI | 20 | PTB: 33 NI: 26 |
Median (range) LTBI: 44 (22–60) TB: 30 (20–63) NI: 25 (22–54) |
LTBI: 80 TB: 38.7 NI: 53.8 |
LTBI: 90 TB: 63.6 NI:5 3.8 |
Clinical, radiological, microbiological, and/or pathological results | TST | IL-2, IL-6, IL-8, IL-10, IL-13, TNF-α, IFN-γ, MIG, IP-10, I-TAG, and MCP-1 | Unstimulated LTBI vs. TB: IL-2, IL-10, IL-13, IL-8, and IFN-γ Stimulated TB vs. NI: IL-2, IL-6, IL-13, MIG, IP-10, I-TAG, MCP-1, and IL-8. TB vs. LTBI: IL-2, IL-6, IL-10, IL-13, TNF-α, MIG, IP-10, I-TAG, INF-γ LTBI vs. NI: IL-8 |
Plasma samples from whole blood (unstimulated, antigen-stimulated, or mitogen-stimulated) | ESAT-6, CFP-10, and TB7.7 Time: 24 hours |
Microbead-based method, BD Biosciences, San Jose, CA, USA |
|
| |||||||||||||||
| Babak Pourakbari, 2015 [48] | Iran | LTBI | Individuals vaccinated and without previous exposure to M. tuberculosis and patients infected with M. tuberculosis, taken at the hospital | 30 | PTB = 30 NI = 30 |
Mean ± SD LTBI: 40.2 ± 15.8 TB: 35.3 ± 18.8 NI: 45.3 ± 5.6 |
LTBI: 27 TB: 13 NI: 73 |
Not reported | Culture | QuantiFERON-TB Gold In-Tube Test, TST | IL-2 | Stimulated LTBI vs. TB and NI: IL-2 |
Plasma samples from whole blood (unstimulated, antigen-stimulated, or mitogen-stimulated) | PE35 (Rv3872) and PPE68 (Rv3873) Time: 3 days |
ELISA, ELISA kit (Mabtech AB, Sweden) |
|
| |||||||||||||||
| Prachi R. Bapat, 2015 [22] |
India | LTBI | Individuals living with TB cases and individuals in the community. Malnourished population | QFT+/TST+ = 26 QFT+/TST− = 12 QFT−/TST+ = 1 Total 39 |
NI = 35 Community = 16 |
Mean (range) 34.4 (12-65) |
All participants: 45.9 | All participants: 30 | N/A | QuantiFERON-TB Gold In-Tube Test, TST | IL-6, IL-10, IL-2, TNF-αR, INF-γ | Stimulated LTBI vs. NI: IL-6 LTBI and NI vs. community: IL-6, IL-10 NI vs. LTBI: IL-10 |
Plasma samples from whole blood (unstimulated or antigen-stimulated) | ESAT-6, CFP-10, and/or TB7.7 Time: 20–24 hours |
Microbead-based method. IMMULITE-1000 Immunoassay System (Siemens Healthcare Global) |
|
| |||||||||||||||
| Yun-Gyoung Hur, 2014 [49] | Korea | LTBI | Adults with TB, individuals recently exposed to M. tuberculosis, healthy participants without M. tuberculosis exposure, and patients with non-TB mycobacteria infections | 51 | PTB = 86 NI = 133 EPTB: 1 (1.7%) |
Median (range) LTBI: 44 (18-82) TB: 32 (20-76) NI: 31 (20-61) MNT: (43-84) |
LTBI: 74.5 TB: 49 NI: 51 MNT: 76.1 |
LTBI: 84.6 TB: 56.9 NI: 63.6 MNT: 60.5 |
Smear/culture or R-rays | QuantiFERON-TB Gold In-Tube Test, TST | IL-1β, IL-2, IL-4, IL-5, IL-6, IL-9, IL-10, IL-12p70, IL-13, IL-17A, IL-22, IFN-γ, TNF-α, IFN-α, sCD40L, CXCL10, VEGF-A | Stimulated TB and LTBI vs. controls: IFN-γ, IL-2, CXCL10 Serum TB vs. NI: IL-22, CXCL10, and VEGF-A. TB vs. LTBI: VEGF-A TB vs. MNT: IL-2, IL-9, IL-13, IL-17, and TNF-α MNT vs. TB: sCD40L |
Plasma samples from whole blood (unstimulated, antigen-stimulated, or mitogen-stimulated) and serum | ESAT-6, CFP-10, and TB7.7 Time: 24 hours |
Microbead-based method, BD FACSVerse (BD Biosciences, San Jose, CA, USA) |
|
| |||||||||||||||
| M. Wei, 2015 [38] | China | LTBI | Controls hospitalized for other causes without radiological signs of TB and patients hospitalized for TB | 40 | PTB = 40 NI = 40 |
Mean ± SD LTBI: 18.0 ± 10.35 TB: 18.47 ± 12.68 NI: 16 ± 9.06 |
LTBI: 55 TB: 47.5 NI: 50 |
Not reported | Clinical diagnosis | T–SPOT®, TST | CCL1, CXCL9, IL-6, IL-10, CSF3, CSF2, IL-1α, IL-8, IL-7, IL-2, TGF-β1, CCL2, TNF-α | Unstimulated TB vs. LTBI and NI: CCL1, CXCL9, IL6, IL-10, CSF3, CSF2, IL-1-α, IL-8, IL-7, IL-2, TGF-β1, CCL2, TNF-α. Stimulated TB vs. LTBI: CCL1 (I-309), CXCL9 (MIG), IL-10, IL-6, CSF2, CSF3, IL-8, IL-1α, IL-7, TGF-β1, CCL2, IL-2, and IL-13 |
Plasma samples from whole blood (unstimulated, antigen-stimulated, or mitogen-stimulated) | ESAT-6 and CFP-10 Time: 20 hours |
Quantitative immunomicroarray (Quantibody Human Cytokine Array 1, RayBiotech, Inc., Norcross, GA) |
|
| |||||||||||||||
| Ji Yeon Lee, 2015 [39] | Korea | LTBI | Healthy and TB patients from the National Medical Center and Community Health Center of Korea | 25 | PTB = 24 | Mean (range) LTBI: 48 (23-59) TB: 48 (28-75) |
LTBI: 44 TB: 37.5 |
Not reported | Smear (ZN) and/or cultures and X-rays | TST | IL-1, IL-6, IL-10, TNF-α, IL-17, GM-CSF, IL-4, IL-1β, INF-γ, LXA4, and PGE2 | Monocyte stimulated MTSA LTBI vs. TB IL-10 MTSA+INF-γ: IL-1, IL-6, IL-10 Stimulated CD4+ T cells and monocytes with PPD: TNF-α. Plasma TB vs. LTBI: LXA4 and PGE2 |
Nonstimulated and antigen-stimulated PBMC culture supernatants and plasma samples | H37Rv soluble antigens Time: 5 days |
Microbead-based method (Bio-Rad Laboratories, Hercules, CA) ELISA for IL-1β, ELISA kit (R&D Systems) EIA for LXA4 (Oxford Biomedical Research, Oxford, MI) EIA for PGE2 (Cayman Chemical, Ann Arbor, MI) Bio-Plex Multiplex Immunoassay Systems (Bio-Rad Laboratories, Hercules, CA) |
|
| |||||||||||||||
| Mulugeta Belay, 2015 [21] | Ethiopia | LTBI | Individuals from health centers in an endemic area to TB | 148 | PTB = 147 NI = 68 |
Mean LTBI: 32 TB: 29.4 NI: 32.4 |
LTBI: 55.5 TB: 41.5 NI: 52.9 |
LTBI: 37 TB: 28.1 NI: 35.3 |
Smear (ZN) | QuantiFERON-TB Gold In-Tube Test | IFN-γ, TNF-α, IL-10 | Stimulated Basal: NI vs. LTBI and TB: IFN-γ, TNF-α, IL-10 NI and TB vs. LTBI: IFN-γ, TNF-α, and IL-10 Six months: TB and LTBI vs. NI: INF-γ. TB and LTBI TNF-α and IL-10: baseline < 6 months < 12 months |
Blood culture supernatant unstimulated or antigen-stimulated | E6C10 and Rv2031 Time: 48 hours |
ELISA, Ready-Set-Go! cytokine ELISA kits (eBioscience, USA) |
|
| |||||||||||||||
| Sunghyun Kim, 2015 [50] | Korea | LTBI | Adult population, contacts of TB cases with and without M. tuberculosis infection | 22 | PTB = 28 NI = 29 |
Mean (range) LTBI: 46.5 (22-69) TB: 32.1 (21-69) NI: 30.1 (22-44) |
LTBI: 86.3 TB: 71.4 NI: 79.3 |
LTBI: 95.5 TB: 32.1 NI: 79.3 |
Culture | QuantiFERON-TB Gold In-Tube Test, TST | IFN-γ, TNF-α, IL-2R, IL-4, IL-10, CXCL9, CXCL10, CXCL11 | Stimulated LTBI vs. NI: IFN-γ, TNF-α, IL-2R, CXCL9, CXCL10 LTBI vs. TB: IL-17 TB vs. NI: INF-γ, TNF-α, IL-2R, CXCL9, CXCL10 TB vs. LTBI: TNF-α, CXCL11 |
RNA from antigen-stimulated whole blood cell pellets | ESAT-6, CFP-10, and TB7.7 Time: 24 hours |
Real-time RT-PCR, TaqMan probe assay, and the ABI 7500 FAST instrument system (Applied Biosystems, Foster City, CA) ELISA |
|
| |||||||||||||||
| Ida Wergeland, 2016 [51] | Norway | LTBI | TB case and people with LTBI from a hospital | 48 | PTB = 14 EPTB: 4 NI = 16 |
Median (range) TB: 32 (18–62) LTBI: 40 (13–67) LTBI borderline: 40 (25–53) NI: 47 (16–68) |
TB: 66.6 LTBI: 63.8 LTBI borderline: 63.6 NI: 75 |
Not reported | Culture or clinical diagnosis and X-ray | QuantiFERON-TB Gold In-Tube Test | IL-1β, IL-1, IL-1ra, IL-2, IL-4, IL-5, IL-6, IL-7, IL-8, IL-9, IL-10, IL-12 (p70), IL-13, IL-15, IL-17, basic FGF, eotaxin, G-CSF, GM-CSF, IFN-γ, IP-10, MCP-1, MIP-1α, MIP-1β, PDGF-BB, RANTES, TNF-α, and VEGF | Unstimulated LTBI vs. TB: IL-1β, IL-1ra, IL-9, and IL-17A. LTBI and TB vs. NI: RANTES NI vs. TB and LTBI: IL-15, eotaxin, and basic FGF NI vs. TB: IL-2, IL-4, IL-13, IL-17A, and IFN-γ. Stimulated TB and LTBI vs. NI: IL-1ra, IL-2, IL-13, IL-15, IFN-γ, IP-10, and MCP-1. LTBI vs. LTBI borderline and NI: IL-1ra, IL-2, IFN-y LTBI vs. NI: IP-10, IL-13, IL-15, IL-17A, MCP-1 |
Plasma samples from whole blood (unstimulated, antigen-stimulated, or mitogen-stimulated) | ESAT-6, CFP-10, and TB 7.7 Time: 16–24 hours |
Microbead-based method, Bio-Plex Pro Human Cytokine Group 27-Plex Panel (Bio-Rad Laboratories Inc., Hercules, CA) |
|
| |||||||||||||||
| Tao Chen, 2016 [52] | China | LTBI | TB case and LTBI medical staff who worked at the institute for TB prevention. People with cancer and pneumonia | 21 | It is not clear | Mean ± SEM NI: 25.5 ± 9.1 LTBI: 38.0 ± 10.4 TB: 32.5 ± 12.7 Others: 48.6 ± 22.1 |
21 | Not reported | Cough with blood-tinged sputum; fever; chest X-rays positive; microbiological test, IGRA positive | T–SPOT®, TST | IL-8, MIG, I-309, eotaxin-2, and ICAM-1 | TB vs. NI and LTBI: IL-8, MIG, and I-309 LTBI vs. NI and others: eotaxin-2, ICAM-1, and MIG |
Serum | N/A | Microarray and quantitative ELISA, Quantibody Human Cytokine Array 1, RayBiotech, Inc., Norcross, GA |
|
| |||||||||||||||
| Fabiana A. Zambuzi, 2016 [40] | Brazil | LTBI | TB case and people with LTBI from a hospital | 14 | PTB = 17 NI = 16 |
Mean LTBI: 31.4 TB: 39.6 NI: 27 |
LTBI: 78.6% TB: 17.6 NI: 81.2 |
Not reported | Microbiology confirmed and clinical diagnosis or X-ray | TST | IL-1b, IL-4, IL-5, IL-6, IL-10, IL-12p70, IFN-a2, TNF-a, IFN-γ, IP-10, RANTES, MCP-1, GM-CSF, IL-17, MIP-1a, MIP-1b, sCD163, and sCD14 | TB vs. NI and LTBI: IL-6, IP-10, TNF-a, sCD163, and sCD14. LTBI vs. TB and NI: RANTES TB vs. LTBI: GMCSF |
Plasma | N/A | DuoSet ELISA for sCD163 and sCD14 and microbead-based method, 16-plex, EMD Millipore Corporation, Billerica, Massachusetts, USA |
|
| |||||||||||||||
| Miguel Santin, 2016 [41] | Spain | LTBI | Adult population recruited at eight TB centers | 43 | PTB = 37 EPTB: 32 (46.4%) NI = 28 |
Median LTBI: 54 (46-64) TB: 41 (31-52) NI: 57 (44.5-77.3) Discordant: 49 (44.5-54) |
Not reported | LTBI: 100 TB: 36.8 NI: 33.3 Discordant: 85.7 |
Microbiology confirmed or compatible when clinical, radiological, and/or ADA and/or histology positive, and cure was achieved after therapy | QuantiFERON-TB Gold In-Tube Test, TST | IFN-γ, IL-2 | Stimulated LTBI vs. NI: IL-2, INF-γ |
Plasma samples from whole blood (unstimulated, antigen-stimulated, or mitogen-stimulated) | ESAT-6, CFP-10, and TB7.7 Time: 72 hours |
Quantitative ELISA, Quantikine® ELISA Human IL-2 Immunoassay (R&D Systems Inc., Minneapolis, MN, USA) |
|
| |||||||||||||||
| Xiangyang Yao, 2017 [42] | China | LTBI | Two cohorts each one with healthcare workers with LTBI and TB case | 10 and 15 | PTB = 40 and 20 NI = 9 and 15 |
Median (range) TB: 34.5 (20-78) and 29 (16-67) LTBI: 38.5 (20-48) and 38 (20-67) NI: 33 (18-56) and 48 (18-68) |
TB: 60 and 45 LTBI: 60 and 60 NI: 44 and 35 |
TB: 35 and 25.8 LTBI: 100 and 100 NI: 100 and 86.7 |
Clinical, radiological, microbiological, and histopathological | QuantiFERON-TB Gold In-Tube Test | sCD40L, EGF, eotaxin, FGF-2, Flt-3 ligand, fractalkine, G-CSF, GM-CSF, GRO, IFN-α2, IFN-γ, IL-1α, IL-1β, IL-1ra, IL-2, IL-3, IL-4, IL-5, IL-6, IL-7, IL-8, IL-9, IL-10, IL-12 (p40), IL-12 (p70), IL-13, IL-15, IL-17, IP-10, MCP-1, MCP-3, MDC, MIP-1α, MIP-1β, TGF-α, TNF-α, TNF-β, VEGF 6Ckine, BCA-1, CTACK, ENA-78, eotaxin-2, eotaxin-3, I-309, IL-16, IL-20, IL-21, IL-23, IL-28A, IL-33, LIF, MCP-2, MCP-4, MIP-1d, SCF,SDF-1A+β, TARC, TPO, TRAIL, TSLP GCP2, I-TAC, IL-11, IL-29, lymphotactin, M-CSF, MIG, MIP-3α, MIP-3β | Unstimulated TB vs. NI and LTBI: sIL-2Ra, IP-10, and MIP-1a TB and NI vs. LTBI: IL-8 Stimulated TB and LTBI vs. NI: G-CSF, GM-CSF, IFN-γ, IL-1a, IL-2, IP-10, BCA-1, and eotaxin-2. TB vs. LTBI: G-CSF. TB vs. LTBI and NI: IL-8, VEGF, MCP-3 |
Plasma samples from whole blood (unstimulated, antigen-stimulated, or mitogen-stimulated) | ESAT-6 and CFP-10 Time: 22 ± 2 hours |
Microbead-based method, Millipore Milliplex map system (EMD Millipore Corporation, Billerica, MA, USA) |
|
| |||||||||||||||
| Jing Wu, 2016 [53] | Not reported | LTBI | Contacts of TB cases with and without LTBI | 36 | PTB = 25 NI = 31 |
Mean (range) LTBI: 48 (7-76) TB: 51 (22-85) NI: 42 (5-80) |
LTBI: 66.9 TB: 28 NI: 65.5 |
LTBI: 86.1 TB: 68 NI: 77.4 |
TB contact history, smear (ZN), culture, clinical diagnosis | T–SPOT®, TST | IL-1β, IL-1, IL-2, IL-4, IL-5, IL-6, IL-7, IL-8, IL-9, IL-10, IL-12(p70), IL-13, IL-15, IL-17, eotaxin, FGF, G-CSF, GM-CSF, IFN-γ, IP-10, MCP-1, MIP-1α, PDGF-BB, MIP-1β, RANTES, TNF-α, VEGF | Unstimulated LTBI vs. TB: IP-10, PDGF-BB, and RANTES. TB vs. LTBI: VEGF Stimulated LTBI vs. TB: IL-2, IL-10, IFN-γ, IP-10, and TNF-α. TB vs. NI: IL-2, IL-10, IP-10 |
Nonstimulated and antigen-stimulated PBMC culture supernatants | PPD Time: 24 hours |
Microbead-based method, Bio-Plex Pro Human Cytokine 27-plex Assay (Bio-Rad, CA, USA) |
|
| |||||||||||||||
| R. Kamakia, 2017 [54] | Kenya | LTBI | Patients with suspected active TB and patients with active TB from Mbagathi District Hospital, Kenya, as well as contacts of people with TB | 16 | PTB = 19 NI = 8 |
Mean (IQR) LTBI: 35.6 (27-39.8) TB: 36.8 (25.8-5.15) NI: 33.5 (23.3-45.3) |
LTBI: 50 TB: 21.1 NI: 75 |
Not reported | ZN, X-ray | QuantiFERON-TB Gold In-Tube Test | IL-17F, IFN-γ, GM-CSF, IL-10, IL-12p70, IL-13, IL-15, IL-17A, IL-22, IL-9, IL-1b, IL-33, IL-2, IL-4, IL-21, IL-23, IL-5, IL-6, IL-17E/IL-25, IL-27, IL-31, MIP-3α, TNF-α, TNF-β, IL-28A | Stimulated LTBI vs. TB: IL-17F, MIP-3α, IL-13, IL-17A, IL-5, INF-γ, IL-9, IL-2. LTBI vs. NI: INF-γ, IL-9, and IL-2 |
Plasma samples from whole blood (unstimulated, antigen-stimulated, or mitogen-stimulated) | ESAT-6, CFP-10, and TB7.7 Time: 2 hours |
Microbead-based method, Milliplex MAP Human Th17 Magnetic Bead Kit (Millipore, St. Louis, MO, USA) |
|
| |||||||||||||||
| Eun-Jeong Won, 2017 [43] | Korea | LTBI | Patients with LTBI, individuals without infection, and cases of TB from a university hospital | 15 | PTB = 48 NI = 13 |
Median (range) LTBI: 52.0 (36-75) NI: 28.9 (16-74) TB QFT+: 73.0 (15-86) TB QFT-: 73.5 (25-89) |
LTBI: 46.7 TB: 58.3 NI: 53.8 |
Not reported | Culture | QuantiFERON-TB Gold In-Tube Test | EGF, eotaxin, G-CSF, GM-CSF, IFN-α2, IFN-γ, IL-1α, IL-1β, IL-1RA, IL-2, IL-3, IL-4, IL-5, IL-6, IL-7, IL-8, IL-10, IL-12p40, IL-12p70, IL-13, IL-15, IL-17, IP-10, MCP-1, MIP-1α, MIP-1β, TNF-α, TNF-β, VEGF | Unstimulated TB vs. LTBI: TNF-α and VEGF. TB vs. LTBI and NI: IL-8, IL-13, INF-γ, IL-2, IP-10, and VEGF. Stimulated LTBI and TB vs. NI: GM-CSF, IFN-γ, IL-1RA, IL-2, IL-3, IL-13, IP-10, and MIP-1β. LTBI vs. TB: EGF, GM-CSF, IL-5, IL-10, and VEGF |
Plasma samples from whole blood (unstimulated, antigen-stimulated, or mitogen-stimulated) | ESAT-6, CFP-10, and TB7.7 Time: 16-24 hours |
Microbead-based method, Milliplex MAP Human Cytokine/Chemokine 29-plex kits (Millipore, Billerica, CA) |
|
| |||||||||||||||
| Ditthawat Nonghanphithak, 2017 [20] | Thailand | LTBI | All individuals were from Srinagarind Hospital, Khon Kaen. Healthy persons with a history of TB contact and healthy individuals with no known TB exposure | 38 | PTB: 48 Early clearance: 162 NI: 39 |
Mean ± SD LTBI: 45 ± 12 TBA: 52 ± 15 EC: 37 ± 16 HC: 40 ± 14 |
TB: 35.4 LTBI: 81.6 EC: 66 HC: 82.1 |
Not reported | Smear (ZN), culture, or a molecular test (Xpert MTB/RIF, clinical diagnosis) | QuantiFERON-TB Gold In-Tube Test | CCL2, CXCL10, IFN-γ | Unstimulated NI vs. TB and LTBI: CCL2 TB vs. NI, EC and LTBI: CXCL10 LTBI vs. HC and EC: CXCL10 Stimulated LTBI vs. TB: INF- γ. TB vs. EC and HC: INF- γ, CXCL10. TB and LTBI vs. NI: CXCL10 |
Nonstimulated and antigen-stimulated PBMC culture supernatants | ESAT-6, CFP-10, and TB7.7 Time: 24 hours |
ELISA, BioLegend (California, USA) |
|
| |||||||||||||||
| Marco Pio La Manna, 2018 [55] | Italy | LTBI | Patients with active TB, health workers, and people with LTBI in a hospital | 32 | PTB: 27 NI: 20 Others non-TB pulmonary infections: 20 |
Range LTBI: 17-84 TB: 17-82 Non-TB: 24-76 NI: 21-68 |
LTBI: 25 TB: 22 Non-TB: 40 NI: 30 |
Not reported | Culture or GeneXpert MTB/RIF from biopsy specimens and/or biological fluids | QuantiFERON-TB Gold In-Tube Test, TST | IL-1α, IL-1β, IL-1ra, IL-2, IL-2Ra, IL-3, IL-4, IL-5, IL-6, IL-7, IL-8, IL-9, IL-10, IL-12(p40), IL-12(p70), IL-13, IL-15, IL-16, IL-17, IL-18, IFN-α2, IFN-γ, TNF-α, TNF-β, TRAIL, CXCL1 (GRO-α), CXCL9 (MIG), CXCL10 (IP-10), CXCL12 (SDF-1α), CCL2 (MCP-1), CCL3 (MIP-1α), CCL4 (MIP-1β), CCL5 (RANTES), CCL7 (MCP-3), CCL11 (eotaxin), CCL27 (CTACK), G-CSF, M-CSF, GM-CSF, SCF, SCGF-β, LIF, MIF, FGF-β, b-NGF, PDGF-BB, VEGF, HGF | Unstimulated LTBI vs. NI and non-TB: IL-1β, IL12p70, and VEGF TB vs. NI and non-TB: PDGF-BB, IL-1β, IL-2, IL-8, IL12p70, MCP-1, and LIF. Stimulated TB/LTBI vs. non-TB: IL12-p40, IL-2ra, SCF, TRAIL, IL-2, IFN-γ, IP-10, b-NGF, LIF, and MIG. TB vs. non-TB: IFNα2, IL-3, and TNF-β. LTBI vs. non-TB: IL-13. LTBI and non-TB vs. TB: MIF |
Plasma samples from whole blood (unstimulated, antigen-stimulated, or mitogen-stimulated) | ESAT-6/CFP-10 Time: 16–24 hours |
Microbead-based method; there is no information |
|
| |||||||||||||||
| Leonar Arroyo, 2018 [44] | Colombia | LTBI | TB case contacts and TB cases | 20 | PTB: 21 | Median (IQR) LTBI: 38.5 (26.75-52.75) TB: 28 (24-41) |
LTBI: 45 TB: not reported | Not reported | Smear (ZN) | Positive response (≥22 pg/ml) to the CFP10 antigen of Mtb and the absence of clinical symptoms compatible with clinical TB | IFN-γ | Stimulated LTBI vs. TB: IFN-γ in response to all antigens |
Nonstimulated and antigen-stimulated PBMC culture supernatants | Mtb DosR (Rv1737c, Rv2029c, and Rv2628) and Rpf (Rv0867c and Rv2389c) antigens Time: 7 days |
Microbead-based method, Millipore (Millipore, Billerica, MA, USA) |
EPTB: extrapulmonary TB; PTB: pulmonary TB; TB/NRCF: the article does not report the clinical form of TB. ∗An example of paper 1 for the row increased immune parameter interpretation: unstimulated, TB vs. NI, and LTBI: IL-10 and IL-6 mean that in an unstimulated sample, IL-10 and IL-6 were increased in TB compared to not infected individuals and persons with LTBI.
3.3. BCG Vaccination Status
Among the 36 included articles, 22 reported BCG vaccination [20–42]. The proportion of BCG vaccination was similar among the groups with LTBI, active TB, and noninfected individuals. Seven papers [26, 28, 36, 38, 42–44] compared the statistical difference between those with and without vaccination, and only two reported statistical significance [45, 20], having a lower percentage in the active TB group compared to healthy controls (healthy persons with no known risk of TB exposure), TB-exposed persons with QFT-negative results, and people with LTBI. One article reported no significant associations between levels of cytokines and BCG scar [21], and one included BCG status in the multinomial regression model getting an adjusted odds ratio increased for TNF-α and IL-6 [22].
3.4. Evaluation of Conversion to LTBI and Progression to Active TB
The majority of the studies did not evaluate progression to active TB and conversion to LTBI. Most of literatures that we reviewed were cross-sectional studies that only have the prevalence or frequency of LTBI [22, 21] and active TB and those who are TST-negative or IGRA-negative. We found two cohort studies, and only one of them evaluated risk of LTBI conversion and progression to diseases, but it reported that they have a low number of convertor (2/101 individuals) and any progressor to active TB [21]; therefore, it is not feasible to identify cytokines that allow to identify progression to either.
3.5. Diagnostic Methods for LTBI and Active TB
The methods used for LTBI diagnosis were the following: 18 studies used TST and IGRAs, nine relied on TST alone, one used TST or IGRAs plus clinical criteria, and eight utilized IGRAs alone. In studies where the two tests were used, the discordant results between the two tests are evident.
In order to establish the diagnosis of active TB, researchers used one or a combination of the following criteria: history of contact with a TB case, smear (Ziehl-Neelsen or auramine rhodamine stain), culture, clinical diagnosis, molecular test, pathology, and/or X-rays.
3.6. Measurement of Immune Parameters
In total, 93 substances (Table 2) were studied, including growth factors; interferons; receptors; tumor necrosis factors; alpha, beta, and delta chemokines; interleukins; and others like sCD40L, MIF, and sCD14.
Table 2.
Substances evaluated for ability to differentiate people with active and latent tuberculosis and uninfected with TB.
| Interleukins | IL-1, IL-1α, IL-1β, IL-2, IL-3, IL-4, IL-5, IL-6, IL-7, IL-8, IL-9, IL-10, IL-12, IL-12 (p40/70), IL-12 (p40), IL-12 (p70), IL-13, IL-15, IL-17, IL-17A, IL-17E, IL-17F, IL-18, IL-21, IL-22, IL-23, IL-25, IL-27, IL-28A, IL-31, IL-33 |
| Growth factors | PDGF, PDGF-BB, EGF, PGF2α, FGF, TGF-α, TGF-β1, G-CSF, CSF2, CSF3, GM-CSF, VEGF, VEGF-A, SFC, β-NGF, basic FGF |
| Interferon | INF-γ, IFN-α, IFN-α2 |
| Receptors | IL-1RA, IL-2R, sIL-2Rα, TNF-αR, IL-1R1, IL-1R2 |
| Tumor necrosis factors | TNF-α, TNF-β, TNF-SF10 (TRAIL) |
| Alpha chemokines | CXCL5 (ENA-78), CXCL6 (GCP-2/LIX), CXCL8 (IL-8), CXCL9 (MIG), CXCL10 (IP-10), CXCL11 (I-TAC), CXCL12 (SDF-1α+β), CXCL13 (BCA-1) |
| Beta chemokines | CCL1 (I-309), CCL2 (MCP-1), CCL3 (MIP-1α), CCL4 (MIP-1β), CCL5 (RANTES), CCL7 (MCP-3), CCL8 (MCP-2), CCL11 (eotaxin), CCL13 (MCP-4), CCL15 (MIP-1δ), CCL17 (TARC), CCL20 (MIP-3α), CCL21 (6Ckine), CCL24 (eotaxin-2), CCL26 (eotaxin-3), CCL27 (CTACK) |
| Delta chemokines | CX3CL1 (fractalkine) |
| Others | ICAM-1 (CD54), sCD163, sCD14, sCD40L, I-TAG, MIF, LIF, LXA4, 15-Epi-LXA4 |
Of these, 24 substances were increased only in active TB, five increased only in the LTBI group, and two increased in uninfected individuals, regardless of the sample analyzed (Figure 2).
Figure 2.
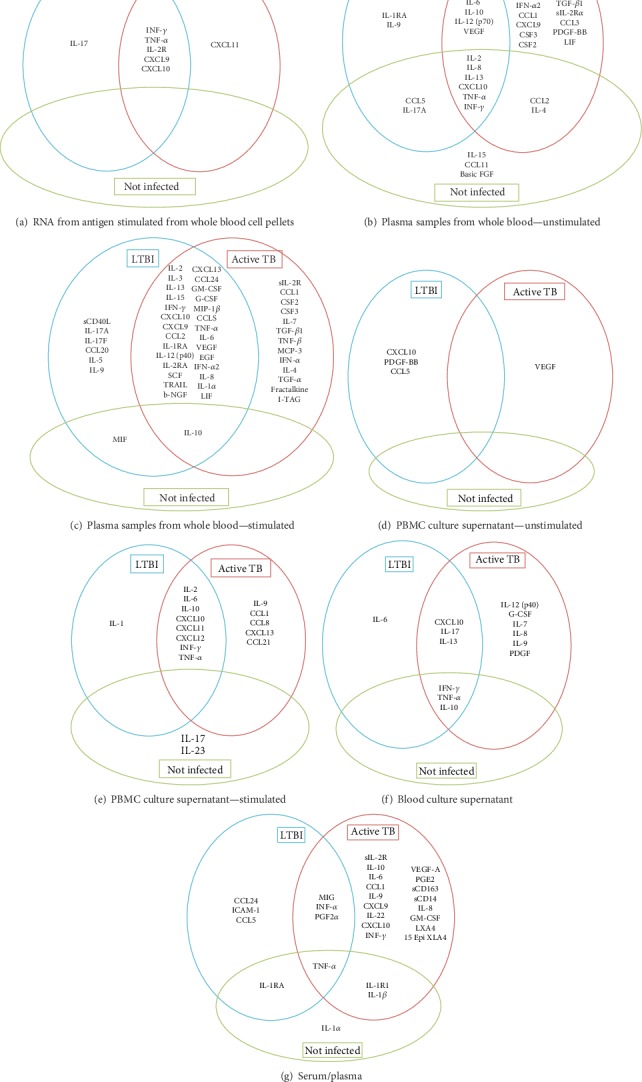
(a–g) Immune cytokine/chemokine mediators statistically different reported in active TB, latent tuberculosis infection, and noninfected individuals in each sample type.
Table 1 shows all the antigens and times used for ex vivo stimulation and cytokines whose concentration was statistically different, by each group. The most frequently measured mediators were IL-6, IL-10, IL-2, TNF-α, INF-γ, and IP-10.
Most of the studies reported mediators after ex vivo stimulation, with most using supernatants of interferon-gamma release assays.
Only eight papers evaluated serum or plasma samples without stimulation. There were no differences between them; only MIG was increased in plasma samples in TB patients, but elevated in serum samples in both, LTBI and active TB.
Among evaluated immune mediators, most were measured in plasma samples from stimulated or unstimulated (as controls) whole blood (Figures 2(a)–2(g)). Other samples utilized were supernatants from PBMC cultures and whole blood culture and RNA from blood cells.
The stimulation antigens used were ESAT-6, PPD, CFP-10, TB7.7 (Rv2654), TB10.4, PE35 (Rv3872), PPE68 (Rv3873), Rv262, FbpB, E6C10, Rv2031, protein fraction 11_24 (Rv2626c), H37Rv soluble antigens, DosR Rv1737c, Rv2029c, Rv2628, Rpf Rv0867c Rv2389c, and inactivated bacteria (Table 1).
The concentration of biomarkers involved in the immune response is dependent on the type of protocol used for in vitro stimulation and the sample evaluated and has high variability between studies. Among the five substances exclusively elevated in LTBI and the two elevated in uninfected individuals, there was inconsistency in the samples processed throughout the studies. For example, IL-5 was evaluated in plasma (1 article), plasma samples from stimulated whole blood (11 articles), PBMC culture supernatant (3 articles), and blood culture supernatant (1 article) but was only increased in two papers that used plasma samples from stimulated whole blood (2/11 articles). In the case of active TB, one substance (CCL1/I-309) was elevated in four different sample types, one (IL-7) in three sample types, and the rest in two (usually plasma samples from whole blood unstimulated and stimulated) or one sample type.
ELISA (n = 17) and microbead-based method (n = 20) were the most frequently used methods for IP measurement (Table 1). Some of the articles used both methods. Table 1 describes details regarding laboratory measurements.
3.7. Risk of Bias of Included Articles
Of the 36 articles reviewed using the Newcastle-Ottawa scales and the Quality Assessment Scale for NIH observational studies, 7 articles had low risk of bias, 29 moderate, and none high risk of bias. Only one study out of the 36 performed calculation of the sample size and took into account the statistical power of their results (Additional files 3 and 4).
3.8. Biases
The main bias identified in the articles reviewed was the absence of a second administration of the tuberculin skin test to detect a possible booster effect, thus leading to the potential inclusion of individuals with false negative results of the TST. Another bias was the analysis of patients with pulmonary and extrapulmonary TB in the same group of active TB as the underlying immune competence and immune response may vary between localized or disseminated disease. In addition, children and adults were included in some studies; however, the analysis was not stratified for each population. Finally, patients with pulmonary TB were included in different phases of treatment; some studies included individuals that completed treatment at the time of IP measurement. The declining microbial burden during or at the end of therapy may contribute to false negative results (Additional files 3 and 4).
Only 4 of the reports took into account the study origin and population's ethnicity as a confounding factor, and these were evaluated by self-reported ethnicity [28, 38, 48, 52].
Of the included manuscripts, 27 articles provided a declaration of conflicts of interest (S3 and S4).
4. Discussion
The immune response against infection and disease caused by M. tuberculosis is mainly mediated by the recruitment and activation of T cells and macrophages, which in turn are regulated by multiple immune mediators such as interleukins and chemokines, possessing a diverse pro- and anti-inflammatory property. The success of the immune response in halting the acquisition of M. tuberculosis is influenced by a myriad of environmental, microbial, and host factors. The host response is measured in order to determine M. tuberculosis infection in the form of skin tests or IGRAs, but this approach is limited by the inability to differentiate LTBI from active TB infection. The ability to refine diagnostics by using assays that incorporate measurement of multiple biomarkers will be critical in order to stride towards TB control and eventual elimination.
Most of the studies analyzed in this review focused on the main pro- and anti-inflammatory interleukins involved in the immune response, mediated mainly by Th1 and Th2 lymphocytes; a few others expanded the markers measured to include chemokine-like substances, growth factors, and receptors as part of the search for new diagnostic biomarkers that can discriminate between LTBI and active TB.
Several immune mediators in addition to INF-γ have been identified. The most frequently evaluated markers are the cytokines IL-6, IL-10, IL-2, TNF-α, and IP-10. Although the response to TB is reliant on Th1 (TNF-α, INF-γ, and IL-2), this has been expanded by the addition of Th2 signature cytokine profile such as IL-6 and IL-10.
Elevated immune mediators and markers that were only detected in active TB share chemoattractant functions involved in trafficking of cells involved in the immune response, among which are T lymphocytes (CD4+ and CD8+), macrophages, dendritic cells, basophils, and eosinophils. These cytokines affect cell growth, maturation, and differentiation (Additional file 5). In LTBI, only interleukins IL17F and IL-5, associated with effector T cell profiles, are overexpressed. The effect of the cytokines found overexpressed in LTBI is related to the increased production of immune substances, chemoattraction, multiplication, and activation of lymphoid cells [56]. Of the cytokines identified in the systematic review, three (IL-12 and TGF-β for active TB and IL-23 for uninfected individuals) are well-established markers involved in immune response to mycobacterial infection (Figure 3: available at http://www.genome.jp/kegg/pathway.html) [57].
Figure 3.
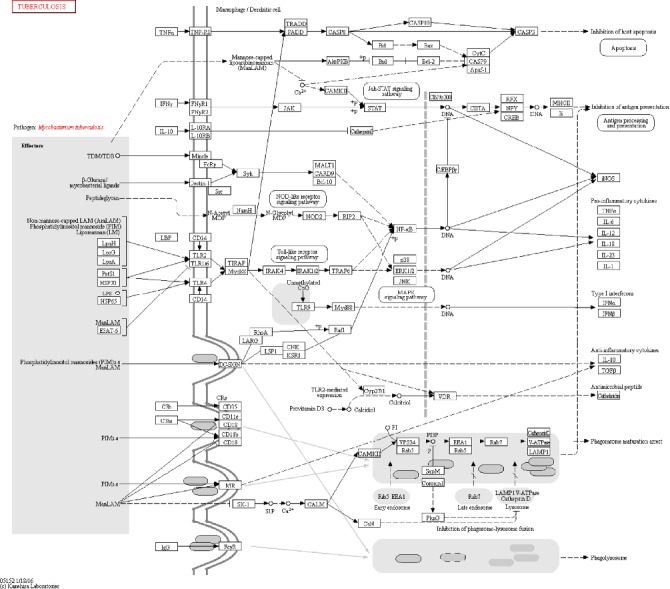
KEGG pathway, highlighting some of the pathways and mediators identified in the reviewed studies. The use of this figure was granted by copyright permission of KEGG [57], and the journal has a copy of the approval.
While most attention has been directed to immune cells, some of the immune substances that participate in the response to M. tuberculosis are produced by epithelial cells, which play a fundamental role in the initiation and expansion of host defense mechanisms in the lung, providing protection against mycobacteria. Epithelial cells participate in activation of innate immunity, as well as adaptive immunity, inducing the recruitment and activation of dendritic cells and T and B lymphocytes, which in turn increase antigen recognition and production of antibodies and other immune substances [58, 59]. These markers merit further investigation for the ability to distinguish early and late mycobacterial infection.
Despite some signals suggesting that the biomarker expression differences between LTBI and active TB can be used for diagnostics, choosing a panel of reproducible, discriminatory markers based on the results of the studies analyzed is quite difficult due to
failure to account for the time/illness of the individuals studied. Not surprisingly, the biology of TB is much more complex than previously thought, and therefore, classification in LTBI and active TB is insufficient. What is considered LTBI actually corresponds to a range of infection status, which may have been recently acquired or present for decades. Recently acquired TB is associated with a higher progression rate to active disease pointing to distinct biological properties. The study of pulmonary immune substances in the animal model reveals changes in the expression of cytokines/chemokines in the cells that make up the granuloma. The diversity of granulomas (diverse functions and architectures and microenvironments) has consequences on the bacterial control [60, 61]. It is suggested that after the in vitro stimulation, changes in the cellular expression due to the phase of infection or tuberculosis disease can lead to a varied response that is evidenced in the analyzed studies. Among the papers included, there were 22 different antigens used for in vitro stimulation, with concentrations that widely varied within the same immune factors and within the same group of patients; for example, INF-γ ranges from 0 to 2640 pg/ml, with overlapping concentrations between the uninfected individuals, LTBI, and active TB group, independent of the sample used (Additional file 6).
Given the heterogeneity of the biology of disease associated with LTBI, the ability to identify the duration of infection remains a challenge for future research. Inability to determine the duration and type of LTBI (i.e., what type of granuloma) might modify the observed response to a mycobacterial antigen leading to blurring of the ability to interpret differences between study groups [60, 61].
different samples and varied cell stimulation protocols. The samples used for the studies were predominantly plasma; however, culture supernatant, serum, and RNA were used introducing variability in measured concentration caused by the matrix used (Table 1 and Additional file 6). Several potential reasons for the variation in immune substance concentrations in plasma and serum from whole blood include inhibition of detection for specific cytokines (e.g., EGF, GM-CSF, IL-3, and IL-4) in the serum [62]; delay in processing of serum or plasma, sample hemolysis, presence of debris, or freeze-thaw cycles, all of which can adversely affect cytokine detection [63]; and the release of several mediators by platelets which can increase cytokine serum levels, especially CCL5 and CD40L [64].
In addition, the wide variety of antigens (ESAT-6, CFP-10, TB7.7, PPD, or Mtb CFA, among others) used to stimulate cells and different incubation times leads to the increases or decreases of the time of cellular exposure to the stimulus and therefore the concentration of the detected immune mediators and other substances. In addition, cellular stimulation adds complexity to the diagnostic utility of detecting biomarkers, especially in areas with limited laboratory infrastructure or access, as is the situation in many of the countries or settings where TB is endemic. In addition, reviewed articles show variations in the results due to the antigen used for stimulation [11–13]. In experiments with whole bacteria, it has been demonstrated that the strain used to carry out stimulation modifies the type of immune response in vitro; for example, the most recent strains in the M. tuberculosis lineage show a lower inflammatory response in macrophages when compared to the older strains [65]. Likewise, Leyten et al. evaluated 25 antigens of latency related to the DosR regulator of M. tuberculosis; it was observed that different antigens can give different cellular responses (measured by the production of INF-γ) after in vitro stimulation, and in addition, this can vary between healthy people, LTBI and active TB cases [11]. This limitation can be overcome in longitudinal studies applying the same measurement at different times along the natural history of M. tuberculosis infection.
the variety of laboratory methods used for detection of substances, which in turn leads to the variable units of measurement and assay sensitivity. The ability to compare the heterogeneous samples is further compounded by use of ELISA, microbead assays, EIA, and real-time PCR—in the absence of an endogenous standard—yielding variable dynamic ranges [66]. The intraindividual variability cannot be assessed, as only 2 studies were longitudinal. This variability results in difficulty to compare and quantify studies
the presence of a selection bias for nonapplication of the booster when individuals are screened using the tuberculin skin test. It is known that the booster effect can occur in individuals and is only detected when a second TST is applied to negative individuals between 1 and 4 weeks after the first administration. The increase in the frequency of positive individuals is notable in the population without any underlying diseases (in prisoners, an increase in positivity was reported from 66% to 77.6% [67]) or in those with other disorders such as rheumatoid arthritis (where the booster positivity changed from 31.3% and 21.7% to 46.5% and 28.8% in early and late rheumatoid arthritis, respectively) [68]. The lack of application of two-step TST may lead to erroneous classification to the uninfected group, resulting in false negative results [67–70]. Equally important, LTBI diagnoses were done using TST and/or IGRAs, which can introduce heterogeneity within the results. Indeed, in many studies when both methods were used, the results demonstrated inconsistent findings, a common theme discussed in literature [71–73]. Additionally, although some articles used TST for LTBI diagnosis, they did not consider the rate of BCG vaccination within children under 10 years old in their analyses [74]
the fact that none of the studies adjusted the analysis for the effect of ethnicity on the association between IP concentrations and the different stages of TB. A study published by Coussens et al. reported that the inflammatory profile differs according to ancestry. Individuals of African descent with TB, despite having similar mycobacterial strains and similar sociodemographic and clinical characteristics, have a different inflammatory profile compared to Eurasian patients with the same disease [75]. Similarly, Mwantembe et al. reported ethnic variation of cytokines (IL-1RA, IL-12) and chemokines (CCL2, CCL5, CCL11, and CXCL8) in South African patients with inflammatory bowel disease [76]. The concentrations of these chemokines and cytokines are determined by allelic frequency and have been involved in response to M. tuberculosis infection. Likewise, genes coding for proteins such as CCL2 [77], IL-17F, IL-17A [78], and IL-12 [37, 79] have been described as polymorphic; variation in allele frequency is affected by ethnic variation, affecting the antimycobacterial response, and thus may be driving the higher risk for development of TB among different populations. The examples emphasize the importance to adjust by ethnicity of the population at the time of reporting the results as these clearly impact biomarker concentrations.
Ethnicity is also related to the response to current M. tuberculosis infection screening tests. Genetic variants associated with the reaction to TST and IGRAs have been described. The TST1 locus is associated with a TST positivity per se (TST1 on 11p14), and the TST2 locus is associated with the intensity of TST reactivity (TST2 on 5p15) [3]. On the other hand, the production of INF-γ has been associated with genetic factors such as the locus located in chromosomal regions 8q12-22n and 3q13-22 [2]. The ethnicity must be considered when performing the immunological analysis in further research.
the heterogeneity of the populations studied. First, the comparison group of active TB included patients with pulmonary TB and extrapulmonary TB together. In these two groups of patients, the presentation of the disease is different, and the main factors of innate immunity, cytokines and chemokines, which play a role in cell-mediated immunity, involved in the dissemination of M. tuberculosis, differ. Mutations have been reported in genes encoding the INF-γ receptor, the IL-12 receptor, and the transcription-activating signal1 (STAT-1) in patients with extrapulmonary TB. Likewise, Yang et al. reported that there are differences in the immunopathogenicity of pulmonary and extrapulmonary infections. The production of CCL2, CXCL9, and CXCL8 modifies the type of tuberculous disease that a patient has, and they play a special role in the formation of granuloma [80]. Patients with pulmonary TB showed lower levels of the cytokines studied than those with extrapulmonary TB. CXCL8 concentration was found to be elevated in fatal TB, increases in CCL2 were observed with disseminated and meningeal TB [81], and TGF-β increased in extrapulmonary TB in children compared to pulmonary TB [14].
Secondly, patients with active TB included in the studies were at different phases of treatment (before, during, and after completion of antituberculous therapy). Several studies have been carried out with the aim of evaluating new biomarkers that allow monitoring the patient's condition after initiating antituberculous therapy. Several of the studies were longitudinal, making it evident that the immune substances changed during the administration of TB treatment [82, 83]. Changes in lung bacterial load related to treatment administration would appear to influence the concentration of cytokines detected in nonstimulated cells, with 17 out of the 27 cytokines/chemokines analyzed (IL-1β, IL-2, IL-4, IL-5, IL-6, IL-9, IL-13, IL-17, eotaxin, IFN-γ, IP-10, MCP-1, MIP-1α, MIP-1β, PDGF, RANTES, and VEGF) being significantly lower in patients with higher bacterial load and levels of IL10, IL15, and TNF-α being higher in the same patients [84].
5. Conclusions
Identification of biomarkers that individually or in combination can differentiate LTBI and active TB has been a research priority; however, a constellation of markers that differentiate between infection and disease is not yet available. The advances in high-throughput technologies for biomarker measurement are promising, but the variability of studies and potential biases that we have highlighted undermines the ability to identify reproducible markers. Although five parameters were exclusively increased in LTBI and 24 in active TB, only a single substance was consistently differential. These substances were not measured in all studies, and results are inconsistent between study groups, prohibiting the desired classification. Undoubtedly, the study of multiple immune substances seems to give better results than the study of a single biomarker; consequently, the search for immune profiles with multiple immune substances should be the goal of future research.
For the results obtained with different immune markers, future research should “harmonize” the methodological conditions to evaluate immune markers as the first step to draw any conclusion about LTBI parameter(s) for use as a diagnostic test. Those aspects include the presence of the booster effect, clinical classification of TB, the ethnicity of participants, and sample size estimation. In addition, cohort studies will allow identification of immune substances related to progression to active TB and conversion to LTBI and variations in the immune response due to the individual's stage of TB, measurement variation for cytokine/chemokines, and hormonal influences [85, 86].
The BCG has been associated with modulating the host's immune system and granting protection against MTB infection and disease [87, 88]. BCG vaccination could potentially modify the concentration of the immune substances in vaccinated adults, considering that it changes the concentrations in children and adolescents [89]. Further studies should evaluate the effect of BCG vaccination in the immune marker response.
It is important to note that our review did not include HIV-infected populations or any other types of immunosuppression, nor children, since those populations have several confounders and particular characteristics that need to be analyzed separately and are beyond the scope of the present review. In addition, as the main goal of our paper was to identify immune markers associated with LTBI, we did not include articles that consider biomarkers for TB before and after the treatment.
Acknowledgments
This systematic review was funded by the Administrative Department of Science, Technology and Innovation (Colciencias), as part of the research project entitled “Host gene expression profile used to identify latent TB infection and the transition to active disease—Perfil de la expresión génica del hospedero para identificar tuberculosis latente y la transición a enfermedad activa,” grant number: 121071249878 and the project “Pro-inflammatory patterns of cytokine/chemokine associated with latent tuberculosis in people deprived of liberty (PDL),” grant number: 639B-06/16-55 funded by Universidad Pontificia Bolivariana. Grupo de Epidemiología at Universidad de Antioquia paid the open access fee of this article.
Abbreviations
- TB:
Tuberculosis
- LTBI:
Latent tuberculosis infection
- EPTB:
Extrapulmonary TB
- PTB:
Pulmonary TB
- NI:
No
- TST:
Tuberculin skin test
- HIV:
Human immunodeficiency virus
- NIH:
National Institutes of Health
- IGRAs:
Interferon-gamma release assays
- INF:
Interferon
- IL:
Interleukins
- TNF:
Tumor necrosis factor
- PRISMA:
Preferred Reporting Items for Systematic reviews and Meta-Analyses
- ESAT-6:
Early secretory antigenic target-6, Rv3875
- CFP-10:
Culture filtrate protein-10, Rv3874
- PPD:
Purified protein derivatives
- IP:
Immune parameter.
Data Availability
All data generated or analyzed during this study are included in this published article [and its Additional files 1, 2, 3, 4 5 and 6].
Conflicts of Interest
The authors declare that they have no competing of interests.
Authors' Contributions
MH, CV, and ZVR contributed substantially to the conception or design of the work. MH and CV acquired data. MH, CV, YK, and ZVR analyzed and interpreted data; drafted the article and revised it critically for important intellectual content; had final approval of the version to be published; and agreed to be accountable for all aspects of the work in ensuring that questions related to the accuracy or integrity of any part of the work are appropriately investigated and resolved. Mariana Herrera and Cristian Vera contributed equally to this work.
Supplementary Materials
Additional file 1: search strategies conducted in the systematic review. Additional file 2: summary of excluded studies and the reasons. Additional file 3: quality of cohort studies (risk of bias evaluation). Additional file 4: quality of cross-sectional studies (risk of bias evaluation). Additional file 5: immune factors, production cell, diana cell, and effect in different tuberculosis stages. Additional file 6: concentration of immune factors in persons with different tuberculosis stages in the papers included in the systematic review.
References
- 1.Pai M., Denkinger C. M., Kik S. V., et al. Gamma interferon release assays for detection of Mycobacterium tuberculosis infection. Clinical Microbiology Reviews. 2014;27(1):3–20. doi: 10.1128/CMR.00034-13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Jabot-Hanin F., Cobat A., Feinberg J., et al. Major loci on chromosomes 8q and 3q control interferon γ production triggered by bacillus Calmette-Guerin and 6-kDa early secretory antigen target, respectively, in various populations. The Journal of Infectious Diseases. 2016;213(7):1173–1179. doi: 10.1093/infdis/jiv757. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Cobat A., Gallant C. J., Simkin L., et al. Two loci control tuberculin skin test reactivity in an area hyperendemic for tuberculosis. The Journal of Experimental Medicine. 2009;206(12):2583–2591. doi: 10.1084/jem.20090892. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Flynn J. L., Chan J., Triebold K. J., Dalton D. K., Stewart T. A., Bloom B. R. An essential role for interferon gamma in resistance to Mycobacterium tuberculosis infection. The Journal of Experimental Medicine. 1993;178(6):2249–2254. doi: 10.1084/jem.178.6.2249. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.WHO, TDR. Priorities for tuberculosis research. October 2017, https://apps.who.int/iris/bitstream/10665/85888/1/9789241505970_eng.pdf.
- 6.Guillén M. A. Avances en el diagnóstico de la infección tuberculosa. Archivos de Bronconeumología. 2011;47(10):521–530. doi: 10.1016/j.arbres.2011.06.018. [DOI] [PubMed] [Google Scholar]
- 7.Cellular and Molecular Immunology, 9th. October 2017, https://www.elsevier.com/books/cellular-and-molecular-immunology/abbas/978-0-323-47978-3.
- 8.Suzukawa M., Akashi S., Nagai H., et al. Combined analysis of IFN-γ, IL-2, IL-5, IL-10, IL-1RA and MCP-1 in QFT supernatant is useful for distinguishing active tuberculosis from latent infection. PLoS One. 2016;11(4, article e0152483) doi: 10.1371/journal.pone.0152483. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Pollock K. M., Whitworth H. S., Montamat-Sicotte D. J., et al. T-cell immunophenotyping distinguishes active from latent tuberculosis. The Journal of Infectious Diseases. 2013;208(6):952–968. doi: 10.1093/infdis/jit265. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Arji N., Busson M., Iraqi G., et al. The MCP-1 (CCL2) -2518 GG genotype is associated with protection against pulmonary tuberculosis in Moroccan patients. Journal of Infection in Developing Countries. 2011;6(1):73–78. doi: 10.3855/jidc.1925. [DOI] [PubMed] [Google Scholar]
- 11.Black G. F., Thiel B. A., Ota M. O., et al. Immunogenicity of novel DosR regulon-encoded candidate antigens of Mycobacterium tuberculosis in three high-burden populations in Africa. Clinical and Vaccine Immunology. 2009;16(8):1203–1212. doi: 10.1128/CVI.00111-09. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Hussain R., Kaleem A., Shahid F., et al. Cytokine profiles using whole-blood assays can discriminate between tuberculosis patients and healthy endemic controls in a BCG-vaccinated population. Journal of Immunological Methods. 2002;264(1-2):95–108. doi: 10.1016/S0022-1759(02)00092-3. [DOI] [PubMed] [Google Scholar]
- 13.Leyten E. M. S., Lin M. Y., Franken K. L. M. C., et al. Human T-cell responses to 25 novel antigens encoded by genes of the dormancy regulon of Mycobacterium tuberculosis. Microbes and Infection. 2006;8(8):2052–2060. doi: 10.1016/j.micinf.2006.03.018. [DOI] [PubMed] [Google Scholar]
- 14.Pavan Kumar N., Anuradha R., Andrade B. B., et al. Circulating biomarkers of pulmonary and extrapulmonary tuberculosis in children. Clinical and Vaccine Immunology. 2013;20(5):704–711. doi: 10.1128/cvi.00038-13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Abou-Setta A. M., Beaupre L. A., Jones C. A., et al. Newcastle-Ottawa Scale Assessment of Cohort Studies. US: Agency for Healthcare Research and Quality; 2011. October 2017, https://www.ncbi.nlm.nih.gov/books/NBK56664/ [Google Scholar]
- 16.Quality assessment tool for observational cohort and cross-sectional studies - NHLBI, NIH. October 2017, https://www.nhlbi.nih.gov/health-pro/guidelines/in-develop/cardiovascular-risk-reduction/tools/cohort.
- 17.Jadad A. R., Moore R. A., Carroll D., et al. Assessing the quality of reports of randomized clinical trials: is blinding necessary? Controlled Clinical Trials. 1996;17(1):1–12. doi: 10.1016/0197-2456(95)00134-4. [DOI] [PubMed] [Google Scholar]
- 18.Pai M., McCulloch M., Gorman J. D., et al. Systematic reviews and meta-analyses: an illustrated, step-by-step guide. National Medical Journal of India. 2004;17(2):86–95. [PubMed] [Google Scholar]
- 19.9.5.1 what is heterogeneity? November 2018, https://handbook-5-1.cochrane.org/chapter_9/9_5_1_what_is_heterogeneity.htm.
- 20.Nonghanphithak D., Reechaipichitkul W., Namwat W., Naranbhai V., Faksri K. Chemokines additional to IFN-γ can be used to differentiate among Mycobacterium tuberculosis infection possibilities and provide evidence of an early clearance phenotype. Tuberculosis. 2017;105:28–34. doi: 10.1016/j.tube.2017.04.005. [DOI] [PubMed] [Google Scholar]
- 21.Belay M., Legesse M., Mihret A., et al. Pro- and anti-inflammatory cytokines against Rv2031 are elevated during latent tuberculosis: a study in cohorts of tuberculosis patients, household contacts and community controls in an endemic setting. PLoS One. 2015;10(4, article e0124134) doi: 10.1371/journal.pone.0124134. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Bapat P. R., Husain A. A., Daginawala H. F., et al. The assessment of cytokines in Quantiferon supernatants for the diagnosis of latent TB infection in a tribal population of Melghat, India. Journal of Infection and Public Health. 2015;8(4):329–340. doi: 10.1016/j.jiph.2015.02.003. [DOI] [PubMed] [Google Scholar]
- 23.Handzel Z. T., Barak V., Altman Y., et al. Increased Th1 and Th2 type cytokine production in patients with active tuberculosis. The Israel Medical Association Journal: IMAJ. 2007;9(6):479–483. [PubMed] [Google Scholar]
- 24.Chegou N. N., Black G. F., Kidd M., van Helden P. D., Walzl G. Host markers in Quantiferon supernatants differentiate active TB from latent TB infection: preliminary report. BMC Pulmonary Medicine. 2009;9(1):p. 21. doi: 10.1186/1471-2466-9-21. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Biselli R., Mariotti S., Sargentini V., et al. Detection of interleukin-2 in addition to interferon-γ discriminates active tuberculosis patients, latently infected individuals, and controls. Clinical Microbiology and Infection. 2010;16(8):1282–1284. doi: 10.1111/j.1469-0691.2009.03104.x. [DOI] [PubMed] [Google Scholar]
- 26.Sutherland J. S., de Jong B. C., Jeffries D. J., Adetifa I. M., Ota M. O. C. Production of TNF-α, IL-12 (p40) and IL-17 can discriminate between active TB disease and latent infection in a West African cohort. PLoS One. 2010;5(8, article e12365) doi: 10.1371/journal.pone.0012365. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Babu S., Bhat S. Q., Kumar N. P., Kumaraswami V., Nutman T. B. Regulatory T cells modulate Th17 responses in patients with positive tuberculin skin test results. The Journal of Infectious Diseases. 2010;201(1):20–31. doi: 10.1086/648735. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Frahm M., Goswami N. D., Owzar K., et al. Discriminating between latent and active tuberculosis with multiple biomarker responses. Tuberculosis. 2011;91(3):250–256. doi: 10.1016/j.tube.2011.02.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Rubbo P.-A., Nagot N., le Moing V., et al. Multicytokine detection improves latent tuberculosis diagnosis in health care workers. Journal of Clinical Microbiology. 2012;50(5):1711–1717. doi: 10.1128/JCM.00117-12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Wang S., Diao N., Lu C., et al. Evaluation of the diagnostic potential of IP-10 and IL-2 as biomarkers for the diagnosis of active and latent tuberculosis in a BCG-vaccinated population. PLoS One. 2012;7(12, article e51338) doi: 10.1371/journal.pone.0051338. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Yu Y., Zhang Y., Hu S., et al. Different patterns of cytokines and chemokines combined with IFN-γ production reflect Mycobacterium tuberculosis infection and disease. PLoS One. 2012;7(9, article e44944) doi: 10.1371/journal.pone.0044944. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Chegou N. N., Essone P. N., Loxton A. G., et al. Potential of host markers produced by infection phase-dependent antigen-stimulated cells for the diagnosis of tuberculosis in a highly endemic area. PLoS One. 2012;7(6, article e38501) doi: 10.1371/journal.pone.0038501. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Anbarasu D., Raja C. P., Raja A. Multiplex analysis of cytokines/chemokines as biomarkers that differentiate healthy contacts from tuberculosis patients in high endemic settings. Cytokine. 2013;61(3):747–754. doi: 10.1016/j.cyto.2012.12.031. [DOI] [PubMed] [Google Scholar]
- 34.Mayer-Barber K. D., Andrade B. B., Oland S. D., et al. Host-directed therapy of tuberculosis based on interleukin-1 and type I interferon crosstalk. Nature. 2014;511(7507):99–103. doi: 10.1038/nature13489. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Sauzullo I., Mastroianni C. M., Mengoni F., et al. Long-term IFN-γ and IL-2 response for detection of latent tuberculosis infection in healthcare workers with discordant immunologic results. Journal of Immunological Methods. 2014;414:51–57. doi: 10.1016/j.jim.2014.07.013. [DOI] [PubMed] [Google Scholar]
- 36.Kim K., Perera R., Tan D. B. A., et al. Circulating mycobacterial-reactive CD4+ T cells with an immunosuppressive phenotype are higher in active tuberculosis than latent tuberculosis infection. Tuberculosis. 2014;94(5):494–501. doi: 10.1016/j.tube.2014.07.002. [DOI] [PubMed] [Google Scholar]
- 37.Jeong Y. H., Hur Y.-G., Lee H., et al. Discrimination between active and latent tuberculosis based on ratio of antigen-specific to mitogen-induced IP-10 production. Journal of Clinical Microbiology. 2015;53(2):504–510. doi: 10.1128/JCM.02758-14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Wei M., Wu Z. Y., Lin J. H., et al. Regulation network of serum cytokines induced by tuberculosis-specific antigens reveals biomarkers for tuberculosis diagnosis. Genetics and Molecular Research. 2015;14(4):17182–17192. doi: 10.4238/2015.December.16.18. [DOI] [PubMed] [Google Scholar]
- 39.Lee J. Y., Jung Y. W., Jeong I., et al. Immune parameters differentiating active from latent tuberculosis infection in humans. Tuberculosis. 2015;95(6):758–763. doi: 10.1016/j.tube.2015.08.003. [DOI] [PubMed] [Google Scholar]
- 40.Zambuzi F. A., Cardoso-Silva P. M., Espindola M. S., et al. Identification of promising plasma immune biomarkers to differentiate active pulmonary tuberculosis. Cytokine. 2016;88:99–107. doi: 10.1016/j.cyto.2016.08.030. [DOI] [PubMed] [Google Scholar]
- 41.Santin M., Morandeira-Rego F., Alcaide F., et al. Detection of interleukin-2 is not useful for distinguishing between latent and active tuberculosis in clinical practice: a prospective cohort study. Clinical Microbiology and Infection. 2016;22(12):1007.e1–1007.e5. doi: 10.1016/j.cmi.2016.09.004. [DOI] [PubMed] [Google Scholar]
- 42.Yao X., Liu Y., Liu Y., et al. Multiplex analysis of plasma cytokines/chemokines showing different immune responses in active TB patients, latent TB infection and healthy participants. Tuberculosis. 2017;107:88–94. doi: 10.1016/j.tube.2017.07.013. [DOI] [PubMed] [Google Scholar]
- 43.Won E.-J., Choi J.-H., Cho Y.-N., et al. Biomarkers for discrimination between latent tuberculosis infection and active tuberculosis disease. The Journal of Infection. 2017;74(3):281–293. doi: 10.1016/j.jinf.2016.11.010. [DOI] [PubMed] [Google Scholar]
- 44.Arroyo L., Marín D., Franken K. L. M. C., Ottenhoff T. H. M., Barrera L. F. Potential of DosR and Rpf antigens from Mycobacterium tuberculosis to discriminate between latent and active tuberculosis in a tuberculosis endemic population of Medellin Colombia. BMC Infectious Diseases. 2018;18(1):p. 26. doi: 10.1186/s12879-017-2929-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Kim S. Y., Park M. S., Kim Y. S., et al. The responses of multiple cytokines following incubation of whole blood from TB patients, latently infected individuals and controls with the TB antigens ESAT-6, CFP-10 and TB7.7. Scandinavian Journal of Immunology. 2012;76(6):580–586. doi: 10.1111/j.1365-3083.2012.02776.x. [DOI] [PubMed] [Google Scholar]
- 46.Hong J. Y., Jung G. S., Kim H., et al. Efficacy of inducible protein 10 as a biomarker for the diagnosis of tuberculosis. International Journal of Infectious Diseases. 2012;16(12):e855–e859. doi: 10.1016/j.ijid.2012.07.013. [DOI] [PubMed] [Google Scholar]
- 47.Hur Y.-G., Gorak-Stolinska P., Ben-Smith A., et al. Combination of cytokine responses indicative of latent TB and active TB in Malawian adults. PLoS One. 2013;8(11, article e79742) doi: 10.1371/journal.pone.0079742. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Pourakbari B., Mamishi S., Marjani M., Rasulinejad M., Mariotti S., Mahmoudi S. Novel T-cell assays for the discrimination of active and latent tuberculosis infection: the diagnostic value of PPE family. Molecular Diagnosis & Therapy. 2015;19(5):309–316. doi: 10.1007/s40291-015-0157-0. [DOI] [PubMed] [Google Scholar]
- 49.Hur Y.-G., Kang Y. A., Jang S.-H., et al. Adjunctive biomarkers for improving diagnosis of tuberculosis and monitoring therapeutic effects. The Journal of Infection. 2015;70(4):346–355. doi: 10.1016/j.jinf.2014.10.019. [DOI] [PubMed] [Google Scholar]
- 50.Kim S., Lee H., Kim H., et al. Diagnostic performance of a cytokine and IFN-γ–induced chemokine mRNA assay after Mycobacterium tuberculosis–specific antigen stimulation in whole blood from infected individuals. The Journal of molecular diagnostics : JMD. 2015;17(1):90–99. doi: 10.1016/j.jmoldx.2014.08.005. [DOI] [PubMed] [Google Scholar]
- 51.Wergeland I., Assmus J., Dyrhol-Riise A. M. Cytokine patterns in tuberculosis infection; IL-1ra, IL-2 and IP-10 differentiate borderline QuantiFERON-TB samples from uninfected controls. PLoS One. 2016;11(9, article e0163848) doi: 10.1371/journal.pone.0163848. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Chen T., Li Z., Yu L., et al. Profiling the human immune response to Mycobacterium tuberculosis by human cytokine array. Tuberculosis (Edinburgh, Scotland) 2016;97:108–117. doi: 10.1016/j.tube.2015.12.007. [DOI] [PubMed] [Google Scholar]
- 53.Wu J., Wang S., Lu C., et al. Multiple cytokine responses in discriminating between active tuberculosis and latent tuberculosis infection. Tuberculosis. 2017;102:68–75. doi: 10.1016/j.tube.2016.06.001. [DOI] [PubMed] [Google Scholar]
- 54.Kamakia R., Kiazyk S., Waruk J., et al. Potential biomarkers associated with discrimination between latent and active pulmonary tuberculosis. The International Journal of Tuberculosis and Lung Disease. 2017;21(3):278–285. doi: 10.5588/ijtld.16.0176. [DOI] [PubMed] [Google Scholar]
- 55.La Manna M. P., Orlando V., Donni P. L., et al. Identification of plasma biomarkers for discrimination between tuberculosis infection/disease and pulmonary non tuberculosis disease. PLoS One. 2018;13(3, article e0192664) doi: 10.1371/journal.pone.0192664. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Lyadova I. V., Panteleev A. V. Th1 and Th17 cells in tuberculosis: protection, pathology, and biomarkers. Mediators of Inflammation. 2015;2015:13. doi: 10.1155/2015/854507.854507 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Kanehisa M., Furumichi M., Tanabe M., Sato Y., Morishima K. KEGG: new perspectives on genomes, pathways, diseases and drugs. Nucleic Acids Research. 2017;45(D1):D353–D361. doi: 10.1093/nar/gkw1092. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Li Y., Wang Y., Liu X. The role of airway epithelial cells in response to mycobacteria infection. Journal of Immunology Research. 2012;2012:11. doi: 10.1155/2012/791392.791392 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Mvubu N. E., Pillay B., McKinnon L. R., Pillay M. Mycobacterium tuberculosis strains induce strain-specific cytokine and chemokine response in pulmonary epithelial cells. Cytokine. 2018;104:53–64. doi: 10.1016/j.cyto.2017.09.027. [DOI] [PubMed] [Google Scholar]
- 60.Cadena A. M., Fortune S. M., Flynn J. A. L. Heterogeneity in tuberculosis. Nature Reviews Immunology. 2017;17(11):691–702. doi: 10.1038/nri.2017.69. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Barry C. E., 3rd, Boshoff H. I., Dartois V., et al. The spectrum of latent tuberculosis: rethinking the biology and intervention strategies. Nature Reviews Microbiology. 2009;7(12):845–855. doi: 10.1038/nrmicro2236. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Rosenberg-Hasson Y., Hansmann L., Liedtke M., Herschmann I., Maecker H. T. Effects of serum and plasma matrices on multiplex immunoassays. Immunologic Research. 2014;58(2-3):224–233. doi: 10.1007/s12026-014-8491-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.de Jager W., Bourcier K., Rijkers G. T., Prakken B. J., Seyfert-Margolis V. Prerequisites for cytokine measurements in clinical trials with multiplex immunoassays. BMC Immunology. 2009;10(1):p. 52. doi: 10.1186/1471-2172-10-52. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Tvedt T. H. A., Rye K. P., Reikvam H., Brenner A. K., Bruserud Ø. The importance of sample collection when using single cytokine levels and systemic cytokine profiles as biomarkers — a comparative study of serum versus plasma samples. Journal of Immunological Methods. 2015;418:19–28. doi: 10.1016/j.jim.2015.01.006. [DOI] [PubMed] [Google Scholar]
- 65.Portevin D., Gagneux S., Comas I., Young D. Human macrophage responses to clinical isolates from the Mycobacterium tuberculosis complex discriminate between ancient and modern lineages. PLoS Pathogens. 2011;7(3, article e1001307) doi: 10.1371/journal.ppat.1001307. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Ledur A., Fitting C., David B., Hamberger C., Cavaillon J. M. Variable estimates of cytokine levels produced by commercial ELISA kits: results using international cytokine standards. Journal of Immunological Methods. 1995;186(2):171–179. doi: 10.1016/0022-1759(95)00184-C. [DOI] [PubMed] [Google Scholar]
- 67.Rueda Z. V., Arroyave L., Marin D., et al. High prevalence and risk factors associated with latent tuberculous infection in two Colombian prisons. The International Journal of Tuberculosis and Lung Disease. 2014;18(10):1166–1171. doi: 10.5588/ijtld.14.0179. [DOI] [PubMed] [Google Scholar]
- 68.Dogan E., Erkoc R., Sayarlioglu H., Uzun K. Tuberculin skin test results and the booster phenomenon in two-step tuberculin skin testing in hemodialysis patients. Renal Failure. 2005;27(4):425–428. doi: 10.1081/JDI-65379. [DOI] [PubMed] [Google Scholar]
- 69.Pérez-Barbosa L., Esquivel-Valerio J. A., Arana-Guajardo A. C., Vega-Morales D., Riega-Torres J., Garza-Elizondo M. A. Increased detection of latent tuberculosis by tuberculin skin test and booster phenomenon in early rheumatoid arthritis patients. Rheumatology International. 2015;35(9):1555–1559. doi: 10.1007/s00296-015-3246-9. [DOI] [PubMed] [Google Scholar]
- 70.Teixeira E. G., Kritski A., Ruffino-Netto A., et al. Two-step tuberculin skin test and booster phenomenon prevalence among Brazilian medical students. The International Journal of Tuberculosis and Lung Disease. 2008;12(12):1407–1413. [PubMed] [Google Scholar]
- 71.Dorman S. E., Belknap R., Graviss E. A., et al. Interferon-γ release assays and tuberculin skin testing for diagnosis of latent tuberculosis infection in healthcare workers in the United States. American Journal of Respiratory and Critical Care Medicine. 2014;189(1):77–87. doi: 10.1164/rccm.201302-0365OC. [DOI] [PubMed] [Google Scholar]
- 72.Overton K., Varma R., Post J. J. Comparison of interferon-γ release assays and the tuberculin skin test for diagnosis of tuberculosis in human immunodeficiency virus: a systematic review. Tuberculosis Respiratory Disease. 2018;81(1):59–72. doi: 10.4046/trd.2017.0072. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Lu P., Chen X., Zhu L.-M., Yang H.-T. Interferon-gamma release assays for the diagnosis of tuberculosis: a systematic review and meta-analysis. Lung. 2016;194(3):447–458. doi: 10.1007/s00408-016-9872-5. [DOI] [PubMed] [Google Scholar]
- 74.Mancuso J. D., Mody R. M., Olsen C. H., Harrison L. H., Santosham M., Aronson N. E. The long-term effect of bacille Calmette-Guérin vaccination on tuberculin skin testing: a 55-year follow-up study. Chest. 2017;152(2):282–294. doi: 10.1016/j.chest.2017.01.001. [DOI] [PubMed] [Google Scholar]
- 75.Coussens A. K., Wilkinson R. J., Nikolayevskyy V., et al. Ethnic variation in inflammatory profile in tuberculosis. PLoS Pathogens. 2013;9(7, article e1003468) doi: 10.1371/journal.ppat.1003468. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Mwantembe O., Gaillard M. C., Barkhuizen M., et al. Ethnic differences in allelic associations of the interleukin-1 gene cluster in South African patients with inflammatory bowel disease (IBD) and in control individuals. Immunogenetics. 2001;52(3-4):249–254. doi: 10.1007/s002510000265. [DOI] [PubMed] [Google Scholar]
- 77.Feng W. X., Flores-Villanueva P. O., Mokrousov I., et al. CCL2−2518 (A/G) polymorphisms and tuberculosis susceptibility: a meta-analysis. International Journal of Tuberculosis and Lung Disease. 2012;16(2):150–156. doi: 10.5588/ijtld.11.0205. [DOI] [PubMed] [Google Scholar]
- 78.Zhao J., Wen C., Li M. Association analysis of interleukin-17 gene polymorphisms with the risk susceptibility to tuberculosis. Lung. 2016;194(3):459–467. doi: 10.1007/s00408-016-9860-9. [DOI] [PubMed] [Google Scholar]
- 79.Liu G., Li G., Xu Y., et al. Association between IL12B polymorphisms and tuberculosis risk: a meta-analysis. Infection, Genetics and Evolution. 2014;21:401–407. doi: 10.1016/j.meegid.2013.12.008. [DOI] [PubMed] [Google Scholar]
- 80.Yang D., Kong Y. The bacterial and host factors associated with extrapulmonary dissemination of Mycobacterium tuberculosis. Frontiers of Biology. 2015;10(3):252–261. doi: 10.1007/s11515-015-1358-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Hasan Z., Jamil B., Khan J., et al. Relationship between circulating levels of IFN-γ, IL-10, CXCL9 and CCL2 in pulmonary and extrapulmonary tuberculosis is dependent on disease severity. Scandinavian Journal of Immunology. 2009;69(3):259–267. doi: 10.1111/j.1365-3083.2008.02217.x. [DOI] [PubMed] [Google Scholar]
- 82.Clifford V., Zufferey C., Street A., Denholm J., Tebruegge M., Curtis N. Cytokines for monitoring anti-tuberculous therapy: a systematic review. Tuberculosis. 2015;95(3):217–228. doi: 10.1016/j.tube.2015.01.003. [DOI] [PubMed] [Google Scholar]
- 83.Ashenafi S., Aderaye G., Bekele A., et al. Progression of clinical tuberculosis is associated with a Th2 immune response signature in combination with elevated levels of SOCS3. Clinical Immunology. 2014;151(2):84–99. doi: 10.1016/j.clim.2014.01.010. [DOI] [PubMed] [Google Scholar]
- 84.Heslop R., Bojang A. L., Jarju S., et al. Changes in host cytokine patterns of TB patients with different bacterial loads detected using 16S rRNA analysis. PLoS One. 2016;11(12, article e0168272) doi: 10.1371/journal.pone.0168272. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Foo Y. Z., Nakagawa S., Rhodes G., Simmons L. W. The effects of sex hormones on immune function: a meta-analysis. Biological Reviews. 2017;92(1):551–571. doi: 10.1111/brv.12243. [DOI] [PubMed] [Google Scholar]
- 86.Klein S. L., Flanagan K. L. Sex differences in immune responses. Nature Reviews. Immunology. 2016;16(10):626–638. doi: 10.1038/nri.2016.90. [DOI] [PubMed] [Google Scholar]
- 87.PPD-induced monocyte mitochondrial damage is associated with a protective effect to develop tuberculosis in BCG vaccinated individuals: a cohort study. 2020, https://journals.plos.org/plosone/article?id=10.1371/journal.pone.0171930. [DOI] [PMC free article] [PubMed]
- 88.Katelaris A. L., Jackson C., Southern J., et al. Effectiveness of BCG vaccination against Mycobacterium tuberculosis infection in adults: a cross-sectional analysis of a UK-based cohort. The Journal of Infectious Diseases. 2020;221(1):146–155. doi: 10.1093/infdis/jiz430. [DOI] [PubMed] [Google Scholar]
- 89.Dockrell H. M., Smith S. G., Lalor M. K. Variability between countries in cytokine responses to BCG vaccination: what impact might this have on protection? Expert Review of Vaccines. 2012;11(2):121–124. doi: 10.1586/erv.11.186. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Additional file 1: search strategies conducted in the systematic review. Additional file 2: summary of excluded studies and the reasons. Additional file 3: quality of cohort studies (risk of bias evaluation). Additional file 4: quality of cross-sectional studies (risk of bias evaluation). Additional file 5: immune factors, production cell, diana cell, and effect in different tuberculosis stages. Additional file 6: concentration of immune factors in persons with different tuberculosis stages in the papers included in the systematic review.
Data Availability Statement
All data generated or analyzed during this study are included in this published article [and its Additional files 1, 2, 3, 4 5 and 6].


