Abstract
Aims
To investigate the distribution of diabetic retinopathy (DR) by sex in patients with type 2 diabetes mellitus (T2DM) in a twelve-province cross-sectional study in China.
Methods
Patients with T2DM, whose ages were ≥18 years, were recruited from 76 cities/counties in 12 provinces in mainland China between January 2015 and December 2018. All participants received a standardized interview, eye examinations, and digital fundus photography. The presence and severity of DR were diagnosed and classified by retina specialists according to the DR domestic typing method.
Results
A total of 12,766 participants (5963 males and 6803 females) were eligible for this study. The total prevalence of DR was 30.1%. Females exhibited a significantly higher prevalence of DR than males (31.1% vs. 29.0%, P = 0.011). A multivariate logistic regression analysis confirmed that female sex was an independent predictor for a higher prevalence of DR after adjusting for age, the duration of diabetes, economic status, and the presence of hypertension (OR: 1.096, 95% CI: 1.013-1.186, P = 0.023). Even after stratification by the diabetic duration, age, and economic status, female sex was still independently associated with the presence of DR in patients whose T2DM history was more than 10 years, whose ages were over 60 years, or who were in a relatively intermediate economic area.
Conclusion
Females had a higher prevalence of DR than males in T2DM patients with a diabetic history of more than 10 years, ages over 60 years, or a relatively intermediate economic status.
1. Introduction
Type 2 diabetes mellitus (T2DM) is highly prevalent worldwide and is increasing rapidly [1]. Diabetic retinopathy (DR) is one of the most frequent and serious microvascular complications in T2DM. The overall prevalence of DR is estimated to be 34.6% [2] and ranges from 11.9% to 43.1% in mainland China [3, 4]. DR remains a leading cause of blindness among working age populations in both developed and developing countries [5]. However, limited treatment options are available for DR when it progresses to late-stage disease, in which vision is already impaired. Late-stage DR requires repeated treatments (laser photocoagulation or intravitreal injections of antivascular endothelial growth factor agents) with unsatisfactory effects, which result in a high socioeconomic burden [6–8]. Therefore, it is valuable to identify the risk factors that will help to prevent the occurrence and delay the progression of DR.
Multiple risk factors contribute to DR, such as chronic hyperglycemia, hypertension, dyslipidemia, a long duration of diabetes, overweight, and age [9, 10]. It remains ambiguous which sex is more susceptible to DR. A study of 120,000 cases from Germany and Australia shows that females are more likely to suffer from DR than are males [11]. Similarly, studies from Britain and Japan show that females are more prone to suffer from visual impairment than are males [12, 13]. However, there are some studies from the United States and India that indicate that male sex is a risk factor for DR [14–16]. In particular, the United Kingdom Prospective Diabetic Study, which is a milestone T2DM study, presented that the progression of retinopathy was associated with male sex [17]. This inconsistency highlights the need for further investigation of the association between DR and sex. Furthermore, there is a lack of data about the Chinese population, which has the largest number of T2DM patients in the world [1]. Therefore, the primary objective of this study was to explore the relationship between sex and DR in T2DM patients; the secondary purpose was to determine the prevalence of DR in mainland China.
2. Materials and Methods
2.1. Study Population and Data Source
This cross-sectional study was a population-based study. Participants were enrolled from 12 provinces, including 76 cities/counties and 381 community health service centers and hospitals in mainland China, between January 2015 and December 2018. The protocol was approved by the Ethics Committee of the Third Affiliated Hospital of Sun Yat-sen University. All participants signed an informed written consent form.
2.2. Study Design
A total of 23,662 participants with T2DM who were at least 18 years old were enrolled in this cross-sectional study. T2DM was diagnosed according to the 1999 criteria of the World Health Organization (WHO) [18]. Twelve thousand eight hundred eighty-four patients were eligible. Patients with serious mental illness and other situations in which the requirements of the agreement cannot be complied with (e.g., patients unable to take care of themselves, alcoholics, and drug abuser) were excluded from the study, as were pregnant and lactating women. One hundred eighteen subjects with missing data or blurry fundus photographs were excluded. A total of 12,766 subjects were finally included in the analysis (Figure 1).
Figure 1.
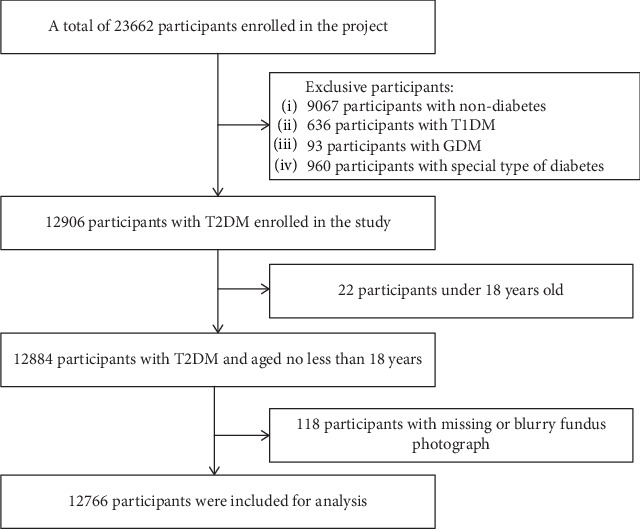
Flowchart of the study.
2.3. Eye Examination
Eye examinations were performed on all participants according to standard operation procedure by specific trained ophthalmologists. The eye examinations included visual acuity measurements, tonometry, and an anterior ocular structure and fundus examination using a standard protocol. Intraocular pressure in both eyes was measured with a noncontact tonometer (VISUPLAN 500 Non-Contact Tonometer, Carl Zeiss Vision Inc., San Diego, USA). The external and anterior ocular segment was examined by slit lamp biomicroscopy (BQ900; Haag-Streit, Bern, Switzerland). Two 45° field digital, colored, nonstereoscopic fundal photographs of each eye were taken in the macula-centered and posterior pole by a nonmydriatic auto fundus camera (Canon CR-DGi retinal camera; Canon, Tokyo, Japan, or TRC-NW400 Non-Mydriatic Retinal Camera, Topcon, Tokyo, Japan).
2.4. Assessment of DR
DR was diagnosed and graded based on fundus photographs according to the guidelines of 1985 and classified into two types (nonproliferative retinopathy and proliferative retinopathy) and six stages, as shown in Supplemental Table 1. Stages 1-3 apply to nonproliferative retinopathy, and stages 4-6 apply to proliferative retinopathy. Details of these guidelines are shown in Supplemental Table 1.
2.5. Data Collection
A standardized questionnaire was applied to collect basic information, such as age, sex, previous eye disease history and eye surgery history, comorbidities, the duration of diabetes, and region. The patients' chief complaint was also recorded by trained doctors. All of physicians and ophthalmologists in this study were trained before research. The economic level variable was categorized into tertiles according to the 2018 per capita gross regional product data from the National Bureau of Statistics for each city, as shown in Fig S1. Hypertension is defined as a blood pressure ≥ 140 mmHg systolic or ≥90 mmHg diastolic or current use of antihypertensive medication according to the European Society of Hypertension (ESH) and the European Society of Cardiology (ESC) [19].
2.6. Statistical Analyses
Database management and statistical analysis were performed using PASW 22.0 for Windows (IBM Inc., Armonk, USA). Descriptive statistics are presented as the mean (standard deviation) or median (interquartile range) for continuous variables and as numbers (percentages) for categorical variables. Continuous variables were compared by t-tests, while categorical variables were compared by Pearson chi-squared tests to determine between sex differences. Univariate logistic regression analysis was performed to assess the nonadjusted relationships between sex and the prevalence of DR. Odds ratios (ORs) and 95% confidence intervals (CIs) were estimated for the association between DR and sex using males as the reference group. After that estimation, age, the duration of diabetes, the presence of hypertension, and the economic level were adjusted in the multivariate logistic regression analysis models. To determine whether the presence of hypertension, the duration of diabetes, age, and economic levels affect the relationship between sex and the prevalence of DR, subgroup analyses were performed based on the presence of hypertension (without and with), the duration of diabetes (<10 years and ≥10 years), age (<60 years old and ≥60 years old), and economic level tertiles. A two-tailed P < 0.05 was considered statistically significant.
3. Results
3.1. Participants' Characteristics
A total of 12,766 participants (5963 males and 6803 females; mean age of 61.9 ± 11.5 years) were eligible for this study. The median duration of diabetes was 5.0 (2.0-10.0) years. There were 6434 (50.4%) patients who had both T2DM and hypertension. The distribution of economic levels among the participants is shown in Table 1. A total of 50.1% of the participants came from intermediate economic areas (Guangdong, Zhejiang, Fujian, Shandong, and Inner Mongolia), 40.3% came from low economic areas (Sichuan, Gansu, Hebei, Shanxi, Jiangxi, Yunnan, Shanxi, Henan, Hubei, Hunan, and Guizhou), and 9.6% came from relatively high economic areas (Beijing, Jiangsu). The characteristics of the participants by sex are shown in Table 1. Compared with males with T2DM, the female population was older (63.3 ± 10.7 vs. 60.2 ± 12.1, P < 0.001), had a longer duration of diabetes (6.0 (2.0-10.0) vs. 5.0 (2.0-10.0), P < 0.001), and had a higher prevalence of hypertension (54.8% vs. 45.4%, P < 0.001).
Table 1.
Characteristics of participants.
| Total (N = 12766) | Male (N = 5963) | Female (N = 6803) | P value | |
|---|---|---|---|---|
| Age (year) | 61.9 (11.5) | 60.2 (12.1) | 63.3 (10.7) | <0.001 |
| Duration of diabetes (years) | 5.0 (2.0-10.0) | 5.0 (2.0-10.0) | 6.0 (2.0-10.0) | <0.001 |
| Hypertension, n (%) | 6434 (50.4) | 2708 (45.4) | 3726 (54.8) | <0.001 |
| Regional economic level, n (%) | <0.001 | |||
| T1 (Sichuan, Gansu, Hebei, Shanxi, Jiangxi, Yunnan, Shaanxi, Henan, Hubei, Hunan, and Guizhou) | 5139 (40.3) | 2237 (37.5) | 2902 (42.7) | |
| T2 (Guangdong, Zhejiang, Fujian, Shandong, and Inner Mongolia) | 6399 (50.1) | 3112 (52.2) | 3287 (48.3) | |
| T3 (Beijing, Jiangsu) | 1228 (9.6) | 614 (10.3) | 614 (9.0) |
Data are the mean (standard deviation), median (25th to 75th percentiles), or n (%); the cities were tertiled by the regional economic level according to the data of 2018 per capita gross regional product from the National Bureau of Statistics. T1 referred to 31,336.13-67,627.83, T2 referred to 67,627.83-103,919.54, and T3 referred to 103,919.54-140,211.24.
3.2. The Prevalence of Diabetic Retinopathy
In the present study, 3847 patients (30.1%) suffered from DR. The females exhibited a significantly higher prevalence of DR than the males (31.1% vs. 29.0%, P = 0.011) (Table 2). No significant difference was observed in the severity of DR between the females and the males.
Table 2.
Prevalence and severity of diabetic retinopathy of participants with gender difference.
| Total (N = 12,766) | Male (N = 5963) | Female (N = 6803) | P value | |
|---|---|---|---|---|
| Diabetic retinopathy, n (%) | 3847 (30.1) | 1731 (29.0) | 2116 (31.1) | 0.011 |
| Nonproliferative, n (%) | ||||
| Stage I | 1822 (14.3) | 843 (14.1) | 981 (14.5) | 0.141 |
| Stage II | 1426 (11.2) | 634 (10.8) | 791 (11.6) | |
| Stage III | 416 (3.3) | 168 (2.8) | 247 (3.6) | |
| Proliferative, n (%) | ||||
| Stage IV | 137 (1.1) | 61 (1.0) | 76 (1.1) | |
| Stage V | 27 (0.2) | 13 (0.2) | 14 (0.2) | |
| Stage VI | 19 (0.1) | 12 (0.2) | 7 (0.1) |
Data are n (%).
3.3. Univariate and Multivariate Logistic Regression Analysis
As shown in Table 3, female sex was found to be potentially correlated with the presence of DR (P < 0.05). The multivariate logistic regression analysis confirmed that female sex was an independent predictor for a higher prevalence of DR after adjusting for age, the duration of diabetes, economic levels, and the presence of hypertension (OR: 1.096, 95% CI: 1.013-1.186, P = 0.023).
Table 3.
Logistic regression analysis assessing the relationships of gender with diabetic retinopathy.
| Univariate analysis | Multivariate analysis | |||||
|---|---|---|---|---|---|---|
| OR | 95% CI | P value | OR | 95% CI | P value | |
| Male | 1 | — | — | — | — | — |
| Female | 1.104 | 1.023-1.191 | 0.011 | 1.096 | 1.013-1.186 | 0.023 |
| Hypertension | 1.215 | 1.120-1.318 | <0.001 | |||
| Age | 0.993 | 0.990-0.997 | 0.001 | |||
| Duration of diabetes | 1.069 | 1.062-1.075 | <0.001 | |||
| Regional economic level—T1 | 1 | — | — | |||
| Regional economic level—T2 | 0.712 | 0.618-0.819 | <0.001 | |||
| Regional economic level—T3 | 0.730 | 0.673-0.792 | <0.001 | |||
Results are given as odds ratios and 95% confidence intervals (OR; 95% CI). The subjects were divided into nondiabetic retinopathy (DR) and DR. Gender was analyzed in the univariate logistic regression analysis, using male as the reference group. Then, a multivariate logistic regression analysis was adjusted for age, duration of diabetes, hypertension, and regional economic level.
3.4. Subgroup Analysis
To preclude the influence of hypertension, the duration of diabetes, age, and economic levels, these four factors were introduced in the subgroup analyses. As shown in Figures 2(a) and 2(b), a remarkable sex difference was observed in patients whose diabetes duration was more than 10 years and in patients who were older than 60 years old. The subgroup logistic regression analysis, which was stratified by the presence of hypertension (without vs. with), the duration of diabetes (<10 years vs. ≥10 years), age (<60 years old vs. ≥60 years old), and economic levels (tertile of per capita gross regional product), was further performed (Table 4). Compared with the male participants, the female participants had a significantly higher prevalence of DR in the diabetic duration ≥ 10 years subgroup (OR: 1.150, 95% CI: 1.012-1.306, P = 0.032), the age ≥ 60 years subgroup (OR: 1.141, 95% CI: 1.031-1.262, P = 0.010), and intermediate economic area subgroup (OR: 1.123, 95% CI: 1.001-1.259, P = 0.048). No sex difference was found in the hypertension, age < 60 years, diabetic duration < 10 years, or high or low economic area subgroups (P > 0.05).
Figure 2.
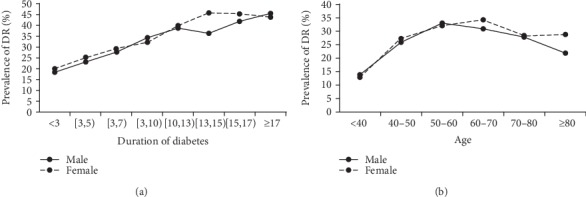
The prevalence of DR in gender difference in subgroup analysis. (a) The trend of prevalence of DR in gender difference with the duration of diabetes. (b) The trend of prevalence of DR in gender difference with age.
Table 4.
Subgroup logistic regression analysis assessing the relationships of gender with diabetic retinopathy.
| Univariate analysis | Multivariate analysis | |||||
|---|---|---|---|---|---|---|
| OR | 95% CI | P value | OR | 95% CI | P value | |
| Stratified by hypertension | ||||||
| Male | 1 | — | — | 1 | — | — |
| Female | ||||||
| Without hypertension | 1.143 | 1.028-1.127 | 0.014 | 1.094 | 0.980-1.222 | 0.109 |
| With hypertension | 1.096 | 0.982-1.223 | 0.102 | 1.091 | 0.974-1.222 | 0.131 |
| Stratified by duration of diabetes | ||||||
| Male | 1 | — | — | 1 | — | — |
| Female | ||||||
| <10 years | 1.090 | 0.987-1.203 | 0.090 | 1.057 | 0.955-1.170 | 0.284 |
| ≥10 years | 1.069 | 0.944-1.211 | 0.291 | 1.150 | 1.012-1.306 | 0.032 |
| Stratified by age | ||||||
| Male | 1 | — | — | 1 | — | — |
| Female | ||||||
| <60 years old | 1.057 | 0.936-1.195 | 0.373 | 0.986 | 0.867-1.122 | 0.830 |
| ≥60 years old | 1.122 | 1.017-1.238 | 0.022 | 1.141 | 1.031-1.262 | 0.010 |
| Stratified by regional economic level | ||||||
| Male | 1 | — | — | 1 | — | — |
| Female | ||||||
| T1 (31,336.13-67,627.83) | 1.051 | 0.935-1.182 | 0.403 | 1.054 | 0.934-1.188 | 0.396 |
| T2 (67,627.83-103,919.54) | 1.135 | 1.017-1.267 | 0.024 | 1.123 | 1.001-1.259 | 0.048 |
| T3 (103,919.54-140,211.24) | 1.040 | 0.814-1.329 | 0.754 | 1.148 | 0.889-1.481 | 0.290 |
Results are given as odds ratios and 95% confidence intervals (OR; 95% CI). The subjects were divided into nondiabetic retinopathy (DR) and DR. Gender was analyzed in the univariate logistic regression analysis in T2DM participants stratified by hypertension, duration of diabetes, age, and regional economic level, using male as the reference group. Then, a multivariate logistic regression analysis in T2DM participants stratified by hypertension was adjusted for age, duration of diabetes, and regional economic level; a multivariate logistic regression analysis in T2DM participants stratified by duration of diabetes was adjusted for age, duration of diabetes, hypertension, and regional economic level; a multivariate logistic regression analysis in T2DM participants stratified by age was adjusted for age, duration of diabetes, hypertension, and regional economic level. A multivariate logistic regression analysis in T2DM participants stratified by the regional economic level was adjusted for age, duration of diabetes, and hypertension.
4. Discussion
This national cross-sectional study with a large sample size showed that the overall prevalence of DR was 30.1% in mainland China. Moreover, the study also indicated that the female participants with T2DM exhibited a higher prevalence of DR than the male participants, particularly for the subjects with a diabetic history of more than 10 years, who were over 60 years old, or who were from areas of a relatively intermediate economic level. This result suggested that female sex was an independent risk factor for DR in T2DM patients.
The prevalence of DR (30.1%) in our study was similar to that in the studies from the United States (33.2%) [20] and Singapore (30.4%-35.0%) [21, 22]. The prevalence of DR in previous studies in Chinese ranged from 11.9% to 43.1% [3, 4]. This discrepancy may be due to study design, DR grading standards, and regional differences. The prevalence of 11.9% came from the general population in the northeast area of China with nonmydriatic retinal photographs [3], whereas the percentage of 43.1% was from a rural population in northern China with retinal photographs obtained after pupil dilation [4]. These two results suggested that DR prevalence varied by region, population, and retinal measurement. A recent multihospital-based population study across China with 16,305 participants showed that the overall age- and sex-standardized prevalence of DR was 27.9% [23], which was similar to our study. The participants in our study came from 76 cities/counties in 12 provinces, including the northern, southern, central, eastern, and western regions of mainland China, which enhanced the results.
The association between age and DR is still controversial. Some previous studies showed that older onset patients with diabetes had a higher prevalence of DR, and the prevalence of DR increased with age at diagnosis [23]. This may be attributed to fact that older patients had longer diabetes duration, which was a strong risk factor for the prevalence of DR [2, 3, 10, 15, 23]. Our study showed that the prevalence of DR increased with age in the age groups below 60 years but decreased with age in both men and women in the groups above 60 years. Consistent with our study, previous study showed that patients were getting less likely to suffer from DR every 10 years after 60 years of age [24]. Moreover, some studies showed that patients with young onset diabetes had a higher prevalence of DR [25–27], even for patients with similar diabetes duration [25]. There are several possible reasons for our result. Firstly, there is an eye problem with reduced vision gradient by age, such as hyperopia. Elderly people tend to consider the vision loss problem caused by ageing, thus would be less motivated for eye checking than younger patients. In addition, the elderly T2DM patients are prone to cataract, which might reduce the detection of DR under nonmydriatic fundus photography. The elderly patients with DR had the likelihood of suffering from other severe chronic complications, such as cardiovascular disease, which made them pay less attention to the eye problem or less motivated to access community health service centers and hospitals for the eye problem. Moreover, DR have higher cardiovascular disease and all-cause mortality in older DR populations [28, 29], which might lead to survival bias. Therefore, the proportion of DR has decreased after 60 years of age in both men and women in our study.
Accumulating evidence indicates that gender appears to be a significant factor in DR [10, 13, 30]. However, it is still debatable which sex is more prone to DR. Some studies have indicated that female sex was an independent risk factor for the incidence and development of overall DR and proliferation DR (PDR) [11–13]. Some studies have shown that the presence and severity of DR are more strongly associated with male sex [10, 14–17]. Other studies suggested that there was no discernible sex difference in the prevalence of DR [2, 31]. This discrepancy may be due to the differences in study designs, patient characteristics (such as diabetes duration and comorbidity), and characteristics of populations sampled (such as race, region, and economic level), which influence DR [2, 10–17, 31]. Interestingly, our study found that sex differences only existed in the T2DM patients who had a longer diabetic duration (≥10 years), who were over 60 years old, or who were from an intermediate economic area. Taken together, these inconsistent results suggest that further investigation into the relationship between sex and DR is required.
The mechanisms by which female sex contributes to the prevalence of DR in T2DM patients are still unknown. There are some potential explanations. First, the outcome may be due to estrogen. A meta-analysis showed that the prevalence of DR in DM patients peaked between the ages of 60 and 69 [32], at which point females were postmenopausal. Our study found that only females over the age of 60 years had a higher prevalence of DR than males. This sex difference was not present in those who were under 60 years of age. These results suggest that estrogen protects the occurrence and development of DR. Indeed, 17β-estradiol (E2) was found to protect RGC-5 cells from high-glucose-induced damage via the mitochondrial pathway [33]. In addition, estrogen was found to be an important regulator of blood flow in the retina and plays a protective role by decreasing vascular resistance in large ocular vessels [34]. A previous study also indicated that hormone therapy was beneficial for ocular vascular disease in postmenopausal females [35]. This finding may be the reason why postmenopausal females with T2DM were more susceptible to DR. Furthermore, estrogen has a protective effect on the occurrence of T2DM and improves its treatment [36–38]. Previous studies showed that the prevalence of DR increased steeply with the duration of DM [32]. As a result, estrogen can benefit DR by preventing the occurrence of T2DM and delaying its progression. Our results were consistent with those of previous studies and further found that females with T2DM and long-term T2DM durations have an elevated risk of DR. Taken together, estrogen benefits DR in many ways. Further studies are required to determine the underlying mechanism by which estrogen influences DR. Second, a recent systematic review reported that lower social economic levels and old age were attributed to diabetic complications [39]. In the present study, sex differences in the prevalence of DR were observed, particularly for those who came from the relatively intermediate economic areas. As a result, the sex difference in our study may be due to the imbalanced distribution of risk factors caused by differences in the social economic levels. There are some socioeconomic factors related to DR prevalence by gender. Among socioeconomic factors, a lower education level was associated with higher DR risk [40]. There is a common health gradient by education. People with lower education levels have weak awareness of self-care and have the higher probability of poor health [41], which affects their health and may increase the risk of DR. Additionally, like those with lower education levels, people with low household income do not have enough healthcare, especially among women. Household income strongly affects the health of elderly women. A higher income to some extent indicates having a healthy lifestyle, more physical exercise, and better access to healthcare services [42]. For low socioeconomic status, women are prone to obesity compared with men, as there are differences in nutritional consumption and stress depending on the level of socioeconomic status, especially for older women, as the 2010-2012 China National Nutrition and Health Survey (CNNHS) showing that women aged 60 years and older had a higher overweight/obesity prevalence than men [43]. And a meta-analysis showed that obesity was a risk factor for DR [44]. Therefore, the gender difference in social-economic factors, such as education, household income, and self-care awareness, may partly lead to the gender difference in the DR risk. Notably, there was no sex difference in the DR patients whose ages were younger than 60 years, where estrogen could protect females from DR. This result suggests that there may be other factors that influence DR, which is worth of further study. Additional research may provide a new potential target for DR prevention and treatment.
There are some factors that limit the extent to which our results can be generalized. First, our sampling methods were not strictly stratified, resulting in insufficient representation. Second, a regional selection bias could not be excluded in this study. The subjects in this study were mainly from northern and southern China. For a more comprehensive understanding of the prevalence of DR in mainland China, nationwide, population-based research studies are needed. Another limitation is that the detailed clinical characteristics are relatively insufficient. As a result, we were not able to analyze more profoundly to find more potential influential factors. In addition, we did not adjust for the potential confounders completely. A large sample size and nationwide enrollment from 76 cities in 12 provinces are the strength of our study, which will add important information about DR in Chinese T2DM patients.
In summary, our study demonstrated that the prevalence of DR in T2DM was 30.1% in mainland China and that female sex was independently associated with the prevalence of DR, particularly for T2DM patients over 60 years of age, who had a diabetic duration of more than 10 years, or who lived in relatively intermediate economic regions. This group of T2DM patients should receive more concern in the clinic, and the underlying mechanism of the female as a risk factor for DR is worth of further study, which may provide a new potential target for DR prevention and treatment.
Acknowledgments
This study was funded by the Key Special Projects of Medical and Health Collaborative Innovation of Guangzhou City (201604020016), the National Key R&D Program of China (2017YFA0105803), the General Program of National Natural Science Foundation of China (81770826), the 5010 Clinical Research Projects of Sun Yat-sen University (2015015), the Key Area R&D Program of Guangdong Province (2019B020227003), and the Science and Technology Plan Projects of Guangdong Province (2016A050502010).
Contributor Information
Zhihui He, Email: 651173682@qq.com.
Yanming Chen, Email: chyanm@mail.sysu.edu.cn.
Data Availability
The data used to support the findings of this study are available from the corresponding author upon request.
Conflicts of Interest
None of the authors has any conflicts of interest to declare.
Authors' Contributions
Mei Li, Yina Wang, and Zifeng Liu contributed to the work equally.
Supplementary Materials
Supplemental Table 1: classification of diabetic retinopathy. Diabetic retinopathy domestic typing (1985). Fig S1: per capita gross regional product of 2018 in China. Data source: National Bureau of Statistics.
References
- 1.Cho N. H., Shaw J. E., Karuranga S., et al. IDF Diabetes Atlas: Global estimates of diabetes prevalence for 2017 and projections for 2045. Diabetes Research and Clinical Practice. 2018;138:271–281. doi: 10.1016/j.diabres.2018.02.023. [DOI] [PubMed] [Google Scholar]
- 2.Yau J. W., Rogers S. L., Kawasaki R., et al. Global prevalence and major risk factors of diabetic retinopathy. Diabetes Care. 2012;35(3):556–564. doi: 10.2337/dc11-1909. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Hu Y., Teng W., Liu L., et al. Prevalence and risk factors of diabetes and diabetic retinopathy in Liaoning province, China: a population-based cross-sectional study. PLoS One. 2015;10(3, article e0121477) doi: 10.1371/journal.pone.0121477. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Wang F. H., Liang Y. B., Zhang F., et al. Prevalence of diabetic retinopathy in rural China: the Handan Eye Study. Ophthalmology. 2009;116(3):461–467. doi: 10.1016/j.ophtha.2008.10.003. [DOI] [PubMed] [Google Scholar]
- 5.Solomon S. D., Chew E., Duh E. J., et al. Diabetic retinopathy: a position statement by the American Diabetes Association. Diabetes Care. 2017;40(3):412–418. doi: 10.2337/dc16-2641. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Ip M. S., Domalpally A., Sun J. K., Ehrlich J. S. Long-term effects of therapy with ranibizumab on diabetic retinopathy severity and baseline risk factors for worsening retinopathy. Ophthalmology. 2015;122(2):367–374. doi: 10.1016/j.ophtha.2014.08.048. [DOI] [PubMed] [Google Scholar]
- 7.Sivaprasad S., Crosby-Nwaobi R., Heng L. Z., Peto T., Michaelides M., Hykin P. Injection frequency and response to bevacizumab monotherapy for diabetic macular oedema (BOLT Report 5) The British Journal of Ophthalmology. 2013;97(9):1177–1180. doi: 10.1136/bjophthalmol-2013-303168. [DOI] [PubMed] [Google Scholar]
- 8.Pearson P. A., Comstock T. L., Ip M., et al. Fluocinolone acetonide intravitreal implant for diabetic macular edema: a 3-year multicenter, randomized, controlled clinical trial. Ophthalmology. 2011;118(8):1580–1587. doi: 10.1016/j.ophtha.2011.02.048. [DOI] [PubMed] [Google Scholar]
- 9.Wat N., Wong R. L., Wong I. Y. Associations between diabetic retinopathy and systemic risk factors. Hong Kong Medical Journal. 2016;22(6):589–599. doi: 10.12809/hkmj164869. [DOI] [PubMed] [Google Scholar]
- 10.Nittala M. G., Keane P. A., Zhang K., Sadda S. R. Risk factors for proliferative diabetic retinopathy in a Latino American population. Retina. 2014;34(8):1594–1599. doi: 10.1097/IAE.0000000000000117. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Awa W. L., Fach E., Krakow D., et al. Type 2 diabetes from pediatric to geriatric age: analysis of gender and obesity among 120 183 patients from the German/Austrian DPV database. European Journal of Endocrinology. 2012;167(2):245–254. doi: 10.1530/EJE-12-0143. [DOI] [PubMed] [Google Scholar]
- 12.Hayward L. M., Burden M. L., Burden A. C., et al. What is the prevalence of visual impairment in the general and diabetic populations: are there ethnic and gender differences? Diabetic Medicine. 2002;19(1):27–34. doi: 10.1046/j.0742-3071.2001.00603.x. [DOI] [PubMed] [Google Scholar]
- 13.Kajiwara A., Miyagawa H., Saruwatari J., et al. Gender differences in the incidence and progression of diabetic retinopathy among Japanese patients with type 2 diabetes mellitus: a clinic-based retrospective longitudinal study. Diabetes Research and Clinical Practice. 2014;103(3):e7–e10. doi: 10.1016/j.diabres.2013.12.043. [DOI] [PubMed] [Google Scholar]
- 14.Varma R., Macias G. L., Torres M., et al. Biologic risk factors associated with diabetic retinopathy: the Los Angeles Latino Eye Study. Ophthalmology. 2007;114(7):1332–1340. doi: 10.1016/j.ophtha.2006.10.023. [DOI] [PubMed] [Google Scholar]
- 15.Pradeepa R., Anitha B., Mohan V., Ganesan A., Rema M. Risk factors for diabetic retinopathy in a South Indian Type 2 diabetic population-the Chennai Urban Rural Epidemiology Study (CURES) Eye Study 4. Diabetic Medicine. 2008;25(5):536–542. doi: 10.1111/j.1464-5491.2008.02423.x. [DOI] [PubMed] [Google Scholar]
- 16.Zhang X., Saaddine J. B., Chou C. F., et al. Prevalence of diabetic retinopathy in the United States, 2005-2008. JAMA. 2010;304(6):649–656. doi: 10.1001/jama.2010.1111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Stratton I. M., Kohner E. M., Aldington S. J., et al. UKPDS 50: risk factors for incidence and progression of retinopathy in type II diabetes over 6 years from diagnosis. Diabetologia. 2001;44(2):156–163. doi: 10.1007/s001250051594. [DOI] [PubMed] [Google Scholar]
- 18.Alberti K. G., Zimmet P. Z. Definition, diagnosis and classification of diabetes mellitus and its complications. Part 1: diagnosis and classification of diabetes mellitus provisional report of a WHO consultation. Diabetic Medicine. 1998;15(7):539–553. doi: 10.1002/(SICI)1096-9136(199807)15:7<539::AID-DIA668>3.0.CO;2-S. [DOI] [PubMed] [Google Scholar]
- 19.Catapano A. L., Graham I., de Backer G., et al. 2016 ESC/EAS guidelines for the management of dyslipidaemias. Revista Española de Cardiología (English Edition) 2017;70(2):p. 115. doi: 10.1016/j.rec.2017.01.002. [DOI] [PubMed] [Google Scholar]
- 20.Wong T. Y., Klein R., Islam F. M. A., et al. Diabetic retinopathy in a multi-ethnic cohort in the United States. American Journal of Ophthalmology. 2006;141(3):446–455.e1. doi: 10.1016/j.ajo.2005.08.063. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Zheng Y., Lamoureux E. L., Lavanya R., et al. Prevalence and risk factors of diabetic retinopathy in migrant Indians in an urbanized society in Asia: the Singapore Indian eye study. Ophthalmology. 2012;119(10):2119–2124. doi: 10.1016/j.ophtha.2012.04.027. [DOI] [PubMed] [Google Scholar]
- 22.Wong T. Y., Cheung N., Tay W. T., et al. Prevalence and risk factors for diabetic retinopathy: the Singapore Malay Eye Study. Ophthalmology. 2008;115(11):1869–1875. doi: 10.1016/j.ophtha.2008.05.014. [DOI] [PubMed] [Google Scholar]
- 23.Zhang G., Chen H., Chen W., Zhang M. Prevalence and risk factors for diabetic retinopathy in China: a multi-hospital-based cross-sectional study. British Journal of Ophthalmology. 2017;101(12):1591–1595. doi: 10.1136/bjophthalmol-2017-310316. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Liu Y., Yang J., Tao L., et al. Risk factors of diabetic retinopathy and sight-threatening diabetic retinopathy: a cross-sectional study of 13 473 patients with type 2 diabetes mellitus in mainland China. BMJ Open. 2017;7(9, article e016280) doi: 10.1136/bmjopen-2017-016280. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Zhang S., Wang J., Song C., Zhu L., Yu Y. Lower prevalence of proliferative diabetic retinopathy in elderly onset patients with diabetes. Diabetes Research and Clinical Practice. 2017;125:47–52. doi: 10.1016/j.diabres.2016.09.009. [DOI] [PubMed] [Google Scholar]
- 26.Yeung R. O., Zhang Y., Luk A., et al. Metabolic profiles and treatment gaps in young-onset type 2 diabetes in Asia (the JADE programme): a cross-sectional study of a prospective cohort. he Lancet Diabetes & Endocrinology. 2014;2(12):935–943. doi: 10.1016/S2213-8587(14)70137-8. [DOI] [PubMed] [Google Scholar]
- 27.Zhang S., Woodward M., Li Q., et al. Impact of age, age at diagnosis and duration of diabetes on the risk of macrovascular and microvascular complications and death in type 2 diabetes. Diabetologia. 2014;57(12):2465–2474. doi: 10.1007/s00125-014-3369-7. [DOI] [PubMed] [Google Scholar]
- 28.Guo V. Y., Cao B., Wu X., Lee J. J. W., Zee B. C. Prospective association between diabetic retinopathy and cardiovascular disease-a systematic review and meta-analysis of cohort studies. Journal of Stroke and Cerebrovascular Diseases. 2016;25(7):1688–1695. doi: 10.1016/j.jstrokecerebrovasdis.2016.03.009. [DOI] [PubMed] [Google Scholar]
- 29.van Hecke M. V., Dekker J. M., Stehouwer C. D., et al. Diabetic retinopathy is associated with mortality and cardiovascular disease incidence: the EURODIAB prospective complications study. Diabetes Care. 2005;28(6):1383–1389. doi: 10.2337/diacare.28.6.1383. [DOI] [PubMed] [Google Scholar]
- 30.Ozawa G. Y., Bearse M. A., Jr., Adams A. J. Male-female differences in diabetic retinopathy? Current Eye Research. 2015;40(2):234–246. doi: 10.3109/02713683.2014.958500. [DOI] [PubMed] [Google Scholar]
- 31.West S. K., Munoz B., Klein R., et al. Risk factors for type ii diabetes and diabetic retinopathy in a mexican-american population: proyecto ver. American Journal of Ophthalmology. 2002;134(3):390–398. doi: 10.1016/s0002-9394(02)01595-7. [DOI] [PubMed] [Google Scholar]
- 32.Song P., Yu J., Chan K. Y., Theodoratou E., Rudan I. Prevalence, risk factors and burden of diabetic retinopathy in China: a systematic review and meta-analysis. Journal of Global Health. 2018;8(1) doi: 10.7189/jogh.08.010803. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Hao M., Li Y., Lin W., et al. Estrogen prevents high-glucose-induced damage of retinal ganglion cells via mitochondrial pathway. Graefe's Archive for Clinical and Experimental Ophthalmology. 2015;253(1):83–90. doi: 10.1007/s00417-014-2771-7. [DOI] [PubMed] [Google Scholar]
- 34.Schmidl D., Schmetterer L., Garhöfer G., Popa-Cherecheanu A. Gender differences in ocular blood flow. Current Eye Research. 2015;40(2):201–212. doi: 10.3109/02713683.2014.906625. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Na K. S., Jee D. H., Han K., Park Y. G., Kim M. S., Kim E. C. The ocular benefits of estrogen replacement therapy: a population-based study in postmenopausal Korean women. PLoS One. 2014;9(9, article e106473) doi: 10.1371/journal.pone.0106473. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Manson J. A. E., Rimm E. B., Colditz G. A., et al. A prospective study of postmenopausal estrogen therapy and subsequent incidence of non-insulin-dependent diabetes mellitus. Annals of Epidemiology. 1992;2(5):665–673. doi: 10.1016/1047-2797(92)90011-E. [DOI] [PubMed] [Google Scholar]
- 37.Andersson B., Mattsson L. Å., Hahn L., et al. Estrogen replacement therapy decreases hyperandrogenicity and improves glucose homeostasis and plasma lipids in postmenopausal women with noninsulin-dependent diabetes mellitus. The Journal of Clinical Endocrinology & Metabolism. 1997;82(2):638–643. doi: 10.1210/jcem.82.2.3746. [DOI] [PubMed] [Google Scholar]
- 38.Margolis K. L., Bonds D. E., Rodabough R. J., et al. Effect of oestrogen plus progestin on the incidence of diabetes in postmenopausal women: results from the Women's Health Initiative Hormone Trial. Diabetologia. 2004;47(7):1175–1187. doi: 10.1007/s00125-004-1448-x. [DOI] [PubMed] [Google Scholar]
- 39.Bekele B. B. The prevalence of macro and microvascular complications of DM among patients in Ethiopia 1990–2017: Systematic review. Diabetes and Metabolic Syndrome: Clinical Research and Reviews. 2019;13(1):672–677. doi: 10.1016/j.dsx.2018.11.046. [DOI] [PubMed] [Google Scholar]
- 40.Cui Y., Zhang M., Zhang L., et al. Prevalence and risk factors for diabetic retinopathy in a cross-sectional population-based study from rural southern China: Dongguan Eye Study. BMJ Open. 2019;9(9, article e023586) doi: 10.1136/bmjopen-2018-023586. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Gumà J., Solé-Auró A., Arpino B. Examining social determinants of health: the role of education, household arrangements and country groups by gender. BMC Public Health. 2019;19(1):p. 699. doi: 10.1186/s12889-019-7054-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Yang Y. T., Iqbal U., Ko H. L., et al. The relationship between accessibility of healthcare facilities and medical care utilization among the middle-aged and elderly population in Taiwan. International Journal for Quality in Health Care. 2015;27(3):222–231. doi: 10.1093/intqhc/mzv024. [DOI] [PubMed] [Google Scholar]
- 43.Wang J., Zhao L., Yu D., et al. Status of nutrition and associated factors among the Chinese aged 60 and above in 2010-2012 in China. Wei Sheng Yan Jiu. 2019;48(2):200–207. [PubMed] [Google Scholar]
- 44.Zhu W., Wu Y., Meng Y. F., Xing Q., Tao J. J., Lu J. Association of obesity and risk of diabetic retinopathy in diabetes patients: a meta-analysis of prospective cohort studies. Medicine. 2018;97(32, article e11807) doi: 10.1097/MD.0000000000011807. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Supplemental Table 1: classification of diabetic retinopathy. Diabetic retinopathy domestic typing (1985). Fig S1: per capita gross regional product of 2018 in China. Data source: National Bureau of Statistics.
Data Availability Statement
The data used to support the findings of this study are available from the corresponding author upon request.


