Abstract
Probiotic bacteria can protect from ovariectomy (ovx)-induced bone loss in mice. Akkermansia muciniphila is considered to have probiotic potential due to its beneficial effect on obesity and insulin resistance. The purpose of the present study was to determine if treatment with pasteurized Akkermansia muciniphila (pAkk) could prevent ovx-induced bone loss. Mice were treated with vehicle or pAkk for 4 wk, starting 3 days before ovx or sham surgery. Treatment with pAkk reduced fat mass accumulation confirming earlier findings. However, treatment with pAkk decreased trabecular and cortical bone mass in femur and vertebra of gonadal intact mice and did not protect from ovx-induced bone loss. Treatment with pAkk increased serum parathyroid hormone (PTH) levels and increased expression of the calcium transporter Trpv5 in kidney suggesting increased reabsorption of calcium in the kidneys. Serum amyloid A 3 (SAA3) can suppress bone formation and mediate the effects of PTH on bone resorption and bone loss in mice and treatment with pAkk increased serum levels of SAA3 and gene expression of Saa3 in colon. Moreover, regulatory T cells can be protective of bone and pAkk-treated mice had decreased number of regulatory T cells in mesenteric lymph nodes and bone marrow. In conclusion, treatment with pAkk protected from ovx-induced fat mass gain but not from bone loss and reduced bone mass in gonadal intact mice. Our findings with pAkk differ from some probiotics that have been shown to protect bone mass, demonstrating that not all prebiotic and probiotic factors have the same effect on bone.
Keywords: Akkermansia, bone mass, gut microbiota, osteoporosis, probiotic
INTRODUCTION
Treatment with probiotic bacteria and/or prebiotics can alter the composition of the gut microbiota or its metabolic activity and thereby confer a health benefit to its host (2). Several studies support that different probiotic bacteria can protect from bone loss due to sex steroid deficiency in rodents (1, 6, 20, 27, 31). In a clinical study, daily treatment with a mixture of three Lactobacillus strains during 1 yr protected against lumbar spine bone mineral density (BMD) decrease in postmenopausal women (14). Moreover, Lactobacillus reuteri was protective of decrease in volumetric BMD in distal tibia in older women (25). Lately, the bacterium Akkermansia muciniphila has received attention due to its beneficial effects on metabolic disorders and it is considered to have probiotic potential (11, 33).
In 2012, our group demonstrated that the gut microbiota is a regulator of bone mass in mice (39). We and others have shown that colonized mice have decreased bone mass associated with an altered immune status in bone compared with germ-free mice (20, 26, 28, 39). In contrast, other groups have demonstrated no effect on bone mass or an increased bone mass and growth associated with increased serum levels of IGF-I after colonization of germ-free mice (34, 37). Differences in genetic background and age of the mice, length of colonization, and diet could all be reasons for the conflicting results. In line with this, Yan et al. demonstrated that the short-term effect of the gut microbiota 1 mo after colonization of germ-free mice was a reduction of bone mass and increased bone resorption while long-term colonization increased overall body growth and serum IGF-I, resulting in enhanced longitudinal and radial bone growth (49). In a study by Li et al. (20), sex steroid deficiency increased intestinal permeability in conventionally raised mice but not in germ-free mice. Mice treated with probiotics were protected from increased intestinal permeability and bone loss, suggesting that part of the effect of sex steroid deficiency on bone mass is mediated by increased gut permeability (20). We recently introduced the term “osteomicrobiology” to describe the cross-disciplinary research field where the role of gut microbiota on bone health is studied (29).
A subset of T cells, regulatory T (Treg) cells, can suppress formation and activity of osteoclasts (15, 22, 50). Ovariectomy (ovx) decreases frequency of Treg cells in bone marrow, but this is reversed by probiotic treatment (6, 27). Different strains of the probiotic bacteria Lactobacillus, Bifidobacterium, and Streptococcus have been shown to differentiate Treg cells both in vitro (17, 30) and in vivo (6, 10, 17, 18, 27). In a recent study, Tyagi et al. showed that treatment of mice with Lactobacillus rhamnosus GG increased bone mass and the production of Treg cells in Peyer's patches, spleen, and bone marrow (43). L. rhamnosus GG treatment increased serum levels of butyrate that has previously been associated with a protective effect on bone, but interestingly Tyagi et al. showed that the effect of butyrate was dependent on Treg cells (21, 43).
Bone mass is maintained by an adequate calcium supply from diet and uptake from the gut. Improved calcium absorption has been demonstrated in both rats and humans after treatment with prebiotics (46, 47). Low serum concentrations of calcium increase serum parathyroid hormone (PTH), which in turn increases bone resorption and reabsorption of calcium in the kidneys to maintain serum calcium levels.
A. muciniphila is a major intestinal species that represents 1–5% of the microbial community in humans (4, 8, 9). Treatment with A. muciniphila was previously shown to protect from metabolic disorders, including fat-mass gain, metabolic endotoxemia, adipose tissue inflammation, and insulin resistance when mice were fed a high-fat diet (11). Interestingly, pasteurized A. muciniphila (pAkk) enhanced protection from metabolic disorders in mice fed high-fat diet (33). Mice treated with pAkk also had increased goblet cell number, suggesting increased mucus layer and improved gut barrier function (33). Moreover, pAkk was recently evaluated in a clinical trial of individuals with the metabolic syndrome. Treatment with pAkk improved insulin sensitivity, reduced insulinemia and plasma total cholesterol, and was demonstrated to be safe and well tolerated (7). The purpose of this study was to determine if treatment with pAkk can protect from ovx-induced bone loss in mice by protecting from increased intestinal permeability and thereby reducing inflammation.
MATERIALS AND METHODS
Ovx mouse model and treatment with pasteurized Akkermansia muciniphila.
Twelve-week-old female C57BL/6 mice from Janvier Laboratories (France) were housed in a standard animal facility under controlled temperature (22°C) and photoperiod (12-h light-dark cycle) and had free access to fresh water and pellet diet (Teklad diet 2016, Envigo). The ovx model is included in the FDA guidelines for preclinical and clinical evaluation for agents used for the treatment of postmenopausal osteoporosis (42). Mice were randomized into four groups (n = 8–12/group) and treatment with pAkk started 3 days before ovx or sham surgery. pAkk (2 × 108 colony-forming units/150 µl with 2.5% glycerol in PBS) or vehicle (PBS with 2.5% glycerol) were given by daily oral gavage for 4 wk. pAkk was produced as previously described (33). In brief, A. muciniphila was pasteurized for 30 min at 70°C, which is a relatively mild pasteurization that limits the denaturation of cellular components (32, 35). A surface protein on A. muciniphila was shown in an earlier study to mediate the beneficial effects of both live A. muciniphila and pAkk (33). The surface protein was stable for temperatures used for the pasteurization. At the end of the study, mice were anesthetized with Ketalar/Dexdomitor vet, bled from the axillary vein, and thereafter killed by cervical dislocation. Tissues for RNA preparation were snap frozen in liquid nitrogen. Bones were excised and fixed in 4% paraformaldehyde. All experimental procedures involving animals were approved by the regional animal ethics committee in Gothenburg, ethics no. 136-2016.
Dual-energy X-ray absorptiometry.
BMD of dissected femurs were analyzed using Faxitron UltraFocus dual-energy X-ray absorptiometry (DXA; Faxitron Bioptics, Tuscon, AZ,). Femurs were scanned using X-ray energy of 40 kV and 0.28 mA for 2.53 s with the spatial resolution 24 µm using ×2 geometric magnification. Images were analyzed using the software VISION DXA (Faxitron Bioptics, Tuscon, AZ).
High-resolution microcomputed tomography.
High-resolution microcomputed tomography (µCT) analyses were performed using Skyscan 1172 scanner (Bruker MicroCT, Aartselaar, Belgium) as previously described (24). Briefly, femur and vertebra (L5) were imaged with an X-ray tube voltage of 50 kV, a current of 200 µA, and a 0.5-mm aluminum filter. The scanning angular rotation was 180°, and the angular increment was 0.70°. The voxel size was 4.5 µm isotropically. NRecon (version 1.6.9) was used to perform the reconstruction after the scans. In femur, the trabecular bone proximal to the distal growth plate was selected for analyses within a conforming volume of interest (cortical bone excluded) commencing at a distance of 650 µm from the growth plate and extending a further longitudinal distance of 134 µm in the proximal direction. Cortical measurements were performed in the diaphyseal region of the femur starting at a distance of 5.2 mm from the growth plate and extending a further longitudinal distance of 134 μm in the proximal direction. In vertebra, the trabecular bone in the vertebral body caudal of the pedicles was selected for analysis within a conforming volume of interest (cortical bone excluded) commencing at a distance of 5 µm caudal of the lower end of the pedicles and extending a further longitudinal distance of 225 µm in the caudal direction.
Gene expression analyses.
RNA from kidney, jejunum, ileum, and colon were isolated using RNeasy Mini QIAcube kit (Qiagen). RNA from the middiaphyseal cortical bone (tibia) was extracted using TRIzol Reagent (Sigma) followed by RNeasy Mini QIAcube kit (Qiagen). Real-time PCR analyses were run using StepOnePlus Real-Time PCR systems (Applied Biosystems). Predesigned probes for Rankl (Tnfsf11, Mm00441908_m1), Opg (Tnfrsf11b, Mm00435452_m1), Trap (Acp5, Mm00475698_m1), Ctsk (Mm00484036_m1), Col1α1 (Mm00801666_g1), Trpv5 (Mm01166037_m1), Saa3 (Mm00441203_m1), Tnfα (Mm0043258_m1), IL17A (Mm00439619_m1), Ocln (Mm00500912_m1), Cldn2 (Mm00516703_s1), Cldn3 (Mm00515499_s1), and Cldn15 (Mm00517635_m1) were used from Applied Biosystems. The mRNA abundance of each gene was calculated using the ΔΔCt method, adjusted for expression of 18s ribosomal RNA (4310893E, Applied Biosystems), and presented as percentage of vehicle-treated sham-operated mice.
Serum and urine analyses.
Analyses were performed according to manufacturer’s directives for serum and urine calcium using QuantiChrom Calcium Assay Kit (Bioassays systems, Hayward, CA) and urine creatinine using Mouse Creatinine Kit (Crystal Chem, Downers Grove, IL). An EIA kit was used to measure serum 25-hydroxy vitamin D (Immunodiagnostic Systems, Herlev, Denmark) and serum collagen type I COOH-terminal telopeptides (Immunodiagnostics Systems, Herlev, Denmark). ELISA kits were used to measure serum osteocalcin using Mouse Osteocalcin Kit (Immutopics, San Clemente, CA), PTH using Mouse-I-PTH ELISA Kit (Elabscience, Houston, TX), and Mouse SAA-3 ELISA Kit (Millipore, Billerica, MA).
Flow cytometry.
Bone marrow cells from one femur and mesenteric lymph nodes cells were isolated and erythrocytes in bone marrow were lysed using 0.83% ammonium chloride. Cells were stained with eBioscience Fixable Viability Dye eFluor 780 according to the manufacturer’s protocol (Invitrogen, ThermoFisher Scientific). Cells were extracellularly stained with anti-CD3-BV510 (Clone 17A2, Nordic BioSite AB, Täby, Sweden), anti-CD4-FITC (Clone RM4-5, Nordic BioSite AB, Täby, Sweden), and anti-CD25-APC (Clone 3C7, BD, Franklin Lakes, NJ). Cells were fixed and permeabilized using the FoxP3 staining buffer kit (Invitrogen, Thermofisher Scientific) and intracellularly stained with anti-Foxp3-PE (Clone FJK-16s, ThermoFisher Scientific) according to manufacturer’s instruction. Treg cells were defined as CD4+CD25+Foxp3+, and results are expressed as frequency of live cells or number of cells. Samples were run on BD FACSVerse (BD, Franklin Lakes, NJ), and data were analyzed using FlowJo software (version 10.4.1).
Statistics.
GraphPad Prism (version 7.03) was used for all statistical analyses. Results are presented as means ± SE. Normal distribution was analyzed using the Shapiro-Wilk test. If the sample distribution did not pass the normality test, values were normalized by log transformation before analysis. The overall effect of treatment (Veh/pAkk) and surgical procedure (Sham/Ovx) and their interaction were calculated using two-way ANOVA followed by Tukey’s or Dunnett’s post hoc test to correct for multiple comparisons between all groups or all groups versus vehicle-treated sham mice. P ≤ 0.05 was considered significant.
RESULTS
Treatment with pasteurized Akkermansia muciniphila reduces fat mass gain.
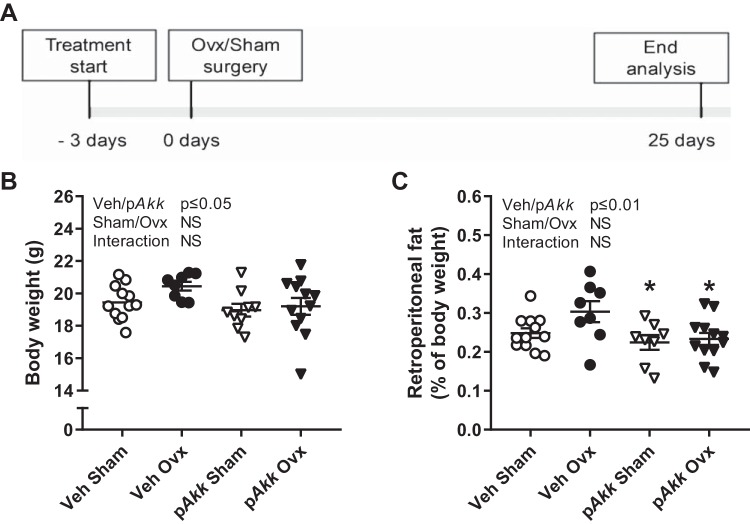
Twelve-week-old female mice were treated with either vehicle (veh) or pAkk by daily oral gavage during 28 days, starting 3 days before ovx or sham surgery (Fig. 1A). Ovx resulted in an expected decrease in uterus weight and increase in thymus weight that was similar in both treatment groups (Table 1). Earlier studies have shown that pAkk has beneficial metabolic effects and protects from fat mass accumulation. In the present study pAkk-treated mice had decreased body weight and retroperitoneal fat independent of ovx (Fig. 1, B and C). The percentage of retroperitoneal fat was reduced for both gonadal intact pAkk-treated mice (−36%) and pAkk-treated ovx-mice (−34%) compared with veh-treated ovx-mice indicating that pAkk protects from ovx-induced fat mass accumulation (Fig. 1C). Treatment with pAkk decreased femur growth indicating a reduced growth of the long bones (Table 2). In contrast, the vertebral height and relative muscle weight were normal in pAkk-treated mice (Tables 1 and 3).
Fig. 1.
Treatment with pasteurized Akkermansia muciniphila reduces fat mass. Outline of study design (A). Twelve-week-old female mice were treated with either vehicle (Veh) or pasteurized Akkermansia muciniphila (pAkk) by daily oral gavage during 28 days, starting 3 days before ovariectomy (Ovx) or sham surgery. At the end of the study, body weight (B) and percentage of retroperitoneal fat (C) were measured. Values are given as means ± SE (n = 8–12). The overall effect of treatment (Veh/pAkk) and surgical procedure (Sham/Ovx) and their interaction were calculated using two-way ANOVA followed by Tukey’s post hoc test to correct for multiple comparisons between all groups, *P ≤ 0.05 for pAkk-treated sham mice and pAkk-treated ovx-mice vs. veh-treated ovx-mice.
Table 1.
Organ weights
| Surgery |
Two-Way ANOVA (P Value) |
||||
|---|---|---|---|---|---|
| Treatment | Sham | Ovx | Sham/Ovx | Veh/pAkk | Interaction |
| Uterus, %BW | |||||
| Veh | 0.332 ± 0.037 | 0.062 ± 0.004 | P ≤ 0.01 | NS | NS |
| pAkk | 0.350 ± 0.070 | 0.054 ± 0.006 | |||
| Thymus, %BW | |||||
| Veh | 0.219 ± 0.006 | 0.284 ± 0.009 | P ≤ 0.01 | NS | NS |
| pAkk | 0.217 ± 0.016 | 0.261 ± 0.010 | |||
| Kidney, %BW | |||||
| Veh | 1.022 ± 0.018 | 1.016 ± 0.019 | P ≤ 0.05 | NS | P ≤ 0.05 |
| pAkk | 1.094 ± 0.034 | 1.003 ± 0.012 | |||
| Liver, %BW | |||||
| Veh | 4.085 ± 0.092 | 4.378 ± 0.062 | NS | NS | NS |
| pAkk | 4.285 ± 0.191 | 4.365 ± 0.070 | |||
| M. quadriceps, %BW | |||||
| Veh | 0.669 ± 0.031 | 0.639 ± 0.031 | NS | NS | NS |
| pAkk | 0.696 ± 0.034 | 0.631 ± 0.029 | |||
Twelve-week-old female mice were treated with either vehicle (Veh) or pasteurized Akkermansia muciniphila (pAkk) by daily oral gavage during 28 days, starting 3 days before ovariectomy (Ovx) or sham surgery. At the end of the study tissues were dissected and weighed. Values are given as means ± SE and expressed relative to body weight (BW) (n = 8–12). Two-way ANOVA to test the effect of treatment (Veh/pAkk) and surgical procedure (Sham/Ovx) and their interaction was used.
Table 2.
Bone parameters in femur
| Treatment | Surgery |
Two-Way ANOVA (P Value) |
|||
|---|---|---|---|---|---|
| Sham | Ovx | Sham/Ovx | Veh/pAkk | Interaction | |
| Tb N, 1/mm | |||||
| Veh | 3.26 ± 0.09 | 3.22 ± 0.15 | NS | NS | NS |
| pAkk | 3.07 ± 0.18 | 3.10 ± 0.10 | |||
| Tb Sp, mm | |||||
| Veh | 0.128 ± 0.001 | 0.126 ± 0.001 | NS | NS | NS |
| pAkk | 0.128 ± 0.002 | 128.2 ± 0.001 | |||
| Crt Thk, mm | |||||
| Veh | 0.197 ± 0.002 | 0.186 ± 0.003 | P ≤ 0.01 | NS | NS |
| pAkk | 0.192 ± 0.002 | 0.184 ± 0.002 | |||
| Femur length, mm | |||||
| Veh | 15.37 ± 0.15 | 15.61 ± 0.08 | NS | P ≤ 0.05 | NS |
| pAkk | 15.19 ± 0.07 | 15.27 ± 0.13 | |||
Twelve-week-old female mice were treated with either vehicle (Veh) or pasteurized Akkermansia muciniphila (pAkk) by daily oral gavage during 28 days, starting 3 days before ovariectomy (Ovx) or sham surgery. At the end of the experiment, dissected femurs were analyzed with high-resolution μCT. Trabecular number (Tb N), trabecular separation (Tb Sp), cortical thickness (Crt Thk), and femur length were analyzed. Values are given as means ± SE, (n = 8–12). Two-way ANOVA to test the effect of treatment (Veh/pAkk) and surgical procedure (Sham/Ovx) and their interaction was used.
Table 3.
Bone parameters in vertebra
| Surgery |
Two-Way ANOVA (P Value) |
||||
|---|---|---|---|---|---|
| Treatment | Sham | Ovx | Sham/Ovx | Veh/pAkk | Interaction |
| Tb N, 1/mm | |||||
| Veh | 5.07 ± 0.11 | 4.78 ± 0.12 | NS | NS | NS |
| pAkk | 5.15 ± 0.19 | 4.95 ± 0.11 | |||
| Tb Sp, mm | |||||
| Veh | 0.150 ± 0.002 | 0.154 ± 0.003 | NS | NS | NS |
| pAkk | 0.146 ± 0.004 | 0.152 ± 0.002 | |||
| Crt Thk, mm | |||||
| Veh | 0.065 ± 0.001 | 0.056 ± 0.001 | P ≤ 0.01 | NS | NS |
| pAkk | 0.060 ± 0.002 | 0.056 ± 0.001 | |||
| Vertebral height, mm | |||||
| Veh | 3.02 ± 0.02 | 3.06 ± 0.01 | NS | NS | NS |
| pAkk | 3.04 ± 0.02 | 3.06 ± 0.02 | |||
Twelve-week-old female mice were treated with either vehicle (Veh) or pasteurized Akkermansia muciniphila (pAkk) by daily oral gavage during 28 days, starting 3 days before ovariectomy (Ovx) or sham surgery. At the end of the experiment, dissected vertebras were analyzed with high-resolution microcomputed tomography (µCT). Trabecular number (Tb N), trabecular separation (Tb Sp), and cortical thickness (Crt Thk) were analyzed. Values are given as means ± SE (n = 8–12). Two-way ANOVA to test the effect of treatment (Veh/pAkk) and surgical procedure (Sham/Ovx) and their interaction was used.
Ovx significantly decreased the relative weights of the kidneys, and this effect was more pronounced in mice treated with pAkk, supported by a significant interaction factor (Table 1).
Treatment with pasteurized Akkermansia muciniphila reduces bone mass in gonadal intact mice and does not protect against bone loss after ovariectomy.
Several probiotic strains have positive effects on bone, and to determine the possible preventive effect of pAkk treatment on ovx-induced bone loss, we measured bone parameters in femur and vertebra by μCT at the end of the study. Dissected femurs were also analyzed by DXA.
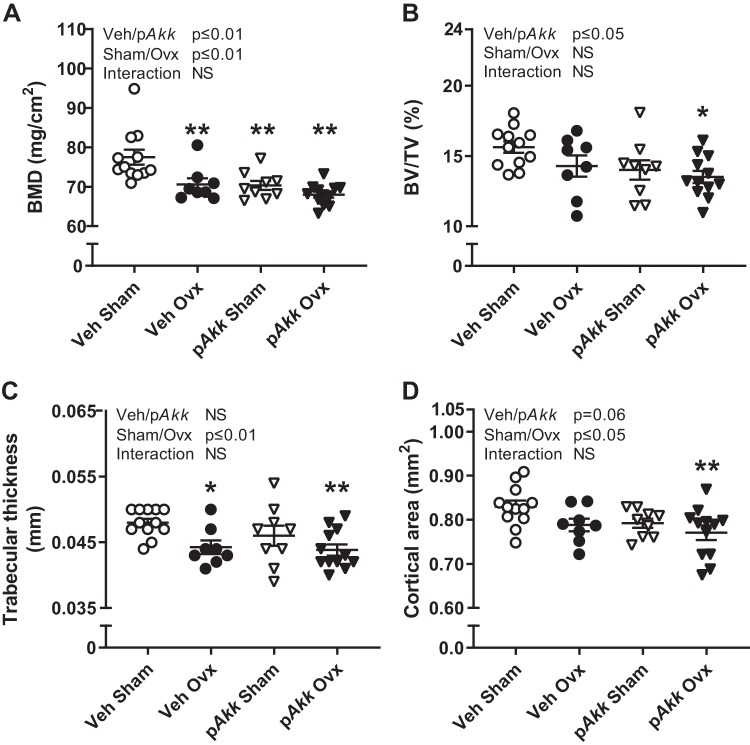
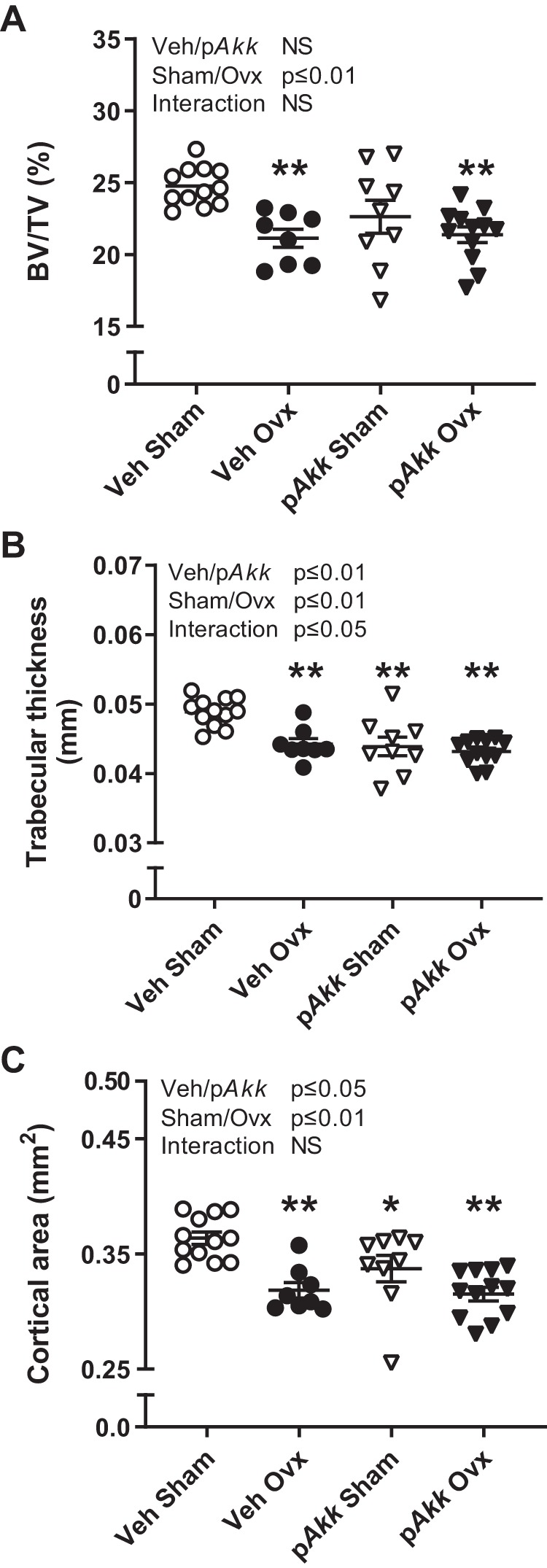
Treatment with pAkk did not protect from ovx-induced decrease in areal BMD (Fig. 2A), trabecular thickness (Fig. 2C), cortical area (Fig. 2D), or cortical thickness in femur (Table 2). On the contrary, treatment with pAkk decreased areal BMD (Fig. 2A) and the trabecular bone volume fraction (BV/TV, Fig. 2B). Moreover, there was a tendency (P = 0.06) for decreased cortical area when treating mice with pAkk (Fig. 2D). The gonadal intact mice treated with pAkk had significantly decreased areal BMD compared with veh-treated control mice (Fig. 2A). The other bone parameters in femur are shown in Table 2.
Fig. 2.
Treatment with pasteurized Akkermansia muciniphila reduces bone mass of gonadal intact mice and does not protect against bone loss in femur after ovariectomy (Ovx). Twelve-week-old female mice were treated with either vehicle (Veh) or pasteurized Akkermansia muciniphila (pAkk) by daily oral gavage during 28 days, starting 3 days before ovx or sham surgery. At the end of the experiment, dissected femurs were analyzed with dual-energy X-ray absorptiometry (DXA) to measure areal bone mineral density (BMD; A) and high-resolution microcomputed tomography (μCT) to measure trabecular bone volume fraction (BV/TV; B), trabecular thickness (C), and cortical area (D). Values are given as means ± SE (n = 8–12). The overall effect of treatment (Veh/pAkk) and surgical procedure (Sham/Ovx) and their interaction were calculated using two-way ANOVA followed by Dunnett’s post hoc test to correct for multiple comparisons between all groups vs. vehicle-treated sham mice, **P ≤ 0.01 and *P ≤ 0.05.
In line with the results in femur, treatment with pAkk did not protect from ovx-induced bone loss in the vertebra (Fig. 3, A–C, and Table 3). Furthermore, treatment with pAkk resulted in decreased trabecular thickness and cortical area in vertebra of gonadal intact mice compared with veh-treated control mice (Fig. 3, B and C).
Fig. 3.
Treatment with pasteurized Akkermansia muciniphila reduces bone mass in vertebra of gonadal intact mice and does not protect against bone loss after ovariectomy (Ovx). Twelve-week-old female mice were treated with either vehicle (Veh) or pasteurized Akkermansia muciniphila (pAkk) by daily oral gavage during 28 days, starting 3 days before ovx or sham surgery. At the end of the experiment, dissected vertebras were analyzed with high-resolution μCT to measure trabecular bone volume fraction (BV/TV; A), trabecular thickness (B), and cortical (C) area. The overall effect of treatment (Veh/pAkk) and surgical procedure (Sham/Ovx) and their interaction were calculated using two-way ANOVA followed by Dunnett’s post hoc test to correct for multiple comparisons between all groups vs. vehicle-treated sham mice, **P ≤ 0.01 and *P ≤ 0.05.
Treatment with pasteurized Akkermansia muciniphila increases serum levels of PTH and SAA3 and affects gene expression in cortical bone.
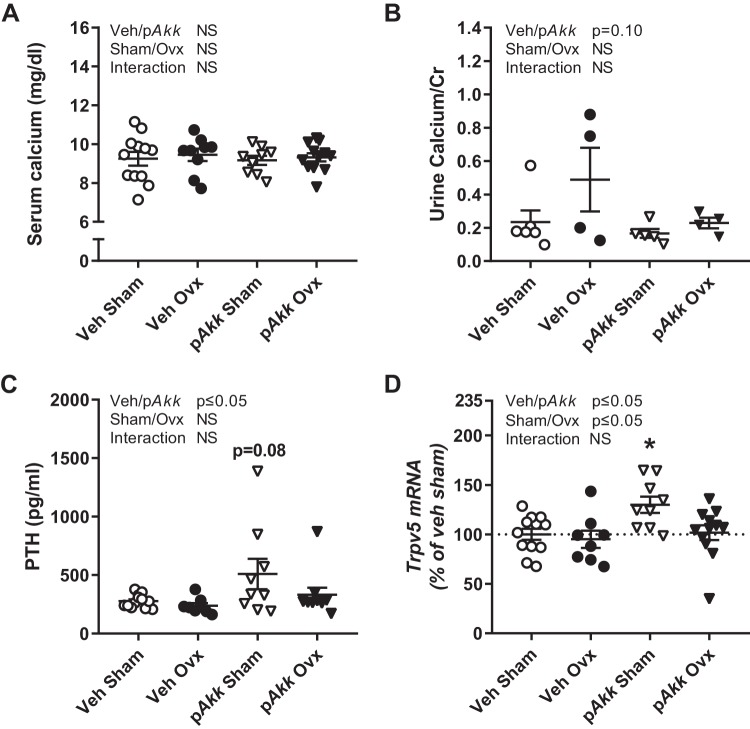
An adequate calcium supply from the diet and sufficient absorption in the gut is of importance to maintain bone mass. Neither ovx nor pAkk treatment affected serum calcium levels (Fig. 4A). There was a tendency for decreased calcium/creatinine ratio in urine in mice treated with pAkk (P = 0.10, Fig. 4B). This is in line with the markedly increased PTH levels in pAkk-treated mice (Fig. 4C). Moreover, pAkk-treated gonadal intact mice had a tendency (P = 0.08) for increased level of PTH compared with veh-treated gonadal intact mice (Fig. 4C). PTH mobilize calcium by increasing bone resorption and reabsorption of calcium in the kidneys. In line with increased serum PTH levels, the mRNA expression of Trpv5, a transporter for uptake of calcium in the kidneys, was increased for gonadal intact mice treated with pAkk (Fig. 4D). The nonactive form of vitamin D, 25 hydroxy vitamin D [25(OH)D3], in serum was not affected by pAkk treatment but increased with ovx (Table 4). Together these data indicate a decreased calcium absorption from the gut in mice treated with pAkk.
Fig. 4.
Treatment with pasteurized Akkermansia muciniphila increases serum levels of PTH. Twelve-week-old female mice treated with either vehicle (Veh) or pasteurized Akkermansia muciniphila (pAkk) by daily oral gavage during 28 days, starting 3 days before ovariectomy (Ovx) or sham surgery. Calcium metabolism was analyzed at the end of the experiment by measuring serum levels of calcium (n = 8–12; A), calcium-creatinine ratio in urine (n = 4–6; B), serum levels of parathyroid hormone (PTH; n = 8–12; C), and mRNA expression of transient receptor potential cation subfamily V member 5 (Trpv5; D) in kidney (n = 8–12). Statistical analysis of serum PTH was performed on log-transformed data. The overall effect of treatment (Veh/pAkk) and surgical procedure (Sham/Ovx) and their interaction were calculated using two-way ANOVA followed by Dunnett’s post hoc test to correct for multiple comparisons between all groups vs. vehicle-treated sham mice, *P ≤ 0.05.
Table 4.
Serum measurements
| Treatment | Surgery |
Two-Way ANOVA (P Value) |
|||
|---|---|---|---|---|---|
| Sham | Ovx | Sham/Ovx | Veh/pAkk | Interaction | |
| 25(OH)D3, ng/ml | |||||
| Veh | 41.7 ± 1.2 | 44.5 ± 1.3 | P ≤ 0.05 | NS | NS |
| pAkk | 38.1 ± 2.8 | 42.9 ± 1.0 | |||
| COOH-terminal telopeptides, ng/ml | |||||
| Veh | 27.2 ± 1.6 | 43.9 ± 8.0 | P ≤ 0.05 | NS | NS |
| pAkk | 31.1 ± 3.2 | 33.3 ± 2.0 | |||
| Osteocalcin, ng/ml | |||||
| Veh | 96.8 ± 3.8 | 116.3 ± 8.8 | P ≤ 0.05 | NS | NS |
| pAkk | 104.1 ± 8.6 | 119.4 ± 7.3 | |||
Twelve-week-old female mice were treated with either vehicle (Veh) or pasteurized Akkermansia muciniphila (pAkk) by daily oral gavage during 28 days, starting 3 days before ovariectomy (Ovx) or sham surgery. At the end of the experiment, serum was analyzed. Values are given as means ± SE (n = 8–12). Two-way ANOVA to test the effect of treatment (Veh/pAkk) and surgical procedure (Sham/Ovx) and their interaction was used.
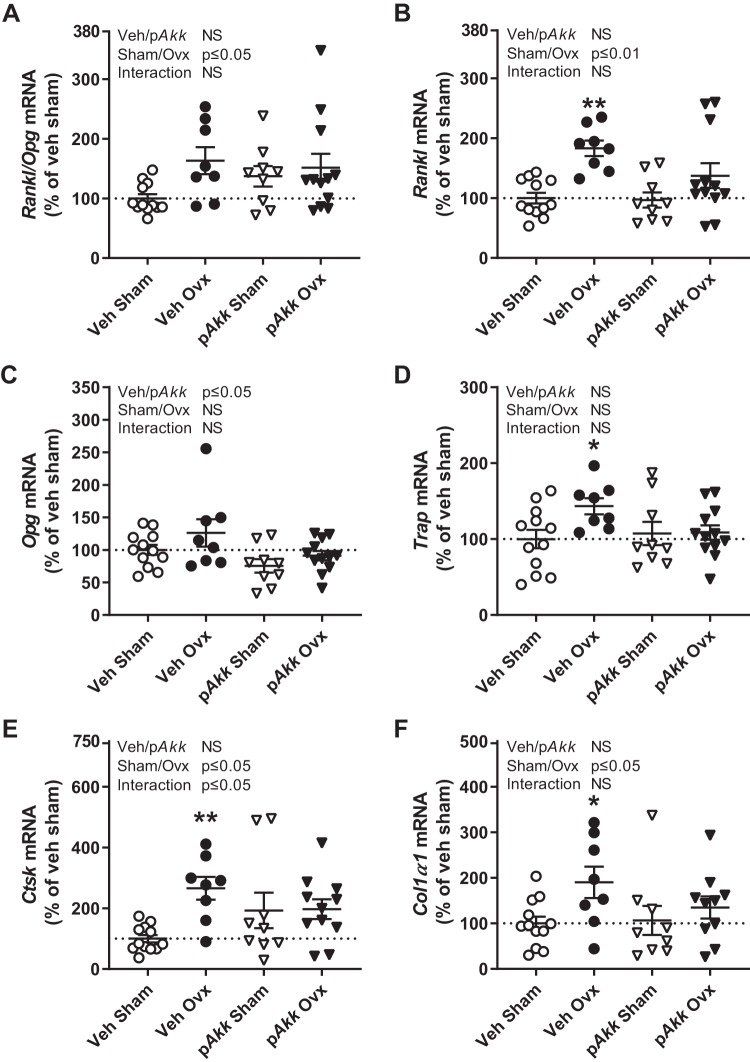
As expected ovx increased bone turnover, reflected both by serum levels of the resorption marker COOH-terminal telopeptides and the formation marker osteocalcin (Table 4). The increased bone turnover was reflected in mRNA expression in cortical bone (Fig. 5, A, B, E, and F). The Rankl/Opg ratio, a major determinant of osteoclastogenesis and bone resorption, was increased by ovx independent of treatment (Fig. 5A). Treatment with pAkk decreased the expression of Opg (Fig. 5C).
Fig. 5.
Treatment with pasteurized Akkermansia muciniphila reduces Opg expression in cortical bone. Twelve-week-old female mice were treated with either vehicle (Veh) or pasteurized Akkermansia muciniphila (pAkk) by daily oral gavage during 28 days, starting 3 days before ovariectomy (Ovx) or sham surgery. Real-time PCR analysis of gene expression known to affect bone turnover in cortical bone of tibia for ratio of receptor activator of nuclear factor-κB ligand (Rankl) and osteoprotegerin (Opg; A) and individual graphs for Rankl (B), Opg (C), tartrate-resistant acid phosphatase (Trap; D), cathepsin K (Ctsk; E), and collagen, type I, α1 (Col1α1; F). Values are given as means ± SE (n = 8–12). The overall effect of treatment (Veh/pAkk) and surgical procedure (Sham/Ovx) and their interaction were calculated using two-way ANOVA followed by Dunnett’s post hoc test to correct for multiple comparisons between all groups vs. vehicle-treated sham mice, **P ≤ 0.01 and *P ≤ 0.05.
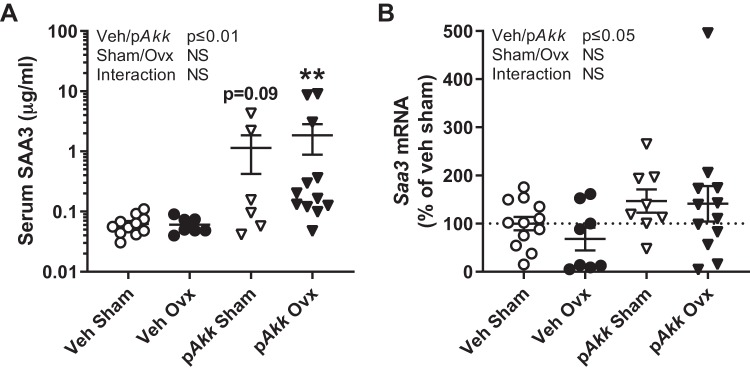
Serum amyloid A (SAA) is a family of apolipoproteins whose levels can rise 1,000-fold in the circulation during inflammation, injury, or infection (44). We originally measured SAA3 as a marker of inflammation to determine the inflammatory effects of ovx and treatment with pAkk. Mice treated with pAkk had a pronounced increase in serum SAA3 levels and increased Saa3 expression in colon indicating increased inflammation (Fig. 6, A and B). However, we observed no differences when treating with pAkk in expression of other inflammatory markers (Tnfα and Il17A) in the intestine (data not shown). Interestingly, SAA3 has been linked with gut microbiota and its production in the colon is associated with the reinforcement of the gut barrier by increasing mucus production signals (41). Accordingly, we found increased Saa3 expression in colon of mice treated with pAkk (Fig. 6B). However, SAA3 has also recently been identified as a potential mediator of the effects of PTH to suppress bone formation and increase bone resorption (3). In line with increased serum levels of PTH, we also found that mice treated with pAkk had increased serum SAA3 levels (Fig. 6A). Moreover, pAkk-treated gonadal intact mice had a tendency (P = 0.09) for an increased level of SAA3 and pAkk-treated ovx-mice had significantly increased levels of SAA3 compared with veh-treated sham mice (Fig. 6A).
Fig. 6.
Treatment with pasteurized Akkermansia muciniphila increases Serum amyloid A 3 (SAA3) levels in serum and Saa3 expression in colon. Twelve-week-old female mice were treated with either vehicle (Veh) or pasteurized Akkermansia muciniphila (pAkk) by daily oral gavage during 28 days, starting 3 days before ovariectomy (Ovx) or sham surgery. SAA3 was analyzed at the end of the experiment by measuring serum levels (A) of SAA3 (n = 6–12) and mRNA expression (B) of Saa3 in colon (n = 8–12). Values are given as means ± SE. Statistical analysis of serum SAA3 was performed on log-transformed data. The overall effect of treatment (Veh/pAkk) and surgical procedure (Sham/Ovx) and their interaction were calculated using two-way ANOVA followed by Dunnett’s post hoc test to correct for multiple comparisons between all groups vs. vehicle-treated sham mice, **P ≤ 0.01.
To evaluate the effect of pAkk treatment in the ovx model on gut permeability we analyzed the expression of tight junction proteins (Ocln, Cldn2, Cldn3, and Cldn15) in jejunum, ileum, and colon. There were no differences between the groups (data not shown).
Treatment with pasteurized Akkermansia muciniphila reduces the frequency of regulatory T cells in the bone marrow.
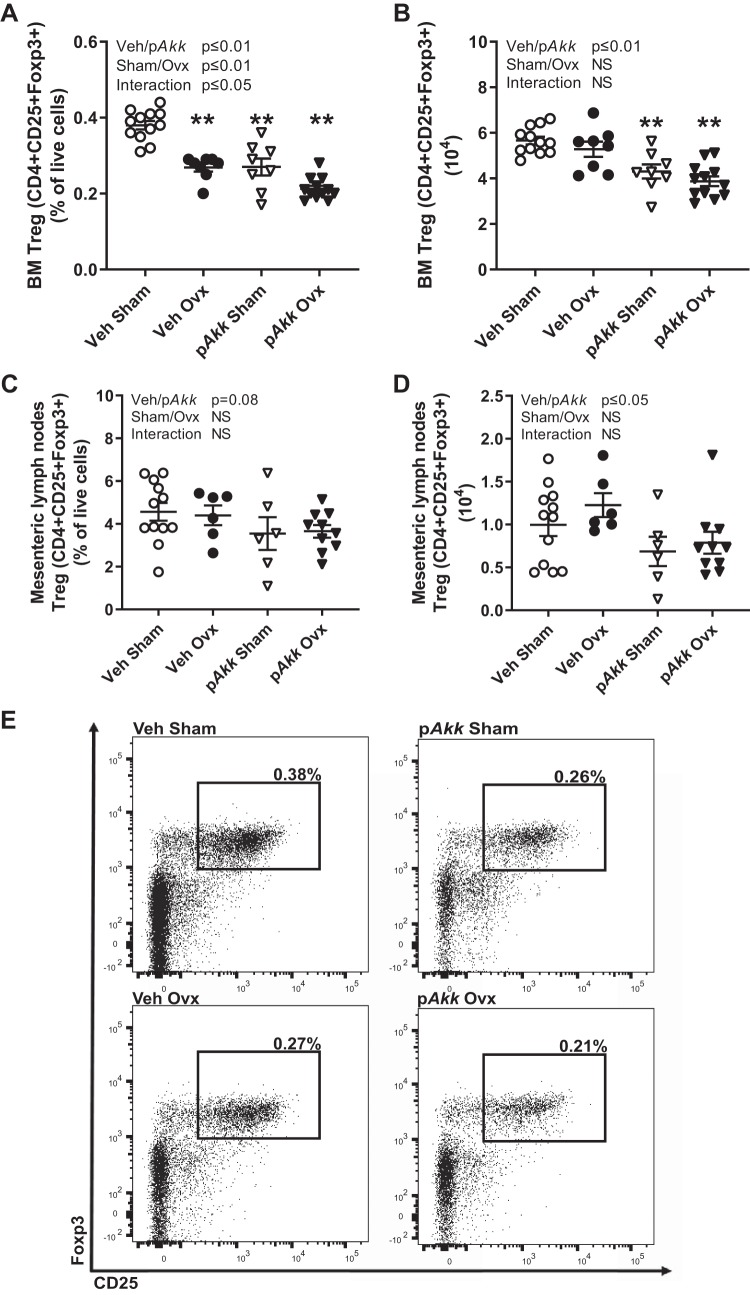
Some probiotic bacteria mediate their anti-inflammatory effects by inducing Treg cells, and the presence of Treg cells can ameliorate bone loss. Earlier studies suggest that Treg cells mediate the beneficial effect of probiotics on bone. Since treatment with pAkk caused reduced bone mass in gonadal intact mice and did not protect against bone loss after ovx, we were interested in the effect on Treg cells. Flow cytometry analysis of bone marrow showed that the frequency of Treg (CD4+CD25+Foxp3+) cells in bone marrow was decreased by ovx (Fig. 7, A and E). Interestingly, treatment with pAkk decreased the frequency of Treg cells in the bone marrow also in gonadal intact mice (Fig. 7, A and E). The decrease was similar in magnitude as the one observed in veh ovx-mice compared with veh sham mice. Since the number of bone marrow cells changes with ovx, we also analyzed the absolute number of Treg cells in bone marrow. Number of Treg cells decreased both in gonadal intact and ovx-mice treated with pAkk while there was no effect in veh ovx-mice compared with veh sham mice (Fig. 7B). To analyze a lymphoid organ closer to the gut where pAkk has its initial effects, we did flow cytometry analysis of cells from mesenteric lymph nodes. There was a tendency (P = 0.08) to a decreased frequency and a significantly decreased absolute number of Treg cells in mesenteric lymph nodes for mice treated with pAkk (Fig. 7, C and D).
Fig. 7.
Treatment with pasteurized Akkermansia muciniphila decreases the frequency and number of regulatory T cells in bone marrow and number of regulatory T cells in mesenteric lymph nodes. Twelve-week-old female mice treated with either vehicle (Veh) or pasteurized Akkermansia muciniphila (pAkk) by daily oral gavage during 28 days, starting 3 days before ovariectomy (Ovx) or sham surgery. At the end of the study bone marrow (BM) cells from dissected femur and cells from mesenteric lymph nodes were stained with antibodies recognizing CD4, Foxp3, and CD25. Values represent the frequency of regulatory T cells (Treg, CD4+CD25+Foxp3+) of gated live cells for BM (A) and mesenteric lymph nodes (C) and absolute number of Treg cells in BM (B) and mesenteric lymph nodes (D). Representative images of Treg populations in bone marrow from flow cytometry analyses (E). Values are given as means ± SE (BM: n = 8–12; mesenteric lymph nodes: n = 6–12). The overall effect of treatment (Veh/pAkk) and surgical procedure (Sham/Ovx) and their interaction were calculated using two-way ANOVA followed by Dunnett’s post hoc test to correct for multiple comparisons between all groups vs. vehicle-treated sham mice, **P ≤ 0.01.
DISCUSSION
In the present study, we show that treatment with pAkk decreased body weight and retroperitoneal fat mass accumulation. However, treatment with pAkk did not protect from ovx-induced bone loss and reduced bone mass in gonadal intact mice. The lower bone mass observed in pAkk-treated mice was associated with increased serum levels of PTH and increased expression of the calcium transporter Trpv5 in kidney and decreased Opg expression in cortical bone suggesting increased turnover of calcium. Moreover, the serum level of SAA3 and gene expression of Saa3 in colon was increased for pAkk-treated mice. In addition, the number of Treg cells, which are known to be protective of bone, was decreased in bone marrow and mesenteric lymph nodes after treatment with pAkk.
Mice treated with pAkk had reduced body weight and retroperitoneal fat mass independent of ovx. This is in line with earlier studies where mice fed a high-fat diet and treated with pAkk had reduced fat mass accumulation (33). Mice normally gains body weight after ovx, but this was not observed in the veh-treated ovx-mice compared with veh-treated sham mice. In a study by Li et al., the ovx-mice also lacked body weight gain when treating by biweekly gavage (20). We believe this could be due to stress of the oral gavage.
In contrast to our hypothesis, treatment with pAkk did not protect from ovx-induced bone loss but reduced bone mass in gonadal intact mice in both femur and vertebra. The effects of pAkk were observed both in trabecular and cortical bone. Moreover, mice treated with pAkk had decreased femur length suggesting that treatment with pAkk suppressed overall longitudinal bone growth. The femur continues to grow slowly after 3 mo of age until 6 mo of age in mice (12). We have previously shown that treatment with three different probiotic Lactobacillus plantarum and Lactobacillus paracasei strains protects from ovx-induced bone loss (27). Moreover, the probiotic Lactobacillus reuteri protected from ovx-induced bone loss and treatment with the probiotic L. rhamnosus GG in sex steroid-deficient mice also protected from bone loss (1, 20). To conclude, different types of bacteria can have completely opposite effects on bone mass. However, it is important to note that in these studies they used live and not pasteurized bacteria.
PTH levels in serum were increased in pAkk-treated mice, in line with the observed tendency for decreased calcium/creatinine levels in urine. Furthermore, the mRNA expression of the calcium transporter Trpv5 in kidney was increased for pAkk-treated mice, suggesting increased reabsorption of calcium in the kidneys. The expression of Trpv5 in the kidneys has previously been shown to be regulated by serum levels of PTH (45). Another mechanism for PTH to mobilize calcium is by promoting bone resorption via increased Rankl and decreased Opg expression (19, 23). The Rankl/Opg ratio is a major determinant of osteoclastogenesis, and the Rankl/Opg ratio was increased for ovx-mice, independent of treatment. Interestingly, treatment with pAkk decreased Opg expression in cortical bone. In contrast, treatment with L. plantarum and L. paracasei strains was found to increase expression of Opg in ovx-mice indicating a completely different mode of action on bone compared with pAkk (27). The decrease in Opg expression in pAkk-treated mice could be due to increased PTH levels and thereby increased resorption. The requirement to mobilize calcium in pAkk-treated mice might be a result of decreased calcium uptake in the intestine in response to pAkk. Saa3 expression in colon is associated with increased expression of Muc2, the key gene involved in mucus production (41). We found increased Saa3 expression in colon of mice treated with pAkk, suggesting increased mucus production. Earlier studies have demonstrated that live A. muciniphila increases the production of mucus and reinforces the gut barrier function (11, 33, 38). Furthermore, the pAkk-treated mice had a pronounced increase in serum SAA3 levels, independent of ovx. Recently, it was shown that SAA3 suppresses bone formation and was necessary for the relative increase in bone resorption and bone loss in response to PTH treatment in mice (3). Our results suggest that SAA3 might be the factor mediating the negative impact of pAkk on bone. SAA3 is also known to be a marker for inflammation, but we observed no differences when treating with pAkk in the expression of other inflammatory markers (Tnfα and IL17A).
Treatment with probiotic L. plantarum and L. paracasei strains in ovx-mice has previously shown to protect from decrease in frequency of Treg cells (6, 27). Treg cells suppress osteoclasts and are suggested to be protective of bone (22, 50). In bone marrow, ovx decreased the frequency of Treg cells in line with earlier publications (6, 27). In contrast to treatment with L. plantarum and L. paracasei strains, pAkk-treated mice had decreased frequency of Treg cells. The gonadal intact mice treated with pAkk had a similar decrease as the veh-treated ovx-mice. Furthermore, the number of Treg cells was also decreased for pAkk-treated mice in bone marrow. The decrease in Treg cells could explain the decrease in bone mass for the mice treated with pAkk. The mechanism of how Treg cells are recruited by probiotics is not fully understood, but it has been shown that different probiotic bacteria directly differentiate Treg cells in vitro (17, 30) and induce Tregs in vivo (10, 17, 18, 43). pAkk seems to counteract the differentiation of Treg cells. We also analyzed a lymphoid tissue close to the intestine, mesenteric lymph nodes. While ovx showed no effect, a significant decrease in number of Treg cells was observed in pAkk-treated mice indicating that pAkk also affects Treg-cell differentiation in tissues in close contact with the intestine. Oral administration of A. muciniphila has earlier been shown to increase the number of Treg cells (Foxp3+) in adipose tissue of obese mice and in pancreas of diabetic prone mice (13, 38). This may be secondary to the improved glucose tolerance and decreased fat mass accumulation in A. muciniphila-treated obese or diabetic prone mice leading to a healthier and less inflamed adipose tissue (48).
In a study by Li et al. (20), germ-free mice were protected from trabecular bone loss induced by sex steroid depletion. Conventionally raised sex steroid-deficient mice lost bone and had increased gut permeability, as well as upregulated osteoclastogenic cytokines in the small intestine and the bone marrow (20). In the same study, ovx-mice treated with the probiotic L. rhamnosus GG were protected from increased intestinal permeability and bone loss, which indicates that an improved gut barrier and thereby decreased antigen load passing through the intestinal barrier activating immune cells are mechanisms for the beneficial effect of probiotics on bone. Furthermore, in a study by Schepper et al., treatment with a mucus supplement prevented from increased gut permeability and bone loss caused by antibiotic treatment, indicating a mechanistic link between increased intestinal permeability and bone loss (36). pAkk-treated mice fed a high-fat diet were also protected from increased intestinal permeability (33). We therefore hypothesized that treatment of ovx-mice with pAkk would decrease gut permeability and protect from bone loss. We found no difference in the expression of tight junction proteins in the gut by ovx or pAkk treatment. This is in line with an earlier study that found that the expression of tight junction proteins in the gut following ovx varied in a spatial and temporal manner with no clear effect in either direction (5). A limitation of our study is that we have not measured gut permeability directly. However, opposite to our hypothesis, pAkk reduced bone mass in gonadal intact mice and did not prevent from ovx-induced bone loss.
It was previously shown that mice treated with live A. muciniphila did not change the composition of the gut microbiota (11). Moreover, neither live A. muciniphila nor pAkk treatment in humans altered the microbiota composition (7). Furthermore, a systematic review found that six out of seven studies where humans were treated with probiotics had no effect on the microbiota composition (16). Therefore, we did not analyze the composition of the microbiota in this study. Taken together, the effect of either the live bacteria or the pasteurized bacteria is likely due to alterations of metabolic activity of the microbiota and not a change in composition.
To conclude, treatment with pAkk reduces the body weight and retroperitoneal fat accumulation in mice. In contrast to our original hypothesis, pAkk did not protect from ovx-induced bone loss. pAkk reduced bone mass in the gonadal intact mice associated with increased serum levels of PTH and SAA3 and decreased frequency and number of Treg cells in bone marrow, suggesting increased bone resorption. This study does not support a protective role of pAkk for bone.
GRANTS
This study was supported by the Swedish Research Council Grant 2018-02589 and by grants from the Swedish government [under the Avtal om Läkarutbildning och Medicinsk Forskning (Agreement for Medical Education and Research) Grant 238261], Lundberg Foundation Grant 2017-0081, Torsten Söderberg Foundation Grant M65115, and Knut and Alice Wallenberg Foundation Grant KAW 2015.0317. The production of Akkermansia muciniphila cells was supported by the unrestricted Spinoza Award of the Netherlands Organization for Scientific Research (to W.M.d.V). P.D.C. is a senior research associate from the FRS-FNRS (Belgium), supported by WELBIO Grant WELBIO-CR-2017C-02) and Funds Baillet Latour (Grant for Medical Research 2015).
DISCLOSURES
H.P., P.D.C., and W.M.d.V. are inventors of patent applications dealing with the use of Akkermansia muciniphila and its components in the context of obesity and related disorders. H.P. is employed by A-Mansia Biotech SA that is commercializing A. muciniphila. P.D.C. and W.M.d.V. are co-founders of and have stocks in A-Mansia Biotech SA. C.O. and K.S. are listed as inventors on a patent application regarding the impact of probiotics on bone metabolism and have received research funding for probiotic-related research from Probi AB.
AUTHOR CONTRIBUTIONS
L.L., J.M.S., H.P., P.D.C., W.M.d.V., C.O., and K.S. conceived and designed research; L.L., J.M.S., K.L.G., P.H., K.H.N., H.C., and K.S. performed experiments; L.L., J.M.S., and K.S. analyzed data; L.L., J.M.S., C.O., and K.S. interpreted results of experiments; L.L. and K.S. prepared figures; L.L., C.O., and K.S. drafted manuscript; L.L., J.M.S., K.L.G., P.H., K.H.N., H.C., U.I., H.P., P.D.C., W.M.d.V., C.O., and K.S. edited and revised manuscript; L.L., J.M.S., K.L.G., P.H., K.H.N., H.C., U.I., H.P., P.D.C., W.M.d.V., C.O., and K.S. approved final version of manuscript.
ACKNOWLEDGMENTS
We thank Andreas Landin and late Anette Hansevi for excellent technical support.
REFERENCES
- 1.Britton RA, Irwin R, Quach D, Schaefer L, Zhang J, Lee T, Parameswaran N, McCabe LR. Probiotic L. reuteri treatment prevents bone loss in a menopausal ovariectomized mouse model. J Cell Physiol 229: 1822–1830, 2014. doi: 10.1002/jcp.24636. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Bron PA, van Baarlen P, Kleerebezem M. Emerging molecular insights into the interaction between probiotics and the host intestinal mucosa. Nat Rev Microbiol 10: 66–78, 2012. doi: 10.1038/nrmicro2690. [DOI] [PubMed] [Google Scholar]
- 3.Choudhary S, Santone E, Yee SP, Lorenzo J, Adams DJ, Goetjen A, McCarthy MB, Mazzocca AD, Pilbeam C. Continuous PTH in male mice causes bone loss because it induces serum amyloid A. Endocrinology 159: 2759–2776, 2018. doi: 10.1210/en.2018-00265. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Collado MC, Derrien M, Isolauri E, de Vos WM, Salminen S. Intestinal integrity and Akkermansia muciniphila, a mucin-degrading member of the intestinal microbiota present in infants, adults, and the elderly. Appl Environ Microbiol 73: 7767–7770, 2007. doi: 10.1128/AEM.01477-07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Collins FL, Rios-Arce ND, Atkinson S, Bierhalter H, Schoenherr D, Bazil JN, McCabe LR, Parameswaran N. Temporal and regional intestinal changes in permeability, tight junction, and cytokine gene expression following ovariectomy-induced estrogen deficiency. Physiol Rep 5: e13263, 2017. doi: 10.14814/phy2.13263. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Dar HY, Shukla P, Mishra PK, Anupam R, Mondal RK, Tomar GB, Sharma V, Srivastava RK. Lactobacillus acidophilus inhibits bone loss and increases bone heterogeneity in osteoporotic mice via modulating Treg-Th17 cell balance. Bone Rep 8: 46–56, 2018. doi: 10.1016/j.bonr.2018.02.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Depommier C, Everard A, Druart C, Plovier H, Van Hul M, Vieira-Silva S, Falony G, Raes J, Maiter D, Delzenne NM, de Barsy M, Loumaye A, Hermans MP, Thissen JP, de Vos WM, Cani PD. Supplementation with Akkermansia muciniphila in overweight and obese human volunteers: a proof-of-concept exploratory study. Nat Med 25: 1096–1103, 2019. doi: 10.1038/s41591-019-0495-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Derrien M, Collado MC, Ben-Amor K, Salminen S, de Vos WM. The mucin degrader Akkermansia muciniphila is an abundant resident of the human intestinal tract. Appl Environ Microbiol 74: 1646–1648, 2008. doi: 10.1128/AEM.01226-07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Derrien M, Vaughan EE, Plugge CM, de Vos WM. Akkermansia muciniphila gen. nov., sp. nov., a human intestinal mucin-degrading bacterium. Int J Syst Evol Microbiol 54: 1469–1476, 2004. doi: 10.1099/ijs.0.02873-0. [DOI] [PubMed] [Google Scholar]
- 10.Ekmekciu I, von Klitzing E, Fiebiger U, Neumann C, Bacher P, Scheffold A, Bereswill S, Heimesaat MM. The probiotic compound VSL#3 modulates mucosal, peripheral, and systemic immunity following murine broad-spectrum antibiotic treatment. Front Cell Infect Microbiol 7: 167, 2017. doi: 10.3389/fcimb.2017.00167. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Everard A, Belzer C, Geurts L, Ouwerkerk JP, Druart C, Bindels LB, Guiot Y, Derrien M, Muccioli GG, Delzenne NM, de Vos WM, Cani PD. Cross-talk between Akkermansia muciniphila and intestinal epithelium controls diet-induced obesity. Proc Natl Acad Sci USA 110: 9066–9071, 2013. doi: 10.1073/pnas.1219451110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Glatt V, Canalis E, Stadmeyer L, Bouxsein ML. Age-related changes in trabecular architecture differ in female and male C57BL/6J mice. J Bone Miner Res 22: 1197–1207, 2007. doi: 10.1359/jbmr.070507. [DOI] [PubMed] [Google Scholar]
- 13.Hänninen A, Toivonen R, Pöysti S, Belzer C, Plovier H, Ouwerkerk JP, Emani R, Cani PD, De Vos WM. Akkermansia muciniphila induces gut microbiota remodelling and controls islet autoimmunity in NOD mice. Gut 67: 1445–1453, 2018. doi: 10.1136/gutjnl-2017-314508. [DOI] [PubMed] [Google Scholar]
- 14.Jansson PA, Curiac D, Lazou Ahrén I, Hansson F, Martinsson Niskanen T, Sjögren K, Ohlsson C. Probiotic treatment using a mix of three Lactobacillus strains for lumbar spine bone loss in postmenopausal women: a randomised, double-blind, placebo-controlled, multicentre trial. Lancet Rheumatol 1: e154–e162, 2019. doi: 10.1016/S2665-9913(19)30068-2. [DOI] [PubMed] [Google Scholar]
- 15.Kelchtermans H, Geboes L, Mitera T, Huskens D, Leclercq G, Matthys P. Activated CD4+CD25+ regulatory T cells inhibit osteoclastogenesis and collagen-induced arthritis. Ann Rheum Dis 68: 744–750, 2009. doi: 10.1136/ard.2007.086066. [DOI] [PubMed] [Google Scholar]
- 16.Kristensen NB, Bryrup T, Allin KH, Nielsen T, Hansen TH, Pedersen O. Alterations in fecal microbiota composition by probiotic supplementation in healthy adults: a systematic review of randomized controlled trials. Genome Med 8: 52, 2016. doi: 10.1186/s13073-016-0300-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Kwon HK, Lee CG, So JS, Chae CS, Hwang JS, Sahoo A, Nam JH, Rhee JH, Hwang KC, Im SH. Generation of regulatory dendritic cells and CD4+Foxp3+ T cells by probiotics administration suppresses immune disorders. Proc Natl Acad Sci USA 107: 2159–2164, 2010. doi: 10.1073/pnas.0904055107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Lavasani S, Dzhambazov B, Nouri M, Fåk F, Buske S, Molin G, Thorlacius H, Alenfall J, Jeppsson B, Weström B. A novel probiotic mixture exerts a therapeutic effect on experimental autoimmune encephalomyelitis mediated by IL-10 producing regulatory T cells. PLoS One 5: e9009, 2010. doi: 10.1371/journal.pone.0009009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Lee SK, Lorenzo JA. Parathyroid hormone stimulates TRANCE and inhibits osteoprotegerin messenger ribonucleic acid expression in murine bone marrow cultures: correlation with osteoclast-like cell formation. Endocrinology 140: 3552–3561, 1999. doi: 10.1210/endo.140.8.6887. [DOI] [PubMed] [Google Scholar]
- 20.Li JY, Chassaing B, Tyagi AM, Vaccaro C, Luo T, Adams J, Darby TM, Weitzmann MN, Mulle JG, Gewirtz AT, Jones RM, Pacifici R. Sex steroid deficiency-associated bone loss is microbiota dependent and prevented by probiotics. J Clin Invest 126: 2049–2063, 2016. doi: 10.1172/JCI86062. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Lucas S, Omata Y, Hofmann J, Böttcher M, Iljazovic A, Sarter K, Albrecht O, Schulz O, Krishnacoumar B, Krönke G, Herrmann M, Mougiakakos D, Strowig T, Schett G, Zaiss MM. Short-chain fatty acids regulate systemic bone mass and protect from pathological bone loss. Nat Commun 9: 55, 2018. doi: 10.1038/s41467-017-02490-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Luo CY, Wang L, Sun C, Li DJ. Estrogen enhances the functions of CD4(+)CD25(+)Foxp3(+) regulatory T cells that suppress osteoclast differentiation and bone resorption in vitro. Cell Mol Immunol 8: 50–58, 2011. doi: 10.1038/cmi.2010.54. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Ma YL, Cain RL, Halladay DL, Yang X, Zeng Q, Miles RR, Chandrasekhar S, Martin TJ, Onyia JE. Catabolic effects of continuous human PTH (1–38) in vivo is associated with sustained stimulation of RANKL and inhibition of osteoprotegerin and gene-associated bone formation. Endocrinology 142: 4047–4054, 2001. doi: 10.1210/endo.142.9.8356. [DOI] [PubMed] [Google Scholar]
- 24.Movérare-Skrtic S, Henning P, Liu X, Nagano K, Saito H, Börjesson AE, Sjögren K, Windahl SH, Farman H, Kindlund B, Engdahl C, Koskela A, Zhang FP, Eriksson EE, Zaman F, Hammarstedt A, Isaksson H, Bally M, Kassem A, Lindholm C, Sandberg O, Aspenberg P, Sävendahl L, Feng JQ, Tuckermann J, Tuukkanen J, Poutanen M, Baron R, Lerner UH, Gori F, Ohlsson C. Osteoblast-derived WNT16 represses osteoclastogenesis and prevents cortical bone fragility fractures. Nat Med 20: 1279–1288, 2014. doi: 10.1038/nm.3654. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Nilsson AG, Sundh D, Bäckhed F, Lorentzon M. Lactobacillus reuteri reduces bone loss in older women with low bone mineral density: a randomized, placebo-controlled, double-blind, clinical trial. J Intern Med 284: 307–317, 2018. doi: 10.1111/joim.12805. [DOI] [PubMed] [Google Scholar]
- 26.Novince CM, Whittow CR, Aartun JD, Hathaway JD, Poulides N, Chavez MB, Steinkamp HM, Kirkwood KA, Huang E, Westwater C, Kirkwood KL. Commensal gut microbiota immunomodulatory actions in bone marrow and liver have catabolic effects on skeletal homeostasis in health. Sci Rep 7: 5747, 2017. doi: 10.1038/s41598-017-06126-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Ohlsson C, Engdahl C, Fåk F, Andersson A, Windahl SH, Farman HH, Movérare-Skrtic S, Islander U, Sjögren K. Probiotics protect mice from ovariectomy-induced cortical bone loss. PLoS One 9: e92368, 2014. doi: 10.1371/journal.pone.0092368. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Ohlsson C, Nigro G, Boneca IG, Bäckhed F, Sansonetti P, Sjögren K. Regulation of bone mass by the gut microbiota is dependent on NOD1 and NOD2 signaling. Cell Immunol 317: 55–58, 2017. doi: 10.1016/j.cellimm.2017.05.003. [DOI] [PubMed] [Google Scholar]
- 29.Ohlsson C, Sjögren K. Effects of the gut microbiota on bone mass. Trends Endocrinol Metab 26: 69–74, 2015. doi: 10.1016/j.tem.2014.11.004. [DOI] [PubMed] [Google Scholar]
- 30.Park JS, Choi JW, Jhun J, Kwon JY, Lee BI, Yang CW, Park SH, Cho ML. Lactobacillus acidophilus improves intestinal inflammation in an acute colitis mouse model by regulation of Th17 and Treg cell balance and fibrosis development. J Med Food 21: 215–224, 2018. doi: 10.1089/jmf.2017.3990. [DOI] [PubMed] [Google Scholar]
- 31.Parvaneh K, Ebrahimi M, Sabran MR, Karimi G, Hwei AN, Abdul-Majeed S, Ahmad Z, Ibrahim Z, Jamaluddin R. Probiotics (Bifidobacterium longum) increase bone mass density and upregulate Sparc and Bmp-2 genes in rats with bone loss resulting from ovariectomy. BioMed Res Int 2015: 897639, 2015. doi: 10.1155/2015/897639. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Peng GC, Hsu CH. The efficacy and safety of heat-killed Lactobacillus paracasei for treatment of perennial allergic rhinitis induced by house-dust mite. Pediatr Allergy Immunol 16: 433–438, 2005. doi: 10.1111/j.1399-3038.2005.00284.x. [DOI] [PubMed] [Google Scholar]
- 33.Plovier H, Everard A, Druart C, Depommier C, Van Hul M, Geurts L, Chilloux J, Ottman N, Duparc T, Lichtenstein L, Myridakis A, Delzenne NM, Klievink J, Bhattacharjee A, van der Ark KC, Aalvink S, Martinez LO, Dumas ME, Maiter D, Loumaye A, Hermans MP, Thissen JP, Belzer C, de Vos WM, Cani PD. A purified membrane protein from Akkermansia muciniphila or the pasteurized bacterium improves metabolism in obese and diabetic mice. Nat Med 23: 107–113, 2017. doi: 10.1038/nm.4236. [DOI] [PubMed] [Google Scholar]
- 34.Quach D, Collins F, Parameswaran N, McCabe L, Britton RA. Microbiota reconstitution does not cause bone loss in germ-free mice. MSphere 3: e00545-17, 2018. doi: 10.1128/mSphereDirect.00545-17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Sakai T, Taki T, Nakamoto A, Shuto E, Tsutsumi R, Toshimitsu T, Makino S, Ikegami S. Lactobacillus plantarum OLL2712 regulates glucose metabolism in C57BL/6 mice fed a high-fat diet. J Nutr Sci Vitaminol (Tokyo) 59: 144–147, 2013. doi: 10.3177/jnsv.59.144. [DOI] [PubMed] [Google Scholar]
- 36.Schepper JD, Collins FL, Rios-Arce ND, Raehtz S, Schaefer L, Gardinier JD, Britton RA, Parameswaran N, McCabe LR. Probiotic Lactobacillus reuteri prevents postantibiotic bone loss by reducing intestinal dysbiosis and preventing barrier disruption. J Bone Miner Res 34: 681–698, 2019. doi: 10.1002/jbmr.3635. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Schwarzer M, Makki K, Storelli G, Machuca-Gayet I, Srutkova D, Hermanova P, Martino ME, Balmand S, Hudcovic T, Heddi A, Rieusset J, Kozakova H, Vidal H, Leulier F. Lactobacillus plantarum strain maintains growth of infant mice during chronic undernutrition. Science 351: 854–857, 2016. doi: 10.1126/science.aad8588. [DOI] [PubMed] [Google Scholar]
- 38.Shin NR, Lee JC, Lee HY, Kim MS, Whon TW, Lee MS, Bae JW. An increase in the Akkermansia spp. population induced by metformin treatment improves glucose homeostasis in diet-induced obese mice. Gut 63: 727–735, 2014. doi: 10.1136/gutjnl-2012-303839. [DOI] [PubMed] [Google Scholar]
- 39.Sjögren K, Engdahl C, Henning P, Lerner UH, Tremaroli V, Lagerquist MK, Bäckhed F, Ohlsson C. The gut microbiota regulates bone mass in mice. J Bone Miner Res 27: 1357–1367, 2012. doi: 10.1002/jbmr.1588. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Tashiro M, Iwata A, Yamauchi M, Shimizu K, Okada A, Ishiguro N, Inoshima Y. The N-terminal region of serum amyloid A3 protein activates NF-κB and up-regulates MUC2 mucin mRNA expression in mouse colonic epithelial cells. PLoS One 12: e0181796, 2017. doi: 10.1371/journal.pone.0181796. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Thompson DD, Simmons HA, Pirie CM, Ke HZ. FDA guidelines and animal models for osteoporosis. Bone 17, Suppl: S125–S133, 1995. doi: 10.1016/8756-3282(95)00285-L. [DOI] [PubMed] [Google Scholar]
- 43.Tyagi AM, Yu M, Darby TM, Vaccaro C, Li JY, Owens JA, Hsu E, Adams J, Weitzmann MN, Jones RM, Pacifici R. The microbial metabolite butyrate stimulates bone formation via t regulatory cell-mediated regulation of WNT10B expression. Immunity 49: 1116–1131.e7, 2018. doi: 10.1016/j.immuni.2018.10.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Uhlar CM, Whitehead AS. Serum amyloid A, the major vertebrate acute-phase reactant. Eur J Biochem 265: 501–523, 1999. doi: 10.1046/j.1432-1327.1999.00657.x. [DOI] [PubMed] [Google Scholar]
- 45.van Abel M, Hoenderop JG, van der Kemp AW, Friedlaender MM, van Leeuwen JP, Bindels RJ. Coordinated control of renal Ca(2+) transport proteins by parathyroid hormone. Kidney Int 68: 1708–1721, 2005. doi: 10.1111/j.1523-1755.2005.00587.x. [DOI] [PubMed] [Google Scholar]
- 46.Weaver CM, Martin BR, Nakatsu CH, Armstrong AP, Clavijo A, McCabe LD, McCabe GP, Duignan S, Schoterman MH, van den Heuvel EG. Galactooligosaccharides improve mineral absorption and bone properties in growing rats through gut fermentation. J Agric Food Chem 59: 6501–6510, 2011. doi: 10.1021/jf2009777. [DOI] [PubMed] [Google Scholar]
- 47.Whisner CM, Martin BR, Schoterman MH, Nakatsu CH, McCabe LD, McCabe GP, Wastney ME, van den Heuvel EG, Weaver CM. Galacto-oligosaccharides increase calcium absorption and gut bifidobacteria in young girls: a double-blind cross-over trial. Br J Nutr 110: 1292–1303, 2013. doi: 10.1017/S000711451300055X. [DOI] [PubMed] [Google Scholar]
- 48.Xu H, Barnes GT, Yang Q, Tan G, Yang D, Chou CJ, Sole J, Nichols A, Ross JS, Tartaglia LA, Chen H. Chronic inflammation in fat plays a crucial role in the development of obesity-related insulin resistance. J Clin Invest 112: 1821–1830, 2003. doi: 10.1172/JCI200319451. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Yan J, Herzog JW, Tsang K, Brennan CA, Bower MA, Garrett WS, Sartor BR, Aliprantis AO, Charles JF. Gut microbiota induce IGF-1 and promote bone formation and growth. Proc Natl Acad Sci USA 113: E7554–E7563, 2016. doi: 10.1073/pnas.1607235113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Zaiss MM, Axmann R, Zwerina J, Polzer K, Gückel E, Skapenko A, Schulze-Koops H, Horwood N, Cope A, Schett G. Treg cells suppress osteoclast formation: a new link between the immune system and bone. Arthritis Rheum 56: 4104–4112, 2007. doi: 10.1002/art.23138. [DOI] [PubMed] [Google Scholar]