Abstract
Insulin pulsatility is important to hepatic response in regulating blood glucose. Growing evidence suggests that insulin-secreting pancreatic β-cells can adapt to chronic disruptions of pulsatility to rescue this physiologically important behavior. We determined the time scale for adaptation and examined potential ion channels underlying it. We induced the adaptation both by chronic application of the ATP-sensitive K+ [K(ATP)] channel blocker tolbutamide and by application of the depolarizing agent potassium chloride (KCl). Acute application of tolbutamide without pretreatment results in elevated Ca2+ as measured by fura-2AM and the loss of endogenous pulsatility. We show that after chronic exposure to tolbutamide (12–24 h), Ca2+ oscillations occur with subsequent acute tolbutamide application. The same experiment was conducted with potassium chloride (KCl) to directly depolarize the β-cells. Once again, following chronic exposure to the cell stimulator, the islets produced Ca2+ oscillations when subsequently exposed to tolbutamide. These experiments suggest that it is the chronic stimulation, and not tolbutamide desensitization, that is responsible for the adaptation that rescues oscillatory β-cell activity. This compensatory response also causes islet glucose sensitivity to shift rightward following chronic tolbutamide treatment. Mathematical modeling shows that a small increase in the number of K(ATP) channels in the membrane is one adaptation mechanism that is compatible with the data. To examine other compensatory mechanisms, pharmacological studies provide support that Kir2.1 and TEA-sensitive channels play some role. Overall, this investigation demonstrates β-cell adaptability to overstimulation, which is likely an important mechanism for maintaining glucose homeostasis in the face of chronic stimulation.
Keywords: β-cells, congenital hyperinsulinemia, intracellular calcium, islets, K(ATP)-channel, oscillations, potassium, persistent hyperinsulinemic hypoglycemia of infancy, PHHI, pulsatility, tolbutamide
INTRODUCTION
Tight regulation of blood glucose and the body’s energy demands requires precise insulin secretion that reflects the blood glucose concentration. This is achieved by secreting periodic large pulses of insulin from pancreatic β-cells when glucose is high following a meal and small pulses when glucose is low during sleep or during a period of fasting (46). The periodic insulin pulses produced at stimulatory glucose levels are due to bursting electrical activity; Ca2+ entry during each burst evokes insulin exocytosis (36). ATP-sensitive K+ [K(ATP)] channels largely dictate the pattern of activity in β-cells (43) and may contribute to packaging electrical impulses into bursts (32). These channels are regulated by glucose via metabolism (4) and thus provide a unique energy-sensing mechanism that serves as a key component in the insulin secretory pathway.
As important as K(ATP) channels are to insulin secretion, it is not surprising that defects in K(ATP) channels are associated with metabolic disorders. A mutation in the genes encoding the K(ATP) channel is responsible for approximately half of all cases of diabetes developing within the first six months of life (18, 41). Specifically, mutations in the KCNJ11 gene, which codes for the pore-forming subunit of the K(ATP) channel (Kir6.2), or mutations in the ABCC8 gene, which codes for the sulfonylurea receptor subunits, can result in neonatal diabetes (1). Another disorder, persistent hypoglycemic hyperinsulinemia (PHHI; also sometimes called congenital hyperinsulinism) is due to constitutive insulin secretion (8, 23). Again, a mutation in the K(ATP) channel is a common cause of the disorder (25). Even mutations with minor effects on K(ATP) channel function can result in diabetes in adulthood (3). These disorders thus highlight the importance of understanding how K(ATP) channels work in health and disease.
In light of these negative consequences of K(ATP) channel mutations in humans it is surprising that, in mouse islets, mutations that eliminate functional K(ATP) channels do not typically interfere with bursting electrical activity and the associated Ca2+ oscillations (14, 42, 48). This is unexpected, since pharmacological manipulations that acutely block K(ATP) channels convert the bursting to tonic spiking with an elevated Ca2+ level (Fig. 1 and Ref. 27). It is indicative of an adaptation mechanism that acts to restore oscillatory islet activity. Such activity results in the secretion of insulin pulses, and it has been demonstrated that the liver responds better to pulsatile insulin than to insulin maintained at a constant level (31). Bursting electrical activity, and accompanying Ca2+ oscillations, are therefore beneficial from a physiological perspective (22, 24, 46).
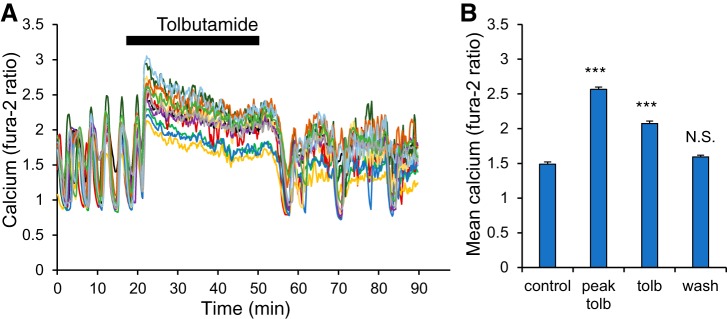
Fig. 1.
Acute tolbutamide application terminates Ca2+ oscillations, leading to a sustained elevated level. A: example of fura-2 traces of the intracellular Ca2+ level from n = 12 islets starting in 11 mM glucose (11G) for 20 min and then exposed to 250 µM tolbutamide for 30 min (plus 11G), followed by a washout back to 11G for 40 min. B: mean Ca2+ values (340/380 nm ratio) for 11G (control, first 20 min), peak value measured during tolbutamide treatment (peak tolb), the mean Ca2+ level during the last 20 min of tolbutamide exposure (tolb), and the last 20 min of washout (wash). ***P < 0.001 and not significant (N.S.) are compared with control (11G).
In this study, we examined how islets respond in vitro to persistent blockade of K(ATP) channel activity by tolbutamide for up to 24 h. In contrast to acute exposure to this β-cell stimulator, we demonstrate that islets chronically exposed to tolbutamide behave very differently during subsequent acute exposure than do those islets without the prior chronic exposure to the stimulator. With prior chronic tolbutamide treatment (12 to 24 h), many islets exhibit Ca2+ oscillations, rather than an elevated Ca2+ level upon subsequent acute tolbutamide exposure. Thus the β-cells adapt during the chronic tolbutamide exposure so as to rescue bursting, and this rescue becomes evident with 12 h of tolbutamide treatment.
A previous study showed that overnight exposure of islets to high glucose (11 mM), rather than low glucose (2.8 mM), caused a left shift in the glucose dose-response curve, and this was attributed to decreased trafficking of K(ATP) channels to the plasma membrane (17). We observed the opposite, a right-shifted glucose dose-response curve with overnight exposure to tolbutamide. Using mathematical modeling, we demonstrate that an increase in the K(ATP) current conductance can account for our data on chronic tolbutamide exposure. The model also predicts that this mechanism can be evoked by chronic exposure of the islets to KCl, which directly depolarizes the β-cells. This prediction was tested and, indeed, chronic exposure to KCl evoked the adaptation mechanism so that islets exhibited Ca2+ oscillations during subsequent acute exposure to tolbutamide. Once again, the islets adapted to chronic stimulation so as to facilitate oscillatory activity.
While mathematical modeling suggests that increased K(ATP) conductance can explain the adaptation data, it is likely that additional ion channels are affected by the chronic tolbutamide or KCl treatment. We tested the effects of several K+ channel blockers on islets with and without prior chronic tolbutamide treatment and found that the responses to the Kir2.1 channel antagonist ML133 and the general voltage-dependent K+-channel blocker tetraethylammonium (TEA) were different in treated versus untreated islets. This suggests that both Kir2.1- and TEA-sensitive currents are affected by the tolbutamide treatment and thus contribute to the adaptation.
Overall, our study reveals that islet β-cells, when chronically stimulated, adapt so as to restore oscillatory activity. Using tolbutamide as the stimulating agent, we found that this adaptation process first becomes evident with 12 h of exposure but is of greater magnitude with longer exposure times (exposures of up to 24 h were investigated). The evidence suggests that changes in K+ conductances are the ionic mechanisms for the adaptation. The findings demonstrate that β-cells have the adaptive capacity to restore oscillatory activity in the face of chronic stimulation, which would be valuable for the maintenance of glucose homeostasis under the challenge of chronic overstimulation.
MATERIALS AND METHODS
Mice.
Adult male CD1 mice at 8–20 wk of age were used for all studies. Mice were purchased from Envigo (Indianapolis, IN) and housed at Ohio University until needed for experiments. All protocols used in these studies were approved by the Ohio University Institutional Animal Care and Use Committee.
Islet isolation.
Pancreatic islets were isolated and cultured as described previously (11). Following isolation, islets were cultured overnight in RPMI-1640 media (Invitrogen) with 11mM glucose, 10% fetal bovine serum, and 1% penicillin/streptomycin. All drug treatments or supplements used for chronic conditions were made up in RPMI-1640, and all experiments were conducted within 1–2 days of islet isolation.
Measurements of intracellular calcium.
Islets were loaded with 1 µM fura-2AM in a mixture of two modified KRB solutions: low glucose solution: 3 mM glucose, 134.5 mM NaCl, 3 mM CaCl2, 5 mM KCl, 2 mM MgCl2, and 10 mM HEPES (pH 7.4); and high glucose solution: 28 mM glucose 122 mM NaCl, 3 mM CaCl2, 5 mM KCl, 2 mM MgCl2, and 10 mM HEPES (pH 7.4). These solutions were mixed in proportional volumes to produce various glucose concentrations as needed. Islets were loaded for 30 min at 37°C and 5% C02 in solutions identical to the starting solution for each recording and then transferred to the recording chamber for an additional 10 min. Intracellular calcium (Ca2+) was measured using the ratiometric Ca2+ indicator fura-2 AM as described previously in greater detail (16). The use of Cell Tracker Red to record control versus treated groups of islets simultaneously is described in Ref. 12.
Data analysis.
A Fisher’s exact test (2 × 2 contingency) was used to compare the percentages of oscillating versus nonoscillating islets either treated with a stimulator or untreated. For glucose dose-response curves, a two-point moving average was used to smooth inter-recording Ca2+ differences between the two separate three-glucose-step recordings used to form the aix-point curve. A two-tailed Student’s t test was used for all other comparisons unless stated otherwise.
Mathematical model.
A mathematical model was used to test whether a small compensating increase in K(ATP) channel conductance could account for the data on chronic tolbutamide exposure and to predict the compensating effects of chronic KCl exposure. The mathematical model is based on the Dual Oscillator Model described in Ref. 9. This includes a first module describing the cellular electrical activity, a second module for glycolysis, and a third one for mitochondrial metabolism. Since we have recently found evidence supporting a key role for Ca2+ activation of the enzyme pyruvate dehydrogenase (33, 34), we add this effect to the model. All equations and parameter values are described in the computer code that can be downloaded from http://www.math.fsu.edu/∼bertram/software/islet.
In the model, the current through K(ATP) channels is described by:
where gK(ATP) is the maximum conductance, V is the plasma membrane potential, VK is the K+ Nernst potential, and o∞ is the fraction of activated channels that depends on the ADP and ATP concentrations and is given by
Here, MgADP− = 0.165 ADP, ADP3− = 0.135 ADP, and ADP4− = 0.05 ADP, while the parameters kdd, ktt, and ktd represent the dissociation constants, which describe the binding equilibrium of the various nucleotide forms. In this paper, acute tolbutamide treatment is simulated by decreasing the constant ktt from 1 μM to 0.91 μM to mimic an increase in the ATP affinity caused by the addition of the drug, leading to channel closure. Chronic tolbutamide adaptation is simulated by increasing the maximum conductance gK(ATP) from 19,700 pS to 21,500 pS, reflecting either increased trafficking of K(ATP) channels to the membrane or increased gene expression of the channels (or a combination of both). Application of 30 mM KCl is simulated by increasing the K+ Nernst potential from VK = −75 mV to −72 mV.
RESULTS
Short-term exposure to tolbutamide terminates islet oscillations and is immediately reversible.
Islets were acutely treated with a 30-min exposure to tolbutamide, and islet activity was measured using fura-2AM fluorescence to measure intracellular Ca2+ ratiometrically (Fig. 1). The recording began in 11 mM glucose (abbreviated 11G) to establish a baseline for normal islet activity. In this condition, the 12 islets were all oscillating, as is typical for islets in 11G. Upon the addition of tolbutamide at 20 min, Ca2+ levels rose, and oscillations ceased. This is the result of K(ATP) channel blockage leading to membrane depolarization. After 30 min in the tolbutamide-treated condition, tolbutamide was washed out with 11G, Ca2+ levels dropped, and the islets resumed oscillatory activity in most cases. The effects on mean Ca2+ levels during each treatment phase are quantified in Fig. 1B. Both the peak response to tolbutamide and the mean response in the final 20 min of treatment were significantly elevated over control and washout (P < 0.001 for each). The mean Ca2+ level during the washout phase (calculated for the last 20 min) did not differ significantly from that of the 20-min control phase. Thus our data show that tolbutamide terminates Ca2+ oscillations and is reversible on the short term.
Ca2+ oscillations are restored following long-term exposure to tolbutamide.
While the acute effect of tolbutamide application on islet activity has been demonstrated previously (27), the chronic effect of the drug on islet oscillations has not been systematically studied. We approached this by incubating normal healthy islets in 11G plus tolbutamide (250 µM) for a range of durations (which we refer to as chronic tolbutamide treatment) and then testing the effects of subsequent 20 min reexposure to 11G plus tolbutamide (acute tolbutamide treatment). After 4 h of chronic tolbutamide treatment (Fig. 2A), islets exhibited a steady elevated Ca2+ level during acute tolbutamide treatment. There are no oscillations while tolbutamide is present, but oscillations soon recover in most islets upon washout with 11G alone, as in Fig. 1. In the 20-h tolbutamide treatment group (Fig. 2B), however, the islets oscillate despite the presence of tolbutamide and do not show the same overstimulation and heightened Ca2+ levels that were observed in the 4-h treatment group. This demonstrates an adaptation that occurred during the longer treatment.
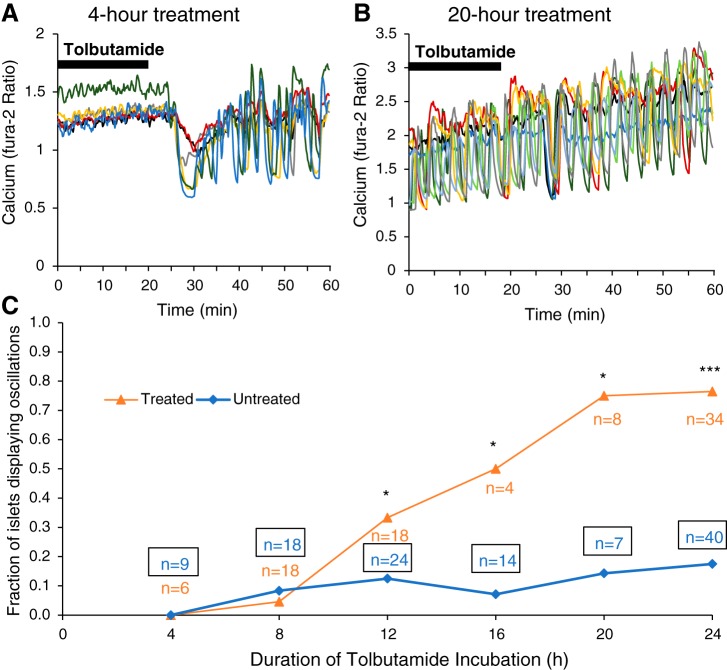
Fig. 2.
Islets show a duration-dependent response to tolbutamide that restores oscillations. A and B: multiple traces are shown for islets incubated in 250 μM tolbutamide and 11 mM glucose (11G) for 4 h (A; n = 6) or 20 h (B; n = 8). These islets were maintained in 11G plus tolbutamide for the first 20 min followed by a washout into 11G. C: the fraction of oscillating islets for multiple tolbutamide treatment durations of 4, 8, 12, 16, and 20 h (±1 h). Untreated islets were maintained in 11G media for the same durations and were recorded simultaneously with the tolbutamide-treated islets. A two-tailed chi-square test was used for statistics: *P < 0.05, ***P < 0.001.
To determine the adaptation time scale, a total of 200 islets were separated into groups with different incubation durations: approximately half had chronic tolbutamide treatment and the remaining islets were in a control group incubated with 11G alone. The fraction of islets exhibiting Ca2+ oscillations is plotted for each incubation duration in Fig. 2C. At 4 and 8 h of tolbutamide exposure, there is no difference between treated and untreated groups in the fraction of islets displaying oscillations in the presence of 250 µM tolbutamide plus 11G. At 12 h, a separation between treated and untreated groups starts to emerge, with 33% of tolbutamide-treated islets oscillating versus only 13% of untreated islets (P = 0.04). The difference between treated and untreated groups grows with the exposure time to chronic tolbutamide. By 20 h, the adaptation appears to reach its maximum with ~75% of chronic tolbutamide-treated islets oscillating compared with 18% of untreated controls.
Modeling shows that increased K(ATP) conductance may mediate adaptation to chronic tolbutamide treatment.
The adaptation to chronic tolbutamide exposure shown in Fig. 2 could, in principle, be due to increased K+ conductance or decreased Ca2+ conductance in the β-cell membrane. Such changes would decrease cell activity so that electrical bursting occurs in the presence of tolbutamide, rather than tonic spiking. However, the adaptation must also be such that in 11G the islet still bursts, as in Fig. 2B during washout. If adaptation increases K+ conductance too much, then the cell would be silent in the absence of tolbutamide (i.e., in 11G alone).
One possibility is that adaptation is achieved by increasing the total K(ATP) channel conductance in the cell membrane, either through increased gene expression of the K(ATP) channel subunits or through increased K(ATP) channel trafficking. To test whether increased K(ATP) channel conductance can both rescue bursting during the acute tolbutamide application and allow bursting to occur in the absence of tolbutamide, we used mathematical modeling. As described in materials and methods, a modification of the Dual Oscillator Model (DOM) was employed (9).
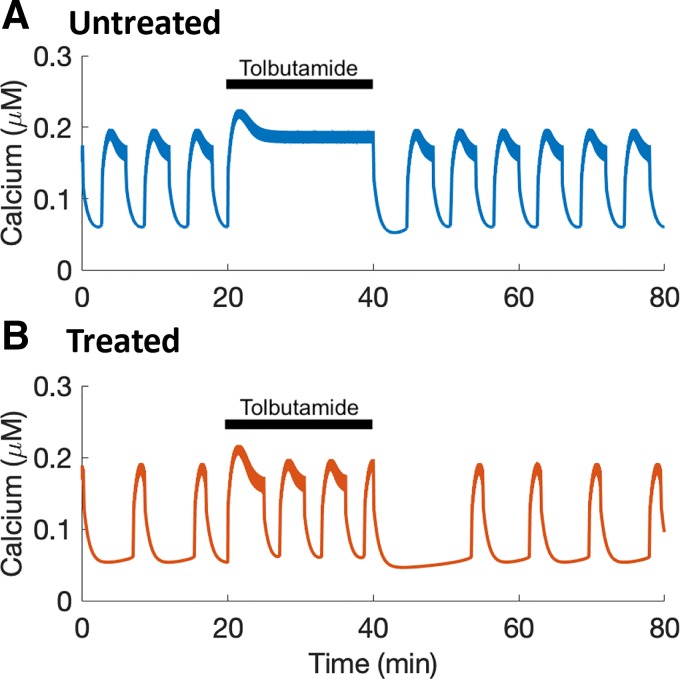
Figure 3A shows a model simulation of an acute application of tolbutamide to an “untreated islet.” In 11G alone the model islet exhibits bursting, and the accompanying Ca2+ oscillations are shown. When tolbutamide is added (simulated by decreasing the dissociation constant ktt), the model islet exhibits tonic spiking, and the Ca2+ concentration is pinned at an elevated level, as in Fig. 1A. When tolbutamide is removed (parameter ktt returned to its baseline value), the model islet returns to a bursting state. In the model “treated islet,” with increased K(ATP) channel conductance, the islet is again bursting before acute application of tolbutamide, and it continues to burst when the tolbutamide is applied (Fig. 3B). When tolbutamide is removed, the model islet again bursts. Therefore, increasing the total K(ATP) conductance is a viable adaptation mechanism that is compatible with the data from Fig. 2.
Fig. 3.
Mathematical modeling demonstrates the feasibility of increased K(ATP) conductance as an adaptation mechanism. A: acute treatment with tolbutamide converts a bursting islet, with accompanying Ca2+ oscillations, into tonic spiking, in which the Ca2+ concentration is pinned to an elevated level. B: in a model “treated islet” (an islet treated chronically with tolbutamide), Ca2+ oscillations persist during an acute tolbutamide application.
Modeling suggests an experiment that excludes the possibility of tolbutamide desensitization.
Another possible explanation for the data of Fig. 2 is that the islet becomes desensitized to tolbutamide with chronic exposure. Our hypothesis, in contrast, is that the sustained activity induced by chronic tolbutamide exposure leads to a compensatory increase in K(ATP) conductance. If so, then this should also be the case in response to chronic exposure of the islet to KCl, which directly depolarizes the islet by reducing the gradient of K+ across the plasma membrane. This treatment excludes the possibility of tolbutamide desensitization, since tolbutamide would only be applied acutely. However, if adaptation occurs so that bursting is produced both during acute tolbutamide application and with 11G alone, what behavior should one expect to see if the treated islet is acutely exposed to KCl?
To answer this question, we again employed the DOM. As before, the adaptation to chronic stimulation (here due to KCl) was simulated by increasing the K(ATP) channel conductance (to the same level as in Fig. 3). In both treated and untreated model islets, acute application of KCl (simulated by decreasing the K+ Nernst potential) converted bursting to tonic spiking, so that the Ca2+ concentration was pinned to an elevated level (Fig. 4). However, when the model islets were subsequently exposed to tolbutamide (and not KCl), the untreated islet still showed a plateau Ca2+ level, while the treated islet exhibited oscillations both during acute tolbutamide treatment and after washout in 11G. Thus the model illustrates that if stimulation through chronic KCl exposure induces the same compensatory effects as stimulation through chronic tolbutamide exposure, then the adaptation would be sufficient to rescue bursting during acute tolbutamide stimulation but insufficient to rescue bursting during KCl stimulation, which affects the current through all K+ channels.
Fig. 4.
Model simulation predicting how an islet exhibiting adaptation through an increase in K(ATP) conductance at the same level as that of Fig. 3 would respond to KCl, tolbutamide, and 11G alone. A: in an untreated islet, the Ca2+ level is at an elevated plateau (reflecting tonic spiking) when stimulated by either KCl or tolbutamide. B: in a treated islet, the Ca2+ level plateaus during stimulation with KCl but oscillates during acute tolbutamide stimulation.
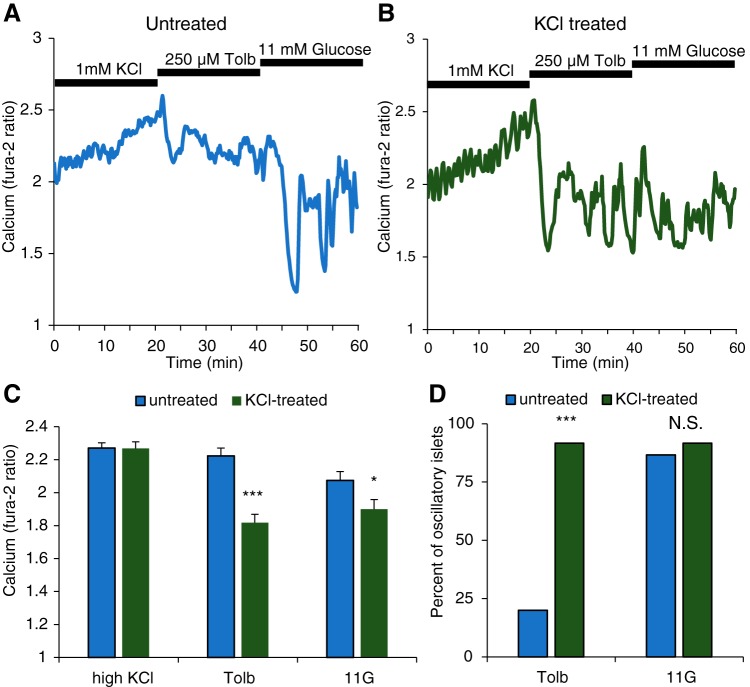
Chronic KCl exposure rescues Ca2+ oscillations during acute tolbutamide exposure.
We tested the model prediction of Fig. 4 by incubating a population of islets in 11G plus 30 mM KCl for 24 h. We then followed the stimulus protocol described in Fig. 4. In a representative example of an untreated islet (Fig. 5A), the Ca2+ concentration is elevated throughout the acute KCl exposure. When exposed to tolbutamide in 11G, the Ca2+ level remained elevated, and oscillations occurred only once the tolbutamide was removed. A representative islet that was pretreated for 24 h with KCl (Fig. 5B) produced similar Ca2+ responses during the acute KCl application. When switched to tolbutamide, however, the treated islet produced a substantial drop in Ca2+ that rebounded into oscillations throughout the acute tolbutamide treatment. The islet continued to show Ca2+ oscillations during the washout in 11G.
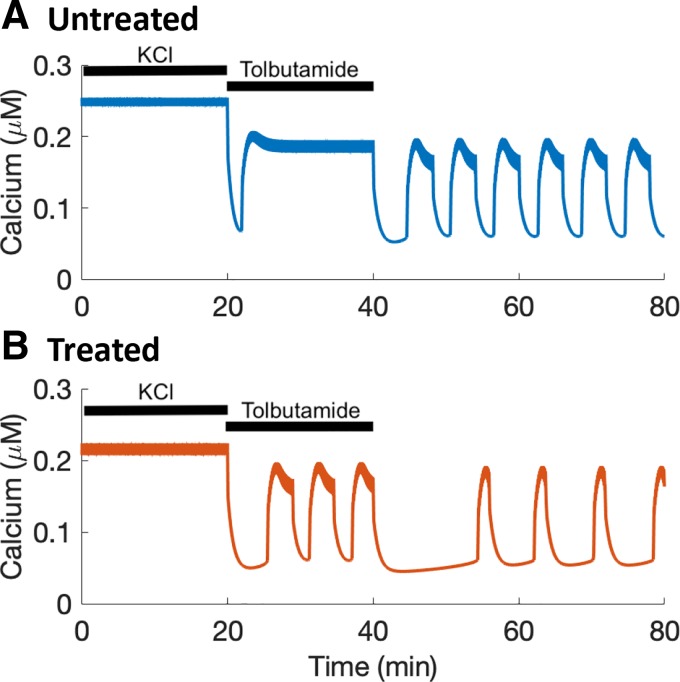
Fig. 5.
Experimental data support model predictions. In all cases, 11 mM glucose (11G) was present. A: representative Ca2+ trace for an untreated islet, exhibiting elevated Ca2+ plateaus when stimulated by either KCl or tolbutamide (Tolb) but oscillating in 11G alone. B: representative Ca2+ trace for an islet with 24-h treatment with 30 mM KCl. As predicted, the islet exhibits an elevated Ca2+ plateau when stimulated with KCl but oscillates when stimulated with tolbutamide or with 11G alone. C: quantification of mean Ca2+ levels for untreated and KCl-treated islets for each acute treatment phase. D: percentage of islets displaying oscillations during each treatment phase. A two-tailed t test was used for statistics in C and chi-square test in D: *P < 0.05, ***P < 0.001; N.S., not significant.
Responses to the stimulus protocol for the population of untreated (n = 15) and 24-h KCl-treated (n = 12) islets are quantified in Fig. 5, C and D. During acute KCl stimulation, the mean Ca2+ level was elevated in both treated and untreated islets (Fig. 5C) and there were no Ca2+ oscillations. With acute tolbutamide stimulation, however, the mean Ca2+ level was significantly higher in the untreated than in the treated islets. This difference was due to the activity patterns in the two populations. Fewer than 25% of the islets in the untreated population showed any evidence of oscillations in tolbutamide. In contrast, the vast majority of the treated islets exhibited oscillations during acute tolbutamide exposure (Fig. 5D). When switched to 11G alone, there was no significant difference in the percentage of islets exhibiting oscillations. Thus the experiments support the model prediction that chronic treatment with KCl could lead to a compensating increase in K(ATP) conductance that rescues oscillations when the treated islet is acutely stimulated by tolbutamide, but this is masked when the greater acute KCl stimulus is applied. Importantly, they also demonstrate that the rescue of Ca2+ oscillations during acute tolbutamide application is not due to tolbutamide desensitization.
Compensatory effects of chronic tolbutamide exposure reduces Ca2+ responses to glucose.
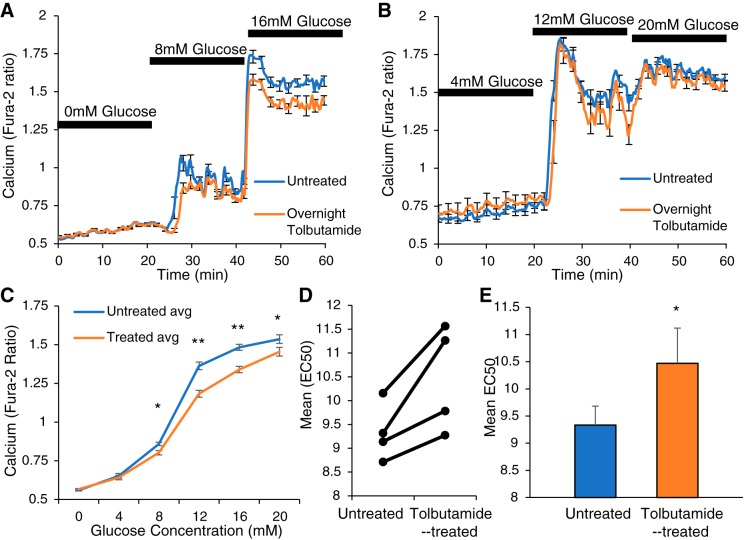
Since pancreatic islets must respond to a wide range of glucose concentrations, it is important to understand the effects that chronic tolbutamide treatment have on the response to glucose throughout this range. We examined Ca2+ responses to glucose stimulation at 4-mM intervals from 0 to 20 mM glucose following 24-h exposure to tolbutamide. The mean Ca2+ traces for both tolbutamide-treated (orange, averaged over n = 13 islets) and untreated (blue, averaged over n = 9 islets) islets are shown for 0, 8, and 16 mM glucose in Fig. 6A and for 4, 12, and 20 mM glucose in Fig. 6B (n = 12 untreated and n = 9 treated islets). The treated groups show substantially lower mean Ca2+ levels at glucose concentrations of 12 and 16 mM. Three additional pairs of glucose trials were conducted and combined to form the glucose dose curve shown in Fig. 6C. The most substantial difference between untreated and chronic tolbutamide-treated islets is again at 12 mM glucose and 16 mM glucose.
Fig. 6.
Islets incubated for 24 h in 250 μM tolbutamide plus 11 mM glucose (11G) display a glucose-dependent reduction in Ca2+ levels versus those incubated in 11G alone. A: averaged Ca2+ traces during 3 consecutive glucose exposures of 0, 8, and 16 mM for n = 13 untreated (blue) and n = 9 treated islets (orange). B: averaged Ca2+ traces during 3 consecutive glucose exposures of 4, 12, and 20 mM for n = 12 untreated (blue) and n = 9 treated islets (orange). Data in A and B represent 1 of 4 trials. C: glucose dose-response curves computed using mean Ca2+ levels over a range of glucose values for all 4 trials. Data were combined and two-tailed t tests were used at each glucose concentration: *P < 0.05, **P < 0.01. D: EC50s for 4 trials show a right shift in EC50 among tolbutamide-treated islets versus those incubated in 11G alone. E: mean of the EC50s from the 4 individual trials. *P < 0.05 determined by paired t test.
To further characterize this change in glucose sensitivity, we examined the effective concentration for half maximal stimulation (EC50) for each set of untreated and tolbutamide-treated islets. Figure 6D shows that there was a shift of 0.5–2 mM glucose in the EC50 between untreated to tolbutamide-treated islets across four separate trials. Figure 6E shows the mean EC50 over the population of untreated islets or tolbutamide-treated islets. Chronic tolbutamide treatment resulted in a statistically significant increase of mM in the EC50 for glucose; a right shift of the glucose dose-response curve.
Several K+ channel types may be involved in adaptation.
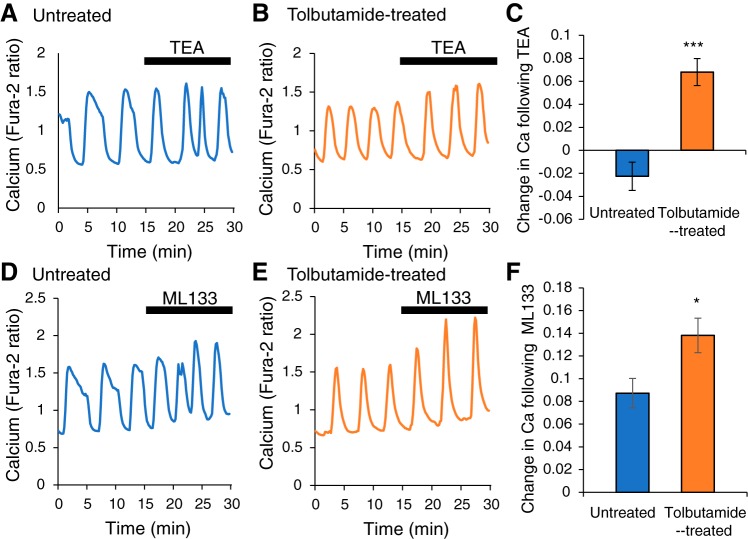
Modeling has provided evidence that adaptation to chronic stimulation could be through increased K(ATP) conductance in the β-cell membrane. However, changes in the conductance level of other K+ channel types could also be involved in the adaptation process. To determine if voltage-dependent K+ channels are involved, we employed the nonspecific voltage-dependent channel blocker tetraethylammonium (TEA). If the conductance level of a voltage-dependent K+ channel is increased as a result of chronic tolbutamide treatment, then the effect of blocking this channel should be greater in the treated islet than in the untreated islet. We tested this by evaluating the effect of TEA on Ca2+ oscillations in 11G.
In an untreated islet (Fig. 7A), TEA has minimal effect on the peak, baseline, or amplitude of the Ca2+ oscillations. In a tolbutamide-treated islet (Fig. 7B), TEA has a more substantial effect on oscillations, noticeably increasing their amplitude. TEA was applied to sets of untreated and sets of treated islets in two separate trials. The mean Ca2+ level was computed before and after TEA application for each set. The difference (after TEA – before TEA) is quantified in Fig. 7C. As in the traces of Fig. 7, A and B, the effect of TEA application is larger in treated versus the untreated islets, suggesting that voltage-sensitive K+ channels are involved in the adaptation to chronic tolbutamide exposure.
Fig. 7.
Voltage-dependent K+ channels and Kir2.1 channels participate in adaptation to chronic (~24 h) tolbutamide treatment. A: in an untreated islet, the voltage-dependent K+ channel blocker TEA does not affect the amplitude of Ca2+ oscillations. B: in a treated islet, TEA increases the oscillation amplitude. C: mean Ca2+ response to TEA between the untreated and treated islets (n = 24 for control; n = 21 for tolbutamide treated). D and E: in both untreated and treated islets, the Kir2.1 channel blocker ML133 increases the amplitude of Ca2+ oscillations. F: the mean Ca2+ response to ML133 is larger in treated islets compared with untreated islets (n = 18 for control and tolbutamide treated). A two-tailed t test was used for statistics: *P < 0.05. ***P < 0.001.
We also tested a blocker of the A-type K+ channel, 4-aminopyridine, but found no statistical difference between tolbutamide-treated and untreated islets in two separate trials. Tolbutamide-treated islets produced a change in calcium as measured by ratio of 340/380 nm signal following 4-AP treatment of 0.09 ± 0.02 (n = 19 islets), whereas untreated islets showed a change of 0.13 ± 0.03 (n = 23 islets, P = 0.21).
Next, we employed an antagonist for inward-rectifying Kir2.1 channels, which have been implicated in adaptation in islets with functional K(ATP) channels genetically knocked out (54). This channel blocker ML133 was applied to both untreated and treated islets, as in the TEA study. In both sets of islets, there was an increase in the Ca2+ oscillation amplitude when ML133 was applied, which is evident in the representative examples (Fig. 7, D and E) and in the quantification of the net change in Ca2+ (Fig. 7F). However, the effect was larger in the tolbutamide-treated islets than in the untreated islets (Fig. 7F). This suggests that increased Kir2.1 conductance could play a role in the adaptation to chronic tolbutamide exposure.
DISCUSSION
Hormone secretion is often pulsatile. For example, luteinizing hormone, follicle stimulating hormone, testosterone, glucagon, growth hormone, and cortisol levels all exhibit oscillations on some time scale. It is therefore of little surprise that insulin levels are also oscillatory in humans and many other species (37, 46). There is evidence that this pulsatility serves a physiological role (15, 29, 30, 39, 46), and is often lost in disease or during aging (26, 38, 40). It is perhaps more surprising that islet β-cells are so good at adapting to manipulations that interfere with the oscillatory activity that normally occurs at stimulatory glucose levels.
K(ATP) channels and the restoration of oscillatory Ca2+.
The ion channels responsible for glucose sensing, K(ATP) channels, are composed of four sulfonylurea-sensitive (SUR1) subunits and four pore-forming Kir6.2 subunits, and functional channels require both sets of subunits (35). In SUR1 homozygous knockout mice, islets typically exhibit bursting activity (14, 48), which result in Ca2+ oscillations (54). This is in spite of the depolarizing effect that removal of these channels should have, which would lead to tonic spiking in the absence of adaptation, as in the case of acute sulfonylurea application (19, 27). Similarly, Ca2+ oscillations have been reported from islets of Kir6.2 knockout mice (42).
Our study was performed to determine whether oscillations could be restored following chronic exposure to the stimulator tolbutamide, a drug used in the management of type 2 diabetes (28). Studies using clonal BRIN-BD11 cells found that 18-h tolbutamide exposure reduced the insulin release in response to secretagogues, including tolbutamide (5), and that chronic exposure reduced K(ATP)-channel-independent insulinotropic actions of sulfonylureas including tolbutamide (7). Another study showed that while overnight treatment with tolbutamide reduced the insulin content of mouse islets, it had a mild attenuation of the mean Ca2+ response to a glucose step from 3 mM to 15 mM, which did not reach significance (2). We observed a similar attenuation of the Ca2+ response to glucose stimulation in the 12- to 20-mM range following chronic tolbutamide exposure. Any Ca2+ oscillations in the study of Annello et al. (2) (if they occurred) were obscured by averaging over several islets. We found that oscillations during acute tolbutamide exposure were often recovered following 12 h or more of chronic tolbutamide exposure (Fig. 2). The extent of recovery was greater with longer exposure, saturating at 20-h exposure, where ~80% of the treated islets exhibited Ca2+ oscillations. When the acute tolbutamide challenge was removed, oscillations persisted (in 11G). Thus the mechanism that rescues oscillations in tolbutamide-stimulated islets also allows for oscillations in 11G alone.
While it is well established that tolbutamide is a potent blocker of K(ATP) channels (43), it has other indirect effects on β cells. For example, the elevated intracellular Ca2+ level that results from acute tolbutamide exposure regulates signaling through phospholipase C, which in turn affects insulin secretion (47). Tolbutamide has also been shown to affect Cl− channels. Whole cell patch clamp (50) and patch clamp from excised membrane patches (51) have shown that tolbutamide reduces the Cl− current through cystic fibrosis transmembrane conductance regulator (CFTR) channels, which are regulated by ATP. Another study showed that tolbutamide potentiates another Cl− channel, a volume-regulated ion channel (VRAC) in rat β cells (10). While such Cl− channel modulation may supplement the blockage of K(ATP) channels, the net effect is a conversion of bursting to tonic spiking with acute tolbutamide exposure and a return to bursting with chronic exposure. We believe that it is the resulting elevated Ca2+ and loss of Ca2+ oscillations that drive the adaptation during chronic tolbutamide exposure, rather than the specific channels that are affected by tolbutamide. Evidence for this is the adaptation that occurs with depolarization through chronic elevated KCl exposure (Fig. 5).
Tolbutamide desensitization does not appear to be a factor in restoring oscillations.
One possibility is that the chronically treated islets become desensitized to tolbutamide. To check this, we used KCl instead of tolbutamide to chronically stimulate islets, and only then applied tolbutamide acutely. Once again, we observed a preponderance of oscillatory activity in the treated islets during acute tolbutamide exposure, in contrast to the control islets incubated in 11G alone (Fig. 5). This supports the conclusion that it is the chronic stimulation, and not tolbutamide desensitization, that accounts for the rescue of oscillations during acute tolbutamide exposure. This conclusion is supported by a prior study that found that while chronic tolbutamide exposure greatly reduced insulin secretion during a later acute tolbutamide challenge, the membrane potential of single β-cells was still responsive to the acute tolbutamide, arguing that any desensitization occurs downstream of the cell’s electrical activity (44).
Multiple mechanisms to maintain pulsatility.
Our mathematical modeling suggests that the adaptation mechanism could be through increased K(ATP) channel conductance (Figs. 3 and 4). This increased conductance is the reason for a transient quiescence following acute tolbutamide exposure in the model simulations (Figs. 3 and 4). This transient quiescence is sometimes seen in experimental traces, although not always. For example, it is present in islets with 4-h tolbutamide treatment (Fig. 2A) but not in islets with 20-h treatment (Fig. 2B). This variability likely reflects the fact that the increased K(ATP) conductance caused by chronic stimulation would be quite small, since if the increase is too large the cell would fail to exhibit electrical activity once the tolbutamide is removed and only glucose remains. A prior study confirmed that the expression level of K(ATP) channel subunits is not adversely affected by chronic tolbutamide treatment in clonal insulin-secreting BRIN-BD11 cells (6), although the resolution of such measurements is not fine enough to pick up the small change in expression levels that would be predicted if in fact the adaptation involved increased K(ATP) channel subunit expression. Rather than increased channel expression, it is also possible that adaptation could be due to increased K(ATP) channel trafficking to the membrane.
There are other potential mechanisms of adaptation to restore pulsatility under chronic stimulation. Genetic manipulation of K(ATP)-channels to eliminate K(ATP)-channel conductance precludes the possibility of increased channel expression or increased numbers of these channels inserted in to the plasma membrane; nevertheless, islets are oscillatory in these models (14, 42, 48). In SUR1 knockout mice, it has been demonstrated that there is increased inward rectifying K+ current, and mathematical modeling was used to show that an inward rectifying Kir2.1 current would be capable of recovering bursting oscillations (54). The upregulation of such a current could be achieved through Ca2+-sensitive gene transcription (49, 52, 53). Consistent with this view, our findings with the Kir2.1-specific inhibitor ML133 suggest that Kir2.1 activity is also increased following ~24 h of chronic stimulation. An additional, as of yet unidentified, TEA-sensitive K+ channel may also play a role. We caution that there are limitations to using pharmacological approaches alone to identify potential mechanisms of adaptation. More definitive and empirical evidence is needed to indicate a role of specific ion channels, but these first-pass studies identify at least two possible candidates.
It is also possible that adaptation for chronic stimulation can be achieved by a mechanism other than gene transcription. It was shown that islets exposed overnight to stimulatory glucose levels have decreased K(ATP) conductance relative to those maintained in low glucose overnight, and that this is due to decreased trafficking of K(ATP) channels to the plasma membrane rather than decreased gene transcription (17).
Possible feedback effects of adaptation on metabolism.
A result of the adaptation is a change in the glucose dose-response curve, such that islets treated overnight with tolbutamide have a smaller response to glucose, as determined by the mean Ca2+ level, at glucose levels >8 mM (Fig. 6). Prior studies have shown that chronic exposure of islets to sulfonylureas result in a large reduction in the glucose-stimulated insulin release as well as release evoked by other stimulators (21, 45). This reduction is more dramatic than the reduction in the mean Ca2+ level shown in Fig. 6 and likely reflects additional negative effects of tolbutamide downstream of Ca2+ (7, 44). This reduced response is protective against overstimulation, and is the opposite of what was seen when islets were treated overnight with 11 mM glucose versus 2.8 mM glucose (17). With tolbutamide exposure, the direct influence is on the cell’s electrical activity and Ca2+ dynamics, and the elevated Ca2+ level associated with tolbutamide exposure likely leads to a reduction in the ATP/ADP ratio due to ATP hydrolysis by Ca2+ pumps in the plasma and endoplasmic reticulum membranes (13). Indeed, data show an increase in ATP/ADP when the islet is hyperpolarized and Ca2+ level reduced with application of the K(ATP) channel activator diazoxide (34). In contrast, incubating an islet in a solution with higher glucose content will lead to an increase in the ATP/ADP ratio due to increased metabolism. It might be expected that chronic treatment with tolbutamide has the opposite effect on the glucose dose-response curve than chronic treatment with a higher glucose level.
Conclusions.
We show that chronic stimulation of pancreatic islets triggers a compensatory hyperpolarizing mechanism to restore normal endogenous oscillatory activity in the presence of overstimulation. This study suggests that maintaining the ability to secrete insulin in a pulsatile manner appears to be very important to overall function as evidenced by the drive to maintain this function despite strong changes in physiological input. We identified several adaptive changes, involving K(ATP) channels and additional K+ channels, and a decrease in glucose sensitivity. The net result is rescue of pulsatility and its regulation by glucose. This rescue of pulsatility, which facilitates the actions of the liver in reducing glucose (31), is countered by the dramatic reduction in glucose-induced insulin secretion that has been demonstrated in previous studies (44, 45). The net effect is a time-dependent decline in the efficacy of treatment with antidiabetic agents (20).
GRANTS
I. Marinelli was supported by the Basque government through BERC 2018-2021 and the Spanish State Research Agency through BCAM Severo Ochoa Excellence Accreditation SEV-2017-0718 and AEI/FEDER-UE Project MULTIQUANT RT12018-093416-B-100. R. Bertram was supported by from National Science Foundation Grant DMS-1853342. C. Nunemaker was supported by National Institute of Diabetes and Digestive and Kidney Diseases Grant R15- DK-121247, the Osteopathic Heritage Foundation, and the Heritage College of Osteopathic Medicine.
DISCLOSURES
No conflicts of interest, financial or otherwise, are declared by the authors.
AUTHOR CONTRIBUTIONS
I.M., R.B., and C.S.N. conceived and designed research; N.C.L., I.M., K.L.C., and C.S. performed experiments; N.C.L., I.M., C.S., and C.S.N. analyzed data; I.M., R.B., and C.S.N. interpreted results of experiments; N.C.L., R.B., and C.S.N. prepared figures; N.C.L., R.B., and C.S.N. drafted manuscript; N.C.L., I.M., R.B., K.L.C., and C.S.N. edited and revised manuscript; N.C.L., R.B., K.L.C., C.S., and C.S.N. approved final version of manuscript.
REFERENCES
- 1.Aguilar-Bryan L, Bryan J. Neonatal diabetes mellitus. Endocr Rev 29: 265–291, 2008. doi: 10.1210/er.2007-0029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Anello M, Gilon P, Henquin JC. Alterations of insulin secretion from mouse islets treated with sulphonylureas: perturbations of Ca2+ regulation prevail over changes in insulin content. Br J Pharmacol 127: 1883–1891, 1999. doi: 10.1038/sj.bjp.0702731. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Ashcroft FM, Puljung MC, Vedovato N. Neonatal diabetes and the KATP channel: from mutation to therapy. Trends Endocrinol Metab 28: 377–387, 2017. doi: 10.1016/j.tem.2017.02.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Ashcroft FM, Rorsman P. K(ATP) channels and islet hormone secretion: new insights and controversies. Nat Rev Endocrinol 9: 660–669, 2013. doi: 10.1038/nrendo.2013.166. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Ball AJ, Flatt PR, McClenaghan NH. Alterations of insulin secretion following long-term manipulation of ATP-sensitive potassium channels by diazoxide and nateglinide. Biochem Pharmacol 69: 59–63, 2005. doi: 10.1016/j.bcp.2004.09.009. [DOI] [PubMed] [Google Scholar]
- 6.Ball AJ, McCluskey JT, Flatt PR, McClenaghan NH. Chronic exposure to tolbutamide and glibenclamide impairs insulin secretion but not transcription of K(ATP) channel components. Pharmacol Res 50: 41–46, 2004. doi: 10.1016/j.phrs.2003.12.001. [DOI] [PubMed] [Google Scholar]
- 7.Ball AJ, McCluskey JT, Flatt PR, McClenaghan NH. Drug-induced desensitization of insulinotropic actions of sulfonylureas. Biochem Biophys Res Commun 271: 234–239, 2000. doi: 10.1006/bbrc.2000.2609. [DOI] [PubMed] [Google Scholar]
- 8.Bennett K, James C, Hussain K. Pancreatic β-cell KATP channels: hypoglycaemia and hyperglycaemia. Rev Endocr Metab Disord 11: 157–163, 2010. doi: 10.1007/s11154-010-9144-2. [DOI] [PubMed] [Google Scholar]
- 9.Bertram R, Satin LS, Pedersen MG, Luciani DS, Sherman A. Interaction of glycolysis and mitochondrial respiration in metabolic oscillations of pancreatic islets. Biophys J 92: 1544–1555, 2007. doi: 10.1529/biophysj.106.097154. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Best L, Davies S, Brown PD. Tolbutamide potentiates the volume-regulated anion channel current in rat pancreatic beta cells. Diabetologia 47: 1990–1997, 2004. doi: 10.1007/s00125-004-1559-4. [DOI] [PubMed] [Google Scholar]
- 11.Carter JD, Dula SB, Corbin KL, Wu R, Nunemaker CS. A practical guide to rodent islet isolation and assessment. Biol Proced Online 11: 3–31, 2009. doi: 10.1007/s12575-009-9021-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Corbin KL, Hall TE, Haile R, Nunemaker CS. A novel fluorescence imaging approach for comparative measurements of pancreatic islet function in vitro. Islets 3: 14–20, 2011. doi: 10.4161/isl.3.1.14133. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Detimary P, Gilon P, Henquin JC. Interplay between cytoplasmic Ca2+ and the ATP/ADP ratio: a feedback control mechanism in mouse pancreatic islets. Biochem J 333: 269–274, 1998. doi: 10.1042/bj3330269. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Düfer M, Haspel D, Krippeit-Drews P, Aguilar-Bryan L, Bryan J, Drews G. Oscillations of membrane potential and cytosolic Ca(2+) concentration in SUR1(−/−) beta cells. Diabetologia 47: 488–498, 2004. doi: 10.1007/s00125-004-1348-0. [DOI] [PubMed] [Google Scholar]
- 15.Freeman ME. Neuroendocrine control of the ovarian cycle of the rat. In: Knobil and Neill’s Physiology of Reproduction, edited by Neill JD. San Diego, CA: Academic, 2006, p. 2327–2388. [Google Scholar]
- 16.Gelin L, Li J, Corbin KL, Jahan I, Nunemaker CS. Metformin inhibits mouse islet insulin secretion and alters intracellular calcium in a concentration-dependent and duration-dependent manner near the circulating range. J Diabetes Res 2018: 1–10, 2018. doi: 10.1155/2018/9163052. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Glynn E, Thompson B, Vadrevu S, Lu S, Kennedy RT, Ha J, Sherman A, Satin LS. Chronic glucose exposure systematically shifts the oscillatory threshold of mouse islets: experimental evidence for an early intrinsic mechanism of compensation for hyperglycemia. Endocrinology 157: 611–623, 2016. doi: 10.1210/en.2015-1563. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Gole E, Oikonomou S, Ellard S, De Franco E, Karavanaki K. A novel KCNJ11 mutation associated with transient neonatal diabetes. J Clin Res Pediatr Endocrinol 10: 175–178, 2018. doi: 10.4274/jcrpe.5166. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Gomis A, Valdeolmillos M. Regulation by tolbutamide and diazoxide of the electrical activity in mouse pancreatic β-cells recorded in vivo. Br J Pharmacol 123: 443–448, 1998. doi: 10.1038/sj.bjp.0701628. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Groop LC, Pelkonen R, Koskimies S, Bottazzo GF, Doniach D. Secondary failure to treatment with oral antidiabetic agents in non-insulin-dependent diabetes. Diabetes Care 9: 129–133, 1986. doi: 10.2337/diacare.9.2.129. [DOI] [PubMed] [Google Scholar]
- 21.Gullo D, Rabuazzo AM, Vetri M, Gatta C, Vinci C, Buscema M, Vigneri R, Purrello F. Chronic exposure to glibenclamide impairs insulin secretion in isolated rat pancreatic islets. J Endocrinol Invest 14: 287–291, 1991. doi: 10.1007/BF03346813. [DOI] [PubMed] [Google Scholar]
- 22.Hellman B. Pulsatility of insulin release–a clinically important phenomenon. Ups J Med Sci 114: 193–205, 2009. doi: 10.3109/03009730903366075. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Huopio H, Shyng SL, Otonkoski T, Nichols CG. KATP channels and insulin secretion disorders. Am J Physiol Endocrinol Metab 283: E207–E216, 2002. doi: 10.1152/ajpendo.00047.2002. [DOI] [PubMed] [Google Scholar]
- 24.Jahan I, Corbin KL, Bogart AM, Whitticar NB, Waters CD, Schildmeyer C, Vann NW, West HL, Law NC, Wiseman JS, Nunemaker CS. Reducing glucokinase activity restores endogenous pulsatility and enhances insulin secretion in islets from db/db mice. Endocrinology 159: 3747–3760, 2018. doi: 10.1210/en.2018-00589. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Kane C, Shepherd RM, Squires PE, Johnson PR, James RF, Milla PJ, Aynsley-Green A, Lindley KJ, Dunne MJ. Loss of functional KATP channels in pancreatic β-cells causes persistent hyperinsulinemic hypoglycemia of infancy. Nat Med 2: 1344–1347, 1996. doi: 10.1038/nm1296-1344. [DOI] [PubMed] [Google Scholar]
- 26.Lang DA, Matthews DR, Peto J, Turner RC. Cyclic oscillations of basal plasma glucose and insulin concentrations in human beings. N Engl J Med 301: 1023–1027, 1979. doi: 10.1056/NEJM197911083011903. [DOI] [PubMed] [Google Scholar]
- 27.Larsson O, Kindmark H, Brandstrom R, Fredholm B, Berggren PO. Oscillations in KATP channel activity promote oscillations in cytoplasmic free Ca2+ concentration in the pancreatic β cell. Proc Natl Acad Sci USA 93: 5161–5165, 1996. doi: 10.1073/pnas.93.10.5161. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Levetan C. Oral antidiabetic agents in type 2 diabetes. Curr Med Res Opin 23: 945–952, 2007. doi: 10.1185/030079907X178766. [DOI] [PubMed] [Google Scholar]
- 29.Lightman SL, Conway-Campbell BL. The crucial role of pulsatile activity of the HPA axis for continuous dynamic equilibration. Nat Rev Neurosci 11: 710–718, 2010. doi: 10.1038/nrn2914. [DOI] [PubMed] [Google Scholar]
- 30.Matthews DR, Naylor BA, Jones RG, Ward GM, Turner RC. Pulsatile insulin has greater hypoglycemic effect than continuous delivery. Diabetes 32: 617–621, 1983. doi: 10.2337/diab.32.7.617. [DOI] [PubMed] [Google Scholar]
- 31.Matveyenko AV, Liuwantara D, Gurlo T, Kirakossian D, Dalla Man C, Cobelli C, White MF, Copps KD, Volpi E, Fujita S, Butler PC. Pulsatile portal vein insulin delivery enhances hepatic insulin action and signaling. Diabetes 61: 2269–2279, 2012. doi: 10.2337/db11-1462. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.McKenna JP, Bertram R. Fast-slow analysis of the Integrated Oscillator Model for pancreatic β-cells. J Theor Biol 457: 152–162, 2018. doi: 10.1016/j.jtbi.2018.08.029. [DOI] [PubMed] [Google Scholar]
- 33.McKenna JP, Ha J, Merrins MJ, Satin LS, Sherman A, Bertram R. Ca2+ effects on ATP production and consumption have regulatory roles on oscillatory islet activity. Biophys J 110: 733–742, 2016. doi: 10.1016/j.bpj.2015.11.3526. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Merrins MJ, Poudel C, McKenna JP, Ha J, Sherman A, Bertram R, Satin LS. Phase analysis of metabolic oscillations and membrane potential in pancreatic islet β cells. Biophys J 110: 691–699, 2016. doi: 10.1016/j.bpj.2015.12.029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Nichols CG. KATP channels as molecular sensors of cellular metabolism. Nature 440: 470–476, 2006. doi: 10.1038/nature04711. [DOI] [PubMed] [Google Scholar]
- 36.Nunemaker CS, Satin LS. Episodic hormone secretion: a comparison of the basis of pulsatile secretion of insulin and GnRH. Endocrine 47: 49–63, 2014. doi: 10.1007/s12020-014-0212-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Nunemaker CS, Zhang M, Wasserman DH, McGuinness OP, Powers AC, Bertram R, Sherman A, Satin LS. Individual mice can be distinguished by the period of their islet calcium oscillations: is there an intrinsic islet period that is imprinted in vivo? Diabetes 54: 3517–3522, 2005. doi: 10.2337/diabetes.54.12.3517. [DOI] [PubMed] [Google Scholar]
- 38.O’Rahilly S, Turner RC, Matthews DR. Impaired pulsatile secretion of insulin in relatives of patients with non-insulin-dependent diabetes. N Engl J Med 318: 1225–1230, 1988. doi: 10.1056/NEJM198805123181902. [DOI] [PubMed] [Google Scholar]
- 39.Paolisso G, Scheen AJ, Giugliano D, Sgambato S, Albert A, Varricchio M, D’Onofrio F, Lefèbvre PJ. Pulsatile insulin delivery has greater metabolic effects than continuous hormone administration in man: importance of pulse frequency. J Clin Endocrinol Metab 72: 607–615, 1991. doi: 10.1210/jcem-72-3-607. [DOI] [PubMed] [Google Scholar]
- 40.Pincus SM, Veldhuis JD, Mulligan T, Iranmanesh A, Evans WS. Effects of age on the irregularity of LH and FSH serum concentrations in women and men. Am J Physiol 273: E989–E995, 1997. doi: 10.1152/ajpendo.1997.273.5.E989. [DOI] [PubMed] [Google Scholar]
- 41.Quan Y, Barszczyk A, Feng ZP, Sun HS. Current understanding of K ATP channels in neonatal diseases: focus on insulin secretion disorders. Acta Pharmacol Sin 32: 765–780, 2011. doi: 10.1038/aps.2011.57. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Ravier MA, Nenquin M, Miki T, Seino S, Henquin JC. Glucose controls cytosolic Ca2+ and insulin secretion in mouse islets lacking adenosine triphosphate-sensitive K+ channels owing to a knockout of the pore-forming subunit Kir6.2. Endocrinology 150: 33–45, 2009. doi: 10.1210/en.2008-0617. [DOI] [PubMed] [Google Scholar]
- 43.Rorsman P, Ashcroft FM. Pancreatic β-cell electrical activity and insulin secretion: Of mice and men. Physiol Rev 98: 117–214, 2018. doi: 10.1152/physrev.00008.2017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Rustenbeck I, Wienbergen A, Bleck C, Jörns A. Desensitization of insulin secretion by depolarizing insulin secretagogues. Diabetes 53, Suppl 3: S140–S150, 2004. doi: 10.2337/diabetes.53.suppl_3.S140. [DOI] [PubMed] [Google Scholar]
- 45.Rustenbeck I, Winkler M, Jörns A. Desensitization of insulin secretory response to imidazolines, tolbutamide, and quinine. I. Secretory and morphological studies. Biochem Pharmacol 62: 1685–1694, 2001. doi: 10.1016/S0006-2952(01)00792-4. [DOI] [PubMed] [Google Scholar]
- 46.Satin LS, Butler PC, Ha J, Sherman AS. Pulsatile insulin secretion, impaired glucose tolerance and type 2 diabetes. Mol Aspects Med 42: 61–77, 2015. doi: 10.1016/j.mam.2015.01.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Schöfl C, Börger J, Mader T, Waring M, von zur Mühlen A, Brabant G. Tolbutamide and diazoxide modulate phospholipase C-linked Ca2+ signaling and insulin secretion in β-cells. Am J Physiol Endocrinol Metab 278: E639–E647, 2000. doi: 10.1152/ajpendo.2000.278.4.E639. [DOI] [PubMed] [Google Scholar]
- 48.Seghers V, Nakazaki M, DeMayo F, Aguilar-Bryan L, Bryan J. Sur1 knockout mice. A model for KATP channel-independent regulation of insulin secretion. J Biol Chem 275: 9270–9277, 2000. doi: 10.1074/jbc.275.13.9270. [DOI] [PubMed] [Google Scholar]
- 49.Sheng M, Thompson MA, Greenberg ME. CREB: a Ca(2+)-regulated transcription factor phosphorylated by calmodulin-dependent kinases. Science 252: 1427–1430, 1991. doi: 10.1126/science.1646483. [DOI] [PubMed] [Google Scholar]
- 50.Sheppard DN, Welsh MJ. Effect of ATP-sensitive K+ channel regulators on cystic fibrosis transmembrane conductance regulator chloride currents. J Gen Physiol 100: 573–591, 1992. doi: 10.1085/jgp.100.4.573. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Venglarik CJ, Schultz BD, DeRoos AD, Singh AK, Bridges RJ. Tolbutamide causes open channel blockade of cystic fibrosis transmembrane conductance regulator Cl− channels. Biophys J 70: 2696–2703, 1996. doi: 10.1016/S0006-3495(96)79839-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.West AE, Chen WG, Dalva MB, Dolmetsch RE, Kornhauser JM, Shaywitz AJ, Takasu MA, Tao X, Greenberg ME. Calcium regulation of neuronal gene expression. Proc Natl Acad Sci USA 98: 11024–11031, 2001. doi: 10.1073/pnas.191352298. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Yildirim V, Bertram R. Calcium oscillation frequency-sensitive gene regulation and homeostatic compensation in pancreatic β-cells. Bull Math Biol 79: 1295–1324, 2017. doi: 10.1007/s11538-017-0286-1. [DOI] [PubMed] [Google Scholar]
- 54.Yildirim V, Vadrevu S, Thompson B, Satin LS, Bertram R. Upregulation of an inward rectifying K+ channel can rescue slow Ca2+ oscillations in K(ATP) channel deficient pancreatic islets. PLOS Comput Biol 13: e1005686, 2017. doi: 10.1371/journal.pcbi.1005686. [DOI] [PMC free article] [PubMed] [Google Scholar]