Abstract
Femoral artery occlusion in rats has been used to study human peripheral artery disease (PAD). Using this animal model, a recent study suggests that increases in levels of tumor necrosis factor-α (TNF-α) and its receptor lead to exaggerated responses of sympathetic nervous activity and arterial blood pressure as metabolically sensitive muscle afferents are activated. Note that voltage-dependent Na+ subtype NaV1.8 channels (NaV1.8) are predominately present in chemically sensitive thin fiber sensory nerves. The purpose of this study was to examine the role played by TNF-α in regulating activity of NaV1.8 currents in muscle dorsal root ganglion (DRG) neurons of rats with PAD induced by femoral artery occlusion. DRG neurons from control and occluded limbs of rats were labeled by injecting the fluorescent tracer DiI into the hindlimb muscles 5 days before the experiments. A voltage patch-clamp mode was used to examine TTX-resistant (TTX-R) NaV currents. Results were as follows: 72 h of femoral artery occlusion increased peak amplitude of TTX-R [1,922 ± 139 pA in occlusion (n = 11 DRG neurons) vs. 1,178 ± 39 pA in control (n = 10), means ± SE; P < 0.001 between the 2 groups] and NaV1.8 currents [1,461 ± 116 pA in occlusion (n = 11) and 766 ± 48 pA in control (n = 10); P < 0.001 between groups] in muscle DRG neurons. TNF-α exposure amplified TTX-R and NaV1.8 currents in DRG neurons of occluded muscles in a dose-dependent manner. Notably, the amplification of TTX-R and NaV1.8 currents induced by TNF-α was attenuated in DRG neurons with preincubation with respective inhibitors of the intracellular signaling pathways p38-MAPK, JNK, and ERK. In conclusion, our data suggest that NaV1.8 is engaged in the role of TNF-α in amplifying muscle afferent inputs as the hindlimb muscles are ischemic; p38-MAPK, JNK, and ERK pathways are likely necessary to mediate the effects of TNF-α.
Keywords: dorsal root ganglion neuron, muscle afferent, NaV1.8 channels, peripheral artery disease, TNF-α
INTRODUCTION
The exercise pressor reflex is a neural feedback engaged in cardiovascular adjustment during exercise (30, 34). This reflex autonomic mechanism is mediated by group III and IV muscle afferent fibers (19, 20). With the enhanced sympathetic activity, blood pressure (BP), heart rate, myocardial contractility, and peripheral vasoconstriction increase (19, 34). The role played by the exercise pressor reflex in modifying BP has been further demonstrated in cardiovascular diseases such as peripheral arterial disease (PAD; 2, 3). In patients with PAD, the exercise pressor reflex is augmented during walking or static exercise (2, 3). Alleviating the blood flow to the ischemic tissues by leg revascularization attenuates the amplified BP in response to exercise in patients with PAD (32). In a rat model of PAD induced by femoral artery occlusion, the exercise pressor reflex is also amplified, and a number of receptors in muscle afferent nerves contribute to the abnormalities in the reflex responses in this disease (25, 27, 49).
PAD is associated with peripheral atherosclerosis, and with atherosclerotic tissues, blood flow to the arteries of the lower extremities is decreased. During this process, numerous cells (i.e., leukocytes, myocytes, microglia, astrocytes, and Schwann cells) produce and release proinflammatory cytokines (PICs; 33), which include interleukins, lymphokines, and cell signaling molecules. Evidence indicates that PICs are involved in regulating physiological functions with their levels increasing in the circulation and in the affected tissues (17, 46). Increased circulating and intramuscular levels of PICs such as interleukin-6 (IL-6) and tumor necrosis factor-α (TNF-α) have also been found in coronary and/or atherosclerotic vascular disorders such as PAD (8, 28, 54). It is speculated that the augmented exercise pressor reflex in PAD is partly due to inflammatory PICs. Our recent study showed that levels of TNF-α and its subtype receptor TNFR1 are upregulated in the dorsal root ganglion (DRG) of rats with femoral artery occlusion (53). This study further demonstrated that TNF-α synthesis suppressor pentoxifylline administered previously into the hindlimb muscles attenuates the exaggerated exercise pressor reflex in PAD rats. However, the precise mechanisms by which the TNF-α pathway regulates the exercise pressor reflex in PAD need to be clarified.
There is a linkage between TNF-α and Na+ current in sensory nerves since TNF-α increases the current densities of tetrodotoxin (TTX)-resistant (TTX-R) Na+ (i.e., voltage-dependent sodium channel NaV1.8) in DRG neurons (6). In general, NaV1.8 channels are the main source in neuronal cells contributing to excitability of afferent nerves. They are responsible for transmitting action potentials along the axons of the group III and IV muscle afferent fibers in evoking the exercise pressor reflex (19). Whereas mRNAs of all NaV channels except NaV1.4 (i.e., NaV1.1–1.3 and NaV1.5–1.9) are present in DRG neurons, functional NaV1.6–1.9 channels have been identified in group III and IV muscle afferent neurons and axons (29, 39, 41). Of these, NaV1.6 and NaV1.7 subtypes are classified as TTX sensitive (TTX-S), whereas NaV1.8 and NaV1.9 are classified as TTX-R (4, 43).
It is noted that NaV1.8 channels are highly expressed in C and Aδ fiber (group III and IV) afferent nerves (23). The role played by NaV1.8 in evoking the exercise pressor reflex was examined using whole animal preparations. A803467, a NaV1.8 blocker, attenuates the pressor response evoked by arterial injection of lactic acid and capsaicin stimulating thin fiber afferents (48). Accordingly, the present study was to determine 1) the role of TNF-α in regulating activity of NaV1.8 currents in DRG neurons innervating the muscles of control limbs and occluded limbs of PAD rats and 2) whether intracellular signaling pathways p38-mitogen-activated protein kinase (p38-MAPK), c-Jun NH2-terminal kinase (JNK), and extracellular signal-regulated kinase (ERK) are engaged in the effects of TNF-α. We hypothesized that blocking NaV1.8 channels attenuates the amplitude of NaV1.8 currents to a greater degree in muscle DRG neurons of occluded limbs than in those of control limbs and TNF-α amplifies NaV1.8 currents via p38-MAPK, JNK, and ERK pathways.
MATERIALS AND METHODS
Ethical approval.
All animal experimental procedures were approved by the Institutional Animal Care and Use Committee of the Pennsylvania State University College of Medicine and complied with the National Institutes of Health guidelines. Thirty-five male Sprague–Dawley rats (4–6 wk old) were obtained from Charles River Laboratories and housed in individual cages with free access to food and water, and they were kept in a temperature-controlled room (25°C) on a 12:12-h light-dark cycle.
Labeling of hindlimb muscle afferent DRG neurons.
The lipophilic dye 1,1′-dioctadecyl-3,3,3′,3′-tetramethylindocarbocyanine perchlorate (DiI) was used for the retrograde labeling of the muscle afferent neurons as described previously (52). Rats were anesthetized by inhalation of an isoflurane-oxygen mixture (2–5% isoflurane in 100% oxygen). With the incision in the calf area, the gastrocnemius muscle was exposed, and DiI (60 mg/mL; Invitrogen) was injected into the white portion of the gastrocnemius muscle. DiI (1 μL) was administered at different locations, with the needle left in the muscle for 1 min to prevent tracer leakage. Then the animals were returned to their cages for the fluorescent DiI tracer to be transported to DRG neurons.
Femoral artery occlusion.
Two days after DiI injection, the rats were anesthetized with an isoflurane-oxygen mixture. The femoral artery on one limb was surgically exposed, dissected, and ligated ~3 mm distal to the inguinal ligament as previously described (52, 53). As the control, the contralateral limb underwent the same procedure except that the suture below the femoral artery was not tied. Then the rats were returned to the cage before the experiments. Buprenorphine hydrochloride (0.05 mg/kg sc) was administered before the surgery for postoperative pain relief. Following the surgery, the animals were kept in the surgery room for 2–3 h for observation and then returned to the animal facility.
Culture of DRG neurons.
Three days after the femoral artery occlusion, the rats were anesthetized with an isoflurane-oxygen mixture followed by cervical dislocation and decapitation. The lumbar (~L4–6) DRG were taken out, dissected, and immediately transferred into ice-cold Hanks’ balanced salt solution (Sigma-Aldrich). After being freed from the connective tissue, the ganglia were enzymatically digested and dissociated in Earle’s balanced salt solution containing collagenase type D (0.6 mg/mL), trypsin (0.30 mg/mL), and DNase (0.1 mg/mL), followed by shaking at 34°C for 40 min. The dissociated neurons were seeded on 10% poly-l-lysine-coated coverslips in a 35-mm culture dish containing 2 mL of minimum essential medium (MEM; Thermo Fisher Scientific) supplemented with 10% FBS, 1% glutamine, and 1% penicillin-streptomycin. The neurons were cultured at 37°C with 5% CO2 and 95% air in the cell culture incubator.
For the TNF-α experiment, the cultured neurons were respectively exposed to 1, 10, and 100 pg/mL of TNF-α (in PBS containing 0.1% bovine serum albumin) for 24 h, and inhibitors of the p38-MAPK, JNK, and ERK1/2 pathways [SB203580 (10 μM), SP600125 (10 μM), and PD98059 (30 μM); all purchased from Tocris Bioscience] were applied to the cultured DRG neurons 2 h before TNF-α application. The concentrations of these inhibitors were selected on the basis of the results in a number of previous reports (6, 14, 21, 26, 44).
Electrophysiology.
DiI-positive neurons were first identified under an inverted microscope (Nikon TE2000), and images were displayed on a video monitor. DiI-positive neurons (cell diameters ≤35 μm) were recorded with a MultiClamp 700B amplifier (Axon Instruments) in a solution containing (in mM) 45 NaCl, 100 N-methyl-d-glucosamine (NMG)-Cl, 10 MnCl2, 10 Na-HEPES, and 10 glucose, pH 7.4 and osmolarity of 330 mosM. The internal solution contained (in mM) 104 NMG-Cl, 14 creatine-PO4, 6 MgCl2, 10 NMG-HEPES, 5 Tris-ATP, 10 NMG-EGTA, and 0.3 Tris-GTP, pH 7.4 and osmolarity of 310 mosM. Seals (~2–8 GΩ) were obtained with ~2–4-MΩ resistance of glass electrodes filled with internal solution. In voltage-clamp mode, the whole cell configuration was applied, and membrane currents of neurons were recorded with a protocol that held at −80 mV, followed by a pulse of 20 mV at room temperature. The recording current was filtered at 2 kHz and sampled at 10 kHz. Voltage errors were minimized by 80% series resistance compensation, and linear leak subtraction was used for all recordings. During the electrophysiological experiment, TTX solution (1 μM; Sigma-Aldrich) was applied to the external solution of voltage clamp to block the TTX-S sodium channels, and 500 nM of A803467 combined with 1 μM of TTX was used in the external solution to block the TTX-R NaV1.8 channels. We assessed NaV1.8 currents as (peak currents after application of TTX − peak currents after both TTX and A803467). All signals were acquired with pClamp 10.1 and analyzed with Clampfit 10.7 software.
Statistical analysis.
Percent inhibition of by A803467 in control and occlusive DRG neurons was analyzed using unpaired t test; other experimental data were analyzed using two-way ANOVA, and Tukey’s post hoc tests were used as appropriate. The data are presented as means ± SE. All statistical analyses were performed using SPSS v25, and differences were considered significant at P < 0.05.
RESULTS
TTX-R and NaV1.8 currents in muscle DRG neurons.
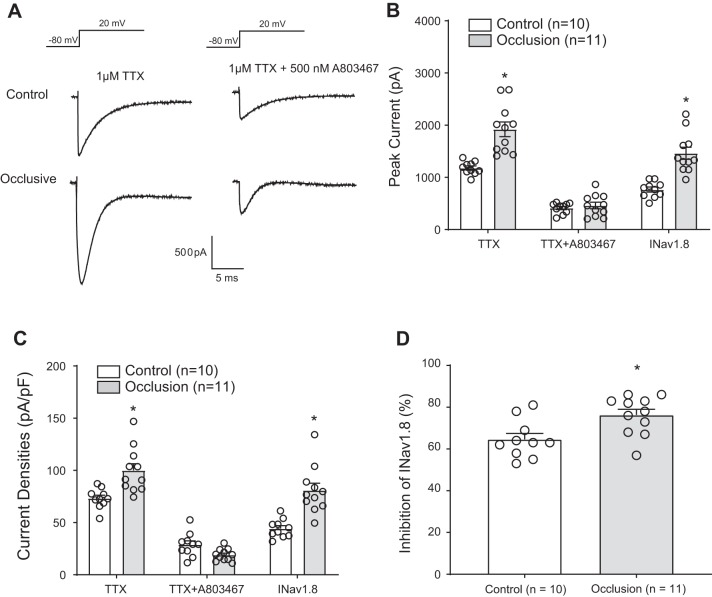
As shown in Fig. 1, A and B, femoral artery occlusion significantly increased peak amplitude of TTX-R currents in DRG neurons compared with the control DRG neurons (P < 0.001, 1,922.02 ± 139.09 pA in the occlusion group vs. 1,178.43 ± 39.22 pA in control; n = 10 DRG neurons in control and n = 11 in occlusion). After application of A803467, blocking the NaV1.8 component of TTX-R, the amplitude of TTX-R currents was largely attenuated in DRG neurons of both groups. was 1,460.78 ± 115.92 pA in the occlusion group and 766.11 ± 48.01 pA in the control group (P < 0.001 between the 2 groups). Moreover, Fig. 1C demonstrates increases in current densities in DRG neurons of occluded limbs compared with those in DRG neurons of control limbs. Likewise, after application of A803467, TTX-R currents were attenuated to a greater degree in DRG neurons of occluded limbs than in DRG neurons of control limbs. Figure 1D shows that percent inhibitory effects of A803467 on were 65 ± 3% in the control group and 76 ± 3% in the occlusion group (P = 0.010 between the 2 groups).
Fig. 1.
Effects of femoral artery occlusion on TTX-resistant (TTX-R) and voltage-dependent Na+ channel subtype NaV1.8 (NaV1.8) currents in rat dorsal root ganglion (DRG) neurons. All rat DRG neurons were dissociated 72 h after femoral occlusion and recorded in voltage patch mode with −80 mV holding followed by a pulse of 20 mV. One micromolar TTX was used to block TTX-sensitive currents, and 500 nM of A803467 was used to block NaV1.8 currents. A: representative current traces after application of TTX and TTX plus A803467 in DRG neurons of control limb and occluded limb. A greater amplitude of currents was observed in DRG neurons of the occluded limb. B: averaged data showing that femoral artery occlusion amplified current amplitude after TTX application. The data also show that the currents were largely decreased after application of TTX and A803467. NaV1.8 currents were assessed as (peak current after TTX − peak currents after both TTX and A803467). was also amplified by femoral artery occlusion. *P < 0.001 between control and occlusion for TTX-R and NaV1.8 current in DRG neurons. C: femoral artery occlusion also increased current densities in DRG neurons compared with the control group. *P = 0.002 between control and occlusion after TTX; *P < 0.001 between control and occlusion for . D: %inhibition of by A803467 was greater in DRG neurons of occluded limbs than that in DRG neurons of control limbs. *P = 0.010 between control and occlusion. Individual data points are also shown; n = 10 DRG neurons in the control group, and n = 11 in the occlusion group.
TNF-α amplified TTX-R and NaV1.8 currents.
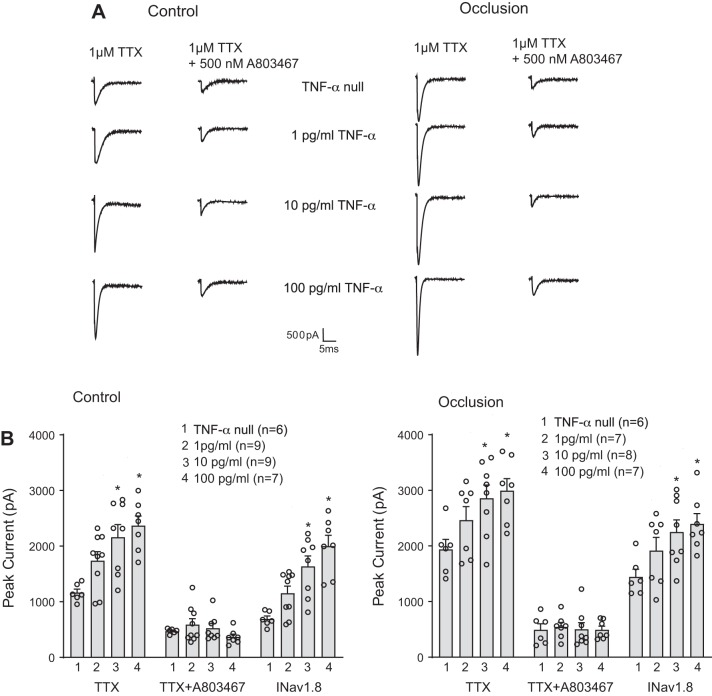
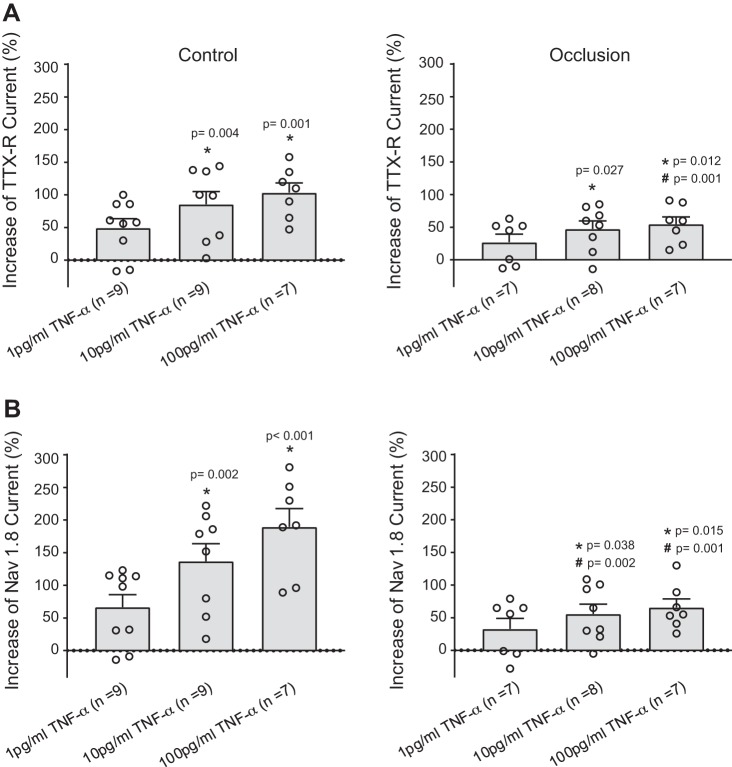
We further examined the effects of TNF-α on TTX-R and NaV1.8 currents. The representative traces and averaged data of Fig. 2, A and B, demonstrate that TNF-α (1, 10, and 100 pg/mL) increased TTX-R currents in a dose-dependent manner in DRG neurons of control and occluded limbs. Figure 2B also shows that TNF-α amplified in a dose-dependent manner in muscle DRG neurons of control limbs and occluded limbs. That is, after being exposed to 10 and 100 pg/mL of TNF-α for 24 h, in DRG neurons of the control limbs, was augmented to 1,151.65 ± 129.70 pA, 1,636.23 ± 187.23 pA (P = 0.002), and 2,000.96 ± 193.15 pA (P < 0.001) compared with its amplitude of 690.98 ± 54.07 pA without application of TNF-α. In DRG neurons of the occluded limbs, was augmented to 1,919.37 ± 234.72 pA, 2,251.23 ± 216.65 pA bx;1(P = 0.047), and 2,393.54 ± 187.59 pA (P = 0.021) compared with 1,444.11 ± 138.49 pA without TNF-α. It is noted that TNF-α amplified the amplitude of TTX-R currents and NaV1.8 currents in DRG neurons of both the control and occluded limbs, but the effects of TNF-α (10 and 100 pg/mL) appeared to a lesser degree in occluded DRG neurons as shown in Fig. 3, A and B.
Fig. 2.
Effects of TNF-α on TTX-resistant (TTX-R) and voltage-dependent Na+ channel subtype NaV1.8 (NaV1.8) currents in rat dorsal root ganglion (DRG) neurons of control and occlusion groups. TTX-R currents were examined in DRG neurons after 24 h of exposure to TNF-α. A: representative current traces showing that TNF-α amplified TTX-R current in a dose-related manner in DRG neurons of both control limbs and occluded limbs. B: averaged data demonstrate that TNF-α amplified TTX-R currents and NaV1.8 currents in DRG neurons of control limbs and occluded limbs. ○, Individual data points; n is the number of DRG neurons. , peak current after TTX − peak currents after both TTX and A803467. In the control group, *P = 0.005 and P = 0.001 compared with TNF-α null after TTX and *P = 0.002 and P < 0.001 for current. In the occlusion group, *P = 0.041 and P = 0.020 compared with TNF-α null after TTX and *P = 0.047 and P = 0.021 for current.
Fig. 3.
Percent effects of TNF-α on TTX-resistant (TTX-R; A) and voltage-dependent Na+ channel subtype NaV1.8 (NaV1.8) currents (B) of dorsal root ganglion (DRG) neurons of control limbs and occluded limbs. TNF-α amplified TTX-R and NaV1.8 currents of DRG neurons in a dose-dependent manner, and the effects of TNF-α appeared to a lesser degree in the occlusion group. ○Individual data points. All the P values are shown in the figure.
Engagement of intracellular signaling pathways.
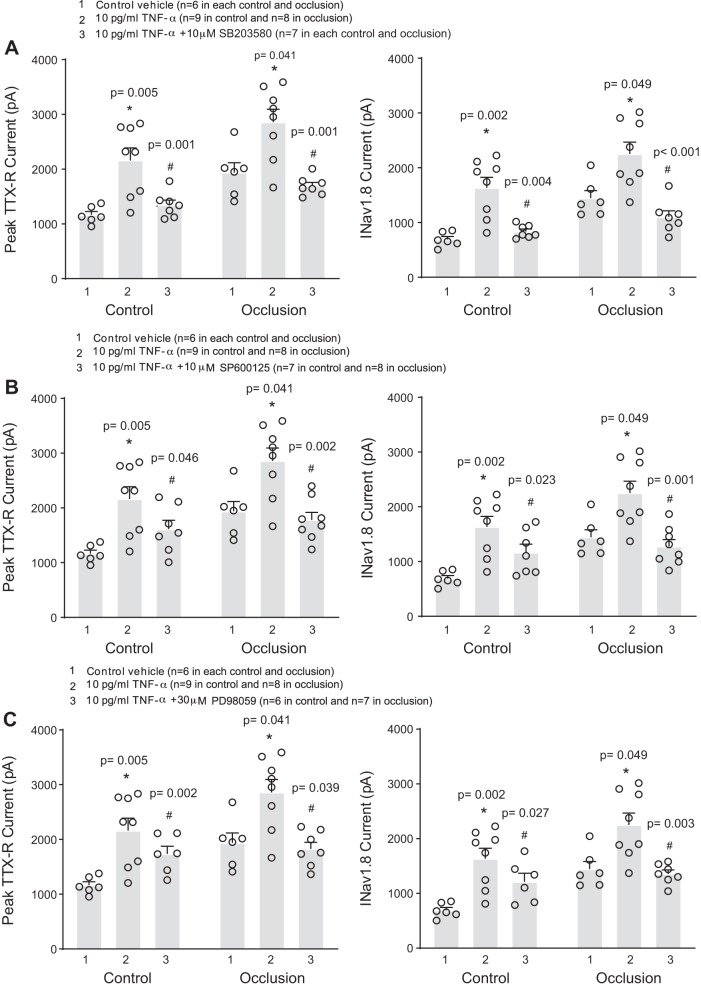
We first examined the potential role of the p38-MAPK pathway in our study by adding 10 μM of SB203580 (p38-MAPK inhibitor) to the DRG neurons for preincubation before application of 10 pg/mL of TNF-α. Figure 4A shows that increases in TTX-R and NaV1.8 currents evoked by TNF-α were attenuated in DRG neurons of control limbs and occluded limbs after SB203580. With application of TNF-α, SB203580 inhibited to a similar degree in the control DRG neurons and occluded DRG neurons (by 47 ± 3% in the control DRG neurons and by 47 ± 6% in the occluded DRG neurons; P = 0.526 between control and occlusion).
Fig. 4.
Involvement of intracellular signaling pathways p38-MAPK, JNK, and ERK in the effects of TNF-α on voltage-dependent Na+ channel (NaV) currents of dorsal root ganglion (DRG) neurons. To inhibit these signaling pathways, before application of 10 pg/mL of TNF-α, SB203580 (10 μM; A), SP600125 (10 μM; B), and PD98059 (30 μM; C) were applied, respectively. TNF-α amplified TTX-resistant (TTX-R) and NaV1.8 currents in rat DRG neurons of the control and occlusion groups compared with the neurons without TNF-α application. Note that the effects of TNF-α were significantly attenuated by respective inhibitors of p38-MAPK, JNK, and ERK. The numbers of DRG neurons (n) are indicated in the figure, and individual data points are also shown. *P < 0.05 compared with the group without application of TNF-α; #P < 0.05 group with TNF-α vs. group with TNF-α plus respective inhibitors SB203580, SP600125, and PD98059.
To evaluate the role of the JNK pathway in the augmentation by TNF-α of TTX-R and NaV1.8 currents in muscle DRG neurons, DRG neurons were preincubated with the JNK inhibitor SP600125 (10 μM) before adding 10 pg/mL of TNF-α. As shown in Fig. 4B, we observed that SP600125 significantly attenuated the increased TTX-R and NaV1.8 currents in DRG neurons of both the control and occluded limbs. There was no significant difference in SP600125’s attenuation of the increased NaV1.8 currents evoked by TNF-α in DRG neurons of the two groups. SP600125 attenuated by 29 ± 6% in the control DRG neurons and by 45 ± 6% in the occluded DRG neurons (P = 0.123 between control and occlusion).
Likewise, 30 μM of PD98059 (ERK1/2 inhibitor) added to the DRG neurons was used to examine the effects of the ERK pathway on the augmentation by TNF-α of TTX-R and NaV1.8 currents in muscle DRG neurons. As shown in Fig. 4C, increases in TTX-R and evoked by TNF-α were significantly attenuated in DRG neurons of control limbs and occluded limbs after application of PD98059. PD98059 attenuated by 24 ± 6% in the control DRG neurons and by 40 ± 5% in the occluded DRG neurons (no significant difference between control and occlusion; P = 0.100 between the 2 groups).
DISCUSSION
It has been reported that NaV1.8 channels are mostly located in DRG neurons with cell diameters ≤35 μM (7, 40). Thus, in this study, we examined NaV currents in DiI-labeled muscle afferent neurons with diameters ≤35 μM. Moreover, we determined the effects of A803467 on TTX-R NaV currents in DRG neurons of control limbs and occluded limbs. As indicated in previous studies (15, 41) for IC50 of A803467 blocking NaV1.8 channels in rat DRG neurons, in the present experiments, we used 500 nM of A803467 to maximally block NaV1.8 channels in DRG neurons. We demonstrated that 1) A803467 applied on DRG neurons attenuates the amplitude of NaV1.8 currents to a greater degree in muscle DRG neurons of occluded limbs than in those of control limbs and 2) TNF-α amplifies NaV1.8 currents and the effects are via the p38-MAPK, JNK, and ERK pathways.
It is noted that TNF-α amplified the amplitude of TTX-R currents and NaV1.8 currents in DRG neurons of both the control and occluded limbs, but the effects of TNF-α appeared to a lesser degree in occluded DRG neurons. This indicates that the TNF-α pathway/TNF-α receptor was likely preactivated/preoccupied in DRG neurons because of 72 h of femoral artery occlusion.
PAD is associated with peripheral atherosclerosis, which is an inflammatory disease and characterized by the initiation and progression involving mononuclear and macrophage cells, which produce and release PICs (33, 42). It is well known that the roles of TNF-α, IL-6, and interleukin-1β (IL-1β) are significant in regulating immune and inflammatory reactions. These PICs modulate the activities of many cell types in various diseases. For example, during diseased states, PICs help to recruit cells to inflammatory sites, stimulating cell survival and division and enhancing proliferation and differentiation (35).
Intermittent claudication is the most common symptom in PAD. Particularly, previous studies have demonstrated that inflammation contributes to the development of intermittent claudication in PAD by adversely affecting skeletal muscle function (36). Elevated inflammatory biomarkers (such as TNF-α and IL-6) are inversely associated with physical performance in patients with PAD. For example, increases in levels of TNF-α and IL-6 in PAD induce skeletal muscle protein breakdown, and they are negatively related to muscle mass and strength (10, 11, 45, 50). Monocyte mRNA expression of TNF-α is associated with maximal walking time in patients with intermittent claudication (36). These prior studies also suggest that circulating levels of PICs are considerably elevated in populations of patients with PAD and correlated with impaired maximal walking time. Also, in large patient populations, elevated inflammatory PICs such as TNF-α and IL-6 are associated with the incidence and severity of PAD (31, 37, 38), and they are considered as potential therapy targets for inflammatory vascular diseases (1).
In our prior study, we observed the appearance of TNF-α in C fibers of muscle afferent nerves supplying DRG neurons and also found that the levels of TNF-α in the DRG are increased by femoral artery occlusion (53). This prior study further provided evidence that increases in levels of TNF-α lead to amplification of muscle metabolite-mediated sympathetic and pressor responses as the hindlimb muscles are ischemic. Thus, in this report, our emphasis was on the role of TNF-α in regulating the activity of NaV1.8 currents in muscle DRG neurons of ischemic limbs.
NaV1.8 channels are predominantly localized in small/medium nociceptive C/Aδ-type DRG neurons (40, 41) and have been identified as a key contributor to the development of the painful sensations associated with chronic inflammation in peripheral tissues (7, 18, 22). Evidence has further confirmed that TNF-α modulates the expression and properties of NaV1.8 channels in DRG neurons or afferent fibers, playing a critical role in nociceptive signaling pathways (6, 9, 12, 13, 24). It was found that chronic TNF-α exposure increases the expression of NaV1.8 channels in DRG neurons with a hyperpolarizing shift in gating properties, along with enhancement of p38-MAPK activity (9). This result suggests an intrinsic linkage between TNF-α and NaV1.8 channels in sensory neurons. Data from our present study demonstrate that TNF-α amplifies the current amplitude of NaV1.8 channels in muscle DRG neurons of occluded limbs and femoral occlusion amplifies NaV1.8 current to a greater degree. Nonetheless, considering the role played by TNF-α in amplifying the BP response to active muscle, we speculate that NaV1.8 channels in muscle sensory DRG neurons are likely to participate in the modulation by TNF-α of the exaggerated exercise pressor reflex in PAD.
On the basis of results of the prior studies indicating that the p38-MAPK pathway is engaged in the modulation by TNF-α of NaV1.8 channels in sensory neurons (5, 6), in our present study we specifically examined the potential role of p38-MAPK in the effects of TNF-α on NaV1.8 channels in DRG neurons of ischemic muscle. We observed that adding 10 μM of SB203580 to the DRG neurons before application of TNF-α attenuated the enhancing effect of TNF-α on NaV1.8 currents in muscle DRG neurons. It has been shown that p38-MAPK phosphorylates specific serine on the NaV1.8 channels and via this mechanism TNF-α leads to slow inactivation of NaV1.8 channels (14, 16). Note that the effects of TNF-α were observed in acute experimental preparations in these previous studies. However, in the present study, we applied TNF-α for 24 h, and our data demonstrated that TNF-α increased the amplitude of peak TTX-R current to a greater degree in DRG neurons of occluded rats compared with that in control rats. In addition, our data showed that current densities of TTX-R and NaV1.8 were increased in DRG neurons of occluded rats consistent with increases in amplitude of peak currents. Thus, it is speculated that TNF-α is likely to lead to an increase in the density of NaV1.8 on the membrane of DRG neurons of occluded rats.
Similarly, we used 10 μM of SP600125 and 30 μM of PD98059 to block JNK and ERK pathways (21, 26, 44) and further examined engagement of those signaling pathways in our study. We observed that inhibition of JNK and ERK in muscle DRG neurons also attenuated the effect of TNF-α on NaV1.8 current. It is noted that inhibition of JNK or ERK tended to lead to a greater effect on NaV1.8 currents in muscle DRG neurons of occluded limbs than that in control limbs. However, on the basis of the statistical results, we cannot suggest that there is a significant difference between the two groups. Our data provide evidence at a cellular level showing the role of TNF-α and NaV1.8 channels in muscle afferent nerves of ischemic limbs in regulating the amplified BP response likely via intracellular signaling pathways including p38-MAPK, JNK, and ERK.
It has been interesting to clarify the role played by TTX-R NaV channels in group III and IV muscle afferent nerves in regulating the exercise pressor reflex. It was found that a blockade of NaV1.7 channels (TTX-S) in muscle afferents by its antagonist (Ssm6a, a peptide from Scolopendra subspinipes mutilans venom) attenuates the peak pressor response in rats (47), whereas inhibition of NaV1.8 channels in muscle afferent nerves by A803467 attenuates the pressor response evoked by arterial injection of lactic acid and capsaicin (48). These results are mainly consistent with the notion that group III and IV muscle afferents containing TTX-S and TTX-R NaV contribute to the mechanosensitive and metabosensitive activation of the exercise pressor reflex. Of note, there are more functional TTX-S and TTX-R NaV channels identified in group III and IV afferent axons and neurons (29, 39, 41). Among them, NaV1.8 and NaV1.9 are TTX-R (4, 43), and they are more likely related to the pressor response to activation of muscle metabolic receptors. Nonetheless, results of our present study suggest that the hindlimb ischemia increases activities of TTX-R NaV1.8 channels and TNF-α can amplify activities of NaV1.8. In our prior study, data have shown that hindlimb ischemia increases the levels of TNF-α and expression of its receptor in the DRG (53). This prior study has also demonstrated that inhibition of TNF-α decreases the pressor response evoked by arterial injection of capsaicin stimulating muscle metabolic receptors, but not by muscle tendon stretch stimulating muscle mechanical receptors (53). Taken together, we suggest that NaV1.8 is engaged in the role of TNF-α in amplifying afferent input of muscle metabolite activation as the hindlimb muscles are ischemic.
Femoral artery occlusion in rats has been used to study human PAD. Although this model does not fully exhibit all the clinical symptoms of PAD, it mimics one of the critical characteristics observed in PAD, namely, intermittent claudication manifested by insufficient blood flow (51). In particular, in occluded rats, the blood flow limitation is observed during exercise, whereas the resting blood flow is maintained (51). This makes it appropriate to investigate exercise physiology in PAD. In particular, in a rat model of PAD induced by femoral artery occlusion, the BP response is augmented during muscle contraction and/or stimulation of muscle metabolic receptors (25). Nonetheless, the usefulness of this model is not a surrogate for study of the slowly developing atherosclerotic condition of PAD; thus, care should be taken when extrapolating the findings of the present study to PAD.
In the previous studies, the effects of TNF-α on TTX-R NaV1.8 current in DRG neurons have been characterized, and the involvement of p38-MAPK and other intracellular kinases in regulating NaV1.8 current was further demonstrated (12, 14, 16). It has also been reported that TNF-α augmented TTX-R currents in DRG neurons following motor nerve injury (6). Nonetheless, results of our present study have defined DRG neurons that innervate the hindlimb skeletal muscle, and we further showed that TNF-α has enhancing effects on TTX-R NaV1.8 current in muscle DRG neurons obtained from the ischemic hindlimb induced by femoral artery occlusion.
In conclusion, femoral artery occlusion increases NaV1.8 currents in muscle DRG neurons, and TNF-α has enhancing effects on activity of NaV1.8 currents. In addition, intracellular signaling pathways, namely, p38-MAPK, JNK, and ERK, are involved in the effects of TNF-α on NaV1.8 currents.
Perspectives and Significance
Within the ischemic hindlimb muscles after femoral artery occlusion, engagement of NaV1.8 in the role of TNF-α in amplifying muscle afferent inputs has the implication that blocking TNF-α-NaV1.8 signaling pathways is likely beneficial to PAD. In particular, these molecular mediators should be targeted in whole animal preparations in future studies examining the exaggerated exercise pressor reflex in PAD.
GRANTS
This study was supported by National Heart, Lung, and Blood Institute Grants P01-HL-134609 and R01-HL-141198.
DISCLOSURES
No conflicts of interest, financial or otherwise, are declared by the authors.
AUTHOR CONTRIBUTIONS
J.L. conceived and designed research; Q.L. performed experiments; Q.L. and L.Q. analyzed data; Q.L. and J.L. interpreted results of experiments; Q.L. prepared figures; Q.L., L.Q., and J.L. drafted manuscript; L.Q. and J.L. edited and revised manuscript; Q.L., L.Q., and J.L. approved final version of manuscript.
ACKNOWLEDGMENTS
We thank C. Yang for outstanding technical assistance.
REFERENCES
- 1.Antonopoulos AS, Papanikolaou E, Vogiatzi G, Oikonomou E, Tousoulis D. Anti-inflammatory agents in peripheral arterial disease. Curr Opin Pharmacol 39: 1–8, 2018. doi: 10.1016/j.coph.2017.11.001. [DOI] [PubMed] [Google Scholar]
- 2.Baccelli G, Reggiani P, Mattioli A, Corbellini E, Garducci S, Catalano M. The exercise pressor reflex and changes in radial arterial pressure and heart rate during walking in patients with arteriosclerosis obliterans. Angiology 50: 361–374, 1999. doi: 10.1177/000331979905000502. [DOI] [PubMed] [Google Scholar]
- 3.Bakke EF, Hisdal J, Kroese AJ, Jørgensen JJ, Stranden E. Blood pressure response to isometric exercise in patients with peripheral atherosclerotic disease. Clin Physiol Funct Imaging 27: 109–115, 2007. doi: 10.1111/j.1475-097X.2007.00720.x. [DOI] [PubMed] [Google Scholar]
- 4.Catterall WA, Goldin AL, Waxman SG. International Union of Pharmacology. XLVII. Nomenclature and structure-function relationships of voltage-gated sodium channels. Pharmacol Rev 57: 397–409, 2005. doi: 10.1124/pr.57.4.4. [DOI] [PubMed] [Google Scholar]
- 5.Chen W, Sheng J, Guo J, Gao F, Zhao X, Dai J, Wang G, Li K. Tumor necrosis factor-α enhances voltage-gated Na+ currents in primary culture of mouse cortical neurons. J Neuroinflammation 12: 126, 2015. doi: 10.1186/s12974-015-0349-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Chen X, Pang RP, Shen KF, Zimmermann M, Xin WJ, Li YY, Liu XG. TNF-α enhances the currents of voltage gated sodium channels in uninjured dorsal root ganglion neurons following motor nerve injury. Exp Neurol 227: 279–286, 2011. doi: 10.1016/j.expneurol.2010.11.017. [DOI] [PubMed] [Google Scholar]
- 7.Djouhri L, Fang X, Okuse K, Wood JN, Berry CM, Lawson SN. The TTX-resistant sodium channel Nav1.8 (SNS/PN3): expression and correlation with membrane properties in rat nociceptive primary afferent neurons. J Physiol 550: 739–752, 2003. doi: 10.1113/jphysiol.2003.042127. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.El-Menyar AA. Cytokines and myocardial dysfunction: state of the art. J Card Fail 14: 61–74, 2008. doi: 10.1016/j.cardfail.2007.09.006. [DOI] [PubMed] [Google Scholar]
- 9.Fischer BD, Ho C, Kuzin I, Bottaro A, O’Leary ME. Chronic exposure to tumor necrosis factor in vivo induces hyperalgesia, upregulates sodium channel gene expression and alters the cellular electrophysiology of dorsal root ganglion neurons. Neurosci Lett 653: 195–201, 2017. doi: 10.1016/j.neulet.2017.05.004. [DOI] [PubMed] [Google Scholar]
- 10.Goodman MN. Interleukin-6 induces skeletal muscle protein breakdown in rats. Proc Soc Exp Biol Med 205: 182–185, 1994. doi: 10.3181/00379727-205-43695. [DOI] [PubMed] [Google Scholar]
- 11.Goodman MN. Tumor necrosis factor induces skeletal muscle protein breakdown in rats. Am J Physiol Endocrinol Metab 260: E727–E730, 1991. doi: 10.1152/ajpendo.1991.260.5.E727. [DOI] [PubMed] [Google Scholar]
- 12.Gudes S, Barkai O, Caspi Y, Katz B, Lev S, Binshtok AM. The role of slow and persistent TTX-resistant sodium currents in acute tumor necrosis factor-α-mediated increase in nociceptors excitability. J Neurophysiol 113: 601–619, 2015. doi: 10.1152/jn.00652.2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.He XH, Zang Y, Chen X, Pang RP, Xu JT, Zhou X, Wei XH, Li YY, Xin WJ, Qin ZH, Liu XG. TNF-α contributes to up-regulation of Nav1.3 and Nav1.8 in DRG neurons following motor fiber injury. Pain 151: 266–279, 2010. doi: 10.1016/j.pain.2010.06.005. [DOI] [PubMed] [Google Scholar]
- 14.Hudmon A, Choi JS, Tyrrell L, Black JA, Rush AM, Waxman SG, Dib-Hajj SD. Phosphorylation of sodium channel NaV1.8 by p38 mitogen-activated protein kinase increases current density in dorsal root ganglion neurons. J Neurosci 28: 3190–3201, 2008. doi: 10.1523/JNEUROSCI.4403-07.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Jarvis MF, Honore P, Shieh CC, Chapman M, Joshi S, Zhang XF, Kort M, Carroll W, Marron B, Atkinson R, Thomas J, Liu D, Krambis M, Liu Y, McGaraughty S, Chu K, Roeloffs R, Zhong C, Mikusa JP, Hernandez G, Gauvin D, Wade C, Zhu C, Pai M, Scanio M, Shi L, Drizin I, Gregg R, Matulenko M, Hakeem A, Gross M, Johnson M, Marsh K, Wagoner PK, Sullivan JP, Faltynek CR, Krafte DS. A-803467, a potent and selective Nav1.8 sodium channel blocker, attenuates neuropathic and inflammatory pain in the rat. Proc Natl Acad Sci USA 104: 8520–8525, 2007. doi: 10.1073/pnas.0611364104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Jin X, Gereau RW IV. Acute p38-mediated modulation of tetrodotoxin-resistant sodium channels in mouse sensory neurons by tumor necrosis factor-alpha. J Neurosci 26: 246–255, 2006. doi: 10.1523/JNEUROSCI.3858-05.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Jonsdottir IH, Schjerling P, Ostrowski K, Asp S, Richter EA, Pedersen BK. Muscle contractions induce interleukin-6 mRNA production in rat skeletal muscles. J Physiol 528: 157–163, 2000. doi: 10.1111/j.1469-7793.2000.00157.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Jurcakova D, Ru F, Kollarik M, Sun H, Krajewski J, Undem BJ. Voltage-gated sodium channels regulating action potential generation in itch-, nociceptive-, and low-threshold mechanosensitive cutaneous C-fibers. Mol Pharmacol 94: 1047–1056, 2018. doi: 10.1124/mol.118.112839. [DOI] [PubMed] [Google Scholar]
- 19.Kaufman MP, Forster HV. Reflexes controlling circulatory, ventilatory and airway responses to exercise. In: Handbook of Physiology, Exercise: Regulation and Integration of Multiple Systems, Control of Respiratory and Cardiovascular Systems, edited by Rowell LB, Shepherd JT. Bethesda, MD: American Physiological Society, 1996, sect. 12, pt. II, chapt. 10, p. 381–447. doi: 10.1002/cphy.cp120110. [DOI] [Google Scholar]
- 20.Kaufman MP, Longhurst JC, Rybicki KJ, Wallach JH, Mitchell JH. Effects of static muscular contraction on impulse activity of groups III and IV afferents in cats. J Appl Physiol 55: 105–112, 1983. doi: 10.1152/jappl.1983.55.1.105. [DOI] [PubMed] [Google Scholar]
- 21.Kayssi A, Amadesi S, Bautista F, Bunnett NW, Vanner S. Mechanisms of protease-activated receptor 2-evoked hyperexcitability of nociceptive neurons innervating the mouse colon. J Physiol 580: 977–991, 2007. doi: 10.1113/jphysiol.2006.126599. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Klein AH, Vyshnevska A, Hartke TV, De Col R, Mankowski JL, Turnquist B, Bosmans F, Reeh PW, Schmelz M, Carr RW, Ringkamp M. Sodium channel Nav1.8 underlies TTX-resistant axonal action potential conduction in somatosensory C-fibers of distal cutaneous nerves. J Neurosci 37: 5204–5214, 2017. doi: 10.1523/JNEUROSCI.3799-16.2017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Lai J, Porreca F, Hunter JC, Gold MS. Voltage-gated sodium channels and hyperalgesia. Annu Rev Pharmacol Toxicol 44: 371–397, 2004. doi: 10.1146/annurev.pharmtox.44.101802.121627. [DOI] [PubMed] [Google Scholar]
- 24.Leo M, Argalski S, Schäfers M, Hagenacker T. Modulation of voltage-gated sodium channels by activation of tumor necrosis factor receptor-1 and receptor-2 in small DRG neurons of rats. Mediators Inflamm 2015: 124942, 2015. doi: 10.1155/2015/124942. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Li J, Xing J. Muscle afferent receptors engaged in augmented sympathetic responsiveness in peripheral artery disease. Front Physiol 3: 247, 2012. doi: 10.3389/fphys.2012.00247. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Lirk P, Haller I, Colvin HP, Lang L, Tomaselli B, Klimaschewski L, Gerner P. In vitro, inhibition of mitogen-activated protein kinase pathways protects against bupivacaine- and ropivacaine-induced neurotoxicity. Anesth Analg 106: 1456–1464, 2008. doi: 10.1213/ane.0b013e318168514b. [DOI] [PubMed] [Google Scholar]
- 27.Liu J, Gao Z, Li J. Femoral artery occlusion increases expression of ASIC3 in dorsal root ganglion neurons. Am J Physiol Heart Circ Physiol 299: H1357–H1364, 2010. doi: 10.1152/ajpheart.00612.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Mann DL. Inflammatory mediators and the failing heart: past, present, and the foreseeable future. Circ Res 91: 988–998, 2002. doi: 10.1161/01.RES.0000043825.01705.1B. [DOI] [PubMed] [Google Scholar]
- 29.Marler TL, Wright AB, Elmslie KL, Heier AK, Remily E, Kim-Han JS, Ramachandra R, Elmslie KS. NaV1.9 channels in muscle afferent neurons and axons. J Neurophysiol 120: 1032–1044, 2018. doi: 10.1152/jn.00573.2017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.McCloskey DI, Mitchell JH. Reflex cardiovascular and respiratory responses originating in exercising muscle. J Physiol 224: 173–186, 1972. doi: 10.1113/jphysiol.1972.sp009887. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.McDermott MM, Greenland P, Green D, Guralnik JM, Criqui MH, Liu K, Chan C, Pearce WH, Taylor L, Ridker PM, Schneider JR, Martin G, Rifai N, Quann M, Fornage M. D-dimer, inflammatory markers, and lower extremity functioning in patients with and without peripheral arterial disease. Circulation 107: 3191–3198, 2003. doi: 10.1161/01.CIR.0000074227.53616.CC. [DOI] [PubMed] [Google Scholar]
- 32.Miller AJ, Luck JC, Kim DJ, Leuenberger UA, Aziz F, Radtka JF III, Sinoway LI, Muller MD. Peripheral revascularization attenuates the exercise pressor reflex and increases coronary exercise hyperemia in peripheral arterial disease. J Appl Physiol (1985) 125: 58–63, 2018. doi: 10.1152/japplphysiol.01046.2017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Miller RJ, Jung H, Bhangoo SK, White FA. Cytokine and chemokine regulation of sensory neuron function. Handb Exp Pharmacol 194: 417–449, 2009. doi: 10.1007/978-3-540-79090-7_12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Mitchell JH, Kaufman MP, Iwamoto GA. The exercise pressor reflex: its cardiovascular effects, afferent mechanisms, and central pathways. Annu Rev Physiol 45: 229–242, 1983. doi: 10.1146/annurev.ph.45.030183.001305. [DOI] [PubMed] [Google Scholar]
- 35.Oppenheim JJ. Cytokines: past, present, and future. Int J Hematol 74: 3–8, 2001. doi: 10.1007/BF02982543. [DOI] [PubMed] [Google Scholar]
- 36.Pande RL, Brown J, Buck S, Redline W, Doyle J, Plutzky J, Creager MA. Association of monocyte tumor necrosis factor α expression and serum inflammatory biomarkers with walking impairment in peripheral artery disease. J Vasc Surg 61: 155–161, 2015. doi: 10.1016/j.jvs.2014.06.116. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Pradhan AD, Rifai N, Ridker PM. Soluble intercellular adhesion molecule-1, soluble vascular adhesion molecule-1, and the development of symptomatic peripheral arterial disease in men. Circulation 106: 820–825, 2002. doi: 10.1161/01.CIR.0000025636.03561.EE. [DOI] [PubMed] [Google Scholar]
- 38.Pradhan AD, Shrivastava S, Cook NR, Rifai N, Creager MA, Ridker PM. Symptomatic peripheral arterial disease in women: nontraditional biomarkers of elevated risk. Circulation 117: 823–831, 2008. doi: 10.1161/CIRCULATIONAHA.107.719369. [DOI] [PubMed] [Google Scholar]
- 39.Ramachandra R, Elmslie KS. Voltage-dependent sodium (NaV) channels in group IV sensory afferents. Mol Pain 12: 1744806916660721, 2016. doi: 10.1177/1744806916660721. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Ramachandra R, McGrew SY, Baxter JC, Howard JR, Elmslie KS. NaV1.8 channels are expressed in large, as well as small, diameter sensory afferent neurons. Channels (Austin) 7: 34–37, 2013. doi: 10.4161/chan.22445. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Ramachandra R, McGrew SY, Baxter JC, Kiveric E, Elmslie KS. Tetrodotoxin-resistant voltage-dependent sodium channels in identified muscle afferent neurons. J Neurophysiol 108: 2230–2241, 2012. doi: 10.1152/jn.00219.2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Ross R. Atherosclerosis: an inflammatory disease. N Engl J Med 340: 115–126, 1999. doi: 10.1056/NEJM199901143400207. [DOI] [PubMed] [Google Scholar]
- 43.Rush AM, Cummins TR, Waxman SG. Multiple sodium channels and their roles in electrogenesis within dorsal root ganglion neurons. J Physiol 579: 1–14, 2007. doi: 10.1113/jphysiol.2006.121483. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Sessenwein JL, Baker CC, Pradhananga S, Maitland ME, Petrof EO, Allen-Vercoe E, Noordhof C, Reed DE, Vanner SJ, Lomax AE. Protease-mediated suppression of DRG neuron excitability by commensal bacteria. J Neurosci 37: 11758–11768, 2017. doi: 10.1523/JNEUROSCI.1672-17.2017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Signorelli SS, Mazzarino MC, Di Pino L, Malaponte G, Porto C, Pennisi G, Marchese G, Costa MP, Digrandi D, Celotta G, Virgilio V. High circulating levels of cytokines (IL-6 and TNFα), adhesion molecules (VCAM-1 and ICAM-1) and selectins in patients with peripheral arterial disease at rest and after a treadmill test. Vasc Med 8: 15–19, 2003. doi: 10.1191/1358863x03vm466oa. [DOI] [PubMed] [Google Scholar]
- 46.Steensberg A, van Hall G, Osada T, Sacchetti M, Saltin B, Klarlund Pedersen B. Production of interleukin-6 in contracting human skeletal muscles can account for the exercise-induced increase in plasma interleukin-6. J Physiol 529: 237–242, 2000. doi: 10.1111/j.1469-7793.2000.00237.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Stone AJ, Copp SW, Kaufman MP. Role played by NaV 1.7 channels on thin-fiber muscle afferents in transmitting the exercise pressor reflex. Am J Physiol Regul Integr Comp Physiol 309: R1301–R1308, 2015. doi: 10.1152/ajpregu.00246.2015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Stone AJ, Kim JS, Yamauchi K, Ruiz-Velasco V, Kaufman MP. Attenuation of autonomic reflexes by A803467 may not be solely caused by blockade of NaV 1.8 channels. Neurosci Lett 543: 177–182, 2013. doi: 10.1016/j.neulet.2013.03.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Tsuchimochi H, McCord JL, Kaufman MP. Peripheral µ-opioid receptors attenuate the augmented exercise pressor reflex in rats with chronic femoral artery occlusion. Am J Physiol Heart Circ Physiol 299: H557–H565, 2010. doi: 10.1152/ajpheart.00387.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Visser M, Pahor M, Taaffe DR, Goodpaster BH, Simonsick EM, Newman AB, Nevitt M, Harris TB. Relationship of interleukin-6 and tumor necrosis factor-α with muscle mass and muscle strength in elderly men and women: the Health ABC Study. J Gerontol A Biol Sci Med Sci 57: M326–M332, 2002. doi: 10.1093/gerona/57.5.M326. [DOI] [PubMed] [Google Scholar]
- 51.Waters RE, Terjung RL, Peters KG, Annex BH. Preclinical models of human peripheral arterial occlusive disease: implications for investigation of therapeutic agents. J Appl Physiol (1985) 97: 773–780, 2004. doi: 10.1152/japplphysiol.00107.2004. [DOI] [PubMed] [Google Scholar]
- 52.Xing J, Lu J, Li J. Acid-sensing ion channel subtype 3 function and immunolabelling increases in skeletal muscle sensory neurons following femoral artery occlusion. J Physiol 590: 1261–1272, 2012. doi: 10.1113/jphysiol.2011.221788. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Xing J, Lu J, Li J. Role of TNF-α in regulating the exercise pressor reflex in rats with femoral artery occlusion. Front Physiol 9: 1461, 2018. doi: 10.3389/fphys.2018.01461. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Yndestad A, Damås JK, Oie E, Ueland T, Gullestad L, Aukrust P. Systemic inflammation in heart failure: the whys and wherefores. Heart Fail Rev 11: 83–92, 2006. doi: 10.1007/s10741-006-9196-2. [DOI] [PubMed] [Google Scholar]