Abstract
Sleep loss contributes to the development of cardiovascular, metabolic, and neurological disorders by promoting a systemic proinflammatory phenotype. The neuroendocrine-immune mechanisms contributing to such pathologies are poorly understood. The sympathetic nervous system (SNS) regulates immunity and is often activated following sleep disturbances. The aims of this study were to determine 1) the effect of SNS inhibition on inflammatory responses to sleep fragmentation (SF) and 2) whether homeostasis can be restored after 1 wk of recovery sleep. We measured stress responses (norepinephrine and corticosterone), gene expression levels of pro- and anti-inflammatory cytokines in peripheral (heart, liver, and spleen) tissues, and protein levels of cytokines and chemokines in serum of female mice that were subjected to acute SF for 24 h, chronic SF for 8 wk, or 7 days of recovery after chronic SF. In each experiment, SF and control mice were chemically sympathectomized with 6-hydroxydopamine (6-OHDA) or injected with vehicle. Both acute and chronic SF elevated mRNA and protein levels of cytokines in peripheral tissues. Changes in inflammatory responses mirrored stress-axes activation, with increased corticosterone and norepinephrine in SF mice. 6-OHDA treatment significantly alleviated SF-induced inflammation, thus providing evidence of SNS regulation of peripheral inflammation from SF. Effects of chronic SF were more severe than acute SF, and 1 wk of recovery from SF sufficiently alleviated peripheral inflammatory responses but not NE responses.
Keywords: cytokines, inflammation, mice, sleep fragmentation, sympathetic nervous system
INTRODUCTION
Sleep is a conserved physiological process that consumes a substantial portion of the lives of most endothermic animals. Among humans, sleep deprivation and/or disruption have become a widespread and serious problem, with an average reduction of nearly 1.5 h of sleep/day in the last century (24). Sleep deprivation and disturbances, like most other stressors, can lead to activation of autonomic and neuroendocrine responses to stress (22). Sleep appears to have suppressive effects on the stress response, and, consequently, sleep dysfunction can increase autonomic sympathetic nervous system (SNS) activation, which culminates in the elevation of norepinephrine (NE) release from sympathetic nerve terminals and increased release of epinephrine from the adrenal medulla. Within minutes, there is an increased activation of the hypothalamic-pituitary-adrenal (HPA) axis, leading to release of glucocorticoids from the adrenal cortices (22, 28, 30). Although the acute effects of sleep deprivation on SNS and HPA activity can be considered mild and potentially adaptive, the chronic effects of disrupted sleep associated with obstructive sleep apnea (OSA), shift work, and modern lifestyle, can be adverse, contributing to the onset of cardiovascular and metabolic diseases, obesity, and neurological disorders (21, 26, 35).
There is increasing evidence that sleep dysfunction promotes inflammatory responses in a variety of tissues. Under normal conditions, immunological function varies with sleep-wake cycles; circulating immune cells fall in the morning and then rise in the early evening (27). It is proposed that this increase in proinflammatory cytokines at the onset of rest is the result of an accumulation of “danger signals” during wakefulness (4). Following sleep deprivation, an increase in proinflammatory cytokines, such as IL-1β, IL-6, and TNF-α, as well as a decrease in immunoregulatory cytokines, such as IL-4 and IL-10, have been reported in mice and humans (2, 6, 13, 18). This induction of inflammation provides a link between sleep disturbances and chronic diseases. For example, a progressive build-up of inflammatory insults in the periphery can promote rupture of plaques and endothelial dysfunction, thus leading to cardiovascular disease (23).
Compared with total sleep deprivation, peripheral and neuroinflammatory responses to sleep fragmentation (SF) have been less explored (7). Chronic sleep fragmentation simulates the interrupted sleep experienced by patients with sleep apnea and other sleep disorders (16). Thus, in the present study, we tested the effects of acute and chronic SF on inflammatory responses in peripheral tissues and sera in female C57BL/6j mice. Given the historical bias toward use of male rodents in research, we decided to use female mice to rectify the incomplete data available that assessed the effects of sleep abnormalities on female physiology. We also accessed whether 7 days of recovery sleep could sufficiently diminish the inflammatory responses from chronic SF. Furthermore, to test a causal linkage between sleep loss-induced SNS hyperactivation and inflammatory responses, we assessed the effect of SF on immune responses in mice that were chemically sympathectomized with 6-hydroxydopamine (6-OHDA). We hypothesized that a successful peripheral sympathectomy would alleviate the inflammatory responses to SF.
MATERIALS AND METHODS
Animals
Female C57BL/6j mice (n = 104) were housed in a colony room (12:12-h light-dark cycle, lights on at 8 AM, 21 ± 1°C) at Western Kentucky University. After being weaned at 21 days of age, mice residing in polypropylene cages with same-sex littermates were provided with corncob bedding and food and water ad libitum. This study was conducted under the approval of the Institutional Animal Care and Use Committee at Western Kentucky University (no. 15-11), and procedures followed the National Institutes of Health’s Guide for the Care and Use of Laboratory Animals and international ethical standards.
Experimental Protocol
Female mice >8 wk of age were selected for experiments with no more than five mice placed in an automated sleep fragmentation chamber (model 80390; Lafayette Instrument Company, Lafayette, IN) with a thin layer of corncob bedding. Food and water were provided ad libitum. Mice were tagged with numbered ear tags and then acclimated to the new caged setting for 72 h before the commencement of sleep fragmentation experiments. A horizontally moving automated sweeping bar at a set interval ensured mice in chambers were subjected to sleep fragmentation and not absolute sleep deprivation (7).
Experiment 1: acute sleep fragmentation.
After an acclimation period of 72 h, 16 mice were chemically sympathectomized with a subcutaneous injection of 6-OHDA (0.1 mg·g body mass−1·day−1) for 5 days while the remaining mice (n = 16) were injected with the same volume of vehicle (100 µL of 0.9% NaCl/day). Importantly, 6-OHDA does not cross the blood-brain barrier in adult mice (14). After the final injection on day 5, experimental mice (n = 16; n = 8 injected with vehicle and n = 8 injected with 6-OHDA) were subjected to acute sleep fragmentation (SF), i.e., a sweeping bar set to move horizontally every 120 s for 24 h. Controls (n = 16; n = 8 injected with vehicle and n = 8 injected with 6-OHDA) received no sweeping bar movements.
Experiment 2: chronic sleep fragmentation.
To induce chronic sleep fragmentation, mice (n = 40) were subjected to a horizontal sweeping bar set to move every 120 s (30 swipes/h) during the light phase (i.e., from 8:00 AM to 8:00 PM) every day for 8 wk. To account for increased activity from daily chronic SF, control mice (n = 20) were subjected to the same number of bar sweeps as experimental mice for 3 h of the light phase (i.e., from 8:00 AM to 11:00 AM), albeit at four times the speed, i.e., 2 swipes/min. During the first light hour (8:00 AM to 9:00 AM) of 5 days before termination of the experiment, mice were subcutaneously injected with either 6-OHDA (0.1 mg·g body mass−1·day−1; n = 10 SF mice, n = 10 control mice) or vehicle (100 µL of 0.9% NaCl/day; n = 10 SF mice, n = 10 control mice). Mice were weighed on an electronic scale (to the nearest 0.1 g) every week.
Experiment 3: chronic sleep fragmentation + recovery.
Mice were subjected to the chronic SF protocol of experiment 2, but post-SF, control (n = 16) and SF mice (n = 16) were subjected to a recovery period (no bar movement) of 7 days during, which they were injected with either 6-OHDA [0.1 mg·g body mass−1·day−1; n = 8 chronic SF + recovery (R) mice, n = 8 control mice] or vehicle (100 µL of 0.9% NaCl/day; n = 8 SF + R mice, n = 8 of control mice) for 5 days. Mice were weighed on an electronic scale (to the nearest 0.1 g) every week.
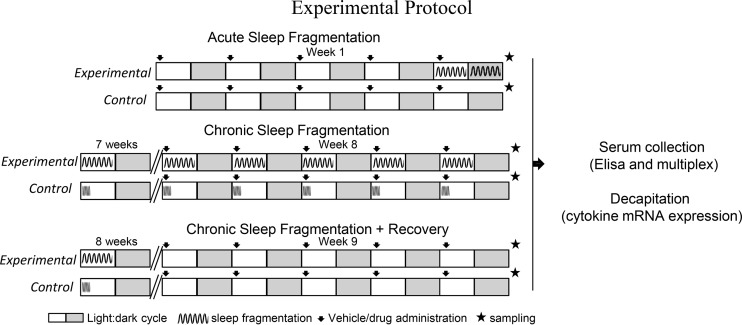
In all three experiments, 24 h following the final drug administration (8:00 AM), mice were deeply anesthetized using isoflurane vapors (<2 min) and then rapidly decapitated in <3 min of initial handling for the serum protein study and tissue gene expression studies. Trunk blood from decapitated mice was collected, kept on ice for <20 min, and then spun at 3,000 g for 30 min at 4°C. The serum was drawn out and stored at −20°C for later ELISA and multiplex analyses. For gene expression studies, heart, liver, and spleen were dissected from decapitated mice and stored in RNAlater solution (Thermo Fischer Scientific). The tissue samples were stored at 4°C until RNA extraction. An experimental timeline is provided in Fig. 1.
Fig. 1.
Experimental protocol. Three experiments were performed with experimental mice subjected to sleep fragmentation (SF) in an automated sleep fragmentation chamber for 24 h (experiment 1: acute SF), 8 wk (experiment 2: chronic SF), or 8 wk of chronic SF followed by undisturbed sleep of 1 wk (experiment 3: chronic SF + recovery). A horizontally moving automated sweeping bar set to swipe across the chamber every 2 min ensured experimental group mice were sleep fragmented. Controls in the chronic SF and recovery experiment were also subjected to a sweeping bar, although at a higher speed and for lesser duration (2 swipes/min for 3 h aligned with lights on) to control for the increased activity-induced effects between groups. During the recovery phase, mice of both control and experimental groups were not subjected to sweeping bar swipes. On the last 5 days of the experiments, mice were injected sc with 0.9% saline or 6-hydroxydopamine. After 24 h of final injection, mice were decapitated for serum protein study and tissue gene expression study. All mice were >8 wk of age and were subjected to 12:12-h light-dark cycles with lights on at 8:00 AM and lights off at 8:00 PM, and provided food and water ad libitum. The white and gray boxes indicate light and dark phase, respectively, of a 24-h day.
Measurement of Gene Expression
RNA was extracted from heart, liver, and spleen (7) using a RNeasy mini kit (Qiagen). RNA concentrations were measured using a NanoDrop 2000 Spectrophotometer (ThermoScientific). Total RNA was reverse transcribed into cDNA using a high-capacity cDNA reverse transcription kit (catalog no. 4368813; Life Technologies). The prepared cDNA was used as a template for determining relative cytokine gene expression using an ABI 7300 RT-PCR system. Cytokine probes [IL1β, IL-6, TNFα, transforming growth factor-β (TGFβ); Applied Biosystems] labeled with fluorescent marker 5-FAM at the 5′-end and quencher MGB at the 3′-end were used for genes of interest along with 18S (VIC-labeled probe) as the endogenous control according to the manufacturer’s instructions. Samples were run in duplicates, and the fold change in mRNA levels was calculated as the relative mRNA expression levels, 2−ΔΔCt (19). Briefly, the cycle threshold (Ct) obtained by fluorescence exceeding background levels was used to calculate ΔCt[Ct(target gene) − Ct(18S)]. Each Ct value was normalized against the lowest Ct value of a control sample. The negative value of this powered to 2 (2−ΔΔCt) was plotted.
Luminex Multiplex Cytokine/Chemokine Assay
Serum levels of cytokine and chemokine concentrations in all three experiments (n = 6/group) were measured in a magnetic 20-plex Luminex assay (catalog no. LMC0006M; Life Technologies) as per the manufacturer’s protocol. A five-parameter logistic curve generated from standards of known concentration was used to convert fluorescent intensity to concentration values. The analyzed molecules were fibroblast growth factor (FGF), interleukin-1α (IL-1α), interleukin-1β (IL-1β), interleukin-2 (IL-2), interleukin-4 (IL-4), interleukin-5 (IL-5), interleukin-6 (IL-6), interleukin-10 (IL-10), interleukin-12 (IL-12), interleukin-13 (IL-13), interleukin-17 (IL-17), macrophage inflammatory protein-α (MIP-1α), monocyte chemoattractant protein-1 (MCP-1), vascular endothelial growth factor (VEGF), interferon-γ (IFN-γ), tumor necrosis factor-α (TNF-α), interferon γ-induced protein 10 (IP-10), monokine induced by interferon-γ (MIG), and keratinocyte chemoattractant (KC).
ELISA
Serum levels of norepinephrine (n = 6/group), corticosterone (cort, n = 8/group) and TGF-β (n = 7/group) were measured in 50, 10, and 5 µL of serum samples, respectively, in all three experiments, as per the manufacturer’s protocols [norepinephrine (catalog no. IB89537; IBL America), corticosterone (catalog no. ADI-900-097; Enzo Life Sciences), and TGF-β (catalog no. ab119557; Abcam)]. Sample sizes varied slightly depending on the remaining volume of the serum sample.
Sleep Recording
Noninvasive real-time monitoring of sleep was performed using piezoelectric technology, as described previously (34). Briefly, mice individually housed in polypropylene cages equipped with a piezoelectric floor (Signal Solutions, Lexington, KY) were injected with vehicle (n = 4) or 6-OHDA (n = 4) for 5 days, similar to the experimental protocols described previously. After the last injection (24 h), sleep was recorded for 48 h to discern the effects of 6-OHDA treatment on sleep. Percent sleep output, i.e., a 100% implying 60 min spent sleeping/60 min duration and 0% implying 0 min spent sleeping/60 min duration, was calculated for 24 h and statistically analyzed to assess the effect of drug treatment on sleep [Supplemental Fig. S1(https://doi.org/10.6084/m9.figshare.11923452.v1)]. Furthermore, in another experiment, mice were subjected to a sweeping bar every 2 min for 12 h to test if the protocol sufficiently induced sleep fragmentation (Supplemental Fig. S1).
Statistical Analysis
Data are presented as means ± SE. All statistical analyses were done using GraphPad prism (version 6.0) and IBM SPSS Statistics version 20 software, as appropriate. A two-way ANOVA assessed the effect of 6-OHDA treatment (factor 1), time-of-day (factor 2), and their interaction (factor 1 × 2) on hourly sleep pattern. A Student’s t test assessed the effect of 6-OHDA on total sleep duration/24 h in the pilot study. Two-way ANOVAs assessed the effect of sleep fragmentation (factor 1), 6-OHDA treatment (factor 2), and their interaction on the serum levels of hormones, cytokines and chemokines, and mRNA expression of cytokines. Bonferroni multiple comparisons were used for post hoc analysis. The effect size estimates are presented as partial eta squared (η2) for parameters tested in sample size of <8 mice/group. P < 0.05 was considered statistically significant.
RESULTS
SF-Induced Effects on Stress Response
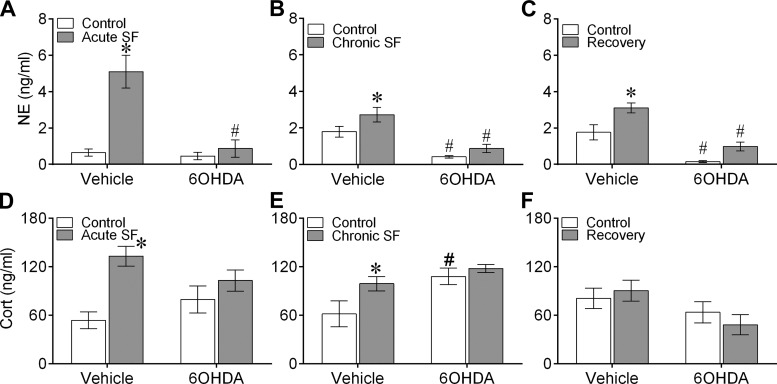
Serum NE and cort levels were significantly affected by SF in all three experiments. Serum NE response was significantly affected by acute SF [F(1,20) = 21.12, P = 0.0002, η2 = 0.64], 6-OHDA treatment [F(1,20) = 17.36, P = 0.0005, η2 = 0.59], and their interaction [F(1,20) = 14.60, P = 0.0011, η2 = 0.55] as determined by two-way ANOVA. Mice subjected to acute SF showed elevated serum NE response compared with controls; however, this SF-induced spike in NE was reduced with pretreatment of 6-OHDA (Bonferroni post hoc test, P < 0.05; Fig. 2A). Similarly, there was a significant effect of chronic SF [F(1,19) = 6.86, P = 0.017, η2 = 0.12] and 6-OHDA treatment [F(1,19) = 37.48, P < 0.0001, η2 = 0.42] on serum NE levels, with mice subjected to chronic SF exhibiting elevated serum NE relative to controls; treatment of 6-OHDA significantly downregulated the serum NE response in both control and chronic SF mice (Bonferroni post hoc test, P < 0.05; Fig. 2B). Interestingly, a recovery period of 1 wk was not sufficient to reduce the elevation of the NE response from chronic SF. There was a significant effect of recovery [F(1,20) = 15.07, P = 0.0009, η2 = 0.26] and 6-OHDA treatment [F(1,20) = 44.38, P < 0.0001, η2 = 0.51], with higher NE levels in recovery mice compared with controls. However, 6-OHDA treatment significantly attenuated the NE response in control and recovery mice (Bonferroni post hoc test, P < 0.05; Fig. 2C).
Fig. 2.
Sleep fragmentation (SF) altered serum catecholamine levels. Mean ± SE serum norepinephrine (NE; A–C) and corticosterone (cort; D–F) levels in vehicle-injected or chemically sympathectomized mice subjected to acute SF (n = 6/group, A and D), chronic SF (n = 6/group, B and E), or 1 wk of recovery following chronic SF (n = 6/group, C and F). 6-OHDA, 6-hydroxydopamine. Significant effect of SF (*) and chemical sympathectomy (#) as determined by Bonferroni post hoc test followed by 2-way ANOVA. For statistical significance, α was set at 0.05.
There was a significant effect of acute SF, but not 6-OHDA, on serum cort levels, as determined by two-way ANOVA [F(1,28) = 13.76, P = 0.001]. Mice subjected to acute SF had significantly higher cort levels than controls (Bonferroni post hoc test, P < 0.05; Fig. 2D). Cort levels were significantly affected by chronic SF [F(1,28) = 4.87, P = 0.036] and 6-OHDA treatment [F(1,28) = 9.23, P = 0.005] in the second experiment, and 6-OHDA treatment alone in the recovery experiment [F(1,28) = 5.39, P = 0.028], as determined by two-way ANOVA. Whereas chronic SF mice had significantly higher cort levels than controls, 6-OHDA treatment in controls significantly augmented the cort response (Bonferroni post hoc test, P < 0.05, Fig. 2E). There was no statistical difference between experimental groups in the recovery experiment (Fig. 2F). Furthermore, body mass did not differ among mice exposed to chronic SF, chronic SF + R, or controls (data not shown).
SF-Induced Effects on Peripheral Inflammatory Responses
Cytokine mRNA expression levels in peripheral tissues.
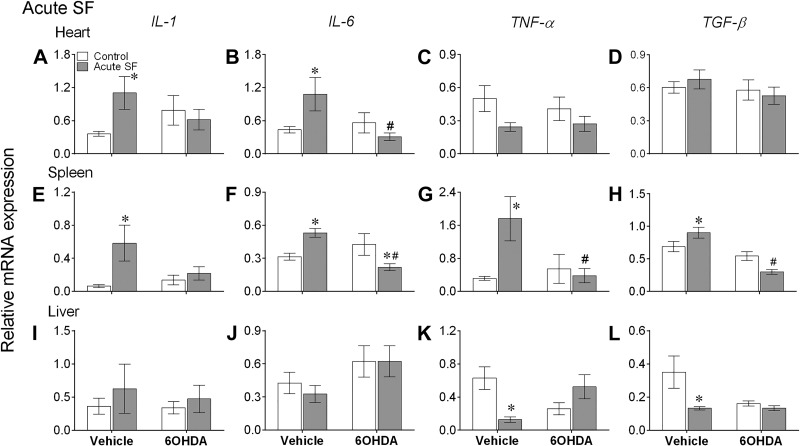
In heart, acute SF significantly affected TNFα expression levels [F(1,28) = 5.04, P = 0.0032], whereas acute SF-induced effects on IL-1β and IL-6 were dependent on 6-OHDA [IL-1β: F(1,28) = 4.34, P = 0.046, IL-6: F(1,28) = 6.47, P = 0.016; 2-way ANOVA]. Likewise, there was a significant effect of acute SF on IL-1β [F(1,28) = 6.29, P = 0.018], 6-OHDA on TGFβ [F(1,28) = 28.48, P < 0.0001] and an acute SF × 6-OHDA interaction on IL-6 [F(1,28) = 11.59, P = 0.000], TNFα [F(1,28) = 5.87, P = 0.023] and TGFβ [F(1,28) = 10.74, P = 0.003, 2-way ANOVA] in spleen. In liver, there was a significant effect of acute SF on TGFβ [F(1,28) = 6.08, P = 0.020], 6-OHDA on IL-6 [F(1,28) = 4.35, P = 0.046], and an effect of acute SF × 6-OHDA interaction on TNFα [F(1,28) = 12.69, P = 0.002], as determined by two-way ANOVA (Fig. 3).
Fig. 3.
Acute sleep fragmentation (SF) altered cytokine mRNA expression levels in peripheral tissues. Mean ± SE gene expression levels of interleukin (IL)-1, IL-6, tumor necrosis factor-α (TNFα), and TGFβ in heart (A–D), spleen (E–H), and liver (I–L) of vehicle-injected or chemically sympathectomized mice subjected to control or acute SF (n = 8/group). 6-OHDA, 6-hydroxydopamine. Significant effect of SF (*) and chemical sympathectomy (#) as determined by Bonferroni post hoc test followed by 2-way ANOVA. For statistical significance, α was set at 0.05.
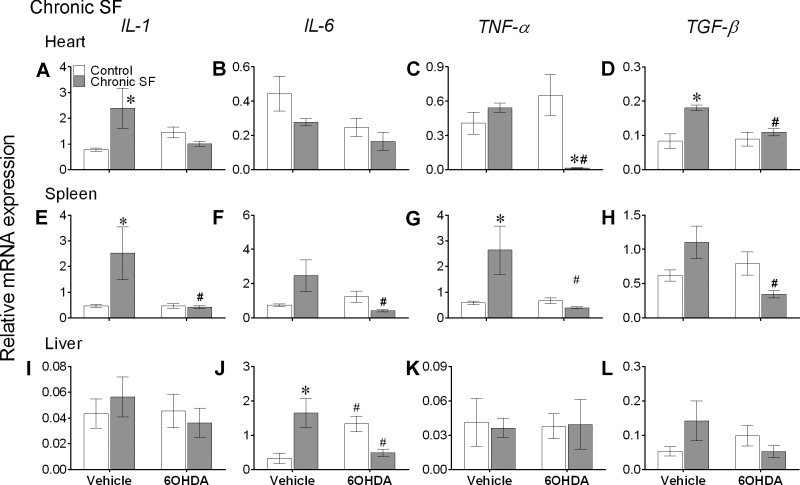
Chronic SF significantly affected IL-6 and TNFα expression in heart [IL-6: F(1,30) = 4.55, P = 0.042; TNFα: F(1,30) = 6.48, P = 0.016], whereas 6-OHDA treatment significantly affected the IL-1β expression in spleen [F(1,30) = 4.16, P = 0.049] and IL-6 in heart [F(1,30) = 7.03, P = 0.013]. In addition, chronic SF induced effects on IL-1β and TNFα expression in heart [IL-1β: F(1,30) = 4.88, P = 0.034; TNFα: F(1,30) = 15.14, P = 0.0005] and spleen [IL-1β: F(1,30) = 4.12, P = 0.050; TNFα: F(1,30) = 4.99, P = 0.033], along with IL-6 in liver and spleen [liver: F(1,26) = 13.55, P = 0.001; spleen: F(1,30) = 5.09, P = 0.031] were dependent on 6-OHDA treatment (2-way ANOVA). TGFβ expression in heart was significantly affected by chronic SF [F(1,30) = 14.49, P = 0.0007], 6-OHDA [F(1,30) = 4.44, P = 0.044], and their interaction [F(1,30) = 6.21, P = 0.018]. There was a significant interaction effect in spleen [F(1,30) = 8.72, P = 0.006], as determined by two-way ANOVA (Fig. 4).
Fig. 4.
Chronic sleep fragmentation (SF) altered cytokine mRNA expression levels in peripheral tissues. Mean ± SE gene expression levels of interleukin (IL)-1, IL-6, tumor necrosis factor-α (TNFα), and transforming growth factor-β (TGFβ) in heart (A–D), spleen (E–H), and liver (I–L) of vehicle-injected or chemically sympathectomized mice subjected to control or chronic SF (n = 8/group except for chronic SF + vehicle group where n = 10 mice). 6-OHDA, 6-hydroxydopamine. Significant effect of SF (*) and chemical sympathectomy (#) as determined by Bonferroni post hoc test followed by 2-way ANOVA. For statistical significance, α was set at 0.05.
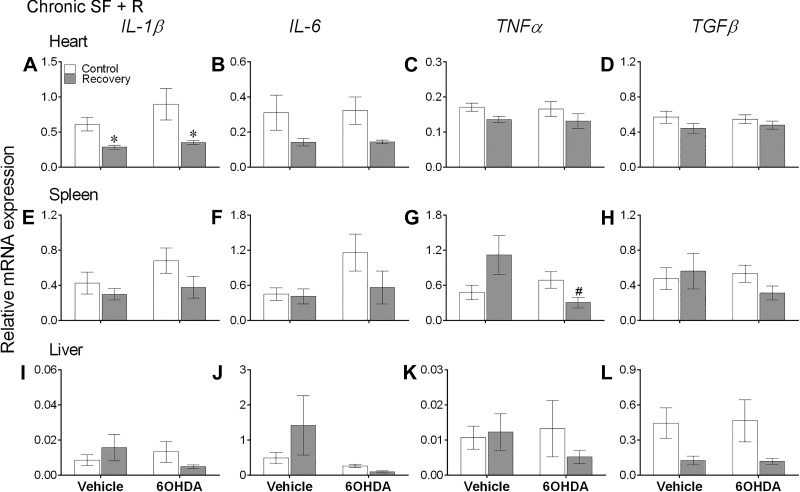
A 1-wk recovery period significantly affected IL-1β, IL-6, and TNFα expression levels in heart [IL-1β: F(1,28) = 12.52, P = 0.001; IL-6: F(1,28) = 7.27, P = 0.012; TNFα: F(1,28) = 4.298, P = 0.047] and TGFβ expression levels in liver [F(1,26) = 8.22, P = 0.008], whereas the recovery-induced effects on TNFα in spleen were dependent on 6-OHDA administration [interaction: F(1,28) = 6.97, P = 0.014, 2-way ANOVA; Fig. 5].
Fig. 5.
One week of recovery altered cytokine mRNA expression levels in peripheral tissues. Mean ± SE gene expression levels of interleukin (IL)-1, IL-6, tumor necrosis factor-α (TNFα), and transforming growth factor-β (TGFβ) in heart (A–D), spleen (E–H), and liver (I–L) of vehicle-injected or chemically sympathectomized mice subjected to control or 1 wk of recovery following chronic sleep fragmentation (SF, n = 8/group). During the recovery phase (R), mice of both control and experimental groups were not subjected to sweeping bar swipes. 6-OHDA, 6-hydroxydopamine. Significant effect of SF (*) and chemical sympathectomy (#) as determined by Bonferroni post hoc test followed by 2-way ANOVA. For statistical significance, α was set at 0.05.
Acute SF significantly increased expression of IL-1β and IL-6 in heart, and all cytokines in spleen, while causing a significant decrease in TNFα and TGFβ in liver (Bonferroni post hoc test, P < 0.05; Fig. 3, A, B, E–H, K, and L). Likewise, chronic SF increased expression of IL-1β and TGFβ in heart, IL-1β and TNFα in spleen, and IL-6 in heart (Bonferroni post hoc test, P < 0.05; Fig. 4, A, D, E, G, and J). There were no significant differences in expression levels of cytokines in peripheral tissues (except IL-1β in heart) of vehicle-treated controls and recovery mice (Bonferroni post hoc test; Fig. 5).
Furthermore, decreased expression of IL-6, TNFα, and TGFβ levels in spleen, along with IL-6 in heart, with the administration of 6-OHDA to acute SF mice indicates that chemical sympathectomy significantly attenuated the inflammatory responses to acute SF (Bonferroni post hoc test, P < 0.05; Fig. 3, B and F–H). A similar effect of 6-OHDA treatment was seen in the chronic SF and recovery experiments; 6-OHDA administered to chronic SF mice significantly reduced expression levels of all cytokines in spleen, TNFα and TGFβ in heart, and IL-6 in liver (Bonferroni post hoc test, P < 0.05; Fig. 4, C, D, E–H, and J), and 6-OHDA administered to recovery mice significantly reduced TNFα levels in spleen (Bonferroni post hoc test, P < 0.05; Fig. 5G).
Serum cytokines and chemokines.
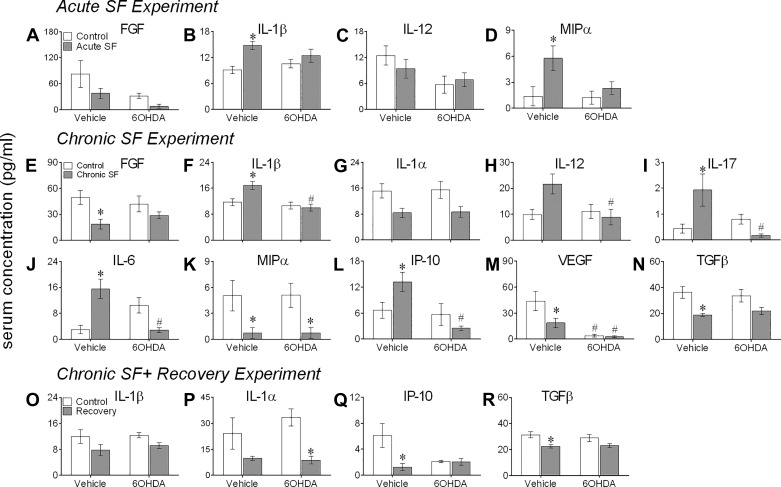
There was a significant effect of 6-OHDA treatment on serum levels of FGF [F(1,20) = 5.58, P = 0.028, η2 = 0.22] and IL-12 [F(1,20) = 5.45, P = 0.030, η2 = 0.21], and of acute SF on IL-1β [F(1,20) = 11.97, P = 0.003, η2 = 0.37] and MIP-α [F(1,20) = 6.78, P = 0.017, η2 = 0.25, 2-way ANOVA], with IL-1β and MIP-α being higher in mice subjected to acute SF compared with controls (Bonferroni post hoc test, P < 0.05; Fig. 6, A–D).
Fig. 6.
Sleep fragmentation (SF) altered serum levels of cytokines and chemokines. Mean ± SE protein levels of cytokines and chemokines in vehicle-injected or chemically sympathectomized mice subjected to acute SF (n = 6/group, A–D), chronic SF (n = 6/group, E–N), or 1 wk of recovery following chronic SF (n = 6/group, O–R). Protein levels were measured in individual serum specimens using multiplex magnetic bead technology for the simultaneous measurement of the 20 cytokines/chemokines. Transforming growth factor-β (TGF-β) levels were measured separately using an ELISA in serum samples (n = 7/group). FGF, fibroblast growth factor; IL-1α, interleukin-1α; IL-1β, interleukin-1β; IL-6, interleukin-6; IL-12, interleukin-12; IL-17, interleukin-17; MIP-α, macrophage inflammatory protein-α; VEGF, vascular endothelial growth factor; IP-10, interferon γ-induced protein 10; 6-OHDA, 6-hydroxydopamine. Shown is the expression of the detected molecules that showed a significant effect of SF and/or chemical sympathectomy, as determined by 2-way ANOVA. Between-group differences were determined by Bonferroni post hoc test, with significant effects of sleep fragmentation (*) and chemical sympathectomy (#) indicated. For statistical significance, α was set at 0.05.
Compared with acute SF, 8 wk of SF had more profound effects on serum cytokine response (Fig. 6, E–N), with significant effects of chronic SF on FGF [F(1,20) = 9.96, P = 0.006, η2 = 0.32], IL-1α [F(1,20) = 10.61, P = 0.004, η2 = 0.35], MIP-α [F(1,20) = 13.28, P = 0.002, η2 = 0.40], VEGF [F(1,20) = 4.52, P = 0.046, η2 = 0.18], and TGF-β [F(1,20) = 15.33, P = 0.007, η2 = 0.43], and its interaction with 6-OHDA treatment on IL-1β [F(1,20) = 6.83, P = 0.016, η2 = 0.25], IL-12 [F(1,20) = 5.54, P = 0.029, η2 = 0.22], IL-17 [F(1,20) = 9.99, P = 0.004, η2 = 0.33], IL-6 [F(1,20) = 24.11, P < 0.0001, η2 = 0.55], and IP-10 [F(1,20) = 6.617, P = 0.018, η2 = 0.25], as determined by two-way ANOVA. There was a significant effect of 6-OHDA treatment on serum levels of IL-1β [F(1,20) = 12.54, P = 0.0020, η2 = 0.39], IP-10 [F(1,20) = 9.64, P = 0.005, η2 = 0.33] and IL17 [F(1,20) = 20.10, P = 0.0002, η2 = 0.33; 2-way ANOVA]; serum FGF, MIP-α, VEGF, and TGF-β levels in mice subjected to chronic SF were significantly lower than the controls (Bonferroni post hoc test, P < 0.05; Fig. 6, E, K, M, and N); and the levels of IL-β, IL-17, IL-6, and IP-10 in chronic SF mice were significantly higher than the controls and chronic SF mice that received 6-OHDA treatment (Bonferroni post hoc test, P < 0.05; Fig. 6, F, I, J, and L). Also, IL-12 and VEGF in mice subjected to chronic SF were significantly higher than their counterparts that received 6-OHDA treatment (Bonferroni post hoc test, P < 0.05; Fig. 6, H and M). Furthermore, MIP-α was significantly higher in 6-OHDA-treated controls compared with 6-OHDA-treated chronic SF mice (Bonferroni post hoc test, P < 0.05; Fig. 6K).
Furthermore, there was a significant effect of recovery on serum levels of IL-1α [F(1,20) = 13.56, P = 0.015], IL-β [F(1,20) = 6.13, P = 0.022], IP-10 [F(1,20) = 6.17, P = 0.022], and TGF-β [F(1,20) = 13.02, P = 0.018], and of its interaction with 6-OHDA on IL-1β [F(1,20) = 5.76, P = 0.026], as determined by two-way ANOVA (Fig. 6, O–R). One week of recovery significantly reduced the serum levels of IP-10 and TGF-β (Bonferroni post hoc test, P < 0.05; Fig. 6, Q and R), and 6-OHDA-treated recovery mice had significantly lower serum levels of IL-1α compared with 6-OHDA-treated controls (Bonferroni post hoc test, P < 0.05; Fig. 6Q).
Effect of 6-OHDA Treatment on Sleep
6-OHDA treatment did not affect sleep, since there were no significant differences in the time spent sleeping per day or the 24-h pattern of sleep between mice injected with vehicle or 6-OHDA (Supplemental Fig. S1). In addition, mice exhibited sleep between intermittent bar sweeps (every 2 min), indicating that the method used to fragment sleep was effective (Supplemental Fig. S1).
DISCUSSION
The present findings show that SF affected the autonomic and neuroendocrine stress systems as evidenced by elevated NE and cort concentrations in sera. Chemically sympathectomized mice exhibited blunted NE responses, which was expected. These results were coupled with an increase in peripheral inflammatory mediators that included elevated proinflammatory gene expression in mice exposed to acute or chronic SF in notably heart and spleen. In addition, proinflammatory cytokines were elevated in the sera of mice exposed to acute SF, and more so in mice exposed to chronic SF. Importantly, pharmacological sympathectomy by 6-OHDA was sufficient to prevent an increase in the majority of proinflammatory cytokines measured among SF mice. Last, mice given an opportunity to sleep normally for 1 wk following chronic SF largely recovered from these peripheral inflammatory responses, except that a recovery period of 1 wk was not sufficient to reduce the elevation of NE response from chronic SF. This suggested that the SF-induced inflammatory phenotype can potentially be reversed, however, with an appropriate recovery period.
Sleep can have suppressive effects on the stress response, and SF-induced activation of stress response is not uncommon (22). Interestingly, the magnitude of the NE response under chronic SF was lower than that under acute SF. Accumulated fatigue from an absence of consolidated sleep for several weeks perhaps attributed to a state of sympathetic exhaustion (22). Alternatively, this decreased sympathoexcitation could be because of chronic SF mice being forced to catch up on sleep more during the night than day. Furthermore, one week of recovery sleep was insufficient to fully alleviate chronic SF-induced activation of the stress response, as reflected by increased levels of NE in recovery mice (11). We suggest that this activation of the stress axis was a consequence of SF alone and not a consequence of the procedure used for inducing SF because both experimental and control mice in each chronic experiment had similar forced locomotion treatments. Furthermore, a significant decrease in NE after 6-OHDA treatment indicates peripheral sympathectomy was successful in all three experiments. In addition, in pilot studies, 6-OHDA treatment did not affect overall sleep duration and daily sleep pattern in pilot studies compared with controls as measured using noninvasive piezoelectric technology (Supplemental Fig. S1). Reduced plasma cort levels in sympathectomized acute SF mice indicated that SNS contributed to the glucocorticoid response under SF (20). On the other hand, plasma cort levels in the controls were similar, irrespective of 6-OHDA treatment, except for control mice of the chronic SF study; this suggested a limited contribution of SNS on circulating glucocorticoids under nonstressed conditions (17, 20).
Elevated sympathetic tone and cort response following exposure to stressors, including sleep deprivation, are known to mediate deleterious physiological and psychological effects through hyperactivation of inflammatory responses in the brain and periphery (21, 26, 36). Even a single night of insufficient sleep increases proinflammatory cytokines, such as IL-1β, IL-6, and TNFα, along with a decrease in anti-inflammatory cytokines, such as IL-4 and IL-10, in mice and humans (6, 7, 13, 18). Similarly, in the present study, we found significant elevation in splenic and cardiac mRNA expression of IL-1β, IL-6, and TNFα (except in heart) after acute SF and in splenic IL-1β and TNFα expression and cardiac IL-1β expression after chronic SF (cf. Figs. 4 and 5). A significant decrease in TGFβ expression suggests a reduction in anti-inflammatory actions, whereas a significant increase in IL-6 indicates an elevated proinflammatory response in the liver of acute and chronic SF mice, respectively. However, contrary to our expectations, hepatic TNFα levels were significantly reduced after acute SF, perhaps attributing to extrainflammatory functions of hepatic TNF-α signaling pathways, including the regulation of hepatocyte proliferation and regeneration (25). Increases in TGFβ expression in spleen and heart of acute and chronic SF mice, respectively, corroborated previous studies suggesting heightened protection from sleep deprivation-induced inflammation being mediated through an elevation in levels of anti-inflammatory cytokines (7, 33).
This SF-induced peripheral immune activation at the transcription level was corroborated by changes in plasma cytokine protein levels, with a significant increase in plasma levels of IL-1β, IL-6, and IL-17 (chronic SF only) in mice subjected to SF. These findings are consistent with elevated serum levels of IL-6 that are associated with sleep disturbances in humans (12). However, it is unclear why mice exposed to acute SF exhibited increases in cardiac and splenic IL-6 mRNA but no elevations in plasma levels. In this study, more serious consequences of SF were evident with chronic exposure, with significant reductions in chemokines such as FGF, VEGF, and MIP-1α and an increase in IP-10. Stress-induced reduction in plasma levels of VEGF have been previously reported (29). Chronic SF-induced adverse effects on the cardiovascular system and muscle atrophy might be consequences of reduced FGF, a key regulator of growth and function of endothelial and smooth muscle cells (31). Similarly, elevated levels of IP-10 have been reported to correlate with sleep deficits associated with metabolic dysfunction in humans (15). This study cannot explain the opposite trends of MIP, however. Well known for its chemotactic and proinflammatory effects (10), MIP was significantly increased and decreased under acute and chronic SF, respectively; thus, further investigation is warranted.
Our initial hypothesis that the SNS contributes to inflammatory responses to sleep loss has been supported, since chemical sympathectomy significantly altered the inflammatory responses in all three SF paradigms examined. However, SF-induced inflammatory processes cannot be attributed to SNS alone, since SF-induced changes in plasma MIP-1α and hepatic IL-6 after chronic SF and cardiac IL-1β after recovery sleep were the same with and without 6-OHDA treatment. These results reiterate the complexities of potential neuro-endocrine-immune interactions by which SF can affect immune functioning (3, 8).
Given the sexual bias in susceptibility to diseases, including neurological and immune diseases (31, 32), this study provides important information on effects of sleep disruption on female peripheral immune responses. Sex steroids regulate transcription of genes relevant to development and maturation of immune cells and responses (31). As a result, basic inflammation response differs between the sexes (9, 32). Thus, we would refrain from suggesting that results reported in this study can be extended to males as well. Paradoxical sleep deprivation has been shown to affect estrous cyclicity and the underlying hormonal altercations in rats (1). Because we did not test the reproductive status of mice in our study, the involvement of reproductive hormone-mediated mechanisms in SF-induced reported effects cannot be ruled out. It is of note that restraint stress has been shown to affect phasing of the estrous cycle in female rats, albeit with great individual variation (5). Given the small sample size in our study, we also speculated a limited representation of animals in different estrous phase. Future studies with a greater sample size to include analysis relative to estrous phases is warranted.
Perspectives and Significance
This study systematically evaluated the SF-associated peripheral inflammatory responses at the gene and protein expression levels in female mice. Changes in inflammatory responses seemed to be reflective of stress-axes activation and were related to the duration of SF. One week of recovery from sleep fragmentation sufficiently alleviated peripheral inflammatory responses but not norepinephrine responses. Unlike previous reports, our experimental approach of exposing mice to 8 wk of SF can more accurately be expected to mimic sleep abnormalities, such as OSA and the diseases associated with it. Finally, chemical sympathectomy significantly alleviated sleep fragmentation-induced inflammation, thus providing evidence of a critical contribution of SNS in development of an inflammatory state. These findings could lead to novel therapeutic interventions that target the SNS for treating inflammation-dependent disorders, such as cardiovascular diseases.
GRANTS
This research was supported by the National Institute of General Medical Sciences Grant R15-GM-117534 to N. T. Ashley.
DISCLOSURES
No conflicts of interest, financial or otherwise, are declared by the authors.
AUTHOR CONTRIBUTIONS
I.M., K.B.P., D.C.T., E.R.P., and B.W.C. performed experiments; I.M., K.B.P., D.C.T., E.R.P., B.W.C., and N.T.A. analyzed data; I.M., D.C.T., E.R.P., A.J.M., B.F.O., G.E.D., and N.T.A. interpreted results of experiments; I.M. prepared figures; I.M. drafted manuscript; I.M., A.J.M., B.F.O., G.E.D., and N.T.A. edited and revised manuscript; I.M., K.B.P., D.C.T., E.R.P., B.W.C., B.F.O., G.E.D., and N.T.A. approved final version of manuscript; N.T.A. conceived and designed research.
ACKNOWLEDGMENTS
We thank Melanie Richter and Nicholas Wheeler for sampling, and Naomi Rowland for assistance with RT-PCR.
REFERENCES
- 1.Antunes IB, Andersen ML, Baracat EC, Tufik S. The effects of paradoxical sleep deprivation on estrous cycles of the female rats. Horm Behav 49: 433–440, 2006. doi: 10.1016/j.yhbeh.2005.09.005. [DOI] [PubMed] [Google Scholar]
- 2.Ashley NT, Sams DW, Brown AC, Dumaine JE. Novel environment influences the effect of paradoxical sleep deprivation upon brain and peripheral cytokine gene expression. Neurosci Lett 615: 55–59, 2016. doi: 10.1016/j.neulet.2016.01.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Ashley NT, Demas GE. Neuroendocrine-immune circuits, phenotypes, and interactions. Horm Behav 87: 25–34, 2017. doi: 10.1016/j.yhbeh.2016.10.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Besedovsky L, Lange T, Born J. Sleep and immune function. Pflugers Arch 463: 121–137, 2012. doi: 10.1007/s00424-011-1044-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Bollinger JL, Bergeon Burns CM, Wellman CL. Differential effects of stress on microglial cell activation in male and female medial prefrontal cortex. Brain Behav Immun 52: 88–97, 2016. doi: 10.1016/j.bbi.2015.10.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Chennaoui M, Sauvet F, Drogou C, Van Beers P, Langrume C, Guillard M, Gourby B, Bourrilhon C, Florence G, Gomez-Merino D. Effect of one night of sleep loss on changes in tumor necrosis factor alpha (TNF-α) levels in healthy men. Cytokine 56: 318–324, 2011. doi: 10.1016/j.cyto.2011.06.002. [DOI] [PubMed] [Google Scholar]
- 7.Dumaine JE, Ashley NT. Acute sleep fragmentation induces tissue-specific changes in cytokine gene expression and increases serum corticosterone concentration. Am J Physiol Regul Integr Comp Physiol 308: R1062–R1069, 2015. doi: 10.1152/ajpregu.00049.2015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Faraut B, Boudjeltia KZ, Vanhamme L, Kerkhofs M. Immune, inflammatory and cardiovascular consequences of sleep restriction and recovery. Sleep Med Rev 16: 137–149, 2012. doi: 10.1016/j.smrv.2011.05.001. [DOI] [PubMed] [Google Scholar]
- 9.Guneykaya D, Ivanov A, Hernandez DP, Haage V, Wojtas B, Meyer N, Maricos M, Jordan P, Buonfiglioli A, Gielniewski B, Ochocka N, Cömert C, Friedrich C, Artiles LS, Kaminska B, Mertins P, Beule D, Kettenmann H, Wolf SA. Transcriptional and translational differences of microglia from male and female brains. Cell Rep 24: 2773–2783.e6, 2018. doi: 10.1016/j.celrep.2018.08.001. [DOI] [PubMed] [Google Scholar]
- 10.Hu J, Chen Z, Gorczynski CP, Gorczynski LY, Kai Y, Lee L, Manuel J, Gorczynski RM. Sleep-deprived mice show altered cytokine production manifest by perturbations in serum IL-1ra, TNFa, and IL-6 levels. Brain Behav Immun 17: 498–504, 2003. doi: 10.1016/j.bbi.2003.03.001. [DOI] [PubMed] [Google Scholar]
- 11.Hurtado-Alvarado G, Pavón L, Castillo-García SA, Hernández ME, Domínguez-Salazar E, Velázquez-Moctezuma J, Gómez-González B. Sleep loss as a factor to induce cellular and molecular inflammatory variations. Clin Dev Immunol 2013: 801341, 2013. doi: 10.1155/2013/801341. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Irwin MR, Olmstead R, Carroll JE. Sleep disturbance, sleep duration, and inflammation: a systematic review of meta-analysis of cohort studies and experimental sleep deprivation. Biol Psychiatry 80: 40–52, 2016. doi: 10.1016/j.biopsych.2015.05.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Irwin MR, Wang M, Campomayor CO, Collado-Hidalgo A, Cole S. Sleep deprivation and activation of morning levels of cellular and genomic markers of inflammation. Arch Intern Med 166: 1756–1762, 2006. doi: 10.1001/archinte.166.16.1756. [DOI] [PubMed] [Google Scholar]
- 14.Jaim-Etcheverry G, Zieher LM. Permanent depletion of peripheral norepinephrine in rats treated at birth with 6-hydroxydopamine. Eur J Pharmacol 13: 272–276, 1971. doi: 10.1016/0014-2999(71)90162-2. [DOI] [PubMed] [Google Scholar]
- 15.Jain SK, Kahlon G, Morehead L, Lieblong B, Stapleton T, Hoeldtke R, Bass PF III, Levine SN. The effect of sleep apnea and insomnia on blood levels of leptin, insulin resistance, IP-10, and hydrogen sulfide in type 2 diabetic patients. Metab Syndr Relat Disord 10: 331–336, 2012. doi: 10.1089/met.2012.0045. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Kaushal N, Ramesh V, Gozal D. TNF-α and temporal changes in sleep architecture in mice exposed to sleep fragmentation. PLoS One 7: e45610, 2012. doi: 10.1371/journal.pone.0045610. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Kawa A, Taniguchi Y, Mizuguchi K, Ryu S, Ariyama T, Kamisaki T, Koreeda F, Kanehisa T. Effect of intraventricular administration of noradrenaline and dopamine on the levels of corticosterone in rats and denervation hypersensitivity resulting from intraventricular administration of 6-hydroxydopamine. Life Sci 23: 991–997, 1978. doi: 10.1016/0024-3205(78)90227-8. [DOI] [PubMed] [Google Scholar]
- 18.Kim J, Hakim F, Kheirandish-Gozal L, Gozal D. Inflammatory pathways in children with insufficient or disordered sleep. Respir Physiol Neurobiol 178: 465–474, 2011. doi: 10.1016/j.resp.2011.04.024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Livak KJ, Schmittgen TD. Analysis of relative gene expression data using real-time quantitative PCR and the 2(-Delta Delta C(T)) Method. Methods 25: 402–408, 2001. doi: 10.1006/meth.2001.1262. [DOI] [PubMed] [Google Scholar]
- 20.Lowrance SA, Ionadi A, McKay E, Douglas X, Johnson JD. Sympathetic nervous system contributes to enhanced corticosterone levels following chronic stress. Psychoneuroendocrinology 68: 163–170, 2016. doi: 10.1016/j.psyneuen.2016.02.027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Mavanji V, Billington CJ, Kotz CM, Teske JA. Sleep and obesity: a focus on animal models. Neurosci Biobehav Rev 36: 1015–1029, 2012. doi: 10.1016/j.neubiorev.2012.01.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Meerlo P, Sgoifo A, Suchecki D. Restricted and disrupted sleep: effects on autonomic function, neuroendocrine stress systems and stress responsivity. Sleep Med Rev 12: 197–210, 2008. doi: 10.1016/j.smrv.2007.07.007. [DOI] [PubMed] [Google Scholar]
- 23.Mullington JM, Haack M, Toth M, Serrador JM, Meier-Ewert HK. Cardiovascular, inflammatory, and metabolic consequences of sleep deprivation. Prog Cardiovasc Dis 51: 294–302, 2009. doi: 10.1016/j.pcad.2008.10.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Rajaratnam SM, Arendt J. Health in a 24-h society. Lancet 358: 999–1005, 2001. doi: 10.1016/S0140-6736(01)06108-6. [DOI] [PubMed] [Google Scholar]
- 25.Schwabe RF, Brenner DA. Mechanisms of liver injury. I. TNF-α-induced liver injury: role of IKK, JNK, and ROS pathways. Am J Physiol Gastrointest Liver Physiol 290: G583–G589, 2006. doi: 10.1152/ajpgi.00422.2005. [DOI] [PubMed] [Google Scholar]
- 26.Schwartz S, McDowell Anderson W, Cole SR, Cornoni-Huntley J, Hays JC, Blazer D. Insomnia and heart disease: a review of epidemiologic studies. J Psychosom Res 47: 313–333, 1999. doi: 10.1016/S0022-3999(99)00029-X. [DOI] [PubMed] [Google Scholar]
- 27.Simpson N, Dinges DF. Sleep and inflammation. Nutr Rev 65: S244–S252, 2007. doi: 10.1301/nr.2007.dec.S244-S252. [DOI] [PubMed] [Google Scholar]
- 28.Suchecki D, Lobo LL, Hipólide DC, Tufik S. Increased ACTH and corticosterone secretion induced by different methods of paradoxical sleep deprivation. J Sleep Res 7: 276–281, 1998. doi: 10.1046/j.1365-2869.1998.00122.x. [DOI] [PubMed] [Google Scholar]
- 29.Suzuki G, Tokuno S, Nibuya M, Ishida T, Yamamoto T, Mukai Y, Mitani K, Tsumatori G, Scott D, Shimizu K. Decreased plasma brain-derived neurotrophic factor and vascular endothelial growth factor concentrations during military training. PLoS One 9: e89455, 2014. doi: 10.1371/journal.pone.0089455. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Tiemeier H, Pelzer E, Jönck L, Möller HJ, Rao ML. Plasma catecholamines and selective slow wave sleep deprivation. Neuropsychobiology 45: 81–86, 2002. doi: 10.1159/000048681. [DOI] [PubMed] [Google Scholar]
- 31.van Leeuwen WM, Lehto M, Karisola P, Lindholm H, Luukkonen R, Sallinen M, Härmä M, Porkka-Heiskanen T, Alenius H. Sleep restriction increases the risk of developing cardiovascular diseases by augmenting proinflammatory responses through IL-17 and CRP. PLoS One 4: e4589, 2009. doi: 10.1371/journal.pone.0004589. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Villa A, Gelosa P, Castiglioni L, Cimino M, Rizzi N, Pepe G, Lolli F, Marcello E, Sironi L, Vegeto E, Maggi A. Sex-Specific features of microglia from adult mice. Cell Rep 23: 3501–3511, 2018. doi: 10.1016/j.celrep.2018.05.048. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Weil ZM, Norman GJ, Karelina K, Morris JS, Barker JM, Su AJ, Walton JC, Bohinc S, Nelson RJ, DeVries AC. Sleep deprivation attenuates inflammatory responses and ischemic cell death. Exp Neurol 218: 129–136, 2009. doi: 10.1016/j.expneurol.2009.04.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Yaghouby F, Donohue KD, O’Hara BF, Sunderam S. Noninvasive dissection of mouse sleep using a piezoelectric motion sensor. J Neurosci Methods 259: 90–100, 2016. doi: 10.1016/j.jneumeth.2015.11.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Zielinski MR, Davis JM, Fadel JR, Youngstedt SD. Influence of chronic moderate sleep restriction and exercise training on anxiety, spatial memory, and associated neurobiological measures in mice. Behav Brain Res 250: 74–80, 2013. doi: 10.1016/j.bbr.2013.04.038. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Zielinski MR, Kim Y, Karpova SA, McCarley RW, Strecker RE, Gerashchenko D. Chronic sleep restriction elevates brain interleukin-1 beta and tumor necrosis factor-alpha and attenuates brain-derived neurotrophic factor expression. Neurosci Lett 580: 27–31, 2014. doi: 10.1016/j.neulet.2014.07.043. [DOI] [PMC free article] [PubMed] [Google Scholar]