Abstract
Based on the cardiac hormone atrial natriuretic peptide (ANP) and its seminal role in blood pressure (BP) homeostasis, we investigated the chronic BP lowering actions of a novel ANP analog currently entering clinical trials for hypertension. Previous reports demonstrate that this analog MANP activates the guanylyl cyclase A receptor (GC-A) and results in more potent biological actions compared with ANP; thus, it may represent a new therapeutic drug for hypertension. A major goal of this study was to establish that chronic subcutaneous delivery of MANP is feasible and hypotensive together with cGMP effects. We investigated the BP-lowering and cGMP-activating actions of acute and chronic subcutaneous delivery in normal and hypertensive rats. Furthermore, we explored vascular mechanisms of MANP in human aortic smooth muscle cells (HASMC) and ex vivo in isolated arteries. In normal rats with a single subcutaneous injection, MANP promoted robust dose-dependent BP-lowering actions and natriuresis, together with cGMP activation. Most importantly in hypertensive rats, once-a-day subcutaneous injection of MANP for 7 days induced cGMP elevation and long-term BP reduction compared with vehicle. Mechanistically, in HASMC, MANP activated cGMP and attenuated angiotensin II-mediated increases in intracellular Ca2+ levels while directly vasorelaxing arterial rings. Our study demonstrates for the first time the effectiveness of subcutaneous administration of MANP for 7 days and provides innovative, vascular mechanisms of BP regulation supporting its continued development as a novel therapeutic for hypertension.
Keywords: 3′,5′-cyclic guanosine monophosphate; atrial natriuretic peptide; guanylyl cyclase A receptor; hypertension; MANP; natriuretic peptide; smooth muscle cells; subcutaneous
INTRODUCTION
The role of the heart as an endocrine organ has been established with the production of atrial natriuretic peptide (ANP), which plays a key role in blood pressure (BP) homeostasis. Specifically, ANP exerts a number of cardiorenal and metabolic actions through the particulate guanylyl cyclase A receptor (GC-A) and its second messenger 3′,5′-cyclic guanosine monophosphate (cGMP) (8, 14, 15, 18, 19). cGMP binds to downstream targets including protein kinase G, via which a variety of beneficial cardiorenal actions are mediated that include BP lowering, natriuretic, and diuretic properties (2). Indeed, in mice with ANP gene disruption, BP was significantly higher than in wild-type mice (17), highlighting the importance of the endogenous ANP/GC-A system in BP regulation. In humans, the ANP genetic variant rs5068, which increases circulating ANP, is associated with lower BP and protection from hypertension (5). Furthermore, hypertension has emerged as a relative ANP deficiency state serving as a rationale for ANP-based therapeutics (21). Although ANP has been regarded as a promising agent for treating hypertension, its rapid enzymatic degradation and the need for continuous intravenous infusion have limited its clinical development.
MANP (also called frameshift ANP or ZD100) is a novel ANP analog that was engineered to overcome the therapeutic limitations of native ANP. Experimental studies have reported that compared with ANP, MANP is highly resistant to neprilysin (13) and has robust and prolonged biological actions that include lowering BP, inducing natriuresis, and increasing cGMP in healthy dogs and in dog models of hypertension during short-term intravenous infusion (22, 24). Moreover, MANP is now entering early clinical trials in human hypertension.
Although short-term actions have been demonstrated in several studies, more long-term effects of MANP on BP, natriuresis, and cGMP activation have not been investigated. Furthermore, chronic once-daily subcutaneous administration of MANP to mediate sustained BP reduction has also not been reported. Finally, no studies have reported direct in vitro mechanisms of action of MANP in vascular smooth muscle cells or its direct vasorelaxing actions in isolated arteries ex vivo.
In the present study, we first confirmed the acute subcutaneous effects of MANP in anesthetized normal rats on BP, natriuresis, and cGMP activation. Importantly, we investigated for the first time the long-term BP lowering actions of subcutaneous MANP for 7 days in conscious rats with elevated BP secondary to Na+ restriction. Additionally, for the first time we also investigated vascular mechanisms of action of MANP by defining cGMP generation in human primary aortic smooth muscle cells (HASMC) together with intracellular Ca2+ regulation in the presence of angiotensin II (ANG II)-simulated increases in intracellular Ca2+. Finally, we assessed the direct vasorelaxing action of MANP in isolated dog arterial rings. We hypothesize that chronic subcutaneous delivery of MANP will mediate sustained BP-lowering actions in an experimental hypertension model in a 7-day study and be associated with sustained activation of cGMP. We also hypothesize that MANP can potently activate the GC-A receptor with cGMP generation, suppress ANG II-induced Ca2+ levels in HASMC in vitro, and induce arterial relaxation ex vivo in isolated dog arteries.
METHODS
Peptide Synthesis
MANP was synthesized by solid-phase peptide synthesis method (Bachem, Torrance, CA). The Cys-Cys disulfide bond was formed by oxidation after synthesis. The full MANP amino acid sequence is SLRRSSCFGGRMDRIGAQSGLGCNSFRYRITAREDKQGWA. The structure was confirmed by mass spectrometry, and high-performance liquid chromatography analysis confirmed purity to be >95%.
In Vivo Rat Studies
Rat studies were conducted in accordance with the Animal Welfare Act and approval from the Mayo Clinic Institutional Animal Care and Use Committee (IACUC). Rats were included if deemed healthy by the investigators and department veterinarians. No rat was excluded in our studies.
Acute studies.
Rats (Sprague-Dawley, male, 250–300 g) were anesthetized with inactin (120 mg/kg, stock solution 100 mg/mL dissolved in saline, intraperitoneal injection, Sigma, St. Louis, MO). A second inactin dose (60 mg/kg) was applied if desired anesthesia was not achieved with the initial dose. Rats were then subjected to vessel and bladder cannulation for saline infusion, BP measurement, blood sampling, and urine collection (11). A polyethylene (PE)-50 tube catheter was placed into the jugular vein for 0.9% saline intravenous infusion. The carotid artery was cannulated with a PE-50 tube catheter for BP measurement (Sonometrics, London, Ontario, Canada) and blood sampling. The bladder was accessed and cannulated with a PE-90 tube catheter for passive urine collection. After completion of the above procedural set up, the continuous infusion of 0.9% saline was initiated. The infusion rate was weight (grams) adjusted and calculated as follows: rat weight/8,571 (unit: mL/min). The infusion throughout the study was only applied to maintain fluid balance and keep the rats from dehydrating, and no drugs were infused intravenously. After 30–35 min, BP was recorded, 0.5 mL blood was sampled, and urine collection was initiated, followed by the subcutaneous injection of 1.0 mL MANP or saline. Rats were randomized to the following three groups: vehicle (saline, n = 5), low-dose MANP (1.94 mg/kg, n = 4), and high-dose MANP (3.88 mg/kg, n = 5). The doses were chosen based on acute intravenous infusion studies, in which we showed in our pilot studies that 300 and 600 pmol·kg−1·min−1 intravenously significantly induced cGMP elevation and BP reduction in rats. The value 1.94 mg/kg is equivalent to 300 pmol·kg−1·min−1 × 1,440 min × 4,493 g/mol, and the same formula was applied to 3.88 mg/kg dose. Blood (0.5 mL) was collected at 0, 30, and 60 min after subcutaneous injection of MANP for the measurement of cGMP. A similar volume of saline was administered after the 0-, 30-, and 60-min samples to compensate for blood loss. Urine was collected at 30, 60, 120, 240, 300, and 360 min postinjection, and cGMP was determined. Urine volume (UV) and urinary Na+ excretion (UNaV) were measured at 30 min. BP was monitored and recorded at 30, 60, 120, 240, 300, and 360 min postinjection before blood sampling. At the end of the study (at 360 min), rats were euthanized by exsanguination. Urinary Na+ was measured with pHOx Ultra (Nova Biomedical, Waltham, MA). Urinary Na+ excretion rate (UNaV) was calculated by urinary Na+ concentration times urine volume rate. Urinary excretion rate of cGMP was calculated by urinary cGMP concentration times urine volume rate. Urinary excretion rate of ANP-like levels (ALL) was calculated by urinary ALL concentration times urine volume rate.
Chronic studies.
Rats (Sprague-Dawley, male, 250–300 g) were first trained for 1 wk for conscious noninvasive BP measurement via tail-cuff method (CODA, Kent Scientific, Torrington, CT) before the formal study. A baseline BP was established before rats were fed with salt-deficient diet (TD.90228, Envigo, East Millstone, NJ) for 7 days to create a model of salt depletion-mediated hypertension, which is known to be associated with activation of ANG II and aldosterone (1, 26, 27). Notably, the Na+ content is 0.01–0.02% (equal to 0.1–0.2 g Na+ per kg of diet) in Na+-deficient diet and 0.3% in normal diet. Daily subcutaneous injection of MANP or vehicle treatment was initiated on the same day as the Na+-deficient diet, and the subcutaneous injections were repeated for 7 days. The MANP (n = 10) dose was 3.88 mg/kg, and saline (n = 9) was used as vehicle. Tail-cuff BP was obtained on day 7 before subcutaneous drug injection (20 h after previous injection on day 6). On day 7, 24-h urine samples from rats were collected after drug injections in metabolic cages. Parameters including UV, UNaV, cGMP, and ALL were measured in the 24-h urine.
Hormone Measurements
Investigators were not blinded to treatments, but biochemical analysis was performed by technicians who were blinded to treatment. Plasma and urinary samples for ALL (ANP-like levels) were measured by a radioimmunoassay (Phoenix Pharmaceuticals) (3) to serve as a surrogate for MANP levels (23). The ANP assay detects the ring structure of ANP, which includes MANP. Plasma and urinary cGMP were determined by ELISA (Enzo Life Sciences, Farmingdale, NY). All assays followed the manufacturer’s instructions.
cGMP Generation in HASMC
Human primary aortic smooth muscle cells (HASMC; ScienCell Research Laboratories, Carlsbad, CA), which express GC-A, were maintained and subcultured according to the manufacturer’s protocols. Passage 4 or 5 was used in the study. Detailed methods have been described previously (11). The cells were treated with concentrations of MANP (10−8 and 10−7 M) for 10 min in the presence of nonspecific phosphodiesterase inhibitor, IBMX (5 × 10−3 M). Intracellular cGMP was measured using a commercial cGMP ELISA kit (Enzo Life Sciences, Farmingdale, NY) with the acetylation method, as instructed by the manufacturer.
Intracellular Calcium Measurement by Fura-2 Method in HASMC
Intracellular Ca2+ was measured with fura-2 method. Briefly, HASMC were seeded with a density of 5×104 cells per slide. Cells were pretreated with MANP (10−7 M) for 5 min before being perfused with 5 mL medium containing ANG II (10−7 M). The groups are as follows: control (medium only), ANG II, or MANP. The calcium signal (F) was normalized to baseline fluorescence (F0) and expressed as a ratio (F/F0) using MetaFluor software (Olympus America Inc., Waltham, MA).
Ex Vivo Arterial Ring Relaxations
Dog artery collection was performed following the guidelines of Animal Welfare Act and with approval of Mayo Clinic IACUC. To assess vasorelaxation, healthy dog femoral arteries were harvested immediately after euthanasia. Detailed methods have been described previously (9). All vessels with intact endothelium were contracted with 10−5 M phenylephrine followed by relaxation with cumulative concentrations (10−9 to 10−5 M) of MANP.
Statistical Analysis
Data are expressed as means ± SE. One-way and two-way ANOVA followed by Bonferroni’s post hoc test was performed in the studies in which multiple time points were involved, and two-tailed t tests were performed for comparisons between two groups. Nonparametric Mann-Whitney test was used for data that were not normally distributed. GraphPad Prism 8 (GraphPad Software, La Jolla, CA) was used for the above calculations, and statistical significance was accepted as P < 0.05.
RESULTS
Acute Study
Blood pressure.
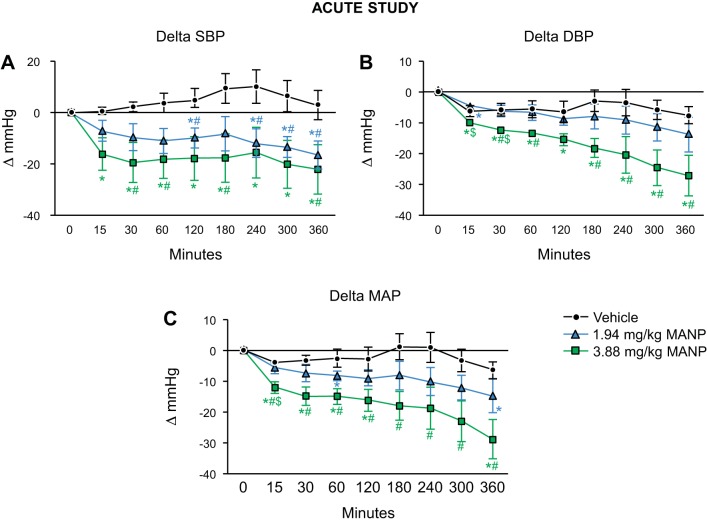
Baseline values for the three groups are as follows. Systolic BP (SBP): vehicle 107 ± 2, low-dose MANP 115 ± 9, high-dose MANP 120 ± 10 mmHg; diastolic BP (DBP): vehicle 78 ± 3, low-dose MANP 87 ± 6, high-dose MANP 94 ± 5 mmHg; and mean arterial pressure (MAP): vehicle 88 ± 3, low-dose MANP 96 ± 7, high-dose MANP 102 ± 7 mmHg. We did not see significant differences in BP values, except that the high-dose group exhibited significantly higher DBP values than the vehicle group. Nevertheless, our results present the absolute changes from baseline values, which served to normalize the variability observed in baseline. Figure 1 illustrates the BP responses to subcutaneous administration of MANP in normal rats. In vivo, a single subcutaneous injection of MANP (low and high dose: 1.94 mg/kg and 3.88 mg/kg, respectively) markedly reduced SBP, DBP, and MAP compared with the vehicle group (Fig. 1, A–C). The overall significance for three groups was achieved in all three parameters: SBP, DBP, and MAP. However, this was not seen between low and high doses. The potent BP effects were sustained and lasted for the entire 6 h of the protocol.
Fig. 1.
Blood pressure changes of single subcutaneous atrial natriuretic peptide analog (MANP) injection in normal male rats: n = 5 vehicle (saline), n = 4 low (1.94 mg/kg), and n = 5 high (3.88 mg/kg) doses of MANP. Changes in systolic blood pressure (SBP; A), diastolic blood pressure (DBP; B), and mean arterial pressure (MAP; C) over 360-min period by single sc injection of vehicle or MANP at low or high dose. *P < 0.05 vs. 0 min, #P < 0.05 vs. vehicle, $P < 0.05 vs. 1.94 mg/kg MANP. Data are expressed as means ± SE. In A–C, 2-way ANOVA and 1-way ANOVA were used for statistical analysis in multiple groups and comparisons within the same group, respectively.
Neurohormones and renal actions.
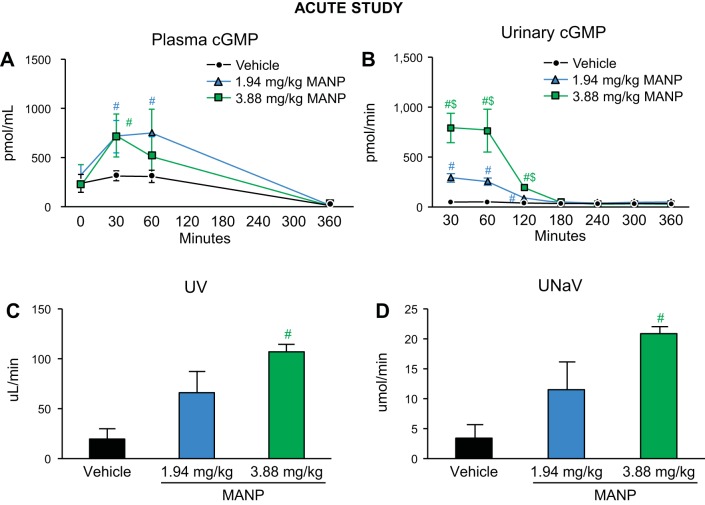
In response to subcutaneous MANP administration, plasma cGMP was significantly elevated in the low- and high-dose groups compared with vehicle at 30 min (Fig. 2A). The duration of plasma cGMP elevation was ~60 min with no difference among groups at 360 min (vehicle: 14 ± 1, low: 15 ± 4, high: 9 ± 2 pmol/mL). As a signal of renal GC-A receptor activation, urinary cGMP excretion was markedly elevated in the first 120 min (Fig. 2B). Furthermore, subcutaneous MANP injection at high dose resulted in significant increases in urine volume (UV) and urinary Na+ excretion (UNaV) in comparison with vehicle at 30 min postinjection (Fig. 2, C and D). Notably, the diuretic and natriuretic actions were only enhanced in the first 30 min with no significant difference observed beyond 30 min, unlike the BP-lowering effects, which were sustained throughout the study.
Fig. 2.
cGMP levels and renal actions with single subcutaneous atrial natriuretic peptide analog (MANP) injection in normal male rats: n = 5 vehicle (saline), n = 4 low (1.94 mg/kg), and n = 5 high (3.88 mg/kg) doses of MANP. Plasma cGMP levels (A) and urinary cGMP levels (B) during the 360-min study and urine output (UV; C) and urinary Na+ excretion rate (UNaV; D) at 30 min postinjection. #P < 0.05 vs. vehicle, $P < 0.05 vs. 1.94 mg/kg MANP. Data are expressed as means ± SE. In A and B, 2-way ANOVA and 1-way ANOVA were used for statistical analysis in multiple groups and comparisons within the same group, respectively. Unpaired t test was performed for C and D.
Seven-Day Chronic Study
Blood pressure.
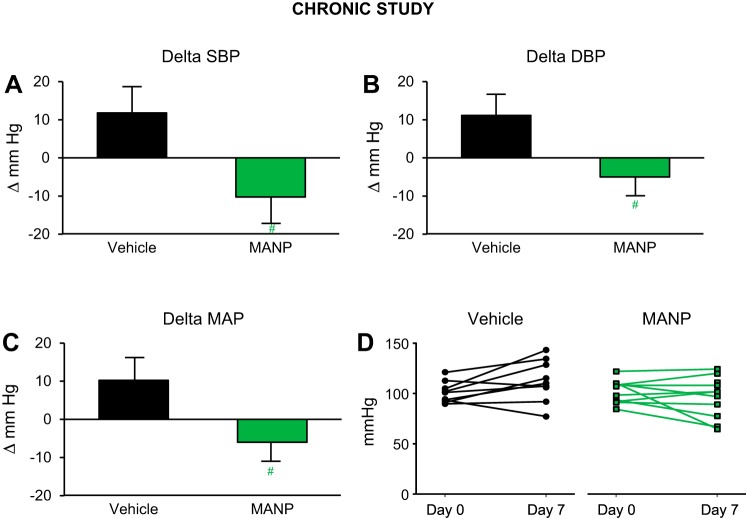
Day 0 baseline BP (measured with tail-cuff method in the conscious state) for rats in both groups were similar as shown in Supplemental Table S1 (see https://doi.org/10.6084/m9.figshare.11353793.v1). In rats subjected to salt depletion for 7 days, BP increased by ~11 mmHg compared with day 0 in vehicle group (Fig. 3, A–D). In comparison, treatment with MANP for 7 days resulted in BP reduction, in which BP at trough (20 h postinjection) was significantly lower on day 7. Notably, daily subcutaneous MANP administration for 7 days significantly reduced SBP (−23 mmHg), DBP (−17 mmHg), and MAP (−17 mmHg) compared with vehicle.
Fig. 3.
Blood pressure (BP) changes in the chronic study in salt-depletion male rats: n = 9 vehicle (saline) and n = 10 atrial natriuretic peptide analog (MANP) (3.88 mg/kg); daily subcutaneous injection for 7 days. Conscious BP was measured via tail-cuff method at 20 h postinjection on day 7. Delta values were recorded as changes from baseline values on day 0. Changes in systolic BP (SBP; A), diastolic BP (DBP; B), and mean arterial pressure (MAP; C) and individual animal MAP values on day 7 from day 0 (D). #P < 0.05 vs. vehicle. Data are expressed as means ± SE. Unpaired t test was used.
Urinary neurohormones and renal actions.
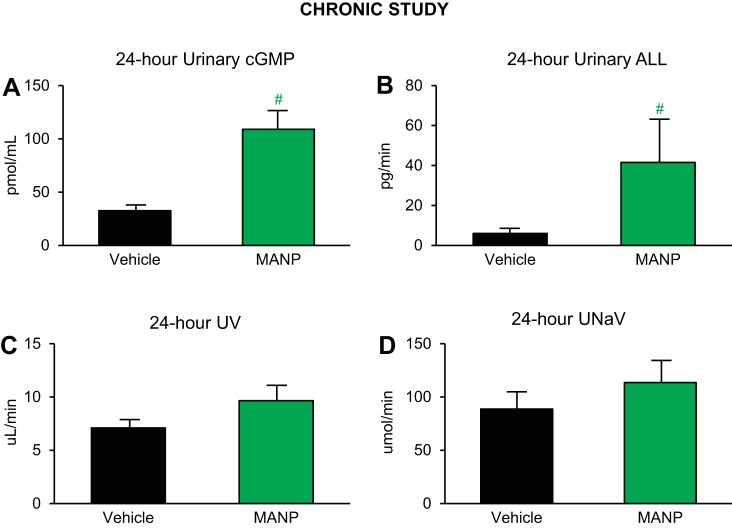
Subcutaneous treatment with MANP for 7 days significantly increased urinary cGMP and ANP-like level (ALL) concentrations (Fig. 4, A and B), which demonstrates that daily injection of MANP is active, consistent with the cGMP results in the aforementioned acute study. Notably, plasma ALL was increased as well (MANP: 479 ± 178, vehicle: 44 ± 9 pg/mL), whereas plasma cGMP did not demonstrate a statistical significance. Furthermore, MANP treatment also displayed a trend to increase UV and UNaV on day 7 (Fig. 4, C and D).
Fig. 4.
Urinary cGMP levels, atrial natriuretic peptide (ANP)-like levels (ALL), and renal actions in salt-depletion male rats: n = 9 vehicle (saline) and n = 10 MANP (3.88 mg/kg); daily subcutaneous injection for 7 days. A–D: 24-h urine analysis beginning after day 7 subcutaneous injection. A: urinary cGMP levels. B: urinary ALL. C: urine volume (UV). D: urinary Na+ excretion rate (UNaV). #P < 0.05 vs. vehicle. Data are expressed as means ± SE. Unpaired t test was performed.
cGMP Generation, Intracellular Ca2+ Levels in HASMC, and Arterial Relaxation in Isolated Dog Femoral Artery Rings
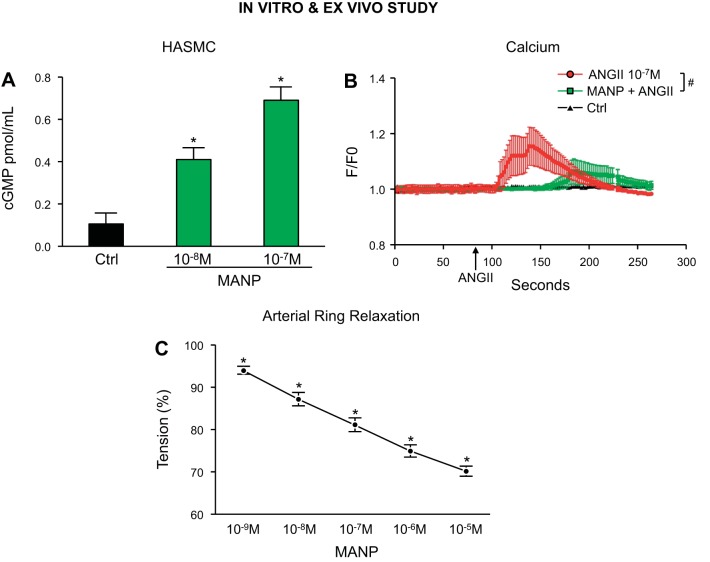
Figure 5A illustrates cGMP activation results in HASMC. MANP at concentrations of 10−8 and 10−7 M increased cGMP production in comparison to control. Furthermore, 10−7 M ANG II significantly increased intracellular Ca2+ levels (red line) in HASMC, whereas pretreatment with 10−7 M MANP (green line) reduced ANG II-stimulated intracellular Ca2+ (Fig. 5B). In isolated dog arterial rings, increasing concentrations of MANP exerted potent arterial vasorelaxation in femoral arteries (Fig. 5C).
Fig. 5.
cGMP generation and intracellular Ca2+ levels by atrial natriuretic peptide analog (MANP) in human primary aortic smooth muscle cells (HASMC) and vasorelaxation in dog arterial rings. A: intracellular cGMP levels by 0 [control (Ctrl)], 10−8, or 10−7 M MANP. *P < 0.05 vs. Ctrl, unpaired t test, n = 3. B: intracellular Ca2+ levels by 10−7 M angiotensin II (ANG II) in the absence or presence of 10−7 M MANP. Cells were preincubated with MANP for 5 min before ANG II stimulation. #P < 0.05 vs. ANG II, 2-way ANOVA followed by Bonferroni’s post hoc test, n = 8. C: ex vivo arterial relaxation by MANP in healthy dog femoral arterial rings. *P < 0.05 vs. baseline 100%, unpaired t test, n = 5.
DISCUSSION
In the present study, we established for the first time the sustained biological actions of chronic subcutaneous administration of MANP, a novel ANP analog targeting the GC-A receptor, chronically in rats. First we confirmed the feasibility of subcutaneous administration of MANP and established the working doses of subcutaneous MANP in normal rats. Most importantly, in a salt-depletion rat model of elevated BP, daily subcutaneous administration of MANP for 7 days reduced BP and increased plasma and urinary ANP-like levels (ALL) and urinary cGMP levels. Furthermore, we demonstrated that MANP potently activated GC-A receptor, suppressed ANG II-stimulated intracellular Ca2+ level increases in HASMC, and mediated arterial vasorelaxation in isolated dog arterial rings. These results therefore elucidated vascular mechanisms of BP-lowering effects in vitro of MANP. Our findings provide a strong foundation for the continued clinical development of MANP as a next generation drug for hypertension.
MANP was designed based on the native GC-A activator ANP, with 12 additional amino acids (AAs) fused to ANP’s carboxyl terminus (23). The extension of these 12 key AAs renders MANP resistance to catabolism in vivo, thereby resulting in greater cGMP production and more pronounced BP reduction in comparison with ANP in acute intravenous infusion studies. Indeed, Dickey et al. (13) have reported that MANP is more resistant to enzymatic degradation by neprilysin (NEP) compared with ANP. In the current study, for the first time we confirmed the sustained actions of MANP with subcutaneous administration in rats, in which we observed a 28-mmHg BP reduction for up to 6 h compared with vehicle treatment in our acute study. These actions were all dose dependent.
In our chronic study, we employed a hypertensive model, which was induced by salt depletion that is known to activate the endogenous renin-angiotensin-aldosterone system (1, 26, 27). Once-daily subcutaneous administration of MANP for 7 days increased both urinary ALL and cGMP levels, which documented the engagement of MANP and GC-A activation. As in the acute study, MANP reduced both systolic and diastolic BP. Most importantly with subcutaneous strategy, the reduction in BP was sustained with MANP and was observed on day 7. The current chronic subcutaneous MANP treatment showed similar physiological changes as a 5-day continuous intravenous infusion of ANP in dogs, documented by sustained BP actions and lack of diuresis and natriuresis (16). Notably, the sustained BP-lowering effect on day 7 occurred 20 h postinjection, which indicates that the trough effect of MANP is potent and sustained. The lack of a sustained effect on water and Na+ excretion may have occurred as a result of Na+ and water depletion during the chronic study. It is also plausible to speculate that MANP may exert diuretic and natriuretic actions chronically in water and Na+ retention states in which a balance is not achieved.
Previous preclinical and clinical studies have strongly supported the use of ANP/GC-A/cGMP pathway for novel antihypertensive therapeutics. First, genetic deletion of ANP or GC-A in mice results in hypertension (17, 20). In general population studies, the ANP gene variant rs5068 is associated with increased plasma ANP together with lower BP and reduced risk of hypertension (5). Notably, the biologically important second messenger cGMP generated from GC-A or soluble GC (sGC) pathway activation has been extensively reported to result in vasodilation (9, 25), which supports the BP-reducing effects observed in our acute and chronic study. Given that there is an urgent need for novel and effective antihypertensive drugs, especially resistant hypertension (4), our study emphasizes the therapeutic potential of MANP as a novel and effective ANP analog targeting the GC-A/cGMP pathway and hypertension.
Our studies are consistent with a preliminary, pilot study abstract report that one single dose of subcutaneous MANP significantly lowered BP in hypertensive patients (6, 7). Thus, we provide the efficacy of subcutaneous MANP in an additional species with molecular mechanism investigations. More importantly, we demonstrated for the first time the sustained 7-day BP-lowering action of MANP, which supports its translation from biology to therapeutics.
Previously, we and others reported that GC-A activators suppress ANG II-stimulated intracellular Ca2+ elevation (10, 12) in HASMC, which results in vasoconstriction in vivo. However, to date there are no reports on the vascular smooth muscle cell actions of MANP. Our current results are consistent with vasorelaxing mechanisms by native GC-A activators such as ANP, which may partially explain the BP-lowering effects we observed in our in vivo studies. Specifically, MANP attenuated ANG II-induced increases in intracellular Ca2+ in HASMC. Additionally, in isolated dog arterial rings, MANP induced dose-dependent vasorelaxation. Future studies investigating the pathways of Ca2+ regulation such as Ca2+ channels and phosphorylase C are warranted.
Our study has limitations. The chronic study lasted 7 days and represents the longest time period for MANP administration to date. Nonetheless, more long-term, e.g., 1-mo, studies, are needed to confirm the antihypertensive actions of MANP to validate and extend our current findings. Another limitation is that we only employed a salt-depletion model of hypertension in the current study. We believe the chronic antihypertensive effects would be comparable if we investigated MANP in other experimental hypertension models, such as ANG II-induced hypertension, as we previously reported in dogs that acute intravenous MANP administration resulted in robust BP-lowering effects (22). Nevertheless, verifying chronic subcutaneous MANP administration in other hypertensive models with spontaneously hypertensive rats or chronic ANG II infusion is warranted. Also, a comparison study with commonly used antihypertensive drug agents such as angiotensin-converting enzyme inhibitors or diuretics is needed.
Perspectives and Significance
These preclinical studies are the first to demonstrate that the novel ANP analog MANP mediates sustained reduction of BP in a model of hypertension. Furthermore, MANP in isolated arterial rings induced vasorelaxation and attenuated ANG II-mediated increases in intracellular Ca2+ in HASMC consistent with vasodilating mechanisms, which in part involve modulation in intracellular Ca2+. These findings highlight the BP regulatory actions of the ANP/GC-A pathway in vitro and in vivo. Most importantly, these studies lay the foundation for chronic use of subcutaneous MANP in human hypertensive patients, especially those who remain uncontrolled with conventional therapies and/or are deficient in ANP.
GRANTS
This research was funded by National Heart, Lung, and Blood Institute (NHLBI) Grant R01 HL136340.
DISCLOSURES
John C. Burnett, Jr. and Horng H. Chen are the inventors for MANP and hold equity in Zumbro Discovery. None of the other authors has any conflicts of interest, financial or otherwise, to disclose.
AUTHOR CONTRIBUTIONS
Y.C., S.J.S., H.H.C., and J.C.B. conceived and designed research; Y.C., J.J.S., S.R.I., G.E.H., and S.P. performed experiments; Y.C., S.R.I., S.P., H.H.C., M.M.R., and J.C.B. analyzed data; Y.C., J.J.S., S.J.S., and J.C.B. interpreted results of experiments; Y.C. prepared figures; Y.C. and J.C.B. drafted manuscript; Y.C., S.J.S., H.H.C., M.M.R., and J.C.B. edited and revised manuscript; Y.C., J.J.S., S.R.I., G.E.H., S.P., S.J.S., H.H.C., M.M.R., and J.C.B. approved final version of manuscript.
ACKNOWLEDGMENTS
We thank Dr. Hon-Chi Lee for providing the expertise and advice on intracellular Ca2+ measurement with fura-2 method.
REFERENCES
- 1.Aguilera G, Catt KJ. Regulation of aldosterone secretion by the renin-angiotensin system during sodium restriction in rats. Proc Natl Acad Sci USA 75: 4057–4061, 1978. doi: 10.1073/pnas.75.8.4057. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Blanton RM. cGMP signaling and modulation in heart failure. J Cardiovasc Pharmacol. In press. doi: 10.1097/FJC.0000000000000749. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Burnett JC Jr, Kao PC, Hu DC, Heser DW, Heublein D, Granger JP, Opgenorth TJ, Reeder GS. Atrial natriuretic peptide elevation in congestive heart failure in the human. Science 231: 1145–1147, 1986. doi: 10.1126/science.2935937. [DOI] [PubMed] [Google Scholar]
- 4.Cai A, Calhoun DA. Resistant hypertension: an update of experimental and clinical findings. Hypertension 70: 5–9, 2017. doi: 10.1161/HYPERTENSIONAHA.117.08929. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Cannone V, Boerrigter G, Cataliotti A, Costello-Boerrigter LC, Olson TM, McKie PM, Heublein DM, Lahr BD, Bailey KR, Averna M, Redfield MM, Rodeheffer RJ, Burnett JC Jr. A genetic variant of the atrial natriuretic peptide gene is associated with cardiometabolic protection in the general community. J Am Coll Cardiol 58: 629–636, 2011. doi: 10.1016/j.jacc.2011.05.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Chen HH, Neutel J, Smith D, Heublein D, Burnett J. A first-in-human trial of a novel designer natriuretic peptide ZD100 in human hypertension. J Am Coll Cardiol 67, Suppl 5: 1946, 2016. doi: 10.1016/S0735-1097(16)31947-7. [DOI] [Google Scholar]
- 7.Chen HH, Neutel JM, Smith DH, Heublein D, Burnett JC. A first-in-human trial of a novel designer natriuretic peptide Zd100 in human hypertension. J Am Soc Hypertens 10: e23, 2016. doi: 10.1016/j.jash.2016.03.051. [DOI] [Google Scholar]
- 8.Chen Y, Burnett JC Jr. Biochemistry, therapeutics, and biomarker implications of neprilysin in cardiorenal disease. Clin Chem 63: 108–115, 2017. doi: 10.1373/clinchem.2016.262907. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Chen Y, Harty GJ, Huntley BK, Iyer SR, Heublein DM, Harders GE, Meems L, Pan S, Sangaralingham SJ, Ichiki T, Burnett JC Jr. CRRL269: a novel designer and renal-enhancing pGC-A peptide activator. Am J Physiol Regul Integr Comp Physiol 314: R407–R414, 2018. doi: 10.1152/ajpregu.00286.2017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Chen Y, Harty GJ, Zheng Y, Iyer SR, Sugihara S, Sangaralingham SJ, Ichiki T, Grande JP, Lee HC, Wang X, Burnett JC Jr. CRRL269: a novel particulate guanylyl cyclase A receptor peptide activator for acute kidney injury. Circ Res 124: 1462–1472, 2019. doi: 10.1161/CIRCRESAHA.118.314164. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Chen Y, Zheng Y, Iyer SR, Harders GE, Pan S, Chen HH, Ichiki T, Burnett JC Jr, Sangaralingham SJ. C53: A novel particulate guanylyl cyclase B receptor activator that has sustained activity in vivo with anti-fibrotic actions in human cardiac and renal fibroblasts. J Mol Cell Cardiol 130: 140–150, 2019. doi: 10.1016/j.yjmcc.2019.03.024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Cornwell TL, Lincoln TM. Regulation of phosphorylase A formation and calcium content in aortic smooth muscle and smooth muscle cells: effects of atrial natriuretic peptide II. J Pharmacol Exp Ther 247: 524–530, 1988. [PubMed] [Google Scholar]
- 13.Dickey DM, Yoder AR, Potter LR. A familial mutation renders atrial natriuretic Peptide resistant to proteolytic degradation. J Biol Chem 284: 19196–19202, 2009. doi: 10.1074/jbc.M109.010777. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Francis GS, Felker GM, Tang WH. A test in context: critical evaluation of natriuretic peptide testing in heart failure. J Am Coll Cardiol 67: 330–337, 2016. doi: 10.1016/j.jacc.2015.10.073. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Goetze JP. B-type natriuretic peptide: from posttranslational processing to clinical measurement. Clin Chem 58: 83–91, 2012. doi: 10.1373/clinchem.2011.165696. [DOI] [PubMed] [Google Scholar]
- 16.Granger JP, Opgenorth TJ, Salazar J, Romero JC, Burnett JC Jr. Long-term hypotensive and renal effects of atrial natriuretic peptide. Hypertension 8: II112–II116, 1986. doi: 10.1161/01.HYP.8.6_Pt_2.II112. [DOI] [PubMed] [Google Scholar]
- 17.John SW, Veress AT, Honrath U, Chong CK, Peng L, Smithies O, Sonnenberg H. Blood pressure and fluid-electrolyte balance in mice with reduced or absent ANP. Am J Physiol 271: R109–R114, 1996. doi: 10.1152/ajpregu.1996.271.1.R109. [DOI] [PubMed] [Google Scholar]
- 18.Kuhn M. Molecular physiology of membrane guanylyl cyclase receptors. Physiol Rev 96: 751–804, 2016. doi: 10.1152/physrev.00022.2015. [DOI] [PubMed] [Google Scholar]
- 19.Levin ER, Gardner DG, Samson WK. Natriuretic peptides. N Engl J Med 339: 321–328, 1998. doi: 10.1056/NEJM199807303390507. [DOI] [PubMed] [Google Scholar]
- 20.Lopez MJ, Wong SK, Kishimoto I, Dubois S, Mach V, Friesen J, Garbers DL, Beuve A. Salt-resistant hypertension in mice lacking the guanylyl cyclase—a receptor for atrial natriuretic peptide. Nature 378: 65–68, 1995. doi: 10.1038/378065a0. [DOI] [PubMed] [Google Scholar]
- 21.Macheret F, Heublein D, Costello-Boerrigter LC, Boerrigter G, McKie P, Bellavia D, Mangiafico S, Ikeda Y, Bailey K, Scott CG, Sandberg S, Chen HH, Malatino L, Redfield MM, Rodeheffer R, Burnett J Jr, Cataliotti A. Human hypertension is characterized by a lack of activation of the antihypertensive cardiac hormones ANP and BNP. J Am Coll Cardiol 60: 1558–1565, 2012. doi: 10.1016/j.jacc.2012.05.049. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.McKie PM, Cataliotti A, Boerrigter G, Chen HH, Sangaralingham SJ, Martin FL, Ichiki T, Burnett JC Jr. A novel atrial natriuretic peptide based therapeutic in experimental angiotensin II mediated acute hypertension. Hypertension 56: 1152–1159, 2010. doi: 10.1161/HYPERTENSIONAHA.110.159210. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.McKie PM, Cataliotti A, Huntley BK, Martin FL, Olson TM, Burnett JC Jr. A human atrial natriuretic peptide gene mutation reveals a novel peptide with enhanced blood pressure-lowering, renal-enhancing, and aldosterone-suppressing actions. J Am Coll Cardiol 54: 1024–1032, 2009. doi: 10.1016/j.jacc.2009.04.080. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.McKie PM, Cataliotti A, Ichiki T, Sangaralingham SJ, Chen HH, Burnett JC Jr. M-atrial natriuretic peptide and nitroglycerin in a canine model of experimental acute hypertensive heart failure: differential actions of 2 cGMP activating therapeutics. J Am Heart Assoc 3: e000206, 2014. doi: 10.1161/JAHA.113.000206. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Sandner P, Zimmer DP, Milne GT, Follmann M, Hobbs A, Stasch JP. Soluble guanylate cyclase stimulators and activators. Handb Exp Pharmacol. In press. doi: 10.1007/164_2018_197. [DOI] [PubMed] [Google Scholar]
- 26.Vallon V, Wead LM, Blantz RC. Renal hemodynamics and plasma and kidney angiotensin II in established diabetes mellitus in rats: effect of sodium and salt restriction. J Am Soc Nephrol 5: 1761–1767, 1995. [DOI] [PubMed] [Google Scholar]
- 27.Webb DJ, Clark SA, Brown WB, Fraser R, Lever AF, Murray GD, Robertson JI. Dietary sodium deprivation raises blood pressure in the rat but does not produce irreversible hyperaldosteronism. J Hypertens 5: 525–531, 1987. doi: 10.1097/00004872-198710000-00003. [DOI] [PubMed] [Google Scholar]