Abstract
Hypercholesterolemia is a major risk factor for adverse cardiovascular outcomes, but its effect on angiogenesis and wound healing is not well understood. In this study, using a combination of mass spectrometry and laurdan two-photon imaging, we show that elevated levels of low-density lipoprotein (LDL), like those seen in hypercholesterolemic patients, lead to an increase in both free cholesterol and cholesterol esters, as well as increase in lipid order of endothelial cell membranes. Notably, these effects are distinct and opposite to the lack of cholesterol loading and the disruption of lipid order observed in our earlier studies in response to oxidized LDL (oxLDL). The same pathological level of LDL leads to a significant inhibition of endothelial proliferation and cell cycle arrest in G2/M phase, whereas oxLDL enhances endothelial proliferation in S phase of the cycle. LDL but not oxLDL suppresses the expression of vascular endothelial growth factor receptor-2 while enhancing the expression of vascular endothelial growth factor (VEGF). Furthermore, we show that aged (8–10 mo) hypercholesterolemic apolipoprotein E-deficient (ApoE−/−) mice display delayed wound closure compared with age-matched C57/BL6 wild-type controls following a skin punch biopsy. The delay in wound healing is associated with a decreased expression of cluster of differentiation 31 platelet endothelial cell adhesion molecule endothelial marker and decreased angiogenesis within the wound bed. Furthermore, decreased endothelial responsiveness to the growth factors VEGF and basic fibroblast growth factor is observed in ApoE−/− mice in Matrigel plugs and in Matrigels with high levels of LDL in wild-type mice. We propose that plasma hypercholesterolemia is antiangiogenic due to elevated levels of LDL.
Keywords: angiogenesis, endothelial proliferation, hypercholesterolemia
INTRODUCTION
Hypercholesterolemia, an increase in the plasma level of cholesterol, is well known to lead to the development of atherosclerosis and adverse cardiovascular outcomes. It is defined as elevation of the plasma levels of low-density lipoprotein (LDL), a lipoprotein particle that is the major physiological cholesterol carrier (4, 9). Indeed, while “normal” LDL level is considered to be below 100 mg/dL or optimally 70 mg/dL, almost 32% of U.S. adults have LDL cholesterol levels of 200 mg/dL or above, which is considered to be pathological and has been closely linked to clogging of the major arteries leading to increased risk of myocardial infarctions and stroke (4, 9). It is also known that the key early stage of hypercholesterolemia-induced vascular dysfunction is impairment of the endothelium, which is typically manifested by the disruption of the endothelial permeability barrier and a decrease in endothelial regulation of vascular tone (14, 24). However, surprisingly little is known about the impact of hypercholesterolemia on angiogenesis and wound healing, a critical process in tissue injury. In this study, we demonstrate that exposure to high levels of LDL in vitro results in cholesterol loading and inhibition of endothelial proliferation and that plasma dyslipidemia in vivo impairs the process of angiogenesis and delays wound healing.
The first major question is what is the relationship between high levels of LDL in the plasma and cholesterol loading of vascular tissues. Numerous studies demonstrated cholesterol loading of macrophages, under hypercholesterolemic conditions in vivo, both in animal models of hypercholesterolemia and in humans (5, 28). Our earlier study also found significant cholesterol loading in vascular endothelial cells (ECs) in aortas of diet-induced hypercholesterolemia in a porcine model of atherosclerosis (13). However, the role of LDL in cholesterol loading of vascular cells is still not well understood. Furthermore, a controversy arose when it was found that exposing macrophages to high levels of LDL in vitro does not lead to significant cholesterol loading. This effect was attributed to the downregulation of the LDL receptor and led to the hypothesis that it is not LDL itself but its oxidized modifications (oxLDL) that lead to cholesterol loading (38). However, our more recent studies demonstrated that exposing endothelial cells to oxLDL does not lead to loading the cells with cholesterol (8, 37) but instead leads to loading the cells with oxidized lipids, including oxysterols and oxidized phospholipids (36). Thus, focusing on vascular endothelial cells, we reexamined the question of whether exposure to physiological levels of LDL may result in cholesterol loading. We show here that this is indeed the case and that LDL-induced cholesterol loading of ECs results in increased lipid packing of the membrane, indicating an increase in free cholesterol in the membrane.
Our next goal was to determine the impact of LDL-mediated cholesterol loading on endothelial proliferation. Several earlier studies, including a recent study from our group, tested the impact of oxLDL on endothelial proliferation and found that the predominant effect in EC exposed to physiological levels of oxLDL was an increase in EC proliferation mediated by RhoA/Akt1 cascade (35, 45). However, in light of our findings that oxLDL and LDL have fundamentally different effects on EC cholesterol, we extended our studies to compare the effects of oxLDL and LDL on EC proliferation. In the current study, we show that, in contrast to oxLDL, exposure to high levels of LDL significantly inhibits EC proliferation.
To extend our studies on the effect of hypercholesterolemia on angiogenesis, we tested the impact of hypercholesterolemia on angiogenesis during the wound healing process in vivo in an apolipoprotein E-deficient (ApoE−/−) mouse model. ApoE-deficient mice represent a model of severe genetically induced hypercholesterolemia, well known to induce endothelial dysfunction and atherosclerosis (34). Only a few earlier studies explored the effect of hypercholesterolemia on wound healing. It was shown that hypercholesterolemia results in a delay in wound closure in ApoE−/− mice (16, 21), but the effect on the wound angiogenesis, a key component of the wound healing process, is not known. Here we show that the delay in wound closure seen in these hypercholesterolemic mice is accompanied with a significant suppression in wound angiogenesis. We also show that this decrease in angiogenesis should be attributed to endothelial cells’ decreased responsiveness to growth factors, as determined by Matrigel plug assay.
MATERIALS AND METHODS
Cell Culture
Human aortic endothelial cells (HAECs; Lonza, Allendale, NJ) were grown between passages 7 and 12 in endothelial growth media 2 (EGM2, Lonza) containing 2% fetal bovine serum (Sigma-Aldrich, St. Louis, MO) and 10 µg/ml penicillin and streptomycin (Life Technologies, Grand Island, NY), unless otherwise indicated. Cell cultures were maintained in a humidified incubator at 37°C, with 5% CO2. Cells were split every 3–4 days.
LDL Isolation and Oxidation
LDL was isolated from human plasma obtained from a local blood bank [Lifesource (now Vitalant), Chicago, IL], and oxLDL was prepared as described previously (32, 45). The plasma was separated by sequential centrifugation in potassium bromide (KBr, Acros Organics; Thermo Fisher Scientific, Waltham, MA) with a final density of 1.063 g/mL. The preparation was then dialyzed two times in PBS containing 1 mM EDTA at 4°C to remove KBr and then subsequently dialyzed in PBS alone for 2 days to remove EDTA. Copper sulfate was then added to LDL with a final concentration of 25 µM for 18 h at 37°C to oxidize LDL. This reaction was stopped by adding 1 mM EDTA (Gibco, Thermo Fisher Scientific, Waltham, MA). The content of thiobarbituric acid-reactive substances (TBARS) in LDL and oxLDL was determined by using a TBARS Assay Kit (ZeptoMetrix, Buffalo, NY) as expressed with malondialdehyde (MDA) equivalent. The Cu-oxLDL used was always between 14 ± 2 MDA equivalents. The lipoxygenase (LPO) oxidized LDL was oxidized using 1 mg/mL LDL that was incubated overnight at 37°C with 5,000 U/mL soybean lipoxygenase (Sigma-Aldrich). The LPO-oxLDL’s oxidation was then measured using a spectrophotometer measuring conjugated dienes at 235 nm, as previously described (32, 45).
Cholesterol Measurements
Total cholesterol was measured with the Amplex Red cholesterol assay kit (Thermo Fisher Scientific) according to the manufacturer's specifications.
For mass spectroscopy, cells were lysed in methanol and stored at −80°C before analysis. Liquid chromatography-electrospray ionization tandem-mass spectrometric (LC-ESI-MS/MS) analysis was performed in the Mass Spectrometry Core and National Jewish Health, as previously described (32). In brief, sterols and oxysterols were quantified using an LC-MS/MS approach with electrospray ionization (ESI) using an AB Sciex 6500 QTRAP mass spectrometer. Internal deuterated cholesterol and oxysterol standards (Avanti Polar Lipids, Alabaster, MO) were used. Cholesterol and oxysterol compositions were quantified using an isotope dilution approach.
Laurdan Two-Photon Microscopy
Analysis of the physical properties of ordered and disordered membrane domains was performed using a laurdan two-photon microscopy, as described earlier (15, 37). Briefly, the images were acquired with a Bio-Rad multiphoton microscope. Cells were loaded with 5 μM laurdan dye (Molecular Probes, Carlsbad, CA) in serum-free medium with DMSO used as a vehicle. Laurdan fluorescence was excited with a mode-locked titanium-sapphire laser with the multiphoton laser excitation set at 800 nm. The images were obtained with a ×63 oil immersion objective (1.3 numerical aperture). The emitted light was collected in the ranges 410–490 and 503–553 nm. The general polarization (GP) was calculated using the equation:
where I is fluorescence signal intensity. The GP averages were obtained from the average values of 20–25 images, normalized (sum = 10,000), and fitted into two Gaussian distributions by using a nonlinear fitting algorithm (Microsoft Excel). The data did not allow an unambiguous separation into more than two populations. The same limitation was noted previously (15, 37). The general range of the GP values is between 0 and 0.8. In earlier studies, lipid-ordered/raft domains were found in the range of 0.25 < GP < 0.55, with disordered domains appearing in the range of 0.05 < GP < 0.25 (15). In liposomes with equal molar ratios of dioleoylphosphatidylcholine, cholesterol, and sphingomyelin, the range was somewhat different, with GP > 0.55 and GP < −0.05 representing membranes in gel and fluid phase, respectively (15). In previous studies in the laboratory, we see a separation between more-ordered and less-ordered domains, with the GP values of 0 < GP < 0.6 and −0.6 < GP < 0, respectively (37). In this study, we see a separation between more-ordered and less-ordered domains, with the GP values of 0.4 < GP < 0.8 and 0.0 < GP < 0.4, respectively. While the difference between this study and previous studies in the absolute GP values is not exactly clear, the most important observation is that, not only is there a clear separation between less- and more-ordered domains but also that pathological levels of LDL induce a significant shift from less-ordered to more-ordered states and that these shifts indicate that the excess cholesterol is clearly loading these endothelial cell membranes with cholesterol.
Proliferation Assays
5-Bromo-2-deoxyuridine proliferation assay.
Proliferation was measured using the 5-bromo-2-deoxyuridine (BrdU) labeling detection kit III (Roche, Pleasanton, CA) following the manufacturer’s instructions with slight modification. Briefly, after exposure to either oxLDL or LDL, BrdU labeling reagent was added for 6 h, and then cells were fixed with precooled ethanol fixative for 20 min in −20°C. The fixed cells were incubated with nuclease working solution followed by anti-BrdU-peroxidase Fab fragments working solution and then anti-mouse-Ig-AP solution (1:100 in PBS with calcium and magnesium). Cells were then covered in color substrate solution and nitroblue tetrazolium chloride/5-bromo-4-chloro-3-indolyl-phosphate toluidine salt (NBT/BCIP) solution 1:50 in substrate buffer (100 mM Tris·HCl buffer, 100 mM NaCl, and 50 mM MgCl2, pH 9.5) at 15–25°C for 15–30 min. Pictures were taken at ×10 with a Zeiss microscope, and at least three pictures per well were captured. After the cells were fixed, all incubations were done for 30 min at 37°C (45).
Celigo BrdU-4′,6-diamidino-2-phenylindole proliferation analysis.
Cells were treated with OxLDL and LDL as described. Following the Celigo protocol (Nexcelom, Lawrence, MA), the cells were then incubated with BrdU (Roche) at 100 μM for 2 h at 37°C. The cells were then fixed using ethanol-glycine fixation for 30 min at room temperature. The cells were incubated with 2N HCl for 10 min and subsequently washed with PBS. The cells were blocked with PBS + 0.5% BSA + 0.1% Tween 20 and subsequently incubated with anti-BrdU antibody (1:250; BD Biosciences, San Jose, CA) for 2 h at room temperature. The cells were then incubated with Alexa Fluor 488 goat anti-mouse antibody (1:400; Life Technologies) for 1 h at room temperature. The cells were then stained with DAPI (1:1,700; Life Technologies) for 30 min at room temperature and then refrigerated in PBS overnight. They were imaged and analyzed the following day using the Celigo System from Nexcelom. In short, cells are scanned using the DAPI and FITC filters. The ratio of the two fluorescent images is used to calculate the percentage of cells in the different stages of the cell cycle (G0/G1, S, or G2/M; see Ref. 7).
Analysis of Cellular Gene Expression
Cells were lysed in TRIzol (Invitrogen, Thermo Fisher Scientific), and RNA was extracted using the Direct-zol RNA miniprep kit from Zymo Research (Irvine, CA). In short, cells are lysed in Trizol (Invitrogen), and then 100% ethanol is added. The lysates are added to the provided spin columns, washed with the provided buffers, and then eluted with DNase-, RNase-free water. The RNA concentration was then measured using the Nanodrop (Nanodrop Technologies, Wilmington, DE). The RNA was then converted to cDNA using the High-Capacity cDNA Reverse Transcription Kit (Applied Biosystems, Foster City, CA), at which point RT-PCR was run to determine gene expression using IDT Primers (Coralville, IA) and SybrGreen Master Mix (Applied Biosystems) on a Viia-7 Real-Time PCR System (Thermo Fisher Scientific; see Ref. 23).
Primers were as follows: vascular endothelial growth factor receptor-2 (VEGFR2), forward 5′-CAAGTGGCTAAGGGCATGGA-3′ and reverse 5′-ATTTCAAAGGGAGGCGAGCA-3′; vascular endothelial growth factor (VEGF), forward 5′-CTCTACCTCCACCATGCCAA-3′ and reverse 5′-GCATGGTGATGTTGGACTCC-3′; angiopoietin-1 (Ang1), forward 5′-CAACAGTGTCCTTCAGAAGCAGC-3′ and reverse 5′-CCAGCTTGATATACATCTGCACAG-3′; angiopoietin-2 (Ang2), forward 5′-ATTCAGCGACGTGAGGATGGCA-3′ and reverse 5′-GCACATAGCGTTGCTGATTAGTC-3′; GAPDH, forward 5′-ACATCGCTCAGACACCATG-3′ and reverse 5′-TGTAGTTGAGGTCAATGAAGGG-3′; and ubiquitin, forward 5′-GTGAGCTTGTTTGTGTCCCT-3′ and reverse 5′-GTGATGGTCTTGCCGGTAA-3′.
Wound Healing Model
Male C57BL6 mice 8–10 mo old (Jax Mice, Sacramento, CA) and ApoE−/− mice (bred in house, with C57BL6 background) were used. The procedure was performed as we previously described (29). Mice were anesthetized by intraperitoneal injections of ketamine (100 mg/kg) and xylazine (5 mg/kg). Full-thickness excisional wounds of 5 mm in diameter were made on the shaved dorsum of the mice with a standard biopsy punch (Acuderm, Ft. Lauderdale, FL). At various time points after injury, the mice were euthanized by carbon dioxide inhalation and cervical dislocation, and the wound with its surrounding tissue was removed. Wounds were embedded in OCT, optimal cutting temperature medium (Fisher Healthcare, Thermo Fisher Scientific, Waltham, MA) for histological analysis or placed in RNAlater (Ambion, Thermo Fisher Scientific) for gene expression analysis. All samples were stored at −80°C until the time of sectioning and analysis. Sex- and age-matched mice were used for all experiments. Animal protocols used in this study were reviewed and approved by the University of Illinois at Chicago Institutional Animal Care and Use Committee. All experiments and procedures were performed in accordance with the approved protocols.
Analysis of Wound Angiogenesis
Fluorescent confocal imaging.
Sections (8 μm thick) were prepared from frozen embedded tissues. To analyze blood vessel density in wound samples, endothelial cells were visualized by cluster of differentiation 31 {CD31 [platelet endothelial cell adhesion molecule (PECAM)], 1:75; BD Biosciences} immunohistochemistry. Anti- PECAM1 rat anti-mouse antibody (1:75) was purchased from BD Biosciences. Alexa Fluor 488 Affini pure goat anti rat secondary (1:800) was purchased from Jackson ImmunoResearch (West Grove, PA). Primary and secondary antibody incubations were for 45 min. DAPI stain (Thermo Fisher Scientific) and Prolong Anti-fade mounting medium (Invitrogen, Thermo Fisher Scientific) were used for 5-min incubations. Images of CD31 (PECAM)-immunostained wound sections were captured using a Zeiss Axiovert 200M or Zeiss laser scanning confocal microscope (LSM) 710 BiG and analyzed using ImageJ (34a). The wound bed was outlined using a freehand drawing tool and the area was measured. The CD31 (PECAM)-positive area within the wound bed was measured and the percent vascularization was calculated (29) as: % vascularization = (CD31-positive area/total wound bed area) × 100.
Histology.
Tissue sections were stained on BondRX autostainer (Leica Biosystems) following a preset protocol. In brief, sections were deparaffinized with Bond Dewax solution and subjected to EDTA-based (Bond ER2 solution, pH 9) antigen retrieval for 20 min at 100°C. Sections were blocked for 5 min with hydrogen peroxide block and washed with Bond Wash Solution. Following 30 min incubation with Background Sniper protein block (no. BS966; Biocare Medical), sections were incubated with anti-CD31 rabbit monoclonal antibody (clone D8V9E, 1:200; Cell Signaling Technology) for 30 min. The detection was performed using the Bond Polymer Refine Detection kit (DS9800; Leica Biosystems) using the following conditions: polymer-HRP incubation time was set to 15 min, and DAB incubation time was 10 min. All slides were counterstained with hematoxylin for 10 min and mounted with Surgipath Micromount Media (Leica Biosystems). Secondary antibody only control was done during the antibody optimization step to confirm the lack of the nonspecific staining from the detection system.
Analysis of Wound CD31/PECAM and VEGF Expression
In short, wounds placed in RNAlater (Ambion, Thermo Fisher Scientific) were homogenized in Trizol (Invitrogen, Thermo Fisher Scientific) using a mechanical homogenizer. RNA was then isolated under RNAse-free conditions using the chloroform extraction method. The RNA concentration was then measured using the Nanodrop (Nanodrop Technologies). The RNA was then converted to cDNA using the High-Capacity cDNA Reverse Transcription Kit (Applied Biosystems), at which point RT-PCR was run to determine gene expression using IDT Primers and SybrGreen Master Mix (Applied Biosystems) on a Viia-7 Real-Time PCR System (Thermo Fisher Scientific; see Ref. 46).
Primers were as follows: PECAM, forward 5′-TGTTGGAGTTCAGAAGTGGAG-3′ and reverse 5′-TCATTGGAGTGGTCATCGC-3′; VEGF-A165, forward 5′-TGCAGGCTGCTGTAACGATG-3′ and reverse 5′-GAACAAGGCTCACAGTGATTTTCT-3′; and GAPDH, forward 5′-GTGGAGTCATACTGGAACATGTAG-3′ and reverse 5′-AATGGTGAAGGTCGGTGTG-3′.
Matrigel Plug Assay Performed in C57BL6 and ApoE−/− Mice
The procedure was performed as we previously described (32). Male C57BL6 mice (8–10 mo old; Jax Mice) and ApoE−/− mice (bred in house) were injected with 250 μL phenol red-free growth factor-reduced Matrigel (BD Biosciences) that had 50 ng/mL VEGF (Fisher Scientific, Thermo Fisher Scientific) and 10 ng/mL bFGF (Fisher Scientific, Thermo Fisher Scientific) added. The Matrigel plugs were maintained in vivo for 7 days. Postexcision, the plugs were immediately placed in OCT (Fisher Healthcare, Thermo Fisher Scientific) on dry ice, or the plugs were fixed for 24 h in 4% paraformaldehyde (PFA) and then embedded in paraffin. Tissues were then sectioned in 5 μm step sections; each section was 100 µm from the previous one. The tissues were then stained, imaged using a Zeiss Axiovert 200M, and analyzed using ImageJ (34a). Anti- PECAM1 rat anti-mouse antibody (1:75) was purchased from BD Biosciences. Alexa Fluor 488 Affini pure goat anti-rat secondary (1:800) was purchased from Jackson ImmunoResearch. Primary and secondary antibody incubations were for 45 min. DAPI stain (Thermo Fisher Scientific) and Prolong Anti-fade mounting medium (Invitrogen, Thermo Fisher Scientific) were used for 5 min. Mouse IgG (Thermo Fisher Scientific) was used as a control (1:2,000).
Additionally, the procedure was then performed in 3-mo-old male C57BL6 mice (Jax Mice) with additional LDL, either 50 mg/dL or 250 mg/dL, added to the Matrigel before injection. The Matrigel plugs were then excised, placed in PFA, embedded in paraffin, and sectioned in 5 μm sections. These sections were then stained with hematoxylin and eosin, or CD31/PECAM (1:200; Cell Signaling, Danvers, MA). Lumens per section were counted for four to six sections, on two separate slides, per plug. There were seven plugs per treatment. The analysis was done in a blinded manner.
Performed in Athymic, Nude Mice
Similarly to the experiments described previously (32), we exposed 7 × 104 HAECs to either 0 mg/dL, 50 mg/dL, or 250 mg/dL LDL. These cells were then used to populate phenol red-free growth factor-reduced Matrigel (BD Biosciences) that had 50 ng/mL VEGF (Fisher Scientific, Thermo Fisher Scientific) and 10 ng/mL bFGF (Fisher Scientific, Thermo Fisher Scientific) added. These Matrigel plugs were then injected subcutaneously into 3-mo-old male athymic nude mice (Jax Mice). Athymic mice are used to prevent an inflammatory response to the xenografted human endothelial cells. As before, the Matrigel plugs were then excised, placed in PFA, embedded in paraffin, and sectioned in 5 μm sections. These sections were then stained with hematoxylin and eosin to look at lumen formation. The analysis was done in a blinded manner. Lumens per section were counted for four to six sections, on two separate slides, per plug. There were six plugs per treatment.
Statistical Analysis
For statistical analysis, Microsoft Excel was used to perform unpaired t tests set with a P value <0.05. All of the analysis was done in a blind fashion with the nature of the experimental group masked to the observer. All mice were age and sex matched. One-way ANOVA followed by Bonferroni post hoc test was performed where appropriate.
Data Availability Statement
All of the data sets and images generated and/or analyzed during the current study are available from the corresponding author upon reasonable request.
RESULTS
LDL Induces Cholesterol Loading and Lipid Ordering in HAECs
Multiple earlier studies have shown that exposing endothelial cells to acetylated LDL, an artificially modified form of LDL used in in vitro research, results in significant cholesterol loading (28). However, little is known about the impact of native LDL on endothelial cholesterol. In this study, therefore, we first tested whether exposing endothelial cells to high levels of LDL results in a significant increase in cellular cholesterol.
Cholesterol was measured in HAECs exposed for 24 h to 50 mg/dL or 250 mg/dL LDL, corresponding to low (normal) and high levels, respectively, as defined by the American Heart Association (4, 9). First, we measured the level of total cellular cholesterol using a fluorometric assay, a well-established method to estimate cell cholesterol (8, 19). There is significant cholesterol loading, with 250 mg/dL yielding nearly two times the cholesterol level in HAECs compared with 50 mg/dL (Fig. 1Aa). It is important to note, however, that total cholesterol includes both free cholesterol residing in the membrane, known to have major effects on numerous cellular functions, and esterified cholesterol, stored intracellularly, which is less biologically active (42). Therefore, we next used mass spectrometry to assess the levels of free cholesterol and cholesterol esters. We found that, while exposure to low/normal LDL level (50 mg/dL) had no effect on the level of free cholesterol in these cells, exposure to high LDL (250 mg/dL) resulted in a significant increase of free cholesterol from 620 ± 51 pmol/nmol Lipid P to 760 ± 38 pmol/nmol Lipid P (Fig. 1Ab). We also detected low but measurable levels of three oxysterols (7-ketocholesterol, 5α,6α-epoxycholestanol, and 27-OH-cholesterol), but there was either no difference between cells exposed to 50 vs. 250 mg/dL LDL (0.44 ± 0.08 vs. 0.45 ± 0.1 pmol/nmol Lipid P for 7-ketocholesterol, 0.26 ± 0.05 vs. 0.33 ± 0.07 pmol/nmol for 5α,6α-epoxycholestanol) or there was even a small decrease (0.11 ± 0.02 vs. 0.04 ± 0.00 pmol/nmol for 27-OH-cholesterol). We also analyzed an array of cholesterol esters and found that 16:0, 16:1, and 18:1 were most abundant and increased significantly in cells exposed to the high LDL level. The total levels of cholesterol esters for the three experimental conditions are shown in Fig. 1Ac. It is noteworthy, however, that, consistent with previous studies (26), the level of cholesterol ester in endothelial cells under control conditions is very low (~2%), increasing to ~15% in cells exposed to 250 mg/dL LDL.
Fig. 1.
Exposure to low-density lipoprotein (LDL) reduces cholesterol loading and membrane lipid ordering in human aortic endothelial cells (HAECs). A: cholesterol loading. a: total cholesterol measured by Amplex Red (n = 4, *P < 0.05. b and c: free cholesterol (b) and total cholesterol (c) esters measured by mass spectroscopy (n = 8, *P < 0.05). B: typical images of HAECs treated with 50 mg/dL (top) or 250 mg/dL LDL (bottom) and imaged using laurdan two-photon microscopy. Scale bars = 20 µm. C: general polarization (GP) histograms for the corresponding images fitted by a two-Gaussian distribution with the curve shifted to the right representing ordered lipid domains (red) and the curve shifted to the left representing disordered lipid domains (yellow). D: average peak values for both ordered and disordered domains in HAECs exposed to 50 mg/dL or 250 mg/dL LDL. *P < 0.05, n = 3.
Additionally, since it is well known that an increase in free cholesterol alters the biophysical properties of biological membranes by increasing lipid order (42), analysis of lipid packing provides an independent way to evaluate the impact of LDL exposure to endothelial membranes. Lipid packing of the membrane is estimated using laurdan two-photon microscopy. Laurdan is a dye sensitive to the degree of lipid order based on how it aligns to the water dipoles within the cell’s membrane, which in turn is a function of lipid packing (15). The probe shifts its emission wavelength from 410 to 490 nm to 500–550 nm based on whether it is in the gel state or the fluid state, respectively. As described earlier, changes in lipid packing are estimated using the GP ratio, a ratio of these two emission wavelengths, with higher GP corresponding to more ordering within the cell membrane and lower GP corresponding to more disordered fluid phase (15, 37). Figure 1B shows typical laurdan images of HAECs exposed to 50 mg/dL LDL (image on top) versus 250 mg/dL LDL (image on bottom), pseudocolored to present the GP values where red corresponds to higher GP and yellow to lower GP. Clearly, the image of a cell exposed to 250 mg/dL LDL has higher GP values (more-ordered membrane) than the image of a cell exposed to 50 mg/dL LDL. This shift in GP (lipid order) is also apparent in the representative histograms shown in Fig. 1C, which are fitted with a two-Gaussian distribution, as described in the earlier studies. The two peaks, within the two-Gaussian distribution, are believed to represent ordered (peak with higher GP values) and disordered (lower GP values) membrane domains. The rightward shift of all three peaks in HAECs exposed to high levels of LDL (250 mg/dL) indicates more ordering within the cell membrane compared with those cells exposed to normal levels of LDL (50 mg/dL). When comparing average GP peak values, we also see a significant increase corresponding to more lipid order, for those cells exposed to high levels of LDL (250 mg/dL; Fig. 1Da). Additionally, it is interesting to note that there is significant increase in the average peak values for both ordered and disordered domains, suggesting that exposing HAECs to high levels of LDL results in cholesterol loading and an increase in lipid packing in both types of domains (Fig. 1D, b and c). The shift in lipid packing to a more-ordered state is consistent with an increase in free cholesterol level shown above using mass spectrometry.
Opposite Effects of LDL and oxLDL on Endothelial Cell Proliferation
Our recent studies showed that exposure to oxLDL results in the disruption of lipid ordering of endothelial membranes (37) and an increase in endothelial proliferation (45). In the current study, after establishing that, in contrast to oxLDL, exposure to high levels of LDL leads to cholesterol loading and membrane ordering of HAECs, we tested the impact of LDL on endothelial proliferation. First, we show that, consistent with our previous study, exposure to 50 μg/mL oxLDL enhances endothelial proliferation, as assessed by counting proliferating cells labeled with BrdU (Fig. 2, A and B). However, exposure to high levels of LDL (250 mg/dL) resulted in a significant decrease in endothelial proliferation compared with cells exposed to normal levels of LDL (50 mg/dL; Fig. 2, A and B).
Fig. 2.
Elevated low-density lipoprotein (LDL) decreases cell proliferation in human aortic endothelial cells (HAECs). A: representative image of 5-bromo-2-deoxyuridine (BrdU) staining of HAECs treated with control, 50 µg/mL oxidized LDL (oxLDL), 50 mg/dL LDL, or 250 mg/dL LDL. B: quantification of BrdU-positive cells for the same experimental conditions. *P < 0.05, n = 4. C: representative Celigo gating for HAECs for the same experimental conditions. D: Celigo analysis indicating percentage of cells in various stages of the cell cycle. *P < 0.05, n = 6.
To delineate initial insight into the mechanism by which LDL inhibits endothelial proliferation, we analyzed the cell cycle using the Celigo BrdU-DAPI protocol. In short, this approach uses a fluorescent BrdU antibody and DAPI nuclear stain to analyze the percentage of cells in three distinct stages of the cell cycle (G0/G1, S, and G2/M). This analysis shows that exposure of endothelial cells to high levels of LDL (250 mg/dL) results in a significant increase in the percentage of cells in the G2/M phase compared with cells exposed to normal levels of LDL (50 mg/dL). In contrast, exposure to oxLDL resulted in a higher percentage of cells in the S phase (Fig. 2, C and D). The diagrams in Fig. 2C show representative 2-D scatter plots on the logarithmic scale of cells that are stained with both DAPI and BrdU. The relative amounts of DAPI and BrdU stains are indicative of the stage of the cell cycle, with the upper quadrant indicating S phase, the lower quadrants indicating G0/G1 phase, and the right quadrant indicating G2/M phase. The quantification of the ratios is shown in Fig. 2D. The viability of the endothelial cells was unaffected by these long 24-h incubations with pathological levels of LDL (Fig. 3).
Fig. 3.
Human aortic endothelial cell (HAEC) viability is unaffected by either low-density lipoprotein (LDL) or oxidized LDL (oxLDL). Quantification of calcein fluorescence in cells exposed to different levels of LDL or oxLDL for 24 h compared with cells exposed to saponin detergent as negative control and untreated cells for positive control (n = 3).
We also quantified gene expression levels of VEGF, a major driver of endothelial proliferation, its receptor VEGFR2, and of angiopoietins 1 and 2 (Fig. 4). Exposure to high levels of LDL (250 mg/dL) significantly suppressed the expression of VEGFR2 and enhanced the expression of VEGF compared with cells exposed to normal levels of LDL (50 mg/dL). No difference was detected in the expression of both VEGFR2 and VEGF in cells exposed to oxLDL. We also found no difference in the expression of angiopoietins 1 and 2 under any of the experimental conditions tested.
Fig. 4.
Effects of low-density lipoprotein (LDL) and oxidized LDL (oxLDL) on gene expression of vascular endothelial growth factor (VEGF), VEGF receptor-2 (VEGFR2), angiopoietin 1 (Ang1), and angiopoietin 2 (Ang2) in human aortic endothelial cells (HAECs). A: relative expression of VEGF, VEGFR2, angiopoietin 1, and angiopoietin 2 in cells exposed to either control conditions or 50 μg/mL oxLDL (n = 4). B: relative expression of VEGF, VEGFR2, angiopoietin 1, and angiopoietin 2 in cells exposed to either 50 mg/dL LDL or 250 mg/dL LDL (n = 4, *P < 0.05).
Furthermore, as we have previously shown that oxLDL-induced increase in endothelial proliferation depends on the activation of RhoA (45) and is associated with increased endothelial stiffening, here we examined whether exposure to LDL had the same or opposite effects. We found that, similar to oxLDL, exposure to a high level of LDL resulted in endothelial stiffening, but the effect of LDL was very mild: ~20% increase in the elastic modulus between cells exposed to 250 vs. 50 mg/dL (data not shown) compared with a more than twofold increase in the elastic modulus in cells exposed to oxLDL (32, 37). No effect of LDL was found for RhoA activation (data not shown).
It is important to note, though, that while aortic endothelial cells are a good model for studying the effects of lipoproteins on cholesterol loading of endothelial cells and endothelial proliferation in general, these cells are usually not associated with angiogenesis in vivo. However, we have validated previously that HAECs form lumens in collagen gels in vitro (37) and functional blood vessels in Matrigel plugs in vivo (32), indicating their angiogenic potential. Additionally, our previous experiments have shown that aortic and microvascular endothelial cells respond similarly to oxidized LDL in a Matrigel plug assay when the Matrigel was prepopulated with either HAECs or human microvascular endothelial cells (32). We expect, therefore, that the sensitivity of HAECs to LDL is similar to that of microvascular endothelial cells.
Hypercholesterolemia Leads to Delayed Wound Closure and Decreased Wound Angiogenesis
Given the way in which high LDL levels affected in vitro endothelial proliferation, we extended the study to investigate how hypercholesterolemia affects angiogenesis in wound healing.
To test the impact of hypercholesterolemia on wound healing, we focused on ApoE−/− mice, a well-known hypercholesterolemic mouse model (34). The wound healing was assessed in mice age 8–10 mo, representing a middle-age group. Age- and sex-matched wild-type controls were used in all experiments. The wound healing process was assessed following 5 mm full-thickness skin punch biopsies, a standard approach to study this process (10, 29). The same protocol was repeated for two independent cohorts of mice. The wounds of both ApoE−/− mice and wild-type mice were allowed to heal for up to 14 days. Wounds were photographed daily, and the wound area was measured. On days 1–7, ApoE−/− mice showed a significantly larger wound size than C57BL6 mice, indicating that the hypercholesterolemic mice show delayed wound closure (Fig. 5, A and B). By day 14, all wounds were fully closed. Over the 14-day course, the mouse weights did not change, nor were they significantly different between the two mouse types (Fig. 5C).
Fig. 5.
Hypercholesterolemia leads to delayed wound closure. A: representative images of mouse wounds on days 0, 3, and 7 for C57BL6 wild-type and apolipoprotein E-deficient (ApoE−/−) male mice aged 8–10 mo. Scale bars = 2.5 mm. B: quantification of wound closure in two experimental groups (*P < 0.05, n = 5–10 mice per time point per group; 4 wounds per mouse). C: time course of average body weights for both wild-type C57BL6 and ApoE−/− mice over the 2-wk course of the wound healing process (n = 5–10 mice per time point per group).
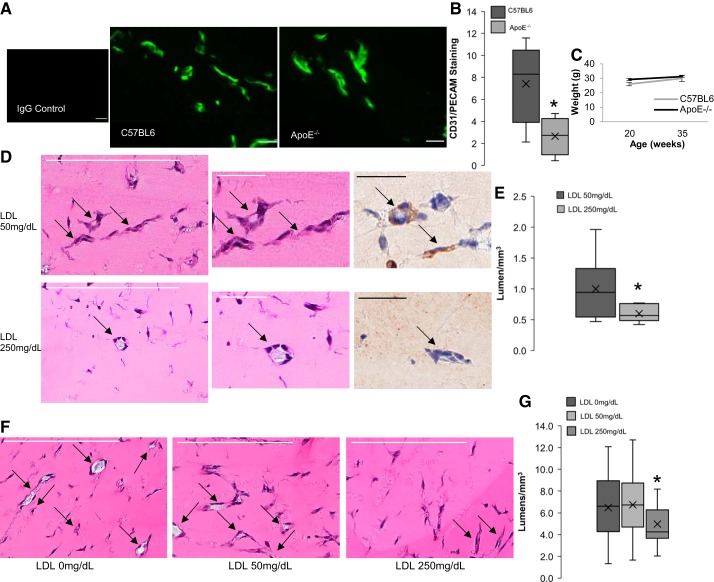
The progression of angiogenesis during the wound healing in ApoE−/− and wild-type (WT) mice was tested by measuring mRNA expression and tissue distribution of an endothelial cell marker, CD31/PECAM. The wounds were excised on days 7, 10, and 14 based on previous studies that have indicated that aged mice will have a delayed wound healing timeline compared with younger mice (18). Figure 6A shows that there was a significance decrease in CD31/PECAM expression in ApoE−/− mice on day 10, corresponding with the general peak of CD31/PECAM expression. A similar decrease in CD31/PECAM staining in ApoE−/− mice is also seen on days 10 and 14 (Fig. 6C). Representative images for day 10 clearly show decreased endothelial cell staining in wounds from ApoE−/− compared with C57BL6 mice (Fig. 6B). The mRNA expression of VEGF-A165 was shown to be not different between the two mouse types (Fig. 6D).
Fig. 6.
Hypercholesterolemia leads to decreased angiogenesis in wound healing. A: relative expression for cluster of differentiation 31 (CD31)/platelet endothelial cell adhesion molecule (PECAM) mRNA in wounds from C57BL6 wild-type and apolipoprotein E-deficient (ApoE−/−) male mice aged 8–10 mo (*P < 0.05, n = 4–6 mice per time point per group). B: representative images of CD31/PECAM-fluorescently labeled and immunostained slides from excised mouse wounds in the two groups of mice on day 10, IgG-stained control image included. Scale bar = 20 μm. C: quantification of CD31/PECAM-immunostaining from excised mouse wounds in C57BL6 and ApoE−/− mice for days 0, 7, 10, and 14 (*P < 0.05, n = 4–6 mice per condition, 4 histological sections per mouse. Sections were taken 50 μm apart). D: relative expression for VEGF-A165 mRNA for the same experimental groups.
Hypercholesterolemia and High Levels of LDL Lead To a Decrease in Angiogenesis in Matrigel Plugs
Matrigel plugs are a well-known in vivo method for assessing endothelial responsiveness to growth factors as well as assessing endothelial angiogenic potential (2). The advantage of this approach is that it allows an assessment of endothelial responsiveness in vivo in adult animals without the significant complications of tissue injury. Following the decreased wound angiogenesis, we focused on a Matrigel plug assay to determine whether hypercholesterolemia inhibits endothelial angiogenic potential. The study was performed on 8- to 9-mo-old male ApoE−/− mice and WT controls, with both groups age and sex matched to the cohorts used for the wound healing/angiogenesis study.
Endothelial cells within the Matrigel plugs were quantified by immunostaining for the endothelial marker CD31/PECAM. Figure 7A shows representative images of the Matrigel sections visualized for CD31/PECAM in the experimental groups described above. Endothelial cells were apparent in Matrigels from both experimental groups, but there was an apparent decrease in endothelial staining under dyslipidemic conditions compared with the wild-type C57BL6 mice. The endothelial responsiveness to both VEGF and bFGF in the animal models was quantified by measuring the percent area stained by CD31/PECAM within the individual sections. Figure 7B shows that there is a significant decrease in endothelial cell staining in ApoE−/− mice compared with WT controls. Because the Matrigel in these experiments was not premixed with endothelial cells, all of the cells seen within the Matrigel plugs are endogenous recruited cells from the host animal and are thus microvascular endothelial cells. Figure 7C indicates that there was no difference in the weight between the two groups over the course of their adult lives.
Fig. 7.
Hypercholesterolemia leads to reduced endothelial responsiveness to growth factors in Matrigel plugs. A: representative images of platelet endothelial cell adhesion molecule (PECAM) 1-immunostained slides from Matrigel plugs in C57BL6 wild-type and apolipoprotein E-deficient (ApoE−/−) male mice aged 8–10 mo. IgG-stained control image is included. Scale bar = 20 μm (n = 4–5 mice per time point per group, 4 histological sections per mouse; sections were taken 50 μm apart). B: quantification of cluster of differentiation 31 (CD31)/PECAM1 staining (*P < 0.05, n = 4–5 mice per time point per group). C: average body weights for the two experimental groups (4–5 mice per group). D: representative images of hematoxylin and eosin stained sections from Matrigels injected into male C57BL6 mice aged 3 mo with 50 mg/dL or 250 mg/dL LDL with arrows depicting lumens (scale bar = 200 μm). Center and left images show close-up images of specific lumen, along with representative CD31/PECAM staining of those lumens (scale bar = 50 μm). E: quantification of the lumen seen in hematoxylin and eosin stained sections from D (n = 7 plugsper treatment with 2 slides/plug, *P < 0.05). F: representative images of hematoxylin and eosin stained sections from Matrigels injected in male athymic nude mice aged 3 mo with human aortic endothelial cells (HAECs) preexposed to 0 mg/dL, 50 mg/dL, or 250 mg/dL LDL with arrows depicting lumens (scale bar = 200 μm). G: quantification of the lumen seen in hematoxylin and eosin stained sections from F (n = 6 plugs/treatment with 2 slides/plug, *P < 0.03 with ANOVA).
Furthermore, to test whether elevated levels of LDL are sufficient to inhibit angiogenesis in Matrigel plugs, we performed two series of experiments: 1) adding LDL to the Matrigel plugs before injection into the mice and 2) populating the Matrigels with endothelial cells preexposed to high levels of LDL before the injection. Both treatments resulted in significant suppression of Matrigel angiogenesis at 250 mg/dL of LDL.
First, we determined whether the addition of 250 mg/dL of LDL to the Matrigel has an inhibitory effect on the capillary formation compared with Matrigels that contained 50 mg/dL. We show here that this is clearly the case: there was significantly less lumen formation in those Matrigels containing 250 mg/dL LDL as opposed to those containing 50 mg/dL (Fig. 7, D and E). This experiment was done in wild-type C57BL6 mice aged 3 mo. Seven plugs in four mice per condition, in two separate cohorts, were analyzed for each condition. Notably, the analysis is done by the observer blind to the nature of the treatment, since all images are coded and blinded for the quantification analysis. Figure 7D shows representative images of hematoxylin and eosin (H&E) staining of the Matrigel sections, with clear lumens indicated by the arrows. Images on the left and middle show specific lumens depicted in H&E, as well as CD31 staining indicating endothelial cells. In addition, we also tested whether preexposure of human endothelial cells to LDL and then populating them into Matrigels injected into athymic nude mice inhibits lumen formation. The rationale for using athymic nude mice is to prevent an inflammatory response to the exogenous xenografted human cells used in this experiment, similar to the study done previously in our laboratory with oxLDL (32). Our results show that indeed this is also the case: Matrigels populated with cells preexposed to 250 mg/dL LDL had significantly fewer lumens than those Matrigels populated with cells preexposed to 50 mg/dL. There was no difference between lumen formation in Matrigels with cells preexposed to either 50 mg/dL or 0 mg/dL LDL (Fig. 7, F and G). As described above, all of the analysis is done with blinded samples.
DISCUSSION
Elevated plasma levels of LDL are the hallmark of hypercholesterolemia, as defined in the clinic, but the impact of excess LDL on cholesterol loading and function of endothelial cells is not well understood. Most of the previous studies, including studies from our laboratory, focused on oxidative modifications of LDL [oxLDL (20, 25, 28, 35, 37, 45), a highly pro-inflammatory form of LDL that is thought to be particularly proatherogenic (41)]. In terms of cholesterol loading, however, our studies showed that, when ECs were exposed to oxLDL, cells were not loaded with cholesterol but instead loaded with different oxidized lipids that are found in the oxLDL particle, including oxysterols and oxidized phospholipids (8, 36). We also showed that incorporation of oxysterols and oxidized lipids disrupts lipid packing of endothelial membrane (3, 36). In this study, we show that, in contrast to oxLDL, exposing human aortic endothelial cells (HAECs) to elevated levels of LDL results in a significant increase in both total and free cholesterol levels. Moreover, we also show here that exposure to high levels of LDL results in tightening of the lipid order of the endothelial membrane, a known effect of cholesterol on membrane structure. Our current finding that exposure of endothelial cells to high levels of LDL has a distinct, and even an opposite, effect on the biophysical properties of endothelial membranes, as compared with oxLDL, suggests that the two forms of LDL also have different effects on endothelial physiological properties.
This study focuses on endothelial proliferation because it plays an important role in development, angiogenesis, and wound healing. Multiple studies explored the effects oxLDL has on endothelial proliferation, but little is still known about the impact of LDL. For oxLDL, the majority of the studies, particularly within the physiological range, showed that exposing endothelial cells to oxLDL results in increased proliferation across several types of endothelial cells (20, 35, 43, 45). Additionally, we showed that oxLDL-induced endothelial proliferation can be reversed by loading the cells with cholesterol, suggesting that the two effects are distinct (45). For LDL, we are not aware of any studies that tested the effect of LDL in the physiological range found in human plasma (50–250 mg/dL) on endothelial proliferation. One study showed that LDL had antiproliferative effects in human umbilical vascular endothelial cells (HUVECs) at very low nonphysiological levels (up to 100 μg/mL; see Ref. 22) and, because of that, the physiological significance of this study is not clear. In our study, therefore, we performed a comparative analysis of the effects of oxLDL and LDL on the proliferation of HAECs, when cells were exposed to normal or high levels of LDL found in human plasma in healthy and hypercholesterolemic individuals. We show that, while oxLDL enhances endothelial proliferation, pathological levels of LDL have significant antiproliferative effect.
In terms of the mechanism, we found that oxLDL and LDL have distinct effects on the different phases of the cell cycle. Exposure to oxLDL had a small, but significant, positive effect on the percentage of cells found in S phase of the cell cycle, the DNA synthesis phase, as expected for a mild proproliferative agent. In contrast, exposure to high levels of LDL had a significant increase in the percentage of cells found in the G2/M phase, a cell growth and checkpoint phase, which is interpreted as cell cycle arrest in the G2 phase. There is also a significant decrease in the percentage of cells in the G0/G1 phase and a trending decrease in the percentage of cells in the S phase. Additionally, cells exposed to high levels of LDL have decreased VEGFR2 expression, which was recently shown to be associated with cell cycle arrest in breast cancer cells. Specifically, recent studies identified a number of compounds that block VEGFR2 in MCF7 cells and induce cell cycle arrest in G2/M phase (30, 39), similar to our observations in endothelial cells exposed to high LDL. Thus, we propose that LDL suppresses endothelial cell proliferation via a decrease in VEGFR2 expression, leading to cell cycle arrest.
The antiproliferative effects of LDL suggest that it is antiangiogenic. Therefore, our next goal was to test the hypothesis that hypercholesterolemia leads to impairment of wound angiogenesis in vivo. In general, previous studies of angiogenesis in hypercholesterolemic animal models gave conflicting results. In context of atherosclerosis, hypercholesterolemia was found to be proatherogenic, which was linked to neovascularization of the plaques (31). The proangiogenic effects of hypercholesterolemia were also linked to the excess oxidized lipids, either by implanting 7-ketocholesterol-containing wafers into the anterior chamber of the eye in a rat (1) or by preexposing endothelial cells to oxLDL in a Matrigel plug assay in mice (32). Interestingly, hypercholesterolemia was also shown to enhance angiogenesis in breast cancer tumors (11). However, studies of several other models found that hypercholesterolemia had an antiangiogenic effect. Specifically, hypercholesterolemia-induced inhibition of angiogenesis was found in the hind limb ischemia model in rabbits (40) and in rats (12), in collateral formation following myocardial infarction in swine (6), and in ischemic brain injury in ApoE−/− mice (44). Our observations that exposure to oxLDL enhances endothelial proliferation while exposure to elevated levels of LDL inhibits endothelial proliferation in the same cells provide a possible explanation for these controversial observations. In this context, a hypercholesterolemic environment rich in oxidized lipids, such as an atherosclerotic plaque, is expected to enhance angiogenesis, whereas hypercholesterolemic plasma, where the levels of oxLDL are relatively low (27, 33), is expected to have the inhibitory effect. Our new observations are consistent with, and are supportive of, this hypothesis.
We show here that plasma hypercholesterolemia, as seen in ApoE−/− mice, inhibits angiogenesis during the wound healing process accompanied with a delay in wound closure. Because it is well known that, in ApoE−/− mice, the serum level of LDL is very high, while the serum level of oxLDL is low, the inhibition of wound angiogenesis in this model is consistent with our hypothesis that plasma hypercholesterolemia outside of the oxLDL-enriched environment of the atherosclerotic plaques is antiangiogenic. This notion is also consistent with the antiproliferative effect of LDL shown in this study. However, it is currently not possible to fully discriminate between the impacts of LDL versus oxLDL in the complex environment of the in vivo wound bed. Additional approaches need to be developed to further differentiate between the roles of LDL and oxLDL in wound angiogenesis.
Notably, our study was performed in “mature” (8–10 mo old) mice, which is believed to correspond to 42–50 human years. This group is frequently ignored in studies using mouse models, which typically focus on either older mice or hypercholesterolemic mice but not on old hypercholesterolemic mice.
Additionally, we saw a decreased endothelial response and decreased angiogenic potential when VEGF- and bFGF-enriched Matrigel was implanted into the hypercholesterolemic ApoE−/− mice. Because there was no difference in VEGF expression in wounds from ApoE−/− or C57BL6 wild-type mice, it suggests that endothelial cells under hypercholesterolemic conditions are less responsive to the growth factors. Furthermore, exogenous addition of high levels of LDL to Matrigel plugs injected into wild-type mice and preexposing human endothelial cells to LDL before populating the Matrigels and injecting them into athymic mice also show significant negative effects. Along with the fact that previous work in the laboratory has shown that, if cells are preexposed to oxLDL and then placed within Matrigel, there is an increased endothelial response (32), this further discriminates between the effects of LDL and oxLDL on endothelial cells. Additionally, this further indicates that, unless the Matrigels or wafers are enriched with oxLDL or oxysterols, the observed effect in this hypercholesterolemic mouse model is significant inhibition of angiogenesis. Taken together, these results suggest that the inhibitory effect of hypercholesterolemia on angiogenesis should be attributed to the negative effect of LDL on endothelial cell responsiveness to growth factors. However, we cannot fully exclude the possibility that other hypercholesterolemia-related factors may contribute to the impairment of wound angiogenesis in ApoE−/− mice, which are known to be highly dyslipidemic, including elevated levels of very low density lipoprotein and triglycerides. More studies are needed to determine the mechanism of hypercholesterolemia-induced impairment of wound healing.
Notably, while there are numerous studies to show that type 2 diabetes and obesity impair wound healing in human patients, there is little information specifically about hypercholesterolemic patients. This is clearly important because, while there is an association between obesity and hypercholesterolemia in the human population, the two factors are distinct, since there are obese patients with normal cholesterol and there are hypercholesterolemic patients with normal body/mass index. In the latter population, hypercholesterolemia most likely has a significant genetic component. Likewise, in animal models, hypercholesterolemia can be induced either by high-fat/high-cholesterol diet or by genetic modification. To discriminate between the effects of obesity and hypercholesterolemia, our studies focus on ApoE−/− mice, a model of severe genetically induced hypercholesterolemia that is not associated with obesity. We observed a clear delay in wound closure of several days, which may occur in humans in several contexts, including aging and stress. The clinical implications of such delays have been associated with increased infection, altered final outcomes (such as changes in scar formation), and delayed return to function, including increased development of chronic wounds (17). Further studies will be needed to evaluate the impact of hypercholesterolemia on wound healing in human populations.
GRANTS
This work was supported in part by National Heart, Lung, and Blood Institute (NHBLI) Grants HL-083298 and HL-073965 (to I. Levitan), NHLBI Training Grant T32-HL-144459 (to V. Aguilar), and National Institute of General Medical Sciences Grant GM-050875 (to L. A. DiPietro).
DISCLOSURES
No conflicts of interest, financial or otherwise, are declared by the authors.
AUTHOR CONTRIBUTIONS
Y.Y.B., L.C., L.A.D., and I.L. conceived and designed research; Y.Y.B., I.S.F., Y.Z., V.A., and M.-J.O. performed experiments; Y.Y.B. and E.L.M. analyzed data; Y.Y.B., E.L.M., I.S.F., L.A.D., and I.L. interpreted results of experiments; Y.Y.B. and I.L. prepared figures; Y.Y.B. and I.L. drafted manuscript; Y.Y.B., K.K.W., and I.L. edited and revised manuscript; Y.Y.B., L.C., E.L.M., I.S.F., Y.Z., V.A., M.-J.O., K.K.W., L.A.D., and I.L. approved final version of manuscript.
ACKNOWLEDGMENTS
We thank Dr. Evgeny Berdyshev and the Mass Spectrometry Core at National Jewish Health in Denver, CO, for performing the mass spectrometry analysis. We are also grateful to Dr. Jonna Frasor for help with the analysis of the cell cycle and Drs. Victor Brovkovych and Dr. Svitlana Brovkovych for technical expertise in the Celigo protocol. Additionally, we are thankful to Alema Jackson for help with the Celigo experiments. We also thank Dr. Michael Cho at the University of Texas, Arlington, for help with the laurdan two-photon microscopy and Dr. Tzu-Pin Shentu for laurdan two-photon expertise. Histology services were provided by the Research Resources Center–Research Histology Core at the University of Illinois at Chicago established with the support of the Vice Chancellor of Research. We thank Gregory Kowalsky for help with preparing the images for the figures.
REFERENCES
- 1.Amaral J, Lee JW, Chou J, Campos MM, Rodríguez IR. 7-Ketocholesterol induces inflammation and angiogenesis in vivo: a novel rat model. PLoS One 8: e56099, 2013. doi: 10.51371/journal.pone.0056099. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Auerbach R, Lewis R, Shinners B, Kubai L, Akhtar N. Angiogenesis assays: a critical overview. Clin Chem 49: 32–40, 2003. doi: 10.1373/49.1.32. [DOI] [PubMed] [Google Scholar]
- 3.Ayee MAA, LeMaster E, Shentu TP, Singh DK, Barbera N, Soni D, Tiruppathi C, Subbaiah PV, Berdyshev E, Bronova I, Cho M, Akpa BS, Levitan I. Molecular-scale biophysical modulation of an endothelial membrane by oxidized phospholipids. Biophys J 112: 325–338, 2017. doi: 10.1016/j.bpj.2016.12.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Baigent C, Blackwell L, Emberson J, Holland LE, Reith C, Bhala N, Peto R, Barnes EH, Keech A, Simes J, Collins R; Cholesterol Treatment Trialists’ (CTT) Collaboration . Efficacy and safety of more intensive lowering of LDL cholesterol: a meta-analysis of data from 170,000 participants in 26 randomised trials. Lancet 376: 1670–1681, 2010. doi: 10.1016/S0140-6736(10)61350-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Bobryshev YV, Ivanova EA, Chistiakov DA, Nikiforov NG, Orekhov AN. Macrophages and their role in atherosclerosis: pathophysiology and transcriptome analysis. BioMed Res Int 2016: 9582430, 2016. doi: 10.1155/2016/9582430. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Boodhwani M, Nakai Y, Mieno S, Voisine P, Bianchi C, Araujo EG, Feng J, Michael K, Li J, Sellke FW. Hypercholesterolemia impairs the myocardial angiogenic response in a swine model of chronic ischemia: role of endostatin and oxidative stress. Ann Thorac Surg 81: 634–641, 2006. doi: 10.1016/j.athoracsur.2005.07.090. [DOI] [PubMed] [Google Scholar]
- 7.Brovkovych V, Izhar Y, Danes JM, Dubrovskyi O, Sakallioglu IT, Morrow LM, Atilla-Gokcumen GE, Frasor J. Fatostatin induces pro- and anti-apoptotic lipid accumulation in breast cancer. Oncogenesis 7: 66, 2018. doi: 10.1038/s41389-018-0076-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Byfield FJ, Tikku S, Rothblat GH, Gooch KJ, Levitan I. OxLDL increases endothelial stiffness, force generation, and network formation. J Lipid Res 47: 715–723, 2006. doi: 10.1194/jlr.M500439-JLR200. [DOI] [PubMed] [Google Scholar]
- 9.Castelli WP, Anderson K, Wilson PW, Levy D. Lipids and risk of coronary heart disease. The Framingham Study. Ann Epidemiol 2: 23–28, 1992. doi: 10.1016/1047-2797(92)90033-M. [DOI] [PubMed] [Google Scholar]
- 10.Chen L, Mirza R, Kwon Y, DiPietro LA, Koh TJ. The murine excisional wound model: Contraction revisited. Wound Repair Regen 23: 874–877, 2015. doi: 10.1111/wrr.12338. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.dos Santos CR, Domingues G, Matias I, Matos J, Fonseca I, de Almeida JM, Dias S. LDL-cholesterol signaling induces breast cancer proliferation and invasion. Lipids Health Dis 13: 16, 2014. doi: 10.1186/1476-511X-13-16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Duan J, Murohara T, Ikeda H, Katoh A, Shintani S, Sasaki K, Kawata H, Yamamoto N, Imaizumi T. Hypercholesterolemia inhibits angiogenesis in response to hindlimb ischemia: nitric oxide-dependent mechanism. Circulation 102, Suppl 3: III370–III376, 2000. doi: 10.1161/01.CIR.102.suppl_3.III-370. [DOI] [PubMed] [Google Scholar]
- 13.Fang Y, Mohler ER III, Hsieh E, Osman H, Hashemi SM, Davies PF, Rothblat GH, Wilensky RL, Levitan I. Hypercholesterolemia suppresses inwardly rectifying K+ channels in aortic endothelium in vitro and in vivo. Circ Res 98: 1064–1071, 2006. doi: 10.1161/01.RES.0000218776.87842.43. [DOI] [PubMed] [Google Scholar]
- 14.Flavahan NA, Vanhoutte PM. Endothelial cell signaling and endothelial dysfunction. Am J Hypertens 8: 28S–41S, 1995. doi: 10.1016/0895-7061(95)00030-S. [DOI] [PubMed] [Google Scholar]
- 15.Gaus K, Zech T, Harder T. Visualizing membrane microdomains by Laurdan 2-photon microscopy. Mol Membr Biol 23: 41–48, 2006. doi: 10.1080/09687860500466857. [DOI] [PubMed] [Google Scholar]
- 16.Gordts SC, Muthuramu I, Amin R, Jacobs F, De Geest B. The impact of lipoproteins on wound healing: topical HDL therapy corrects delayed wound healing in apolipoprotein E deficient mice. Pharmaceuticals (Basel) 7: 419–432, 2014. doi: 10.3390/ph7040419. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Gould L, Abadir P, Brem H, Carter M, Conner-Kerr T, Davidson J, DiPietro L, Falanga V, Fife C, Gardner S, Grice E, Harmon J, Hazzard WR, High KP, Houghton P, Jacobson N, Kirsner RS, Kovacs EJ, Margolis D, McFarland Horne F, Reed MJ, Sullivan DH, Thom S, Tomic-Canic M, Walston J, Whitney JA, Williams J, Zieman S, Schmader K. Chronic wound repair and healing in older adults: current status and future research. J Am Geriatr Soc 63: 427–438, 2015. doi: 10.1111/jgs.13332. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Guo S, Dipietro LA. Factors affecting wound healing. J Dent Res 89: 219–229, 2010. doi: 10.1177/0022034509359125. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Han H, Rosenhouse-Dantsker A, Gnanasambandam R, Epshtein Y, Chen Z, Sachs F, Minshall RD, Levitan I. Silencing of Kir2 channels by caveolin-1: cross-talk with cholesterol. J Physiol 592: 4025–4038, 2014. doi: 10.1113/jphysiol.2014.273177. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Heinloth A, Heermeier K, Raff U, Wanner C, Galle J. Stimulation of NADPH oxidase by oxidized low-density lipoprotein induces proliferation of human vascular endothelial cells. J Am Soc Nephrol 11: 1819–1825, 2000. [DOI] [PubMed] [Google Scholar]
- 21.Hiebert PR, Wu D, Granville DJ. Granzyme B degrades extracellular matrix and contributes to delayed wound closure in apolipoprotein E knockout mice. Cell Death Differ 20: 1404–1414, 2013. doi: 10.1038/cdd.2013.96. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Jin F, Hagemann N, Brockmeier U, Schäfer ST, Zechariah A, Hermann DM. LDL attenuates VEGF-induced angiogenesis via mechanisms involving VEGFR2 internalization and degradation following endosome-trans-Golgi network trafficking. Angiogenesis 16: 625–637, 2013. doi: 10.1007/s10456-013-9340-2. [DOI] [PubMed] [Google Scholar]
- 23.Keylock KT, Vieira VJ, Wallig MA, DiPietro LA, Schrementi M, Woods JA. Exercise accelerates cutaneous wound healing and decreases wound inflammation in aged mice. Am J Physiol Regul Integr Comp Physiol 294: R179–R184, 2008. doi: 10.1152/ajpregu.00177.2007. [DOI] [PubMed] [Google Scholar]
- 24.Landmesser U, Hornig B, Drexler H. Endothelial function: a critical determinant in atherosclerosis? Circulation 109, Suppl 1: II27–II33, 2004. doi: 10.1161/01.CIR.0000129501.88485.1f. [DOI] [PubMed] [Google Scholar]
- 25.LeMaster E, Huang RT, Zhang C, Bogachkov Y, Coles C, Shentu TP, Sheng Y, Fancher IS, Ng C, Christoforidis T, Subbaiah PV, Berdyshev E, Qain Z, Eddington DT, Lee J, Cho M, Fang Y, Minshall RD, Levitan I. Pro-atherogenic flow increases endothelial stiffness via enhanced CD36-mediated uptake of oxidized low-density lipoproteins. Arterioscler Thromb Vasc Biol 38: 64–75, 2018. doi: 10.1161/ATVBAHA.117.309907. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Levitan I, Christian AE, Tulenko TN, Rothblat GH. Membrane cholesterol content modulates activation of volume-regulated anion current in bovine endothelial cells. J Gen Physiol 115: 405–416, 2000. doi: 10.1085/jgp.115.4.405. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Levitan I, Fancher SI, Berdyshev E. Association of circulating oxidized lipids with cardiovascular outcomes. In: Clinical Lipidomics, edited by Wang X. London, UK: Springer Nature, 2017. [Google Scholar]
- 28.Levitan I, Volkov S, Subbaiah PV. Oxidized LDL: diversity, patterns of recognition, and pathophysiology. Antioxid Redox Signal 13: 39–75, 2010. doi: 10.1089/ars.2009.2733. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Low QE, DiPietro LA. Quantification of wound angiogenesis. Methods Mol Med 78: 319–327, 2003. doi: 10.1385/1-59259-332-1:319. [DOI] [PubMed] [Google Scholar]
- 30.Mghwary AE, Gedawy EM, Kamal AM, Abuel-Maaty SM. Novel thienopyrimidine derivatives as dual EGFR and VEGFR-2 inhibitors: design, synthesis, anticancer activity and effect on cell cycle profile. J Enzyme Inhib Med Chem 34: 838–852, 2019. doi: 10.1080/14756366.2019.1593160. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Moulton KS, Vakili K, Zurakowski D, Soliman M, Butterfield C, Sylvin E, Lo K-M, Gillies S, Javaherian K, Folkman J. Inhibition of plaque neovascularization reduces macrophage accumulation and progression of advanced atherosclerosis. Proc Natl Acad Sci USA 100: 4736–4741, 2003. doi: 10.1073/pnas.0730843100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Oh M-J, Zhang C, LeMaster E, Adamos C, Berdyshev E, Bogachkov Y, Kohler EE, Baruah J, Fang Y, Schraufnagel DE, Wary KK, Levitan I. Oxidized LDL signals through Rho-GTPase to induce endothelial cell stiffening and promote capillary formation. J Lipid Res 57: 791–808, 2016. doi: 10.1194/jlr.M062539. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Pawlak K, Mysliwiec M, Pawlak D. oxLDL - the molecule linking hypercoagulability with the presence of cardiovascular disease in hemodialyzed uraemic patients. Thromb Res 134: 711–716, 2014. doi: 10.1016/j.thromres.2014.07.007. [DOI] [PubMed] [Google Scholar]
- 34.Reardon CA, Getz GS. Mouse models of atherosclerosis. Curr Opin Lipidol 12: 167–173, 2001. doi: 10.1097/00041433-200104000-00010. [DOI] [PubMed] [Google Scholar]
- 34a.Schneider CA, Rasband WS, Eliceiri KW. NIH Image to ImageJ: 25 years of image analysis. Nat Methods 9: 671–675, 2012. doi: 10.1038/nmeth.2089. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Seibold S, Schürle D, Heinloth A, Wolf G, Wagner M, Galle J. Oxidized LDL induces proliferation and hypertrophy in human umbilical vein endothelial cells via regulation of p27Kip1 expression: role of RhoA. J Am Soc Nephrol 15: 3026–3034, 2004. doi: 10.1097/01.ASN.0000146425.58046.6A. [DOI] [PubMed] [Google Scholar]
- 36.Shentu TP, Singh DK, Oh M-J, Sun S, Sadaat L, Makino A, Mazzone T, Subbaiah PV, Cho M, Levitan I. The role of oxysterols in control of endothelial stiffness. J Lipid Res 53: 1348–1358, 2012. doi: 10.1194/jlr.M027102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Shentu TP, Titushkin I, Singh DK, Gooch KJ, Subbaiah PV, Cho M, Levitan I. oxLDL-induced decrease in lipid order of membrane domains is inversely correlated with endothelial stiffness and network formation. Am J Physiol Cell Physiol 299: C218–C229, 2010. doi: 10.1152/ajpcell.00383.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Steinberg D. Atherogenesis in perspective: hypercholesterolemia and inflammation as partners in crime. Nat Med 8: 1211–1217, 2002. doi: 10.1038/nm1102-1211. [DOI] [PubMed] [Google Scholar]
- 39.Tamura D, Arao T, Tanaka K, Kaneda H, Matsumoto K, Kudo K, Aomatsu K, Fujita Y, Watanabe T, Saijo N, Kotani Y, Nishimura Y, Nishio K. Bortezomib potentially inhibits cellular growth of vascular endothelial cells through suppression of G2/M transition. Cancer Sci 101: 1403–1408, 2010. doi: 10.1111/j.1349-7006.2010.01544.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Van Belle E, Rivard A, Chen D, Silver M, Bunting S, Ferrara N, Symes JF, Bauters C, Isner JM. Hypercholesterolemia attenuates angiogenesis but does not preclude augmentation by angiogenic cytokines. Circulation 96: 2667–2674, 1997. doi: 10.1161/01.CIR.96.8.2667. [DOI] [PubMed] [Google Scholar]
- 41.Witztum JL, Steinberg D. The oxidative modification hypothesis of atherosclerosis: does it hold for humans? Trends Cardiovasc Med 11: 93–102, 2001. doi: 10.1016/S1050-1738(01)00111-6. [DOI] [PubMed] [Google Scholar]
- 42.Yeagle PL. Cholesterol and the cell membrane. Biochim Biophys Acta 822: 267–287, 1985. doi: 10.1016/0304-4157(85)90011-5. [DOI] [PubMed] [Google Scholar]
- 43.Yu S, Wong SL, Lau CW, Huang Y, Yu C-M. Oxidized LDL at low concentration promotes in-vitro angiogenesis and activates nitric oxide synthase through PI3K/Akt/eNOS pathway in human coronary artery endothelial cells. Biochem Biophys Res Commun 407: 44–48, 2011. doi: 10.1016/j.bbrc.2011.02.096. [DOI] [PubMed] [Google Scholar]
- 44.Zechariah A, ElAli A, Hagemann N, Jin F, Doeppner TR, Helfrich I, Mies G, Hermann DM. Hyperlipidemia attenuates vascular endothelial growth factor-induced angiogenesis, impairs cerebral blood flow, and disturbs stroke recovery via decreased pericyte coverage of brain endothelial cells. Arterioscler Thromb Vasc Biol 33: 1561–1567, 2013. doi: 10.1161/ATVBAHA.112.300749. [DOI] [PubMed] [Google Scholar]
- 45.Zhang C, Adamos C, Oh MJ, Baruah J, Ayee MAA, Mehta D, Wary KK, Levitan I. OxLDL induces endothelial proliferation via Rho/ROCK/Akt/p27kip1 signaling: prevention by cholesterol loading. Am J Physiol Cell Physiol: 313: C34–C351, 2017. doi: 10.1152/ajpcell.00249.2016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Zhao Y, Bao L, Chan LS, DiPietro LA, Chen L. Aberrant wound healing in an epidermal interleukin-4 transgenic mouse model of atopic dermatitis. PLoS One 11: e0146451, 2016. doi: 10.1371/journal.pone.0146451. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
All of the data sets and images generated and/or analyzed during the current study are available from the corresponding author upon reasonable request.