Abstract
We first tested the hypothesis that consuming a high-fructose corn syrup (HFCS)-sweetened soft drink augments kidney vasoconstriction to sympathetic stimulation compared with water (study 1). In a second study, we examined the mechanisms underlying these observations (study 2). In study 1, 13 healthy adults completed a cold pressor test, a sympathoexcitatory maneuver, before (preconsumption) and 30 min after drinking 500 mL of decarbonated HFCS-sweetened soft drink or water (postconsumption). In study 2, venous blood samples were obtained in 12 healthy adults before and 30 min after consumption of 500 mL water or soft drinks matched for caffeine content and taste, which were either artificially sweetened (Diet trial), sucrose-sweetened (Sucrose trial), or sweetened with HFCS (HFCS trial). In both study 1 and study 2, vascular resistance was calculated as mean arterial pressure divided by blood velocity, which was measured via Doppler ultrasound in renal and segmental arteries. In study 1, HFCS consumption increased vascular resistance in the segmental artery at rest (by 0.5 ± 0.6 mmHg·cm−1·s−1, P = 0.01) and during the cold pressor test (average change: 0.5 ± 1.0 mmHg·cm−1·s−1, main effect: P = 0.05). In study 2, segmental artery vascular resistance increased in the HFCS trial (by 0.8 ± 0.7 mmHg·cm−1·s−1, P = 0.02) but not in the other trials. Increases in serum uric acid were greater in the HFCS trial (0.3 ± 0.4 mg/dL, P ≤ 0.04) compared with the Water and Diet trials, and serum copeptin increased in the HFCS trial (by 0.8 ± 1.0 pmol/L, P = 0.06). These findings indicate that HFCS acutely increases vascular resistance in the kidneys, independent of caffeine content and beverage osmolality, which likely occurs via simultaneous elevations in circulating uric acid and vasopressin.
Keywords: Doppler ultrasound, renal blood velocity, renal vascular resistance, soda, vasopressin, uric acid
INTRODUCTION
People who regularly consume sugar-sweetened soft drinks (i.e., high-calorie, high-fructose beverages) have an ~60% increased risk of developing chronic kidney disease (11). The pathophysiology of high fructose intake and the risk of kidney disease may be related to fructose-induced increases in serum uric acid (i.e., hyperuricemia), elevations in circulating vasopressin, and/or relative postprandial hypertension (6, 21, 22, 34). For instance, high blood pressure is an established risk factor for the development of kidney disease (27), while elevations in serum uric acid are associated with a two- to threefold increased risk, and accelerated progression, of kidney disease (23, 31). Moreover, high baseline levels of plasma copeptin (a surrogate measure for vasopressin) are associated with kidney disease progression (5). Notably, vasopressin release can be augmented by fructose intake, and increased stimulation of the vasopressin receptors can promote endogenous fructose production during physiological stressors, such as dehydration and/or heat stress (36, 41, 47). This endogenous fructose production is mediated by activation of the uric acid-producing, polyol-fructokinase pathway in the kidneys (25). Our laboratory has previously observed greater increases in serum uric acid and copeptin in healthy adults when they consumed a soft drink with a high absolute concentration of fructose compared with consumption of an equal volume of water following 4 h of physical exertion in a hot environment (9). Additionally, this commercially available soft drink elevated blood pressure and exacerbated reductions in kidney function compared with water. The precise mechanisms underlying these observations remain unknown (40). However, it is possible that a soft drink-mediated reduction in renal blood flow may have contributed to reductions in kidney function. This is supported by data demonstrating that increasing fructose intake in rats exacerbates renal vasoconstriction (38). It is unknown, however, whether these findings translate to humans in an acute setting.
Our laboratory has used the cold pressor test (CPT), a sympathoexcitatory stimulus, to provide insights into the acute vasoconstrictor responsiveness of the renal vasculature, as measured with Doppler ultrasound (7, 39). When administered during rest, the CPT elicits an ~20% reduction in blood velocity and an ~70% increase in vascular resistance in the segmental artery (7). The effect of consuming a soft drink on changes in renal hemodynamics in response to a sympathetic stimulus has not been investigated. Such information could provide valuable insights regarding the link between sugar-sweetened soft drink consumption and deleterious renal outcomes. Thus, the purpose of the present study was to test the hypothesis that soft drink consumption augments vascular resistance in the segmental artery during the CPT compared with drinking an equivalent volume of water (study 1). On the basis of the findings of study 1, we then sought to determine the mechanisms underlying these observations. Specifically, we aimed to elucidate the mechanisms by which segmental artery vascular resistance is augmented with soft drink consumption. In this second study, we tested the hypothesis that elevations in segmental artery vascular resistance were due to high-fructose corn syrup (HFCS), which are mediated through increases in circulating uric acid and vasopressin (study 2).
METHODS
Participants
Thirteen healthy adults (3 women and 10 men) participated in study 1 and 12 healthy adults (2 women and 10 men) participated in study 2. Only three participants completed both studies. Therefore, these studies were considered independent of each other, and no direct comparisons were made between studies. Participant characteristics for study 1 were age: 22 ± 2 yr, height: 175 ± 9 cm, and weight: 76 ± 15 kg and for study 2 were age: 24 ± 4 yr, height: 177 ± 8 cm, and weight: 76 ± 9 kg. Participants self-reported to regularly consume a weekly volume of soft drinks equal to 575 ± 837 mL in study 1 and 346 ± 481 mL in study 2. Participants provided written consent after being fully informed of the experimental procedures and possible risks. Participants were physically active, nonsmokers, and reported to be free from any known cardiovascular, metabolic, renal, or neurological diseases. Female participants self-reported to be normally menstruating, were confirmed to not be pregnant via a urine pregnancy test, and were tested in the first 10 days following menstruation. These studies were approved by the Institutional Review Board at the University at Buffalo and performed in accordance with the standards set by the latest revision of the Declaration of Helsinki.
Instrumentation and Measurements
In both study 1 and study 2, nude body weight and height were measured using a stadiometer and scale (Sartorius, Bohemia, NY). Urine specific gravity was measured in duplicate using a refractometer (Atago, Bellevue, WA). Heart rate was continually measured via a 3-lead ECG (DA100C, Biopac Systems, Goleta, CA).
Blood velocity was measured in the middle portion of the same segmental artery in the right kidney (segmental artery) for that participant and the distal segment of the right renal artery (renal artery) using methods previously described in detail (8). Briefly, segmental and renal artery blood velocities were obtained via Doppler ultrasound (GE Vivid iQ, Chicago, IL) with a phased-array transducer (2.5–3.5 MHz) and while participants were in the left lateral recumbent position. Indelible ink was used to mark the transducer location during baseline measurements, and this location was used for all subsequent measurements. Participants were instructed to perform a midexhalation, non-Valsalva breath hold for no longer than 10 s during the ultrasound measurements and were appropriately familiarized with this technique before beginning the study. Mean segmental and renal artery blood velocities were indexed from the waveform envelope by the time-averaged maximum velocity, and mean blood velocity was measured and averaged over three cardiac cycles (8). Previous work from our laboratory suggested that there is a greater increase in vascular resistance to the CPT in the segmental artery compared with the renal artery (7). Because of this, and the practical limitations associated with optimizing both renal and segmental artery measurements during the same CPT, blood velocity was only measured in the segmental artery during the CPT. Blood velocity was measured in both the segmental and renal artery during pre-CPT baseline periods at pre- and post-500 mL fluid consumption (Fig. 1). The transducer was only removed from the body after completing the preconsumption CPT, but it remained in place throughout the duration of pre-CPT and CPT measurements. All renal measurements were obtained by the same sonographer (C. L. Chapman), and were extracted by a separate member of the investigative team (T. Grigoryan, L. D. Pietrafesa, and A. C. Bloomfield). This approach yielded a within-subject test-retest coefficient of variation for renal blood velocity measurements of 3.9 ± 0.8% (renal artery) and 3.9 ± 1.2% (segmental artery). Although it is not possible to accurately measure vessel wall diameter given the depth of the renal and segmental arteries, pharmacologically induced reductions in renal blood flow have been shown to be due to changes in blood velocity and not diameter (28). Therefore, renal and segmental blood velocities were interpreted to reflect changes in renal blood flow, as has been previously done (7, 8, 10, 13, 29, 39, 46).
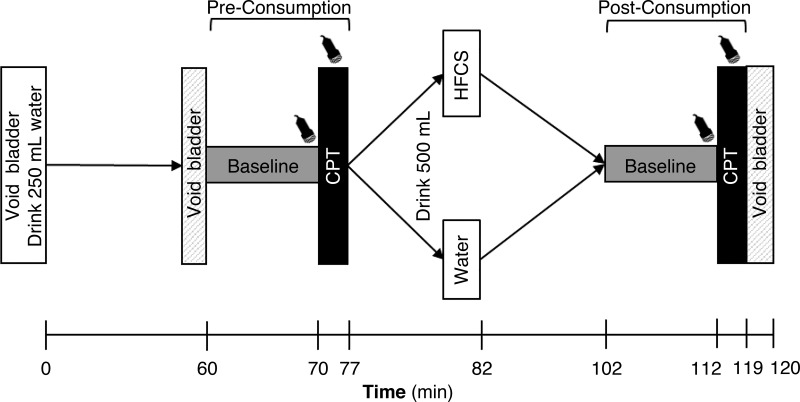
Fig. 1.
Study 1: schematic of the study protocol. The transducer symbol indicates when renal and cardiovascular measurements were taken. Ultrasound measurements were taken both for the middle portion of the segmental artery and distal segment of the right renal artery immediately before the cold pressor test (CPT) during the end of the baseline periods at preconsumption and postconsumption. During the CPT, ultrasound measurements were only performed in the segmental artery. HFCS and Water indicate the two trials [consumption of 500 mL of high-fructose corn syrup-sweetened soft drink (HFCS trial) or an equal volume of water (Water trial)].
In study 1, changes in beat-to-beat blood pressure over time were measured via the Penaz method (Finometer Pro, FMS, Amsterdam, The Netherlands) and were corrected to the blood pressure immediately before the CPT (pre-CPT) from auscultation of the brachial artery by electrosphygmomanometry (Tango M2, SunTech, Raleigh, NC). Stroke volume was calculated using ModelFlow (45).
In study 2, blood and urine were collected and analyzed. Blood pressure in study 2 was measured manually in duplicate immediately before kidney measurements were obtained. Hemoglobin was measured in duplicate using the Hemopoint H2 (Alere, Orlando, FL), and hematocrit was measured in triplicate using microcentrifugation. Serum osmolality was measured in duplicate via freezing point depression (model 3250, Advanced Instruments, Norwood, MA). Serum measurements of sodium, potassium, creatinine, and uric acid were measured via standard clinical techniques by Kaleida Health, Department of Pathology and Laboratory Medicine (Williamsville, NY). Fructose in serum and urine was measured using a commercially available fructose ELISA kit (LifeSpan BioSciences, Seattle, WA). Serum copeptin was measured to indirectly assess vasopressin release using a human copeptin ELISA kit (LifeSpan BioSciences) (4).
Experimental Protocol
Study 1: effect of HFCS-sweetened soft drink consumption on renal and segmental artery hemodynamics during the CPT.
overview.
During two randomized experimental visits, participants completed a CPT (described below) before and 30 min after 500-mL fluid ingestion in two trials (Fig. 1). Participants consumed filtered tap water in the control trial (Water trial) and a decarbonated HFCS-sweetened soft drink (Mountain Dew, PepsiCo, Purchase, NY) in the experimental trial (HFCS trial).
pre-500 ml fluid consumption.
Participants reported to the temperature-controlled laboratory (HFCS trial: 24 ± 1°C, 32 ± 5% relative humidity; Water trial: 25 ± 2°C, 32 ± 8% relative humidity, P ≥ 0.16) after abstaining from exercise, caffeine, and alcohol for 12 h, and food for 3 h. For the second experimental visit, participants arrived at the laboratory at the same time of day as their first visit to control for any potential diurnal effects. Upon arrival, participants provided a urine sample by completely voiding their bladder in a collection urinal. Euhydration was confirmed at this time via a urine specific gravity level of <1.020 (2). After this, participants drank 250 mL of water to promote urine production over the subsequent 60 min. Following water consumption, participants sat and rested quietly for 60 min. After this rest period, participants voided their bladder in a separate urinal to establish a baseline urine flow rate over this 1-h period. Participants then laid in the left lateral recumbent position and were instrumented as detailed above. After instrumentation, a 10-min pre-500 mL fluid consumption baseline (preconsumption) was established. Cardiovascular and renal measurements were taken during the last 2 min of preconsumption. Immediately following preconsumption measurements, the CPT commenced. The CPT is a sympathoexcitatory maneuver in which the participant’s hand is submerged in an agitated ice slurry mixture up to the styloid processes for 2 min. The CPT stimulates nociceptors and increases both vascular resistance and blood pressure (12). Cardiovascular and renal ultrasound data were collected immediately before commencing the CPT (pre-CPT), at 1 min of the CPT, at the end of the CPT, and at 1 min post-CPT (recovery). At the end of the CPT, participants rated their perceived hand pain (from 0 = no pain to 10 = worst pain imaginable) to the nearest 0.5 increment. Recovery continued until 5 min post-CPT.
post-500 ml fluid consumption.
Following recovery, participants were given 5 min to consume 500 mL of water or HFCS. Water was used as the control beverage in study 1 to control for the potential effects of the fluid volume ingested on changes in renal hemodynamics. Mountain Dew is a high-calorie, high-fructose, caffeinated soft drink that we have demonstrated accentuates reductions in renal function when consumed during and after physical work in the heat (9). Mountain Dew has one of the highest free fructose concentrations among commercially available soft drinks, with fructose and glucose each representing 59.5% and 39.7%, respectively, of the total sugar content in the beverage (44). According to an analysis from Walker et al. (44), ~36 g of fructose and ~25 g of glucose are present in 500 mL of Mountain Dew. The osmolality and on-label contents of the beverage are shown in Table 1. In pilot testing, renal vascular imaging via Doppler ultrasound was impossible when Mountain Dew was consumed as packaged because of the profound acoustic shadowing of the kidney associated with carbonation. Further pilot testing indicated that 48 h of decarbonation procedures were necessary to reliably image the renal vasculature. This decarbonation process was accomplished by pouring the soft drink into a wide-mouthed bottle with the top loosely fastened yet tight enough to prevent contamination of the drink. After ingesting 500 mL of the assigned beverage within 5 min, participants laid supine for 20 min. A postconsumption baseline (postconsumption) then commenced, so that the CPT was initiated at 30 min postconsumption. Testing was completed 30 min after fluid consumption because previous data have shown that increases in fructose in the blood following oral ingestion can be detected at this time, thus ensuring that the high-fructose soft drink was in the circulation (26). Cardiovascular and renal measurements during the postconsumption baseline and CPT occurred in the same manner as preconsumption. Following postconsumption CPT measurements, participants voided into a collection urinal for measurements of urine specific gravity and urine flow rate.
Table 1.
Properties of soft drinks
| Diet | Sucrose | HFCS | |
|---|---|---|---|
| Osmolality, mosmol/kgH2O | |||
| Fresh | 69 (4) | 741 (3) | 861 (2) |
| 24-h decarbonated | 61 (2) | 738 (3) | 850 (2) |
| 48-h decarbonated | 59 (2) | 730 (2) | 846 (2) |
| Total energy, kcal | 5 | 240 | 240 |
| Sugar, g | <1 | 62 | 65 |
| Sodium, mg | 70 | 92 | 85 |
| Caffeine, mg | 76 | 76 | 77 |
Soft drinks were matched for caffeine content and taste and were either artificially-sweetened (Diet; Diet Mountain Dew), sucrose-sweetened (Sucrose; Mountain Dew Throwback), or sweetened with high-fructose corn syrup (HFCS; regular Mountain Dew). Drink values are per 500 mL.
Study 2: mechanisms by which segmental artery hemodynamics change with soft drink consumption.
In four randomized, double-blinded experimental trials, participants consumed 500 mL of water (Water trial) or soft drinks matched for taste and caffeine content that were either artificially sweetened [Diet Mountain Dew (Diet trial)], sucrose-sweetened [Mountain Dew Throwback (Sucrose trial)] or sweetened with HFCS [i.e., the exact beverage from study 1 (HFCS trial)]. The timeline of study 2 was kept the same as study 1 to examine the blood marker response to the various soft drinks. The use of these soft drinks allowed for direct insights into the cardiovascular and renal effects of changing the sweetener in the soft drink, independent of the caffeine content. The osmolality and on-label contents of each soft drink are shown in Table 1. Notably, the HFCS-sweetened soft drink contained ~60% fructose (44) and provided the ability to isolate the effects of increasing the fructose content compared with the sucrose-sweetened soft drink, which was composed of 50% fructose and 50% glucose, because the soft drinks were matched for caffeine and total calories and both were hyperosmotic (Table 1).
In study 2, venous blood samples were taken immediately before drink consumption and 30 min after consumption. Additionally, renal and segmental artery blood velocities were obtained before drink consumption and after collection of the final venous blood sample. Urine samples were collected immediately before and 1 h after drink consumption in accordance with the timeline of data collection in study 1. Participants logged their food and fluid intakes for the 24 h preceding the experimental trial and were instructed to keep a similar diet before each visit. Dietary logs were analyzed for fluid volume and amount of total energy, fat, protein, carbohydrate, sugar, and sodium using online software (myfitnesspal, Under Armour, Baltimore, MD).
Data and Statistical Analyses
In study 1, we were unable to obtain segmental artery measurements in one participant due to acoustic shadowing of the kidney during the HFCS trial. Thus, ultrasound-based data in study 1 are reported as n = 13 for the renal artery and n = 12 for the segmental artery. In study 2, we were unable to measure renal measurements in one participant each during the Water, Diet, and HFCS trials due to the availability of the ultrasound machine. Additionally, renal artery measurements were not obtained in one participant in the Water trial and two participants in the HFCS trial due to acoustic shadowing of the kidney. Thus, ultrasound based-data in study 2 are reported as Water (n = 10), Diet (n = 11), Sucrose (n = 11), and HFCS (n = 9) for the renal artery and Water (n = 11), Diet (n = 11), Sucrose (n = 12), and HFCS (n = 11) for the segmental artery.
All nonultrasound data were sampled continuously at 1 kHz via a data acquisition system (Biopac MP150, Goleta, CA). Heart rate (studies 1 and 2) and mean arterial pressure (study 1 only) were extracted during the same three cardiac cycles for all renal measurements described above. In study 1, cardiac output was calculated as the product of stroke volume and heart rate, and total peripheral resistance was calculated as the quotient of mean arterial pressure and cardiac output. In study 2, mean arterial pressure was calculated as one third pulse pressure plus diastolic pressure. In both studies 1 and 2, renal and segmental artery vascular resistance were calculated as the quotient of mean arterial pressure and renal and segmental artery blood velocity. Urine fructose excretion was calculated by correcting urine fructose to urine flow rate.
All data were analyzed using Prism software (version 8, GraphPad Software, La Jolla, CA). In study 1, comparisons between preconsumption and postconsumption baseline data within a trial were made using two-tailed paired t tests. During the CPT, data were analyzed as the absolute change from pre-CPT using two-way ANOVA. CPT data were analyzed as the absolute change to examine the responses to the CPT independent from differences that were observed pre-CPT. Additionally, the absolute difference between preconsumption and postconsumption during the CPT was compared between HFCS and Water trials using two-way ANOVA. This analysis was deemed the most appropriate because it isolated the effect of water and soft drink consumption while controlling for any day-to-day differences in measurement or physiological variability. In study 2, two-way ANOVA was used to examine differences between drink type (condition) and across time. Twenty-four-hour fluid volume and food intakes were analyzed using one-way ANOVA. In all instances, when ANOVA revealed a significant main effect or interaction, post hoc Holm-Sidak test pairwise comparisons were made. A priori statistical significance was set at P ≤ 0.05, and actual P values are reported where possible. Data are reported as means ± SD.
RESULTS
Study 1: Effect of HFCS-Sweetened Soft Drink Consumption on Renal and Segmental Artery Hemodynamics During the CPT
Resting physiological responses to 500 mL fluid consumption.
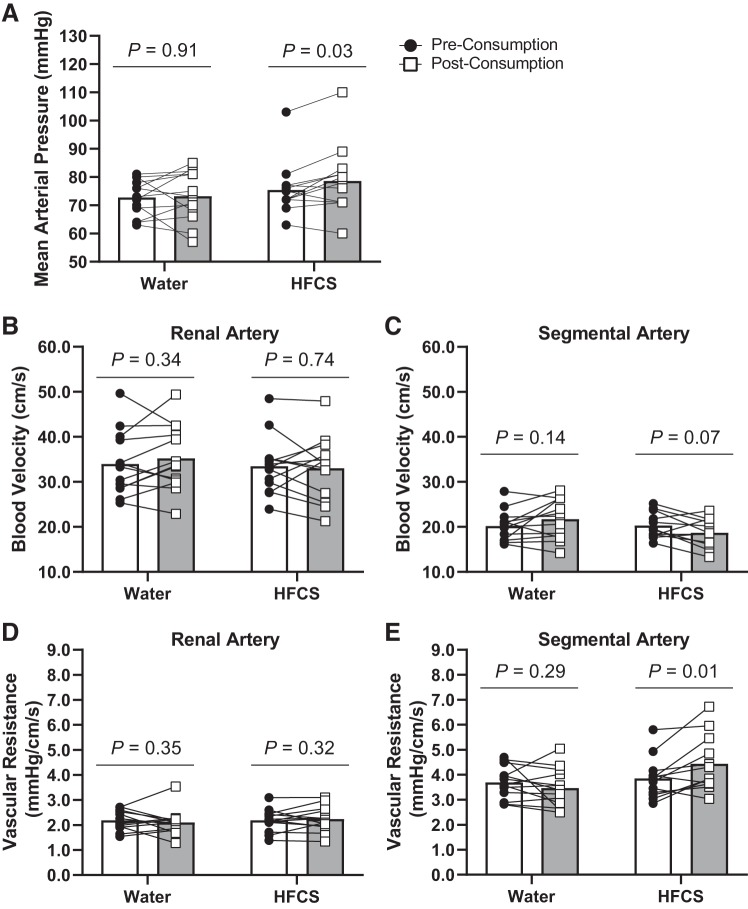
There were no changes in heart rate, stroke volume, cardiac output, and total peripheral resistance from preconsumption to postconsumption in either the Water or HFCS trials (P ≥ 0.08; Table 2). Mean arterial pressure increased in the HFCS trial at postconsumption (by 3 ± 5 mmHg, P = 0.03; Fig. 2A), but it did not change with the Water trial (P = 0.91; Fig. 2A). Renal (P = 0.74; Fig. 2B) and segmental (P = 0.07; Fig. 2C) artery blood velocities did not change with either drink at postconsumption. HFCS consumption increased vascular resistance in the segmental artery (by 0.5 ± 0.6 mmHg·cm−1·s−1, P = 0.01; Fig. 2E) but did not change with water (P = 0.29; Fig. 2E) or either drink in the renal artery (P ≥ 0.32; Fig. 2D). There were no differences in urine specific gravity or urine flow rate between HFCS and Water trials (P ≥ 0.66; Table 2).
Table 2.
Responses to 500 mL of fluid consumption for study 1
| Water |
HFCS |
|||
|---|---|---|---|---|
| Parameter | Pre | Post | Pre | Post |
| Cardiovascular | ||||
| Heart rate, bpm | 61 (11) | 59 (11) | 63 (12) | 64 (10) |
| Stroke volume, mL | 83 (22) | 92 (22) | 73 (14) | 79 (20) |
| Cardiac output, L/min | 4.9 (1.7) | 5.2 (1.4) | 4.4 (1.0) | 5.0 (1.1) |
| Systolic blood pressure, mmHg | 102 (14) | 102 (11) | 109 (9) | 113 (16) |
| Diastolic blood pressure, mmHg | 59 (7) | 58 (9) | 57 (7) | 60 (10) |
| Total peripheral resistance, mmHg·L−1·min | 20.5 (6.1) | 20.2 (5.8) | 17.8 (4.1) | 16.5 (4.0) |
| Renal function | ||||
| Urine specific gravity | 1.003 (0.004) | 1.001 (0.002) | 1.003 (0.005) | 1.001 (0.002) |
| Urine flow rate, mL/min | 5.3 (2.7) | 5.6 (1.5) | 5.6 (2.9) | 5.4 (2.6) |
| Perception to cold pressor test | ||||
| Hand pain, AU | 6.8 (1.2) | 7.0 (1.2) | 6.9 (0.8) | 7.3 (0.9) |
Data are presented as absolute values and mean (SD); n = 13. Physiological responses preconsumption (Pre) and 30 min postconsumption (Post) of 500 mL of high-fructose corn syrup-sweetened soft drink (HFCS trial) or water (Water trial) are shown (study 1). The perceptual response to hand pain immediately after the cold pressor test is included at preconsumption and postconsumption in each trial. Preconsumption and postconsumption comparisons were made using separate paired two-tailed t tests for HFCS and Water trials.
Fig. 2.
Study 1: physiological responses to 500 mL of fluid consumption of high-fructose corn syrup-sweetened soft drink (HFCS trial) or water (Water trial) at preconsumption and 30 min postconsumption. A−E: mean arterial pressure (A), renal artery blood velocity (B), segmental artery blood velocity (C), vascular resistance in the renal artery (D), and vascular resistance in the segmental artery (E). Data are presented as means with individual responses. Preconsumption and postconsumption comparisons were made using separate paired t tests for HFCS and Water trials. n = 12 for the segmental artery in the HFCS trial and n = 13 for all remaining variables.
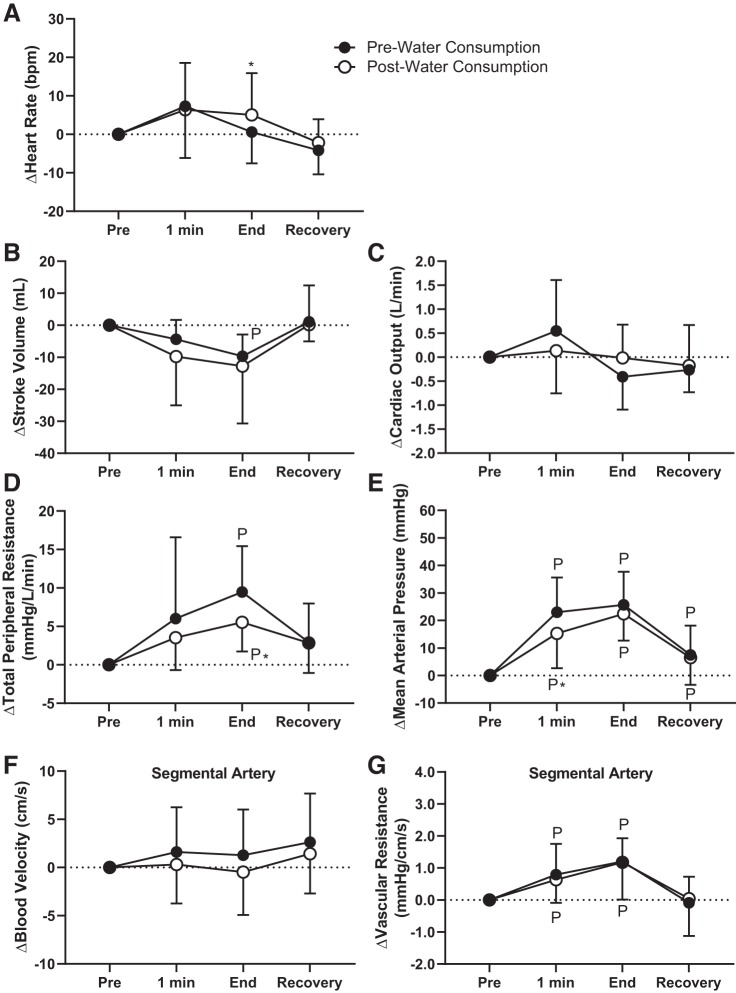
Physiological responses to water consumption during the CPT.
The increase in heart rate was higher at the end of the CPT at postconsumption compared with preconsumption in the Water trial (pre: 1 ± 8 beats/min and post: 5 ± 11 beats/min, P < 0.05; Fig. 3A). The CPT did not elicit differential changes in stroke volume and cardiac output between preconsumption and postconsumption in the Water trial (P ≥ 0.08; Fig. 3, B and C). At the end of the CPT, increases in total peripheral resistance were attenuated at postconsumption in the Water trial (pre: 9.5 ± 6.0 mmHg·L−1·min and post: 5.5 ± 3.8 mmHg·L−1·min, P < 0.04; Fig. 3D). In the Water trial, mean arterial pressure was higher at 1 min of the CPT at preconsumption compared with postconsumption during the CPT (by 8 ± 15 mmHg, P = 0.01), but there were no differences at the end of the CPT (P = 0.43; Fig. 3E). There were no differences in blood velocity or vascular resistance in the segmental artery between preconsumption and postconsumption in the Water trial (P ≥ 0.32; Fig. 3, F and G).
Fig. 3.
Study 1: effect of pre- and post-500 mL water consumption on physiological responses to the cold pressor test (CPT). A−G: heart rate (A), stroke volume (B), cardiac output (C), total peripheral resistance (D), mean arterial pressure (E), segmental artery blood velocity (F), and vascular resistance in the segmental artery (G). 1 min, 1 min of the CPT; End, end of the CPT. Statistical analyses from post hoc Holm-Sidak’s test pairwise comparisons completed after two-way repeated-measures ANOVA. PSignificantly different from pre-CPT (P < 0.04); *significantly different from pre-water consumption (P < 0.05). n = 13.
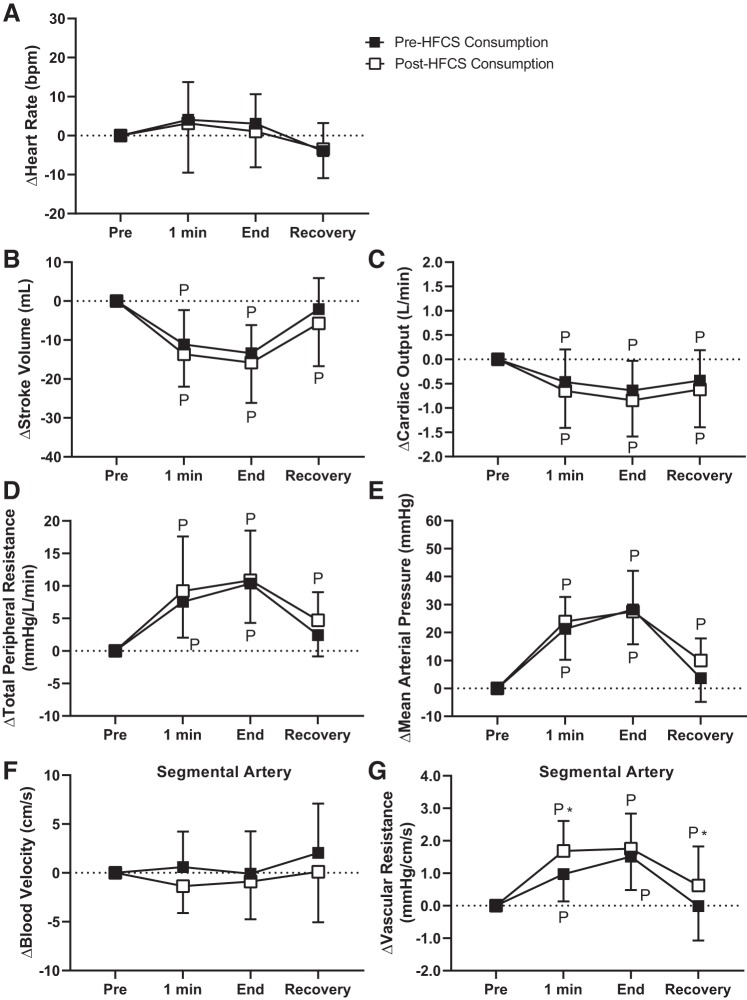
Physiological responses to soft drink consumption during the CPT.
The HFCS trial did not elicit differential changes in heart rate, stroke volume, cardiac output, total peripheral resistance, mean arterial pressure, or segmental artery blood velocity during the CPT compared with the Water trial (P ≥ 0.10; Fig. 4). However, the increase in vascular resistance in the segmental artery was higher at postconsumption compared with preconsumption in the HFCS trial at 1 min of the CPT (pre: 1.0 ± 0.8 mmHg·cm−1·s−1 and post: 1.6 ± 0.9 mmHg·cm−1·s−1, P < 0.02) and at recovery (pre: −0.0 ± 1.1 mmHg·cm−1·s−1 and Post: 0.6 ± 1.2 mmHg·cm−1·s−1, P < 0.03; Fig. 4G).
Fig. 4.
Study 1: effect of pre- and post-500 mL high-fructose corn syrup-sweetened soft drink (HFCS) consumption on physiological responses to the cold pressor test (CPT). A−G: heart rate (A), stroke volume (B), cardiac output (C), total peripheral resistance (D), mean arterial pressure (E), segmental artery blood velocity (F), and vascular resistance in the segmental artery (G). 1 min, 1 min of the CPT; End, end of the CPT. Statistical analyses from post hoc Holm-Sidak’s test pairwise comparisons completed after two-way repeated-measures ANOVA. PSignificantly different from pre-CPT (P ≤ 0.05); *significantly different from pre-soft drink consumption (P < 0.03). n = 12 for segmental artery and n = 13 for all remaining variables.
CPT differences between pre- and post-500 mL fluid consumption compared between HFCS and Water trials.
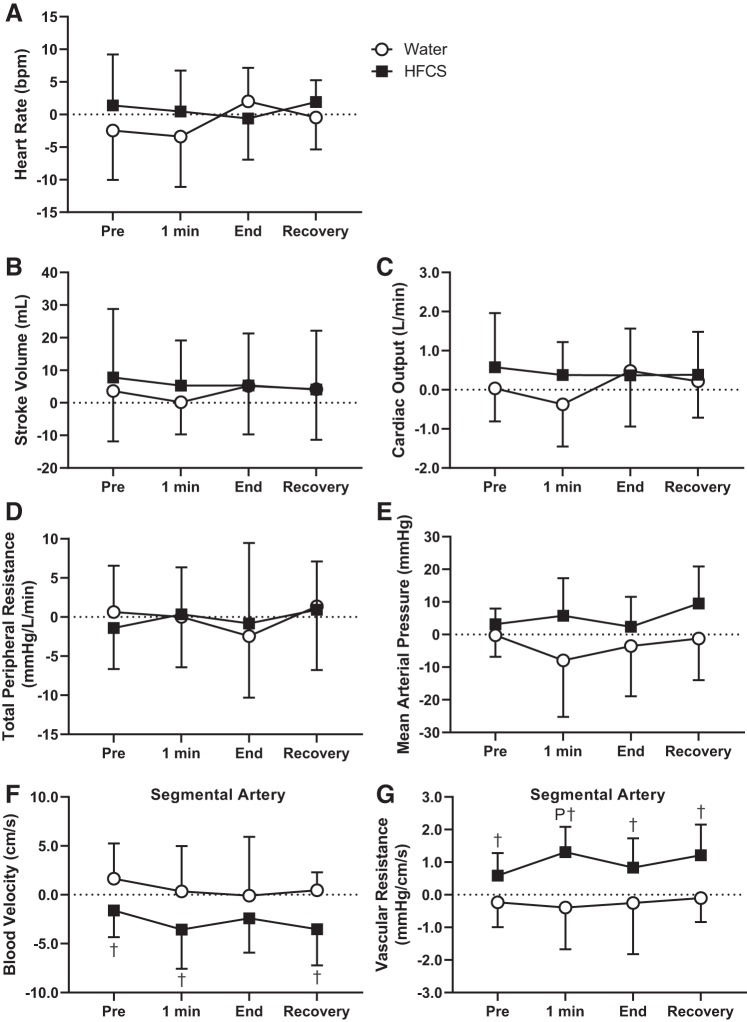
At all time points during the CPT, there were no differences between preconsumption and postcconsumption in HFCS and Water trials in the change in heart rate (P ≥ 0.50; Fig. 5A), stroke volume (P ≥ 0.78; Fig. 5B), cardiac output (P ≥ 0.35; Fig. 5C), total peripheral resistance (P ≥ 0.86; Fig. 5D), and mean arterial pressure (P ≥ 0.10; Fig. 5E). The change from preconsumption to postconsumption in segmental artery blood velocity was lower in the HFCS trial compared with the Water trial at pre-CPT (−1.6 ± 2.7 vs. 1.6 ± 3.6 cm/s; P < 0.05), 1 min of the CPT (−3.6 ± 4.0 vs. 0.3 ± 4.6 cm/s, P < 0.03), and recovery (−3.5 ± 3.7 vs. 0.5 ± 1.8 cm/s; P < 0.03; Fig. 5F). Additionally, the change from preconsumption to postconsumption in vascular resistance in the segmental artery was higher in the HFCS trial compared with the Water trial at pre-CPT (0.6 ± 0.7 vs. −0.2 ± 0.8 mmHg·cm−1·s−1, P < 0.03), 1 min of the CPT (1.3 ± 0.8 vs. −0.4 ± 1.3 mmHg·cm−1·s−1, P < 0.01), end of the CPT (0.8 ± 0.9 vs. −0.3 ± 1.6 mmHg·cm−1·s−1, P = 0.05), and recovery (1.2 ± 0.9 vs. −0.1 ± 0.7 mmHg·cm−1·s−1, P < 0.01; Fig. 5G).
Fig. 5.
Study 1: comparison of the calculated difference between preconsumption and postconsumption during each time point during the cold pressor test (CPT). HFCS and Water indicate the two trials [consumption of 500 mL of high-fructose corn syrup-sweetened soft drink (HFCS trial) or an equal volume of water (Water trial)]. A−G: heart rate (A), stroke volume (B), cardiac output (C), total peripheral resistance (D), mean arterial pressure (E), segmental artery blood velocity (F), and vascular resistance in the segmental artery (G). 1 min, 1 min of the CPT; End, end of the CPT. Statistical analyses from post hoc Holm-Sidak’s test pairwise comparisons were completed after two-way repeated measures ANOVA. PSignificantly different from pre-CPT (P < 0.04); †significantly different from the Water trial (P ≤ 0.03). n = 12 for the segmental artery in the HFCS trial and n = 13 for all remaining variables.
Study 2: Mechanisms by Which Segmental Artery Hemodynamics Change With Soft Drink Consumption
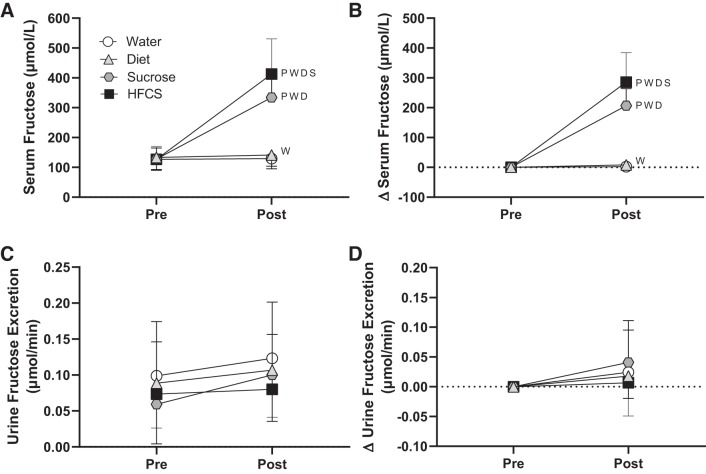
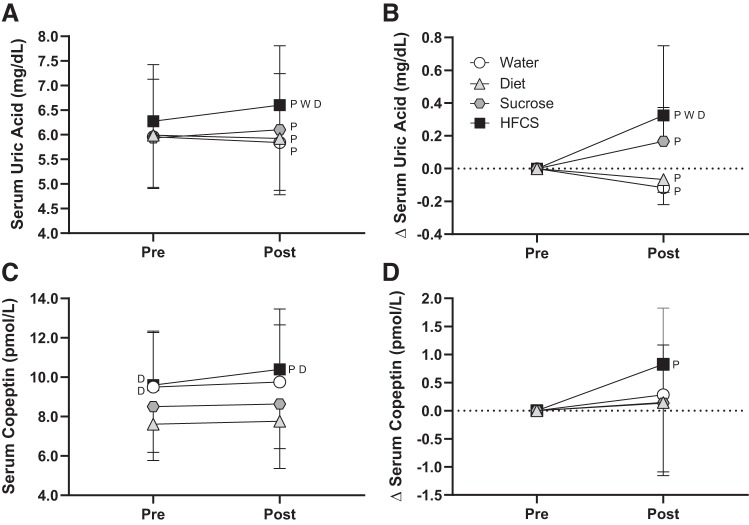
There were no differences in total fluid volume or food intakes in the 24 h before the experimental trials (P ≥ 0.13; see Supplemental Table S1 in the Supplemental Data; Supplemental Data are available online at http://doi.org/10.5281/zenodo.3674087). The change in heart rate, stroke volume, blood pressure, and total peripheral resistance from preconsumption did not differ between trials (P ≥ 0.15; Table 3). The change in renal artery blood velocity was reduced in the HFCS trial (by −3.4 ± 6.2 cm/s; P ≤ 0.04) compared with Water (by 4.1 ± 6.1 cm/s) and Sucrose (by 4.5 ± 4.5 cm/s; Table 3) trials. The change in vascular resistance in the renal artery in the HFCS trial was greater than that in the Water trial (P = 0.02; Table 3). Segmental artery blood velocity only decreased in the HFCS trial (by −3.2 ± 2.8 cm/s, P = 0.01), and vascular resistance in the segmental artery increased in the HFCS trial (by 0.8 ± 0.7 mmHg·cm−1·s−1, P = 0.02, Table 4). Segmental artery vascular resistance increased to a greater extent in the HFCS trial compared with the Water trial (P = 0.04; Table 3). Percent changes in plasma volume did not differ between trials (P ≥ 0.11; Table 4). Plasma osmolality and serum glucose increased (P < 0.04) in the HFCS and Sucrose trials only, but the magnitude of increase did not differ between these trials (P ≥ 0.43; Table 4). At 30 min postconsumption, increases in serum fructose were greatest in the HFCS trial (by 286 ± 99 µmol/L) compared with other trials (P < 0.05; Fig. 6B). There were no differences between trials in urine flow rate at preconsumption (Water: 7.6 ± 5.0 mL/min, Diet: 8.3 ± 3.5 mL/min, Sucrose: 7.7 ± 3.4 mL/min, and HFCS: 7.9 ± 3.3 mL/min, P ≥ 0.94) or postconsumption (Water: 10.4 ± 3.5 mL/min, Diet: 10.0 ± 2.8 mL/min, Sucrose: 8.7 ± 3.4 mL/min, and HFCS: 8.7 ± 1.9 mL/min, P ≥ 0.22). Urine flow rate increased from preconsumption in the Diet trial (by 1.7 ± 2.0 mL/min, P = 0.05), but there were no changes in the other trials (P ≥ 0.21). There were no effects of time or drink on urine fructose excretion (P ≥ 0.20; Fig. 6D). Increases in serum uric acid at 30 min postconsumption in the HFCS trial (0.3 ± 0.4 mg/dL, P ≤ 0.04) were greater than in the Water (−0.1 ± 0.1 mg/dL) and Diet (−0.1 ± 0.1 mg/dL) trials but did not differ from the Sucrose trial (0.2 ± 0.2 mg/dL, P = 0.26; Fig. 7B). Serum copeptin increased from preconsumption only in the HFCS trial (by 0.8 ± 1.0 pmol/L, P = 0.06), but these increases did not differ from the other trials (P ≥ 0.60; Fig. 7D).
Table 3.
Cardiovascular and renal hemodynamic responses to 500 mL drink consumption for study 2
| Water |
Diet |
Sucrose |
HFCS |
|||||
|---|---|---|---|---|---|---|---|---|
| Pre | Post | Pre | Post | Pre | Post | Pre | Post | |
| Cardiovascular | ||||||||
| Heart rate, beats/min | 54 (5) | 53 (5) | 59 (7) | 54 (5)* | 58 (8) | 59 (6)†‡ | 59 (8) | 60 (7)†‡ |
| ∆ | −1 (4) | −5 (5)* | 0 (6) | 0 (5) | ||||
| Stroke volume, mL | 109 (12) | 107 (15) | 109 (14) | 107 (17) | 104 (19) | 106 (16) | 107 (20) | 104 (13) |
| ∆ | −2 (10) | −2 (7) | 1 (9) | −3 (9) | ||||
| Cardiac output, L/min | 6.0 (0.7) | 5.8 (0.9) | 6.2 (0.7) | 5.8 (0.8) | 5.9 (1.0) | 6.1 (0.8) | 6.3 (1.2) | 6.3 (0.8) |
| ∆ | −0.2 (0.7) | −0.5 (0.6) | 0.2 (0.5) | −0.1 (0.7) | ||||
| Systolic blood pressure, mmHg | 124 (8) | 121 (10) | 123 (7) | 126 (7) | 121 (7) | 126 (9)* | 124 (7) | 128 (7) |
| ∆ | −3 (5) | 2 (4) | 4 (5)*† | 3 (6) | ||||
| Diastolic blood pressure, mmHg | 77 (8) | 79 (6) | 79 (5) | 83 (3)* | 77 (5) | 81 (7) | 77 (6) | 81 (4) |
| ∆ | 1 (5) | 5 (4)* | 4 (6) | 4 (8) | ||||
| Mean arterial pressure, mmHg | 93 (7) | 93 (6) | 94 (5) | 98 (4)* | 92 (5) | 96 (7)* | 93 (5) | 96 (4) |
| ∆ | 0 (5) | 4 (3)* | 4 (4)* | 4 (6) | ||||
| Total peripheral resistance, mmHg·L−1·min | 16.2 (2.0) | 17.1 (2.9) | 16.3 (3.5) | 18.2 (4.8) | 16.4 (3.1) | 16.6 (2.8) | 15.9 (3.8) | 16.0 (2.7) |
| ∆ | 1.0 (3.1) | 2.2 (2.3) | 0.3 (1.7) | 0.1 (2.4) | ||||
| Renal Artery | ||||||||
| Blood velocity, cm/s | 36.5 (8.8) | 40.7 (9.2) | 40.9 (8.2) | 41.3 (7.5) | 37.9 (8.8) | 41.7 (9.3)* | 43.6 (8.7)† | 40.2 (7.8) |
| ∆ | 4.1 (6.1) | 0.3 (5.1) | 4.5 (4.5)* | −3.4 (6.2)†§ | ||||
| Vascular resistance, mmHg·cm−1·s | 2.7 (0.7) | 2.3 (0.5) | 2.4 (0.4) | 2.4 (0.4) | 2.6 (0.6) | 2.4 (0.4) | 2.2 (0.4) | 2.5 (0.5) |
| ∆ | −0.3 (0.5) | 0.1 (0.4) | −0.2 (0.3) | 0.2 (0.4)† | ||||
| Segmental Artery | ||||||||
| Blood velocity, cm/s | 25.5 (6.3) | 26.5 (5.3) | 24.8 (4.4) | 24.4 (6.0) | 21.4 (5.5)‡ | 21.5 (5.4) | 24.8 (6.6) | 21.6 (6.1)* |
| ∆ | 1.0 (5.1) | −0.4 (4.0) | 0.2 (5.3) | −3.2 (2.8)* | ||||
| Vascular resistance, mmHg·cm−1·s | 3.8 (0.9) | 3.6 (0.6) | 3.9 (0.8) | 4.2 (0.8) | 4.5 (1.0) | 4.7 (1.2) | 3.9 (0.8) | 4.7 (1.1)*† |
| ∆ | −0.2 (0.6) | 0.3 (0.8) | 0.2 (1.1) | 0.8 (0.7)*† | ||||
Data are absolute values and change values (∆) from preconsumption (Pre). Physiological responses to 500 mL drink consumption at baseline (Pre) and 30 min after consumption of the beverage (Post) are shown. Beverages were composed of water (Water trial) or soft drinks matched for caffeine content and taste that were either artificially sweetened (Diet trial), sucrose-sweetened (Sucrose trial), or sweetened with high-fructose corn syrup (HFCS trial) (study 2). For the renal artery, n = 10 (Water), 11 (Diet), 11 (Sucrose), and 9 (HFCS); for the segmental artery, n = 11 (Water), 11 (Diet), 12 (Sucrose), and 11 (HFCS); n = 12 for the remaining variables. Statistical analysis was performed by two-way ANOVA and post hoc Holm-Sidak test pairwise comparisons.
Significantly different from Pre (P ≤ 0.05);
significantly different from the Water trial (P ≤ 0.04);
significantly different from the Diet trial (P < 0.01);
significantly different from the Sucrose trial (P = 0.04).
Table 4.
Blood profile response to soft drink consumption for study 2
| Water |
Diet |
Sucrose |
HFCS |
|||||
|---|---|---|---|---|---|---|---|---|
| Pre | Post | Pre | Post | Pre | Post | Pre | Post | |
| ∆Plasma volume, % | −1 (2)* | −3 (3) | 1 (3)‡ | −1 (2) | ||||
| Plasma osmolality, mosmol/kg H2O | 284 (2) | 283 (2) | 285 (2) | 284 (2) | 284 (2) | 286 (3)* | 285 (3) | 286 (3)*†‡ |
| ∆ | −1 (2) | 0 (1) | 2 (2)*†‡ | 2 (2)*‡ | ||||
| Serum glucose, mg/dL | 88 (6) | 84 (6) | 88 (5) | 89 (5)† | 85 (7) | 126 (20)*†‡ | 88 (6) | 124 (25)*†‡ |
| ∆ | −4 (9) | 1 (5) | 42 (21)*†‡ | 36 (28)*†‡ | ||||
| Serum sodium, mmol/L | 139 (1) | 139 (1) | 139 (1) | 139 (1) | 138 (1) | 139 (2) | 139 (2) | 139 (2) |
| ∆ | 0 (1) | 0 (1) | 0 (1) | 0 (2) | ||||
| Serum potassium, mmol/L | 4.2 (0.3) | 4.3 (0.3) | 4.4 (0.4) | 4.4 (0.3) | 4.3 (0.3) | 4.1 (0.3) | 4.2 (0.2) | 4.0 (0.4)‡ |
| ∆ | 0.0 (0.2) | 0.0 (0.3) | −0.2 (0.2) | −0.1 (0.2) | ||||
| Serum creatinine, mg/dL | 1.0 (0.2) | 0.9 (0.2) | 0.9 (0.1) | 0.9 (0.1) | 0.9 (0.1) | 1.0 (0.1) | 0.9 (0.1) | 1.0 (0.1) |
| ∆ | 0.0 (0.0) | 0.0 (0.0) | 0.1 (0.0) | 0.1 (0.0 | ||||
Data are absolute values and change values (∆) from preconsumption (Pre); n = 12. Blood marker responses to 500 mL drink consumption at baseline (Pre) and 30 min after consumption of the beverage (Post) are shown. Beverages were composed of water (Water trial) or soft drinks matched for caffeine content and taste that were either artificially sweetened (Diet trial), sucrose-sweetened (Sucrose trial), or sweetened with high-fructose corn syrup (HFCS trial) (study 2). Statistical analysis was performed by two-way ANOVA and post hoc Holm-Sidak test pairwise comparisons.
Significantly different from Pre (P ≤ 0.04);
significantly different from the Water trial (P ≤ 0.05);
significantly different from the Diet trial (P ≤ 0.03).
Fig. 6.
Study 2: effect of consumption of 500 mL of water (Water trial) or soft drinks matched for caffeine content and taste that were either artificially sweetened (Diet trial), sucrose-sweetened (Sucrose trial), or sweetened with high-fructose corn syrup (HFCS trial) on serum fructose at 30 min postconsumption (A and B) and urine fructose excretion at 60 min postconsumption (C and D). Absolute values (A and C) and change values (∆, B and D) are shown. Statistical analysis was performed by two-way ANOVA and post hoc Holm-Sidak test pairwise comparisons. PSignificantly different from preconsumption (P < 0.01); Wsignificantly different from the Water trial (P ≤ 0.04); Dsignificantly different from the Diet trial (P ≤ 0.04); Ssignificantly different from the Sucrose trial (P ≤ 0.05). n = 12.
Fig. 7.
Study 2: effect of consumption of 500 mL of water (Water trial) or soft drinks matched for caffeine content and taste that were either artificially sweetened (Diet trial), sucrose sweetened (Sucrose trial), or sweetened with high-fructose corn syrup (HFCS trial) on serum uric acid (A and B) and serum copeptin (C and D) at 30 min postconsumption. Absolute values (A and C) and change values (∆, B and D) are shown. Statistical analysis was performed by two-way ANOVA and post hoc Holm-Sidak test pairwise comparisons. PSignificantly different from preconsumption (P < 0.04 for serum uric acid and P = 0.06 for serum copeptin); Wsignificantly different from the Water trial (P < 0.04); Dsignificantly different from the Diet trial (P < 0.05). n = 12.
DISCUSSION
In support of our first hypothesis (study 1), consumption of 500 mL of a commercially available soft drink sweetened with HFCS increased segmental artery vascular resistance 30 min postconsumption and accentuated the increase in segmental artery vascular resistance during the CPT, a sympathetic stimulus. Study 2 was designed to examine the mechanisms by which HFCS-sweetened soft drink consumption increased segmental artery vascular resistance. The primary findings from study 2 were 1) consumption of a HFCS-sweetened soft drink acutely increased vascular resistance in the segmental artery, but this did not occur after drinking sucrose-sweetened or artificially sweetened soft drinks; and 2) the HFCS-sweetened soft drink elicited the greatest increases in serum fructose and stimulated simultaneous increases in both serum uric acid and copeptin, a stable surrogate measure of vasopressin.
Consumption of a Soft Drink Sweetened With HFCS Acutely Elevates Vascular Resistance in the Segmental Artery at Rest and During Sympathetic Stimulation (Study 1)
In a previous study, we observed augmented increases in blood pressure, reductions in renal function, and greater increases in serum uric acid and copeptin when participants consumed Mountain Dew during and after 4 h of exercise in the heat compared with drinking water (9). In explanation of these findings, we speculated that soft drink consumption during this hyperadrenergic state (37) may have provoked greater reductions in renal blood flow. However, understanding the mechanisms of these observations was confounded by the multiple sympathetic stressors involved, which included heat strain (i.e., increase in core body temperature) and exercise. Notably, both heat strain (16) and exercise (14) activate the sympathetic nervous system. Our laboratory has used the CPT to stimulate the sympathetic nervous system and examine the subsequent renal vascular responses (7, 39). In the present study, we aimed to determine the isolated effects of soft drink consumption without the addition of heat and exercise on the renal hemodynamic response during sympathetic activation. Compared with drinking water, HFCS consumption augmented increases in vascular resistance in the segmental artery to the CPT. Notably, vascular resistance in the segmental artery was elevated following HFCS consumption by ~16%, and further augmented increases in segmental artery vascular resistance were observed during the CPT. Thus, HFCS consumption likely increased both baseline renal vasoconstrictor tone and the renal vasoconstrictor response to sympathetic activation.
The mechanism by which this augmented increase in segmental artery vascular resistance occurred with HFCS consumption is likely multifactorial, as there are many redundant and opposing pathways by which vasoconstriction is elicited in the kidneys (20). However, a relatively moderate bolus of fluid is not likely responsible, as the vascular resistance response to the CPT in the segmental artery did not change with water consumption. Some of the mechanisms underlying the observations in study 1 were investigated in study 2.
Elevations in Segmental Artery Vascular Resistance With Soft Drink Consumption Are Likely Caused by HFCS (Study 2)
Study 2 used commercially available taste and caffeine-matched soft drinks that differed by the type of sweetener. This permitted the unique exploration of the beverage constituents likely contributing to the increased segmental artery vascular resistance following HFCS-sweetened soft drink consumption. By also obtaining blood and urine samples, study 2 allowed for assessment of some of the potential physiological mechanisms mediating any renal vascular responses. Increases in segmental artery vascular resistance postconsumption occurred only in the HFCS trial and not in the Sucrose, Diet, or Water trials. Moreover, despite that the HFCS-sweetened soft drink had a higher beverage osmolality than the sucrose-sweetened soft drink, there were no differences in the increase in plasma osmolality or changes in plasma volume between trials. Thus, it is unlikely that beverage osmolality explains our findings. Because the soft drinks were matched for caffeine content (and likely most other additives, e.g., food color), we believe that the increased fructose content in the HFCS-sweetened beverage elicited the increased segmental artery vascular resistance. This is supported by our findings for a greater increase in serum fructose concentration at 30 min post-HFCS consumption compared with all other trials.
Le et al. (26) previously reported increases in serum uric acid occurring within 30 min of consuming a soft drink sweetened with HFCS. This fructose-induced postprandial hyperuricemia (15) is likely accompanied by increased vasopressin release, independent of changes in osmolality. For instance, Wolf et al. (47) previously showed that hypertonic fructose infusion increases vasopressin independent of osmolality, as no changes in vasopressin were observed with an equimolar infusion of hypertonic glucose. The findings of the present study extend this work, demonstrating that at 30 min post-HFCS consumption, serum uric acid and serum copeptin are simultaneously elevated, which did not occur in the other trials. Notably, increases in both serum uric acid (43) and vasopressin (3) induce renal vasoconstriction. Our data support that increases in segmental artery vascular resistance were observed only when both serum uric acid and copeptin were elevated. Interestingly, increases in serum uric acid in the absence of any increases in copeptin, such as what occurred with consumption of a sucrose-sweetened soft drink, did not elicit increases in segmental artery vascular resistance. This may be suggestive of a potential synergistic effect, whereby increases in circulating uric acid and vasopressin may be necessary to stimulate renal vasoconstriction. However, direct evidence of this interaction between uric acid and vasopressin is required.
The observed increases in segmental artery vascular resistance following consumption of a HFCS-sweetened soft drink were not likely due to caffeine content because the caffeine content was the same in the three soft drinks. This is further supported by evidence that caffeine does not change renal blood flow in doses of <360 mg (33) and has no acute effect on vasopressin secretion (30). Furthermore, data in animals indicate that renal vasoconstrictor tone may be decreased following intrarenal infusion of methylxanthines (i.e., a class of adenosine receptor antagonists including caffeine) (32). There may also be a role for other circulating vasoconstrictors in response to consumption of HFCS-sweetened soft drinks. Further investigation is warranted regarding exploring the potential contributions of endothelin-1 and/or angiotensin II for the increased vascular resistance in the segmental artery occurring subsequent to consumption of HFCS-sweetened soft drinks. Such involvement is likely, given evidence that these vasoconstrictor agents are upregulated in rats chronically fed high-fructose diets (42). Furthermore, angiotensin II is also stimulated by increases in uric acid (49), such as fructose-induced hyperuricemia (15, 26, 35), which occurred in the HFCS trial. The role of renal sympathetic nerve activity on HFCS-sweetened soft drink-induced increases in vascular resistance in the segmental artery cannot be elucidated by the present experimental design. In study 1, we did not observe acute changes in total peripheral resistance, which can sometimes be used as an indirect measure of sympathetic activation, following consumption of a HFCS-sweetened soft drink. That said, it is unknown whether this is also indirect evidence that renal sympathetic nerve activity was unchanged. We are only aware of one study that directly examined the effect of fructose consumption on renal sympathetic nerve activity. This study reported no changes in basal renal sympathetic nerve activity in rats fed a high-fructose diet for 2–4 wk (48). In addition to the mechanisms by which renal vasoconstriction may be promoted by consumption of a HFCS-sweetened soft drink, there is the potential that HFCS ingestion impacted vasodilatory actions in the kidneys. The vasodilator, nitric oxide, can be produced in the kidneys by endothelial nitric oxide synthase in the glomeruli and by neuronal nitric oxide synthase in the macula densa (1). Glushakova et al. (17) have previously reported that fructose reduces nitric oxide derived from endothelial nitric oxide synthase. Collectively, this suggests that consumption of a HFCS-sweetened soft drink may shift the vasodilator-vasoconstrictor balance toward vasoconstriction by both reducing vasodilatory substance release and promoting vasoconstrictor substance release. However, this remains speculative and warrants further investigation.
Considerations
There are a few methodological considerations that warrant mentioning. First, we and others are not able to directly assess renal and segmental artery vessel wall diameter via Doppler ultrasound. Although it has been previously shown that changes in renal blood flow were due to changes in renal blood velocity, as vessel wall diameter did not change during pharmacologically induced vasoconstriction (28), we cannot be certain that diameter did not change. Despite this, we used rigorous controls to ensure the highest quality and reliable renal ultrasound measurements were obtained (8). The limitations associated with technique have been extensively outlined previously (8). Second, we decarbonated the soft drinks to reduce the risk of acoustic shadowing of the kidney during our renal ultrasound measurements. Thus, we do not know if the renal vascular response is altered with a higher carbon dioxide concentration in an ingested beverage. Third, we performed the CPT 30 min after consumption of the soft drink, and we do not know whether this augmented renal vascular response exists at longer durations postconsumption. Data from Le et al. (26) indicate that soft drink consumption increases the concentration of fructose in the blood for 2 h and elevates serum uric acid for 3 h. On the basis of these data, it is conceivable that the renal vascular response to sympathetic activation would be augmented for at least 2 h postconsumption. Fourth, we strictly tested the acute effects of soft drink consumption among casual drinkers. Thus, we do not know the effects of chronic soft drink consumption on these responses or how our results would change in frequent consumers of soft drinks. Fifth, it remains possible that caffeine interacts with HFCS to elicit changes in renal hemodynamics. Future work should consider using experimental models with a caffeine-free, HFSC-sweetened soft drink to further tease out the independent effects of soft drinks. Sixth, we acknowledge the differences in osmolality between the sucrose- and HFCS-sweetened beverages. Thus, it remains possible that there are additional components within the HFCS-sweetened beverage (including, but not limited to, a higher beverage osmolality) that may have elicited or modulated the changes in renal hemodynamics observed in the present study.
Perspectives and Significance
There is much interest in renal and cardiovascular health outcomes associated with soft drink consumption. Our study indicates that a soft drink sweetened with HFCS elevates vascular resistance in the kidneys and elicits a greater renal vasoconstrictor response to sympathetic activation. These findings provide insight into the acute effects of soft drink consumption on renal vascular control. Furthermore, our data also provide insights into the responsiveness of the kidneys during a sympathetic stressor, which can be regularly encountered in activities of daily living (e.g., opening a jar or walking upstairs). Notably, there are also a number of states characterized by heightened basal sympathetic activity, including chronic kidney disease (24), type II diabetes (19), and obesity (18). Whether consumption of a HFCS-sweetened soft drink has similar responses in the renal vasculature of these groups is not known. In theory, regular consumption of HFCS-sweetened soft drinks could chronically exacerbate increases in renal vasoconstrictor tone that may predispose them to an increased risk of nephropathy occurring subsequent to localized renal ischemia. In support of this, chronic excessive fructose intake has been linked to kidney disease (22).
Conclusions
Consumption of 500 mL of a commercially available soft drink sweetened with HFCS increased vascular resistance in the kidneys within 30 min. We also found that increases in segmental artery vascular resistance were exacerbated during the CPT compared with water consumption. Furthermore, in a followup study, we determined that these changes in segmental artery hemodynamics were elicited by the sweetener HFCS and were not due to the caffeine content or osmolality of the beverage. These increases in segmental artery vascular resistance were likely due to simultaneous increases in serum uric acid and copeptin. Collectively, our findings indicate that HFCS-sweetened soft drink consumption increased renal vasoconstrictor tone at rest and during sympathetic activation.
GRANTS
This work was partially supported by United States Centers for Disease Control and Prevention Grant R01-OH-011528.
DISCLOSURES
No conflicts of interest, financial or otherwise, are declared by the authors.
AUTHOR CONTRIBUTIONS
C.L.C., B.D.J., and Z.J.S. conceived and designed research; C.L.C., T.G., N.T.V., E.L.R., P.J.K., L.D.P., A.C.B., B.D.J., and Z.J.S. performed experiments; C.L.C., T.G., P.J.K., L.D.P., A.C.B., and Z.J.S. analyzed data; C.L.C., B.D.J., and Z.J.S. interpreted results of experiments; C.L.C. prepared figures; C.L.C. and Z.J.S. drafted manuscript; C.L.C., T.G., N.T.V., E.L.R., P.J.K., L.D.P., A.C.B., B.D.J., and Z.J.S. edited and revised manuscript; C.L.C., T.G., N.T.V., E.L.R., P.J.K., L.D.P., A.C.B., B.D.J., and Z.J.S. approved final version of manuscript.
ACKNOWLEDGMENTS
We thank the individuals who participated in this study. We also extend our gratitude toward Aaron Gonzalez for the efforts with data collection. We thank Elizabeth Mietlicki-Baase and Todd Rideout for generosity with providing supplies.
REFERENCES
- 1.Ahmad A, Dempsey SK, Daneva Z, Azam M, Li N, Li PL, Ritter JK. Role of nitric oxide in the cardiovascular and renal systems. Int J Mol Sci 19: E2605, 2018. doi: 10.3390/ijms19092605. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.American College of Sports Medicine, Sawka MN, Burke LM, Eichner ER, Maughan RJ, Montain SJ, Stachenfeld NS. American College of Sports Medicine position stand. Exercise and fluid replacement. Med Sci Sports Exerc 39: 377–390, 2007. [DOI] [PubMed] [Google Scholar]
- 3.Azzawi SA, Shirley DG. The effect of vasopressin on renal blood flow and its distribution in the rat. J Physiol 341: 233–244, 1983. doi: 10.1113/jphysiol.1983.sp014803. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Balanescu S, Kopp P, Gaskill MB, Morgenthaler NG, Schindler C, Rutishauser J. Correlation of plasma copeptin and vasopressin concentrations in hypo-, iso-, and hyperosmolar states. J Clin Endocrinol Metab 96: 1046–1052, 2011. doi: 10.1210/jc.2010-2499. [DOI] [PubMed] [Google Scholar]
- 5.Bankir L, Bouby N, Ritz E. Vasopressin: a novel target for the prevention and retardation of kidney disease? Nat Rev Nephrol 9: 223–239, 2013. doi: 10.1038/nrneph.2013.22. [DOI] [PubMed] [Google Scholar]
- 6.Brown CM, Dulloo AG, Yepuri G, Montani J-P. Fructose ingestion acutely elevates blood pressure in healthy young humans. Am J Physiol Regul Integr Comp Physiol 294: R730–R737, 2008. doi: 10.1152/ajpregu.00680.2007. [DOI] [PubMed] [Google Scholar]
- 7.Chapman CL, Benati JM, Johnson BD, Vargas NT, Lema PC, Schlader ZJ. Renal and segmental artery hemodynamics during whole body passive heating and cooling recovery. J Appl Physiol 127: 974–983, 2019. doi: 10.1152/japplphysiol.00403.2019. [DOI] [PubMed] [Google Scholar]
- 8.Chapman CL, Johnson BD, Hostler D, Lema PC, Schlader ZJ. Reliability and agreement of human renal and segmental artery hemodynamics measured using Doppler ultrasound. J Appl Physiol 128: 627–636, 2020. doi: 10.1152/japplphysiol.00813.2019. [DOI] [PubMed] [Google Scholar]
- 9.Chapman CL, Johnson BD, Sackett JR, Parker MD, Schlader ZJ. Soft drink consumption during and following exercise in the heat elevates biomarkers of acute kidney injury. Am J Physiol Regul Integr Comp Physiol 316: R189–R198, 2019. doi: 10.1152/ajpregu.00351.2018. [DOI] [PubMed] [Google Scholar]
- 10.Chapman CL, Johnson BD, Vargas NT, Hostler D, Parker MD, Schlader ZJ. Hyperthermia and dehydration during physical work in the heat both contribute to the risk of acute kidney injury. J Appl Physiol. In press. doi: 10.1152/japplphysiol.00787.2019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Cheungpasitporn W, Thongprayoon C, O’Corragain OA, Edmonds PJ, Kittanamongkolchai W, Erickson SB. Associations of sugar-sweetened and artificially sweetened soda with chronic kidney disease: a systematic review and meta-analysis. Nephrology (Carlton) 19: 791–797, 2014. doi: 10.1111/nep.12343. [DOI] [PubMed] [Google Scholar]
- 12.Cui J, Shibasaki M, Low DA, Keller DM, Davis SL, Crandall CG. Heat stress attenuates the increase in arterial blood pressure during the cold pressor test. J Appl Physiol 109: 1354–1359, 2010. doi: 10.1152/japplphysiol.00292.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Drew RC, Muller MD, Blaha CA, Mast JL, Heffernan MJ, Estep LE, Cui J, Reed AB, Sinoway LI. Renal vasoconstriction is augmented during exercise in patients with peripheral arterial disease. Physiol Rep 1: e00154, 2013. doi: 10.1002/phy2.154. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Fisher JP, Young CN, Fadel PJ. Autonomic adjustments to exercise in humans. Compr Physiol 5: 475–512, 2015. doi: 10.1002/cphy.c140022. [DOI] [PubMed] [Google Scholar]
- 15.Fox IH, Kelley WN. Studies on the mechanism of fructose-induced hyperuricemia in man. Metabolism 21: 713–721, 1972. doi: 10.1016/0026-0495(72)90120-5. [DOI] [PubMed] [Google Scholar]
- 16.Gagnon D, Schlader ZJ, Crandall CG. Sympathetic activity during passive heat stress in healthy aged humans. J Physiol 593: 2225–2235, 2015. doi: 10.1113/JP270162. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Glushakova O, Kosugi T, Roncal C, Mu W, Heinig M, Cirillo P, Sánchez-Lozada LG, Johnson RJ, Nakagawa T. Fructose induces the inflammatory molecule ICAM-1 in endothelial cells. J Am Soc Nephrol 19: 1712–1720, 2008. doi: 10.1681/ASN.2007121304. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Grassi G, Seravalle G, Quarti-Trevano F, Scopelliti F, Dell’Oro R, Bolla G, Mancia G. Excessive sympathetic activation in heart failure with obesity and metabolic syndrome: characteristics and mechanisms. Hypertension 49: 535–541, 2007. doi: 10.1161/01.HYP.0000255983.32896.b9. [DOI] [PubMed] [Google Scholar]
- 19.Huggett RJ, Scott EM, Gilbey SG, Stoker JB, Mackintosh AF, Mary DA. Impact of type 2 diabetes mellitus on sympathetic neural mechanisms in hypertension. Circulation 108: 3097–3101, 2003. doi: 10.1161/01.CIR.0000103123.66264.FE. [DOI] [PubMed] [Google Scholar]
- 20.Johns EJ, Kopp UC, DiBona GF. Neural control of renal function. Compr Physiol 1: 731–767, 2011. [DOI] [PubMed] [Google Scholar]
- 21.Johnson RJ, Bakris GL, Borghi C, Chonchol MB, Feldman D, Lanaspa MA, Merriman TR, Moe OW, Mount DB, Sanchez Lozada LG, Stahl E, Weiner DE, Chertow GM. Hyperuricemia, acute and chronic kidney disease, hypertension, and cardiovascular disease: report of a scientific workshop organized by the National Kidney Foundation. Am J Kidney Dis 71: 851–865, 2018. doi: 10.1053/j.ajkd.2017.12.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Johnson RJ, Segal MS, Sautin Y, Nakagawa T, Feig DI, Kang DH, Gersch MS, Benner S, Sánchez-Lozada LG. Potential role of sugar (fructose) in the epidemic of hypertension, obesity and the metabolic syndrome, diabetes, kidney disease, and cardiovascular disease. Am J Clin Nutr 86: 899–906, 2007. doi: 10.1093/ajcn/86.4.899. [DOI] [PubMed] [Google Scholar]
- 23.Kang DH, Nakagawa T, Feng L, Watanabe S, Han L, Mazzali M, Truong L, Harris R, Johnson RJ. A role for uric acid in the progression of renal disease. J Am Soc Nephrol 13: 2888–2897, 2002. doi: 10.1097/01.ASN.0000034910.58454.FD. [DOI] [PubMed] [Google Scholar]
- 24.Kaur J, Young BE, Fadel PJ. Sympathetic overactivity in chronic kidney disease: consequences and mechanisms. Int J Mol Sci 18: E1682, 2017. doi: 10.3390/ijms18081682. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Lanaspa MA, Ishimoto T, Cicerchi C, Tamura Y, Roncal-Jimenez CA, Chen W, Tanabe K, Andres-Hernando A, Orlicky DJ, Finol E, Inaba S, Li N, Rivard CJ, Kosugi T, Sanchez-Lozada LG, Petrash JM, Sautin YY, Ejaz AA, Kitagawa W, Garcia GE, Bonthron DT, Asipu A, Diggle CP, Rodriguez-Iturbe B, Nakagawa T, Johnson RJ. Endogenous fructose production and fructokinase activation mediate renal injury in diabetic nephropathy. J Am Soc Nephrol 25: 2526–2538, 2014. doi: 10.1681/ASN.2013080901. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Le MT, Frye RF, Rivard CJ, Cheng J, McFann KK, Segal MS, Johnson RJ, Johnson JA. Effects of high-fructose corn syrup and sucrose on the pharmacokinetics of fructose and acute metabolic and hemodynamic responses in healthy subjects. Metabolism 61: 641–651, 2012. doi: 10.1016/j.metabol.2011.09.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Levey AS, Eckardt KU, Tsukamoto Y, Levin A, Coresh J, Rossert J, De Zeeuw D, Hostetter TH, Lameire N, Eknoyan G. Definition and classification of chronic kidney disease: a position statement from Kidney Disease: Improving Global Outcomes (KDIGO). Kidney Int 67: 2089–2100, 2005. doi: 10.1111/j.1523-1755.2005.00365.x. [DOI] [PubMed] [Google Scholar]
- 28.Marraccini P, Fedele S, Marzilli M, Orsini E, Dukic G, Serasini L, L’Abbate A. Adenosine-induced renal vasoconstriction in man. Cardiovasc Res 32: 949–953, 1996. doi: 10.1016/S0008-6363(96)00128-9. [DOI] [PubMed] [Google Scholar]
- 29.Momen A, Leuenberger UA, Ray CA, Cha S, Handly B, Sinoway LI. Renal vascular responses to static handgrip: role of muscle mechanoreflex. Am J Physiol Heart Circ Physiol 285: H1247–H1253, 2003. doi: 10.1152/ajpheart.00214.2003. [DOI] [PubMed] [Google Scholar]
- 30.Nussberger J, Mooser V, Maridor G, Juillerat L, Waeber B, Brunner HR. Caffeine-induced diuresis and atrial natriuretic peptides. J Cardiovasc Pharmacol 15: 685–691, 1990. doi: 10.1097/00005344-199005000-00001. [DOI] [PubMed] [Google Scholar]
- 31.Obermayr RP, Temml C, Gutjahr G, Knechtelsdorfer M, Oberbauer R, Klauser-Braun R. Elevated uric acid increases the risk for kidney disease. J Am Soc Nephrol 19: 2407–2413, 2008. doi: 10.1681/ASN.2008010080. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Osswald H, Schnermann J. Methylxanthines and the kidney. Handb Exp Pharmacol 200: 391–412, 2011. doi: 10.1007/978-3-642-13443-2_15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Passmore AP, Kondowe GB, Johnston GD. Renal and cardiovascular effects of caffeine: a dose-response study. Clin Sci (Lond) 72: 749–756, 1987. doi: 10.1042/cs0720749. [DOI] [PubMed] [Google Scholar]
- 34.Perez-Pozo SE, Schold J, Nakagawa T, Sánchez-Lozada LG, Johnson RJ, Lillo JL. Excessive fructose intake induces the features of metabolic syndrome in healthy adult men: role of uric acid in the hypertensive response. Int J Obes 34: 454–461, 2010. doi: 10.1038/ijo.2009.259. [DOI] [PubMed] [Google Scholar]
- 35.Perheentupa J, Raivio K. Fructose-induced hyperuricaemia. Lancet 2: 528–531, 1967. doi: 10.1016/S0140-6736(67)90494-1. [DOI] [PubMed] [Google Scholar]
- 36.Roncal-Jimenez CA, Milagres T, Andres-Hernando A, Kuwabara M, Jensen T, Song Z, Bjornstad P, Garcia GE, Sato Y, Sanchez-Lozada LG, Lanaspa MA, Johnson RJ. Effects of exogenous desmopressin on a model of heat stress nephropathy in mice. Am J Physiol Renal Physiol 312: F418–F426, 2017. doi: 10.1152/ajprenal.00495.2016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Rowell LB. Hyperthermia: a hyperadrenergic state. Hypertension 15: 505–507, 1990. doi: 10.1161/01.HYP.15.5.505. [DOI] [PubMed] [Google Scholar]
- 38.Sánchez-Lozada LG, Tapia E, Jiménez A, Bautista P, Cristóbal M, Nepomuceno T, Soto V, Avila-Casado C, Nakagawa T, Johnson RJ, Herrera-Acosta J, Franco M. Fructose-induced metabolic syndrome is associated with glomerular hypertension and renal microvascular damage in rats. Am J Physiol Renal Physiol 292: F423–F429, 2007. doi: 10.1152/ajprenal.00124.2006. [DOI] [PubMed] [Google Scholar]
- 39.Schlader ZJ, Chapman CL, Benati JM, Gideon EA, Vargas NT, Lema PC, Johnson BD. Renal hemodynamics during sympathetic activation following aerobic and anaerobic exercise. Front Physiol 9: 1928, 2019. doi: 10.3389/fphys.2018.01928. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Schlader ZJ, Hostler D, Parker MD, Pryor RR, Lohr JW, Johnson BD, Chapman CL. The potential for renal injury elicited by physical work in the heat. Nutrients 11: E2087, 2019. doi: 10.3390/nu11092087. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Song 宋志林 Z, Roncal-Jimenez CA, Lanaspa-Garcia MA, Oppelt SA, Kuwabara M, Jensen T, Milagres T, Andres-Hernando A, Ishimoto T, Garcia GE, Johnson G, MacLean PS, Sanchez-Lozada LG, Tolan DR, Johnson RJ. Role of fructose and fructokinase in acute dehydration-induced vasopressin gene expression and secretion in mice. J Neurophysiol 117: 646–654, 2017. doi: 10.1152/jn.00781.2016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Tran LT, MacLeod KM, McNeill JH. Endothelin-1 modulates angiotensin II in the development of hypertension in fructose-fed rats. Mol Cell Biochem 325: 89–97, 2009. doi: 10.1007/s11010-008-0023-z. [DOI] [PubMed] [Google Scholar]
- 43.Uedono H, Tsuda A, Ishimura E, Yasumoto M, Ichii M, Ochi A, Ohno Y, Nakatani S, Mori K, Uchida J, Nakatani T, Inaba M. Relationship between serum uric acid levels and intrarenal hemodynamic parameters. Kidney Blood Press Res 40: 315–322, 2015. doi: 10.1159/000368507. [DOI] [PubMed] [Google Scholar]
- 44.Walker RW, Dumke KA, Goran MI. Fructose content in popular beverages made with and without high-fructose corn syrup. Nutrition 30: 928–935, 2014. doi: 10.1016/j.nut.2014.04.003. [DOI] [PubMed] [Google Scholar]
- 45.Wesseling KH, Jansen JR, Settels JJ, Schreuder JJ. Computation of aortic flow from pressure in humans using a nonlinear, three-element model. J Appl Physiol 74: 2566–2573, 1993. doi: 10.1152/jappl.1993.74.5.2566. [DOI] [PubMed] [Google Scholar]
- 46.Wilson TE, Sauder CL, Kearney ML, Kuipers NT, Leuenberger UA, Monahan KD, Ray CA. Skin-surface cooling elicits peripheral and visceral vasoconstriction in humans. J Appl Physiol 103: 1257–1262, 2007. doi: 10.1152/japplphysiol.00401.2007. [DOI] [PubMed] [Google Scholar]
- 47.Wolf JP, Nguyen NU, Dumoulin G, Berthelay S. Influence of hypertonic monosaccharide infusions on the release of plasma arginine vasopressin in normal humans. Horm Metab Res 24: 379–383, 1992. doi: 10.1055/s-2007-1003340. [DOI] [PubMed] [Google Scholar]
- 48.Wong PS, Johns EJ. Effect of acute saline volume loading on renal sympathetic nerve activity in anaesthetised fructose-fed and fat-fed rats. J Auton Nerv Syst 75: 60–69, 1999. doi: 10.1016/S0165-1838(98)00180-5. [DOI] [PubMed] [Google Scholar]
- 49.Yu MA, Sánchez-Lozada LG, Johnson RJ, Kang DH. Oxidative stress with an activation of the renin-angiotensin system in human vascular endothelial cells as a novel mechanism of uric acid-induced endothelial dysfunction. J Hypertens 28: 1234–1242, 2010. doi: 10.1097/HJH.0b013e328337da1d. [DOI] [PubMed] [Google Scholar]