Abstract
Disruption of mitochondrial dynamics is an important pathogenic event in both acute and chronic kidney diseases, but the underlying mechanism remains poorly understood. Here, we report the regulation of mitofusin-2 (Mfn2; a key mitochondrial fusion protein) by microRNA-214 (miR-214) in renal ischemia-reperfusion that contributes to mitochondrial fragmentation, renal tubular cell death, and ischemic acute kidney injury (AKI). miR-214 was induced, whereas Mfn2 expression was decreased, in mouse ischemic AKI and cultured rat kidney proximal tubular cells (RPTCs) following ATP depletion treatment. Overexpression of miR-214 decreased Mfn2. Conversely, inhibition of miR-214 with anti-miR-214 prevented Mfn2 downregulation in RPTCs following ATP depletion. Anti-miR-214 further ameliorated mitochondrial fragmentation and apoptosis, whereas overexpression of miR-214 increased apoptosis, in ATP-depleted RPTCs. To test regulation in vivo, we established a mouse model with miR-214 specifically deleted from kidney proximal tubular cells (PT-miR-214−/−). Compared with wild-type mice, PT-miR-214−/− mice had less severe tissue damage, fewer apoptotic cells, and better renal function after ischemic AKI. miR-214 induction in ischemic AKI was suppressed in PT-miR-214−/− mice, accompanied by partial preservation of Mfn2 in kidneys. These results unveil the miR-214/Mfn2 axis that contributes to the disruption of mitochondrial dynamics and tubular cell death in ischemic AKI, offering new therapeutic targets.
Keywords: ischemia-reperfusion; kidney; microRNA-214, mitochondria; mitofusin
INTRODUCTION
Ischemia-reperfusion injury leads to ischemic diseases in various tissues, such as stroke in the brain, myocardial infarction, and ischemic acute kidney injury (AKI). Ischemic AKI is associated with high rates of morbidity and mortality, and effective therapies are currently unavailable (7, 37). A key pathological feature of ischemic AKI is the injury and death of proximal tubular cells (21, 24). Although the mechanism of tubular cell injury and death is complex and multifactorial, mitochondrial damage is one of the critical events (5, 11, 32, 33, 35, 39, 41, 48, 50).
Mitochondria are dynamic organelles that undergo fission and fusion to maintain their viability and function (5, 10, 30, 49, 50). Interestingly, mitochondrial fission and fusion are governed by two classes of proteins. Although fusion involves mitofusin-1 (Mfn1), mitofusin-2 (Mfn2) at the mitochondrial outer membrane, and optic atrophy 1 at the mitochondrial inner membrane, mitochondrial fission involves dynamin-related protein 1, fission 1, and newly identified Bax interacting factor-1 (5, 10, 12, 30, 49, 50). Recent research has recognized that mitochondrial dynamics are disrupted during cell stress, resulting in mitochondrial fragmentation and membrane permeabilization followed by cell death in various diseases (2, 8, 15, 18, 31, 34, 42, 45, 51). In kidneys, mitochondrial fragmentation as a result of the disruption of mitochondrial dynamics is an important pathogenic event for AKI (8) as well as chronic kidney diseases, such as diabetic nephropathy (16, 29, 42, 51).
MicroRNAs are short, noncoding RNAs that play important roles in the regulation of gene expression by inducing target gene mRNA degradation or repressing target gene translation. Numerous studies have demonstrated various regulatory roles of microRNAs in kidney diseases as a whole class of regulatory molecules or individually (17, 22). For example, mice with Dicer (an enzyme for microRNA biogenesis) specifically deleted from kidney proximal tubules showed microRNA depletion from kidneys and were resistant to ischemic AKI (43), but these mice were sensitive to kidney injury and renal fibrosis associated with unilateral ureteral obstruction, diabetes nephropathy, and cisplatin nephrotoxicity (28). Individually, miR-21, miR-668, miR-687, and miR-17-5p protected against ischemic AKI (6, 20, 38, 45), whereas some other microRNAs, including miR-155, miR-377, and miR-182, were shown to promote ischemic AKI (23, 25, 52). One microRNA that is induced in a variety of kidney disease models is miR-214, which is induced in unilateral ureteral obstruction (13), ischemic AKI (53), albumin overload-induced chronic kidney disease (3), and diabetic kidney disease (4).
There is emerging evidence for the regulation of mitochondria by microRNAs. In cisplatin-induced kidney injury, Guo et al. (19) demonstrated that miR-709 was induced to mediate mitochondrial damage and renal tubular cell death by repressing mitochondrial transcriptional factor A. Our recent work further demonstrated the upregulation of miR-668 in AKI models and human patients, and, upon induction, miR-668 was shown to repress mitochondrial protein 18 to prevent mitochondrial fragmentation and protect renal tubular cells from apoptosis in ischemic AKI (45). In the present study, we identified the induction of miR-214 in both cell culture and mouse models of ischemic AKI. Functionally, we show that miR-214 targets or represses Mfn2 to promote mitochondrial fragmentation, contributing to tubular cell apoptosis and AKI. Together, these results provide new insights into the molecular regulation of mitochondria and tubular cell death in AKI, suggesting novel therapeutic strategies.
MATERIALS AND METHODS
Cells and treatment.
The rat kidney proximal tubular cell line (RPTCs) was originally obtained from Dr. Hopfer (Case Western Reserve University, Cleveland, OH) and cultured for the experiment as previously described (8, 45). miR-214 expression plasmid and its control empty vector (Origene, Rockville, MD) as well as anti-miR-214 locked nucleic acid (LNA) and scramble sequence LNA (Exiqon) were transfected into RPTCs using Lipofectamine 2000. For the ATP depletion model, cells were incubated with 10 mM sodium azide (mitochondrial respiration inhibitor) in glucose-free buffer for 3 h and 15 min followed by 2 h of reperfusion in full culture medium, as previously described (27).
Evaluation of apoptosis in cell culture.
Cells were stained with 10 μg/mL Hoechst 33342, and apoptotic cells were then identified by cellular condensation, blebbing, nuclear condensation, and fragmentation with phase-contrast microscopy and fluorescent microscopy. Cells with apoptotic morphologies were counted to determine the percentage of apoptosis.
Mouse model of ischemia-reperfusion.
Mice were anesthetized with pentobarbital sodium via intraperitoneal injection (50 mg/kg). After hair removal, mice were kept on a thermostatic blanket with a rectal probe to maintain body temperature at 36.5°C for surgery. The kidney pedicles were clamped with microvessel clips to block blood supply of kidneys for ischemia. Clips were released after ischemia for reperfusion. Sham mice received operation but were not subjected to kidney pedicle clamping. Mice were euthanized 48 h later for analysis.
Quantitative PCR analysis of miR-214.
Total RNA was extracted form kidney tissues or cells with the mirVana miRNA Isolation Kit (Life Technologies). After reverse transcription, the level of miR-214 was detected by the TaqMan microRNA assay (Life Technologies) as previously described (43). Small nuclear RNA 202 was used as internal control, and quantification was done using ΔCt values (where Ct is threshold cycle) (6, 45)
Immunoblot analysis.
Cultured cells were lysed in SDS lysis buffer, and protein concentration was then measured using the BCA Protein Assay Kit (ThermoFisher Scientific). Protein samples were loaded for electrophoresis on SDS-polyacrylamide gel and then transferred to PVDF membrane. After being blocked in 5% milk, the membrane was incubated with primary antibody at 4°C overnight. The blot was washed with PBS containing 0.1% of Tween 20 and incubated with secondary antibody for 1 h at room temperature. Finally, the blot was incubated with SuperSignal West Pico Chemiluminescent Substrate (ThermoFisher Scientific) and scanned with MyECL Imager (ThermoFisher Scientific) to detect signals. The primary antibodies used were anti-β-actin (A5441, Sigma-Aldrich), anti-active-caspase-3 (no. 9664, Cell Signaling Technology), anti-Mfn2 (M6444, Sigma-Aldrich), and anti-cyclophilin B (no. 43603, Cell Signaling Technology).
Analysis of mitochondrial fragmentation.
RPTCs were stained with 0.1 mM MitoTracker red (ThermoFisher Scientific) for 30 min and then subjected to treatment. After treatment, cells were fixed with 4% paraformaldehyde for 15 min followed by a PBS rinse, and slides were then evaluated for mitochondrial morphology by confocal microscopy. Fragmented mitochondria showed punctate structure, whereas filamentous mitochondria showed a thread- or worm-like morphology.
Statistical analysis.
All data are presented as means ± SD. GraphPad Prism 8 and Microsoft Excel 2012 were used for calculation and statistical analysis. Student’s t test was used to show the difference between two groups, and one-way ANOVA was used to show the difference between multiple groups. P values of <0.05 were considered significant.
RESULTS
miR-214 is induced in both in vivo and in vitro models of ischemic AKI.
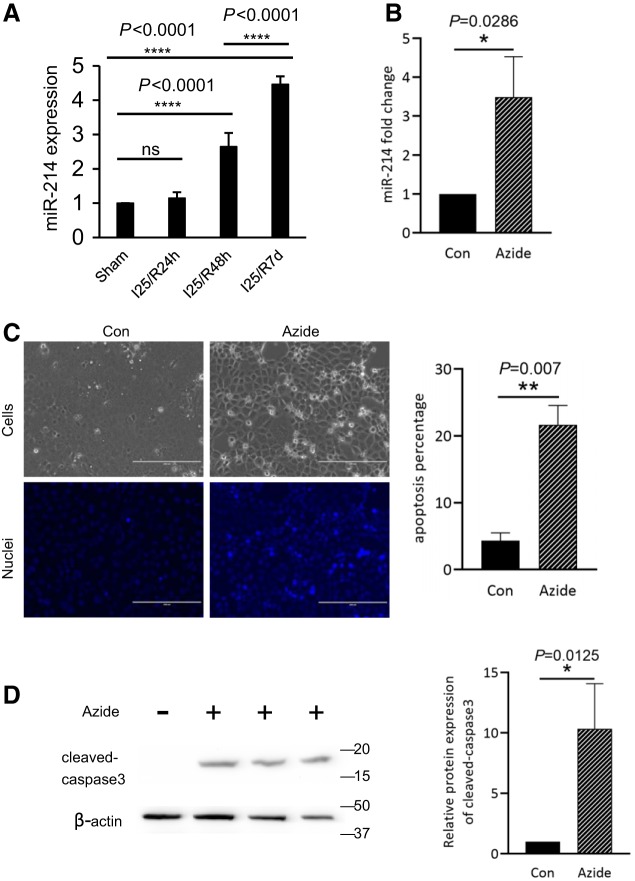
First, miR-214 expression was examined in the kidneys after renal ischemia-reperfusion by Taqman-based quantitative real-time PCR. As shown in Fig. 1A, compared with sham controls, no significant changes of miR-214 expression were detected after ischemia with 24 h of reperfusion. The miR-214 level was significantly increased to ~2.6-fold over sham controls after ischemia with 48 h of reperfusion, which was further increased to 4.5-fold over controls at 7 days of reperfusion (Fig. 1A).
Fig. 1.
MicroRNA-214 (miR-214) is induced in both in vivo and in vitro models of renal ischemia-reperfusion. A: C57BL/6J male mice were subjected to 25 min of bilateral renal ischemia (I25) with 24 h (R24h), 48 h (R48h), 72 h, or 7 days (R7d) of reperfusion or were subjected to sham operation. miR-214 was quantified by quantitative PCR showing the induction of miR-214 in kidney tissues after ischemia followed by reperfusion of 48 h to 7 days (n = 6). B: rat kidney proximal tubular cells (RPTCs) were treated with 10 mM azide for 3 h for ATP depletion followed by 2 h of recovery in full culture medium (n = 3). miR-214 was quantified by quantitative PCR, showing the induction of miR-214 after ATP depletion in RPTCs. C: representative images and quantitative analysis of apoptotic cells. The percentage of apoptotic cells was determined by evaluating the cells with typical apoptotic morphology. D: representative immunoblots and densitometry analysis of cleaved caspase 3 in RPTCs. β-actin was used as a loading control. Con, control; ns, not significant. *P < 0.05; **P < 0.01; ****P < 0.0001.
We further examined miR-214 expression in the in vitro model of ATP depletion of renal tubular cells followed by recovery to mimic in vivo ischemia-reperfusion. To this end, RPTCs were treated with the cellular respiration inhibitor azide in plain buffer to induce ATP depletion and then returned to normal culture medium for recovery. As shown in Fig. 1B, miR-214 expression was significantly increased in azide-treated cells. Meanwhile, these cells also showed an increase of apoptosis, which was confirmed by cell morphology (Fig. 1C) and the appearance of cleaved/active caspase 3 (Fig. 1D). Together, these results suggest that miR-214 is induced in both in vivo and in vitro models of renal ischemia-reperfusion and is accompanied by tubular cell apoptosis.
Inhibition of miR-214 with anti-miR-214 LNA protects RPTC from apoptosis following ATP depletion.
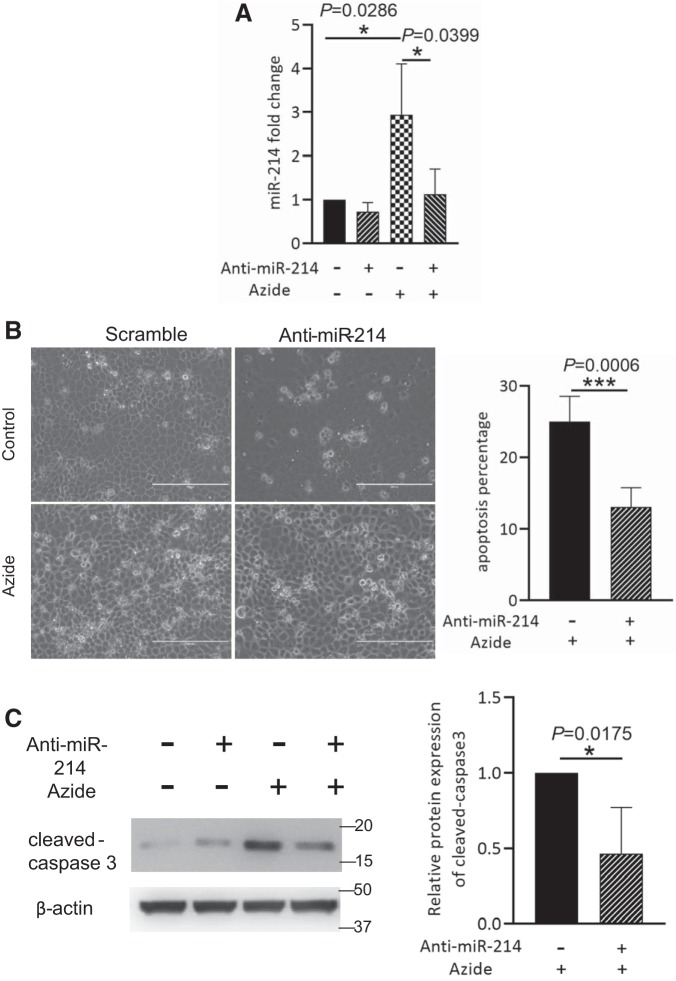
To explore the functional effect of miR-214 induction, we initially tested anti-miR-214 LNA (sequence-based antagonist of miR-214) on apoptosis induced by ATP depletion. Anti-miR-214 was transfected into RPTCs before ATP depletion to block miR-214. As expected, the induction of miR-214 following ATP depletion was remarkably inhibited by anti-miR-214. Importantly, compared with scramble sequence control, anti-miR-214 reduced apoptosis in morphological analysis (Fig. 2B) and in immunoblot analysis of cleaved caspase 3 (Fig. 2C), suggesting that miR-214 promotes apoptosis in this model.
Fig. 2.
Anti-microRNA-214 (miR-214) protects rat kidney proximal tubular cells (RPTCs) from apoptosis induced by ATP depletion. RPTCs were transfected by scrambled sequence or anti-miR-214 for 24 h and then treated with 10 mM azide for 3 h to induce ATP depletion followed by recovery of 2 h in full culture medium (n = 3). A: quantitative PCR analysis of miR-214 showing the inhibitory effect of anti-miR-214 on miR-214 expression. B: representative images and quantitative analysis of apoptotic cells showing the inhibitory effect of anti-miR-214 on ATP depletion-induced apoptosis. C: representative immunoblots and densitometry analysis of cleaved caspase 3 in ATP-depleted RPTCs. β-Actin was used as a loading control. *P < 0.05; ***P < 0.001.
Overexpression of miR-214 increases apoptosis following ATP depletion.
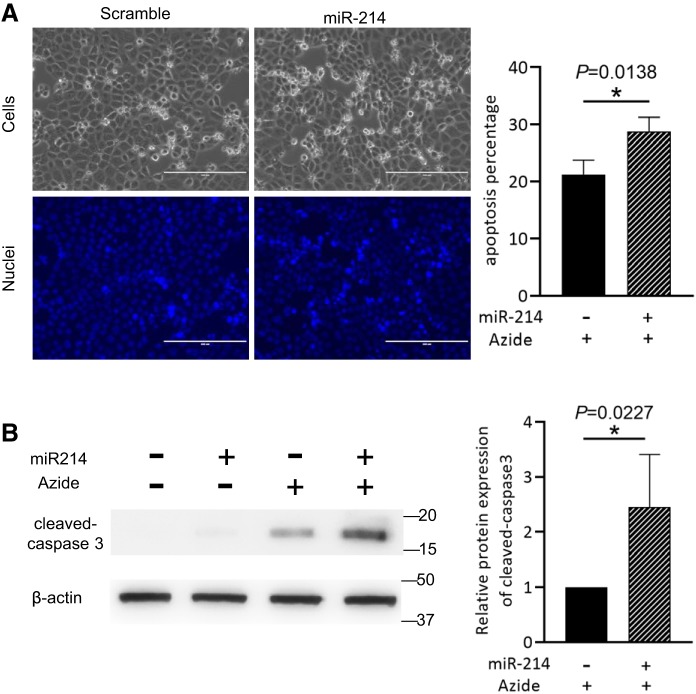
We further examined the effect of miR-214 overexpression on cell apoptosis. To this end, miR-214 was overexpressed in RPTCs before cells were subjected to ATP depletion and reperfusion. The results showed that the number of apoptotic cells (Fig. 3A) and the cleaved caspase 3 level (Fig. 3B) were further increased in miR-214-overexpressing RPTCs after ATP depletion and reperfusion, further verifying the proapoptotic role of miR-214 in this model.
Fig. 3.
MicroRNA-214 (miR-214) increases ATP depletion-induced apoptosis in rat kidney proximal tubular cells (RPTCs). RPTCs were transfected with empty vector or miR-214 plasmid before being subjected to ATP depletion (n = 3). A: representative images and quantitative analysis of apoptotic cells showing miR-214 overexpression increased apoptosis following ATP depletion in RPTCs. B: representative immunoblots and densitometry analysis of cleaved caspase 3 showing miR-214 increased active caspase 3 following ATP depletion in RPTCs. β-Actin was used as a loading control. I25R48h, 25 min of bilateral renal ischemia followed by 48 h of reperfusion. *P < 0.05.
Mfn2 is downregulated in both in vivo and in vitro models of ischemic AKI.
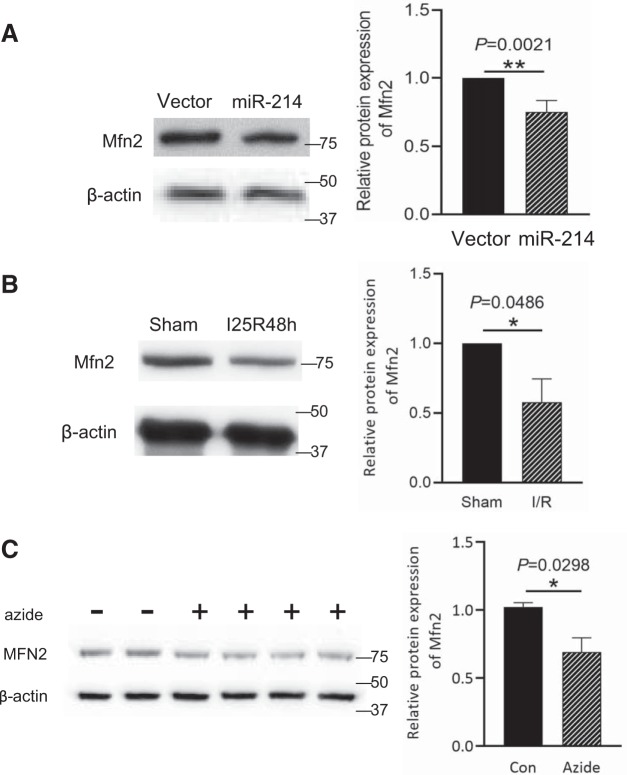
To understand how miR-214 promotes tubular cell apoptosis, we considered the potential targets of this microRNA, and Mfn2 came to our attention. Mfn2 is a critical protein for mitochondrial fusion, and its repression by miR-214 is expected to prevent mitochondrial fusion and, in turn, induce mitochondrial fragmentation, which would provide a logical explanation for the apoptosis-promoting effect of miR-214. Notably, a recent study (47) has suggested that Mfn2 might be a direct target of miR-214. To initially verify the regulatory effect of miR-214 on Mfn2 expression, miR-214 was overexpressed in human embryonic kidney cells. As shown in Fig. 4A, miR-214 overexpression significantly reduced Mfn2 protein expression. The sequence position 952–958 in the 3′-untranslated regions of Mfn2 is the putative miR-214 binding site, which is conserved in the human, mouse, and rat (40).
Fig. 4.
Mitofusin-2 (Mfn2) is downregulated in mouse ischemic acute kidney injury and in ATP-depleted rat kidney proximal tubular cells (RPTCs). A: human embryonic kidney (HEK)-293 cells were transfected with empty vector or microRNA-214 (miR-214) plasmids. Representative immunoblots and densitometry analysis of Mfn2 demonstrated that miR-214 suppressed Mfn2 expression in HEK-293 cells compared with empty vector (n = 3). B: male C57BL/6J mice were subjected to 25 min of bilateral renal ischemia followed by 48 h of reperfusion (I25/48h) or were subjected to sham operation. Representative immunoblots and densitometry analysis demonstrated the decrease of Mfn2 expression after ischemia-reperfusion (n = 5). β-Actin was used as a loading control. C: RPTCs were treated with 10 mM azide for 3 h to induce ATP depletion and then recovered for 2 h in full culture medium. Representative immunoblots and densitometry analysis of Mfn2 demonstrated Mfn2 downregulation in ATP-depleted RPTCs (n = 3). β-Actin was used as a loading control. Con, control; I/R, ischemia-reperfusion. *P < 0.05; **P < 0.01.
We further observed the changes of Mfn2 expression in kidney tissues after renal ischemia-reperfusion. As shown in Fig. 4B, Mfn2 expression in kidney tissues was decreased after renal ischemia-reperfusion. Consistently, Mfn2 expression was decreased in RPTCs following ATP depletion in vitro. The results are consistent with the possibility that miR-214 is induced to repress Mfn2 in these models.
Anti-miR-214 reduces Mfn2 downregulation and mitochondrial fragmentation during ATP depletion.
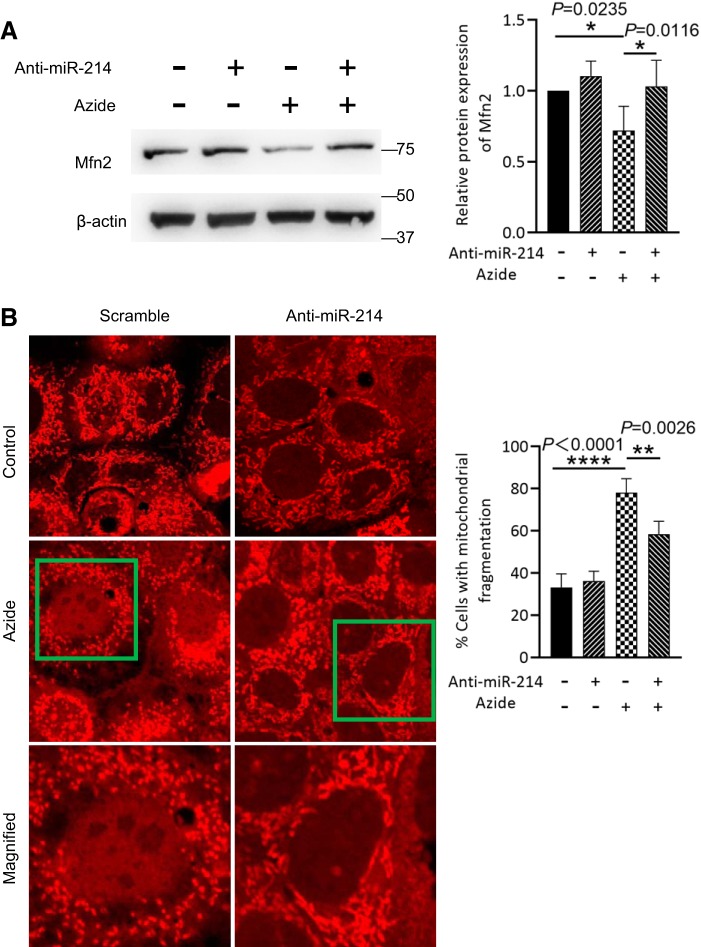
To further explore the possible regulatory effect of miR-214 on Mfn2 expression during ATP depletion, anti-miR-214 LNA or scrambled sequence LNA was transfected into RPTCs before the induction of ATP depletion. As shown in Fig. 5A, ATP depletion induced Mfn2 downregulation, which was reversed by anti-miR-214.
Fig. 5.
Anti-microRNA-214 (miR-214) prevents mitofusin-2 (Mfn2) downregulation and mitochondrial fragmentation in rat kidney proximal tubular cells (RPTCs) following ATP depletion. RPTCs were transfected with anti-miR-214 or scrambled sequence locked nucleic acids before being subjected to ATP depletion and recovery. A: representative immunoblots and densitometry analysis of Mfn2 showing that ATP depletion-induced Mfn2 downregulation was reduced by anti-miR-214 (n = 3). β-Actin was used as a loading control. B: RPTCs were stained with MitoTrackerer red and then subjected to 10 mM azide treatment. Representative images and quantitative analysis of mitochondrial fragmentation in RPTCs demonstrated mitochondrial fragmentation induced by ATP depletion and the inhibitory effect of anti-miR-214. The bottom images are magnified images of the boxed areas in the middle images. *P < 0.05; **P < 0.01; ****P < 0.0001.
To observe the effect on mitochondrial dynamics or morphology, we stained mitochondria with MitoTracker red. Consistent with Mfn2 downregulation, there was a significant increase of cells with fragmented mitochondria following ATP depletion. Notably, mitochondrial fragmentation was partially reduced by anti-miR-214 (Fig. 5B), indicating the regulatory effect of miR-214 on Mfn2 expression and mitochondrial dynamics during ATP depletion.
Mfn2 downregulation and ischemic AKI are suppressed in proximal tubule-specific miR-214−/− mice.
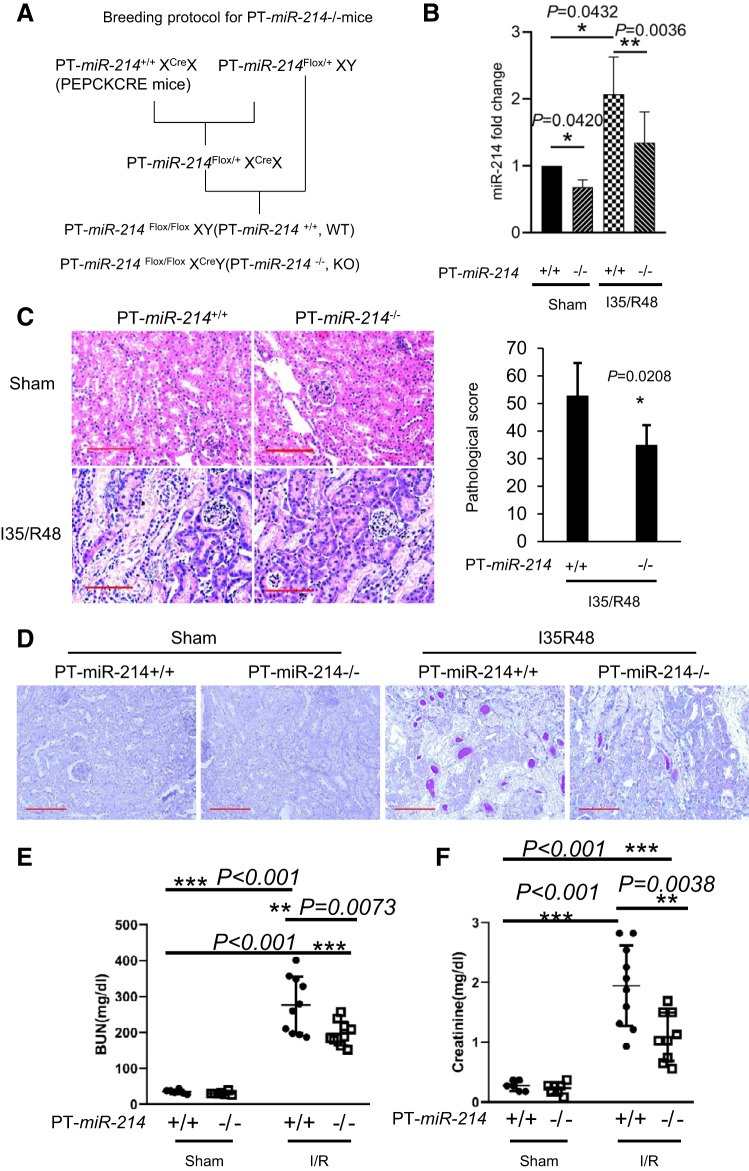
To elucidate the pathogenic role of miR-214 in ischemic AKI, we established a proximal tubule-specific miR-214 knockout (PT-miR-214−/−) mouse model by breeding miR-214 floxed mice (1) with phosphoenolpyruvate carboxykinase-Cre mice (36). The breeding protocol is shown in Fig. 6A. PT-miR-214−/− mice showed less miR-214 expression in kidney tissues than PT-miR-214+/+ mice, validating miR-214 deficiency in PT-miR-214−/− mouse kidneys (Fig. 6B). miR-214 was significantly induced in PT-miR-214+/+ mice subjected to renal ischemia-reperfusion, and this induction was markedly reduced in PT-miR-214−/− mice (Fig. 6B). Histological examination detected obvious renal tubular damage in PT-miR-214+/+ mice, which was significantly lower in PT-miR-214−/− mice (Fig. 6C). As shown in Fig. 6D, the decreased tubular damage in PT-miR-214−/− mice was further confirmed by period acid-Schiff staining. Functionally, blood urea nitrogen and creatinine levels were significantly lower in PT-miR-214−/− mice than in PT-miR-214+/+ mice (Fig. 6, E and F), further confirming a role of miR-214 in ischemic AKI.
Fig. 6.
Ischemic acute kidney injury (AKI) is suppressed in proximal tubule-specific microRNA-214-deficient (PT-miR-214−/−) mice. PT-miR-214−/− and PT-miR-214+/+ mice were subjected to 35 min of bilateral renal ischemia followed by 48 h of reperfusion (I35/R48) or were subjected to sham operation. A: breeding protocol for generating PT-miR-214−/− and PT-miR-214+/+ mice. B: quantitative PCR measurement showing decreased miR-214 expression in the kidneys of PT-miR-214−/− mice under control and ischemic AKI than PT-miR-214+/+ mice (n ≥ 5). C: representative images of renal histology by hematoxylin and eosin staining with semiquantification of tubular damage scores (n = 7). Scale bar = 50 μm. D: representative images of renal histology by periodic acid-Schiff staining. Scale bar = 100 μm. E: blood urea nitrogen (BUN) levels showing the protective effect of miR-214 deletion from kidney proximal tubules (n ≥ 6). F: serum creatinine measurement showing the protective effect of miR-214 deletion from kidney proximal tubules (n ≥ 6). PEPCK, phosphoenolpyruvate carboxykinase; KO, knockout; WT, wild type. *P < 0.05; **P < 0.01; ***P < 0.001.
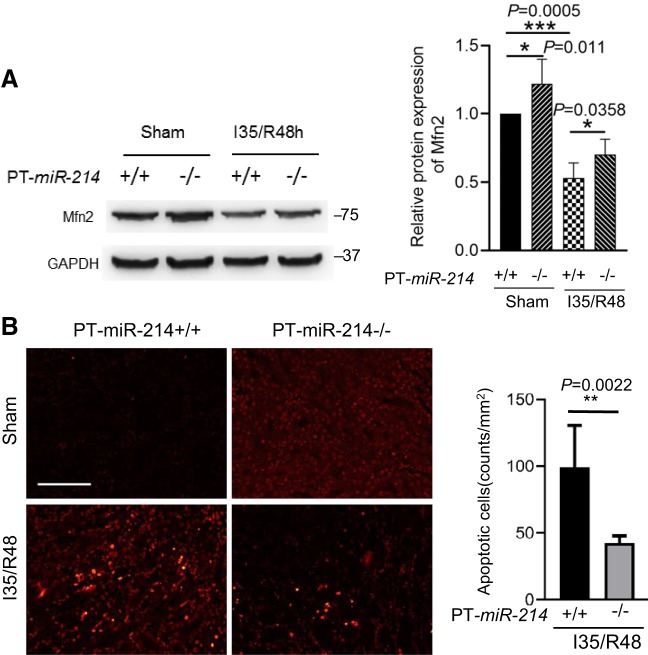
To test whether miR-214 participates in ischemic AKI via Mfn2, we analyzed Mfn2 expression in kidney tissues of PT-miR-214−/− and PT-miR-214+/+ mice. As shown in Fig. 7A, PT-miR-214−/− mice showed higher Mfn2 expression in kidney tissues than PT-miR-214+/+ mice under the control condition. During renal ischemia-reperfusion, Mfn2 was dramatically decreased in PT-miR-214+/+ mice, and this decrease was partially prevented in PT-miR-214−/− mice. Correspondingly, TUNEL staining showed that renal apoptosis in ischemic AKI was significantly reduced in PT-miR-214−/− mice compared with PT-miR-214+/+ mice (Fig. 7B). Together, these results suggest that miR-214 deficiency in proximal tubules reduces Mfn2 downregulation and alleviates renal apoptosis and damage, thus protecting against ischemic AKI.
Fig. 7.
Mitofusin-2 (Mfn2) downregulation and apoptosis in renal ischemia-reperfusion are ameliorated in proximal tubule-specific microRNA-214-deficient (PT-miR-214−/−) mice. A: representative immunoblots and densitometry analysis of Mfn2 showing that Mfn2 downregulation in renal ischemia-reperfusion was ameliorated in PT-miR-214−/− mice (n = 3). B: representative images of TUNEL staining and quantitative analysis showing that apoptosis in renal ischemia-reperfusion is ameliorated in PT-miR-214−/− mice (n ≥ 5). Scale bar = 50 μm. I35/R48, 35 min of bilateral renal ischemia followed by 48 h of reperfusion. *P < 0.05; **P < 0.01; ***P < 0.001.
DISCUSSION
In the present study, we demonstrated a remarkable induction of miR-214 in ischemic AKI in mice and in an ATP depletion-associated tubular cell injury model. Functionally, we show that miR-214 overexpression promoted ATP depletion-associated apoptosis, whereas anti-miR-214 reduced apoptosis, in ATP-depleted RPTCs. Moreover, miR-214 deficiency in proximal tubules showed reduced renal apoptosis, less tubular damage, and better renal function in mice during renal ischemia-reperfusion, further providing evidence for the pathological role of miR-214 in ischemic AKI.
Mechanistically, we show that miR-214 induction promoted mitochondrial fragmentation. Mitochondrial fragmentation, as a result of the disruption of mitochondrial fission-fusion dynamics, is a critical pathogenic event in kidney tubular damage and apoptosis in both acute and chronic kidney diseases (8, 12, 16, 42, 44, 51). In the present study, anti-miR-214 abrogated mitochondrial fragmentation in RPTCs with ATP depletion, which was accompanied by reduced apoptosis, providing direct evidence for miR-214 functions as a cell injury factor via promoting mitochondrial fragmentation.
At the molecular level, our results suggest that miR-214 may promote mitochondrial fragmentation and kidney cell death at least partly by targeting Mfn2, an important mitochondrial fusion protein. In other cell types, there is evidence that Mfn2 may be a direct target gene of miR-214 (26, 47). In the present study, overexpression of miR-214 repressed Mfn2 expression both in human embryonic kidney cells (Fig. 3A) and RPTCs (Fig. 4A), and Mfn2 was increased in kidney tissues in PT-miR-214−/− mice compared with PT-miR-214+/+ mice (Fig. 7A), further verifying the regulation of Mfn2 by miR-214. In addition, anti-miR-214 could prevent Mfn2 downregulation in ATP-depleted RPTCs. Furthermore, the preservation of Mfn2 by anti-miR-214 was accompanied by reduced apoptosis, linking miR-214 regulation of Mfn2 to cell injury and death. Consistently, deletion of miR-214 from proximal tubules partially preserved Mfn2 in ischemic AKI and reduced renal apoptosis in mice (Fig. 7). Together, these results indicate that miR-214 is induced in conditions of ischemic AKI and, upon induction, miR-214 represses Mfn2, contributing to the disruption of mitochondrial dynamics to exacerbate kidney cell injury and death in ischemic AKI.
Disruption of mitochondrial dynamics is one of the pathogenic events leading to mitochondrial damage and cell death in various diseases (5, 10, 30, 49, 50). A critical molecular change is the activation of dynamin-related protein 1, a mitochondrial fission protein. However, changes in mitochondrial fusion proteins have also been demonstrated. For example, Bak, a proapoptotic Bcl-2 family member at the mitochondrial outer membrane, has been shown to dissociate from Mfn2 and associate with Mfn1 during cell stress, resulting in the arrest of mitochondrial fusion for mitochondrial fragmentation (9). Our recent work has indicated a role of Bax interacting factor-1 in proteolytic cleavage and inactivation of the mitochondrial inner membrane fusion protein optic atrophy 1, contributing to the cleavage of the inner membrane for mitochondrial fragmentation (12). In 2012, Funk and Schnellmann (14) documented the decrease of Mfn2 in ischemic AKI, although the underlying mechanism was not investigated. The present study has further verified Mfn2 downregulation in both in vitro and in vivo models of ischemic AKI and provided evidence for the involvement of miR-214 in this downregulation. Together, these studies provide new insights into the molecular mechanism of mitochondrial damage in cell injury and death.
Regarding the role of miR-214 in ischemic AKI, there is an apparent discrepancy between the present study and a recent study by Zhu et al. (53), in which miR-214 was examined in a rat model of ischemic AKI and in hypoxia-induced NRK-52E cells. miR-214 was induced in those models, an observation consistent with our results. However, in those models, miR-214 was shown to be protective against hypoxia-induced apoptosis in NRK-52E cells and ischemic AKI in rats. The discrepancy may be caused by the different models examined in these two studies, including different cell types (RPTCs vs. NRK-52E cells), different in vitro treatments (ATP depletion vs. hypoxia), and animal species (mouse vs. rat). In addition, for in vivo experiments, Zhu et al. (53) administered miR-214 via intraperitoneal injection, whereas we examined and compared proximal tubule-specific miR-214-deficient versus proficient mice. Therefore, the systemic effect of miR-214 and the renal tubule-specific effect may be different. This is particularly important to consider in view of the multiple potential targets of miR-214 in various disease conditions.
In addition to Mfn2, multiple target genes have been reported for miR-214. For example, in a recent study, Bai et al. (3) showed that miR-214 may regulate mitochondrial encoded NADH dehydrogenase 6 and 4L, resulting in mitochondrial oxidative disruption in experimental models of chronic kidney disease, whereas Zhu et al. (53) suggested that miR-214 may target Dickkopf WNT signaling pathway inhibitor 3 in ischemic AKI. Considering the documented pathological role of miR-214 in multiple kidney diseases, it is important to elucidate the specific target genes of miR-214 in these diseases in future studies to gain an indepth understanding of the pathogenesis and design new therapeutic approaches.
Although the present study was focused on acute injury, miR-214 was persistently induced after renal ischemia-reperfusion (Fig. 1), suggesting a possible regulation of kidney repair after injury. The persistent induction of miR-214 in renal ischemia-reperfusion may suggest a possible regulation of the AKI to chronic kidney disease transition by this microRNA as well. In fact, miR-214 has been shown to participate in renal fibrosis in unilateral ureteral obstruction (13) and disruption of mitochondrial oxidation (3).
In conclusion, this study has demonstrated miR-214 induction in ischemic AKI in mice and in ATP-depleted renal tubular cells. Upon induction, miR-214 may repress the mitochondrial fusion protein Mfn2 to disturb mitochondrial dynamics to promote mitochondrial fragmentation and tubular cell death, contributing to ischemic AKI. Identification of the miR-214/Mfn2 axis in ischemic AKI adds new understanding to mitochondrial damage under this disease condition and suggests new therapeutic targets for ischemic AKI.
GRANTS
This work was supported in part by Department of Veterans Affairs Merit Review Award I01 BX000319 and National Institute of Diabetes and Digestive and Kidney Diseases Grants DK-058831 and DK-087843. Z. Dong is a recipient of the Senior Research Career Scientist award from the Department of Veterans Affairs.
DISCLOSURES
No conflicts of interest, financial or otherwise, are declared by the authors.
AUTHOR CONTRIBUTIONS
Y.Y., Z.M., and Z.D. conceived and designed research; Y.Y. and Z.M. performed experiments; Y.Y., Z.M., J.Z., M.Z., and H.L. analyzed data; Y.Y., Z.M., J.Z., M.Z., H.L., and Z.D. interpreted results of experiments; Y.Y. and Z.M. prepared figures; Y.Y. and Z.M. drafted manuscript; H.L. and Z.D. edited and revised manuscript; Y.Y., Z.M., J.Z., M.Z., H.L., and Z.D. approved final version of manuscript.
ACKNOWLEDGMENTS
We thank Dr. Volker Haase (Vanderbilt University Medical Center) for providing the phosphoenolpyruvate carboxykinase-Cre mouse line and Dr. Eric Olson (University of Texas Southwestern Medical Center, Dallas, TX) for providing the miR-214 floxed mouse line.
REFERENCES
- 1.Aurora AB, Mahmoud AI, Luo X, Johnson BA, van Rooij E, Matsuzaki S, Humphries KM, Hill JA, Bassel-Duby R, Sadek HA, Olson EN. MicroRNA-214 protects the mouse heart from ischemic injury by controlling Ca2+ overload and cell death. J Clin Invest 122: 1222–1232, 2012. doi: 10.1172/JCI59327. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Ayanga BA, Badal SS, Wang Y, Galvan DL, Chang BH, Schumacker PT, Danesh FR. Dynamin-related protein 1 deficiency improves mitochondrial fitness and protects against progression of diabetic nephropathy. J Am Soc Nephrol 27: 2733–2747, 2016. doi: 10.1681/ASN.2015101096. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Bai M, Chen H, Ding D, Song R, Lin J, Zhang Y, Guo Y, Chen S, Ding G, Zhang Y, Jia Z, Huang S, He JC, Yang L, Zhang A. MicroRNA-214 promotes chronic kidney disease by disrupting mitochondrial oxidative phosphorylation. Kidney Int 95: 1389–1404, 2019. doi: 10.1016/j.kint.2018.12.028. [DOI] [PubMed] [Google Scholar]
- 4.Bera A, Das F, Ghosh-Choudhury N, Mariappan MM, Kasinath BS, Ghosh Choudhury G. Reciprocal regulation of miR-214 and PTEN by high glucose regulates renal glomerular mesangial and proximal tubular epithelial cell hypertrophy and matrix expansion. Am J Physiol Cell Physiol 313: C430–C447, 2017. doi: 10.1152/ajpcell.00081.2017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Bhargava P, Schnellmann RG. Mitochondrial energetics in the kidney. Nat Rev Nephrol 13: 629–646, 2017. doi: 10.1038/nrneph.2017.107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Bhatt K, Wei Q, Pabla N, Dong G, Mi QS, Liang M, Mei C, Dong Z. MicroRNA-687 induced by hypoxia-inducible factor-1 targets phosphatase and tensin homolog in renal ischemia-reperfusion injury. J Am Soc Nephrol 26: 1588–1596, 2015. doi: 10.1681/ASN.2014050463. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Bonventre JV, Yang L. Cellular pathophysiology of ischemic acute kidney injury. J Clin Invest 121: 4210–4221, 2011. doi: 10.1172/JCI45161. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Brooks C, Wei Q, Cho SG, Dong Z. Regulation of mitochondrial dynamics in acute kidney injury in cell culture and rodent models. J Clin Invest 119: 1275–1285, 2009. doi: 10.1172/JCI37829. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Brooks C, Wei Q, Feng L, Dong G, Tao Y, Mei L, Xie ZJ, Dong Z. Bak regulates mitochondrial morphology and pathology during apoptosis by interacting with mitofusins. Proc Natl Acad Sci USA 104: 11649–11654, 2007. doi: 10.1073/pnas.0703976104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Chan DC. Fusion and fission: interlinked processes critical for mitochondrial health. Annu Rev Genet 46: 265–287, 2012. doi: 10.1146/annurev-genet-110410-132529. [DOI] [PubMed] [Google Scholar]
- 11.Che R, Yuan Y, Huang S, Zhang A. Mitochondrial dysfunction in the pathophysiology of renal diseases. Am J Physiol Renal Physiol 306: F367–F378, 2014. doi: 10.1152/ajprenal.00571.2013. [DOI] [PubMed] [Google Scholar]
- 12.Cho SG, Xiao X, Wang S, Gao H, Rafikov R, Black S, Huang S, Ding HF, Yoon Y, Kirken RA, Yin XM, Wang HG, Dong Z. Bif-1 interacts with prohibitin-2 to regulate mitochondrial inner membrane during cell stress and apoptosis. J Am Soc Nephrol 30: 1174–1191, 2019. doi: 10.1681/ASN.2018111117. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Denby L, Ramdas V, Lu R, Conway BR, Grant JS, Dickinson B, Aurora AB, McClure JD, Kipgen D, Delles C, van Rooij E, Baker AH. MicroRNA-214 antagonism protects against renal fibrosis. J Am Soc Nephrol 25: 65–80, 2014. doi: 10.1681/ASN.2013010072. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Funk JA, Schnellmann RG. Persistent disruption of mitochondrial homeostasis after acute kidney injury. Am J Physiol Renal Physiol 302: F853–F864, 2012. doi: 10.1152/ajprenal.00035.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Gall JM, Wang Z, Bonegio RG, Havasi A, Liesa M, Vemula P, Borkan SC. Conditional knockout of proximal tubule mitofusin 2 accelerates recovery and improves survival after renal ischemia. J Am Soc Nephrol 26: 1092–1102, 2015. doi: 10.1681/ASN.2014010126. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Galvan DL, Long J, Green N, Chang BH, Lin JS, Schumacker P, Truong LD, Overbeek P, Danesh FR. Drp1S600 phosphorylation regulates mitochondrial fission and progression of nephropathy in diabetic mice. J Clin Invest 129: 2807–2823, 2019. doi: 10.1172/JCI127277. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Guo C, Dong G, Liang X, Dong Z. Epigenetic regulation in AKI and kidney repair: mechanisms and therapeutic implications. Nat Rev Nephrol 15: 220–239, 2019. doi: 10.1038/s41581-018-0103-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Guo X, Disatnik MH, Monbureau M, Shamloo M, Mochly-Rosen D, Qi X. Inhibition of mitochondrial fragmentation diminishes Huntington’s disease-associated neurodegeneration. J Clin Invest 123: 5371–5388, 2013. doi: 10.1172/JCI70911. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Guo Y, Ni J, Chen S, Bai M, Lin J, Ding G, Zhang Y, Sun P, Jia Z, Huang S, Yang L, Zhang A. MicroRNA-709 mediates acute tubular injury through effects on mitochondrial function. J Am Soc Nephrol 29: 449–461, 2018. doi: 10.1681/ASN.2017040381. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Hao J, Wei Q, Mei S, Li L, Su Y, Mei C, Dong Z. Induction of microRNA-17-5p by p53 protects against renal ischemia-reperfusion injury by targeting death receptor 6. Kidney Int 91: 106–118, 2017. doi: 10.1016/j.kint.2016.07.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Havasi A, Borkan SC. Apoptosis and acute kidney injury. Kidney Int 80: 29–40, 2011. doi: 10.1038/ki.2011.120. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Kato M, Natarajan R. Epigenetics and epigenomics in diabetic kidney disease and metabolic memory. Nat Rev Nephrol 15: 327–345, 2019. doi: 10.1038/s41581-019-0135-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Li H, Ma Y, Chen B, Shi J. miR-182 enhances acute kidney injury by promoting apoptosis involving the targeting and regulation of TCF7L2/Wnt/β-catenins pathway. Eur J Pharmacol 831: 20–27, 2018. doi: 10.1016/j.ejphar.2018.05.001. [DOI] [PubMed] [Google Scholar]
- 24.Linkermann A, Chen G, Dong G, Kunzendorf U, Krautwald S, Dong Z. Regulated cell death in AKI. J Am Soc Nephrol 25: 2689–2701, 2014. doi: 10.1681/ASN.2014030262. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Liu Z, Yang Q, Wei Q, Chang Y, Qu M, Yu L. The protective effect of miR-377 inhibitor against renal ischemia-reperfusion injury through inhibition of inflammation and oxidative stress via a VEGF-dependent mechanism in mice. Mol Immunol 106: 153–158, 2019. doi: 10.1016/j.molimm.2018.12.028. [DOI] [PubMed] [Google Scholar]
- 26.Lv JW, Wen W, Jiang C, Fu QB, Gu YJ, Lv TT, Li ZD, Xue W. Inhibition of microRNA-214 promotes epithelial-mesenchymal transition process and induces interstitial cystitis in postmenopausal women by upregulating Mfn2. Exp Mol Med 49: e357, 2017. doi: 10.1038/emm.2017.98. [DOI] [PMC free article] [PubMed] [Google Scholar] [Retracted]
- 27.Ma Z, Wei Q, Dong G, Huo Y, Dong Z. DNA damage response in renal ischemia-reperfusion and ATP-depletion injury of renal tubular cells. Biochim Biophys Acta 1842: 1088–1096, 2014. doi: 10.1016/j.bbadis.2014.04.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Ma Z, Wei Q, Zhang M, Chen JK, Dong Z. Dicer deficiency in proximal tubules exacerbates renal injury and tubulointerstitial fibrosis and upregulates Smad2/3. Am J Physiol Renal Physiol 315: F1822–F1832, 2018. doi: 10.1152/ajprenal.00402.2018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Mallipattu SK, Horne SJ, D’Agati V, Narla G, Liu R, Frohman MA, Dickman K, Chen EY, Ma’ayan A, Bialkowska AB, Ghaleb AM, Nandan MO, Jain MK, Daehn I, Chuang PY, Yang VW, He JC. Krüppel-like factor 6 regulates mitochondrial function in the kidney. J Clin Invest 125: 1347–1361, 2015. doi: 10.1172/JCI77084. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Nunnari J, Suomalainen A. Mitochondria: in sickness and in health. Cell 148: 1145–1159, 2012. doi: 10.1016/j.cell.2012.02.035. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Ong SB, Subrayan S, Lim SY, Yellon DM, Davidson SM, Hausenloy DJ. Inhibiting mitochondrial fission protects the heart against ischemia/reperfusion injury. Circulation 121: 2012–2022, 2010. doi: 10.1161/CIRCULATIONAHA.109.906610. [DOI] [PubMed] [Google Scholar]
- 32.Pan JS, Sheikh-Hamad D. Mitochondrial dysfunction in acute kidney injury and sex-specific implications. Med Res Arch 7: 1898, 2019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Parikh SM, Yang Y, He L, Tang C, Zhan M, Dong Z. Mitochondrial function and disturbances in the septic kidney. Semin Nephrol 35: 108–119, 2015. doi: 10.1016/j.semnephrol.2015.01.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Perry HM, Huang L, Wilson RJ, Bajwa A, Sesaki H, Yan Z, Rosin DL, Kashatus DF, Okusa MD. Dynamin-related protein 1 deficiency promotes recovery from AKI. J Am Soc Nephrol 29: 194–206, 2018. doi: 10.1681/ASN.2017060659. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Ralto KM, Parikh SM. Mitochondria in acute kidney injury. Semin Nephrol 36: 8–16, 2016. doi: 10.1016/j.semnephrol.2016.01.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Rankin EB, Tomaszewski JE, Haase VH. Renal cyst development in mice with conditional inactivation of the von Hippel-Lindau tumor suppressor. Cancer Res 66: 2576–2583, 2006. doi: 10.1158/0008-5472.CAN-05-3241. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Sharfuddin AA, Molitoris BA. Pathophysiology of ischemic acute kidney injury. Nat Rev Nephrol 7: 189–200, 2011. doi: 10.1038/nrneph.2011.16. [DOI] [PubMed] [Google Scholar]
- 38.Song N, Zhang T, Xu X, Lu Z, Yu X, Fang Y, Hu J, Jia P, Teng J, Ding X. miR-21 protects against ischemia/reperfusion-induced acute kidney injury by preventing epithelial cell apoptosis and inhibiting dendritic cell maturation. Front Physiol 9: 790, 2018. doi: 10.3389/fphys.2018.00790. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Sun J, Zhang J, Tian J, Virzì GM, Digvijay K, Cueto L, Yin Y, Rosner MH, Ronco C. Mitochondria in sepsis-induced AKI. J Am Soc Nephrol 30: 1151–1161, 2019. doi: 10.1681/ASN.2018111126. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Sun M, Yu H, Zhang Y, Li Z, Gao W. MicroRNA-214 mediates isoproterenol-induced proliferation and collagen synthesis in cardiac fibroblasts. Sci Rep 5: 18351, 2015. doi: 10.1038/srep18351. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Szeto HH. Pharmacologic approaches to improve mitochondrial function in AKI and CKD. J Am Soc Nephrol 28: 2856–2865, 2017. doi: 10.1681/ASN.2017030247. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Wang W, Wang Y, Long J, Wang J, Haudek SB, Overbeek P, Chang BH, Schumacker PT, Danesh FR. Mitochondrial fission triggered by hyperglycemia is mediated by ROCK1 activation in podocytes and endothelial cells. Cell Metab 15: 186–200, 2012. doi: 10.1016/j.cmet.2012.01.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Wei Q, Bhatt K, He HZ, Mi QS, Haase VH, Dong Z. Targeted deletion of Dicer from proximal tubules protects against renal ischemia-reperfusion injury. J Am Soc Nephrol 21: 756–761, 2010. doi: 10.1681/ASN.2009070718. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Wei Q, Liu Y, Liu P, Hao J, Liang M, Mi QS, Chen JK, Dong Z. MicroRNA-489 induction by hypoxia-inducible factor-1 protects against ischemic kidney injury. J Am Soc Nephrol 27: 2784–2796, 2016. doi: 10.1681/ASN.2015080870. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Wei Q, Sun H, Song S, Liu Y, Liu P, Livingston MJ, Wang J, Liang M, Mi QS, Huo Y, Nahman NS, Mei C, Dong Z. MicroRNA-668 represses MTP18 to preserve mitochondrial dynamics in ischemic acute kidney injury. J Clin Invest 128: 5448–5464, 2018. doi: 10.1172/JCI121859. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Wu J, Li J, Chen WK, Liu S, Liu JH, Zhang JS, Fang KW. MicroRNA-214 affects fibroblast differentiation of adipose-derived mesenchymal stem cells by targeting mitofusin-2 during pelvic floor dysfunction in SD rats with birth trauma. Cell Physiol Biochem 42: 1870–1887, 2017. doi: 10.1159/000479570. [DOI] [PubMed] [Google Scholar]
- 48.Ying Y, Padanilam BJ. Regulation of necrotic cell death: p53, PARP1 and cyclophilin D-overlapping pathways of regulated necrosis? Cell Mol Life Sci 73: 2309–2324, 2016. doi: 10.1007/s00018-016-2202-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Youle RJ, van der Bliek AM. Mitochondrial fission, fusion, and stress. Science 337: 1062–1065, 2012. doi: 10.1126/science.1219855. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Zhan M, Brooks C, Liu F, Sun L, Dong Z. Mitochondrial dynamics: regulatory mechanisms and emerging role in renal pathophysiology. Kidney Int 83: 568–581, 2013. doi: 10.1038/ki.2012.441. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Zhan M, Usman IM, Sun L, Kanwar YS. Disruption of renal tubular mitochondrial quality control by Myo-inositol oxygenase in diabetic kidney disease. J Am Soc Nephrol 26: 1304–1321, 2015. doi: 10.1681/ASN.2014050457. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Zhang XB, Chen X, Li DJ, Qi GN, Dai YQ, Gu J, Chen MQ, Hu S, Liu ZY, Yang ZM. Inhibition of miR-155 ameliorates acute kidney injury by apoptosis involving the regulation on TCF4/Wnt/β-Catenin pathway. Nephron 143: 135–147, 2019. doi: 10.1159/000501038. [DOI] [PubMed] [Google Scholar]
- 53.Zhu X, Li W, Li H. miR-214 ameliorates acute kidney injury via targeting DKK3 and activating of Wnt/β-catenin signaling pathway. Biol Res 51: 31, 2018. doi: 10.1186/s40659-018-0179-2. [DOI] [PMC free article] [PubMed] [Google Scholar]