Abstract
Cisplatin is a widely used chemotherapy drug with notorious nephrotoxicity. Na+-glucose cotransporter 2 inhibitors are a class of novel antidiabetic agents that may have other effects in the kidneys besides blood glucose control. In the present study, we demonstrated that canagliflozin significantly attenuates cisplatin-induced nephropathy in C57BL/6 mice and suppresses cisplatin induced renal proximal tubular cell apoptosis in vitro. The protective effect of canagliflozin was associated with inhibition of p53, p38 and JNK activation. Mechanistically, canagliflozin partially reduced cisplatin uptake by kidney tissues in mice and renal tubular cells in culture. In addition, canagliflozin enhanced the activation of Akt and inhibited the mitochondrial pathway of apoptosis during cisplatin treatment. The protective effect of canagliflozin was diminished by the phosphatidylinositol 3-kinase/Akt inhibitor LY294002. Notably, canagliflozin did not affect the chemotherapeutic efficacy of cisplatin in A549 and HCT116 cancer cell lines. These results suggest a new application of canagliflozin for renoprotection in cisplatin chemotherapy. Canagliflozin may protect kidneys by reducing cisplatin uptake and activating cell survival pathways.
Keywords: acute kidney injury, Akt, canagliflozin, cisplatin, nephrotoxicity
INTRODUCTION
Cisplatin is one of the most commonly used chemotherapy agents for the treatment of solid tumors such as non-small cell lung cancer, ovarian cancer, and prostate cancer (22, 27, 38). However, the use of cisplatin is limited by its side effects, including nephrotoxicity. Clinically, more than 25% patients develop nephrotoxicity including acute kidney injury (AKI) after cisplatin administration (4, 27). In the past few decades, researches have indicated the involvement of multiple signaling pathways and factors in cisplatin-induced nephrotoxicity including tubular cell injury and death (12, 22, 27, 44). Despite those intensive studies, the effective approach to block nephrotoxicity during cisplatin-based chemotherapy is lacking.
Na+-glucose cotransporter 2 (SGLT2) inhibitors are newly developed antidiabetic drugs that reduce glucose reabsorption by specifically inhibiting SGLT2 in renal proximal tubules. In addition to their efficacy in glycemic control and glycosylated hemoglobin reduction, recent studies have indicated that SGLT2 inhibitors have beneficial effects in the kidneys, including those regarding estimated glomerular filtration rate, slowdown of albuminuria progression and onset of renal replacement therapy, and a reduction of mortality by renal causes in patients with type 2 diabetes (9, 25, 30, 40, 42). The potential underlying mechanism may involve the regulation of nonglycemic pathways, including the modulation of glomerular hemodynamics and the change of renal metabolism and energy requirement (42). Those changes can lead to the inhibition of renal inflammation, oxidative stress, and endoplasmic reticulum stress (8, 20, 36), suggesting potential benefits of SGLT2 inhibitors in other renal injury conditions beyond diabetes, which needs further investigation.
Along with the use of SGLT2 inhibitors in clinical practice, their effects of suppressing cancer progression have also been reported (32, 35, 36). Can the combined use of SGLT2 inhibitors protect the kidneys while suppressing tumor growth during cisplatin chemotherapy? To answer this question, we examined the effect of canagliflozin (a clinically used SGLT2 inhibitor) on cisplatin-induced nephrotoxicity and cancer cell death. Our results demonstrate the significant renal protection of canagliflozin against cisplatin-induced nephrotoxicity without obvious effects on cancer cell death. The renal protective effect of canagliflozin may depend on the reduced uptake of cisplatin in kidney and Akt activation. This novel pharmacological function of canagliflozin implicates its potential use in combinational therapy with cisplatin for patients with cancer.
MATERIALS AND METHODS
Reagents and antibodies.
Canagliflozin was purchased from Cayman Chemical (Ann Arbor, MI). Cisplatin and sodium carboxymethyl cellulose were purchased from Sigma-Aldrich (St. Louis, MO). LY294002 was from Cell Signaling Technology. Primary antibodies were from the following sources: cleaved caspase-3, poly(ADP-ribose) polymerase-1 (PARP-1), p53, phospho-p53 (Ser15), JNK, phospho-JNK (Thr183/Tyr185), ERK1/2, phospho-ERK1/2 (Thr202/Tyr204), p38, phospho-p38 (Thr180/Tyr182), phospho-Akt (Ser473), Akt, p53-upregulated modulator of apoptosis (PUMA), Bax, cyclophilin B, GAPDH, and heat shock protein 60 antibodies were from Cell Signaling Technology (Danvers, MA); cytochrome c antibody was from BD Pharmingen. All secondary antibodies were from Jackson ImmunoResearch (West Grove, PA).
Animals and cisplatin treatment.
All animal procedures were performed under an animal protocol approved by the Institutional Animal Care and Use Committee of Augusta University. Male C57BL/6 mice were purchased from Jackson Laboratory (Bar Harbor, ME). Animals were housed with a 12:12-h light-dark cycle and fed with standard diet and tap water ad libitum in controlled room temperature (22 ± 1°C) and humidity (50 ± 5%) in the animal facility of Augusta University. Mice (8–10 wk old) were used and randomly separated into four groups. For cisplatin treatment, mice were administered with a dose of cisplatin (30 mg/kg ip) as described in a previous study (26, 37, 44). Canagliflozin dissolved in 0.1% carboxymethyl cellulose (55) or vehicle solution was delivered by oral gavage for 7 consecutive days before cisplatin treatment and then 1 day after cisplatin treatment. Control animals without cisplatin treatment were injected with a comparable volume of saline. Animals were euthanized at 72 h after treatment to collect blood and kidneys for analysis.
Measurement of renal function.
Blood urea nitrogen (BUN) and serum creatinine were measured as previously described (45). Briefly, blood samples were collected from abdominal vessels at the time of euthanasia. Serum was collected by centrifugation after clotting at room temperature. BUN and serum creatine were determined with a kit from Stanbio Laboratory (Boerne, TX).
Renal histology.
Fresh kidneys were collected and fixed with 4% paraformaldehyde overnight at 4°C for paraffin embedding. The paraffin-embedded tissues were sliced into 4-µm sections and stained with hematoxylin and eosin. Tubular damage was indicated by loss of the brush border, cast formation, and cell lysis. The kidney damage score was obtained by histopathologist in a blind manner. Histological damage was scored by renal tubular injury estimation as follows: 0, no damage; 1, <25%; 2, 25–50%; 3, 50 –75%; and 4, >75%.
Cell culture and treatments.
Rat kidney proximal tubular cells (RPTCs) were originally obtained from Dr. Ulrich Hopfer (Case Western Reserve University). RPTCs were cultured in Ham’s F-12-DMEM supplemented with 10% FBS as previously described (46). RPTCs were seeded in 35-mm collagen-coated dishes to reach more than 90% confluence by the next day. Cells were treated as incubated with or without 20 µM cisplatin for 16 h in the presence or absence of 15 μM canagliflozin. Cells were analyzed by morphology for apoptosis and collected for further biochemical analyses.
Examination of apoptosis in kidney tissues and cell cultures.
Apoptosis in renal tissue was detected by TUNEL assay using the In Situ Cell Death Detection kit (Roche Applied Science, Indianapolis, IN) (43, 44). For quantification, positive cells were counted under a fluorescence microscope in a random, blinded fashion in 10–20 nonoverlapping fields from each section. The apoptosis rate in RPTCs was analyzed morphologically. Cell nuclei were stained by Hoechst33342. Cell morphology was assessed by phase-contrast and fluorescence microscopy. The percentage of cells with typical apoptotic morphology as previously described (18, 44) was estimated in 4 fields with ~200 cells/field.
Measurement of caspase activity.
The enzymatic activity of caspase was measured using DEVD-AFC, a fluorogenic peptide substrate of caspases, as previously described (18). Briefly, whole cell lysate was collected with 1% Triton X-100 and incubated with enzymatic reactions to convert DEVD-AFC to free AFC. After 1 h of reactions at 37°C, the fluorescence at 360-nm excitation/530-nm emission was measured by a GENios plate-reader (Tecan US) to examine free AFC released. Caspase activity was calculated as the release of nanomoles of free AFC per hour per milligram of protein based on the standard curve.
Platinum measurement.
Cells were incubated with cisplatin for 12 h in the absence or presence of canagliflozin and lysed in a lysis buffer containing 0.5% SDS with proteinase K and benzonase. Lysates were incubated at 60°C for 2 h followed by 37°C incubation overnight. Subsequently, these lysates were further dissolved in 70% nitric acid. Similarly, the kidneys with or without canagliflozin at 1 day or 3 days after cisplatin administration were homogenized in lysis buffer and further dissolved in nitric acid. Cells or kidneys without cisplatin treatment were used as blanks for measurement. All samples were analyzed by Optima 8000 ICP-OES in the Chemical and Biomolecular Analysis Facility at Augusta University. The results are shown as nanograms of platinum per milligram of protein.
Cellular fractionation.
To examine Bax and cytochrome c translocation, cells were fractionated into cytosolic and membrane-bound organellar fractions using buffer containing a low concentration of digitonin (13, 17). Briefly, cells were incubated with 0.05% digitonin in isotonic sucrose buffer (250 mM sucrose, 10 mM HEPES-NaOH, 10 mM KCl, 1.5 mM MgCl2, 1 mM EDTA, 1 mM EGTA, and 0.5 mM proteinase inhibitor cocktail; pH 7.2) for 3 min at room temperature as previously described (5, 26). The soluble extract was collected as the cytosolic fraction. The digitonin-insoluble part was washed with isotonic buffer and further dissolved in SDS buffer as the mitochondria-rich organellar fraction.
Immunoblot analysis.
The protein concentration of cultured cells or kidney lysates in SDS lysis buffer was determined using Pierce bicinchoninic acid reagent (ThermoFisher Scientific). Equal amounts of protein samples were loaded for SDS-PAGE followed by electroblotting to PVDF membrane. After being blocked in 5% nonfat milk or BSA, the blots were incubated with primary antibody in 4°C overnight followed by horseradish peroxidase-conjugated secondary antibody incubation. The blots were then incubated with Super Signal West Pico Chemiluminescent Substrate (ThermoFisher Scientific) to record signals by scanning with MyECL Imager (ThermoFisher Scientific) or KwikQuant Imager (Kindle Bioscience).
Immunofluorescence staining.
Cells grown on coverslips and fresh kidney tissue were fixed with 4% paraformaldehyde at room temperature. Paraffin-embedded renal sections were rehydrated and steamed for 1 h in sodium citrate buffer for antigen retrieval. The fixed cells or rehydrated tissue sections were then permeabilized with Triton X-100 followed by 1 h of blocking. Cells or tissue sections were subsequently exposed to primary antibodies at 4°C overnight followed by FITC-labeled or Cy3-labeled secondary antibody incubation. The signal was examined by Zeiss 780 Upright confocal microscopy in the Image Core Facility at Augusta University.
Statistics.
Data are expressed as means ± SD. GraphPad Prism 8 was used for statistical analysis. Student’s t test was used to show the statistical differences between two groups, and ANOVA was used for multigroup statistical differences analysis. P values of <0.05 were considered significantly different.
RESULTS
Canagliflozin attenuates cisplatin-induced nephrotoxicity.
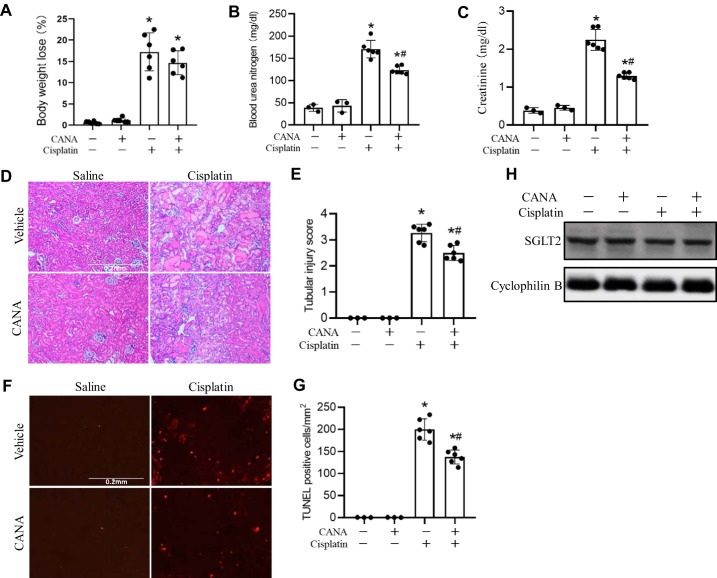
To investigate the effect of canagliflozin on cisplatin-induced nephropathy, C57BL/6 mice were pretreated with canagliflozin (20 mg·kg−1·day−1) orally for 7 days before cisplatin injection and posttreated with canagliflozin 1 day after cisplatin injection. Cisplatin induced significant toxicity in mice, as indicated by less activity and loss of body weight at 72 h (Fig. 1A). Kidney injury was indicated by the loss of renal function with significantly elevated BUN (Fig. 1B) and serum creatinine (Fig. 1C). Notably, canagliflozin reduced BUN from ~171 to ~124 mL/dL and serum creatinine from ~2.25 to ~1.29 mg/dL in cisplatin-treated mice. (Fig. 1, A and B). In renal histology, cisplatin induced severe renal damage, including hyaline cast formation, vacuolization, and renal tubule lysis in kidney tissues, which was significantly suppressed by canagliflozin (Fig. 1D), and the tubular injury score was reduced from 3.3 to 2.5 (Fig. 1E). Kidney cell apoptosis was detected after cisplatin treatment (Fig. 1F). TUNEL staining revealed remarkable apoptosis in the cortex and outer medullar area after cisplatin treatment. Quantification of TUNEL-positive cells showed that cisplatin-induced renal cell death was reduced from 200 to 138 cells/mm2 with canagliflozin treatment (Fig. 1F). Cisplatin or canagliflozin treatment did not significantly affect SGLT2 expression in mice (Fig. 1G). Based on occasional tests, canagliflozin did not significantly affect blood glucose, but it had an obvious diuretic effect after several days of treatment (not shown).
Fig. 1.
Protective effect of canagliflozin (CANA) on cisplatin-induced nephrotoxicity. C57BL/6 mice were administered with CANA (20 mg·kg−1·day−1) orally for 8 consecutive days, and intraperitoneal injection of 30 mg/kg cisplatin or saline was done on the seventh day of treatment. Mice were euthanized at 72 h after cisplatin treatment to collect kidney tissues for histology and blood samples for measurements of blood urea nitrogen and creatinine. A: body weight loss. B: blood urea nitrogen. C: serum creatinine. D: representative histology of hematoxylin and eosin staining (scale bar = 0.2 mm). E: histology scores of tubular injury. F: representative images of TUNEL staining (scale bar = 0.2 mm). G: quantification of TUNEL-positive cells in kidney tissues. H: immunoblots of Na+-glucose cotransporter 2 (SGLT2). Immunoblots are representative of 3 independent experiments. Cyclophilin B was used as the internal loading control. Data are expressed as means ± SD; n = 3 for control groups and n = 6 for treatment groups. *P < 0.05 vs. control groups; #P < 0.05 vs. the cisplatin-treated group with CANA.
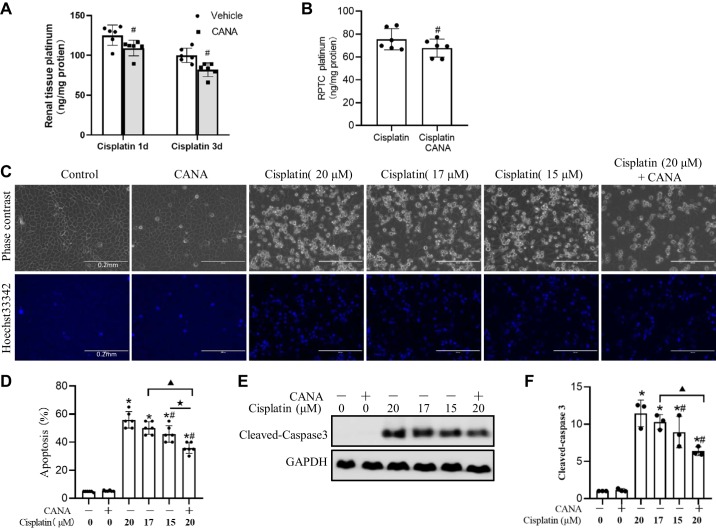
Canagliflozin ameliorates cisplatin-induced proximal tubular apoptosis in vitro.
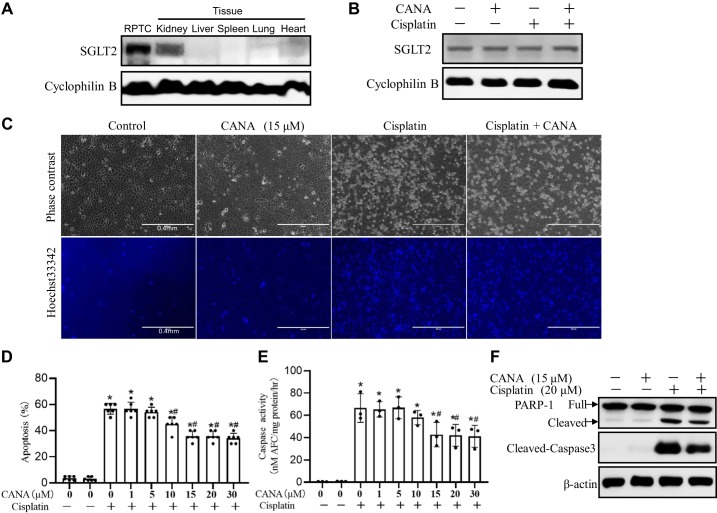
The proximal tubule is the major targeted renal compartment in the kidney after cisplatin injury. Thus, we examined whether canagliflozin can protect cisplatin-induced proximal tubular cell apoptosis in vitro. RPTCs were incubated with 20 μM cisplatin in the presence or absence of canagliflozin. Immunoblot analysis showed SGLT2 expression in RPTCs (Fig. 2A), which was not changed by cisplatin or canagliflozin treatment (Fig. 2B). Cisplatin induced ~55% apoptosis after 16 h, which was inhibited by canagliflozin in a dose-dependent manner (Fig. 2, A and B). The antiapoptotic effect of canagliflozin started from 10 μM and reached maximum at 15 μM, which was confirmed by both morphological assessment (Fig. 2B) and caspase activity measurement (Fig. 2C). The inhibition of cisplatin-induced RPTC apoptosis by 15 μM canagliflozin was confirmed by morphological examination (Fig. 2, A and B), caspase activity (Fig. 2C), and the reduction of caspase 3 and PARP-1 cleavage (Fig. 2D).
Fig. 2.
Canagliflozin (CANA) attenuates cisplatin-induced apoptosis in rat kidney proximal tubular cells (RPTCs). RPTCs were treated with 20 μM cisplatin in the absence or presence of CANA. A: immunoblots of Na+-glucose cotransporter 2 (SGLT2) in RPTCs and kidney, liver, spleen, and heart tissues. B: immunoblots of SGLT2. RPTCs with or without CANA were treated with 20 μM cisplatin or vehicle for 16 h. Cyclophilin B was used as an internal loading control. C: representative images of cell morphology and nuclear morphology by Hoechst 33342 staining (scale bar = 0.4 mm). D: percentage of apoptosis (n = 6). E: caspase activity. Data are expressed as means ± SD; n = 3. F: immunoblots of cleaved caspase-3 and poly(ADP-ribose) polymerase-1 (PARP-1). β-Actin was used as the internal loading control. Immunoblots are representative of at least 3 independent experiments. *P < 0.05 vs. control groups; #P < 0.05 vs. the cisplatin-treated group without CANA.
Canagliflozin suppresses cisplatin-induced p53 phosphorylation and MAPK signaling activation.
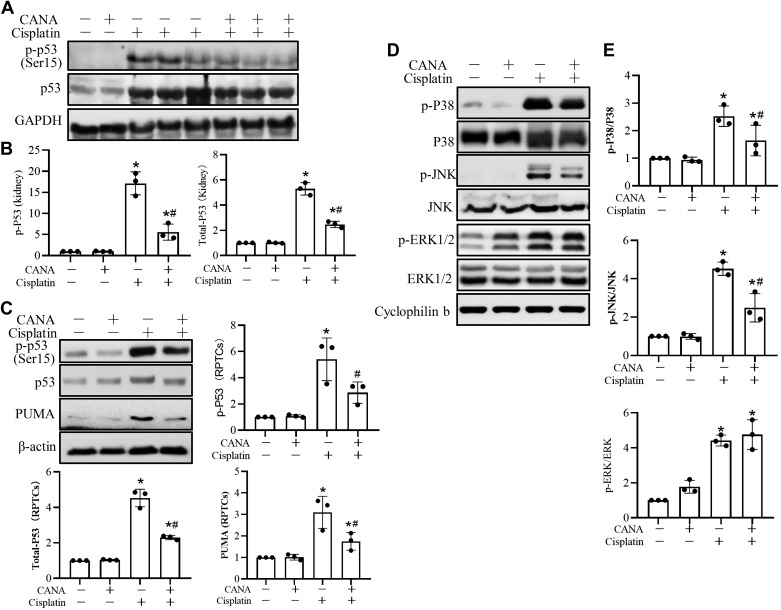
Our previous studies have implicated a critical role of p53 phosphorylation and upregulation in cisplatin-induced kidney injury (17, 44, 53). Thus, we examined the effect of canagliflozin on p53 activation during cisplatin treatment. As shown in Fig. 3, A and B, cisplatin induced p53 upregulation and phosphorylation in the kidney in mice, which were significantly suppressed by canagliflozin treatment. Similarly, canagliflozin suppressed the induction of p53 and its downstream target gene, PUMA (17), during cisplatin treatment of RPTCs (Fig. 3C). Thus, both in vivo and in vitro evidence indicated inhibition of the p53 pathway by canagliflozin.
Fig. 3.
Canagliflozin (CANA) suppresses cisplatin-induced p53 and MAPK activation. A: representative immunoblots of total p53 and phosphorylated (p-)p53 (Ser15) in mouse kidneys. C57BL/6 mice with or without CANA were treated with 30 mg/kg cisplatin or saline for 3 days. Kidney cortical tissue lysates were extracted for analysis. GAPDH was used as an internal loading control. B: densitometric analysis of p53 and p-p53 (Ser15) normalized to GAPDH. C: representative immunoblots of total p53, p-p53 (Ser15), and p53-upregulated modulator of apoptosis (PUMA) in rat kidney proximal tubular cells (RPTCs) with densitometric analysis. RPTCs were treated with 15 μM CANA and coincubated with or without 20 μM cisplatin for 16 h to collect cell lysates for immunoblot analysis normalized by β-actin, the internal control. Values are means ± SD; n = 3. D: representative immunoblots of p-p38, total p38, p-JNK, total JNK, p-ERK1/2, and total ERK1/2 in RPTCs. Cyclophilin B was used as an internal loading control. E: densitometry analysis of p-p38 normalized to p38, p-JNK normalized to JNK, and p-ERK normalized to ERK. Data are expressed as means ± SD; n = 3. *P < 0.05 vs. the control group; #P < 0.05 vs. the cisplatin-treated group without CANA.
In addition to p53, activation of the MAPK signaling pathway has been reported to be associated with cisplatin-induced renal injury (3, 31, 39, 50, 52). We subsequently analyzed the effects of canagliflozin on MAPK activation. As shown in Fig. 3, D and E, cisplatin induced phosphorylation of p38, JNK, and ERK1/2, whereas expression levels of total ERK, JNK, and p38 proteins were not changed. Canagliflozin suppressed the phosphorylation of p38 and JNK but not ERK1/2 (Fig. 3, D and E).
Canagliflozin suppresses Bax translocation and cytochrome c release in cisplatin-induced RPTC injury.
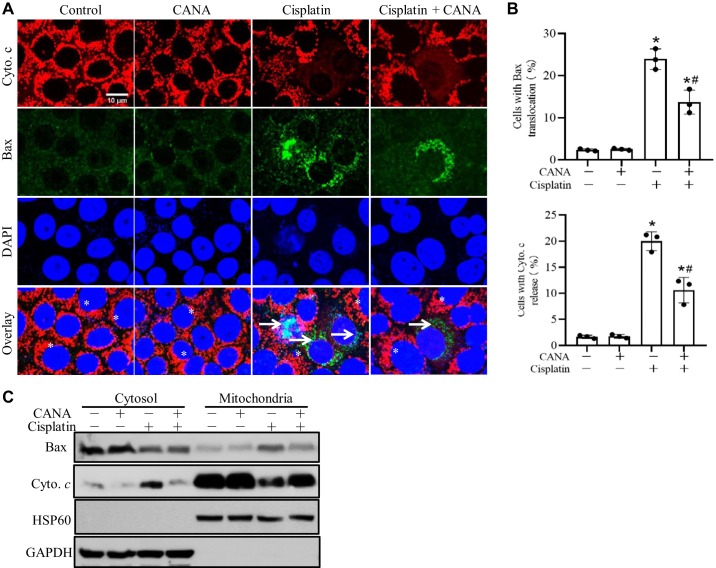
The mitochondrial pathway of apoptosis plays a indispensable role in cisplatin-induced nephrotoxicity (13, 14, 43), which is characterized by the translocation of Bax to mitochondria and the release cytochrome c into the cytosol. As shown in Fig. 4A, RPTCs without cisplatin treatment showed a diffuse cytosolic signal of Bax and mitochondrial signal of cytochrome c. After cisplatin treatment, some cells showed mitochondrial staining of Bax with cytochrome c released into cytosol. The counting of cells with Bax translocation and cytochrome c release indicated that canagliflozin substantially inhibited mitochondrial accumulation of Bax and cytochrome c release during cisplatin treatment (Fig. 4B). The immunoblots of Bax and cytochrome c in cytosolic and membrane fractions further confirmed the inhibitory effect of canagliflozin on Bax translocation and cytochrome c release during cisplatin treatment of RPTCs (Fig. 4C).
Fig. 4.
Canagliflozin (CANA) suppresses Bax translocation and cytochrome c (Cyto c) release during cisplatin treatment of rat kidney proximal tubular cells (RPTCs). RPTCs were incubated with 20 μM cisplatin in the absence or presence of 15 μM CANA for 12 h. Cells were fixed for dual immunofluorescence of Cyto c and Bax or fractioned for cytosolic and mitochondria-enriched membrane parts for immunoblot analysis. A: dual immunofluorescence of Cyto c and active Bax. *Cells with Bax in the cytosol and Cyto c in mitochondria. Arrows indicate cells with Bax in mitochondria and Cyto c released into the cytosol. Scale bar = 10 μm. B: quantification of cells with Bax translocation and Cyto c release. Percentages of cells with Bax and Cyto c translocation were calculated in 250–300 cells from 8 randomly selected areas for each slide. *P < 0.05 vs. the control group; #P < 0.05 vs. the cisplatin-treated group without CANA. C: representative immunoblots to show Bax translocation to mitochondria and Cyto c release to the cytosol. GAPDH and heat shock protein (HSP)60 were used as internal protein loading controls for cytosolic and membrane fractions, respectively. Immunoblots are representative of 3 independent experiments.
Canagliflozin partially reduces cisplatin uptake by the kidneys.
Because canagliflozin is a transporter inhibitor and cisplatin needs to get into cells to induce injury, we further tested whether canagliflozin affects cisplatin uptake in renal cells. To this end, we measured the content of platinum in mouse kidneys and RPTCs after cisplatin treatment. In the kidney, the platinum concentration rapidly increased at 1 day after cisplatin administration in mice (Fig. 5A), although no obvious histology abnormalities and renal function decline were observed (data not shown). The platinum concentration slightly decreased at 3 days due to cisplatin excretion. Canagliflozin reduced cisplatin accumulation in the kidney by ~15% and 16% at 1 and 3 days after cisplatin treatment, respectively (Fig. 5A). Consistent with the in vivo results, RPTCs had ~75 ng/mg platinum accumulation after 10 h of cisplatin treatment, and canagliflozin reduced the accumulation by~9% (Fig. 5B). To determine whether the reduced accumulation of cisplatin is responsible for the protective effect of canagliflozin, we compared apoptosis induced by cisplatin with canagliflozin to that induced by lower doses of cisplatin. When the cisplatin concentration was reduced to 17 μM (15% lower than 20 μM) and 15 μM (25% lower than 20 μM), there was still ~50% and ~46% apoptosis, which was marginally lower than the apoptosis (~55%) induced by 20 μM cisplatin (Fig. 5, C and D). In contrast, canagliflozin reduced the apoptosis to 35% apoptosis. Immunoblot analysis of caspase 3 activation further confirmed the cell morphology results (Fig. 5, E and F). Therefore, the reduction of platinum accumulation by canagliflozin can only partially account for its antiapoptotic effect in cisplatin nephrotoxicity.
Fig. 5.
Partial inhibitory effect of canagliflozin (CANA) on cisplatin uptake by kidney tissues and cells. A: platinum quantification in mouse kidneys. C57BL/6 mice with or without CANA treatment were euthanized at 1 day (1d) or 3 days (3d) after cisplatin treatment. Data are presented as means ± SD; n = 6. #P < 0.05 vs.the cisplatin only-treated group at the same time point. B: platinum quantification in rat kidney proximal tubular cells (RPTCs). RPTCs were incubated with 20 μM cisplatin in the absence or presence of CANA for 10 h. Values are means ± SD; n = 6. #P < 0.05 vs. the cisplatin-treated group. C: representative cell and nuclear images after Hoechst 33342 staining. Scale bar = 0.2 mm. D: percentage of apoptosis in RPTCs. E: representative immunoblots of cleaved caspase-3. GAPDH was used as an internal loading control. F: densitometric analysis of cleaved caspase 3 normalized to GAPDH. *P < 0.05 vs. the control group; #P < 0.05 vs. the 20 μM cisplatin-treated group; ▲P < 0.05 vs. the 17 μM cisplatin-treated group; ★P < 0.05 vs. the 15 μM cisplatin-treated group.
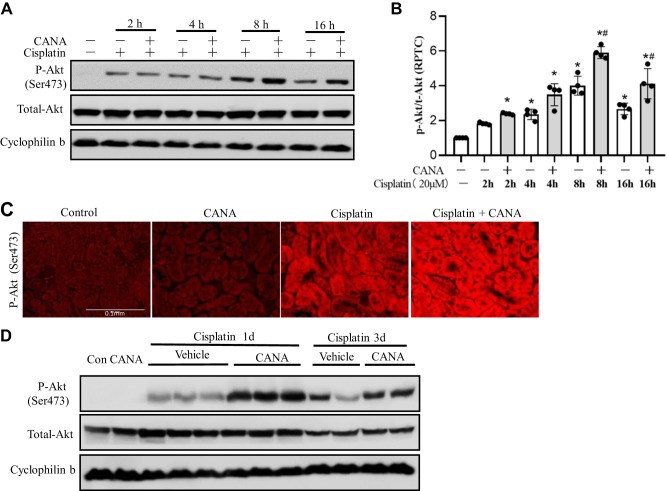
Canagliflozin increases Akt activation to prevent cisplatin-induced renal cell apoptosis.
In cisplatin nephrotoxicity, the phospatidylinositol 3-kinase (PI3K)/Akt signaling pathway is activated for cellular survival by suppressing the mitochondrial pathway of apoptosis (19, 21). To further explore the mechanism underlying the cytoprotective effect of canagliflozin, we investigated Akt activation. Akt activation was induced by cisplatin in RPTCs in a time-dependent manner, as shown by increased Akt phosphorylation at Ser473 (p-Ser473-Akt), and, interestingly, canagliflozin further increased p-Ser473-Akt during cisplatin treatment (Fig. 6A). Densitometry analysis confirmed that the enhancement of p-Ser473-Akt by canagliflozin started from 4 h, peaked at 8 h, and persisted to 16 h during cisplatin treatment (Fig. 6B). Consistently, in mouse kidneys, the positive cytosolic staining of p-Ser473-Akt was drastically enhanced in the cortex and outer medulla (mainly proximal tubules) at 24 h after the cisplatin injection, which was also further upregulated by canagliflozin (Fig. 6C). Immunoblot analysis confirmed that canagliflozin could further enhance renal p-Ser473-Akt at 1 and 3 days in kidney tissue lysates after cisplatin administration, whereas total Akt was not significantly affected (Fig. 6D).
Fig. 6.
Canagliflozin (CANA) increases AKT activation in cisplatin-induced renal injury. A and B: rat kidney proximal tubular cells (RPTCs) were incubated with 20 μM cisplatin in the absence or presence of 15 μM CANA for 0–16 h. Whole cell lysates were collected for immunoblot analysis of phosphorylated (p-)Akt (Ser473) and total Akt with cyclophilin B as an internal loading control. A: representative immunoblots. B: densitometric analysis normalized to cyclophilin B. Data are expressed as means ± SD; n = 4. *P < 0.05 vs. the control group; #P < 0.05 vs. the cisplatin-treated group without CANA at the same time point. C and D: C57BL/6 mice were administered with CANA (20 mg·kg−1·day−1) orally for 8 consecutive days, and intraperitoneal injection of 30 mg/kg cisplatin or saline was given on the seventh day of treatment. Mice were euthanized at 1 day (1d) or 3 days (3d) after cisplatin injection. C: representative immunofluorescence image of p-Akt (Ser473) at 1 day after cisplatin or saline treatment (scale bar = 0.1 mm). D: representative immunoblots of p-Akt (Ser473) and total Akt with cyclophilin B as an internal loading control.
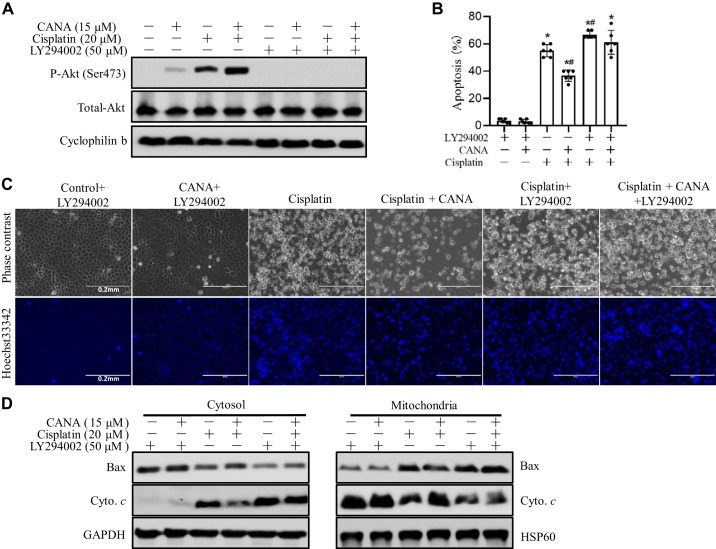
To further confirm the involvement of Akt activation in the protective effect of canagliflozin during cisplatin nephrotoxicity, LY294002 was used to inhibit the PI3K/Akt pathway. As expected, LY294002 blocked Akt activation (p-Ser473-Akt) during cisplatin treatment regardless of canagliflozin (Fig. 7A). Next, we examined cell morphology for apoptosis. As shown in Fig. 7, B and C, LY294002 reversed the cytoprotective effect of canagliflozin during cisplatin treatment of RPTCs. Because Akt may prevent the mitochondrial pathway of apoptosis (21), we further examined the effect on cisplatin-induced Bax translocation to mitochondria and cytochrome c release from mitochondria to the cytosol. As shown in Fig. 7D, canagliflozin suppressed Bax translocation and cytochrome c release during cisplatin treatment, and the suppressive effect of canagliflozin was reversed by LY294002. These results indicate that canagliflozin may protect kidney tubule cells during cisplatin nephrotoxicity at least partially by activating Akt.
Fig. 7.
LY294002 antagonizes the protective effect of canagliflozin (CANA) on cisplatin-induced apoptosis in rat kidney proximal tubular cells (RPTCs). RPTCs were treated with 20 μM cisplatin in the absence or presence of 15 μM CANA. LY294002 (50 μM) was used to inhibit Akt. A: representative immunoblots of phosphorylated Akt (Ser473) and total Akt with cyclophilin B as an internal loading control. B: percentage of apoptosis. *P < 0.05 vs. the control group; #P < 0.05 vs. the cisplatin only-treated group. C: representative images of cell and nuclear morphology after staining with Hoechst 33342. Scale bar = 0.2 mm. D: representative immunoblots of Bax and cytochrome c (Cyto c) in cytosol and mitochondrial fractions. Heat shock protein (HSP)60 and GAPDH were used as internal loading controls for mitochondrial and cytosol fractions, respectively.
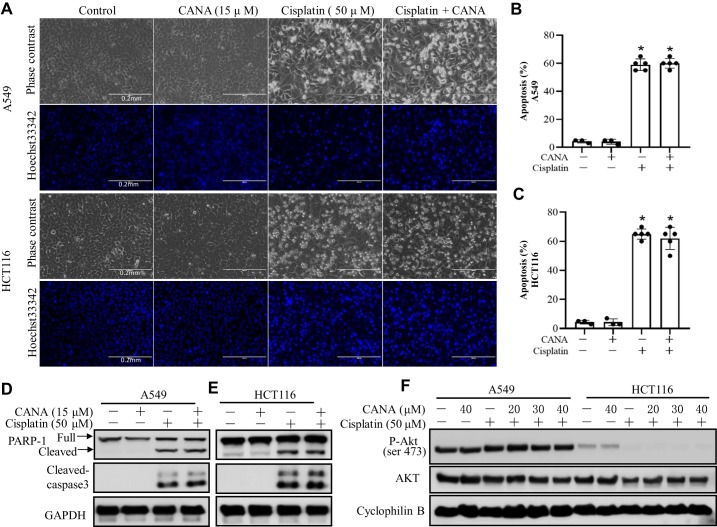
No obvious effect of canagliflozin on cisplatin-induced apoptosis in cancer cells.
To determine whether canagliflozin affects the antitumor efficacy of cisplatin, we examined the effects on the A549 non-small cell lung cancer cell line and HCT116 colon cancer cell line. A549 and HCT116 cancer cells were treated with 50 μM cisplatin in the absence or presence of canagliflozin (15 μM) for 24 h. As shown in Fig. 8, A–C, cisplatin induced ~60% apoptosis in both cell lines. In A549 cells, canagliflozin slightly increased apoptosis during cisplatin treatment, although without statistical significance. Consistently, canagliflozin did not affect cisplatin-induced apoptosis in HCT116 cells. Immunoblot analysis of PARP-1 and cleaved caspase 3 also confirmed that canagliflozin did not suppress the anticancer effect of cisplatin.
Fig. 8.
Canagliflozin (CANA) does not affect cisplatin-induced apoptosis in A549 and HCT116 cancer cells. A549 and HCT116 cancer cells were treated with 50 μM cisplatin in the absence or presence of 15 μM CANA for 24 h. A: representative images of cell and nuclear morphology after staining with Hoechst 33342. Scale bar = 0.2 mm. B: percentages of apoptosis in A549 cells. C: percentages of apoptosis in HCT116 cells. Values are means ± SD; n = 6. *P < 0.05 vs. the respective control group; #P < 0.05 vs. the cisplatin-treated group. D and E: representative immunoblots of cleaved caspase-3 and poly(ADP-ribose) polymerase-1 (PARP-1) in A539 cells (D) and HCT116 cells (E). GAPDH was used as an internal loading control. F: representative immunoblots of phosphorylated (p-)Akt (Ser473) and AKT in A539 and HCT116 cells. Cyclophilin B was used as internal loading control.
DISCUSSION
SGLT2 inhibitors are a novel class of blood glucose-lowering agents that reduce glucose reabsorption via SGLT2 in kidney proximal tubules. Emerging evidence from large randomized control trials has indicated that SGLT2 inhibitors also have renal beneficial effects beyond their blood glucose control effect (25, 40, 57). Indeed, SGLT2 inhibition promotes natriuresis that regulates renal and systemic hemodynamic functions, including lowering blood pressure and glomerular hyperfiltration and plasma volume, and proteinuria (9, 25, 42). The pleiotropic effects of SGLT2 inhibitors suggest that they may also be therapeutics for nondiabetic kidney diseases, although their renal beneficial effect is controversial.
In SGLT2 inhibitors, dapagliflozin has been reported to protect against ischemia-reperfusion-induced kidney injury (7). In mice with protein-overload proteinuria, dapagliflozin can reduce proteinuria and glomerular lesions (6). In addition, empagliflozin attenuates renal fibrosis in rats exposed to unilateral ureteric obstruction (1), and luseogliflozin prevents renal fibrosis after renal ischemia-reperfusion injury by attenuating renal capillary injury (55). However, there are also studies that have shown no protective effect of SGLT2 inhibitors in kidney injury models, such as luseogliflozin in renal ischemia-reperfusion injury (55), empagliflozin in oxalate-induced nephrocalcinosis (23), and dapagliflozin in the 5/6 (subtotally) nephrectomized rat model (56). Canagliflozin is the first SGLT2 inhibitor approved by the Federal Drug Administration, and it has shown clear clinical renal and cardiovascular protection in patients with type 2 diabetes in large clinical trials (25, 30). One study reported the protective effect of canagliflozin on cisplatin nephrotoxicity (2), but the underlying mechanism is unclear, and its effect on the anticancer efficacy of cisplatin is unknown. In the present study, we demonstrated that canagliflozin significantly ameliorated cisplatin-induced nephrotoxicity in both in vitro and in vivo models. Mechanistically, we have shown that canagliflozin may protect kidney cells by reducing cisplatin uptake and by enhancing Akt activation. Furthermore, canagliflozin did not antagonize cisplatin-induced apoptosis in cancer cells, indicating its potential as a new renoprotective approach during cisplatin-based cancer therapy.
Previous studies from our and other laboratories have shown that cisplatin nephrotoxicity is mainly caused by the uptake of cisplatin from the basolateral transporters into renal proximal tubular cells (28, 51). Copper transporter 1 and organic cation transporter 2 are two important transporters for cisplatin located on the basolateral side. After cisplatin uptake, the subsequent efflux of cisplatin into the renal lumen is mediated by multidrug and toxin extrusion protein 1 (24). However, it is unknown whether cisplatin can be reabsorbed by the proximal tubule from the lumen and how this reabsorption will affect nephrotoxicity. A recent study has shown that megalin, a large glycoprotein at the apical membranes of proximal tubule epithelial cells, could mediate the uptake of cisplatin (10), and inhibition of megalin by cilastatin can significantly limit cisplatin nephrotoxicity. In addition to the well-known role of SGLT2 in mediating the uptake of Na+ and glucose, whether other substances can be reabsorbed through SGLT2 is unknown. Interestingly, it has been reported that SGLT2 may be involved in the cellular uptake of gentamicin and can exacerbate gentamicin-induced cytotoxicity (15). Hence, we investigated whether canagliflozin can mediate cisplatin reabsorption in proximal tubular cells (Fig. 5). We found that cisplatin retention rapidly increased at 24 h in the kidney and decreased slightly 72 h after cisplatin administration. Canagliflozin reduced the renal platinum concentration by 15% and 16% at 24 and 72 h after cisplatin treatment, respectively. Consistent with this in vivo result, canagliflozin reduced platinum accumulation in RPTCs by ~9% after 10 h of cisplatin treatment. The exact mechanism responsible for the reduction of cisplatin accumulation in kidney tissues and cells is currently unknown. One possibility is that cisplatin may compete with glucose to be reabsorbed through SGLT2, as an earlier study showed that cisplatin decreased the affinity of d-glucose to its transport carriers (48). Another possibility is that canagliflozin may directly function on organic cation transporter 2 or copper transporter 1 to inhibit cisplatin uptake. In addition, canagliflozin may indirectly affect cisplatin reabsorption by increasing urine volume, which may happen in vivo.
While the reduction of cisplatin uptake in kidney cells contributes to the renal protective effect of canagliflozin, it cannot fully account for the effect (Fig. 5, C–F). In our study, canagliflozin inhibited p53 and MAPK signaling pathways (Fig. 3) and led to mitochondrial protection and cell death suppression (Fig. 4). We further tried to explore the upstream protective mechanisms of canagliflozin. Previous studies have shown that autophagy activation in the kidney can ameliorate cisplatin injury by affecting p53 and JNK activation (16, 29, 49). Canagliflozin may activate autophagy through the regulation of glucose metabolism (47). However, we did not detect obvious effect of canagliflozin on autophagy during cisplatin treatment of RPTCs (not shown). Furthermore, recent studies have demonstrated that the PI3K/Akt pathway activation can suppress renal cell apoptosis, lessen cisplatin-induced acute kidney injury, and play a key role in the maintenance of renal function (19, 21). Akt activation by canagliflozin has been reported in myocardial ischemia-reperfusion injury (33). Consistent with previous reports (19, 21), we detected Akt activation during cisplatin-induced kidney injury, primarily in proximal tubules. Importantly, canagliflozin could further enhance Akt activation during cisplatin treatment both in vivo and in vitro (Fig. 6). Enhanced Akt activation was also associated with reduced Bax translocation to mitochondria and cytochrome c release during cisplatin treatment. The PI3K/Akt inhibitor LY294002 suppressed Akt phosphorylation and totally abrogated the cytoprotective effect by canagliflozin (Fig. 7). Therefore, Akt activation by canagliflozin may be the major mechanism for its renoprotective effect during cisplatin treatment.
To use canagliflozin to protect kidney clinically, it is important to determine whether it affects the chemotherapy efficacy of cisplatin in tumors. Interestingly, some previous studies have indicated that SGLT2 promotes tumorigenesis in pancreatic, prostate, colon and lung cancer (11, 32, 34, 54). Hence, SGLT2 inhibitors may be beneficial for kidney as well as cancer treatment. When we treated C57BL/6 mice with canagliflozin and cisplatin, our data indicated that canagliflozin did not change the body weight decline after cisplatin administration (data not shown), while it ameliorated kidney injury. Notably, canagliflozin did not diminish cisplatin-induced apoptosis in the HCT116 colon cancer cell line and A549 non-small cell lung cancer cell line (Fig. 8), indicating the potential clinical use of canagliflozin for renal protection during cisplatin-mediated chemotherapy.
In conclusion, our study provides evidence of the renoprotective effect of canagliflozin in cisplatin nephrotoxicity. Mechanistically, the renal protection of canagliflozin mainly depends on Akt activation as well as reduced uptake of cisplatin in the kidneys. In contrast, canagliflozin does not diminish the chemotherapeutic effect of cisplatin in cancer cells, suggesting a novel strategy to use SGLT2 inhibitors for renoprotection in cisplatin-based cancer therapy.
GRANTS
This work was supported in part by National Natural Science Foundation of China Grant 81600567, Department of Veterans Affairs Merit Review Award I01 BX000319, and National Institute of Diabetes and Digestive and Kidney Diseases Grants DK-058831 and DK-087843. Z. Dong is a recipient of a Senior Research Career Scientist award from the Department of Veterans Affairs.
DISCLOSURES
No conflicts of interest, financial or otherwise, are declared by the authors.
AUTHOR CONTRIBUTIONS
Z.S., J.Z., and Z.D. conceived and designed research; Z.S. and J.Z. performed experiments; Z.S., J.Z., and Z.D. analyzed data; Z.S., J.Z., Q.W., G.D., and Z.D. interpreted results of experiments; Z.S. and J.Z. prepared figures; Z.S. and J.Z. drafted manuscript; Z.S., J.Z., Q.W., G.D., and Z.D. edited and revised manuscript; Z.S., J.Z., Q.W., G.D., and Z.D. approved final version of manuscript.
REFERENCES
- 1.Abbas NAT, El Salem A, Awad MM. Empagliflozin, SGLT2 inhibitor, attenuates renal fibrosis in rats exposed to unilateral ureteric obstruction: potential role of klotho expression. Naunyn Schmiedebergs Arch Pharmacol 391: 1347–1360, 2018. doi: 10.1007/s00210-018-1544-y. [DOI] [PubMed] [Google Scholar]
- 2.Abdelrahman AM, Al Suleimani Y, Shalaby A, Ashique M, Manoj P, Nemmar A, Ali BH. Effect of canagliflozin, a sodium glucose co-transporter 2 inhibitor, on cisplatin-induced nephrotoxicity in mice. Naunyn Schmiedebergs Arch Pharmacol 392: 45–53, 2019. doi: 10.1007/s00210-018-1564-7. [DOI] [PubMed] [Google Scholar]
- 3.Arany I, Megyesi JK, Kaneto H, Price PM, Safirstein RL. Cisplatin-induced cell death is EGFR/src/ERK signaling dependent in mouse proximal tubule cells. Am J Physiol Renal Physiol 287: F543–F549, 2004. doi: 10.1152/ajprenal.00112.2004. [DOI] [PubMed] [Google Scholar]
- 4.Arany I, Safirstein RL. Cisplatin nephrotoxicity. Semin Nephrol 23: 460–464, 2003. doi: 10.1016/S0270-9295(03)00089-5. [DOI] [PubMed] [Google Scholar]
- 5.Brooks C, Wei Q, Cho SG, Dong Z. Regulation of mitochondrial dynamics in acute kidney injury in cell culture and rodent models. J Clin Invest 119: 1275–1285, 2009. doi: 10.1172/JCI37829. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Cassis P, Locatelli M, Cerullo D, Corna D, Buelli S, Zanchi C, Villa S, Morigi M, Remuzzi G, Benigni A, Zoja C. SGLT2 inhibitor dapagliflozin limits podocyte damage in proteinuric nondiabetic nephropathy. JCI Insight 3: e98720, 2018. doi: 10.1172/jci.insight.98720. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Chang YK, Choi H, Jeong JY, Na KR, Lee KW, Lim BJ, Choi DE. Dapagliflozin, SGLT2 inhibitor, attenuates renal ischemia-reperfusion injury. PLoS One 11: e0158810, 2016. [Erratum in PLoS One 11: e160478, 2016.] doi: 10.1371/journal.pone.0158810. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Heerspink HJL, Perco P, Mulder S, Leierer J, Hansen MK, Heinzel A, Mayer G. Canagliflozin reduces inflammation and fibrosis biomarkers: a potential mechanism of action for beneficial effects of SGLT2 inhibitors in diabetic kidney disease. Diabetologia 62: 1154–1166, 2019. doi: 10.1007/s00125-019-4859-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Hocher B, Tsuprykov O. Diabetic nephropathy: renoprotective effects of GLP1R agonists and SGLT2 inhibitors. Nat Rev Nephrol 13: 728–730, 2017. doi: 10.1038/nrneph.2017.140. [DOI] [PubMed] [Google Scholar]
- 10.Hori Y, Aoki N, Kuwahara S, Hosojima M, Kaseda R, Goto S, Iida T, De S, Kabasawa H, Kaneko R, Aoki H, Tanabe Y, Kagamu H, Narita I, Kikuchi T, Saito A. Megalin blockade with cilastatin suppresses drug-induced nephrotoxicity. J Am Soc Nephrol 28: 1783–1791, 2017. doi: 10.1681/ASN.2016060606. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Ishikawa N, Oguri T, Isobe T, Fujitaka K, Kohno N. SGLT gene expression in primary lung cancers and their metastatic lesions. Jpn J Cancer Res 92: 874–879, 2001. doi: 10.1111/j.1349-7006.2001.tb01175.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Jiang M, Dong Z. Regulation and pathological role of p53 in cisplatin nephrotoxicity. J Pharmacol Exp Ther 327: 300–307, 2008. doi: 10.1124/jpet.108.139162. [DOI] [PubMed] [Google Scholar]
- 13.Jiang M, Pabla N, Murphy RF, Yang T, Yin XM, Degenhardt K, White E, Dong Z. Nutlin-3 protects kidney cells during cisplatin therapy by suppressing Bax/Bak activation. J Biol Chem 282: 2636–2645, 2007. doi: 10.1074/jbc.M606928200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Jiang M, Wang CY, Huang S, Yang T, Dong Z. Cisplatin-induced apoptosis in p53-deficient renal cells via the intrinsic mitochondrial pathway. Am J Physiol Renal Physiol 296: F983–F993, 2009. doi: 10.1152/ajprenal.90579.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Jiang M, Wang Q, Karasawa T, Koo JW, Li H, Steyger PS. Sodium-glucose transporter-2 (SGLT2; SLC5A2) enhances cellular uptake of aminoglycosides. PLoS One 9: e108941, 2014. doi: 10.1371/journal.pone.0108941. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Jiang M, Wei Q, Dong G, Komatsu M, Su Y, Dong Z. Autophagy in proximal tubules protects against acute kidney injury. Kidney Int 82: 1271–1283, 2012. doi: 10.1038/ki.2012.261. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Jiang M, Wei Q, Wang J, Du Q, Yu J, Zhang L, Dong Z. Regulation of PUMA-alpha by p53 in cisplatin-induced renal cell apoptosis. Oncogene 25: 4056–4066, 2006. doi: 10.1038/sj.onc.1209440. [DOI] [PubMed] [Google Scholar]
- 18.Jiang M, Yi X, Hsu S, Wang CY, Dong Z. Role of p53 in cisplatin-induced tubular cell apoptosis: dependence on p53 transcriptional activity. Am J Physiol Renal Physiol 287: F1140–F1147, 2004. doi: 10.1152/ajprenal.00262.2004. [DOI] [PubMed] [Google Scholar]
- 19.Kaushal GP, Kaushal V, Hong X, Shah SV. Role and regulation of activation of caspases in cisplatin-induced injury to renal tubular epithelial cells. Kidney Int 60: 1726–1736, 2001. doi: 10.1046/j.1523-1755.2001.00026.x. [DOI] [PubMed] [Google Scholar]
- 20.Kimura Y, Kuno A, Tanno M, Sato T, Ohno K, Shibata S, Nakata K, Sugawara H, Abe K, Igaki Y, Yano T, Miki T, Miura T. Canagliflozin, a sodium-glucose cotransporter 2 inhibitor, normalizes renal susceptibility to type 1 cardiorenal syndrome through reduction of renal oxidative stress in diabetic rats. J Diabetes Investig 10: 933–946, 2019. doi: 10.1111/jdi.13009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Kuwana H, Terada Y, Kobayashi T, Okado T, Penninger JM, Irie-Sasaki J, Sasaki T, Sasaki S. The phosphoinositide-3 kinase gamma-Akt pathway mediates renal tubular injury in cisplatin nephrotoxicity. Kidney Int 73: 430–445, 2008. doi: 10.1038/sj.ki.5002702. [DOI] [PubMed] [Google Scholar]
- 22.Li Z, Xu K, Zhang N, Amador G, Wang Y, Zhao S, Li L, Qiu Y, Wang Z. Overexpressed SIRT6 attenuates cisplatin-induced acute kidney injury by inhibiting ERK1/2 signaling. Kidney Int 93: 881–892, 2018. doi: 10.1016/j.kint.2017.10.021. [DOI] [PubMed] [Google Scholar]
- 23.Ma Q, Steiger S, Anders HJ. Sodium glucose transporter-2 inhibition has no renoprotective effects on non-diabetic chronic kidney disease. Physiol Rep 5: e13228, 2017. doi: 10.14814/phy2.13228. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Nakamura T, Yonezawa A, Hashimoto S, Katsura T, Inui K. Disruption of multidrug and toxin extrusion MATE1 potentiates cisplatin-induced nephrotoxicity. Biochem Pharmacol 80: 1762–1767, 2010. doi: 10.1016/j.bcp.2010.08.019. [DOI] [PubMed] [Google Scholar]
- 25.Neal B, Perkovic V, Mahaffey KW, de Zeeuw D, Fulcher G, Erondu N, Shaw W, Law G, Desai M, Matthews DR; CANVAS Program Collaborative Group . Canagliflozin and cardiovascular and renal events in type 2 diabetes. N Engl J Med 377: 644–657, 2017. doi: 10.1056/NEJMoa1611925. [DOI] [PubMed] [Google Scholar]
- 26.Pabla N, Dong G, Jiang M, Huang S, Kumar MV, Messing RO, Dong Z. Inhibition of PKCδ reduces cisplatin-induced nephrotoxicity without blocking chemotherapeutic efficacy in mouse models of cancer. J Clin Invest 121: 2709–2722, 2011. doi: 10.1172/JCI45586. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Pabla N, Dong Z. Cisplatin nephrotoxicity: mechanisms and renoprotective strategies. Kidney Int 73: 994–1007, 2008. doi: 10.1038/sj.ki.5002786. [DOI] [PubMed] [Google Scholar]
- 28.Pabla N, Murphy RF, Liu K, Dong Z. The copper transporter Ctr1 contributes to cisplatin uptake by renal tubular cells during cisplatin nephrotoxicity. Am J Physiol Renal Physiol 296: F505–F511, 2009. doi: 10.1152/ajprenal.90545.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Periyasamy-Thandavan S, Jiang M, Wei Q, Smith R, Yin XM, Dong Z. Autophagy is cytoprotective during cisplatin injury of renal proximal tubular cells. Kidney Int 74: 631–640, 2008. doi: 10.1038/ki.2008.214. [DOI] [PubMed] [Google Scholar]
- 30.Perkovic V, Jardine MJ, Neal B, Bompoint S, Heerspink HJL, Charytan DM, Edwards R, Agarwal R, Bakris G, Bull S, Cannon CP, Capuano G, Chu PL, de Zeeuw D, Greene T, Levin A, Pollock C, Wheeler DC, Yavin Y, Zhang H, Zinman B, Meininger G, Brenner BM, Mahaffey KW; CREDENCE Trial Investigators . Canagliflozin and renal outcomes in type 2 diabetes and nephropathy. N Engl J Med 380: 2295–2306, 2019. doi: 10.1056/NEJMoa1811744. [DOI] [PubMed] [Google Scholar]
- 31.Ramesh G, Reeves WB. p38 MAP kinase inhibition ameliorates cisplatin nephrotoxicity in mice. Am J Physiol Renal Physiol 289: F166–F174, 2005. doi: 10.1152/ajprenal.00401.2004. [DOI] [PubMed] [Google Scholar]
- 32.Saito T, Okada S, Yamada E, Shimoda Y, Osaki A, Tagaya Y, Shibusawa R, Okada J, Yamada M. Effect of dapagliflozin on colon cancer cell [Rapid Communication]. Endocr J 62: 1133–1137, 2015. doi: 10.1507/endocrj.EJ15-0396. [DOI] [PubMed] [Google Scholar]
- 33.Sayour AA, Korkmaz-Icöz S, Loganathan S, Ruppert M, Sayour VN, Oláh A, Benke K, Brune M, Benkő R, Horváth EM, Karck M, Merkely B, Radovits T, Szabó G. Acute canagliflozin treatment protects against in vivo myocardial ischemia-reperfusion injury in non-diabetic male rats and enhances endothelium-dependent vasorelaxation. J Transl Med 17: 127, 2019. doi: 10.1186/s12967-019-1881-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Scafoglio C, Hirayama BA, Kepe V, Liu J, Ghezzi C, Satyamurthy N, Moatamed NA, Huang J, Koepsell H, Barrio JR, Wright EM. Functional expression of sodium-glucose transporters in cancer. Proc Natl Acad Sci USA 112: E4111–E4119, 2015. doi: 10.1073/pnas.1511698112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Shiba K, Tsuchiya K, Komiya C, Miyachi Y, Mori K, Shimazu N, Yamaguchi S, Ogasawara N, Katoh M, Itoh M, Suganami T, Ogawa Y. Canagliflozin, an SGLT2 inhibitor, attenuates the development of hepatocellular carcinoma in a mouse model of human NASH. Sci Rep 8: 2362, 2018. doi: 10.1038/s41598-018-19658-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Shibusawa R, Yamada E, Okada S, Nakajima Y, Bastie CC, Maeshima A, Kaira K, Yamada M. Dapagliflozin rescues endoplasmic reticulum stress-mediated cell death. Sci Rep 9: 9887, 2019. doi: 10.1038/s41598-019-46402-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Sun L, Liu J, Yuan Y, Zhang X, Dong Z. Protective effect of the BET protein inhibitor JQ1 in cisplatin-induced nephrotoxicity. Am J Physiol Renal Physiol 315: F469–F478, 2018. doi: 10.1152/ajprenal.00527.2017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Wang D, Lippard SJ. Cellular processing of platinum anticancer drugs. Nat Rev Drug Discov 4: 307–320, 2005. doi: 10.1038/nrd1691. [DOI] [PubMed] [Google Scholar]
- 39.Wang S, Wei Q, Dong G, Dong Z. ERK-mediated suppression of cilia in cisplatin-induced tubular cell apoptosis and acute kidney injury. Biochim Biophys Acta 1832: 1582–1590, 2013. doi: 10.1016/j.bbadis.2013.05.023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Wanner C, Inzucchi SE, Lachin JM, Fitchett D, von Eynatten M, Mattheus M, Johansen OE, Woerle HJ, Broedl UC, Zinman B; EMPA-REG OUTCOME Investigators . Empagliflozin and progression of kidney disease in type 2 diabetes. N Engl J Med 375: 323–334, 2016. doi: 10.1056/NEJMoa1515920. [DOI] [PubMed] [Google Scholar]
- 42.Warren AM, Knudsen ST, Cooper ME. Diabetic nephropathy: an insight into molecular mechanisms and emerging therapies. Expert Opin Ther Targets 23: 579–591, 2019. doi: 10.1080/14728222.2019.1624721. [DOI] [PubMed] [Google Scholar]
- 43.Wei Q, Dong G, Franklin J, Dong Z. The pathological role of Bax in cisplatin nephrotoxicity. Kidney Int 72: 53–62, 2007. doi: 10.1038/sj.ki.5002256. [DOI] [PubMed] [Google Scholar]
- 44.Wei Q, Dong G, Yang T, Megyesi J, Price PM, Dong Z. Activation and involvement of p53 in cisplatin-induced nephrotoxicity. Am J Physiol Renal Physiol 293: F1282–F1291, 2007. doi: 10.1152/ajprenal.00230.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Wei Q, Sun H, Song S, Liu Y, Liu P, Livingston MJ, Wang J, Liang M, Mi QS, Huo Y, Nahman NS, Mei C, Dong Z. MicroRNA-668 represses MTP18 to preserve mitochondrial dynamics in ischemic acute kidney injury. J Clin Invest 128: 5448–5464, 2018. doi: 10.1172/JCI121859. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Woost PG, Orosz DE, Jin W, Frisa PS, Jacobberger JW, Douglas JG, Hopfer U. Immortalization and characterization of proximal tubule cells derived from kidneys of spontaneously hypertensive and normotensive rats. Kidney Int 50: 125–134, 1996. doi: 10.1038/ki.1996.295. [DOI] [PubMed] [Google Scholar]
- 47.Xu C, Wang W, Zhong J, Lei F, Xu N, Zhang Y, Xie W. Canagliflozin exerts anti-inflammatory effects by inhibiting intracellular glucose metabolism and promoting autophagy in immune cells. Biochem Pharmacol 152: 45–59, 2018. doi: 10.1016/j.bcp.2018.03.013. [DOI] [PubMed] [Google Scholar]
- 48.Yanase M, Uyama O, Nakanishi T, Shiratsuki N, Sugita M. Decreased sodium dependent D-glucose transport across renal brush-border membranes in cis-diamminedichloride platinum induced acute renal failure. Ren Fail 14: 23–30, 1992. doi: 10.3109/08860229209039113. [DOI] [PubMed] [Google Scholar]
- 49.Yang C, Kaushal V, Shah SV, Kaushal GP. Autophagy is associated with apoptosis in cisplatin injury to renal tubular epithelial cells. Am J Physiol Renal Physiol 294: F777–F787, 2008. doi: 10.1152/ajprenal.00590.2007. [DOI] [PubMed] [Google Scholar]
- 50.Yano T, Itoh Y, Matsuo M, Kawashiri T, Egashira N, Oishi R. Involvement of both tumor necrosis factor-alpha-induced necrosis and p53-mediated caspase-dependent apoptosis in nephrotoxicity of cisplatin. Apoptosis 12: 1901–1909, 2007. doi: 10.1007/s10495-007-0110-8. [DOI] [PubMed] [Google Scholar]
- 51.Yokoo S, Yonezawa A, Masuda S, Fukatsu A, Katsura T, Inui K. Differential contribution of organic cation transporters, OCT2 and MATE1, in platinum agent-induced nephrotoxicity. Biochem Pharmacol 74: 477–487, 2007. doi: 10.1016/j.bcp.2007.03.004. [DOI] [PubMed] [Google Scholar]
- 52.Zhang B, Ramesh G, Uematsu S, Akira S, Reeves WB. TLR4 signaling mediates inflammation and tissue injury in nephrotoxicity. J Am Soc Nephrol 19: 923–932, 2008. doi: 10.1681/ASN.2007090982. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Zhang D, Liu Y, Wei Q, Huo Y, Liu K, Liu F, Dong Z. Tubular p53 regulates multiple genes to mediate AKI. J Am Soc Nephrol 25: 2278–2289, 2014. doi: 10.1681/ASN.2013080902. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Zhang X, Zhang X, Liu X, Qi P, Wang H, Ma Z, Chai Y. MicroRNA-296, a suppressor non-coding RNA, downregulates SGLT2 expression in lung cancer. Int J Oncol 54: 199–208, 2019. doi: 10.3892/ijo.2018.4599. [DOI] [PubMed] [Google Scholar]
- 55.Zhang Y, Nakano D, Guan Y, Hitomi H, Uemura A, Masaki T, Kobara H, Sugaya T, Nishiyama A. A sodium-glucose cotransporter 2 inhibitor attenuates renal capillary injury and fibrosis by a vascular endothelial growth factor-dependent pathway after renal injury in mice. Kidney Int 94: 524–535, 2018. doi: 10.1016/j.kint.2018.05.002. [DOI] [PubMed] [Google Scholar]
- 56.Zhang Y, Thai K, Kepecs DM, Gilbert RE. Sodium-glucose linked cotransporter-2 inhibition does not attenuate disease progression in the rat remnant kidney model of chronic kidney disease. PLoS One 11: e0144640, 2016. doi: 10.1371/journal.pone.0144640. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Zinman B, Wanner C, Lachin JM, Fitchett D, Bluhmki E, Hantel S, Mattheus M, Devins T, Johansen OE, Woerle HJ, Broedl UC, Inzucchi SE; EMPA-REG OUTCOME Investigators . Empagliflozin, cardiovascular outcomes, and mortality in type 2 diabetes. N Engl J Med 373: 2117–2128, 2015. doi: 10.1056/NEJMoa1504720. [DOI] [PubMed] [Google Scholar]